Abstract
Members of the highly conserved pleiotropic CK1 family of serine/threonine-specific kinases are tightly regulated in the cell and play crucial regulatory roles in multiple cellular processes from protozoa to human. Since their dysregulation as well as mutations within their coding regions contribute to the development of various different pathologies, including cancer and neurodegenerative diseases, they have become interesting new drug targets within the last decade. However, to develop optimized CK1 isoform-specific therapeutics in personalized therapy concepts, a detailed knowledge of the regulation and functions of the different CK1 isoforms, their various splice variants and orthologs is mandatory. In this review we will focus on the stress-induced CK1 isoform delta (CK1δ), thereby addressing its regulation, physiological functions, the consequences of its deregulation for the development and progression of diseases, and its potential as therapeutic drug target.
Keywords: Casein kinase 1, CSNK1D, Phosphorylation, S, Small molecule inhibitor, R, P, Site-specific phosphorylation, Stress-induced kinase, p53, Wnt signaling pathway, Hedgehog pathway, Cancer, N
1. Introduction
CK1δ, a member of the CK1 (formerly named casein kinase 1) family, has been first isolated by Graves and colleagues in the early 1990s. The human gene encoding CK1δ (CSNK1D) is located on the long arm of the chromosome 17 (17q25.3) (Graves et al., 1993). In the last decades the roles of CK1δ have been characterized more and more, both in physiological and in pathologic conditions. In fact, dysregulation of the expression and activity of CK1δ has been observed in different types of cancers, as well as in different neurological disorders, among them Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Amyotrophic Lateral Sclerosis (ALS). CK1 isoforms δ and ε exhibit high identity within their kinase domains but no differences of their functional roles were detected in several early studies. Even though it still remains challenging to assign distinct functions to one of the human CK1 isoforms (α, γ1–3, δ, ε), new studies are now focusing on this issue. Considering the fact that even CK1δ transcription variants (TVs) exhibit different functions, there is a need to clearly state, which isoform or even TV has been used to receive detailed information and to increase reproducibility of the data by other researchers (Fustin et al., 2018; Narasimamurthy et al., 2018). From this point of view there is a high demand to develop highly specific, isoform- or even TV-specific therapeutics which could be of use in personalized therapy concepts for the treatment of neurodegenerative diseases and cancer. The present review will focus on CK1δ, its regulation, functions, its relevance in human diseases, and as a target for drug development.
2. Genetic coding of CK1δ
Historically, the initial gene sequence of CK1δ was isolated by Graves et al. in form of cDNA from the testicles of rats in the year 1993. The gene construct was sequenced and characterized as a 1284 nucleotide sequence transcribing into a 49 kDa protein consisting of 428 amino acids (aa) (Graves et al., 1993). This sequence was followed by the human gene construct with 1245 nucleotides describing a protein containing 415 aa (Kusuda et al., 1996). In the following years CK1δ was discovered and described in yeasts, various animals, plants, and even parasites (Barik et al., 1997; Donald et al., 2005; Allocco et al., 2006; Urbaniak, 2009; Rachidi et al., 2014; Dorin-Semblat et al., 2015) (Table 1).
Table 1.
Chronological overview of NCBI data bank entries of CK1δ in various organisms. The list includes the species, the name of the protein, the GI number, the amount of amino acids, and the reference, where the sequence was first published. Aa, amino acid; TV, transcription variant.
| Species | Name | GI-number | aa | Reference |
|---|---|---|---|---|
| Saccharomyces cerevisiae JAY291 | HRR25 | 256272813 | 494 | (DeMaggio et al., 1992) |
| Rattus norvegicus | CK1δ | 294525 | 428 | (Graves et al., 1993) |
| Caenorhabditis elegans | CK1δ | 379657083 | 315 | (Consortium, 1998) |
| Drosophila melanogaster | CK1 homolog | 3335146 | 440 | (Kloss et al., 1998) |
| Rattus norvegicus | CK1δ | 14422451 | 415 | (Takano and Nagai, 2001) |
| Homo sapiens | CK1δ TV1 | 13097702 | 415 | (Strausberg et al., 2002) |
| Homo sapiens | CK1δ TV2 | 16041786 | 409 | (Strausberg et al., 2002) |
| Mus musculus | CK1δ CRA a | 148702868 | 415 | (Mural et al., 2002) |
| Mus musculus | CK1δ CRA b | 148702869 | 428 | (Mural et al., 2002) |
| Mus musculus | CK1δ CRA c | 148702870 | 409 | (Mural et al., 2002) |
| Xenopus laevis | CK1δ | 148229581 | 415 | (Klein et al., 2002) |
| Danio rerio | CK1δa | 157280839 | 403 | (Albornoz et al., 2007) |
| Danio rerio | CK1δb | 157280837 | 409 | (Albornoz et al., 2007) |
| Homo sapiens | CK1δ TV3 | 1393428169 | 427 | (Huang et al., 2018) |
Although there are various variants of CK1δ in different organisms, three different TVs of CK1δ are present in humans, in rat (Rattus norvegicus) and in mice (Mus musculus).
All three different CK1δ TVs expressed in human, rat, and mouse do not differ in their amino acid sequences until position 381. After position 399 three distinct sequences for the respective TVs can clearly be distinguished as illustrated in Fig. 1.
Fig. 1.
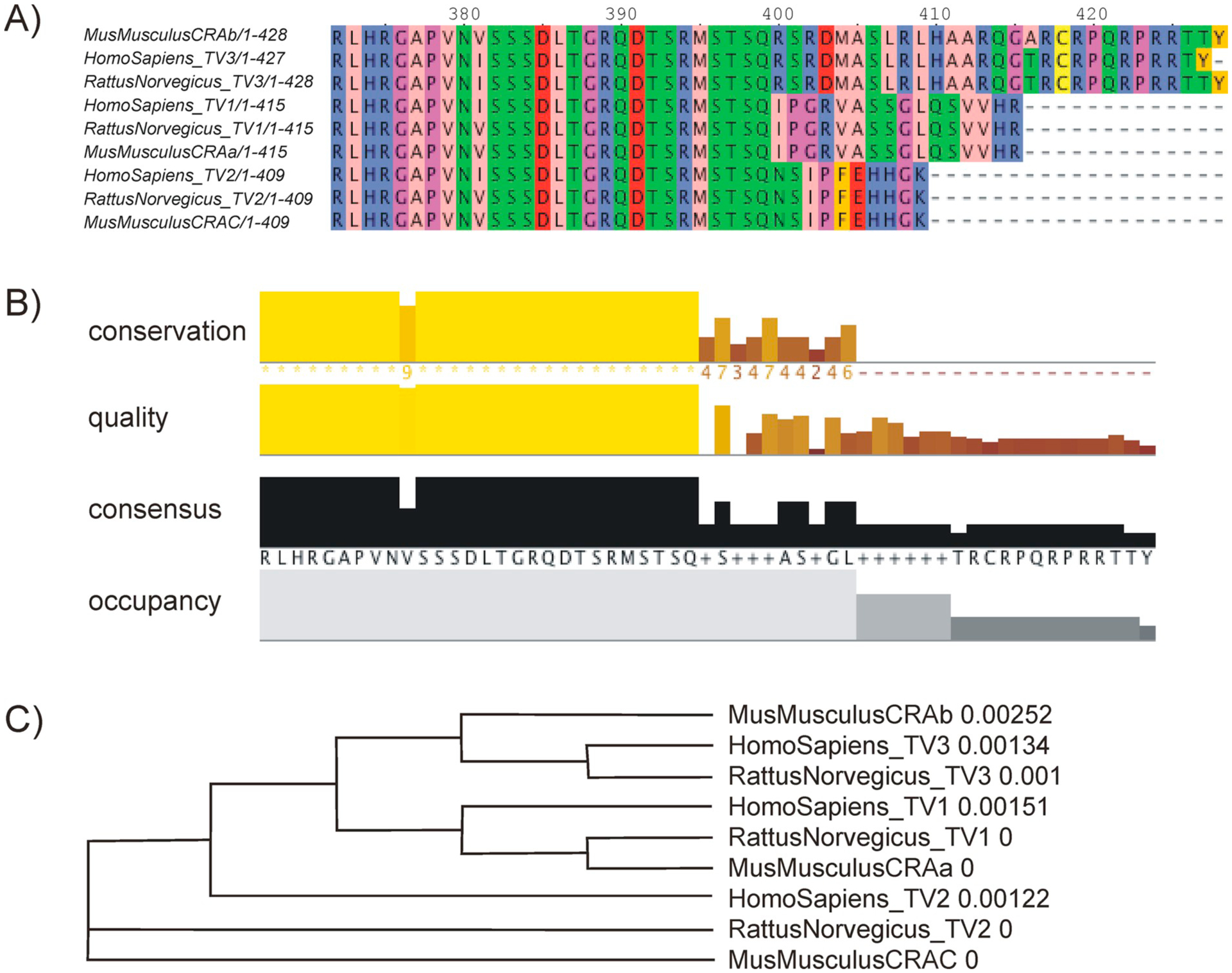
Comparison of the amino acid sequences of three different CK1δ sequences in human, rat, and mouse. The alignment presented in (A) is followed by a display of the conserved regions, the quality, the consensus sequence as well as the occupancy of the alignment. The conservation as well as the quality is displayed with a color code ranging from yellow (highly conserved, good quality) to brown (poorly conserved, poor quality) (B). Alignments presented in panels A and B are shown starting from amino acid 372. The sequences are also displayed in a phylogenetic tree, indicating the relationship between the various CK1δ variants. The alignments as well as the phylogenic tree were generated using the alignment information from the Clustal Omega algorithm (Madeira et al., 2019) (C). TV, transcription variant.
The Clustal Omega algorithm (Madeira et al., 2019) identifies three distinct sequences that are shared between the three different organisms: the shortest sequence containing 409 aa (TV2 in humans and rat as well as CRAc in mouse), followed by a sequence consisting of 415 aa (TV1 in humans and rats/CRAa in the mouse), and a sequence containing 428 aa for the mouse (CRAb) and the rat (TV3) homolog as well as a human TV3 sequence, which is missing one aa at the second to last position, resulting in a length of 427 aa.
In addition to the shown sequences of human, rat, and mouse, CK1δ sequences in most eukaryotic organisms are homologous. The phylogenetic tree displayed in Supplementary Fig. 1 resembles the degree of evolutionary relationship between various sequences of CK1δ.
2.1. Analysis of transcription variants of CK1δ
Various TVs of the gene CSNK1D have been described in the “Mammalian Gene Collection”. Two different TVs of CK1δ were postulated during the early analysis of human and murine genes in 2002 (Strausberg et al., 2002). Since then TV1 (accession number: AAH03558.1, GI: 13097702) and TV2 (accession number: AAH15775.1, GI: 16041786) have been well described in literature. Recently, it could be clearly shown that both variants exhibit opposite functions in the circadian rhythm (Fustin et al., 2018). Both sequences are highly homologous and are alike for the first 399 aa, but differ at the following 16 aa for TV1 and ten aa for TV2, respectively. They do share the first eight exons. The variance occurs due to the fact that TV1 is finished by exon 10, while TV2 uses exon 9. TV2 also includes exon 10, but after the first ten amino acids of exon 9 a stop codon halts the translation and prevents the translation of exon 10 (Fig. 2).
Fig. 2.
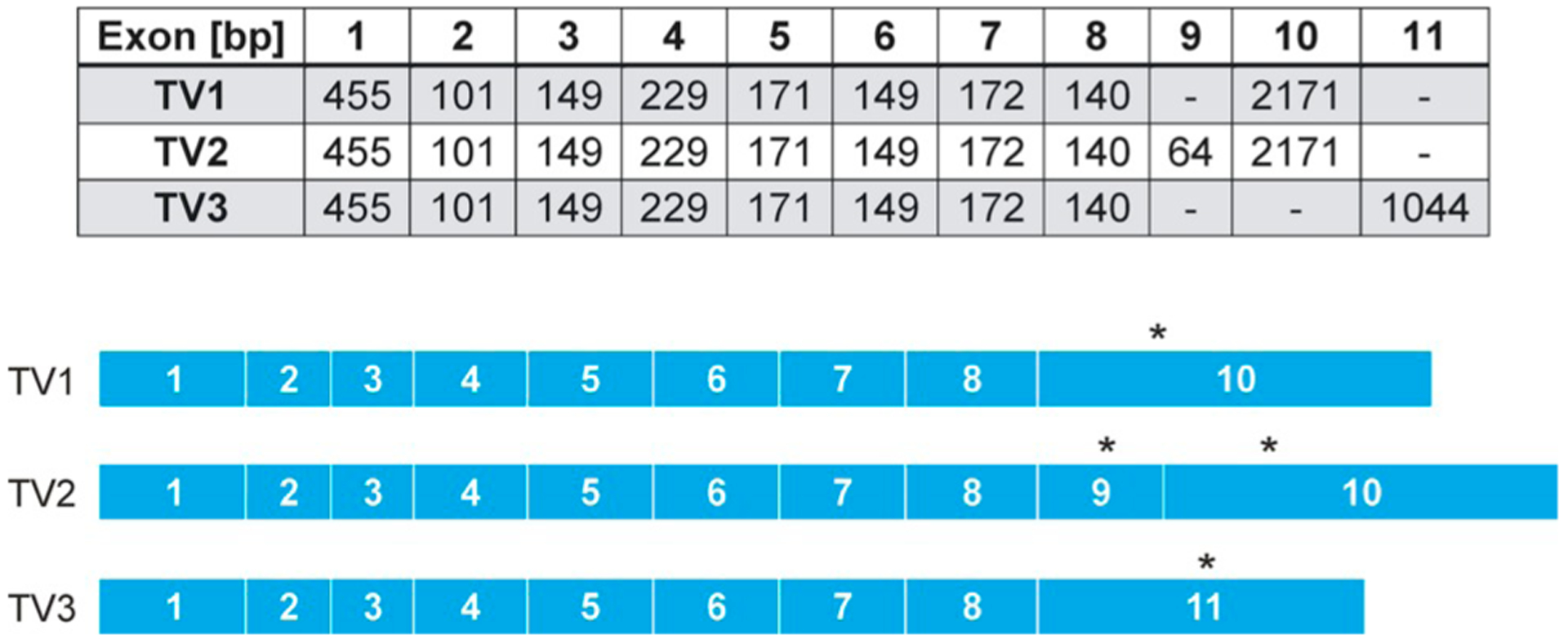
Exon structure of the three transcription variants of CK1δ in humans. The stop codon position of each variant is marked with the asterisk. The information of TV1, TV2, and TV3 can be found using the data bank NCBI (GI: 13097702, 16041786, and 1393428169). Bp, base pairs; TV transcription variant.
Additionally, a third human TV (TV3, accession number: NP_001350678.1, GI: 1393428169), which is very similar to the original CK1δ rat sequence published by Graves and colleagues (Graves et al., 1993), can be found on chromosome 17 (accession number: NG_012828.2, GI: 1428083528). TV3 uses exon 11 instead of 9 and 10. Exon 11 is located downstream of exon 10 and is overlapping with the gene Slc16a3. However, exon 11 is located in a non-coding area. The use of the different exons resulting in the three transcription variants is depicted in Fig. 2.
The inclusion of exon 11 used for TV3 increases the initially postulated length of the CK1δ gene (CSNK1D) from 29.3 kb to approximately 35 kb, which means the length is similar to the rat CSNK1D gene with 34.6 kb.
All three transcription variants were identified in 2014 by the data bank analysis approach by Ezkurdia et al. (2014). Even though the study combined the detection of cellular protein expression by peptide mass spectrometry with the protein-coding potential of the genome, at that time no specific evidence was provided indicating that all three TVs are actually translated. Furthermore, TV3 is listed as “unreviewed” entry in the UniProtKB data base (H7BYT1_HUMAN).
2.2. Polyadenylation patterns of transcription variants
Analysis of polyadenylation sites using the tool RegRNA 2.0 for identification of functional RNA motifs (Chang et al., 2013) revealed that TV1 and TV2 share the same polyadenylation pattern downstream of the stop codon on exon 10, starting at position 1246. The identified motif is 32 nucleotides long (AGUAGAGUCUGCGCUGUGACCUUCUGUUGGGC).
Since exon 10 is not present in TV3, a 32 nucleotides long sequence (AGUGGCUUGUUCCACCUCAGCUCCCAUCUAAC) located downstream of the stop codon on exon 11, at the starting nucleotide 320, is used. The various motifs result in different minimum free energy values (−28.70 kcal/mol for TV1 and TV2, and −16.03 kcal/mol for TV3). The predicted RNA folding structures of the respective motifs with flanking regions are depicted in Fig. 3.
Fig. 3.
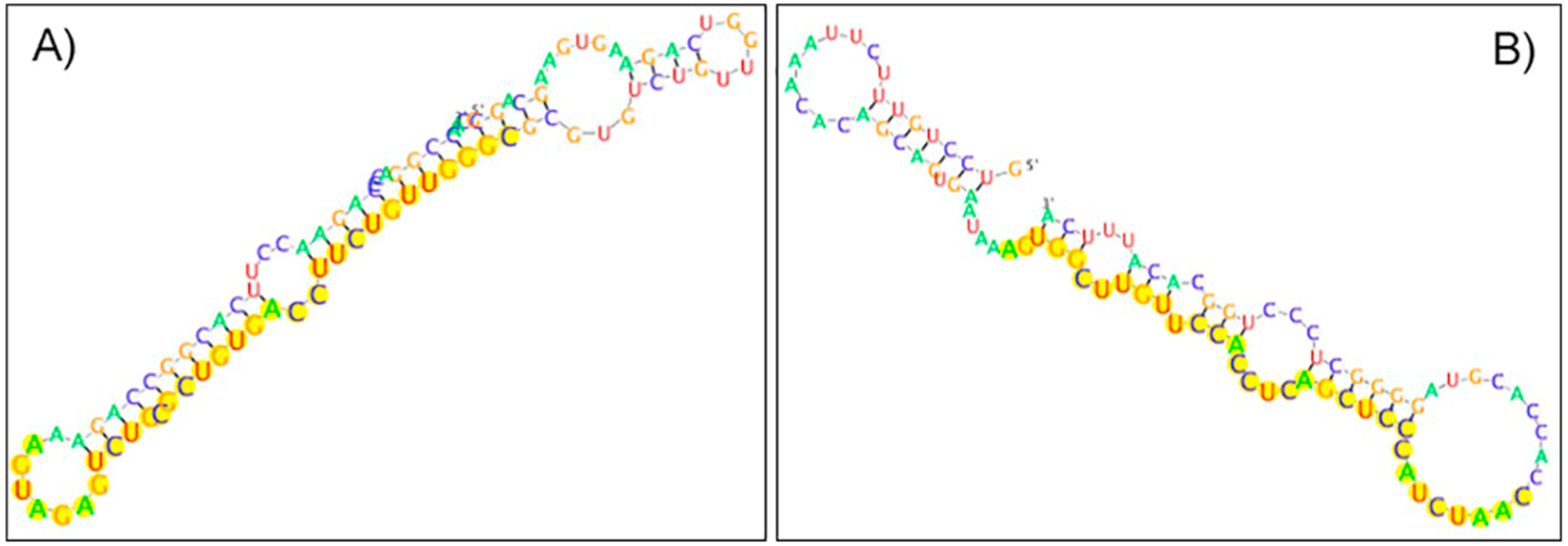
Predicted RNA folding structure of the polyadenylation motif and the flanking regions of TV1 and TV2 on exon 10 (A) as well as TV3 on exon 11 (B). The minimum free energy values for TV1/TV2 and TV3 are −28.70 kcal/mol and −16.03 kcal/mol, respectively. This might indicate that TV1 and TV2 are less polyadenylated compared to TV3 based on the observation that stable secondary structures decrease the polyadenylation of the specific site (Klasens et al., 1998).
3. CK1δ structure and domains
As an own family within the superfamily of serine/threonine-specific kinases all CK1 members are composed by two lobes, basically building all eukaryotic protein kinases (ePKs): the N-terminal lobe (N-lobe) mainly consists of β-sheet strands while the larger C-terminal lobe (C-lobe) is mainly composed by α-helices and loop structures (Fig. 4). Apart from five twisted antiparallel β-strands (β1-β5), the N-lobe also contains a prominent α-helix (αA) crucial for conformational regulation of kinase activity. Within the C-lobe, loop L-78 has previously been attributed to the modulation of CK1 inhibitor selectivity (Peifer et al., 2009) and binding of a tungstate derivative (as a phosphate analog) identified a recognition motif (W1) for the binding of phosphorylated substrates. Major sites involved in mediating substrate recognition are Arg-178, Gly-215, and Lys-224. Positively charged side chains of Arg-178 and Lys-224, but also Lys-217, Lys-221, and Arg-222 enable the formation of ionic interactions with acidic or phospho-primed substrates. This also applies for binding of the C-terminal domain to the substrate binding region for the purpose of autoregulatory function (Longenecker et al., 1996). Upon substrate binding the phospho-acceptor group of the targeted Ser/Thr residue is directed to the γ-phosphate of simultaneously bound ATP, located in close proximity within H-bonding range. Also loop L-EF is putatively involved in substrate recognition and binding, although some residues of this loop could not be modelled in several studies due to poor electron density. Therefore, this loop has been found to be partially disordered in previously performed analyses of CK1δ structure (Longenecker et al., 1996; Zeringo et al., 2013). Additionally, residues Asn-172 and Thr-176 seem to be crucial for substrate binding and/or kinetic activity of CK1δ. The amino acid exchange N172D was identified by analyzing the CK1δ sequence of the simian virus 40 (SV40)-minimally transformed rat fibroblast cell line Rev2. The substitution of asparagine by aspartic acid is supposed to significantly alter the electrostatic potential of the protein surface from neutral to acidic, resulting in impaired kinase activity (Hirner et al., 2012). Within the same region of this substrate binding area also residue Thr-176 can be found. A point mutation leading to the amino acid replacement T176I was originally identified in Hrr25, the CK1δ ortholog in Saccharomyces cerevisiae (Murakami et al., 1999). This amino acid exchange resulted in a loss-of-function phenotype with only little residual kinase activity and has been used in various studies in order to create mutants of CK1δ with insufficient kinase activity (Murakami et al., 1999; Milne et al., 2001; Mehlgarten and Schaffrath, 2003).
Fig. 4.
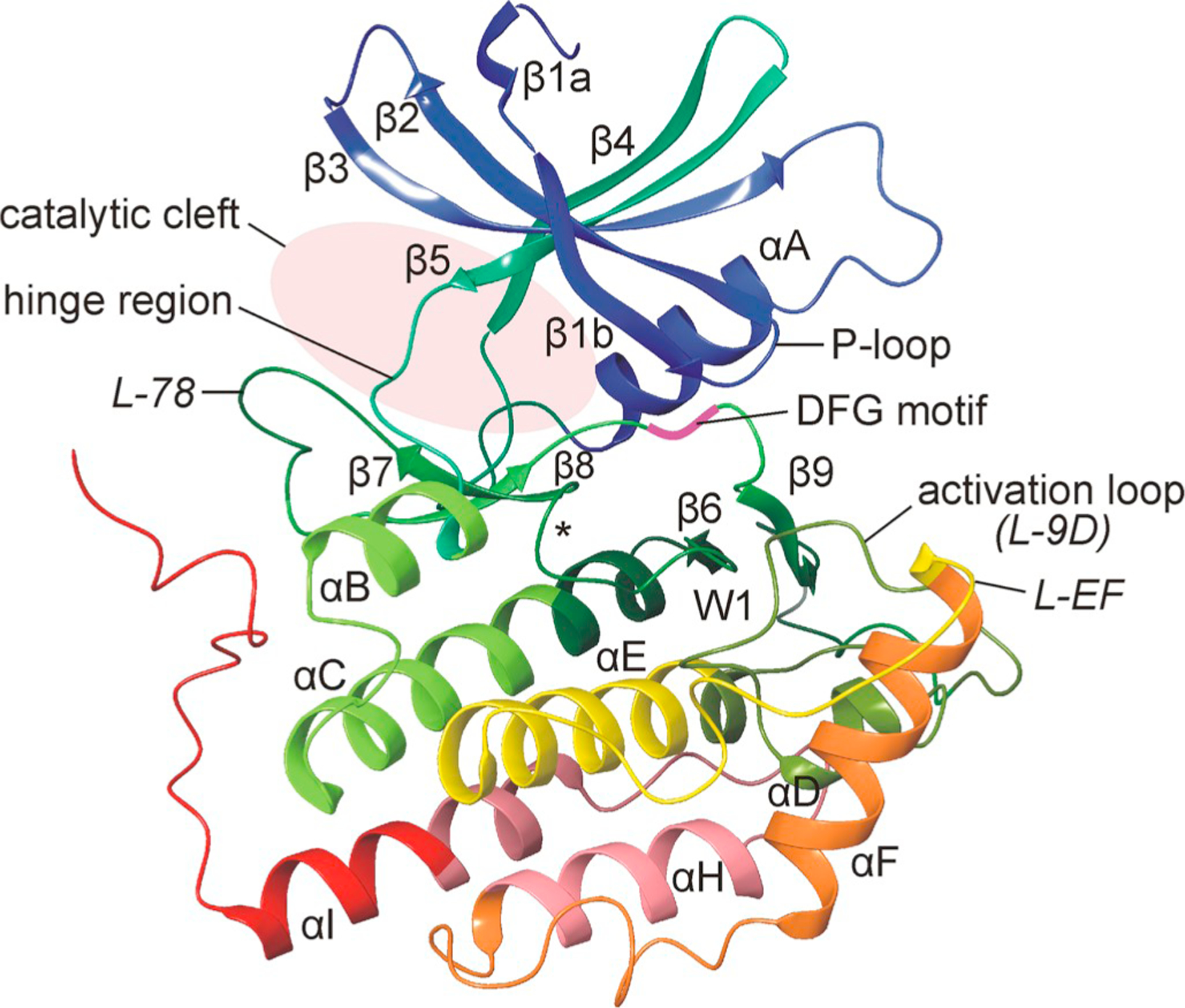
Three-dimensional structure of human CK1δ. Representation of the three-dimensional structure of human CK1δ. The structure of the N-lobe mainly consists of β-sheet strands while the larger C-terminal lobe is mainly composed by α-helices and loop structures. Structural elements are labeled according to Xu et al. (1995). Domains and residues of functional importance are labeled accordingly. Within loop L-89 the DFG motif is located with its aspartate residue being crucial for kinase activity and enzymatic function. Identification of a tungstate binding domain, indicated by W1, led to the identification of a recognition motif for the binding of phosphorylated substrates. The position of the catalytic loop (L-67) is marked with the asterisk (Xu et al., 1995; Longenecker et al., 1996). The figure was created by using CK1δ crystallization data deposited in the protein data bank (PDB) with ID 6GZM (Minzel et al., 2018).
A catalytic cleft for binding of substrates and ATP is formed between the N- and C-terminal lobe of CK1, which are connected via a hinge region (Xu et al., 1995, Longenecker et al., 1996). The ATP active site is formed by two binding regions: a deep hydrophobic pocket (HPI, also known as selectivity pocket) and a second spacious hydrophobic region (HRII). Furthermore, sugar and phosphate binding domains can be found in the ATP active site (Peifer et al., 2009). The glycine-rich P-loop contains the motif Gly-X-Gly-X-X-Gly and bridges strands β1 and β2 (L-12) by forming a β-strand-turn-β-strand motif. This P-loop builds the top cover of the ATP binding site, usually holds the non-transferable phosphate of ATP in place, and is assumed to be more flexible as long as no ATP is present in the ATP binding pocket (Hantschel and Superti-Furga, 2004; Zeringo et al., 2013).
Residues directly involved in forming interactions in the ATP binding site have been analyzed by Singh and Gupta (Singh and Gupta, 2015). In this study the incomplete structure of CK1δ has initially been completed by homology modeling (starting from the structure of CK1α). By using FTsiteserver active site residues Gly-18, Ser-19, Phe-20, Asp-22, Lys-38, Leu-39, Glu-40, Gln-48, Leu-49, and Glu-52 were predicted for the newly created CK1δ structure, termed dCK1-M (Singh and Gupta, 2015). Lysine residue 38 is also conserved in isoforms CK1α (Lys-46) and CK1ε (Lys-38) and is crucial for ATP binding and kinase activity, since substitution of this residue, usually by arginine or methionine, completely abolishes kinase activity creating a kinase-dead CK1δ mutant (Rivers et al., 1998; Budd et al., 2000; Milne et al., 2001; Zeringo et al., 2013; Singh and Gupta, 2015). Furthermore, the so called catalytic loop (L-67) with the sequence Asp-Val-Lys-Pro-Asp-Asn (amino acids 128–133) is also essential for ATP binding. Residue Asp-128 can even be considered as catalytic base (Xu et al., 1995; Zeringo et al., 2013) (Fig. 5).
Fig. 5.
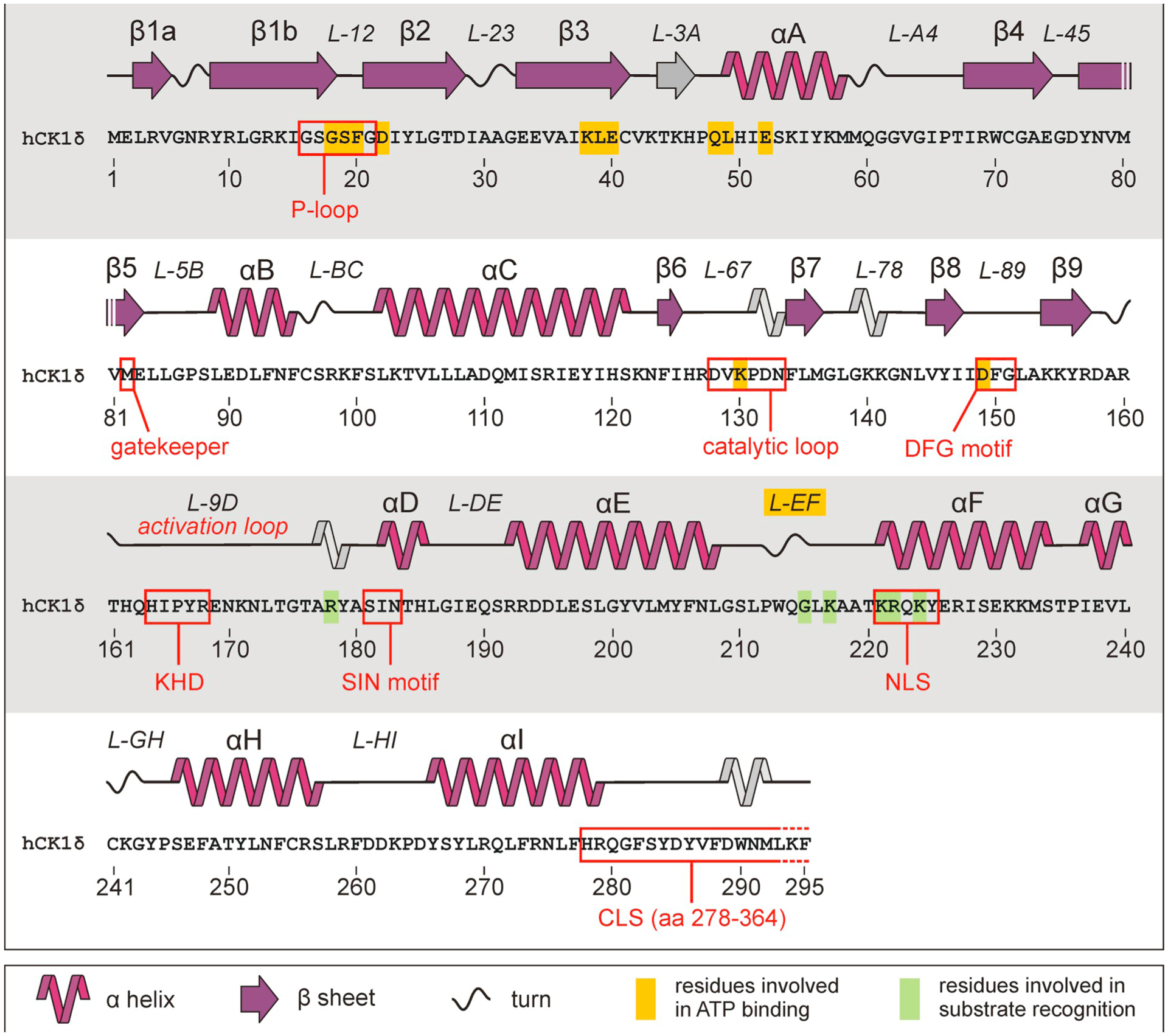
Detailed representation of secondary structure, functional domains, and functional amino acid residues in the kinase domain of human CK1δ. Localization of structural elements building the CK1δ kinase domain is shown for α-helices, β-sheets, turns, and loop-structures. Nomenclature of elements is indicated as first published by Xu et al. (1995). Structures not described in the initial publication are shown in grey. Domains of functional importance are marked with red boxes while amino acid residues involved in ATP binding or substrate recognition are marked with yellow or green background, respectively. Because human CK1δ TV1, 2, and 3 are fully conserved in the N-terminal domain and the kinase domain, the depicted protein sequence is representative for all three variants. Unfortunately, data regarding three-dimensional structure of the C-terminal domain is not available. CLS, centrosome localization signal; KHD, kinesin homology domain; NLS, nuclear localization signal; TV, transcription variant.
Kinase activity of eukaryotic protein kinases in general can be regulated by conformational changes affecting the so called activation loop, which in the case of CK1δ is represented by loop L-9D (Xu et al., 1995, Longenecker et al., 1996). Switching to an active conformation, the activation loop moves out of the catalytic site and the aspartate residue of the DFG motif (residues Asp-149, Phe-150, and Gly-151 in the case of CK1δ, located in loop L-89) shifts to an internal position. There, the aspartate of this highly conserved motif chelates a Mg2+ ion essential for ATP binding and orientation (Endicott et al., 2012). Another conserved eukaryotic protein kinase motif, the APE motif at the end of the activation loop, is modified in CK1δ and is represented by the SIN motif in helix αD (Hanks and Hunter, 1995). In conclusion, residues Lys-38, Lys-130 (catalytic loop residue), Asp-149 (DFG residue), and the P-loop act together to form interactions with the triphosphate moiety of ATP.
In addition, especially for the regulation of kinase activity, as well as for forming interactions with small molecule inhibitors, several residues of CK1δ encoded by exon 3 are essentially involved (Long et al., 2012, Richter, Ullah et al. 2015). One of them is the so called gatekeeper residue located directly within the ATP binding pocket. While the catalytic activity of the kinase usually is not affected by the size of the gatekeeper side chain, the affinity of small molecules, and their access to certain binding pockets (selectivity pockets) beyond the gatekeeper position can be limited by gatekeeper mutations. In the case of CK1δ residue Met-82 (located in loop L-5B) takes the role of the gatekeeper and substitution to the more space-filling residue phenylalanine (M82F) actually abolished binding of a certain class of imidazole-based inhibitors to the selectivity pocket (HPI) in the ATP active site of CK1δ (Peifer et al., 2009).
In other studies however, increased affinity of benzimidazole- and benzothiazole-based inhibitors to the CK1δ M82F gatekeeper mutant could be observed, caused by additional π-hydrogen bonds or π-stacking interactions between Phe-82 and the inhibitor molecules (Bischof et al., 2012; Garcia-Reyes et al., 2018). For another CK1δ point mutation localized in exon 3 resulting in S97C substitution the loss of ATP binding affinity has been detected by molecular dynamics simulations and docking studies, respectively (Kumar et al., 2014). In contrast, a CK1δ T67S mutant displayed increased kinase activity in vitro and enhanced oncogenic potential in cell culture experiments and in a subcutaneous tumor xenotransplantation model. Moreover, CK1δ T67S showed increased sensitivity toward CK1-specific inhibitors (Richter, Ullah et al. 2015).
Besides substrate and ATP binding regions further functional domains being involved in mediating protein-protein interactions are located in the CK1δ protein. Additionally, the kinase domain, which extends between amino acids 9 to 277, contains a kinesin homology domain (KHD) as well as a putative dimerization domain (DD). The DD includes residues from β-strands β1, β2, β3, and β7, as well as from loops L-12, L-78, helix αB, and the hinge region (Longenecker et al., 1998). The KHD, located within the activation-loop (L-9D), is supposed to mediate interactions between CK1 isoforms and components of the cytoskeleton (Roof et al., 1992, Xu et al., 1995, Behrend et al., 2000b). At the junction between L-EF and the αF-helix also a nuclear localization signal sequence (NLS) is located which, however, is not sufficient for nuclear localization of CK1δ (Hoekstra et al., 1991; Graves et al., 1993). Finally, a centrosome localization signal (CLS) domain can be found between residues 278 and 364 (Greer and Rubin, 2011).
Because sequences of human CK1δ TV1, 2, and 3 are fully conserved up to amino acid 399 structural elements described so far can be found in all variants (sequence alignment of human CK1δ TV1, 2, and 3 can be found in Fig. 1A). Unfortunately, analysis of the three-dimensional structure of CK1δ has only been performed with truncated proteins e.g. terminating at amino acids 295 or 318, respectively. This procedure is necessary in order to circumvent problems occurring due to C-terminal proteolysis and variable (auto-)phosphorylation states of the full-length protein (Longenecker et al., 1996). Although these proteins still contain the full kinase domain, however, no validated structural data regarding the C-terminal domain could be made available so far.
4. Regulation of CK1δ activity
Because CK1δ is ubiquitously expressed and its activity is essential for proper function of several important cellular signal transduction pathways, its expression and activity needs to be strictly controlled. First of all, expression of CK1δ varies between different tissues and cell types and is related to certain physiological and pathophysiological conditions and stimuli (Lohler et al., 2009). DNA-damaging substances like the topoisomerase inhibitors etoposide and camptothecin, as well as γ-irradiation, resulted in tumor protein 53 (p53)-dependent increased levels of CK1δ mRNA (Knippschild et al., 1997; Behrend et al., 2000b). Furthermore, gastrin has been shown to induce CK1δ/ε-mediated phosphorylation of protein kinase D2 (PKD2) (von Blume et al., 2007), while elevated CK1-specific activity in general has been detected in cells upon stimulation with insulin (Cobb and Rosen, 1983) or viral transformation (Elias et al., 1981).
Apart from transcriptional or translational control of CK1δ protein expression, its kinase activity can also be regulated at protein level by sequestration to particular subcellular compartments, interaction with other proteins, and posttranslational modifications.
By sequestration of CK1δ to distinct subcellular compartments well defined pools of substrates can be made available to the kinase in order to guide its activity toward specific functions (Wang et al., 1992; Vancura et al., 1994; Sillibourne et al., 2002). Subcellular sequestration is mainly mediated by scaffolding structures containing distinct interaction motifs. In general, the purpose of these scaffolds is to bring interacting molecules into close proximity within interaction complexes in order to enable their orchestrated function in certain signal transduction pathways. Additionally, these scaffolds are supposed to allosterically control the activity of their interaction partners (Locasale et al., 2007; Good et al., 2011).
One of these scaffolds involved in sequestration of CK1 is A-kinase anchor protein (AKAP) 450 (also known as AKAP350, AKAP9, or centrosomal and Golgi N-kinase anchoring protein [CG-NAP]). AKAP450 has been shown to specifically interact with CK1δ and ε, resulting in recruitment of both isoforms to the centrosome to exert centrosomespecific functions contributing to cell cycle regulation (Sillibourne et al., 2002; Greer and Rubin, 2011). Moreover, interaction of AKAP450 and CK1δ has been demonstrated as one mechanism essential for primary ciliogenesis (Greer et al., 2014). As another example, previously identified as a scaffolding adaptor activating the inhibitor κB kinase (IKK), X-linked DEAD-box RNA helicase 3 (DDX3X) has been shown to promote CK1ε-mediated phosphorylation of Dishevelled (Dvl) in the canonical Wingless/Int-1 (Wnt) signal transduction pathway (Cruciat et al., 2013; Gu et al., 2013). Apart from CK1ε, DDX3X also has the potential to increase the activity of CK1δ by up to five orders of magnitude, thereby representing an important co-factor for CK1-mediated Wnt-specific functions (Cruciat et al., 2013). Additional interacting proteins were identified in a study screening for interaction partners that direct CK1δ to ubiquitinated lesions of Alzheimer’s disease. These all contained a common single open reading frame which was termed casein kinase-1 binding protein (CK1BP). Sequence alignment demonstrated that CK1BP is structurally homologous to the acidic domain of dysbindin, a component of dystrophin-associated protein complex (DPC) and biogenesis of lysosome-related organelles complex-1 (BLOC-1). CK1BP inhibits CK1δ kinase activity in a dose-dependent manner as demonstrated for the in vitro phosphorylation of tau and α-synuclein (Yin et al., 2006). Interestingly, interaction with heparin has been reported to activate CK1δ in vitro. Heparin presumably interacts with the C-terminal regulatory domain and finally prevents autoinhibition. Although being unlikely to represent a physiological regulator of CK1δ kinase activity, experiments performed with heparin impressively demonstrate the autoinhibitory potential of the C-terminal domain (Graves et al., 1993; Cegielska et al., 1998).
Dimerization of CK1δ can also be seen as a mechanism for regulation of enzymatic activity and is made possible via an extensive dimer interface inside the DD. In more detail, an α-helix from the CK1δ C-lobe binds to the hydrophobic cavity in the N-lobe of a second CK1δ protein (Ye et al., 2016). Finally, the binding of ATP and perhaps also the binding of large substrates is prevented by Arg-13 inserting into the adenine binding pocket upon dimerization (Longenecker et al., 1998). In solution, CK1δ is always purified as a monomer leading to the question if dimerization can actually be considered biologically relevant. However, dimerization of CK1δ has been confirmed by studies in which kinase activity of endogenous CK1δ was down-regulated by binding of a transfected/transgenic dominant-negative and less active CK1δ mutant (Hirner et al., 2012). Interestingly, also a kinase-dead mutant of CK1ε demonstrated dominant-negative potential, leading to the conclusion that dimerization as a regulatory mechanism is not restricted to CK1δ (Cerda et al., 2015). Results of a study analyzing the interaction between the yeast (Saccharomyces cerevisiae) CK1δ ortholog Hrr25 and the meiosis-specific monopolin subunit Mam1 even led to the hypothesis that the hydrophobic dimerization site may be a conserved site for the binding of recruiters or regulators in general (Ye et al., 2016).
As a measure to reversibly modulate and fine-tune kinase activity also in the short term, CK1δ can be postranslationally modified either by intramolecular autophosphorylation or site-specific phosphorylation by upstream cellular kinases (Fig. 6). Generally, CK1 kinase activity is decreased upon (C-terminal) phosphorylation and can be regulated in vivo by the action of kinases and phosphatases (Rivers et al., 1998). Autophosphorylation processes result in the generation of sequences with the motif pSer/pThr-X-X-Y (X stands for any amino acid while Y stands for any amino acid except serine or threonine). These motifs can subsequently act as pseudo-substrates blocking the catalytic center of the kinase. Within the C-terminal domain of CK1δ residues Ser-318, Thr-323, Ser-328, Thr-329, Ser-331, and Thr-337 were identified as candidate sites targeted by autophosphorylation (Graves and Roach, 1995; Rivers et al., 1998). The presence of an autoinhibitory domain is furthermore confirmed by the observation that proteolytic cleavage of the C-terminal domain results in increased CK1 kinase activity in vitro (Carmel et al., 1994; Graves and Roach, 1995). As a mechanism leading to dephosphorylation of inhibitory C-terminal autophosphorylation sites activation of group I metabotropic glutamate receptors (mGluRs) was identified. Following activation of mGluRs Ca2+-dependent stimulation of the phosphatase calcineurin finally initiates dephosphorylation. Although the underlying study was performed using CK1ε the respective target sites are also conserved in CK1δ (Liu et al., 2002).
Fig. 6.
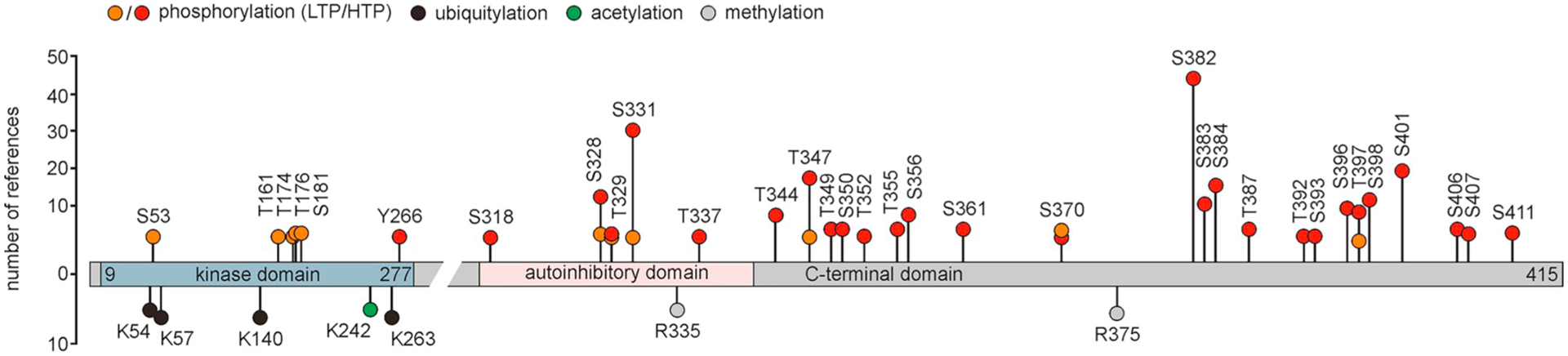
Posttranslational modification of human CK1δ. Identified posttranslational modifications of CK1δ TV1 are indicated at their reported positions. Because most modifications have been reported for the C-terminal domain, this domain is depictured in a stretched presentation compared to the kinase domain. In the case of phosphorylation the distinction is made between reports of low-throughput studies and high-throughput studies. The figure was created based on information provided for CK1δ by PhosphoSitePlus® (Hornbeck et al., 2015). HTP, high-throughput studies, LTP, low-throughput studies, TV, transcription variant.
Within the last decade also site-specific phosphorylation of CK1δ by upstream kinases has been extensively described and target sites have been identified. However, most of these studies were only performed in vitro and final proof for site-specific phosphorylation in vivo is pending in many cases. Within the C-terminal domain of CK1δ Ser-370 could be detected as major residue targeted by protein kinase A (PKA), protein kinase B (PKB/Akt), CDC-like kinase 2 (CLK2), protein kinase C α (PKCα), and checkpoint kinase 1 (Chk1) (Giamas et al., 2007; Bischof et al., 2013; Meng et al., 2016). CK1δ kinase activity was enhanced after mutation of Ser-370 to alanine (S370A) and the key role of Ser-370 was furthermore demonstrated in vivo by expressing a S370A mutant of CK1δ in Xenopus laevis embryos. By affecting Wnt/β-catenin-mediated signal transduction overexpression of the hyperactive S370A mutant led to enhanced formation of an ectopic dorsal axis in Xenopus embryos (Giamas et al., 2007).
Apart from Ser-370 site-specific phosphorylation of CK1δ by Checkpoint kinase 1 (Chk1) has additionally been identified for Ser-328, Ser-331, and Thr-397. Also for these sites mutation to alanine resulted in significantly increased kinase activity (Bischof et al., 2013). Ser-328 and Thr-329 were also identified to be targeted by PKCα. Mutation of Ser-328, Thr-329, and Ser-370 to alanine significantly affected the kinetic parameters of CK1 and resulted in an increase of the catalytic efficiency (kcat/Km) as well, which was most impressive for S328A and T329A (Meng et al., 2016).
Cyclin dependent kinase 2/cyclin E (CDK2/E) and cyclin dependent kinase 5/p35 (CDK5/p35) were also identified as cellular kinases able to modulate CK1δ activity through site-specific phosphorylation within the C-terminal domain. Phosphorylation of Ser-331 and Thr-344 by CDK2/E has been shown by two independent methods, while phosphorylation of Thr-329, Thr-347, Ser-356, Ser-361, and Thr-397 finally could not be confirmed. Furthermore, no clear evidence could be generated confirming phosphorylation of Thr-329, Thr-344, Thr-347, Ser-356, Thr-397 by CDK5/p35 (Ianes et al., 2016). Recently however, Eng et al. identified Thr-347 to be phosphorylated by CDKs (Eng et al., 2017).
While most studies focused on the identification of phosphorylation sites in the C-terminal domain of CK1δ, potential targeted sites for cellular kinases can also be found in the kinase domain. Recent reports demonstrate that residues Ser-53, Thr-176, and Ser-181 can be phosphorylated by PKCα (Meng et al., 2019, Bohm et al., 2019). Furthermore, phosphorylation of residues Thr-161, Thr-174, Thr-176, and Ser-181 has recently also been demonstrated for Chk1 (Bohm et al., 2019). Subsequently performed enzyme kinetic analysis and experiments pre-incubating CK1δ with either PKCα or Chk1 led to identification of domain-specific effects of PKCα- or Chk1-mediated phosphorylation: while kinase activity can be effectively regulated by site-specific phosphorylation within the C-terminal domain (Bischof et al., 2013, Meng et al., 2016), phosphorylation events occurring in the kinase domain might be involved in fine-tuning kinase activity by regulating interactions of the kinase with different substrates or ATP (Bohm et al., 2019, Meng et al., 2019, Bohm et al., 2019).
When being incubated together with Chk1, PKCα, CDK2/E, or CDK5/p35 the in vitro kinase activity of CK1δ is significantly reduced (Bischof et al., 2013; Ianes et al., 2016; Meng et al., 2016). While these observations were only generated in vitro, there is also evidence from cell culture-based analyses demonstrating inhibitory effects of site-specific phosphorylation on CK1δ kinase activity. CK1-specific kinase activity was reduced in the fibrosarcoma cell line HT1080 after activating Chk1 by hydroxyurea treatment (Bischof et al., 2013). After treating COLO357 pancreatic cancer cells with the PKC-specific inhibitor Gö-6983 cellular CK1-specific kinase activity was increased (Meng et al., 2016). A similar effect could be observed after treating COLO357 cells with the pan-CDK inhibitor dinaciclib (Ianes et al., 2016). Furthermore, the CDK-targeted phosphorylation sites Thr-344 and Thr-347 are essential for regulation of CK1δ activity toward period circadian protein homolog 2 (PER2) and degradation of PER2 was promoted when Thr-344 and Thr-347 were mutated to alanine (Eng et al., 2017).
Although much is known about localization of site-specific phosphorylation of CK1δ, only very few studies were performed using low-throughput methods and mechanistic investigations. Several phosphorylation sites within CK1δ were only identified by using high-throughput approaches and proteome-wide screenings without further analysis of the effect of phosphorylation or even the identification of the associated upstream kinase.
By performing proteome-wide analysis also additional posttranslational modifications could be identified for CK1δ like ubiquitination (Lys-54, Lys-57, Lys-140, and Lys-263), acetylation (Lys-242), and methylation (Arg-335 and Arg-375) (information obtained from PhosphoSitePlus®, Hornbeck et al., 2015) (see Fig. 6). However, until now no specific functions or effects have been linked to the observed modifications.
Recently, the expression of all three transcription variants in human and mouse was clearly shown by using primers specific for TV1, TV2, and TV3, respectively (own unpublished results). Furthermore, Michaelis-Menten kinetics clearly revealed that Km and Vmax of the three mouse TVs differ in regard to phosphorylation of both, substrates either containing a canonical (α-casein) (Fig. 7A and Table 2) or a non-canonical consensus sequence (GST-β-catenin1–181) (Fig. 7B and Table 2). TV3 is able to phosphorylate α-casein significantly stronger than TV1. Moreover, β-catenin is significantly stronger phosphorylated by TV3 in comparison to TV1 as well as TV2. These variances could be explained by differences in the degree of autophosphorylation mainly occurring within the C-terminal domains of the different transcription variants ((Bischof et al., 2012) and Fig. 7C). In addition, it also has to be considered that due to sequence differences within their C-terminal regulatory domains the presence of consensus sequences for CK1δ targeting kinases is varying, resulting in differences of site-specific phosphorylation and consequently, also in altered kinase activity (Bischof et al., 2013). Additionally, the phosphorylation state of CK1δ is not only modulating kinase activity and functions of the different CK1δ splice variants, it has also been shown to influence the binding of specific ATP-competitive inhibitors e.g. resulting in different IC50 values associated to different phosphorylation states (Bischof et al., 2012; Bischof et al., 2013; Richter et al., 2014).
Fig. 7.
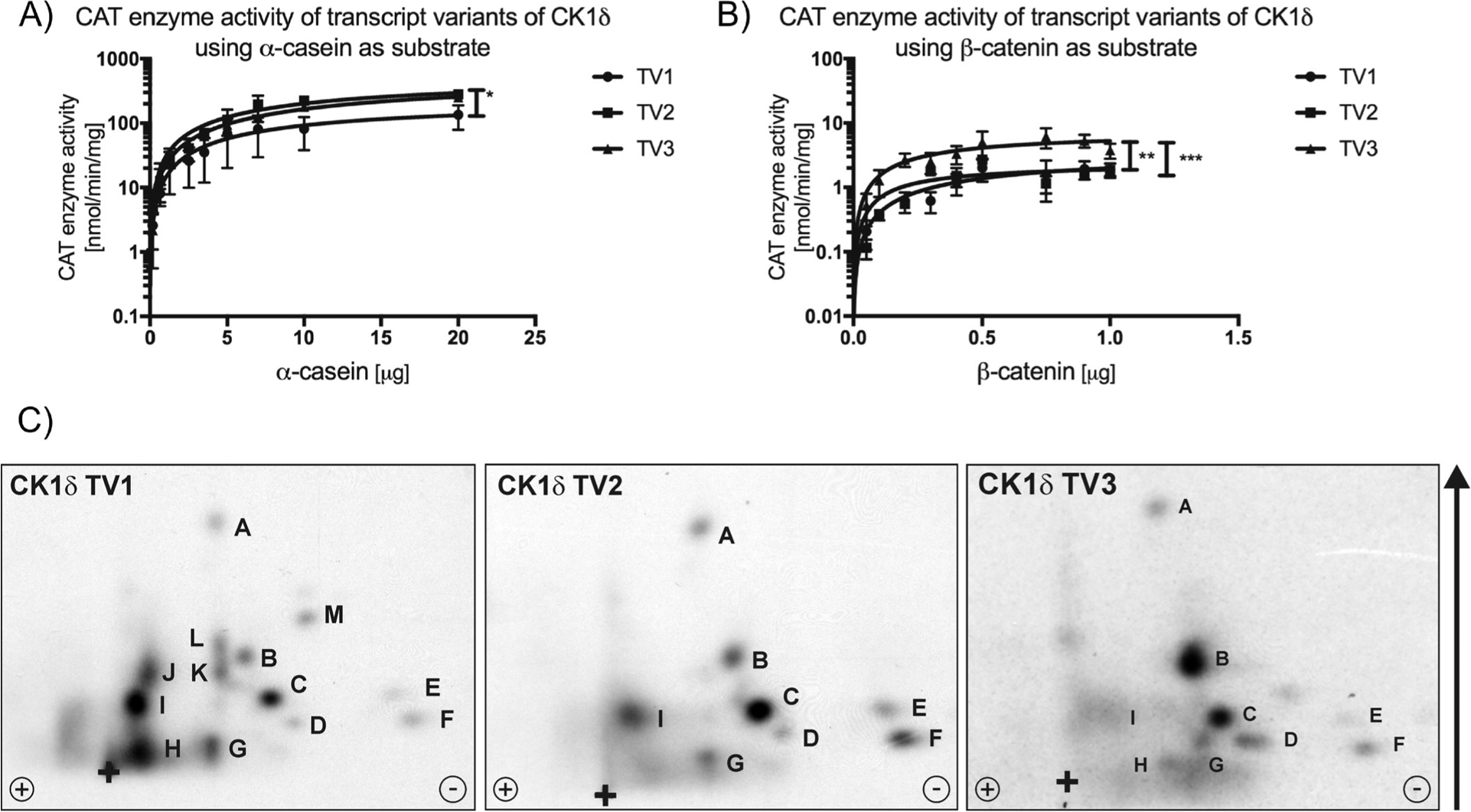
The three transcription variants of CK1δ show significant differences in their kinetic parameters and their (auto-)phosphorylation status. Catalytical (CAT) enzyme activity [nmol/min/mg] was used as readout after having performed in vitro kinase reactions using radioactively labeled ATP to identify the amount of phosphorylated α-casein (A) and β-catenin (GST-β-catenin1–181) (B) of the three identified CK1δ TVs. Statistically significant differences between the whole curves of the TVs were tested by a Kruskal-Wallis test using an uncorrected Fisher’s LSD test as follow-up. * indicates p ≤ 0.05, ** indicates p ≤ 0.01, and *** indicates p < 0.001. (C) Analysis of the phosphorylation status of the different CK1δ transcription variants after autophosphorylation by two dimensional phosphopeptide analysis. The phosphopeptide analysis of TV3 clearly shows differences in major and minor phosphopeptides compared to the phosphopeptide maps of TV1 and TV2. Phosphopeptides A-E are present in all three CK1δ transcription variants, whereas phosphopeptides L, K, J, and M were only observed for TV1. Phosphopeptides O and N are only present in TV3. Figure panels in (C) showing phosphopeptide maps of CK1δ TV1 and TV2 are a derivative of “Fig. 2” published in Bischof et al. (2012), used under CC BY 4.0 (http://creativecommons.org/licenses/by/4.0/). CAT, catalytical; TV, transcription variant.
Table 2.
Km [μg] and Vmax [nmol/min/mg] of CK1δ TV1, TV2, and TV3 using α-casein as well as β-catenin as substrates. Values were calculated using a non-linear Michaelis-Menten fit. Km, Michaelis constant; TV, transcription variant; Vmax, maximum enzyme reaction velocity.
| CK1δ variant | α-Casein | β-Catenin | ||
|---|---|---|---|---|
| Km [μg] | Vmax [nmol/min/mg] | Km [μg] | Vmax [nmol/min/mg] | |
| TV1 | 15.54 | 236.02 | 0.8121 | 3.63 |
| TV2 | 17.21 | 548.88 | 0.2527 | 2.37 |
| TV3 | 24.86 | 576.60 | 0.3885 | 7.33 |
5. Substrates and substrate recognition
At physiological pH CK1δ carries positive charges at residues Arg-178 and Lys-224, which are mainly involved in substrate binding, preferentially enabling interactions with acidic substrates. Whereas mammalian CK1 isoforms have so far only been shown to exclusively phosphorylate serine and threonine residues of their substrates, the Saccharomyces cerevesiae CK1δ homolog Hrr25 and Xenopus laevis CK1α have been shown to additionally phosphorylate tyrosine residues (deMaggio et al., PNAS, 1992; Pulgar et al., 1996). Substrates being primed by previous phosphorylation represent the canonical CK1 consensus sequence motif pSer/pThr-X-X-(X)-Ser/Thr with X standing for any amino acid and pSer/pThr for the phospho-primed residue. The Ser/Thr residue to be phosphorylated by CK1δ is located three to four residues upstream to the primed residue. The primed residue can also be replaced by a cluster of negatively charged acidic residues like aspartic (Asp) or glutamic acid (Glu) (three or four residues). However, phospho-primed motifs are favored over Asp or Glu containing motifs and Asp is favored over Glu (Agostinis et al., 1989; Flotow et al., 1990; Flotow and Roach, 1991; Meggio et al., 1991; Graves et al., 1993).
In addition to the canonical consensus motif alternative sequence motifs have been identified, which can also represent targets for CK1-mediated phosphorylation. One of these motifs is the so-called SLS (Ser-Leu-Ser) motif in which the first serine residue is phosphorylated by CK1. This motif can be found in β-catenin and nuclear factor of activated T cells (NFAT). In both cases the SLS motif is followed by a cluster of acidic amino acids essential for efficient binding to CK1 (Marin et al., 2003). Phosphorylation of β-catenin at Ser-45 has been demonstrated for CK1 isoforms α and δ (Amit et al., 2002) while CK1 isoforms α, δ, and ε co-fractionated with the sensitivity to red light reduced 1 (SRR-1) domain of NFAT1 (Okamura et al., 2004). Moreover, the consensus motif Lys/Arg-X-Lys/Arg-X-X-Ser/Thr has been identified in several sulfatide and cholesterol-3-sulfate (SCS)-binding proteins, among them myelin basic protein (MBP), the Ras homolog family member A (RhoA), and tau. SCS-mediated stimulation of CK1δ-mediated phosphorylation could be observed for the two basic brain proteins MBP and tau, and also for the acidic protein RhoA (Kawakami et al., 2008).
To date, > 150 substrates have been identified to be phosphorylated by members of the CK1 family, at least in vitro. Although many substrates can be phosphorylated by more than one CK1 isoform and in many cases it is not possible to assign particular isoforms to particular substrates, Table 3 aims to list substrates, which were identified to be phosphorylated by CK1δ.
Table 3.
CK1δ-specific substrates. Substrates reported for CK1δ-targeted phosphorylation (in vitro and in vivo) are listed and grouped according to their associated functions. Only substrates reported for human CK1δ or appropriate orthologous proteins (e.g. in yeast species) are included.
| Substrates | References |
|---|---|
| Cancer-associated proteins: | |
| – Adenomatous polyposis coli (APC) | (Gao et al., 2002) |
| – Axin | (Gao et al., 2002) |
| – β-Catenin | (Amit et al., 2002) |
| – Full-length cubitus interruptus (Ci-155) | (Price and Kalderon, 2002) |
| – Dishevelled (Dvl) | (Gao et al., 2002) |
| – Nucleoside diphosphate kinase A (nm23-H1) | (Garzia et al., 2008) |
| – Dapper1a (Dpr1a) | (Teran et al., 2009) |
| – Fat | (Sopko et al., 2009) |
| – Deoxycytidine kinase (dCK) | (Smal et al., 2010) |
| – Yes-associated protein (YAP) | (Zhao et al., 2010) |
| – Metastasis suppressor 1 (MTSS1) | (Zhong et al., 2013) |
| – Neural precursor cell expressed developmentally down-regulated protein 4 (NEDD4) | (Liu et al., 2014) |
| – Sprouty2 (SPRY2) | (Yim et al., 2014) |
| Control of mitotic or meiotic processes: | |
| – Meiotic recombination protein Rec8 (Rec8) | (Ishiguro et al., 2010) |
| – Endogenous meiotic inhibitor 2 (Emi2) | (Isoda et al., 2011) |
| – Wee1 | (Penas et al., 2014) |
| Cytoskeleton-associated and scaffolding proteins: | |
| – Annexin II/lipocortin II | (Gao et al., 2000) |
| – Desmoglein 2 | (Gao et al., 2000) |
| – Keratin 17 | (Gao et al., 2000) |
| – Microtubule-associated protein 1A (MAP1A) | (Wolff et al., 2005) |
| – Microtubule-associated protein 4 (MAP4) | (Behrend et al., 2000b) |
| – Stathmin | (Behrend et al., 2000b) |
| – Tau | (Behrend et al., 2000b) |
| – α/β-Tubulin | (Behrend et al., 2000b) |
| – γ-Tubulin | (Behrend et al., 2000b) |
| – Connexin-43 (Cx43) | (Cooper and Lampe, 2002) |
| – Ras homolog family members A and B | (Kawakami et al., 2008) |
| – End-binding 1 (EB1) | (Zyss et al., 2011) |
| – Sid4 | (Johnson et al., 2013) |
| – Ran-binding protein in the microtubule-organizing center (RanBPM) | (Wolff et al., 2015) |
| DNA-/RNA-associated proteins: | |
| – Chromatin-associated protein swi6 (Swi6) | (Ho et al., 1997) |
| – Heterogeneous nuclear ribo-nucleoprotein A1 (hnRNP A1) | (Gao et al., 2000) |
| – Putative RNA helicase | (Gao et al., 2000) |
| – Nuclear factor of activated T cells 1, 2, and 4 (NFAT1, 2, 4) | (Okamura et al., 2004) |
| – Forkhead box G1 (FoxG1) | (Regad et al., 2007) |
| – Topoisomerase Iiα (TOP2A) | (Grozav et al., 2009) |
| – DNA methyltransferase 1 (Dnmt1) | (Sugiyama et al., 2010) |
| – Ubiquitin-like containing PHD and RING finger domains 1 protein (UHRF1) | (Chen et al., 2013) |
| – Yeast sterol regulatory element-binding protein homolog (Sre1N) | (Brookheart et al., 2014) |
| Golgi- and vesicle-associated proteins: | |
| – ADP-ribosylation factor GTPase-activating protein (ARF GAP1) | (Yu and Roth, 2002) |
| – Snapin | (Wolff et al., 2006) |
| – Protein kinase D2 (PKD2) | (von Blume et al., 2007) |
| – Synaptic vesicle protein 2A (SV2A) | (Zhang et al., 2015a) |
| Mediators of cellular stress: | |
| – Tumor protein 53 (p53) | (Knippschild et al., 1997) |
| – Murine double minute 2 homolog (Mdm2) | (Winter et al., 2004) |
| – Hypoxia-inducible factor 1α (HIF-1α) | (Kalousi et al., 2010) |
| Proteins associated to neurodegenerative processes: | |
| – Presenilin-2 (PS-2) | (Walter et al., 1998) |
| – Cyclin-dependent kinase 5 (CDK5) | (Sharma et al., 1999) |
| – α-Synuclein | (Okochi et al., 2000) |
| – β-Secretase (BACE1) | (Walter, Fluhrer et al. 2001) |
| – Parkin | (Yamamoto et al., 2005) |
| – Cyclic AMP response element-binding protein (CREB) | (Shanware et al., 2007) |
| – Myelin basic protein (MBP) | (Kawakami et al., 2008) |
| – TAR DNA-binding protein of 43 kDa (TDP-43) | (Nonaka et al., 2016) |
| Receptors and receptor-associated proteins: | |
| – Transmembrane tumor necrosis factor α (mTNFα) | (Watts et al., 1999) |
| – Amplified in breast cancer 1 (AIB1) | (Giamas et al., 2009) |
| – Estrogen receptor α (Erα) | (Giamas et al., 2009) |
| – Adiponectin | (Xu et al., 2015) |
| Regulation of circadian rhythm: | |
| – Period circadian protein homolog 1–2 | (Camacho et al., 2001) |
| – Cryptochromes 1 (CRY1) and 2 (CRY2) | (Walton et al., 2009) |
| – Proliferator-activated receptor γ co-activator 1α (PGC-1α) | (Li et al., 2011b) |
| Ribosome-related proteins: | |
| – Nucleolar protein 56 (Nop56) | (Gao et al., 2000) |
| – Ribosomal proteins L4 (RPL4), L8 (RPL8), L13 (RPL13) | (Gao et al., 2000) |
| – Eukaryotic initiation factor 6 (eIF6)/Tif6p49 | (Biswas et al., 2011) |
| – Essential nuclear protein 1 (ENP1)/bystin-like protein (BYSL) | (Zemp et al., 2014) |
| – LTV1 | (Zemp et al., 2014) |
| Viral proteins: | |
| – Human cytomegalovirus phosphoprotein (ppUL44) | (Alvisi et al., 2011) |
| – Human herpes virus (HHV) E3 ubiquitin ligase (ICP0) | (Chaurushiya et al., 2012) |
| – Simian virus 40 large T-antigen (SV40 T-Ag) | (Hirner et al., 2012) |
6. Subcellular localization of CK1δ and interaction with cellular proteins
In cells CK1δ is distributed within both, the cytoplasm and the nucleus. Increased levels of CK1δ can permanently be detected in peri-nuclear regions in close proximity to the Golgi apparatus and the trans Golgi network (TGN) (Behrend et al., 2000b; Milne et al., 2001; Greer et al., 2014; Stoter et al., 2014). Depending on cellular conditions CK1δ can also be temporarily associated to membranes and receptors, transport vesicles, components of the cytoskeleton, centrosomes, or spindle poles (Behrend et al., 2000b; Milne et al., 2001; Lohler et al., 2009; Greer et al., 2014; Wang et al., 2015). Studies using kinase-dead mutants or truncated CK1δ variants demonstrated that the CK1δ kinase domain is required for nuclear localization of CK1δ. However, the present nuclear localization signal (NLS) alone is not sufficient for nuclear localization. Moreover, not only the presence of a kinase domain but also its enzymatic activity is essential for correct distribution of CK1δ within the cell (Hoekstra et al., 1991; Graves et al., 1993; Milne et al., 2001). This hypothesis has been confirmed for the localization of CK1δ and its yeast ortholog Hrr25 to centrosomes (Milne et al., 2001, Peng et al., 2015a, Peng et al., 2015b). However, there are also contradicting reports showing that kinase activity is dispensable to target CK1δ to the centrosome (Qi et al., 2015; Elmore et al., 2018).
Further guidance to distinct subcellular compartments is achieved by interaction of CK1δ with interacting and scaffolding proteins. These interactions are mediated by docking motifs like the Phe-X-X-X-Phe motif, which has been identified in NFAT, β-catenin, and PER proteins, or the Ser-Gln-Ile-Pro motif present in microtubule plus-end-binding protein 1 (EB1) (Vielhaber et al., 2000; Okamura et al., 2004; Bustos et al., 2006; Etchegaray et al., 2009; Zyss et al., 2011). Although some of these proteins are also substrates for CK1δ-mediated phosphorylation the docking motifs are not necessarily localized adjacent to the phosphorylated residues, but contribute to proper orientation of the interaction partners and to stabilization of the interaction (Bustos et al., 2006). Another protein containing the Phe-X-X-X-Phe interaction motif is family with sequence similarity 83 member H (FAM83H), which has originally been identified as a protein involved in the formation of enamel. By its interaction with FAM83H nuclear CK1δ is localized to nuclear speckles, which supply splicing factors to active transcription sites (Kuga et al., 2016; Wang et al., 2016). FAM83H contains four Phe-X-X-X-Phe interaction motifs, one of them located in a conserved domain of unknown function (DUF1669). This domain is common to other FAM83 family members as well and interactions with CK1δ could also be found for FAM83A, FAM83B, and FAM83E (Fulcher et al., 2018). Although FAM83 proteins are substrates for CK1δ-mediated phosphorylation they are rather considered as regulatory partners directing CK1 isoforms to specific cellular compartments and substrate pools (reviewed in Bozatzi and Sapkota, 2018).
In addition to interaction partners, which have already been mentioned above, numerous additional interacting proteins have been described for CK1δ within recent years (Table 4). These proteins are not (only) targets for CK1δ-mediated phosphorylation, but are also involved in more complex regulatory processes and form strong interactions with CK1δ. Apart from the already mentioned scaffolding protein AKAP450 also the Ran-binding protein in the microtubule-organizing center (MTOC) (RanBPM) was identified as a centrosome-targeted interacting protein for CK1δ. The proteins interacted in a yeast two-hybrid screen, partially co-localized, and RanBPM was even phosphorylated by CK1δ (Wolff et al., 2015). Furthermore, interaction between CK1δ and microtubule-associated protein 1A (MAP1A) has been demonstrated by yeast two-hybrid screen and co-immunoprecipitation. Consequently, microtubule dynamics might be changed via CK1δ-mediated phosphorylation of the light chain LC2 of MAP1A (Wolff et al., 2005). CK1δ is also involved in the regulation of vesicle transport and synaptic functions, underlined by the fact that CK1δ interacts with snapin, a protein associated with SNAP25, regulating neurotransmitter release in neuronal cells (Wolff et al., 2006). In developmental processes CK1δ has been found to interact with the pro-neural basic helix-loop-helix (bHLH) transcription factor Atoh1, which plays a key role in sensory hair development. Following phosphorylation by CK1δ degradation of Atoh1 is initiated by the E3 ubiquitin ligase Huwe1 (Cheng et al., 2016). As another developmental-associated factor also lymphocyte enhancer factor (LEF-1) can be bound and phosphorylated by CK1δ, resulting in disruption of binding between LEF-1 and β-catenin while DNA-binding of LEF-1 is not impaired (Hammerlein et al., 2005). Association of CK1δ has also been reported for the regulatory and complex-building/-initiating molecule 14-3-3 ζ, which also interacts with CK1α and CK1ε (Dubois et al., 1997; Zemlickova et al., 2004). In order to mediate this interaction CK1δ contains the sequence Leu-Gly-Ser-Leu-Pro, which is quite similar to a putative motif described for 14-3-3 binding (Muslin et al., 1996). Finally, almost two decades ago interaction between CK1δ and the circadian clock proteins PER and cryptochrome (CRY) has been demonstrated by Lee and colleagues. In the circadian cycle CK1 isoforms facilitate the translocation of PERs and CRYs to the nucleus (Lee et al., 2001).
Table 4.
Interaction partners of CK1δ. In case interaction was confirmed by co-immunoprecipitation, yeast two-hybrid screen, or similar approaches reported interaction partners for CK1δ are listed in the following table. Furthermore, information is provided if the respective protein is target for CK1δ-mediated phosphorylation (●: yes, -: no). In case the respective protein is only phosphorylated by isoforms other than CK1δ or only single family members of the indicated interaction partners are target for CK1δ-mediated phosphorylation this information is provided in parentheses.
| Protein | Phosphorylation by CK1(S) | References |
|---|---|---|
| 14-3-3 ζ | - (● CK1α) | (Dubois et al., 1997, Zemlickova et al., 2004) |
| AKAP450 (A-kinase anchor protein 450) | - | (Sillibourne et al., 2002) |
| Atoh1 (atonal bHLH transcription F factor1) | ● | (Cheng et al., 2016) |
| Axin 1 and 2 | ● | (Amit et al., 2002, Gao et al., 2002) |
| Chk1 (checkpoint kinase 1) | - | (Bischof et al., 2013) |
| CK1BP (casein kinase-1 binding protein) | ● | (Yin et al., 2006) |
| CPI-17 (protein kinase C-potentiated myosin phosphatase inhibitor of 17 kDa) | - | (Zemlickova et al., 2004) |
| DDX3X (DEAD-box RNA helicase 3 X-linked) | - (● CK1ε) | (Cruciat et al., 2013, Dolde et al., 2018) |
| Dvl1 (dishevelled 1) | ● | (Gao et al., 2002) |
| EB1 (microtubule plus-end-binding protein1) | ● | (Zyss et al., 2011) |
| FAM83A, B, E, and H | ● (FAM83H) | (Kuga et al., 2016, Wang et al., 2016, Fulcher et al., 2018) |
| LEF-1 (lymphocyte enhancer factor-1) | ● | (Hammerlein et al., 2005) |
| MAP1A (microtubule-associated protein 1A) | ● | (Wolff et al., 2005) |
| NFAT1, 2, and 4 (nuclear factor of activated T cells) | ● (NFAT1) | (Okamura et al., 2004) |
| p53 (tumor protein 53) | ● | (Milne et al., 1992, Venerando et al., 2010) |
| PER1 and 2 (period circadian protein homolog 1 and 2) | ● | (Vielhaber et al., 2000, Lee et al., 2001, Miyazaki et al., 2004, Etchegaray et al., 2009, Shanware et al., 2011) |
| RanBPM (Ran-binding protein in the MTOC) | ● | (Wolff et al., 2015) |
| Snapin (SNARE-associated protein snapin) | ● | (Wolff et al., 2006) |
| SPRY2 (sprouty2) | ● | (Yim et al., 2014) |
| Tau (MAPT, microtubule-associated protein tau) | ● | (Behrend et al., 2000b, Li et al., 2004, Kawakami et al., 2008) |
7. CK1δ-associated cellular functions
7.1. CK1δ in the circadian rhythm
The circadian rhythm is controlled by the cellular clock, permitting a cellular rhythm of approximately 24 h. Alterations in the circadian rhythm could be observed in different disorders such as sleeping, metabolic, and neurological disorders, which will be discussed in more detail in the following paragraphs (Ferrell and Chiang, 2015; De Lazzari et al., 2018; Leng et al., 2019; Stenvers et al., 2019).
The circadian rhythm is characterized by a negative feedback loop mediated by PER and CRY proteins, whose expression levels oscillate over the circadian clock. PERs and CRYs can form heterodimers, shuttle into the nucleus (Lee et al., 2001; Aryal et al., 2017), and inhibit their own expression through the inhibition of the CLOCK/BMAL1-responsive circadian gene transcription (Virshup et al., 2007). Once in the nucleus, CK1δ seems to enhance the inhibition of circadian-driven transcription by reducing CLOCK/BMAL1 binding affinity to DNA (Aryal et al., 2017). Since mRNA levels of CK1δ/ε could vary in light-induced phase-shift in mice, CK1δ/ε seems to play an important role in balancing the circadian rhythm (Ishida et al., 2001). In fact, the regulation of PER degradation is mainly influenced by reversible phosphorylation controlled by kinases and phosphatases (so called “phosphoswitch”) (Gallego and Virshup, 2007; Virshup et al., 2007). CK1δ and ε are among the most important kinases, controlling PER phosphorylation (Camacho et al., 2001; Lee et al., 2001; Xu et al., 2005; Narasimamurthy et al., 2018). CK1δ seems to have a more important and essential role in the circadian clock compared to CK1ε (Etchegaray et al., 2009; Walton et al., 2009). When CK1δ is disrupted or inactivated, an elongation in the circadian clock has been observed (Etchegaray et al., 2009, Isojima et al., 2009, Lee et al., 2011b, Mieda et al., 2016), while overexpression of CK1δ leads to a shortening of the circadian rhythm (Lee et al., 2009; Mieda et al., 2016). Inhibition of CK1δ/ε by PF-670462 also has shown inhibitory effects on the expression of clock genes such as Bmal1, Per1, Per2 and Nr1d1 in rats (Kennaway et al., 2015).
After inhibition of N6-methylation (m6A) of CK1δ mRNA, enhancement of CK1δ translation permits the expression of CK1δ TV1 and TV2, which seem to differently influence the circadian rhythm, first by acceleration following increased degradation of PER2 and secondly by deceleration consequently leading to PER2 stabilization (Fustin et al., 2018). Moreover, a recent study identified CK1δ TV2 with a stronger role in priming phosphorylation of PER2 compared to CK1δ TV1, which permits subsequent phosphorylation events on PER2 (Narasimamurthy et al., 2018).
Robustness of the circadian clock has been analyzed by Nakajima and colleagues, who confirmed that the inhibition of PER2 phosphorylation by CK1δ/ε leads to destabilization of the oscillation (Nakajima et al., 2015). Moreover, CK1δ-mediated phosphorylation of PER2 seems to be a temperature-insensitive process, being responsible for the robustness of this process (Isojima et al., 2009). Nevertheless, the activity of CK1δ in phosphorylating PER2 seems to be controlled by upstream kinases, likely by cyclin-dependent kinases or proline-directed kinases, which can phosphorylate CK1δ on Thr-344 or Thr-347, thereby reducing its activity (Ianes et al., 2016; Eng et al., 2017). Interestingly, the circadian rhythm seems to be coupled with – and also be influenced by – the cell cycle (Unsal-Kacmaz et al., 2005). In fact, mutations and deregulation of circadian rhythm components have been observed in cancer (Wood et al., 2008).
7.2. CK1δ in DNA damage and cellular stress
Stress-conditions, among them genotoxic stress and DNA damage, lead to p53-dependent activation of CK1δ, finally resulting in CK1δ-mediated phosphorylation of key regulatory proteins involved in these processes, like p53 and Mdm2 (Knippschild et al., 1997). CK1δ phosphorylates p53 within its N-terminal domain on residues Ser-4, Ser-6, Ser-9 (Knippschild et al., 1997; Higashimoto et al., 2000), and Ser-20 (MacLaine et al., 2008; Venerando et al., 2010). When p53 is phosphoprimed on Ser-15 additional CK1δ-mediated phosphorylation on Thr-18 results in lower binding affinity between p53 and Mdm2 and consequently elevated p53 activity (Dumaz et al., 1999; Alsheich-Bartok et al., 2008). Moreover, not only p53 but also its negative regulator Mdm2 can be phosphorylated by CK1δ. Under normal conditions, CK1δ-mediated phosphorylation of Mdm2 on Ser-240, Ser-242, Ser-246, and Ser-383 stabilizes the Mdm2-p53 complex and consequently leads to increased degradation of p53 (Blattner et al., 2002; Winter et al., 2004). Interestingly, REGγ (11S regulatory particles, 28-kDa proteasome activator γ) seems to have a role in promoting degradation of CK1δ, finally leading to stabilization of Mdm2 protein levels and therefore decreased p53 activity (Li et al., 2013). Upon DNA damage, ataxia telangiectasia mutated (ATM)-mediated phosphorylation of CK1δ leads to phosphorylation of Mdm2, permitting SCFβ-TrCP (skp cullin F-box containing complex beta-transducin repeat containing protein)-mediated ubiquitination of Mdm2 and its proteasomal degradation (Inuzuka et al., 2010a, Inuzuka et al., 2010b, Wang et al., 2012). Under hypoxia, a hypoxia-inducible factor 1 (HIF-1) heterodimer (HIF-1α and aryl hydrocarbon receptor nuclear translocator (ARNT)) can form and activate the transcription of hypoxia-responsive genes. CK1δ plays a role in phosphorylating HIF-1α on residue Ser-247, thereby interfering with its binding to ARNT and resulting in decreased HIF-1 activity (Kalousi et al., 2010). Interestingly, under hypoxic conditions CK1δ can also reduce cell proliferation and lipid droplet formation by reducing HIF-1α/ARNT complex formation (Kourti et al., 2015). The activity of topoisomerase II α (TOPOII-α), another important regulator of DNA replication and cell division, is also influenced by CK1δ-mediated phosphorylation on residue Ser-1106, finally resulting in increased TOPOII-α function (Grozav et al., 2009). Furthermore, CK1δ phosphorylates the ubiquitin-like containing PHD and RING finger domains 1 protein (UHRF1), which plays an important role in the maintenance of DNA methylation during DNA replication. Upon DNA damage, enhanced degradation of UHRF1 can be observed due to increased CK1δ-mediated phosphorylation on Ser-108, permitting binding of the SCFβ-TrCP E3 ligase and subsequent proteasomal degradation of UHRF1 (Chen et al., 2013).
7.3. CK1δ in cell cycle, mitosis, and meiosis
CK1δ was shown to have important roles in microtubule dynamics, cell cycle progression, genomic stability, mitosis, and meiosis (Behrend et al., 2000a, Behrend et al., 2000b, Sillibourne et al., 2002, Stoter et al., 2005, Johnson et al., 2013, Greer et al., 2014, Penas et al., 2014, Penas et al., 2015, Phadnis et al., 2015, Sakuno and Watanabe, 2015, Chan et al., 2017, Greer et al., 2017). As already mentioned before, CK1δ is anchored to the centrosome via its interaction with AKAP450 (Sillibourne et al., 2002), allowing CK1δ-mediated phosphorylation of EB1, a component relevant for centrosome positioning during T cell activation (Zyss et al., 2011). In order to ensure centrosomal integrity and maintenance of genomic stability centrosome-associated CK1δ might also cooperate with a centrosomal subpopulation of p53 (Wahl et al., 1996; Meek, 2000; Tarapore and Fukasawa, 2002). Consequently, CK1δ/ε play an important role in cell cycle progression as well as in genomic stability. Following its inhibition by IC261 cells undergo a transient mitotic arrest (Behrend et al., 2000a), even though additional studies reported that cell cycle arrest upon treatment with IC261 is a result of severe off-target effects mediated by IC261 (Cheong et al., 2011; Stoter et al., 2014). Nevertheless, mouse embryonic fibroblasts (MEFs) lacking CK1δ are characterized by multiple centrosomes and the presence of micronuclei, indicators of genomic instability (Greer et al., 2017). Accordingly, silencing of CK1δ leads to lower amounts of Chk1 and cell division cycle 2 (CDC2)/CDK1, both having important roles in DNA damage response and mitotic checkpoints (Greer et al., 2017). Interestingly, Chk1-mediated regulation of CK1δ as well as their physical binding have previously been shown, confirming interaction of CK1δ with Chk1 (Bischof et al., 2013). Moreover, CK1δ-mediated phosphorylation of Wee1-G2 checkpoint kinase (Wee1) can lead to its proteasomal degradation resulting in increased levels of active CDK1 and the consequent entrance of cells into mitosis (Penas et al., 2014). In line with these results, inhibition of CK1δ or its destruction after APC/CCdh1 (adenomatous polyposis coli/cyclosome cadherine 1)-mediated ubiquitination increases the stability of Wee1 kinase, which elevates CDK1 phosphorylation, leading to cell cycle exit (Penas et al., 2014; Penas et al., 2015). On the other hand, CK1δ is also able to phosphorylate phosphoprimed septation initiation protein 4 (Sid4), thereby initiating the recruitment of Chk2/Cds1 (checkpoint kinase 2/replication checkpoint kinase Cds1) and a subsequent mitotic commitment (Chan et al., 2017). On the other hand, under mitotic stress, Hhp1 and Hhp2 (Hhp1/2), orthologous forms of CK1 in Schizosaccharomyces pombe, have been identified to play an important role in the mitotic checkpoint by delaying cytokinesis. By co-localization with spindle pole bodies (SPBs), CK1 can phosphorylate Sid4, thereby inducing Dma1-mediated ubiquitination and degradation of Sid4, leading to cytokinesis suspension (Johnson et al., 2013; Elmore et al., 2018). Inhibition of CK1 isoforms by D4476 also increases the mitotic cell rate via increased stability of β-catenin and elevated β-catenin-mediated transcription, confirming an important role of CK1 in mitosis and cell cycle progression (Benham-Pyle et al., 2016).
Apart from its involvement in mitosis CK1δ also plays an important role in the regulation of meiotic processes. CK1δ and its ortholog Hrr25 have been detected within P-bodies, cytoplasmic RNA-protein granules present in meiotic cells. This binding seems to reduce CK1 turnover into the cytoplasm, thereby preserving protein integrity for subsequent stages of meiosis (Zhang et al., 2016; Zhang et al., 2018). Localization of Hrr25 to P-bodies is necessary for completion of the meiotic program (Zhang et al., 2018). Hrr25 has also been observed to be involved in the induction of nuclear division of meiosis as well as in the synthesis of membranes to engulf newly synthetized nuclei, thereby mediating the exit from meiosis II (Arguello-Miranda et al., 2017). In oocytes in metaphase I and in metaphase II CK1δ co-localizes with γ-tubulin at the spindle poles (Qi et al., 2015). However, CK1δ seems not to be essential for spindle organization and meiotic progression in human cells. This is in contrast to earlier studies performed in yeast. Ishiguro and colleagues showed that Hhp2, the CK1δ/ε ortholog in S. pombe, acts as cohesion kinase promoting cleavage of the cohesion subunit Rec8 (meiotic recombination protein Rec8) during meiosis (Ishiguro et al., 2010; Katis et al., 2010). This observation is supported by a report from Rumpf and colleagues showing that the CK1δ/ε orthologs Hhp1 and Hhp2 in S. pombe are essential to achieve full phosphorylation of Rec8 and subsequent efficient removal of Rec8 during meiosis I (Rumpf et al., 2010). This data impressively shows that results based on investigations in yeast models cannot necessarily be transferred to mammalian cells. However, stromal antigen 3 (STAG3), the mammalian ortholog of meiotic recombination protein Rec11, is also phosphorylated by CK1, thereby confirming the conservation of this process and the observations made in yeast, showing that Rec11 is phosphorylated by Hhp1 and Hhp2, subsequently permitting DNA breakage and consequent meiotic recombination (Phadnis et al., 2015; Sakuno and Watanabe, 2015).
7.4. CK1δ-specific functions associated with cytoskeleton components
Numerous studies report cellular localization of CK1δ to components of the cytoskeleton, where CK1δ exercises essential regulatory tasks: CK1δ is able to modulate microtubule polymerization and stability at the spindle apparatus and the mitotic centrosome by directly phosphorylating α-, β-, and γ-tubulin (Behrend et al., 2000b; Stoter et al., 2005). Not only tubulin itself, but also microtubule-associated proteins (MAPs) are interacting with – and are phosphorylated by CK1δ, subsequently resulting in modulated interaction of MAPs with microtubules and altered microtubule dynamics (Brouhard and Rice, 2018). So far, site-specific phosphorylation by CK1δ has been demonstrated for MAP4, MAP1A, and tau, as well as for the microtubule-destabilizing protein stathmin (Behrend et al., 2000b; Li et al., 2004; Wolff et al., 2005; Hanger et al., 2007; Leon-Espinosa et al., 2013). Microtubule nucleation at the Golgi apparatus was inhibited by siRNA-mediated knock-down of CK1δ. Furthermore, the localization of CK1δ to the centrosome plays a crucial role in ciliogenesis: based on a study specifically knocking-down CK1δ kinase activity (by either using a CK1δ-specific inhibitor or specific siRNA) CK1δ has been shown to mediate primary ciliogenesis by interaction with AKAP450 proteins as well as by maintaining Golgi organization and directed protein trafficking (Greer et al., 2014). This is supported by another study suggesting a role for CK1δ in the assembly of primary cilia (Lee et al., 2012). In contrast, the same study impressively showed an involvement of the highly related isoform CK1ε in a Wnt5a-CK1ε-Dvl2-Plk1-mediated pathway for cilia disassembly.
Ciliogenesis in general can be linked to the activity of distinct cellular signal transduction pathways. By compartmentalization of essential signal molecules the cilium has been shown to restrain signaling via the canonical Wnt as well as the Hedgehog (Hh) pathway (Rohatgi et al., 2007; Corbit et al., 2008; Lancaster et al., 2011). Furthermore, ciliogenesis can be promoted by activity of the Hippo pathway (Kim et al., 2014). In conclusion, with its role in direct regulation of ciliogenesis, CK1δ might not only able to directly modulate the activity of the Wnt, Hh, and Hippo pathways (see chapters below), but also to indirectly control their activity by regulating (dis-)assembly of the primary cilium as a physical signal transduction platform used by the mentioned pathways.
7.5. CK1δ in the Wnt pathway
The Wnt signaling pathway plays an important role in developmental processes, regeneration, cell proliferation and tissue homeostasis. In many types of cancers alterations or mutations in the Wnt pathway have been observed (Clevers and Nusse, 2012; Polakis, 2012; Nusse and Clevers, 2017; Krishnamurthy and Kurzrock, 2018). Briefly, in absence of Wnt ligand, β-catenin is phosphorylated and ubiquitinated by the β-catenin destruction complex, leading to its proteasomal degradation. Upon Wnt binding to Frizzled (Fzd), the co-receptor LRP5/6 can be phosphorylated by CK1α and glycogen synthase kinase 3 (GSK3). Subsequently, Axin binds to LRP5/6 and recruits the β-catenin destruction complex, permitting higher stability of β-catenin. β-catenin shuttles into the nucleus, associates with TCF transcription factor, and induces the transcription of Wnt target genes (Clevers and Nusse, 2012; Nusse and Clevers, 2017).
As previously summarized in different focused review articles, CK1 isoforms are involved in different ways in the Wnt signaling pathway (Cruciat, 2014; Knippschild et al., 2014; Price, 2006). CK1δ has been shown to phosphorylate the Wnt pathway components Dishevelled 1 (Dvl1) as well as Axin, APC, and β-catenin (Amit et al., 2002; Gao et al., 2002; Xing et al., 2003; Ha et al., 2004). Accordingly, CK1δ was also observed to have an essential role in neurite formation and dopaminergic neuron differentiation by phosphorylating Dvl (Bryja et al., 2007; Greer and Rubin, 2011). Moreover, CK1δ/ε phosphorylate Dvl2, thereby playing an important role for intestinal stem cell (ISC) maintenance. In fact, co-ablation of CK1δ and ε leads to the elimination of ISCs (Morgenstern et al., 2017). In addition, CK1δ seems to be important for the regulation of planar cell polarity (PCP), which depends on proper phosphorylation of planar cell polarity protein Van Gogh-like 2 (Vangl2), mediated by CK1δ/ε following Wnt-5a-mediated induction of the non-canonical Wnt signaling pathway (Yang et al., 2017).
CK1δ can either (i) positively or (ii) negatively influence the Wnt pathway: (i) CK1δ can stabilize β-catenin after the phosphorylation of lipoprotein receptor-related protein 6 (LRP6) and the subsequent recruitment of Axin and the β-catenin destruction complex, thereby avoiding β-catenin phosphorylation and ubiquitination (Zeng et al., 2005; Wu et al., 2009). Moreover, CK1δ/ε activity as well as stability are influenced by the tumor promoter TPA (12-O-tetra-decanoylphorbol-13-acetate), which also permits increased binding of β-catenin to TCF4E in a CK1δ/ε-dependent manner, resulting in an activation of Wnt target genes (Su et al., 2018). (ii) CK1δ negatively influences the Wnt pathway by directly phosphorylating β-catenin on Ser-45, thereby priming β-catenin for further GSK3β-mediated phosphorylation and subsequent degradation (Amit et al., 2002).
7.6. CK1δ in the Hedgehog pathway
The Hedgehog (Hh) signaling pathway plays an important role in embryonic development by regulating cell differentiation, regeneration, proliferation, and organogenesis. Deregulation and mutations of main players of this pathway can influence tumorigenesis and cancer development (Jiang and Hui, 2008; Yao and Chuang, 2015; Wu et al., 2017). Briefly, in absence of Hh ligand (Sonic Hedgehog (Shh), Indian hedgehog (Ihh), or Desert hedgehog (Dhh)) the twelve-pass-membrane receptor Patched (Ptch) inhibits the seven-pass membrane receptor Smoothened (Smo). In this context, cubitus interruptus/glioma-associated oncogene (Ci/Gli) transcription factor can be phosphorylated, undergoes partial proteasomal degradation, and shuttles into the nucleus where it can act as a repressor of Hh-targeted gene transcription. Once Hh binds to Ptch, Smo receptor can be phosphorylated and Ci/Gli transcription factor can migrate into the nucleus to activate Hh target genes (Jiang and Hui, 2008; Heretsch et al., 2010; Wu et al., 2017). CK1 isoforms play different roles in the Hh pathway. CK1δ has recently been shown to increase Smo activity by phosphorylating Smo at Ser-683 after priming phosphorylation mediated by PKC (Jiang et al., 2014). Upon Hh stimulation, CK1δ phosphorylates the full-length Cubitus interruptus positive transcription factor (CiA), protecting it from proteasomal degradation (Shi et al., 2014). In contrast, site-specific phosphorylation of Ci by CK1 results in enhanced binding of SCFSlimb E3 ubiquitin ligase to Ci, subsequently leading to proteasomal degradation of Ci (Smelkinson et al., 2007). Additionally, after priming phosphorylation by PKA, CK1 phosphorylates CiA, thereby increasing its proteolysis and generation of the repressive form of Ci (CiR) (Price and Kalderon, 2002).
7.7. CK1δ in the Hippo pathway
The Hippo pathway is involved in embryonic development to determine organ size by influencing cell proliferation, apoptosis, and tissue homeostasis (Zeng and Hong, 2008; Bae and Luo, 2018; Moon et al., 2018). Hippo signaling is activated by high cell density. A phosphorylation cascade is induced after phosphorylation of mammalian sterile-20 like kinase 1/2 (MST1/2), which further phosphorylates large tumor suppressor kinase 1/2 (LATS1/2), ultimately phosphorylating yes-associated protein (YAP)/tafazzin (TAZ). As major downstream target YAP/TAZ can either be ubiquitinated and degraded or retained in the cytoplasm after binding to 14–3–3. Under conditions of low cell density, the Hippo-signaling cascade is not activated and YAP/TAZ is able to translocate into the nucleus and to bind TEAD (TEA domain)/SMAD (SMA/mothers against decapentaplegic) transcription factors, inducing Hippo target gene transcription for growth and differentiation (Zeng and Hong, 2008, Bae and Luo, 2018, Moon et al., 2018). YAP degradation is also influenced by CK1δ/ε-mediated phosphorylation on Ser-381, after its priming phosphorylation by LATS on Ser-127, which permits the recruitment of the E3 ubiquitin ligase SCFβ-TrCP, ubiquitination, and subsequent degradation of LATS (Zhao et al., 2010). Interestingly, the Hippo pathway seems to be connected to the Wnt pathway in different ways, for instance by interaction of YAP/TAZ with DVL, β-catenin, and the β-catenin destruction complex (Varelas et al., 2010; Heallen et al., 2011; Azzolin et al., 2012; Imajo et al., 2012; Rosenbluh et al., 2012; Konsavage Jr. and Yochum, 2013; Azzolin et al., 2014; Wang et al., 2018), as well as with regulation of p53 (Ferraiuolo et al., 2017; Furth et al., 2018). Hippo signaling can negatively influence Wnt signaling via Dvl protein, which in presence of Wnt ligand binding is phosphorylated by CK1δ/ε and inhibits the β-catenin destruction complex. In this context, YAP/TAZ binds Dvl, thereby reducing its CK1δ/ε-mediated phosphorylation as well as the subsequent transduction of Wnt signaling (Varelas et al., 2010; Imajo et al., 2012). Moreover, phosphorylated YAP/TAZ is able to bind β-catenin, which is then retained in the cytoplasm, resulting in a decreased transcription of Wnt target genes (Heallen et al., 2011; Imajo et al., 2012). Interestingly, YAP/TAZ have also been identified as downstream effectors of the non-canonical Wnt signaling pathway, whose target genes seem to have inhibitory potential on canonical Wnt signaling (Park et al., 2015).
8. Involvement of CK1δ in pathological processes
Most studies in regard to the involvement of CK1δ in the development and progression of certain diseases and disorders concentrate on the relation of CK1δ to cancer and neurologic diseases. Apart from those, also disorders affecting cell cycle, metabolism, and stem cell functions associated with CK1δ are another main topic. Furthermore, metabolic diseases as well as inflammatory and infectious diseases related to CK1δ-specific functions are also reported in some studies and will be discussed in the following sections.
8.1. CK1δ in tumorigenesis and tumor progression
Cancer-associated functions of CK1δ are closely related to the above described roles of CK1δ in Wnt/β-catenin-, p53-, Hh-, and Hippo-related signaling. Meanwhile numerous studies described the oncogenic features of CK1δ in different types of cancer. These include, among others, gastrointestinal tumors, breast cancer, kidney cancer, hematological malignancies, and skin cancer. A database research analyzing microarray datasets generated from the analysis of certain tumor cell lines and tumor tissues revealed that CK1δ mRNA is overexpressed in many cancer types like bladder cancer, brain cancer, breast cancer, colorectal cancer, kidney cancer, lung cancer, melanoma, ovarian cancer, pancreatic cancer, and prostate cancer, as well as in hematopoietic malignancies (Fig. 8 and Table 5) (Schittek and Sinnberg, 2014).
Fig. 8.
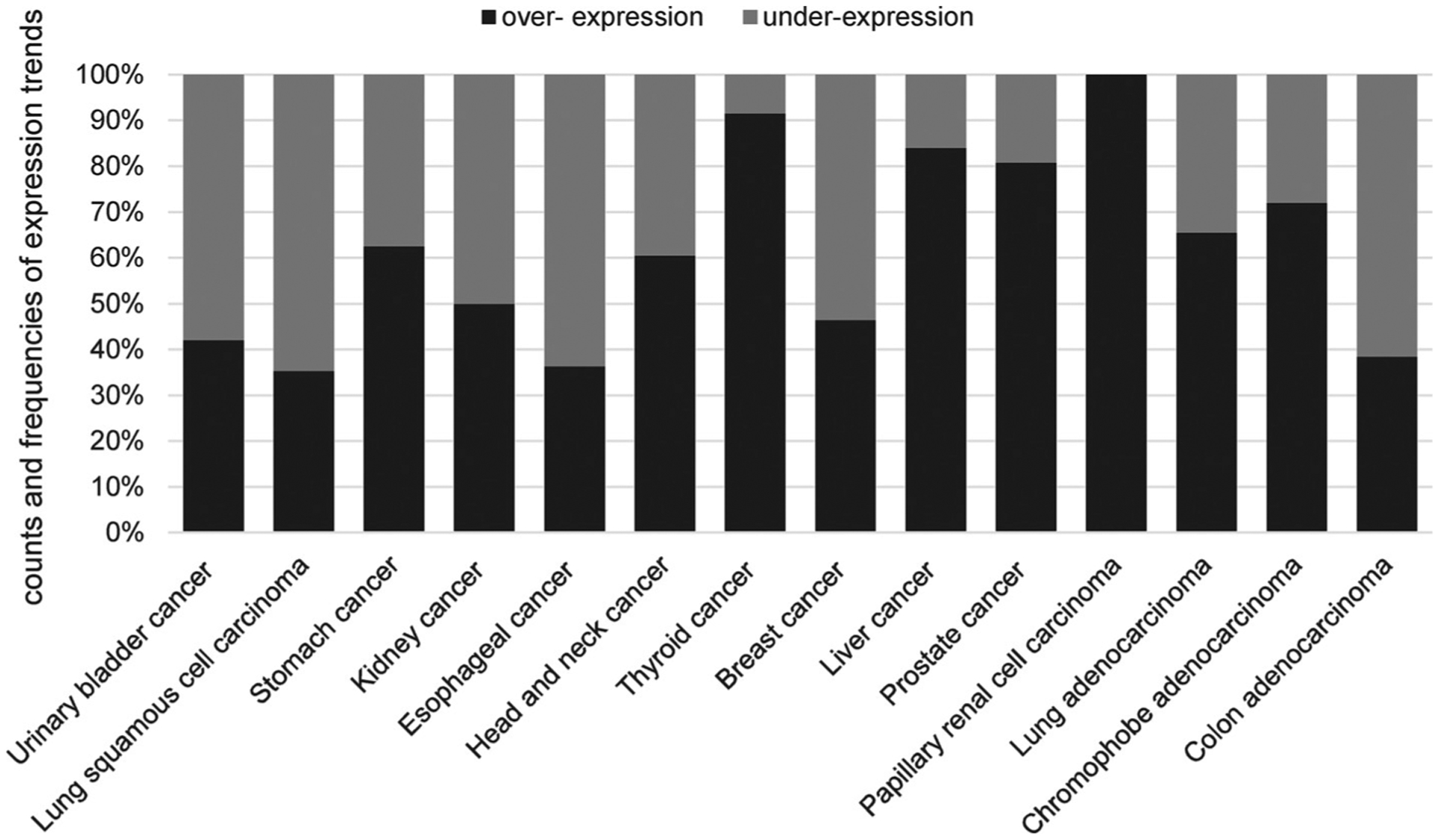
Expression trend frequency of different cancer types. Frequencies of patients following each expression trend for CK1δ are presented for all relevant cancer types. For each patient, log2 fold-change (log2FC) values greater than zero were considered to follow an over-expression trend, less than zero to follow an under-expression trend. Patients with log2FC = 0 were excluded from the dataset. Note that all patients are included in this graphic, irrespective of statistical significance of the trend. Data is based on the BioXpress online tool (Wan et al., 2015; Dingerdissen et al., 2018).
Table 5.
Expression levels of CK1δ in different types of cancer. Differential expression tendency of CK1δ is presented for different cancers. Subject ratio was calculated by comparing the number of patients significantly over- or under-expressing CK1δ to the number of patients analyzed in total. For each patient, log2 fold-change (log2FC) values greater than zero were considered to follow an over-expression trend, less than zero to follow an under-expression trend. Significance was justified based on adjusted P value (P value cutoff of 0.05). Data was obtained from BioXpress online tool (Wan et al., 2015, Dingerdissen et al., 2018).
| Cancer type | Subjects ratio | log2FC | P value | Adjusted P value | Significance | Expression trend |
|---|---|---|---|---|---|---|
| DOID:11054/urinary bladder cancer | 11/19 (57.89) | −0.25 | 0.046 | 0.095 | No | Down |
| DOID:3907/lung squamous cell carcinoma | 33/51 (64.71) | −0.06 | 0.351 | 0.402 | No | Down |
| DOID:10534/stomach cancer | 12/32 (37.5) | −0.1 | 0.224 | 0.406 | No | Down |
| DOID:263/kidney cancer | 36/72 (50.0) | −0.05 | 0.344 | 0.419 | No | Down |
| DOID:5041/esophageal cancer | 7/11 (63.64) | −0.11 | 0.435 | 0.59 | No | Down |
| DOID:11934/head and neck cancer | 17/43 (39.53) | −0.01 | 0.919 | 0.941 | No | Down |
| DOID:1781/thyroid cancer | 54/59 (91.53) | 0.16 | 0 | 0.002 | Yes | Up |
| DOID:1612/breast cancer | 53/114 (46.49) | 0.11 | 0.016 | 0.029 | Yes | Up |
| DOID:3571/liver cancer | 42/50 (84.0) | 0.21 | 0.008 | 0.049 | Yes | Up |
| DOID:10283/prostate cancer | 42/52 (80.77) | 0.09 | 0.04 | 0.071 | No | Up |
| DOID:4465/papillary renal cell carcinoma | 32/32 (100.0) | 0.26 | 0.051 | 0.176 | No | Up |
| DOID:3910/lung adenocarcinoma | 38/58 (65.52) | 0.08 | 0.207 | 0.717 | No | Up |
| DOID:4471/chromophobe adenocarcinoma | 18/25 (72.0) | 0.03 | 0.741 | 0.977 | No | Up |
| DOID:234/colon adenocarcinoma | 10/26 (38.46) | 0.04 | 0.663 | 1.000 | No | Up |
Animal experiments and cell culture-based analysis confirmed increased expression of CK1δ in cells of hyperplastic B cell follicles and B cell lymphoma in p53-deficient mice, but also in choriocarcinoma cells and pancreatic ductal adenocarcinoma (Maritzen et al., 2003; Stoter et al., 2005; Brockschmidt et al., 2008). The function of CK1δ in lymphoid neoplasms was first described using a mouse model leading to the observation that the expression level of CK1δ is increased in cells of hyperplastic B follicles and advanced B cell lymphomas in p53-deficient mice. Later, CK1δ mRNA and protein levels were also analyzed in 18 lymphoma cell lines and strong expression of CK1δ could be found in all analyzed cell lines (Maritzen et al., 2003; Winkler et al., 2015).
Interestingly, in some other studies low expression of CK1δ in cancer tissues has also been described (Fig. 8 and Table 5). Decreased expression levels of CK1δ may result in cell cycle arrest and apoptosis in various cancer cell lines. These effects are independent of Wnt/β-catenin-mediated signal transduction. However, they seem to depend on activation of RAS and inactivation of p53 (Cheong et al., 2011; Cheong and Virshup, 2011). Decreased activity of CK1δ has also been characterized in SV40-transformed cells in vitro and in SV40-induced mammary carcinogenesis in a bitransgenic mouse model in vivo. In this model CK1δ kinase activity is impaired by site-specific mutations (e.g. N172D) and transgenic, mutant CK1δ exercises a dominant-negative effect on endogenous CK1δ. The resulting partial inhibition of CK1δ activity reduced transformation of SV40-transformed cells, decelerated tumor progression, and prolonged survival of WAP-mutCK1δ/WAP-T bitransgenic mice (Hirner et al., 2012). In contrast, the R324H mutation in the C-terminal region of CK1δ is associated with increased oncogenic potential and results in promotion of the development of adenomas in the intestinal mucosa (Tsai et al., 2007). Furthermore, the T67S mutant identified in colorectal cancer tissue exhibits increased kinase activity also resulting in higher oncogenic potential. Overexpression of CK1δT67S leads to enhanced colony formation of HT29 cells and increased tumor formation in xenografts (Richter et al., 2015).
Studies on the analysis of a correlation of CK1δ and the overall survival of cancer patients revealed that high expression levels of CK1δ are associated with shorter patient survival in glioblastoma, lung cancer, and colorectal cancer patients (Schittek and Sinnberg, 2014; Richter et al., 2016). In contrast, a high expression of CK1δ with longer patient survival time can be found in breast cancer, chronic lymphocytic leukemia, and astrocytic glioma patients (Schittek and Sinnberg, 2014).
8.2. Neurologic diseases and disorders
Immunohistochemistry and gene expression studies have demonstrated that CK1 isoforms are expressed to a different extend in various parts of the nervous tissue (Schwab et al., 2000; Camacho et al., 2001; Yasojima et al., 2001; Chergui et al., 2005; Lohler et al., 2009). Other studies have been conducted to examine the relationship of CK1δ in brain tissue to specific pathological hallmarks by immunohistochemistry. Those studies found that CK1δ is associated with the corresponding specific pathological hallmarks of Alzheimer’s disease (AD), Down syndrome (DS), progressive supranuclear palsy (PSP), parkinsonism dementia complex of Guam (PDC), Pick’s disease (PiD), pallido-ponto-nigral degeneration (PPND) (Schwab et al., 2000), and familial advanced sleep phase syndrome (FASPS) (Xu et al., 2005; Ebisawa, 2007).
Alzheimer’s disease is a progressive neurodegenerative disease characterized by two specific brain lesions. One lesion is described by the appearance of neurofibrillary tangles (NFTs), abnormal neurites, known as neuropil threads, and neuritic plaques (NPs) in nerve cells (Buee et al., 2000). The second lesion is represented by granulovacuolar degeneration bodies (GVBs) formed in the hippocampus (Okamoto et al., 1991). Filaments of accumulated microtubule-associated tau protein can be considered as reliable marker for neurofibrillary degeneration (Flament et al., 1990). Localization analysis of CK1δ in pathological tissue of patients with Alzheimer’s disease revealed that the highest expression levels of CK1δ in pathological tissues can be detected in GVBs, and the second highest levels were detected in NPs. Low CK1δ expression levels could be detected in NFTs. In addition, hippocampal expression levels of CK1δ in Alzheimer’s patients were higher than in the control group. Interestingly, analysis of expression levels of CK1δ in different organs of AD patients revealed that elevated levels of CK1δ could only be found in the brain but not in peripheral organs. In brain tissue, the expression levels of CK1δ are remarkably increased in brain areas with increased tau pathology, while the expression levels of CK1δ are modestly elevated in areas with low NFT burden (Yasojima et al., 2000). Moreover, co-localization studies confirmed that CK1δ is co-localized with its interaction and substrate protein tau in the specific pathological tissue lesions like NFTs or GVBs (Schwab et al., 2000).
Typically, NFTs are composed of aggregated filaments formed by hyperphosphorylated tau. Tau can be phosphorylated by CK1δ at Ser-202, Thr-205, Ser-396, and Ser-404 in vitro. Furthermore, exogenous expression of CK1δ in HEK293 cells induced tau phosphorylation and resulted in reduced binding of tau to microtubules (Li et al., 2004). Mass spectrometry-based analysis of tau phosphorylation revealed that CK1δ and GSK3β are responsible for phosphorylation of most sites detected in hyperphosphorylated tau protein and that CK1δ together with GSK3β might play an important role in the pathogenesis of AD (Hanger et al., 2007). Therefore, CK1δ could be a potential target for AD treatment and recently, different inhibitors have been developed and may be therapeutically useful in the future. Interestingly, [11C]-labeled CK1 inhibitors have been developed as derivatives of already published difluoro-dioxolo-benzoimidazol-benzamide compounds (Richter et al., 2014), and have been used as radiotracers for CK1 in positron emission tomography (PET) imaging for both, diagnosis and therapeutic follow up (Gao et al., 2018). TDP-43 (transactive response DNA-binding protein of 43 kDa) has been characterized as a hallmark of frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) (Amador-Ortiz et al., 2007; Bigio, 2011). Moreover; some studies have also shown that TDP-43 is deposited in GVBs and can also be strongly associated with the clinical phenotype of AD. Here, TDP-43 contributes to the AD neurodegenerative process through beta-amyloid (Aβ)-dependent and Aβ-independent pathways (Chang et al., 2016). The relationship between CK1δ and TDP-43 is first reflected in their localization to GVBs. Moreover, by studying different stages of granulovacuolar degeneration the increased distribution areas of TDP-43 in brain lesions were found to be consistent with the distribution of CK1δ (Thal et al., 2011). In ALS, phosphorylation of TDP-43 by CK1δ has been indicated in vitro and by liquid chromatography-ion trap mass spectrometry (LC–MS/MS), thereby identifying 29 sites within TDP-43 being targeted by CK1δ (Kametani et al., 2009). TDP-43 intracellular accumulation and mislocalization seems to be influenced by CK1δ-mediated phosphorylation (Nonaka et al., 2016). Accordingly, inhibition of CK1δ-mediated phosphorylation of TDP-43 by different CK1δ inhibitors in a neuronal cell model as well as in a Drosophila model resulted in prevention of neurotoxicity and rescue of cells from cell death (Alquezar et al., 2016). Recently, a newly developed CK1δ inhibitor (IGS3.27) could also reduce phosphorylation of TDP-43 and additionally led to normalization of TDP-43 translocation (Posa et al., 2018). Therefore, CK1δ seems to be an interesting target of inhibition for ALS therapy concepts and a lot of effort has been made to improve CK1δ-selectivity as well as permeability of CK1δ-specific inhibitors through the blood-brain-barrier (BBB) (Alquezar et al., 2016; Joshi et al., 2016; Wager et al., 2017). Interestingly, the FDA-approved drug riluzole, which is already used as a clinical treatment for ALS, can inhibit CK1δ/ε, even though with a quite high half maximal inhibitory concentration (IC50) value (~16 μM) (Bissaro et al., 2018).
CK1δ is also involved in Parkinson’s disease (PD) as indicated by its ability to phosphorylate α-synuclein, one of the main component of Lewy bodies (LB), on Ser-87 and Ser-129 (Okochi et al., 2000). However, priming phosphorylation of α-synuclein on Tyr-125 seems to be necessary for CK1δ-mediated phosphorylation on Ser-129 (Kosten et al., 2014). Different mutations of α-synuclein have been characterized in PD, among them E46K, which seems to enhance Ser-129 phosphorylation (Mbefo et al., 2015).
Familial advanced sleep phase syndrome (FASPS) is a circadian rhythm sleeping disorder (CRSD) characterized by very early sleep onset and offset, as well as by a short τ period (the period in which behavior continues to oscillate in the absence of external cues) (Jones et al., 1999). The mammalian clock protein PER2 plays a key role in the regulation of circadian rhythms in FASPS. Etiology studies carried out by screening patients’ genes detected a missense mutation (T44A) in the PER2-binding domain of human CK1δ or a S662G mutation in the clock gene PER2 to be causative for FASPS (Xu et al., 2005; Xu et al., 2007). Introduction of human CK1δ T44A changed the circadian rhythm in both, transgenic Drosophila and transgenic mice. However, only changes observed in the mouse model were similar to the shortened circadian period observed in human. Changes observed in transgenic Drosophila were associated with a lengthened circadian period (Xu et al., 2005). A subsequently performed study confirmed in vivo phosphorylation of PER2 by CK1δ at Ser-662. Following site-specific phosphorylation of PER2 at Ser-662 the protein is characterized by increased stability and longer half-life compared to non-phosphorylated PER2 (Shanware et al., 2011). Hyeong-Min Lee and colleagues could show that orchestrated action of CK1δ and protein phosphatase 1 (PP1) can determine cell circadian periodicity thorough their influence on PER phosphorylation state. In CK1δ/ε-deficient fibroblasts, PER phosphorylation was significantly reduced and cell rhythm disappeared. In contrast, inhibition of PP1 is able to induce high phosphorylation levels of PER resulting in shortened cell rhythm (Lee et al., 2011a). Furthermore, different multi-phosphorylation site mutants of CK1δ were characterized to have different activity on PER2 stability, while the most significant impact on PER2 stability was observed for the CK1δ T347A mutant. These results indicate that PER2 stability can be influenced by site-specific phosphorylation of CK1δ at Thr-347, performed by other intracellular kinases (Eng et al., 2017).
8.3. CK1δ in the mediation of drug addiction
Via modulation of the circadian rhythm-regulating PER proteins or the dopaminoceptive signal integrator dopamine and cAMP-regulated neuronal phosphoprotein 32 (DARPP-32), CK1δ (but also CK1ε) can be involved in the development of drug addiction (Nairn et al., 2004; Falcon and McClung, 2009). Certain mutations in the gene coding for PER2 proteins have previously been linked to conditioned place preference (CPP) of cocaine and increased alcohol consumption, highlighting the role of PER2 in the development of addictive behavior (Abarca et al., 2002; Spanagel et al., 2005). Using the CK1δ/ε-specific inhibitor PF-670462 relapse-like alcohol consumption in rats could successfully be prevented by centrally mediated modulation of PER2 phosphorylation (Perreau-Lenz et al., 2012).
The neurotransmission integrator protein DARPP-32 is involved in mediating the motoric and rewarding effects of drugs like heroin (Fernandez et al., 2006; Mahajan et al., 2009). Following activation of dopamine D1 receptors by heroin PKA-mediated phosphorylation of DARPP-32 is induced, finally leading to inhibition of PP1 by phosphorylated DARPP-32 and subsequent increase in drug-related rewarding properties (Fernandez et al., 2006). In this pathway CK1δ and ε can be involved by phosphorylating DARPP-32 at Ser-130, resulting in increased phosphorylation of Thr-34 by PKA and subsequent DARPP-32/PP1 signaling (Nairn et al., 2004; Svenningsson et al., 2005). Also here, treatment with the CK1δ/ε-specific inhibitor PF-670462 attenuated methamphetamine-induced locomotor activity by inhibition of DARPP-32 phosphorylation mediated by CK1δ and ε (Bryant et al., 2009). Furthermore, treatment with the highly potent CK1δ/ε-specific inhibitor PF-5006739 resulted in attenuation of drug-seeking behavior in rats trained to self-administer the synthetic opioid fentanyl (Wager et al., 2014). In conclusion, specific inhibitors of CK1δ (and ε) might be of future use for the treatment of drug addiction or alcohol abuse.
8.4. Metabolic diseases
Apart from sleeping disorders also the onset and severity of metabolic diseases, including obesity and type 2 diabetes, can be linked to circadian clock disorders. Therefore, as a key regulator of the circadian clock, CK1δ can also affect metabolic dysfunction and can serve as promising drug target. In this context, glucose tolerance was improved by daily administration of a CK1δ- (and ε-)specific inhibitor (PF-5006739) to mice in a model for diet-induced obesity or in a genetic mouse model for obesity (ob/ob) (Cunningham et al., 2016). CK1δ can furthermore be linked to circadian metabolic regulation via PGC-1α (peroxisome proliferator-activated receptor γ co-activator 1α), a transcriptional coactivator coordinating circadian metabolic rhythms by simultaneously regulating the expression of metabolic and clock genes. Phosphorylation of PGC-1α by CK1δ enhances its proteasomal degradation, thereby inhibiting the transcriptional function of PGC-1α in cultured hepatocytes, resulting in decreased gluconeogenesis gene expression and glucose secretion (Li et al., 2011b). In contrast to this observation, the treatment of a human adipocyte cell line with CK1δ-specific inhibitors resulted in increased basal and insulin-stimulated glucose uptake (Xu et al., 2015). Xu and colleagues reported that the expression of adiponectin, an important adipokine secreted by adipose tissue, was associated with CK1δ expression in adipose tissue of morbidly obese patients. Furthermore, in vitro studies revealed that Ser-174 and Thr-235 of adiponectin are phosphorylated by CK1δ in vitro, contributing to modulation of the ability of adiponectin to form biologically active high molecular complexes (Xu et al., 2015).
8.5. Hijacking of mammalian CK1 pathways by CK1s from parasites
There is increasing evidence suggesting that CK1 is associated with infectious diseases by the manipulation of host cell CK1 signaling pathways by intracellular parasites, mediated through the export of their own CK1 into the host cell (Sacerdoti-Sierra and Jaffe, 1997; Silverman et al., 2010a; Silverman et al., 2010b; Dorin-Semblat et al., 2015; Jiang et al., 2018). Their survival depends on their ability to subvert the host cell to acquire nutrients or to evade the immune response (Lamotte et al., 2017). For instance, Leishmania resides in the parasitophorous vacuole of macrophages from where it modifies macrophage biology and attenuates the immune response (Lamotte et al., 2017). Plasmodium infects red blood cells (RBCs), and modifies their cell membrane to favor the adhesion of the infected RBCs to the vascular endothelium and thus avoid clearance by the spleen (Zhang et al., 2015b). In these two parasites, CK1 has been shown to be excreted and could thus contribute to the reprogramming of the respective host cells (Sacerdoti-Sierra and Jaffe, 1997; Silverman et al., 2010a; Dorin-Semblat et al., 2015). The identified host functions of parasitic CK1s imply that these kinases could replace mammalian CK1s to ensure similar functions, including phosphorylation of human CK1 substrates and regulation of human CK1 signaling pathways (Liu et al., 2009). The BLAST and reverse BLAST of the parasitic CK1s with human CK1 shows that they are closely related to human CK1 (see Supplementary Fig. 2 for alignment). More specifically, these kinases display a higher level of identity toward human CK1δ TV1, suggesting that they might be preferentially hijacking the functions of this paralog. The protein sequences of these orthologs is absolutely conserved within each different species for Trypanosoma brucei, Trypanosoma cruzi, Plasmodium, and Leishmania (Rachidi et al., 2014). This finding suggests that there is a strong evolutionary selection pressure to maintain the protein sequence unchanged and as closely related to its human counterpart as possible.
The protein organization of parasitic CK1s is very similar to that of human CK1δ. Indeed, all the residues involved in ATP binding, the gatekeeper (Met-82), the DFG, the KHD, and the SIN motifs, as already defined in Fig. 5, are conserved, suggesting that they are crucial for CK1 function. In contrast, other motifs like the CLS are less conserved. The glycine-rich ATP binding loop is conserved in all the selected CK1 sequences, except for TcCK1. The amino acid substitution present in TcCK1 does not affect ATP binding as those residues are conserved, but instead its potential regulation is affected. Indeed, the GSGSFG domain is replaced by GAGSFG in TcCK1. However, in Leishmania CK1.2, the two serines of this motif are phosphorylated during differentiation, suggesting that they could have a regulatory effect for LmCK1.2 ((Tsigankov et al., 2014) and own unpublished data); in Trypanosoma brucei, Plasmodium falciparum, and in Toxoplasma gondii, the first serine of the motif is phosphorylated (Nett et al., 2009; Treeck et al., 2011). However, despite phosphorylation of the glycine triad during differentiation, it has been shown that LmCK1.2 is still active, although its activity is lower after differentiation (Rachidi et al., 2014). The catalytic loop motif represents a domain for which a T. cruzi-specific substitution has been identified. In all parasitic CK1s except for T. cruzi the motif DVKPDN (present in human CK1δ) is replaced by the motif DIKPDN, which is similar to that of human CK1α. In T. cruzi CK1 the human motif is replaced by the motif DMKPDN. These changes could be important, as they seem to be conserved. Altogether, these findings indicate that parasitic CK1s are closely related to CK1δ, but additionally have characteristics of CK1α and CK1ε.
However, still very little is known about the functions of these kinases in the parasites and more importantly about their functions in the host cell. The most studied CK1s are that of Plasmodium and Leishmania:
Plasmodium falciparum has only one CK1, PfCK1 (PF3D7_1136500), which presents 69% of identity with human CK1 in the kinase domain (Barik et al., 1997). PfCK1 was shown to be essential for completion of the asexual intra-erythrocytic cycle (Solyakov et al., 2011). It is expressed throughout blood stage, localizes in the parasite but also in the host red blood cell, especially associated to the surface of the RBC during the first step of infection (Dorin-Semblat et al., 2015). Indeed, data from Magowan et al. suggests that PfCK1 could be phosphorylating protein 4.1 and the mature-parasite-infected erythrocyte surface antigen (MESA), two components of the erythrocyte membrane skeleton (Magowan et al., 1998). Moreover it has been shown to be secreted in the culture medium. This finding suggests that PfCK1 could be involved in priming other RBCs for subsequent invasion (Dorin-Semblat et al., 2015). A study of the PfCK1 interactome indicates that, similarly to other CK1s, it has multiple binding partners and thus regulates multiple pathways, including transcription, translation, and protein trafficking. Finally, PfCK1 function is likely to be essential for parasite proliferation in erythrocytes.
Leishmania donovani has six CK1 paralogs. Two paralogs, LdBPK_351020.1 and LdBPK_351030.1 (LmCK1.2), are closely related (67% identity), but mainly differ within their C-terminal domains (Martel et al., 2017). They display the highest identity to human CK1. Recently, Martel et al. showed that LdBPK_351020.1 is not essential for parasite survival and could have a role during stationary phase (Martel et al., 2017). The four other paralogs, LdBPK_303530.1, LdBPK_251640.1, LdBPK_041230.1, and LdBPK_271680.1, are extremely divergent from the first two (Rachidi et al., 2014). Little is known about the functions of the six paralogs, except for LdBPK_271680.1, which contains an excretion signal and is important for regulating growth of cultured parasites and virulence (Dan-Goor et al., 2013), and LdBPK_351030.1, which is the most abundant CK1 in Leishmania and the only paralog described as having a function in the host cell (Rachidi et al., 2014; Silverman et al., 2010a). LdBPK_351030.1 (LmCK1.2) is active in both, promastigotes and amastigotes, and can be inhibited by D4476, a CK1-specific inhibitor (Rachidi et al., 2014). LmCK1.2 seems to have been selected for its capacity to interact with and phosphorylate host proteins to modulate macrophage-mediated processes to favor the survival of the parasite. Four pieces of evidence support this hypothesis: (i) LmCK1.2 is released into extracellular compartments inside vesicles (Silverman et al., 2010a), (ii) LmCK1.2 is strictly conserved between Leishmania species and closely related to human CK1δ, (iii) Leishmania CK1 phosphorylates human interferon alpha/beta receptor 1 (IFNAR1), which leads to the attenuation of the cellular response to interferon alpha (Liu et al., 2009), and (iv) LmCK1.2 is essential for Leishmania intracellular survival in the mammalian host. Little is known about the functions of LmCK1.2 in the parasite, as only few substrates have been found, among them heat shock proteins 90 and 70 (Hsp90 and Hsp70), and no interaction partners ((Hombach-Barrigah et al., 2019) and Martel et al., manuscript in preparation). LmCK1.2 is sensitive to known human CK1 inhibitors and is inhibited by CKI-7, IC261, and D4476 (Rachidi et al., 2014). Interestingly, results obtained for IC261 (IC50 of 10 μM for LmCK1.2 versus 4.8 μM for mammalian CK1) suggest that the ATP binding pocket of LmCK1.2 might be structurally closer to that of CK1α than to that of CK1δ (Rachidi et al., 2014). As Leishmania CK1 is essential for parasite survival in the macrophage, it constitutes a perfect target for anti-leishmanial therapy. Several screens looking for small molecules able to inhibit LmCK1.2 and thus kill the parasites were performed and despite the high identity to human CK1, small molecules were identified to more specifically target Leishmania CK1 (Allocco et al., 2006; Marhadour et al., 2012; Durieu et al., 2016).
Altogether, by being exported into the host cells, parasitic CK1s take over functions normally performed by human CK1. Studying these orthologs could bring more knowledge on the functions of human CK1s and lead to new therapeutic strategies preventing hijacking of the host cell by parasites.
9. Modulating CK1δ activity
Recently, interest in modulating the activity of CK1δ has increased enormously. The main focus is directed to the development of highly effective small molecule inhibitors (SMIs) since deregulation of CK1δ expression and activity levels contributes to the pathogenesis of severe disorders, among them cancer and neurologic diseases like AD, PD, ALS, and sleeping disorders. However, due to the existence of several highly conserved CK1 isoforms (CK1α, γ1–3, δ, ε) with overlapping or opposed physiological and pathophysiological functions, it is very challenging to design SMIs being selective for CK1δ only. Furthermore, there is increasing evidence that posttranslational modifications of CK1δ, especially site-specific phosphorylation within its C-terminal regulatory domain, modulates the efficiency of SMIs (Bischof et al., 2012; Bischof et al., 2013; Richter et al., 2014). It is also very challenging and of high clinical interest to develop SMIs with higher affinity toward CK1δ mutants with increased oncogenic potential since their use would expand the therapeutic window and decrease the occurrence of severe side effects enormously.
Within the last 30 years more and more CK1-specific SMIs have been developed. Since most of the compounds described so far are classified as ATP-competitive inhibitors (type I inhibitors), it cannot be excluded that due to structural similarities within the ATP binding site additional kinases and other ATP binding proteins are also inhibited to some extent.
Whereas the first CK1 inhibitor, CKI-7 (N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide), did not show any CK1 isoform-specificity (Chijiwa et al., 1989), IC261 (3-[(2,4,6-trimethoxyphenyl)-methylidenyl]-indolin-2-one) (Mashhoon et al., 2000) and D4476 (4-[4-(2,3-dihydro-benzo)[1,4]dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl]-benzamide) (Rena et al., 2004) exhibit a higher selectivity toward CK1δ and ε. Although CK1δ/ε-specific inhibition by IC261 affects site-specific phosphorylation of Bid and induces apoptosis in tumor cells (Izeradjene et al., 2004), several cellular effects of IC261 are not due to inhibition of CK1δ/ε. Among these effects are the inhibition of microtubule polymerization, which is due to the direct binding of IC261 to tubulin (Cheong et al., 2011; Stoter et al., 2014), and the blocking of voltage-gated sodium channels, which are implicated in tumor progression (Fohr et al., 2017). Furthermore, due to the fact that many CK1-specific inhibitor compounds are classified as ATP-competitive inhibitors (type I inhibitors) comparison of their efficacy is difficult since their IC50 values have been assessed at different ATP concentrations (Supplementary Table 1) (reviewed in Knippschild et al., 2014).
Several structure-based virtual screens resulted in the identification of various CK1δ-specific inhibitors, among them amino-anthraquinone analogues (Cozza et al., 2008), N6-phenyl-1H-pyrazolo[3,4-d]pyrimidine-3,6-diamine derivatives (Yang et al., 2012), and the dihydroxyquinoline sulphonamide NSC45572 (Myrianthopoulos et al., 2017). A group-based quantitative structure and activity relationship (GQSAR) model based on N-benzothiazolyl-2-phenyl acetamide derivatives (Salado et al., 2014) was generated by Joshi and co-workers. Using this model, site-specific molecular fragments of individual compounds allow for the interpretation of observed differences in the biological activity of these compounds. Compounds identified using this approach could be used as lead substances against CK1δ, able to block CK1δ-mediated site-specific phosphorylation of TDP-43 and might be useful for developing new therapeutic concepts for the treatment of ALS (Joshi et al., 2016).
In addition, several benzimidazole-based CK1-specific inhibitors have demonstrated their potential to specifically inhibit CK1δ and ε (SR-3029 and SR-2890 (Bibian et al., 2013); Bischof-5 and Bischof-6 (Bischof et al., 2012); Richter-2 (Richter et al., 2014); IWP-2, IWP-4, and compound 19 (Garcia-Reyes et al., 2018)). Although Bischof-5, a 2-benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivative with a trifluoromethyl substitution on the phenyl ring, exhibits IC50 values toward rat CK1δ and human CK1δ transcription variants 1 and 2 between 22 and 42 nM, its ability to inhibit proliferation of various tumor cell lines is limited (Bischof et al., 2012). However, the specificity, activity, and anti-proliferative activity has further been increased by a difluoromethyldioxolo group on the benzimidazole, finally leading to the development of Richter-2 (IC50 CK1δ = 0.14 μM vs. IC50 CK1ε = 0.52 μM (Richter et al., 2014)).
Optimization of benzimidazole derivatives by Bibian and coworkers included enhancement of the interaction of compounds with Arg-13 of CK1δ, thereby reducing off-target effects by altering the substituents on the benzimidazole unit (either R1 or R2) and increasing inhibition of CK1δ, which could be achieved by using piperazine or N-methyl piperazine as R4 and thiophene, furan, and 3-fluorophenyl groups as R3. These modifications finally resulted in development of the highly CK1δ/ε-selective inhibitors SR-2890 and SR-3029 with improved inhibitory and anti-proliferative features (Bibian et al., 2013).
Interestingly, due to structural similarities to benzimidazole-based CK1 inhibitors, especially to Bischof-5 (Bischof et al., 2012), inhibitors of Wnt production (IWPs), known to be antagonists of the Wnt pathway by preventing Wnt ligand palmitoylation through inhibition of the membrane-bound O-acyltransferase porcupine (Porcn), have recently been described to specifically inhibit CK1δ. This led to the development of improved IWP-based ATP-competitive inhibitors of CK1δ and to the conclusion that the effects observed for IWPs are not only due to Porcn inhibition, but also to effects on CK1δ/ε-related signaling (Garcia-Reyes et al., 2018).
Quite recently, [11C] labeled highly potent difluoro-dioxolo-benzoimidazol-benzamides have been generated, which have a high potential to be prognostically used as PET radiotracers for imaging of AD (Gao et al., 2018).
Several additional small molecule inhibitors, which have been described to exhibit dual specificity, will be introduced in the following paragraph. Compound (R)-DFR053 ((R)-2-(1-hydroxybut-2-ylamino)-6-[3-(2-pyridyl)phenylamino]-9-isopropylpurine), a roscovitine derivative, specifically targets CK1δ and CDK5, and could be useful for the analysis of pathways involved in neurodegeneration and therapeutic applications in AD (Oumata et al., 2008). The pyrazolo-pyridine analogues MRT00055778 (N1-[4-(5-methyl-3-phenylisoxazol-4-yl)pyrimidin-2-yl]acetamide) and MRT00033659 (5-(3-acetamidophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine) have been shown to inhibit CK1δ and Chk1, finally resulting in stabilization of the p53 pathway (Huart et al., 2013). In addition, the Clk-specific inhibitor TG003, a benzothiazole derivative (Muraki et al., 2004), has been described to inhibit Clk and CK1 isoforms in a mouse model for mechanical allodynia and thermal hyperalgesia (Kurihara et al., 2014). Based on structural similarity between N-(benzo[d]thiazol-2-yl)-2-phenylacetamide derivatives with CK1 inhibitory activity and 1-(benzo[d]thiazol-2-yl)-3-phenylureas which have been designed as Aβ-binding alcohol dehydrogenase (ABAD) inhibitors, several benzothiazolylphenylureas could be identified as dual-specific inhibitors, among them K690 and K691, presenting an interesting class of dual-specific anti-AD therapeutics (Benek et al., 2018).
Several diaryl-isoxazoles and -imidazoles have been described as dual inhibitors against p38α MAPK and CK1δ/ε. Whereas PF-670462 (4-[3-cyclohexyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl]-pyrimidin-2-ylamine) possesses only poor CK1 isoform selectivity compound PF-4800567 exhibits a much stronger inhibition of CK1ε than CK1δ (Badura et al., 2007; Walton et al., 2009).
Seerden and colleagues could demonstrate structure-activity relationship of 4-(40-fluorophenyl)imidazoles as specific inhibitors of p38α MAPK, CK1δ, or JAK2 with IC50 values lower than 100 nM (Seerden et al., 2014).
In addition, substituted isoxazoles 1 and 2 with the typical vicinal pyridin-4-yl/4-F-phenyl pharmacophore (Peifer et al., 2009), which have been originally generated and characterized as ATP-competitive inhibitors for p38α MAPK (IC50 p38α MAPK 1 = 0.45 μM and p38α MAPK 2 = 2.2 μM) (Peifer et al., 2007; Peifer et al., 2008), exhibited significant inhibition of CK1δ at 10 μM. Molecular modeling of compound 1 resulted in an optimizing strategy to develop (E)-3-(2,4-dimethoxy-phenyl)-N-(4-[5-(4-fluoro-phenyl)-2-methylsulfanyl-3H-imidazol-4-yl]-pyridin-2-yl)-acrylamide (compound 17) and (E)-3-(2,4-dimethoxy-phenyl)-N-(4-[5-(4-fluoro-phenyl)-2-methanesulfinyl-3H-imidazol-4-yl]-pyridin-2-yl)-acrylamide) (compound 18) as dual-specific inhibitors with high selectivity toward CK1δ at an ATP concentration of 100 μM (compound 17: IC50 p38α MAPK = 19 nM, IC50 CK1δ = 4 nM, IC50 CK1ε = 73 nM; compound 18: IC50 p38α MAPK = 41 nM, IC50 CK1δ = 5 nM, IC50 CK1ε = 447 nM) (Peifer et al., 2009).
Recently, 3-(2,5-dimethoxyphenyl)-N-(4-(5-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-4-yl)-pyridin-2-yl)-propanamide (compound 11b) and 4-(2,5-dimethoxyphenyl)-N-(4-(5-(4-fluorphenyl)-2-(methylthio)-1H-imidazol-4-yl)-pyridin-2-yl)-1-methyl-1H-pyrrole-2-carboxamide (compound 16b) have been developed with high specificity toward CK1δ (compound 11b: IC50 CK1δ = 4 nM, IC50 CK1ε = 25 nM, IC50 p38α MAPK = 10 nM), 16b (IC50 CK1δ = 8 nM, IC50 CK1ε = 81 nM, p38α MAPK = 10 nM) and remarkable efficacy for inhibiting the growth of various pancreatic tumor cell lines (Halekotte et al., 2017).
In order to develop a CK1δ/ε-specific inhibitor able to cross the blood-brain-barrier (BBB) structure-based drug design resulted in synthesis of PF-5006739 (4-{4-(4-fluorophenyl)-1-[1-(1,2-oxazol-3-ylmethyl)piperidin-4-yl]-1H-imidazol-5-yl}pyrimidin-2-amine), exhibiting an IC50 value of 3.9 nM for CK1δ and 7 nM for CK1ε and showing centrally mediated delay of the circadian rhythm in animal models (Wager et al., 2014).
Furthermore, N-(1H-pyrazol-3-yl)-quinazolin-4-amines have been developed to specifically inhibit CK1δ/ε. Compounds 3c and 3d exhibited good binding interactions with CK1δ/ε and could therefore serve for optimizing their ability to specifically inhibit CK1δ/ε (Karthikeyan et al., 2017).
Finally, natural products with inhibitory activity against CK1δ/ε, like chloromethylhalicyclamine B, isolated from the marine sponge Acanthostrongylophora ingens, allow for the development of highly active CK1δ/ε-specific inhibitors, structurally different to the inhibitors known so far (Esposito et al., 2016).
Since SMIs often exhibit low bioavailability, off-target effects, and severe side effects, there are only some reports demonstrating biological activity of CK1δ-specific inhibitors in animal models (Table 6). Therefore, interest in identification and validation of synthetic peptides able to inhibit CK1δ kinase activity or to block the interaction of CK1δ with cellular proteins has increased remarkably.
Table 6.
Biological impact of CK1δ-specific inhibitors in animal models.
| Inhibitor | Effects |
|---|---|
| PF-670462 |
|
| Wager-6 PF-5006739 |
|
| SR-3029 |
|
| Salado-20, 24, 35 |
|
In this context a peptide library based on the amino acid sequences of human CK1δ TV1 and 2 was recently used to determine the dominant contacts of the interaction surface between α-tubulin and CK1δ. Peptide P39 (also named peptide δ−361 = peptide sequence starting at CK1δ amino acid 361) showed the strongest interaction with GST-α-tubulin. Furthermore, binding of δ−361 to α-tubulin also resulted in selective inhibition of CK1δ-mediated phosphorylation of GST-α-tubulin, whereas phosphorylation of α-casein was not affected. In cells, δ−361 is able to disturb the CK1δ/α-tubulin interaction, finally leading to microtubule destabilization and cell death (Kruger et al., 2016).
Previously, interaction of CK1 isoforms with the DEAD-box RNA helicase DDX3X has been reported to result in stimulation of CK1 kinase activity and Wnt signaling (Cruciat et al., 2013). Moreover, Wnt-associated mutations of DDX3X can be associated with medulloblastoma. Using a CK1δ/ε-derived peptide library to fine-map the interaction domains for DDX3X on CK1δ and ε, several CK1δ- or ε-derived peptides were identified being able to interact with DDX3X, among them peptides δ−1, δ−41, ε−1, and ε−41. Furthermore, in vitro kinase reactions revealed the ability of peptides δ−1, δ−41, and ε−41 (but not ε−1) to block the activation of CK1δ/ε by DDX3X, probably due to inhibition of the activating interaction of DDX3X with CK1δ/ε. Furthermore, peptides δ−1, δ−41, and ε−41 were able to inhibit stimulation of CK1 kinase activity in cell culture. Considering that mutations in DDX3X identified in medulloblastoma patients increase the activity of CK1 in living cells, leading to aberrant stimulation of CK1-mediated pathways, such as Wnt/β-catenin and Hh signaling, the identified interaction-blocking peptides could provide a powerful tool for new therapeutic therapy concepts for the treatment of Wnt/β-catenin- or Hh-driven cancers (Dolde et al., 2018).
Recently, a peptide microarray was also used for mapping the interaction regions within intrinsically disordered regions of human Axin 1, a scaffolding protein playing central roles in Wnt signaling. The interaction domains necessary for Axin 1 to interact with CK1δ/ε were identified and it could be shown that Axin 1 and Dvl compete for CK1δ/ε-mediated site-specific phosphorylation thereby pointing to an important role of Axin 1 in modulating phosphorylation of Dvl by CK1δ/ε and activation of the canonical Wnt pathway (Harnos et al., 2018).
While selective inhibition of CK1δ is desired in the case of cancer or neurodegenerative disease, in the context of regenerative processes also activation of CK1 kinase activity could be of therapeutic benefit. For instance, kinase activity of CK1δ and ε has been shown to be essential in a model for neuronal regeneration (Bischof et al., 2011). However, no small molecule-based activators of CK1δ have been available so far, and while promising results have initially been published reporting the activation of CK1α by the small molecule pyrvinium (Thorne et al., 2010) a later performed study disproved the described effects. Instead of directly activating CK1α pyrvinium mediates its effects via a complex mechanism involving down-regulation of PKB/Akt and activation of GSK3 (Venerando et al., 2013).
10. Concluding remarks
Numerous studies mainly performed within the last two decades provide strong evidence demonstrating that CK1 isoform δ is a key player in several cellular signal transduction pathways. Consequently, if control mechanisms regulating expression and activity of CK1δ are rendered ineffective, dysregulation of CK1δ can contribute to the pathogenesis of certain diseases. Due to this reason numerous CK1-specific small molecule inhibitors have been developed in recent years. Although the development of CK1 isoform-specific inhibitors remains a major challenge to the scientific community, some compounds already showed promising results in cell culture- and animal-based studies analyzing efficacy e.g. for the treatment of cancer or neurodegenerative diseases.
Supplementary Material
Acknowledgements
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series – a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The authors would like to thank Martin Stöter for scientific and experimental input and discussion of data presented in chapter two.
The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org/wiki/CSNK1D.
Funding
This work was supported by a grant to Uwe Knippschild from the Deutsche Forschungsgemeinschaft (DFG) [grant number KN356/9–1], a grant to Joachim Bischof from the Else Kröner-Fresenius-Stiftung [grant number 2017_A142], and a grant to Najma Rachidi [grant number ANR-13-ISV3–0009]. The sponsors had no influence on study design, analysis and interpretation of data, on the writing of the report, and on the decision to submit the article for publication.
Abbreviations:
- A
alanine
- aa
amino acids
- ABAD
Aβ-binding alcohol dehydrogenase
- AD
Alzheimer’s disease
- AIB1
amplified in breast cancer 1
- AKAP
A-kinase anchor protein
- ALS
amyotrophic lateral sclerosis
- APC
adenomatous polyposis coli
- APC/C Cdh1
anaphase-promoting complex/cyclosome in complex with activator protein CDH1
- ARF GAP1
ADP-ribosylation factor GTPase-activating protein
- Arg or R
arginine
- ARNT
aryl hydrocarbon receptor nuclear translocator
- Asn or N
asparagine
- Asp or D
aspartic acid
- ATM
ataxia telangiectasia mutated
- Atoh1
proneural basic helix-loop-helix (bHLH) transcription factor
- ATP
adenosine triphosphate
- Aβ
beta-amyloid
- BACE1
β-secretase
- BBB
blood-brain-barrier
- bHLH
basic helix-loop-helix
- BLAST
basic local alignment search tool
- BLOC-1
biogenesis of lysosome-related organelles complex-1
- BMAL1
Brain and Muscle ARNT-Like 1
- BYSL
bystin-like protein
- CAT
catalytical
- CCdh1
cyclosomecadherin1
- CDC
cell division cycle
- CDK
cyclin-dependent kinase
- CG-NAP
Golgi N-kinase anchoring protein
- Chk1
checkpoint kinase 1
- Chk2/Cds1
checkpoint kinase 2/replication checkpoint kinase Cds1
- Ci
cubitus interruptus
- Ci-155
full-length cubitus interruptus
- CiA
cubitus interruptus positive transcription factor
- CiR
cubitus interruptus repressive transcription factor
- CK1BP
casein kinase 1 binding protein
- CK1α/γ1–3/δ/ε
casein kinase 1 alpha/gamma 1–3/delta/epsilon
- CKL2
CDC-like kinase 2
- CLL
chronic lymphocytic leukemia
- C-lobe
C-terminal lobe
- CLS
centrosome localization signal
- CPI-17
protein kinase C-potentiated myosin phosphatase inhibitor of 17 kDa
- CPP
conditioned place preference
- CREB
cyclic AMP response element-binding protein
- CRY
cryptochrome
- CSNK1D
casein kinase 1 delt
- C-terminus
carboxy-terminus
- Cx43
connexin-43
- Cys or C
cysteine
- DARPP-32
dopamine and cAMP-regulated neuronal phosphoprotein 32
- dCK
deoxycytidine kinase
- DD
dimerization domain
- DDX3X
DEAD-box RNA helicase 3 X-linked
- Dhh
desert hedgehog
- Dma1
E3 ubiquitin-protein ligase Dma1
- Dnmt1
DNA methyltransferase 1
- DPC
dystrophin-associated protein complex
- Dpr1a
dapper1a
- DS
Down syndrome
- DUF1669
domain of unknown function 1669
- Dvl
dishevelled
- EB1
microtubule plus-end-binding protein 1
- eIF6/Tif6p49
eukaryotic initiation factor 6
- Emi2
endogenous meiotic inhibitor 2
- ENP1
essential nuclear protein 1
- ePK
eukaryotic protein kinase
- ERα
estrogen receptor α
- FAM83
family with sequence similarity 83
- FASPS
familial advanced sleep phase syndrome
- FoxG1
forkhead box G1
- FTLD
frontotemporal lobar degeneration
- Fzd
frizzled
- Gli
glioma-associated oncogene
- Gln
glutamine
- Glu
glutamic acid
- Gly or G
glycine
- GSK3β
glycogen synthase kinase 3β
- GST
glutathione S-transferase
- GVBs
granulovacuolar degeneration bodys
- Hh
hedgehog
- HIF-1α
hypoxiainducible factor 1α
- hnRNP A1
heterogeneous nuclear ribo-nucleoprotein A1
- HPI
hydrophobic pocket I
- HRII
hydrophobic region II
- Hsp90/70
heat shock protein 90/70
- HTP
high-throughput
- Huwe1
E3 ubiquitin-protein ligase Huwe1
- IC50
half maximal inhibitory concentration
- ICP0
human herpes virus (HHV) E3 ubiquitin ligase
- IFNAR1
interferon alpha/beta receptor 1
- Ihh
Indian hedgehog
- IKK
inhibitor of κB (IκB) kinase
- Ile or I
isoleucine
- ISC
intestinal stem cell
- IWP
inhibitor of Wnt production
- kcat
catalyst rate constant
- KD
kinase domain
- KHD
kinesin homology domain
- Km
Michaelis constant
- LATS1/2
large tumor suppressor kinase 1/2
- LEF-1
lymphocyte enhancer factor-1
- Leu or L
leucine
- LRP
lipoprotein receptor-related protein
- LTP
low-throughput
- Lys or K
lysine
- M
molar
- m6A
N6-methylation
- Mam1
Monopolin complex subunit Mam1
- MAP
microtubule-associated protein
- MAPK
mitogen activated protein kinase
- MAPT
microtubule-associated protein tau
- MBP
myelin basic protein
- MBP
myelin basic protein
- MDM2
murine double minute 2 homolog
- MEF
mouse embryonic fibroblast
- Met or M
methionine
- MESA
mature-parasite-infected erythrocyte surface antigen
- mGluR
group I metabotropic glutamate receptor
- MST1/2
mammal sterile-20 like kinase 1/2
- mTNFα
transmembrane tumor necrosis factor α
- MTOC
microtubule-organizing center
- MTSS1
metastasis suppressor 1
- NEDD4
neural precursor cell expressed developmentally down-regulated protein 4
- NFAT1
nuclear factor of activated T-cells 1
- NFTs
neurofibrillary tangles
- N-lobe
N-terminal lobe
- NLS
nuclear localization signal
- nm23-H1
nucleoside diphosphate kinase A
- Nop56
nucleolar protein 56
- NPs
neuritic plaques
- N-terminus
amino-terminus
- p53
tumor protein 53
- P-bodies
processing bodies
- PCP
planar cell polarity
- PD
Parkinson’s disease
- PDB
protein data bank
- PDC
parkinsonism dementia complex of Guam
- PET
positron emission tomography
- PER
period circadian protein homolog
- PGC-1α
proliferator-activated receptor γ co-activator 1α
- Phe or F
phenylalanine
- PiD
Pick’s disease
- PKA
protein kinase A
- PKB/Akt
protein kinase B
- PKCa
protein kinase C alpha
- PKD2
protein kinase D2
- Plk1
polo-like kinase 1
- Porcn
porcupine
- PP1
protein phosphatase 1
- PPND
pallido-ponto-nigral degeneration
- ppUL44
human cytomegalovirus phosphoprotein
- Pro or P
proline
- PS-2
presenilin-2
- pSer
phospho-serine
- PSP
progressive supranuclear palsy
- Ptch
patched
- pThr
phospho-threonine
- RanBPM
Ran-binding protein in the microtubule-organizing center (MTOC)
- RBC
red blood cell
- Rec11
meiotic recombination protein Rec11
- Rec8
meiotic recombination protein Rec8
- REGγ
11S regulatory particles, 28-kDa proteasome activator γ
- RhoA
Ras homolog family member A
- RPL4/8/13
ribosomal protein L4/8/13
- SCF
skp cullin F-box containing complex
- SCS
sulfatide and cholesterol-3-sulfate
- Ser or S
serine
- Shh
sonic Hedgehog (Shh)
- Sid4
septation initiation protein sid4
- Sid4
septation initiation protein Sid4
- SMAD
SMA/mothers against decapentaplegic
- SMI
small molecule inhibitor
- Smo
smoothened
- SNAP25
synaptosomal nerve-associated protein 25
- SPBs
spindle pole bodies
- SPRY2
sprouty2
- Sre1N
yeast sterol regulatory element-binding protein homolog
- SRR-1
sensitivity to red light reduced 1
- STAG3
stromal antigen 3
- SV2A
synaptic vesicle protein 2A
- SV40 T-Ag
simian virus 40 large T-antigen
- SV40
simian virus 40
- Swi6
chromatin-associated protein swi6
- TAZ
tafazzin
- TDP-43
TAR DNA-binding protein of 43 kDa
- TEAD
TEA domain
- TGN
trans Golgi network
- Thr or T
threonine
- TOP2A
topoisomerase IIα
- TOPOII-α
topoisomerase II α
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TV
transcription variant
- Tyr or Y
tyrosine
- UHRF1
ubiquitin-like containing PHD and RING finger domains 1 protein
- UVB
ultraviolet B radiation
- Val
valine
- Vangl2
planar cell polarity protein Van Gogh-like 2
- Vmax
maximum enzyme reaction velocity
- Wee1
Wee1 G2 checkpoint kinase
- Wnt
Wingless/Int-1
- X
any amino acid
- Y
any amino acid except serine or threonine
- YAP
yes-associated protein
- β-TrCP
beta-transducin repeat containing protein
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gene.2019.144005.
References
- Abarca C, Albrecht U, Spanagel R, 2002. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc. Natl. Acad. Sci. U. S. A 99 (13), 9026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis P, Pinna LA, Meggio F, Marin O, Goris J, Vandenheede JR, Merlevede W, 1989. A synthetic peptide substrate specific for casein kinase I. FEBS Lett. 259 (1), 75–78. [DOI] [PubMed] [Google Scholar]
- Albornoz A, Yanez JM, Foerster C, Aguirre C, Pereiro L, Burzio V, Moraga M, Reyes AE, Antonelli M, 2007. The CK1 gene family: expression patterning in zebrafish development. Biol. Res 40 (2), 251–266. [DOI] [PubMed] [Google Scholar]
- Allocco JJ, Donald R, Zhong T, Lee A, Tang YS, Hendrickson RC, Liberator P, Nare B, 2006. Inhibitors of casein kinase 1 block the growth of Leishmania major promastigotes in vitro. Int. J. Parasitol 36 (12), 1249–1259. [DOI] [PubMed] [Google Scholar]
- Alquezar C, Salado IG, de la Encarnacion A, Perez DI, Moreno F, Gil C, de Munain AL, Martinez A, Martin-Requero A, 2016. Targeting TDP-43 phosphorylation by casein kinase-1delta inhibitors: a novel strategy for the treatment of frontotemporal dementia. Mol. Neurodegener 11 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsheich-Bartok O, Haupt S, Alkalay-Snir I, Saito S, Appella E, Haupt Y, 2008. PML enhances the regulation of p53 by CK1 in response to DNA damage. Oncogene 27 (26), 3653–3661. [DOI] [PubMed] [Google Scholar]
- Alvisi G, Marin O, Pari G, Mancini M, Avanzi S, Loregian A, Jans DA and Ripalti A (2011). “Multiple phosphorylation sites at the C-terminus regulate nuclear import of HCMV DNA polymerase processivity factor ppUL44.” Virology 417(2): 259–267. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW, 2007. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann. Neurol 61 (5), 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I, 2002. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16 (9), 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello-Miranda O, Zagoriy I, Mengoli V, Rojas J, Jonak K, Oz T, Graf P, Zachariae W, 2017. Casein kinase 1 coordinates Cohesin cleavage, gametogenesis, and Exit from M phase in meiosis II. Dev. Cell 40 (1), 37–52. [DOI] [PubMed] [Google Scholar]
- Aryal RP, Kwak PB, Tamayo AG, Gebert M, Chiu PL, Walz T and Weitz CJ (2017). “Macromolecular Assemblies of the Mammalian Circadian Clock.” Mol Cell 67(5): 770–782.e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S, 2012. Role of TAZ as mediator of Wnt signaling. Cell 151 (7), 1443–1456. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S, 2014. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158 (1), 157–170. [DOI] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L, Germain K. St, Schaeffer E, Tate B, Sprouse J, 2007. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J. Pharmacol. Exp. Ther 322 (2), 730–738. [DOI] [PubMed] [Google Scholar]
- Bae SJ, Luo X, 2018. Activation mechanisms of the hippo kinase signaling cascade. Biosci. Rep 38 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S, Taylor RE, Chakrabarti D, 1997. Identification, cloning, and mutational analysis of the casein kinase 1 cDNA of the malaria parasite, Plasmodium falciparum. Stage-specific expression of the gene. J. Biol. Chem 272 (42), 26132–26138. [DOI] [PubMed] [Google Scholar]
- Behrend L, Milne DM, Stoter M, Deppert W, Campbell LE, Meek DW, Knippschild U, 2000a. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene 19 (47), 5303–5313. [DOI] [PubMed] [Google Scholar]
- Behrend L, Stoter M, Kurth M, Rutter G, Heukeshoven J, Deppert W, Knippschild U, 2000b. Interaction of casein kinase 1 delta (CK1delta) with post-Golgi structures, microtubules and the spindle apparatus. Eur. J. Cell Biol 79 (4), 240–251. [DOI] [PubMed] [Google Scholar]
- Benek O, Hroch L, Aitken L, Gunn-Moore F, Vinklarova L, Kuca K, Perez DI, Perez C, Martinez A, Fisar Z, Musilek K, 2018. 1-(Benzo[d]thiazol-2-yl)-3-phenylureas as dual inhibitors of casein kinase 1 and ABAD enzymes for treatment of neurodegenerative disorders. J Enzyme Inhib Med Chem 33 (1), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle BW, Sim JY, Hart KC, Pruitt BL and Nelson WJ (2016). “Increasing beta-catenin/Wnt3A activity levels drive mechanical strain-induced cell cycle progression through mitosis.” Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibian M, Rahaim RJ, Choi JY, Noguchi Y, Schurer S, Chen W, Nakanishi S, Licht K, Rosenberg LH, Li L, Feng Y, Cameron MD, Duckett DR, Cleveland JL, Roush WR, 2013. Development of highly selective casein kinase 1delta/1epsilon (CK1delta/epsilon) inhibitors with potent antiproliferative properties. Bioorg. Med. Chem. Lett 23 (15), 4374–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigio EH, 2011. TDP-43 variants of frontotemporal lobar degeneration. J. Mol. Neurosci 45 (3), 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Muller A, Fander M, Knippschild U, Fischer D, 2011. Neurite outgrowth of mature retinal ganglion cells and PC12 cells requires activity of CK1delta and CK1epsilon. PLoS One 6 (6), e20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Leban J, Zaja M, Grothey A, Radunsky B, Othersen O, Strobl S, Vitt D, Knippschild U, 2012. 2-Benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivatives as potent inhibitors of CK1delta/epsilon. Acids, Amino. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Randoll SJ, Sussner N, Henne-Bruns D, Pinna LA, Knippschild U, 2013. CK1delta kinase activity is modulated by Chk1-mediated phosphorylation. PLoS One 8 (7), e68803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissaro M, Federico S, Salmaso V, Sturlese M, Spalluto G, Moro S, 2018. Targeting protein kinase CK1delta with Riluzole: could it be one of the possible missing bricks to interpret its effect in the treatment of ALS from a molecular point of view? ChemMedChem 13 (24), 2601–2605. [DOI] [PubMed] [Google Scholar]
- Biswas A, Mukherjee S, Das S, Shields D, Chow CW, Maitra U, 2011. Opposing action of casein kinase 1 and calcineurin in nucleo-cytoplasmic shuttling of mammalian translation initiation factor eIF6. J. Biol. Chem 286 (4), 3129–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner C, Hay T, Meek DW, Lane DP, 2002. Hypophosphorylation of Mdm2 augments p53 stability. Mol. Cell. Biol 22 (17), 6170–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm T, Meng Z, Haas P, Henne-Bruns D, Rachidi N, Knippschild U and Bischof J (2019). “The kinase domain of CK1delta can be phosphorylated by Chk1.” Biosci Biotechnol Biochem: 1–13. [DOI] [PubMed] [Google Scholar]
- Bozatzi P, Sapkota GP, 2018. The FAM83 family of proteins: from pseudo-PLDs to anchors for CK1 isoforms. Biochem. Soc. Trans 46 (3), 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschmidt C, Hirner H, Huber N, Eismann T, Hillenbrand A, Giamas G, Radunsky B, Ammerpohl O, Bohm B, Henne-Bruns D, Kalthoff H, Leithauser F, Trauzold A, Knippschild U, 2008. Anti-apoptotic and growth-stimulatory functions of CK1 delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut 57 (6), 799–806. [DOI] [PubMed] [Google Scholar]
- Brookheart RT, Lee CY, Espenshade PJ, 2014. Casein kinase 1 regulates sterol regulatory element-binding protein (SREBP) to control sterol homeostasis. J. Biol. Chem 289 (5), 2725–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard GJ, Rice LM, 2018. Microtubule dynamics: an interplay of biochemistry and mechanics. Nat Rev Mol Cell Biol 19 (7), 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, Sokoloff G, Palmer AA, 2009. A role for casein kinase 1 epsilon in the locomotor stimulant response to methamphetamine. Psychopharmacology 203 (4), 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Schulte G, Rawal N, Grahn A, Arenas E, 2007. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J. Cell Sci 120 (Pt 4), 586–595. [DOI] [PubMed] [Google Scholar]
- Budd DC, McDonald JE, Tobin AB, 2000. Phosphorylation and regulation of a Gq/11-coupled receptor by casein kinase 1alpha. J. Biol. Chem 275 (26), 19667–19675. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR, 2000. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev 33 (1), 95–130. [DOI] [PubMed] [Google Scholar]
- Bustos VH, Ferrarese A, Venerando A, Marin O, Allende JE, Pinna LA, 2006. The first armadillo repeat is involved in the recognition and regulation of beta-catenin phosphorylation by protein kinase CK1. Proc. Natl. Acad. Sci. U. S. A 103 (52), 19725–19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho F, Cilio M, Guo Y, Virshup DM, Patel K, Khorkova O, Styren S, Morse B, Yao Z, Keesler GA, 2001. Human casein kinase Idelta phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 489 (2–3), 159–165. [DOI] [PubMed] [Google Scholar]
- Carmel G, Leichus B, Cheng X, Patterson SD, Mirza U, Chait BT, Kuret J, 1994. Expression, purification, crystallization, and preliminary x-ray analysis of casein kinase-1 from Schizosaccharomyces pombe. J. Biol. Chem 269 (10), 7304–7309. [PubMed] [Google Scholar]
- Cegielska A, Gietzen KF, Rivers A, Virshup DM, 1998. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J. Biol. Chem 273 (3), 1357–1364. [DOI] [PubMed] [Google Scholar]
- Cerda O, Caceres M, Park KS, Leiva-Salcedo E, Romero A, Varela D, Trimmer JS, Stutzin A, 2015. Casein kinase-mediated phosphorylation of serine 839 is necessary for basolateral localization of the Ca(2)(+)-activated non-selective cation channel TRPM4. Pflugers Arch. 467 (8), 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Alonso-Nunez M, Grallert A, Tanaka K, Connolly Y, Smith DL, Hagan IM, 2017. Dialogue between centrosomal entrance and exit scaffold pathways regulates mitotic commitment. J. Cell Biol 216 (9), 2795–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TH, Huang HY, Hsu JB, Weng SL, Horng JT and Huang HD (2013). “An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs.” BMC Bioinformatics 14 Suppl 2: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang XL, Tan MS, Tan L, Yu JT, 2016. The role of TDP-43 in Alzheimer’s disease. Mol. Neurobiol 53 (5), 3349–3359. [DOI] [PubMed] [Google Scholar]
- Chaurushiya MS, Lilley CE, Aslanian A, Meisenhelder J, Scott DC, Landry S, Ticau S, Boutell C, Yates III JR, Schulman BA, Hunter T, Weitzman MD, 2012. Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain. Mol. Cell 46 (1), 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ma H, Inuzuka H, Diao J, Lan F, Shi YG, Wei W, Shi Y, 2013. DNA damage regulates UHRF1 stability via the SCF(beta-TrCP) E3 ligase. Mol. Cell. Biol 33 (6), 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YF, Tong M, Edge AS, 2016. Destabilization of Atoh1 by E3 ubiquitin ligase Huwe1 and casein kinase 1 is essential for Normal sensory hair cell development. J. Biol. Chem 291 (40), 21096–21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JK, Virshup DM, 2011. Casein kinase 1: complexity in the family. Int. J. Biochem. Cell Biol 43 (4), 465–469. [DOI] [PubMed] [Google Scholar]
- Cheong JK, Nguyen TH, Wang H, Tan P, Voorhoeve PM, Lee SH, Virshup DM, 2011. IC261 induces cell cycle arrest and apoptosis of human cancer cells via CK1delta/varepsilon and Wnt/beta-catenin independent inhibition of mitotic spindle formation. Oncogene 30 (22), 2558–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P, 2005. Physiological role for casein kinase 1 in glutamatergic synaptic transmission. J. Neurosci 25 (28), 6601–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Hagiwara M, Hidaka H, 1989. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J. Biol. Chem 264 (9), 4924–4927. [PubMed] [Google Scholar]
- Clevers H, Nusse R, 2012. Wnt/beta-catenin signaling and disease. Cell 149 (6), 1192–1205. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Rosen OM, 1983. Description of a protein kinase derived from insulin-treated 3T3-L1 cells that catalyzes the phosphorylation of ribosomal protein S6 and casein. J. Biol. Chem 258 (20), 12472–12481. [PubMed] [Google Scholar]
- Consortium, C. e. S, 1998. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282 (5396), 2012–2018. [DOI] [PubMed] [Google Scholar]
- Cooper CD, Lampe PD, 2002. Casein kinase 1 regulates connexin-43 gap junction assembly. J. Biol. Chem 277 (47), 44962–44968. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF, 2008. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol 10 (1), 70–76. [DOI] [PubMed] [Google Scholar]
- Cozza G, Gianoncelli A, Montopoli M, Caparrotta L, Venerando A, Meggio F, Pinna LA, Zagotto G, Moro S, 2008. Identification of novel protein kinase CK1 delta (CK1delta) inhibitors through structure-based virtual screening. Bioorg. Med. Chem. Lett 18 (20), 5672–5675. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, 2014. Casein kinase 1 and Wnt/beta-catenin signaling. Curr. Opin. Cell Biol 31, 46–55. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Dolde C, de Groot RE, Ohkawara B, Reinhard C, Korswagen HC, Niehrs C, 2013. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt/beta-catenin signaling. Science 339 (6126), 1436–1441. [DOI] [PubMed] [Google Scholar]
- Cunningham PS, Ahern SA, Smith LC, da Silva Santos CS, Wager TT, Bechtold DA, 2016. Targeting of the circadian clock via CK1delta/epsilon to improve glucose homeostasis in obesity. Sci. Rep 6, 29983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan-Goor M, Nasereddin A, Jaber H, Jaffe CL, 2013. Identification of a secreted casein kinase 1 in Leishmania donovani: effect of protein over expression on parasite growth and virulence. PLoS One 8 (11), e79287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lazzari F, Bisaglia M, Zordan MA, Sandrelli F, 2018. Circadian rhythm abnormalities in Parkinson’s disease from humans to flies and Back. Int. J. Mol. Sci 19 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaggio AJ, Lindberg RA, Hunter T, Hoekstra MF, 1992. The budding yeast HRR25 gene product is a casein kinase I isoform. Proc. Natl. Acad. Sci. U. S. A 89 (15), 7008–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingerdissen HM, Torcivia-Rodriguez J, Hu Y, Chang TC, Mazumder R, Kahsay R, 2018. BioMuta and BioXpress: mutation and expression knowledgebases for cancer biomarker discovery. Nucleic Acids Res. 46 (D1), D1128–d1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolde C, Bischof J, Gruter S, Montada A, Halekotte J, Peifer C, Kalbacher H, Baumann U, Knippschild U, Suter B, 2018. A CK1 FRET biosensor reveals that DDX3X is an essential activator of CK1epsilon. J. Cell Sci 131 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RG, Zhong T, Meijer L, Liberator PA, 2005. Characterization of two T. gondii CK1 isoforms. Mol. Biochem. Parasitol 141 (1), 15–27. [DOI] [PubMed] [Google Scholar]
- Dorin-Semblat D, Demarta-Gatsi C, Hamelin R, Armand F, Carvalho TG, Moniatte M, Doerig C, 2015. Malaria parasite-infected erythrocytes secrete PfCK1, the Plasmodium homologue of the pleiotropic protein kinase casein kinase 1. PLoS One 10 (12), e0139591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois T, Rommel C, Howell S, Steinhussen U, Soneji Y, Morrice N, Moelling K, Aitken A, 1997. 14-3-3 is phosphorylated by casein kinase I on residue 233. Phosphorylation at this site in vivo regulates Raf/14-3-3 interaction. J. Biol. Chem 272 (46), 28882–28888. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Milne DM, Meek DW, 1999. Protein kinase CK1 is a p53-threonine 18 kinase which requires prior phosphorylation of serine 15. FEBS Lett. 463 (3), 312–316. [DOI] [PubMed] [Google Scholar]
- Durieu E, Prina E, Leclercq O, Oumata N, Gaboriaud-Kolar N, Vougogiannopoulou K, Aulner N, Defontaine A, No JH, Ruchaud S, Skaltsounis AL, Galons H, Spath GF, Meijer L, Rachidi N, 2016. From drug screening to target deconvolution: a target-based drug discovery pipeline using Leishmania casein kinase 1 isoform 2 to identify compounds with Antileishmanial activity. Antimicrob. Agents Chemother 60 (5), 2822–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, 2007. Circadian rhythms in the CNS and peripheral clock disorders: human sleep disorders and clock genes. J. Pharmacol. Sci 103 (2), 150–154. [DOI] [PubMed] [Google Scholar]
- Elias L, Li AP, Longmire J, 1981. Cyclic adenosine 3′:5′-monophosphate-dependent and -independent protein kinase in acute myeloblastic leukemia. Cancer Res. 41 (6), 2182–2188. [PubMed] [Google Scholar]
- Elmore ZC, Guillen RX, Gould KL, 2018. The kinase domain of CK1 enzymes contains the localization cue essential for compartmentalized signaling at the spindle pole. Mol. Biol. Cell 29 (13), 1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott JA, Noble ME, Johnson LN, 2012. The structural basis for control of eukaryotic protein kinases. Annu. Rev. Biochem 81, 587–613. [DOI] [PubMed] [Google Scholar]
- Eng GWL, Edison, Virshup DM, 2017. Site-specific phosphorylation of casein kinase 1 delta (CK1delta) regulates its activity towards the circadian regulator PER2. PLoS One 12 (5), e0177834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Bourguet-Kondracki ML, Mai LH, Longeon A, Teta R, Meijer L, Van Soest R, Mangoni A, Costantino V, 2016. Chloromethylhalicyclamine B, a marine-derived protein kinase CK1delta/epsilon inhibitor. J. Nat. Prod 79 (11), 2953–2960. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Machida KK, Noton E, Constance CM, Dallmann R, Di Napoli MN, DeBruyne JP, Lambert CM, Yu EA, Reppert SM, Weaver DR, 2009. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol 29 (14), 3853–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML, 2014. Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum. Mol. Genet 23 (22), 5866–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, McClung CA, 2009. A role for the circadian genes in drug addiction. Neuropharmacology 56 (Suppl. 1), 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Schiappa R, Girault JA, Le Novere N, 2006. DARPP-32 is a robust integrator of dopamine and glutamate signals. PLoS Comput. Biol 2 (12), e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo M, Verduci L, Blandino G, Strano S, 2017. Mutant p53 protein and the hippo transducers YAP and TAZ: a critical oncogenic node in human cancers. Int. J. Mol. Sci 18 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JM, Chiang JY, 2015. Circadian rhythms in liver metabolism and disease. Acta Pharm. Sin. B 5 (2), 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament S, Delacourte A, Delaere P, Duyckaerts C, Hauw JJ, 1990. Correlation between microscopical changes and Tau 64 and 69 biochemical detection in senile dementia of the Alzheimer type. Tau 64 and 69 are reliable markers of the neurofibrillary degeneration. Acta Neuropathol. 80 (2), 212–215. [DOI] [PubMed] [Google Scholar]
- Flotow H, Roach PJ, 1991. Role of acidic residues as substrate determinants for casein kinase I. J. Biol. Chem 266 (6), 3724–3727. [PubMed] [Google Scholar]
- Flotow H, Graves PR, Wang AQ, Fiol CJ, Roeske RW, Roach PJ, 1990. Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem 265 (24), 14264–14269. [PubMed] [Google Scholar]
- Fohr KJ, Knippschild U, Herkommer A, Fauler M, Peifer C, Georgieff M, Adolph O, 2017. State-dependent block of voltage-gated sodium channels by the casein-kinase 1 inhibitor IC261. Investig. New Drugs 35 (3), 277–289. [DOI] [PubMed] [Google Scholar]
- Fulcher LJ, Bozatzi P, Tachie-Menson T, Wu KZL, Cummins TD, Bufton JC, Pinkas DM, Dunbar K, Shrestha S, Wood NT, Weidlich S, Macartney TJ, Varghese J, Gourlay R, Campbell DG, Dingwell KS, Smith JC, Bullock AN, Sapkota GP, 2018. The DUF1669 domain of FAM83 family proteins anchor casein kinase 1 isoforms. Sci. Signal 11 (531). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth N, Aylon Y, Oren M, 2018. p53 shades of Hippo. Cell Death Differ. 25 (1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fustin JM, Kojima R, Itoh K, Chang HY, Ye S, Zhuang B, Oji A, Gibo S, Narasimamurthy R, Virshup D, Kurosawa G, Doi M, Manabe I, Ishihama Y, Ikawa M, Okamura H, 2018. Two Ck1delta transcripts regulated by m6A methylation code for two antagonistic kinases in the control of the circadian clock. Proc. Natl. Acad. Sci. U. S. A 115 (23), 5980–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Virshup DM, 2007. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8 (2), 139–148. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Metherall J, Virshup DM, 2000. Identification of casein kinase I substrates by in vitro expression cloning screening. Biochem. Biophys. Res. Commun 268 (2), 562–566. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM, 2002. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl. Acad. Sci. U. S. A 99 (3), 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Wang M, Zheng QH, 2018. Synthesis of carbon-11-labeled CK1 inhibitors as new potential PET radiotracers for imaging of Alzheimer’s disease. Bioorg. Med. Chem. Lett 28 (13), 2234–2238. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, Henne-Bruns D, Pichlo C, Brunstein E, Baumann U, Wesseler F, Rathmer B, Schade D, Peifer C, Knippschild U, 2018. Discovery of inhibitor of Wnt production 2 (IWP-2) and related compounds as selective ATP-competitive inhibitors of casein kinase 1 (CK1) delta/epsilon. J. Med. Chem 61 (9), 4087–4102. [DOI] [PubMed] [Google Scholar]
- Garzia L, D’Angelo A, Amoresano A, Knauer SK, Cirulli C, Campanella C, Stauber RH, Steegborn C, Iolascon A, Zollo M, 2008. Phosphorylation of nm23-H1 by CKI induces its complex formation with h-prune and promotes cell motility. Oncogene 27 (13), 1853–1864. [DOI] [PubMed] [Google Scholar]
- Giamas G, Hirner H, Shoshiashvili L, Grothey A, Gessert S, Kuhl M, Henne-Bruns D, Vorgias CE, Knippschild U, 2007. Phosphorylation of CK1delta: identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem. J 406 (3), 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamas G, Castellano L, Feng Q, Knippschild U, Jacob J, Thomas RS, Coombes RC, Smith CL, Jiao LR, Stebbing J, 2009. CK1delta modulates the transcriptional activity of ERalpha via AIB1 in an estrogen-dependent manner and regulates ERalpha-AIB1 interactions. Nucleic Acids Res. 37 (9), 3110–3123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Good MC, Zalatan JG, Lim WA, 2011. Scaffold proteins: hubs for controlling the flow of cellular information. Science 332 (6030), 680–686.21551057 [Google Scholar]
- Graves PR, Roach PJ, 1995. Role of COOH-terminal phosphorylation in the regulation of casein kinase I delta. J. Biol. Chem 270 (37), 21689–21694. [DOI] [PubMed] [Google Scholar]
- Graves PR, Haas DW, Hagedorn CH, DePaoli-Roach AA, Roach PJ, 1993. Molecular cloning, expression, and characterization of a 49-kilodalton casein kinase I isoform from rat testis. J. Biol. Chem 268 (9), 6394–6401. [PubMed] [Google Scholar]
- Greer YE, Rubin JS, 2011. Casein kinase 1 delta functions at the centrosome to mediate Wnt-3a-dependent neurite outgrowth. J. Cell Biol 192 (6), 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer YE, Westlake CJ, Gao B, Bharti K, Shiba Y, Xavier CP, Pazour GJ, Yang Y, Rubin JS, 2014. Casein kinase 1 delta functions at the centrosome and Golgi to promote ciliogenesis. Cell, Mol Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer YE, Gao B, Yang Y, Nussenzweig A, Rubin JS, 2017. Lack of casein kinase 1 Delta promotes genomic instability - the accumulation of DNA damage and Down-regulation of checkpoint kinase 1. PLoS One 12 (1), e0170903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozav AG, Chikamori K, Kozuki T, Grabowski DR, Bukowski RM, Willard B, Kinter M, Andersen AH, Ganapathi R, Ganapathi MK, 2009. Casein kinase I delta/epsilon phosphorylates topoisomerase IIalpha at serine-1106 and modulates DNA cleavage activity. Nucleic Acids Res. 37 (2), 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Fullam A, Brennan R, Schroder M, 2013. Human DEAD box helicase 3 couples IkappaB kinase epsilon to interferon regulatory factor 3 activation. Mol. Cell. Biol 33 (10), 2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI, 2004. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell 15 (4), 511–521. [DOI] [PubMed] [Google Scholar]
- Halekotte J, Witt L, Ianes C, Kruger M, Buhrmann M, Rauh D, Pichlo C, Brunstein E, Luxenburger A, Baumann U, Knippschild U, Bischof J, Peifer C, 2017. Optimized 4,5-diarylimidazoles as potent/selective inhibitors of protein kinase CK1delta and their structural relation to p38alpha MAPK. Molecules 22 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerlein A, Weiske J, Huber O, 2005. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell. Mol. Life Sci 62 (5), 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH, 2007. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem 282 (32), 23645–23654. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Hunter T, 1995. Protein kinases 6. The eukaryotic protein kinase super-family: kinase (catalytic) domain structure and classification. FASEB J. 9 (8), 576–596. [PubMed] [Google Scholar]
- Hantschel O, Superti-Furga G, 2004. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 5 (1), 33–44. [DOI] [PubMed] [Google Scholar]
- Harnos J, Rynes J, Viskova P, Foldynova-Trantirkova S, Bajard-Esner L, Trantirek L, Bryja V, 2018. Analysis of binding interfaces of the human scaffold protein AXIN1 by peptide microarrays. J. Biol. Chem 293 (42), 16337–16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF, 2011. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332 (6028), 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heretsch P, Tzagkaroulaki L, Giannis A, 2010. Modulators of the hedgehog signaling pathway. Bioorg. Med. Chem 18 (18), 6613–6624. [DOI] [PubMed] [Google Scholar]
- Higashimoto Y, Saito S, Tong XH, Hong A, Sakaguchi K, Appella E, Anderson CW, 2000. Human p53 is phosphorylated on serines 6 and 9 in response to DNA damage-inducing agents. J. Biol. Chem 275 (30), 23199–23203. [DOI] [PubMed] [Google Scholar]
- Hiramoto K, Yamate Y, Kasahara E, Sato EF, 2018. An inhibitor of casein kinase 1epsilon/delta (PF670462) prevents the deterioration of dextran sodium sulfate-induced ulcerative colitis caused by UVB eye irradiation. Int. J. Biol. Sci 14 (9), 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirner H, Gunes C, Bischof J, Wolff S, Grothey A, Kuhl M, Oswald F, Wegwitz F, Bosl MR, Trauzold A, Henne-Bruns D, Peifer C, Leithauser F, Deppert W, Knippschild U, 2012. Impaired CK1 delta activity attenuates SV40-induced cellular transformation in vitro and mouse mammary carcinogenesis in vivo. PLoS One 7 (1), e29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Mason S, Kobayashi R, Hoekstra M, Andrews B, 1997. Role of the casein kinase I isoform, Hrr25, and the cell cycle-regulatory transcription factor, SBF, in the transcriptional response to DNA damage in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A 94 (2), 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra MF, Liskay RM, Ou AC, DeMaggio AJ, Burbee DG, Heffron F, 1991. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science 253 (5023), 1031–1034. [DOI] [PubMed] [Google Scholar]
- Hombach-Barrigah A, Bartsch K, Smirlis D, Rosenqvist H, MacDonald A, Dingli F, Loew D, Spath GF, Rachidi N, Wiese M, Clos J, 2019. Leishmania donovani 90 kD heat shock protein - impact of phosphosites on parasite fitness, infectivity and casein kinase affinity. Sci. Rep 9 (1), 5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E, 2015. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43 (Database issue), D512–D520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LY, Zhao J, Chen H, Wan L, Inuzuka H, Guo J, Fu X, Zhai Y, Lu Z, Wang X, Han ZG, Sun Y, Wei W, 2018. SCF(FBW7)-mediated degradation of Brg1 suppresses gastric cancer metastasis. Nat. Commun 9 (1), 3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huart AS, Saxty B, Merritt A, Nekulova M, Lewis S, Huang Y, Vojtesek B, Kettleborough C, Hupp TR, 2013. A casein kinase 1/checkpoint kinase 1 pyrazolopyridine protein kinase inhibitor as novel activator of the p53 pathway. Bioorg. Med. Chem. Lett 23 (20), 5578–5585. [DOI] [PubMed] [Google Scholar]
- Ianes C, Xu P, Werz N, Meng Z, Henne-Bruns D, Bischof J, Knippschild U, 2016. CK1delta activity is modulated by CDK2/E- and CDK5/p35-mediated phosphorylation. Amino Acids 48 (2), 579–592. [DOI] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E, 2012. A molecular mechanism that links hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 31 (5), 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka H, Fukushima H, Shaik S, Wei W, 2010a. Novel insights into the molecular mechanisms governing Mdm2 ubiquitination and destruction. Oncotarget 1 (7), 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S, Wan L, Ang XL, Mock C, Yin H, Stommel JM, Gygi S, Lahav G, Asara J, Xiao ZX, Kaelin WG Jr., Harper JW, Wei W, 2010b. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 18 (2), 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Yagita K, Fukuyama T, Nishimura M, Nagano M, Shigeyoshi Y, Yamaguchi S, Komori T, Okamura H, 2001. Constitutive expression and delayed light response of casein kinase Iepsilon and Idelta mRNAs in the mouse suprachiasmatic nucleus. J. Neurosci. Res 64 (6), 612–616. [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Tanaka K, Sakuno T, Watanabe Y, 2010. Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat. Cell Biol 12 (5), 500–506. [DOI] [PubMed] [Google Scholar]
- Isoda M, Sako K, Suzuki K, Nishino K, Nakajo N, Ohe M, Ezaki T, Kanemori Y, Inoue D, Ueno H, Sagata N, 2011. Dynamic regulation of Emi2 by Emi2-bound Cdk1/Plk1/CK1 and PP2A-B56 in meiotic arrest of Xenopus eggs. Dev. Cell 21 (3), 506–519. [DOI] [PubMed] [Google Scholar]
- Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto KH, Kiuchi R, Ishida M, Ukai-Tadenuma M, Minami Y, Kito R, Nakao K, Kishimoto W, Yoo SH, Shimomura K, Takao T, Takano A, Kojima T, Nagai K, Sakaki Y, Takahashi JS, Ueda HR, 2009. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. U. S. A 106 (37), 15744–15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izeradjene K, Douglas L, Delaney AB, Houghton JA, 2004. Casein kinase I attenuates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by regulating the recruitment of fas-associated death domain and procaspase-8 to the death-inducing signaling complex. Cancer Res. 64 (21), 8036–8044. [DOI] [PubMed] [Google Scholar]
- Janovska P, Verner J, Kohoutek J, Bryjova L, Gregorova M, Dzimkova M, Skabrahova H, Radaszkiewicz T, Ovesna P, Vondalova Blanarova O, Nemcova T, Hoferova Z, Vasickova K, Smyckova L, Egle A, Pavlova S, Poppova L, Plevova K, Pospisilova S, Bryja V, 2018. Casein kinase 1 is a therapeutic target in chronic lymphocytic leukemia. Blood 131 (11), 1206–1218. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC, 2008. Hedgehog signaling in development and cancer. Dev. Cell 15 (6), 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Liu Y, Fan J, Epperly G, Gao T, Jiang J, Jia J, 2014. Hedgehog-regulated atypical PKC promotes phosphorylation and activation of smoothened and Cubitus interruptus in drosophila. Proc. Natl. Acad. Sci. U. S. A 111 (45), E4842–E4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Zhang M, Sun J, Yang X, 2018. Casein kinase 1alpha: biological mechanisms and theranostic potential. Cell Commun Signal 16 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Chen JS, Gould KL, 2013. CK1 is required for a mitotic checkpoint that delays cytokinesis. Curr. Biol 23 (19), 1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ, 1999. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat. Med 5 (9), 1062–1065. [DOI] [PubMed] [Google Scholar]
- Joshi K, Goyal S, Grover S, Jamal S, Singh A, Dhar P, Grover A, 2016. Novel group-based QSAR and combinatorial design of CK-1delta inhibitors as neuroprotective agents. BMC Bioinformatics 17 (Suppl. 19), 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousi A, Mylonis I, Politou AS, Chachami G, Paraskeva E, Simos G, 2010. Casein kinase 1 regulates human hypoxia-inducible factor HIF-1. J. Cell Sci 123 (Pt 17), 2976–2986. [DOI] [PubMed] [Google Scholar]
- Kametani F, Nonaka T, Suzuki T, Arai T, Dohmae N, Akiyama H, Hasegawa M, 2009. Identification of casein kinase-1 phosphorylation sites on TDP-43. Biochem. Biophys. Res. Commun 382 (2), 405–409. [DOI] [PubMed] [Google Scholar]
- Karthikeyan C, Jharia P, Waiker DK, Nusbaum AC, Amawi H, Kirwen EM, Christman R, Arudra SKC, Meijer L, Tiwari AK, Trivedi P, 2017. N-(1H-Pyrazol-3-yl)quinazolin-4-amines as a novel class of casein kinase 1delta/epsilon inhibitors: synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. Lett 27 (12), 2663–2667. [DOI] [PubMed] [Google Scholar]
- Katis VL, Lipp JJ, Imre R, Bogdanova A, Okaz E, Habermann B, Mechtler K, Nasmyth K, Zachariae W, 2010. Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev. Cell 18 (3), 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami F, Suzuki K, Ohtsuki K, 2008. A novel consensus phosphorylation motif in sulfatide- and cholesterol-3-sulfate-binding protein substrates for CK1 in vitro. Biol. Pharm. Bull 31 (2), 193–200. [DOI] [PubMed] [Google Scholar]
- Keenan CR, Langenbach SY, Jativa F, Harris T, Li M, Chen Q, Xia Y, Gao B, Schuliga MJ, Jaffar J, Prodanovic D, Tu Y, Berhan A, Lee PVS, Westall GP, Stewart AG, 2018. Casein kinase 1delta/epsilon inhibitor, PF670462 attenuates the Fibrogenic effects of transforming growth factor-beta in pulmonary fibrosis. Front. Pharmacol 9, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Varcoe TJ, Voultsios A, Salkeld MD, Rattanatray L, Boden MJ, 2015. Acute inhibition of casein kinase 1delta/epsilon rapidly delays peripheral clock gene rhythms. Mol. Cell. Biochem 398 (1–2), 195–206. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim M, Lee MS, Kim CH, Lim DS, 2014. The MST1/2-SAV1 complex of the hippo pathway promotes ciliogenesis. Nat. Commun 5, 5370. [DOI] [PubMed] [Google Scholar]
- Klasens BI, Das AT, Berkhout B, 1998. Inhibition of polyadenylation by stable RNA secondary structure. Nucleic Acids Res. 26 (8), 1870–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P, 2002. Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev. Dyn 225 (4), 384–391. [DOI] [PubMed] [Google Scholar]
- Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW, 1998. The drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94 (1), 97–107. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF, Meek DW, 1997. p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene 15 (14), 1727–1736. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Kruger M, Richter J, Xu P, Garcia-Reyes B, Peifer C, Halekotte J, Bakulev V, Bischof J, 2014. The CK1 family: contribution to cellular stress response and its role in carcinogenesis. Front. Oncol 4, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsavage WM Jr., Yochum GS, 2013. Intersection of hippo/YAP and Wnt/beta-catenin signaling pathways. Acta Biochim. Biophys. Sin. Shanghai 45 (2), 71–79. [DOI] [PubMed] [Google Scholar]
- Kosten J, Binolfi A, Stuiver M, Verzini S, Theillet FX, Bekei B, van Rossum M, Selenko P, 2014. Efficient modification of alpha-synuclein serine 129 by protein kinase CK1 requires phosphorylation of tyrosine 125 as a priming event. ACS Chem. Neurosci 5 (12), 1203–1208. [DOI] [PubMed] [Google Scholar]
- Kourti M, Ikonomou G, Giakoumakis NN, Rapsomaniki MA, Landegren U, Siniossoglou S, Lygerou Z, Simos G, Mylonis I, 2015. CK1delta restrains lipin-1 induction, lipid droplet formation and cell proliferation under hypoxia by reducing HIF-1alpha/ARNT complex formation. Cell. Signal 27 (6), 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy N, Kurzrock R, 2018. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat. Rev 62, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Kalbacher H, Kastritis PL, Bischof J, Barth H, Henne-Bruns D, Vorgias C, Sarno S, Pinna LA, Knippschild U, 2016. New potential peptide therapeutics perturbing CK1delta/alpha-tubulin interaction. Cancer Lett. 375 (2), 375–383. [DOI] [PubMed] [Google Scholar]
- Kuga T, Kume H, Adachi J, Kawasaki N, Shimizu M, Hoshino I, Matsubara H, Saito Y, Nakayama Y, Tomonaga T, 2016. Casein kinase 1 is recruited to nuclear speckles by FAM83H and SON. Sci. Rep 6, 34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rajendran V, Sethumadhavan R, Purohit R, 2014. Relationship between a point mutation S97C in CK1delta protein and its affect on ATP-binding affinity. J. Biomol. Struct. Dyn 32 (3), 394–405. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Sakurai E, Toyomoto M, Kii I, Kawamoto D, Asada T, Tanabe T, Yoshimura M, Hagiwara M, Miyata A, 2014. Alleviation of behavioral hypersensitivity in mouse models of inflammatory pain with two structurally different casein kinase 1 (CK1) inhibitors. Mol. Pain 10 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuda J, Hidari N, Hirai M, Hashimoto K, 1996. Sequence analysis of the cDNA for the human casein kinase I delta (CSNK1D) gene and its chromosomal localization. Genomics 32 (1), 140–143. [DOI] [PubMed] [Google Scholar]
- Lamotte S, Spath GF, Rachidi N, Prina E, 2017. The enemy within: targeting host-parasite interaction for antileishmanial drug discovery. PLoS Negl. Trop. Dis 11 (6), e0005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG, 2011. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat. Cell Biol 13 (6), 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM, 2001. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107 (7), 855–867. [DOI] [PubMed] [Google Scholar]
- Lee H, Chen R, Lee Y, Yoo S, Lee C, 2009. Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc. Natl. Acad. Sci. U. S. A 106 (50), 21359–21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C, 2011a. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc. Natl. Acad. Sci. U. S. A 108 (39), 16451–16456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Hirota T, Peters EC, Garcia M, Gonzalez R, Cho CY, Wu X, Schultz PG, Kay SA, 2011b. A small molecule modulates circadian rhythms through phosphorylation of the period protein. Angew Chem Int Ed Engl 50 (45), 10608–10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Johmura Y, Yu LR, Park JE, Gao Y, Bang JK, Zhou M, Veenstra TD, Yeon Kim B, Lee KS, 2012. Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J. 31 (14), 3104–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K, 2019. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 18 (3), 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Espinosa G, Garcia E, Garcia-Escudero V, Hernandez F, Defelipe J, Avila J, 2013. Changes in tau phosphorylation in hibernating rodents. J. Neurosci. Res 91 (7), 954–962. [DOI] [PubMed] [Google Scholar]
- Li G, Yin H, Kuret J, 2004. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J. Biol. Chem 279 (16), 15938–15945. [DOI] [PubMed] [Google Scholar]
- Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, Loweth JA, Vezina P, 2011a. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phosphorylation. J. Neurochem 118 (2), 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen XW, Yu L, Saltiel AR, Lin JD, 2011b. Circadian metabolic regulation through crosstalk between casein kinase 1delta and transcriptional coactivator PGC-1alpha. Mol. Endocrinol 25 (12), 2084–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao D, Wei H, Yao L, Dang Y, Amjad A, Xu J, Liu J, Guo L, Li D, Li Z, Zuo D, Zhang Y, Huang S, Jia C, Wang L, Wang Y, Xie Y, Luo J, Zhang B, Luo H, Donehower LA, Moses RE, Xiao J, O’Malley BW, Li X, 2013. REGgamma deficiency promotes premature aging via the casein kinase 1 pathway. Proc. Natl. Acad. Sci. U. S. A 110 (27), 11005–11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Virshup DM, Nairn AC, Greengard P, 2002. Mechanism of regulation of casein kinase I activity by group I metabotropic glutamate receptors. J. Biol. Chem 277 (47), 45393–45399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Carvalho LP, Bhattacharya S, Carbone CJ, Kumar KG, Leu NA, Yau PM, Donald RG, Weiss MJ, Baker DP, McLaughlin KJ, Scott P, Fuchs SY, 2009. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol. Cell. Biol 29 (24), 6401–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wan L, Liu P, Inuzuka H, Liu J, Wang Z, Wei W, 2014. SCF(beta-TRCP)-mediated degradation of NEDD4 inhibits tumorigenesis through modulating the PTEN/Akt signaling pathway. Oncotarget 5 (4), 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, Shaw AS, Chakraborty AK, 2007. Scaffold proteins confer diverse regulatory properties to protein kinase cascades. Proc. Natl. Acad. Sci. U. S. A 104 (33), 13307–13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohler J, Hirner H, Schmidt B, Kramer K, Fischer D, Thal DR, Leithauser F, Knippschild U, 2009. Immunohistochemical characterisation of cell-type specific expression of CK1delta in various tissues of young adult BALB/c mice. PLoS One 4 (1), e4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long A, Zhao H, Huang X, 2012. Structural basis for the interaction between casein kinase 1 delta and a potent and selective inhibitor. J. Med. Chem 55 (2), 956–960. [DOI] [PubMed] [Google Scholar]
- Longenecker KL, Roach PJ, Hurley TD, 1996. Three-dimensional structure of mammalian casein kinase I: molecular basis for phosphate recognition. J. Mol. Biol 257 (3), 618–631. [DOI] [PubMed] [Google Scholar]
- Longenecker KL, Roach PJ, Hurley TD, 1998. Crystallographic studies of casein kinase I delta toward a structural understanding of auto-inhibition. Acta Crystallogr D Biol Crystallogr 54 (Pt 3), 473–475. [DOI] [PubMed] [Google Scholar]
- MacLaine NJ, Oster B, Bundgaard B, Fraser JA, Buckner C, Lazo PA, Meek DW, Hollsberg P, Hupp TR, 2008. A central role for CK1 in catalyzing phosphorylation of the p53 transactivation domain at serine 20 after HHV-6B viral infection. J. Biol. Chem 283 (42), 28563–28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R, 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47 (W1), W636–w641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magowan C, Liang J, Yeung J, Takakuwa Y, Coppel RL, Mohandas N, 1998. Plasmodium falciparum: influence of malarial and host erythrocyte skeletal protein interactions on phosphorylation in infected erythrocytes. Exp. Parasitol 89 (1), 40–49. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Sykes DE, Hu Z, Bonoiu A, Ding H, Prasad PN, Schwartz SA, 2009. Therapeutic targeting of “DARPP-32”: a key signaling molecule in the dopiminergic pathway for the treatment of opiate addiction. Int. Rev. Neurobiol 88, 199–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhadour S, Marchand P, Pagniez F, Bazin MA, Picot C, Lozach O, Ruchaud S, Antoine M, Meijer L, Rachidi N, Le Pape P, 2012. Synthesis and biological evaluation of 2,3-diarylimidazo[1,2-a]pyridines as antileishmanial agents. Eur. J. Med. Chem 58, 543–556. [DOI] [PubMed] [Google Scholar]
- Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE, 2003. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc. Natl. Acad. Sci. U. S. A 100 (18), 10193–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritzen T, Lohler J, Deppert W, Knippschild U, 2003. Casein kinase I delta (CKIdelta) is involved in lymphocyte physiology. Eur. J. Cell Biol 82 (7), 369–378. [DOI] [PubMed] [Google Scholar]
- Martel D, Beneke T, Gluenz E, Spath GF, Rachidi N, 2017. Characterisation of casein kinase 1.1 in Leishmania donovani using the CRISPR Cas9 toolkit. Biomed. Res. Int 2017, 4635605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF, Kuret J, 2000. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J. Biol. Chem 275 (26), 20052–20060. [DOI] [PubMed] [Google Scholar]
- Mbefo MK, Fares MB, Paleologou K, Oueslati A, Yin G, Tenreiro S, Pinto M, Outeiro T, Zweckstetter M, Masliah E, Lashuel HA, 2015. Parkinson disease mutant E46K enhances alpha-synuclein phosphorylation in mammalian cell lines, in yeast, and in vivo. J. Biol. Chem 290 (15), 9412–9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek DW, 2000. The role of p53 in the response to mitotic spindle damage. Pathol Biol (Paris) 48 (3), 246–254. [PubMed] [Google Scholar]
- Meggio F, Perich JW, Reynolds EC, Pinna LA, 1991. A synthetic beta-casein phosphopeptide and analogues as model substrates for casein kinase-1, a ubiquitous, phosphate directed protein kinase. FEBS Lett. 283 (2), 303–306. [DOI] [PubMed] [Google Scholar]
- Mehlgarten C, Schaffrath R, 2003. Mutant casein kinase I (Hrr25p/Kti14p) abrogates the G1 cell cycle arrest induced by Kluyveromyces lactiszymocin in budding yeast. Mol. Gen. Genomics 269 (2), 188–196. [DOI] [PubMed] [Google Scholar]
- Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, Dupre SM, Chesham JE, Rajamohan F, Knafels J, Sneed B, Zawadzke LE, Ohren JF, Walton KM, Wager TT, Hastings MH, Loudon AS, 2010. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl. Acad. Sci. U. S. A 107 (34), 15240–15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Bischof J, Ianes C, Henne-Bruns D, Xu P, Knippschild U, 2016. CK1delta kinase activity is modulated by protein kinase C alpha (PKCalpha)-mediated site-specific phosphorylation. Amino Acids 48 (5), 1185–1197. [DOI] [PubMed] [Google Scholar]
- Meng Z, Bohm T, Xu P, Henne-Bruns D, Peifer C, Witt L, Knippschild U, Bischof J, 2019. Kinase activity of casein kinase 1 delta (CK1delta) is modulated by protein kinase C alpha (PKCalpha) by site-specific phosphorylation within the kinase domain of CK1delta. Biochim. Biophys. Acta, Proteins Proteomics 1867 (7–8), 710–721. [DOI] [PubMed] [Google Scholar]
- Mieda M, Okamoto H, Sakurai T, 2016. Manipulating the cellular circadian period of arginine vasopressin neurons alters the behavioral circadian period. Curr. Biol 26 (18), 2535–2542. [DOI] [PubMed] [Google Scholar]
- Milne DM, Palmer RH, Campbell DG, Meek DW, 1992. Phosphorylation of the p53 tumour-suppressor protein at three N-terminal sites by a novel casein kinase I-like enzyme. Oncogene 7 (7), 1361–1369. [PubMed] [Google Scholar]
- Milne DM, Looby P, Meek DW, 2001. Catalytic activity of protein kinase CK1 delta (casein kinase 1delta) is essential for its normal subcellular localization. Exp. Cell Res 263 (1), 43–54. [DOI] [PubMed] [Google Scholar]
- Minzel W, Venkatachalam A, Fink A, Hung E, Brachya G, Burstain I, Shaham M, Rivlin A, Omer I, Zinger A, Elias S, Winter E, Erdman PE, Sullivan RW, Fung L, Mercurio F, Li D, Vacca J, Kaushansky N, Shlush L, Oren M, Levine R, Pikarsky E, Snir-Alkalay I and Ben-Neriah Y (2018). “Small Molecules Co-targeting CKIalpha and the Transcriptional Kinases CDK7/9 Control AML in Preclinical Models.” Cell 175(1): 171–185.e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Nagase T, Mesaki M, Narukawa J, Ohara O, Ishida N, 2004. Phosphorylation of clock protein PER1 regulates its circadian degradation in normal human fibroblasts. Biochem. J 380 (Pt 1), 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Yeon Park S, Woo Park H, 2018. Regulation of the hippo pathway in cancer biology. Cell. Mol. Life Sci 75 (13), 2303–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern Y, Das Adhikari U, Ayyash M, Elyada E, Toth B, Moor A, Itzkovitz S, Ben-Neriah Y, 2017. Casein kinase 1-epsilon or 1-delta required for Wnt-mediated intestinal stem cell maintenance. EMBO J. 36 (20), 3046–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A, Kimura K, Nakano A, 1999. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. Implication of negative regulation by the Hrr25 kinase in the vesicle budding from the endoplasmic reticulum. J. Biol. Chem 274 (6), 3804–3810. [DOI] [PubMed] [Google Scholar]
- Muraki M, Ohkawara B, Hosoya T, Onogi H, Koizumi J, Koizumi T, Sumi K, Yomoda J, Murray MV, Kimura H, Furuichi K, Shibuya H, Krainer AR, Suzuki M, Hagiwara M, 2004. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J. Biol. Chem 279 (23), 24246–24254. [DOI] [PubMed] [Google Scholar]
- Mural RJ, Adams MD, Myers EW, Smith HO, Miklos GL, Wides R, Halpern A, Li PW, Sutton GG, Nadeau J, Salzberg SL, Holt RA, Kodira CD, Lu F, Chen L, Deng Z, Evangelista CC, Gan W, Heiman TJ, Li J, Li Z, Merkulov GV, Milshina NV, Naik AK, Qi R, Shue BC, Wang A, Wang J, Wang X, Yan X, Ye J, Yooseph S, Zhao Q, Zheng L, Zhu SC, Biddick K, Bolanos R, Delcher AL, Dew IM, Fasulo D, Flanigan MJ, Huson DH, Kravitz SA, Miller JR, Mobarry CM, Reinert K, Remington KA, Zhang Q, Zheng XH, Nusskern DR, Lai Z, Lei Y, Zhong W, Yao A, Guan P, Ji RR, Gu Z, Wang ZY, Zhong F, Xiao C, Chiang CC, Yandell M, Wortman JR, Amanatides PG, Hladun SL, Pratts EC, Johnson JE, Dodson KL, Woodford KJ, Evans CA, Gropman B, Rusch DB, Venter E, Wang M, Smith TJ, Houck JT, Tompkins DE, Haynes C, Jacob D, Chin SH, Allen DR, Dahlke CE, Sanders R, Li K, Liu X, Levitsky AA, Majoros WH, Chen Q, Xia AC, Lopez JR, Donnelly MT, Newman MH, Glodek A, Kraft CL, Nodell M, Ali F, An HJ, Baldwin-Pitts D, Beeson KY, Cai S, Carnes M, Carver A, Caulk PM, Center A, Chen YH, Cheng ML, Coyne MD, Crowder M, Danaher S, Davenport LB, Desilets R, Dietz SM, Doup L, Dullaghan P, Ferriera S, Fosler CR, Gire HC, Gluecksmann A, Gocayne JD, Gray J, Hart B, Haynes J, Hoover J, Howland T, Ibegwam C, Jalali M, Johns D, Kline L, Ma DS, MacCawley S, Magoon A, Mann F, May D, McIntosh TC, Mehta S, Moy L, Moy MC, Murphy BJ, Murphy SD, Nelson KA, Nuri Z, Parker KA, Prudhomme AC, Puri VN, Qureshi H, Raley JC, Reardon MS, Regier MA, Rogers YH, Romblad DL, Schutz J, Scott JL, Scott R, Sitter CD, Smallwood M, Sprague AC, Stewart E, Strong RV, Suh E, Sylvester K, Thomas R, Tint NN, Tsonis C, Wang G, Wang G, Williams MS, Williams SM, Windsor SM, Wolfe K, Wu MM, Zaveri J, Chaturvedi K, Gabrielian AE, Ke Z, Sun J, Subramanian G, Venter JC, Pfannkoch CM, Barnstead M, Stephenson LD, 2002. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science 296 (5573), 1661–1671. [DOI] [PubMed] [Google Scholar]
- Muslin AJ, Tanner JW, Allen PM, Shaw AS, 1996. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84 (6), 889–897. [DOI] [PubMed] [Google Scholar]
- Myrianthopoulos V, Lozach O, Zareifi D, Alexopoulos L, Meijer L, Gorgoulis VG, Mikros E, 2017. Combined virtual and experimental screening for CK1 inhibitors identifies a modulator of p53 and reveals important aspects of in silico screening performance. Int. J. Mol. Sci 18 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P, 2004. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology 47 (Suppl. 1), 14–23. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Koinuma S, Shigeyoshi Y, 2015. Reduction of translation rate stabilizes circadian rhythm and reduces the magnitude of phase shift. Biochem. Biophys. Res. Commun 464 (1), 354–359. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R, Hunt SR, Lu Y, Fustin JM, Okamura H, Partch CL, Forger DB, Kim JK, Virshup DM, 2018. CK1delta/epsilon protein kinase primes the PER2 circadian phosphoswitch. Proc. Natl. Acad. Sci. U. S. A 115 (23), 5986–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett IR, Martin DM, Miranda-Saavedra D, Lamont D, Barber JD, Mehlert A, Ferguson MA, 2009. The phosphoproteome of bloodstream form Trypanosoma brucei, causative agent of African sleeping sickness. Mol. Cell. Proteomics 8 (7), 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Suzuki G, Tanaka Y, Kametani F, Hirai S, Okado H, Miyashita T, Saitoe M, Akiyama H, Masai H, Hasegawa M, 2016. Phosphorylation of TAR DNA-binding protein of 43 kDa (TDP-43) by truncated casein kinase 1delta triggers Mislocalization and accumulation of TDP-43. J. Biol. Chem 291 (11), 5473–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Clevers H, 2017. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169 (6), 985–999. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Hirai S, Iizuka T, Yanagisawa T, Watanabe M, 1991. Reexamination of granulovacuolar degeneration. Acta Neuropathol 82 (5), 340–345. [DOI] [PubMed] [Google Scholar]
- Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A, 2004. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol. Cell. Biol 24 (10), 4184–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C, 2000. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J. Biol. Chem 275 (1), 390–397. [DOI] [PubMed] [Google Scholar]
- Oumata N, Bettayeb K, Ferandin Y, Demange L, Lopez-Giral A, Goddard ML, Myrianthopoulos V, Mikros E, Flajolet M, Greengard P, Meijer L, Galons H, 2008. Roscovitine-derived, dual-specificity inhibitors of cyclin-dependent kinases and casein kinases 1. J. Med. Chem 51 (17), 5229–5242. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL, 2015. Alternative Wnt signaling activates YAP/TAZ. Cell 162 (4), 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer C, Kinkel K, Abadleh M, Schollmeyer D, Laufer S, 2007. From five- to sixmembered rings: 3,4-diarylquinolinone as lead for novel p38MAP kinase inhibitors. J. Med. Chem 50 (6), 1213–1221. [DOI] [PubMed] [Google Scholar]
- Peifer C, Urich R, Schattel V, Abadleh M, Rottig M, Kohlbacher O, Laufer S, 2008. Implications for selectivity of 3,4-diarylquinolinones as p38alphaMAP kinase inhibitors. Bioorg. Med. Chem. Lett 18 (4), 1431–1435. [DOI] [PubMed] [Google Scholar]
- Peifer C, Abadleh M, Bischof J, Hauser D, Schattel V, Hirner H, Knippschild U, Laufer S, 2009. 3,4-Diaryl-isoxazoles and -imidazoles as potent dual inhibitors of p38alpha mitogen activated protein kinase and casein kinase 1delta. J. Med. Chem 52 (23), 7618–7630. [DOI] [PubMed] [Google Scholar]
- Penas C, Ramachandran V, Simanski S, Lee C, Madoux F, Rahaim RJ, Chauhan R, Barnaby O, Schurer S, Hodder P, Steen J, Roush WR, Ayad NG, 2014. Casein kinase 1delta-dependent Wee1 protein degradation. J. Biol. Chem 289 (27), 18893–18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penas C, Govek EE, Fang Y, Ramachandran V, Daniel M, Wang W, Maloof ME, Rahaim RJ, Bibian M, Kawauchi D, Finkelstein D, Han JL, Long J, Li B, Robbins DJ, Malumbres M, Roussel MF, Roush WR, Hatten ME, Ayad NG, 2015. Casein kinase 1delta is an APC/C(Cdh1) substrate that regulates cerebellar granule cell neurogenesis. Cell Rep. 11 (2), 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Grassart A, Lu R, Wong CC, Yates III J, Barnes G, Drubin DG, 2015a. Casein kinase 1 promotes initiation of clathrin-mediated endocytosis. Dev. Cell 32 (2), 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Moritz M, Han X, Giddings TH, Lyon A, Kollman J, Winey M, Yates III J, Agard DA, Drubin DG, Barnes G, 2015b. Interaction of CK1delta with gammaTuSC ensures proper microtubule assembly and spindle positioning. Mol. Biol. Cell 26 (13), 2505–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Vengeliene V, Noori HR, Merlo-Pich EV, Corsi MA, Corti C, Spanagel R, 2012. Inhibition of the casein-kinase-1-epsilon/delta/prevents relapse-like alcohol drinking. Neuropsychopharmacology 37 (9), 2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis N, Cipak L, Polakova S, Hyppa RW, Cipakova I, Anrather D, Karvaiova L, Mechtler K, Smith GR, Gregan J, 2015. Casein kinase 1 and phosphorylation of Cohesin subunit Rec11 (SA3) promote meiotic recombination through linear element formation. PLoS Genet. 11 (5), e1005225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P, 2012. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol 4 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posa D, Martinez-Gonzalez L, Bartolome F, Nagaraj S, Porras G, Martinez A, Martin-Requero A, 2018. Recapitulation of Pathological TDP-43 Features in Immortalized Lymphocytes from Sporadic ALS Patients. Neurobiol, Mol. [DOI] [PubMed] [Google Scholar]
- Price MA, 2006. CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev 20 (4), 399–410. [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D, 2002. Proteolysis of the hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108 (6), 823–835. [DOI] [PubMed] [Google Scholar]
- Pulgar V, Tapia C, Vignolo P, Santos J, Sunkel CE, Allende CC, Allende JE, 1996. The recombinant alpha isoform of protein kinase CK1 from Xenopus laevis can phosphorylate tyrosine in synthetic substrates. Eur J Biochem 242 (3), 519–528. [DOI] [PubMed] [Google Scholar]
- Qi ST, Wang ZB, Huang L, Liang LF, Xian YX, Ouyang YC, Hou Y, Sun QY, Wang WH, 2015. Casein kinase 1 (alpha, delta and epsilon) localize at the spindle poles, but may not be essential for mammalian oocyte meiotic progression. Cell Cycle 14 (11), 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi N, Taly JF, Durieu E, Leclercq O, Aulner N, Prina E, Pescher P, Notredame C, Meijer L, Spath GF, 2014. Pharmacological assessment defines Leishmania donovani casein kinase 1 as a drug target and reveals important functions in parasite viability and intracellular infection. Antimicrob. Agents Chemother 58 (3), 1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regad T, Roth M, Bredenkamp N, Illing N, Papalopulu N, 2007. The neural progenitor-specifying activity of FoxG1 is antagonistically regulated by CKI and FGF. Nat. Cell Biol 9 (5), 531–540. [DOI] [PubMed] [Google Scholar]
- Rena G, Bain J, Elliott M, Cohen P, 2004. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 5 (1), 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Bischof J, Zaja M, Kohlhof H, Othersen O, Vitt D, Alscher V, Pospiech I, Garcia-Reyes B, Berg S, Leban J, Knippschild U, 2014. Difluoro-dioxolo-benzoimidazol-benzamides as potent inhibitors of CK1delta and epsilon with nano-molar inhibitory activity on cancer cell proliferation. J. Med. Chem 57 (19), 7933–7946. [DOI] [PubMed] [Google Scholar]
- Richter J, Ullah K, Xu P, Alscher V, Blatz A, Peifer C, Halekotte J, Leban J, Vitt D, Holzmann K, Bakulev V, Pinna LA, Henne-Bruns D, Hillenbrand A, Kornmann M, Leithauser F, Bischof J, Knippschild U, 2015. Effects of altered expression and activity levels of CK1delta and varepsilon on tumor growth and survival of colorectal cancer patients. Int. J. Cancer 136 (12), 2799–2810. [DOI] [PubMed] [Google Scholar]
- Richter J, Rudeck S, Kretz AL, Kramer K, Just S, Henne-Bruns D, Hillenbrand A, Leithauser F, Lemke J, Knippschild U, 2016. Decreased CK1delta expression predicts prolonged survival in colorectal cancer patients. Tumour Biol. 37 (7), 8731–8739. [DOI] [PubMed] [Google Scholar]
- Rivers A, Gietzen KF, Vielhaber E, Virshup DM, 1998. Regulation of casein kinase I epsilon and casein kinase I delta by an in vivo futile phosphorylation cycle. J. Biol. Chem 273 (26), 15980–15984. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP, 2007. Patched1 regulates hedgehog signaling at the primary cilium. Science 317 (5836), 372–376. [DOI] [PubMed] [Google Scholar]
- Roof DM, Meluh PB, Rose MD, 1992. Kinesin-related proteins required for assembly of the mitotic spindle. J. Cell Biol 118 (1), 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg LH, Lafitte M, Quereda V, Grant W, Chen W, Bibian M, Noguchi Y, Fallahi M, Yang C, Chang JC, Roush WR, Cleveland JL and Duckett DR (2015). “Therapeutic targeting of casein kinase 1delta in breast cancer.” Sci Transl Med 7(318): 318ra202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC, 2012. Beta-catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151 (7), 1457–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpf C, Cipak L, Dudas A, Benko Z, Pozgajova M, Riedel CG, Ammerer G, Mechtler K, Gregan J, 2010. Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle 9 (13), 2657–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdoti-Sierra N, Jaffe CL, 1997. Release of ecto-protein kinases by the protozoan parasite Leishmania major. J. Biol. Chem 272 (49), 30760–30765. [DOI] [PubMed] [Google Scholar]
- Sakuno T, Watanabe Y, 2015. Phosphorylation of cohesin Rec11/SA3 by casein kinase 1 promotes homologous recombination by assembling the meiotic chromosome axis. Dev. Cell 32 (2), 220–230. [DOI] [PubMed] [Google Scholar]
- Salado IG, Redondo M, Bello ML, Perez C, Liachko NF, Kraemer BC, Miguel L, Lecourtois M, Gil C, Martinez A, Perez DI, 2014. Protein kinase CK-1 inhibitors as new potential drugs for amyotrophic lateral sclerosis. J. Med. Chem 57 (6), 2755–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittek B, Sinnberg T, 2014. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol. Cancer 13, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C, DeMaggio AJ, Ghoshal N, Binder LI, Kuret J, McGeer PL, 2000. Casein kinase 1 delta is associated with pathological accumulation of tau in several neurodegenerative diseases. Neurobiol. Aging 21 (4), 503–510. [DOI] [PubMed] [Google Scholar]
- Seerden JP, Leusink-Ionescu G, Woudenberg-Vrenken T, Dros B, Molema G, Kamps JA, Kellogg RM, 2014. Synthesis and structure-activity relationships of 4-fluorophenyl-imidazole p38alpha MAPK, CK1delta and JAK2 kinase inhibitors. Bioorg. Med. Chem. Lett 24 (15), 3412–3418. [DOI] [PubMed] [Google Scholar]
- Shanware NP, Trinh AT, Williams LM, Tibbetts RS, 2007. Coregulated ataxia telangiectasia-mutated and casein kinase sites modulate cAMP-response element-binding protein-coactivator interactions in response to DNA damage. J. Biol. Chem 282 (9), 6283–6291. [DOI] [PubMed] [Google Scholar]
- Shanware NP, Hutchinson JA, Kim SH, Zhan L, Bowler MJ, Tibbetts RS, 2011. Casein kinase 1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. J. Biol. Chem 286 (14), 12766–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Sharma M, Amin ND, Albers RW, Pant HC, 1999. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc. Natl. Acad. Sci. U. S. A 96 (20), 11156–11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Li S, Li S, Jiang A, Chen Y, Jiang J, 2014. Hedgehog-induced phosphorylation by CK1 sustains the activity of ci/Gli activator. Proc. Natl. Acad. Sci. U. S. A 111 (52), E5651–E5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillibourne JE, Milne DM, Takahashi M, Ono Y, Meek DW, 2002. Centrosomal anchoring of the protein kinase CK1delta mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450. J. Mol. Biol 322 (4), 785–797. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE, 2010a. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci 123 (Pt 6), 842–852. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE, 2010b. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol 185 (9), 5011–5022. [DOI] [PubMed] [Google Scholar]
- Singh SP, Gupta DK, 2015. A comparative study of structural and conformational properties of casein kinase-1 isoforms: insights from molecular dynamics and principal component analysis. J. Theor. Biol 371, 59–68. [DOI] [PubMed] [Google Scholar]
- Smadja Storz S, Tovin A, Mracek P, Alon S, Foulkes NS, Gothilf Y, 2013. Casein kinase 1delta activity: a key element in the zebrafish circadian timing system. PLoS One 8 (1), e54189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smal C, Vertommen D, Amsailale R, Arts A, Degand H, Morsomme P, Rider MH, Neste EV, Bontemps F, 2010. Casein kinase 1delta activates human recombinant deoxycytidine kinase by Ser-74 phosphorylation, but is not involved in the in vivo regulation of its activity. Arch. Biochem. Biophys 502 (1), 44–52. [DOI] [PubMed] [Google Scholar]
- Smelkinson MG, Zhou Q, Kalderon D, 2007. Regulation of ci-SCFSlimb binding, ci proteolysis, and hedgehog pathway activity by ci phosphorylation. Dev. Cell 13 (4), 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, Demarta C, Bouza Y, Sicard A, Nivez MP, Eschenlauer S, Lama T, Thomas DC, Sharma P, Agarwal S, Kern S, Pradel G, Graciotti M, Tobin AB, Doerig C, 2011. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat. Commun 2, 565. [DOI] [PubMed] [Google Scholar]
- Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, Varelas X, Arbouzova NI, Shaw S, Saburi S, Matakatsu H, Blair S, McNeill H, 2009. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr. Biol 19 (13), 1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U, 2005. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat. Med 11 (1), 35–42. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Swanson TA, Engwall M, 2009. Inhibition of casein kinase I epsilon/delta produces phase shifts in the circadian rhythms of Cynomolgus monkeys. Psychopharmacology 204 (4), 735–742. [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A, 2019. Circadian clocks and insulin resistance. Nat Rev Endocrinol 15 (2), 75–89. [DOI] [PubMed] [Google Scholar]
- Stoter M, Bamberger AM, Aslan B, Kurth M, Speidel D, Loning T, Frank HG, Kaufmann P, Lohler J, Henne-Bruns D, Deppert W, Knippschild U, 2005. Inhibition of casein kinase I delta alters mitotic spindle formation and induces apoptosis in trophoblast cells. Oncogene 24 (54), 7964–7975. [DOI] [PubMed] [Google Scholar]
- Stoter M, Kruger M, Banting G, Henne-Bruns D, Knippschild U, 2014. Microtubules depolymerization caused by the CK1 inhibitor IC261 may be not mediated by CK1 blockage. PLoS One 9 (6), e100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA, 2002. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. U. S. A 99 (26), 16899–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Song J, Wang Z, Zhou L, Xia Y, Yu S, Sun Q, Liu SS, Zhao L, Li S, Wei L, Carson DA, Lu D, 2018. Tumor promoter TPA activates Wnt/beta-catenin signaling in a casein kinase 1-dependent manner. Proc. Natl. Acad. Sci. U. S. A 115 (32), E7522–e7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Hatano N, Sueyoshi N, Suetake I, Tajima S, Kinoshita E, Kinoshita-Kikuta E, Koike T, Kameshita I, 2010. The DNA-binding activity of mouse DNA methyltransferase 1 is regulated by phosphorylation with casein kinase 1delta/epsilon. Biochem. J 427 (3), 489–497. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P, 2005. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 7 (2), E353–E360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A and Nagai K (2001). Casein kinase 1 delta.
- Tarapore P, Fukasawa K, 2002. Loss of p53 and centrosome hyperamplification. Oncogene 21 (40), 6234–6240. [DOI] [PubMed] [Google Scholar]
- Teran E, Branscomb AD, Seeling JM, 2009. Dpr acts as a molecular switch, inhibiting Wnt signaling when unphosphorylated, but promoting Wnt signaling when phosphorylated by casein kinase Idelta/epsilon. PLoS One 4 (5), e5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Del Tredici K, Ludolph AC, Hoozemans JJ, Rozemuller AJ, Braak H, Knippschild U, 2011. Stages of granulovacuolar degeneration: their relation to Alzheimer’s disease and chronic stress response. Acta Neuropathol. 122 (5), 577–589. [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller III DM, Lee E, 2010. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol 6 (11), 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Elias JE, Boothroyd JC, 2011. The phosphoproteomes of Plasmodium falciparum and toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe 10 (4), 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai IC, Woolf M, Neklason DW, Branford WW, Yost HJ, Burt RW, Virshup DM, 2007. Disease-associated casein kinase I delta mutation may promote adenomatous polyps formation via a Wnt/beta-catenin independent mechanism. Int. J. Cancer 120 (5), 1005–1012. [DOI] [PubMed] [Google Scholar]
- Tsigankov P, Gherardini PF, Helmer-Citterich M, Spath GF, Myler PJ, Zilberstein D, 2014. Regulation dynamics of Leishmania differentiation: deconvoluting signals and identifying phosphorylation trends. Mol. Cell. Proteomics 13 (7), 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A, 2005. Coupling of human circadian and cell cycles by the timeless protein. Mol. Cell. Biol 25 (8), 3109–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak MD, 2009. Casein kinase 1 isoform 2 is essential for bloodstream form Trypanosoma brucei. Mol. Biochem. Parasitol 166 (2), 183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancura A, Sessler A, Leichus B, Kuret J, 1994. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J. Biol. Chem 269 (30), 19271–19278. [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, Wrana JL, Attisano L, 2010. The hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 18 (4), 579–591. [DOI] [PubMed] [Google Scholar]
- Venerando A, Marin O, Cozza G, Bustos VH, Sarno S, Pinna LA, 2010. Isoform specific phosphorylation of p53 by protein kinase CK1. Cell. Mol. Life Sci 67 (7), 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerando A, Girardi C, Ruzzene M, Pinna LA, 2013. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down-regulation and GSK3 activation. Biochem. J 452 (1), 131–137. [DOI] [PubMed] [Google Scholar]
- Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM, 2000. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol. Cell. Biol 20 (13), 4888–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup DM, Eide EJ, Forger DB, Gallego M, Harnish EV, 2007. Reversible protein phosphorylation regulates circadian rhythms. Cold Spring Harb. Symp. Quant. Biol 72, 413–420. [DOI] [PubMed] [Google Scholar]
- von Blume J, Knippschild U, Dequiedt F, Giamas G, Beck A, Auer A, Van Lint J, Adler G, Seufferlein T, 2007. Phosphorylation at Ser244 by CK1 determines nuclear localization and substrate targeting of PKD2. EMBO J. 26 (22), 4619–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TT, Chandrasekaran RY, Bradley J, Rubitski D, Berke H, Mente S, Butler T, Doran A, Chang C, Fisher K, Knafels J, Liu S, Ohren J, Marconi M, DeMarco G, Sneed B, Walton K, Horton D, Rosado A, Mead A, 2014. Casein kinase 1delta/epsilon inhibitor PF-5006739 attenuates opioid drug-seeking behavior. ACS Chem. Neurosci 5 (12), 1253–1265. [DOI] [PubMed] [Google Scholar]
- Wager TT, Galatsis P, Chandrasekaran RY, Butler TW, Li J, Zhang L, Mente S, Subramanyam C, Liu S, Doran AC, Chang C, Fisher K, Grimwood S, Hedde JR, Marconi M, Schildknegt K, 2017. Identification and profiling of a selective and brain penetrant Radioligand for in vivo target occupancy measurement of casein kinase 1 (CK1) inhibitors. ACS Chem. Neurosci 8 (9), 1995–2004. [DOI] [PubMed] [Google Scholar]
- Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, Galloway DA, 1996. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat. Med 2 (1), 72–79. [DOI] [PubMed] [Google Scholar]
- Walter J, Grunberg J, Schindzielorz A, Haass C, 1998. Proteolytic fragments of the Alzheimer’s disease associated presenilins-1 and −2 are phosphorylated in vivo by distinct cellular mechanisms. Biochemistry 37 (17), 5961–5967. [DOI] [PubMed] [Google Scholar]
- Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C, 2001. Phosphorylation regulates intracellular trafficking of beta-secretase. J. Biol. Chem 276 (18), 14634–14641. [DOI] [PubMed] [Google Scholar]
- Walton KM, Fisher K, Rubitski D, Marconi M, Meng QJ, Sladek M, Adams J, Bass M, Chandrasekaran R, Butler T, Griffor M, Rajamohan F, Serpa M, Chen Y, Claffey M, Hastings M, Loudon A, Maywood E, Ohren J, Doran A, Wager TT, 2009. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J. Pharmacol. Exp. Ther 330 (2), 430–439. [DOI] [PubMed] [Google Scholar]
- Wan Q, Dingerdissen H, Fan Y, Gulzar N, Pan Y, Wu TJ, Yan C, Zhang H and Mazumder R (2015). “BioXpress: an integrated RNA-seq-derived gene expression database for pan-cancer analysis.” Database (Oxford) 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PC, Vancura A, Mitcheson TG, Kuret J, 1992. Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol. Biol. Cell 3 (3), 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Inuzuka H, Zhong J, Fukushima H, Wan L, Liu P, Wei W, 2012. DNA damage-induced activation of ATM promotes beta-TRCP-mediated Mdm2 ubiquitination and destruction. Oncotarget 3 (9), 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Davis S, Menon S, Zhang J, Ding J, Cervantes S, Miller E, Jiang Y, Ferro-Novick S, 2015. Ypt1/Rab1 regulates Hrr25/CK1delta kinase activity in ERGolgi traffic and macroautophagy. J. Cell Biol 210 (2), 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SK, Hu Y, Yang J, Smith CE, Richardson AS, Yamakoshi Y, Lee YL, Seymen F, Koruyucu M, Gencay K, Lee M, Choi M, Kim JW, Hu JC, Simmer JP, 2016. Fam83h null mice support a neomorphic mechanism for human ADHCAI. Mol Genet Genomic Med 4 (1), 46–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huai G, Wang H, Liu Y, Qi P, Shi W, Peng J, Yang H, Deng S, Wang Y, 2018. Mutual regulation of the hippo/Wnt/LPA/TGFbeta signaling pathways and their roles in glaucoma (review). Int. J. Mol. Med 41 (3), 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AD, Hunt NH, Wanigasekara Y, Bloomfield G, Wallach D, Roufogalis BD, Chaudhri G, 1999. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in ‘reverse signalling’. EMBO J. 18 (8), 2119–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Oltmer F, Richter J, Bischof J, Xu P, Burster T, Leithauser F, Knippschild U, 2015. CK1delta in lymphoma: gene expression and mutation analyses and validation of CK1delta kinase activity for therapeutic application. Front Cell Dev Biol 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M, Milne D, Dias S, Kulikov R, Knippschild U, Blattner C, Meek D, 2004. Protein kinase CK1delta phosphorylates key sites in the acidic domain of murine double-minute clone 2 protein (MDM2) that regulate p53 turnover. Biochemistry 43 (51), 16356–16364. [DOI] [PubMed] [Google Scholar]
- Wolff S, Xiao Z, Wittau M, Sussner N, Stoter M, Knippschild U, 2005. Interaction of casein kinase 1 delta (CK1delta) with the light chain LC2 of microtubule associated protein 1A (MAP1A). Biochim. Biophys. Acta 1745 (2), 196–206. [DOI] [PubMed] [Google Scholar]
- Wolff S, Stoter M, Giamas G, Piesche M, Henne-Bruns D, Banting G, Knippschild U, 2006. Casein kinase 1 delta (CK1delta) interacts with the SNARE associated protein snapin. FEBS Lett. 580 (27), 6477–6484. [DOI] [PubMed] [Google Scholar]
- Wolff S, García-Reyes B, Henne-Bruns D, Bischof J, Knippschild U, 2015. Protein kinase CK1 interacts with and phosphorylates RanBPM in vitro. Journal of Molecular Biochemistry 4, 11–19. [Google Scholar]
- Wood PA, Yang X, Taber A, Oh EY, Ansell C, Ayers SE, Al-Assaad Z, Carnevale K, Berger FG, Pena MM, Hrushesky WJ, 2008. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol. Cancer Res 6 (11), 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang H, Garcia Abreu J, He X, 2009. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One 4 (3), e4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhang Y, Sun B, McMahon AP, Wang Y, 2017. Hedgehog signaling: from basic biology to Cancer therapy. Cell Chem Biol 24 (3), 252–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Kimelman D, Xu W, 2003. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 17 (22), 2753–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RM, Carmel G, Sweet RM, Kuret J, Cheng X, 1995. Crystal structure of casein kinase-1, a phosphate-directed protein kinase. EMBO J. 14 (5), 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, Fu YH, 2005. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434 (7033), 640–644. [DOI] [PubMed] [Google Scholar]
- Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptacek LJ, 2007. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128 (1), 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Fischer-Posovszky P, Bischof J, Radermacher P, Wabitsch M, Henne-Bruns D, Wolf AM, Hillenbrand A, Knippschild U, 2015. Gene expression levels of casein kinase 1 (CK1) isoforms are correlated to adiponectin levels in adipose tissue of morbid obese patients and site-specific phosphorylation mediated by CK1 influences multimerization of adiponectin. Mol. Cell. Endocrinol 406, 87–101. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Friedlein A, Imai Y, Takahashi R, Kahle PJ, Haass C, 2005. Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J. Biol. Chem 280 (5), 3390–3399. [DOI] [PubMed] [Google Scholar]
- Yang LL, Li GB, Yan HX, Sun QZ, Ma S, Ji P, Wang ZR, Feng S, Zou J, Yang SY, 2012. Discovery of N6-phenyl-1H-pyrazolo[3,4-d]pyrimidine-3,6-diamine derivatives as novel CK1 inhibitors using common-feature pharmacophore model based virtual screening and hit-to-lead optimization. Eur. J. Med. Chem 56, 30–38. [DOI] [PubMed] [Google Scholar]
- Yang W, Garrett L, Feng D, Elliott G, Liu X, Wang N, Wong YM, Choi NT, Yang Y, Gao B, 2017. Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development. Cell Res. 27 (12), 1466–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao E, Chuang PT, 2015. Hedgehog signaling: from basic research to clinical applications. J. Formos. Med. Assoc 114 (7), 569–576. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Kuret J, DeMaggio AJ, McGeer E, McGeer PL, 2000. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 865 (1), 116–120. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Akiyama H, McGeer EG, McGeer PL, 2001. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci. Lett 297 (2), 97–100. [DOI] [PubMed] [Google Scholar]
- Ye Q, Ur SN, Su TY, Corbett KD, 2016. Structure of the Saccharomyces cerevisiae Hrr25:Mam1 monopolin subcomplex reveals a novel kinase regulator. EMBO J. 35 (19), 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim DG, Ghosh S, Guy GR, Virshup DM, 2014. Casein kinase 1 regulates Sprouty2 in FGF-ERK signaling. Oncogene. 34 (4), 474–484. [DOI] [PubMed] [Google Scholar]
- Yin H, Laguna KA, Li G, Kuret J, 2006. Dysbindin structural homologue CK1BP is an isoform-selective binding partner of human casein kinase-1. Biochemistry 45 (16), 5297–5308. [DOI] [PubMed] [Google Scholar]
- Yu S, Roth MG, 2002. Casein kinase I regulates membrane binding by ARF GAP1. Mol. Biol. Cell 13 (8), 2559–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemlickova E, Johannes FJ, Aitken A, Dubois T, 2004. Association of CPI-17 with protein kinase C and casein kinase I. Biochem. Biophys. Res. Commun 316 (1), 39–47. [DOI] [PubMed] [Google Scholar]
- Zemp I, Wandrey F, Rao S, Ashiono C, Wyler E, Montellese C, Kutay U, 2014. CK1delta and CK1epsilon are components of human 40S subunit precursors required for cytoplasmic 40S maturation. J. Cell Sci 127 (Pt 6), 1242–1253. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Hong W, 2008. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13 (3), 188–192. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X, 2005. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438 (7069), 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeringo NA, Murphy L, McCloskey EA, Rohal L, Bellizzi III JJ, 2013. A monoclinic crystal form of casein kinase 1 delta. Acta Crystallogr Sect F Struct Biol Cryst Commun 69 (Pt 10), 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gordon SL, Fritsch MJ, Esoof N, Campbell DG, Gourlay R, Velupillai S, Macartney T, Peggie M, van Aalten DM, Cousin MA, Alessi DR, 2015a. Phosphorylation of synaptic vesicle protein 2A at Thr84 by casein kinase 1 family kinases controls the specific retrieval of synaptotagmin-1. J. Neurosci 35 (6), 2492–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang C, Kim S, Golkaram M, Dixon MW, Tilley L, Li J, Zhang S, Suresh S, 2015b. Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite. Proc. Natl. Acad. Sci. U. S. A 112 (19), 6068–6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Shi Q, Varia SN, Xing S, Klett BM, Cook LA, Herman PK, 2016. The activity-dependent regulation of protein kinase stability by the localization to P-bodies. Genetics 203 (3), 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Butler AM, Shi Q, Xing S, Herman PK, 2018. P-body localization of the Hrr25/casein kinase 1 protein kinase is required for the completion of meiosis. Mol. Cell. Biol 38 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL, 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24 (1), 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Shaik S, Wan L, Tron AE, Wang Z, Sun L, Inuzuka H, Wei W, 2013. SCFbeta-TRCP targets MTSS1 for ubiquitination-mediated destruction to regulate cancer cell proliferation and migration. Oncotarget 4 (12), 2339–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyss D, Ebrahimi H, Gergely F, 2011. Casein kinase I delta controls centrosome positioning during T cell activation. J. Cell Biol 195 (5), 781–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


