ABSTRACT
Mantle cell lymphoma (MCL) is an aggressive form of B cell non-Hodgkin’s lymphoma and remains incurable under current treatment modalities. One of the main reasons for treatment failure is the development of drug resistance. Accumulating evidence suggests that B cell activating factor (BAFF) and BAFF receptor (BAFF-R) play an important role in the proliferation and survival of malignant B cells. High serum BAFF levels are often correlated with poor drug response and relapse in MCL patients. Our study shows that BAFF-R is expressed on both MCL patient cells and cell lines. BAFF-R knockdown leads to MCL cell death showing the importance of BAFF-R signaling in MCL survival. Moderate knockdown of BAFF-R in MCL cells did not affect its viability, but sensitized them to cytarabine treatment in vitro and in vivo, with prolonged mice survival. Anti-BAFF-R antibody treatment promoted drug-induced MCL cell death. Conversely, the addition of recombinant BAFF (rhBAFF) to MCL cells protected them from cytarabine-induced apoptosis. We tested the efficacy of a humanized defucosylated ADCC optimized anti-BAFF-R antibody in killing MCL. Our data show both in vitro and in vivo efficacy of this antibody for MCL therapy. To conclude, our data indicate that BAFF/BAFF-R signaling is crucial for survival and involved in drug resistance of MCL. Targeting BAFF-R using BAFF-R antibody might be a promising therapeutical strategy to treat MCL patients resistant to chemotherapy.
KEYWORDS: Mantle Cell Lymphoma, drug resistance, baff, adcc, nk cells, therapy
Introduction
Mantle cell lymphoma (MCL) is a highly aggressive rare subtype of B cell non-Hodgkin’s lymphoma (NHL) and considered an incurable disease.1,2 It is the result of the rearrangement of the cyclin D1 (CCND1) locus to that of the immunoglobulin heavy chain (11q13-14q32). It leads to overexpression and activation of CCND1 important for the development and maintenance of the MCL disease.3 The induction regimen containing rituximab and high doses of cytarabine followed by autologous stem cell transplantation and maintenance of rituximab is adapted as a standard care procedure for young fit patients of MCL.4 The addition of high-dose cytarabine to the treatment of MCL has improved the survival, however, limitations include hematological toxicities and relapse. The recent advances have witnessed the development of many efficacious targeted therapies such as ibrutinib, bortezomib, and lenalidomide that has improved the health condition of MCL relapsed patients.5,6 However, the prognosis of these relapsed MCL patients still remains as a great concern. Therefore, it becomes imperative to thoroughly understand the development of drug resistance mechanisms to open new treatment avenues for relapsed patients.
BAFF, a member of tumor necrosis factor (TNF) superfamily is important for survival and maturation of B cells. It is either released as a soluble factor or displayed on the cell surface of various cell types such as monocytes, dendritic cells, and bone marrow stromal cells.7 It exerts its biological effect by binding to the three identified receptors: BAFF-receptor (BAFF-R), B cell maturation antigen (BCMA), and transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI).8 The BAFF/BAFF-R signaling is required for the maturation and maintenance of normal B cells. Furthermore, BAFF/BAFF-R axis plays a key role in the pathogenesis and progression of malignant B cells.9 Several studies have also reported the higher expression of BAFF in NHL, precursor B acute lymphoblastic leukemia (pre-B ALL), B-cell chronic lymphocytic leukemia (B-CLL), and multiple myeloma (MM) cells relative to the normal B- cells.10–13 Moreover, the serum level of BAFF was found to be elevated in patients with NHL, B-CLL, and MM cases.14–17 The NHL patients with high BAFF serum levels showed an aggressive form of the disease and poor drug response.18 The malignant B-cells and/or the cells present in the microenvironment are known to facilitate their growth and survival through altered BAFF production. Lwin et al. have earlier demonstrated that the high levels of BAFF secreted by bone marrow stromal cells (BMSC) have protected the NHL-B lymphoma cells from spontaneous and drug-induced apoptosis.10 Moreover, various other studies have indicated the key role of BAFF-R in the pathogenesis and drug resistance in malignant B-cells. Parameswaran et al. have shown the expression of BAFF-R in pre-B ALL cells implicating their contribution to the development and progression of the disease.19 Furthermore, another report has shown a higher percentage of MCL, follicular lymphoma (FL), CLL, and diffuse large B-cell lymphoma (DLBCL) cells to be BAFF-R positive.20 An interesting study by Zhou group demonstrated that the dual functional BAFF receptor aptamers can inhibit ligand-induced proliferation and deliver siRNAs specific to STAT3 in NHL cells.21 However, the pathological roles of BAFF-R in MCL cells and its therapeutic targeting need more attention.
The present study aims to investigate the significance of BAFF-R expression in growth and survival of MCL cells and ways to target BAFF-R for MCL therapy. Here, we demonstrate that BAFF-R is essential for the growth and survival of MCL cells. Moreover, targeting of BAFF-R in MCL cells sensitizes them to cytarabine treatment both in vitro and in vivo. Finally, we used a BAFF-R antibody (VAY-736) optimized for antibody-dependent cell cytotoxicity (ADCC), in combination with allogeneic NK cells for MCL therapy using xenograft mouse models.
Material and methods reagents
Recombinant human BAFF (rhBAFF), APC anti-human CD19 antibody, APC Annexin V apoptosis detection kit with PI (BioLegend), Anti-BAFF-R VAY-736 (Novartis), Anti-BAFF- R antibody clone 11c1 (Pharmingen), cytarabine, Fluorescein isothiocyanate (FITC) anti-human immunoglobulin G (IgG) (Sigma Aldrich), neutralizing anti-human BAFF R (R&D Systems), GAPDH, Lamin A/C, α-tubulin, cleaved caspase 3, total-ERK antibodies (Santa Cruz Biotechnology), NF-kB p100/p52, phospho-AKT(Ser473), phospho- ERK1/2(Thr202/Tyr204), total-AKT antibodies (Cell Signaling Technology) were used for the study.
Cell culture
Jeko-1, Mino, and HEK293T cells were purchased from American Type Culture Collection (ATCC). JVM2 was kindly provided by Dr. Distelhorst. JVM2, Mino, and Jeko-1 cells were cultured in RPMI1640 medium supplemented with 10%, 15%, and 20% FBS, respectively. HEK293T cells were maintained in DMEM medium supplemented with 10% FBS. All the cell lines were maintained in a 5% CO2 incubator at 37 oC.
Patient samples
Primary lymphoma cells were isolated from pleural effusions of MCL patients obtained from the University Hospital Case Medical Center. Human Peripheral Blood Mononuclear Cells (PBMCs) from healthy donors were obtained from the Hematopoietic Stem Cell Core Facility, Case Western Reserve University. MCL patient blood and bone marrow samples were obtained from Dr. Rose Beck. Only discarded samples were used and approved by the University Hospitals Case Medical Center Institutional Review Board.
Lentivirus production and BAFF-R knockdown
Human BAFF-R shRNAs constructs (shBR3-3: TRCN0000058891, shBR3-4: TRCN0000058892, Sigma Aldrich) were co-transfected with lentivirus packaging plasmids psPAX2 and pMD2G into HEK293T cells. Virus supernatants were collected after 48 hours’ post-transfection. For viral transduction, 1 × 106 cells resuspended in 1 ml virus-containing medium with 10 µg/ml of polybrene and transferred to a 14 ml round bottom test tube and centrifuged at 2,250 rpm for 90 minutes in a centrifuge that was prewarmed at 32°C. Cell pellets were resuspended in RPMI 1640 growth media and grown for 24 hours before selection with 0.5 µg/ml puromycin.
Western blotting
Cells were lysed with 9 M urea buffer prepared in 20 mM Tris (pH 7.5) followed by cellular DNA fragmentation by sonication. Later, the supernatant was collected after centrifugation at 12,000 rpm (10 minutes) and protein concentration was determined. Nuclear extracts were prepared using the Nuclei EZ Prep kit (Sigma). SDS-PAGE was performed using 20–50 µg of proteins and transferred to the nitrocellulose membrane (Bio-Rad). The membrane was blocked in 5% nonfat dry milk (1 hour). Membranes were probed with primary antibodies overnight at 4°C and with secondary antibodies for 1 hour. Blots were developed by using the Clarity ECL substrate (Bio-Rad).
Cell proliferation and viability assay
Jeko-1 or Mino cells were plated (1 × 105/ml) in triplicates in a 24-well plate under different conditions. Cell proliferation and viability were measured by staining cells with 0.4% trypan blue and counting in an automated hemocytometer (Bio-Rad).
Apoptosis assay
Jeko-1, Mino, or JVM2 cells were infected with shGFP or shBR3-3 or shBR3-4 for 96 hours to knockdown the BAFF-R expression. The cells were harvested and washed with PBS and resuspended in Annexin V binding buffer. Subsequently, cells were stained with Annexin V/propidium iodide (PI). The Annexin V and PI-positive cells were analyzed on the Accuri C6 flow cytometry system.
In vitro antibody treatment
For competition assays, JVM2 cells were pre-incubated with different concentrations of rhBAFF for 2 hours, washed with PBS and incubated with 5 µg/ml anti-BAFF-R VAY-736 for 30 minutes at room temperature (RT), followed by incubation with FITC-conjugated anti-human IgG antibody, and analyzed by fluorescence-activated cell sorting (FACS; Accuri Flow Cytometers Inc). The assessment of BAFF-R antibody binding was performed by incubating JVM2 cells in different concentrations of anti-BAFF-R VAY-736 antibody for 30 minutes at RT, washed with PBS, and incubated with PE-labeled anti-BAFF-R and analyzed by FACS.
ADCC assays
NK cells from normal human blood donors were isolated by MACS negative separation column (Miltenyi Biotech). 1 × 106 cells of JVM2 or Jeko-1 were labeled with calcein-AM (Life science Technologies) for 30 minutes at 37°C. After washing with PBS, control human IgG Ab or 5 ug/mL of anti-BAFF-R VAY-736 was added to JVM2 or Jeko-1 cells and incubated for 1 hour at 37°C. A total of 10,000 JVM2 or Jeko-1 cells/well were plated in 96-well plate (triplicates) and then 50,000 of purified NK cells were added per well. After 4 hours of incubation, 100 µl of culture supernatant was transferred to a Black View 96-well plate and arbitrary fluorescent units (AFU) were measured on Tecan SPECTRAFLUOR.
In vivo tumor growth
All animal experiments were as per the Institutional Animal Care and Use Committee and NIH guidelines. NOD/SCID Mice were purchased from the Jackson Laboratory. 3 × 106 of JVM2 cells or 10 × 106/8 × 106 of Jeko-1 or 10 × 106 of Mino cells were injected subcutaneously in the dorsal flanks of 8-week old NOD/SCID mice (3 or 5 mice/experimental group). Cells were allowed to proliferate for 10–20 days until tumors reached the measurable size (50 mm3). Subsequently, mice were injected with PBS, 3 × 106 NK, or 3 × 106 NK mixed with anti- BAFF-R VAY736 antibody (10 mg/kg) intratumorally twice a week. Tumor sizes were measured by caliper (3 times/week).
Statistical analysis
Data were analyzed using the two-way ANOVA and unpaired Student’s t-test. All in vitro experiments were done in triplicates (N = 3). Three independent experiments with triplicates done for in vitro experiments and one representative experiment shown. P values; ns = not significant, *p < .05, **p < .01, ***p < .001, ****p < .0001. Statistical analysis of mice survival curve was done by Log-Rank (Mantel-Cox) test.
Results
BAFF-R antibody sensitizes MCL cells to chemotherapy
First, we detected and confirmed the presence of the BAFF-R expression in three clinical diagnosed MCL patient samples (gated CD19+ CD5+) and three MCL cell lines JVM2, Jeko-1, and Mino by flow cytometry analysis, using the anti-BAFF-R 11c1 antibody (Figure 1(a,b)). Primarily, we asked if BAFF stimulation protects MCL cells from chemotherapy-induced cell death. As cytarabine is commonly used in the treatment of MCL, we initially studied its effect on MCL cells in combination with added recombinant BAFF. Recombinant BAFF treatment alone didn’t alter proliferation rate and viability in Jeko-1 cells. However, BAFF protected cytarabine-induced cell death, as evidenced from the cell proliferation and viability graphs (Figure 1(c) and Supplementary Fig 1A). Subsequently, we tested the effect of a neutralizing anti-BAFF-R antibody in combination with cytarabine.
Figure 1.
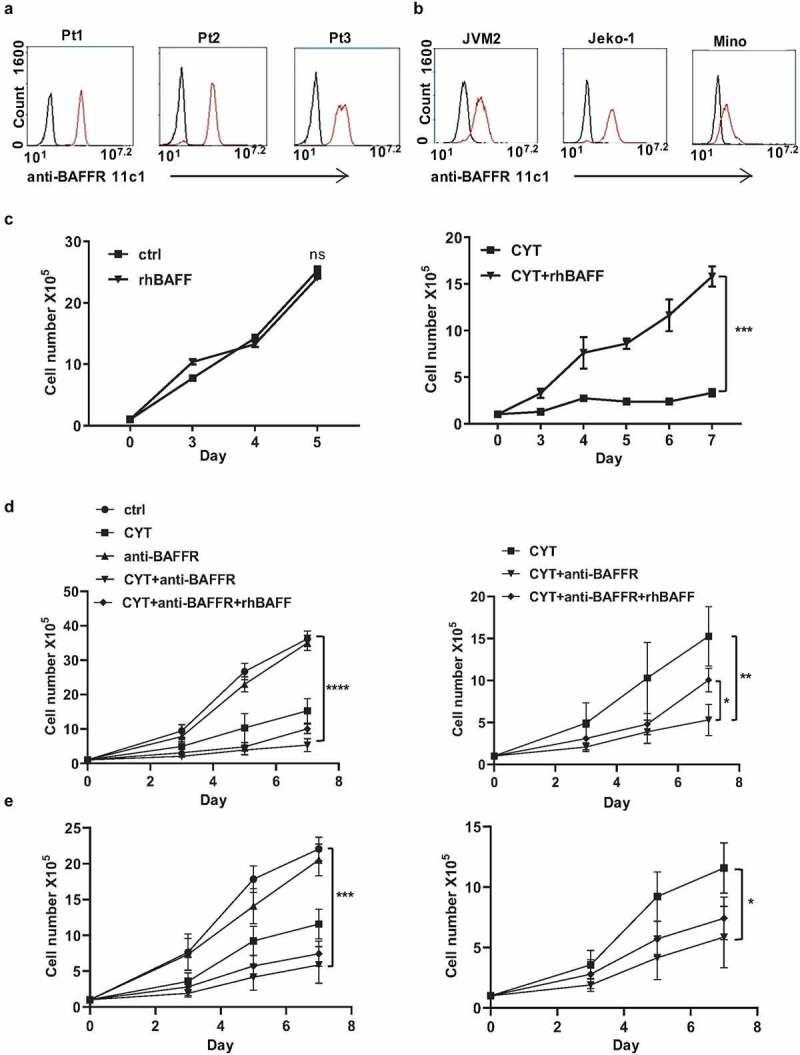
BAFF-R antibody sensitizes MCL cells to chemotherapy. (a) Flow cytometry analysis on three liquid MCL patient samples (Pt1, Pt2, and Pt3). Black histograms, control isotype; red histograms, anti-BAFFR 11c1. (b) BAFF-R expression in three MCL cell lines JVM2, Jeko-1, and Mino cells by flow cytometry. Black histograms, control isotype; red histograms, anti-BAFFR 11c1. (c) 1 × 105 Jeko-1 cells were seeded in 24-well plate in triplicates and cultured in the absence or presence of 200 ng/ml recombinant BAFF (rhBAFF) with or without 20 nM of cytarabine (CYT). The cell number was measured for the time indicated, not significant (ns) p > .05 for control Jeko-1 cells compared to rhBAFF-treated cells at day 5. ***p < .001 for Jeko-1 cells with cytarabine (CYT)+rhBAFF treatment compared with cells treated with CYT alone at day 7. One of the three experiments with triplicate samples shown. (d, left) 1 × 105 Jeko-1 cells or (e, left) Mino cells were seeded in 24-well plate in triplicates and cultured under different conditions such as untreated (ctrl) or 20 nM cytarabine (CYT), 5 μg/ml of neutralizing anti-BAFFR antibody (anti-BAFFR), CYT+anti-BAFFR antibody, and CYT+ anti-BAFFF-R antibody+200 ng/ml rhBAFF. The cell number was measured for the time indicated, ****p < .0001 Jeko-1 or Mino cells treated with anti BAFF-R antibody as compared to combined treatment of cytarabine and anti-BAFFR antibody. *p < .05 Jeko-1 cells treated with cytarabine+anti-BAFFR antibody compared to cytarabine+anti-BAFFR antibody+recombinant BAFF-treated Jeko-1 cells. *p < .05 for Jeko-1 cells treated with cytarabine versus combined treatment of cytarabine and anti-BAFFR antibody. Error bars are from single experiment done with triplicate samples representative of three such experiments done with triplicates
The treatment of antibody alone had only minor effects on the proliferation and viability of Jeko-1 cells. However, the combined treatment of antibody and cytarabine reduced the proliferation and viability of Jeko-1 cells. Moreover, the combined treatment presented a synergistic action as compared to the monotreatments (Figure 1(d) and Supplementary Figure 1B). Interesting enough, recombinant BAFF partially reversed this synergistic effect (Figure 1(d)). Mino cells also behaved similarly to these treatments. The treatment of anti-BAFFR antibody didn’t induce any changes in the proliferation of Mino cells. However, the combination of anti-BAFF-R antibody and cytarabine decreased the proliferation of MCL cells significantly (Figure 1(e)). The cell viability of Jeko-1 and Mino cells were reduced from 44 to 25% and 48% to 34%, respectively, in the combined treatment of cytarabine and BAFF-R antibody as compared to cytarabine alone (Supplementary Figure 1D, E).
Modest knockdown of BAFF-R expression sensitizes MCL cells to cytarabine treatment
In order to explore the importance of BAFF-R in MCL survival and maintenance we generated BAFF-R (BR3) knockdown MCL cells by transducing with BAFF-R shRNAs, namely, shBR3-3 and shBR3-4. These shRNAs found to efficiently knockdown the BAFF-R expression in Jeko-1 cells by 35% (shBR3-3) and 55% (shBR3-4) (figure 2(a)). The Annexin V/PI staining surprisingly showed 63% cell death by shBR3-4 while it was 4% for shBR3-3 knockdown cells which was closer to control cells (2%) (figure 2(b)), after 72 hours of transduction. The western blot analysis displayed the presence of robust cleaved caspase 3 band indicating apoptosis-mediated cell death induced by shBR3-4 (Supplementary Figure 2A). The percentage of cell death in Mino and JVM2 was found to be 95% and 76%, respectively, after shBR3-4-mediated knockdown of BAFF-R which too was due to apoptosis as evinced by the presence of cleaved caspase 3 band (Supplementary Figure 2B). We next asked if modest knockdown of BAFF-R in MCL cells (shBR3-3) have any effect on cell viability, proliferation, or sensitization to drug treatment. In line with the previous observation, BAFF-R knockdown by shBR3-3 in Jeko-1 and Mino cells does not have any significant effects on cell growth and viability (figure 2(c-d) left and Supplementary Figure 2C-D left). The treatment of cytarabine (20 nM) to shBR3-3 Jeko-1 cells and ShBR3-3 Mino cells decreased the proliferation rate significantly compared to respective controls shGFP Jeko-1 and shGFP Mino cells right (figure 2(c-d). The cell viability of shGFP Jeko-1 cells treated with cytarabine eventually dropped to 52% on day 7 (Supplementary Figure 2C left), while cell viability of shBR3-3 Jeko-1 cells were significantly reduced to 18% (Supplementary Figure 2C right). This shows that even moderate knockdown of BAFF-R makes MCL cells more sensitized to cytarabine without developing drug resistance. Moreover, the same trend was also observed in Mino cells where cell proliferation and viability was significantly reduced in cytarabine-treated shBR3-3 cells in comparison to shGFP Mino cells (figure 2(d) and Supplementary Figure 2D). Furthermore, we confirmed cell death using Annexin V/PI staining-based FACS analysis of Jeko-1 shBR3-3 and shGFP cells treated with cytarabine (figure 2(e), left).
Figure 2.
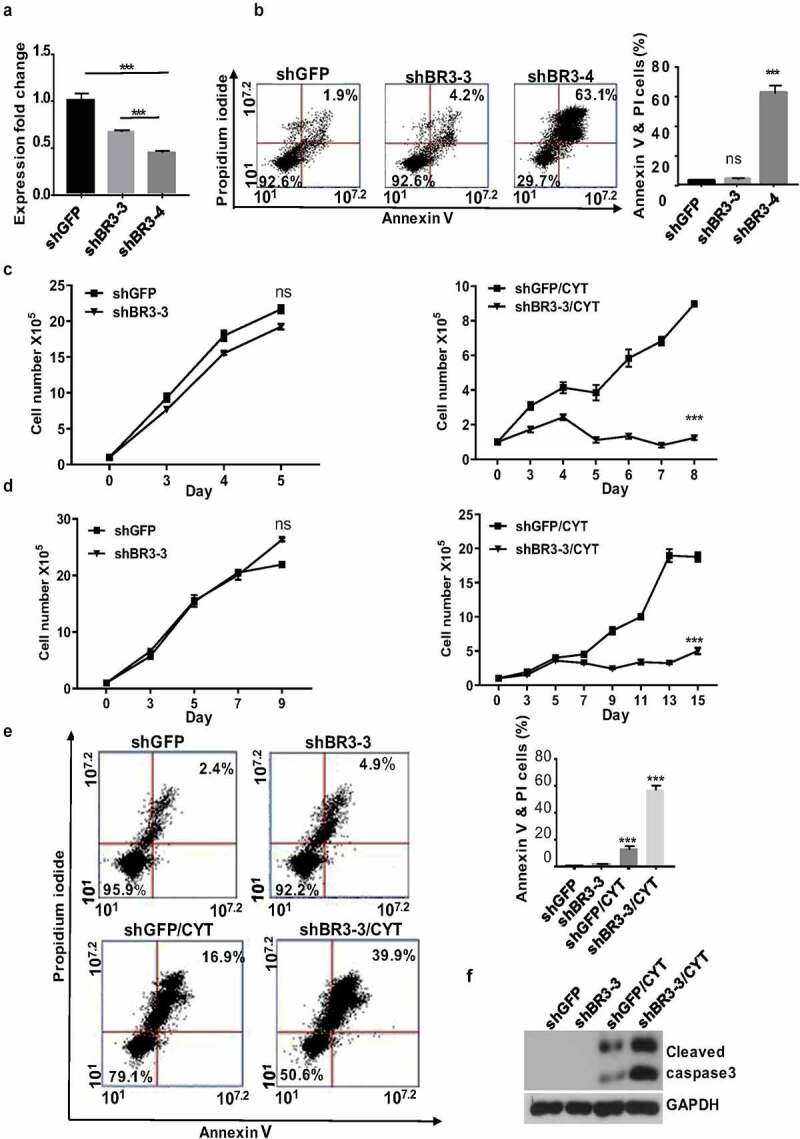
Modest knockdown of BAFF-R expression sensitizes MCL cells to cytarabine treatment in vitro. (a) Jeko-1 cells were infected with lentivirus shGFP, shBR3-3, or shBR3-4 for 72 hours. The transcriptional level of BAFF-R in Jeko-1 cells expressing shGFP, shBR3-3, or shBR3-4 was determined by RT-qPCR. ***p < .001 for Jeko-1 cells expressing shGFP compared to shBR3-3 and shBR3-3 compared to shBR3-4. (b) Graphical representation (on the left panel) showing the total population of early (Annexin V) and late apoptotic cells (PI) in one of three experiments with triplicate samples 96 hours after shGFP, shBR3-3, or shBR3-4 lentivirus infection in Jeko-1. The quantification data was shown on the right panel. ***p < .001 for Jeko-1 cells expressing shBR3-4 compared to shGFP; ns for Jeko-1 cells expressing shBR3-3 compared to shGFP. (c, left) 1 × 105 Jeko-1 cells or Mino cells (d, left) expressing shGFP or shBR3-3 were seeded in 24-well plate in triplicates and the cell numbers were plotted. p > .05 is considered not significant (ns). Cell counts from (c, right) Jeko-1 shGFP and shBR3-3 cells or (d, right) Mino cells shGFP and shBR3-3 treated with cytarabine ***p < .001. One of the three experiments done with triplicate samples shown. (e, left) The apoptotic cell population was stained by Annexin V and PI followed by flow cytometry analysis Dot plot graph represents the total population of early and late apoptotic cells from (c). Apoptotic cell quantification data shown on the right panel. ***p < .001 for cells expressing shGFP and CYT treatment for 96 hours compared to cells expressing shGFP alone. ***p < .001 for cells expressing shBR3-3 compared to cells expressing shGFP after CYT treatment for 96 hours. Error bars are from single experiment done with triplicate samples representative of three such experiments done with triplicates. (f) Western blot analysis for the apoptotic marker of cleaved caspase-3 in Jeko-1 cells expressing shGFP or shBR3-3 after CYT treatment for 96 hours. GAPDH was used as a loading control
The percentage of cell death in shBR3-3 expressing untreated and cytarabine-treated Jeko-1 cells was 18% and 50%, respectively (figure 2(e), right). The presence of cleaved caspase 3 confirmed apoptosis-mediated cell death in these cells (figure 2(f)).
BAFF protects MCL cells from chemotherapy-induced cell death
While BAFF-R antibody treatment and BAFF-R knockdown sensitized MCL cells to cytarabine, addition of recombinant BAFF protected them from cytarabine-induced cell death. To further confirm this protective effect is indeed contributed by BAFF signaling, we stimulated control (shGFP) and BAFF-R knockdown (shBR3-3) Jeko-1 and Mino cells with BAFF. As we expected, cytarabine treatment has more pronounced effect in BAFF-R knockdown Jeko-1 (Figure 3(a-b)) and Mino cells (Figure 3(c-d)). as compared to control cells. In case of Jeko-1 cells, the treatment of cytarabine resulted in reduced proliferation of cells reaching to 11.2 million cells in shGFP cells compared to 4.5 million cells in shBR3-3 cells (Figure 3(a-b)). In line with this observation, the cell viability also was reduced more in cytarabine-treated shBR3-3 than shGFP cells (Supplementary Figure 3A-B). As expected, rhBAFF protected shGFP Jeko-1 and shGFP Mino cells from cytarabine-induced apoptosis, while addition of BAFF had no significant effect in the proliferation rate of shBR3-3 Jeko-1 and shBR3-3 Mino cells treated with cytarabine (Figure 3(a-d) and Supplementary Figure 3A-D). This confirms the role of BAFF/BAFF-R signaling in chemo-protection of MCL cells.
Figure 3.
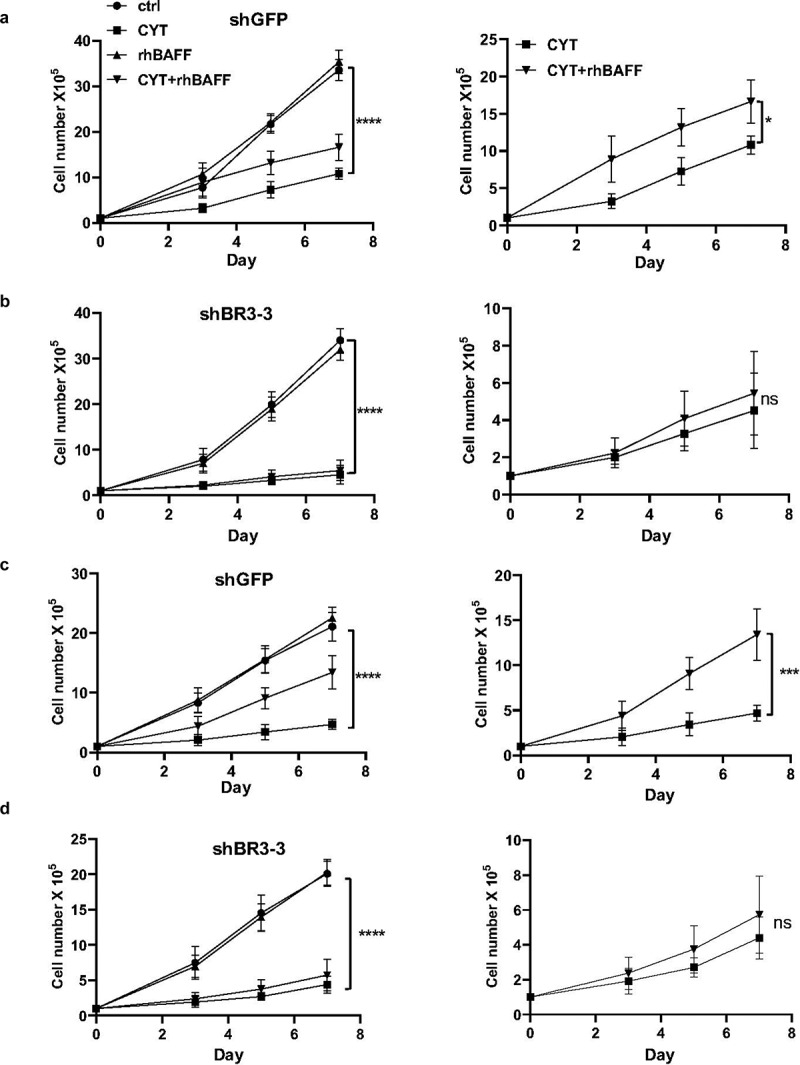
BAFF stimulation failed to antagonize cytarabine-induced BAFF-R knockdown MCL cell death. (a) 1 × 105 Jeko-1 cells expressing shGFP were seeded in 24-well plate in triplicates for a week. The cells were treated with different combination of components such as untreated (ctrl), CYT, rhBAFF, and CYT+rhBAFF. The cell number was plotted. ****p < .0001 represents untreated shGFP cells as compared to cytarabine-treated cells. *p < .05 represents cytarabine-treated shGFP cells in comparison to combined treatment of cytarabine and recombinant BAFF. Experiments shown are representative of three replicates from one experiment. (b) 1 × 105 Jeko-1 cells expressing shBR3-3 were seeded in 24-well plate in triplicates for a week. The cells were treated with different combination of components such as ctrl, CYT, rhBAFF and CYT+rhBAFF. The cell number was plotted. ****p < .0001 represents untreated shBR3-3 cells as compared to cytarabine-treated cells. Experiments shown are one representative experiment of three experiments done with replicates; Not significant (ns) for the comparison of cytarabine-treated shBR3-3 cells in comparison to combined treatment of cytarabine and recombinant BAFF. (c) 1 × 105 Mino cells expressing shGFP were seeded in 24-well plate in triplicates for a week. The cells were treated with different combination of components such as ctrl, CYT, rhBAFF, and CYT+rhBAFF. The cell number was plotted. ****p < .0001 represents untreated shGFP cells as compared to cytarabine-treated cells. ***p < .001 represents cytarabine-treated shGFP cells in comparison to combined treatment of cytarabine and recombinant BAFF. Experiments shown are representative of three replicates from one experiment. (d) 1 × 105 Mino cells expressing shBR3-3 were seeded in 24-well plate in triplicates for a week. The cells were treated with different combination of components such as ctrl, CYT, rhBAFF, and anti-BAFFR. The cell number was plotted. ****p < .0001 represents untreated shBR3-3 cells as compared to cytarabine-treated cells; ns shows the comparison of cytarabine-treated shBR3-3 cells in comparison to combined treatment of cytarabine and recombinant BAFF. Error bars are from single experiment done with triplicate samples representative of three such experiments done with triplicates
To understand the mechanisms of BAFF-R dependent MCL survival and drug sensitivity, we performed western blot analysis to dissect the downstream signaling in Jeko-1 cells. Consistent with other observations, our result indicated that BAFF-R was associated with the activation of canonical and non-canonical NF-kB pathways, ERK, and AKT signaling pathways. It was found that knockdown of BAFF-R by shBR3-4 in Jeko-1 cells reduced p100 processing into p52, eradicated AKT phosphorylation, decreased total AKT level, and eliminated ERK1 phosphorylation (Supplementary Figure 3E). Modest knockdown of BAFF-R by shBR3-3 in Jeko-1 cells have a minimal effect on basal levels of p52, p-AKT, and p-ERK, but significantly reduced the production of p52, p-AKT, and p-ERK in response to BAFF stimulation (Supplementary Figure 3F). In response to BAFF stimulation, Jeko-1 shBR3-3 cells have less p100 processing into p52 in the cytoplasm and less translocation of p52 into nucleus compared to control cells (Supplementary Figure 3G).
BAFF-R knockdown sensitizes MCL to cytarabine treatment in vivo and prolongs mice survival
We used the Jeko-1 and Mino xenograft NSG mouse model to determine whether the BAFF-R knockdown MCL cells are more sensitized to chemotherapy, compared to control cells. Control (shGFP) cells and shBR3-3 Jeko-1 and Mino cells were subcutaneously injected into NSG mice. Mice were treated with cytarabine when they had a palpable tumor (Figure 4(a)). Similar to in vitro observations (Figure 2(c-d), left), there was no significant difference in the relative tumor growth rate between control shGFP and shBR3-
Figure 4.
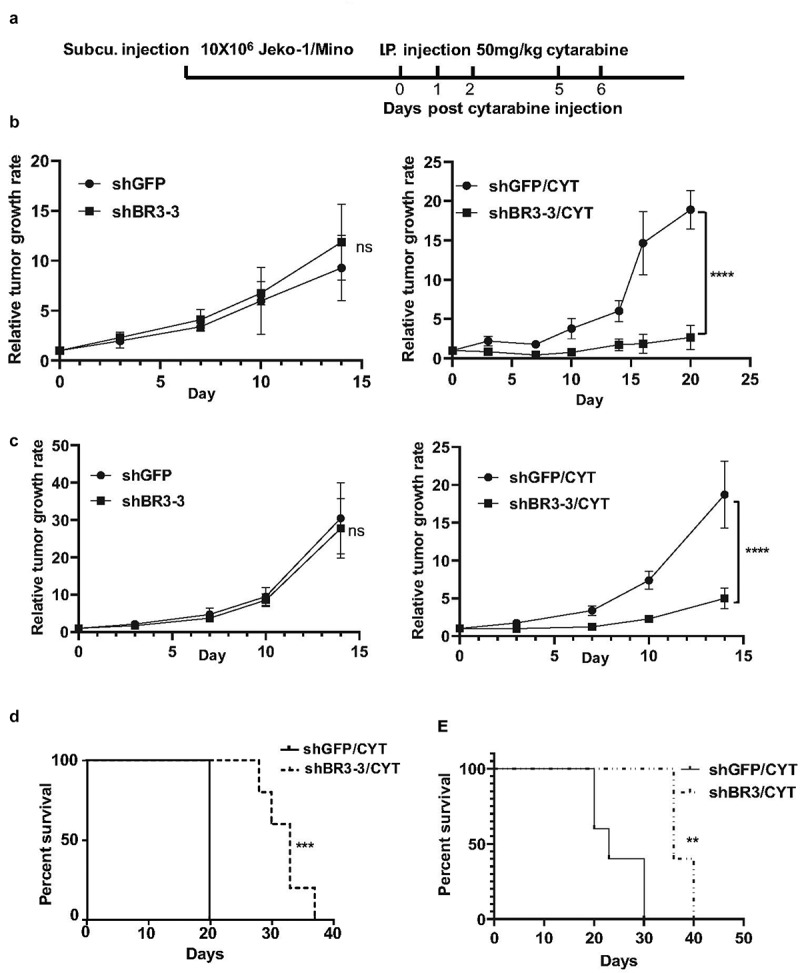
Modest knockdown BAFF-R expression significantly sensitizes the tumor to cytarabine treatment in vivo and prolongs survival of mice in the xenograft model. (a) Schematic representation of in vivo administration of cytarabine in Jeko-1 and Mino xenograft mice model. Jeko-1 and Mino cells were subcutaneously injected into NSG mice to generate solid tumor. When the tumor grew to measurable size, cytarabine was injected intraperitoneal for 3 consecutive days followed by 2 days’ interval gap and another 2 consecutive days’ injection as indicated. (b) Tumor volume was measured twice a week for two weeks and accordingly relative tumor growth rate was estimated for Jeko-1 xenograft cells. Left panel shows relative tumor growth rate of shGFP mice with shBR3-3. ****p < .0001 for relative tumor growth rate of mice with Jeko-1 cells expressing shBR3-3 and CYT treatment compared with shGFP and CYT treatment at different time points (right panel). N = 5. (c) Tumor volume was measured twice a week for two weeks and accordingly relative tumor growth rate was estimated for Mino xenograft cells. Left panel shows relative tumor growth rate of shGFP mice with shBR3-3. ****p < .0001 for relative tumor growth rate of mice with Mino cells expressing shBR3-3 and CYT treatment compared with shGFP and CYT treatment at different time points (right panel). N = 5. (d) Kaplan-Meier survival curve was graphed when the Jeko-1 cells tumor volume in mice reached 2000 mm3 and sacrificed the mice. ***p < .001 for survival time of mice with shBR3-3 and CYT treatment compared to mice with shGFP and CYT treatment. N = 5. (e) Kaplan-Meier survival curve was graphed when the Mino cells tumor volume in mice reached 2000 mm3 and sacrificed the mice. **<0.01 for survival time of mice with shBR3-3 and CYT treatment compared to mice with shGFP and CYT treatment. N = 5
3 injected mice (Figure 4(b-c), left).
The cytarabine treatment regime was given 5 times for 3 consecutive days with a 2-day interval followed by another 2 consecutive days’ drug treatment. The cytarabine treatment resulted in a significant decrease in tumor growth rate in Jeko-1 shBR3-3 and Mino shBR3-3 mice as compared to Jeko-1 shGFP and Mino shGFP mice, respectively (Figure 4(b-c), right). After the withdrawal of the drug, the tumor started to grow aggressively in both shGFP group mice, while in shBR3-3 group mice, significant reduced tumor growth was observed (Figure 4(b-c), right). When tumor volume reached around 2000 mm3, the mice were sacrificed and the Kaplan-Meier survival curve was plotted. Analysis of tumor volume and survival were done in same experiment. Both Jeko-1 shBR3-3 and Mino shBR3-3 injected mice treated with cytarabine exhibited significant prolonged survival compared to respective cytarabine-shGFP cells injected mice (Figure 4(d-e)).
VAY-736 antibody binds to MCL cells
Our data show the importance of BAFF-R in MCL cell survival and the BAFF-R targeting holds immense potential in MCL treatment. Recently we reported the efficacy of VAY-736, a humanized defucosylated engineered antibody targeting BAFF-R, in mediating ADCC of pre-B ALL cells. Efficacy of VAY-736 has been also shown in human CLL effectively by blocking the BAFF-mediated survival signaling and by sensitizing them to ibrutinib. We first tested the binding of VAY-736 antibody to MCL cells. The VAY-736 binding to the BAFF-R expressed on Jeko-1 and JVM2 cells was detected using FITC human IgG antibody (Figure 5(a)). The pre-incubation of JVM2 cells with rhBAFF inhibited the binding of VAY-736 in a dose-dependent manner (Figure 5(b)). VAY-736 antibody also inhibited the binding of different concentrations of anti-BAFF-R antibody in a dose-dependent manner (Figure 5(c)). The binding of VAY-736 to BAFF-R in JVM2 cells was observed to be well sustained until 96 hours of incubation (Figure 5(d)). Since VAY-736 competes with BAFF for binding the BAFF-R, we tested its effect on cytarabine treatment in Jeko-1 cells. VAY-736 treatment only had minor effects on the viability and proliferation of Jeko-1 cells after treatment with cytarabine (Figure 5(e)), but it can antagonize BAFF-mediated apoptosis protection in Jeko-1 cells (Figure 5(f)).
Figure 5.
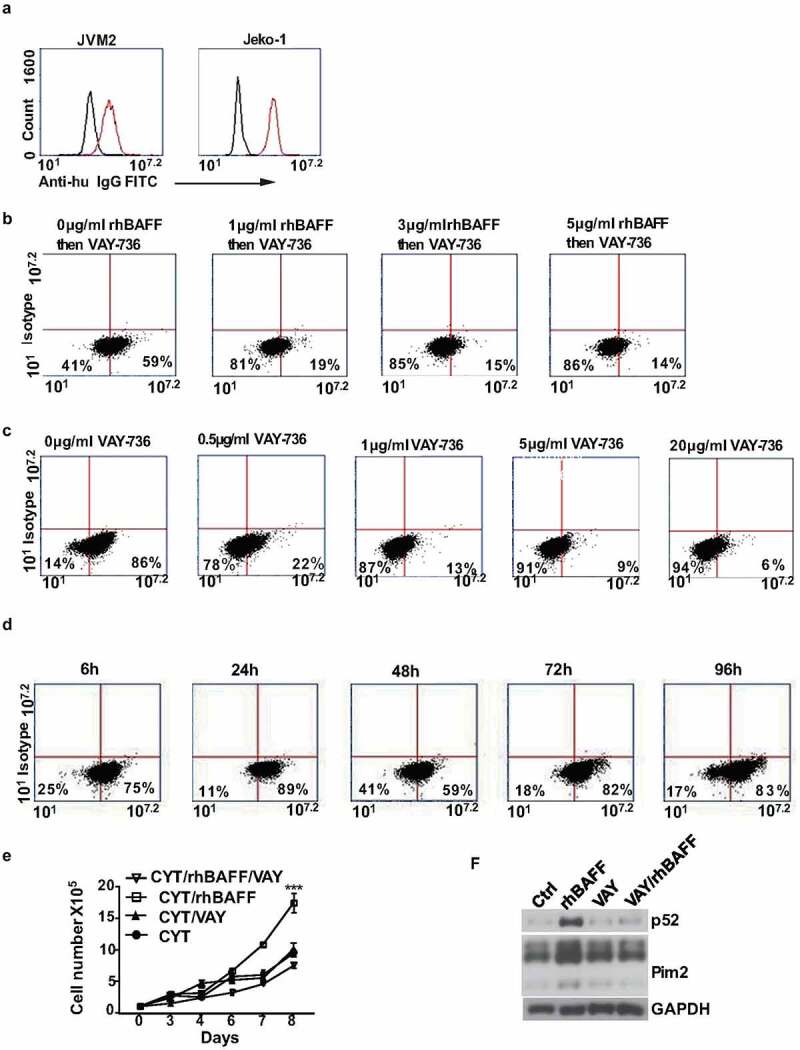
The binding of the VAY-736 to BAFF-R was competed by recombinant BAFF and anti-BAFF-R 11c1 antibody in MCL cell lines. (a) The binding of the VAY-736 antibody to JVM2 and Jeko-1 cells was detected by FITC anti-human IgG. Black histogram, control isotype; red histogram, VAY-736. (b) Inhibition of VAY-736 binding to the BAFF-R by rhBAFF in JVM2 cells as detected by FITC-anti-human IgG antibody. (c) VAY-736 binding to BAFF-R was competed by anti-BAFFR 11c1 in JVM2 cells. (d) Binding of VAY-736 to BAFFR was persistent in JVM2 as detected by FITC-anti-human IgG antibody (e) 1 × 105 Jeko-1 cells were seeded in 24-well plate in triplicate and treated with CYT in the absence or presence of 10 μg/ml VAY-736 antibody. The cell number was measured for the time indicated. ***p < .001 for Jeko-1 cells with CYT and rhBAFF treatment compared with CYT alone on day 8. One of two experiments on triplicate samples was shown. (f) 4 × 105/ml Jeko-1 cells were pre-incubated with PBS or VAY-736 for 72 hours and then stimulated with rhBAFF for 20 hours. Whole-cell lysates were made and western blot analysis was performed to detect protein levels of p52 and Pim2. GAPDH was used as the loading control
VAY-736 enhances NK cell-mediated MCL cell killing both in vitro and in vivo
VAY-736 antibody is optimized for the FcRγIII-mediated, ADCC by NK cells, and has been effectively used against ALL and CLL cells previously. This prompted us to investigate the therapeutic effect of VAY-736 in MCL cells. We initially analyzed the efficacy of different ratios of NK effector cells to target (E:T) in inducing MCL cell death. Remarkably, the percentage of cell death was more when NK cells are combined with VAY-736 than the NK cells alone (Figure 6(a)). The E:T ratio of 5:1 was found to induce cell death up to 40% in JVM2 cells and this ratio was used for further ADCC experiments. The killing capacity of NK cells was raised from 37 to 61% and 41 to 67% when combined with VAY-736 against the two patients-derived MCL cells (Figure 6(b)). We also explored the ADCC-promoting activity of the VAY-736 on NK cells isolated
Figure 6.
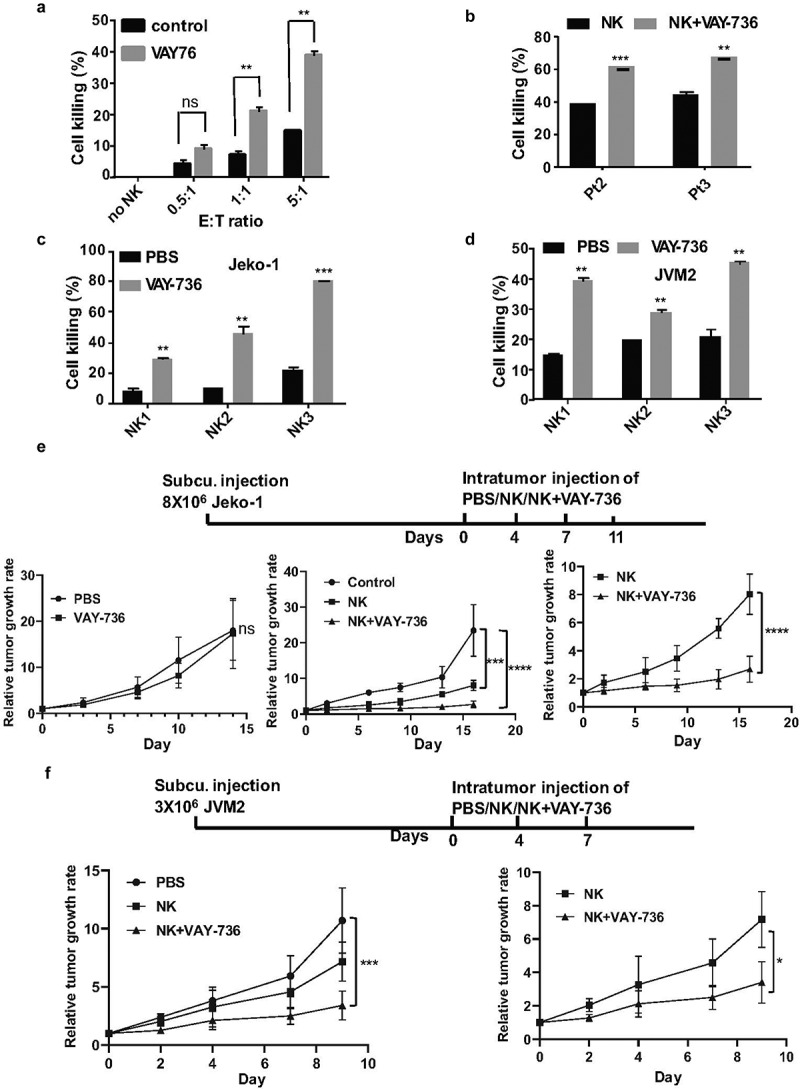
VAY-736 enhances ADCC mediated by NK cells in vitro and in vivo. (a) Calcein-AM labeled JVM2 cells pre-incubated for 1 hour with control human IgG or VAY-736 were incubated with different ratio of NK cells (E:T) for 4 hours (b) Calcein-AM labeled Pt2 and Pt3 cells were pre-incubated 1 hour with control human IgG, VAY-736 followed by incubating with NK cells at (E:T of 5:1) for 4 hours. Statistical significance was calculated using unpaired student’s t-test. N = 3; **p < .01, ***p < .001. (c) Percentage of Jeko-1 cell lysis mediated by NK cells from 3 different normal donors. Cells were all pre-incubated with 10 ug/mL of VAY-736 by using E:T ratio of 5:1. Statistical significance was calculated using unpaired student’s t-test. N = 3; **p < .01, ***p < .001. (d) Percentage of JVM2 cell lysis mediated by NK cells from three different normal donors. Cells were all pre-incubated with 10 ug/mL of VAY-736 by using E:T ratio of 5:1. Statistical significance was calculated using unpaired student’s t-test. N = 3; **p < .01, ***p < .001. (e) Top, schematic depiction of Jeko-1 cells xenograft and VAY-736 treatment in mice. Bottom, relative tumor growth rate after different treatments. On the left panel, PBS group compare to VAY-736; On the middle panel, NK or NK+VAY-736 treatment compare to PBS control; on the right panel, NK/VAY-736 versus NK treatment. Statistical significance was calculated using two-way ANOVA. ****p < .0001 represents statistical significance between PBS versus NK+VAY-736 group, and NK in comparison to NK+VAY-736 group, ***p < .001 represents statistical significance between PBS versus NK group. N = 5 for left panel and N = 3 for middle and right panel. (f) Top, schematic depiction of JVM2 cells xenograft and VAY-736 treatment in mice. Bottom, relative tumor growth rate after different treatments. Statistical significance was calculated using two-way ANOVA. ***p < .0001 represents statistical significance between PBS versus NK+VAY-736 group, *p < .05 represents statistical significance between NK versus NK+VAY-736 group. N = 3
from three different donors. After 4 hours of incubation of effector and target cells, 25% to 80% of Jeko-1 and 17% to 47% of JVM2 cell deaths were induced by NK cells from different donors (Figure 6(c)). To determine whether VAY-736 monotreatment has any effect on MCL growth in vivo, we used the Jeko-1 xenograft NSG mouse model. We subcutaneously transplanted Jeko-1 cells into NSG mice and injected VAY736 or PBS. The analysis of tumor volume indicated that there was no significant change in the tumor growth rate among these two groups (Figure 6(e) left bottom). Subsequently, we used JVM2 and Jeko-1 cells that were subcutaneously injected into NSG mice (Figure 6(e,f)). When the tumors attained a measurable volume, mice were injected intratumorally with PBS, NK cells, or NK cells plus VAY-736 for different time intervals. The tumor volume was found significantly decreased in NK plus VAY-736 treated group as compared to the PBS treated control group in the JVM2 xenograft mice model (Figure 6(d)). In the Jeko-1 xenograft mice model, NK cells alone and NK plus VAY-736 treatment group both significantly reduce tumor burden than the control group. There was a significant reduction of tumor burden in NK and VAY-736 combined treatment groups than NK cells treatment group (Figure 6(e)). A similar effect was also observed in case of JVM2 cells where the combined treatment of NK and VAY-736 antibody efficiently reduced the tumor growth rate in comparison to NK-treated group alone (Figure 6(f)). Thus, both Jeko-1 and JVM2 xenografts treated with NK and VAY-736 showed slowing of tumor growth compared to NK cell alone treated mice.
Discussion
The role of BAFF signaling in normal and malignant B-cell survival is well known. Whether BAFF signaling is autocrine or paracrine, its significance in B-cell cancers is evident from the literature. Autocrine BAFF signaling in B-cell lymphomas is known to stimulate cell growth and survival.11,22 BAFF is also reported to protect ALL and CLL cells as well as lymphoma and multiple myeloma cells from spontaneous and drug-induced apoptosis.23
Conversely, cell killing was enhanced by soluble BCMA-Fc, anti-BAFF-R, anti-BAFF, or anti-APRIL antibodies specifying the crucial role of BAFF signaling in rescuing B cancer from apoptosis.24,25 However, the exact role of BAFF in MCL is not fully characterized. Since MCL cells are known to express both BAFF and BAFF-R.18 It might be possible that autocrine signaling plays a significant function in the survival of B-cells as earlier reports have suggested that BAFF can increase the survival of malignant B-cell via both autocrine and paracrine signaling pathways.18,26,27 Our data shows that even a 60% BAFF-R knockdown leads to spontaneous cell death implying that BAFF-R is essential for MCL survival and signifies the importance of autocrine BAFF signaling in MCL survival. Primary MCL cells from patients die spontaneously and are difficult to keep alive in culture, which shows that autocrine BAFF signaling alone is not enough to keep primary MCL cells alive. There are other factors crucial for MCL survival and proliferation. In humans, it might be that these cells are dependent on both autocrine and paracrine signaling as BAFF is produced by the majority of cells in the tumor microenvironment. In this study, we detailed the role of BAFF-R in regulating MCL cell survival and its response to cytotoxic agents. The cytarabine has been effectively used in the treatment of AML and has shown promising outcomes in the treatment of MCL that acts by hindering DNA chain elongation.28–35
Here in our study, we show that the addition of BAFF protects MCL cells from cytarabine-induced cell death and blocking BAFF-R by BAFF-R antibody sensitized MCL cells to cytarabine treatment. As evident from our data, there is a quantitative balance between BAFF-R expression levels and MCL survival, as 40% BAFF-R KD leads to cell survival versus 60% BAFF-R KD leads to cell death. We further defined the role of BAFF-R in MCL survival by injecting BAFF-R KD cells into immunocompromised mice. Those mice injected with BAFF-R KD MCL cells developed tumor similar to control MCL injected mice, but they were sensitized to cytarabine treatment, confirming the importance of inhibiting BAFF signaling as a therapeutic tool to sensitize chemotherapy-resistant patients. The bone marrow microenvironment plays an essential role in biology and therapy of B-cell lymphomas.36 The bone marrow stromal cells and myeloid cells can produce BAFF in the lymphoma microenvironment.37–39 Since inhibiting a soluble protein like BAFF is difficult, we targeted its receptor expressed on the cell surface of MCL cells, using neutralizing BAFF-R antibody in combination with chemotherapy. This will be a potential therapeutic strategy for treating MCL patients, especially for treating chemo-resistant patients. The combination therapy can induce chemosensitization that can increase the efficacy and reduce the undesired cytotoxicity of chemotherapeutic agents.40 We demonstrated another strategy to therapeutically target MCL cells using an ADCC optimized BAFF-R antibody. Our recent report showed the efficacy of this antibody in enhancing NK cell-mediated ALL killing.41 Here we extend our observations to characterize the efficacy of this antibody in targeting MCL cells in vitro and in vivo. One way to improve the observed ADCC in these in vivo experiments might be increasing numbers of NK cells injected into these mice, as our in vitro data show a direct correlation with tumor cell killing and E:T ratio. We reported TGF-beta as a factor inhibiting CD16 expression and hence ADCC efficacy of NK cells, in advanced stages of ALL.41 Previous reports have also suggested that the tumor microenvironment often restrains NK cell activity against different cancer cells.42,43 So it is important to study the microenvironmental factors in MCL whether they inhibit NK cells’ ADCC efficacy in patients. Inhibiting such factors, if any, as a combination approach will enhance killing of MCL cells. VAY-736 antibody showed binding to MCL cell lines and patient cells and it stayed on the surface of cells for about 96 hours, which is favorable for an optimal ADCC performance. High serum BAFF levels are often correlated with poor drug response and relapse in lymphoma patients.18,44 Therefore, we tested competition of binding of this antibody to MCL cells in the presence of exogenously added BAFF. Binding of BAFF-R antibody was compromised only in the presence of extreme BAFF concentrations, which is unrealistic for endogenous BAFF levels. This is in accordance with our past observations using ALL cells.41 A chimeric antibody developed against natively folded BAFF-R protein was also reported to perform ADCC against B cell cancers.45 This antibody might have a different BAFF-R epitope binding compared to VAY-736 as it performs anti-tumor activity as a mono-treatment unlike VAY-736. It will be interesting to do a comparative performance study of both antibodies.
Our data show a high in vitro and in vivo efficacy of BAFF-R antibody in killing MCL cells. It is important to analyze NK cells from these patients to analyze whether its ADCC efficacy is compromised. If NK cells are dysfunctional in these patients, it is advisable to adoptively transfer NK cells either autologous or allogeneic NK cells, after ex-vivo expansion, as both cells showed efficacy in mediating MCL killing. Based on our results, we strongly believe that a combination of either neutralizing BAFF-R antibody or BAFF-R inhibitors along with cytotoxic drugs might be a more optimal therapeutic regimen for drug-resistant MCL patients. Our data also confirm ADCC optimized anti-BAFF-R antibody along with the adoptive transfer of NK cells as a potential approach to target MCL.
Supplementary Material
Acknowledgments
Financial support for this work obtained from NIH 1R21CA201775- 01A1 (RP), St. Baldrick’s Foundation (RP), Andrew McDonough B+ (Be Positive) Foundation (RP). This work was supported by the Athymic Animal, Hematopoietic Biorepository and Cellular Therapy, Radiation Core facilities, Flow Cytometry and other Common Resources of the Case Comprehensive Cancer Center.
Funding Statement
NIH 1R21CA201775-01A1 (RP), St. Baldrick’s Foundation (RP), The Andrew McDonough B+ (Be Positive) Foundation (RP);National Institutes of Health [1R21CA201775–01A1];
Contribution
K.Z and N.K.R performed major experiments and wrote the manuscript; Y.V also performed one experiment and analyzed results; M.D.L, R.B, and P.C. involved in research design and analysis; D.F performed one experiment and analyzed data. R.P conceived the concept, analyzed data, wrote manuscript and supervised the research.
Disclosure statement
J.W is an employee at Novartis pharma. R.P is a scientific advisory board member of Luminary Therapeutics
Key Points
BAFF-R plays key role in the MCL cell survival and its modest knockdown sensitizes MCL cells to cytarabine treatment
VAY-736 binds to BAFF-R and antagonizes BAFF-mediated apoptosis protection in MCL cells and NK cell-mediated cell cytotoxicity.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Reed DR, Portell CA.. Mantle cell lymphoma. Novel therapeutics for rare lymphomas. Springer, Cham. 2020;69–13.
- 2.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha CK, Praulich I, Gehrke I, Hallek M, Kreuzer KA. A rare case of t(11;22) in a mantle cell lymphoma like B-cell neoplasia resulting in a fusion of IGL and CCND1: case report. Mol Cytogenet. 2011;4(1):8. doi: 10.1186/1755-8166-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye H, Desai A, Huang S, Jung D, Champlin R, Zeng D, Yan F, Nomie K, Romaguera J, Ahmed M, et al. Paramount therapy for young and fit patients with mantle cell lymphoma: strategies for front-line therapy. J Exp Clin Cancer Res. 2018;37(1):150. doi: 10.1186/s13046-018-0800-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyling M, Ferrero S. European mantle cell lymphoma network. The role of targeted treatment in mantle cell lymphoma: is transplant dead or alive? Haematologica. 2016;101(2):104–114. doi: 10.3324/haematol.2014.119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner RE, Romaguera J, Wang M. Current trials for frontline therapy of mantle cell lymphoma. J Hematol Oncol. 2018;11(1):13. doi: 10.1186/s13045-018-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer J-L, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai D, Kanno Y, Hase H, Kojima H, Okumura K, Kobata T. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur J Immunol. 2007;37(1):110–118. doi: 10.1002/eji.200636623. [DOI] [PubMed] [Google Scholar]
- 9.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244(1):115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lwin T, Crespo LA, Wu A, Dessureault S, Shu HB, Moscinski LC, Sotomayor E, Dalton WS, Tao J. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non- Hodgkin’s B lymphoma cells from apoptosis. Leukemia. 2009;23(1):170–177. doi: 10.1038/leu.2008.266. [DOI] [PubMed] [Google Scholar]
- 11.Parameswaran R, Müschen M, Kim YM, Groffen J, Heisterkamp N. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res. 2010;70(11):4346–4356. doi: 10.1158/0008-5472.CAN-10-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paiva C, Rowland TA, Sreekantham B, Godbersen C, Best SR, Kaur P, Loriaux MM, Spurgeon SEF, Danilova OV, Danilov AV, et al. SYK inhibition thwarts the BAFF - B- cell receptor crosstalk and thereby antagonizes Mcl-1 in chronic lymphocytic leukemia. Haematologica. 2017;102(11):1890–1900. doi: 10.3324/haematol.2017.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Sun Y, Zhang N, Li J, Ta F, Wei W, Yu S, Ai L. Characteristics of BAFF and APRIL factor expression in multiple myeloma and clinical significance. Oncol Lett. 2017;14(3):2657–2662. doi: 10.3892/ol.2017.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA, Greipp PR, Jelinek DF. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103(2):689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 15.Shen X, Wang M, Guo Y, Ju S. The correlation between Non-Hodgkin lymphoma and expression levels of B-cell activating factor and its receptors. Adv Clin Exp Med. 2016;25(5):837–844. doi: 10.17219/acem/29182. [DOI] [PubMed] [Google Scholar]
- 16.Molica S, Digiesi G, Battaglia C, Cutrona G, Antenucci A, Molica M, Giannarelli D, Sperduti I, Gentile M, Morabito F, et al. Baff serum level predicts time to first treatment in early chronic lymphocytic leukemia. Eur J Haematol. 2010;85(4):314–320. doi: 10.1111/j.1600-0609.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 17.Fragioudaki M, Boula A, Tsirakis G, Psarakis F, Spanoudakis M, Papadakis IS, Pappa CA, Alexandrakis MG. B cell-activating factor: its clinical significance in multiple myeloma patients. Ann Hematol. 2012;91(9):1413–1418. doi: 10.1007/s00277-012-1470-x. [DOI] [PubMed] [Google Scholar]
- 18.Novak AJ, Grote DM, Stenson M, Ziesmer SC, Witzig TE, Habermann TM, Harder B, Ristow KM, Bram RJ, Jelinek DF, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104(8):2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 19.Parameswaran R, Lim M, Fei F, Abdel-Azim H, Arutyunyan A, Schiffer I, McLaughlin ME, Gram H, Huet H, Groffen J, et al. Effector-mediated eradication of precursor B acute lymphoblastic leukemia with a novel Fc-engineered monoclonal antibody targeting the BAFF-R. Mol Cancer Ther. 2014;13(6):1567–1577. doi: 10.1158/1535-7163.MCT-13-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodig SJ, Shahsafaei A, Li B, Mackay CR, Dorfman DM. BAFF-R, the major B cell- activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol. 2005;36(10):1113–1119. doi: 10.1016/j.humpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Tiemann K, Chomchan P, Alluin J, Swiderski P, Burnett J, Zhang X, Forman S, Chen R, Rossi J, et al. Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells. Nucleic Acids Res. 2013;41(7):4266–4283. doi: 10.1093/nar/gkt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smulski CR, Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol. 2018;9:2285. doi: 10.3389/fimmu.2018.02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan MC, Grewal IS. Targeting of BAFF and APRIL for autoimmunity and oncology. Adv Exp Med Biol. 2009;647:52–63. [DOI] [PubMed] [Google Scholar]
- 24.Kowalczyk-Quintas C, Chevalley D, Willen L, Jandus C, Vigolo M, Schneider P. Inhibition of membrane-bound BAFF by the Anti-BAFF antibody belimumab. Front Immunol. 2018;9:2698. doi: 10.3389/fimmu.2018.02698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haiat S, Billard C, Quiney C, Ajchenbaum-Cymbalista F, Kolb JP. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006;118(3):281–292. doi: 10.1111/j.1365-2567.2006.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B, Chadburn A, Jou E, Schattner EJ, DM Knowles, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL [published correction appears in J Immunol. 2004;172(8):5127]. J Immunol. 2004;172(8):5127. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 27.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, Defrance T, Ajchenbaum-Cymbalista F, Simonin P-Y, Feldblum S, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 28.Ellison RR, Holland JF, Weil M, Jacquillat C, Boiron M, Bernard J, Sawitsky A, Rosner F, Gussoff B, Silver RT; Ellison RR, Holland JF, Weil M, et al . Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood. 1968;32(4):507–523. doi: 10.1182/blood.V32.4.507.507. [DOI] [PubMed] [Google Scholar]
- 29.Cadman E, Farber L, Berd D, Bertino J. Combination therapy for diffuse histiocytic lymphoma that includes antimetabolites. Cancer Treat Rep. 1977;61:1109–1116. [PubMed] [Google Scholar]
- 30.Merryman RW, Edwin N, Redd R, Bsat J, Chase M, LaCasce A, Freedman A, Jacobson C, Fisher D, Ng S, et al. Rituximab/bendamustine and rituximab/cytarabine induction therapy for transplant-eligible mantle cell lymphoma. Blood Adv. 2020;4(5):858–867. doi: 10.1182/bloodadvances.2019001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motokura MT. Turning point in the treatment of mantle cell lymphoma. Yonago Acta Med. 2019;62(1):1–7. doi: 10.33160/yam.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima H, Iida M, Miyazaki H, Koga T, Moriyama H, Manome Y. Enhancement of cytarabine sensitivity in squamous cell carcinoma cell line transfected with deoxycytidine kinase. Arch Otolaryngol Head Neck Surg. 2002;128(6):708–713. doi: 10.1001/archotol.128.6.708. [DOI] [PubMed] [Google Scholar]
- 33.Graham FL, Whitmore GF. Studies in mouse L-cells on the incorporation of 1-beta- D-arabinofuranosylcytosine into DNA and on inhibition of DNA polymerase by 1- beta-D-arabinofuranosylcytosine 5ʹ-triphosphate. Cancer Res. 1970;30:2636–2644. [PubMed] [Google Scholar]
- 34.Kufe DW, Major PP, Egan EM, Beardsley GP. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem. 1980;255(19):900–8997. doi: 10.1016/S0021-9258(19)70512-2. [DOI] [PubMed] [Google Scholar]
- 35.Kufe D, Spriggs D, Egan EM, Munroe D. Relationships among Ara-CTP pools, formation of (Ara-C)DNA, and cytotoxicity of human leukemic cells. Blood. 1984;64(1):54–58. doi: 10.1182/blood.V64.1.54.54. [DOI] [PubMed] [Google Scholar]
- 36.Sircar A, Chowdhury SM, Hart A, Bell W, Singh S, Sehgal L, Epperla N. Impact and intricacies of bone marrow microenvironment in B-cell lymphomas: from biology to therapy. Int J Mol Sci. 2020;21(3):904. doi: 10.3390/ijms21030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517‐534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 38.Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34(36):4673‐4682. doi: 10.1038/onc.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jona A, Szodoray P, Illés A. Immunologic pathomechanism of Hodgkin’s lymphoma. Exp Hematol. 2013;41(12):995‐1004. doi: 10.1016/j.exphem.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Wcislo G. Resveratrol inhibitory effects against a malignant tumor: a molecular insight. In: Polyphenols: prevention and treatment of human disease. Academic Press; 2018. p. 217–229. [Google Scholar]
- 41.Vicioso Y, Gram H, Beck R, et al . Combination therapy for treating advanced drug-resistant acute lymphoblastic leukemia. Cancer Immunol Res. 2019;7(7):1106‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Shmuel A, Biber G, Unleashing Natural B-SM. Killer cells in the tumor microenvironment-the next generation of immunotherapy? Front Immunol. 2020;11:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jewett A, Kos J, Kaur K, Safaei T, Sutanto C, Chen W, Wong P, Namagerdi AK, Fang C, Fong Y, et al. Natural killer cells: diverse functions in tumor immunity and defects in pre-neoplastic and neoplastic stages of tumorigenesis. Mol Ther Oncolytics. 2019;16:41–52. doi: 10.1016/j.omto.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SJ, Lee SJ, Choi IY, Park Y, Choi CW, Kim IS, Yu W, Hwang HS, Kim BS. Serum BAFF predicts prognosis better than APRIL in diffuse large B-cell lymphoma patients treated with rituximab plus CHOP chemotherapy. Eur J Haematol. 2008;81(3):177–184. doi: 10.1111/j.1600-0609.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 45.Qin H, Wei G, Sakamaki I, Dong Z, Cheng WA, Smith DL, Wen F, Sun H, Kim K, Cha S, et al. Novel BAFF-receptor antibody to natively folded recombinant protein eliminates drug-resistant human B-cell malignancies In Vivo. Clin Cancer Res. 2018;24(5):1114–1123. doi: 10.1158/1078-0432.CCR-17-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


