Abstract
Mesenchymal stem/stromal cells (MSCs) have long been recognized to help regenerate tissues, by exploiting their intrinsic potentials for differentiation and secretion of therapeutic paracrine factors together with feasibility for cell banking. These unique MSC properties are attractive to provide effective new cell-based therapies for unmet medical needs. Currently, the infusion of suspended MSCs is accepted as a promising therapy to treat systemic inflammatory diseases. However, low cell engraftment/retention in target organs and off-target entrapment using conventional cell infusion must be improved to provide reliable localized disease treatments. Cell sheet technology offers an alternative: three-dimensional (3D) tissue-like structures can be harvested from culture using mild temperature reduction, and transplanted directly onto target tissue sites without suturing, yielding stable cell engraftment and prolonged cell retention in situ without off-target losses. Engineered MSC sheets directly address two major cell therapy strategies based on their therapeutic benefits: (1) tissue replacements based on mult-ilineage differentiation capacities, focusing on cartilage regeneration in this review, and (2) enhancement of tissue recovery via paracrine signaling, employing their various secreted cytokines to promote neovascularization. MSCs also have production benefits as a promising allogeneic cell source by exploiting their reliable proliferative capacity to facilitate expansion and sustainable cell banking for off-the-shelf therapies. This article reviews the advantages of both MSCs as allogeneic cell sources in contrast with autologous cell sources, and allogeneic MSC sheets engineered on thermo-responsive cell dishes as determined in basic studies and clinical achievements, indicating promise to provide robust new cell therapies to future patients.
Keywords: allogeneic cell therapy, cell bank, preclinical research, tissue engineering, tissue regeneration, translational research
Introduction
Medical treatments continuously advance with new technology developments. Newly introduced and approved biologic drugs provide increasing examples of improved efficacies and fewer side effects for biological molecules compared with conventional small-molecule synthetic drugs. Nevertheless, many clinical pathologies remain unaddressed, including diverse inextirpable degenerative diseases, such as neurodegenerative disorders, some cardiovascular diseases, osteoarthritis, and acute/chronic fibrosis, due to lack of effective therapeutic approaches.
In a healthy body, various cell types, including somatic stem cells, continuously orchestrate normal tissue and organ functions through appropriate proliferation–differentiation-aging processes to maintain homeostasis. Deteriorations of cellular function, especially stem/progenitor cell deficiencies, promote degenerative diseases since these normal maintenance pathways are disrupted or abnormal. Cell therapies have been arduously attempted to treat some of these intractable diseases. Currently, embryonic stem (ES) cells, induced pluripotent stem (iPS) cells, and somatic stem/progenitor cells, such as mesenchymal stem/stromal cells (MSCs) and hematopoietic stem cells, have been investigated as promising cell sources for new therapies. MSCs in particular have been widely applied to address various diseases by exploiting their known multipotency (e.g. bone, cartilage, and adipose) and abundant paracrine secretome [1]. MSCs also exhibit high growth potential that facilitates a reliable, sustainable cell banking and supply system amenable to produce substantial numbers of human doses [2,3]. However, few MSC-based therapies have progressed beyond preclinical studies to clinical trials evaluating their safety and therapeutic efficacy. One reason for this struggle is thought to derive from their intrinsically poor cell engraftment/retention in target tissues and substantial off-target loss of infused cells, which remains a daunting, significant hurdle for many MSC therapies [4–10]. To overcome this challenge, cell delivery systems with or without biomaterials are designed to produce tissue-like structures that are implanted or adherent locally at host target tissue sites, and improve cell retention without off-target problems, and therefore considered more efficient as culture-expanded cell-based therapies.
In this review, we discuss cell delivery to tissue sites exploiting ‘cell sheet technology’ allowing local cell transplantation with thermo-responsive cell cultureware that allows confluently cultured cells to yield readily harvested three-dimensional (3D) tissue-like sheet structures. Sheets are harvested with minor temperature reduction to below 32°C from 37°C (i.e. that of a normal cell culture incubator) (Figure 1). Cell sheets retain endogenous cell matrix and membrane proteins and enhance healthy cultured cell yields for immediate use as engineered patches [11,12]. This enables direct cell sheet transplantation onto target tissues without supporting scaffolds or suturing [13,14]. Decades of investigations developing cell sheet technology reveal that scaffold-free cell sheets exhibit both safety and efficacy in treating seven different organs using autologous cell sources in clinical settings: cornea, esophagus, heart, lung, middle ear, periodontal membrane, and cartilage regeneration [15–21]. Moreover, recent reports describing use of allogeneic cell sheet technology suggest a high potential for clinical utility, especially with MSCs isolated from adipose tissue, bone marrow, periodontal membrane, and cartilage [22–25]. This review discusses the advantages of combining cell sheet technology and MSCs; effective cell sourcing, expansion and cell delivery using cell sheets engineered from MCSs, and resulting numerous attractive features and reliable outcomes in preclinical and clinical studies. MSC sheet technology will offer new opportunities for diverse disease treatments in the near future. Furthermore, we discuss the scaling and economic benefits of forthcoming allogeneic MSC sheet-based regenerative therapies.
Figure 1. Cell sheet technology using TRCDs.
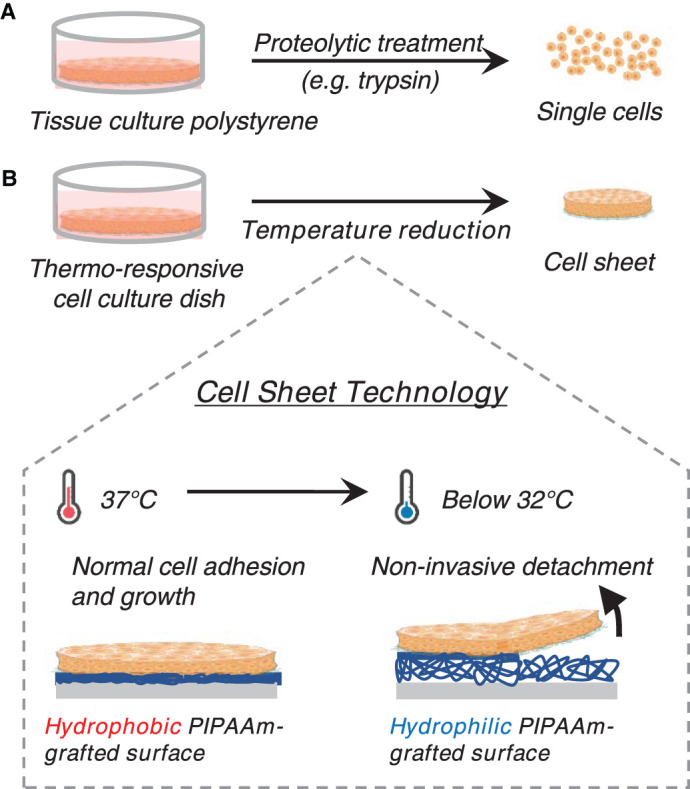
(A) Conventional single-cell recovery method by proteolytic treatment. (B) Non-proteolytic cell sheet recovery using thermo-responsive cell culture dish (TRCD). Mild temperature reduction facilitates spontaneous cell sheet detachment.
Allogeneic cell sourcing: MSCs with high proliferative capacity enabling sustainable cell banking for off-the-shelf sourcing as therapy
Clinically feasible cell-based regenerative therapies rely on consistent, available and potent cell sources. Several clinical trials of ES cells have been conducted, followed by recent iPS cell-based early-stage clinical trials [26–29]. Nonetheless, the crucial hurdles for commercialization of pluripotent stem cells, including costs of long-term cell culture, must be cleared [30] and reliable methods for tumorigenic cell elimination must be established and validated [31]. On the other hand, somatic stem/progenitor cells isolated from various tissues are valuable to replenish deficient host cells to restore homeostasis [32] by exploiting their lower possibilities of tumorigenicity compared with pluripotent stem cells [33,34]. Autologous somatic cell sourcing is an advantage when cells must avoid host immune rejection for stable cell engraftment and retention [35]. However, autologous cell therapy usually requires multi-stage surgeries for obtaining patients’ own cells, expanding them ex situ, and transplanting the harvested cells back to targeted tissue sites. Additionally, variability due to patient individual difference is a major obstacle for product quality control and reliability [36–39].
To overcome these obstacles, allogeneic cell transplantation has frequently been attempted as a promising next-generation cell therapy. In this regard, MSCs are a primary allogeneic cell source amenable to cell banking due to their high proliferative potential [2,3]. This robust proliferative capacity allows use of a single qualified cell line showing high efficacy for expansion to accommodate substantial numbers of patient treatments. Moreover, MSCs show no/low-MHC class II expression, suggesting tolerance to host immune rejection [40,41]. Therefore, MSCs are frequently proposed as a strategy to overcome current major problematic issues surrounding allogeneic cell immune rejection while yielding large cell banks for therapeutic applications. Beyond cell banking advantages, MSCs exhibit unique multi-lineage differentiation both in vitro and in vivo, and therapeutic cytokine secretions to heal damaged tissues [1]. Based on these remarkable properties, MSC-based therapy can be a game-changing strategy to provide new treatments to address unmet medical needs.
Conventional cell delivery systems —advantages and disadvantages
Cultured MSCs are a major allogeneic cell source of current interest due to their immunomodulatory effects against excessive immunoreactions in systemic diseases such as graft-versus-host disease (GVHD), Crohn's disease, and severe acute pancreatitis [42–46]. While single-cell administration by intravenous infusion is advantageous to deliver cells and their paracrine factors to the entire body via the blood stream, this strategy is unsuitable to treat non-systemic, localized diseases due to low cell engraftment and survival rates at target sites and high off-target localization [4–9]. Intra-arterial and local injections show slightly better homing to target sites, though injected MSC accumulation is still high in lung early post-transplantation [10,47,48]. To date, both systemic and local injection of cell suspensions have been used to position MSCs in target organs for localized disease treatments, but insufficient MSC retention can limit local exposures to therapeutically beneficial paracrine factors and compromise the potency and duration of the desired therapeutic effect [5]. Increasing MSC dose to enhance therapeutic effects concomitantly expands the risks of embolism in lung and liver by off-target MSCs, exposing the difficulties of dose control [7,10]. This is one reason currently limiting clinical trials to small-cohort safety and efficacy studies [5]. Therefore, alternative cell delivery methods are needed to establish localized disease treatments.
To facilitate and improve cell engraftment in target host tissues, numerous biomaterial-based scaffold approaches using seeded MSCs are continuously reported. However, biomaterial designs often focus on manipulating a single parameter of the cell transplantation (e.g. biocompatibility, biodegradability, and donor cell viability or differentiation), and among myriad types of materials applied, few examples of knowledge transfer from in vitro experiments are translated successfully to in vivo cell transplantation experiments [49]. An alternative approach is scaffold-free tissue engineering utilizing biological cell–cell binding architectures as typified by spheroid/aggregate culture or cell-dense culture in non-adherent wells to facilitate ‘self-assembly’. This approach can deliver cells at high density to target sites while avoiding interference by and rejection of scaffold biomaterials and their degradation products [50,51]. This strategy removes certain confounding cell-implant variables to enhance tissue regeneration and integration in host disease sites.
Cell sheet engineering —key enabling features of thermo-responsive cell cultureware
Among diverse cell sheet engineering methods more recently reported, Okano and co-workers originally invented the thermo-responsive culture dish, thermo-responsive cell dishes (TRCD), to produce the first scaffold-free cell sheets, enabling cultured cells to form native tissue-like structure compared with other scaffold-free methods (cell sheet technology, Figure 1) [52,53]. Culture plastic grafted with ultrathin layers of the temperature-responsive polymer, poly(N-isopropylacrylamide), transitions from hydrophobic in culture conditions, 37°C, to hydrophilic below its lower critical solution temperature of 32°C. Below 32°C, grafted poly(N-isopropylacrylamide) swells and hydrates, forming a swollen polymer layer between the culture surface and adherent cells, enabling harvest of cultured cells as a confluent sheet retaining instructive extracellular matrix (ECM) and cell-cell interactions (Figure 1) [12,52–55]. The approach yields transplantable tissue-like constructs that spontaneously adhere to target organs without sutures or complex procedures, safety concerns in transplanted cells, and show therapeutic effects [56,57]. In addition to TRCD-based methods, electro-responsive [58,59], pH-responsive [60], and magnetic-responsive systems [61–63] are employed to harvest cells from culture surfaces as cell sheets. However, the pioneering TRCD-based cell sheet technology has now been widely applied to treat diverse diseases with various cell types, requiring no additional devices in laboratory and clinical situations, limiting safety concerns caused by cell labeling systems or pH reduction methods that affect cell property changes when harvested as cell sheets. Significantly, several types of autologous human cell sheets have been successfully delivered to small numbers of patients using this TRCD approach to date, demonstrating clinical safety and efficacy in cell sheet therapies in seven tissue sites [15–21].
Extending this strategy to MSCs as a prospective allogeneic cell source, two major cell sheet-based strategies have been proposed in clinical and preclinical studies: (1) tissue replacement, especially focusing on cartilage regeneration in this review to introduce a unique allogeneic cell source, based on cell sheet differentiation into chondrocytes, and (2) tissue recovery employing cell sheet therapeutic cytokine production and paracrine signaling.
Tissue replacement: cartilage-derived MSC/chondrocyte sheet treatment
Cartilage regeneration has been an active target of diverse tissue engineering efforts due to the increasing incidence of cartilage injury, its lack of innate regenerative capacity [64], and outcomes from surgical bone marrow induction approaches (e.g. microfracture) that commonly result in fibrocartilage in contrast with hyaline cartilage found in native healthy articular surfaces [65–67]. Native cartilage contains self-renewing chondrocyte progenitors expressing MSC-related markers [68]. Isolated cells from cartilage de-differentiate into a fibroblastic morphology in vitro, and exhibit the capacities of colony-forming unit fibroblast (CFU-F) formation and multi-lineage differentiation [69,70]. Culture-expanded chondrocytes can re-differentiate into mature cartilaginous cells expressing cartilage-specific molecules under chondrogenic differentiation conditions [71,72]. These reports strongly suggest that cultured chondrocytes are one MSC type possessing strong chondrogenic capacity. Although nomenclature of cultured-expanded chondrocytes varies by schools (e.g. cartilage-derived stem/progenitor cells, chondroprogenitor cells, etc.), ‘chondrocytes’ in this review particularly denote in vitro-expanded cells derived from cartilage as one MSCs type, distinct from mature/differentiated chondrocytes existing in vivo.
Various tissue engineering methods, either combined with or without biomaterials, have been developed with myriad clinical studies ongoing seeking to reliably regenerate cartilage [73–75]. However, to date, most human studies utilize autologous cells, such as matrix-associated autologous chondrocyte implantation (MACI). No gold standard clinical practice for cell-based chondral defect treatment exists due to the various limitations of current approaches, including the necessity for and expense of multiple surgeries, inconsistent donor tissue availability, donor cell quality, and potency [76,77].
Cell sheet engineering for cartilage repair
MACI has demonstrated better outcomes compared with bone marrow induction approaches [78]. However, its cost compared with microfracture is high, and reported graft delamination and fibrocartilage formation [79,80] may be attributed to low density, and dissociated chondrocyte colonization in the porcine collagen grafting matrix. Cell sheet technology can better address these clinical issues, grafting cell-dense scaffold-free patches directly to target tissue surfaces, preserving cell-cell communication and endogenous ECM presumed to promote chondrogenic re-differentiation based on previous basic studies [81,82]. Additionally, cell sheets conform to the shapes of various defects to better facilitate graft integration with host tissue, avoiding alignment and defect space-filling issues seen in grafts such as osteochondral allograft [83].
As one MSC type, cultured chondrocytes isolated from articular cartilage have been investigated as a primary cell source in autologous cell sheet therapy for cartilage regeneration. Articular cartilage-derived chondrocyte sheets secrete cartilage protective humoral factors [84]. The formation of human articular chondrocyte sheets from these cells enhances gene expression of aggrecan and type 2 collagen in vitro compared with conventionally cultured cells [85,86]. Interestingly, extended culture after layering of three human articular chondrocyte sheets enhances gene expression levels of type 2 collagen while suppressing type 1 collagen expression compared with single-cell sheets [85,86]. Human articular cartilage-derived cell sheets spontaneously engraft at transplanted tissue sites and regenerate cartilage tissue, expressing type 2 collagen in rat and rabbit xenogeneic transplantation models [87,88]. Moreover, the safety and efficacy of chondrocyte sheets is shown in large animal model studies using mini pigs [89] and rabbits [90]. Targeted biodistribution to the knee [91] and genomic stability of human-derived chondrocytes after in vitro cultivation were certified by G-band staining and array CGH [92]. Significantly, both safety and prominent clinical improvements were demonstrated in human patients transplanted to unloaded cartilage areas in eight autologous cases using patient-derived chondrocyte sheets combined with alignment surgery [21]. However, the two-stage surgical procedure and patient-specific cell quality variations still remain challenging issues in autologous cell-based approaches.
Juvenile cartilage-derived chondrocytes
Cultured chondrocytes as an MSC sourced from young donor cartilage show a higher proliferative ability and chondrogenic potential compared with adult donor cells [93,94]. One prominent juvenile cartilage cell source is from polydactyly surgical discards (incidence: approximately 1 per 1,000–2,000 live births [95,96], which can be easily isolated, expanded to a thousand times in a few weeks. Cryopreserved human allogeneic cell bank from one donor from both Japanese origin [25] and US origin (Kondo et al., submitted) can cover large populations of the applicable patients. Polydactyly-derived cell characteristics, including high transforming growth factor (TGF) beta secretion, in juvenile chondrocyte sheets, has been reported [25]. Hyaline cartilage regenerative capacity using human juvenile polydactyly cartilage-derived chondrocyte sheets was confirmed in an immunosuppressed rabbit osteochondral defect model [97] and nude rat focal chondral defect model (Kondo et al., submitted). The rat chondral defect model without intentional bone marrow induction particularly demonstrated that regenerated hyaline cartilage forms from human origins with no gaps found at host–donor tissue interfaces (Figure 2). This suggests the advantage of juvenile chondrocyte sheets for native tissue-like repair by exploiting highly proliferative and chondrogenic sheet characteristics. These reports support application of potent juvenile chondrocyte sheets to serve as next-generation single-stage cartilage regenerative therapy. Strategies that can reliably validate regenerative capacity and minimize variability of human juvenile chondrocyte sheets with proper cell/donor selection criteria will be critical for designing and conducting successful large-scale studies.
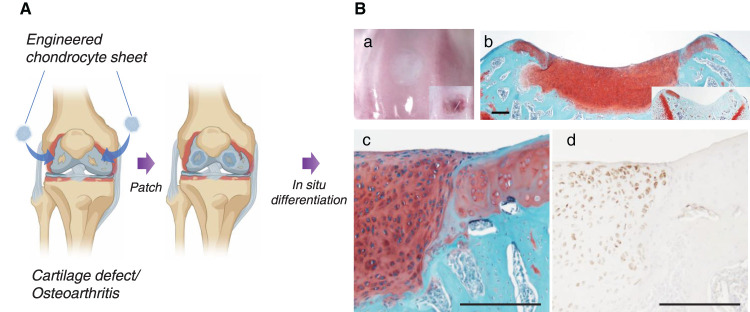
Figure 2. Cartilage regeneration with engineered human juvenile polydactyly cartilage-derived chondrocyte sheets.
(A) Schematic images of chondrocyte sheet transplantation to knee defects. (B) Repaired trochlear groove cartilage with human juvenile polydactyly cartilage-derived chondrocyte sheet. In situ hyaline cartilage maturation occurs within 4 weeks in rodent focal cartilage defect models. (a) Macroscopic image (right corner box shows defect only control). (b) Safranin O staining of nude rat trochlear groove (right corner box shows defect only control with fibrotic tissue formation). (c) Magnified image of Safranin O staining at regenerated cartilage and host tissue interface. Note no gap is observable at the interface. (d) Human vimentin antigen-specific immunostaining, suggesting that regenerated cartilage originates from transplanted human cells. Bars: 200 μm.
Tissue recovery: MSC sheets promote tissue regeneration in seven different organs via secreted paracrine factors
In addition to recognized intrinsic multi-lineage differentiation potential (e.g. osteogenic, adipogenic, and chondrogenic), MSCs also produce a remarkable array of paracrine factors that elicit both immunomodulatory effects and enhance tissue regeneration [98–100]. Cell sheets engineered from human MSCs (i.e. MSC sheets) have been studied in vitro [101,102] and in vivo to employ those functional properties. Significantly, cell sheet technology enables transplantation of tissue-like structures containing contiguous MSCs grown in their endogenous matrix onto target sites without off-target distribution. This results in stable cell engraftment and target site retention over time compared with single-cell administration [7,23,103,104]. Local MSC sheet application better facilitates direct, local therapeutic factor delivery to damaged tissue sites, resulting in continuous support for tissue recovery from various diseases.
MSC sheet treatments in multiple organ disease models, such as heart [22], periodontal membrane [20,24,105–107], skin [108], bone [23], esophagus [109], intestine [110], kidney [7], artery [104], and brain [111], have been studied using the TRCD approach, and their details are summarized in Table 1. These studies reveal that MSCs isolated from bone marrow, adipose tissue, and periodontal ligament consistently exhibit common characteristics such as multi-lineage differentiation potential, adipogenesis and osteogenesis, and colony-forming ability in vitro. These isolated MSCs can be employed to prepare transplantable MSC sheets to address various cell culture conditions (Table 1). In addition, in vivo analyses show that MSC sheets remain localized on target tissue surfaces up to 2 months, depending on the target sites and/or their assays [7,20,22–24,104–111] (Table 1), although long-term studies are required to verify sheet safety and efficacy. Furthermore, prolonged MSC local engraftment in cell sheets distinguishes their capabilities to enhance therapeutic benefits compared with single-cell administration [7,23,104]; local MSC sheet transplantation is reported to improve organ functionality on seven different tissue/organ disease models. Notably, transplanted MSC sheets adhere spontaneously and directly to host tissue surfaces, influencing the cells in damaged and surrounding area through local autocrine and paracrine effects that promote both neovascularization and tissue regeneration with host cell recruitment (Figure 3) [7,22,23,104,108,109,111–113]. Interestingly, some reports using GFP-labeled cell tracking systems also indicate that transplanted MSCs from TRCD-prepared cell sheets migrate into the host tissue and express markers of endothelial cells, pericytes, and/or other cell types after transplantation, suggesting direct support for neovascularization in addition to indirect contributions from MSC-secreted paracrine factors in local tissue regeneration [22,108,111].
Table 1. Preclinical models reporting TRCD-based allogeneic MSC cell sheet therapy in various tissue sites.
| Target site | Disease model | MSC sheet preparation and properties in vitro | In vivo observation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Origin | Size | Seeding density in 35 mm dish | Culture duration | Total grafted cell number | In vitro properties | Grafted MSC sheets | Therapeutic effects in host tissue | ||
| Heart [22] | Rat myocardial infraction model | Adipose tissue | 24 × 24 mm2 | 7.8 × 105 cells/dish | 3 days | 1 × 106 cells/rat | VEGF and HGF secretion | High cell viability at 2-day post-transplantation Cell sheet retention at least for 4 weeks |
Enhanced angiogenesis Suppression of fibrosis Improvement of cardiac function |
| Periodontal membrane [24,105,106] | Dog three-wall infrabony defect model | Periodontal ligament tissue | 8.8 cm2 (35 mm dish) | 3–4 × 104 cells/dish | 5 days | N.A. (trimmed triple-layered sheets) | Alkaline phosphatase activity Osteoblast/cementoblast markers Periodontal markers |
Cell sheet retention at least for 2 months | Periodontal regeneration including alveolar bone, cementum, well-oriented fibers, and nerve filament |
| Skin [108] | Rat wound-healing model of type 2 diabetes and obesity | Adipose tissue | 8.8 cm2 (35 mm dish) | 1.5 × 105 cells/dish | 7–8 days | 1.5 × 105 cells/rat | VEGF, HGF, TGF-ß1, IGF-1, EGF, and KGF secretion | Cell sheet retention at least for 2 months | Improved skin wound healing Enhanced angiogenesis |
| Bone [23] | Rat bisphosphonate-related osteonecrosis of the jaw model | Bone marrow | 8.8 cm2 (35 mm dish) | 2.5 × 105 cells/dish | 7 days | 1.5 × 106 cells/rat | VEGF and HGF secretion Bone regeneration marker (RANKL and OPG) gene expression |
Cell sheet retention at least for 2 months | Improved skin wound healing Bone regeneration Enhanced angiogenesis |
| Esophagus [109] | Porcine esophageal endoscopic submucosal dissection model | Adipose tissue | 3.5 cm2 (12 well plate) | 3.8 × 106 cells/dish | 12 h | 1.2 × 107 cells/rat (double-layered sheets x4) | N.A. | Cell sheet retention at least for 3 days | Less alimentary trouble and higher weight gain Reduced stricture and fibrosis formation |
| Intestine [110] | Porcine intestial anastomosis delayed wound-healing model | Adipose tissue | 8.8 cm2 (35 mm dish) | 2.4 × 106 cells/dish | 4 days | 2.4 × 106 cells/rat | Gene expression levels of FGF2 and TGF-ß1 | Cell sheet retention at least for 1 week | Enhanced collagen synthesis Increased the stiffness of intestinal anatomosis |
| Artery [104] | Rat femoral artery injury model | Adipose tissue | 8.8 cm2 (35 mm dish) | 1 × 106 cells/dish | 1–2 days | 6 × 106 cells/rat (triple-layered sheets x2) | N.A. | Cell sheet retention at least for 2 weeks | Artery reendothelialization Suppression of myofibroblast proliferation |
| Kidney [7] | Rat ischemia — reperfusion — injury model | Bone marrow | 8.8 cm2 (35 mm dish) | 1.2 × 106 cells/dish | 2 days | 7.2 × 106 cells/rat | VEGF and HGF secretion | Cell sheet retention at least for 2 weeks | Enhanced angiogenesis Suppression of microvascular injury Suppression of fibrosis Improvement of renal function |
| Neuron [111] | Rat stroke model | Adipose tissue | 8.8 cm2 (35 mm dish) | 1 × 106 cells/dish | 2 days | 3 × 106 cells/rat (triple-layered sheets) | IGF-1, HGF, VEGF, and TGF-ß1 secretion single-cell condition) |
Cell sheet retention at least for 2 weeks | Enhanced angiogenesis Enhanced neurogenesis Behavior improvement |
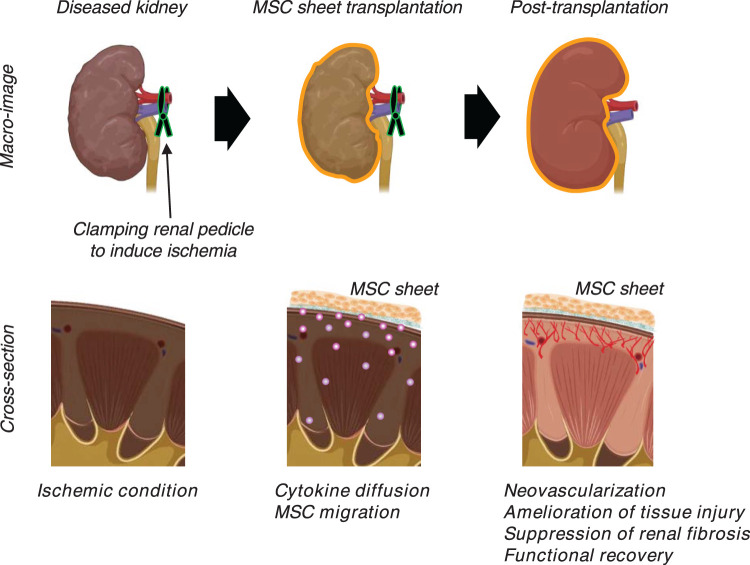
Figure 3. Enhanced tissue recovery after MSC sheet transplantation onto diseased kidney.
Schematic depiction of mesenchymal stem/stromal cell (MSC) sheet strategy. MSC sheets are transplanted over diseased kidney sites in an ischemia-reperfusion injury (IRI) model. Two-weeks post-transplantation, MSC sheets remain on kidney cortex surface and improve kidney functions as evaluated by levels of serum creatinine and blood urea nitrogen. In addition, renal tubule and epithelial cell injury in this IRI kidney model are ameliorated, and renal fibrosis, the final common product of chronic kidney disease, is significantly suppressed with enhanced neovascularization in MSC sheet transplantation group compared with non-treatment and single-cell administration groups. These findings suggest that MSC sheets might provide new therapy options for treating kidney disease.
Based on distinct, promising therapeutic effects reported for TRCD-based MSC sheet transplantation (Table 1), a first-in-human clinical study to verify their safety and efficacy has demonstrated periodontal regeneration using autologous human MSC sheets [20]. To permit expanded patient treatments for diverse diseases, allogeneic MSC sources exhibiting broad histocompatibility and consistent paracrine factor secretion profiles are required to produce cell banks that yield validated, efficient MSC therapeutic properties. As a next-step to scaling more effective, local MSC therapy for economic off-the-shelf use, allogeneic human MSC sheet transplantation exploiting their innate high proliferative capacity and therapeutic efficacy is timely; yet, challenges defining MSC sheet critical quality and functional attributes still remain. Nonetheless, human allogeneic MSC sheet fabrication and transplantation is rapidly developing as a rational and economically attractive process, capable of possibly addressing both past issues with inconsistent cell therapy outcomes, and also diverse unmet medical needs in multiple organ diseases. Additionally, establishing human MSC sheet treatments and therapeutic mechanisms in one initial target disease could support expansion of related new MSC-based therapeutic strategies for other diseases in the future.
Conclusions
Cell sheet therapy strategies are well-established in numerous preclinical and clinical applications using diverse cell types. TRCD-based MSC sheet technology in particular is now described in diverse preclinical models and some early pilot human clinical reports. While autologous human MSC sources are first-in-human for cell sheet therapeutic use, allogeneic human MSC sources are currently more attractive to produce new scalable, affordable, histocompatible and widely distributable cell sheet-based therapies for tissue regeneration, exploiting their recognized differentiation potential, immunomodulatory capacity, and diverse paracrine secretome at transplanted specific organs. Moreover, allogeneic human MSC therapies are shown scalable due to readily accessible cell sourcing and cell banking systems, enabling phenotypic and genomic stability for safety and efficacy control and off-the-shelf cell sheet availability for broad clinical use. Thus, allogeneic MSC sheets represent an attractive cell therapy strategy to provide more reliable, novel therapies to address diverse unmet medical needs.
Summary
Cell therapies in conventional injectable cell suspension forms currently lack sufficient homing, disease site retention, reliable potency, and durable therapeutic responses for local diseases.
Human MSCs offer substantial therapeutic benefits if quality control features for allogeneic sourcing and disease use are known.
Juvenile chondrocytes harvested from routine polydactyly surgical discards are an attractive source of MSCs amenable to scaling, banking and allogeneic cell sheet use to regenerate/replace the damaged human cartilage.
MSC sheet transplantation rapidly engrafts and elicits therapeutic signaling in situ via secreted paracrine factors to provide a new strategy for tissue regeneration in various diseases.
TRCD-based MSC sheet technology is a feasible approach as demonstrated by its safety and efficacy in multiple preclinical and clinical studies.
Acknowledgement
Schematic representations were created with Biorender.com.
Abbreviations
- ECM
xtracellular matrix
- ES cell
embryonic stem cell
- GFP
green fluorescent protein
- iPS cell
induced pluripotent stem cell
- MACI
matrix-associated autologous chondrocyte implantation
- MSC
mesenchymal stem/stromal cell
- TGF
transforming growth factor
- TRCD
thermo-responsive culture dish
Competing Interests
Teruo Okano holds equity in CellSeed, Inc. (Japan) and is an inventor/developer designated on the patent for CellSeed's commercialized temperature-responsive cultureware. No other competing financial interests exist and all authors declare that they have no other competing interests.
Funding
This work was supported in part by University of Utah Health Sciences translational research partnerships, and the University Technology Acceleration Grant from the Utah Science, Technology, and Research (USTAR) program, Utah, U.S.A.
Author Contributions
S.K. and M.K. wrote the manuscript and equally contributed to this work. D.W.G. and T.O. reviewed and edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Pittenger, M.F., Discher, D.E., Peault, B.M., Phinney, D.G., Hare, J.M. and Caplan, A.I. (2019) Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechanteur, C., Briquet, A., Giet, O., Delloye, O., Baudoux, E. and Beguin, Y. (2016) Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J. Transl. Med. 14, 145 10.1186/s12967-016-0892-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquez-Curtis, L.A., Janowska-Wieczorek, A., McGann, L.E. and Elliott, J.A. (2015) Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology 71, 181–197 10.1016/j.cryobiol.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Nenasheva, T., Nikolaev, A., Diykanov, D., Sukhanova, A., Tcyganov, E., Panteleev, A.et al. (2017) The introduction of mesenchymal stromal cells induces different immunological responses in the lungs of healthy and M. tuberculosis infected mice. PLoS One 12, e0178983 10.1371/journal.pone.0178983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy, O., Kuai, R., Siren, E.M., Bhere, D., Milton, Y., Nissar, N.et al. (2020) Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 6, eaba6884 10.1126/sciadv.aba6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, R.H., Pulin, A.A., Seo, M.J., Kota, D.J., Ylostalo, J., Larson, B.L.et al. (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63 10.1016/j.stem.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imafuku, A., Oka, M., Miyabe, Y., Sekiya, S., Nitta, K. and Shimizu, T. (2019) Rat mesenchymal stromal cell sheets suppress renal fibrosis via microvascular protection. Stem Cells Transl. Med. 8, 1330–1341 10.1002/sctm.19-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatsumi, K., Ohashi, K., Matsubara, Y., Kohori, A., Ohno, T., Kakidachi, H.et al. (2013) Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem. Biophys. Res. Commun. 431, 203–209 10.1016/j.bbrc.2012.12.134 [DOI] [PubMed] [Google Scholar]
- 9.Gao, J., Dennis, J.E., Muzic, R.F., Lundberg, M. and Caplan, A.I. (2001) The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169, 12–20 10.1159/000047856 [DOI] [PubMed] [Google Scholar]
- 10.Furlani, D., Ugurlucan, M., Ong, L., Bieback, K., Pittermann, E., Westien, I.et al. (2009) Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 77, 370–376 10.1016/j.mvr.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 11.Yamato, M., Utsumi, M., Kushida, A., Konno, C., Kikuchi, A. and Okano, T. (2001) Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 7, 473–480 10.1089/10763270152436517 [DOI] [PubMed] [Google Scholar]
- 12.Kushida, A., Yamato, M., Konno, C., Kikuchi, A., Sakurai, Y. and Okano, T. (1999) Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 45, 355–362 [DOI] [PubMed] [Google Scholar]
- 13.Nishida, K. (2003) Tissue engineering of the cornea. Cornea 22, S28–S34 10.1097/00003226-200310001-00005 [DOI] [PubMed] [Google Scholar]
- 14.Nishida, K., Yamato, M., Hayashida, Y., Watanabe, K., Maeda, N., Watanabe, H.et al. (2004) Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 77, 379–385 10.1097/01.TP.0000110320.45678.30 [DOI] [PubMed] [Google Scholar]
- 15.Nishida, K., Yamato, M., Hayashida, Y., Watanabe, K., Yamamoto, K., Adachi, E.et al. (2004) Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196 10.1056/NEJMoa040455 [DOI] [PubMed] [Google Scholar]
- 16.Ohki, T., Yamato, M., Ota, M., Takagi, R., Murakami, D., Kondo, M.et al. (2012) Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 143, 582–588.e2 10.1053/j.gastro.2012.04.050 [DOI] [PubMed] [Google Scholar]
- 17.Sawa, Y., Miyagawa, S., Sakaguchi, T., Fujita, T., Matsuyama, A., Saito, A.et al. (2012) Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg. Today 42, 181–184 10.1007/s00595-011-0106-4 [DOI] [PubMed] [Google Scholar]
- 18.Kanzaki, M., Takagi, R., Washio, K., Kokubo, M. and Yamato, M. (2017) Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen. Med. 2, 26 10.1038/s41536-017-0031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto, K., Yamato, M., Morino, T., Sugiyama, H., Takagi, R., Yaguchi, Y.et al. (2017) Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen. Med. 2, 6 10.1038/s41536-017-0010-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata, T., Yamato, M., Washio, K., Yoshida, T., Tsumanuma, Y., Yamada, A.et al. (2018) Periodontal regeneration with autologous periodontal ligament-derived cell sheets–a safety and efficacy study in ten patients. Regen. Ther. 9, 38–44 10.1016/j.reth.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato, M., Yamato, M., Mitani, G., Takagaki, T., Hamahashi, K., Nakamura, Y.et al. (2019) Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen. Med. 4, 1–11 10.1038/s41536-019-0069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyahara, Y., Nagaya, N., Kataoka, M., Yanagawa, B., Tanaka, K., Hao, H.et al. (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 12, 459–465 10.1038/nm1391 [DOI] [PubMed] [Google Scholar]
- 23.Kaibuchi, N., Iwata, T., Yamato, M., Okano, T. and Ando, T. (2016) Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 42, 400–410 10.1016/j.actbio.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 24.Tsumanuma, Y., Iwata, T., Kinoshita, A., Washio, K., Yoshida, T., Yamada, A.et al. (2016) Allogeneic transplantation of periodontal ligament-derived multipotent mesenchymal stromal cell sheets in canine critical-size supra-alveolar periodontal defect model. BioResearch Open Access 5, 22–36 10.1089/biores.2015.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maehara, M., Sato, M., Toyoda, E., Takahashi, T., Okada, E., Kotoku, T.et al. (2017) Characterization of polydactyly-derived chondrocyte sheets versus adult chondrocyte sheets for articular cartilage repair. Inflamm. Regen. 37, 22 10.1186/s41232-017-0053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin, U. (2017) Therapeutic application of pluripotent stem cells: challenges and risks. Front. Med. 4, 229 10.3389/fmed.2017.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eguizabal, C., Aran, B., Chuva de Sousa Lopes, S., Geens, M., Heindryckx, B., Panula, S.et al. (2019) Two decades of embryonic stem cells: a historical overview. Hum. Reprod. Open 2019, hoy024 10.1093/hropen/hoy024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandai, M., Watanabe, A., Kurimoto, Y., Hirami, Y., Morinaga, C., Daimon, T.et al. (2017) Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, J. (2019) Preparing for first human trial of induced pluripotent stem cell-derived cells for Parkinson's disease: an interview with Jun Takahashi. Regen. Med. 14, 93–95 10.2217/rme-2018-0158 [DOI] [PubMed] [Google Scholar]
- 30.Chen, K.G., Mallon, B.S., McKay, R.D. and Robey, P.G. (2014) Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 14, 13–26 10.1016/j.stem.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato, Y., Bando, H., Di Piazza, M., Gowing, G., Herberts, C., Jackman, S.et al. (2019) Tumorigenicity assessment of cell therapy products: the need for global consensus and points to consider. Cytotherapy 21, 1095–1111 10.1016/j.jcyt.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 32.Blau, H.M. and Daley, G.Q. (2019) Stem cells in the treatment of disease. N. Engl. J. Med. 380, 1748–1760 10.1056/NEJMra1716145 [DOI] [PubMed] [Google Scholar]
- 33.Lee, A.S., Tang, C., Rao, M.S., Weissman, I.L. and Wu, J.C. (2013) Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 19, 998–1004 10.1038/nm.3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barkholt, L., Flory, E., Jekerle, V., Lucas-Samuel, S., Ahnert, P., Bisset, L.et al. (2013) Risk of tumorigenicity in mesenchymal stromal cell-based therapies–bridging scientific observations and regulatory viewpoints. Cytotherapy 15, 753–759 10.1016/j.jcyt.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 35.Zakrzewski, J.L., Van Den Brink, M.R. and Hubbell, J.A. (2014) Overcoming immunological barriers in regenerative medicine. Nat. Biotechnol. 32, 786–794 10.1038/nbt.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camilleri, E.T., Gustafson, M.P., Dudakovic, A., Riester, S.M., Garces, C.G., Paradise, C.R.et al. (2016) Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res. Ther. 7, 107 10.1186/s13287-016-0370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo, E., Fajka-Boja, R., Kriston-Pal, E., Hornung, A., Makra, I., Kudlik, G.et al. (2015) Licensing by inflammatory cytokines abolishes heterogeneity of immunosuppressive function of mesenchymal stem cell population. Stem Cells Dev. 24, 2171–2180 10.1089/scd.2014.0581 [DOI] [PubMed] [Google Scholar]
- 38.Lei, J., Hui, D., Huang, W., Liao, Y., Yang, L., Liu, L.et al. (2013) Heterogeneity of the biological properties and gene expression profiles of murine bone marrow stromal cells. Int. J. Biochem. Cell Biol. 45, 2431–2443 10.1016/j.biocel.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 39.Francois, M., Romieu-Mourez, R., Li, M.Y. and Galipeau, J. (2012) Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195 10.1038/mt.2011.189 [DOI] [PubMed] [Google Scholar]
- 40.Adkisson, H., Milliman, C., Zhang, X., Mauch, K., Maziarz, R. and Streeter, P. (2010) Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem Cell Res. 4, 57–68 10.1016/j.scr.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 41.Huang, X.P., Ludke, A., Dhingra, S., Guo, J., Sun, Z., Zhang, L.et al. (2016) Class II transactivator knockdown limits major histocompatibility complex II expression, diminishes immune rejection, and improves survival of allogeneic bone marrow stem cells in the infarcted heart. FASEB J. 30, 3069–3082 10.1096/fj.201600331R [DOI] [PubMed] [Google Scholar]
- 42.Makhija, R. and Kingsnorth, A.N. (2002) Cytokine storm in acute pancreatitis. J. Hepato-Biliary-Pancreat. Surg. 9, 401–410 10.1007/s005340200049 [DOI] [PubMed] [Google Scholar]
- 43.Panés, J., García-Olmo, D., Van Assche, G., Colombel, J.F., Reinisch, W., Baumgart, D.C.et al. (2016) Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet 388, 1281–1290 10.1016/S0140-6736(16)31203-X [DOI] [PubMed] [Google Scholar]
- 44.Panés, J., García-Olmo, D., Van Assche, G., Colombel, J.F., Reinisch, W., Baumgart, D.C.et al. (2018) Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn's disease. Gastroenterology 154, 1334–1342.e4 10.1053/j.gastro.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 45.Le Blanc, K., Rasmusson, I., Sundberg, B., Götherström, C., Hassan, M., Uzunel, M.et al. (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 10.1016/S0140-6736(04)16104-7 [DOI] [PubMed] [Google Scholar]
- 46.Le Blanc, K., Frassoni, F., Ball, L., Locatelli, F., Roelofs, H., Lewis, I.et al. (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 10.1016/S0140-6736(08)60690-X [DOI] [PubMed] [Google Scholar]
- 47.Zonta, S., De Martino, M., Bedino, G., Piotti, G., Rampino, T., Gregorini, M.et al. (2010) Which is the most suitable and effective route of administration for mesenchymal stem cell-based immunomodulation therapy in experimental kidney transplantation: endovenous or arterial? Transplant. Proc. 42, 1336–1340 10.1016/j.transproceed.2010.03.081 [DOI] [PubMed] [Google Scholar]
- 48.Togel, F., Yang, Y., Zhang, P., Hu, Z. and Westenfelder, C. (2008) Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am. J. Physiol. Renal. Physiol. 295, F315–F321 10.1152/ajprenal.00098.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitrousis, N., Fokina, A. and Shoichet, M.S. (2018) Biomaterials for cell transplantation. Nat. Rev. Mater. 3, 441–456 10.1038/s41578-018-0057-0 [DOI] [Google Scholar]
- 50.DuRaine, G.D., Brown, W.E., Hu, J.C. and Athanasiou, K.A. (2015) Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Ann. Biomed. Eng. 43, 543–554 10.1007/s10439-014-1161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee, J.K., Link, J.M., Hu, J.C. and Athanasiou, K.A. (2017) The self-assembling process and applications in tissue engineering. Cold Spring Harb. Perspect. Med. 7, a025668 10.1101/cshperspect.a025668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okano, T., Yamada, N., Okuhara, M., Sakai, H. and Sakurai, Y. (1995) Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 16, 297–303 10.1016/0142-9612(95)93257-E [DOI] [PubMed] [Google Scholar]
- 53.Yamato, M. and Okano, T. (2004) Cell sheet engineering. Mater. Today 7, 42–47 10.1016/S1369-7021(04)00234-2 [DOI] [Google Scholar]
- 54.Yamada, N., Okano, T., Sakai, H., Karikusa, F., Sawasaki, Y. and Sakurai, Y. (1990) Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 11, 571–576 10.1002/marc.1990.030111109 [DOI] [Google Scholar]
- 55.Kikuchi, A., Okuhara, M., Karikusa, F., Sakurai, Y. and Okano, T. (1998) Two-dimensional manipulation of confluently cultured vascular endothelial cells using temperature-responsive poly (N-isopropylacrylamide)-grafted surfaces. J. Biomater. Sci. 9, 1331–1348 10.1163/156856298X00424 [DOI] [PubMed] [Google Scholar]
- 56.Matsuura, K., Utoh, R., Nagase, K. and Okano, T. (2014) Cell sheet approach for tissue engineering and regenerative medicine. J. Control Release 190, 228–239 10.1016/j.jconrel.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi, J., Kikuchi, A., Aoyagi, T. and Okano, T. (2019) Cell sheet tissue engineering: cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed. Mater. Res. A 107, 955–967 10.1002/jbm.a.36627 [DOI] [PubMed] [Google Scholar]
- 58.Inaba, R., Khademhosseini, A., Suzuki, H. and Fukuda, J. (2009) Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials 30, 3573–3579 10.1016/j.biomaterials.2009.03.045 [DOI] [PubMed] [Google Scholar]
- 59.Guillaume-Gentil, O., Akiyama, Y., Schuler, M., Tang, C., Textor, M., Yamato, M.et al. (2008) Polyelectrolyte coatings with a potential for electronic control and cell sheet engineering. Adv. Mater. 20, 560–565 10.1002/adma.200700758 [DOI] [Google Scholar]
- 60.Guillaume-Gentil, O., Semenov, O.V., Zisch, A.H., Zimmermann, R., Vörös, J. and Ehrbar, M. (2011) pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials 32, 4376–4384 10.1016/j.biomaterials.2011.02.058 [DOI] [PubMed] [Google Scholar]
- 61.Ito, A., Hayashida, M., Honda, H., Hata, K.-I., Kagami, H., Ueda, M.et al. (2004) Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 10, 873–880 10.1089/1076327041348446 [DOI] [PubMed] [Google Scholar]
- 62.Ito, A., Ino, K., Kobayashi, T. and Honda, H. (2005) The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials 26, 6185–6193 10.1016/j.biomaterials.2005.03.039 [DOI] [PubMed] [Google Scholar]
- 63.Ito, A., Jitsunobu, H., Kawabe, Y. and Kamihira, M. (2007) Construction of heterotypic cell sheets by magnetic force-based 3-D coculture of HepG2 and NIH3T3 cells. J. Biosci. Bioeng. 104, 371–378 10.1263/jbb.104.371 [DOI] [PubMed] [Google Scholar]
- 64.Huey, D.J., Hu, J.C. and Athanasiou, K.A. (2012) Unlike bone, cartilage regeneration remains elusive. Science (New York, NY) 338, 917–921 10.1126/science.1222454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armiento, A.R., Alini, M. and Stoddart, M.J. (2019) Articular fibrocartilage-Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 146, 289–305 10.1016/j.addr.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 66.Cole, B.J., Pascual-Garrido, C. and Grumet, R.C. (2009) Surgical management of articular cartilage defects in the knee. J. Bone Joint Surg. Am. 91, 1778–1790 PMID: [PubMed] [Google Scholar]
- 67.Martín, A.R., Patel, J.M., Zlotnick, H.M., Carey, J.L. and Mauck, R.L. (2019) Emerging therapies for cartilage regeneration in currently excluded ‘red knee'populations. NPJ Regen. Med. 4, 12 10.1038/s41536-019-0074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang, Y. and Tuan, R.S. (2015) Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 11, 206 10.1038/nrrheum.2014.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alsalameh, S., Amin, R., Gemba, T. and Lotz, M. (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 50, 1522–1532 10.1002/art.20269 [DOI] [PubMed] [Google Scholar]
- 70.Jiang, Y., Cai, Y., Zhang, W., Yin, Z., Hu, C., Tong, T.et al. (2016) Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cells Transl. Med. 5, 733–744 10.5966/sctm.2015-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jakob, M., Demarteau, O., Schäfer, D., Hintermann, B., Dick, W., Heberer, M.et al. (2001) Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell Biochem. 81, 368–377 [DOI] [PubMed] [Google Scholar]
- 72.Benya, P.D. and Shaffer, J.D. (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30, 215–224 10.1016/0092-8674(82)90027-7 [DOI] [PubMed] [Google Scholar]
- 73.Kwon, H., Brown, W.E., Lee, C.A., Wang, D., Paschos, N., Hu, J.C.et al. (2019) Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 15, 550–570 10.1038/s41584-019-0255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Negoro, T., Takagaki, Y., Okura, H. and Matsuyama, A. (2018) Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen. Med. 3, 17 10.1038/s41536-018-0055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang, B.J., Hu, J.C. and Athanasiou, K.A. (2016) Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98, 1–22 10.1016/j.biomaterials.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li, Y., Wei, X., Zhou, J. and Wei, L. (2013) The age-related changes in cartilage and osteoarthritis. BioMed Res. Int. 2013, 916530 10.1155/2013/916530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grebenik, E.V., Gornostaeva, E.A., Telpuhov, S.N., Lychagin, V.I., Timashev, A.V. and et al, P.S. (2018) Repair of damaged articular cartilage: current approaches and future directions. Int. J. Mol. Sci. 19, 2366 10.3390/ijms19082366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basad, E., Ishaque, B., Bachmann, G., Stürz, H. and Steinmeyer, J. (2010) Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg. Sports Traumatol. Arthrosc. 18, 519–527 10.1007/s00167-009-1028-1 [DOI] [PubMed] [Google Scholar]
- 79.Harris, J., Siston, R., Brophy, R., Lattermann, C., Carey, J. and Flanigan, D. (2011) Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthr. Cartil. 19, 779–791 10.1016/j.joca.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 80.Basad, E., Wissing, F.R., Fehrenbach, P., Rickert, M., Steinmeyer, J. and Ishaque, B. (2015) Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surg. Sports Traumatol. Arthrosc. 23, 3729–3735 10.1007/s00167-014-3295-8 [DOI] [PubMed] [Google Scholar]
- 81.Watt, F.M. (1988) Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J. Cell Sci. 89, 373–378 PMID: [DOI] [PubMed] [Google Scholar]
- 82.Schulze-Tanzil, G., De Souza, P., Castrejon, H.V., John, T., Merker, H.-J., Scheid, A.et al. (2002) Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 308, 371–379 10.1007/s00441-002-0562-7 [DOI] [PubMed] [Google Scholar]
- 83.Goldring, M.B. and Berenbaum, F. (2015) Emerging targets in osteoarthritis therapy. Curr. Opin. Pharmacol. 22, 51–63 10.1016/j.coph.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamahashi, K., Sato, M., Yamato, M., Kokubo, M., Mitani, G., Ito, S.et al. (2015) Studies of the humoral factors produced by layered chondrocyte sheets. J. Tissue Eng. Regen. Med. 9, 24–30 10.1002/term.1610 [DOI] [PubMed] [Google Scholar]
- 85.Mitani, G., Sato, M., Lee, J.I., Kaneshiro, N., Ishihara, M., Ota, N.et al. (2009) The properties of bioengineered chondrocyte sheets for cartilage regeneration. BMC Biotechnol. 9, 17 10.1186/1472-6750-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaneshiro, N., Sato, M., Ishihara, M., Mitani, G., Sakai, H., Kikuchi, T.et al. (2007) Cultured articular chondrocytes sheets for partial thickness cartilage defects utilizing temperature-responsive culture dishes. Eur. Cells Mater. 13, 87–92 10.22203/eCM.v013a09 [DOI] [PubMed] [Google Scholar]
- 87.Takahashi, T., Sato, M., Toyoda, E., Maehara, M., Takizawa, D., Maruki, H.et al. (2018) Rabbit xenogeneic transplantation model for evaluating human chondrocyte sheets used in articular cartilage repair. J. Tissue Eng. Regen. Med. 12, 2067–2076 10.1002/term.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takizawa, D., Sato, M., Okada, E., Takahashi, T., Maehara, M., Tominaga, A.et al. (2020) Regenerative effects of human chondrocyte sheets in a xenogeneic transplantation model using immune-deficient rats. J. Tissue Eng. Regen. Med. 14, 1296–1306 10.1002/term.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ebihara, G., Sato, M., Yamato, M., Mitani, G., Kutsuna, T., Nagai, T.et al. (2012) Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials 33, 3846–3851 10.1016/j.biomaterials.2012.01.056 [DOI] [PubMed] [Google Scholar]
- 90.Ito, S., Sato, M., Yamato, M., Mitani, G., Kutsuna, T., Nagai, T.et al. (2012) Repair of articular cartilage defect with layered chondrocyte sheets and cultured synovial cells. Biomaterials 33, 5278–5286 10.1016/j.biomaterials.2012.03.073 [DOI] [PubMed] [Google Scholar]
- 91.Takaku, Y., Murai, K., Ukai, T., Ito, S., Kokubo, M., Satoh, M.et al. (2014) In vivo cell tracking by bioluminescence imaging after transplantation of bioengineered cell sheets to the knee joint. Biomaterials 35, 2199–2206 10.1016/j.biomaterials.2013.11.071 [DOI] [PubMed] [Google Scholar]
- 92.Yokoyama, M., Sato, M., Umezawa, A., Mitani, G., Takagaki, T., Yokoyama, M.et al. (2016) Assessment of the safety of chondrocyte sheet implantation for cartilage regeneration. Tissue Eng. Part C Methods 22, 59–68 10.1089/ten.tec.2015.0254 [DOI] [PubMed] [Google Scholar]
- 93.Cavalli, E., Levinson, C., Hertl, M., Broguiere, N., Brück, O., Mustjoki, S.et al. (2019) Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 9, 1–15 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adkisson IV, H.D., Martin, J.A., Amendola, R.L., Milliman, C., Mauch, K.A., Katwal, A.B.et al. (2010) The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am. J. Sports Med. 38, 1324–1333 10.1177/0363546510361950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Woolf, C.M. and Woolf, R.M. (1970) A genetic study of polydactyly in utah. Am. J. Hum. Genet. 22, 75 PMID: [PMC free article] [PubMed] [Google Scholar]
- 96.Castilla, E., Paz, J., Mutchinick, O., Muñoz, E., Giorgiutti, E. and Gelman, Z. (1973) Polydactyly: a genetic study in South America. Am. J. Hum. Gen. 25, 405 PMID: [PMC free article] [PubMed] [Google Scholar]
- 97.Toyoda, E., Sato, M., Takahashi, T., Maehara, M., Okada, E., Wasai, S.et al. (2020) Transcriptomic and proteomic analyses reveal the potential mode of action of chondrocyte sheets in hyaline cartilage regeneration. Int. J. Mol. Sci. 21, 149 10.3390/ijms21010149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caplan, A.I. and Correa, D. (2011) The MSC: an injury drugstore. Cell Stem Cell 9, 11–15 10.1016/j.stem.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caplan, A.I. and Bruder, S.P. (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol. Med. 7, 259–264 10.1016/S1471-4914(01)02016-0 [DOI] [PubMed] [Google Scholar]
- 100.Vizoso, F.J., Eiro, N., Cid, S., Schneider, J. and Perez-Fernandez, R. (2017) Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18, 1852 10.3390/ijms18091852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim, K., Bou-Ghannam, S., Thorp, H., Grainger, D.W. and Okano, T. (2019) Human mesenchymal stem cell sheets in xeno-free media for possible allogenic applications. Sci. Rep. 9, 14415 10.1038/s41598-019-50430-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakao, M., Kim, K., Nagase, K., Grainger, D.W., Kanazawa, H. and Okano, T. (2019) Phenotypic traits of mesenchymal stem cell sheets fabricated by temperature-responsive cell culture plate: structural characteristics of MSC sheets. Stem Cell Res. Ther. 10, 353 10.1186/s13287-019-1431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sekine, H., Shimizu, T., Dobashi, I., Matsuura, K., Hagiwara, N., Takahashi, M.et al. (2011) Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng. Part A 17, 2973–2980 10.1089/ten.tea.2010.0659 [DOI] [PubMed] [Google Scholar]
- 104.Homma, J., Sekine, H., Matsuura, K., Kobayashi, E. and Shimizu, T. (2018) Mesenchymal stem cell sheets exert antistenotic effects in a rat arterial injury model. Tissue Eng. Part A 24, 1545–1553 10.1089/ten.tea.2018.0030 [DOI] [PubMed] [Google Scholar]
- 105.Iwata, T., Yamato, M., Tsuchioka, H., Takagi, R., Mukobata, S., Washio, K.et al. (2009) Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 30, 2716–2723 10.1016/j.biomaterials.2009.01.032 [DOI] [PubMed] [Google Scholar]
- 106.Tsumanuma, Y., Iwata, T., Washio, K., Yoshida, T., Yamada, A., Takagi, R.et al. (2011) Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 32, 5819–5825 10.1016/j.biomaterials.2011.04.071 [DOI] [PubMed] [Google Scholar]
- 107.Iwata, T., Yamato, M., Ishikawa, I., Ando, T. and Okano, T. (2014) Tissue engineering in periodontal tissue. Anat. Rec. 297, 16–25 10.1002/ar.22812 [DOI] [PubMed] [Google Scholar]
- 108.Kato, Y., Iwata, T., Morikawa, S., Yamato, M., Okano, T. and Uchigata, Y. (2015) Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes 64, 2723–2734 10.2337/db14-1133 [DOI] [PubMed] [Google Scholar]
- 109.Perrod, G., Rahmi, G., Pidial, L., Camilleri, S., Bellucci, A., Casanova, A.et al. (2016) Cell sheet transplantation for esophageal stricture prevention after endoscopic submucosal dissection in a porcine model. PLoS One 11, e0148249 10.1371/journal.pone.0148249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maruya, Y., Kanai, N., Kobayashi, S., Koshino, K., Okano, T., Eguchi, S.et al. (2017) Autologous adipose-derived stem cell sheets enhance the strength of intestinal anastomosis. Regen. Ther. 7, 24–33 10.1016/j.reth.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryu, B., Sekine, H., Homma, J., Kobayashi, T., Kobayashi, E., Kawamata, T.et al. (2019) Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J. Neurosurg. 132, 442–455 10.3171/2018.11.JNS182331 [DOI] [PubMed] [Google Scholar]
- 112.Chen, L., Xing, Q., Zhai, Q., Tahtinen, M., Zhou, F., Chen, L.et al. (2017) Pre-vascularization enhances therapeutic effects of human mesenchymal stem cell sheets in full thickness skin wound repair. Theranostics 7, 117–131 10.7150/thno.17031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Werner, S., Krieg, T. and Smola, H. (2007) Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 127, 998–1008 10.1038/sj.jid.5700786 [DOI] [PubMed] [Google Scholar]