Summary
Macrophages promote an early host response to infection by releasing pro-inflammatory cytokines such as interleukin-1β (IL-1β), TNF, and IL-6. The bioactivity of IL-1β is classically dependent on NLRP3 inflammasome activation, which culminates in caspase-1 activation and pyroptosis. Recent studies suggest a role for NLRP3 inflammasome activation in lung inflammation and fibrosis in both COVID-19 and SARS, and there is evidence of NLRP3 involvement in HIV-1 disease. Here, we show that GU-rich single-stranded RNA (GU-rich RNA) derived from SARS-CoV-2, SARS-CoV-1, and HIV-1 trigger a TLR8-dependent pro-inflammatory cytokine response from human macrophages in the absence of pyroptosis, with GU-rich RNA from the SARS-CoV-2 spike protein triggering the greatest inflammatory response. Using genetic and pharmacological inhibition, we show that the induction of mature IL-1β is through a non-classical pathway dependent on caspase-1, caspase-8, the NLRP3 inflammasome, potassium efflux, and autophagy while being independent of TRIF (TICAM1), vitamin D3, and pyroptosis.
Subject areas: Immunology, Virology
Graphical abstract
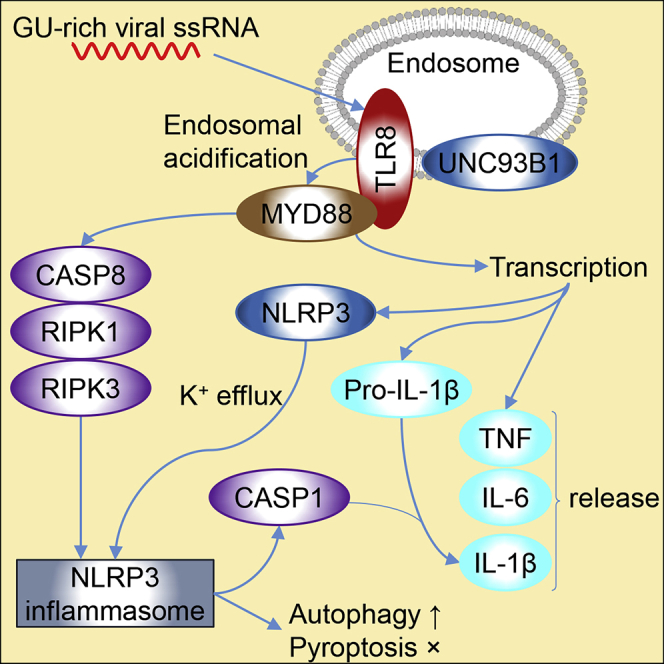
Highlights
-
•
GU-rich ssRNA induces IL-1β, TNF, and IL-6 from human macrophages through TLR8
-
•
SARS-CoV-2 RNA elicits a greater inflammatory response than SARS-CoV-1 or HIV-1 RNA
-
•
SARS-CoV-2 RNA elicits TLR8-mediated IL-1β production in the absence of pyroptosis
-
•
TLR8-mediated IL-1β release is dependent on CASP8, K+ efflux, NLRP3, and autophagy
Immunology; Virology
Introduction
Coronavirus-19 disease (COVID-19) is a highly contagious and sometimes fatal respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a betacoronavirus closely related to SARS-CoV-1, which causes severe acute respiratory syndrome (SARS). COVID-19 symptoms can include fever and pneumonia, and in a small percentage of cases an acute respiratory distress syndrome (ARDS) reminiscent of the cytokine release syndrome (CRS)-induced ARDS and secondary hemophagocytic lymphohistiocytosis previously observed in both SARS and Middle East respiratory syndrome (Huang et al., 2020). High concentrations of pro-inflammatory cytokines, including interleukin (IL) 1β, IL-6, and tumor necrosis factor (TNF), characterize this “cytokine storm,” which can lead to alveolar damage, pulmonary fibrinolysis, and pulmonary injury. Increased levels of IL-6 correlate with increased mortality, and IL-6, IL-10, and TNF concentrations inversely correlate with lymphocyte count, suggesting that SARS-CoV-2-mediated CRS potentially hampers the adaptive immune response (Pedersen and Ho, 2020). Human immunodeficiency virus type 1 (HIV-1) pathogenesis is also associated with immune activation and chronic inflammation despite effective viral suppression from antiretroviral treatment (Antiretroviral Therapy Cohort Collaboration, 2008), which contributes to non-AIDS related inflammatory diseases activity (Catalfamo et al., 2012).
Virally infected cells release antigens, including RNA (collectively termed pathogen-associated molecular patterns [PAMPs]), which can induce inflammatory responses in neighboring cells such as the transcription and release of proinflammatory cytokines. A variety of pattern recognition receptors, including toll-like receptors (TLR) recognize these PAMPs. There are 10 human TLRs, each of which recognizes a distinct group of PAMPs. TLRs 1, 2, 4, 5, 6, and 10 are located at the plasma membrane and recognize bacterial membrane components, whereas TLRs 3, 7, 8, and 9 are predominantly located within endosomes and recognize microbial nucleic acids. TLR8 is phylogenetically and structurally related to TLR7, is expressed as a homodimer in macrophages and myeloid dendritic cells, and is activated by viral or bacterial uridine-rich short single-stranded RNA during infection or through phagocytosis (Cervantes et al., 2013; Heil et al., 2004; Moen et al., 2019; Vierbuchen et al., 2017). Upon ligand-induced conformational change that is required for its activation (Zhu et al., 2009), TLR8 associates and interacts with the adapter protein MYD88, which recruits the IL-1 receptor-associated kinases, leading to the nuclear factor-κB-dependent transcription of numerous proinflammatory mediators including IL-6, IL-12, IL-27, TNF, interferon (IFN) γ, and IL-1β (Akira et al., 2006; Ghosh et al., 2006; Vierbuchen et al., 2017).
IL-1β is synthesized as an inactive pro-protein that must be proteolytically cleaved by caspase (CASP)-1 to generate the active pro-inflammatory cytokine (Sutterwala et al., 2006). One of the complexes that participates in this process is the NLRP3 (NLR family pyrin domain containing 3) inflammasome. This multimeric cytosolic protein complex canonically requires two distinct steps for activation (Elliott and Sutterwala, 2015). The first is a priming step resulting in the production of pro-IL-1β, and transcription and posttranslational modification of NLRP3. The second remains elusive; a common denominator of its activation is the cytosolic efflux of K+ (Muñoz-Planillo et al., 2013), which appears to be necessary and sufficient to trigger NLRP3 to interact with and recruit PYCARD (pyrin domain [PYD] and C-terminal caspase-recruitment domain [CARD] containing protein; umquhile ASC). The CARD of PYCARD then recruits the pro-CASP1 zymogen via its CARD to form an NLRP3-PYCARD-pro-CASP1 complex. The pro-CASP1 zymogen then self-activates through proteolytic cleavage. The enzymatically active heterodimer of CASP1 then cleaves pro-interleukin-1β into mature IL-1β. Upon activation, the NLRP3 inflammasome classically promotes pyroptosis, an inflammatory form of cell death. Little is known regarding the role of TLR8 in the innate immune response against infection with SARS-CoV-2, SARS-CoV-1, and HIV-1. In this study, we investigated the mechanism through which GU-rich single-stranded RNA sequences (GU-rich RNA) derived from SARS-CoV-2, SARS-CoV-1, and HIV-1 trigger pro-inflammatory responses from human macrophages.
Results
SARS-CoV-2-, SARS-CoV-1-, and HIV-1-derived GU-rich RNA treatment of macrophages results in IL-1β release through a TLR8-dependent mechanism
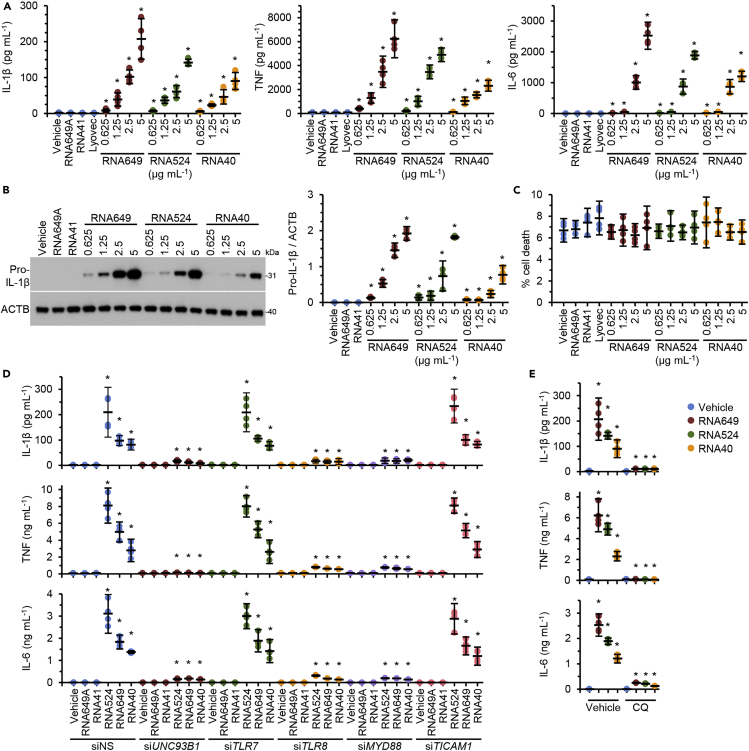
The importance of identifying the activity and mechanism(s) by which innate immunity is activated by viral single-stranded RNA has become magnified as serious COVID-19, SARS, and AIDS are all associated with a dramatic increase in inflammatory cytokines. To address this, we identified a GU-rich RNA from the spike protein of SARS-CoV-2 (RNA649 [5′-GUCAGAGUGUGUACUUG-3′; position 24649–24665 nt; accession number: NC_045512.2]) (Wu et al., 2020), and together with RNA524 (also derived from the spike protein of SARS-CoV-1) (Li et al., 2013) and RNA40 (Heil et al., 2004), we evaluated their ability to induce IL-1β production from primary human macrophages (see transparent methods). All three GU-rich RNAs induced the expression of pro-IL-1β and the release of mature IL-1β in a dose-dependent manner (Figures 1A and 1B). Of note, macrophages exposed to RNA from SARS-CoV-2 (RNA649) released significantly greater levels of IL-1β than RNAs from SARS-CoV-1 (p = 0.03) and HIV-1 (p = 0.04) (RNA649 > RNA524 > RNA40). Additionally, GU-rich RNA-mediated IL-1β secretion was evident in the absence of an additional exogenously added stimulus for the NLRP3 inflammasome such as what is required for lipopolysaccharide (LPS)-mediated IL-1β production (Gaidt et al., 2016). We observed a similar pattern for the release of TNF and IL-6, with RNA649 inducing the greatest amount of both cytokines (p < 0.05; Figure 1A). Importantly, neither RNA41 nor RNA649A (inactive derivatives of RNA40 and RNA524/649, respectively, in which adenosine replaces all uracil nucleotides) had any effect on either the expression or secretion of IL-1β, TNF, or IL-6. Moreover, in agreement with our previous studies, we observed no increase in cell death with any of the GU-rich RNAs (Figure 1C) (Campbell and Spector, 2012; Campbell et al., 2019).
Figure 1.
GU-rich RNAs induce the release of proinflammatory cytokines in the absence of pyroptosis through TLR8 signaling
(A–C) Macrophages were treated for 24 h with GU-rich RNA. GU-rich RNA sequences are shown in Table S1. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05. (A) Cell supernatants were collected and analyzed for cytokines. (B) Left, representative western blots of pro-IL-1β and ACTB. Right, densitometric analysis of blots. (C) Aliquots of supernatants were spectrophotometrically tested for LDH.
(D) Macrophages were transfected with UNC93B1 small interfering RNA (siRNA) (siUNC93B1), TLR7 siRNA (siTLR7), TLR8 (siTLR8), MYD88 (siMYD88), TICAM1 (siTICAM1), or scrambled siRNA (siNS) for 48 h then treated with 5 μg mL−1 GU-rich RNA or controls for 24 h. Supernatants were collected and analyzed for cytokine secretion. Silencing is shown in Figure S1. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
(E) Macrophages were pre-treated with 10 μM chloroquine (CQ) or vehicle control (vehicle) for 2 h and then treated for 24 h with 5 μg mL−1 GU-rich RNA. Cell supernatants were collected and analyzed for cytokines. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
As all human nucleic acid-specific TLRs (TLR3, 7, 8, and 9) are located in the endosomal compartment and rely upon UNC93B1 for their trafficking from the endoplasmic reticulum (ER) to the endosomes (Lee and Barton, 2014), we used RNAi for UNC93B1 (Figure S1) to confirm that viral GU-rich RNAs are recognized by endosomal TLRs. UNC93B1 silencing abolished GU-rich RNA-mediated production of IL-1β, TNF, and IL-6 (Figure 1D). We next investigated whether either of TLR7 or TLR8 was required for GU-rich RNA-mediated production of IL-1β, TNF, and IL-6 using RNAi for TLR7 and TLR8 (Figure S1). TLR8 silencing abolished GU-rich RNA-induced IL-1β, TNF, and IL-6 production from macrophages, whereas TLR7 silencing had no effect (Figure 1D). Additionally, RNAi for MYD88 (Figure S1) also attenuated IL-1β, TNF, and IL-6 production (Figure 1D). Conversely, RNAi for TICAM1 (toll-like receptor adaptor molecule 1; TRIF) had no effect on IL-1β, TNF, and IL-6 production, indicating that TLR3 is not involved in GU-rich RNA signaling. Thus the TLR8/MYD88 pathway is required for viral GU-rich RNA-induced IL-1β, TNF, and IL-6 expression from human macrophages similar to what was previously observed for RNA derived from the archaeon Methanosphaera stadtmanae in transdifferentiated BLaER1 cells (Vierbuchen et al., 2017).
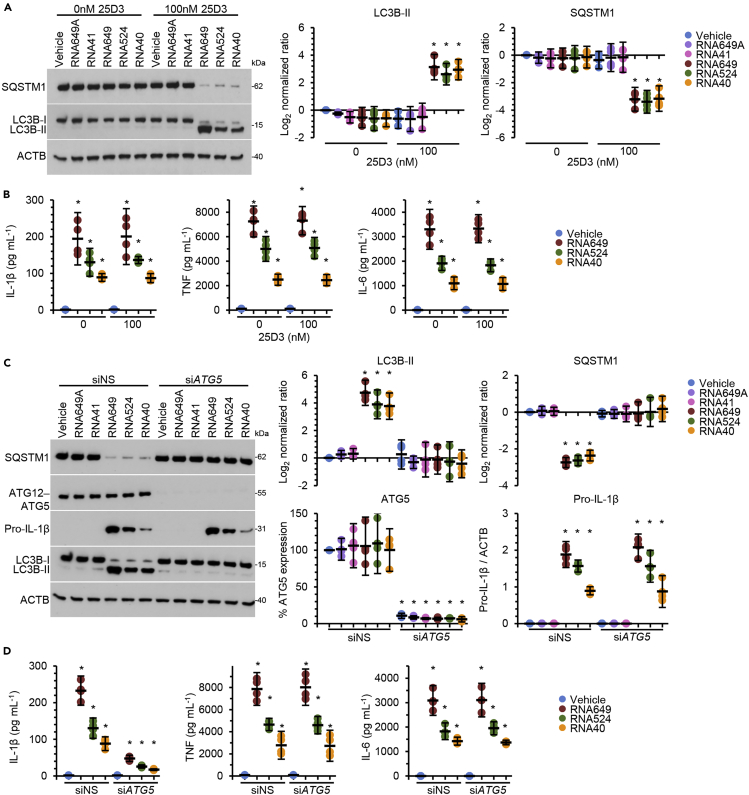
Preventing endosomal acidification inhibits endosomal TLR activation and signaling (Hart et al., 2005; Rutz et al., 2004). Therefore, we blocked endosomal acidification using chloroquine and observed that this inhibited GU-rich RNA-induced IL-1β, TNF, and IL-6 production (Figure 1E). However, chloroquine also blocks autophagic flux, and previous studies have demonstrated that autophagy is involved in both the AIM2-inflammasome- (Wang et al., 2014) and NLRP3-inflammasome-induced secretion of IL-1β (Dupont et al., 2011). Moreover, as both RNA40 and the imidazoquinoline CL097 promote autophagic responses in human macrophages through a TLR8-, beclin-1 (BECN1)-, and vitamin D3-dependent mechanism (Campbell and Spector, 2012), we investigated the role for autophagy in GU-rich RNA-mediated IL-1β release. Although we observed the suppression of GU-rich RNA-mediated autophagy under vitamin D3-deficient conditions in accordance with our previous data (Campbell and Spector, 2012) (Figure 2A), we observed no significant decrease in secreted IL-1β, TNF, and IL-6 from those cells indicating that cytokine secretion is not dependent on induced autophagy (Figure 2B). To address whether constitutive autophagic flux is required for IL-1β secretion, we silenced ATG5, which inhibits not only GU-rich RNA-mediated autophagy (Figure 2C) but also constitutive autophagy (Ye et al., 2018). Interestingly, ATG5 silencing markedly reduced the secretion of IL-1β induced by GU-rich RNA, but it did not impact pro-IL-1β synthesis or the release of TNF or IL-6 (Figures 2C and 2D), indicating that constitutive autophagy is required for IL-1β release from macrophages.
Figure 2.
GU-rich RNA-induced IL-1β secretion is dependent on autophagy
(A) Macrophages were treated for 24 h with 5 μg mL−1 GU-rich RNA in the presence or absence of vitamin D3 (calcifediol; 25-hydroxycholecalciferol [25D3]). Left, representative western blots of LC3B isoforms and SQSTM1. Right, densitometric analysis of blots. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
(B) Supernatants from cells in (A) were collected and analyzed for cytokines. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
(C) Macrophages transfected with ATG5 siRNA (siATG5) or scrambled siRNA (siNS) were treated for 24 h with 5 μg mL−1 GU-rich RNA in macrophage media. Left, representative western blots of LC3B isoforms, SQSTM1, pro-IL-1β, and ATG5. Right, densitometric analysis of blots. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
(D) Supernatants from cells in (C) were collected and analyzed for cytokines. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
SARS-CoV-2-, SARS-CoV-1-, and HIV-1-derived GU-rich RNA treatment of macrophages results in IL-1β release through the NLRP3 inflammasome
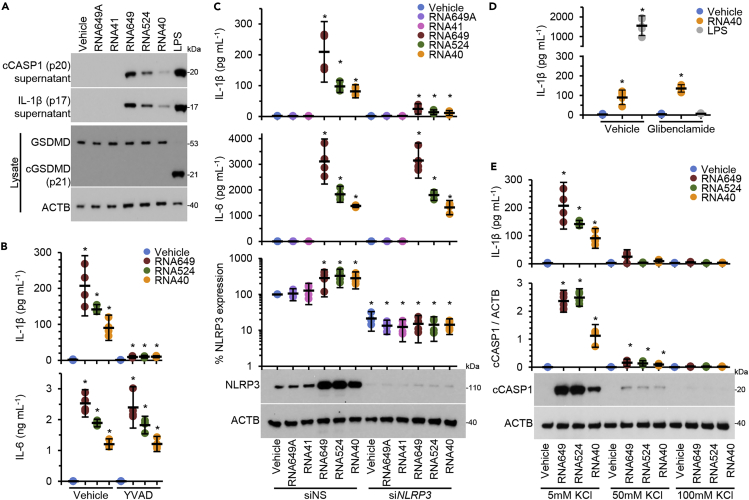
We detected CASP1 p20 and IL-1β p17 release in the supernatant of primary human macrophages after 24-h treatment with GU-rich RNA indicative of inflammasome activation and IL-1β processing and release (Figure 3A). To confirm a role for CASP1, we used Ac-YVAD-cmk, a specific inhibitor of CASP1. We show that Ac-YVAD-cmk completely suppressed IL-1β release induced by GU-rich RNA but had no effect on IL-6 release (Figure 3B). In addition to cleaving IL-1 family cytokines, active CASP1 cleaves the cytosolic protein gasdermin D (GSDMD). Upon cleavage, the N-terminal 30-kDa fragment of GSDMD forms a pore in the plasma membrane, which leads to lysis of the cell through pyroptosis (Shi et al., 2015). As lactate dehydrogenase (LDH) release is a marker of pyroptosis and we observed no increase in macrophage LDH release post-GU-rich RNA exposure (Figure 1C), we examined whether GU-rich RNA treatment of macrophages resulted in GSDMD processing. In contrast to LPS treatment, which triggers GSDMD-dependent release of IL-1β from human macrophages, GU-rich RNA exposure did not induce the processing of GSDMD to its 30-kDa fragment (Figure 3A).
Figure 3.
GU-rich RNAs activate the NLRP3 inflammasome
(A) Macrophages were treated for 24 h with 5 μg mL−1 GU-rich RNA. Cell supernatant was collected and cells lysed. Top, a representative western blot of extracellular cleaved CASP1 (cCASP1) and IL-1β. Bottom, a representative western blot of cleaved GSDMD (cGSDMD) and ACTB. n = 4.
(B) Macrophages were pre-treated with a CASP1 inhibitor (100 μM Ac-YVAD-cmk; YVAD) or vehicle control (vehicle) for 2 h. Macrophages were then treated for 24 h with 5 μg mL−1 GU-rich RNA. Cell supernatants were collected and analyzed for cytokines. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
(C) Macrophages were transfected with NLRP3 siRNA (siNLRP3) or scrambled siRNA (siNS). Macrophages were then treated for 24 h with GU-rich RNA and supernatants were collected and analyzed for cytokine secretion. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
(D) Macrophages were pre-treated with a 100 μM glibenclamide or vehicle control (vehicle) for 2 h. Macrophages were then treated for 24 h with 5 μg mL−1 GU-rich RNA or 10 ng mL−1 lipopolysaccharide (LPS) + 2.5 mM ATP. Cell supernatants were collected and analyzed for cytokines. Data are shown as a scatter plot with means ± 95% confidence interval. n = 6. ∗p < 0.05.
(E) Macrophages were cultured under different concentrations of KCl for 2 h. Macrophages were then treated for 24 h with 5 μg mL−1 GU-rich RNA under these same conditions. Top, cell supernatants were collected and analyzed for IL-1β. Bottom, cells were lysed and analyzed for cleaved CASP1 (cCASP1) by western blot. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
To identify the type of inflammasome activated by GU-rich RNA, we used RNAi to silence NLRP3. NLRP3 silencing abolished IL-1β, but not IL-6, release from GU-rich RNA-treated macrophages (Figure 3C), indicating that that GU-rich RNA activates the NLRP3 inflammasome in macrophages. As potassium efflux is a common feature of NLRP3 inflammasome activation (Muñoz-Planillo et al., 2013), we next applied glibenclamide, a sulfonylurea drug that acts upstream of NLRP3 and downstream of the P2X7 receptor, a purinergic type 2 ligand-gated ion channel that allows the passage of molecules of up to 900 Da, Na+ and Ca2+ influx and K+ efflux, and is used to inhibit LPS-induced NLRP3 inflammasome-mediated CASP1 activation, secretion of IL-1β, and pyroptosis in human macrophages (Lamkanfi et al., 2009). Glibenclamide had no effect on reducing RNA40-mediated IL-1β secretion despite severely attenuating the LPS-stimulated release of IL-1β (Figure 3D). We next applied an extracellular high potassium solution (KCl), which directly inhibits K+ efflux (Muñoz-Planillo et al., 2013). We observed a dose-dependent inhibition of GU-rich RNA-mediated IL-1β release and CASP1 cleavage in macrophages pre-treated with KCl (Figure 3E). These data indicate that K⁺ efflux is necessary and sufficient for GU-rich RNA-mediated CASP1 activation and IL-1β release from macrophages, and that it is independent of the glibenclamide-sensitive K+ efflux.
The alternative inflammasome pathway is also involved in GU-rich RNA NLRP3-mediated release of IL-1β from macrophages
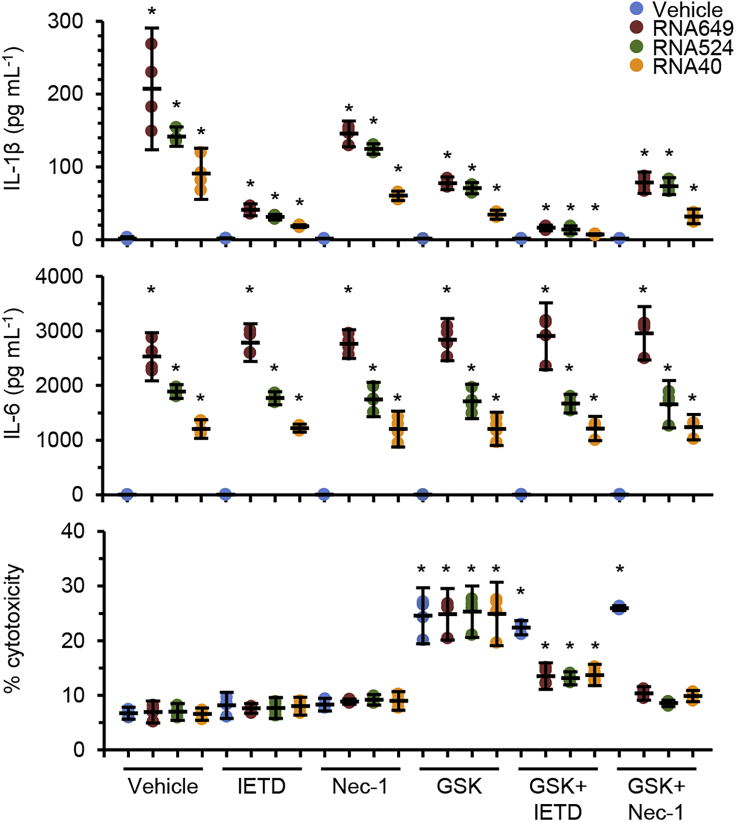
As GU-rich RNA-induced IL-1β processing and secretion is devoid of the classic NLRP3 inflammasome characteristic of pyroptosis and is released from living cells, and as LPS triggers an alternative NLRP3 inflammasome activation pathway in human monocytes in the absence of a secondary stimulus of ATP K+ efflux (Gaidt et al., 2016), we investigated the possibility that the alternative pathway using necroptotic mediators may play a role in GU-rich RNA-induced IL-1β secretion from human macrophages using inhibitors for CASP8 (Z-IETD-FMK), RIPK1 (necrostatin-1) and RIPK3 (GSK′872). Inhibition of CASP8 decreased GU-rich RNA-induced IL-1β without inducing cell death (Figure 4). Inhibition of RIPK1 had no significant reduction of GU-rich RNA-induced IL-1β, whereas inhibition of RIPK3 increased cell death and inhibited GU-rich RNA-induced IL-1β. When we co-treated macrophages with Z-IETD-FMK and GSK′872, we observed no change in cell death in the vehicle-treated macrophages, but in the GU-rich RNA-treated cells, we observed a significant reduction in both cell death and IL-1β production. Of note, no combination of these inhibitors perturbed GU-rich RNA-stimulated IL-6 production (Figure 4). Collectively, these data indicate that, unlike LPS in monocytes, GU-rich RNA-stimulated macrophages do not respond with necroptotic protein-driven inflammasome activation upon CASP8 perturbation.
Figure 4.
GU-rich RNA activation of the NLRP3 inflammasome involves the alternative pathway
Macrophages were pre-treated with inhibitors for CASP8 (10 μM Z-IETD-FMK; IETD), RIPK1 (10 μM necrostatin-1; Nec-1), or RIPK3 (5 μM GSK′872) or vehicle control (vehicle) for 2 h. Macrophages were then treated for 24 h with 5 μg mL−1 GU-rich RNA. Cell supernatants were collected and analyzed for cytokines and LDH. Data are shown as scatter plots with means ± 95% confidence interval. n = 4. ∗p < 0.05.
Discussion
Classical modes of LPS-mediated NLRP3 activation (canonical and non-canonical) in murine macrophages comprise a priming step that enables NLRP3 activation (signal 1) followed by a K+ efflux-dependent activation signal (signal 2) that triggers lytic cell death in the form of pyroptosis, necessary for IL-1β release (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015), whereas in human monocytes, LPS can induce the transcription and release of IL-1β in the absence of the K+ efflux signal 2. Named the “alternative inflammasome” (Gaidt et al., 2016), it relies upon TLR4-TICAM1-RIPK1-FADD-CASP8 to activate NLRP3 and secrete IL-1β in the absence of pyroptosis. In the present study, we demonstrate that in addition to inducing the transcription and release of pro-inflammatory cytokines IL-6 and TNF, GU-rich RNA from SARS-CoV-2, SARS-CoV-1, and HIV-1 all activate the NLRP3 inflammasome and induce the transcription and release of IL-1β. Moreover, RNA derived from the spike protein of SARS-CoV-2 induces the greatest inflammatory response. Although we cannot rule out the contribution of other inflammasomes, GU-rich RNA-mediated secretion of IL-1β is completely lost in the NLRP3-silenced cells, indicating an NLRP3-dependent pathway. With the use of inhibitors and RNAi, we also demonstrate that activation of the NLRP3 inflammasome by GU-rich RNA is specifically TLR8-dependent and RNA-mediated, leading to CASP1 activation and the conversion of pro-IL-1β to its active form. Furthermore, we demonstrate that this mechanism proceeds in a CASP8- and K+ efflux-dependent mechanism that is independent of TICAM1 and pyroptosis. Thus we have identified that components of both the non-canonical pathway and the alternative activation pathway are involved in GU-rich RNA-mediated IL-1β release from human macrophages.
Demonstrating the species- and ligand-specific role and impact on IL-1β secretion, the TLR7/8 agonists imiquimod and CL097 both activate the NLRP3 inflammasome in LPS-primed murine bone-marrow-derived dendritic cells independent of K+ efflux and induce all the classical downstream effects including PYCARD oligomerization, GSDMD-driven pyroptosis, and secretion of IL-1β (Groβ et al., 2016). This specificity extends to the virus species and strain from whence we obtain the GU-rich RNA. Specifically, we observed that both betacoronavirus-derived GU-rich RNA induced significantly more IL-1β, IL-6, and TNF than that induced by the well-studied RNA40 derived from HIV-1. More interestingly, we also observed that within the species of severe acute respiratory syndrome-related coronavirus, the GU-rich RNA derived from SARS-CoV-2 was more inflammatory than that derived from SARS-CoV-1. There are a number of variables to consider when comparing these single-stranded RNAs, including the difference in length (the coronavirus-derived RNAs are 17-mers, whereas the HIV-1-derived RNA is a 20-mer) and sequence, but the differences are, nonetheless, interesting considering the different pathologies. In both COVID-19 and SARS, there is associated lung damage believed to be the result of a fulminant and sustained virally triggered inflammatory reaction mediated by a host of cytokines including IL-1β, IL-6, and TNF leading to alveolar infiltration by macrophages and monocytes (Hsueh et al., 2004; Ye et al., 2020; Zhang et al., 2020). In sicker patients, the levels of these pro-inflammatory cytokines are higher and lead to a potent inflammatory response, respiratory decompensation, and multi-organ failure that can lead to death (Herold et al., 2020; Ng et al., 2004). The overwhelming inflammatory response has led investigators to examine the potential beneficial immunomodulatory effects of vitamin D and the cholinergic pathway (Farsalinos et al., 2020; Turrubiates-Hernández et al., 2021).
It was previously thought that IL-1β release and pyroptosis could not be uncoupled (Cullen et al., 2015; Evavold et al., 2018). However, we observe IL-1β release from live, metabolically active cells following CASP1 activation, similar to previous studies (Conos et al., 2016; Gaidt et al., 2016). In response to both GU-rich RNA and HIV-1-infection, human macrophages upregulate their expression of TREM1, which is essential for macrophage survival under these conditions (Campbell et al., 2019; Yuan et al., 2017). It is thus possible that human macrophages are intrinsically refractory to GU-rich RNA-induced cell death due to TREM1 upregulation, a phenomenon consistent with the abundance of monocyte-derived macrophages in the bronchoalveolar lavage of patients with severe COVID-19 (Liao et al., 2020) and macrophage survival during HIV-1-infection, in contrast to the massive cell death observed in HIV-1-infected CD4+ T cells (Van Lint et al., 2013).
Considering that inflammation and hyper-immune activation are markers for HIV-1 pathogenesis, the study of inflammasomes is still a relatively new field of study in HIV-1 research (Paiardini and Müller-Trutwin, 2013; Sereti and Altfeld, 2016). Despite this, there is emerging evidence for NLRP3 involvement in HIV-1 pathogenesis as (1) NLRP3 may promote HIV-1 disease progression during gut-associated lymphoid tissue (GALT) destruction as its overexpression is speculated to favor HIV-1 replication and cell death (Feria et al., 2018); (2) single-nucleotide polymorphisms in NLRP3 increase susceptibility to HIV-1 infection (Zhang et al., 2015); (3) the expression of NLRP3 inflammasome-associated genes are upregulated in the brains of HIV-1-infected patients (Walsh et al., 2014); and (4) the NLRP3 inflammasome is a driver of HIV-1-mediated cardiovascular disease (Kearns et al., 2019). Moreover, productive HIV-1 infection is required for NLRP3 inflammasome activation as antiretroviral therapy decreases the transcription and release of IL-1β (Guo et al., 2014).
In summary, GU-rich RNA derived from SARS-CoV-2, SARS-CoV-1, and HIV-1 induce the rapid induction of IL-6, TNF, and IL-1β from human primary macrophages in the absence of viral infection. The IL-1β release in response to these GU-rich RNA is dependent upon NLRP3 with K+ efflux acting as signal 2. These data suggest that TLR8 and and/or NLRP3 may prove to be excellent therapeutic targets to ameliorate SARS-CoV-2- and HIV-1-associated chronic inflammation.
Limitations of the study
In this study, we demonstrate that viral GU-rich RNA oligonucleotides elicit the expression and maturation of IL-1β from human macrophages through the TLR8-dependent activation of the NLRP3 inflammasome in the absence of pyroptosis. The major limitation of the research presented is that we have not demonstrated that TLR8 is triggered during active viral infection. However, we have previously shown that exposure of human macrophages to purified live, heat-inactivated, or RNase/DNase I/2,2′-dithiodipyridine-treated (which inactivates the infectivity of retroviruses by covalently modifying the nucleocapsid zinc finger motifs) HIV-1 induces TLR8-dependent transcription factor EB nuclear translocation and autophagy similar to what we observe using LyoVec-complexed RNA40 (Campbell et al., 2015). Thus, it is likely that SARS-CoV-2 and SARS-CoV-1 trigger TLR8 signaling and NLRP3 inflammasome activation during active infection.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Grant Campbell (gcampbell@ucsd.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze datasets/code.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
This work was supported, in whole or in part, by the National Institute of Neurological Disorders and Stroke of the NIH (www.ninds.nih.gov) (R01 NS104015 to S.A.S.), and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (impaactnetwork.org). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network is provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Conceptualization, G.R.C., R.K.T., and S.A.S.; methodology, G.R.C., R.K.T., and S.A.S.; investigation, G.R.C., R.K.T., and J.H.; validation, G.R.C., R.K.T., and J.H.; formal analysis, G.R.C., R.K.T., J.H., and S.A.S.; writing – original draft, G.R.C., R.K.T., and S.A.S.; writing – review & editing, G.R.C. and S.A.S.; visualization, G.R.C.; funding acquisition, S.A.S.; resources, S.A.S.; supervision, G.R.C. and S.A.S.
Declaration of interests
The authors declare no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102295.
Contributor Information
Grant R. Campbell, Email: gcampbell@ucsd.edu.
Stephen A. Spector, Email: saspector@ucsd.edu.
Supplemental information
References
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G.R., Spector S.A. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8:e1003017. doi: 10.1371/journal.ppat.1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G.R., Rawat P., Bruckman R.S., Spector S.A. Human immunodeficiency virus type 1 Nef inhibits autophagy through transcription factor EB sequestration. PLoS Pathog. 2015;11:e1005018. doi: 10.1371/journal.ppat.1005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G.R., To R.K., Spector S.A. TREM-1 protects HIV-1-infected macrophages from apoptosis through maintenance of mitochondrial function. mBio. 2019;10 doi: 10.1128/mBio.02638-19. e02638–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfamo M., Le Saout C., Lane H.C. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev. 2012;23:207–214. doi: 10.1016/j.cytogfr.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes J.L., La Vake C.J., Weinerman B., Luu S., O'Connell C., Verardi P.H., Salazar J.C. Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes. J. Leukoc. Biol. 2013;94:1231–1241. doi: 10.1189/jlb.0413206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conos S.A., Lawlor K.E., Vaux D.L., Vince J.E., Lindqvist L.M. Cell death is not essential for caspase-1-mediated interleukin-1β activation and secretion. Cell Death Differ. 2016;23:1827–1838. doi: 10.1038/cdd.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S.P., Kearney C.J., Clancy D.M., Martin S.J. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Rep. 2015;11:1535–1548. doi: 10.1016/j.celrep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Dupont N., Jiang S., Pilli M., Ornatowski W., Bhattacharya D., Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011;30:4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E.I., Sutterwala F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44.e36. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Eliopoulos E., Leonidas D.D., Papadopoulos G.E., Tzartos S., Poulas K. Nicotinic cholinergic system and COVID-19: in silico identification of an interaction between SARS-CoV-2 and nicotinic receptors with potential therapeutic targeting implications. Int. J. Mol. Sci. 2020;21:5807. doi: 10.3390/ijms21165807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feria M.G., Taborda N.A., Hernández J.C., Rugeles M.T. HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS One. 2018;13:e0192845. doi: 10.1371/journal.pone.0192845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt M.M., Ebert T.S., Chauhan D., Schmidt T., Schmid-Burgk J.L., Rapino F., Robertson A.A., Cooper M.A., Graf T., Hornung V. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Ghosh T.K., Mickelson D.J., Fink J., Solberg J.C., Inglefield J.R., Hook D., Gupta S.K., Gibson S., Alkan S.S. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Groß C.J., Mishra R., Schneider K.S., Médard G., Wettmarshausen J., Dittlein D.C., Shi H., Gorka O., Koenig P.A., Fromm S. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45:761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Guo H., Gao J., Taxman D.J., Ting J.P., Su L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014;289:21716–21726. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.M., Athié-Morales V., O'Connor G.M., Gardiner C.M. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-γ production. J. Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.H., Zhong C.Q., Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh P.R., Chen P.J., Hsiao C.H., Yeh S.H., Cheng W.C., Wang J.L., Chiang B.L., Chang S.C., Chang F.Y., Wong W.W. Patient data, early SARS epidemic, Taiwan. Emerg. Infect. Dis. 2004;10:489–493. doi: 10.3201/eid1003.030571. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Kearns A.C., Liu F., Dai S., Robinson J.A., Kiernan E., Tesfaye Cheru L., Peng X., Gordon J., Morgello S., Abuova A. Caspase-1 activation is related with HIV-associated atherosclerosis in an HIV transgenic mouse model and HIV patient cohort. Arterioscler. Thromb. Vasc. Biol. 2019;39:1762–1775. doi: 10.1161/ATVBAHA.119.312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M., Mueller J.L., Vitari A.C., Misaghi S., Fedorova A., Deshayes K., Lee W.P., Hoffman H.M., Dixit V.M. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.L., Barton G.M. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014;24:360–369. doi: 10.1016/j.tcb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen M., Cao H., Zhu Y., Zheng J., Zhou H. Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013;15:88–95. doi: 10.1016/j.micinf.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Moen S.H., Ehrnström B., Kojen J.F., Yurchenko M., Beckwith K.S., Afset J.E., Damås J.K., Hu Z., Yin H., Espevik T. Human toll-like receptor 8 (TLR8) is an important sensor of pyogenic bacteria, and is attenuated by cell surface TLR signaling. Front. Immunol. 2019;10:1209. doi: 10.3389/fimmu.2019.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B.L., Rajendiran T.M., Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P.C., Lam C.W., Li A.M., Wong C.K., Cheng F.W., Leung T.F., Hon E.K., Chan I.H., Li C.K., Fung K.S. Inflammatory cytokine profile in children with severe acute respiratory syndrome. Pediatrics. 2004;113:e7–e14. doi: 10.1542/peds.113.1.e7. [DOI] [PubMed] [Google Scholar]
- Paiardini M., Müller-Trutwin M. HIV-associated chronic immune activation. Immunol. Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz M., Metzger J., Gellert T., Luppa P., Lipford G.B., Wagner H., Bauer S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- Sereti I., Altfeld M. Immune activation and HIV: an enduring relationship. Curr. Opin. HIV AIDS. 2016;11:129–130. doi: 10.1097/COH.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Sutterwala F.S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G.S., Grant E.P., Bertin J., Coyle A.J., Galan J.E., Askenase P.W. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Turrubiates-Hernández F.J., Sánchez-Zuno G.A., González-Estevez G., Hernández-Bello J., Macedo-Ojeda G., Muñoz-Valle J.F. Potential immunomodulatory effects of vitamin D in the prevention of severe coronavirus disease 2019: an ally for Latin America (Review) Int. J. Mol. Med. 2021;47:32. doi: 10.3892/ijmm.2021.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint C., Bouchat S., Marcello A. HIV-1 transcription and latency: an update. Retrovirology. 2013;10:67. doi: 10.1186/1742-4690-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T., Bang C., Rosigkeit H., Schmitz R.A., Heine H. The human-associated archaeon Methanosphaera stadtmanae is recognized through its RNA and induces TLR8-dependent NLRP3 inflammasome activation. Front. Immunol. 2017;8:1535. doi: 10.3389/fimmu.2017.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J.G., Reinke S.N., Mamik M.K., McKenzie B.A., Maingat F., Branton W.G., Broadhurst D.I., Power C. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. 2014;11:35. doi: 10.1186/1742-4690-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.J., Huang H.Y., Huang M.P., Liou W., Chang Y.T., Wu C.C., Ojcius D.M., Chang Y.S. The microtubule-associated protein EB1 links AIM2 inflammasomes with autophagy-dependent secretion. J. Biol. Chem. 2014;289:29322–29333. doi: 10.1074/jbc.M114.559153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Zhou X.J., Zhang H. Exploring the role of autophagy-related gene 5 (ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front Immunol. 2018;9:2334. doi: 10.3389/fimmu.2018.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Fan X., Staitieh B., Bedi C., Spearman P., Guidot D.M., Sadikot R.T. HIV-related proteins prolong macrophage survival through induction of triggering receptor expressed on myeloid cells-1. Sci. Rep. 2017;7:42028. doi: 10.1038/srep42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Fan H.W., Zhang J.Z., Wang Y.M., Xing H.J. NLRP3 rs35829419 polymorphism is associated with increased susceptibility to multiple diseases in humans. Genet. Mol. Res. 2015;14:13968–13980. doi: 10.4238/2015.October.29.17. [DOI] [PubMed] [Google Scholar]
- Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand. J. Clin. Lab Invest. 2020;6:441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Brownlie R., Liu Q., Babiuk L.A., Potter A., Mutwiri G.K. Characterization of bovine Toll-like receptor 8: ligand specificity, signaling essential sites and dimerization. Mol. Immunol. 2009;46:978–990. doi: 10.1016/j.molimm.2008.09.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.