Abstract
Background
Cerebral microbleeds are increasingly reported in critical ill patients with respiratory failure in need of mechanical ventilation and/or extracorporeal membrane oxygenation (ECMO). Typically, these critical illness-associated microbleeds involve the juxtacortical white matter and corpus callosum. Recently, this pattern was reported in patients with respiratory failure, suffering from COVID-19.
Materials and methods
In this retrospective single-center study, we listed patients from March 11, 2020 to September 2, 2020, with laboratory-confirmed COVID-19, critical illness and cerebral microbleeds. Literature research was conducted through a methodical search on Pubmed databases on critical illness-associated microbleeds and cerebral microbleeds described in patients with COVID-19.
Results and discussion
On 279 COVID-19 admissions, two cases of cerebral microbleeds were detected in critical ill patients with respiratory failure due to COVID-19. Based on review of existing literature critical illness-associated microbleeds tend to predominate in subcortical white matter and corpus callosum. Cerebral microbleeds in patients with COVID-19 tend to follow similar patterns as reported in critical illness-associated microbleeds. Hence, one patient with typical critical illness-associated microbleeds and COVID-19 is reported. However, a new pattern of widespread cortico-juxtacortical microbleeds, predominantly in the anterior vascular territory with relative sparing of deep gray matter, corpus callosum and infratentorial structures is documented in a second case. The possible etiologies of these microbleeds include hypoxia, hemorrhagic diathesis, brain endothelial erythrophagocytosis and/or cytokinopathies. An association with COVID-19 remains to be determined.
Conclusion
Further systematic investigation of microbleed patterns in patients with neurological impairment and COVID-19 is necessary.
Keywords: Microbleed, Critical care, Respiratory failure, COVID-19, Extra-corporeal membrane oxygenation
1. Introduction
Chronic hypertension, cerebral amyloid angiopathy and diffuse axonal injury are well acknowledged causes of cerebral microbleeds [1]. Increasingly, microbleeds have been reported in patients with critical illness associated with respiratory failure [1]. Only recently, microbleeds are reported in patients with Covid-19 [2]. We report two cases of cerebral microbleeds in patients diagnosed with COVID-19 with respiratory failure and provide a review of literature.
2. Materials and methods
2.1. Data collection
From March 11, 2020 until September 2, 2020 a total amount of 279 patients with COVID-19 were admitted in University Hospitals Ghent of which 34 patients died. During this period cerebral microbleeds were detected in two critical ill patients suffering from COVID-19.
2.1.1. Case 1
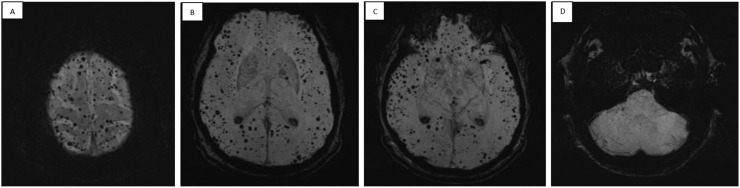
A 54-year-old male patient was admitted to the intensive care unit (ICU) because of respiratory failure with need for mechanical ventilation, due to COVID-19 pneumonia. He developed severe acute respiratory distress syndrome (ARDS) with respiratory failure for which veno-venous extracorporeal membrane oxygenation (ECMO) was initiated. After 19 days the patient was successfully weaned from ECMO. Limited strength in the right arm was noticed and magnetic resonance imaging (MRI) of the brain was performed. Fluid-attenuated inversion recovery (FLAIR) imaging showed multiple white matter hyperintensities and mild brain atrophy. There was no restricted diffusion, excluding acute cerebral ischemia. Susceptibility weighted imaging (SWI) showed multiple hypo-intense lesions located in the corpus callosum, compatible with microbleeds and not corresponding to the hyperintensities seen on FLAIR ( Fig. 1). Nerve conduction studies showed symmetric decreased compound motor action potentials and sensory nerve action potentials, suggestive for critical illness sensorimotor polyneuropathy. An electromyography (EMG) disclosed a superimposed posterior cord lesion of the brachial plexus; explaining the clinical characteristics of this patient, probably secondary to prone ventilation..
Fig. 1.
Maximal intensity projection (MIP) of susceptibility weighted imaging (SWI) showing remarkable hypo-intense lesions located in the corpus callosum, compatible with microbleeds.
2.1.2. Case 2
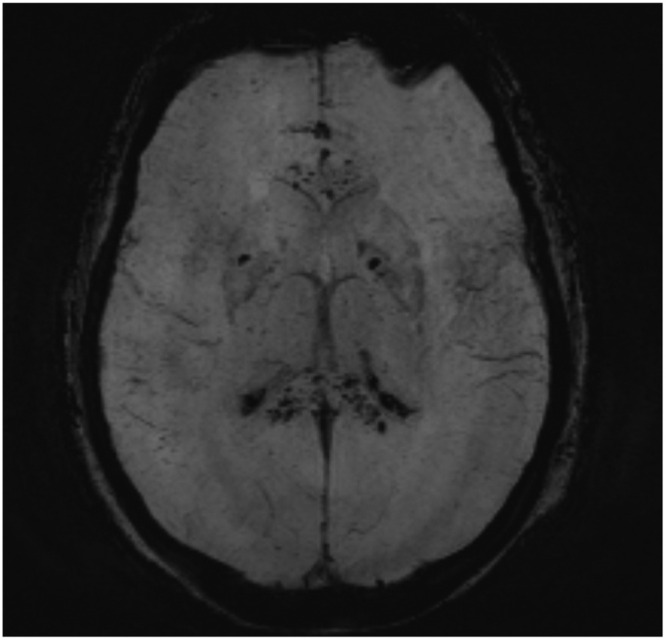
A 72-year-old male patient without any relevant medical history was diagnosed with COVID-19. He developed respiratory insufficiency, requiring mechanical ventilation. Because of delayed awakening, neurologic evaluation was warranted. Clinical neurologic examination demonstrated a Glasgow Coma Scale (GCS) of 5/15 (E1M3V1), symmetric exaggerated tendon reflexes over the four limbs, normal plantar reflexes, slowed vestibulo-oculocephalic reflexes and miotic isocoric pupils reactive to light. Because of the clinical features, the use of long-acting sedative drugs, acute kidney failure and high probability of accumulation of sedative drugs and a high bilirubinemia (total bilirubin 10.2 mg/dL [reference value: 0.2–1.1 mg/dL], direct bilirubin 7.96 mg/dL [reference value: < 0.45 mg/dL]) a toxic-metabolic and/or septic etiology of the clinical presentation of encephalopathy was suspected. EEG showed diffuse slowing of the background rhythm without epileptiform elements. Cerebrospinal fluid (CSF) was normal, except for elevated bilirubin. Additional brain MRI showed no restricted diffusion and limited microvascular FLAIR-hyperintensities, predominantly in the parieto-occipital white matter. T2-imaging showed enlarged perivascular spaces in the lenticulostriate territory and some minor cortical T2-hyperintensities. SWI showed abundant cortico-juxtacortical hypointensities compatible with diffuse microbleeds, and a remarkable posterior-anterior gradient with relative sparing of infratentorial structures, deep gray matter and corpus callosum ( Fig. 2). Four weeks later, a subtle increase of T2-hyperintensities surrounding cortical microbleeds was found in absence of restricted diffusion..
Fig. 2.
Maximal intensity projection (MIP) of susceptibility weighted imaging (SWI) showing diffuse cortico-juxtacortical hypo-intense lesions compatible with microbleeds, predominantly in the anterior vascular territory with relative sparing of deep gray matter, corpus callosum and infratentorial structures.
2.2. Literature research
A bibliographic Pubmed search was conducted using the keywords ‘critical illness’, ‘COVID-19′, ‘cerebral microbleeds’ and ‘cerebral microhemorrhages’. Case reports and case series describing patients with cerebral microbleeds were included. Also, one general review on the topic of cerebral microbleeds was selected [3]. Articles discussing stroke, macrobleeds, subarachnoid hemorrhage or other vascular pathology occurring in patients with COVID-19 were discarded. The ongoing nature of this pandemic led to continuous monitoring of new publications and updating of our literature list. We concluded our literature search on October 11, 2020.
3. Results and discussion
Cerebral microbleeds are a well-known consequence of diffuse axonal injury and small vessel disease due to chronic hypertension or cerebral amyloid angiopathy (CAA) [1]. Particular regions in the brain are affected predominantly in relation to different etiologies. Microbleeds due to arterial hypertension are most often seen in the basal ganglia, thalamus, brainstem and cerebellum, whereas parieto-occipital cortical microbleeds predominate in cerebral amyloid angiopathy [3].
More recently, microbleeds have been reported in critically ill patients [1], [4], [5], [6]. Based on our literature search we found 6 articles relating critical illness-associated microbleeds [1], [4], [5], [6], [7], [8]. In 2015, Riech et al. described 3 patients with severe ARDS with 2 of them receiving ECMO [6]. In all 3 patients, brain MRI showed microhemorrhages, particularly in the splenium of the corpus callosum [6]. In 2017, Fanou et al. published a case series of 12 patients who underwent brain MRI during or immediately after an ICU-admission [1]. Respiratory failure was present in all cases; 11 patients received mechanical ventilation and 3 patients received ECMO [1]. Brain MRI in all 12 patients showed extensive microbleeds, diffusely involving the juxtacortical white matter and corpus callosum, sparing the cortex, deep and periventricular white matter, basal ganglia and thalami [1]. Kuo reported a pattern of cerebral microbleeds, predominantly in the corpus callosum and juxtacortical white matter and to a lesser extent in the internal capsule in a patient who presented with a pneumonia, followed by respiratory failure requiring mechanical ventilation [7]. More specifically in the context of ECMO, Shah et al. reported a pattern of microhemorrhages predominantly in the corpus callosum [5]. In contrast to the cases presented above, these microbleeds were accompanied by corresponding FLAIR-hyperintensities [5]. Riech et al. found that microbleeds with emphasis in the corpus callosum were present in 30% of patients who suffered from severe ARDS requiring ECMO and underwent brain MRI [8]. Establishing the etiopathogenesis of cerebral microbleeds in critically ill patients often is hampered by the lack of previous brain imaging. Interestingly, Hall et al. presented a patient who developed hypoxia and ARDS during hospitalization in whom multiple microhemorrhages were discovered that were not present on brain MRI at admission, suggesting a relationship with the clinical presentation of respiratory failure [4].
De Stefano et al. were the first to report a case of critical illness-associated cerebral microbleeds in the context of COVID-19, present in juxtacortical white matter, corpus callosum and internal capsule without any ischemic or necrotizing lesion [2]. A similar pattern has recently been described in critical ill patients with COVID-19 in different cases and case series, based on our literature search. [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. The number of microbleeds varied from a handful to innumerable and involved the juxtacortical white matter and corpus callosum [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. In their retrospective study, Kremer et al. found that extensive and isolated microbleeds were present in 9 of the 37 patients with COVID-19 and abnormal brain MRI’s (with exclusion of ischemic infarcts), mainly affecting the juxtacortical white matter, corpus callosum, internal capsule and cerebellar peduncles [17]. All 9 patients suffered from serious ARDS [17]. One retrospective study reports an increased mortality and worse functional outcome in patients with microbleeds and COVID-19 [18].
In our first case, a similar pattern of cerebral microbleeds was found following MRI brain imaging, adding a case of critical illness-associated microbleeds to the existing literature, particularly in COVID-19-related ARDS.
The pathogenesis of this condition remains unclear, but is thought to be related to hypoxia, as a similar pattern of microbleeds has been reported in patients with high-altitude cerebral edema [6], [19], [20]. Hackett et al. analyzed cerebral imaging of 8 patients with high-altitude cerebral edema and found extensive microbleeds mainly involving the corpus callosum and subcortical white matter [19]. The similarity with critical illness-associated microbleeds suggest a common pathogenesis, with hypoxia as a common factor [1], [19], [21]. One hypothesis suggests that hypoxemia leads to increased blood flow, which might exceed venous resistance and contribute to capillary stress, inducing a leak in the blood-brain barrier [6]. However, the continuous heparin infusion in patients treated with ECMO might cause a hemorrhagic diathesis and contribute to the pathogenesis of these microbleeds [5], [6].
It remains unclear why microbleeds in ARDS are accentuated at the corpus callosum [5], [17]. In high-altitude cerebral edema, the associated T2- and FLAIR-hyperintensities and restricted diffusion are explained by the known vulnerability of the corpus callosum and particularly the splenium to cytokinopathy, due to a higher density of cytokine, glutamate, toxin, and other excitatory amino acid receptors, leading to a tendency for cytotoxic edema of the corpus callosum when cytokinopathy occurs [1], [22]. However, in context of ARDS – as in our case – no edema was found on FLAIR-, T2- or diffusion-weighted images [1].
The differential diagnosis of our second case has been challenging, because of absence of previous brain imaging. Pre-existent alterations not related to the current episode might be a possible explanation. He had no history of chronic hypertension, head trauma, high-altitude exposure or brain radiation therapy. For pre-existing lesions cerebral microangiopathy seems to be possible given the concomitant presence of limited white matter hyperintensities with a posterior predominance and enlarged perivascular spaces on T2-imaging. According to the Boston-criteria for CAA, there is a lack of clinical suspicion for a diagnosis of a probable CAA [23]. Of note is the absence of lobar hemorrhage, cortical superficial siderosis and the relatively sparing of occipital lobe, features one might expect in case of CAA given the abundant lesion load. However, a subclinical presentation of sporadic CAA or other microvascular genetic pathology cannot be ruled out completely.
Given the peculiar predilection site of the microbleeds, one might consider these lesions not to be a pre-existent finding. Microbleeds might be secondary to disseminated intravascular coagulation (DIC) or a hemorrhagic diathesis [24]. In this case there was no evidence for thrombocytopenia or thrombotic micro-angiopathy (TMA), nor did the patient meet the criteria for DIC. A hemorrhagic diathesis could not be excluded given the dosing of low molecular weight heparin at 1 mg/kg divided over two daily doses. An etiopathologic relation with COVID-19 or an indirect pathophysiological mechanism through cytokine release could not be documented but remains a possibility, as the subtle increase of T2-hyperintensities surrounding cortical microbleeds on follow up imaging suggests an active pathology.
Alternatively, brain endothelial erythrophagocytosis in the context of oxidative stress across brain endothelium, causing translocation of iron-rich red blood cells or degradation products across brain endothelium might be a promising concept to be considered regarding the development of imaging features of microbleeds without disruption of microvasculature and thus posing the idea of pseudo-microbleeds [25]. Infection has been described as a possible trigger for erythrophagocytosis, but it needs to be determined whether this might be elicited by Covid-19 [26].
Our second case is the first case reporting a pattern of widespread cortico-juxtacortical microbleeds, predominantly in the anterior vascular territory with relative sparing of deep gray matter, corpus callosum and infratentorial structures and not meeting the clinical characteristics for CAA [23].
In conclusion, the clinical relevance of critical illness-associated microbleeds is currently unknown and remains to be elucidated. Further research and systematic investigation of microbleeds, their patterns and their clinical impact in patients with neurological impairment and COVID-19 is necessary.
Financial support
None.
Potential conflicts of interest
Nothing to report.
Integrity of research and reporting
Patients or family of the patients gave orally their informed consent prior to the inclusion of this study for the collection of data following their admission at the intensive care department.
CRediT authorship contribution statement
Jonas Toeback: Conception, first and third draft, editing and approval of the final draft. Sofie DR Depoortere: second draft, editing and approval of the final draft. Joris Vermassen: editing and approval of the final draft. Elke LH Vereecke: editing and approval of the final draft. Veroniek Van Driessche: editing and approval of the final draft. Dimitri M Hemelsoet: editing of first, second and third draft and approval of the final draft.
References
- 1.Fanou E.M., Coutinho J.M., Shannon P., Kiehl T.R., Levi M.M., Wilcox M.E., Aviv R.I., Mandell D.M. Critical illness-associated cerebral microbleeds. Stroke. 2017;48(4):1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 2.De Stefano P., Nencha U., De Stefano L., Mégevand P., Seeck M. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Clin. Neurophysiol. Pract. 2020;5:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouw A.A., Seewann A., van der Flier W.M., Barkhof F., Rozemuller A.M., Scheltens P., Geurts J.J.G. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry. 2011;82(2):126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 4.Hall J.P., Minhas P., Kontzialis M., Jhaveri M.D. Teaching neuroImages: distinct brain microhemorrhage pattern in critical illness associated with respiratory failure. Neurology. 2018;90(22) doi: 10.1212/WNL.0000000000005609. [DOI] [PubMed] [Google Scholar]
- 5.Shah J., Armstrong M.J. Extracorporeal membrane oxygenation: uncommon cause of corpus callosal microhemorrhage. Neurology. 2015;84(6):630. doi: 10.1212/WNL.0000000000001227. [DOI] [PubMed] [Google Scholar]
- 6.Riech S., Kallenberg K., Moerer O., Hellen P., Bärtsch P., Quintel M., Knauth M. The pattern of brain microhemorrhages after severe lung failure resembles the one seen in high-altitude cerebral edema. Crit. Care Med. 2015;43(9):e386–e389. doi: 10.1097/CCM.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 7.Kuo K.H., Yu Y.B., Lai T.H. Cerebral microbleeds associated with critical illness. Acta Neurol. Taiwan. 2019;28(1):25–26. [PubMed] [Google Scholar]
- 8.Riech S., Hellen P., Moerer O., Kallenberg K., Müller M., Quintel M., Knauth M. Microhemorrhages in the corpus callosum after treatment with extracorporeal membrane oxygenation. Crit. Care. 2015;19(Suppl 1):P277. [Google Scholar]
- 9.Cannac O., Martinez-Almoyna L., Hraiech S. Critical illness-associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology. 2020;95(11):498–499. doi: 10.1212/WNL.0000000000010537. [DOI] [PubMed] [Google Scholar]
- 10.Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M., Borja M.J., Zan E., Fatterpekar G.M. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297:E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vattoth S., Abdelhady M., Alsoub H., Own A., Elsotouhy A. Critical illness-associated cerebral microbleeds in COVID-19. Neuroradiol. J. 2020;33(5):374–376. doi: 10.1177/1971400920939229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J. Neuroimaging. 2020;30(5):593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta N.A., Lien C., Iv M. Critical illness-associated cerebral microbleeds in severe COVID-19 infection. Clin. Imaging. 2020;68:239–241. doi: 10.1016/j.clinimag.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin E., Lantos J.E., Strauss S.B., Phillips C.D., Campion T.R., Jr., Navi B.B., Parikh N.S., Merkler A.E., Mir S., Zhang C., Kamel H., Cusick M., Goyal P., Gupta A. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am. J. Neuroradiol. 2020;41:2001–2008. doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoskes A., Migdady I., Fernandez A., Ruggieri P., Rae-Grant A. Cerebral microhemorrhage and purpuric rash in COVID-19: the case for a secondary microangiopathy. J. Stroke Cereb. Dis. 2020;29(10):105–111. doi: 10.1016/j.jstrokecerebrovasdis.2020.105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kihira S., Delman B.N., Belani P., Stein L., Aggarwal A., Rigney B., Schefflein J., Doshi A.H., Pawha P.S. Imaging features of acute encephalopathy in patients with COVID-19: a case series. AJNR Am. J. Neuroradiol. 2020;41(10):1804–1808. doi: 10.3174/ajnr.A6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kremer S., Lersy F., de Sèze J., Ferré J.-C., Maamar A., Carsin-Nicol B., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020:202–222. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal S., Jain R., Dogra S., Krieger P., Lewis A., Nguyen V., Melmed K., Galetta S. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51:2649–2655. doi: 10.1161/STROKEAHA.120.030940. STROKEAHA120030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackett P.H., Yarnell P.R., Weiland D.A., Reynard K.B. Acute and evolving MRI of high-altitude cerebral edema: microbleeds, edema, and pathophysiology. AJNR Am. J. Neuroradiol. 2019;40(3):464–469. doi: 10.3174/ajnr.A5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagawa Y., Madokoro S., Matsunami T., Nagasawa H., Takeuchi I., Jitsuiki K., Takahashi N., Ohsaka H., Ishikawa K., Omori K. Mountain sickness with delayed signal changes in the corpus callosum on magnetic resonance imaging: a case report. J. Rural Med. 2019;14(2):253–257. doi: 10.2185/jrm.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oster J., Doherty C., Grant P.E., Simon M., Cole A.J. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia. 2003;44(6):852–854. doi: 10.1046/j.1528-1157.2003.40902.x. [DOI] [PubMed] [Google Scholar]
- 22.Starkey J., Kobayashi N., Numaguchi Y., Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017;37(2):562–576. doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
- 23.Linn J., Halpin A., Demaerel P., Ruhland J., Giese A.D., Dichgans M., van Buchem M.A., Bruckmann H., Greenberg S.M. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi M., Toh C.H., Thachil J., Watson H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 25.Chang R., Castillo J., Zambon A.C., Krasieva T.B., Fisher M.J., Sumbria R.K. Brain endothelial erythrophagocytosis and hemoglobin transmigration across brain endothelium: implications for pathogenesis of cerebral microbleeds. Front Cell Neurosci. 2018;12:279. doi: 10.3389/fncel.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fens M.H.A.M., van Wijk R., Andringa G., van Rooijen K.L., Dijstelbloem H.M., Rasmussen J.T., de Vooght K.M.K., Schiffelers R.M., Gaillard C.A.J.M., van Solinge W.W. A role for activated endothelial cells in red blood cell clearance: implications for vasopathology. Haematologica. 2012;97(4):500–508. doi: 10.3324/haematol.2011.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]