Abstract
Recent advances in exosome biology have uncovered a significant role of exosomes in cancer and make them a determining factor in intercellular communication. Exosomes are types of extracellular vesicles that are involved in the communication between cells by exchanging various signaling molecules between the surrounding cells. Among various signaling molecules, long non-coding RNAs (lncRNAs), a type of non-coding RNA having a size of more than 200 nt in length and lacking protein-coding potential, have emerged as crucial regulators of intercellular communication. Tumor-derived exosomes containing various lncRNAs, known as exosomal lncRNAs, reprogram the microenvironment by regulating numerous cellular functions, including the regulation of gene transcription that favors cancer growth and progression, thus significantly determining the biological effects of exosomes. In addition, deregulated expression of lncRNAs is found in various human cancers and serves as a diagnostic biomarker to predict cancer type. The present review discusses the role of exosomal lncRNAs in the crosstalk between tumor cells and the surrounding cells of the microenvironment. Furthermore, we also discuss the involvement of exosomal lncRNAs within the tumor microenvironment in favoring tumor growth, metabolic reprogramming of tumor cells, and tumor-supportive autophagy. Therefore, lncRNAs can be used as a therapeutic target in the treatment of various human cancers.
Keywords: exosomes, long non-coding RNAs, microRNAs, cancer, tumor microenvironment, autophagy, chemotherapy, cancer therapy
Graphical Abstract
lncRNAs are >200 nt long RNAs that are transcribed but not translated into proteins. Cells secrete lncRNAs into extracellular vesicles called exosomes to communicate with their microenvironment or distant cells. Cancer-related exosomal lncRNAs mediate tumor interaction with their microenvironment, which plays an important role in its reprogramming into a tumor-promoting microenvironment.
Main Text
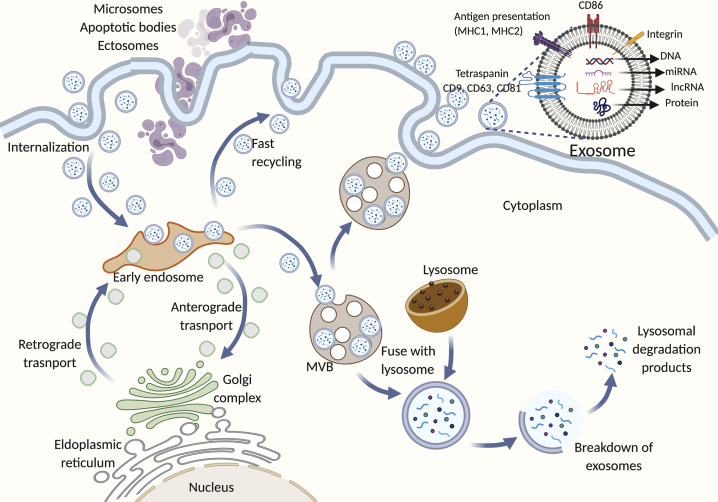
Exosomes are single-membrane vesicles of about 30–150 nm in diameter that are formed from endocytic vesicles.1,2 During endocytosis, the target extracellular material is surrounded by an area of the plasma membrane, which invaginates and forms a vesicle containing the ingested material.3 Once internalized, this membrane-bound vesicle fuses with the early endosome, which serves as a sorting station for this cargo containing mainly proteins and lipids.4 Early endosomes are highly dynamic vesicles that form either multivesicular bodies (MVBs), also called late endosomes, or recycling endosomes that are part of the multiple tubulovesicular recycling system.5,6 This recycling system transports cargo from early endosomes to the plasma membrane, Golgi network, or endoplasmic reticulum. Recycling of cargo from early endosomes to the plasma membrane via recycling endosomes is called a slow recycling pathway.7,8 Cargoes can be delivered directly from the early endosomes to the plasma membrane, which is known as a fast recycling pathway.7,9 The transport of cargo from early endosomes to the Golgi network or endoplasmic reticulum is called the retrograde pathway.10 Most proteins that are recycled back to the plasma membrane are cell surface receptors, adhesion molecules, ion channels, or proteins involved in cell polarization,11 whereas there is a wide range of functionally diverse proteins that utilize a retrograde pathway.12 Late endosomes fuse with the lysosomes to form endolysosomes, resulting in the degradation of their cargo through lysosomal hydrolases. When early endosomes mature into late endosomes, they accumulate small vesicles inside their lumen that are formed by inward budding of early endosomes and contain proteins, lipids, and nucleic acids that are specifically sorted.13 The presence of intraluminal vesicles (ILVs) inside late endosomes gives them a multivesicular appearance. Therefore, they are often referred to MVBs.14 Not all ILVs are destined for degradation; some are secreted out in the extracellular space due to the fusion of MVBs with the plasma membrane in a highly regulated process. These small, secreted ILVs are referred to as exosomes. They are different from other types of similar-sized extracellular vesicles (EVs) secreted by cells, including microvesicles, ectosomes, and apoptotic bodies based on their origin, structure, and presence of specific markers on their membranes.15, 16, 17 Microvesicles, ectosomes, and apoptotic bodies originate from the plasma membrane and have irregular shapes, whereas exosomes are endosomal in origin, cup-shaped, homologous, and carry specific markers, including Alix, TSG101, and tetraspanins CD63, CD9, and CD81 (Figure 1).18, 19, 20, 21
Figure 1.
Release and Composition of Exosomes
Extracellular cargo is internalized from the plasma membrane and packed in membrane-bound vesicles that later fuse with early endosomes. Early endosomes serve as a sorting station from which either these vesicles (carrying mostly cell surface receptors) are recycled back to the plasma membrane or form MVBs that later fuse with lysosomes and undergo degradation. Importantly, not all MVBs undergo degradation; some are secreted outside extracellular space by fusion with the plasma membrane and have some specific markers on their membrane. They are exosomes and carry various signaling molecules as shown in the figure.
The term exosome was first coined in 1987 by Johnstone et al.22 in sheep reticulocytes, where they reported the secretion and loss of transferrin receptor into small-sized vesicles (50 nm) to the extracellular space during reticulocyte maturation into erythrocytes. The group harvested these vesicles from sheep reticulocytes by centrifugation at 100,000 × g for 90 min. The lysed vesicles contained transferrin receptor and had many activities that were characteristic of the reticulocyte plasma membrane and were reduced or absent in mature erythrocytes. These activities include acetylcholinesterase, cytochalasin B binding (glucose transporter), nucleoside binding (i.e., nucleoside transporter), and Na+-independent amino acid transport, suggesting that proteins performing these activities are secreted out through exosomes during reticulocyte maturation.22 Since then, exosomes have been discovered in most cell types, such as immune cells (T lymphocytes,23 B lymphocytes,24 natural killer cells,25 dendritic cells,26 and mast cells27), cancer cells,28 embryonic29 and mesenchymal stem cells,30 adipocytes,31 and glial cells,32 and also in biological fluids such as blood, urine, semen, and breast milk.33 Exosomes perform a wide range of functions, including cell-to-cell communications through the delivery of DNA, RNA, lipids, or proteins from target to recipient cells,34 immune regulation,35 and selective loss of biological macromolecules during cell maturation,22 and they are involved in the development and progression of many diseases, including cancer,36 diabetes,31 and cardiovascular37 and neurodegenerative38 diseases. Cancer-derived exosomes are vital in cancer cell survival and play an important role in the modulation of the tumor microenvironment to favor cancer progression and metastasis.39,40
Cancer-Derived Exosomes
Many cancer cell types secrete exosomes that help to communicate with surrounding cancer or normal cells such as immune cells, stromal cells, vascular endothelial cells, and cancer-associated fibroblasts (CAFs). This helps in the establishment of a favorable tumor microenvironment, which promotes immune escape,41 tumor invasion,42, migration,43 and formation of premetastatic niches in secondary organs.44 Exosomes secreted by cancer cells suppress T cell receptors (TCRs) and inhibit the proliferation of CD8+ cytotoxic T lymphocytes (CTLs) that specifically target cancer cells and kill them. Coincubation of activated CD8+ T cells with exosomes isolated from tumor cell lines, including head and neck squamous cell carcinoma PCL-13 and melanoma cell line Mel-SW, inhibits their proliferation.45 CD8+ Jurkat cells (T lymphocyte cell line) coincubated with tumor exosomes undergo apoptosis and have fragmented DNA,45 suggesting immune suppression by cancer-derived exosomes. The stem-like brain tumor-initiating cells (BTICs) secrete high amounts of extracellular matrix protein tenascin-C (TNC) in exosomes, which inhibits T cell proliferation and activity by binding to α5β1 and αvβ6 integrins on T lymphocytes that blocks TCR signaling.46 TNC promotes glioblastoma invasion and is associated with an immunosuppressive phenotype and worse overall survival in glioblastoma patients.46,47 Another important pro-tumorigenic and immunosuppressive protein secreted by cancer cells in exosomes is programmed death-ligand 1 (PD-L1), which binds to the programmed cell death-1 (PD-1) receptor on T cells and inhibits its anti-tumor function and effectively protects the tumor from immune surveillance.48 Exosomal PD-L1 isolated from cell culture supernatants of breast cancer cell lines MDA-MB-231 (human) and 4T1 (mouse mammary tumor cells), colon cancer cell line RKO, and lung cancer cell line HCC827 blocks T cell functions by inhibiting the CD3/CD28-triggered T cell activation signaling pathway.48 Interestingly, the exosomes can transfer functional PD-L1 to other cells with low or no PD-L1 expression, suggesting the role of tumor-derived exosomes in enhancing the anti-tumor response.48 Moreover, PD-L1 knockdown in mouse 4T1 cells injected into mice shows tumor regression, whereas PD-L1 knockdown tumors injected with exosomal PD-L1 shows high tumor growth and reduced cytotoxic T cell activity.48 Except for proteins, exosomes are enriched in lipids, DNA, RNA, and metabolites that are specific to the cell of origin and play an important role in exosome functions.18 The RNA content in the exosomes has gained considerable attention in recent years due to their role in promoting a favorable tumor microenvironment,49,50 tumor progression,51 angiogenesis,52 and metastasis53 and their use as diagnostic markers in many cancer types.54, 55, 56 The types of RNAs that have been detected in tumor exosomes are coding mRNAs and non-coding circular RNAs, microRNAs (miRNAs), and long non-coding RNAs (lncRNAs). Non-coding RNAs (ncRNAs) that represent a large segment of the human genome and do not code for proteins are the major contributors in RNA-mediated carcinogenesis. In this review, we will focus on the role of lncRNAs secreted through exosomes in the modulation of the tumor microenvironment and its role in cancer development, growth, and response to therapy.
Non-coding RNAs
Non-coding RNAs are functional RNA molecules that do not code for proteins. Only a few RNAs (less than 2%) are translated into proteins or are mRNAs, even though 90% of the human genome is transcribed.57, 58, 59, 60 The rest of the transcribed RNAs are called non-coding RNAs that play a central role in regulating gene expression at the transcriptional, post-transcriptional, and translational levels. The non-coding RNAs are divided into two main groups based on the length of the transcript. There are short non-coding RNAs that are less than 40 nt in length, and there are lncRNAs that are comprised of more than 200 nt. The short non-coding RNAs include miRNAs (18–25 nt in length),61 short interfering RNAs (siRNAs, 19–23 nt in length),62,63 PIWI-interacting RNAs (piRNAs, 21–36 nt in length),64 and transfer RNA fragments (tRFs, 17–26 nt in length).65 The lncRNAs include a wide range of RNAs performing various biological functions and are found extensively in a large diversity of species, including animals,66 plants,67 bacteria,68 fungi,69 and viruses.70 lncRNAs are primarily classified based on size, but due to recent advances in transcriptome sequencing and analysis, lncRNAs can be further classified into different classes.71
lncRNAs: Biogenesis, Classification, and Functions
lncRNAs are transcribed based on their location in the genome and, similar to mRNAs, are polyadenylated, capped, and can carry genetic information. However, due to the complexity of the non-coding transcriptome, there is no well-defined relationship between lncRNA structure and function. As a result, two similar-structure lncRNAs can perform different functions and, due to the advances in RNA sequencing technologies that have led to the addition of many new unannotated transcripts, most of the lncRNAs lack well-characterized functions. The commonly used lncRNA classifications are based on their genomic location and the direction of transcription to protein-coding genes, functions, the resemblance with mRNA, and the presence of associated repeat elements. Based on their genomic location and the direction of transcription to mRNA, a lncRNA can be (1) intergenic, if it is located between two genes,72 (2) intronic, if it is present within the intron of a protein-coding gene,73 or (3) sense or anti-sense, if it is transcribed in the same74 or opposite direction of complementary protein-coding genes.75 lincRNA-p21 is an example of a large intergenic non-coding RNA induced by tumor suppressor protein p53 to relay its tumor-suppressive functions during DNA damage. lincRNA-p21 binds with heterogeneous nuclear ribonucleoprotein K (hnRNP-K), a transcriptional repressor, and localizes it to p53 target genes that inhibit their transcription.72 ANRASSF1 is an intronic lncRNA that targets the promoter region of tumor suppressor gene RASSF1A and inhibits its functions. ANRASSF1 is an unspliced lncRNA that is transcribed from the opposite strand on the RASSF1 gene. ANRASSF1 recruits polycomb repressive complex 2 (PRC2) to the nearby promotor region of the RASSF1 while binding DNA at its transcription site. PRC2 recruitment induces tri-methylation of histone 3 on lysine 27 (H3K27me3) in that region and induces transcriptional downregulation of the RASSF1A gene.73 lncRNA SYSIL is a Synaptopodin 2 (SYNPO2, muscle-associated protein) intron sense-overlapping lncRNA that directly binds to the enhancer of zeste homolog 2 (EZH2) protein, a component of PRC2, and recruits this complex to the promoter of its target genes, including muscle-specific genes such as myogenin (MyoG), muscle creatine kinase (MCK), and myosin heavy chain 4 (Myh4) and cyclin-dependent kinase inhibitor p21. PRC2 recruitment induces H3K27me3 regions in promoters of these genes that result in their epigenetic silencing and the inhibition of myogenesis.74 Tsix is an anti-sense lncRNA to the Xist gene that plays an essential role in X-inactivation during dosage compensation. Tsix is present 15 kb downstream of the Xist gene and transcribed across the Xist locus that results in its silencing. In this way, Tsix influences the random choice mechanism that decides which of the two X chromosomes will be inactivated.75,76
Based on functions, lncRNAs can be classified into those that act on genes present near the site of their transcription (cis-lncRNAs) or those that leave the site of transcription and act on distant genes (trans). Cis-acting lncRNAs can regulate gene expression by (1) recruiting regulatory factors that can influence neighboring gene functions, (2) regulate DNA elements such as enhancers or silencers that control gene expression that are present within the lncRNA locus, and (3) assemble transcription or spliceosome machinery on lncRNAs that affect the transcription of the nearby gene by interfering in the binding of RNA polymerase or transcription factors for that gene. In cases 2 and 3, the lncRNA-mediated gene regulation is independent of its sequence or production. Cis-acting lncRNA ROCK1 (regulator of cytokines and inflammation), which is induced by the activation of Toll-like receptors, regulates its cognate gene’s (MARCKS) expression by recruiting histone deacetylase (HDAC1) to its promotor region.77 ROCK1 binds to the MARCKS promotor and apurinic/apyrimidinic endodeoxyribonuclease 1 (APEX1) protein and forms a ROCK1-APEX complex on the MARCKS promotor. The ROCK1-APEX ribonucleoprotein complex recruits HDAC1 on the MARCKS promotor that removes the activating histone H3 lysine 27 acetylation (H3K27ac3) and reduces its transcription. MARCKS is an important regulator of inflammatory cytokine expression and calcium signaling.77 The example of cis-acting lncRNA that contains the regulatory elements of its target gene is plasmacytoma variant translocation 1 (PVT1), which is 1,716 nt in length and located near the Myc gene at human chromosome 8q24.78 The PVT1 locus contains four intragenic enhancers for MYC, and its promotor competes with the MYC promotor for these regulatory elements. The PVT1 promotor inhibits MYC expression via promotor competition, where it blocks the accessibility of these enhancers for MYC transcription. Genetic silencing or mutations in the PVT promotor increases MYC expression and, hence, cell proliferation. Interestingly, knockdown of PVT lncRNA does not affect MYC expression and cell growth, suggesting that it is the PVT promotor region that controls MYC transcription and not the lncRNA itself.78 The lncRNA Airn (antisense Igf2r RNA noncoding) transcription process, and not its spliced or unspliced RNA products, silences the cis Igf2r (insulin-like growth factor 2 receptor) gene.79
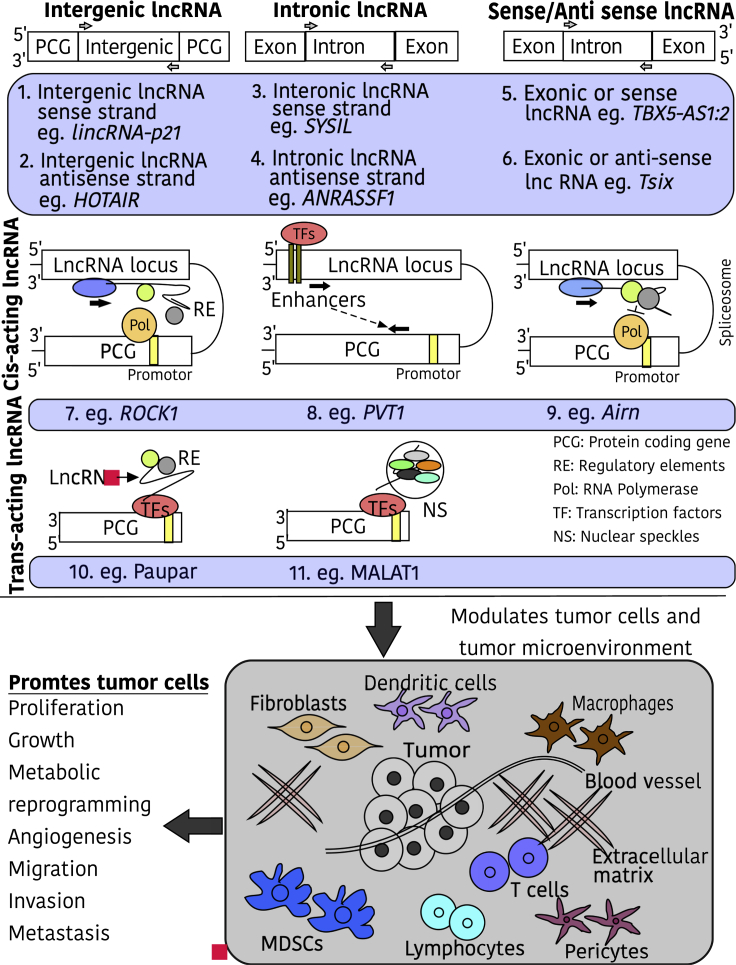
Trans-acting lncRNAs can influence gene expression similar to cis-acting lncRNAs by interacting with regulatory elements of the target gene. Trans-acting lncRNA Paupar regulates neural proliferation and differentiation by directly binding with the epigenetic regulator protein KAP1. Paupar interacts with KAP1 and paired box protein PAX6 to form a ternary ribonucleoprotein complex on the regulatory regions of its distant target genes, including E2F transcription factor 2 (E2F2), Mab-21-like 2 (Mab21L2), and macrophage stimulating 1 (Mst1). These genes are involved in neural functions and proliferation. The DNA-bound Paupar-KAP1-PAX6 ternary complex induces H3K9me3 histone modifications at the regulatory regions of these genes and regulates their transcription.80 Moreover, trans-acting lncRNAs can also be the part of nuclear speckles that are self-organizing, dynamic nuclear structures present in the interchromatin region of the nucleus and contain numerous proteins and RNA involved in RNA modifications, DNA repair, and chromatin organization.81 Some lncRNAs can influence nuclear speckles and affect the genetic and epigenetic regulation of their target genes. Metastasis-associated lung adenocarcinoma transcript 1, or MALAT1, is a nuclear speckle-associated lncRNA that interacts with several precursor (pre-)mRNA splicing factors and influences their distribution in nuclear speckles. MALAT1 interacts with serine/arginine splicing factors, predominantly present in the nuclear speckles, and modulates pre-mRNA splicing of hundreds of genes. Furthermore, MALAT1 knockdown induces aberrant mitosis and cell death, suggesting its role in controlling gene expression.82 Another important lncRNA that localizes to nuclear speckle overactive chromatin regions and regulates the transcription of numerous distant genes is nuclear-enriched abundant transcript 1 (NEAT1). MALAT1 and NEAT1 bind to the transcriptionally active genes, and their binding is responsive to their target genes. NEAT1 binds to transcriptional start sites and transcriptional termination sites, whereas MALAT1 localizes across gene bodies and transcriptional termination sites.83 Figure 2 describes the classification of lncRNAs.
Figure 2.
Types and Functions of lncRNAs
lncRNAs are either classified on the basis of their genomic location and the direction of transcription (1 and 2, intergenic, if located between two genes; 3 and 4, intronic, if present within the intron of the protein-coding gene; 5 and 6, sense or antisense, if transcribed in same or opposite direction of the complementary protein-coding gene) or functions (7, 8, and 9, cis-acting lncRNAs, regulate genes present nearby the site of their transcription; 10 and 11, trans-acting lncRNAs, regulate genes present nearby the site of their transcription). lncRNAs regulate crosstalk between cancer cells and the surrounding microenvironment cells, including dendritic cells, macrophages, endothelial cells, extracellular components, immune cells, myeloid-derived suppressor cells, and fibroblasts. This regulation modulates various functions in tumor cells as shown in the figure.
lncRNAs and the Tumor Microenvironment
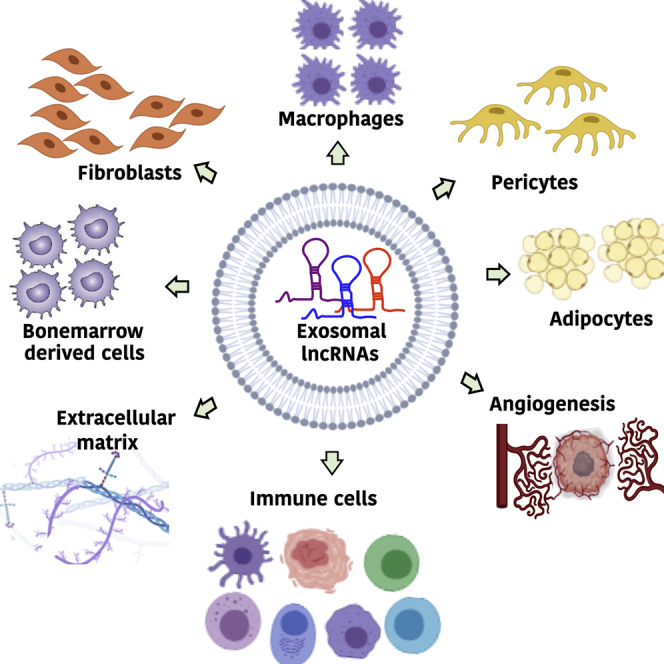
The tumor microenvironment is the local environment surrounding tumor cells and is comprised of the extracellular matrix, blood vessels, immune cells, stromal cells, and bioactive agents, including cytokines and hormones. The tumor microenvironment acts as a scaffold around tumor cells that helps in immune escape and promotes tumor growth, angiogenesis, and metastasis.84, 85, 86, 87 Cancer cells reprogram the tumor microenvironment into a tumor-favorable and immune-suppressive environment through the secretion of various factors, including cytokines, chemokines, metabolites, and growth-inhibitory or growth-promoting proteins.84 Cancer cells use exosomes as communication mediators for surrounding cells. Cancer-derived exosomes contain various signaling molecules that play an important role in the reprogramming of the tumor microenvironment via a plethora of cell-cell communications.88 lncRNAs emerge as important players in mediating these communications that contribute to tumor growth and progression.89 Cancer cells that secrete lncRNAs communicate with stromal cells, macrophages, components of the extracellular matrix, endothelial cells, immune cells, and myeloid-derived suppressor cells (MDSCs).89 This communication helps cancer cells generate a tumor-supportive microenvironment that is important for cancer growth, progression, and metastasis (Figure 2). Cancer cells secrete lncRNAs in exosomes (exosomal lncRNAs) that are taken by nearby or distant cells via endocytosis and regulate their functions.90 Aberrant levels of exosomal lncRNAs are found in many cancers, and their expression levels are associated with a favorable tumor microenvironment that makes them promising biomarkers and therapeutic targets in cancer.91, 92, 93
Exosomic lncRNAs: Promoters of a Tumor-Favorable Microenvironment
During tumor development, cancer cells and the surrounding microenvironment have restricted access to oxygen supply due to the presence of poor vasculature inside the growing solid tumor mass. Reduced oxygen availability, also called hypoxia, led to the activation of hypoxia-inducible factor (HIF)-1α pathway in tumor cells, which acts as an adaptive mechanism during oxygen stress.94 The hypoxic condition induces the transcription of many lncRNAs that contributes to cell survival.95,96 Bladder cancer cells secrete lncRNA urothelial cancer-associated 1 (UCA1) during hypoxia in exosomes that promote tumor progression through an epithelial-to-mesenchymal transition. Induction of hypoxia in bladder cancer cell line 5637 culture enhances exosomal-mediated secretion of lncRNA-UCA1. Exosomes isolated from lncRNA-UCA1 shRNA (UCA silenced)-treated 5637 cells growing under hypoxia decreases cell proliferation, migration, and invasion in the UMUC2 bladder cancer cell line. Moreover, when these exosomes were injected into 5637 tumor-bearing mice, the size of the tumor grew slowly compared to controls, suggesting the role of exosomal lncRNA-UCA1 in promoting tumor growth.96 The CAFs secrete lncRNA CCAL (colorectal cancer-associated lncRNA) in exosomes that promote oxaliplatin resistance in colorectal cancer cells (CRCs).97 The oxaliplatin is used to treat patients with metastasized colorectal cancer. CCAL is highly expressed in CRC tissues compared to adjacent non-cancerous tissues. CAFs express much higher CCAL than do other cell populations in CRC tissues, suggesting their role in the development of oxaliplatin resistance. CRCs grown in CAF conditioned medium show resistance to oxaliplatin-induced apoptosis compared to cells grown in normal fibroblast conditioned medium. Treatment of CAFs with exosomal secretion inhibitor GW4869 prevents oxaliplatin resistance, thus implying the role of CAF-derived exosomes in inducing CRC resistance. These exosomes contain CCAL that activates the Wnt/β-catenin pathway in the recipient CRCs and promotes chemoresistance. CCAL indirectly stabilizes β-catenin mRNA by directly interacting with the mRNA stabilizing protein HuR.97 Moreover, CAFs transfer exosomal lncRNA H19 to CRCs that promote their stemness and oxaliplatin resistance. lncRNA H19 acts as a competing endogenous RNA (ceRNA) for miR-141 and masks its activity. miR-141 inhibits the stemness of CRCs by inhibiting the Wnt/β-catenin pathway.98 Exosomal lncRNA homeobox transcript antisense RNA (HOTAIR) decreases laryngeal cancer radiosensitivity by regulating miRNA miR-454-3p. Laryngeal cancer cells cocultured with laryngeal cancer cell-derived exosomes show reduced radiosensitivity and apoptosis compared to controls. Genetic silencing of HOTAIR in the exosomes decreases cancer cell colonies and reduces apoptosis, whereas its overexpression does the opposite. HOTAIR competes with miR-454-3p for cell cycle regulatory E2F2 and inhibits its activity.99 Additionally, Feng et al.100 identified lncRNAs in breast cancer cell-derived exosomes that are involved in lung fibroblasts proliferation and migration, thus facilitating tumor pre-metastatic niche formation. Table 1 summarizes the role of exosomal lncRNAs in the modulation of the tumor microenvironment.
Table 1.
Exosomal lncRNAs and Their Roles in the Tumor Microenvironment
| Exosomal lncRNAs | Secretory Cells | Recipient Cells | Function and Mechanism in Recipient Cells |
|---|---|---|---|
| GS1-600G8.5 | breast cancer metastatic cells MDABR3 and its parent cell line MDA-MB-231 | brain microvascular endothelial cells (BMECs) | destroys blood-brain barrier composed of BMECs, pericytes, and astrocytes and promotes the passage of cancer cells across it101 |
| SBF2-AS1 (SET binding factor 2) | M2 macrophage | pancreatic cancer cell line PANC-1 | induces tumor progression in tumor xenografts of PANC-1; SBF2-AS1 inhibits miR-122-5p and upregulates its target gene X-linked inhibitor of apoptosis (XIAP) that contributes to pancreatic cancer development102 |
| MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) | metastatic colorectal cancer cell (CRC) lines SW620 and LoVo | primary CRCs | increases proliferation, migration, and invasion; MALAT1 inhibits miR-26a/26b and upregulates in target fucosyltransferase 4 (FUT4) expression that led to fucosylation and activation of the PI3K/Akt/mTOR pathway103 |
| H19 | mesenchymal stem cells | trophoblast cells HTR-8/SVneo | increases cell invasion and migration and inhibits apoptosis; H19 inhibits let-7b expression and prevents its inhibitory binding with FOXO1, causing FOXO1 induction, which promotes cell malignancy and inhibits apoptosis104 |
| SNHG9 (small nucleolar RNA host gene 9) | adipocyte-derived stem cells | human umbilical vein endothelial cells | alleviates inflammation-induced apoptosis in endothelial cells; SNHG9 forms a dimer with TRADD mRNA and inhibits its translation; TRADD silencing inhibits inflammation and apoptosis in HUVECs105 |
| PVT1 (plasmacytoma variant translocation 1) | bone marrow mesenchymal stem cells (BMSCs) | osteosarcoma cell lines Saos-2, MG-63, and MNNG/HOS | promotes osteosarcoma growth and metastasis; PVT1 sponges miR-183-5p and releases its inhibitory effects on oncogene ERG, which promotes cell proliferation, growth, migration, and metastasis106 |
| LNMAT2 (lymph node metastasis-associated transcript 2) | bladder cancer cell lines UM-UC-3, 5637 and T24 | human lymphatic endothelial cells (HLECs) | stimulates HLEC tube formation and migration, induces tumor lymphangiogenesis, and promotes lymph mode (LN) metastasis; exosomes containing LNMAT2 and heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) are internalized by HLEC; LNMAT2 recruits hnRNPA2B1 to the PROX1 promoter and increase its H3K4 trimethylation, leading to prospero homeobox 1 (PROX1) overexpression that contributes to lymphangiogenesis and LN metastasis53 |
| Gm26809 | melanoma cell line B16F0 | fibroblast NIH/3T3 | reprogrammed fibroblasts into CAMs.Gm26809 increases α-smooth muscle actin and fibroblast activation protein (FAP) expression and promotes fibroblasts migration; Gm26809-induced CAFs facilitate melanoma cell proliferation and migration107 |
| TU339 | hepatocellular carcinoma cell (HCC) line PLC/PRF/5 | macrophage cell line THP-1 and U937 | macrophage activation and polarization; TU339 decreases pro-inflammatory cytokine production IL-1β and TNF-α, CD86 expression, and phagocytosis; TU339 reduces IL-12 p40 expression (M1 marker), whereas it increases CCL17 and CD206 expression (M2 markers)92 |
Exosomic lncRNAs: Metabolic Programmers in the Tumor Microenvironment
Cancer cells have altered metabolic activities relative to normal cells. The alterations in the cancer genome and its microenvironment support cancer cell metabolism that plays a critical role in the acquisition and maintenance of malignant properties.108,109 Cancer cells heavily rely on aerobic glycolysis, also known as the Warburg effect, which normal cells do during hypoxic conditions.110 This generates high amounts of lactate and ATPs from glucose in much less time compared to completing the oxidation of glucose in mitochondria. Rapid ATP synthesis is required by the continuously proliferating cancer cells. Additionally, this generates many glycolytic intermediates that go to subsidiary pathways and support the energy demands of proliferating cancer cells.111 Many signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/Akt, Ras, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), are involved in the metabolic reprogramming of cancer cells.112 lncRNAs play an important role in the regulation of these pathways and, hence, could be a relevant player in regulating metabolism in cancer cells or the surrounding microenvironment. One of the examples is exosomal lncRNA-p21 that promotes angiogenesis and tumor cell adhesion to endothelial cells. lncRNA-p21 expression increases in non-small-cell lung cancer (NSCLC) cell-derived exosomes under hypoxic conditions. Genetic silencing of lncRNA-p21 in NSCLC cells decreases the secretion of lncRNA-p21 and its regulated miRNAs, including miR-23a, miR-146b, miR-330, and miR-494 in exosomes. Treatment of the endothelial cell line HUVECs (human umbilical vein endothelial cells) with these exosomes shows downregulation in metabolism-related genes, including glucose transporter 1 (GLUT1), PFKFB3, and GAPDH, which is important in endothelial cell activation.113 Conversely, lncRNA-p21 silencing in prostate cancer cells increases their proliferation and makes them more tumorigenic.114 lncRNA-p21 knockdown induces the upregulation of pyruvate kinase M2 (PKM2), an isoenzyme involved in aerobic glycolysis and preferentially expressed in proliferating cells.115 PKM2 upregulation is associated with enhanced glucose consumption, pyruvate concentration, and lactate production, which are critical for proliferating cancer cells. The knockdown of PKM2 overcomes the inhibition of cell proliferation and tumorigenicity induced by lncRNA-p21 silencing in prostate cancer cells. Moreover, PKM2 upregulation in lncRNA-p21 knockdown cells depends on PTEN/AKT/mammalian target of rapamycin (mTOR) signaling, as inhibition of this pathway using PI3K inhibitor LY294002 or the mTOR inhibitor rapamycin rescues the increase in PKM2. This suggests that lncRNA-p21 inhibits cell proliferation and tumorigenicity by suppressing PKM2-mediated cell survival, which requires the PI3K/Akt pathway.114
Another important lncRNA, SNHG3 (small nucleolar RNA host gene 3), reprograms the metabolic pathways in breast tumor cells. lncRNA SNHG3 is secreted from CAFs in exosomes and promotes breast cancer cell proliferation by suppressing the expression of miRNA miR-330. The knockdown of SNHG3 in CAFs increases miR-330 expression and downregulates PKM2 in breast cancer cells. SNHG3 sponges miR-330-5p activity to positively regulate PKM2, which increases glycolysis carboxylation, decreases mitochondrial oxidative phosphorylation, and enhances breast tumor cell proliferation.116 CAFs promote the metastatic activity of breast cancer cells by activating the expression of HOTAIR in breast cancer cells. CAFs secrete transforming growth factor (TGF)-β1, which phosphorylates and activates the SMAD (group of intracellular proteins that deliver extracellular signals from TGF-β ligands to the nucleus) pathway in recipient cancer cells. Activated SMADs, including SMAD2, SMAD3, and SMAD4, directly bind with the promoter region of lncRNA HOTAIR and induce its transcriptional activation. Activated lncRNA HOTAIR induces an epithelial-to-mesenchymal transition and metastasis of breast cancer cells by activating CDK5 signaling.117 Circulatory exosomes collected from breast cancer patient plasma contains lncRNA HOTAIR that correlates with the ErbB2/HER2 expression in primary breast tumor tissues.118 High circulating exosomal HOTAIR levels are associated with less response to neoadjuvant chemotherapy and worse prognosis in breast cancer patients.119 lncRNA HOTAIR promotes glycolysis in hepatocellular carcinoma (HCC) cells by directly binding and upregulating GLUT1 expression. The GLUT1 upregulation is associated with an increase in glucose consumption and lactate production. However, the mTOR inhibitor rapamycin significantly reduces these effects, suggesting that HOTAIR-mediated increased glycolysis depends on mTOR signaling.120 Moreover, HOTAIR was found to be an upstream regulator of a glycolytic enzyme, hexokinase 2 (HK2), in pancreatic adenocarcinoma cells.121 HK2 is expressed higher in cancer cells, and its depletion inhibits glycolysis and oncogenic transformation.122,123 HOTAIR overexpression increases lactate production, glucose uptake, and ATP production in pancreatic adenocarcinoma cells.121 Ma et al.124 reported that HOTAIR-mediated HK2 regulation depends on miR-125 and miR-143 activity. HOTAIR directly binds and inhibits miR-125- and miR-143-mediated HK2 downregulation and promotes glucose metabolism and cell growth. Tumor-associated macrophages (TAMs) release HIF-1α-stabilizing lncRNA (HISLA) in the extracellular vesicles, which regulate aerobic glycolysis and apoptotic resistance of breast cancer cells.125 HISLA is expressed much higher in polarized TAMs made by treating human monocyte-derived macrophages (MDMs) with the conditioned medium of the breast cancer cell line MDA-MB-231 compared to primary TAMs from human breast cancer tissues. Lactate secreted in the tumor microenvironment by breast cancer cells upregulates HISLA in TAMs by activating ERK-ELK1 signaling. HISLA increases HIF-1α expression and aerobic glycolysis denoted by increased glucose consumption, lactate production, and extracellular acidification rates in TAMs. HISLA blocks the interaction of HIF-1α with prolyl hydroxylase domain-containing protein 2 (PHD2) that causes the hydroxylation of proline-564 and proline-402 of HIF-1α and makes it a target of ubiquitin-mediated proteasomal degradation. The RNA stem loop at nucleotides 190–248 of HISLA is critical for its binding with PHD2, which prevents PHD2-HIF-1α interaction. TAM releases HISLA in the microenvironment into exosomes that enhances glycolysis in the recipient cancer cells and protects them from chemotherapy-induced apoptosis. In addition, recipient cells are more metastatic to lungs, and this effect is rescued by silencing HISLA or inhibiting extracellular vesicle secretion in TAMs. Hence, there is a feedback loop between cancer and stromal cells where lactate secretion from cancer cells enhances HISLA production in TAMs, which releases it in the extracellular vesicles. These extracellular vesicles are taken by cancer cells that promote tumor glycolysis and chemoresistance.125 Moreover, high HISLA and GLUT3 expression are associated with poor disease-free survival and a poor chemotherapeutic response in breast cancer patients.125 lncRNA profiling of high-grade serous carcinoma patients reveals the presence of lncRNA LINK-A in exosomes isolated from effusion supernatants.126 LINK-A correlates with the immunosuppression in triple-negative breast cancer (TNBC) patients. TNBC patients who respond to chemotherapy have lower expression of LINK-A and more tumor infiltration of CD8+ T cells and antigen-presenting cells (APCs).127 LINK-A binds with phosphatidylinositol-(3,4,5)-trisphosphate and facilitates its interaction with inhibitory G protein-coupled receptor (GPCR) pathways, resulting in reduced cyclic AMP (cAMP) concentrations, which attenuate protein kinase A-mediated serine 3 phosphorylation of the E3 ubiquitin ligase TRIM71. Hypophosphorylated TRIM71 catalyzes K48-linked polyubiquitin chain formation on tumor suppressor proteins Rb and p53, and the components of antigen peptide-loading complex (PLC), which facilitates the folding and loading of antigenic peptides onto major histocompatibility complex (MHC) class I molecules. This leads to their ubiquitin-mediated proteasomal degradation, causing more tumorigenicity and immune suppression. lncRNAs expressing mammary tumors are enriched with metabolites of glycolysis and redox balance, whereas genes involved in lipid metabolism, T cell activation, and antigen presentation are downregulated.127 In glioma cells, LINK-A promotes their growth, migration, and invasion by increasing lactate dehydrogenase (LDH)-A. LINK-A silencing downregulates LDH-A expression, glucose uptake, and lactate production, suggesting the role of LDH-A in promoting LINK-A oncogenic functions.128 Furthermore, lncRNA PTENP1 participates in a normal cell-to-cancer cell communication via exosomes to suppress carcinogenesis.129 Normal cells secrete PTENP1 in exosomes that are taken by bladder cancer cells. Exosomal PTENP1 inhibits the malignant behavior of bladder cancer cells by increasing cell apoptosis and inhibiting cell migration and invasion. PTENP1 prevents the binding of tumor suppressor PTEN with inhibitory miRNAs that inhibit PTEN translation.129 PTEN decreases glycolysis and promotes oxidative phosphorylation, thus preventing the metabolic reprogramming in cancer cells that play an essential role in PTEN-mediated cancer suppression.130
Exosomal lncRNAs and Autophagy: Role in the Tumor Microenvironment
Autophagy is a self-degradative or catabolic process that removes misfolded or aggregated proteins, damaged organelles, or intracellular pathogens, and it maintains cell homeostasis in response to nutrient deficiency. Autophagy is an important phenomenon in cancer, as cancer cells upregulate autophagy to protect them from environmental stress and replenish their fuel supply.131 Recent studies have discovered lncRNAs as important regulators of autophagy that protect cancer cells from cellular stress and chemotherapy. lncRNA-induced autophagy plays an important role in CAF-mediated tumor cell survival and proliferation.132 lncRNA-CAF prevents the degradation of interleukin (IL)-33, a protumorigenic cytokine by inhibiting p62-dependent autophagy in CAFs. Overexpression of lncRNA-CAF in normal fibroblasts transforms them into CAFs by inducing the expression of α-smooth muscle actin, vimentin, and N-cadherin, and promotes a mesenchymal phenotype. However, IL-33 knockdown prevents these CAF-related changes, suggesting that IL-33 is a critical mediator of lncRNA-CAF effects. lncRNA-CAF is secreted by oral squamous cell carcinoma (OSCC) cells in exosomes that are taken by stromal fibroblasts in the microenvironment. Upregulated lncRNA-CAF levels in normal stromal fibroblasts promote a CAF phenotype that further supports tumor proliferation. This suggests that there is a positive feedback loop where cancer cells secrete exosomal lncRNA-CAF in the tumor microenvironment that is taken by stromal fibroblasts, leading to lncRNA-CAF-mediated IL-33 stability in fibroblasts that promote their transformation into CAFs and, hence, support tumor growth. In addition, higher lncRNA-CAF levels are associated with poor prognosis in OSCC patients, whereas lncRNA-CAF/IL-33 levels in the tumor microenvironment correlate with high tumor-node-metastasis stage.132
Antisense non-coding RNA (ANRIL) induces autophagy via beclin-1 upregulation in endothelial cells to promote angiogenesis. ANRIL acts as a miRNA sponge where it competes with miR-99a and miR-449a and inhibits their binding with beclin-1 in endothelial cells. This induces beclin-1-dependent autophagy that promotes endothelial cell proliferation and angiogenesis.133 ANRIL expression is significantly higher in exosomes isolated from the urine of bone cancer patients compared to healthy controls.134 Another exosomal lncRNA, H19, that is released from stem-like CD90+ liver cancer cells influences the tumor microenvironment by promoting endothelial cell proliferation, angiogenic phenotype, and cell-to-cell adhesion.135 lncRNA H19-induced autophagy in liver cancer cells causes hypoxia/reoxygenation injury via activation of the PI3K/Akt pathway.136 Furthermore, glioma stem cells (GSCs) regulate the inflammatory response in the surrounding microglial cells by lncRNA MALAT1. Microglial cells account for a considerable portion of glioma mass, and their density correlates with metastasis and progression of the disease. GSCs secrete MALAT1 in exosomes that are taken by surrounding microglial cells. MALAT1 inhibits miR-129 expression by sponging its activity, causing the upregulation of high mobility group box-1 (HMGB1) protein. HMGB1 upregulation triggers an inflammatory response by inducing the secretion of IL-6, IL-8, and tumor necrosis factor (TNF)-α in microglial cells. HMGB1-induced inflammation could contribute to GSC survival and glioma progression. Also, MALAT1 induces autophagy in glioma cells and promotes their cell migration and invasion.137 MALAT1 directly binds and inhibits miR-384, which targets Golgi membrane protein 1 (GOLM1), a Golgi-related protein that is oncogenic in many cancers, including gliomas.137,138 miR-384 inhibition increases GOLM1 expression, which induces autophagy and promotes cell migration and invasion. This suggests that MALAT1-induced autophagy could be a vital process in glioma survival that is important for glioma-mediated microenvironment remodeling for its growth.137
Targeting Exosomal lncRNA Transfer in Chemotherapy
Therapeutic Potential
Recent reports have suggested that exosomes can be a vital player in mediating therapy resistance by delivering proteins, miRNAs, or lncRNAs to either cancer cells or the microenvironment and can increase tumor cell survival and promote malignancy.139 High levels of lncRNAs in serum exosomes are associated with a poor response to chemotherapy treatment, suggesting that targeting exosomal lncRNAs could be a potential therapy for drug-resistant tumors.140,141 lncRNA-based anticancer therapies could involve the use of lncRNA antagonists against oncogenic lncRNAs or the introduction of tumor-suppressive lncRNA mimics. However, the strategy of delivering lncRNAs to their target cells and their safety profiles remain to be determined. There are many small RNAs that are in preclinical or clinical trials for cancer treatment, supporting the notion that non-coding RNAs could be used as therapeutic drugs.142,143
Drug Resistance
lncRNAs have been shown to promote drug resistance in many cancer types. One example is lncRNA SBF2 (SET binding factor 2), an antisense RNA to SBF2 protein that transfers temozolomide resistance to chemoresponsive glioblastoma cells.140 Temozolomide-resistant glioblastoma cells release lncRNA SBF2 in exosomes that are taken by neighboring chemoresponsive glioma cells. lncRNA SBF2 acts as a ceRNA for miR-151-3p and blocks its inhibitory binding with X-ray repair cross complementing 4 (XRCC4), an important protein that repairs double-stranded breaks in gliomas and thus contributes to chemoresistance.140 Mesenchymal stem cells release lncRNA histocompatibility leukocyte antigen complex P5 (HCP5) in the microenvironment, which contributes to stemness and chemoresistance in the recipient gastric carcinoma cells.144 lncRNA HCP5 interacts and inhibits miR-3619-5p, which targets PPARG coactivator 1α (PPARGC1A), an important gene in AMP-activated protein kinase (AMPK) regulation and fatty acid oxidation.145 PPARGC1A codes for protein peroxisome proliferator-activated receptor (PPAR) coactivator-1α (PGC1α), which forms a transcriptional complex with CCAAT enhancer-binding protein β (CEBPB) and induces carnitine palmitoyltransferase 1 (CPT1) transcription to enhance fatty acid oxidation. lncRNA HCP5 interaction with miR-3619-5p later releases an inhibitory effect on PPARGC1A, prompting CPT1-mediated fatty acid oxidation that contributes to stemness and chemoresistance in gastric carcinoma cells.144 lncRNA H19 is transferred via exosomes from doxorubicin-resistant to doxorubicin-sensitive breast cancer cells, making sensitive cells more resistant to drug treatment.146 Moreover, trastuzumab-resistant breast cancer cells secrete lncRNA AGAP2 antisense RNA (AGAP2-AS1) in exosomes, which promotes chemoresistance in drug-sensitive cells.147 Another lncRNA small nucleolar RNA host gene 14 (SNHG14) was found to be upregulated in trastuzumab-resistant breast cancer cells compared to parental breast cancer cells.148 Trastuzumab-resistant HER2+ breast cancer cells secrete lncRNA-SNHG14 in exosomes that induce the anti-apoptotic Bcl-2 signaling pathway in recipient trastuzumab-sensitive cells and promote drug resistance.148
Exosomal lncRNA ARSR (lncRNA activated in renal cell carcinoma [RCC] with sunitinib resistance) is highly expressed in sunitinib-resistant RCC cells and correlated with a clinically poor sunitinib response.149 lncRNA ARSR is transmitted by sunitinib-resistant RCC cells to sunitinib-sensitive RCC or endothelial cells via exosomes to facilitate drug resistance. Endothelial cells incubated with lncRNA ARSR containing exosomes show a poor response to anti-angiogenic effects of sunitinib, including tube formation inhibition. lncRNA ARSR acts as a ceRNA for miR-34 and miR-449, leading to the liberation and activation of their corresponding target miRNAs AXL and c-MET in RCC cells. Activation of cell surface receptor tyrosine kinases AXL and c-MET induces AKT, ERK, and STAT-3 phosphorylation, which contributes to sunitinib resistance in RCC cells.149 Furthermore, chemotherapeutic drugs, including sorafenib, camptothecin, and doxorubicin, induce lncRNA VLDLR antisense RNA 1 (VLDLR) in HCC cells that contribute to drug resistance.150 Drug-resistant HCC cells release lncRNA VLDLR in exosomes that promote resistance in recipient drug-sensitive HCC cells by promoting the expression of ATP-binding cassette (ABC) transporters, including ABCG2 (ATP-binding cassette, subfamily G member 2).150 ABC transporters pump a variety of drugs out of cells at the expense of ATP hydrolysis.151 Furthermore, mesenchymal stem cells induce proteasomal inhibitor and chemotherapy drug bortezomib resistance in multiple myeloma cells by secreting proteasome subunit α3 (PSMA3) and lncRNA PSMA3-AS1 (arises from the antisense strand of PSMA3) in exosomes.152 PSMA3 mRNA forms a duplex with the lncRNA PSMA3-AS1 transcript that promotes PSMA3 mRNA stability by reducing its decay. PSMA3 stability enhances bortezomib resistance in recipient myeloma cells. Furthermore, circulating exosomes containing PSMA3 and PSMA3-AS1 in multiple myeloma patients correlate with overall survival and progression-free survival.152 This suggests that exosomal lncRNAs are vital components in drug resistance and could be used as a target to enhance chemotherapeutic response.
Conclusions
It is clear from the above discussion that exosomal-mediated lncRNA transfer between tumor cells and the microenvironment is an important phenomenon that contributes to cancer cell survival, growth, and migration, reprogramming the tumor microenvironment, and the development of resistance mechanisms against chemotherapeutic drugs. In addition, exosomal lncRNAs could be used as a biomarker for many cancers, as their expressions correlate with the malignancy of the disease and response to therapy. Therefore, identification and evaluation of exosomal lncRNAs that contribute to cancer progression and drug resistance may be a valuable tool in the context of cancer treatment. To target exosomes is not feasible because they act as intercellular messengers that facilitate vital cell communication, but we can target these specific lncRNAs that play a vital role in exosome-mediated carcinogenesis.
Author Contributions
A.S.P. and K.B.C. wrote, edited, and reviewed the manuscript. Both authors approved the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
K.B.C.’s laboratory is supported in whole or part by NIH/National Cancer Institute (NCI) grant 1K22CA197074; Buffet Pilot & Pediatric Cancer Research Grants at the University of Nebraska Medical Center (UNMC), LB506 (Nebraska Department of Health and Human Services) and Leukemia Research Foundation, LB506 (Nebraska Department of Health and Human Services); and the Department of Biochemistry & Molecular Biology start-up grants. The authors would like to thank Jeffrey Patterson, University of Nebraska Medical Center (Omaha, NE, USA), for editorial assistance. Figures were prepared using EazyDraw and BioRender tools.
References
- 1.Zaborowski M.P., Balaj L., Breakefield X.O., Lai C.P. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65:783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares Martins T., Catita J., Martins Rosa I., A B da Cruz E Silva O., Henriques A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE. 2018;13:e0198820. doi: 10.1371/journal.pone.0198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumari S., Mg S., Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20:256–275. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovic M., Sharma M., Rahajeng J., Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol. Histopathol. 2010;25:99–112. doi: 10.14670/hh-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piper R.C., Katzmann D.J. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenring J.R. Recycling endosomes. Curr. Opin. Cell Biol. 2015;35:117–122. doi: 10.1016/j.ceb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheff D.R., Daro E.A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sönnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi T. Emerging roles of recycling endosomes. J. Biochem. 2013;153:505–510. doi: 10.1093/jb/mvt034. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer S.R. Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett. 2009;583:3811–3816. doi: 10.1016/j.febslet.2009.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannes L., Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Huotari J., Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piper R.C., Luzio J.P. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic. 2001;2:612–621. doi: 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- 15.Holme P.A., Solum N.O., Brosstad F., Røger M., Abdelnoor M. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and western blotting. Thromb. Haemost. 1994;72:666–671. [PubMed] [Google Scholar]
- 16.Hess C., Sadallah S., Hefti A., Landmann R., Schifferli J.A. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 1999;163:4564–4573. [PubMed] [Google Scholar]
- 17.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 19.Théry C., Boussac M., Véron P., Ricciardi-Castagnoli P., Raposo G., Garin J., Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 20.Simpson R.J., Lim J.W., Moritz R.L., Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 21.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 23.Wahlgren J., Karlson Tde.L., Glader P., Telemo E., Valadi H. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS ONE. 2012;7:e49723. doi: 10.1371/journal.pone.0049723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wubbolts R., Leckie R.S., Veenhuizen P.T., Schwarzmann G., Möbius W., Hoernschemeyer J., Slot J.W., Geuze H.J., Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 25.Lugini L., Cecchetti S., Huber V., Luciani F., Macchia G., Spadaro F., Paris L., Abalsamo L., Colone M., Molinari A. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012;189:2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 26.Segura E., Amigorena S., Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol. Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Skokos D., Le Panse S., Villa I., Rousselle J.C., Peronet R., David B., Namane A., Mécheri S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 28.Bae S., Brumbaugh J., Bonavida B. Exosomes derived from cancerous and non-cancerous cells regulate the anti-tumor response in the tumor microenvironment. Genes Cancer. 2018;9:87–100. doi: 10.18632/genesandcancer.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y.H., Shao L., Zhang Y., Zhou J., Liu B., Pan X., Geng Y.J., Yu X.Y., Li Y. Exosomes derived from embryonic stem cells as potential treatment for cardiovascular diseases. Adv. Exp. Med. Biol. 2017;998:187–206. doi: 10.1007/978-981-10-4397-0_13. [DOI] [PubMed] [Google Scholar]
- 30.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark. Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng Z.B., Poliakov A., Hardy R.W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frühbeis C., Fröhlich D., Kuo W.P., Krämer-Albers E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller S., Ridinger J., Rupp A.K., Janssen J.W., Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Li X.B., Zhang Z.R., Schluesener H.J., Xu S.Q. Role of exosomes in immune regulation. J. Cell. Mol. Med. 2006;10:364–375. doi: 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azmi A.S., Bao B., Sarkar F.H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellin G., Gardin C., Ferroni L., Chachques J.C., Rogante M., Mitrečić D., Ferrari R., Zavan B. Exosome in cardiovascular diseases: a complex world full of hope. Cells. 2019;8:166. doi: 10.3390/cells8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sardar Sinha M., Ansell-Schultz A., Civitelli L., Hildesjö C., Larsson M., Lannfelt L., Ingelsson M., Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-mediated metastasis: communication from a distance. Dev. Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Kim D.H., Kim H., Choi Y.J., Kim S.Y., Lee J.E., Sung K.J., Sung Y.H., Pack C.G., Jung M.K., Han B. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019;51:1–13. doi: 10.1038/s12276-019-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Z., Yang F., Yu L., Yu Z., Jiang L., Wang Q., Yang Y., Wang L., Cao X., Wang J. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J. Immunol. 2012;188:5954–5961. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 43.Harada T., Yamamoto H., Kishida S., Kishida M., Awada C., Takao T., Kikuchi A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108:42–52. doi: 10.1111/cas.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng W., Dean D.C., Hornicek F.J., Shi H., Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol. Cancer. 2019;18:124. doi: 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieckowski E.U., Visus C., Szajnik M., Szczepanski M.J., Storkus W.J., Whiteside T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirzaei R., Sarkar S., Dzikowski L., Rawji K.S., Khan L., Faissner A., Bose P., Yong V.W. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. OncoImmunology. 2018;7:e1478647. doi: 10.1080/2162402X.2018.1478647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia S., Lal B., Tung B., Wang S., Goodwin C.R., Laterra J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro-oncol. 2016;18:507–517. doi: 10.1093/neuonc/nov171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y., Li C.W., Chan L.C., Wei Y., Hsu J.M., Xia W., Cha J.H., Hou J., Hsu J.L., Sun L., Hung M.C. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28:862–864. doi: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Challagundla K.B., Wise P.M., Neviani P., Chava H., Murtadha M., Xu T., Kennedy R., Ivan C., Zhang X., Vannini I. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J. Natl. Cancer Inst. 2015;107:djv135. doi: 10.1093/jnci/djv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Q., Peng F., Chen J. The role of exosomal microRNAs in the tumor microenvironment of breast cancer. Int. J. Mol. Sci. 2019;20:3884. doi: 10.3390/ijms20163884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshii S., Hayashi Y., Iijima H., Inoue T., Kimura K., Sakatani A., Nagai K., Fujinaga T., Hiyama S., Kodama T. Exosomal microRNAs derived from colon cancer cells promote tumor progression by suppressing fibroblast TP53 expression. Cancer Sci. 2019;110:2396–2407. doi: 10.1111/cas.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu H.Y., Yu C.H., Zhang H.H., Zhang S.Z., Yu W.Y., Yang Y., Chen Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int. J. Biol. Macromol. 2019;132:470–477. doi: 10.1016/j.ijbiomac.2019.03.221. [DOI] [PubMed] [Google Scholar]
- 53.Chen C., Luo Y., He W., Zhao Y., Kong Y., Liu H., Zhong G., Li Y., Li J., Huang J. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Invest. 2020;130:404–421. doi: 10.1172/JCI130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M., Xu R., Rai A., Suwakulsiri W., Izumikawa K., Ishikawa H., Greening D.W., Takahashi N., Simpson R.J. Distinct shed microvesicle and exosome microRNA signatures reveal diagnostic markers for colorectal cancer. PLoS ONE. 2019;14:e0210003. doi: 10.1371/journal.pone.0210003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen D., Wu X., Xia M., Wu F., Ding J., Jiao Y., Zhan Q., An F. Upregulated exosomic miR-23b-3p plays regulatory roles in the progression of pancreatic cancer. Oncol. Rep. 2017;38:2182–2188. doi: 10.3892/or.2017.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hannafon B.N., Trigoso Y.D., Calloway C.L., Zhao Y.D., Lum D.H., Welm A.L., Zhao Z.J., Blick K.E., Dooley W.C., Ding W.Q. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90. doi: 10.1186/s13058-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 58.Venter J.C., Adams M.D., Myers E.W., Li P.W., Mural R.J., Sutton G.G., Smith H.O., Yandell M., Evans C.A., Holt R.A. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 59.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., FANTOM Consortium. RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group) The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 60.Saha S., Sparks A.B., Rago C., Akmaev V., Wang C.J., Vogelstein B., Kinzler K.W., Velculescu V.E. Using the transcriptome to annotate the genome. Nat. Biotechnol. 2002;20:508–512. doi: 10.1038/nbt0502-508. [DOI] [PubMed] [Google Scholar]
- 61.Kim Y.K., Kim V.N. Processing of intronic microRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasquinelli A.E., Ruvkun G. Control of developmental timing by microRNAs and their targets. Annu. Rev. Cell Dev. Biol. 2002;18:495–513. doi: 10.1146/annurev.cellbio.18.012502.105832. [DOI] [PubMed] [Google Scholar]
- 63.Ambros V., Lee R.C., Lavanway A., Williams P.T., Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr. Biol. 2003;13:807–818. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 64.Girard A., Sachidanandam R., Hannon G.J., Carmell M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- 65.Lee Y.S., Shibata Y., Malhotra A., Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu C., Huang Y.H. Progress in long non-coding RNAs in animals. Yi Chuan. 2017;39:1054–1065. doi: 10.16288/j.yczz.17-120. [DOI] [PubMed] [Google Scholar]
- 67.Wang H.V., Chekanova J.A. Long noncoding RNAs in plants. Adv. Exp. Med. Biol. 2017;1008:133–154. doi: 10.1007/978-981-10-5203-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris K.A., Breaker R.R. Large noncoding RNAs in bacteria. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.RWR-0005-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Till P., Mach R.L., Mach-Aigner A.R. A current view on long noncoding RNAs in yeast and filamentous fungi. Appl. Microbiol. Biotechnol. 2018;102:7319–7331. doi: 10.1007/s00253-018-9187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi K., Zhang Y., Wang Y., Wang Z., Xie M., Jin Z., Zhao T. Long noncoding RNA and its role in virus infection and pathogenesis. Front. Biosci. 2019;24:777–789. doi: 10.2741/4750. [DOI] [PubMed] [Google Scholar]
- 71.St Laurent G., Wahlestedt C., Kapranov P. The landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D., Khalil A.M., Zuk O., Amit I., Rabani M. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beckedorff F.C., Ayupe A.C., Crocci-Souza R., Amaral M.S., Nakaya H.I., Soltys D.T., Menck C.F., Reis E.M., Verjovski-Almeida S. The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation. PLoS Genet. 2013;9:e1003705. doi: 10.1371/journal.pgen.1003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin J.J., Lv W., Xia P., Xu Z.Y., Zheng A.D., Wang X.J., Wang S.S., Zeng R., Luo H.M., Li G.L., Zuo B. Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc. Natl. Acad. Sci. USA. 2018;115:E9802–E9811. doi: 10.1073/pnas.1801471115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J.T., Davidow L.S., Warshawsky D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nat. Genet. 1999;21:400–404. doi: 10.1038/7734. [DOI] [PubMed] [Google Scholar]
- 76.Panning B. X-chromosome inactivation: the molecular basis of silencing. J. Biol. 2008;7:30. doi: 10.1186/jbiol95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Q., Chao T.C., Patil V.S., Qin Y., Tiwari S.K., Chiou J., Dobin A., Tsai C.M., Li Z., Dang J. The long noncoding RNA ROCKI regulates inflammatory gene expression. EMBO J. 2019;38:e100041. doi: 10.15252/embj.2018100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho S.W., Xu J., Sun R., Mumbach M.R., Carter A.C., Chen Y.G., Yost K.E., Kim J., Jing He J., Nevins S.A. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell. 2018;173:1398–1412.e22. doi: 10.1016/j.cell.2018.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latos P.A., Pauler F.M., Koerner M.V., Şenergin H.B., Hudson Q.J., Stocsits R.R., Allhoff W., Stricker S.H., Klement R.M., Warczok K.E. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–1472. doi: 10.1126/science.1228110. [DOI] [PubMed] [Google Scholar]
- 80.Pavlaki I., Alammari F., Sun B., Clark N., Sirey T., Lee S., Woodcock D.J., Ponting C.P., Szele F.G., Vance K.W. The long non-coding RNA Paupar promotes KAP1-dependent chromatin changes and regulates olfactory bulb neurogenesis. EMBO J. 2018;37:e98219. doi: 10.15252/embj.201798219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spector D.L., Lamond A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011;3:3. doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.West J.A., Davis C.P., Sunwoo H., Simon M.D., Sadreyev R.I., Wang P.I., Tolstorukov M.Y., Kingston R.E. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol. Cell. 2014;55:791–802. doi: 10.1016/j.molcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrone S., Whiteside T.L. Tumor microenvironment and immune escape. Surg. Oncol. Clin. N. Am. 2007;16:755–774. doi: 10.1016/j.soc.2007.08.004. viii. [DOI] [PubMed] [Google Scholar]
- 85.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watnick R.S. The role of the tumor microenvironment in regulating angiogenesis. Cold Spring Harb. Perspect. Med. 2012;2:a006676. doi: 10.1101/cshperspect.a006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maia J., Caja S., Strano Moraes M.C., Couto N., Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen D., Lu T., Tan J., Li H., Wang Q., Wei L. Long non-coding RNAs as communicators and mediators between the tumor microenvironment and cancer cells. Front. Oncol. 2019;9:739. doi: 10.3389/fonc.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni C., Fang Q.Q., Chen W.Z., Jiang J.X., Jiang Z., Ye J., Zhang T., Yang L., Meng F.B., Xia W.J. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+γδ1 Treg cells. Signal Transduct. Target. Ther. 2020;5:41. doi: 10.1038/s41392-020-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Işın M., Uysaler E., Özgür E., Köseoğlu H., Şanlı Ö., Yücel O.B., Gezer U., Dalay N. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front. Genet. 2015;6:168. doi: 10.3389/fgene.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X., Lei Y., Wu M., Li N. Regulation of macrophage activation and polarization by HCC-derived exosomal lncRNA TUC339. Int. J. Mol. Sci. 2018;19:2958. doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang Y., Song X., Li Y., Chen B., Zhao W., Wang L., Zhang H., Liu Y., Han D., Zhang N. lncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol. Cancer. 2020;19:85. doi: 10.1186/s12943-020-01206-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Masoud G.N., Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi K., Yan I.K., Haga H., Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J. Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xue M., Chen W., Xiang A., Wang R., Chen H., Pan J., Pang H., An H., Wang X., Hou H., Li X. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol. Cancer. 2017;16:143. doi: 10.1186/s12943-017-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng X., Ruan H., Zhang X., Xu X., Zhu Y., Peng H., Zhang X., Kong F., Guan M. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int. J. Cancer. 2020;146:1700–1716. doi: 10.1002/ijc.32608. [DOI] [PubMed] [Google Scholar]
- 98.Ren J., Ding L., Zhang D., Shi G., Xu Q., Shen S., Wang Y., Wang T., Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui X., Xiao D., Cui Y., Wang X. Exosomes-derived long non-coding RNA HOTAIR reduces laryngeal cancer radiosensitivity by regulating microRNA-454-3p/E2F2 axis. OncoTargets Ther. 2019;12:10827–10839. doi: 10.2147/OTT.S224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng T., Zhang P., Sun Y., Wang Y., Tong J., Dai H., Hua Z. High throughput sequencing identifies breast cancer-secreted exosomal lncRNAs initiating pulmonary pre-metastatic niche formation. Gene. 2019;710:258–264. doi: 10.1016/j.gene.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Lu Y., Chen L., Li L., Cao Y. Exosomes derived from brain metastatic breast cancer cells destroy the blood-brain barrier by carrying lncRNA GS1-600G8.5. BioMed Res. Int. 2020;2020:7461727. doi: 10.1155/2020/7461727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin Z., Zhou Y., Ma T., Chen S., Shi N., Zou Y., Hou B., Zhang C. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J. Cell. Mol. Med. 2020;24:5028–5038. doi: 10.1111/jcmm.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu J., Xiao Y., Liu B., Pan S., Liu Q., Shan Y., Li S., Qi Y., Huang Y., Jia L. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2020;39:54. doi: 10.1186/s13046-020-01562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Y., Ding H., Wei M., Zha W., Guan S., Liu N., Li Y., Tan Y., Wang Y., Wu F. MSC-secreted exosomal H19 promotes trophoblast cell invasion and migration by downregulating let-7b and upregulating FOXO1. Mol. Ther. Nucleic Acids. 2020;19:1237–1249. doi: 10.1016/j.omtn.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song Y., Li H., Ren X., Li H., Feng C. SNHG9, delivered by adipocyte-derived exosomes, alleviates inflammation and apoptosis of endothelial cells through suppressing TRADD expression. Eur. J. Pharmacol. 2020;872:172977. doi: 10.1016/j.ejphar.2020.172977. [DOI] [PubMed] [Google Scholar]
- 106.Zhao W., Qin P., Zhang D., Cui X., Gao J., Yu Z., Chai Y., Wang J., Li J. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany NY) 2019;11:9581–9596. doi: 10.18632/aging.102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu T., Hu J. Melanoma-derived exosomes induce reprogramming fibroblasts into cancer-associated fibroblasts via Gm26809 delivery. Cell Cycle. 2019;18:3085–3094. doi: 10.1080/15384101.2019.1669380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sinkala M., Mulder N., Patrick Martin D. Metabolic gene alterations impact the clinical aggressiveness and drug responses of 32 human cancers. Commun. Biol. 2019;2:414. doi: 10.1038/s42003-019-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liberti M.V., Locasale J.W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Papa S., Choy P.M., Bubici C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene. 2019;38:2223–2240. doi: 10.1038/s41388-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castellano J.J., Marrades R.M., Molins L., Viñolas N., Moises J., Canals J., Han B., Li Y., Martinez D., Monzó M., Navarro A. Extracellular vesicle lincRNA-p21 expression in tumor-draining pulmonary vein defines prognosis in NSCLC and modulates endothelial cell behavior. Cancers (Basel) 2020;12:734. doi: 10.3390/cancers12030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X., Xu Y., Wang X., Jiang C., Han S., Dong K., Shen M., Xu D. lincRNA-p21 suppresses development of human prostate cancer through inhibition of PKM2. Cell Prolif. 2017;50:e12395. doi: 10.1111/cpr.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 2011;43:969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Li Y., Zhao Z., Liu W., Li X. SNHG3 functions as miRNA sponge to promote breast cancer cells growth through the metabolic reprogramming. Appl. Biochem. Biotechnol. 2020;191:1084–1099. doi: 10.1007/s12010-020-03244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren Y., Jia H.H., Xu Y.Q., Zhou X., Zhao X.H., Wang Y.F., Song X., Zhu Z.Y., Sun T., Dou Y. Paracrine and epigenetic control of CAF-induced metastasis: the role of HOTAIR stimulated by TGF-β1 secretion. Mol. Cancer. 2018;17:5. doi: 10.1186/s12943-018-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y.L., Liu L.C., Hung Y., Chen C.J., Lin Y.Z., Wu W.R., Wang S.C. Long non-coding RNA HOTAIR in circulatory exosomes is correlated with ErbB2/HER2 positivity in breast cancer. Breast. 2019;46:64–69. doi: 10.1016/j.breast.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 119.Lu R., Zhang J., Zhang W., Huang Y., Wang N., Zhang Q., Qu S. Circulating HOTAIR expression predicts the clinical response to neoadjuvant chemotherapy in patients with breast cancer. Cancer Biomark. 2018;22:249–256. doi: 10.3233/CBM-170874. [DOI] [PubMed] [Google Scholar]
- 120.Wei S., Fan Q., Yang L., Zhang X., Ma Y., Zong Z., Hua X., Su D., Sun H., Li H., Liu Z. Promotion of glycolysis by HOTAIR through GLUT1 upregulation via mTOR signaling. Oncol. Rep. 2017;38:1902–1908. doi: 10.3892/or.2017.5840. [DOI] [PubMed] [Google Scholar]
- 121.Ma Y., Hu M., Zhou L., Ling S., Li Y., Kong B., Huang P. Long non-coding RNA HOTAIR promotes cancer cell energy metabolism in pancreatic adenocarcinoma by upregulating hexokinase-2. Oncol. Lett. 2019;18:2212–2219. doi: 10.3892/ol.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W., Chandel N., Laakso M., Muller W.J., Allen E.L. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeWaal D., Nogueira V., Terry A.R., Patra K.C., Jeon S.M., Guzman G., Au J., Long C.P., Antoniewicz M.R., Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018;9:446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma J., Fan Y., Feng T., Chen F., Xu Z., Li S., Lin Q., He X., Shi W., Liu Y. HOTAIR regulates HK2 expression by binding endogenous miR-125 and miR-143 in oesophageal squamous cell carcinoma progression. Oncotarget. 2017;8:86410–86422. doi: 10.18632/oncotarget.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen F., Chen J., Yang L., Liu J., Zhang X., Zhang Y., Tu Q., Yin D., Lin D., Wong P.P. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019;21:498–510. doi: 10.1038/s41556-019-0299-0. [DOI] [PubMed] [Google Scholar]
- 126.Filippov-Levy N., Cohen-Schussheim H., Tropé C.G., Hetland Falkenthal T.E., Smith Y., Davidson B., Reich R. Expression and clinical role of long non-coding RNA in high-grade serous carcinoma. Gynecol. Oncol. 2018;148:559–566. doi: 10.1016/j.ygyno.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 127.Hu Q., Ye Y., Chan L.C., Li Y., Liang K., Lin A., Egranov S.D., Zhang Y., Xia W., Gong J. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat. Immunol. 2019;20:835–851. doi: 10.1038/s41590-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu D., Zhao B., Cao X., Wan J. Long non-coding RNA LINK-A promotes glioma cell growth and invasion via lactate dehydrogenase A. Oncol. Rep. 2017;38:1525–1532. doi: 10.3892/or.2017.5806. [DOI] [PubMed] [Google Scholar]
- 129.Zheng R., Du M., Wang X., Xu W., Liang J., Wang W., Lv Q., Qin C., Chu H., Wang M. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer. 2018;17:143. doi: 10.1186/s12943-018-0880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ortega-Molina A., Serrano M. PTEN in cancer, metabolism, and aging. Trends Endocrinol. Metab. 2013;24:184–189. doi: 10.1016/j.tem.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun K., Deng W., Zhang S., Cai N., Jiao S., Song J., Wei L. Paradoxical roles of autophagy in different stages of tumorigenesis: protector for normal or cancer cells. Cell Biosci. 2013;3:35. doi: 10.1186/2045-3701-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ding L., Ren J., Zhang D., Li Y., Huang X., Hu Q., Wang H., Song Y., Ni Y., Hou Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via lncRNA-CAF/interleukin-33. Carcinogenesis. 2018;39:397–406. doi: 10.1093/carcin/bgy006. [DOI] [PubMed] [Google Scholar]
- 133.Zeng R., Song X.J., Liu C.W., Ye W. lncRNA ANRIL promotes angiogenesis and thrombosis by modulating microRNA-99a and microRNA-449a in the autophagy pathway. Am. J. Transl. Res. 2019;11:7441–7448. [PMC free article] [PubMed] [Google Scholar]
- 134.Abbastabar M., Sarfi M., Golestani A., Karimi A., Pourmand G., Khalili E. Tumor-derived urinary exosomal long non-coding RNAs as diagnostic biomarkers for bladder cancer. EXCLI J. 2020;19:301–310. doi: 10.17179/excli2019-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cui C., Li Z., Wu D. The long non-coding RNA H19 induces hypoxia/reoxygenation injury by up-regulating autophagy in the hepatoma carcinoma cells. Biol. Res. 2019;52:32. doi: 10.1186/s40659-019-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma R., Zhang B.W., Zhang Z.B., Deng Q.J. lncRNA MALAT1 knockdown inhibits cell migration and invasion by suppressing autophagy through miR-384/GOLM1 axis in glioma. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2601–2615. doi: 10.26355/eurrev_202003_20529. [DOI] [PubMed] [Google Scholar]
- 138.Xu R., Ji J., Zhang X., Han M., Zhang C., Xu Y., Wei Y., Wang S., Huang B., Chen A. PDGFA/PDGFRα-regulated GOLM1 promotes human glioma progression through activation of AKT. J. Exp. Clin. Cancer Res. 2017;36:193. doi: 10.1186/s13046-017-0665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang C., Robbins P.D. The roles of tumor-derived exosomes in cancer pathogenesis. Clin. Dev. Immunol. 2011;2011:842849. doi: 10.1155/2011/842849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Z., Yin J., Lu C., Wei Y., Zeng A., You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J. Exp. Clin. Cancer Res. 2019;38:166. doi: 10.1186/s13046-019-1139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tong Y., Yang L., Yu C., Zhu W., Zhou X., Xiong Y., Wang W., Ji F., He D., Cao X. Tumor-secreted exosomal lncRNA POU3F3 promotes cisplatin resistance in ESCC by inducing fibroblast differentiation into CAFs. Mol. Ther. Oncolytics. 2020;18:1–13. doi: 10.1016/j.omto.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.(2019). Delivering the promise of RNA therapeutics. Nat. Med. 25, 1321. [DOI] [PubMed]
- 143.Zhou L.Y., Qin Z., Zhu Y.H., He Z.Y., Xu T. Current RNA-based therapeutics in clinical trials. Curr. Gene Ther. 2019;19:172–196. doi: 10.2174/1566523219666190719100526. [DOI] [PubMed] [Google Scholar]
- 144.Wu H., Liu B., Chen Z., Li G., Zhang Z. MSC-induced lncRNA HCP5 drove fatty acid oxidation through miR-3619-5p/AMPK/PGC1α/CEBPB axis to promote stemness and chemo-resistance of gastric cancer. Cell Death Dis. 2020;11:233. doi: 10.1038/s41419-020-2426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng C.F., Ku H.C., Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018;19:3447. doi: 10.3390/ijms19113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X., Pei X., Guo G., Qian X., Dou D., Zhang Z., Xu X., Duan X. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J. Cell. Physiol. 2020;235:6896–6904. doi: 10.1002/jcp.29585. [DOI] [PubMed] [Google Scholar]
- 147.Zheng Z., Chen M., Xing P., Yan X., Xie B. Increased expression of exosomal AGAP2-AS1 (AGAP2 antisense RNA 1) in breast cancer cells inhibits trastuzumab-induced cell cytotoxicity. Med. Sci. Monit. 2019;25:2211–2220. doi: 10.12659/MSM.915419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong H., Wang W., Chen R., Zhang Y., Zou K., Ye M., He X., Zhang F., Han J. Exosome-mediated transfer of lncRNA-SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int. J. Oncol. 2018;53:1013–1026. doi: 10.3892/ijo.2018.4467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Qu L., Ding J., Chen C., Wu Z.J., Liu B., Gao Y., Chen W., Liu F., Sun W., Li X.F. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi K., Yan I.K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fletcher J.I., Williams R.T., Henderson M.J., Norris M.D., Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updat. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 152.Xu H., Han H., Song S., Yi N., Qian C., Qiu Y., Zhou W., Hong Y., Zhuang W., Li Z. Exosome-transmitted PSMA3 and PSMA3-AS1 promote proteasome inhibitor resistance in multiple myeloma. Clin. Cancer Res. 2019;25:1923–1935. doi: 10.1158/1078-0432.CCR-18-2363. [DOI] [PubMed] [Google Scholar]