Abstract
Trimeric intracellular cation (TRIC) channels have been identified as monovalent cation channels that are located in the ER/SR membrane. Two isoforms discovered in mammals are TRIC-A (TMEM38a) and TRIC-B (TMEM38b). TRIC-B ubiquitously expresses in all tissues, and TRIC-B−/− mice is lethal at the neonatal stage. TRIC-A mainly expresses in excitable cells. TRIC-A−/− mice survive normally but show abnormal SR Ca2+ handling in both skeletal and cardiac muscle cells. Importantly, TRIC-A mutations have been identified in human patients with stress-induced arrhythmia. In the past decade, important discoveries have been made to understand the structure and function of TRIC channels, especially its role in regulating intracellular Ca2+ homeostasis. In this review article, we focus on the potential roles of TRIC-A in regulating cardiac function, particularly its effects on intracellular Ca2+ signaling of cardiomyocytes and discuss the current knowledge gaps.
Keywords: Heart, RyR, Mitochondria, Nuclear envelope
Introduction
Ca2+ signaling plays a central role in cardiac physiology [18]. The regulation of Ca2+ signaling shows a great dynamic range in terms of frequency and spatial-temporal relationship, forming a variety of patterns from localized brief Ca2+ bursts to long-lasting, global Ca2+ transients [8, 17, 18, 53, 68, 81]. On the other hand, sustained elevation of intracellular Ca2+ is known to be cytotoxic, which would lead to mitochondria damage, dysregulation of gene expression, and apoptotic and necrotic cell death. Thus, physiological Ca2+ homeostasis and signaling must be tightly regulated. To assure this, many molecules and proteins, including signaling ligands and receptors, are coupled together and function in a coordinated manner [6, 7]. Compromise of such Ca2+ homeostasis and signaling has been linked to different human diseases, including muscle dysfunction and heart failure [8, 17, 53, 68, 81].
In cardiomyocytes, rhythmic electrical excitation travelling through the cellular membrane triggers the entry of Ca2+ through L-type Ca2+ channels that mediates the systolic release of Ca2+ from the sarcoplasmic reticulum (SR) to the cytosol through ryanodine receptor 2 (RyR2) channels [7, 12, 18, 95]. One of the fundamental goals of cardiac physiology is to understand the detailed mechanisms behind Ca2+ handling and to find approaches to correct defective Ca2+ cycling associated with heart failure and arrhythmias.
Trimeric intracellular cation (TRIC) channels have been identified as monovalent cation channels that are located in the ER/SR membrane [101, 109]. Two isoforms discovered in mammals are TRIC-A (TMEM38a) and TRIC-B (TMEM38b). While TRIC-B ubiquitously expresses in all tissues, TRIC-A mainly expresses in excitable cells. The aggravated embryonic lethality in TRIC-A and TRIC-B double knockout animals suggests the crucial role of TRIC channels in embryonic development [101]. TRIC-A−/− mice survive normally but show abnormal SR Ca2+ handling in skeletal muscle, characterized by irregular contractile force during fatigue, compromised Ca2+ sparks, and Ca2+ overload in the SR [105]. Additionally, TRIC-A ablation causes hypertension in mice due to altered Ca2+ signaling in smooth muscle [98]. On the other hand, the absence of TRIC-B is lethal in mice at the neonatal stage [101]. Later studies demonstrated the crucial function of TRIC-B in inositol trisphosphate receptor (IP3R)–mediated Ca2+ signaling from the endoplasmic reticulum (ER) in non-excitable cells [97]. Takeshima’s group demonstrated the important function of TRIC-B in the development of bone and identified several genetic mutations within the TRIC-B locus that are associated with osteogenesis imperfecta [40, 107].
The crystal structure of TRIC has been resolved and provided insights into their functional mechanism [43, 65, 82, 91, 100]. Yang et al. resolved the nematode TRIC homologs and demonstrated that the channel consists of seven transmembrane domains, predicted by a previous bioinformatic approach [79], and forms a homotrimeric complex within the lipid membrane [100]. Each monomer contains an hourglass-shaped, fluid-filled, cation-permeable pore. This important feature has subsequently been confirmed in the structures of other types of prokaryotic TRIC channels [43, 82], and in vertebrate TRIC-A and TRIC-B channels [91].
TRIC-A functions as a counter-current channel in SR/ER Ca2+ signaling
During excitation-contraction (EC) coupling, the opening of RyR allows a massive amount of Ca2+ moving from the SR to the cytosol due to the electrochemical gradient of Ca2+ ions. Since Ca2+ is a cation, this instant efflux of Ca2+ would result in the accumulation of negative charges inside the SR. This potential asymmetry would hinder the subsequent Ca2+ release. Similarly, during relaxation, the uptake of Ca2+ into the ER/SR also requires a similar opposite ionic counter movement. In the absence of an additional flow of counter-ions to balance the charge, the Ca2+ release or uptake during EC coupling would be compromised. Thus, robust counter-ion flux across the SR/ER is crucial to compensate the potential changes and promotes efficient Ca2+ release and uptake during EC coupling [9, 19, 27, 28, 85, 89, 90]. Identification of the molecular identity of those counter-ion channels are crucial for understanding the Ca2+ regulation and may provide potential molecular targets for developing therapeutic means for cardiac and skeletal muscle diseases.
Yazawa et al. first unveiled the molecular identity of such K+-permeable counter-ion channels as trimeric intracellular cation channels (TRICs) [101]. Sitsapesan’s group conducted several studies concerning the biophysical characteristics of TRIC-A and TRIC-B channels. They found that TRIC-A displayed different conductance properties [67, 88]. Unlike TRIC-A, TRIC-B preferably opened at sub-conductance levels [88]. Further experiments unveiled other differences between these two isoforms. For TRIC-A channels, the conductance property did not change regardless of whether they were isolated or clustered in space. But for TRIC-B channels, grouped trimmers displayed a much higher open probability comparing to isolated trimmers. Thus, they proposed that physical interactions between multiple TRIC-B trimeric channels changed the gating behavior of the channels [64]. The activation of TRIC-A channel was regulated by both voltage and Ca2+ binding [67, 101]. Ca2+ regulation of TRIC channels was investigated in depth by Chen’s group in a recent study [91]. At resting state, Ca2+ binds to G74 residue on the threefold axis, which is located in the luminal side of TRIC-A, and stabilizes the closure of the pore. During systolic Ca2+ release, depletion of luminal Ca2+ results in the dissociation of Ca2+ binding to the channel. This change enables the Ca2+-dependent gating of the TRIC-A channel. They also found K129 was critical for the TRIC-A pore conductance. K129A mutation resulted in constant opening of the channels while TRIC-A channels with K129Q mutation mostly stayed closed. Taken all together, TRIC channels ideally meet all expectations of the counter-current channels for the ER/SR Ca2+ release, due to their voltage-dependent, luminal Ca2+-regulated, and potassium-permeable characteristics [91].
However, the assumption that TRIC channels carry the essential counter-current for SR charge compensation was challenged by Gillespie and Fill, who argued that RyR channels are poorly Ca2+ selective and exhibit high conductance of monovalent and divalent cations such as K+ and Mg2+, which could carry the counter-current [31]. Using a Poisson-Nernst-Planck/density functional theory (PNP/DFT) model, their calculation demonstrated that large K+ and Mg2+ counter-current through RyR could clamp the SR membrane potential far from the Nernst potential for Ca2+, nullifying the need for additional counter-current channels [30, 31]. Moreover, Guo et al. studied the isolated cardiac SR microsomes and saponin-permeabilized cardiomyocytes. They revealed that replacement of cytosolic K+ with Na+ or Cs+ failed to affect single RyR2 channel currents, open probability, or Ca2+ sparks. Thus, they rejected the idea that SR K+ channels have significant contribution to the counter-current supporting RyR2 Ca2+ release [35]. On the other hand, recent mathematical modeling suggests that no single channel type is essential for the counter-current. Instead, all possible sources of channel-mediated cation or anion counter-current would be utilized to form a cascading network of counter-currents in the entire SR [112]. This network would ensure an efficient counter-current to support Ca2+ release in different physiological conditions. In this case, TRIC-A may not represent an indispensable prerequisite for efficient SR Ca2+ release as presumed earlier but still contribute to the ion equilibrium across SR during repetitive cycles of release events. This conclusion, together with the earlier observation that cardiomyocytes from TRIC-A/TRIC-B double knockout mice exhibit significantly elevated caffeine-induced Ca2+ release [101], raised an intriguing question: could TRIC channels have additional Ca2+ regulatory roles for RyR other than the counter-current function?
TRIC-A regulates SR Ca2+ signaling through direct modulation of RyR activity
RyRs are high conductance Ca2+ release channels located on the junctional SR in striated muscles [60]. In addition to small molecules like Ca2+, Mg2+, and ATP that regulates RyR2 directly, many other RyR2 modulators have been identified and extensively studied, including FKBP12.6 [83], calmodulin (CaM) [61], sorcin [51], protein kinase A (PKA) [55], protein phosphatase 1 and 2A (PP1, and PP2A) [56], and Ca2+/calmodulin-dependent protein kinase type II (CaMKII) [93]. It is worth noting that almost all of those RyR2 regulatory proteins function as stabilizers of RyR2 activities, whose defects would commonly lead to Ca2+ leakage through RyR2 channels [4, 14].
A study from Sitsapesan’s group found that in skeletal muscle, the RyR1 channel from the TRIC-A−/− mice displayed increased sensitivity to Mg2+ inhibition and a defective response to protein kinase A phosphorylation [26]. Meanwhile, physiological activators such as ATP are less effective in activating individual RyR1 channels reconstituted into the lipid bilayer membrane. However, they also reported that the Ca2+-dependent control of RyR1 channel was not altered in the absence of TRIC-A [26]. These findings are consistent with the potential role of TRIC-A as an enhancer of RyR channels, such that the absence of TRIC-A leads to reduced RyR channel function.
Recently, Zhou et al. presented evidence of a direct modulation of RyR2 activity by TRIC-A [110]. We observed that cardiomyocytes derived from TRIC-A−/− mice have a lower basal Ca2+ spark frequency and a higher SR Ca2+ content. These myocytes also have slow-rising and prolonged intracellular Ca2+ transient profiles. We further explored the role of TRIC-A in HEK293 cells with inducible expression of RyR2 as a model of store-overload induced Ca2+ release (SOICR) [41, 110]. Overexpression of TRIC-A in HEK293 cells leads to decreased spontaneous Ca2+ oscillations and hence help mitigate SOICR as a result of reduced ER Ca2+ content. This phenotype is specific to TRIC-A as co-expression of TRIC-B does not alter the amplitude or kinetics of SOICR in the same setting. Furthermore, our biochemical and immunohistochemical data demonstrated a direct interaction between TRIC-A and RyR2, possibly via the carboxyl-terminal tail (CTT) domain of TRIC-A (CTT-A). This result is consistent with the data from Dr. Zorzato’s group where they reported that TRIC-A (named SRP-27 in their paper) directly binds to RyR in their pulled-down and coimmunoprecipitation assays [10]. We further unveiled that CTT-A is a crucial structure responsible for the RyR2 modulation as evidenced by both HEK293 SOICR assay as well as measuring the effect of CTT-A on RyR2 channel from cardiac SR vesicles in reconstituted lipid bilayer assay [110]. Interestingly, the CTT domains are absent in all of the recent structural studies. The investigators claim this domain may decrease the stability of the crystal [43, 65, 82, 100] and suggest it could be a flexible function domain.
Further computational modeling predicted several potential binding sites of TRIC-A on RyR2 [110]. Remarkably, one of the potential binding sites is located at the SPRY domain of RyR. It is known that the SPRY domain of RyR could bind to the dihydropyridine receptor for control of RyR channel activity [21]. Thus, the hypothesized interaction of CTT-A and SPRY domain could be a potential target site for therapeutic regulation of RyR activity. Together, these data revealed a novel role of TRIC-A as a direct modulator of RyR2, in addition to its counter-ion channel function. Both functions would enhance RyR2-mediated Ca2+ release in cardiac muscle.
Mutations in RyR2 linked to cardiac arrhythmia, including catecholaminergic polymorphic ventricular tachycardia (CPVT), have been identified as gain of function mutations such as N4104K, R4497C, and N4895D [41, 69]. Those mutations enhance the activity of RyR2, elevate Ca2+ leakage, and subsequently lead to cardiac dysfunction [4, 41]. Therefore, stabilizing RyR2 activity to prevent the leakage of Ca2+ has been a major target for the potential treatment of cardiac diseases. Interestingly, several novel CPVT mutations, including I4855M [73] and A4860G [42], have been identified as loss-of-function mutations. Moreover, phosphorylation of RyR2 at S2808 has been shown to promote the hyperactivity of RyR2 and contribute to the pathological condition of the heart [76]. However, the ablation of the RyR2 phosphorylation at Ser-2808 did not reverse the cardiac phenotypes but rather exacerbated the disease phenotype by reducing the survival rate and impairing in vivo cardiac function [49]. Thus, accumulating evidence shows that RyR2 requires tight and balanced regulations not only by negative regulators but also by positive modulators. Either hyper- or de- activation of RyR2 could result in pathological consequences. The phenotypes of TRIC-A−/− heart show some similar characteristics to those in RyR2-S2808A [87], RyR2-A4860G [106], and RyR2-Ex3-del+/− mice [50], which further suggests the importance of the proper regulation of RyR2 channels. Potential therapeutic interventions can be used to target the functional interaction between TRIC-A and RyR2 to restore defective Ca2+ signaling in cardiovascular diseases.
The functional crosstalk between IP3R and RyR-mediated Ca2+ signaling has also been implicated in muscle and heart cells under physiological and pathological conditions [24, 44, 46, 84, 102, 103, 111]. Dissecting the role of TRIC-A and TRIC-B in RyR/IP3R crosstalk regulation and Ca2+ signaling regulation in physiological and pathological condition will be another essential task of future research.
TRIC-A regulates SOCE through interaction with STIM1/Orai1 complex
ER/SR Ca2+ modulation has been the primary focus of TRIC studies in the past decade. However, a new study from Shrestha et al. unveiled a new role of TRIC-A in the regulation of Ca2+ entry mechanism [78]. They found that TRIC-A modulated store operated Ca2+ entry (SOCE) by interacting with stromal interaction molecule 1 (STIM1)/Ca2+-release activated Ca2+ channel 1(Orai1) complex [78]. STIM1 is an ER/SR-resident transmembrane protein with a Ca2+ binding domain. Upon [Ca2+]ER depletion, dissociation of Ca2+ results in STIM1 oligomerization and its translocation to ER–plasma membrane (PM) junctions. These STIM1 clusters would recruit and activate the Orai1 channels on the plasma membrane and trigger Ca2+ entry into the cytosol [71, 94]. Using mutant Orai1 or Orai1 blocker BTP2, Shrestha et al. showed that the RyR2-induced Ca2+ oscillations in HEK293 cells required the SOCE machinery. Upon ER Ca2+ store depletion, TRIC-A channels co-clustered with the STIM1/Orai1 complex within ER–PM junctions. The association of STIM1 with TRIC-A reduced the co-localization of STIM1 and Orai in the cells, thus suppressing SOCE. Furthermore, they demonstrated that knocking down of TRIC-A in HL-1 would promote SOCE, mimicking the effects of STIM1 overexpression.
Several studies have established the physiological and pathological relevance of STIM1 in the heart [66, 72]. Silencing of STIM1 results in compromised cardiac function as well as reduced cardiomyocyte size [5]. On the other hand, STIM1 overexpression in the transgenic mice leads to cardiac hypertrophy and sudden death [20]. Although STIM1 is abundantly expressed in neonatal cardiomyocytes as well as in HL-1 cells, the level in adult cardiomyocytes is low [86]. Moreover, Hill’s group demonstrated that SOCE abundantly presents in neonatal cardiomyocytes; however, SOCE is absent in adult cardiomyocytes [52]. Therefore, the role of TRIC-A interaction with STIM1 in the control of SOCE in cardiomyocytes needs further investigation.
Potential role of TRIC-A in mitochondrial metabolism and SR-mitochondria crosstalk
The crosstalk between intracellular organelles has drawn great attention in cell biology and physiology studies in recent years. The crosstalk between ER/SR and mitochondria-mediated Ca2+ signaling plays an important role in physiological and pathological conditions [74, 75]. Li et al. presented evidence implicating TRIC-A in regulation of mitochondrial metabolism through directly or indirectly modulating mitochondrial Ca2+ signaling [47]. Mitochondria play a crucial role in oxidative metabolism that produces 95% energy required for the cell function [3]. In cardiomyocytes, Ca2+ uptake by mitochondria is an important messenger for matching energy supply to demand during various physiological workloads. This is achieved through Ca2+-induced activation of Krebs cycle dehydrogenases and pyruvate dehydrogenase [15, 16, 36, 58]. Additionally, mitochondrial Ca2+ has also been indicated to enhance the activities of complexes I, II, IV, and V along the respiratory chain [22, 32]. However, persistent augmentation of Ca2+ handling in cardiomyocytes, triggered by repetitive isoproterenol treatment, chronic pressure overload, or ischemia/reperfusion, would lead to pathological mitochondrial Ca2+ overload, which is not a simple compensatory mechanism to increase energy output [37, 48, 75, 77, 80, 96, 108]. This pathological overload usually occurs concomitantly with increased production of mitochondrial reactive oxygen species (ROS), which is causally related to progressive heart failure [57, 63]. Moreover, recent studies revealed that mitochondrial Ca2+ overload enhanced the activity of Na+/Ca2+/Li+ exchanger (NCLX), leading to Ca2+ extrusion at the cost of increased matrix Na+. Matrix Na+ interacts with phospholipids, such as phosphatidylcholine, of the inner mitochondria membrane, leads to reduced membrane fluidityand, the diffusion of ubiquinol (coenzyme Q) from glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or complex II to complex III of the respiratory chain, and elevation of ROS production of complex III at Qo site [38]. ROS thus activates hypertrophic signaling through the oxidation of histone deacetylase 4 and the activation of other redox-sensitive pathways [1]. Furthermore, ROS impairs EC-coupling by altering the function of RyRs, SR Ca2+-ATPase, NCX, and other Ca2+ signaling–related proteins [29, 33, 99]. Toxicity of mitochondrial Ca2+ overload is not only about excessive ROS generation but also related to the activation of apoptotic and necrotic cell death. Mitochondrial Ca2+ overload induces sustained opening of the mitochondrial permeability transition pore (mPTP), a pore complex in the IMM that allows passage of ions and solutes up to 1.5 kDa. This sudden increase of IMM permeability causes the loss of mitochondrial membrane potential and promotes the release of pro-apoptotic factors, which triggers the downstream apoptotic cascade, leading to apoptotic cell death [13, 23, 39, 92]. Furthermore, osmotic influx of water through these pores swells mitochondrial matrix and ceases ATP production, disabling ATP-dependent ion exchangers/pumps. The devastated cellular ion homeostasis eventually leads to plasma membrane rupture and necrotic cell death [2, 11].
There are no significant abnormalities in the heart under basal conditions in TRIC-A−/− mice. However, heart from TRIC-A-deficient mice showed altered SR Ca2+ regulation such as (SR Ca2+ overload) under stress conditions. For example, chronic treatment with β-adrenergic receptor agonist isoproterenol (ISO) leads to extensive fibrosis development in the TRIC-A−/− heart. Such effect is likely caused by increased death of cardiomyocytes and fibrotic remodeling, which may be linked to the overload of SR Ca2+ associated with the ablation of TRIC-A. SR Ca2+ overload due to TRIC-A depletion likely exacerbates mitochondria Ca2+ overload, promoting mitochondria ROS production and facilitating apoptosis and/or necrosis of the TRIC-A−/− cardiomyocytes. However, the underlying mechanism is still largely unknown. Therefore, it is worthwhile to further explore the role of TRIC-A in Ca2+ mediated SR-mitochondrial crosstalk.
Localization of TRIC-A in the nuclear envelope suggests potential transcriptional function
TRIC-A is not only located in the ER/SR membrane but also heavily expressed in the membrane of the nuclear envelope of muscle cells [101]. Although the exact function of TRIC-A in the nuclear envelope remains unknown, pioneer studies by Schirmer’s group provided important insights [70]. They screened the nuclear envelope transmembrane proteins (NETs) that mediated the global changes in actively transcribing genomic loci during myoblast differentiation and identified TRIC-A as one of the NETs directing specific chromosomal regions to the nuclear periphery for transcriptional repression during myogenesis [70]. To investigate whether the dysfunction of NETs like TRIC-A contributes to the pathogenesis of hereditary muscular diseases, they acquired primary myoblasts/fibroblasts culture derived from Emery–Dreifuss muscular dystrophy (EDMD) patients. Interestingly, TRIC-A displayed the most notable change of distribution among the 8 candidate NETs in those patients. Most patient samples exhibited moderate redistribution of TRIC-A favoring the SR localization [45]. Recently, they also identified TRIC-A mutations (N260D and N260 deletion) which alter the redistribution of target genes to the nuclear periphery during myogenesis. This result further confirms that abnormalities in TRIC-A-mediated chromosomal repositioning can be an etiological factor of EDMD [59].
Another possible role of TRIC-A on the nuclear envelope could be related to nuclear Ca2+ regulation. Since TRIC-A is known to regulate ER/SR Ca2+ signaling, its role in the nuclear Ca2+ regulation is plausible. It has been shown that Ca2+ signaling in the nucleoplasm regulates events that are distinct from the ones mediated by cytosolic Ca2+ [25]. For example, cardiomyocyte nucleus contains its own Ca2+ store called nucleoplasmic reticulum that expresses RyR and IP3R [25, 54]. IP3-induced Ca2+ release from nucleoplasmic reticulum facilitates protein kinase C (PKC) translocation to the nuclear envelope [25]. Abnormal nuclear Ca2+ signaling is also involved in cardiomyocyte hypertrophy. Diminishing Ca2+ level in the nuclei leads to swelling of the nuclei in neonatal cardiomyocytes, accompanied by increased calcineurin expression and increased nuclear enrichment of NFAT [34]. It is well known that calcineurin/NFAT signaling cascade plays a critical role in activating the transcription of genes involved in cardiac hypertrophy, such as β-myosin heavy chain (β-MHC) [34, 62]. Recently, we confirmed the noticeable expression of TRIC-A on the periphery and the membrane invaginations of the nuclear envelope of cardiomyocytes (unpublished data). Unlike the skeletal muscle cells, which form a smooth oval shape of the nuclear envelope, cardiomyocytes develop apparent membrane invaginations on the nuclear envelope. Researchers have speculated that such invagination could play a role in gene regulation [104], although the detailed mechanism is still lacking. TRIC-A locates directly on the invagination membrane structure of the nuclear envelope, suggesting it may play a role in nuclear Ca2+ regulation and gene translational regulation. Further studies are required for understanding the exact role that TRIC-A plays in the nuclear envelope of cardiomyocytes. The potential of TRIC-A regulating nuclear Ca2+ signaling would be an interesting direction for future investigation.
Table 1 summarizes our current understanding for the role of TRIC-A in cardiac, smooth and skeletal muscles, and the phenotypes associated with knockout of TRIC-A or genetic mutation in TRIC-A. In addition to supporting the counter current movement associated with intracellular Ca release, TRIC-A can also interact with RyR channels to directly or indirectly modulate ER/SR Ca homeostasis and crosstalk with mitochondria.
Table 1.
Phenotypes and mechanism in different tissues
| Tissue | Mechanism | Phenotype | Publication |
|---|---|---|---|
| Cardiac muscle |
a) Counter-current b) Regulation of RyR2 c) Regulation of STIM1 mediated SOCE. |
Stress-induced cardiac arrhythmia Stress-induced cardiac fibrosis |
Zhou et al., 2020 Shrestha et al., 2020 |
| Smooth muscle |
a) Counter-current b) Regulation of RyR-IP3R |
Hypertension | Yamazaki et al., 2011 |
| Skeletal muscle |
a) Counter-current b) Regulation of RyR1 c) Nuclear action for gene transcription |
Irregular muscular contractile force Emery–Dreifuss muscular dystrophy |
Zhao et al., 2010 El-Ajouz et al., 2017 Robson et al., 2016 |
Conclusion
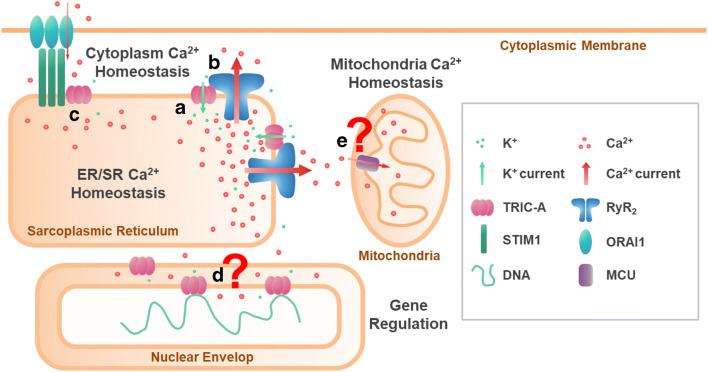
We depict a diagram to demonstrate the proposed functions of TRIC-A in cardiomyocytes (Fig. 1). Although there are still debates about whether TRIC channels carry the essential counter-current during RyR channel–mediated Ca2+ release, the identification of direct interactions between TRIC-A and RyR2 support a pivotal role of TRIC channels in regulating ER/SR Ca2+ homeostasis. Due to the growing interest in the physio-pathological roles of TRIC channels, novel regulatory mechanisms mediated by TRIC-A have started to emerge, including direct control of RyR2 activity, SOCE, SOICR functions, and its potential role in modulating mitochondria Ca2+ signaling. In addition to SR/ER localization, TRIC-A is also identified on the nuclear envelope and may participate in excitation-transcriptional regulation of genes involved in myogenesis and adaptive responses of the muscle and heart under physiologic and pathophysiologic settings.
Fig. 1.
Multi-functional role of TRIC-A in regulating Ca2+ signaling in cardiomyocytes. (a) TRIC-A channels are predominantly localized to the ER/SR, providing counter-current K+ movement (green arrows) for SR charge compensation during RyR2-mediated Ca2+ release (thick red arrows). (b) TRIC-A channels directly interact with RyR2 through its carboxyl terminal tail to modulate RyR2 opening status. (c) Association between TRIC-A and STIM1 has been proposed to control store-operated Ca2+ entry. (d) TRIC-A is found in the nuclear envelope, while its role in modulation of nuclear Ca2+ signaling and gene transcription remains largely unknown. (e) TRIC-A may function to modulate Ca2+ signaling crosstalk from SR to mitochondria to match the metabolic demand of myocardial workload under physiologic or pathologic conditions
Funding
This work is supported by NIH grants AR061385, AR070752, DK106394, AG056919, and HL138570 as well as by DOD grant W81XWH-18-1-0787 to JM; NIH grants R01NS105621 and R01HL138570 to JZ.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This article is part of the special issue on Calcium Signal Dynamics in Cardiac Myocytes and Fibroblasts: Mechanisms in Pflügers Archiv—European Journal of Physiology
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinyu Zhou and Ang Li contributed equally to this work.
References
- 1.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 2.Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr Cardiol. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- 3.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benard L, Oh JG, Cacheux M, Lee A, Nonnenmacher M, Matasic DS, Kohlbrenner E, Kho C, Pavoine C, Hajjar RJ, Hulot JS. Cardiac Stim1 silencing impairs adaptive hypertrophy and promotes heart failure through inactivation of mTORC2/Akt signaling. Circulation. 2016;133:1458–1471. doi: 10.1161/CIRCULATIONAHA.115.020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–17. doi: 10.1161/res.90.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Best PM, Abramcheck CW. Potassium efflux from single skinned skeletal muscle fibers. Biophys J. 1985;48:907–913. doi: 10.1016/S0006-3495(85)83853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleunven C, Treves S, Jinyu X, Leo E, Ronjat M, De Waard M, Kern G, Flucher BE, Zorzato F. SRP-27 is a novel component of the supramolecular signalling complex involved in skeletal muscle excitation-contraction coupling. Biochem J. 2008;411:343–349. doi: 10.1042/BJ20070906. [DOI] [PubMed] [Google Scholar]
- 11.Bonora M, Wieckowsk MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34:1608. doi: 10.1038/onc.2014.462. [DOI] [PubMed] [Google Scholar]
- 12.Bootman MD, Higazi DR, Coombes S, Roderick HL. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci. 2006;119:3915–3925. doi: 10.1242/jcs.03223. [DOI] [PubMed] [Google Scholar]
- 13.Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 14.Bovo E, Mazurek SR, Blatter LA, Zima AV. Regulation of sarcoplasmic reticulum Ca 2+ leak by cytosolic Ca 2+ in rabbit ventricular myocytes. J Physiol. 2011;589:6039–6050. doi: 10.1113/jphysiol.2011.214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996;71:1024–1035. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandes R, Bers DM. Analysis of the mechanisms of mitochondrial NADH regulation in cardiac trabeculae. Biophys J. 1999;77:1666–1682. doi: 10.1016/S0006-3495(99)77014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capes EM, Loaiza R, Valdivia HH. Ryanodine receptors. Skelet Muscle. 2011;1:18. doi: 10.1186/2044-5040-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Coronado R, Miller C. Decamethonium and hexamethonium block K+ channels of sarcoplasmic reticulum. Nature. 1980;288:495–497. doi: 10.1038/288495a0. [DOI] [PubMed] [Google Scholar]
- 20.Correll RN, Goonasekera SA, van Berlo JH, Burr AR, Accornero F, Zhang H, Makarewich CA, York AJ, Sargent MA, Chen X, Houser SR, Molkentin JD. STIM1 elevation in the heart results in aberrant Ca(2)(+) handling and cardiomyopathy. J Mol Cell Cardiol. 2015;87:38–47. doi: 10.1016/j.yjmcc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Tae HS, Norris NC, Karunasekara Y, Pouliquin P, Board PG, Dulhunty AF, Casarotto MG. A dihydropyridine receptor alpha1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int J Biochem Cell Biol. 2009;41:677–686. doi: 10.1016/j.biocel.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Das AM, Harris DA. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res. 1990;24:411–417. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- 23.De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ, Ichas F. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- 24.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- 25.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Ajouz S, Venturi E, Witschas K, Beech M, Wilson AD, Lindsay C, Eberhardt D, O’Brien F, Iida T, Nishi M, Takeshima H, Sitsapesan R. Dampened activity of ryanodine receptor channels in mutant skeletal muscle lacking TRIC-A. J Physiol. 2017;595:4769–4784. doi: 10.1113/JP273550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink RH, Stephenson DG. Ca2 + -movements in muscle modulated by the state of K + -channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987;409:374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- 28.Fink RH, Veigel C. Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle. Acta Physiol Scand. 1996;156:387–396. doi: 10.1046/j.1365-201X.1996.212000.x. [DOI] [PubMed] [Google Scholar]
- 29.Flesch M, Maack C, Cremers B, Baumer AT, Sudkamp M, Bohm M. Effect of beta-blockers on free radical-induced cardiac contractile dysfunction. Circulation. 1999;100:346–353. doi: 10.1161/01.CIR.100.4.346. [DOI] [PubMed] [Google Scholar]
- 30.Gillespie D. Energetics of divalent selectivity in a calcium channel: the ryanodine receptor case study. Biophys J. 2008;94:1169–1184. doi: 10.1529/biophysj.107.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillespie D, Fill M. Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys J. 2008;95:3706–3714. doi: 10.1529/biophysj.108.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013;52:2793–2809. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–H833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 34.Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gomez-Viquez NL, Rodrigues MA, Gomes DA, Martins-Cruz J, Lederer WJ, Leite MF. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium. 2008;44:230–242. doi: 10.1016/j.ceca.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Guo T, Nani A, Shonts S, Perryman M, Chen H, Shannon T, Gillespie D, Fill M. Sarcoplasmic reticulum K(+) (TRIC) channel does not carry essential countercurrent during Ca(2+) release. Biophys J. 2013;105:1151–1160. doi: 10.1016/j.bpj.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansford RG. Dehydrogenase activation by Ca2+ in cells and tissues. J Bioenerg Biomembr. 1991;23:823–854. doi: 10.1007/BF00786004. [DOI] [PubMed] [Google Scholar]
- 37.Hemalatha KL, Mainzen Prince PS. Preventive effects of zingerone on cardiac mitochondrial oxidative stress, calcium ion overload and adenosine triphosphate depletion in isoproterenol induced myocardial infarcted rats. RSC Adv. 2016;6:112332–112339. doi: 10.1039/C6RA23330A. [DOI] [Google Scholar]
- 38.Hernansanz-Agustin P, Choya-Foces C, Carregal-Romero S, Ramos E, Oliva T, Villa-Pina T, Moreno L, Izquierdo-Alvarez A, Cabrera-Garcia JD, Cortes A, Lechuga-Vieco AV, Jadiya P, Navarro E, Parada E, Palomino-Antolin A, Tello D, Acin-Perez R, Rodriguez-Aguilera JC, Navas P, Cogolludo A, Lopez-Montero I, Martinez-Del-Pozo A, Egea J, Lopez MG, Elrod JW, Ruiz-Cabello J, Bogdanova A, Enriquez JA, Martinez-Ruiz A. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature. 2020;586:287–291. doi: 10.1038/s41586-020-2551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch T, Marzo I, Kroemer G. Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep. 1997;17:67–76. doi: 10.1023/a:1027339418683. [DOI] [PubMed] [Google Scholar]
- 40.Ichimura A, Takeshima H. TRIC-B mutations causing osteogenesis imperfecta. Biol Pharm Bull. 2016;39:1743–1747. doi: 10.1248/bpb.b16-00612. [DOI] [PubMed] [Google Scholar]
- 41.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasuya G, Hiraizumi M, Maturana AD, Kumazaki K, Fujiwara Y, Liu K, Nakada-Nakura Y, Iwata S, Tsukada K, Komori T, Uemura S, Goto Y, Nakane T, Takemoto M, Kato HE, Yamashita K, Wada M, Ito K, Ishitani R, Hattori M, Nureki O. Crystal structures of the TRIC trimeric intracellular cation channel orthologues. Cell Res. 2016;26:1288–1301. doi: 10.1038/cr.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Thanh P, Meinke P, Korfali N, Srsen V, Robson MI, Wehnert M, Schoser B, Sewry CA, Schirmer EC. Immunohistochemistry on a panel of Emery-Dreifuss muscular dystrophy samples reveals nuclear envelope proteins as inconsistent markers for pathology. Neuromuscul Disord. 2017;27:338–351. doi: 10.1016/j.nmd.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 47.Li A, Li XJ, Yi JX, Zhou XY, Park KH, Nishi M, Takashima H, Ma JJ, Zhou JS. TRIC-A channel modulates Ca2+ homeostasis in mitochondria. Biophys J. 2020;118:448a. doi: 10.1016/j.bpj.2019.11.2502. [DOI] [Google Scholar]
- 48.Li A, Yi J, Li X, Zhou J (2020) Physiological Ca 2+ transients vs pathological steady-state Ca 2+ elevation, who flips the ROS coin in skeletal muscle mitochondria. Front Physiol 11. 10.3389/fphys.2020.595800 [DOI] [PMC free article] [PubMed]
- 49.Liu B, Ho HT, Velez-Cortes F, Lou Q, Valdivia CR, Knollmann BC, Valdivia HH, Gyorke S. Genetic ablation of ryanodine receptor 2 phosphorylation at Ser-2808 aggravates Ca(2+)-dependent cardiomyopathy by exacerbating diastolic Ca2+ release. J Physiol. 2014;592:1957–1973. doi: 10.1113/jphysiol.2013.264689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Wang R, Sun B, Mi T, Zhang J, Mu Y, Chen J, Bround MJ, Johnson JD, Gillis AM, Chen SR. Generation and characterization of a mouse model harboring the exon-3 deletion in the cardiac ryanodine receptor. PLoS One. 2014;9:e95615. doi: 10.1371/journal.pone.0095615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lokuta AJ, Meyers MB, Sander PR, Fishman GI, Valdivia HH. Modulation of cardiac ryanodine receptors by sorcin. J Biol Chem. 1997;272:25333–25338. doi: 10.1074/jbc.272.40.25333. [DOI] [PubMed] [Google Scholar]
- 52.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:136–147. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacLennan DH. Ca2+ signalling and muscle disease. Eur J Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- 54.Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium. 2006;39:65–73. doi: 10.1016/j.ceca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 56.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara T, Oikawa S, Kinugawa S, Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 58.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 59.Meinke P, Kerr ARW, Czapiewski R, de Las Heras JI, Dixon CR, Harris E, Kolbel H, Muntoni F, Schara U, Straub V, Schoser B, Wehnert M, Schirmer EC. A multistage sequencing strategy pinpoints novel candidate alleles for Emery-Dreifuss muscular dystrophy and supports gene misregulation as its pathomechanism. EBioMedicine. 2020;51:102587. doi: 10.1016/j.ebiom.2019.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meissner G. Ryanodine receptor Ca2+ release channels and their regulation by endogenous effectors. Ann Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 61.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987;262:3065–3073. doi: 10.1016/S0021-9258(18)61469-3. [DOI] [PubMed] [Google Scholar]
- 62.Morkin E. Control of cardiac myosin heavy chain gene expression. Microsc Res Tech. 2000;50:522–531. doi: 10.1002/1097-0029(20000915)50:6<522::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 63.Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CR, Blacker TS, Hall AR, Duchen MR, Kastner L, Lipp P, Zeller T, Muller C, Knopp A, Laufs U, Bohm M, Hoth M, Maack C. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab. 2015;22:472–484. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien F, Eberhardt D, Witschas K, El-Ajouz S, Iida T, Nishi M, Takeshima H, Sitsapesan R, Venturi E. Enhanced activity of multiple TRIC-B channels: an endoplasmic reticulum/sarcoplasmic reticulum mechanism to boost counterion currents. J Physiol. 2019;597:2691–2705. doi: 10.1113/JP277241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ou X, Guo J, Wang L, Yang H, Liu X, Sun J, Liu Z. Ion- and water-binding sites inside an occluded hourglass pore of a trimeric intracellular cation (TRIC) channel. BMC Biol. 2017;15:31. doi: 10.1186/s12915-017-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parks C, Alam MA, Sullivan R, Mancarella S. STIM1-dependent Ca(2+) microdomains are required for myofilament remodeling and signaling in the heart. Sci Rep. 2016;6:25372. doi: 10.1038/srep25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitt SJ, Park KH, Nishi M, Urashima T, Aoki S, Yamazaki D, Ma J, Takeshima H, Sitsapesan R. Charade of the SR K + -channel: two ion-channels, TRIC-A and TRIC-B, masquerade as a single K + -channel. Biophys J. 2010;99:417–426. doi: 10.1016/j.bpj.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 70.Robson MI, de Las Heras JI, Czapiewski R, Le Thanh P, Booth DG, Kelly DA, Webb S, Kerr ARW, Schirmer EC. Tissue-specific gene repositioning by muscle nuclear membrane proteins enhances repression of critical developmental genes during myogenesis. Mol Cell. 2016;62:834–847. doi: 10.1016/j.molcel.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenberg P, Katz D, Bryson V. SOCE and STIM1 signaling in the heart: timing and location matter. Cell Calcium. 2019;77:20–28. doi: 10.1016/j.ceca.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roston TM, Guo W, Krahn AD, Wang R, Van Petegem F, Sanatani S, Chen SR, Lehman A. A novel RYR2 loss-of-function mutation (I4855M) is associated with left ventricular non-compaction and atypical catecholaminergic polymorphic ventricular tachycardia. J Electrocardiol. 2017;50:227–233. doi: 10.1016/j.jelectrocard.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res. 2010;88:30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- 75.Santulli G, Xie WJ, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shintani-Ishida K, Inui M, Yoshida K. Ischemia-reperfusion induces myocardial infarction through mitochondrial Ca2+ overload. J Mol Cell Cardiol. 2012;53:233–239. doi: 10.1016/j.yjmcc.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 78.Shrestha N, Bacsa B, Ong HL, Scheruebel S, Bischof H, Malli R, Ambudkar IS, Groschner K. TRIC-A shapes oscillatory Ca2+ signals by interaction with STIM1/Orai1 complexes. PLoS Biol. 2020;18:e3000700. doi: 10.1371/journal.pbio.3000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silverio AL, Saier MH., Jr Bioinformatic characterization of the trimeric intracellular cation-specific channel protein family. J Membr Biol. 2011;241:77–101. doi: 10.1007/s00232-011-9364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silverman HS, Stern MD. Ionic basis of ischaemic cardiac injury: insights from cellular studies. Cardiovasc Res. 1994;28:581–597. doi: 10.1093/cvr/28.5.581. [DOI] [PubMed] [Google Scholar]
- 81.Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su M, Gao F, Yuan Q, Mao Y, Li DL, Guo Y, Yang C, Wang XH, Bruni R, Kloss B, Zhao H, Zeng Y, Zhang FB, Marks AR, Hendrickson WA, Chen YH. Structural basis for conductance through TRIC cation channels. Nat Commun. 2017;8:15103. doi: 10.1038/ncomms15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Timerman AP, Jayaraman T, Wiederrecht G, Onoue H, Marks AR, Fleischer S. The ryanodine receptor from canine heart sarcoplasmic-reticulum is associated with a novel Fk-506 binding-protein. Biochem Biophys Res Commun. 1994;198:701–706. doi: 10.1006/bbrc.1994.1101. [DOI] [PubMed] [Google Scholar]
- 84.Tjondrokoesoemo A, Li N, Lin PH, Pan Z, Ferrante CJ, Shirokova N, Brotto M, Weisleder N, Ma J. Type 1 inositol (1,4,5)-trisphosphate receptor activates ryanodine receptor 1 to mediate calcium spark signaling in adult mammalian skeletal muscle. J Biol Chem. 2013;288:2103–2109. doi: 10.1074/jbc.M112.425975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tomlins B, Williams AJ, Montgomery RA. The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol. 1984;80:191–199. doi: 10.1007/BF01868775. [DOI] [PubMed] [Google Scholar]
- 86.Touchberry CD, Elmore CJ, Nguyen TM, Andresen JJ, Zhao XL, Orange M, Weisleder N, Brotto M, Claycomb WC, Wacker MJ. Store-operated calcium entry is present in HL-1 cardiomyocytes and contributes to resting calcium. Biochem Biophys Res Commun. 2011;416:45–50. doi: 10.1016/j.bbrc.2011.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ullrich ND, Valdivia HH, Niggli E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol. 2012;53:33–42. doi: 10.1016/j.yjmcc.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venturi E, Matyjaszkiewicz A, Pitt SJ, Tsaneva-Atanasova K, Nishi M, Yamazaki D, Takeshima H, Sitsapesan R. TRIC-B channels display labile gating: evidence from the TRIC-A knockout mouse model. Pflugers Arch. 2013;465:1135–1148. doi: 10.1007/s00424-013-1251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volpe P, Palade P, Costello B, Mitchell RD, Fleischer S. Spontaneous calcium release from sarcoplasmic reticulum. Effect of local anesthetics. J Biol Chem. 1983;258:12434–12442. doi: 10.1016/S0021-9258(17)44194-9. [DOI] [PubMed] [Google Scholar]
- 90.Walker JM, Rinne A, Littwitz C, Bender K, Kienitz M-C, Pott L. Adenovirus-mediated delivery of short hairpin RNA (shRNA) mediates efficient gene silencing in terminally differentiated cardiac myocytes. In: Hicks BW, editor. Viral Applications of Green Fluorescent Protein. Totowa: Humana Press; 2009. pp. 107–123. [DOI] [PubMed] [Google Scholar]
- 91.Wang XH, Su M, Gao F, Xie W, Zeng Y, Li DL, Liu XL, Zhao H, Qin L, Li F, Liu Q, Clarke OB, Lam SM, Shui GH, Hendrickson WA, Chen YH. Structural basis for activity of TRIC counter-ion channels in calcium release. Proc Natl Acad Sci U S A. 2019;116:4238–4243. doi: 10.1073/pnas.1817271116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 2012;8:863–884. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 94.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto S, Matsui K, Ohashi N. Protective effect of Na+ /H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J Cardiovasc Pharmacol. 2002;39:569–575. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 97.Yamazaki D, Komazaki S, Nakanishi H, Mishima A, Nishi M, Yazawa M, Yamazaki T, Taguchi R, Takeshima H. Essential role of the TRIC-B channel in Ca2+ handling of alveolar epithelial cells and in perinatal lung maturation. Development. 2009;136:2355–2361. doi: 10.1242/dev.036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamazaki D, Tabara Y, Kita S, Hanada H, Komazaki S, Naitou D, Mishima A, Nishi M, Yamamura H, Yamamoto S, Kakizawa S, Miyachi H, Yamamoto S, Miyata T, Kawano Y, Kamide K, Ogihara T, Hata A, Umemura S, Soma M, Takahashi N, Imaizumi Y, Miki T, Iwamoto T, Takeshima H. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011;14:231–241. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 99.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 100.Yang H, Hu M, Guo J, Ou X, Cai T, Liu Z. Pore architecture of TRIC channels and insights into their gating mechanism. Nature. 2016;538:537–541. doi: 10.1038/nature19767. [DOI] [PubMed] [Google Scholar]
- 101.Yazawa M, Ferrante C, Feng J, Mio K, Ogura T, Zhang M, Lin PH, Pan Z, Komazaki S, Kato K, Nishi M, Zhao X, Weisleder N, Sato C, Ma J, Takeshima H. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature. 2007;448:78–82. doi: 10.1038/nature05928. [DOI] [PubMed] [Google Scholar]
- 102.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 103.Zhang WM, Lin MJ, Sham JS. Endothelin-1 and IP3 induced Ca2+ sparks in pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S121–S124. doi: 10.1097/01.fjc.0000166226.03712.4f. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W, Bai T, Zhang S, Xu S, Chen H, Li C. Isoforms of the nuclear envelope protein Nurim are differentially expressed during heart development in mice. Gene. 2017;627:123–128. doi: 10.1016/j.gene.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 105.Zhao X, Yamazaki D, Park KH, Komazaki S, Tjondrokoesoemo A, Nishi M, Lin P, Hirata Y, Brotto M, Takeshima H, Ma J. Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. J Biol Chem. 2010;285:37370–37376. doi: 10.1074/jbc.M110.170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao YT, Valdivia CR, Gurrola GB, Powers PP, Willis BC, Moss RL, Jalife J, Valdivia HH. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc Natl Acad Sci U S A. 2015;112:E1669–E1677. doi: 10.1073/pnas.1419795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao C, Ichimura A, Qian N, Iida T, Yamazaki D, Noma N, Asagiri M, Yamamoto K, Komazaki S, Sato C, Aoyama F, Sawaguchi A, Kakizawa S, Nishi M, Takeshima H. Mice lacking the intracellular cation channel TRIC-B have compromised collagen production and impaired bone mineralization. Sci Signal. 2016;9:ra49. doi: 10.1126/scisignal.aad9055. [DOI] [PubMed] [Google Scholar]
- 108.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou X, Lin P, Yamazaki D, Park KH, Komazaki S, Chen SR, Takeshima H, Ma J. Trimeric intracellular cation channels and sarcoplasmic/endoplasmic reticulum calcium homeostasis. Circ Res. 2014;114:706–716. doi: 10.1161/CIRCRESAHA.114.301816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou X, Park KH, Yamazaki D, Lin PH, Nishi M, Ma Z, Qiu L, Murayama T, Zou X, Takeshima H, Zhou J, Ma J. TRIC-A channel maintains store calcium handling by interacting with type 2 ryanodine receptor in cardiac muscle. Circ Res. 2020;126:417–435. doi: 10.1161/CIRCRESAHA.119.316241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zsolnay V, Fill M, Gillespie D. Sarcoplasmic reticulum Ca(2+) release uses a cascading network of intra-SR and channel countercurrents. Biophys J. 2018;114:462–473. doi: 10.1016/j.bpj.2017.11.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]