Abstract
Alzheimer’s disease has a preclinical stage when cerebral amyloid-β deposition occurs before symptoms emerge, and when amyloid-β-targeted therapies may have maximum benefits. Existing amyloid-β status measurement techniques, including amyloid PET and CSF testing, are difficult to deploy at scale, so blood biomarkers are increasingly considered for screening. We compared three different blood-based techniques—liquid chromatography-mass spectrometry measures of plasma amyloid-β, and single molecule array (Simoa) measures of plasma amyloid-β and phospho-tau181—to detect cortical 18F-florbetapir amyloid PET positivity (defined as a standardized uptake value ratio of >0.61 between a predefined cortical region of interest and eroded subcortical white matter) in dementia-free members of Insight 46, a substudy of the population-based British 1946 birth cohort. We used logistic regression models with blood biomarkers as predictors of amyloid PET status, with or without age, sex and APOE ε4 carrier status as covariates. We generated receiver operating characteristics curves and quantified areas under the curves to compare the concordance of the different blood tests with amyloid PET. We determined blood test cut-off points using Youden’s index, then estimated numbers needed to screen to obtain 100 amyloid PET-positive individuals. Of the 502 individuals assessed, 441 dementia-free individuals with complete data were included; 82 (18.6%) were amyloid PET-positive. The area under the curve for amyloid PET status using a base model comprising age, sex and APOE ε4 carrier status was 0.695 (95% confidence interval: 0.628–0.762). The two best-performing Simoa plasma biomarkers were amyloid-β42/40 (0.620; 0.548–0.691) and phospho-tau181 (0.707; 0.646–0.768), but neither outperformed the base model. Mass spectrometry plasma measures performed significantly better than any other measure (amyloid-β1-42/1-40: 0.817; 0.770–0.864 and amyloid-β composite: 0.820; 0.775–0.866). At a cut-off point of 0.095, mass spectrometry measures of amyloid-β1-42/1-40 detected amyloid PET positivity with 86.6% sensitivity and 71.9% specificity. Without screening, to obtain 100 PET-positive individuals from a population with similar amyloid PET positivity prevalence to Insight 46, 543 PET scans would need to be performed. Screening using age, sex and APOE ε4 status would require 940 individuals, of whom 266 would proceed to scan. Using mass spectrometry amyloid-β1-42/1-40 alone would reduce these numbers to 623 individuals and 243 individuals, respectively. Across a theoretical range of amyloid PET positivity prevalence of 10–50%, mass spectrometry measures of amyloid-β1-42/1-40 would consistently reduce the numbers proceeding to scans, with greater cost savings demonstrated at lower prevalence.
Keywords: Alzheimer’s disease, amyloid imaging, dementia, beta-amyloid, tau, epidemiology
Keshavan et al. show that plasma Aβ1-42/1-40 and a plasma Aβ composite measured by liquid chromatography–mass spectrometry perform better than various blood biomarkers measured by single molecular array, and better than age, sex and APOE ε4, in screening for preclinical Alzheimer’s disease in the British 1946 birth cohort.
Introduction
A core early feature of Alzheimer’s disease is brain deposition of amyloid-β, which is detectable in vivo using amyloid PET ligands binding fibrillar amyloid-β (Klunk et al., 2004; Morris et al., 2016), and by CSF testing showing reduced concentrations of amyloid-β42 (Motter et al., 1995; Olsson et al., 2016) or amyloid-β42/amyloid-β40 (Slaets et al., 2013; Toledo et al., 2013; Pannee et al., 2016). These methods now have consensus appropriate use criteria (Johnson et al., 2013; Shaw et al., 2018), are incorporated into current clinical guidelines (National Institute for Health and Care Excellence, 2018) and research criteria for Alzheimer’s disease diagnosis (Dubois et al., 2014; Jack et al., 2018), and are increasingly used in clinical trials to either confirm Alzheimer’s disease pathology in symptomatic individuals, or to identify asymptomatic individuals at risk, who are now defined as having preclinical Alzheimer’s disease. However, amyloid PET is expensive, limited in accessibility and involves ionizing radiation. CSF sampling is relatively invasive, confers risk to individuals with coagulopathies, and requires suitably trained personnel. As neither method is likely to be viable for population-based screening, several blood-based approaches have been developed.
A meta-analysis of studies published until 2015 (Olsson et al., 2016) yielded conflicting results on the ability of plasma amyloid-β to distinguish Alzheimer’s disease dementia from controls, or mild cognitive impairment (MCI) progressing to Alzheimer’s disease dementia from stable MCI. These mixed findings were related particularly to heterogeneity of comparisons; older studies compared clinically diagnosed Alzheimer’s disease cases with various non-pathologically defined control groups, while newer studies compared amyloid-positive and -negative groups as defined by PET or CSF. Initially, methods used to quantify plasma amyloid-β also lacked sensitivity, but from 2016 onward, highly sensitive immunoassays and high-throughput mass spectrometry methods were developed for measuring plasma amyloid-β. Several studies, utilizing either of these methods, probed the ability of blood-based assays to distinguish individuals with in vivo gold standard biomarker evidence of Alzheimer pathology (i.e. either cortical amyloid PET uptake or lowered CSF amyloid-β1-42 or amyloid-β1-42/amyloid-β1-40) from controls without evidence of Alzheimer pathology. Some publications focused on memory clinic populations (mixed cohorts of cognitively healthy elderly subjects, MCI and dementia), such as the Japanese National Center for Geriatrics and Gerontology, the Australian Imaging, Biomarker and Lifestyle Study of Ageing (Nakamura et al., 2018), the Swedish BioFINDER study (Palmqvist et al., 2018), and the Washington University cohort (Schindler et al., 2019). Some studies specifically prospectively investigated individuals reporting subjective cognitive decline, such as the Amsterdam Dementia Cohort (Verberk et al., 2018), and Insight pre-AD (Vergallo et al., 2019). Taken together, in memory clinic populations, plasma amyloid-β42/amyloid-β40 was able to predict concurrent PET or CSF amyloid status with sensitivities of 70–76% and specificities of 72–78% (accuracy varied from 68% to 97% across cohorts measured by different assays). Plasma amyloid-β42/amyloid-β40 measured by an immunoprecipitation–mass spectrometry (IP-MS) method was also able to predict conversion from PET-negative to PET-positive status more than 1.5 years later, with individuals who had plasma amyloid-β42/amyloid-β40 < 0.1218 being 15 times more likely to convert from PET-negative to PET-positive than those above this cut-off point (Schindler et al., 2019).
More recently, plasma phospho-tau181 (p-tau181) has emerged as a potential biomarker of amyloid-β positivity, with studies reporting an accuracy of 76–88% for predicting amyloid PET status (Mielke et al., 2018; Karikari et al., 2020). Plasma p-tau181 may have some additive predictive value with plasma amyloid-β42/40 measured by immunoassays (Janelidze et al., 2020). Validation of these approaches has largely focused on mixed cohorts (of individuals who are cognitively normal, those with MCI and those with dementia).
Methods for identifying amyloid-β-positive individuals at scale will take priority if disease-modifying therapies are licensed. A key possible reason for the negative results seen in many of the earlier amyloid-lowering therapy trials was lack of biomarker evidence of Alzheimer’s disease in the full recruited study populations (Doody et al., 2014; Salloway et al., 2014; Vandenberghe et al., 2016). More recent trials have therefore turned to ensuring that participants have Alzheimer’s pathology as defined by mutation carrier status in dominantly inherited disease or by amyloid biomarkers. Examining results of 4-year follow-up in the Dominantly Inherited Alzheimer’s Network Trial Unit (DIAN-TU) study of solanezumab and gantenerumab, where the presence of Alzheimer’s disease was defined by mutation carrier status, primary cognitive endpoints were not met (Bateman, 2020). In mild symptomatic sporadic Alzheimer’s disease, the ENGAGE trial of aducanumab also did not meet its primary end point but the similarly designed EMERGE trial did show benefit at 78 weeks (Biogen, 2019), which has led to a submission for regulatory approval to the US Food and Drug Administration. Among the key limitations of the amyloid-lowering trials in even mild symptomatic sporadic Alzheimer’s disease is the possibility that the window of opportunity for preventing cognitive decline in such individuals has already passed. With primary prevention trials such as the Anti-Amyloid Treatment in Asymptomatic Alzheimer disease (A4) Study of solanezumab (Sperling et al., 2020) aiming to target those who may still be in that window, population screening for amyloid-β positivity will need to shift focus to asymptomatic individuals.
The main aim of our study was therefore to compare directly the ability of three blood-based candidates for detecting cerebral amyloid-β deposition—liquid chromatography-mass spectrometry (LC-MS) measures of plasma amyloid-β, and single molecule array (Simoa) measures of plasma amyloid-β and phospho-tau181—to determine amyloid PET status in a population-based sample of dementia-free individuals at age ∼70 years, drawn from the longest continuously participating British birth cohort study. In addition, we assessed correlations between biomarkers, probed associations of demographic factors with blood biomarkers, and examined the potential cost savings of deploying each screening method across a range of amyloid PET-positivity prevalence.
Materials and methods
Study design and participants
The Medical Research Council (MRC) National Survey for Health and Development (NSHD; the British 1946 birth cohort) followed an initial sample of 5362 individuals from their birth in mainland Britain during 1 week in March 1946, over 24 waves of data collection. Insight 46 is a prospective substudy of 502 members at age 69–71 years, undertaken at University College London (UCL); previous publications have detailed the eligibility criteria and substudy protocol (Lane et al., 2017), and compared the characteristics of Insight 46 participants to those of the larger NSHD cohort (James et al., 2018). Ethical approval for Insight 46 was given by the National Research Ethics Service Committee London (reference 14/LO/1173). All participants gave written informed consent according to the Declaration of Helsinki.
Neuroimaging
Dynamic PET-MRI data were acquired simultaneously on a single Biograph mMR 3 T PET-MRI scanner, as described previously (Lane et al., 2017). Amyloid-β burden was assessed by analysing the PET data acquired at a 10-min period, 50 min after intravenous injection of 370 MBq 18F-florbetapir. Global standardized uptake value ratio (SUVR) was calculated by normalizing the uptake in a predefined cortical region of interest (Landau et al., 2013) to that in eroded subcortical white matter. Amyloid PET status was obtained by fitting a two-component Gaussian mixture model of SUVR in all participants with adequate PET data, and taking the 99th percentile of the lower (amyloid PET-negative) Gaussian as the cut-off point (SUVR = 0.61, equivalent to 17 centiloids). SUVR ≥ 0.61 was defined as PET positive and SUVR < 0.61 as PET negative. Although all study assessments were designed to be completed in a single day, 59 individuals (13.4% of those included in the analysis) had their PET scans on a different day to blood sampling due to PET tracer availability or scanner maintenance issues; the median delay between the blood test and the scan in these individuals was 0.131 years [interquartile range (IQR): 0.060–0.211 years].
Whole brain volume, subcortical white matter hyperintensity volume and total intracranial volume were extracted from the MRI data for use as covariates in models examining the associations of age with plasma amyloid-β biomarkers. Whole brain volume was extracted from T1-weighted images using the automated Brain Multi-Atlas Propagation and Segmentation (BMAPS) technique (Leung et al., 2011). Subcortical white matter hyperintensity volume was extracted from T1 and FLAIR images using the automated Bayesian Model Selection (BaMoS) algorithm (Sudre et al., 2015), which excludes infratentorial regions, followed by visual quality control to exclude individuals with white matter lesions characteristic of demyelination or large cortical infarcts inappropriately segmented as subcortical white matter hyperintensities. Total intracranial volume was extracted using Statistical Parametric Mapping software version 12 (Malone et al., 2015).
Cognitive assessment
Neuropsychological testing (Lane et al., 2017; Lu et al., 2019) included a summary measure of cognition [the Mini-Mental State Examination (MMSE); Folstein et al., 1975], tests of episodic memory [Wechsler memory scale-revised logical memory test (Wechsler, 1987) and 12-item Face-Name Associative Memory Exam (FNAME-12); Papp et al., 2014], and processing speed (Wechsler adult intelligence scale-revised digit symbol substitution test (DSS); Wechsler, 1981]. The preclinical Alzheimer’s cognitive composite (PACC) was derived as the sum of z-scores of the MMSE, delayed logical memory (LMD), FNAME-12 and DSS (Lu et al., 2019). Participant and informant history of cognitive concerns and of prior neurological diagnoses was also elicited. Dementia was defined by expert consensus (informed by clinical history, informant history and MMSE score < 26). MCI was defined as participant or informant concern regarding participant’s cognition, and either LMD score ≥1.5 standard deviations (SD) below the mean, or DSS score ≥1.5 SD below the mean.
Blood sampling and processing
Non-fasted venous blood was sampled between 09:30 and 11:00 h, using a tourniquet and 21G or 23G butterfly needle with a BD Vacutainer collecting system, into 8.5 ml gel separator serum tubes and 10 ml EDTA plasma tubes. Whole blood was transported and centrifuged at room temperature, at 2000g for 10 min, within 30 min of sampling. Plasma and serum supernatant aliquots of 0.5 ml were stored in polypropylene screw-top cryovials at −80°C within 60 min of sampling.
APOE ε4 genotyping of the single nucleotide polymorphisms rs439358 and rs7412 (Rawle et al., 2018) was used to define APOE ε4 carrier status as carrier (one or two ε4 alleles) or non-carrier.
All blood assays were performed blinded to clinical information.
Nomenclature for amyloid-β assays
Throughout this manuscript we refer to amyloid-β42 and amyloid-β40 where assays do not specify the starting amino acid of the relevant peptides, and to amyloid-β1-42 and amyloid-β1-40 where assays quantify the specific peptides starting at the first amino acid residue of the amyloid-β sequence.
Simoa assays
Plasma amyloid-β40 and amyloid-β42
One 0.5 ml aliquot of plasma from each individual was thawed to room temperature over 1 h and vortexed for 2 s. Next, 0.3 ml was pipetted into a 1.5 ml polypropylene tube for centrifugation at 13 000g for 10 min; 0.1 ml of the supernatant was pipetted onto each of two plates, for analysing amyloid-β40 and amyloid-β42, respectively. Samples were analysed in duplicate, using the same batch of reagents (Simoa Aβ40 and Aβ42 kits), on the same HD-1 Analyser (Quanterix) at UCL. Results were accepted if the intra-assay coefficient of variation (CV) across the duplicates was <15%. Run validation controls prepared using stock peptide solutions from the kits indicated inter-assay CV < 30%.
Plasma p-tau181
Plasma p-tau181 was measured on the Simoa HD-1 instrument at the University of Gothenburg (Quanterix) using a recently published method that has been validated both analytically and clinically (Benussi et al., 2020; Karikari et al., 2020). Briefly, the assay used the p-tau181-specific monoclonal antibody AT270 for capture and the N-terminal mouse monoclonal antibody Tau12 that binds the N-terminal epitope 6-QEFEVMEDHAGT-18 on full-length human tau for detection. The calibrator was recombinant full-length recombinant tau-441 phosphorylated in vitro by glycogen synthase kinase 3β (#TO8-50FN, SignalChem). The specificity of the assay for tau forms that contain the indicated epitopes was previously demonstrated by mass spectrometry (Karikari et al., 2020). All Insight 46 samples measured above the assay’s lower limit of quantification of 1.0 pg/ml. For further details, see the Supplementary material.
LC-MS plasma amyloid-β assay
Extended methods are available in the Supplementary material. Calibrators were prepared using recombinant amyloid-β1-38, amyloid-β1-40 and amyloid-β1-42 (rPeptide) added to 8% bovine serum albumin in phosphate-buffered saline. Recombinant ‘heavy’ peptides (15N-uniformly labelled amyloid-β1-38, amyloid-β1-40 and amyloid-β1-42; rPeptide) were added to samples and calibrators prior to preparation and used as internal standards. Pooled plasma samples from the University of Gothenburg were used to track assay performance over different days, and showed inter-assay CV < 5%.
After a single thaw, amyloid-β peptides were immunoprecipitated from 0.25 ml of each sample, with anti-amyloid-β antibodies 4G8 (epitope 17–27 in the amyloid-β sequence) and 6E10 (epitope 1–16, both antibodies from BioLegend) coupled to Dynabeads™ M-280 Sheep Anti-Mouse IgG magnetic beads (Thermo Fisher Scientific), performed using a KingFisher™ Flex Purification System (Thermo Fisher Scientific). After immunoprecipitation, eluates in 0.1 ml of 0.5% formic acid were vacuum centrifuged and stored at −80°C.
Prior to liquid chromatography-tandem mass spectrometry (LC-MS/MS), the dried eluates were resuspended in 20% acetonitrile and 4% concentrated ammonia in water, and injected into the LC-MS system (a Dionex UltiMate LC system and a Thermo Scientific Q ExactiveTM Quadrupole-OrbitrapTM hybrid mass spectrometer). Chromatographic separation was achieved using basic mobile phases and a reversed-phase monolith column at a flow rate of 0.3 ml/min. The mass spectrometer, operating in parallel reaction monitoring mode, was set to isolate the 4+ charge state precursors of the amyloid-β peptides. Product ions specific for each precursor (Supplementary Table 1) were selected and summed to calculate the chromatographic areas for each peptide and its corresponding internal standard. The area ratio of each analyte to its internal standard was used for quantification in samples and calibrators. Peptides analysed included amyloid-β1-38, amyloid-β1-40, amyloid-β1-42 and amyloid-β−3-40 (also known as APP669–711). An LC-MS composite was also generated by taking the average of the z-scores of the amyloid-β−3-40/amyloid-β1-42 and amyloid-β1-40/amyloid-β1-42 ratios, after the method of Nakamura et al. (2018).
Statistical analysis
Analyses were performed in Stata Version 14.2 (Stata Corporation, Texas, USA).
Descriptive statistics
Mann-Whitney U-tests for non-normally distributed continuous variables, Student’s t-test for normally distributed continuous variables and χ2 tests for categorical variables were used to compare the amyloid PET-positive and PET-negative groups, for all who had a high-quality amyloid scan.
Inter-biomarker correlations
Correlations between blood biomarker values were assessed in all individuals for whom blood biomarker data were available. As blood biomarker values were positively skewed, logarithmic transformation was used before assessing Pearson correlations between assays.
Associations between blood biomarkers and demographics, accounting for imaging outcomes and cognition
For analyses of the associations of demographic factors with blood biomarkers, individuals were included if they had a full set of blood biomarker data, a high-quality amyloid PET scan, and were dementia-free. Figure 2 shows the process of inclusion and exclusion of participants to derive the dementia-free group. Although all individuals were born in the same week, age at the time of blood testing indicated time of attendance within the testing period of 2.6 years. Associations of blood biomarkers with age were tested using univariable linear regression, subsequently additionally adjusting for sex, APOE ε4 carrier status, SUVR, PACC, whole brain volume and subcortical white matter hyperintensity volume. Models including whole brain volume or subcortical white matter hyperintensity volume also adjusted for total intracranial volume. Unadjusted differences in blood biomarkers between the sexes were assessed by Mann-Whitney U-tests. For all these analyses, the outcome variables were log-transformed blood biomarkers or ratios, apart from the LC-MS composite which was examined without transformation.
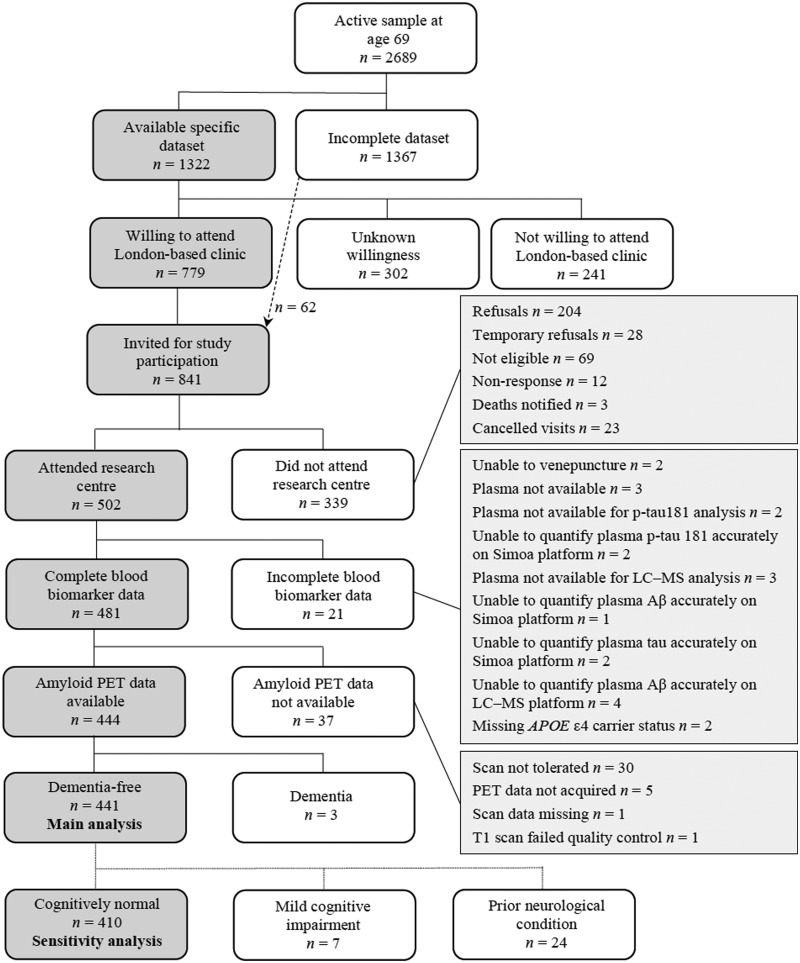
Figure 2.
Recruitment flow chart. The chart shows an overview of Insight 46 recruitment from the MRC NSHD and summary of blood biomarker data available [modified with permission (James et al., 2018) under the terms of the Creative Commons Attribution 4.0 International Licence (http://creativecommons.org/licenses/by/4.0/)]. The derivation of the dementia free group (used for the main analyses) and the cognitively normal group (used in the sensitivity analysis for prediction of amyloid PET status) is shown. Aβ = amyloid-β.
Linear regression was used to investigate the associations of SUVR and APOE ε4 carrier status, adjusted for age and sex, with log-transformed blood biomarkers (or non-transformed LC-MS composite) as the dependent variable. Possible interactive effects of sex and SUVR, sex and APOE ε4 carrier status, and SUVR and APOE ε4 carrier status were investigated by including appropriate interaction terms. Model assumptions of linearity were checked by examination of residuals.
Blood biomarker concordance with amyloid PET and relative costs of screening
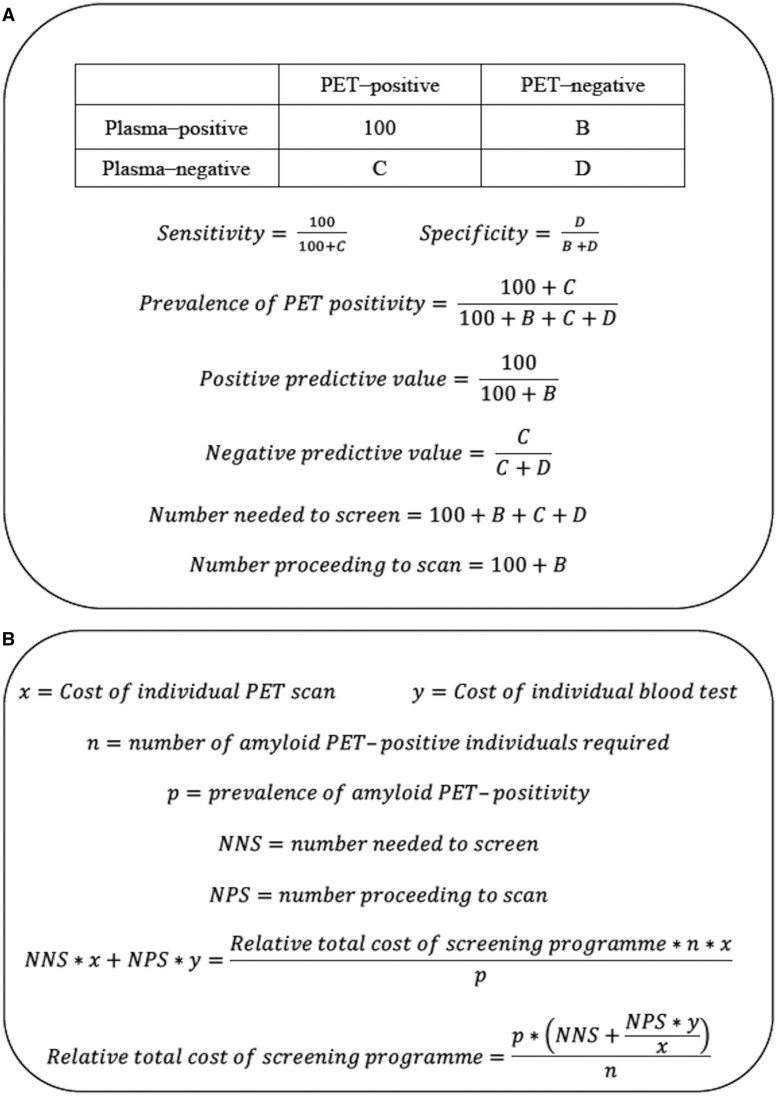
To assess the contribution of blood biomarkers to prediction of binary amyloid PET status, logistic regression models were constructed using blood biomarkers as predictors, with and without inclusion of age, sex and APOE ε4 carrier status. The model predictions were then used in receiver operating characteristics (ROC) analyses and the areas under the ROC curves (AUC) were compared using DeLong tests The best model derived from each assay platform was defined as having the highest AUC. Plasma cut-off points were determined using Youden’s index as provided by the output of the ROC analysis. The resulting values of sensitivity and specificity were applied across a range of 10–50% theoretical prevalence of amyloid PET positivity to calculate the positive and negative predictive values of the blood test, the total number needed to screen by blood test, and number proceeding to PET scan, to identify 100 amyloid PET-positive individuals. These calculations were performed by solving for the contents of the 2 × 2 table generated in each case, using the equations shown in Fig. 1A. The relative costs of screening programmes using the different blood tests were examined by solving the equations in Fig. 1B, at different theoretical cost ratios of individual blood tests to PET scan, and across a range of 10–50% theoretical prevalence of amyloid PET positivity.
Figure 1.
Formulae. (A) Calculations for number needed to screen to obtain 100 amyloid PET-positive individuals. Sensitivity and specificity are independent of prevalence, and are fixed once the cut-off point is chosen (e.g. at Youden’s index). If prevalence is specified, the first three simultaneous equations can be solved for B–D. The positive and negative predictive values of the plasma test and the number needed to screen can then be calculated by the following three equations. (B) Calculations for relative cost of the screening programme. These calculations are based on specified costs of an individual PET scan (x) and blood test (y), number needed to prescreen (NNS) with blood test and number proceeding to scan (NPS), to obtain a specified number of amyloid PET-positive individuals (n) in the context of a known estimated population prevalence of amyloid PET positivity (p). It is assumed that x and y include initial setup costs.
Sensitivity analyses
ROC analyses were performed for amyloid PET status within APOE ε4 subgroups (non-carrier versus carrier) and after further excluding those with prior neurological diagnoses and those who fulfilled criteria for MCI (Fig. 2). The contribution of time between blood test and scan, and of educational attainment, were also ascertained.
To examine whether the choice of amyloid PET cut-off point significantly affected the performance of the best-performing unadjusted plasma tests, sensitivity analyses were undertaken using a range of definitions of amyloid PET status either side of the cut-off point of 0.61 that had previously been determined by mixture modelling (SUVR cut-off points between 0.57 and 0.65), with recalculation of the Youden’s index plasma cut-off point in each case.
Data availability
The data sharing policy is available on the NSHD Data Sharing website (see https://skylark.ucl.ac.uk/NSHD/doku.php).
Results
Participants: descriptive statistics
Of 502 participants assessed in Insight 46, 481 had a complete set of blood biomarker data, 444 had a high-quality amyloid PET scan, of whom 441 (92% of those with complete blood biomarker data) were dementia-free (Fig. 2). Table 1 summarizes the characteristics of all individuals included in the analysis; 82 of 441 individuals (18.6%) were amyloid PET positive. Compared with PET-negative individuals, PET-positive individuals had lower MMSE (median PET positive 29 versus PET negative 30, P = 0.018) and PACC scores [mean (SD): −0.122 (0.726) versus 0.046 (0.668), P = 0.044], were more likely to be APOE ε4 carriers (57.3% versus 22.0%, P < 0.0001), and had higher whole brain volumes (median (IQR), ml: 1126 (1965–1187) versus 1096 (1026–1153), P = 0.018). Supplementary Table 2 summarizes the characteristics of all dementia-free individuals, including those with missing data, and all those excluded from analysis.
Table 1.
Characteristics of dementia-free participants with full blood biomarker and amyloid PET data
| Characteristic |
Amyloid PET-negative
(n = 359) |
Amyloid PET-positive
(n = 82) |
P |
All dementia-free with amyloid scan
(n = 441) |
|---|---|---|---|---|
| Age at blood sampling, years | 70.7 (0.7) | 70.6 (0.6) | 0.521 | 70.7 (0.7) |
| Sex, % female | 49.6 | 54.9 | 0.367 | 50.6 |
| APOE ε4 status, % carrier | 22.0 | 57.3 | <0.0001 | 28.6 |
| MMSE | 30 (29, 30) | 29 (28, 30) | 0.018 | 30 (29, 30) |
| PACC (z-score) | 0.046 (0.668) | −0.122 (0.726) | 0.044 | 0.015 (0.682) |
| Educational attainment by age 26, n (%) | 0.571 | |||
| No qualification | 52 (14.5) | 13 (15.9) | 65 (14.7) | |
| Vocational | 18 (5.0) | 5 (6.1) | 23 (5.2) | |
| O-level/equivalent | 91 (25.4) | 21 (25.6) | 112 (25.4) | |
| A-level/equivalent | 129 (35.9) | 29 (35.4) | 159 (35.8) | |
| Degree/equivalent | 69 (19.2) | 14 (17.1) | 83 (18.8) | |
| n (%) individuals with blood sample and amyloid PET scan not done on same day | 52 (14.5) | 7 (8.5) | 0.621 | 59 (13.4) |
| Time between blood sample and amyloid PET scan for individuals who did not have them on the same day, y |
0.126 [0.063, 0.210] n = 52 |
0.134 [0.038, 0.350] n = 7 |
0.672 |
0.131 [0.060, 0.211] n = 59 |
| Total intracranial volume, ml | 1421 [1335, 1507] | 1448 [1381, 1542] | 0.076 | 1427 [1341, 1517] |
| Whole brain volume, ml |
1096 [1026, 1153] n = 357 |
1126 [1065, 1187] | 0.018 |
1100 [1034, 1162] n = 439 |
| WMHV, ml |
2.8 [1.5, 6.6] n = 348 |
3.5 [1.8, 7.0] n = 79 |
0.329 |
3.1 [1.6, 6.8] n = 427 |
| Serum creatinine, µmol/l | 73 [63, 83] | 73 [64, 87] | 0.375 | 73 [63.5, 84] |
| Body mass index | 27.4 [24.3, 30.4] | 26.2 [24.1, 28.8] | 0.054 | 27.3 [24.3, 30.2] |
| Simoa plasma Aβ40, pg/ml | 289 [255, 319] | 285 [257, 328] | 0.601 | 288 [256, 322] |
| Simoa plasma Aβ42, pg/ml | 19.9 [17.1, 22.6] | 18.1 [15.5, 22.9] | 0.011 | 19.6 [16.7, 22.7] |
| Simoa plasma Aβ42/Aβ40 | 0.068 [0.059, 0.078] | 0.061 [0.052, 0.072] | 0.001 | 0.066 [0.058, 0.077] |
| Simoa plasma p-tau181, pg/ml | 8.5 [6.1, 12.2] | 12.8 [9.2, 16.0] | <0.0001 | 9.2 [6.4, 12.9] |
| LC-MS plasma Aβ1-38, pg/ml | 24.8 [21.6, 27.9] | 24.5 [21.6, 28.7] | 0.960 | 24.8 [21.6, 8.0] |
| LC-MS plasma Aβ1-40, pg/ml | 284 [255, 314] | 285 [260, 311] | 0.976 | 284 [257, 314] |
| LC-MS plasma Aβ1-42, pg/ml | 29.6 [24.9, 34.2] | 23.7 [20.3, 27.2] | <0.0001 | 28.6 [23.4, 33.3] |
| LC-MS plasma Aβ−3-40, pg/ml | 30.1 [24.4, 35.7] | 29.9 [24.4, 34.9] | 0.911 | 30.1 [24.4, 35.7] |
| LC-MS plasma Aβ1-42/Aβ1-40 | 0.104 [0.093, 0.116] | 0.082 [0.075, 0.090] | <0.0001 | 0.099 [0.087, 0.113] |
| LC-MS plasma composite | −0.154 (0.736) | 0.667 (0.718) | <0.0001 | −0.001 (0.799) |
Values are expressed as mean (SD) for normally distributed variables, median [interquartile range] for skewed variables, and percentages for binary and categorical variables. The significance of differences between amyloid PET-negative and PET-positive participants was determined by Mann-Whitney U-tests (for continuous variables excepting age and LC-MS plasma composite), t-tests (for age and LC-MS plasma composite), two sample tests of proportions (for binary variables) and Kruskal-Wallis equality of proportions rank test (for educational attainment). Aβ = amyloid-β; MMSE = Mini-Mental State Examination; NFL = neurofilament light chain; NSHD = National Survey of Health and Development; PACC = preclinical Alzheimer’s cognitive composite; p-tau181 = phospho-tau181; WMHV = white matter hyperintensity volume.
Inter-biomarker correlations
Weak positive correlations were observed between Simoa ln amyloid-β42 and LC-MS ln amyloid-β1-42 (r = 0.207, P = 0.001), Simoa ln amyloid-β40 and LC-MS ln amyloid-β1-40 (r = 0.406, P < 0.0001), and Simoa ln amyloid-β42/amyloid-β40 and LC-MS ln amyloid-β1-42/amyloid-β1-40 (r = 0.189, P = 0.003). Simoa ln p-tau181 was weakly negatively correlated with LC-MS ln amyloid-β1-42 (r = −0.204, P = 0.001) and LC-MS ln amyloid-β1-42/amyloid-β1-40 (r = −0.303, P < 0.0001) but not with Simoa amyloid-β markers. All measured individual LC-MS amyloid-β markers were moderately positively correlated (r = 0.547–0.698, P < 0.0001) and the LC-MS composite was strongly negatively correlated with LC-MS ln amyloid-β1-42/amyloid-β1-40 (r = −0.863, P < 0.0001). Supplementary Table 3 provides Pearson correlations across all measured biomarkers with Bonferroni-corrected P-values.
Associations of demographic factors with blood biomarkers
Significant sex differences were seen only for Simoa plasma amyloid-β40 [median (IQR), pg/ml: females 282 (255–315) versus males 296 (258–327), P = 0.025] as shown in Supplementary Table 4.
All plasma amyloid-β species and ratios excepting Simoa plasma amyloid-β40 showed significant associations with age, even within the very narrow age range of this cohort. The direction of the association was inconsistent between the two methods; both Simoa plasma amyloid-β42 and Simoa plasma amyloid-β42/amyloid-β40 were positively associated with age, but the LC-MS measures were negatively associated with age (excepting the composite which had a positive association). For every year increase in age, there was a 0.1 log-fold decrease in LC-MS amyloid-β1-42/amyloid-β1-40, and a 0.2-fold increase in LC-MS composite. Simoa plasma p-tau181 was also positively associated with age, showing a 0.4 log-fold increase per year. These associations persisted despite adjustment for sex, APOE ε4 carrier status and SUVR, and were not attenuated by further adjustment for whole brain volume, subcortical white matter hyperintensity volume or PACC (Supplementary Table 5).
Considering cerebral amyloid as a continuous variable (SUVR), as expected higher SUVR was associated with lower Simoa plasma amyloid-β42 and amyloid-β42/amyloid-β40. Higher SUVR and being an APOE ε4 carrier were independently associated with lower LC-MS plasma amyloid-β1-42, amyloid-β1-42/amyloid-β1-40 and higher composite (Supplementary Table 6). A model incorporating age, sex, APOE ε4 carrier status and SUVR explained 1.2% of the variance in Simoa amyloid-β42, 3.4% for amyloid-β42/amyloid-β40 and 23.7% for p-tau181. For LC-MS methods this model explained 29.2%, 23.0% and 22.4% of the variance in amyloid-β1-42, amyloid-β1-42/amyloid-β1-40 and composite respectively.
There were no significant interactions (at P < 0.05) between sex and SUVR, or sex and APOE ε4 carrier status, in their associations with the investigated blood biomarkers.
Time between blood sampling and scan did not have any significant associations with blood biomarkers, or confound their associations with SUVR.
Concordance with amyloid PET status
Table 2 shows the AUC from ROC analyses for amyloid PET status in dementia-free individuals incorporating blood biomarkers as predictors, either alone or combined with age, sex and APOE ε4 carrier status. LC-MS amyloid-β1-42/amyloid-β1-40 (AUC 0.817, 95% confidence interval 0.770–0.864) and LC-MS composite (0.820, 0.775–0.866) performed significantly better than the best-performing Simoa biomarker (p-tau181: 0.707, 0.646–0.768; P = 0.002 and P = 0.001, respectively) and also significantly better than a model incorporating age, sex and APOE ε4 carrier status alone (0.695, 0.628–0.762; P = 0.004 and P = 0.002, respectively). Inclusion of these covariates did not significantly improve the performance of LC-MS amyloid-β1-42/amyloid-β1-40 or LC-MS composite. Sensitivity analyses in cognitively normal individuals without prior neurological conditions showed similar results (Supplementary Table 7). Combining Simoa p-tau181 with either Simoa or LC-MS amyloid-β did not contribute significantly to model performance (Supplementary Table 8). Further inclusion of educational attainment and time between blood sampling and scan also did not contribute significantly (data not shown). The best-performing Simoa and LC-MS biomarkers all had better concordance with amyloid PET status in APOE ε4 non-carriers than carriers (Supplementary Table 9).
Table 2.
AUC from ROC analyses of amyloid PET status
| Predictor(s) | Biomarker alone |
Biomarker + Age + sex + APOEε4 carrier status |
||
|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | |
| Age + sex + APOE ε4 carrier status | – | – | 0.695 | 0.628, 0.762 |
| Simoa plasma Aβ42/Aβ40 | 0.620 | 0.548, 0.691 | 0.727 | 0.665, 0.788 |
| Simoa plasma p-tau181 | 0.707 | 0.646, 0.768 | 0.778 | 0.727, 0.828 |
| LC-MS plasma Aβ1-42/Aβ1-40 | 0.817a,b | 0.770, 0.864 | 0.841c | 0.796, 0.886 |
| LC-MS plasma composite | 0.820b,d | 0.775, 0.866 | 0.843c | 0.798, 0.887 |
The results of analyses using the two best performing blood biomarkers from each platform are shown. Models incorporated blood biomarkers with and without inclusion of age, sex and APOE ε4 carrier status, in dementia-free individuals (n = 441).
DeLong test results indicated where P < 0.05:
P = 0.004 compared to age + sex + APOE ε4 carrier status; P = 0.002 compared to Simoa plasma p-tau181.
P < 0.0001 compared to Simoa plasma Aβ42/Aβ40.
P < 0.0001 compared to age + sex + APOE ε4 carrier status.
P = 0.002 compared age + sex + APOE ε4 carrier status; P = 0.001 compared to Simoa plasma p-tau181.
Aβ = amyloid-β; AUC = area under the receiver operating characteristics curve; CI = confidence interval; p-tau181 = phospho-tau181.
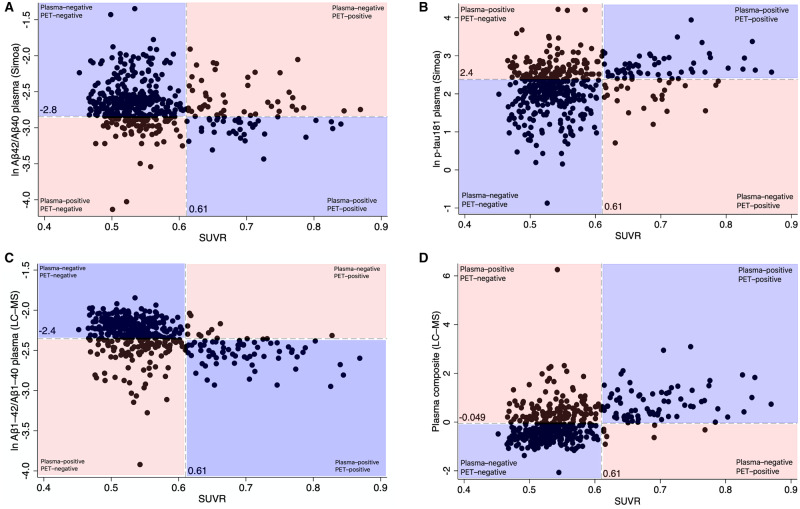
The percentage of individuals who were discordant for plasma and PET ranged from 26% to 32% depending on the plasma test used. Most discordant individuals were ‘plasma-positive, PET-negative’ using both the LC-MS biomarkers [104/115 = 90.4% of individuals discordant for LC-MS amyloid-β42/amyloid-β40 versus PET (Fig. 3C); and 124/130 = 94.6% of individuals discordant for the LC-MS composite versus PET (Fig. 3D)], but both types of discordance were seen using the Simoa biomarkers (Fig. 3A and B). The patterns were similar if different SUVR cut-off points were used (Supplementary Tables 10–13).
Figure 3.
Concordance of blood biomarkers with amyloid PET SUVR in dementia-free individuals in Insight 46 (n = 441). (A) ln Simoa plasma amyloid-β42/amyloid-β40. (B) ln Simoa plasma p-tau181. (C) ln LC-MS plasma amyloid-β1-42/amyloid-β1-40. (D) LC-MS plasma composite. Dashed vertical lines represent the SUVR cut-off point for PET positivity. Dashed horizontal lines represent the LC-MS biomarker cut-off points corresponding with Youden’s index derived by ROC analyses. Concordant classification by PET and plasma is represented by the blue area and discordant classification by the orange area on each graph. The non-log transformed cut-off points are 0.058 for Simoa plasma amyloid-β42/amyloid-β40, 10.8 pg/ml for Simoa plasma p-tau181, 0.095 for LC-MS plasma amyloid-β1-42/amyloid-β1-40 and −0.049 for LC-MS plasma composite. Aβ = amyloid-β; p-tau181 = phospho-tau181.
Blood tests for screening prior to amyloid PET scan
Table 3 shows the potential outcomes of screening with the two best-performing biomarkers from each platform, in a population with amyloid PET positivity prevalence similar to Insight 46. Without plasma screening, 543 scans would be required to obtain 100 amyloid PET-positive individuals. Screening 940 individuals by using a base model of age, sex and APOE ε4 carrier status would reduce the number of scans required to 266 (i.e. by 50.0%). Neither Simoa plasma amyloid-β42/amyloid-β40 nor p-tau181 could outperform the base model, allowing for 41.3% and 44.4% reduction in scan numbers, respectively. However, screening 623 individuals with LC-MS amyloid-β1-42/amyloid-β1-40 using a cut-off point of 0.095 would reduce the number of scans required to 243 (i.e. by 54.4%) and screening 588 individuals with the LC-MS composite using a cut-off point of −0.049 would reduce the number of scans required to 264 (i.e. by 50.4%). Conversely, using multivariable models incorporating age, sex and APOE ε4 carrier status, LC-MS amyloid-β1-42/amyloid-β1-40 and composite would allow the number of scans to be reduced by 54.8% and 60.9%, respectively.
Table 3.
Use of blood biomarkers for screening prior to amyloid PET scan
| Model | Sensitivity, % | Specificity, % | Accuracy, % | Number needed to screen | Number proceeding to amyloid PET scan | Number of scans saved relative to no screening | % Scans saved relative to no screening | % Scans saved relative to Age + sex + APOEε4 carrier status |
|---|---|---|---|---|---|---|---|---|
| Age + sex + APOE ε4 carrier status | 57.3 | 78.3 | 74.4 | 940 | 266 | 272 | 50.0 | – |
| Simoa plasma Aβ42/Aβ40 | 45.1 | 78.0 | 71.9 | 1192 | 314 | 224 | 41.3 | −17.6 |
| Simoa plasma p-tau181 | 70.7 | 68.3 | 68.7 | 762 | 297 | 241 | 44.4 | −11.4 |
| LC-MS plasma Aβ1-42/Aβ1-40 | 86.6 | 71.9 | 74.6 | 623 | 243 | 295 | 54.4 | 8.5 |
| LC-MS plasma composite | 91.5 | 65.7 | 70.5 | 588 | 264 | 274 | 50.4 | 0.7 |
| Simoa plasma Aβ42/Aβ40 + age + sex + APOE ε4 carrier status | 68.3 | 72.1 | 71.4 | 794 | 280 | 258 | 47.4 | −5.1 |
| Simoa plasma p-tau181 + age + sex + APOE ε4 carrier status | 90.2 | 52.4 | 59.4 | 596 | 331 | 207 | 38.1 | −23.9 |
| LC-MS plasma Aβ1-42/Aβ1-40 + age + sex + APOE ε4 carrier status | 86.6 | 72.1 | 74.8 | 620 | 241 | 297 | 54.8 | 9.2 |
| LC-MS plasma composite + age + sex + APOE ε4 carrier status | 76.8 | 81.1 | 80.3 | 697 | 207 | 331 | 60.9 | 21.7 |
Values for sensitivity, specificity and accuracy were obtained by using Youden’s index cut-off points from each model for discriminating amyloid PET-positive from PET-negative dementia-free individuals (n = 441). The penultimate column shows the percentage of scans saved relative to the number of scans that would be required without screening in a population with similar prevalence of amyloid PET-positivity to Insight 46 (18.6% prevalence of amyloid PET positivity would mean 538 scans would be required to obtain 100 amyloid PET-positive individuals). The last column shows the percentage of scans saved relative to the base model incorporating age + sex + APOE ε4 carrier status, where negative values indicate worse screening test performance than the base model. Aβ = amyloid-β; p-tau181 = phospho-tau181.
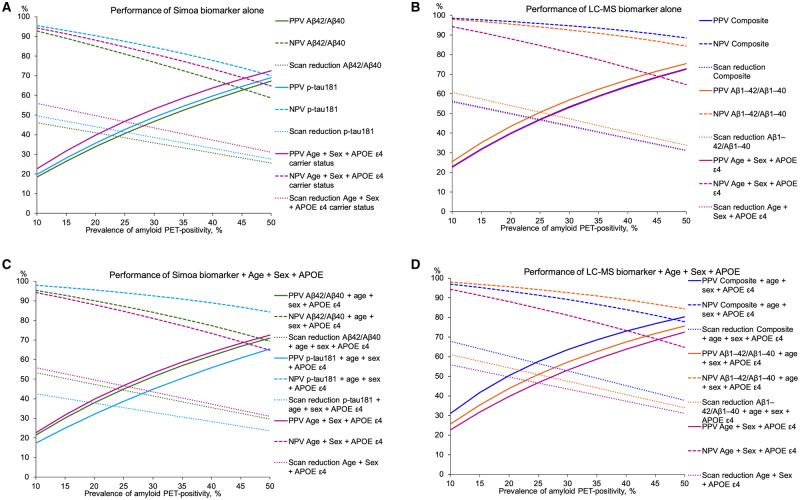
Extending the findings to hypothetical populations with differing prevalence of amyloid PET positivity, Fig. 4 shows that regardless of the screening test used, greater scan reduction would be afforded in populations with lower prevalence of amyloid PET positivity. Of the two Simoa platform tests, only plasma p-tau181 would have better negative predictive value (NPV) than the base model, but neither Simoa test would perform better than the base model in terms of positive predictive value (PPV) or scan reduction (Fig. 4A). The greatest PPV and scan reduction would be afforded by LC-MS amyloid-β1-42/amyloid-β1-40, while the NPV would be greatest for the LC-MS composite (Fig. 4B). If added to age, sex and APOE ε4 carrier status, LC-MS composite would have an improvement in PPV and scan reduction at the expense of a worsening of NPV, whereas LC-MS amyloid-β1-42/amyloid-β1-40 would have an improvement in NPV without much change in PPV or scan reduction (Fig. 4D).
Figure 4.
Hypothetical performance of the best-performing Simoa and LC-MS tests by prevalence of amyloid PET positivity. (A) Simoa biomarker alone. (B) LC-MS biomarker alone. (C) Simoa biomarker + age + sex + APOE ε4 carrier status. (D) LC-MS biomarker + age + sex + APOE ε4 carrier status. Lines were modelled by computing the positive (PPV) and negative predictive values (NPV) of the tests and the scan reduction afforded at specified values of amyloid PET positivity prevalence over the range 10–50%, in 5% intervals. Scan reduction is the percentage of scans saved relative to the number of scans that would be required without screening (calculated according to the specified prevalence of amyloid PET positivity). Solid lines show PPV, dashed lines show NPV and dotted lines show scan reduction. Aβ = amyloid-β; p-tau181 = phospho-tau181.
Relative costs of blood screening
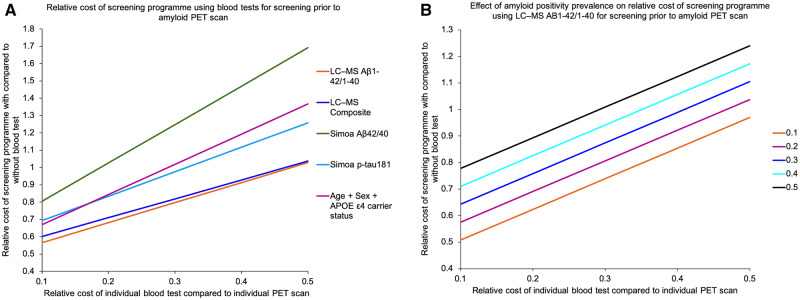
Using the equations in Fig. 1, in a population of similar prevalence of amyloid PET positivity to Insight 46, the LC-MS amyloid-β1-42/amyloid-β1-40 test would yield the lowest relative cost of screening with versus without using blood tests (Fig. 5A) across a range of theoretical fractional costs of individual blood tests compared to PET scans. However, with higher prevalence of amyloid PET positivity, as may be seen with increasing age, the screening programme would be rendered less economical for a given blood test (Fig. 5B shows the relationship with amyloid PET positivity for the LC-MS amyloid-β1-42/amyloid-β1-40 test).
Figure 5.
Relative costs of screening programmes using different blood tests. (A) Using different blood tests, at fixed prevalence of amyloid PET positivity. (B) Using LC-MS plasma amyloid-β1-42/amyloid-β1-40 over a range of 10–50% of theoretical prevalence of amyloid PET positivity. Aβ = amyloid-β; p-tau181 = phospho-tau181.
Discussion
In more than 400 dementia-free individuals of near-identical age, we show that LC-MS methods for measuring plasma amyloid-β1-42/amyloid-β1-40 and a plasma composite outperform the Simoa amyloid-β42/amyloid-β40 and p-tau181 assays in their ability to discern amyloid PET status. Using either LC-MS method to screen before PET scanning has the potential to yield significant savings for clinical trial recruitment, affording further reductions in the required number of PET scans compared to the number of scans needed without pre-screening or when using age, sex and APOE ε4 carrier status for screening.
Despite our cohort’s narrow age range, lower LC-MS amyloid-β1-42/amyloid-β1-40 was associated with increased age, and this was not attenuated by further adjustment for PACC, white matter hyperintensity volume or whole brain volume. A more age-diverse study (Schindler et al., 2019) has reported a negative association, but its magnitude was much smaller (a reduction of ∼3% per year of age). In our age-restricted study it is therefore possible that this finding either represents a true age effect, or reflects some other source of variation between those attending near the start and the end of the study (James et al., 2018), or both.
We found associations between APOE ε4 carrier status and LC-MS amyloid-β1-42 and amyloid-β1-42/amyloid-β1-40 independent of SUVR. However, while other groups using mass spectrometry-based techniques for quantifying plasma amyloid-β to predict cerebral amyloid status (Ovod et al., 2017; Nakamura et al., 2018; Schindler et al., 2019) commonly included age and APOE ε4 carrier status in predictive models, we found that including these variables made no material difference to the ability to predict amyloid status when using LC-MS amyloid-β1-42/amyloid-β1-40, and only small improvements using the LC-MS composite proposed by Nakamura et al. (2018). Noting that amyloid PET results are not routinely adjusted for APOE ε4 carrier status, this provides further confidence that the LC-MS assay is directly reflecting brain amyloid-β. Our finding that the majority of discordant cases were ‘plasma-positive, PET-negative’, and this persisted despite changing the PET-positivity cut-off, is consistent with prior studies showing similar results for plasma (Schindler et al., 2019) and CSF (Palmqvist et al., 2016), suggesting that as with CSF, plasma amyloid-β markers may become abnormal before a threshold for cortical amyloid-β positivity is reached.
Our results were not significantly altered by using different PET cut-off points. However, Youden’s index cut-off points for plasma amyloid-β, which we chose to maximize the combination of sensitivity and specificity, may not be the most useful in every screening setting. For example, in recruitment to a clinical trial it may be desirable to choose a cut-off point that maximizes sensitivity, although this increases the false positive rate. In contrast, for population screening for clinical purposes it may be desirable to minimize false positives, even if at the expense of the false negative rate.
In addition to cost reduction, plasma testing may allow for screening of more diverse populations at scale, and reductions in screen failures, resulting in faster clinical trial recruitment. We demonstrate that the greatest potential savings would be made at lower prevalence of amyloid-β positivity. The prevalence of amyloid PET positivity is highly age-dependent; as shown in a 2015 meta-analysis it was 16% at 60 years, 23% at 70 years and 33% at 80 years (Jansen et al., 2015). In more recent data from the Mayo Clinic Study of Aging the prevalence was 18% at age 60–69 and 32% at age 70–79 (Roberts et al., 2018) and the former figure is consistent with the prevalence of 18.6% we note in Insight 46. As with any screening test, false positives will be expected, and a general consideration with all amyloid biomarker studies of asymptomatic individuals is that progression from amyloid positivity to clinical signs or symptoms may take many years (Vos et al., 2013; Donohue et al., 2017; Roberts et al., 2018), so amyloid-positive individuals may never develop cognitive symptoms in their lifetime. Any use of plasma biomarker-based screening will require clear protocols for counselling and communicating plasma test results to prospective participants (Harkins et al., 2015), including that a positive result is likely to require confirmation with another more definitive modality (PET or CSF).
The accuracy of the Simoa plasma p-tau181 assay in predicting amyloid PET status in Insight 46 was lower than that previously published in cohorts comprising mixed populations of cognitively unimpaired individuals, MCI and Alzheimer’s disease dementia; the AUC were 0.707 in Insight 46, 0.881 in the Translational Biomarkers of Aging and Dementia (TRIAD) cohort and 0.761 in the BIOFINDER-2 cohort in which the same assay was used (Karikari et al., 2020), and 0.79–0.81 in the BioFINDER-2 cohort in which a different Mesoscale Discovery assay was used (Janelidze et al., 2020). In the TRIAD cohort, when analysis was limited to cognitively unimpaired older individuals, the AUC for prediction of amyloid PET status was 0.772. The differences observed may relate to differences in amyloid PET tracers (18F-florbetapir in Insight 46, 18F-AZD-4694 in TRIAD and 18F-flutemetamol in BioFINDER) but may also be influenced by differences in the cohorts in terms of burden of tau pathology, which is also highly correlated with plasma p-tau181 (Mielke et al., 2018; Janelidze et al., 2020; Karikari et al., 2020). As these cohorts had wider age ranges, including some individuals up to a decade older than those in Insight 46, amyloid PET-positive individuals in those cohorts may have had a greater burden of cerebral tau pathology at the time of blood sampling than those in Insight 46, resulting in higher plasma p-tau181 levels. At this phase of Insight 46 we did not have a measure of cerebral tau pathology such as tau PET, and therefore were not able to investigate the relative predictive capacity of plasma p-tau181 for amyloid PET versus tau PET. Since performing our study, an immunoassay for plasma p-tau217 has been developed that shows improved predictive capacity for neuropathologically defined Alzheimer’s disease versus non-Alzheimer’s disease dementias (AUC 0.98 for plasma p-tau217 and 0.85 for plasma p-tau181), improved performance for biomarker-supported in vivo diagnosis of Alzheimer’s disease versus non-Alzheimer’s disease (AUC 0.96 and 0.81 respectively), good concordance with cerebral tau deposition defined by tau PET, and elevations in presenilin-1 mutation carriers with presymptomatic dominantly inherited Alzheimer’s disease compared to non-carriers (Palmqvist et al., 2020). A mass spectrometry-based plasma p-tau217 assay (Barthélemy et al., 2020) has also shown good predictive capacity for CSF amyloid status in two smaller cohorts consisting of a mixture of younger and older controls, and individuals with cognitive impairment with and without CSF amyloid pathology (AUC 0.92–0.99 for plasma p-tau217 as opposed to 0.75–0.98 for plasma p-tau181). Future work will therefore benefit from comparing the plasma p-tau217 and p-tau181 assays in their utility for predicting amyloid status in preclinical cohorts like Insight 46.
While our study design has strengths, including general population-representativeness at initial recruitment, direct comparison of blood testing platforms, and concurrent prospectively-collected plasma and PET amyloid data, we acknowledge certain limitations. Our participants are exclusively white British, so these findings cannot be extrapolated directly to ethnically diverse populations. The narrow age range of our study might also have underestimated predictive capacity of models of amyloid PET status that incorporated age, sex and APOE ε4 carrier status. Using global cortical amyloid PET as an outcome may underestimate the true number of participants with cerebral amyloid deposition, as CSF changes and region-specific amyloid PET deposition (Palmqvist et al., 2016), may occur earlier than when participants become ‘amyloid PET-positive’ by global cortical PET SUVR. CSF was not available for this phase of data collection in Insight 46. Using CSF as the comparator instead of PET might reduce the cost savings of blood tests, as CSF testing is likely to be more economical than PET, both in terms of initial setup and ongoing costs (Wittenberg et al., 2019). Our assessment of the relative costs of screening using blood tests is simplified, as it absorbs any differences in setup costs between the two blood test platforms into one theoretical cost estimate for each test. Both platforms require expensive instruments and specialist operators, and it is likely that real-world differences in setup and maintenance costs would play an important role in choosing one platform over another, in addition to differences in accuracy between platforms.
In conclusion, we demonstrate superior performance of the LC-MS platform over Simoa assays for plasma amyloid-β and p-tau181, and over a model incorporating age, sex and APOE ε4 carrier status, in screening for concurrent PET amyloid status in a large number of dementia-free individuals. Our study strengthens a growing body of evidence that plasma screening can reduce the numbers of amyloid PET scans required to identify amyloid-β-positive individuals, for recruitment to clinical trials or ultimately for giving anti-amyloid therapies, and suggests that this may be feasible in a preclinical cohort.
Supplementary Material
Acknowledgements
We are very grateful to those study members who helped in the design of the study through focus groups, and to the participants both for their contributions to Insight 46 and for their commitments to research over the past seven decades. We are grateful to the radiographers and nuclear medicine physicians at the University College London Institute of Nuclear Medicine, the staff at the Leonard Wolfson Experimental Neurology Centre at University College London, and neuroradiologists Dr Chandrashekar Hoskote and Dr Sachit Shah for providing clinical reads for the MRI scans.
Funding
This research was funded by a Wolfson Clinical Research Fellowship awarded to A.K., and a Weston Brain Institute and Selfridges Group Foundation award (UB17005), with leveraged funding from Alzheimer’s Research UK (ARUK-PG2014–1946, ARUK-PG2017–1946), Medical Research Council Dementia Platforms UK (CSUB19166) and the Wolfson Foundation (PR/ylr/18575). The genetic analyses were funded by the Brain Research Trust (UCC14191). Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly, kindly provided the 18F-florbetapir tracer (AmyvidTM) free of cost but had no role in the design, conduct, analysis or reporting of Insight 46 study findings. We are particularly indebted to the support of the late Chris Clark of Avid Radiopharmaceuticals who championed this study from its outset. T.K.K. holds a Brightfocus fellowship (#A2020812F), and is further supported by the Swedish Alzheimer Foundation (Alzheimerfonden; #AF-930627), the Swedish Brain Foundation (Hjärnfonden; #FO2020-0240), the Swedish Dementia Foundation (Demensförbundet), Gamla Tjänarinnor, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, the Gun and Bertil Stohnes foundation, and the Anna Lisa and Brother Björnsson’s Foundation. N.J.A. is supported by the Wallenberg Centre for Molecular and Translational Medicine, the Swedish Alzheimer Foundation (Alzheimerfonden), the Swedish Dementia Foundation (Demensförbundet), Hjärnfonden, Sweden and the Anna Lisa and Brother Björnsson’s Foundation. T.D.P. was supported by a Wellcome Trust Clinical Research Fellowship (200109/Z/15/Z). The NSHD, MR, and AW are funded by the Medical Research Council (MC_UU_00019/1, MC_UU_00019/3). N.C.F. is supported by UK Dementia Research Institute at University College London, Medical Research Council, National Institute for Health Research (Senior Investigator award), and Engineering and Physical Sciences Research Council. K.B. is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986). H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), ADDF (#201809-2016862), Swedish State Support for Clinical Research (#ALFGBG-720931), the Leonard Wolfson Experimental Neurology Centre and the UK Dementia Research Institute at UCL. J.M.S. is supported by University College London Hospitals Biomedical Research Centre, Engineering and Physical Sciences Research Council (EP/J020990/1), British Heart Foundation (PG/17/90/33415), and EU’s Horizon 2020 research and innovation programme (666992). N.C.F. and J.M.S. are supported by the National Institute for Health Research Queen Square Dementia Biomedical Research Unit and the Leonard Wolfson Experimental Neurology Centre. The HD-1 Analyser at UCL was funded by a Multi-User Equipment grant from the Wellcome Trust.
Competing interests
N.C.F.’s research group has received payment for consultancy or for conducting studies from Biogen, Eli Lilly Research Laboratories, GE Healthcare, and Roche. N.C.F. receives no personal compensation for the aforementioned activities. J.M.S. has received research funding from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly), has consulted for Roche Pharmaceuticals, Biogen, Merck, and Eli Lilly, given educational lectures sponsored by GE Healthcare, Eli Lilly, and Biogen, and serves on a Data Safety Monitoring Committee for Axon Neuroscience SE. H.Z. has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Alzecure, Fujirebio and Biogen. K.B. has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis and Roche Diagnostics. H.Z. and K.B. are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- LC-MS
liquid chromatography-mass spectrometry
- MCI
mild cognitive impairment
- Simoa
single molecule array
- SUVR
standardized uptake value ratio
References
- Barthélemy NR, Horie K, Sato C, Bateman RJ.. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med 2020; 217: e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ. Overview of dominantly inherited AD and top-line DIAN-TU results of solanezumab and gantenerumab. In: Alzheimer’s Association Internation Conference, Amsterdam, 2020.
- Benussi A, Karikari TK, Ashton NJ, Gazzina S, Premi E, Benussi L, et al. Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2020; 91: 960–7. [DOI] [PubMed] [Google Scholar]
- Biogen. Biogen Q3 2019 Earnings Presentation. 2019. http://investors.biogen.com/static-files/40565136-b61f-4473-9e58-9be769bbac6c (24 December 2019, date last accessed).
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS; for the Alzheimer’s Disease Neuroimaging Initiative. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017; 317: 2305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med 2014; 370: 311–21. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–29. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Harkins K, Sankar P, Sperling R, Grill JD, Green RC, Johnson KA, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther 2015; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SN, Lane CA, Parker TD, Lu K, Collins JD, Murray-Smith H, et al. Using a birth cohort to study brain health and preclinical dementia: recruitment and participation rates in Insight 46. BMC Res Notes 2018; 11: 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med 2020; 26: 379–86. [DOI] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015; 313: 1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the Alzheimer's association. Alzheimers Dement 2013; 9: e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020; 19: 422–33. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004; 55: 306–19. [DOI] [PubMed] [Google Scholar]
- Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med 2013; 54: 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane CA, Parker TD, Cash DM, Macpherson K, Donnachie E, Murray-Smith H, et al. Study protocol: insight 46—a neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol 2017; 17: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KK, Barnes J, Modat M, Ridgway GR, Bartlett JW, Fox NC, et al. Brain MAPS: an automated, accurate and robust brain extraction technique using a template library. Neuroimage 2011; 55: 1091–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Nicholas JM, Collins JD, James SN, Parker TD, Lane CA, et al. Cognition at age 70: life course predictors and associations with brain pathologies. Neurology 2019; 93: e2144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 2015; 104: 366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018; 14: 989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E, Chalkidou A, Hammers A, Peacock J, Summers J, Keevil S.. Diagnostic accuracy of (18)F amyloid PET tracers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2016; 43: 374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol 1995; 38: 643–8. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature 2018; 554: 249–54. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers. NICE Guideline 97; London: National Institute for Health and Care Excellence (UK); 2018. https://www.nice.org.uk/guidance/ng97/evidence/full-guideline-pdf-4852695709. [PubMed] [Google Scholar]
- Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol 2016; 15: 673–84. [DOI] [PubMed] [Google Scholar]
- Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017; 13: 841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020; 324: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Janelidze S, Stomrud E, Zetterberg H, Karl J, Mattsson N, et al. Detecting brain amyloid status using fully automated plasma Aβ biomaker assays. Alzheimer’s Dement 2018; 14: 1670. [Google Scholar]
- Palmqvist S, Mattsson N, Hansson O; for the Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain 2016; 139: 1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannee J, Portelius E, Minthon L, Gobom J, Andreasson U, Zetterberg H, et al. Reference measurement procedure for CSF amyloid beta (Abeta)1-42 and the CSF Abeta1-42/Abeta1-40 ratio—a cross-validation study against amyloid PET. J Neurochem 2016; 139: 651–8. [DOI] [PubMed] [Google Scholar]
- Papp KV, Amariglio RE, Dekhtyar M, Roy K, Wigman S, Bamfo R, et al. Development of a psychometrically equivalent short form of the face-name associative memory exam for use along the early Alzheimer's disease trajectory. Clin Neuropsychol 2014; 28: 771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M.. Apolipoprotein-E (Apoe) epsilon4 and cognitive decline over the adult life course. Transl Psychiatry 2018; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Aakre JA, Kremers WK, Vassilaki M, Knopman DS, Mielke MM, et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol 2018; 75: 970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med 2014; 370: 322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019; 93: e1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Arias J, Blennow K, Galasko D, Molinuevo JL, Salloway S, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement 2018; 14: 1505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaets S, Le Bastard N, Martin JJ, Sleegers K, Van Broeckhoven C, De Deyn PP, et al. Cerebrospinal fluid Abeta1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J Alzheimers Dis 2013; 36: 759–67. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Donohue MC, Raman R, Sun C-K, Yaari R, Holdridge K, et al. ; for the A4 Study Team. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol 2020; 77: 735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Cardoso MJ, Bouvy WH, Biessels GJ, Barnes J, Ourselin S.. Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging 2015; 34: 2079–102. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. ; the Alzheimer’s Disease Neuroimaging Initiative. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun 2013; 1: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Rinne JO, Boada M, Katayama S, Scheltens P, Vellas B, et al. ; Bapineuzumab 3000 and 3001 Clinical Study Investigators. Bapineuzumab for mild to moderate Alzheimer's disease in two global, randomized, phase 3 trials. Alzheimers Res Ther 2016; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018; 84: 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergallo A, Megret L, Lista S, Cavedo E, Zetterberg H, Blennow K, et al. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer's disease. Alzheimers Dement 2019; 15: 764–75. [DOI] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013; 12: 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale—revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler memory scale—revised: manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Wittenberg R, Knapp M, Karagiannidou M, Dickson J, Schott J.. Economic impacts of introducing diagnostics for mild cognitive impairment Alzheimer's disease patients. Alzheimers Dement 2019; 5: 382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy is available on the NSHD Data Sharing website (see https://skylark.ucl.ac.uk/NSHD/doku.php).