Abstract
Background
We aim to assess the value of locoregional treatment (LRT) including breast‐conserving surgery (BCS), mastectomy (MAST), and radiotherapy (RT) in patients with de novo stage IV breast cancer.
Methods
Patients with de novo stage IV breast cancer were retrospectively identified from the Surveillance, Epidemiology, and End Results database between 2004 and 2014. Kaplan‐Meier analysis, log‐rank tests, propensity score matching (PSM), and the multivariate Cox proportional model were used for statistical analysis.
Results
A total of 5798 patients were identified including 849 (14.6%), 763 (13.2%), 2338 (40.3%), and 1848 (31.9%) who received BCS alone, BCS+RT, MAST alone, and MAST+RT, respectively. The proportions of receiving BCS decreased from 35.9% in 2004 to 26.2% in 2014 (p = 0.002), and the probability of patients receiving MAST increased from 64.1% in 2004 to 74.8% in 2014 (p = 0.002). Before PSM, there was a significant difference in breast cancer‐specific survival (BCSS) among the treatment arms. Patients who received RT had better BCSS, the 5‐year BCSS was 40.5%, 52.3%, 41.5%, and 47.7% in patients treated with BCS alone, BCS+RT, MAST alone, and MAST+RT, respectively (p < 0.001). In the PSM cohort, patients treated with BCS alone had lower 5‐year BCSS compared to those treated with BCS+RT (43.9% and 52.1%, p = 0.002). However, there were comparable 5‐year BCSS between BCS+RT and MAST alone groups (51.3% and 50.1%, p = 0.872), and BCS+RT and MAST+RT cohorts (51.5% and 55.7%, p = 0.333). Similar results were confirmed in multivariate analysis.
Conclusions
Postoperative RT improves BCSS in patients with de novo stage IV breast cancer, and BCS+RT shows a non‐inferior outcome compared to MAST+RT. BCS+RT may be the optimal local management of de novo stage IV breast cancer.
Keywords: breast‐conserving surgery, mastectomy, metastatic breast cancer, radiotherapy, survival
In this population‐based retrospective study, we aim to compare the differences on survival between breast‐conserving surgery (BCS) and mastectomy (MAST) in de novo stage IV breast cancer. In addition, we further analyzed the effect of additional radiotherapy (RT) on survival outcomes in this patient subset. Our results showed that postoperative RT improves BCSS in patients with de novo stage IV breast cancer, and BCS+RT shows non‐inferior outcome compared to MAST+RT. BCS+RT may be the optimal local management of de novo stage IV breast cancer.
1. INTRODUCTION
Approximately 2 million women were newly diagnosed with breast cancer in 2018 worldwide, accounting for 11.6% of all cancer types, and breast cancer is the leading cause of cancer death in females. 1 De novo stage IV breast cancer, which is defined by metastasis from the breast and axilla to distant sites, was approximately 3.6%–6% of all newly diagnosed breast cancer. 2 , 3 Bone (66.3%), lung (30.3%), liver (26.1%), and brain (7.3%) were the most common sites of distant metastasis. 4 The clinical outcome in patients with de novo stage IV breast cancer was significantly poor with median survival ranging from 16 to 29 months. 5 , 6 , 7 Systemic therapy was a mainstay of treatment in patients with de novo stage IV breast cancer. 5 , 8 , 9 , 10 , 11 Locoregional treatments (LRTs), including surgery or radiotherapy (RT), are recommended just for relieving symptoms, and whether LRT should be administrated to primary tumor remains controversial. Two large prospective studies of local surgery have yielded conflicting results 7 , 12 ; however, most of the current retrospective studies showed a survival advantage with local surgery to the primary tumor. 13 , 14 , 15 , 16
RT is still deemed to be one of the significant ways of LRT, whereas related studies are rare and inconclusive compared with surgery. A retrospective study from France regarded the effect of RT in de novo stage IV breast cancer, and they found that RT alone provided comparable metastasis progression‐free survival and overall survival (OS) rates compared to the combination of RT and surgery. 17 However, another population‐based study including 2207 patients with de novo stage IV breast cancer showed that post‐surgery RT increased breast cancer‐specific survival (BCSS) rates compared to surgery alone. 18 Given the controversy, several prospective randomized trials are in progress to sort out this concept. 19 , 20 , 21 However, these trials all focused on the value of surgery in de novo stage IV breast cancer. To the best of our knowledge, the survival benefit of RT has been rarely elucidated in the prospective study; therefore, it is worth exploring the value of postoperative RT in this disease. In addition, the optimal procedure of surgical approaches and whether additional RT after surgery has survival benefit remain unclear. In this population‐based retrospective study, we aim to compare the differences in survival between breast‐conserving surgery (BCS) and mastectomy (MAST) in de novo stage IV breast cancer. In addition, we further analyzed the effect of postoperative RT on survival outcomes in this patient subset.
2. METHODS AND MATERIALS
2.1. Database source and patient's collection
The Surveillance, Epidemiology, and End Results (SEER) database was used to identify patients. The SEER database is a population‐based, open‐access resource collecting information on cancer prevalence, management, and survival for approximately 28% of the United States population. 22 We included patients who met the following criteria: (a) female patients with newly diagnosed metastatic breast cancer from 2004 to 2014; (b) receiving systemic chemotherapy and local therapy including BCS and MAST; and (c) availability of data on age, race/ethnicity, tumor (T) stage, nodal (N) stage, estrogen receptor (ER), progesterone receptor (PR), surgical approaches, and RT records. Patients without positive pathological diagnosis and receiving non‐beam irradiation were excluded. Non‐beam irradiation was defined as RT technologies including radioactive implants and radioisotopes. We obtained the access to the SEER database, and using the data was exempt from the approval of the Institutional Review Board for not involving any private information.
2.2. Variables
The following baseline variables were identified: age at diagnosis (<65 years, ≥65 years), race/ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic, and Other), histology (invasive ductal carcinoma [IDC], invasive lobular carcinoma [ILC], and other), grade (well‐differentiated, moderately differentiated, and poorly differentiated/undifferentiated), T stage (T1, T2, T3, and T4), N stage (N0, N1, N2, and N3), ER status (ER positive and ER negative), PR status (PR positive and PR negative), treatment procedures (BCS alone, BCS+RT, MAST alone, and MAST+RT). The TNM stage was defined using the 7th edition of the American Joint Committee on Cancer staging system. 23 The primary objective of this study was BCSS, defined as the time interval from diagnosis to death from breast cancer.
2.3. Statistical analysis
Chi‐square test was used to analyze the differences in demographic and clinicopathological characteristics among the treatment groups. Survival curves were delineated with the Kaplan‐Meier analysis and compared with the log‐rank test. Propensity score matching (PSM) was performed using 1:1 nearest neighbor matching to balance the distribution of most demographic and clinical characteristics including age at diagnosis, race/ethnicity, histology, grade, T stage, N stage, ER status, and PR status. Cox proportional hazards multivariable regression was used to calculate the independent risk factors of BCSS. All statistical results were conducted using SPSS statistical software (version 22.0, IBM Corporation, Armonk, NY, USA). A p < 0.05 (two‐tailed) was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
In totality, 5798 patients were identified with a median age of 55 years (range, 19–94 years). The patient baseline characteristics are shown in Table 1. All of the patients received systematic chemotherapy. Majority of the patients were non‐Hispanic White (62.1%), IDC (77.2%) subtype, poor differentiation/undifferentiated (63.9%), and ER positive (62.3%) disease. In addition, 48.8% and 45.5% of the patients experienced T3‐4 and N2‐3 disease, respectively. With regarding to LRT, 14.6% (n = 849), 13.2% (n = 763), 40.3% (n = 2338), and 31.9% (n = 1848) of patients were treated with BCS alone, BCS+RT, MAST alone, and MAST+RT, respectively.
TABLE 1.
Baseline characteristics of the patients with de novo stage IV breast cancer before PSM
| Variables | N (%) | BCS | BCS+RT | MAST | MAST+RT | p |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| <65 | 4415 (76.1) | 613 (72.2) | 617 (80.9) | 1719 (73.5) | 1466 (79.3) | <0.001 |
| ≥65 | 1383 (23.9) | 236 (27.8) | 146 (19.1) | 619 (26.5) | 382 (20.7) | |
| Race/ethnicity | ||||||
| Non‐Hispanic White | 3603 (62.1) | 542 (63.8) | 475 (62.3) | 1438 (61.5) | 1148 (62.1) | 0.361 |
| Non‐Hispanic Black | 1011 (17.4) | 148 (17.4) | 117 (15.3) | 433 (18.5) | 313 (16.9) | |
| Hispanic | 701 (12.2) | 103 (12.1) | 102 (13.4) | 273 (11.7) | 223 (12.1) | |
| Other | 483 (8.3) | 56 (6.6) | 69 (9) | 194 (8.3) | 164 (8.9) | |
| Pathological subtype | ||||||
| IDC | 4474 (77.2) | 681 (80.2) | 638 (83.6) | 1771 (75.7) | 1384 (74.9) | <0.001 |
| ILC | 373 (6.4) | 52 (6.1) | 34 (4.5) | 158 (6.8) | 129 (7) | |
| Other | 951 (16.4) | 116 (13.7) | 91 (11.9) | 409 (17.5) | 335 (18.1) | |
| Grade | ||||||
| Well differentiated | 284 (4.9) | 58 (6.8) | 47 (6.2) | 104 (4.4) | 75 (4.1) | 0.001 |
| Moderately differentiated | 1807 (31.2) | 245 (28.9) | 263 (34.5) | 701 (30) | 598 (32.4) | |
| Poorly differentiated/ undifferentiated | 3707 (63.9) | 546 (64.3) | 453 (59.3) | 1533 (65.6) | 1175 (63.5) | |
| Tumor stage | ||||||
| T1 | 812 (14) | 218 (25.7) | 208 (27.3) | 247 (10.6) | 139 (7.5) | <0.001 |
| T2 | 2155 (37.2) | 404 (47.5) | 381 (49.9) | 827 (35.4) | 543 (29.4) | |
| T3 | 1049 (18.1) | 89 (10.5) | 76 (10) | 485 (20.7) | 339 (21.6) | |
| T4 | 1782 (30.7) | 138 (16.3) | 98 (12.8) | 779 (33.3) | 767 (41.5) | |
| Nodal stage | ||||||
| N0 | 979 (16.9) | 241 (28.4) | 202 (26.5) | 382 (16.3) | 154 (8.3) | <0.001 |
| N1 | 2182 (37.6) | 327 (38.5) | 297 (38.9) | 865 (37) | 693 (35.7) | |
| N2 | 1188 (20.5) | 135 (15.9) | 121 (15.9) | 490 (21) | 442 (23.8) | |
| N3 | 1449 (25) | 146 (17.2) | 143 (18.7) | 601 (25.7) | 559 (30.2) | |
| ER status | ||||||
| Negative | 2183 (37.7) | 347 (40.9) | 256 (33.6) | 945 (40.4) | 635 (34.4) | <0.001 |
| Positive | 3615 (62.3) | 502 (59.1) | 507 (66.4) | 1393 (59.6) | 1213 (65.6) | |
| PR status | ||||||
| Negative | 2977 (51.3) | 457 (53.8) | 365 (47.8) | 1261 (53.9) | 894 (48.4) | <0.001 |
| Positive | 2821 (48.7) | 392 (46.2) | 398 (52.2) | 1077 (46.1) | 954 (51.6) | |
Abbreviations: BCS, breast‐conserving surgery; ER, estrogen receptor; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma; MAST, mastectomy; N, number; PR, progesterone receptor; RT, radiotherapy.
Patients with IDC (p < 0.001) and early T and N stage (p < 0.001) were more likely to receive BCS, while patients with poorly differentiated/undifferentiated (p = 0.001) and advanced T/N stage (p < 0.001) had more possibility to receive MAST. In addition, patients with younger age, advanced T/N stage, and ER/PR‐positive diseases were more likely to receive postoperative RT (Table 1).
3.2. Trends of local treatment receipt
Figure S1 shows the trends of different therapeutic modalities from 2004 to 2014. The proportions of receiving BCS decreased from 35.9% in 2004 to 26.2% in 2014 (p = 0.002), whereas RT receipt had no statistically significant tendency with 54.1% in 2004 and 53.8% in 2014 (p = 0.65) of the patients treated with BCS. In addition, the probability of patients receiving MAST increased from 64.1% in 2004 to 74.8% in 2014 (p = 0.002). However, there was no statistical significance in the probability of RT administration in patients treated with MAST, with 40.8% in 2004 and 46.6% in 2014 (p = 0.073), respectively.
3.3. Survival and prognostic analyses before PSM
With a median follow‐up time of 37 months (range, 0–155 months), a total of 3723 deaths occurred, including 3281 breast cancer‐specific deaths, and the 5‐year BCSS was 44.8%. The 5‐year BCSS was 46.2% and 44.3% (p = 0.67) between BCS and MAST groups, respectively (Figure 1). When further stratified by surgical approach and RT, the 5‐year BCSS was 40.5%, 52.3%, 41.5%, and 47.7% in patients treated with BCS alone, BCS+RT, MAST alone, and MAST+RT, respectively (p < 0.001) (Figure 2).
FIGURE 1.
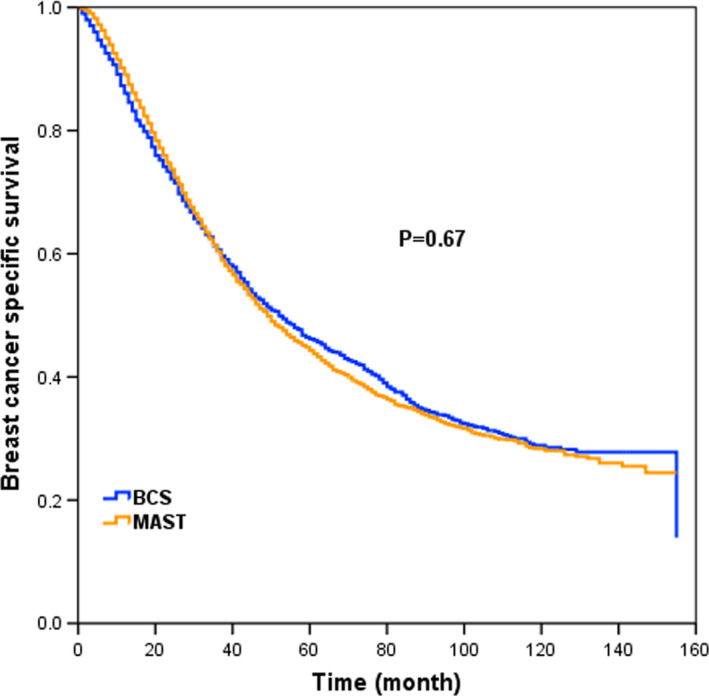
Breast cancer‐specific survival (BCSS) in patients treated with BCS and MAST for the whole cohort before propensity score matching (PSM)
FIGURE 2.
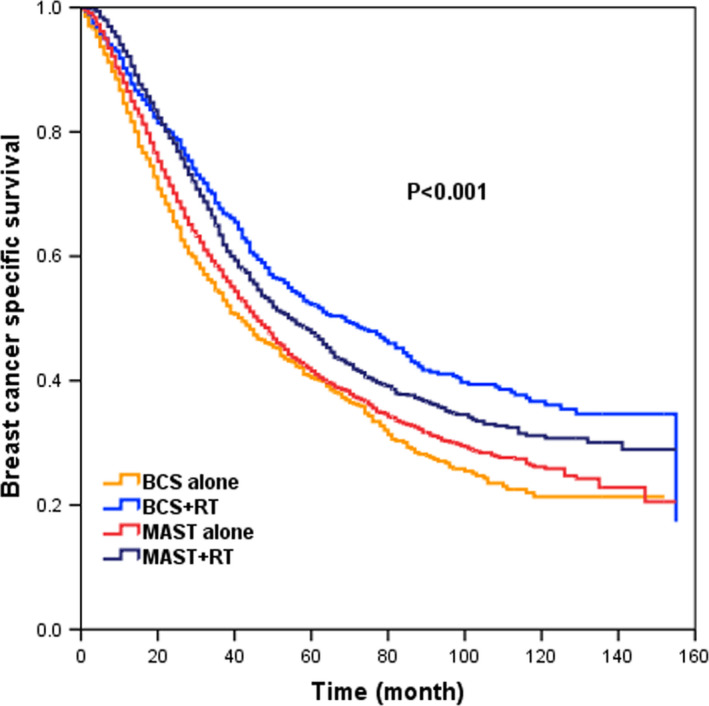
BCSS in patients receiving BCS alone, BCS+radiotherapy (RT), MAST alone, and MAST+RT for the whole cohort before PSM
On multivariate Cox regression analysis for BCSS (Table 2), old age (≥65 years), non‐Hispanic black, ILC subtype, poorly differentiated/undifferentiated, and advanced T/N stage were independent adverse prognostic factors, while ER‐positive and PR‐positive were associated with favorable prognosis. In addition, patients receiving BCS+RT (hazard ratio [HR]: 0.701, 95% confidence interval [CI]: 0.614–0.8, p < 0.001), MAST alone (HR: 0.794, 95% CI: 0.716–0.88, p < 0.001), and MAST+RT (HR: 0.635, 95% CI: 0.569–0.71, p < 0.001) had better BCSS when using BCS as a reference.
TABLE 2.
Multivariate analysis of cancer‐specific survival in patients with stage IV breast cancer before PSM
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Age (years) | |||
| <65 | 1 | ||
| ≥65 | 1.14 | 1.052–1.236 | 0.001 |
| Race/ethnicity | |||
| Non‐Hispanic white | 1 | ||
| Non‐Hispanic black | 1.295 | 1.185–1.419 | <0.001 |
| Hispanic | 0.988 | 0.887–1.100 | 0.825 |
| Other | 0.829 | 0.723–0.949 | 0.007 |
| Pathological subtype | |||
| IDC | 1 | ||
| ILC | 1.242 | 1.073–1.438 | 0.004 |
| Other | 1.023 | 0.932–1.124 | 0.632 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.147 | 0.943–1.394 | 0.169 |
| Poorly differentiated/ undifferentiated | 1.49 | 1.228–1.808 | <0.001 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.229 | 1.092–1.384 | <0.001 |
| T3 | 1.471 | 1.287–1.682 | <0.001 |
| T4 | 1.792 | 1.583–2.030 | <0.001 |
| Nodal stage | |||
| N0 | 1 | ||
| N1 | 0.973 | 0.876–1.080 | 0.604 |
| N2 | 1.058 | 0.941–1.190 | 0.347 |
| N3 | 1.211 | 1.083–1.353 | <0.001 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.774 | 0.703–0.852 | <0.001 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.709 | 0.644–0.780 | <0.001 |
| Treatments | |||
| BCS | 1 | ||
| BCS+RT | 0.701 | 0.614–0.800 | <0.001 |
| MAST | 0.794 | 0.716–0.880 | <0.001 |
| MAST+RT | 0.635 | 0.569–0.710 | <0.001 |
Abbreviations: BCS, breast‐conserving surgery; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma; MAST, mastectomy; PR, progesterone receptor; RT, radiotherapy.
3.4. Survival and prognostic analyses after PSM
When performing PSM, a total of 1227 pairs were completely matched between BCS and MAST cohorts. The patient characteristics for the whole patients after PSM are shown in Table S1. After adjusting age, ethnicity, grade, histology, T/N stage, ER/PR status, patients who received BCS were associated with worse BCSS compared with those who received MAST in the whole cohort (HR: 0.82, 95% CI: 0.736–0.915, p < 0.001, Table S2). The survival curve is shown in Figure 3 (p < 0.001).
FIGURE 3.
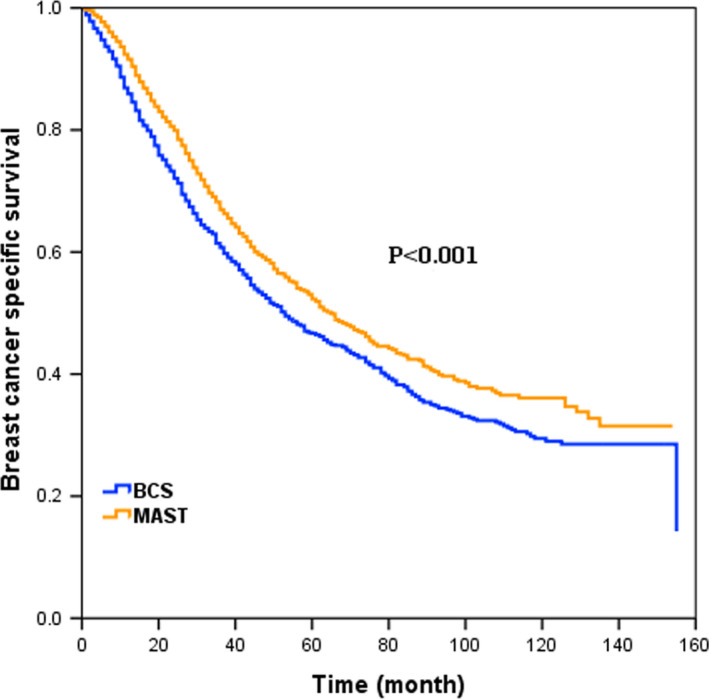
BCSS in patients treated with BCS and MAST for the whole patients after PSM
When further stratifying by surgery and RT, 415, 578, and 464 pairs were completely matched between BCS alone and BCS+RT, BCS+RT and MAST alone, and BCS+RT and MAST+RT groups, respectively. The patient characteristics in the three groups are shown in Tables S3–S5, respectively. After adjusting age, ethnicity, grade, histology, T/N stage, ER/PR status, the 5‐year BCSS rates in patients receiving BCS alone and BCS+RT were 43.9% and 52.1%, respectively (p = 0.002, Figure 4A). Patients treated with BCS+RT had comparable survival compared with MAST alone group (51.3% and 50.1%, p = 0.872, Figure 4B), and MAST+RT group (51.5% and 55.7%, p = 0.333, Figure 4C). In multivariate analysis, patients treated with BCS alone had poorer BCSS than those treated with BCS+RT (HR: 0.732, 95% CI: 0.609–0.880, p < 0.001) (Table 3). However, there were comparable BCSS between BCS+RT and MAST alone groups (HR: 1.103, 95% CI: 0.862–1.191, p = 0.875, Table 4), and BCS+RT and MAST+RT groups (HR: 0.897, 95% CI: 0.747–1.078, p = 0.247) (Table 5).
FIGURE 4.
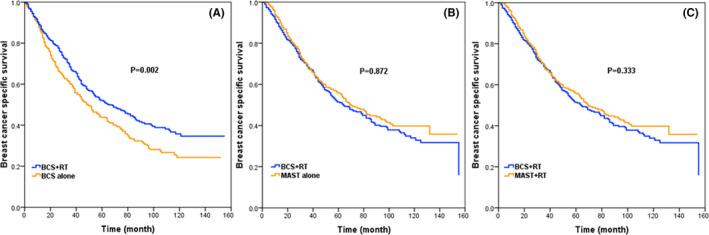
BCSS between BCS alone and BCS+RT groups (A), BCS+RT and MAST alone groups (B), and BCS+RT and MAST+RT groups (C) after PSM
TABLE 3.
Multivariate analysis of BCSS in patients with stage IV breast cancer after PSM matched between BCS alone and BCS+RT groups
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Age (years) | |||
| <65 | 1 | ||
| ≥65 | 0.798 | 0.619–1.029 | 0.082 |
| Race/ethnicity | |||
| Non‐Hispanic White | 1 | ||
| Non‐Hispanic Black | 1.171 | 0.893–1.536 | 0.253 |
| Hispanic | 0.809 | 0.596–1.098 | 0.174 |
| Other | 1.058 | 0.653–1.715 | 0.818 |
| Pathological subtype | |||
| IDC | 1 | ||
| ILC | 1.793 | 0.871–3.689 | 0.113 |
| Other | 0.99 | 0.691–1.420 | 0.958 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.58 | 0.798–3.127 | 0.19 |
| Poorly differentiated/ undifferentiated | 1.87 | 0.938–3.731 | 0.076 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.267 | 0.996–1.611 | 0.054 |
| T3 | 1.667 | 1.124–2.473 | 0.11 |
| T4 | 1.809 | 1.285–2.545 | <0.001 |
| Nodal stage | |||
| N0 | 1 | ||
| N1 | 1.016 | 0.809–1.275 | 0.891 |
| N2 | 1.03 | 0.764–1.387 | 0.847 |
| N3 | 0.997 | 0.734–1.354 | 0.984 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.82 | 0.608–1.106 | 0.194 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.659 | 0.492–0.883 | 0.005 |
| Treatment | |||
| BCS | 1 | ||
| BCS+RT | 0.732 | 0.609–0.88 | <0.001 |
Abbreviations: BCS, breast‐conserving surgery; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma; PR, progesterone receptor; RT, radiotherapy.
TABLE 4.
Multivariate analysis of BCSS in patients with de novo stage IV breast cancer after PSM matched between BCS+RT and MAST alone groups
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Age (years) | |||
| <65 | 1 | ||
| ≥65 | 1.035 | 0.823–1.301 | 0.77 |
| Race/ethnicity | |||
| Non‐Hispanic White | 1 | ||
| Non‐Hispanic Black | 1.28 | 1.025–1.597 | 0.029 |
| Hispanic | 0.873 | 0.661–1.154 | 0.341 |
| Other | 0.837 | 0.590–1.189 | 0.321 |
| Pathological subtype | |||
| IDC | 1 | ||
| ILC | 1.505 | 0.984–2.304 | 0.059 |
| Other | 0.944 | 0.7–1.273 | 0.704 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.78 | 0.960–3.300 | 0.067 |
| Poorly differentiated/ undifferentiated | 2.242 | 1.208–4.163 | 0.011 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.126 | 0.888–1.429 | 0.328 |
| T3 | 1.619 | 1.178–2.225 | 0.003 |
| T4 | 1.957 | 1.470–2.606 | <0.001 |
| Nodal stage | |||
| N0 | 1 | ||
| N1 | 1.181 | 0.944–1.477 | 0.146 |
| N2 | 1.296 | 0.979–1.715 | 0.07 |
| N3 | 1.249 | 0.962–1.621 | 0.096 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.934 | 0.724–1.203 | 0.596 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.622 | 0.485–0.798 | <0.001 |
| Treatments | |||
| BCS | 1 | ||
| MAST+RT | 1.013 | 0.862–1.191 | 0.875 |
Abbreviations: BCS, breast‐conserving surgery; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma; MAST, mastectomy; PR, progesterone receptor, RT, radiotherapy.
TABLE 5.
Multivariate analysis of BCSS in patients with de novo stage IV breast cancer after PSM matched between BCS+RT and MAST+RT groups
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Age | |||
| <65 | 1 | ||
| ≥65 | 1.06 | 0.801–1.402 | 0.683 |
| Race/ethnicity | |||
| Non‐Hispanic white | 1 | ||
| Non‐Hispanic black | 1.326 | 1.028–1.711 | 0.3 |
| Hispanic | 1.194 | 0.883–1.614 | 0.249 |
| Other | 0.842 | 0.580–1.221 | 0.364 |
| Pathological subtype | |||
| IDC | 1 | ||
| ILC | 1.471 | 0.859–2.520 | 0.16 |
| Other | 0.955 | 0.692–1.316 | 0.776 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.446 | 0.662–3.158 | 0.355 |
| Poorly differentiated/ undifferentiated | 2.016 | 0.920–4.415 | 0.08 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.013 | 0.823–1.477 | 0.513 |
| T3 | 1.211 | 0.833–1.76 | 0.316 |
| T4 | 1.537 | 1.104–2.141 | 0.11 |
| Nodal stage | |||
| N0 | 1 | ||
| N1 | 1.033 | 0.766–1.393 | 0.831 |
| N2 | 0.832 | 0.585–1.183 | 0.305 |
| N3 | 1.137 | 0.824–1.569 | 0.434 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.858 | 0.648–1.137 | 0.286 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.651 | 0.49–0.865 | 0.003 |
| Treatments | |||
| BCS+RT | 1 | ||
| MAST+RT | 0.897 | 0.747–1.078 | 0.247 |
Abbreviations: BCS, breast‐conserving surgery; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; IDC, infiltrating duct carcinoma; ILC, infiltrating lobular carcinoma; MAST, mastectomy; PR, progesterone receptor; RT, radiotherapy.
4. DISCUSSION
In the present study, we aimed to assess the effect of surgical approaches and additional RT on patients with de novo stage IV breast cancer, and we found that BCS alone was significantly associated with poorer survival than BCS+RT, while additional RT to BCS achieved comparable BCSS compared with RT to MAST and MAST alone.
Traditionally, the primary therapeutic tactics in de novo stage IV breast cancer focus on systemic therapy; however, the application status of LRT including local surgery and RT remains unclear. In previous studies, 35–77.6% of the patients were treated with LRT, and of the patients receiving local surgery, approximately 12–51.4% received BCS, while 48.6–87% were treated with MAST. 7 , 13 , 14 , 17 However, the distribution of receiving RT had a significant difference in de novo stage IV breast cancer. In the study by Soran et al., postoperative RT was administrated to all BCS patients, while only 38% of the patients were treated with postoperative RT in the MAST cohort. 7 In another two studies, 10.8% and 20.7% of the patients received post‐surgery RT, respectively. However, the details of the relationship between surgical procedures and postoperative RT were not recorded in these two studies. 14 , 17 Therefore, there was no consensus regarding the operation patterns and RT selection in de novo stage IV breast cancer, and decisions to administrate LRT were generally made by each institution according to their treatment protocols. In our study, the probability of receiving MAST (72.2%) was similar to previous studies. 7 , 13 , 14 , 17 However, significant differences were found in RT administration with 13.2% and 31.9% of patients treated with BCS+RT and MAST+RT, respectively. It is noteworthy that patients who received BCS decreased by 9.7%, while the patients treated with MAST increased by 10.7% over time. However, there was no significance in the probability of receiving post‐surgery RT. The reasons for the changing trends are unclear, and a possible explanation is that systemic therapy has achieved the best long‐term survival, resulting in decreased BCS administration and stable post‐surgery RT receipt. In addition, patients receiving MAST had more advanced T/N stage and poorer grade, leading to increased MAST selection for improving BCSS rate. 18
The effect of surgical procedures on survival outcomes in de novo stage IV breast cancer remains unclear. Numerous previous studies with large cohorts have suggested that LRT could improve survival in this disease. 13 , 14 , 15 , 16 However, all of them were retrospective studies, and few regarded the survival difference between BCS and MAST. A retrospective study identified 566 patients with metastatic breast cancer who received surgery and no surgery therapy, and patients treated with MAST were associated with an improved OS compared with those who received BCS (37% vs. 20%, p = 0.04). 24 However, 34% of the MAST group had preoperative chemotherapy, while only 15% of the BCS cohort received chemotherapy in their study. Another study from the National Cancer Database showed that patients receiving BCS were associated with poorer 3‐year overall survival (27.7% vs. 31.8%) than those receiving MAST. 2 Interestingly, a similar result was found in the above studies that both BCS groups were more likely to have positive margins than MAST groups (55% vs. 27% by Khan et al.) (26% vs. 3% by McGuire et al.). 2 , 24 According to a previous study, patients with positive margins had significantly unfavorable survival than those with negative margins (p < 0.001), 2 which might be a relatively reasonable explanation that BCS had poor survival than MAST. The same trend was found in our study. Despite this, the result of our study is more convincing for a large sample size of 5798 patients and chemotherapy receipt in the whole cohort.
For LRT in de novo stage IV breast cancer, most studies focused on surgical treatment, while rare studies regarded the value of RT in this patient subset. Two retrospective studies had drawn conflicting conclusions, 17 , 25 and there are currently no prospective studies assessing the effect of RT in de novo stage IV breast cancer. A study by Le Scodan et al. showed a 3‐year OS benefit in the LRT cohort compared with the non‐LRT cohort (43.4% vs. 26.7%, p < 0.001), and 91% of the patients were treated with RT (RT alone: 81%, surgery followed by RT: 13%) in LRT group. 25 However, the patients receiving RT had smaller tumor size, lower nodal burden, more bone‐only metastases, less visceral and brain metastases, and more chemotherapy and endocrine therapy receipt. 25 Another retrospective analysis have found that patients treated with surgery alone had comparable local recurrence‐free survival or OS compared with surgery+RT. 26 However, the details of lymph node (LN) status were not recorded in the surgery alone and surgery+RT cohort in their study, which could affect RT administration. In our population‐based study with a large sample size, additional RT to BCS or MAST both achieved better survival than surgery alone in the premise of receiving chemotherapy, which was similar to previous results. 18 , 27 The most important is that our data had detailed information of LN status with 80.5% and 86.3% had positive LNs in the surgery alone and surgery+RT cohort, respectively. In non‐metastatic breast cancer with positive LNs, post‐surgery RT could reduce the locoregional recurrence rate and improve survival. 28 , 29 Therefore, RT may also play an important role in LRT of metastatic breast cancer with positive LNs.
Advances in systemic management including taxane‐based chemotherapy, targeted therapy, and endocrine therapy allow patients to live longer with their disease. 5 , 6 , 7 Quality of life in cancer patients is increasingly regarded as a clinically relevant goal that warrant consideration, and the advantage of smaller surgery and longer survival is gradually highlighted. As our study showed, BCS+RT had non‐ inferior survival than MAST+RT or surgery alone; therefore, additional RT to BCS could be the optimal choice in de novo stage IV breast cancer. There are many ascendancies regarding BCS+RT as the LRT patterns in stage IV breast cancer. Firstly, patients with BCS suffer less treatment‐related side effects such as inflammatory response and tissue damage than those with MAST. 30 Secondly, patients treated with BCS have a better self‐image and sexual well‐being, leading to better psychological health and life satisfaction than those treated with MAST. 31 , 32 Thirdly, BCS procedures are more likely to be carried out by experienced surgeons in teaching hospitals with detailed discussion, which is associated with a better outcome. 33 Fourthly, patients receiving BCS have better treatment compliance and tolerance, and they may have more possibilities of receiving post‐surgery medical surveillance. Therefore, cooperative multidisciplinary care is highly significant in the management of de novo stage IV breast carcinoma, and the addition of RT to BCS might be the optimal treatment mode.
There are several limitations to acknowledge in our study. Firstly, the data were obtained from the SEER database, and selection biases that retrospective study inherently exists could not be ruled out, even though PSM analysis was used. Secondly, detailed treatment information including chemotherapy regimen, endocrine therapy, anti‐HER2 targeted therapies, RT technique, RT dose, target volume, and sequential surgery, chemotherapy, and RT data was unavailable in the SEER database. Fourthly, metastasis sites and treatment patterns after relapse were not recorded, which could also interfere with survival outcomes. The strong point of our study is that our study detailedly assessed the effect of BCS, MAST, and additional RT in de novo stage IV breast cancer based on a large population in the premise of chemotherapy, especially few scholars to explore the effect of surgical approach in this patient subset.
In conclusion, our study suggests that postoperative RT improves BCSS in patients with de novo stage IV breast cancer, and BCS+RT shows a non‐inferior outcome compared to MAST+RT. BCS+RT may be the optimal local management of de novo stage IV breast cancer. More studies are needed to confirm our results.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Jun Wang, Shi‐Ping Yang, Ping Zhou, San‐Gang Wu, and Zhen‐Yu He: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing–original draft, and writing–review and editing. Chen‐Lu Lian, Jian Lei, and Li Hua: Investigation, and writing–review and editing.
Supporting information
Fig S1
Table S1‐S5
Jun Wang, Shi‐Ping Yang, and Ping Zhou contributed equally to this work.
Funding information
This work was partly supported by the National Natural Science Foundation of China (No. 81872459, 81803050), the Commission Young and Middle‐aged Talents Training Project of Fujian Health Commission (No. 2019‐ZQNB‐25), the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070), and the Natural Science Foundation of Hainan Province, China (No. 819QN345).
Contributor Information
Zhen‐Yu He, Email: hezhy@sysucc.org.cn.
San‐Gang Wu, Email: wusg@xmu.edu.cn.
DATA AVAILABILITY STATEMENT
The data sets generated for this study are available in the SEER database (https://seer.cancer.gov/about/overview.html).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132:620‐626; discussion 626–627. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Wang H, Zhang C, Zhang J, et al. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget. 2017;8:26368‐26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: Trends in survival during a 14‐year period. J Clin Oncol. 2004;22:3302‐3308. [DOI] [PubMed] [Google Scholar]
- 6. Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol. Biomarkers Prev. 2017;26:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soran A, Ozmen V, Ozbas S, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07‐01. Ann Surg Oncol. 2018;25:3141‐3149. [DOI] [PubMed] [Google Scholar]
- 8. Chia SK, Speers CH, Dyachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population‐based cohort of women with metastatic breast cancer. Cancer. 2007;110:973‐979. [DOI] [PubMed] [Google Scholar]
- 9. Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542‐5551. [DOI] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network (NCCN) . Clinical Care Guidelines. Breast Cancer Version 1. 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 25, 2019.
- 11. Untch M, Augustin D, Ettl J, et al. ABC3 Consensus Commented from the Perspective of the German Guidelines: Third International Consensus Conference for Advanced Breast Cancer (ABC3). Lisbon, 07. 11. 2015. Geburtshilfe Frauenheilkd. 2016;76:156‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open‐label randomised controlled trial. Lancet Oncol. 2015;16:1380‐1388. [DOI] [PubMed] [Google Scholar]
- 13. Gultekin M, Yazici O, Eren G, et al. Impact of locoregional treatment on survival in patients presented with metastatic breast carcinoma. Breast. 2014;23:775‐783. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen DH, Truong PT, Alexander C, et al. Can locoregional treatment of the primary tumor improve outcomes for women with stage IV breast cancer at diagnosis? Int J Radiat Oncol Biol Phys. 2012;84:39‐45. [DOI] [PubMed] [Google Scholar]
- 15. Ruiterkamp J, Ernst MF, van de Poll‐Franse LV, et al. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol. 2009;35:1146‐1151. [DOI] [PubMed] [Google Scholar]
- 16. Lang JE, Tereffe W, Mitchell MP, et al. Primary tumor extirpation in breast cancer patients who present with stage IV disease is associated with improved survival. Ann Surg Oncol. 2013;20:1893‐1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bourgier C, Khodari W, Vataire AL, et al. Breast radiotherapy as part of loco‐regional treatments in stage IV breast cancer patients with oligometastatic disease. Radiother Oncol. 2010;96:199‐203. [DOI] [PubMed] [Google Scholar]
- 18. Kim YJ, Jung SY, Kim K. Survival benefit of radiotherapy after surgery in de novo stage IV breast cancer: a population‐based propensity‐score matched analysis. Sci Rep. 2019;9:8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Early surgery or standard palliative therapy in treating patients with stage IV breast cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT01242800?termZsurgeryþstageþIVþbreastþcancer%26rankZ3. Accessed: March 1, 2017.
- 20. Primary operation in synchronous metastasized invasive breast cancer (POSYTIVE). Available at: https://clinicaltrials.gov/ct2/show/NCT01015625?termZNCT01015625%26rankZ1. Accessed: March 1, 2017.
- 21. Bone metastasis and surgery in breast cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT02125630?termZsurgery. Accessed: March 1, 2017.
- 22. Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54:e55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gospodarowicz MK, Brierley JD, Wittekind C. TNM classification of malignant tumours. Chichester: Wiley; 2017. [Google Scholar]
- 24. McGuire KP, Eisen S, Rodriguez A, et al. Factors associated with improved outcome after surgery in metastatic breast cancer patients. Am J Surg. 2009;198:511‐515. [DOI] [PubMed] [Google Scholar]
- 25. Le Scodan R, Stevens D, Brain E, et al. Breast cancer with synchronous metastases: survival impact of exclusive locoregional radiotherapy. J Clin Oncol. 2009;27:1375‐1381. [DOI] [PubMed] [Google Scholar]
- 26. Choi SH, Kim JW, Choi J, et al. Locoregional treatment of the primary tumor in patients with de novo stage IV breast cancer: a radiation oncologist's perspective. Clin Breast Cancer. 2018;18:e167‐e178. [DOI] [PubMed] [Google Scholar]
- 27. Mauro GP, de Andrade CH, Stuart SR, et al. Effects of locoregional radiotherapy in patients with metastatic breast cancer. Breast. 2016;28:73‐78. [DOI] [PubMed] [Google Scholar]
- 28. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247‐253. [DOI] [PubMed] [Google Scholar]
- 29. National Comprehensive Cancer Network (NCCN) . Clinical Practice guidelines in oncology, Breast Cancer. Version 1, 2011. 2011[2012‐6‐6]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.
- 30. Retsky M, Demicheli R, Hrushesky WJ, et al. Reduction of breast cancer relapses with perioperative non‐steroidal anti‐inflammatory drugs: new findings and a review. Curr Med Chem. 2013;20:4163‐4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cipora E, Konieczny M, Karwat ID, et al. Surgical method of treatment and level of satisfaction with life among women diagnosed with breast cancer, according to time elapsed since performance of surgery. Ann Agric Environ Med. 2018;25:453‐459. [DOI] [PubMed] [Google Scholar]
- 32. Klassen AF, Pusic AL, Scott A, et al. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health. 2009;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu J, Groot G, Boden C, et al. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. 2018;18:e539‐554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S5
Data Availability Statement
The data sets generated for this study are available in the SEER database (https://seer.cancer.gov/about/overview.html).


