Abstract
Background
Intermittent treatment with TKIs is an option for the great majority (70%–80%) of CML patients who do not achieve a stable deep molecular response and are not eligible for treatment discontinuation. For these patients, the only alternative is to assume TKI continuously, lifelong.
Methods
The Italian phase III multicentric randomized OPTkIMA study started in 2015, with the aim to evaluate if a progressive de‐escalation of TKIs (imatinib, nilotinib, and dasatinib) is able to maintain the molecular response (MR3.0) and to improve Health Related Quality of Life (HRQoL).
Results
Up to December 2018, 166/185 (90%) elderly CML patients in stable MR3.0/MR4.0 completed the first year of any TKI intermittent schedule 1 month ON and 1 month OFF. The first year probability of maintaining the MR3.0 was 81% and 23.5% of the patients who lost the molecular response regained the MR3.0 after resuming TKI continuously. Patients’ HRQoL at baseline was better than that of matched peers from healthy population. Women was the only factor independently associated with worse baseline HRQoL (p > 0.0001). Overall, global HRQoL worsened at 6 (p < 0.001) but returned to the baseline value at 12 months and it was statistically significantly worse in women (p = 0.001).
Conclusions
De‐escalation of any TKI by 1 month ON/OFF schedule maintains the MR3.0/MR4.0 in 81% of the patients during the first 12–24 months. No patients progressed to accelerated/blastic phase, all the patients (23.5%) losing MR3.0 regained the MR3.0 and none suffered from TKI withdrawn syndrome. The study firstly report on HRQoL in elderly CML patients moving from a continuous daily therapy to a de‐escalated intermittent treatment.
Keywords: chronic myeloid leukaemia, intermittent, quality of life, tyrosine kinase inhibitor
The 1‐year probability of maintaining the MR3.0 while on intermittent 1 month ON/OFF TKI therapy was 81%. Patients’ QoL at baseline was better than that of matched peers from healthy population. Diarrhea significantly improved at 6 and 12 months (p = 0.022) and fatigue worsened at 6 (p = 0.001) and 12 months (p = 0.022). Overall, global QoL worsened at 6 (p < 0.001) but returned to the baseline value at the 12 months

1. INTRODUCTION
Tyrosine Kinase Inhibitors (TKIs) significantly improved the life expectancy of patients with Philadelphia‐positive Chronic Myeloid Leukaemia (CML).1, 2, 3
The current treatment strategy with TKIs aims to prevent CML progression to accelerated/blastic phase (AP/BP) and to access drug discontinuation and treatment‐free remission (TFR). Molecular response (≤0.1% BCR‐ABL1%IS) is achieved in 80–90% of patients and 30–50% of them obtain deep molecular response (DMR) (≥MR4.0 ≤0.01% BCR‐ABL1%IS or 10.000 copies of transcript as minimum sum of reference gene). Patients in stable DMR have access to TFR, but, invariably, half of them loses molecular response with need of restarting treatment.4
Although no more than 20%–25% of the whole CML patients population can get and maintain the TFR,5 TKIs discontinuation has become the current paradigm of CML management and TFR the main goal of TKI therapy.6, 7, 8, 9
This current approach, nowadays recommended,10, 11 is far from being optimal and, in absence of concrete strategies to increase the number of deep molecular responders, it makes approximately 80% of the patients to have no alternative but to continue the daily treatment lifelong.6, 12, 13, 14
For these latter patients, this expectation raises a number of important questions concerning the adherence and tolerance to therapy, including the late and unexpected side effects, the quality of life, and the sustainable costs of therapy. These questions are particularly relevant in the CML elderly population, in whom these issues are likely to be not only dose dependent but also age related.15
Furthermore, these questions are also clinically and socially relevant because it is known that the incidence of CML progresses with age and, in the next years, the prevalence of CML in elderly population is expected to increase.16, 17, 18, 19
The previous phase II INTERIM study20 demonstrated that a policy of intermittent imatinib treatment (1 month ON–one month OFF) in elderly CML patients is feasible and successful, at long term. After 6 years of follow up, neither progression to blastic phase nor CML‐related deaths were recorded, the patients who had lost the complete cytogenetic response (CCyR) regained the CCyR after resuming imatinib continuously and 60% are on intermittent treatment in CCyR and MR3.0 or MR4.0.21 Furthermore, grade I‐II side effects disappeared in more than 50% of the patients on intermittent treatment.
The European Forum for Good Clinical Practice (EFGCP) pointed out the need to increase participation of elderly people in clinical trials, particularly in the case of patients with hematologic malignancies, where health‐related quality of life (HRQoL) issues have been understudied.22 Since little data exist on the effect of TKIs on quality of life in CML patients,23, 24 new specific clinical trials, which also include HRQoL, may provide the lacking information in elderly CML patients.
To this purpose, in July 2015, an Italian prospective multicentric randomized phase III trial was started with the aim to validate the policy of the intermittent de‐escalation treatment and to explore the impact of this strategy on the HRQoL. In this first interim report, we focused on the patients who, by intention to treat, have completed the first year of the study, to achieve information on maintenance of MR3.0/MR4.0 molecular response and also HRQoL.
2. PATIENTS AND METHODS
2.1. OPTkIMA study design
OPTkIMA study is an ongoing Italian phase III multicentric randomized study, where a “fixed” intermittent administration (1 month ON/OFF) of TKI (control arm), the same of the previous INTERIM Study, is compared with a “progressive” intermittent administration (1 month ON–1 month OFF for the 1st year; 1 month ON–2 months OFF for the 2nd year; and 1 month ON–3 months OFF for the 3rd year) (experimental arm). The study was approved by the local Ethical Committees of all the participating Centers, and was registered at ClinicalTrials.gov (NCT02326311).
Upon signing of written informed consent, chronic‐phase (CP) Ph+ CML patients older than 60 years and in MR3.0 or MR4.0 after ≥2 years of daily treatment with imatinib (IM), nilotinib (NIL), or dasatinib (DAS) have been randomized 1:1 to receive “fixed” or “progressive” intermittent administration. Randomization has been stratified by type of TKI (IM, NIL, or DAS) and by depth of molecular response (MR3.0 or MR4.0), according to the IS, as recommended by the International Experts Panel's Guide Lines.21 IM, NIL, or DAS have been administered intermittently at the same daily dose given at the time of the enrollment.
The study aims to validate “fixed” intermittent administration (1 month ON/OFF) of TKI, previously explored in the INTERIM Trial,21 and to evaluate if a “progressive” increase in intermittent treatment discontinuation until 3 months is able to maintain the MR3.0 / MR4.0 molecular response and improve HRQoL outcomes. Patients’ self‐reported European Organization for Research and Treatment of Cancer (EORTC) outcome measures have been assessed throughout the 3 years follow‐up period.
Molecular monitoring was performed according to the 2013 ELN guidelines every 3 months by RT‐qPCR on peripheral blood.25 In case of MR3.0 loss, checked in two monthly consecutive RT‐qPCR analysis, patients were planned to resume TKI daily and continued to be followed.
2.2. Definition of molecular response
RT‐qPCR assessments were carried out at the Reference Laboratory of each participating Center, according to ELN Guidelines. At each time point scheduled for the MRD monitoring, 10 ml of peripheral blood was sampled for RT‐qPCR analysis. Molecular response (MR) by RT‐qPCR was defined according to the latest laboratory recommendations and using ABL1 as reference gene. Measurable MR was assigned following the international scale (IS) and scored MR3.0 if ≤0.1% BCR‐ABL1%IS, MR4.0 if ≤0.01% BCR‐ABL1%IS, MR4.5 if ≤0.0032% BCR‐ABL1%IS, and MR5.0 if ≤0.001 BCR‐ABL1%IS. Minimum sum of ABL1 reference gene transcripts, irrespective of whether BCR‐ABL1 was detected or not, 10.000, 32.000, and 100.000 for MR4.0, MR4.5, and MR5.0, respectively. The participating Reference Labs belonged to the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) Labnet and accredited by the GIMEMA Labnet Quality Committee to release the results of RT‐qPCR analysis, since certified for the quantification of BCR‐ABL1 according to the IS, as recommended by the International Experts Panel's Guide Lines.26
2.3. Procedures for HRQoL assessment
Health‐related quality of life (HRQoL) was assessed with the EORTC Quality of Life Questionnaire‐Core 30 (EORTC QLQ‐C30)27 and its QLQ‐CML24 28 and the QLQ‐ELD‐14 29 modules. During the first year (i.e., when treatment schedule between arms was not different, 1 month ON/OFF), the protocol stipulated that HRQoL had to be assessed at baseline and then at 3, 6, and at 12 months. Afterwards, HRQoL was assessed at 18, 24, 30, and 36 months and these time points were chosen to maximize the sensitivity to possible effects of treatments being tested.
2.4. Statistical analysis
The HRQoL compliance for each time point was calculated as the percentage of returned questionnaires out of those expected from patients still on study. Proportions, means, standard deviation, and medians were used to summarize patients’ characteristics. We used uni‐ and multivariable linear regression analysis to estimate the association of baseline global health status/QoL with key sociodemographic and clinical factors such as age, gender, comorbidities (at least two vs. one or less), type of TKI (first vs. second generation), and the duration of therapy with TKIs at study entry (months). We also used a linear mixed model for repeated measures to estimate mean HRQoL trajectories over time, testing the null hypothesis of no change from baseline by an overall F‐test. We used the same approach to estimate the mean HRQoL trajectories by sex, testing the null hypothesis of no difference between men and women. For descriptive purposes, we also assessed the prevalence of clinically important problems and symptoms by gender, as defined as in previously published work.30 All statistical tests were two‐sided, with statistical significance set as α = 0.05. Due to the exploratory nature of the analyses, we did not adjust for multiple testing.
3. RESULTS
3.1. Interim report
In this first interim report, the patients who, by intention to treat, have completed the first year of the study were evaluated. Since the treatment intermittent schedule is not different during the 1st year of therapy for both the patients randomized in the “fixed” and in the “progressive” arms, it is expected that no differences will be found up to the end of the 1st year in the two arms, but information on loosing of MR3.0/MR4.0 molecular response and HRQoL can be obtained.
3.2. Treatment and molecular results
Up to December 2018, 185 patients have been enrolled by 26 Italian Hematological Centers (first patient randomized in July 2015) and 166/185 patients (90%) completed the first year of follow up in the OPTkIMA study. Table 1 reports the most important clinical and biological characteristics of the 185 patients. The median age was 71 years (range 60–89) and 61% of the patients belonged to the Sokal intermediate‐/high‐risk group. A total of 140/185 (76%), 25/185 (13%), and 20/185 (11%) patients were receiving IM, NIL, and DAS, at the time of enrollment, respectively. Overall, 99/185 (54%) and 86/185 (46%) patients have been randomized in the “fixed” and “progressive” arms, respectively.
TABLE 1.
Clinical and biological characteristics of the 185 patients enrolled in OPTkIMA trial
| Variable |
Total (n = 185) |
Fixed (n = 99 – 54%) |
Progressive (n = 86 – 46%) |
p |
|---|---|---|---|---|
| M/F | 106/79 (57% / 43%) | 51/48 (52% ‐ 48%) | 55/31 (64% ‐ 36%) | 0.10 |
| Median age (range) | 71 (60–89) | 70 (60–89) | 72.5 (60–85) | 0.02 |
| Type of transcript | 185 | 99 | 85 | 0.24 |
| b3a2 | 121 (65%) | 61 (62%) | 59 (69%) | |
| b2a2 | 64 (35%) | 38 (38%) | 26 (31%) | |
| Sokal | 0.09 | |||
| L | 73 (40%) | 37 (37%) | 36 (42) | 0.09 |
| I | 82 (44%) | 42 (42%) | 41 (48%) | |
| H | 30 (16%) | 21 (21%) | 9 (10%) | |
| TKI | 0.66 | |||
|
IMA median dose (range) |
140 (76%) 400 mg (100–600) |
72 (73%) 400 mg (100–600) |
68 (79%) 400 mg (100–400) |
0.54 |
|
NILO median dose (range) |
25 (13%) 600 mg (200–800) |
15 (15%) 600 mg (200–800) |
10 (12%) 600 mg (200–600) |
0.32 |
|
DAS median dose (range) |
20 (11%) 100 mg (50–100) |
12 (12%) 100 mg (50–100) |
8 (9%) 100 mg (50–100) |
0.12 |
| Median duration of TKI (mo) | 87.5 (24–245) | 85 (24–245) | 90 (24–194) | 0.79 |
| Molecular response at enrollment | 0.48 | |||
| MR3.0 | 37 (20%) | 18 (18%) | 19 (22%) | |
| MR4.0 | 146 (79%) | 78 (79%) | 67 (78%) | |
| > MR4.0 | 2 (1%) | 2 (2%) | 0 (0%) | |
| Pts with at least 2 comorbidities | 121 (65%) | 58 (58%) | 63 (73%) | 0.07 |
| Pts with at least 2 drugs other than TKI | 117 (63%) | 60 (61%) | 57 (66%) | 0.10 |
L = Sokal Low risk; I = Sokal Intermediate risk; H = Sokal High risk; IMA = imatinib; NILO = nilotinib; DAS = dasatinib.
A total of 47/166 patients (28%) abandoned the intermittent treatment during the 1st year. The reasons of intermittent schedule discontinuation were as follows: informed consent withdrawn (4/166–2%), second cancer (4/166–2%), and loss of MR3.0 (39/166–23.5%) (Table 2). No patient progressed to AP/BP.
TABLE 2.
Patients and causes of OPTkIMA discontinuation in the 1st year of intermittent treatment 1 month ON and 1 month OFF
| TOT | IC withdrawn | Second Cancer | Loss of MR3.0 | |
|---|---|---|---|---|
| 3°Month | 14 | 3 | 1 | 10 |
| 6°Month | 21 | 1 | 1 | 19 |
| 9°Month | 4 | 0 | 1 | 3 |
| 12°Month | 8 | 0 | 1 | 7 |
| TOT | 47/166 (28%) | 4/166 (2%) | 4/166 (2%) |
39a/166 (23%) |
At the time of enrollment: 17 in MR3.0 and 22 in MR4.0.
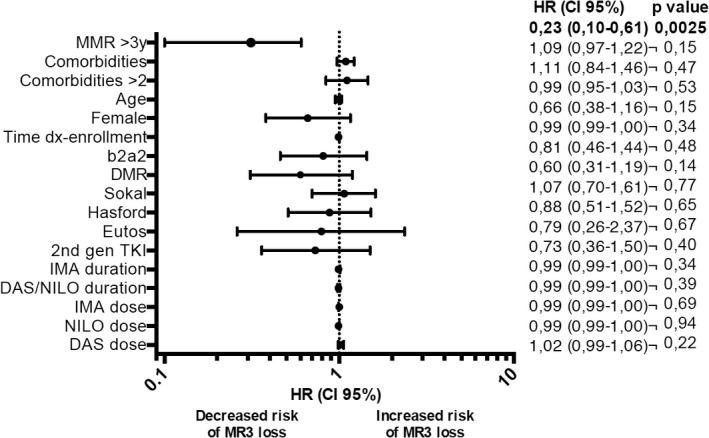
Considering these last 39 patients who lost MR3.0 in the 1st year, 22 and 17 were in MR4.0 and MR3.0 when they were enrolled into the study, respectively. Thus, the probability of maintaining the MR3.0 while on OPTkIMA was 81% at 1 year (95% CI 0.75–0.87) (Figure 1). Moreover, of the 166 patients who completed the first year of OPTkIMA, 136 (82%) and 30 (18%) were in MR4.0 and MR3.0 at baseline, respectively. As a consequence, 22/136 (16%) and 17/30 (57%) patients in MR4.0 and MR3.0 at baseline lost the molecular response during the 1st year (p < 0.00001). The impact of the most important clinical and biological variables (age, gender, disease risk according to Sokal, EURO, and EUTOS score, time from diagnosis to enrollment, type of BCR‐ABL transcript, dose of TKI, duration of TKI, depth of molecular response, and duration of MR3.0) on the probability to maintain the MR3.0 was analyzed by univariate analysis. The only factor associated with a higher probability to maintain the MR3.0 was a duration of MR3.0 greater than 3 years [HR 0.23, 95%CI 0.10–0.61), p = 0.0025] (Table 3). All the 39 patients resumed the same TKI continuously and all but 2 (95%) obtained at least the MR3.0 response, within 6 months. The mutational analysis was performed by denaturing high‐performance liquid cromatography (DHPLC) in all the cases and in 2/39 patients (5%) an ABL mutation was detected (D363Y and Y320C). Both of these 2 patients did not re‐achieve the MR3.0 with imatinib: one shifted to nilotinib after 3 months and is currently in follow up; the other one died for progressive rheumatologic disease not in MR3.0.
FIGURE 1.
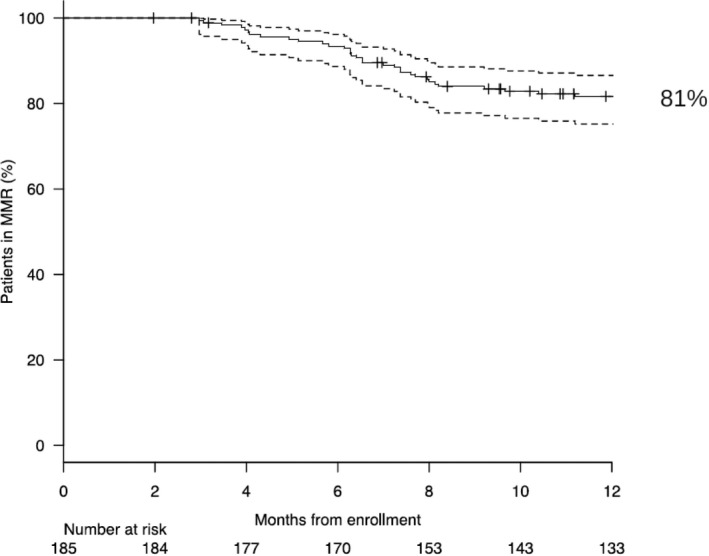
Probability of maintaining MR3.0 at 1st year of OPTkIMA
TABLE 3.
Forest plot analysis on the impact of the clinical and biological variables on the probability of MR3.0 loss—Univariate analysis
3.3. Treatment and tolerance
The adverse events registered in the electronic CRFs and managed according to the published ELN guidelines31 are reported in Table 4. Overall, the intermittent treatment was well tolerated, with 6 serious adverse events (2 appendicitis, 1 peri‐anal abscess, 1 heart‐failure, 1 hip fracture, and 1 severe artheriopathy) and 27 nonsevere adverse, of which 13 were reported in the first 9 months of the study time and 14 at the 12th month. None of these events were treatment related. According to the data reported in the electronic CRFs, none of the patients experienced the TKI withdrawn syndrome.
TABLE 4.
AEs reported during the 1st year of OPTkIMA Study
| AE | Severe | Month of OPTkIMA | ON/OFF |
|---|---|---|---|
| Artheriopathy | Yes | 1st | ON |
| Heart failure | Yes | 2nd | OFF |
| Appendicitis | Yes | 4th | OFF |
| Appendicitis | Yes | 11th | ON |
| Hip fracture | Yes | 11th | ON |
| Peri‐anal abscess | Yes | 12th | OFF |
| Ascites | No | 1st | ON |
| Arthritis | No | 2nd | OFF |
| Chills | No | 2nd | OFF |
| Cramps | No | 3rd | ON |
| Hand pain | No | 3rd | ON |
| Chills | No | 5th | ON |
| Legs edema | No | 6th | OFF |
| Atrial Fibrillation | No | 6th | OFF |
| Inguinal Hernia | No | 7th | ON |
| Conjunctiva bleeding | No | 9th | ON |
| Hypotension | No | 9th | ON |
| Dyspnea | No | 9th | ON |
| Arthritis | No | 9th | ON |
| Pneumonia | No | 10th | OFF |
| Peri‐orbital edema | No | 10th | OFF |
| Bronchitis | No | 10th | OFF |
| Diarrhea | No | 11th | ON |
| Influenza | No | 12th | OFF |
| Fever | No | 12th | OFF |
| Itch | No | 12th | OFF |
| Fever | No | 12th | OFF |
| Drowsiness | No | 12th | OFF |
| Fever | No | 12th | OFF |
| Post‐vitreous detachment | No | 12th | OFF |
| Diarrohes | No | 12th | OFF |
| Cramps | No | 12th | OFF |
| Hypertension | No | 12th | OFF |
3.4. Update beyond the 1st year
After the 1st year of intermittent treatment (1 month ON/OFF), 119 patients entered the 2nd year, of whom 63 (54%) and 56 (47%) belonged to the “fixed “and “progressive” arm, respectively. Overall, 105/119 patients (88%) completed the 2nd year, of whom 59 (56%) in the “fixed” and 46 (44%) in the “progressive” arm.
Of 63 patients randomized to the “fixed” arm who entered the 2nd year, 4 (6%) discontinued the intermittent schedule because of death for second cancer (2 cases) and senectus (2 cases). No patient lost the MR3.0.
3.5. Health‐related quality of Life (HRQoL) results
3.5.1. Compliance and baseline QoL Profile
Compliance with HRQoL questionnaires was as follows: 96%, 91%, 92%, and 80% at baseline, 3, 6, and 12 months, respectively. As reported in Figure 2, baseline symptoms profile of OPTkIMA patients generally showed better reported symptoms with respect to gender‐ and age‐matched peers from the general population.32, 33 Female gender was the only factor independently associated with worse baseline HRQoL in multivariable analysis (p <.0001), while controlling for other key potential confounding factors, including age, number of comorbidities, type of TKI, and duration of previous treatment (Table 5). The prevalence of clinically relevant problems was higher in female patients across all EORTC QLQ‐C30 scales with the largest difference found for physical functioning, being 36.6% and 62% for male and female patients, respectively. Details are reported in Figure 3.30
FIGURE 2.
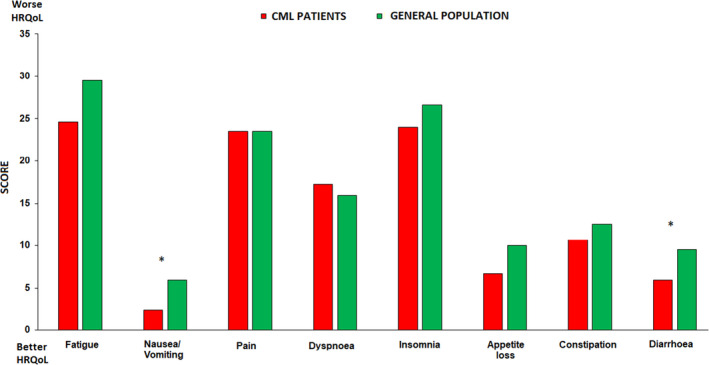
Baseline symptom profile of OPTkIMA patients versus sex and age matched peers from general population. Legend: * = clinically meaningful difference. The figure represents the age‐sex adjusted mean level of symptom burden per group
TABLE 5.
Multivariable regression analysis of baseline Global Quality of Life (EORTC QLQ‐C30)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Estimate (95% CI) | p‐value | Estimate (95% CI) | p‐value | |
| Age (y) | −0.28 (−0.70; 0.14) | 0.192 | −0.23 (−0.63; 0.18) | 0.273 |
| Being female | −11.96 (−17.82; −6.11) | <0.0001 | −12.51 (−18.32; −6.70) | <0.0001 |
| At least 2 comorbidities | −5.16 (−11.24; 0.92) | 0.096 | −5.28 (−11.17; 0.61) | 0.079 |
| 2nd‐generation TKIa | −2.20 (−9.40; 5.01) | 0.548 | −0.31 (−7.33; 6.7) | 0.930 |
| TKI duration (months) | 0.07 (0.01; 0.13) | 0.036 | 0.06 (0.00; 0.13) | 0.062 |
The bold values represent the significant parameters associated with global HRQoL.
Nilotinib or dasatinib (reference category is the first‐generation TKI imatinib). CI, confidence interval.
FIGURE 3.
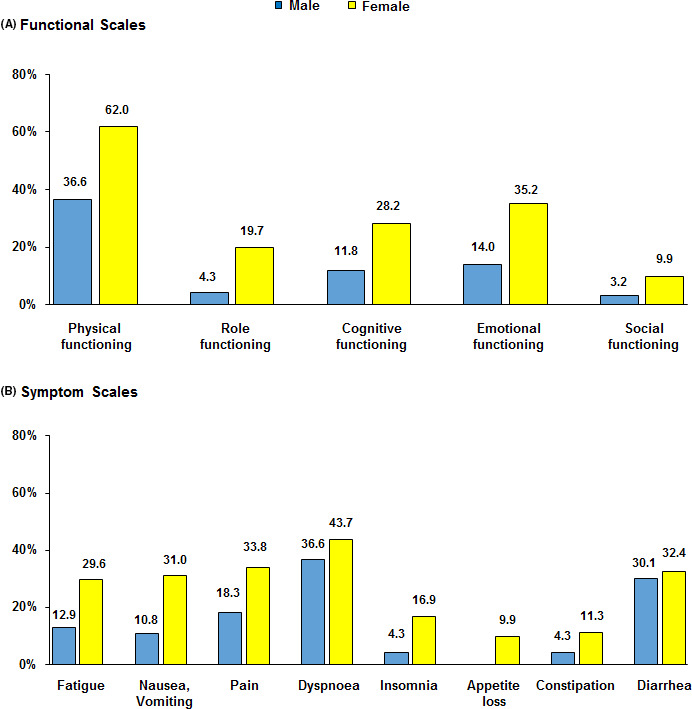
Prevalence of clinically important problems and symptoms by gender. (A) Functional scales, (B) Symptom scales. Legend: Clinically important problems and symptoms (Giesinger et al. J Clin Epidemiol. 2019 Oct 19;118:1–8)
3.5.2. Longitudinal QoL profile
Statistically significant improvements over time were found for diarrhea at 6 (p = 0.022) and 12 months (p = 0.022), with mean score decreasing from 11.5 points at baseline to 5.4 points at 12 months. These changes were also clinically meaningful across all time points (Figure 4). Nausea and vomiting scale also improved at 3 months (p =0.006) and then returned to baseline levels (Figure 4). There was a statically significant worsening in fatigue severity at 6 (p = 0.001) and 12 months (p = 0.022) (Figure 4). Global HRQoL also decreased at 6 months (p < 0.001) but then returned to baseline levels at 12 months (Figure 4). However, global HRQoL over time was statically significantly different (p = 0.001) by gender with female patients reporting worse outcomes during the 12 months of observation (Figure 5). No other scales of the EORTC QLQ‐C30 showed a statistically significant difference from baseline at any time point.
FIGURE 4.
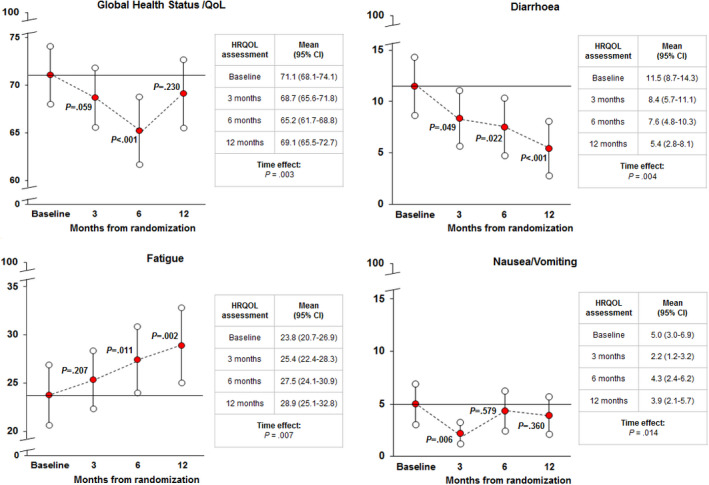
Trajectories of change in quality of life outcomes for selected EORTC QLQ‐C30 scales. Legend: For the global QoL scale higher scores indicate better QoL outcomes, whereas lower scores indicate worse QoL outcomes. For diarrhoea, fatigue, and nausea/vomiting higher scores indicate higher symptom severity, whereas lower scores indicate lower symptom severity. P‐values refer to change from baseline
FIGURE 5.
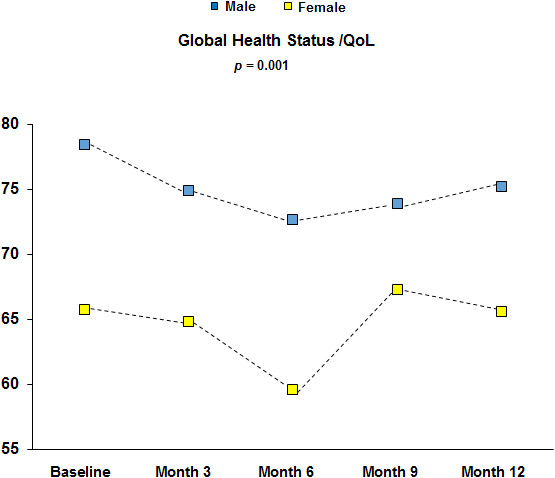
Trajectories of gender differences in Global Quality of Life up to 12 months
4. DISCUSSION
OPTkIMA Study is a phase III Italian randomized trial, started in July 2015, where elderly (≥60 years) CML patients in stable molecular response (MR3.0 or MR4.0) after ≥2 years of standard treatment with IM, NIL, or DAS are randomized 1:1 to receive “fixed” or “progressive” intermittent TKI administration until MR3.0 is lost.
The main objectives of the study are to assess the changing of HRQoL in the patients moving from a continuous to a progressive de‐escalated intermittent TKIs treatment and to find the TKI minimum effective dose able to maintain the major molecular response (MR3.0).
It is known that persistent or recurrent low‐grade adverse events (AEs) are common in CML patients during chronic treatment with TKIs. These events alter the patient's HRQoL and adherence to therapy and may cause lifelong comorbidities, especially in the elderly.
This first interim analysis was made to acquire information on HRQoL and on the maintenance of MR3.0/MR4.0 in those patients of the both arms who received IM, NIL, or DAS “one month ON/OFF” for the 1st year.
Although patients’ accrual was lower than expected, the compliance in reporting the HRQoL questionnaires was extremely satisfying (more than 90% in the first 6 months and 80% at the 12th month). Analyzing the HRQoL, the baseline profile of OPTkIMA patients was better than that of gender‐ and age‐ matched healthy participants (Figure 2).32, 33 We were surprised of that, and even more by the fact that female was the only factor independently associated with worse baseline HRQoL in multivariable analysis (p < 0.0001) (Table 2).
Concerning these findings, we can speculate that elderly patients with CML who are in stable molecular response have a good HRQoL, being confident with their disease, and considering themselves as cancer Survivors could tend to minimize some symptoms associated with aging, that, on the contrary, patients without cancer may consider as important and detrimental for HRQoL. In the second case, the prevalence of clinically relevant symptoms was higher in women (62%) than in men (36.6%), and the largest difference was found in the physical functioning (Figure 3).
Other hematologic and oncologic studies investigating the HRQoL report a worst HRQoL in the female with respect to the male gender,34 and different factors have been discussed as possible causes. The modern society strongly leans on elderly women more than elderly men (e.g., daily management of the family, housekeeping, grandchildren management, etc.). As a consequence, a cancer diagnosis, its treatment, as well as the participation in a clinical trial, such as OPTkIMA, may impair the physical and mental functioning of an elderly female patient more significantly than those of elderly male patient.
Monitoring the symptoms, a statistically significant improvement over time was found for diarrhea at 6 (p = 0.022) and 12 months (p = 0.022) (Figure 4). For other symptoms, such as nausea and vomiting, the scale also improved at 3 months (p = 0.006) and then returned to baseline levels (Figure 4). On the contrary, there was a statically significant worsening in fatigue severity at 6 (p = 0.001) and 12 months (p = 0.022) (Figure 4).
Global HRQoL also decreased at 6 months (p < 0.001) but then returned to baseline levels at 12 months (Figure 4).). Once again, when the global HRQoL over time was evaluated, it was statistically significantly different (p = 0.001) by gender with female patients reporting worse outcomes during the 12 months of observation (Figure 5).
The interpretation of these data is indeed difficult and intriguing. The amelioration of symptoms such as diarrhea or nausea can be related to the intermittent TKI administration that, particularly in the first months after its initiation, can significantly reduce symptoms associated with the continuous long‐lasting TKI administration (notably the median duration of continuous TKI administration ranges between 85 and 90 months in our patients).
The worsening in the fatigue scale at 6th and 12th month and the decrease in the global HRQoL at 6th month could be induced by the stress in patients who are normally well confident with their disease, its control, and its remission. Furthermore, even though no physician reported in the electronic CRF the typical TKI withdrawn syndrome, it is possible that some mild symptoms associated with the month OFF may have affected the patient's reported outcome. However, we did not observe any significant reduction in hemoglobin level and none of the patients retired consent, suggesting that this lack of “improvement” in HRQol was not significantly deemed by patients.
From the hematologic point of view, our analysis confirmed that the intermittent therapy is effective and safe. During the 1st year, 39/166 patients (23.5%) lost MR3.0 and all of them re‐gained the major molecular response within 6 months from resumption of continuous treatment. The probability of MR3.0 maintenance while on OPTkIMA at 1 year was 81% (95% CI 0.75–0.87; Figure 1), quite comparable with the 80% MR3.0 maintenance at 1 year observed in the previous INTERIM trial.20, 21
Of note, the depth of MR at baseline (MR3.0 and MR4.0) had an impact on the probability to maintain the MR while on intermittent schedule. Focusing on the 166 patients who completed the 1st year, 30 and 136 were in MR3.0 and MR4.0 at baseline; 17/30 (57%) and 22/136 (16%) lost the molecular response during the 1st year, respectively (p < 0.00001). The only factor associated with a higher probability to maintain the MR3.0 by univariate analysis was a duration of MR3.0 greater than 3 years [HR 0.23, 95%CI 0.10–0.61), p = 0.0025] (Table 3). The study is ongoing, and after the 1st year of intermittent treatment (1 month ON/OFF), 119 patients entered the 2nd year, 63 in the “fixed”, and 56 in the “progressive” arm, respectively, and none of them lost the MR3.0. Only another study explored a policy of TKI dose reduction. It is the DESTINY trial, in which a 50% daily dose reduction of IM in the first year followed by discontinuation of TKI was adopted [DESTINY trial 35]. Although DESTINY and OPTkIMA trials seem similar, they have substantial differences. OPTkIMA trial includes elderly patients and is not aimed to treatment permanent discontinuation, but is planned to identify the minimum effective dose of TKI (IM, NIL, and DAS) able to maintain the MR3.0, which is a surrogate marker of survival. DESTINY trial enrolled younger patients and a 50% dose de‐escalation for 12 months is scheduled as a bridge to IM discontinuation. This “clinical” selection may be improved by monitoring strictly the slope of the MR and by selecting for TD those patients with a MR slope showing a stability of BCR‐ABL1 transcript levels.36
In conclusion, this first interim analysis showed that the intermittent therapy, 1 month ON/OFF, is equally effective and safe across all the three TKIs. It also allowed to acquire basal and relevant information on HRQoL in elderly CML patients moving from standard daily therapy to experimental intermittent treatment. Significant differences in the HRQoL were observed in comparison with healthy peers matched for gender and age and between men and women. The study is ongoing and relevant information on the comparison of HRQoL between the “fixed” and the “progressive” randomization arms will be available in the next future.
CONFLICTS OF INTEREST
AI—speaker honoraria from Novartis, Pfizer, and Incyte; EA—consultancy and advisory for Novartis, Bristol Myers Squibb, Incyte, and Pfizer; GR—speaker honoraria from Novartis, Pfizer, and Incyte; MB—consultant and receiving honoraria from Novartis, Incyte, and Takeda; DR—speaker honoraria: MSD, Novartis, Gilead; advisory committees: MSD, Janssen, Gilead. All the other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
DR is the PI of the study. DR, MM, and MB designed the study; DR, MM, AI, EA, MB, SB, and NP analyzed the data and wrote the study; ADV generated the electronic database; all the Authors collected the data; and all the authors revised the final version of the paper and approved it before its submission.
[Corrections added on 14 August 2021, after first online publication: Monica Bocchia has been added as the 24th author and Lara Aprile's affiliation has been corrected]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request, considering that the study is ongoing.
REFERENCES
- 1.García‐Gutiérrez V, Hernández‐Boluda JC. Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851‐2857. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first‐line treatment of chronic myeloid leukemia: 10‐year survival results of the randomized CML study IV and impact of non‐CML determinants. Leukemia. 2017;31(11):2398‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih YCT, Cortes JE, Kantarjian HM. Treatment value of second‐generation BCR‐ABL1 tyrosine kinase inhibitors compared with imatinib to achieve treatment‐free remission in patients with chronic myeloid leukaemia: a modelling study. Lancet Haematol. 2019;6(8):e398‐e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousselot P, Charbonnier A, Cony‐Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic‐phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424‐430. [DOI] [PubMed] [Google Scholar]
- 6.Hernández‐Boluda JC, Pereira A, Pastor‐Galán I, et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J. 2018;8(10):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laneuville P. When to stop tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Curr Treat Options Oncol. 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saglio G, Sharf G, Almeida A, et al. Considerations for treatment‐free remission in patients with chronic myeloid leukemia: a joint patient‐physician perspective. Clin Lymphoma Myeloma Leuk. 2018;18:375‐379. [DOI] [PubMed] [Google Scholar]
- 9.Hehlmann R, Cortes JE, Zyczynski T, et al. Tyrosine kinase inhibitor interruptions, discontinuations and switching in patients with chronic‐phase chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2019;94(1):46‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baccarani M, Abruzzese E, Accurso V, et al. Managing chronic myeloid leukemia for treatment‐free remission: a proposal from the GIMEMA CML WP. Blood Adv. 2019;3(24):4280‐4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong JH, Winton EF, Heffner LT, et al. Does the frequency of molecular monitoring after tyrosine kinase inhibitor discontinuation affect outcomes of patients with chronic myeloid leukemia? Cancer. 2017;123(13):2482‐2488. [DOI] [PubMed] [Google Scholar]
- 13.Spiess B, Rinaldetti S, Naumann N, et al. Diagnostic performance of the molecular BCR‐ABL1 monitoring system may impact on inclusion of CML patients in stopping trials. Branford S, editor. PLoS One. 2019;14(3):e0214305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardi S, Malagola M, Zanaglio C, et al. Digital PCR improves the quantitation of DMR and the selection of CML candidates to TKIs discontinuation. Cancer Med. 2019;8(5):2041‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto C, Nakashima H, Ikeda T, et al. Analysis of the cost‐effectiveness of treatment strategies for CML with incorporation of treatment discontinuation. Blood Adv. 2019;3(21):3266‐3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulon S, Cony‐Makhoul P, Guerci‐Bresler A, et al. Using healthcare claims data to analyze the prevalence of BCR‐ABL‐positive chronic myeloid leukemia in France: a nationwide population‐based study. Cancer Med. 2019;8(6):3296‐3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauseker M, Gerlach R, Tauscher M, Hasford J. Improved survival boosts the prevalence of chronic myeloid leukemia: predictions from a population‐based study. J Cancer Res Clin Oncol. 2016;142(7):1441‐1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunnarsson N, Sandin F, Höglund M, et al. Population‐based assessment of chronic myeloid leukemia in Sweden: striking increase in survival and prevalence. Eur J Haematol. 2016;97(4):387‐392. [DOI] [PubMed] [Google Scholar]
- 19.Delord M, Foulon S, Cayuela JM, Rousselot P, Bonastre J. The rising prevalence of chronic myeloid leukemia in France. Leuk Res. 2018;69:94‐99. [DOI] [PubMed] [Google Scholar]
- 20.Russo D, Martinelli G, Malagola M, et al. Effects and outcome of a policy of intermittent imatinib treatment in elderly patients with chronic myeloid leukemia. Blood. 2013;121(26):5138‐5144. [DOI] [PubMed] [Google Scholar]
- 21.Russo D, Malagola M, Skert C, et al. Managing chronic myeloid leukaemia in the elderly with intermittent imatinib treatment. Blood Cancer J. 2015;5(9):e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efficace F, Cottone F, Sommer K, et al. Validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 Summary Score in Patients With Hematologic Malignancies. Value Heal. 2019;22(11):1303‐1310. [DOI] [PubMed] [Google Scholar]
- 23.Efficace F, Castagnetti F, Martino B, et al. Health‐related quality of life in patients with chronic myeloid leukemia receiving first‐line therapy with nilotinib. Cancer. 2018;124(10):2228‐2237. [DOI] [PubMed] [Google Scholar]
- 24.Efficace F, Stagno F, Iurlo A, et al. Health‐related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first‐line dasatinib versus imatinib therapy. Leukemia. 2019;34(2):488‐498. [DOI] [PubMed] [Google Scholar]
- 25.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross NCP, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365‐376. [DOI] [PubMed] [Google Scholar]
- 28.Efficace F, Baccarani M, Breccia M, et al. International development of an EORTC questionnaire for assessing health‐related quality of life in chronic myeloid leukemia patients: the EORTC QLQ‐CML24. Qual Life Res. 2014;23(3):825‐836. [DOI] [PubMed] [Google Scholar]
- 29.Wheelwright S, Darlington AS, Fitzsimmons D, et al. International validation of the EORTC QLQ‐ELD14 questionnaire for assessment of health‐related quality of life elderly patients with cancer. Br J Cancer. 2013;109(4):852‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giesinger JM, Loth FLC, Aaronson NK, et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ‐C30 in clinical practice and research. J Clin Epidemiol. 2020;118:1‐8. [DOI] [PubMed] [Google Scholar]
- 31.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szende A, Williams A, EuroQol Group . Measuring self‐reported population health: an international perspective based on EQ‐5D. [Budapest] Hungary: SpringMed Publishing; 2004:115. [Google Scholar]
- 33.Stauder R, Yu GE, Koinig KA, et al. Health‐related quality of life in lower‐risk MDS patients compared with age‐ and sex‐matched reference populations: a European LeukemiaNet Study. Leukemia. 2018;32(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Yan J, Chen J, et al. Factors associated with quality of life of adult patients with acute leukemia and their family caregivers in China: a cross‐sectional study. Health Qual Life Outcomes. 2020;18(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark RE, Polydoros F, Apperley JF, et al. De‐escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non‐randomised, phase 2 trial. Lancet Haematol. 2019;6(7):e375‐e383. [DOI] [PubMed] [Google Scholar]
- 36.Kunbaz A, Eskazan AE. An alternative way – tyrosine kinase inhibitor (TKI) de‐escalation – to discontinue TKIs in order to achieve treatment‐free remission. Exp Rev of Hematol. 2019;477‐480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request, considering that the study is ongoing.


