Abstract
Background
Absolute monocyte count (AMC) is often used to be assessed in cancer follow‐up, which has regained interest as a potential prognostic indicator in many solid tumors, though not consistently or comprehensively. In the present study, we set out to perform a comprehensive meta‐analysis of all available data regarding the prognostic significance of AMC in solid tumors. We also evaluated the association between AMC and clinical features in solid tumors.
Methods
A hazard ratio (HR) and corresponding 95% confidence interval (CI) or a p value (p) from eligible studies were extracted and subsequently pooled analyzed. Subgroup analyses and meta‐regression analyses were conducted according to the confounders of included studies. In addition, the relationships between AMC and clinical characteristics were also explored in the meta‐analysis.
Results
Overall, ninety‐three articles comprising 104 studies with 32229 patients were finally included. The results showed that elevated AMC was associated with worse overall survival (OS) (HR = 1.615; 95% CI: 1.475‐1.768; p < 0.001), disease‐free survival (DFS) (HR:1.488; 95% CI: 1.357‐1.633; p < 0.001), progressive‐free survival (PFS) (HR: 1.533; 95% CI: 1.342‐1.751; p < 0.001) and cancer‐specific survival (CSS) (HR: 1.585; 95% CI: 1.253‐2.006; p < 0.001) in non‐hematological tumors. Subgroup analyses according to each confounder further proved the consistent prognostic value of AMC in solid tumor outcomes. Moreover, elevated AMC was more likely to be observed in male group and patients with smoking history, and associated with longer tumor length and advanced T stage.
Conclusion
In short, the meta‐analysis found that elevated AMC might indicate poor long‐term outcomes in non‐hematologic cancers, thus AMC may be a valuable marker in the prognosis for patients with solid tumors.
Keywords: inflammation, monocyte, prognosis, solid tumor
Elevated AMC indicates poor long‐term outcomes in solid tumors, which could be applied in the prognosis assessment.

Abbreviations
- AMC
absolute monocyte count
- CCL2
C‐C chemokine ligand 2
- CCR2
the receptor for chemokine CCL2
- CI
confidence interval
- CSS
cancer‐specific survival
- DFS
disease‐free survival
- HR
hazard ratio
- MCP‐1
monocyte chemoattractant protein‐1
- MDSC
myeloid‐derived suppressor cell
- NK cell
natural killer cell
- NOS
Newcastle–Ottawa Quality Assessment Scale
- OR
odds ratio
- OS
overall survival
- PD‐L1
programmed cell death ligand 1
- PFS
progressive‐free survival
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines
- RFS
recurrence‐free survival
- ROC
receiver operating characteristic curve
- SAR
survival after recurrence
- SMD
standardized mean difference
- TAM
tumor‐associated macrophage
- TME
tumor microenvironment
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Cancer remains one of the leading causes of death and a major health care challenge worldwide. 1 The prominent differences of cancer profiles in individual countries are associated with marked geographic diversity. 1 Despite substantial progress, cancer morbidity and mortality rates have been rapidly growing in both developing and developed countries. 1 Based on the statistics, 1806,590 new cancer cases and 606,520 cancer deaths are projected to occur in the United States in 2020. 2 Similarly, the Chinese national cancer registries reported about 3929,000 new cancer cases and 2338,000 cancer deaths in China in 2015. 3
In the last decade, there have been the paradigms of chemotherapy being transformed from empiric therapy to individual pharmacogenomics‐ and genetics‐based personalized medicine, coinciding with rapid technological advances and brilliant discoveries in many fields such as genetics and molecular biomarkers. 4 These markers have been recognized to reflect the characteristics of cancer signatures, optimize therapy decisions and provide timeliness information about the response to personalized treatment. 4 While several biomarkers specific to particular cancer types have been applied routinely, such as PSA for prostate cancer, general decisive biomarkers used for overall cancers are unavailable. 5 Thus, the identification of new potential tumor biomarkers with improved test convenience and sensitivity carries great significance to provide quicker diagnosis and more accurate prognosis.
With the increased understanding of tumor immunology, the dual interaction between cancer and the immune system has been recognized and the microenvironment in which the cancer cells grow has been highlighted. 6 , 7 , 8 For a broad point of view, tumor microenvironment (TME) is a highly complicated heterogeneous ecosystem containing not only tumor cells, but also a variety of non‐immune and immune cells. 8 , 9 The immune contexture describes the density, function orientation, and spatial organization of the immune cell populations, including innate immune cells (e.g., macrophage, neutrophils, or natural killer cell (NK cell)), adaptive immune cells (e.g., T and B lymphocytes), and myeloid and lymphoid lineages. 9 Immune cells play a pivotal role in the cytokine‐ and chemokine‐ mediated imbalanced immune response, which attributes to their inherent functions and the molecules they express. 10 These cells have been reported to participate in the manifestation of tumor recognition and the consecutive steps of malignancy initiation, progression, and metastasis. 11 , 12 In addition, previous study indicated that tumor cells may modify the immunophenotype of immune cells and extracellular microenvironment, thus enhancing the deterioration of the immune contexture which determines tumor outcomes. 13 The quantitative assessment of immunological status based on immune cells was applied in the prediction of some solid tumors. 14
Tumor‐associated macrophages (TAMs), derived from the infiltrating bone marrow‐derived monocytes, are a major component in TME and therefore were considered as conspicuous stromal targets in many types of solid tumors. 6 , 7 Tumor‐associated macrophages could differentiate into “proinflammatory” M1 phenotypes with antitumor activity or “proangiogenic and immunosuppressive” M2 phenotypes according to the microenvironment. The dominant phenotype M2 was thought to be important in tumor progression, angiogenesis, and immune tolerance through promoting fibroblast proliferation, extracellular matrix deposition, and immunosuppression in the late stages. 15 , 16 , 17 Recently, the rapid development of biotechnologies has boosted the understanding of the interplay between cancer cells and monocytes/macrophages. 18 It is suggested that monocyte subpopulation distribution and transcriptomes are significantly perturbed by cancer, subsequently reflecting patients outcomes. 18 In mouse models of cancer, monocytes have been shown to contribute to tumor progression, metastasis, and anti‐vascular endothelial growth factor therapy resistance. 19 , 20 Monocytes have been shown to be associated with cancer prognosis in patients with subdividing cancers, such as follicular lymphoma and colorectal cancer, although this line of research is still subject to debate. 21 , 22 Some data showed an insignificant prognostic value of monocytes in cervical cancer or malignant pleural mesothelioma. 23 , 24 Much more attention was paid to hematological tumors and lymphocyte‐to‐monocyte ratio. 25 , 26 Hence, the present researches available on the association between absolute monocyte count (AMC) and solid tumors have not been systematically analyzed so far, and evidence for the use of the peripheral AMC as a predictor of clinical outcome in solid tumors remained controversial.
Therefore, we performed a meta‐analysis with in order to validate the role of monocyte as a predictor in solid tumors. In addition, we also integrated data to demonstrate the relevant clinicopathological factors in relation to peripheral monocyte count, which is of the essence to tailor the personalized cancer treatment strategy.
2. METHODS
2.1. Data sources and search strategy
The present study was performed in accordance the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (PRISMA). 27 An electronic literature search was conducted in the databases of Medline (PubMed), Embase, and the Web of Science on April 14, 2020, by two investigators independently, with the following search terms: “absolute monocyte count,” “monocyte count,” “cancer,” “carcinoma,” and “neoplasm.” In addition, manual searches were supplemented in all citation lists of the retrieved articles for further investigation of potentially relevant studies. Language was restricted to English and Chinese.
2.2. Criteria for inclusion and exclusion
Studies included in this meta‐analysis meet the following criteria: (1) patients with solid tumors were studied; (2) the prognostic impact of AMC on overall survival (OS), cancer‐specific survival (CSS), progressive‐free survival (PFS), disease‐free survival (DFS) and/or recurrence‐free survival (RFS) was evaluated; (3) a hazard ratio (HR) with 95% confidence interval (CI) could be extracted in univariate or multivariate analysis of Cox hazard model, or could be estimated by Parmar's method 28 ; (4) AMC was calculated as a dichotomized variable by a cut‐off value. Studies were excluded if they match any of the following: (1) reference abstracts, case reports, conference abstracts, reviews or meta‐analysis; (2) studies on hematological malignancies; (3) insufficient data for estimating HR and 95% CI; (4) studies reporting AMC only as a continuous variable; (5) duplicate publications or repeated analysis. Moreover, if studies with overlapping patients were identified, the study with the most information and most recent publication was included. The full‐texts of the relevant articles were retrieved to assess eligibility.
2.3. Data extraction and quality assessment
Two investigators performed data extraction independently with a standard extraction form. The following data were extracted: first author's surname, publication year, country, region, patient sources, cancer type, study design, characteristics of cancer (distant metastasis, TNM stage, treatment), characteristics of the study cohort (sample size, mean age, gender), testing time of monocyte, cut‐off value defining low monocyte, method for the selection of cut‐off value, outcome measures (OS, DFS, CSS, RFS, PFS assessed as HRs and corresponding 95% CI and/or p values) model of survival analysis (multivariate or univariate).
The reviewers independently assessed the methodological quality of the included studies by the Newcastle–Ottawa Quality Assessment Scale (NOS). 29 The 9‐point scoring system comprised three domains of quality including selection, comparability, and outcome assessment, and studies with NOS scores of more than six were defined as high‐quality studies. Any discrepancies between reviewers were resolved by discussion until a consensus was reached.
2.4. Statistical analysis
This meta‐analysis was performed with STATA version 14.0 (STATA Corporation, College Station, TX, USA). The survival data were measured by HR and 95% CI. The aggregated HRs and 95%CIs, either directly extracted or presented in the form of Kaplan‐Meier survival curves, were calculated to evaluate the prognostic value of AMC on the long‐term prognosis (OS/DFS/PFS/CSS) using low AMC group as a reference. The relationships between the AMC and certain clinical features of patients were also assessed with STATA version 14.0. Odds ratios (ORs) for dichotomous variables and standardized mean differences (SMDs) for continuous variables with their 95% CIs were regarded as summarized statistics, respectively. Statistical significance was indicated when p < 0.05 in data synthesis. Besides, we used the outcomes in multivariate analysis whenever the univariate and multivariate analyses were available. The heterogeneity of pooled studies was measured by Cochran's Q test and Higgins I‐squared (I2) statistic; p < 0.1 or I 2 > 50% was defined as significant heterogeneity. The random‐effects model was used in the analysis. Subgroup analysis was performed based on region, sample size, cut‐off value, cancer type, distant metastatic status, TNM stage, testing time of blood, analysis method, and study quality to explore the heterogeneity sources. Then, meta‐regression analyses were conducted to determine the hazard effects of covariates. Publication bias was evaluated by Begg's and Egger's test. Sensitivity analysis was conducted to explore the influence on the pooled effect size after removing a single study each time. Two‐sided p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Studies characteristics
The initial literature search identified a total of 6016 potentially relevant publications. After thoroughly screening the titles and abstracts by two investigators independently, the full‐texts of 173 potential studies were selected for further identification. Finally, 102 retrospective studies and 2 retrospective‐prospective studies from 93 eligible papers that met the inclusion criteria were included in this meta‐analysis. 22 , 23 , 24 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 The flow diagram of the selection procedure is presented in Figure 1.
FIGURE 1.
Flow chart of the literature search
Of those 104 studies, 82 were based on Asians and 22 on non‐Asians. According to the types of cancer, 41 studies were on abdominal cancers, 30 studies on thoracic cancers, 14 studies on pelvic cancers, 12 studies on head and neck cancers, 6 studies on melanoma, and 1 study remained unknown. Based on the testing time of blood, majority of the included studies were pre‐treatment (n = 59) and pre‐operative (n = 42), while one study was post‐treatment and two studies remained unknown. There were 65 studies estimated as high‐quality studies and 39 low‐quality studies. The endpoints OS, DFS, CSS PFS, and survival after recurrence (SAR) were addressed in 88, 41, 23, 10, and 1 studies, respectively. As shown in Table 1, the receiver operating characteristic curves (ROC) were applied to detect the optimal cut‐off values in 40 included studies, 23 studies used median values and 4 studies used mean values, 12 studies chosen cut‐off values based on previous studies, 9 studies used cut‐offs with significant value of different statistical methods such as log‐rank test, 3 studies used a certain normal value, 3 studies used normal upper limits of monocyte count and 10 studies did not report methods determining the cut‐off values of AMC. The major characteristics of the meta‐analysis are shown in Table 1. The detailed extracted data are shown in Table S1 and detailed NOS scores of each included study are presented in Table S2.
TABLE 1.
The baseline characteristics of included studies
| Author, year | Region | Cancer type | Distant metastasis | TMN stage | Test time | N a | Mean age | Cut‐off value (/mm3) | Method e | Endpoint | Analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head and neck cancers | ||||||||||||
| Bobdey, 2017 | Asian | Oral cavity cancer | Both | I‐IV | Pre‐treatment | 471 | 50 | 500 | ROC | OS | MV | 8 |
| Chen, 2009 | Asian | HNC | Both | I‐IV | Pre‐treatment | 270 | 56.5 b | 1000 | Previous study | OS | MV | 7 |
| Furukawa, 2019 | Asian | Tongue cancer | NA | I‐IV | Pre‐operative | 103 | 63 b | 320 | Mean value | OS | MV | 7 |
| Jiang, 2015 | Asian | NPC | Yes | IV | Pre‐treatment | 672 | 46 | 665 | ROC | OS | MV | 5 |
| Li, 2013 | Asian | NPC | No | I‐IV | Pre‐treatment | 1547 | 51 | 475 | ROC | OS/DFS | MV/MV | 6 |
| Lin, 2014 | Asian | NPC | Yes | IV | Pre‐treatment | 256 | 53.6 | 350 | ROC | OS | MV | 6 |
| Huang, 2015(1) | Non‐Asian | OPC | NA | I‐IV | Pre‐treatment | 510 | 57.5 | 600 | Median value | OS/RFS | UV/UV | 8 |
| Huang, 2015(2) | Non‐Asian | OPC | NA | I‐IV | Pre‐treatment | 192 | 65 | 700 | Median value | OS/RFS | UV/UV | 8 |
| Takahashi, 2019 | Asian | OPC | Both | I‐IV | Pre‐treatment | 75 | 65 b | 485 | ROC | OS/PFS | MV/MV | 6 |
| Tsia, 2014 | Asian | Oral cavity cancer | Both | I‐IV | Pre‐treatment | 202 | 53 | 403 | Median value | CSS | MV | 8 |
| Yang, 2018 | Asian | HPC | No | I‐IV | Pre‐treatment | 197 | NA | 630 | P value* | OS/DFS/CSS | UV/UV/UV | 7 |
| Yokato, 2020 | Asian | Thyroid cancer | No | I‐IVA | Pre‐operative | 570 | 58 b | 260 | ROC | RFS | UV | 6 |
| Thoracic cancers | ||||||||||||
| Botta, 2013(1) | Asian | Lung cancer | Both | III‐IV | Pre‐treatment | 73 | 58.57 | 600 | ULN | PFS | UV | 7 |
| Botta, 2013(2) | Non‐Asian | Lung cancer | Both | III‐IV | Pre‐treatment | 39 | 67.85 | 600 | ULN | PFS | UV | 7 |
| Charrier, 2019 | Non‐Asian | Lung cancer | Both | III‐IV | Pre‐treatment | 148 | 62 b | 800 | NA | OS/PFS | MV/MV | 8 |
| Chen, 2020 | Asian | Breast cancer | No | II‐III | Pre‐treatment | 262 | 48 b | 340 | ROC | OS/DFS | UV/UV | 7 |
| Go, 2015 | Asian | Lung cancer | Both | I‐IV | Pre‐treatment | 134 | 68.5 | 640 | Median value | OS | MV | 6 |
| Hai, 2018 | Asian | Lung cancer | No | I‐IIIA | Pre‐operative | 433 | 60.6 | 375 | ROC | OS/DFS | UV/UV | 9 |
| Han, 2016 | Asian | ESCC | No | I‐III | Pre‐operative | 218 | 60.5 | 420 | ROC | OS/DFS | MV/MV | 9 |
| Huang, 2015 | Asian | ESCC | No | NA | Pre‐operative | 348 | 59.2 | 550 | ROC | CSS | MV | 7 |
| Huang, 2019(1) | Asian | Breast cancer | Both | I‐IV | Pre‐treatment | 133 | NA | 440 | NA | OS/DFS | UV/UV | 7 |
| Huang, 2019(2) | Asian | Breast cancer | Both | I‐IV | Pre‐treatment | 317 | NA | 440 | NA | OS/DFS | UV/UV | 7 |
| Huang, 2019(3) | Asian | Breast cancer | Both | I‐IV | Pre‐treatment | 57 | NA | 440 | NA | OS/DFS | UV/UV | 7 |
| Huang, 2019(4) | Asian | Breast cancer | No | I‐III | Pre‐treatment | 94 | NA | 440 | NA | OS/DFS | UV/UV | 7 |
| Kumagai, 2014 | Asian | Lung cancer | No | I‐III | Pre‐operative | 302 | 67 | 430 | ROC | OS/RFS | MV/MV | 8 |
| Lee, 2017 | Asian | Lung cancer | Both | III‐IV | Pre‐treatment | 135 | NA | 800 | Previous study | OS | MV | 7 |
| Lee, 2018(1) | Asian | Breast cancer | No | I‐III | Pre‐operative | NA | NA | 360 | Median value | DFS | UV | 7 |
| Lee, 2018(2) | Asian | Breast cancer | No | I‐III | Pre‐operative | 37 | NA | 360 | Median value | DFS | UV | 7 |
| Lee, 2018 (3) | Asian | Breast cancer | No | I‐III | Pre‐operative | NA | NA | 360 | Median value | DFS | UV | 7 |
| Lin, 2014 | Asian | Lung cancer | Both | III‐IV | Pre‐treatment | 370 | 63.6 | 450 | ROC | OS/PFS | MV/MV | 6 |
| Ni, 2014 | Asian | Breast cancer | No | I‐III | Pre‐treatment | 542 | 49 | 400 | Mean value | DFS | MV | 7 |
| Sakin, 2019 | Asian | Lung cancer | Yes | IV | Pre‐treatment | 113 | 65 b | 860 | Normal value | OS | UV | 6 |
| Schernberg, 2017 | Non‐Asian | ESCC | No | I‐III | Pre‐treatment | 126 | 62 | 1000 | NA | OS/PFS | UV/MV | 7 |
| Song, 2019 | Asian | ESCC | No | I‐III | Pre‐operative | 686 | 61 b | 500 | Median value | OS/DFS | MV/UV | 7 |
| Soyano, 2018 | Non‐Asian | Lung cancer | Both | NA | Pre‐treatment | 157 | 66 b | 630 | P value** | OS/PFS | MV/MV | 7 |
| Tang, 2016 | Asian | Lung cancer | No | III | Pre‐treatment | 78 | 57 | 600 | Previous study | OS | UV | 5 |
| Tanizaki, 2018 | Asian | Lung cancer | Both | III‐IV | Pre‐treatment | 134 | 68 | 650 | Previous study | OS/PFS | UV/UV | 7 |
| Tanrikulu, 2016 | Non‐Asian | MPM | Both | I‐IV | Pre‐treatment | 292 | 58.4 | 550 | ROC | OS | MV | 6 |
| Wang, 2019 | Asian | ESCC | Both | I‐IV | Pre‐treatment | 43 | 62 b | 330 | NA | OS | MV | 6 |
| Wen, 2015 | Asian | Breast cancer | No | I‐III | Pre‐operative | 2000 | 49.4 | 480 | ROC | OS | MV | 8 |
| Zhang, 2017 | Asian | MPM | NA | NA | Pre‐treatment | 105 | 56 | 545 | ROC | OS | MV | 8 |
| Zhu, 2017 | Asian | ESCC | No | IIB | Pre‐operative | 220 | NA | 630 | Previous study | OS/DFS | UV/UV | 9 |
| Abdominal cancers | ||||||||||||
| Abu‐Shawer, 2019 | Asian | Gastric cancer | Both | I‐IV | Pre‐treatment | 502 | 54 b | 660 | Median value | OS | UV | 6 |
| Cong, 2016 | Asian | Gastric cancer | No | II‐III | Pre‐operative | 188 | 77 b | 350 | ROC | OS | MV | 7 |
| Feng, 2018 | Asian | Gastric cancer | No | I‐III | Pre‐operative | 3243 | 58 b | 510 | X‐tile | OS | MV | 6 |
| Fujiwara, 2019 | Asian | DEBDC | Both | I‐IV | Pre‐operative | 121 | 66.7 | 300 | ROC | OS/DFS | UV/UV | 8 |
| Giacomelli, 2017 | Non‐Asian | Pancreatic cancer | No | I‐III | Pre‐treatment | 57 | 62 b | 800 | NA | PFS | UV | 6 |
| Gu, 2019 | Asian | HCC | No | 1‐III | Pre‐treatment | 116 | 51 | 400 | Median value | OS/RFS | UV/UV | 7 |
| Haruki, 2017 | Asian | Colorectal cancer | Yes | IV | Pre‐operative | 89 | 64 | 300 | Mean value | OS/DFS | UV/UV | 7 |
| Hu, 2016 | Asian | Colorectal cancer | Both | I‐IV | Pre‐operative | 210 | 56.1 b | 505 | ROC | OS | MV | 7 |
| Inamoto, 2019 | Asian | Colorectal cancer | Both | I‐IV | Pre‐operative | 448 | 69 | 400 | ROC | OS/DFS/CSS | MV/MV/MV | 6 |
| Ishihara, 2019 | Asian | Renal cell cancer | Yes | IV | Pre‐treatment | 58 | NA | 650 | Previous study | OS/PFS | UV/UV | 7 |
| Iwase, 2013 | Asian | Gallbladder cancer | No | NA | Post‐operative | 34 | 67 | 300 | Previous study | OS/DFS | UV/UV | 7 |
| Kim, 2014 | Asian | HCC | No | NA | Pre‐operative | 256 | 54 | 300 | ROC | RFS | UV | 8 |
| Krakowska, 2018 | Non‐Asian | Colorectal cancer | Both | III‐IV | Pre‐treatment | 295 | 63 b | NA | Normal value | OS/PFS | UV/UV | 7 |
| Lee, 2016 | Asian | Colorectal cancer | Yes | IV | Pre‐treatment | 120 | NA | 413.3 | ROC | OS | UV | 6 |
| Leith, 2007(1) | Non‐Asian | Colorectal cancer | No | I‐III | NA | 149 | NA | 900 | Previous study | OS/CSS | UV/UV | 6 |
| Leith, 2007(2) | Non‐Asian | Colorectal cancer | Yes | IV | NA | 84 | NA | 900 | Previous study | CSS | UV | 6 |
| Li, 2016 | Asian | Pancreatic cancer | No | I‐III | Pre‐operative | 144 | 62 | 400 | ROC | OS/RFS | UV/UV | 6 |
| Li, 2018 | Asian | Colon cancer | No | I‐III | Pre‐operative | 216 | 64 | 350 | Mean value | OS/PFS | MV/MV | 7 |
| Lin, 2014 | Asian | HCC | Both | I‐IV | Pre‐treatment | 216 | 64.8 | 380 | ROC | OS | MV | 9 |
| Lin, 2016 | Asian | Colorectal cancer | Yes | IV | Pre‐treatment | 488 | 54 | 550 | ROC | OS/PFS | MV/MV | 6 |
| Neal, 2015 | Non‐Asian | Colorectal cancer | Yes | IV | Pre‐operative | 302 | 64.8 | 700 | ROC | OS/CSS | UV/UV | 7 |
| Oh, 2016 | Asian | Colorectal cancer | No | II | Pre‐operative | 261 | 65 | 520 | ROC | OS/DFS | UV/UV | 7 |
| Paik, 2014 | Asian | Colorectal cancer | Both | I‐IV | Pre‐operative | 600 | 62.3 | 900 | ROC | OS/DFS | MV/MV | 6 |
| Pan, 2018 | Asian | Gastric cancer | No | I‐III | Pre‐operative | 870 | 60 b | 230 | ROC | OS | UV | 7 |
| Qi, 2015(1) | Asian | Pancreatic cancer | Both | III‐IV | Pre‐treatment | 211 | 61.2 | 400 | Median value | OS | MV | 5 |
| Qi, 2015(2) | Asian | Pancreatic cancer | Both | III‐IV | Pre‐treatment | 110 | 60.8 | 400 | Median value | OS | UV | 5 |
| Ren, 2016 | Asian | HCC | No | NA | Pre‐operative | 101 | 49.2 | 456 | ROC | OS/DFS | UV/UV | 8 |
| Saito, 2019 | Asian | Gastric cancer | No | I‐III | Pre‐operative | 445 | NA | 658.5 | ROC | OS | UV | 6 |
| Sasaki, 2006 | Asian | HCC | NA | NA | Pre‐operative | 198 | 63 | 300 | Median value | DFS/CSS | MV/MV | 9 |
| Sasaki, 2007 | Asian | Colorectal cancer | Yes | IV | Pre‐operative | 97 | 62.6 | 300 | Median value | CSS | MV | 9 |
| Shen, 2014 | Asian | HCC | No | NA | Pre‐operative | 351 | 50.1 | 545 | ROC | OS/DFS | MV/MV | 8 |
| Shibutani, 2017 | Asian | Colorectal cancer | No | I‐III | Pre‐operative | 189 | 68 | 300 | Previous studies | OS/RFS | UV/UV | 6 |
| Tanio, 2019 | Asian | Colorectal cancer | Both | I‐IV | Pre‐operative | 361 | NA | 421.5 | ROC | OS | UV | 7 |
| Urakawa, 2019 | Asian | Gastric cancer | No | II‐III | Pre‐operative | 278 | 68 b | 401 | Median value | OS/RFS | MV/MV | 8 |
| Wang, 2018 | Asian | Gastric cancer | No | I‐III | Pre‐treatment | 104 | 60 | 330 | Median value | OS | UV | 8 |
| Wang, 2019 | Asian | Gastric cancer | No | III | Pre‐operative | 182 | 55.7 | 440 | Median value | OS/DFS | UV/UV | 7 |
| Wu, 2019 | Asian | Colorectal cancer | No | I‐III | Pre‐treatment | 153 | 56 b | 330 | Median value | OS | UV | 8 |
| Yamamoto, 2020 | Asian | Colorectal cancer | No | I‐III | Pre‐operative | 463 | NA | 455.5 | ROC | OS | UV | 6 |
| Yang, 2017 | Asian | Colon cancer | Yes | IV | Pre‐treatment | 95 | 56 | 370 | Median value | OS/PFS | MV/MV | 7 |
| Zhang, 2015 | Asian | Rectal cancer | No | IIB | Pre‐operative | 270 | NA | 595 | ROC | OS/DFS | MV/MV | 8 |
| Zhang, 2016 | Asian | ICC | Both | NA | Pre‐treatment | 187 | 60 b | 500 | Median value | OS | MV | 6 |
| Pelvic cancers | ||||||||||||
| Abu‐Shawer, 2019 | Asian | GNC | Both | III‐IV | Pre‐treatment | 259 | 57 | 590 | ROC | OS/EFS | UV | 6 |
| Burgess, 2020 | Non‐Asian | Endometrial cancer | Both | I‐IV | Pre‐operative | 310 | 63.8 | 700 e | Previous study | OS/ DFS/ PFS | MV/MV/UV | 7 |
| Eo, 2018 | Asian | Cervical cancer | No | I‐II | Pre‐treatment | 233 | 51 b | 800 | Previous study | OS/PFS | MV/MV | 8 |
| Hayashi, 2017 | Asian | Prostate cancer | No | I‐III | Pre‐operative | 248 | 66 | 369 | Median value | RFS | MV | 6 |
| Ittiamornlert, 2019 | Asian | Cervical cancer | Both | I‐IV | Pre‐treatment | 355 | 52.5 | 970 | NA | OS/PFS | UV/UV | 6 |
| Lee, 2012 | Asian | Cervical cancer | No | I‐IVA | Pre‐treatment | 788 | 51 | 349 | Median value | OS/PFS | MV/MV | 6 |
| Lee, 2020 | Asian | Cervical cancer | No | II‐III | Pre‐treatment | 125 | 53.67 | 330 | ROC | OS/DFS | UV/UV | 6 |
| Li, 2016 | Asian | Cervical cancer | No | II‐IVA | Pre‐treatment | 424 | 47 | 380 | ROC | OS/PFS | MV/MV | 6 |
| Machida, 2017 | Non‐Asian | Endometrial cancer | Both | I‐IV | Pre‐treatment | 141 | 58.2 | 400 | NA | SAR | UV | 8 |
| Matsuo, 2015 | Non‐Asian | Endometrial cancer | NA | I‐IV | Pre‐operative | 541 | 52.1 | 700 | P value*** | OS/DFS | MV/MV | 8 |
| Shigeta, 2016(1) | Asian | Prostate cancer | Yes | IV | Pre‐treatment | 106 | 73 | 400 | ROC | OS/PFS | MV/MV | 6 |
| Shigeta, 2016(2) | Asian | Prostate cancer | Yes | IV | Pre‐treatment | 108 | 71 | 400 | ROC | OS/PFS | MV/MV | 6 |
| Singh, 2017 | Non‐Asian | Cervical cancer | No | I‐IVA | Pre‐treatment | 181 | 52 | 660 | Median value | OS/PFS | UV/UV | 8 |
| Wang, 2017 | Asian | Prostate cancer | Both | I‐IV | Pre‐treatment | 290 | 75 | 425 | ROC | OS/PFS/CSS | MV/UV/MV | 8 |
| Other cancers | ||||||||||||
| Gandini, 2016 | Non‐Asian | Melanoma | Yes | IV | Pre‐treatment | 127 | 55 | 460 | ULN | OS | MV | 7 |
| Martens, 2016 | Non‐Asian | Melanoma | Yes | IV | Pre‐treatment | 209 c | 58 | 413.3 | P value* | OS | UV | 6 |
| Rochet, 2015(1) | Non‐Asian | Melanoma | No | III | Pre‐operative | 153 | 59 b | 600 | P value**** | OS/RFS | MV/MV | 6 |
| Rochet, 2015(2) | Non‐Asian | Melanoma | Yes | IV | Pre‐operative | 74 | 56 b | 600 | P value**** | OS/RFS | MV/MV | 6 |
| Schmidt, 2005 | Non‐Asian | Melanoma | Yes | IV | Pre‐treatment | 321 | 51 | NA | Normal value | OS | UV | 5 |
| Shi, 2020 | Asian | Malignant cancers d | Both | NA | Pre‐treatment | 193 | 65 b | 370 | P value*** | OS | MV | 7 |
| Wagner, 2020 | Non‐Asian | Melanoma | No | I‐II | Pre‐operative | 1412 c | 63 | 810 | P value*** | OS | MV | 7 |
NOS: Newcastle‐Ottawa Quality Assessment Scale; OS: overall survival; DFS: disease‐free survival; PFS: progression‐free survival; SAR: survival after the recurrence of cancer; MV: multivariate analysis; UV: univariate analysis; ULN: upper limit of normal; DEBDC: distal extrahepatic bile duct cancer; ESCC: esophageal squamous cell carcinoma; GNC: Gynecological cancers; HCC: hepatocellular carcinoma; HPC: hypopharyngeal squamous cell carcinoma; ICC: intrahepatic cholangiocarcinoma; MPE: malignant pleural effusion; MPM: malignant pleural mesothelioma; NPC: nasopharyngeal carcinoma; OPC: oropharyngeal cancer; TET: thymic epithelial tumor; ROC: receiver operating characteristic curve; NA: not available information.
Number of included patients.
Median.
Existing missing data.
Malignant cancers with pleural effusion.
Method of determining cut‐off value.
p value: the lowest significant log‐rank p‐value of all analyzed eccentric cut‐off points.
p value: the cut‐off value was assessed by Contal and O'Quigley.
p value: the cut‐off value to maximize the survival outcome for DFS and OS.
p value: the significant value of χ 2 test of the log‐rank test analysis for different cut‐off points between the 25% and 75% quartile values.
3.2. Overall survival
A total of 88 studies including 29,130 patients provided suitable data for OS analysis. Comparing with low monocyte count, the elevated AMC showed a significant relevance with poorer survival (HR = 1.615; 95% CI: 1.475‐1.768; p < 0.001) (Figure 2). The test of heterogeneity was significant and random‐effects model was used (I2 = 83.50%; p < 0.001). Subgroup analyses were performed based on the available data. The subgroup analyses revealed a significant association between higher AMC and unfavorable cancer prognosis in Asian patients, studies with large sample size, studies with low cut‐off value, studies with multivariate analysis, and low‐quality studies with decreased heterogeneity. Similar associations were seen in analyses stratified by TNM stage and distant metastatic status, as well as in pre‐treatment studies and pre‐operative studies. Considering various cancer types may lead to inconsistent results, a subgroup analysis according to cancer type was conducted. Most subgroups showed a negative prognostic effect of elevated AMC. This includes the subgroups of breast cancer, cervical cancer, colorectal cancer, esophageal cancer, hepatocellular cancer, head and neck cancer, lung cancer, melanoma, pancreatic cancer, and prostate cancer. Of subgroups stratified by cancer type, the highest effect on OS was found in cervical cancer (HR: 2.917; 95% CI: 1.186‐7.175; p = 0.020) and no heterogeneity was found in the prostate cancer subgroup (HR: 2.253; 95% CI: 1.665‐3.048; p < 0.001) (Figure 3). When stratified by primary tumor sites, the pooled highest effect on OS was found in pelvic cancers (HR:2.111, 95% CI: 1.480‐3.011, p < 0.001). (Table 2). Meta‐regression analyses were performed, while none of an individual parameter was identified as the cause of heterogeneity (Table 2).
FIGURE 2.
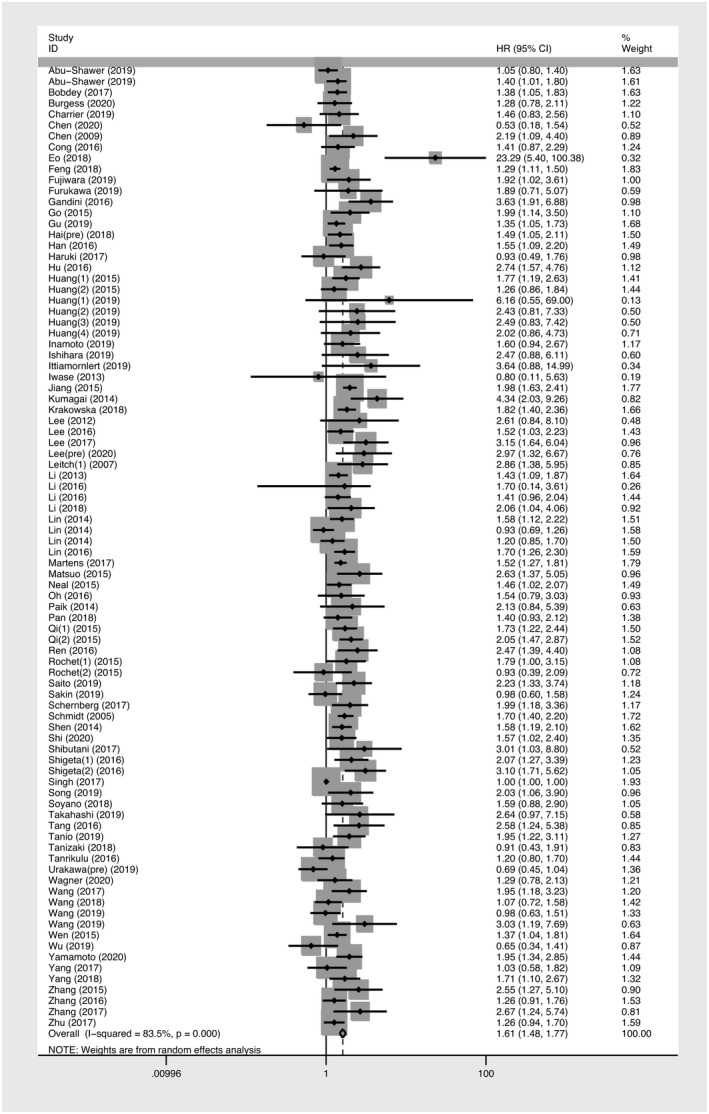
Forest plot of meta‐analysis of the prognostic role of absolute monocyte count for overall survival with random‐effects model
FIGURE 3.
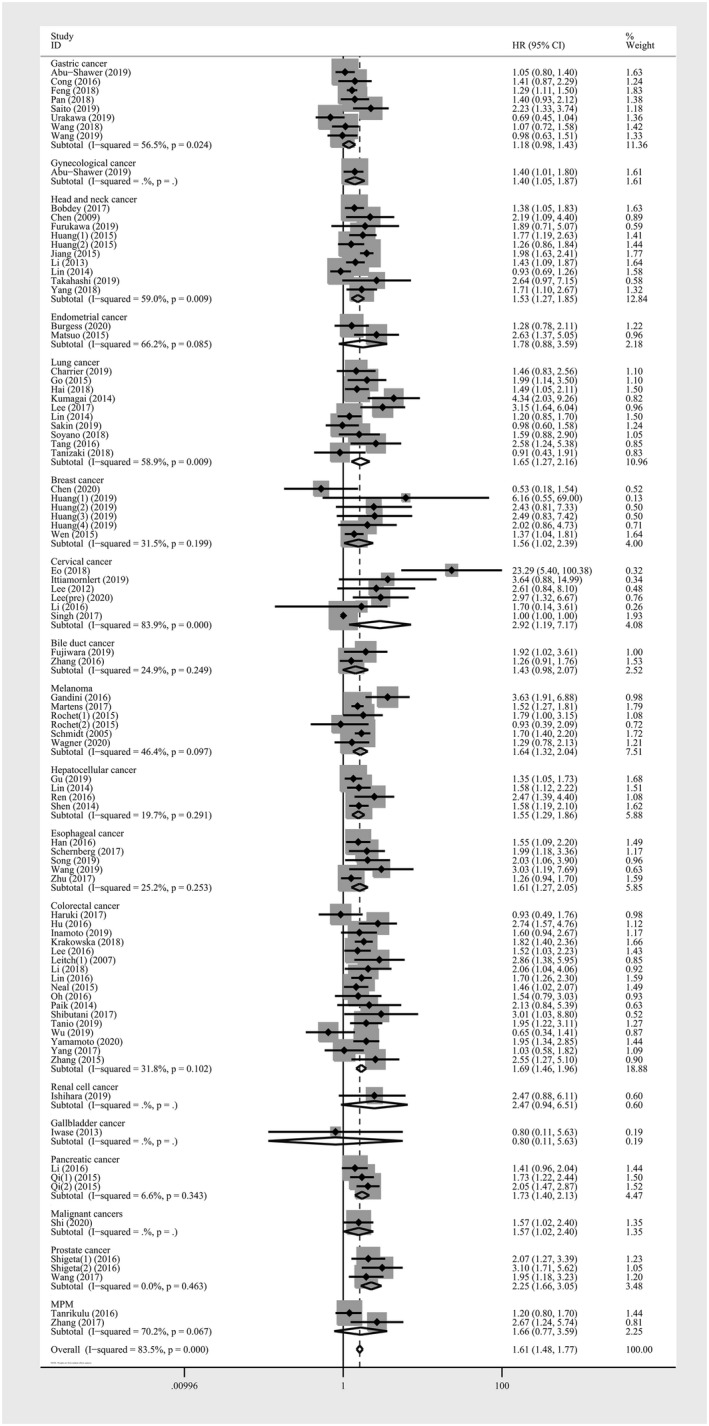
Forest plot of meta‐analysis of the prognostic role of absolute monocyte count for subgroup analysis of overall survival stratified by cancer type in solid tumors
TABLE 2.
The pooled data on the survival of the meta‐analysis
| Variables | N a | Case b | Pooled data | Heterogeneity | Meta‐regression (p value) | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | I2 | Ph | ||||
| (High level vs. low level) | |||||||
| Overall survival | |||||||
| Overall | 88 | 29,130 | 1.615 (1.475‐1.768) | <0.001 | 83.50% | <0.001 | |
| By region | |||||||
| Asian | 70 | 23,730 | 1.589 (1.462‐1.728) | <0.001 | 53.90% | <0.001 | 0.791 |
| Non‐Asian | 18 | 5400 | 1.580 (1.307‐1.910) | <0.001 | 86.40% | <0.001 | N. E. |
| By sample size | |||||||
| >200 | 46 | 23,899 | 1.560 (1.431‐1.700) | <0.001 | 52.30% | 0.001 | 0.886 |
| ≤200 | 42 | 5231 | 1.640 (1.419‐1.895) | <0.001 | 80.20% | <0.001 | N. E. |
| By cut‐off value | |||||||
| >500 | 37 | 13,786 | 1.657 (1.440‐1.907) | <0.001 | 84.50% | <0.001 | 0.664 |
| ≤500 | 48 | 14,728 | 1.543 (1.401‐1.700) | <0.001 | 49.50% | <0.001 | 0.543 |
| NA | 2 | 616 | 1.750 (1.475‐2.077) | <0.001 | 0% | 0.699 | N. E. |
| By cancer type | |||||||
| Bile duct cancer | 2 | 308 | 1.427 (0.984‐2.070) | 0.061 | 24.90% | 0.249 | 0.428 |
| Breast cancer | 6 | 2863 | 1.564 (1.023‐2.391) | 0.039 | 31.50% | 0.199 | 0.464 |
| Cervical cancer | 6 | 2106 | 2.917 (1.186‐7.175) | 0.020 | 83.90% | <0.001 | 0.571 |
| Colorectal cancer | 17 | 4709 | 1.689 (1.456‐1.958) | <0.001 | 31.80% | 0.102 | 0.533 |
| Endometrial cancer | 2 | 851 | 1.777 (0.880‐3.588) | 0.109 | 66.20% | 0.085 | 0.598 |
| Esophageal cancer | 5 | 1293 | 1.614 (1.268‐2.053) | <0.001 | 25.20% | 0.253 | 0.543 |
| Gallbladder cancer | 1 | 34 | 0.800 (0.114‐5.632) | 0.823 | 0.376 | ||
| Gastric cancer | 8 | 5812 | 1.181 (0.978‐1.426) | 0.084 | 56.50% | 0.024 | 0.233 |
| Gynecological cancer | 1 | 259 | 1.400 (1.049‐1.869) | 0.022 | 0.404 | ||
| Hepatocellular cancer | 4 | 784 | 1.551 (1.292‐1.861) | <0.001 | 19.70% | 0.291 | 0.496 |
| Head and neck cancer | 9 | 4293 | 1.530 (1.265‐1.850) | <0.001 | 59.00% | 0.009 | 0.439 |
| Lung cancer | 10 | 2004 | 1.654 (1.265‐2.162) | <0.001 | 58.90% | 0.009 | 0.499 |
| Melanoma | 6 | 2197 | 1.644 (1.325‐2.040) | <0.001 | 46.40% | 0.097 | 0.519 |
| MPM | 2 | 397 | 1.663 (0.771‐3.589) | 0.195 | 70.20% | 0.067 | 0.479 |
| Pancreatic cancer | 3 | 465 | 1.731 (1.404‐2.135) | <0.001 | 6.60% | 0.343 | 0.565 |
| Prostate cancer | 3 | 504 | 2.253 (1.665‐3.048) | <0.001 | 0% | 0.463 | 0.903 |
| Renal cell cancer | 1 | 58 | 2.470 (0.937‐6.508) | 0.067 | N. E. | ||
| Unknown malignant cancer | 1 | 193 | 1.567 (1.023‐2.399) | 0.039 | 0.516 | ||
| By primary tumor site | |||||||
| Abdominal cancers | 36 | 12,170 | 1.510 (1.366‐1.671) | <0.001 | 50.20% | <0.001 | 0.598 |
| Head and neck cancers | 10 | 4293 | 1.530 (1.265‐1.850) | <0.001 | 59.00% | 0.009 | 0.704 |
| Thoracic cancers | 23 | 6557 | 1.609 (1.381‐1.876) | <0.001 | 42.60% | 0.017 | 0.934 |
| Pelvic cancers | 12 | 3720 | 2.111 (1.480‐3.011) | <0.001 | 85.30% | <0.001 | 0.581 |
| Other cancers | 7 | 2390 | 1.628 (1.359‐1.950) | <0.001 | 35.80% | 0.155 | N. E. |
| By distant metastasis (DM) | |||||||
| No DM | 38 | 17,142 | 1.553 (1.361‐1.773) | <0.001 | 80.80% | <0.001 | 0.349 |
| DM | 15 | 3133 | 1.554 (1.313‐1.839) | <0.001 | 66.40% | <0.001 | 0.445 |
| Both | 30 | 7404 | 1.609 (1.450‐1.784) | <0.001 | 28.80% | 0.073 | 0.617 |
| NA | 5 | 1451 | 1.780 (1.329‐2.385) | <0.001 | 27.40% | 0.239 | N. E. |
| By TNM stage | |||||||
| <IV | 28 | 13,268 | 1.513 (1.316‐1.738) | <0.001 | 62.00% | <0.001 | 0.443 |
| IV | 15 | 3133 | 1.554 (1.313‐1.839) | <0.001 | 66.40% | <0.001 | 0.611 |
| I‐IV | 37 | 11,457 | 1.693 (1.464‐1.958) | <0.001 | 81.00% | <0.001 | 0.837 |
| NA | 8 | 1254 | 1.615 (1.374‐1.898) | <0.001 | 0% | 0.433 | N. E. |
| By the time of blood testing | |||||||
| Pre‐treatment | 52 | 13,024 | 1.596 (1.418‐1.795) | <0.001 | 84.70% | <0.001 | 0.538 |
| Pre‐operative | 34 | 15,923 | 1.596 (1.418‐1.796) | <0.001 | 53.40% | <0.001 | 0.550 |
| Post‐operative | 1 | 34 | 0.800 (0.114‐5.632) | 0.823 | N. E. | ||
| NA | 1 | 149 | 2.860 (1.377‐5.939) | 0.005 | 0.294 | ||
| By analysis method | |||||||
| MV | 46 | 19,650 | 1.660 (1.495‐1.843) | <0.001 | 57.80% | <0.001 | 0.244 |
| UV | 42 | 9480 | 1.524 (1.344‐1.728) | <0.001 | 81.60% | <0.001 | N. E. |
| By NOS score | |||||||
| High quality | 52 | 15,300 | 1.596 (1.414‐1.800) | <0.001 | 80.70% | <0.001 | N. E. |
| Low quality | 36 | 13,830 | 1.590 (1.441‐1.754) | <0.001 | 50.50% | <0.001 | 0.680 |
| Disease‐free survival | |||||||
| Overall | 41 | 11,514 | 1.488 (1.357‐1.633) | <0.001 | 32.00% | 0.028 | |
| By region | |||||||
| Asian | 35 | 9734 | 1.478 (1.329‐1.644) | <0.001 | 39.80% | 0.009 | N. E. |
| Non‐Asian | 6 | 1780 | 1.648 (1.335‐2.036) | <0.001 | 0% | 0.996 | 0.394 |
| By sample size | |||||||
| >200 | 21 | 9170 | 1.499 (1.304‐1.723) | <0.001 | 48.90% | 0.006 | N. E. |
| ≤200 | 20 | 2344 | 1.452 (1.301‐1.621) | <0.001 | 3.30% | 0.415 | 0.803 |
| By cut‐off value | |||||||
| >500 | 12 | 3679 | 1.538 (1.357‐1.744) | <0.001 | 0% | 0.506 | 0.312 |
| ≤500 | 29 | 7835 | 1.453 (1.286‐1.642) | <0.001 | 40.00% | 0.015 | N. E. |
| By cancer | |||||||
| Breast cancer | 9 | 1550 | 1.465 (1.129‐1.900) | <0.001 | 0% | 0.646 | 0.043 |
| Cervical cancer | 1 | 125 | 2.076 (1.063‐4.053) | 0.032 | 0.160 | ||
| Colorectal cancer | 6 | 1857 | 1.738 (1.279‐2.362) | <0.001 | 42.70% | 0.120 | 0.063 |
| Endometrial cancer | 2 | 851 | 1.669 (1.213‐2.295) | <0.001 | 0% | 0.847 | 0.067 |
| Esophageal cancer | 3 | 1124 | 1.331 (1.077‐1.643) | 0.002 | 0% | 0.403 | 0.031 |
| Extrahepatic bile duct cancer | 1 | 121 | 1.939 (1.047‐3.591) | 0.048 | 0.130 | ||
| Gallbladder cancer | 1 | 34 | 2.084 (0.529‐8.218) | 0.294 | 0.280 | ||
| Gastric cancer | 2 | 460 | 0.805 (0.536‐1.209) | 0.295 | 48.50% | 0.163 | 0.006 |
| Hepatocellular cancer | 4 | 906 | 1.536 (1.116‐2.112) | 0.008 | 65.70% | 0.033 | 0.033 |
| Head and neck cancer | 6 | 3132 | 1.570 (1.291‐1.909) | 0.026 | 32.70% | 0.191 | 0.043 |
| Lung cancer | 2 | 735 | 1.474 (1.152‐1.888) | 0.006 | 0% | 0.417 | 0.045 |
| Melanoma | 2 | 227 | 1.530 (1.004‐2.332) | 0.048 | 0% | 0.907 | 0.056 |
| Pancreatic cancer | 1 | 144 | 1.471 (1.014‐2.135) | 0.042 | 0.048 | ||
| Prostate cancer | 1 | 248 | 1.894 (0.998‐3.595) | 0.051 | 0.126 | ||
| By primary tumor site | |||||||
| Head and neck cancers | 5 | 3016 | 1.585 (1.203‐2.088) | 0.001 | 41.60% | 0.144 | N. E. |
| Thoracic cancers | 13 | 2867 | 1.418 (1.226‐1.641) | <0.001 | 0% | 0.709 | 0.703 |
| Abdominal cancers | 16 | 3638 | 1.489 (1.244‐1.782) | <0.001 | 61.90% | 0.001 | 0.610 |
| Pelvic cancers | 5 | 1766 | 1.623 (1.305‐2.017) | <0.001 | 0% | 0.800 | 0.798 |
| Other cancers | 2 | 227 | 1.530 (1.004‐2.332) | 0.048 | 0% | 0.907 | 0.937 |
| By distant metastasis (DM) status | |||||||
| No DM | 30 | 8196 | 1.468 (1.312‐1.642) | <0.001 | 41.40% | 0.010 | 0.455 |
| DM | 1 | 89 | 1.143 (0.694‐1.883) | 0.600 | N. E. | ||
| Both | 7 | 1986 | 1.627 (1.315‐2.013) | <0.001 | 0% | 0.518 | 0.282 |
| NA | 3 | 1243 | 1.727 (1.269‐2.351) | 0.001 | 0% | 0.932 | 0.270 |
| By TNM stage | |||||||
| <IV stage | 9 | 4743 | 1.436 (1.239‐1.664) | <0.001 | 42.00% | 0.026 | 0.703 |
| IV stage | 2 | 163 | 1.279 (0.855‐1.914) | 0.231 | 0% | 0.453 | N. E. |
| I‐IV stage | 7 | 6019 | 1.524 (1.359‐1.710) | <0.001 | 1.30% | 0.436 | 0.411 |
| NA | 4 | 589 | 1.931 (1.066‐3.498) | 0.030 | 66.80% | 0.029 | 0.555 |
| By the time of blood testing | |||||||
| Pre‐treatment | 12 | 4092 | 1.498 (1.323‐1.697) | <0.001 | 0% | 0.859 | 0.688 |
| Pre‐operative | 28 | 7388 | 1.500 (1.321‐1.703) | <0.001 | 47.60% | 0.003 | 0.692 |
| Post‐operative | 1 | 34 | 2.084 (0.529‐8.218) | 0.294 | N. E. | ||
| By analysis method | |||||||
| MV | 16 | 6277 | 1.420 (1.248‐1.616) | <0.001 | 44.40% | 0.029 | 0.274 |
| UV | 25 | 5237 | 1.566 (1.375‐1.784) | <0.001 | 18.10% | 0.209 | N. E. |
| By NOS score | |||||||
| High quality | 29 | 7126 | 1.438 (1.293‐1.599) | <0.001 | 32.50% | 0.048 | 0.236 |
| Low quality | 12 | 4388 | 1.665 (1.371‐2.021) | <0.001 | 31.10% | 0.142 | N. E. |
| Progressive‐free survival | |||||||
| Overall | 23 | 5126 | 1.533 (1.342‐1.751) | <0.001 | 45.50% | 0.010 | |
| By region | |||||||
| Asian | 16 | 3852 | 1.672 (1.380‐2.026) | <0.001 | 48.60% | 0.015 | 0.126 |
| Non‐Asian | 7 | 1274 | 1.290 (1.145‐1.454) | <0.001 | 0% | 0.531 | N. E. |
| By sample size | |||||||
| >200 | 10 | 3769 | 1.655 (1.348‐2.031) | <0.001 | 50.50% | 0.033 | 0.409 |
| ≤200 | 13 | 1357 | 1.442 (1.209‐1.720) | <0.001 | 38.30% | 0.078 | N. E. |
| By cut‐off value | |||||||
| >500 | 13 | 2359 | 1.439 (1.184‐1.749) | <0.001 | 54.60% | 0.009 | 0.967 |
| ≤500 | 9 | 2472 | 1.700 (1.442‐2.004) | <0.001 | 1.30% | 0.423 | N. E. |
| NA | 1 | 295 | 1.380 (1.062‐1.793) | 0.016 | 0.152 | ||
| By cancer type | |||||||
| Cervical cancer | 5 | 1981 | 2.338 (1.091‐5.011) | 0.029 | 81.90% | <0.001 | 0.460 |
| Colorectal cancer | 4 | 1094 | 1.487 (1.259‐1.756) | <0.001 | 0% | 0.667 | 0.610 |
| Endometrial cancer | 1 | 310 | 1.397 (0.938‐2.080) | 0.100 | 0.471 | ||
| Esophageal cancer | 1 | 126 | 1.900 (1.110‐3.251) | 0.019 | 0.761 | ||
| Head and neck cancer | 1 | 75 | 2.923 (1.214‐7.036) | 0.017 | 0.259 | ||
| Lung cancer | 6 | 921 | 1.381 (1.161‐1.642) | <0.001 | 0% | 0.445 | 0.805 |
| Pancreatic cancer | 1 | 57 | 1.188 (0.419‐3.369) | 0.746 | 0.963 | ||
| Prostate cancer | 3 | 504 | 1.791 (1.310‐2.449) | <0.001 | 33.20% | 0.224 | 0.439 |
| Renal cell cancer | 1 | 58 | 1.140 (0.521‐2.497) | 0.743 | N. E. | ||
| By primary tumor site | |||||||
| Head and neck cancers | 1 | 75 | 2.923 (1.214‐7.036) | 0.017 | 0.176 | ||
| Thoracic cancers | 6 | 921 | 1.318 (1.161‐1.642) | <0.001 | 0% | 0.445 | N. E. |
| Abdominal cancers | 7 | 1335 | 1.494 (1.281‐1.743) | <0.001 | 61.90% | 0.811 | 0.501 |
| Pelvic cancers | 9 | 2795 | 1.842 (1.339‐2.533) | <0.001 | 72.80% | <0.001 | 0.248 |
| By distant metastatic status | |||||||
| No DM | 7 | 2025 | 1.876 (1.173‐3.000) | 0.009 | 72.20% | 0.001 | N. E. |
| DM | 5 | 855 | 1.640 (1.361‐1.975) | <0.001 | 0% | 0.423 | 0.736 |
| Both | 11 | 2246 | 1.436 (1.253‐1.646) | <0.001 | 12.20% | 0.328 | 0.694 |
| By TNM stage | |||||||
| <IV | 3 | 506 | 2.892 (0.945‐8.850) | 0.063 | 75.80% | 0.016 | N. E. |
| IV | 5 | 855 | 1.640 (1.361‐1.975) | <0.001 | 0% | 0.423 | 0.250 |
| I‐IV | 13 | 3482 | 1.378 (1.187‐1.600) | <0.001 | 33.90% | 0.111 | 0.077 |
| NA | 2 | 283 | 1.608 (1.202‐2.152) | 0.001 | 0% | 0.469 | 0.265 |
| By the time of blood testing | |||||||
| Pre‐treatment | 20 | 4367 | 1.469 (1.302‐1.658) | <0.001 | 30.90% | 0.094 | 0.272 |
| Pre‐operative | 3 | 759 | 2.717 (1.093‐6.757) | 0.032 | 81.30% | 0.005 | N. E. |
| By analysis method | |||||||
| MV | 13 | 3334 | 1.762 (1.478‐2.102) | <0.001 | 35.90% | 0.095 | 0.011 |
| UV | 10 | 1792 | 1.276 (1.112‐1.465) | 0.001 | 14.00% | 0.314 | N. E. |
| By NOS score | |||||||
| High quality | 14 | 1992 | 1.396 (1.221‐1.596) | <0.001 | 23.70% | 0.198 | 0.172 |
| Low quality | 9 | 3134 | 1.805 (1.377‐2.366) | <0.001 | 56.50% | 0.019 | N. E. |
| Cancer‐specific survival | |||||||
|
<0.001 |
57.00% | 0.013 | 1.585 (1.253‐2.006) | ||||
| By region | |||||||
| Asian | 7 | 1780 | 1.593 (1.179‐2.152) | 0.002 | 64.90% | 0.009 | 0.952 |
| Non‐Asian | 3 | 535 | 1.621 (1.055‐2.491) | 0.028 | 41.30% | 0.182 | N. E. |
| By sample size | |||||||
| >200 | 5 | 1590 | 1.780 (1.197‐2.648) | 0.004 | 62.80% | 0.029 | N. E. |
| ≤200 | 5 | 725 | 1.454 (1.074‐1.970) | 0.016 | 52.80% | 0.076 | 0.549 |
| By cut‐off value | |||||||
| >500 | 5 | 1080 | 1.484 (1.195‐1.843) | <0.001 | 9.30% | 0.353 | 0.754 |
| ≤500 | 5 | 1235 | 1.772 (1.106‐2.839) | 0.017 | 75.20% | 0.003 | N. E. |
| By cancer type | |||||||
| Colorectal cancer | 5 | 1080 | 1.524 (1.209‐1.921) | <0.001 | 0% | 0.482 | 0.651 |
| Hepatocellular cancer | 1 | 198 | 1.070 (0.835‐1.371) | 0.593 | 0.369 | ||
| Head and neck cancer | 2 | 399 | 3.339 (0.742‐15.026) | 0.116 | 83.50% | 0.014 | 0.821 |
| Lung cancer | 1 | 348 | 1.282 (0.897‐1.832) | 0.173 | 0.496 | ||
| Prostate cancer | 1 | 290 | 2.240 (1.282‐3.914) | <0.001 | N. E. | ||
| By primary tumor site | |||||||
| Head and neck cancers | 2 | 399 | 3.339 (0.742‐15.026) | 0.116 | 83.50% | 0.014 | 0.313 |
| Thoracic cancers | 1 | 348 | 1.282 (0.897‐1.832) | 0.173 | N. E. | ||
| Abdominal cancers | 6 | 1278 | 1.373 (1.087‐1.734) | 0.008 | 34.60% | 0.177 | 0.815 |
| Pelvic cancers | 1 | 290 | 2.240 (1.282‐3.914) | 0.005 | 0.451 | ||
| By metastatic status | |||||||
| No DM | 2 | 497 | 1.975 (0.699‐5.579) | 0.199 | 74.30% | 0.049 | 0.322 |
| DM | 4 | 680 | 1.508 (1.208‐1.881) | 0.019 | 0% | 0.916 | 0.176 |
| Both | 3 | 940 | 2.579 (1.168‐5.695) | <0.001 | 68.90% | 0.040 | 0.043 |
| NA | 1 | 198 | 1.070 (0.835‐1.371) | 0.593 | N. E. | ||
| By TNM stage | |||||||
| <IV | 1 | 149 | 3.780 (1.372‐10.415) | 0.010 | N. E. | ||
| IV | 4 | 680 | 1.508 (1.208‐1.881) | <0.001 | 0% | 0.916 | 0.143 |
| I‐IV | 3 | 940 | 2.579 (1.168‐5.695) | 0.019 | 68.90% | 0.040 | 0.648 |
| NA | 2 | 546 | 1.135 (0.926‐1.391) | 0.224 | 0% | 0.415 | 0.071 |
| By the time of blood testing | |||||||
| Pre‐treatment | 3 | 689 | 2.577 (1.331‐4.989) | 0.005 | 67.20% | 0.048 | N. E. |
| Pre‐operative | 5 | 1393 | 1.253 (1.066‐1.473) | 0.006 | 0% | 0.526 | 0.111 |
| NA | 2 | 233 | 2.038 (0.738‐5.626) | 0.169 | 69.10% | 0.072 | 0.570 |
| By analysis method | |||||||
| MV | 6 | 1583 | 1.600 (1.126‐2.272) | 0.009 | 69.10% | 0.006 | 0.915 |
| UV | 4 | 732 | 1.595 (1.210‐2.101) | 0.001 | 14.90% | 0.317 | N. E. |
| By NOS score | |||||||
| High quality | 7 | 1634 | 1.577 (1.196‐2.078) | 0.001 | 65.10% | 0.009 | N. E. |
| Low quality | 3 | 681 | 1.680 (0.978‐2.884) | 0.060 | 41.00% | 0.183 | 0.751 |
NOS: Newcastle‐Ottawa Quality Assessment Scale; OS: overall survival; DFS: disease‐free survival; PFS: progression‐free survival; CSS: cancer‐specific survival; HR: hazard ratio; MPM: malignant pleural mesothelioma; DM: distant metastasis; NA: not available information; MV: multivariate analysis; UV: univariate analysis; 95%CI: confidence interval; P: p value of pooled HR; I 2: value of Higgins I‐squared statistics; Ph: p value of Heterogeneity test; N. E.: not estimation.
Numbers of studies included in the meta‐analysis.
Number of included patients.
3.3. Disease‐free survival
There were 41 studies, comprising 11,514 patients, reporting HRs for DFS. Overall, high AMC was significantly associated with worse DFS (HR:1.488; 95% CI: 1.357‐1.633; p < 0.001) (Figure 4). Low heterogeneity was shown between these studies (I2 = 32.00%; p = 0.028). The analyses demonstrated that elevated AMC was positively related to pooled HR for DFS when stratified by region, sample size, cut‐off value, analysis method, and study quality. In subgroup analyses according to cancer type, studies on breast cancer, endometrial cancer, esophageal cancer, lung cancer, and melanoma also demonstrated the negative effect of high AMC on outcomes with consistency. In addition, we found poor DFS in studies with IV stage cancers, but the estimate was insignificant (HR:1.279; 95% CI: 0.855‐1.914; p = 0.231) (Table 2).
FIGURE 4.
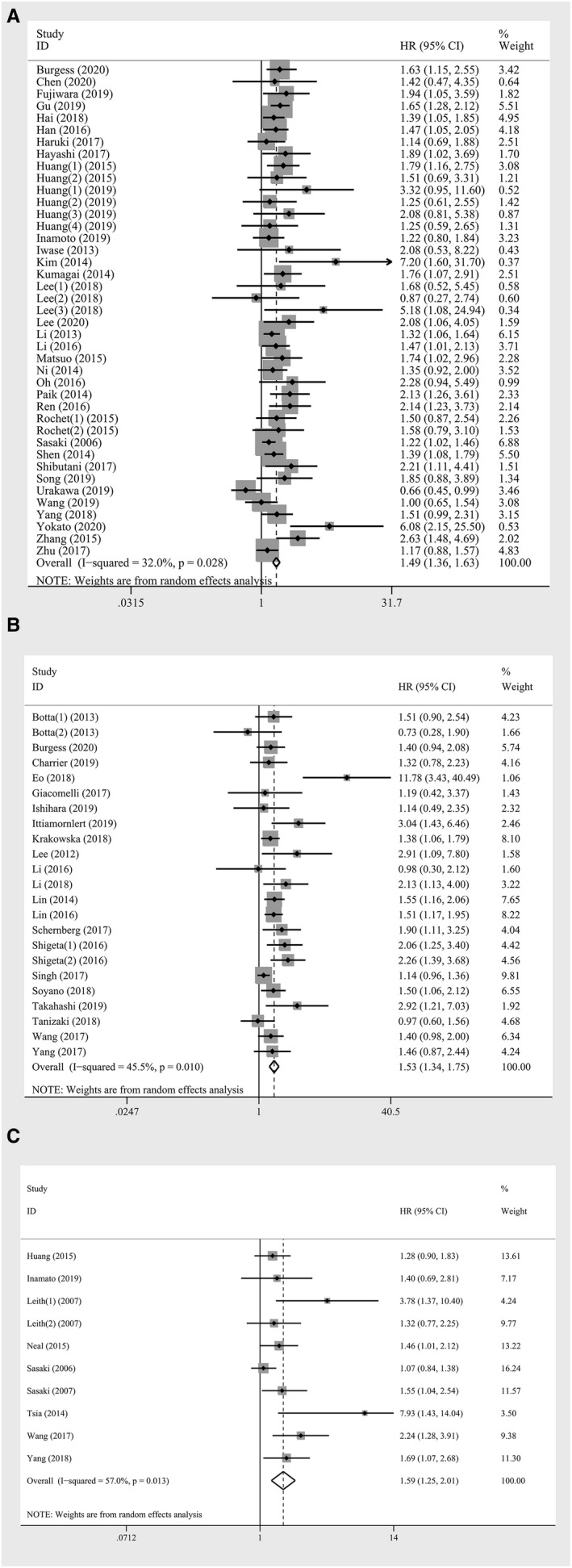
Forest plot of meta‐analysis of the prognostic role of absolute monocyte count for (A) disease‐free survival (B) progression‐free survival (C) cancer‐specific survival in solid tumors
3.4. Progressive‐free survival
Data on the association between AMC and PFS were derived from 23 studies involving 5,126 patients. Overall higher AMC associated with worse prognosis (HR: 1.533; 95% CI: 1.342‐1.751; p < 0.001), with moderate heterogeneity (I2 = 45.50%; p = 0.010) (Table 2, Figure 4). Exploratory subgroup analyses were performed according to region, cancer type and TNM stages, and a prognostic role of AMC was observed in non‐Asian patients (HR: 1.274, 95% CI: 1.145‐1.454; p < 0.001), patients with colorectal cancer (HR: 1.487; 95% CI: 1.259‐1.756; p < 0.001) and lung cancer (HR: 1.381; 95% CI: 1.161‐1.642; p < 0.001), patients with stage IV cancer (HR: 1.640; 95% CI: 1.361‐1.975; p < 0.001) with no heterogeneity. The adverse effect of higher AMC was also seen when stratified by sample size, cut‐off value, distant metastatic status, the time of blood testing, analysis method, and study quality (Table 2).
3.5. Cancer‐specific survival
Ten studies involving 2,315 patients reported suitable data for CSS analysis. Overall, an increase in the monocyte showed associations with worse CSS (HR: 1.585; 95% CI: 1.253‐2.006; p < 0.001) (Table 2; Figure 4). A relatively high heterogeneity was observed across the studies (I2 = 57.00%; p = 0.013). When stratified by cancer type, distant metastatic status, and the time of blood testing, elevated AMC was associated with worse outcome in patients with colorectal cancer (HR: 1.523; 95% CI: 1.209‐1.921; p < 0.001), distant metastatic cancer (HR: 1.508; 95% CI: 1.208‐1.881; p < 0.001) and studies with pre‐operative AMC (HR: 1.253; 95% CI: 1.066‐1.473; p = 0.006) without heterogeneity. The subgroup analyses also revealed that elevated AMC might be a potential biomarker in non‐Asian patients, studies with high cut‐off value, and studies with univariate analysis or low quality (Table 2).
3.6. Relationships of AMC and clinical features
Association between monocyte count and clinicopathological parameters were evaluated among 28 studies. There was a significantly positive associations between elevated AMC and advanced T stage (OR:1.298; 95% CI:1.035‐1.629; p = 0.024), microvascular invasion (OR:1.896; 95% CI:1.240‐2.900; p = 0.003), macrovascular invasion (OR:4.713; 95% CI: 1.293‐17.177; p = 0.019) and larger tumor length (OR:1.783; 95% CI:1.378‐2.308; p < 0.001). The pooled analysis revealed that the AMC of male with solid tumors was more likely higher (OR:2.147; 95% CI: 1.650‐2.795, p < 0.001). (Table 3, Figure S1) We also evaluated that smoking was positively associated with higher AMC (OR:1.684, 95% CI: 1.104‐2.570, p = 0.016). Patients with elevated pre‐operative monocyte has lower albumin (SMD: 0.264; 95% CI: 0.084‐0.444; p = 0.004), while higher platelet (SMD:0.455; 95% CI:0.166‐0.743; p = 0.002) (Table 3).
TABLE 3.
Meta‐analysis of the association between elevated AMC and clinicopathological features of cancers
| Variables | Studies | Patients | Pooled OR | 95% CI | p value* | Heterogeneity I 2 | Ph value** |
|---|---|---|---|---|---|---|---|
| Dichotomous variables | |||||||
| Gender (male vs. female) | 19 | 4340 | 2.147 | 1.650‐2.795 | <0.001 | 65.50% | <0.001 |
| APF (high vs. low) | 5 | 1102 | 1.265 | 0.965‐1.659 | 0.089 | 4.60% | 0.381 |
| Distant metastasis | 5 | 1405 | 1.179 | 0.921‐1.510 | 0.192 | 0% | 0.718 |
| T stage (T2+ vs. <T2) | 9 | 4222 | 1.298 | 1.035‐1.629 | 0.024 | 18.70% | 0.277 |
| N stage (N1+ vs. N0) | 9 | 4324 | 1.080 | 0.910‐1.281 | 0.379 | 20.00% | 0.265 |
| TMN stage (III+IV vs. I+II) | 9 | 2503 | 1.224 | 0.950‐1.576 | 0.118 | 47.50% | 0.055 |
| Microvascular invasion (yes vs. no) | 3 | 555 | 1.896 | 1.240‐2.900 | 0.003 | 0% | 0.620 |
| Macrovascular invasion (yes vs. no) | 2 | 452 | 4.713 | 1.293‐17.177 | 0.019 | 83.90% | 0.013 |
| Vascular invasion (yes vs. no) | 3 | 740 | 1.580 | 0.975‐2.561 | 0.063 | 0% | 0.700 |
| Lymphatic permeation (yes vs. no) | 2 | 520 | 0.943 | 0.537‐1.655 | 0.837 | 18.80% | 0.267 |
| Tumor length (high vs. low) | 8 | 1520 | 1.783 | 1.378‐2.308 | <0.001 | 8.20% | 0.367 |
| Differentiation (well vs. poor/moderate) | 10 | 2625 | 0.968 | 0.784‐1.196 | 0.762 | 9.40% | 0.356 |
| Adjuvant therapy a | 6 | 1422 | 0.900 | 0.715‐1.133 | 0.370 | 0% | 0.670 |
| Adjuvant therapy b | 3 | 883 | 0.905 | 0.675‐1.214 | 0.505 | 0% | 0.651 |
| Smoking (yes vs. no) | 6 | 1858 | 1.684 | 1.104‐2.570 | 0.016 | 72.80% | 0.002 |
| Continuous variables | |||||||
| Age | 11 | 3072 | 0.019 | −0.077‐0.116 | 0.693 | 39.10% | 0.088 |
| Hemoglobin | 4 | 1025 | 0.004 | −0.119‐0.128 | 0.946 | 0% | 0.707 |
| Platelet | 4 | 1008 | 0.455 | 0.166‐0.743 | 0.002 | 72.00% | 0.013 |
| Albumin (low vs. high) | 3 | 790 | 0.264 | 0.084‐0.444 | 0.004 | 0% | 0.471 |
| Hematocrit | 3 | 807 | −0.014 | −0.156‐0.124 | 0.841 | 0% | 0.690 |
OR: odd ratio; SMD: standardized mean difference; CI: confidence interval; I 2: the value of I‐squared statistics; vs.: versus.
Studies with adjuvant therapy versus studies without adjuvant therapy.
Studies with radiotherapy versus studies with concurrent chemoradiotherapy.
p value of pooled HR.
p value of Heterogeneity test.
p value of SMD.
3.7. Publication bias and sensitivity analysis
For OS, the funnel plot was visibly asymmetrical, indicating the presence of publication bias (Figure S2). In accord with the plot, the results of Begg's test (p = 0.028) and Egger's test (p < 0.001) further confirmed that the asymmetry was mainly attributed to the publication bias. The “trim‐and‐fill” analysis was performed and no significant change in our results was found, further suggesting the stability of the meta‐analysis (HR: 1.461; 95% CI: 1.343‐1.588; p < 0.001) (Supplementary Figure 3). For DFS, the results of Begg's test (p = 0.003) and Egger's test (p = 0.001) showed publication bias. The adjusted random effect HR of 1.363 (95% CI: 1.234‐1.506, p < 0.001) was obtained using the “trim‐and‐fill” analysis, which was consistent with the primary analysis. For PFS, there were no evidence of asymmetry, and no trimming was performed after applying the “trim‐and‐fill” analysis. For CSS, the results of Begg's test (p = 0.007) and Egger's test (p = 0.001) indicated publication bias, and there was no trimming performed after applying the “trim‐and‐fill” analysis.
In addition, sensitivity analysis was carried out and the results showed that the pooled HRs were not significantly affected by omitting an individual study.
4. DISCUSSION
To the best of our knowledge, we performed a comprehensive meta‐analysis for the first time to assess the prognostic value of AMC in various solid tumors. The systematic review and meta‐analysis involving data on 32,229 participants from 104 studies provided robust evidence that elevated the level of monocyte count might be an independent prognostic factor for poor OS, DFS, PFS, and CSS in non‐hematologic tumors. Subgroup analyses focused on clinical outcomes were conducted and further proved the predictor role of elevated AMC on long‐term cancer outcomes. In addition, we found a tendency that an elevated AMC was significantly associated with some clinicopathological characteristics including gender, T stage, vascular invasion, tumor length, and smoking, as well as higher platelet counts and lower albumin.
Since Virchow described the role of inflammation in 1863, inflammatory response has gone beyond the marker of infection. It has been hypothesized that cancer arises from the background of inflammation, which has been supported by a multitude of evidence in the last decade. 10 , 11 , 12 So far, it has been widely accepted that the inflammatory components, which constitute a major part of the TME, may be triggered by the conditions that predisposes to cancer or by genetic events. 6 What is more, as a binary “anti‐tumor” or “pro‐tumor” environment, the dominant function of TME is determined by the cross‐regulation of immune cells and non‐immune cells by perceiving signaling molecules to induce the proliferative activity of the tumor, metastasis, cell migration, and immune response against therapy. 7 , 11 Recent research efforts have shed light on the prognostic significance of immune cells in various cancers. 21 , 22 , 23 , 24 , 25 , 26
Of these immune cells, monocytes are a subset of circulating blood cell originated from myeloid progenitors in the bone marrow and subsequently can be attracted to peripheral tissues via bloodstream. 120 Circulating monocytes perform versatile functions both in antimicrobial defense and chronic inflammation. In TME, peripheral monocytes constantly enter the tumor sites and inhibit the tumor‐related immune defense function by expressing inhibitory molecules and/or releasing soluble inhibitory factors via tumor‐derived signals. 121 , 122 As monocyte measurement is easily standardized and available in blood routine examination, monocyte could be a potentially helpful and convenient serum biomarker in clinical practice even be added as a new item in the immunoscore system. 14 The qualitative and quantitative changes of the monocyte count in tumor have been attracting research attention. In accordance with our finding, recent meta‐analyses also pooled the data of individual tumor types and demonstrated that elevated peripheral monocyte appeared to be synonymous with an increased risk of mortality in the context of several malignancies. 123 , 124 However, the effect of elevated AMC on outcomes has not been synthetically and comprehensively analyzed in solid tumors and the variation of the effects according to tumor type has not been explained. In addition, the direct evidence for some cancers, such as melanoma and malignant pleural mesothelioma, was sparse due to limited studies and small sample sizes. Therefore, we conducted the present meta‐analysis to sort through currently available data and concluded that elevated AMC was associated with poor clinical outcomes in non‐hematologic tumors.
The exact underlying mechanisms of the association between elevated AMC and unfavorable outcomes have not been fully clarified and might be multifactorial. It is recognized that monocytes originate from mononuclear myeloid cells in bone marrow, which come into play in response to pathogenic stimuli such as cancer by myelopoiesis which largely manifest in the expansion of monocytes and neutrophils. 125 , 126 In the study, our results aggregated previous studies and revealed the positive associations between elevated AMC and local invasion of tumor cells and tumor length. Besides, TAMs plays a vital role at the crossroads of inflammation and cancer. 6 , 7 Monocytes travel to peripheral tissue and move directionally to the tumor sites owning to tumor‐derived signals, subsequently differentiate to TAMs. 12 , 18 Anti‐tumor M1 macrophages are characterized by the induction of lipopolysaccharide (LPS) and IFN‐γ, and are able to withstand intracellular pathogens and cancer cells. 15 , 16 In contrast, polarized pro‐tumor M2 macrophages are a source and target of a distinct pattern of cytokines, chemokines, and growth factors generally exerting tumor‐promoting and immune escape effects, and impairs anti‐cancer therapies. 127 , 128 Therefore, the understanding of the balance between M1 and M2 polarization provides a theoretical foundation for better rational manipulation of monocytes differentiation and macrophage polarization switching in TME. 128 Monocyte subpopulations have different functions in TME. 129 Previous study described that inflammatory monocytes could be predominantly divided into classical inflammatory monocytes (CCR2highLy6C++CD43+ in mice, homologous to CCR2highCD14++CD16− in human), intermediate monocytes (Ly6C++CD43++ in mice, homologous to CD14++CD16+ in human) and nonclassical patrolling monocytes (CX3CR1highLy6C+CD43++ in mice, homologous to CX3CRhighCD14+CD16++ in human). (The + denotes an expression level that is 10‐fold above the isotype control and ++ is 100‐fold above the isotype control). 129 , 130 Prat et al. found a significant increase in intermediate monocyte subpopulations that performed protumor function through the proangiogenic capacities in ovarian cancer. 131 The study also explored the correlation between intermediate monocytes and protumor immunosuppressive microenvironment in ascites. 131 It was hypothesized that the circulating monocytes might be modulated by secreted factors produced by stromal cells and tumor cells in TME, such as IL‐10 and CCL2. 131
In addition, the monocyte chemotactic factor monocyte chemoattractant protein‐1 /C‐C chemokine ligand 2 (MCP‐1/CCL2) secreted by tumor cells is a chemokine with potent monocyte chemotactic activity via binding to CCR2 (the receptor for chemokine CCL2), has been shown to directly or indirectly enhance immunosuppression and metastasis by vascular endothelial growth factor (VEGF) secretion and other tumor‐secreted factors like IFNγ in murine cancer models. 132 , 133 , 134 Yoshimura T. expatiated on the correlation between MCP‐1 production and TAMs and indicated that MCP‐1 production regulated the vicious cycle between host cells and tumor cells thus promoting cancer progression. 134 Additionally, recent studies reported the NK cell‐monocyte interactions enhanced NK cell antitumor activity in cancer prognosis and the response to monoclonal antibody therapy. 135 , 136 It has been reported that tumor‐infiltrating monocytes/macrophages induced NK cell dysfunction via TGFβ1, thus impairing the expression of IFNα, TNFγ, and Ki‐67 in tumor progression in gastric cancer. 136 Kubo et al. also demonstrated that patrolling monocytes contributing to the prevention of primary tumor by producing IL‐15, which is the key mediator to activate the anti‐metastatic role of NK cells. 137 Moreover, myeloid‐derived suppressor cells (MDSCs) generated from myeloid cells in TME and were similar but functionally distinct from monocytes and neutrophils. 125 Chae et al. demonstrated that MDSC could also arose directly from monocytes and displayed the immune suppressive activity in tumor progression in the murine model. 138 However, cells with MDSC features could be easily mistaken for monocytes in some studies. 138 Otherwise, Kuang et al. found monocytes activated by tumors strongly express programmed cell death ligand 1 (PD‐L1) and effectively suppressed tumor‐specific T cell immunity in HCC in vivo. 139 Taube et al. also demonstrated some factors such as IL‐10 and IL‐32γ induced PD‐L1 expression on monocytes in melanoma. 140 Therefore, the PD‐L1 expression on the monocytes could be a novel mechanism explaining the association between monocyte and cancer. All the proposed mechanisms about monocyte might inspire potential molecular targets for personalized treatment strategy of solid tumors.
It has been reported that a therapeutic regimen such as chemotherapy could modulate the pro‐tumor and anti‐tumor ability of monocytes/macrophages lineages. 141 Different oncotherapy regimens have been supposed to be associated with various changes in the biology and function of these cells. 141 In the subgroup analyses, nevertheless, studies on the prognostic value of AMC in patients with different treatments have not been evaluated due to the variety and complexity of treatment programs. In addition, our meta‐analysis has demonstrated that elevated AMC was associated with poor prognosis. However, a precise cut‐off value in clinical practice is a matter of broad discussion. At present, ROC curves were widely used in the threshold selection in diagnostic or screening tests considering the optimization of false‐positive and false‐negative interpretation. For this reason, most of the included studies chose ROC curves to define their threshold cut‐off values. In the paper, we split studies with a cut‐off >500 or ≤500, both studies with high cut‐off and with low cut‐off were associated with an increased risk of worse outcomes.
Our study possesses several strengths. Distinctively, we focused on all non‐hematological tumors and provided comprehensive evidence for the prognostic value of AMC, which might be a weighted one involved in the immunoscore system. Moreover, because of the diversity of cancer types and studies, the research into subgroup analysis were lucubrated. It is the first time that pre‐treatment AMC, pre‐operative AMC, and post‐operative AMC are compared. Based on subgroup analyses stratified by cut‐off values of monocytes, higher cut‐off values for OS and DFS seem to be more discriminative effective on prognosis, while inversely in PFS and CSS. Additionally, we studied the relationships between AMC and gender, T stage, tumor length, microvascular invasion, smoking, and other clinic parameters, which provide potential implications in clinical practice in the future.
Nevertheless, there were some limitations to the present study. A key limit was that cut‐off levels of AMC were set based on ROC, median value, previous studies or other methods, making the routine application less practical. Although the cut‐off >500/mm3 may enable us to better identify the poor outcomes in the present study, the optimal AMC cut‐off awaits standardization. Besides, as Walker SP said, “the unpredictability of the diseases undermined the ability to plan ahead.” 142 In practice, the dynamic change in prognostic indicators might be more valuable for one patient with solid tumor. Future studies on changes in cancer biomarkers and cut‐off values defining the contributions of each cancer type are required. Additionally, we failed to find the sources of the heterogeneity of overall survival analyses with subgroup analyses and meta‐regression analyses. Due to the variations of study quality and sample size among included studies, the statistical methods might be refined and a weighted mean might be computed. As mentioned before, publication bias existed in OS, DFS, and CSS analyses, and we were unable to extract the unreported data in some studies. Moreover, numerous confounding factors influence the post‐operative AMC, such as surgical stress, bleeding, sepsis, even wound healing. Therefore, the prognostic value of post‐operative AMC is relatively rarely reported. In addition, the relationships between monocyte and the features of tumor patients were not well defined in our study because of the lack of original data, hence the results may be less suitable in clinical practice.
5. CONCLUSIONS
In conclusion, our comprehensive meta‐analysis strongly supported that elevated AMC was remarkably associated with poor prognosis of patients with solid cancer. Monocyte, the relatively accessible low‐cost cancer biomarker, could have widespread clinical implications for surgical management, treatment strategy, and prognosis assessment. Further multicenter studies in a randomized and prospective manner with optimal AMC cut‐offs were warranted to refine our results and to advance the clinical applications of the monocyte counts in the future. Finally, given the different functions of monocyte subsets in TME, the monitoring of blood monocyte subpopulations could be further explored and applied to follow‐up treatment response.
6. DATA AVAILABLE STATEMENT
This study reports a systematic review for which all data are already available within the public realm in the form of scientific publications, references for which are provided.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Shu Wen, Ying Hu, and Liangzhi Xu conceived and designed the experiments; Litao Huang, Jin Peng, and Nan Chen collected the data; Meina Yang and Xiaoyang Shen analyzed the data; Shu Wen and Nan Chen contributed the materials/analysis tools and wrote the manuscript. Ying Hu and Song Yang revised the manuscript. All authors reviewed and approved the manuscript prior to submission.
Supporting information
Fig S1
Fig S2
Fig S3
ACKNOWLEDGMENT
We are particularly grateful to Deying Kang for his revision in the methodological section.
Shu Wen, Nan Chen are contributed equally to this work as first authors.
Contributor Information
Ying Hu, Email: huyingmyworld@126.com.
Liangzhi Xu, Email: xuliangzhi_art@126.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3. Sun K, Zheng R, Zhang S, et al. Report of cancer incidence and mortality in different areas of China, 2015. China Cancer. 2019;28:1‐11. 10.11735/j.issn.1004-0242.2019.01.A001. [DOI] [Google Scholar]
- 4. Nalejska E, Mączyńska E, Lewandowska MA Prognostic and predictive biomarkers: tools in personalized oncology. Mol Diagn Ther. 2014;18(3):273‐284. 10.1007/s40291-013-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vickers AJ, Thompson IM, Klein E, Carroll PR, Scardino PT A commentary on PSA velocity and doubling time for clinical decisions in prostate cancer. Urology. 2014;83(3):592‐596. 10.1016/j.urology.2013.09.075. [DOI] [PubMed] [Google Scholar]
- 6. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A Cancer‐related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073‐1081. 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8. Finn OJ Immuno‐oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;8:viii6‐viii9. 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galon J, Fridman WH, Pagès F The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67(5):1883‐1886. 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 10. Jarnicki A, Putoczki T, Ernst M Stat3: linking inflammation to epithelial cancer: more than a “gut” feeling? Cell Div. 2010;5:14. 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swartz MA, Iida N, Roberts EW, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72(10):2473‐2480. 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanahan D, Coussens LM Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309‐322. 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 13. Zadka Ł, Grybowski DJ, Dzięgiel P Modeling of the immune response in the pathogenesis of solid tumors and its prognostic significance. Cell Oncol. 2020;43(4):539‐575. 10.1007/s13402-020-00519-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128‐2139. 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 15. Zhang M, He Y, Sun X, et al. A high M1/M2 ratio of tumor‐associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bygd HC, Forsmark KD, Bratlie KM The significance of macrophage phenotype in cancer and biomaterials. Clin Transl Med. 2014;3(1):62. 10.1186/s40169-014-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P Tumour‐associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399‐416. 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassetta L, Fragkogianni S, Sims AH, et al. Human tumor‐associated macrophage and monocyte transcriptional landscapes reveal cancer‐specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588‐602. 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arwert EN, Harney AS, Entenberg D, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23(5):1239‐1248. 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung K, Heishi T, Khan OF, et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti‐VEGFR2 cancer therapy. J Clin Invest. 2017;127(8):3039‐3051. 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma. 2012;53(4):575‐580. 10.3109/10428194.2011.637211. [DOI] [PubMed] [Google Scholar]
- 22. Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97(9):1266‐1270. 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li SW, Yuan W, Zhao B, et al. Positive effect of HPV status on prognostic value of blood lymphocyte‐to‐monocyte ratio in advanced cervical carcinoma. Cancer Cell Int. 2016;16:54. 10.1186/s12935-016-0334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanrikulu AC, Abakay A, Komek H, Abakay O Prognostic value of the lymphocyte‐to‐monocyte ratio and other inflammatory markers in malignant pleural mesothelioma. Environ Health Prev Med. 2016;21(5):304‐311. 10.1007/s12199-016-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y Prognostic value of lymphocyte‐to‐monocyte ratio in patients with solid tumors: a systematic review and meta‐analysis. Cancer Treat Rev. 2015;41(10):971‐978. 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 26. Xia WK, Lin QF, Shen D, Liu ZL, Su J, Mao WD Prognostic significance of lymphocyte‐to‐monocyte ratio in diffuse large B‐cell lymphoma: a systematic review and meta‐analysis. FEBS Open Bio. 2016;6(6):558‐565. 10.1002/2211-5463.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parmar MK, Torri V, Stewart L Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815‐2834. . [DOI] [PubMed] [Google Scholar]
- 29. Stang A Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 30. Abu‐Shawer O, Abu‐Shawer M, Haimour A, et al. Hematologic markers of distant metastases in gastric cancer. Gastrointest Oncol. 2019;10(3):529‐536. 10.21037/jgo.2019.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abu‐Shawer O, Abu‐Shawer M, Hirmas N, et al. Hematologic markers of distant metastases and poor prognosis in gynecological cancers. BMC Cancer. 2019;19(1):141. 10.1186/s12885-019-5326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bobdey S, Ganesh B, Mishra P, Jain A Role of monocyte count and neutrophil‐to‐lymphocyte ratio in survival of oral cancer patients. Int Arch Otorhinolaryngol. 2017;21(1):21‐27. 10.1055/s-0036-1587318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Botta C, Barbieri V, Ciliberto D, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non‐small cell lung cancer patients. Cancer Biol Ther. 2013;14(6):469‐475. 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgess B, Levine B, Taylor RN, Kelly MG Preoperative circulating lymphocyte and monocyte counts correlate with patient outcomes in type I and type II endometrial cancer. Reprod Sci. 2020;27(1):194‐203. 10.1007/s43032-019-00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Charrier M, Mezquita L, Lueza B, et al. Circulating innate immune markers and outcomes in treatment‐naive advanced non‐small cell lung cancer patients. Eur J Cancer. 2019;108:88‐96. 10.1016/j.ejca.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 36. Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res. 2020;12:1543‐1567. 10.2147/CMAR.S235519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen MH, Chang PM, Chen PM, et al. Prognostic significance of a pretreatment hematologic profile in patients with head and neck cancer. J Cancer Res Clin Oncol. 2009;135(12):1783‐1790. 10.1007/s00432-009-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cong X, Li S, Xue Y Impact of preoperative lymphocyte to monocyte ratio on the prognosis of the elderly patients with stage II‐III gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19(10):1144‐1148. 10.3760/cma.j.issn.1671-0274.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 39. Eo WK, Kwon BS, Kim KH, et al. Monocytosis as a prognostic factor for survival in stage IB and IIA cervical cancer. J cancer. 2018;9(1):64‐70. 10.7150/jca.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feng F, Zheng G, Wang Q, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018;18(1):148. 10.1186/s12876-018-0877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujiwara Y, Haruki K, Shiba H, et al. The comparison of inflammation‐based prognostic scores in patients with extrahepatic bile duct cancer after pancreaticoduodenectomy. J Surg Res. 2019;238:102‐112. 10.1016/j.jss.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 42. Furukawa K, Kawasaki G, Naruse T, Umeda M Prognostic significance of pretreatment lymphocyte‐to‐monocyte ratio in patients with tongue cancer. Anticancer Res. 2019;39(1):405‐412. 10.21873/anticanres.13126. [DOI] [PubMed] [Google Scholar]
- 43. Gandini S, Ferrucci PF, Botteri E, et al. Prognostic significance of hematological profiles in melanoma patients. Int J Cancer. 2016;139(7):1618‐1625. 10.1002/ijc.30215. [DOI] [PubMed] [Google Scholar]
- 44. Giacomelli I, Scartoni D, Mohammadi H, Regine WF, Chuong MD Does lymphocyte‐to‐monocyte ratio before, during, or after definitive chemoradiation for locally advanced pancreatic cancer predict for clinical outcomes? J Gastrointest Oncol. 2017;8(4):721‐727. 10.21037/jgo.2017.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Go SI, Kim RB, Song HN, et al. Prognostic significance of the absolute monocyte counts in lung cancer patients with venous thromboembolism. Tumour Biol. 2015;36(10):7631‐7639. 10.1007/s13277-015-3475-2. [DOI] [PubMed] [Google Scholar]
- 46. Gu J, Zhang X, Cui R, et al. Prognostic predictors for patients with hepatocellular carcinoma receiving adjuvant transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2019;31(7):836‐844. 10.1097/MEG.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 47. Hai Y, Chen N, Wu W, et al. High postoperative monocyte indicates inferior clinicopathological characteristics and worse prognosis in lung adenocarcinoma or squamous cell carcinoma after lobectomy. BMC Cancer. 2018;18(1):1011. 10.1186/s12885-018-4909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han L, Jia Y, Song Q, et al. Prognostic significance of preoperative absolute peripheral monocyte count in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(7):740‐746. 10.1111/dote.12401. [DOI] [PubMed] [Google Scholar]
- 49. Haruki K, Shiba H, Fujiwara Y, et al. Preoperative peripheral blood neutrophil count predicts long‐term outcomes following hepatic resection for colorectal liver metastases. Oncol Lett. 2017;13(5):3688‐3694. 10.3892/ol.2017.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hayashi T, Fujita K, Nojima S, et al. Peripheral blood monocyte count reflecting tumor‐infiltrating macrophages is a predictive factor of adverse pathology in radical prostatectomy specimens. Prostate. 2017;77(14):1383‐1388. 10.1002/pros.23398. [DOI] [PubMed] [Google Scholar]
- 51. Hu S, Zou Z, Li H, et al. The preoperative peripheral blood monocyte count is associated with liver metastasis and overall survival in colorectal cancer patients. PLoS One. 2016;11(6):e0157486. 10.1371/journal.pone.0157486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121(4):545‐555. 10.1002/cncr.29100. [DOI] [PubMed] [Google Scholar]
- 53. Huang Y, Feng JF Low preoperative lymphocyte to monocyte ratio predicts poor cancer‐specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2015;8:137‐145. 10.2147/OTT.S73794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Z, Li Z, Yao Z, et al. Clinical prognostic evaluation of immunocytes in different molecular subtypes of breast cancer. J Cell Physiol. 2019;234(11):20584‐20602. 10.1002/jcp.28662. [DOI] [PubMed] [Google Scholar]
- 55. Inamoto S, Kawada K, Okamura R, Hida K, Sakai Y Prognostic impact of the combination of neutrophil‐to‐lymphocyte ratio and Glasgow prognostic score in colorectal cancer: a retrospective cohort study. Int J Colorectal Dis. 2019;34(7):1303‐1315. 10.1007/s00384-019-03316-z. [DOI] [PubMed] [Google Scholar]
- 56. Ishihara H, Tachibana H, Takagi T, et al. Predictive impact of peripheral blood markers and c‐reactive protein in nivolumab therapy for metastatic renal cell carcinoma. Target Oncol. 2019;14(4):453‐463. 10.1007/s11523-019-00660-6. [DOI] [PubMed] [Google Scholar]
- 57. Ittiamornlert P, Ruengkhachorn I Neutrophil‐lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. BMC Cancer. 2019;19(1):51. 10.1186/s12885-019-5269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iwase R, Shiba H, Haruki K, et al. Post‐operative lymphocyte count may predict the outcome of radical resection for gallbladder carcinoma. Anticancer Res. 2013;33(8):3439‐3444. [PubMed] [Google Scholar]
- 59. Jiang R, Cai XY, Yang ZH, et al. Elevated peripheral blood lymphocyteto‐monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer. 2015;34(6):237‐246. 10.1186/s40880-015-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim YK, Kim SH, Lee SD, Lee SA, Park SJ Pretransplant absolute monocyte count in peripheral blood predicts posttransplant tumor prognosis in patients undergoing liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2014;13(3):250‐258. 10.1016/s1499-3872(14)60251-4. [DOI] [PubMed] [Google Scholar]
- 61. Krakowska M, Dębska‐Szmich S, Czyżykowski R, Zadrożna‐Nowak A, Potemski P The prognostic impact of neutrophil‐to‐lymphocyte ratio, lymphocyte‐to‐monocyte ratio, and platelet‐to‐lymphocyte ratio in patients with advanced colorectal cancer treated with first‐line chemotherapy. Prz gastroenterol. 2018;13(3):218‐222. 10.5114/pg.2018.78287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kumagai S, Marumo S, Shoji T, et al. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer. 2014;85(3):457‐464. 10.1016/j.lungcan.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 63. Lee HJ, Kim JM, Chin YJ, et al. Prognostic value of hematological parameters in locally advanced cervical cancer patients treated with concurrent chemoradiotherapy. Anticancer Res. 2020;40(1):451‐458. 10.21873/anticanres.13973. [DOI] [PubMed] [Google Scholar]
- 64. Lee KH, Kim EY, Yun JS, et al. The prognostic and predictive value of tumor‐infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18(1):938. 10.1186/s12885-018-4832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee S, Eo W, Jeon H, Park S, Chae J Prognostic significance of host‐related biomarkers for survival in patients with advanced non‐small cell lung cancer. J Cancer. 2017;8(15):2974‐2983. 10.7150/jca.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee S, Song A, Eo W Serum ferritin as a prognostic biomarker for survival in relapsed or refractory metastatic colorectal cancer. J Cancer. 2016;7(8):957‐964. 10.7150/jca.14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee YY, Choi CH, Sung CO, et al. Prognostic value of pre‐treatment circulating monocyte count in patients with cervical cancer: comparison with SCC‐Ag level. Gynecol Oncol. 2012;124(1):92‐97. 10.1016/j.ygyno.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 68. Li GJ, Xu HW, Ji JJ, Yang F, Gao BQ Prognostic value of preoperative lymphocyte‐to‐monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther. 2016;9:1085‐1092. 10.2147/OTT.S96707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li J, Jiang R, Liu WS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte‐to‐monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One. 2013;8(12):e83069. 10.1371/journal.pone.0083069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li Z, Xu Z, Huang Y, et al. The predictive value and the correlation of peripheral absolute monocyte count, tumor‐associated macrophage and microvessel density in patients with colon cancer. Medicine (Baltimore). 2018;97(21):e10759. 10.1097/MD.0000000000010759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lin GN, Liu PP, Liu DY, Peng JW, Xiao JJ, Xia ZJ Prognostic significance of the pre‐chemotherapy lymphocyte‐to‐monocyte ratio in patients with previously untreated metastatic colorectal cancer receiving FOLFOX chemotherapy. Chin J Cancer. 2016;35:5. 10.1186/s40880-015-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lin GN, Jiang XM, Peng JW, Xiao JJ, Liu DY, Xia ZJ Prognostic significance of the peripheral blood absolute monocyte count in patients with locally advanced or metastatic hepatocellular carcinoma receiving systemic chemotherapy. Asian Pac J Cancer Prev. 2014;15(15):6387‐6390. 10.7314/apjcp.2014.15.15.6387. [DOI] [PubMed] [Google Scholar]
- 73. Lin GN, Peng JW, Liu DY, Xiao JJ, Chen YQ, Chen XQ Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumor Biol. 2014;35(11):10849‐10854. 10.1007/s13277-014-2362-6. [DOI] [PubMed] [Google Scholar]
- 74. Lin GN, Peng JW, Xiao JJ, Liu DY, Xia ZJ Prognostic impact of circulating monocytes and lymphocyte‐to‐monocyte ratio on previously untreated metastatic non‐small cell lung cancer patients receiving platinum‐based doublet. Med Oncol. 2014;31(7):70. 10.1007/s12032-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 75. Machida H, De Zoysa MY, Takiuchi T, Hom MS, Tierney KE, Matsuo K Significance of monocyte counts at recurrence on survival outcome of women with endometrial cancer. Int J Gynecol Cancer. 2017;27(2):302‐310. 10.1097/IGC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martens A, Wistuba‐Hamprecht K, Foppen MG, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908‐2918. 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Matsuo K, Hom MS, Moeini A, et al. Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol. 2015;138(2):332‐338. 10.1016/j.ygyno.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Neal CP, Cairns V, Jones MJ, et al. Prognostic performance of inflammation‐based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol. 2015;32(5):144. 10.1007/s12032-015-0590-2. [DOI] [PubMed] [Google Scholar]
- 79. Ni XJ, Zhang XL, Ou‐Yang QW, et al. An elevated peripheral blood lymphocyte‐to‐monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9(11):e111886. 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Oh SY, Kim YB, Suh KW Prognostic significance of systemic inflammatory response in stage II colorectal cancer. J Surg Res. 2017;208:158‐165. 10.1016/j.jss.2016.08.100. [DOI] [PubMed] [Google Scholar]
- 81. Paik KY, Lee IK, Lee YS, Sung NY, Kwon TS Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat. 2014;46(1):65‐73. 10.4143/crt.2014.46.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pan YC, Jia ZF, Cao DH, et al. Preoperative lymphocyte‐to‐monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine. 2018;97(52):e13896. 10.1097/MD.0000000000013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qi Q, Geng Y, Sun M, Wang P, Chen Z Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15(2):145‐150. 10.1016/j.pan.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 84. Ren QQ, Fu SJ, Zhao Q, et al. Prognostic value of preoperative peripheral monocyte count in patients with hepatocellular carcinoma after liver transplantation. Tumor Biol. 2016;37(7):8973‐8978. 10.1007/s13277-015-4758-3. [DOI] [PubMed] [Google Scholar]
- 85. Rochet NM, Kottschade LA, Grotz TE, Porrata LF, Markovic SN The prognostic role of the preoperative absolute lymphocyte count and absolute monocyte count in patients with resected advanced melanoma. Am J Clin Oncol. 2015;38(3):252‐258. 10.1097/COC.0b013e31829b5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Saito H, Shimizu S, Kono Y, et al. Score of the preoperative absolute number of lymphocytes, monocytes, and neutrophils as a prognostic indicator for patients with gastric cancer. Surg today. 2019;49(10):850‐858. 10.1007/s00595-019-01817-6. [DOI] [PubMed] [Google Scholar]
- 87. Sakin A, Sahin S, Yasar N, et al. The relation between hemogram parameters and survival in extensive‐stage small cell lung cancer. Oncol Res Treat. 2019;42(10):506‐515. 10.1159/000501595. [DOI] [PubMed] [Google Scholar]
- 88. Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139(6):755‐764. 10.1016/j.surg.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 89. Sasaki A, Kai S, Endo Y, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11(5):596‐602. 10.1007/s11605-007-0140-0. [DOI] [PubMed] [Google Scholar]
- 90. Schernberg A, Moureau‐Zabotto L, Rivin Del Campo E, et al. Leukocytosis and neutrophilia predict outcome in locally advanced esophageal cancer treated with definitive chemoradiation. Oncotarget. 2017;8(7):11579‐11588. 10.18632/oncotarget.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273‐278. 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shen SL, Fu SJ, Huang XQ, et al. Elevated preoperative peripheral blood monocyte count predicts poor prognosis for hepatocellular carcinoma after curative resection. BMC Cancer. 2014;14:744. 10.1186/1471-2407-14-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shi XY, Yi FS, Wang Z, Qiao X, Zhai K Prognostic value of a new score using serum alkaline phosphatase and pleural effusion lactate dehydrogenase for patients with malignant pleural effusion. Thoracic Cancer. 2020;11(2):320‐328. 10.1111/1759-7714.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K Prognostic significance of the preoperative lymphocyte‐to‐monocyte ratio in patients with colorectal cancer. Oncol Lett. 2017;13(2):1000‐1006. 10.3892/ol.2016.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shigeta K, Kosaka T, Kitano S, et al. High absolute monocyte count predicts poor clinical outcome in patients with castration‐resistant prostate cancer treated with docetaxel chemotherapy. Ann Surg Oncol. 2016;23(12):4115‐4122. 10.1245/s10434-016-5354-5. [DOI] [PubMed] [Google Scholar]
- 96. Singh S, Himler J, Nagel CI, Resnick K The prognostic value of baseline lymphocyte, neutrophil, and monocyte counts in locally advanced cervical carcinoma treated with radiation. Obstet Gynecol Int. 2017;2017:8584605. 10.1155/2017/8584605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Song Q, Wu JZ, Wang S Postoperative monocyte count change is a better predictor of survival than preoperative monocyte count in esophageal squamous cell carcinoma. Biomed Res Int. 2019;2019:2702719. 10.1155/2019/2702719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Soyano AE, Dholaria B, Marin‐Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non‐small cell lung Cancer patients treated with anti‐PD‐1 antibodies. J Immunother Cancer. 2018;6(1):129. 10.1186/s40425-018-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Takahashi H, Sakakura K, Tada H, Kaira K, Oyama T, Chikamatsu K Prognostic significance and population dynamics of peripheral monocytes in patients with oropharyngeal squamous cell carcinoma. Head Neck. 2019;41(6):1880‐1888. 10.1002/hed.25625. [DOI] [PubMed] [Google Scholar]
- 100. Tang H, Ma H, Peng F, et al. Prognostic performance of inflammation‐based prognostic indices in locally advanced non‐small‐lung cancer treated with endostar and concurrent chemoradiotherapy. Mol Clin Oncol. 2016;4(5):801‐806. 10.3892/mco.2016.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tanio A, Saito H, Uejima C, et al. A prognostic index for colorectal cancer based on preoperative absolute lymphocyte, monocyte, and neutrophil counts. Surg Today. 2019;49(3):245‐253. 10.1007/s00595-018-1728-6. [DOI] [PubMed] [Google Scholar]
- 102. Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in non‐small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97‐105. 10.1016/j.jtho.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 103. Tsai YD, Wang CP, Chen CY, et al. Pretreatment circulating monocyte count associated with poor prognosis in patients with oral cavity cancer. Head Neck. 2014;36(7):947‐953. 10.1002/hed.23400. [DOI] [PubMed] [Google Scholar]
- 104. Urakawa S, Yamasaki M, Goto K, et al. Peri‐operative monocyte count is a marker of poor prognosis in gastric cancer: increased monocytes are a characteristic of myeloid‐derived suppressor cells. Cancer Immunol Immunother. 2019;68(8):1341‐1350. 10.1007/s00262-019-02366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wagner NB, Luttermann F, Gassenmaier M, et al. Absolute and relative differential blood count predicts survival of AJCC stage I‐II melanoma patients scheduled for sentinel lymph node biopsy. Austr J Dermatol. 2020;6:e310‐e318. 10.1111/ajd.13248. [DOI] [PubMed] [Google Scholar]
- 106. Wang Q, Zhu D The prognostic value of systemic immune‐inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. 2019;10(5):965‐978. 10.21037/jgo.2019.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang X, Zhang B, Chen X, et al. Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR‐1210), a novel anti‐PD‐1 antibody. Thoracic Cancer. 2019;10(6):1395‐1401. 10.1111/1759-7714.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang YL, Ge XX, Wang Y, et al. The values of applying classification and counts of white blood cells to the prognostic evaluation of resectable gastric cancers. BMC Gastroenterol. 2018;18(1):99. 10.1186/s12876-018-0812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang YQ, Zhu YJ, Pan JH, et al. Peripheral monocyte count: AN independent diagnostic and prognostic biomarker for prostate cancer‐A Large Chinese Cohort Study. Asian J Androl. 2017;19(5):579‐585. 10.4103/1008-682X.186185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wen J, Ye F, Huang X, et al. Prognostic significance of preoperative circulating monocyte count in patients with breast cancer based on a large cohort study. Medicine. 2015;94(49):e2266. 10.1097/MD.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wu J, Ge XX, Zhu W, et al. Values of applying white blood cell counts in the prognostic evaluation of resectable colorectal cancer. Mol Med Rep. 2019;19(3):2330‐2340. 10.3892/mmr.2019.9844. [DOI] [PubMed] [Google Scholar]
- 112. Yamamoto M, Saito H, Hara K, et al. Combination of C‐reactive protein and monocyte count is a useful prognostic indicator for patients with colorectal cancer. In Vivo. 2020;34(1):299‐305. 10.21873/invivo.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yang J, Guo X, Wang M, Ma X, Ye X, Lin P Pre‐treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild‐type RAS. Sci Rep. 2017;7(1):17166. 10.1038/s41598-017-17130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang J, Hsueh C‐Y, Cao W, Zhou L Pretreatment lymphocyte‐to‐monocyte ratio as an independent prognostic factor for hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2018;138(8):734‐740. 10.1080/00016489.2018.1449965. [DOI] [PubMed] [Google Scholar]
- 115. Yokota M, Katoh H, Nishimiya H, et al. Lymphocyte‐monocyte ratio significantly predicts recurrence in papillary thyroid cancer. J Surg Research. 2020;246:535‐543. 10.1016/j.jss.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 116. Zhang A, Cao S, Jin S, et al. Elevated aspartate aminotransferase and monocyte counts predict unfavorable prognosis in patients with malignant pleural mesothelioma. Neoplasma. 2017;64(1):114‐122. 10.4149/neo_2017_114. [DOI] [PubMed] [Google Scholar]
- 117. Zhang C, Wang H, Ning Z, et al. Prognostic value of systemic inflammatory response markers in patients with intrahepatic cholangiocarcinoma. Int J Clin Exp Med. 2016;9:11502‐11509. [Google Scholar]
- 118. Zhang LN, Xiao W, OuYang PY, et al. The prognostic impact of preoperative blood monocyte count in pathological T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy. Tumour Biol. 2015;36(10):8213‐8219. 10.1007/s13277-015-3560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhu Y, Li M, Bo C, et al. Prognostic significance of the lymphocyte‐to‐monocyte ratio and the tumor‐infiltrating lymphocyte to tumor‐associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2017;66(3):343‐354. 10.1007/s00262-016-1931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. van Furth R, Cohn ZA The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415‐435. 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chanmee T, Ontong P, Konno K, Itano N Tumor‐associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670‐1690. 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Peranzoni E, Zilio S, Marigo I, et al. Myeloid‐derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238‐244. 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 123. Peng H, Luo X Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta‐analysis. Cancer Cell Int. 2019;19:70. 10.1186/s12935-019-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wen S, Chen N, Peng J, et al. Peripheral monocyte counts predict the clinical outcome for patients with colorectal cancer: a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol. 2019;31(11):1313‐1321. [DOI] [PubMed] [Google Scholar]
- 125. Gabrilovich DI Myeloid‐derived suppressor cells. Cancer Immunol Res. 2017;5(1):3‐8. 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ouzounova M, Lee E, Piranlioglu R, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun. 2017;8:14979. 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677‐686. 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 128. Kratochvill F, Neale G, Haverkamp JM, et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12(11):1902‐1914. 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ziegler‐Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74‐e80. 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 130. Gordon S, Taylor PR Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953‐964. 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 131. Prat M, Le Naour A, Coulson K, et al. Circulating CD14high CD16low intermediate blood monocytes as a biomarker of ascites immune status and ovarian cancer progression. J Immunother Cancer. 2020;8(1):e000472. 10.1136/jitc-2019-000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL IFN‐γ and CCL2 cooperate to redirect tumor‐infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 2016;6(4):400‐413. 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vakilian A, Khorramdelazad H, Heidari P, Sheikh Rezaei Z, Hassanshahi G CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int. 2017;103:1‐7. 10.1016/j.neuint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 134. Yoshimura T The chemokine MCP‐1 (CCL2) in the host interaction with cancer: a foe or ally? Cell Mol Immunol. 2018;15(4):335‐345. 10.1038/cmi.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Campbell AR, Duggan MC, Suarez‐Kelly LP, et al. MICA‐expressing monocytes enhance natural killer cell fc receptor‐mediated antitumor functions. Cancer Immunol Res. 2017;5(9):778‐789. 10.1158/2326-6066.CIR-16-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Peng LS, Zhang JY, Teng YS, et al. Tumor‐associated monocytes/macrophages impair NK‐Cell function via TGFβ1 in human gastric cancer. Cancer Immunol Res. 2017;5(3):248‐256. 10.1158/2326-6066.CIR-16-0152. [DOI] [PubMed] [Google Scholar]
- 137. Kubo H, Mensurado S, Gonçalves‐Sousa N, Serre K, Silva‐Santos B Primary tumors limit metastasis formation through induction of IL15‐mediated cross‐talk between patrolling monocytes and NK cells. Cancer Immunol Res. 2017;5(9):812‐820. 10.1158/2326-6066.CIR-17-0082. [DOI] [PubMed] [Google Scholar]
- 138. Chae M, Peterson TE, Balgeman A, et al. Increasing glioma‐associated monocytes leads to increased intratumoral and systemic myeloid‐derived suppressor cells in a murine model. Neuro Oncol. 2015;17(7):978‐991. 10.1093/neuonc/nou343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD‐L1. J Exp Med. 2009;206(6):1327‐1337. 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Taube JM, Young GD, McMiller TL, et al. Differential expression of immune‐regulatory genes associated with PD‐L1 display in melanoma: implications for PD‐1 pathway blockade. Clin Cancer Res. 2015;21(17):3969‐3976. 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J Interaction of tumor‐associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8(7):1596004. 10.1080/2162402X.2019.1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Walker SP The ROC curve redefined — optimizing sensitivity (and specificity) to the lived reality of cancer. N Engl J Med. 2019;380(17):1594–1595. 10.1056/NEJMp1814951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3


