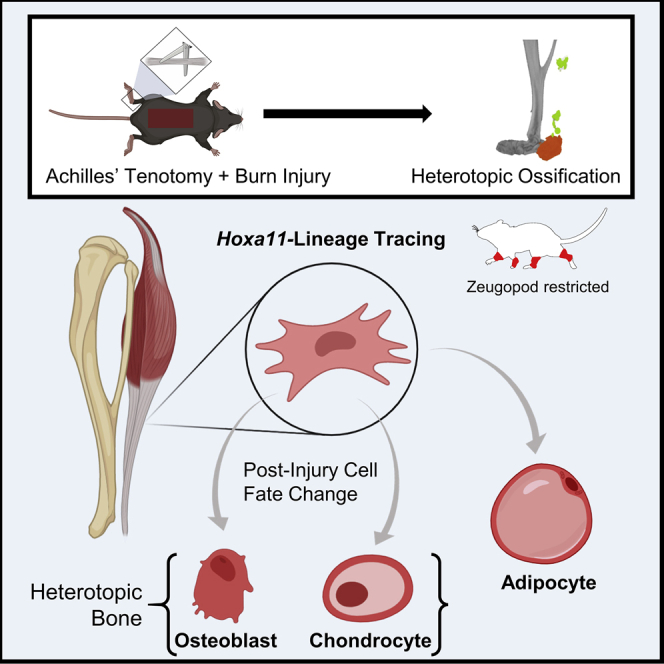
Summary
Heterotopic ossification (HO) is a form of pathological cell-fate change of mesenchymal stem/precursor cells (MSCs) that occurs following traumatic injury, limiting range of motion in extremities and causing pain. MSCs have been shown to differentiate to form bone; however, their lineage and aberrant processes after trauma are not well understood. Utilizing a well-established mouse HO model and inducible lineage-tracing mouse (Hoxa11-CreERT2;ROSA26-LSL-TdTomato), we found that Hoxa11-lineage cells represent HO progenitors specifically in the zeugopod. Bioinformatic single-cell transcriptomic and epigenomic analyses showed Hoxa11-lineage cells are regionally restricted mesenchymal cells that, after injury, gain the potential to undergo differentiation toward chondrocytes, osteoblasts, and adipocytes. This study identifies Hoxa11-lineage cells as zeugopod-specific ectopic bone progenitors and elucidates the fate specification and multipotency that mesenchymal cells acquire after injury. Furthermore, this highlights homeobox patterning genes as useful tools to trace region-specific progenitors and enable location-specific gene deletion.
Keywords: mesenchymal progenitors, heterotopic ossification, Hoxa11, aberrant differentiation, tendon
Graphical Abstract
Highlights
-
•
Lineage tracing, single-cell RNA-seq and single cell ATAC enable cell specific analysis of in vivo cell fate
-
•
Hoxa11 lineage marks distinct mesenchymal precursors in the zeugopod
-
•
Hoxa11 lineage mesenchymal precursors undergo an aberrant cell fate change towards ectopic bone and cartilage
Here, Pagani et al. use the Hoxa11 patterning gene, expressed exclusively in the limb zeugopod, to demonstrate by bioinformatic single cell transcriptomic, epigenomic analyses, and ex vivo confocal microscopy that extra-skeletal cells originating from Hoxa11 lineage cells are a population of mesenchymal cells with the ability to undergo aberrant differentiation towards chondrocytes, osteoblasts or adipocytes after injury
Introduction
The correct programming of adult precursor cells is critical to enable normal wound healing after injury. Burn injuries, soft-tissue wounds, and fractures all require a highly orchestrated response of inflammatory, vascular, neural, fibroblast, and mesenchymal stem/progenitor/precursor cells (MSCs) to coordinate proper healing. However, in some cases of severe trauma, proper precursor cell programming can be maladaptive, causing pathological healing. This altered programming of precursor cells is manifested in the process of heterotopic ossification (HO). HO is characterized by aberrant differentiation of adult tissue-resident MSCs that are ectopic from the adult skeleton. Recent studies have begun to elucidate bone progenitor cells during normal bone development and repair. The process by which tissue-resident mesenchymal cells undergo aberrant osteogenic and chondrogenic differentiation, however, has yet to be defined (Agarwal et al., 2016a, 2017a; Chan et al., 2018; Comazzetto et al., 2019; Genet et al., 2015; Hsieh et al., 2019; Hwang et al., 2019; Matsushita et al., 2020; Torossian et al., 2017; Wang et al., 2018).
In recent years, Cre/LoxP-based lineage-tracing approaches have provided the technical ability to follow the fates of stably marked native cell populations within the injury site and assess these cells' osteogenic capacity (Agarwal et al., 2016a; Kan et al., 2018). Although lineage studies have characterized HO as an endochondral process (Foley et al., 2018), studies have yet to elucidate whether several cell populations differentiate into aberrant cartilage and bone or whether HO is derived from one progenitor population. Mouse lines that mark local MSCs have been used to study HO (Agarwal et al., 2016b, 2017b; Dey et al., 2016; Kan et al., 2013, 2018). However, these Cre models have limitations. For example, Prrx1Cre marks the entire lateral plate mesoderm, and each Cre allele is found at many sites outside of the musculoskeletal system (Logan et al., 2002). Given the lack of specificity, use of these Cre systems will not precisely identify the cells likely playing a role in bone formation or in homeostasis (Agarwal et al., 2016a). Cre systems have also been used to conditionally delete genes during development; however, this can lead to native skeletal abnormalities such as shortened limbs or altered bone mineral density, introducing confounding variables into the measurement of ectopic bone. Therefore, inducible systems are preferred. The available alleles that mark MSCs include PdgfraCreER, Gli1CreER, and ScxCreER lines; however, these alleles also mark MSCs in tissues outside of the extremities (Li et al., 2018; O'Rourke et al., 2016; Qian et al., 2017; Sugimoto et al., 2013b; Zhao et al., 2015). Importantly, these cell lineages are not specific to one anatomic region, making it difficult to interpret any lineage specific gene-deletion studies.
Recent studies have utilized the embryonic patterning gene Hoxa11 to mark the progenitor cells responsible for fracture healing (Pineault et al., 2019). MSCs are mesoderm-derived and Hox genes have been shown to be expressed in a region-specific manner in these cells (Pineault et al., 2019). Hoxa11 is expressed specifically in the zeugopod, the radius-ulna and tibia-fibula region of the limb. Hoxa11-CreERT2;ROSA26-LSL-TdTomato lineage-trace reporter mouse model marks Hoxa11-lineage cells during skeletal development and growth. At 24 h following tamoxifen induction at postnatal day 3, Hoxa11-lineage cells are found within perichondrium at the distal growth plate, periosteal, endosteal, and trabecular bone surfaces. After 8 weeks, these cells are found in the periosteum, endosteum, and bone marrow space (Pineault et al., 2019). However, Hoxa11-expressing cells are not isolated to the skeleton. It was found that Hoxa11 cells expressing endogenous GFP are located in tendon and muscle interstitial tissue at embryonic day 14.5, yet it remains unclear whether these cells persist in the adult tendon and muscle (Pineault et al., 2019). Furthermore, using Hoxa11-CreERT2;ROSA26-LSL-TdTomato mice, it was shown that TdTomato+ (Hoxa11iTom+) MSCs were marked prior to Sox9CreER and OsxCreER lineage-marked cells, indicating that these Hoxa11-expressing cells serve as progenitors for osteoblastogenesis and chondrogenesis (Pineault et al., 2019). These results suggest that Hoxa11 marks the primitive MSCs that may be responsible for the formation of aberrant ectopic bone.
In the current study, we utilize a burn/tenotomy (BT) injury model in Hoxa11-CreERT2;ROSA-LSL-TdTomato mice to identify, lineage trace, and analyze the cell fate of the HO progenitor cells. This model reproducibly results in HO formation, which allows for the evaluation of the precursor cells responsible for aberrant bone formation. Using single-cell RNA sequencing (scRNA-seq) and single-nucleus assay for transposase-accessible chromatin using sequencing (snATAC-seq), we were able to specifically identify and analyze MSCs marked by TdTomato transcript and MSCs not marked by TdTomato. We found that TdTomato-expressing MSCs were also identified by markers of chondrocytes and osteoblasts. Using trajectory analyses, we identify the TdTomato+ population responsible for chondrogenic and osteogenic differentiation. Our findings also suggest that these cells can differentiate into adipocytes following injury. Our results demonstrate that Hoxa11-lineage cells are a unique population of precursor cells that undergo an aberrant fate change toward a chondrogenic and osteogenic cell type.
Results
Hoxa11-lineage cells express genes associated with mesenchymal cells at baseline and after injury
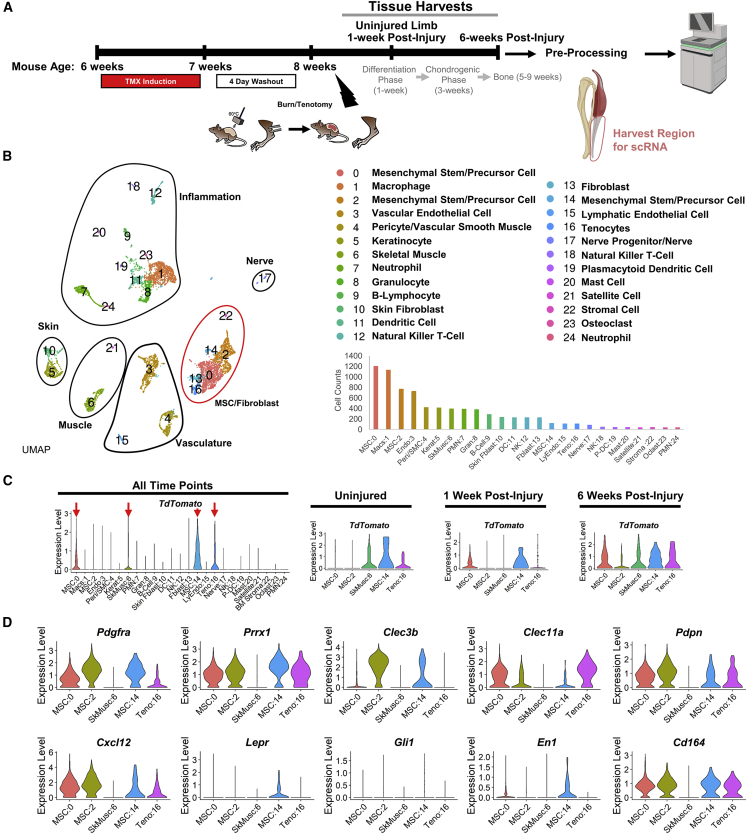
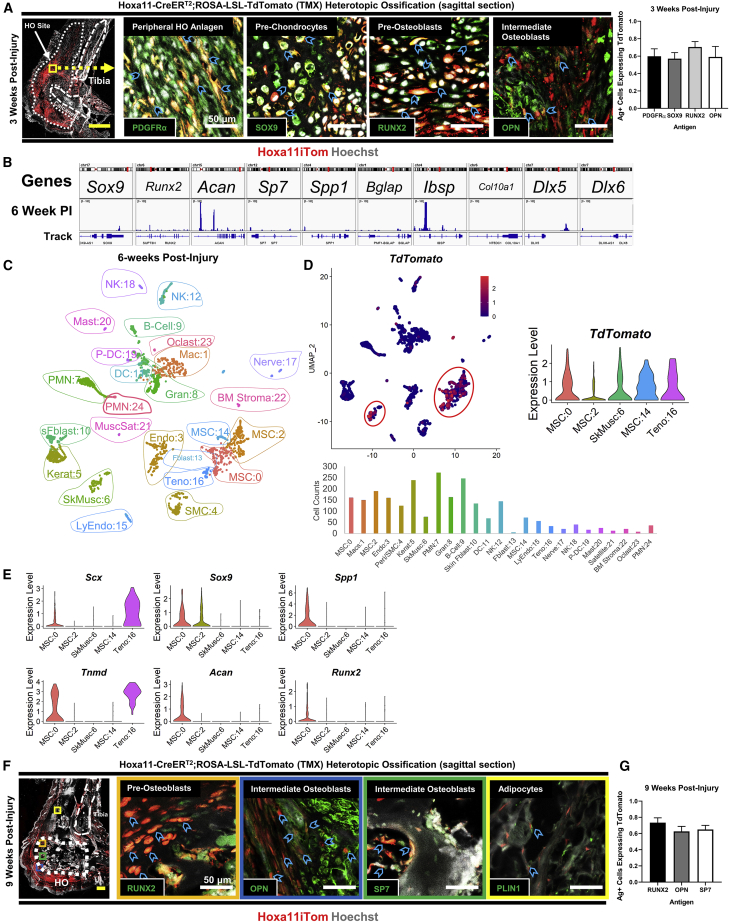
To characterize the cells at the HO site, we performed scRNA-seq on cells harvested from the distal hindlimb of an uninjured mouse, injured left hindlimb 1 week after BT injury, and injured hindlimb 6 weeks after BT injury of Hoxa11-CreERT2;ROSA-LSL-TdTomato mice to determine dynamic molecular changes in the Hoxa11-lineage cells (Figure 1A). Specifically, the Achilles’ tendon and surrounding soft tissue posterior to the tibia and fibula were harvested (Figure 1A). Native bone, such as the tibia, fibula, tarsals, metatarsals, and phalanges, were not harvested for analysis. Twenty-five unique clusters were created by UMAP analysis. Clusters were identified by uniquely expressed upregulated genes (Figure 1B and Table S1). Cell-cycle phase, G2/M score, and S score were calculated (Tirosh et al., 2016) (Figure S1A). Because we used Hoxa11-CreERT2;ROSA-LSL-TdTomato mice, expression of the TdTomato RNA transcript was used to identify clusters of the Hoxa11 lineage. Combined analysis of all conditions and time points identified the following clusters as TdTomato expressing: MSC:0, SkMusc:6, MSC:14, and Teno:16 (Figure 1C). Following individual time-point analysis, cluster MSC:2 also showed TdTomato expression at the 6-week time point (Figure 1C). These five clusters were the focus of our study due to their expression of the TdTomato transcript marking them as Hoxa11-lineage cells. Additional genes frequently used to study bone progenitors were included for comparison. SkMusc:6 showed no expression of bone progenitor lineage markers. Pdgfra and Prrx1 mark mesenchymal progenitor cells (Miwa and Era, 2018), and were expressed by clusters MSC:0, MSC:2, MSC:14, and Teno:16 (Figure 1D). Clec11a, which has known roles in osteogenic differentiation and bone fracture healing (Yue et al., 2016), was also expressed in clusters MSC:0, MSC:2, MSC:14, and Teno:16 (Figure 1D). Cxcl12, a marker commonly used for hematopoietic stem cells (Ding and Morrison, 2013) and osteoblasts (Shahnazari et al., 2013), was expressed in clusters MSC:0, MSC:2, MSC:14, and Teno:16 (Figure 1D). Clec3b, previously shown to be important in mineralization (Iba et al., 2001), was expressed in MSC:0, MSC:2, and MSC:14 (Figure 1D). Of the Hoxa11 lineage clusters, only MSC:14 displayed Lepr expression (Figure 1D). Lepr encodes the leptin receptor and is frequently used as a marker for bone marrow mesenchymal stem cells (Zhou et al., 2014), suggesting that the MSC:14 cells are of bone origin (Figure 1D). En1, which is important in cranial morphogenesis and calvarial defect healing (Deckelbaum et al., 2012), showed expression in MSC:0 and MSC:14 (Figure 1D). Interestingly, Gli1, a marker found to be important in bone fracture healing (Shi et al., 2017), showed no expression in TdTomato-expressing clusters in the combined time-point analysis (Figure 1D). Lastly, two markers that have been used to mark multipotent skeletal stem cells in humans, Pdpn and Cd164, were expressed in MSC:0, MSC:2, MSC:14, and Teno:16 (Chan et al., 2018; Walmsley et al., 2015) (Figure 1D). These findings suggest that Hoxa11-lineage cells may be responsible for forming ectopic bone following injury; however, it remains unclear how these cells progress from normal physiology toward an aberrant cell fate.
Figure 1.
Combined time-point analysis of scRNA sequencing shows distinct TdTomato-expressing clusters
(A) Experimental schematic showing injury model and cells harvested in the uninjured limb (n = 1), 1 week following BT injury (n = 1), and 6 weeks following BT injury (n = 2) from Hoxa11-CreERT2:ROSA-TdTomato mice that were processed and sequenced. Mouse schematic adapted from our group's previous work (Agarwal et al., 2016a).
(B) UMAP cluster, cluster definitions, and cell counts of combined time-point analysis.
(C) Violin plots of combined time-point and individual time-point TdTomato expression.
(D) Violin plots of gene markers for mesenchymal and bone progenitors.
Hoxa11-lineage cells turn on transcriptional profiles associated with osteogenic and chondrogenic cell states during HO progression
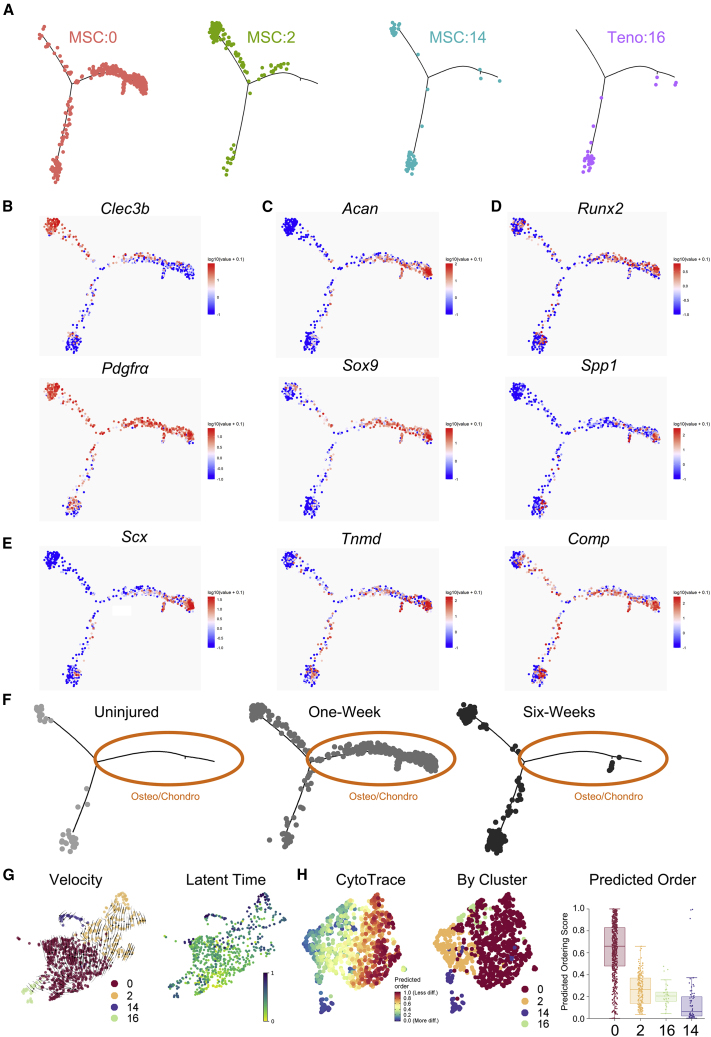
Following initial scRNA-seq analyses, pseudo-time analysis was used to determine how the transcriptional profiles of Hoxa11-lineage cells were changing across the conditions and time points (uninjured, 1 week, and 6 weeks). Cells expressing over at least one count of the TdTomato transcript within the four mesenchymal TdTomato-expressing clusters were used for pseudo-time analyses (Figure 2A). SkMusc:6 was excluded from the analysis as it formed a separate trajectory (Figure S2). Three major branches were identified by visualizing gene expression across the trajectory: a precursor-like state characterized by Pdgfra and Clec3b (Figure 2B), a combined osteogenesis/chondrogenesis-like state characterized by bone (Spp1, Runx2), chondrocyte (Sox9, Acan), and tenocyte (Scx, Tnmd) differentiation markers (Figures 2C–2E), and lastly, a branch characterized by tendon markers Scx, Tnmd, and Comp (Figure 2E). Interestingly, Pdgfra expression is maintained in the osteo/chondro branch of the analysis. Tendon markers Scx, Tnmd, and Comp are expressed in the osteo/chondro branch, suggesting the Hoxa11-lineage cells that comprise HO have tendon origin, which is consistent with previous work (Agarwal et al., 2017b). Importantly, cluster MSC:0 was the only cluster that occupies the terminal end of the osteo/chondro branch (Figure 2A), suggesting that this unique cluster of cells differentiates into heterotopic bone. Acan and Fbn2 were uniquely and highly expressed in MSC:0. Confocal microscopy images showed co-localization of TdTomato fluorophore with ACAN and FBN2 protein in the HO anlagen 1 week following injury (Figure S3). When the trajectory was separated by time after injury, there were no cells occupying the osteo/chondro branch in the uninjured sample, while many cells occupied this branch of the analysis 1 week post injury. A small population, which occupied a small separate branch on the trajectory, remained at 6 weeks (Figure 2F). Few cells are likely found at the 6-week time point as they are entrapped in HO bone and difficult to isolate for scRNA-seq. This dynamic analysis highlights that Hoxa11-lineage cells express osteogenic and chondrogenic gene differentiation markers only after injury (Figure 2F).
Figure 2.
Trajectory analysis of TdTomato+ cells shows osteo/chondro branch following traumatic injury
(A) Monocle 2 pseudo-time analysis separated by TdTomato-expressing cluster.
(B) Feature plots of Pdgfra and Clec3b gene expression levels.
(C) Feature plots of chondrocyte differentiation genes Acan and Sox9 expression levels.
(D) Feature plots of osteoblast differentiation genes Runx2 and Spp1 expression levels.
(E) Feature plots of tendon differentiation genes Scx, Tnmd, and Comp expression levels.
(F) Pseudo-time plots separated by time point with osteo/chondro branch circled in orange.
(G) UMAP steam plot of RNA velocity of TdTomato+ cells in clusters MSC:0, MSC:2, MSC:14, and Teno:16 (left) with arrows showing velocity and UMAP latent time plot (right).
(H) CytoTrace analysis of TdTomato-expressing cells including a UMAP plot with predicted ordering score (left), a UMAP plot colored by cluster (center), and a box plot showing predicted score of clusters (right).
To delineate the lineage kinetics in the MSCs, we performed trajectory analysis using the estimated RNA velocity of TdTomato-positive cells in clusters MSC:0, MSC:2, MSC:14, and Teno:16 (La Manno et al., 2018; Bergen et al., 2020) (Figure 2H). The vectors of individual estimated velocity indicated the origin as cluster MSC:0 with a dual outflow toward MSC:2 and Teno:16. Moreover, there was a minor bifurcation in MSC:2 that pointed toward MSC:14 (Figure 2H). We further inferred the position of cells in the fate commitment process by computing the latent time. The results showed a comparable origin in MSC:0 with four distinct endpoints terminated in MSC:0, MSC:2, MSC:14, and Teno:16 (Figure 2H). Cell-cycle scores, feature counts, and RNA counts were also visualized on the trajectory (Figure S1B). RNA features increased along the right branch of the trajectory. CytoTrace is a trajectory analysis that considers variations in RNA features as a factor in determining differentiation state (Gulati et al., 2020). MSC:0 was the least differentiated compared with other MSC clusters based on CytoTrace predicted ordering (Figure 2H). When taken together, velocity, CytoTrace, and pseudo-time analyses suggest that MSC:0 is able to differentiate into chondrogenic and osteogenic lineages compared with more differentiated MSC:2, MSC:14, and Teno:16 clusters.
To provide a clearer picture of how Hoxa11-lineage cells transition throughout the progression of HO, we utilized individual time-point analyses of snATAC, scRNA, and immunofluorescence (IF) microscopy.
Hoxa11-lineage cells do not express markers of osteogenesis or chondrogenesis in uninjured leg
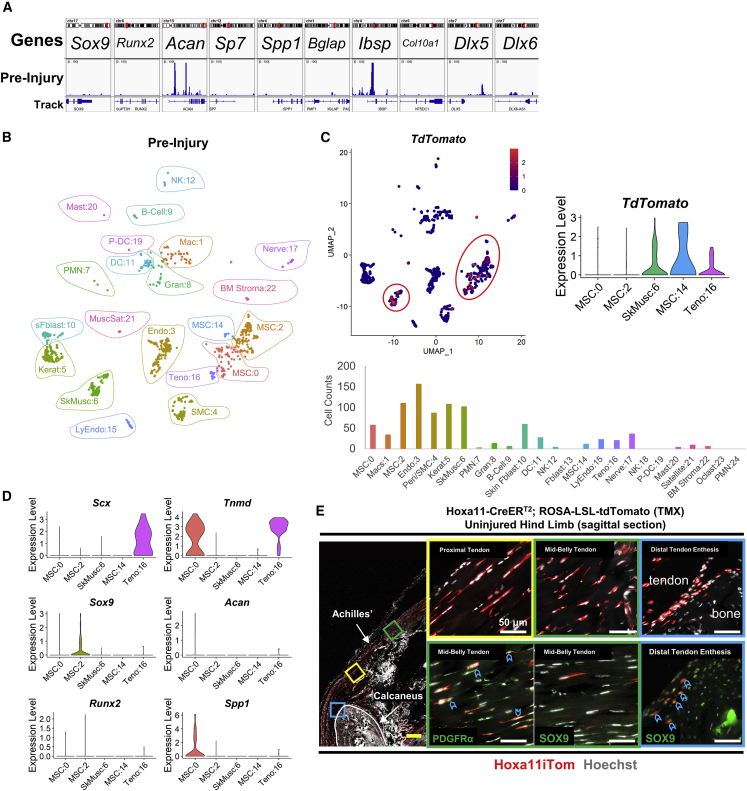
snATAC was utilized to characterize the chromatin states of genes important in chondrogenic and osteogenic differentiation. Euchromatin, or “open” chromatin, is important for the transcription of gene loci. snATAC is specifically useful for isolating unique clusters of cells mapped from the scRNA-seq—a technique not available to bulk ATAC. Cluster MSC:0 was the focus of snATAC-seq as this cluster likely contains the cells that differentiate into HO, based on genetic expression at the 1-week post-injury time point. MSC:0 cells showed very low levels of open chromatin reads near promoters of genes important in chondrogenesis and osteogenesis in the uninjured limb (Figure 3A). Chondrogenic gene Sox9 had a low number of open chromatin reads near promoter regions, suggesting decreased expression (Figure 3A). Chondrogenic gene Acan had a higher level of open chromatin reads; however, it was not expressed in scRNA-seq data (Figures 3A and 3D). Genes Dlx5 and Dlx6, which are critical in mammalian skeletal development (Robledo et al., 2002), and Runx2 had low levels of open chromatin regions (Figure 3A). Later-stage osteoblast differentiation genes Sp7 and Spp1 (Komori, 2006, 2017) also had low levels of open chromatin reads. Cells of MSC:0 showed open chromatin regions within and surrounding bone matrix protein-encoding gene Ibsp, but not Bglap (Komori, 2010) (Figure 3A). As open chromatin is integral for the transcription of genes, this suggests that in the uninjured limb Hoxa11-lineage cells are not primed to differentiate toward chondrocytes and osteoblasts. While useful, accessibility to open promoter regions is not the only determinant of gene transcription (Chereji et al., 2019). To account for this, we also characterized the Hoxa11-lineage cells in an uninjured limb by analyzing the scRNA transcriptome profiles of Hoxa11-lineage clusters and IF data.
Figure 3.
Hoxa11-lineage cells are found within tendon and enthesis and do not express chondro/osteo differentiation genes in the uninjured limb
(A) SnATAC-seq of TdTomato-expressing cluster MSC:0 in the uninjured limb (n = 1). Genes shown are important in chondrogenesis and osteogenesis. Bars represent mean value of reads.
(B) UMAP plot of cells in the uninjured limb.
(C) UMAP feature plot depicting TdTomato expression in the uninjured limb (top left), violin plot showing TdTomato expression across clusters (top right, from Figure 1C), and cell counts across all clusters (bottom).
(D) Violin plots of genes necessary in differentiation of tenocytes (top), chondrocytes (middle), and osteoblasts (bottom).
(E) Confocal microscopy images of the uninjured hindlimb with tile scan (left) and 63× total zoom inset images of regions of interest (n = 2 mice/antigen of interest, n = 3 images/mouse). Colored boxes in tile show region of inset. Sections were immunolabeled with PDGFRα and SOX9 (bottom). Blue chevrons mark Hoxa11iTom cells co-labeled with specified antibodies. Yellow scale bar, 500 μm; white scale bars, 50 μm.
We found that the majority of TdTomato expression was limited to SkMusc:6, MSC:14, and Teno:16, while MSC:0 and MSC:2 showed minimal expression (Figures 3B and 3C). Scx expression was only expressed in Teno:16, while Tnmd expression was expressed in both MSC:0 and Teno:16. Scx regulates Tnmd expression (Shukunami et al., 2018), which suggested that in the uninjured limb, Teno:16 was more stem in nature. Chondrogenic and osteogenic genes showed little to no expression within Hoxa11-lineage clusters in the uninjured limb (Figure 3D). These findings suggest that in the uninjured limb, Hoxa11-lineage cells are within tendon and muscle but are not primed for aberrant osteogenic and chondrogenic cell fate.
The location and expression of Hoxa11-lineage marking by the TdTomato fluorophore was confirmed by confocal microscopy (Figure 3E). TdTomato expression was limited to the limb zeugopod following BT injury (Figure S4). Expression was found throughout the Achilles' tendon as well as at the tendon enthesis. IF labeling of mesenchymal progenitor cell marker PDGFRα showed co-localization of TdTomato within the Achilles' tendon. Histological sections were also immunolabeled by SOX9, which only showed expression at the tendon enthesis, not the mid-belly tendon (Figure 3E), which is consistent with previous studies (Sugimoto et al., 2013a). SOX9 immunolabeling also co-localized with TdTomato+ cells at the tendon enthesis.
Hoxa11-lineage cells within tendon enter an osteogenic and chondrogenic state following injury
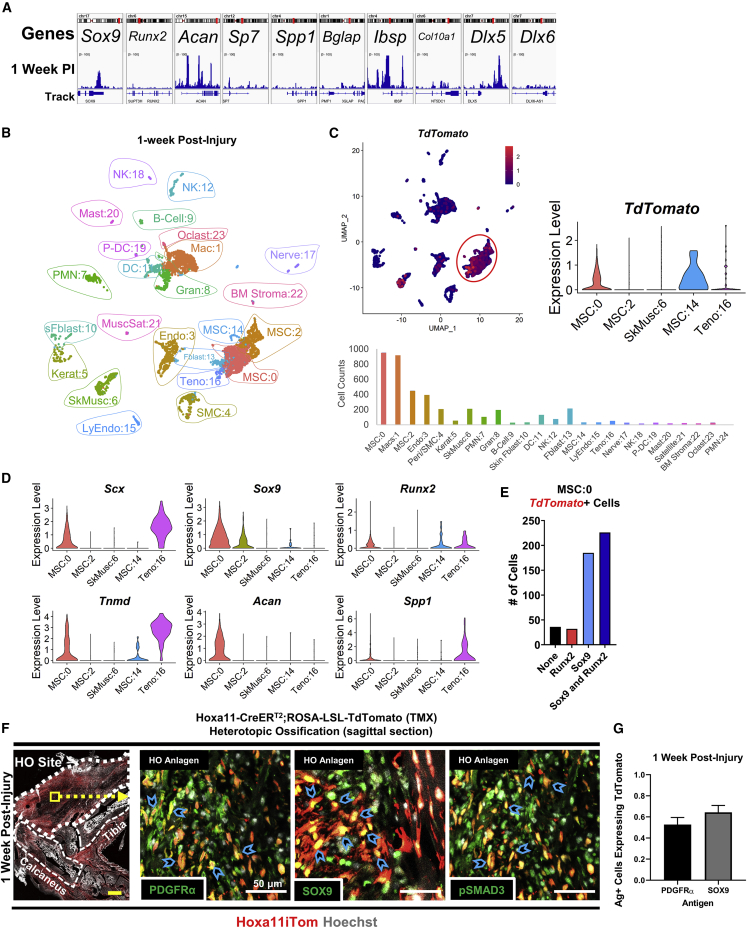
To delineate the gene expression changes after injury, we analyzed snATAC data and scRNA data of the 1-week post-injury cells in the TdTomato-expressing cluster MSC:0 (Figures 4A and 4B). SnATAC showed increased levels of open chromatin at chondrogenic genes Sox9, Acan, and Col10a1 (Figure 4A). Osteogenic differentiation genes Runx2, Sp7, Bglap, Ibsp, Spp1, Dlx5, and Dlx6 all showed increased levels of open chromatin reads (Figure 4A). In the scRNA data, TdTomato expression was altered following injury, expressed mainly in clusters MSC:0, MSC:14, and at very low levels in Teno:16 (Figure 4C). MSC:0 was characterized by Scx, Tnmd, Sox9, Acan, Runx2, and Spp1 (Figure 4D). There was no difference in Sox9 expression in MSC:2 between the uninjured and injured hindlimb. Interestingly, osteogenic differentiation markers showed increased expression in MSC:14 and Teno:16 following injury (Figure 4D).
Figure 4.
Hoxa11 marks a unique osteo/chondro/teno cell type following injury
(A) snATAC-seq of TdTomato-expressing cluster MSC:0 1 week post injury. Genes shown are important in chondrogenesis and osteogenesis. Bars represent mean value of reads.
(B) UMAP plot of cells 1 week following BT injury.
(C) UMAP feature plot depicting TdTomato expression (top left), violin plot showing TdTomato expression at 1 week following BT injury for reference (top right, from Figure 1C), and cell counts of all clusters (bottom).
(D) Violin plots of genes necessary in differentiation of tenocytes (left), chondrocytes (middle), and osteoblasts (right).
(E) Bar chart visualizing the number of TdTomato+ cells in MSC cluster 0 expressing Sox9, Runx2, Sox9 and Runx2, or neither.
(F) Confocal microscopy images of the hindlimb 1 week following BT injury with tile scan (n = 2 mice/antigen of interest, n = 3 images/mouse) (left). Dotted line shows the HO site while dashed line shows labeled calcaneus and tibia bones. The yellow box highlights region of 63× total zoom inset images (right). Sections were immunolabeled with PDGFRα, SOX9, and pSMAD3 (bottom). Blue chevrons mark Hoxa11iTom cells co-labeled with specified antibodies. Yellow scale bar, 500 μm; white scale bars, 50 μm.
(G) Bar chart showing mean with SEM of cell-count quantification of antigen-expressing cells co-labeled with TDTOMATO fluorophore.
Cells were also scored by their expression of Sox9, Runx2, both Sox9 and Runx2, or neither, to determine whether cells in MSC:0 are undergoing an endochondral ossification process whereby chondrocytes are formed first, which are replaced by osteoblasts, or whether the cells are differentiating directly toward osteoblasts, a process more commonly found in intramembranous bone formation. Of all TdTomato+ MSC:0 cells at the 1-week time point (N = 479), 7% expressed only Runx2 (n = 32), 47% expressed both Sox9 and Runx2 (n = 226), 39% expressed only Sox9 (n = 185), and 8% had no expression of either (n = 36) (Figure 4E). This suggests that a small percentage of the cells within this cluster differentiate directly toward osteoblasts by the 1-week time point and contribute to HO through intramembranous ossification, while the remaining cells progress through endochondral ossification.
IF labeling of PDGFRα, SOX9, and pSMAD3, signal transducer of the canonical transforming growth factor-β pathway that is critical for HO formation (Sorkin et al., 2020; Wang et al., 2018), was performed within the HO anlagen at the injury site at the 1-week time point. All antigens showed co-localization with TdTomato fluorophore marking the Hoxa11-lineage cells (Figure 4F). PDGFRα+ TDTOMATO+ cells showed elongated morphology, suggesting a predifferentiated state, while SOX9+ TdTomato+ cells had rounded, condensed morphology consistent with chondrocytes. Over half of the PDGFRα- and SOX9-expressing cells were marked by the TDTOMATO fluorophore (Figure 4G). These results suggest that at the 1-week time point, Hoxa11-lineage cells begin to differentiate toward heterotopic bone.
Hoxa11-lineage cells differentiate into mature ectopic bone
Finally, to determine whether Hoxa11-lineage cells differentiate and contribute to the ectopic bone formation seen after injury, we analyzed IF labeling at weeks 3 and 9 as well as the snATAC and scRNA transcriptome data from MSCs that were derived from cells 6 weeks post injury (Figure 5).
Figure 5.
Hoxa11-lineage cells differentiate into ectopic chondrocyte, osteoblasts, and adipocytes
(A) Confocal microscopy images of the hindlimb 3 weeks following BT injury, including tile scan image with dashed lines marking native bone and dotted line marking HO (left) and 63× insets of region of interest (n = 2 mice/antigen of interest, n = 3 images/mouse). Sections were immunolabeled with indicated antibodies (bottom). Blue chevrons mark Hoxa11iTom cells co-labeled with specified antibodies. Yellow scale bar, 500 μm; white scale bars, 50 μm. Right: bar chart showing mean with SEM of cell-count quantification of antigen-expressing cells co-labeled with TDTOMATO fluorophore.
(B) snATAC-seq of TdTomato-expressing cluster MSC:0 6 weeks post injury. Genes shown are important in chondrogenesis and osteogenesis. Bars represent mean value of reads.
(C) UMAP plot of cells 6 weeks following BT injury.
(D) UMAP feature plot depicting TdTomato expression with clusters expressing TdTomato circled in red (top left), violin plot showing TdTomato expression 6 weeks following BT injury for reference (top right, from Figure 1C), and cell counts across all clusters (bottom).
(E) Violin plots of genes necessary in differentiation of tenocytes (left), chondrocytes (middle), and osteoblasts (right).
(F) Confocal microscopy images of the hindlimb 9 weeks (bottom) following BT injury with tile scan for reference (n = 2 mice/antigen of interest, n = 3 images/mouse) (left). Dotted line shows the HO site while dashed line shows labeled tibia bone. Calcaneus not labeled at 9-week time point as it is surrounded by heterotopic bone. The colored boxes highlight regions of 63× total zoom inset images (right). Sections were immunolabeled with indicated antibodies. Blue chevrons mark Hoxa11iTom cells co-labeled with specified antibodies. Yellow scale bar, 500 μm; white scale bars, 50 μm.
(G) Bar chart showing mean with SEM of cell-count quantification of antigen-expressing cells co-labeled with TDTOMATO fluorophore.
IF labeling and imaging performed at the 3-week time point demonstrated that Hoxa11-lineage cells differentiated into an osteogenic/chondrogenic state. PDGFRα co-localized with TDTOMATO-expressing cells near the periphery of condensing HO anlagen (Figure 5A). SOX9 expression was paired with chondrocyte morphology of TDTOMATO-expressing cells within the HO anlagen (Figure 5A). Osteoblast markers RUNX2 and OPN co-localized with TDTOMATO expression within the HO anlagen as well at the 3-week time point (Figure 5A). Over 50% of the antigen-expressing cells were marked by the Hoxa11-lineage tracer at 3 weeks (Figure 5A).
Analysis of snATAC data showed that while the mean reads of open chromatin had decreased since the 1-week time point, chondrogenic and osteogenic gene loci remained partially open (Figure 5B).
Analysis of the scRNA transcriptome of the TdTomato-expressing clusters post injury demonstrated TdTomato expression in all five clusters at 6 weeks (Figures 5C and 5D). Scx and Tnmd were expressed in Hoxa11-lineage MSC:0 and Teno:16 at 6 weeks; however, chondrogenic and osteogenic differentiation gene expression was limited to MSC:0 (Figure 5E). While Sox9 was expressed in MSC:2 at 6 weeks post injury, the expression level remained constant throughout the uninjured, 1-week post-injury, and 6-week post-injury samples (Figures 5E, 3D, and 4D). The osteogenic and chondrogenic genes that were upregulated in MSC:14 and Teno:16 at the 1-week post-injury time point resolved back to the uninjured baseline at 6 weeks post injury, while MSC:0 maintained osteogenic and chondrogenic expression (Figure 5E). These results suggest that MSC:0 has formed the heterotopic bone and remains chondrogenic and osteogenic.
IF labeling at the 9-week time point, when mature bone is present, showed Hoxa11-lineage TdTomato-expressing cells within fully formed ectopic bone. Near the periphery of the ectopic bone, TdTomato+ cells were marked by RUNX2 (Figure 5F). OPN and SP7 marked TdTomato+ cells and were entrapped within the mature HO (Figure 5F). Lastly, TdTomato+ cells were co-localized with PLIN1, a lipid support protein within adipocytes, suggesting that Hoxa11-lineage cells can differentiate into adipocytes. This is also evidenced by snATAC results of adipogenic genes in MSC:0 cells (Figure S5). At the 9-week time point, over 60% of antigen-expressing cells were marked by the Hoxa11 TDTOMATO lineage tracer (Figure 5G). These results confirmed that Hoxa11-lineage cells differentiated into ectopic bone following injury through a paired chondrogenic and osteogenic phenotype.
Discussion
HO is a tightly orchestrated process regulated by various transcription factors. The expression of Hox genes and proteins help define regional formation of structures throughout development. However, recent studies have found that Hox genes may play a role that extends beyond embryonic patterning and formation of the musculoskeletal system in murine limbs, specifically within the limb zeugopod (Pineault et al., 2019; Rux et al., 2016; Swinehart et al., 2013). Hoxa11-lineage cells were found to be self-renewing and persistent throughout embryonic development and adulthood, serving a critical role in fracture repair (Pineault et al., 2019).
In the current study, we investigated whether cells that were previously described as self-renewing Hoxa11-lineage adult skeletal progenitor-enriched MSCs (Pineault et al., 2019) were involved in the aberrant cell fate and ectopic bone formation after traumatic injury. To analyze this, we used a Hoxa11 lineage-tracing mouse line in conjunction with a mouse model of traumatic injury in the zeugopod. We found that in the uninjured limb, Hoxa11-lineage cells were located within tendon and muscle and were not primed to differentiate into cartilage or bone. TdTomato expression in muscle fibers is a novel finding; however, it is outside the scope of this work. Previous research has identified Hoxa11eGFP expression within tendon but not muscle fibers (Rux et al., 2016; Swinehart et al., 2013); however, they did not use the lineage-tracing model used in this study. Previous research has found that local MSCs differentiate into HO and contribute to wound healing (Agarwal et al., 2017b; Dey et al., 2016; Eming et al., 2014). These cells are already present and awaiting signals from the surrounding tissue to undergo proliferation and differentiation to repair the injured tissue, rather than stemming from circulation (Loder et al., 2018). After injury, we found upregulation of osteogenic and chondrogenic genes in the Hoxa11-lineage cells as well as increased levels of open chromatin in genes responsible for osteochondral MSC differentiation. After 9 weeks, osteoblasts were positive for the TDTOMATO reporter, demonstrating that Hoxa11-lineage cells were responsible for the ectopic bone formation after injury.
The use of scRNA transcriptome analysis of cells from Hoxa11-lineage reporter in the uninjured limb and 1 week and 6 weeks after injury allowed us to identify five clusters that expressed the TdTomato reporter gene. Furthermore, this system allowed us to track the differentiation of these cells after trauma. Hoxa11-lineage clusters were very heterogeneous, containing markers that have previously been reported to mark cells contributing to the formation of healed bone and ectopic bone. Four clusters—MSC:0, MSC:2, MSC:14, and Teno:16—expressed the mesenchymal markers Pdgfra and Prrx1 (Agarwal et al., 2016a; Farahani and Xaymardan, 2015). Additionally, we found genes related to osteoblasts and osteocyte differentiation including Cxcl12 (Ding and Morrison, 2013; clusters MSC:0, MSC:2, MSC:14, and Teno:16), Clec11a (Yue et al., 2016; clusters MSC:0, MSC:2, MSC:14, and MSC:16), Clec3b (Iba et al., 2001; clusters MSC:0, MSC:2, and MSC:14), and En1 (Deckelbaum et al., 2012; clusters MSC:0 and MSC:14). Furthermore, we noted expression of Pdpn and Cd164 in clusters MSC:0, MSC:2, MSC:14, and Teno:16 that have been described to mark multipotent human skeletal stem cells (Chan et al., 2018; Walmsley et al., 2015). Although characterization of bone progenitor cells has posed a challenge due to overlapping and conflicting findings (Ono et al., 2019), our results identified previously described marker gene expression found within the Hoxa11-lineage cells within our single-cell dataset. Leptin receptor, which is expressed by the gene Lepr, has been previously used to mark bone marrow mesenchymal stromal cells that regenerate bone following injury (Zhou et al., 2014) and was expressed in MSC:14. This is particularly interesting, as these cells were previously known to reside within the bone marrow. As the cells that were sequenced by scRNA were harvested from the Achilles' tendon and surrounding soft tissue, purposefully excluding cells of the bone marrow, this would suggest that a population of Lepr+ cells exists outside of the bone marrow prior to injury.
Previous studies have utilized various genetic models to study the cells that contribute to HO, yet these models have had significant limitations, including a lack of region specificity complicating interpretations regarding cell origin. Additionally, the many cell-specific reporters tend to mark terminally differentiated cell types but do not allow the characterization of the more primitive cells of interest. Cre mouse lines Prrx1Cre, Nfatc1Cre, Glast1Cre, Gli1Cre, and ScxCre, while useful in marking specific HO progenitor cells that eventually form HO, do not mark the most primitive MSCs (Agarwal et al., 2016b, 2017b; Dey et al., 2016; Kan et al., 2013, 2018). Thus, there remains a need to develop a model that reliably marks and traces site-specific, multipotent lineage stem cells that form heterotopic bone. In this study, we characterized a novel inducible Hoxa11 mouse line that marks zeugopod MSCs and traced these cells through ectopic bone formation. The Hoxa11 cells expressed chrondrogenic marker SOX9 and early osteogenic markers RUNX2 and OPN shortly after BT injury. A later osteogenic marker, SP7, was observed after bony growth was observed. These findings suggest that the Hoxa11-CreERT2 mouse is an effective and reliable method to mark progenitor cells to evaluate the pathogenesis of HO restricted to MSCs in the zeugopod.
There are several benefits to the Hoxa11-CreERT2 model for studying HO formation. Performing experiments with the BT mouse model within the Hoxa11-CreERT2;ROSA26-LSL-TdTomato mice allows for consistent and reliable visualization of cells that differentiate into HO by an endogenous fluorophore, which avoids the complications of IF staining. Furthermore, this mouse line also ensures that cells visualized, or modifications made with the Cre recombinase, are restricted to the zeugopod due to the isolated expression of the Hoxa11 patterning gene.
This system is useful for evaluating other tissue-resident stem cells that give rise to mature tendon or adipose tissue. Lineage-traced Hoxa11 cells were found in abundance in uninjured and injured Achilles’ tendon. Current methods to evaluate the stem cells that differentiate into tendon are limited to ScxCre; however, these cells are classified as differentiated progenitors rather than the more primitive Hoxa11-expressing cells (Yoshimoto et al., 2017), which is consistent with trajectory analyses. We found an Scx+ cluster that expressed TdTomato in the uninjured limb (Teno:16). Following injury, TdTomato and Scx expression were found in MSC:0 as well. Additionally, our study demonstrated the co-localization of TDTOMATO and perilipin, suggesting that Hoxa11 cells likely can give rise to mature adipocytes, which was supported by snATAC findings. This model may also be useful in studying the differentiation of mesenchymal precursors to adipocytes, as AdiponectinCre and PdgfraCre models have both been utilized to study adipogenesis (Lee et al., 2012; Wang et al., 2010) and share similar disadvantages in systemic deletion of genes.
Lastly, the combined chondrogenic and osteogenic cell state provides additional insight into the process by which MSCs differentiate into ectopic bone following injury. These findings suggest that one Hoxa11-lineage cell type, described in this paper as MSC:0, begins in a stem state that, following injury, progresses toward a combined osteogenic and chondrogenic cell, highlighted by the expression of Sox9, Acan, Runx2, and Spp1. Expression of Runx2 at the 1-week time point suggests for the first time that HO is formed by intramembranous ossification (Takarada et al., 2016), which is the ossification directly from preosteogenic conditions, as well as endochondral ossification, which requires a chondrogenic state before ossification.
In conclusion, we utilize the Hoxa11-CreERT2;ROSA-LSL-TdTomato mouse to study the contribution of site-specific MSCs to HO. This validated and reliable model can be used to further study the MSCs responsible for HO, as well as studying stem/precursor cells that contribute to various extremity pathologies within fat, muscle, tendon, and bone. These techniques can be utilized to evaluate the efficacy of pharmacological and therapeutic interventions to limit HO formation.
Experimental procedures
Mouse model
All animal procedures were carried out in accordance with the guidelines provided in the Guide for the Use and Care of Laboratory Animals from the Institute for Laboratory Animal Research (ILAR, 2011), and conducted in compliance with State and Federal law and standards of the US Department of Health and Human Services, and were approved by the Institutional Animal Care and Use Committee of the University of Michigan (PRO0007930). All animals were housed in the University of Michigan's Unit for Laboratory Animal Medicine-supervised, Assessment and Accreditation of Laboratory Animal Care-accredited facilities at 18°C–22°C, on a 12:12-h light/dark cycle with ad libitum access to food and water. Hoxa11-CreERT2;ROSA-TdTomato mice were provided generously by the Wellik Laboratory (University of Wisconsin, Madison, WI) and bred in-house (Pineault et al., 2019).
Additional experimental procedures
Methodology for the tamoxifen induction, BT surgery, scRNA-seq, snATAC-seq, bioinformatics, IF histology, confocal microscopy, and image analysis can be found in Supplemental information.
Author contributions
C.A.P.: Authorship of manuscript, developed experimental design, performed and analyzed IF histology microscopy, performed and interpreted scRNA-seq and snATAC-seq results. A.K.H.: Contributed to manuscript, developed experimental design, executed mouse surgery, isolated and prepared scRNA-seq and snATAC-seq samples. C.H.: Performed IF histology and microscopy, contributed to experimental design. S.M.: Contributed to manuscript and performed bioinformatics analyses. K.P.: Performed snATAC analysis and contributed to manuscript. N.L.: Assisted in tissue sectioning, histology staining, confocal imaging, and image analysis. J.N.: Assisted in histology staining. Y.S.: Assisted in histology staining. Y.-H.C.: Performed Velocyto analysis. N.E.: Assisted in experimental design. N.V.: Performed tissue harvests and sectioning. P.Y.: Performed tissue harvests and sectioning. N.P.: Assisted in IF histology and microscopy. J.G.: Assisted in image quantification. H.R.: Assisted in image quantification. R.N.: Assisted in image quantification. K.K.: Bred and maintained mouse colony. K.V.: General support of laboratory techniques and laboratory management. A.L.S.: Contributed to manuscript. G.E.H.: Contributed to manuscript. J.Y.S.: Provided mouse breeding pairs and contributed to manuscript. D.M.W.: Provided mouse breeding pairs and contributed to manuscript. B.L.: Principal investigator of the laboratory. Developed experimental design and contributed to manuscript.
Acknowledgments
The authors would like to acknowledge Dr. Stephen Weiss of the University of Michigan for providing insightful guidance on experimental design. Some figure elements were generated in BioRender.com.
B.L. is supported by funding from NIH/NIAMS; NIH1R01AR071379, NIHR01GM123069, American College of Surgeons Clowes Award, and International FOP Association.
Published: February 18, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2021.01.011.
Accession numbers
scRNA-seq and snATAC-seq data can be accessed on the Gene Expression Omnibus at accession number GEO: GSE150995.
Supplemental information
References
- Agarwal S., Loder S., Brownley C., Cholok D., Mangiavini L., Li J., Breuler C., Sung H.H., Li S., Ranganathan K. Inhibition of Hif1alpha prevents both trauma-induced and genetic heterotopic ossification. Proc. Natl. Acad. Sci. U S A. 2016;113:E338–E347. doi: 10.1073/pnas.1515397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Loder S.J., Breuler C., Li J., Cholok D., Brownley C., Peterson J., Hsieh H.H., Drake J., Ranganathan K. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol. Ther. 2017;25:1974–1987. doi: 10.1016/j.ymthe.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Loder S.J., Cholok D., Peterson J., Li J., Breuler C., Cameron Brownley R., Hsin Sung H., Chung M.T., Kamiya N. Scleraxis-lineage cells contribute to ectopic bone formation in muscle and tendon. Stem Cells. 2017;35:705–710. doi: 10.1002/stem.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Loder S.J., Sorkin M., Li S., Shrestha S., Zhao B., Mishina Y., James A.W., Levi B. Analysis of bone-cartilage-stromal progenitor populations in trauma induced and genetic models of heterotopic ossification. Stem Cells. 2016;34:1692–1701. doi: 10.1002/stem.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen V., Lange M., Peidli S., Wolf F.A., Theis F.J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 2020;38:1408–1414. doi: 10.1038/s41587-020-0591-3. [DOI] [PubMed] [Google Scholar]
- Chan C.K.F., Gulati G.S., Sinha R., Tompkins J.V., Lopez M., Carter A.C., Ransom R.C., Reinisch A., Wearda T., Murphy M. Identification of the human skeletal stem cell. Cell. 2018;175:43–56 e21. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereji R.V., Eriksson P.R., Ocampo J., Prajapati H.K., Clark D.J. Accessibility of promoter DNA is not the primary determinant of chromatin-mediated gene regulation. Genome Res. 2019;29:1985–1995. doi: 10.1101/gr.249326.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comazzetto S., Murphy M.M., Berto S., Jeffery E., Zhao Z., Morrison S.J. Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin receptor+ niche cells in the bone marrow. Cell Stem Cell. 2019;24:477–486 e476. doi: 10.1016/j.stem.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckelbaum R.A., Holmes G., Zhao Z., Tong C., Basilico C., Loomis C.A. Regulation of cranial morphogenesis and cell fate at the neural crest-mesoderm boundary by engrailed 1. Development. 2012;139:1346–1358. doi: 10.1242/dev.076729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D., Bagarova J., Hatsell S.J., Armstrong K.A., Huang L., Ermann J., Vonner A.J., Shen Y., Mohedas A.H., Lee A. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci. Transl. Med. 2016;8:366ra163. doi: 10.1126/scitranslmed.aaf1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6:265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani R.M., Xaymardan M. Platelet-derived growth factor receptor alpha as a marker of mesenchymal stem cells in development and stem cell biology. Stem Cells Int. 2015;2015:362753. doi: 10.1155/2015/362753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K.L., Hebela N., Keenan M.A., Pignolo R.J. Histopathology of periarticular non-hereditary heterotopic ossification. Bone. 2018;109:65–70. doi: 10.1016/j.bone.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Genet F., Kulina I., Vaquette C., Torossian F., Millard S., Pettit A.R., Sims N.A., Anginot A., Guerton B., Winkler I.G. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J. Pathol. 2015;236:229–240. doi: 10.1002/path.4519. [DOI] [PubMed] [Google Scholar]
- Gulati G.S., Sikandar S.S., Wesche D.J., Manjunath A., Bharadwaj A., Berger M.J., Ilagan F., Kuo A.H., Hsieh R.W., Cai S. Single-cell transcriptional diversity is a hallmark of developmental potential. Science. 2020;367:405–411. doi: 10.1126/science.aax0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H.H.S., Agarwal S., Cholok D.J., Loder S.J., Kaneko K., Huber A., Chung M.T., Ranganathan K., Habbouche J., Li J. Coordinating tissue regeneration through transforming growth factor-beta activated kinase 1 inactivation and reactivation. Stem Cells. 2019;37:766–778. doi: 10.1002/stem.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C., Marini S., Huber A.K., Stepien D.M., Sorkin M., Loder S., Pagani C.A., Li J., Visser N.D., Vasquez K. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 2019;7:36. doi: 10.1038/s41413-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K., Durkin M.E., Johnsen L., Hunziker E., Damgaard-Pedersen K., Zhang H., Engvall E., Albrechtsen R., Wewer U.M. Mice with a targeted deletion of the tetranectin gene exhibit a spinal deformity. Mol. Cell. Biol. 2001;21:7817–7825. doi: 10.1128/MCB.21.22.7817-7825.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan C., Chen L., Hu Y., Ding N., Li Y., McGuire T.L., Lu H., Kessler J.A., Kan L. Gli1-labeled adult mesenchymal stem/progenitor cells and hedgehog signaling contribute to endochondral heterotopic ossification. Bone. 2018;109:71–79. doi: 10.1016/j.bone.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L., Peng C.Y., McGuire T.L., Kessler J.A. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013;53:194–203. doi: 10.1016/j.bone.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- Komori T. Roles of runx2 in skeletal development. Adv. Exp. Med. Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- La Manno G., Soldatov R., Zeisel A., Braun E., Hochgerner H., Petukhov V., Lidschreiber K., Kastriti M.E., Lonnerberg P., Furlan A. RNA velocity of single cells. Nature. 2018;560:494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Petkova A.P., Mottillo E.P., Granneman J.G. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Bernau K., Sandbo N., Gu J., Preissl S., Sun X. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. eLife. 2018;7:e36865. doi: 10.7554/eLife.36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder S.J., Agarwal S., Chung M.T., Cholok D., Hwang C., Visser N., Vasquez K., Sorkin M., Habbouche J., Sung H.H. Characterizing the circulating cell populations in traumatic heterotopic ossification. Am. J. Pathol. 2018;188:2464–2473. doi: 10.1016/j.ajpath.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M., Martin J.F., Nagy A., Lobe C., Olson E.N., Tabin C.J. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Matsushita Y., Nagata M., Kozloff K.M., Welch J.D., Mizuhashi K., Tokavanich N., Hallett S.A., Link D.C., Nagasawa T., Ono W. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 2020;11:332. doi: 10.1038/s41467-019-14029-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Era T. Tracing the destiny of mesenchymal stem cells from embryo to adult bone marrow and white adipose tissue via PDGFRalpha expression. Development. 2018;145:dev155879. doi: 10.1242/dev.155879. [DOI] [PubMed] [Google Scholar]
- O'Rourke M., Cullen C.L., Auderset L., Pitman K.A., Achatz D., Gasperini R., Young K.M. Evaluating tissue-specific recombination in a PDGFRalpha-CreERT2 transgenic mouse line. PLoS one. 2016;11:e0162858. doi: 10.1371/journal.pone.0162858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono N., Balani D.H., Kronenberg H.M. Stem and progenitor cells in skeletal development. Curr. Top. Dev. Biol. 2019;133:1–24. doi: 10.1016/bs.ctdb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineault K.M., Song J.Y., Kozloff K.M., Lucas D., Wellik D.M. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat. Commun. 2019;10:3168. doi: 10.1038/s41467-019-11100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C., Wong C.W.Y., Wu Z., He Q., Xia H., Tam P.K.H., Wong K.K.Y., Lui V.C.H. Stage specific requirement of platelet-derived growth factor receptor-alpha in embryonic development. PLoS one. 2017;12:e0184473. doi: 10.1371/journal.pone.0184473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo R.F., Rajan L., Li X., Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–1101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux D.R., Song J.Y., Swinehart I.T., Pineault K.M., Schlientz A.J., Trulik K.G., Goldstein S.A., Kozloff K.M., Lucas D., Wellik D.M. Regionally restricted hox function in adult bone marrow multipotent mesenchymal stem/stromal cells. Dev. Cell. 2016;39:653–666. doi: 10.1016/j.devcel.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnazari M., Chu V., Wronski T.J., Nissenson R.A., Halloran B.P. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013;27:3505–3513. doi: 10.1096/fj.12-225763. [DOI] [PubMed] [Google Scholar]
- Shi Y., He G., Lee W.C., McKenzie J.A., Silva M.J., Long F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun. 2017;8:2043. doi: 10.1038/s41467-017-02171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukunami C., Takimoto A., Nishizaki Y., Yoshimoto Y., Tanaka S., Miura S., Watanabe H., Sakuma T., Yamamoto T., Kondoh G. Scleraxis is a transcriptional activator that regulates the expression of Tenomodulin, a marker of mature tenocytes and ligamentocytes. Sci. Rep. 2018;8:3155. doi: 10.1038/s41598-018-21194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin M., Huber A.K., Hwang C., Carson W.F.t., Menon R., Li J., Vasquez K., Pagani C., Patel N., Li S. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020;11:722. doi: 10.1038/s41467-019-14172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y., Shukunami C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Hiraki Y., Shukunami C. Generation and characterization of ScxCre transgenic mice. Genesis. 2013;51:275–283. doi: 10.1002/dvg.22372. [DOI] [PubMed] [Google Scholar]
- Swinehart I.T., Schlientz A.J., Quintanilla C.A., Mortlock D.P., Wellik D.M. Hox11 genes are required for regional patterning and integration of muscle, tendon and bone. Development. 2013;140:4574–4582. doi: 10.1242/dev.096693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T., Nakazato R., Tsuchikane A., Fujikawa K., Iezaki T., Yoneda Y., Hinoi E. Genetic analysis of Runx2 function during intramembranous ossification. Development. 2016;143:211–218. doi: 10.1242/dev.128793. [DOI] [PubMed] [Google Scholar]
- Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., 2nd, Treacy D., Trombetta J.J., Rotem A., Rodman C., Lian C., Murphy G. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torossian F., Guerton B., Anginot A., Alexander K.A., Desterke C., Soave S., Tseng H.W., Arouche N., Boutin L., Kulina I. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight. 2017;2:e96034. doi: 10.1172/jci.insight.96034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley G.G., Atashroo D.A., Maan Z.N., Hu M.S., Zielins E.R., Tsai J.M., Duscher D., Paik K., Tevlin R., Marecic O. High-throughput screening of surface marker expression on undifferentiated and differentiated human adipose-derived stromal cells. Tissue Eng. Part A. 2015;21:2281–2291. doi: 10.1089/ten.tea.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li F., Xie L., Crane J., Zhen G., Mishina Y., Deng R., Gao B., Chen H., Liu S. Inhibition of overactive TGF-beta attenuates progression of heterotopic ossification in mice. Nat. Commun. 2018;9:551. doi: 10.1038/s41467-018-02988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.V., Deng Y., Wang Q.A., Sun K., Scherer P.E. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology. 2010;151:2933–2939. doi: 10.1210/en.2010-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y., Takimoto A., Watanabe H., Hiraki Y., Kondoh G., Shukunami C. Scleraxis is required for maturation of tissue domains for proper integration of the musculoskeletal system. Sci. Rep. 2017;7:45010. doi: 10.1038/srep45010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R., Shen B., Morrison S.J. Clec11a/osteolectin is an osteogenic growth factor that promotes the maintenance of the adult skeleton. Elife. 2016;5:e18782. doi: 10.7554/eLife.18782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Feng J., Ho T.V., Grimes W., Urata M., Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015;17:386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.O., Yue R., Murphy M.M., Peyer J.G., Morrison S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.