Abstract
We aimed to estimate the nonselective live birth prevalence, actual live birth prevalence, reduction percentage because of selective terminations, and population prevalence for Down syndrome (DS) in European countries. The number of people with DS alive in a country was estimated by first modeling the number of live births of children with DS by year of birth. Subsequently, for these different years of birth, survival curves for people with DS were constructed and then applied to these yearly estimates of live births with DS. For Europe, 2011–2015, we estimate 8,031 annual live births of children with DS, which would have been around 17,331 births annually, absent selective terminations. The estimated reduction of live birth prevalence was, on average, 54%, varying between 0% in Malta and 83% in Spain. As of 2015, we estimate 419,000 people with DS are living in Europe; without elective terminations, there would have been about 574,000 people with DS, which corresponds to a population reduction rate of 27%. Such statistics can be important barometers for prenatal testing trends and resource allocation within countries. Disability awareness initiatives and public policy initiatives can also be better grounded with these more precise estimates.
Subject terms: Neurological disorders, Population genetics, Aneuploidy
Introduction
Down syndrome (DS) is increasingly shifting from a postnatal to a prenatal diagnosis, especially as noninvasive prenatal screens (NIPS) are expanding worldwide. NIPS can signal the likelihood that a fetus has a chromosomal condition, like DS, as early as 9 weeks of gestation, after which an expectant couple can elect to pursue chorionic villus sampling or amniocentesis [1, 2]. With DS-related elective terminations increasing in the U.S., there are an estimated 30% fewer live births with DS [3]. As of 2010, this has resulted in an estimated 19% reduction in the overall DS population in the U.S [4].
There have been multiple studies on the basis of EUROCAT data about DS birth prevalence in European countries [5–8]. In contrast, the population size and population prevalence of people with DS alive in European countries have only been roughly estimated (see Supplementary S8) [9]. More precise estimates of population size and population prevalence have been published for just a few European countries—that is, England/Wales [10–12], the United Kingdom [13], Ireland [13], the Netherlands [13], and Denmark [14]. Yet, recent news reports have offered speculation. “Few countries have come as close to eradicating DS births as Iceland,” per one report [15]. Accurately estimating the live birth and population prevalence for any genetic condition matters. Such statistics can be important barometers for prenatal testing trends and resource allocation within countries. Disability awareness initiatives and public policy initiatives can also be better grounded with more precise estimates. Here we report detailed results of multi-country analyses, incorporating a variety of precise birth registries and modeling techniques.
Materials and methods
The number of people with DS alive in a country can be estimated by modeling the number of LBs of children with DS, and, subsequently, constructing DS-specific survival curves. For the European countries, we have used an adaptation of this method, as developed by de Graaf et al. for the U.S. and specific U.S. states [3, 16, 17].
Estimating nonselective live birth prevalence
Nonselective LB prevalence for DS is what would have occurred absent DS-related elective terminations. This can be estimated by applying the maternal-age-specific chances for a live birth with DS, derived from Morris et al. [18], to the maternal ages in the general population. Country-specific data on maternal age for 1950–2015 are available in the World Population Prospects of the United Nations [19]. For estimating nonselective prevalence before 1950, we used the Demographic Yearbook Collection of the United Nations [20]. For missing data, we used extrapolations on basis of the World Population Prospects of the United Nations Population Division [19]. Procedures and sources are detailed in Supplementary S1A, S1B, S3A, and S3B.
Estimating actual live birth prevalence
Prenatal screening, if followed by elective DS-related termination, changes the actual LB prevalence of DS. To estimate actual live birth prevalence, we have collected data on actual numbers of LBs of children with DS, from EUROCAT and other sources (Supplementary S1D) [21]. For missing data, we have used extrapolations of trends. For some countries, we have used additional data (for instance, the number of invasive procedures), which can be used in a more indirect way (Supplementary S1C).
Modeling survival
We adapted an earlier model from de Graaf et al. [4, 10, 16, 17]. On the basis of multiple DS-specific historical studies in developed countries, de Graaf et al. constructed and validated a model with different survival curves for people with DS for each year of birth [4]. In addition, they constructed separate curves for different ethnic groups in the U.S. based on the relationship between 1-year survival within these ethnic groups in general population and the 1-year survival in people with DS in recent years. De Graaf et al. extrapolated this relation back in time [4]. De Graaf et al. used the same strategy to differentiate U.S. states [17].
In our European study, we have assumed that a lower 1-year survival in the general population (Supplementary S4B) will be indicative for a less well-developed medical care system, which will concomitantly impact the survival of children with DS. We used a similar strategy as de Graaf et al. [4, 17]. to correct for this probable effect. Subsequently, we used relationships between 1-year survival in DS and 5- and 10-year survival in DS from historical studies [4] to predict 5-year and 10-year survival (Supplementary S4A).
Like de Graaf et al., for modeling survival rates beyond 10 years of age, we made use of the average of the (highly similar) survival curves for DS from seven different historical studies [4]. For the period before 1950, de Graaf et al. [4]. used a survival curve beyond 10 years of age with a more rapid decrease, based on the work of Penrose [22]. De Graaf et al. used this more hazardous survival curve for predicting the survival until the calendar year 1950. In 1950, 60% of children with DS in the U.S. were estimated to survive their first year of live. If another country had an estimated 1-year survival rate for DS <60%, we assumed that survival beyond 10 years of age in earlier cohorts followed the more hazardous survival age up to that year.
Validating and adjusting the survival modeling
First, there are a few studies in which the number of people with DS by age group has been estimated using population counts. We compared these with our modeled predictions (Supplementary S5A). Secondly, we compared our age distribution of deaths of people with DS with information on this age distribution from national statistical offices as reported in the WHO Mortality DataBase (MDB) [23] (Supplementary S5C).
The initial model matched the population counts of people with DS for four different former West bloc countries (a term we use to refer to European countries that were not formerly under communism). However, the initial model (“Model A”) had a very poor fit for four different former East bloc countries (a term we use to refer to European countries formerly under communism; Supplementary S5A).
For the former East bloc countries, we developed three alternative models for survival. “Model B” has been made to fit the East bloc data on people alive (Supplementary S5A). However, Model B (in which survival to 10 years of age and above 10 years of age is adapted) might be too extreme in lowering the historical estimates of 1-year survival in people with DS. As an alternative, we developed “Model C” (Supplementary S5B) in which 1-year survival is unaltered (in comparison to Model A), but the survival rates above 10 years of age up to 1980 are lowered, as this adaptation led to a distribution of age at death (Supplementary S5C) that was much more similar to the empirical data than following Model A. However, the assumption that only survival above 10 years of age would historically be less favorable in former East bloc countries does not seem fully logical. As a final exploration, we developed “Model D,” in which survival above 10 years of age up to 1980 is less favorable (as it is in Model C), but 1-year survival is adapted to a value that is the mean of the value of model A (which is the same value as in Model C) and Model B.
In Supplementary S5C, data on the distribution of age at death of people with DS from national statistics are compared with our models’ predictions. The initial model (Model A) has a good fit for former West bloc countries. For the former East bloc, Model D appears to have the best fit.
Estimating population prevalence
For estimating population prevalence, we made use of the estimated number of people in general population, derived from the World Population Prospects of the United Nations [19].
For the former West bloc countries, we have used Model A for survival in people with DS (Supplementary S4 and S5). For the former East bloc countries, we present the results of Model D. In Supplementary S6 and S7, the results of the other Models are presented as well (with Models B and D in detail).
Results
LBs and LB prevalence of DS
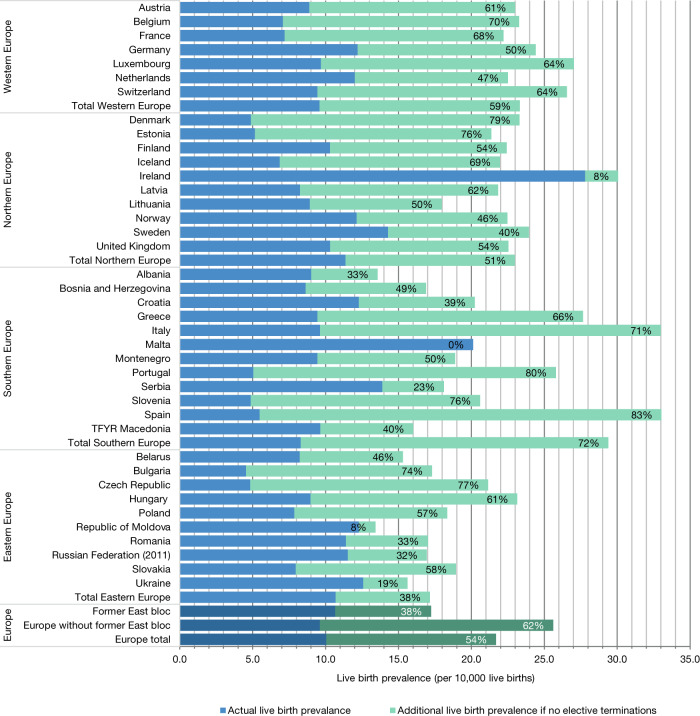
For Europe, between 2011–2015, we estimate 8031 annual live births of children with DS and a live birth prevalence of 10.1 per 10,000 live births. Without elective terminations, LB prevalence would have been around 21.7 per 10,000 live births, or 17,331 births annually. The estimated reduction of LB prevalence by elective terminations during this period was, on average, 54%, varying between 0% in Malta to 83% in Spain (Fig. 1; Supplementary S6 and S7).
Fig. 1. Live birth prevalence estimates of people with Down syndrome (DS) per 10,000 live births (2011–2015) and the effect of elective terminations.
Percentages denote the reduction due to DS-specific elective terminations.
Among the four geographic European regions, between 2011 and 2015, the nonselective LB prevalence was highest (29.4 per 10,000 LBs) and the actual LB prevalence was lowest (8.3 per 10,000 LBs) in Southern Europe (Fig. 1). Southern Europe had the highest reduction percentage (71%), followed by Western Europe (59%), Northern Europe (51%), and Eastern Europe (38%). However, inside regions, there are considerable differences between countries (Fig. 1, and Supplementary S6).
Differences between former East bloc and former West bloc countries were clear. For 2011–2015, nonselective LB prevalence for former East bloc countries ranged between 13 and 22 per 10,000 LBs and for former West bloc countries ranged between 22 and 33 per 10,000 LBs. Reduction percentage was lower in the former East bloc countries (38%) than in the former West bloc countries (62%), but the range was wide (Fig. 1).
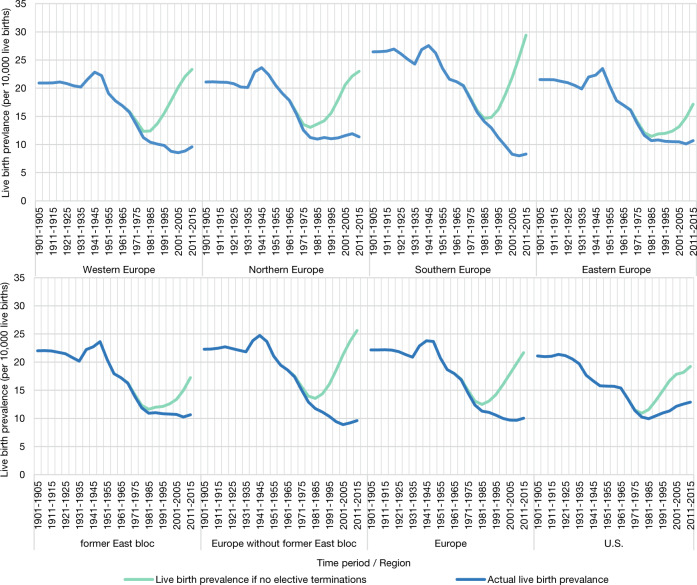
For 1945 (see Fig. 2; Supplementary S6 and S7), nonselective LB prevalence is estimated at 24.4 per 10,000 LBs in Europe versus only 18.0 per 10,000 LBs in the U.S. After World War II, in the U.S. and Europe alike (Fig. 2), nonselective LB prevalence of DS decreased until around 1980 and rose again afterwards. In the U.S., the nadir of LB prevalence (of 10.9 per 10,000 LBs) was reached in 1978; in Europe as a whole (12.3 per 10,000) in 1982. Between the four European regions, and between countries, there was variation between nadirs (Supplementary S6 and S7).
Fig. 2. Estimates of nonselective and actual live birth prevalence of children with Down syndrome per 10,000 live births, 1901–2015.
US comparison based on previously reported modelling [3, 4, 17], updated with recent data.
From 1980 to 2015, the changes in nonselective LB prevalence of DS in Europe and the U.S. were fairly similar (in Europe increasing from 12.5 per 10,000 in 1981–1985 to 21.7 per 10,000 in 2011–2015; in the US from 11.6 per 10,000 to 19.2 per 10,000). However, the difference in the changes of actual LB prevalence was more striking. In Europe, actual LB prevalence decreased between 1980 and 2000 to around 10.1 per 10,000; in the US, by contrast, actual LB prevalence had been slowly increasing from 1980 onwards, reaching around 12.9 per 10,000 in 2011–2015. For 2011–15, reduction percentage in LB prevalence was estimated at 54% in Europe, versus only 33% in the U.S.
Population numbers and population prevalence of DS
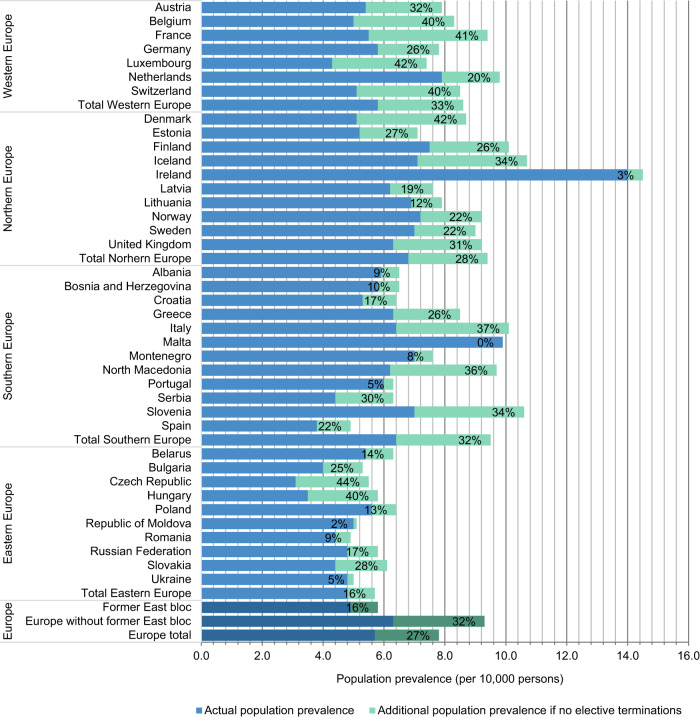
Using Model D for survival in former East bloc countries, the DS population prevalence as of 2015 in Europe is estimated at 5.7 per 10,000 (Fig. 3). This corresponds to an estimation of 419,000 people with DS as of 2015 (see Table 1 for numbers by country), of whom 32% are under the age of 20 and 35% above the age of 40 (Fig. 4). Without elective terminations, nonselective population prevalence would have been about 7.7 per 10,000, or 574,000 people with DS, which corresponds to a population reduction rate of 27%. By comparison, as of 2015 in the U.S., as estimated on basis of the method of De Graaf et al. [4], there were around 215,000 people with DS (including 5,800 foreign born) living in the U.S. (6.7 per 10,000). This would have been 271,000 (8.5 per 10,000) (including 5,800 foreign born) without elective terminations, corresponding to a 21% reduction due to elective terminations (Fig. 4).
Fig. 3. Population prevalence estimates of people with Down syndrome (DS) per 10,000 inhabitants (as of 2015) and the effect of elective terminations.
Model D used for former East bloc countries. Percentages denote the reduction due to DS-specific elective terminations.
Table 1.
2015 estimates of number of people with Down syndrome in European countries.
| Region/country | Number |
|---|---|
| Western Europe | 111,304 |
| Austria | 4716 |
| Belgium | 5646 |
| France | 35,684 |
| Germany | 47,465 |
| Luxembourg | 243 |
| Netherlands | 13,309 |
| Switzerland | 4241 |
| Northern Europe | 69,760 |
| Denmark | 2887 |
| Estoniaa | 679 |
| Finland | 4130 |
| Iceland | 234 |
| Ireland | 6557 |
| Latviaa | 1226 |
| Lithuaniaa | 2020 |
| Norway | 3725 |
| Sweden | 6792 |
| United Kingdom | 41,511 |
| Southern Europe | 97,964 |
| Albaniaa | 1729 |
| Bosnia and Herzegovinaa | 2063 |
| Croatiaa | 2232 |
| Greece | 7035 |
| Italy | 38,330 |
| Malta | 423 |
| Montenegroa | 440 |
| North Macedoniaa | 780 |
| Portugal | 6421 |
| Serbia + Kosovoa | 5275 |
| Sloveniaa | 913 |
| Spain | 32,323 |
| Eastern Europe | 139,997 |
| Belarusa | 5161 |
| Bulgariaa | 2879 |
| Czech Republica | 3299 |
| Hungarya | 3463 |
| Polanda | 21,328 |
| Republic of Moldovaa | 2041 |
| Romaniaa | 8736 |
| Russian Federationa | 69,220 |
| Slovakiaa | 2396 |
| Ukrainea | 21,474 |
aModel D used for estimates for former East bloc countries.
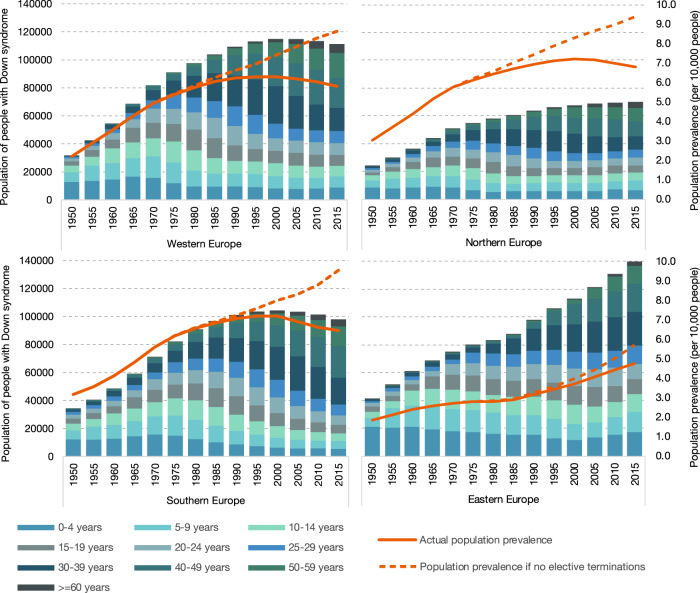
Fig. 4. Estimates of the actual numbers of people with Down syndrome (DS) by age, along with nonselective and actual population prevalence per 10,000 inhabitants.
Model D used for former East bloc countries.
Development in population prevalence of DS has been fairly similar in Western, Northern, and Southern Europe (Fig. 4). That is, nonselective population prevalence has been increasing from 1950 onwards; actual population prevalence increased up to 2000 and slightly decreased since (Fig. 4), as a result of rising numbers of DS-related terminations (Fig. 2). The high reduction percentage in LBs of children with DS in Southern Europe in recent years is reflected in the relatively low percentage of people with DS under the age of 20 in this region—that is 23% people with DS, whereas there were 29% in Western Europe and 35% in Northern Europe.
Eastern Europe shows a different development. First, reduction of population prevalence as a result of DS-related terminations is relatively low, 16% as of 2015. By comparison, this was 28% in Northern Europe, 33% in Western Europe and 32% in Southern Europe. Second, empirical results suggest that up to 2000, the survival of people with DS in former East bloc countries had been significantly poorer than in former West bloc countries. As a result, population prevalence in East bloc countries has been estimated to be much lower than it would have been had we applied former West bloc survival rates. With the survival of people with DS being poor in the past, but having improved strongly in recent years, a relatively high percentage (39% as of 2015) of people with DS in Eastern Europe are estimated to be under 20 years of age.
Detailed results by country, and by region, including the results for other models of survival, can be found in Supplementary S6 and S7 (see also Table 1).
Discussion
Development of LB prevalence
Though there are some differences in timing and magnitude, nonselective LB prevalence decreased in all countries after 1945, and, for most countries, increased after around 1980. The same development is found in the U.S., a result of decreasing family sizes, earlier marriage, and the earlier birth of the first child in the period after WWII. In more recent years, families started to postpone the birth of the first child [4]. In most European countries, rising maternal age after 1980 did not lead to increases in actual LB prevalence. Between 1981 and 1985 and 2011 and 2015, out of 37 countries permitting terminations, 11 countries had some increase in actual LB prevalence (maximum of 16% increase) and 26 had a decrease, as DS-related terminations following prenatal diagnosis outweighed the effects of rising maternal age (Supplementary S6).
Reduction rates have increased in almost all European countries following the introduction of prenatal screening. However, rates vary between countries and regions and can be influenced by legal policies. For example, reduction rates are very low in Malta and Ireland where terminations are highly restricted. A country’s wealth might also play a role. Between 2011 and 2015, the overall reduction in former East bloc countries was 38%, compared to 63% for the rest of Europe. Former East bloc countries have lower incomes and, in general, their screening programs have been introduced more recently and are less widely available. We found a strong correlation between Gross Domestic Product per capita and reduction rates in these countries (r = 0.73; p < 0.000; Supplementary S9) [24], suggesting that prenatal screening availability and uptake increases as countries and citizens become wealthier.
However, we also found differences in reduction rates in wealthier countries where prenatal screening is universally available and is largely funded by health insurers or governments. In Denmark, for example, prenatal services are considered a routine part of health care, both by counselors and pregnant women, whereas in the Netherlands, counselors stress that prenatal screening should be a personal and well informed choice, and many women abstain from screening because they do not see DS as a reason for terminating their pregnancy [25, 26]. As such, cultural differences seem to play a role in a country’s reduction percentage.
There also seems to be a tendency that high maternal ages, and thus high nonselective prevalence, are often accompanied by relatively high reduction percentages. This might be a result of older women more often being inclined to use prenatal screening, reimbursement policies more favorable for older maternal age groups, prenatal tests having better performance in older women, countries’ wealth (which correlates with both higher maternal ages and a wider availability of prenatal services), or some combination thereof. Overall, Europe has, on average, a much higher reduction percentage than the U.S. Differences in reimbursement policies and cultural considerations likely play a role.
Our research catalogues the epidemiology up to 2015, which is before the introduction of NIPS as a national screening strategy in European countries. If NIPS becomes widely available, higher reduction percentages will likely follow. NIPS has fewer false negatives than traditional prenatal screens, and some women who might otherwise abstain from former prenatal screens that they had considered too unreliable, will choose for NIPS [25].
Development of population prevalence and survival
The size of the population of people with DS is influenced by developments in survival rates. During the last 100 years, survival of people with DS has improved dramatically. In the U.S. and in former West bloc countries, after 1950, survival improved quickly. Increased access to better health care has led to the survival of people with DS well into their 50’s and 60’s. The earlier onset of Alzheimer’s disease in this population is now one of the key determinants of mortality in this adult population [27, 28]. However, in the former East bloc states, survival appears not to have improved significantly until after the 1990s. This implies that in estimating population prevalence of DS in less developed non-Western countries, it is not valid to apply Western survival rates of DS. Survival was, and still will be, much lower in these countries.
Limitations of the method
First, there is uncertainty in the estimates of actual LB prevalence due to incompleteness of data. In the Supplementary S1, each country’s sources and uncertainties are specified.
Secondly, the estimates of survival were based on the pattern of survival in diverse historical studies, which can lead to population estimates being too high or too low. However, modeled population estimates by age groups and projections of age distribution of age at death match available empirical data well for former West bloc countries (Supplementary S5). In contrast, for former East bloc countries, empirical data suggest that our initial model assumes a far too high survival before 2000. Though one could argue that these empirical data might be flawed, the pattern is very consistent for the different East bloc countries, which suggests there is a systematic effect and not a random effect of empirical errors. We had developed Model D as the best fitting model for the distribution of age at death. However, historical survival in East bloc countries might even have been worse, as in Model B, which was made to fit the counts of people with DS in four East bloc countries. Model D projects around 157,000 people with DS in former East bloc countries as of 2015; Model B projects 28% less, or around 113,000 people.
Third, we assumed that the net effect of migration of people with DS has been zero. De Graaf et al. have demonstrated that even in a country with significant immigration, as the U.S., the effect on population prevalence of DS due to migration of people with DS is small for both the country as a whole and for individual states [4, 17].
Finally, our modeling does not include years in which NIPS has been implemented [29], as data from these recent years are not yet available. Recent cultural and legal changes (e.g., the revision of pregnancy termination laws in Ireland [30] and Northern Ireland [31]) might also have potential effects on live birth prevalence.
Conclusion
Overall, our research has produced estimates of the population size and age distribution of people with DS in all the European countries. These data should prove valuable to researchers and policy makers, while also establishing a baseline by which the effects of prenatal testing can be measured in future years.
Supplementary information
Acknowledgements
We would like to thank Sophia Campbell for contacting Down syndrome organizations with our requests for country-specific data. We also thank Dinka Vukovic (Croatian Down Syndrome Association), Emanuela Zaimi (Down Syndrome Albania Foundation), Sergey Kuryanov (Ukrainian Charitable Organization “Down Syndrome”), Maria Madalina Turza (European Centre for the Rights of Children with Disabilities) for providing information on numbers of people with Down syndrome from registries in Croatia, Albania, Ukraine and Romania, respectively. We thank Liina Lokko and Marge Kato (Down Syndrome Association in Estonia), Ida Charlotte Bay Lund (Aarhus Universitetshospital), and Zoya Talitskaya (Downside Up Charity Fund) in providing information on number of live births of children with Down syndrome from registers in Estonia, Denmark, and Russia, respectively. We appreciate the contributors from Down syndrome associations all over Europe who gave information on screening and for the medical and social support they provide for people with Down syndrome in their countries.
Compliance with ethical standards
Conflict of interest
Dr BGS occasionally consults on the topic of Down syndrome through Gerson Lehrman Group. He receives remuneration from Down syndrome non-profit organizations for speaking engagements and associated travel expenses. Dr BGS receives annual royalties from Woodbine House, Inc., for the publication of his book, Fasten Your Seatbelt: A Crash Course on Down Syndrome for Brothers and Sisters. Within the past 2 years, he has received research funding from F. Hoffmann-La Roche, Inc. and LuMind IDSC Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. Dr BGS is occasionally asked to serve as an expert witness for legal cases where Down syndrome is discussed. Dr BGS serves in a non-paid capacity on the Honorary Board of Directors for the Massachusetts Down Syndrome Congress and the Professional Advisory Committee for the National Center for Prenatal and Postnatal Down Syndrome Resources. Dr BGS has a sister with Down syndrome. Dr GdG had a daughter with DS, who passed away in 2005 at the age of 15. He works as science and education officer at the Dutch Down Syndrome Foundation, a non-profit organization. FB serves a CEO of Down Syndrome Education International and Down Syndrome Education USA, nonprofits engaged in research and support for young people with Down syndrome. He has a sister with Down syndrome.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/7/2022
A Correction to this paper has been published: 10.1038/s41431-022-01124-8
Supplementary information
The online version of this article (10.1038/s41431-020-00748-y) contains supplementary material, which is available to authorized users.
References
- 1.Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18:1056–65. doi: 10.1038/gim.2016.97. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Practice Bulletins—Obstetrics, Committee on Genetics, and the Society for Maternal-Fetal Medicine Practice bulletin no. 163 summary: screening for fetal Aneuploidy. Obstet Gynecol. 2016;127:979–81. doi: 10.1097/AOG.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 3.de Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet A. 2015;167:756–67. doi: 10.1002/ajmg.a.37001. [DOI] [PubMed] [Google Scholar]
- 4.de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017;19:439–47. doi: 10.1038/gim.2016.127. [DOI] [PubMed] [Google Scholar]
- 5.Boyd PA, Devigan C, Khoshnood B, Loane M, Garne E, Dolk H, et al. Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down’s syndrome. BJOG Int J Obstet Gynaecol. 2008;115:689–96. doi: 10.1111/j.1471-0528.2008.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoshnood B, De Vigan C, Vodovar V, Bréart G, Goffinet F, Blondel B. Advances in medical technology and creation of disparities: the case of Down syndrome. Am J Public Health. 2006;96:2139–44. doi: 10.2105/AJPH.2005.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JK, Garne E, Wellesley D, Addor M-C, Arriola L, Barisic I, et al. Major congenital anomalies in babies born with Down syndrome: a EUROCAT population-based registry study. Am J Med Genet A. 2014;164A:2979–86. doi: 10.1002/ajmg.a.36780. [DOI] [PubMed] [Google Scholar]
- 8.Loane M, Morris JK, Addor M-C, Arriola L, Budd J, Doray B, et al. Twenty-year trends in the prevalence of Down syndrome and other trisomies in Europe: impact of maternal age and prenatal screening. Eur J Hum Genet. 2013;21:27–33. doi: 10.1038/ejhg.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond Engl. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Graaf G, Vis JC, Haveman M, van Hove G, de Graaf EAB, Tijssen JGP, et al. Assessment of prevalence of persons with down syndrome: a theory-based demographic model. J Appl Res Intellect Disabil. 2011;24:247–62. doi: 10.1111/j.1468-3148.2010.00593.x. [DOI] [Google Scholar]
- 11.Wu J, Morris JK. The population prevalence of Down’s syndrome in England and Wales in 2011. Eur J Hum Genet. 2013;21:1016–9. doi: 10.1038/ejhg.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Morris JK. Erratum: the population prevalence of Down’s syndrome in England and Wales in 2011. Eur J Hum Genet. 2013;21:1033–4. doi: 10.1038/ejhg.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander M, Ding Y, Foskett N, Petri H, Wandel C, Khwaja O. Population prevalence of Down’s syndrome in the United Kingdom. J Intellect Disabil Res. 2016;60:874–8. doi: 10.1111/jir.12277. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JL, Hasle H, Correa A, Schendel D, Friedman JM, Olsen J, et al. Survival among people with Down syndrome: a nationwide population-based study in Denmark. Genet Med J Am Coll Med Genet. 2013;15:64–69. doi: 10.1038/gim.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinones J, August 14 ALCN, 2017, Pm 4:00. ‘What kind of society do you want to live in?’: Inside the country where Down syndrome is disappearing. https://www.cbsnews.com/news/down-syndrome-iceland/ (accessed 13 Jan 2020).
- 16.de Graaf G, Buckley F, Skotko BG. Live births, natural losses, and elective terminations with Down syndrome in Massachusetts. Genet Med J Am Coll Med Genet. 2016;18:459–66. doi: 10.1038/gim.2016.15. [DOI] [PubMed] [Google Scholar]
- 17.de Graaf G, Buckley F, Dever J, Skotko BG. Estimation of live birth and population prevalence of Down syndrome in nine U.S. states. Am J Med Genet A. 2017;173:2710–9. doi: 10.1002/ajmg.a.38402. [DOI] [PubMed] [Google Scholar]
- 18.Morris JK, Mutton DE, Alberman E. Revised estimates of the maternal age specific live birth prevalence of Down’s syndrome. J Med Screen. 2002;9:2–6. doi: 10.1136/jms.9.1.2. [DOI] [PubMed] [Google Scholar]
- 19.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects:The 2017 Revision, DVD Edition. https://population.un.org/wpp/Download/Standard/Fertility/ (accessed 9 Nov 2018).
- 20.United Nations, Department of Economic and Social Affairs, Statistic Division. Demographic Yearbook System. https://unstats.un.org/unsd/demographic-social/products/dyb/#statistics (accessed 7 Jun 2018).
- 21.EUROCAT. Table A5 - Down Syndrome (per 10,000 births). https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence (accessed 4 Jul 2018).
- 22.Penrose LS. The incidence of mongolism in the general population. J Ment Sci. 1949;95:685–8. doi: 10.1192/bjp.95.400.685. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Raw data files of the WHO Mortality Database. https://www.who.int/healthinfo/statistics/mortality_rawdata/en/ (accessed 17 Sep 2019).
- 24.International Monetary Fund. World Economic Outlook Database April 2019. https://www.imf.org/external/pubs/ft/weo/2019/01/weodata/index.aspx (accessed 3 Mar 2020).
- 25.Crombag NM, Page-Christiaens GC, Skotko BG, de Graaf G. Receiving the news of Down syndrome in the era of prenatal testing. Am J Med Genet A. 2020;182:374–85. doi: 10.1002/ajmg.a.61438. [DOI] [PubMed] [Google Scholar]
- 26.Crombag NMTH. Vellinga YE, Kluijfhout SA, Bryant LD, Ward PA, Iedema-Kuiper R, et al. Explaining variation in Down’s syndrome screening uptake: comparing the Netherlands with England and Denmark using documentary analysis and expert stakeholder interviews. BMC Health Serv Res. 2014;14:437. doi: 10.1186/1472-6963-14-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019;15:135–47. doi: 10.1038/s41582-018-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hithersay R, Startin CM, Hamburg S, Mok KY, Hardy J, Fisher EMC, et al. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. 2019;76:152–60. doi: 10.1001/jamaneurol.2018.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadsbøll K, Petersen OB, Gatinois V, Strange H, Jacobsson B, Wapner R, et al. Current use of noninvasive prenatal testing in Europe, Australia and the USA: a graphical presentation. Acta Obstet Gynecol Scand. 2020;99:722–30. doi: 10.1111/aogs.13841. [DOI] [PubMed] [Google Scholar]
- 30.Book (eISB) electronic IS. electronic Irish Statute Book (eISB). http://www.irishstatutebook.ie/eli/2018/act/31/enacted/en/html (accessed 21 Sep 2020).
- 31.The Abortion (Northern Ireland) Regulations 2020. https://www.legislation.gov.uk/uksi/2020/345/contents/made (accessed 21 Sep 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.