Abstract
Purpose:
To review the clinical features and treatment-associated outcomes of primary orbital melanoma among cases reported in the literature and to present a case treated with orbital exenteration and post-operative radiotherapy.
Methods:
Case reports and case series on primary orbital melanoma published in the literature between 1980 and 2020 were reviewed. Data collected included patient demographics, presenting ocular symptoms, diagnostic imaging, histology, management, and outcomes.
Results:
Eighty-eight cases of primary orbital melanoma were reviewed. The average age at presentation was 45 years and 58% of patients were male. The most common presenting symptoms and signs were proptosis (73%), decreased visual acuity (32%), pain (14%), diplopia (15%), and palpable mass (9%). Imaging frequently showed a well-circumscribed enhancing lesion. Diagnosis was made by histology in all cases, and orbital blue nevus was identified in 42%. In the majority of cases, treatment consisted of orbital exenteration (54%) or excision (38%). Adjuvant radiotherapy was given in 47% of cases. For the 72 patients with reported outcomes, 36% had metastases, 15% had local recurrence, and 32% died of metastatic disease. Patients who received surgery and radiotherapy had improved survival compared to those who received surgery alone (p = .01). There was no difference in survival between those who underwent orbital exenteration or excision (p = .16).
Conclusions:
Primary orbital melanoma is a rare malignancy and should be considered in patients with a history of unilateral proptosis and a well-defined orbital mass on imaging. Surgery remains the mainstay of treatment. Adjuvant radiotherapy may improve patient survival.
Keywords: Cancer, exenteration, orbit, primary orbital melanoma, radiotherapy
Introduction
Orbital melanoma can occur as either primary disease, as secondary disease due to local extension from a uveal, conjunctival or eyelid primary melanoma, or as metastatic disease.1 Melanoma accounts for 5–20% of metastatic and secondary orbital malignancies.2-4 Primary orbital melanomas are extremely rare, encompassing less than 1% of orbital neoplasms.5 They are often reported in association with predisposing melanocytic lesions, such as blue nevus, oculodermal melanocytosis (nevus of Ota), or orbital melanocytosis.6-8 In a case series by Tellado et al, 47.5% of patients with primary orbital melanoma had congenital melanosis and intraorbital blue nevus was present in 90%.5 Primary orbital melanoma is thought to arise from melanocytes of the leptomeninges or ciliary nerves or from ectopic nests of melanocytes in the orbit.9 Patients with primary orbital melanoma generally present with painless proptosis; however, associated changes in visual acuity, diplopia, or pain may be present.5
Surgery remains the mainstay of treatment for orbital melanoma. Surgical options include local resection, debulking, or exenteration,10,11 and local adjuvant radiotherapy is commonly given.2 Generally, the disease is associated with poor prognosis, although there have been a few reports of long survival.5,11 Recent studies have suggested that certain genetic alterations in primary orbital melanoma, namely SF3B1 and E1F1AX mutations, may indicate a better prognosis.12,13 Due to the paucity of literature on primary orbital melanoma and small sample sizes of existing studies, the optimal treatment has not yet been defined for this rare disease. In this study, we report a case of primary orbital melanoma treated with orbital exenteration and adjuvant proton beam radiotherapy and a review of the literature on this rare entity.
Case
A 38-year-old white man was referred for evaluation of 2 months of slowly progressive proptosis of the left eye. He also complained of a pressure sensation behind his left eye and diplopia when looking upwards, downwards and to the right, associated with dizziness. He had a history of psoriasis, treated with UV radiation and acitretin 7 months ago, with no prior history of malignancy. An MRI scan revealed a homogenously enhancing soft tissue lesion replacing the left superior rectus muscle (Figure 1). The lesion extended through the left superior orbital fissure and abutted the optic canal without definite extension. The mass compressed and displaced the optic nerve but there was no evidence of intracranial extension. Visual acuity was 20/15 in the right eye and 20/20 in the left eye and no afferent pupillary defect was detected. There was an associated restriction of upgaze and downgaze. Exophthalmometry measured 18 mm on the right and 23 mm on the left. There was no evidence of oculodermal melanosis, conjunctival melanoma, or uveal melanoma.
Figure 1.
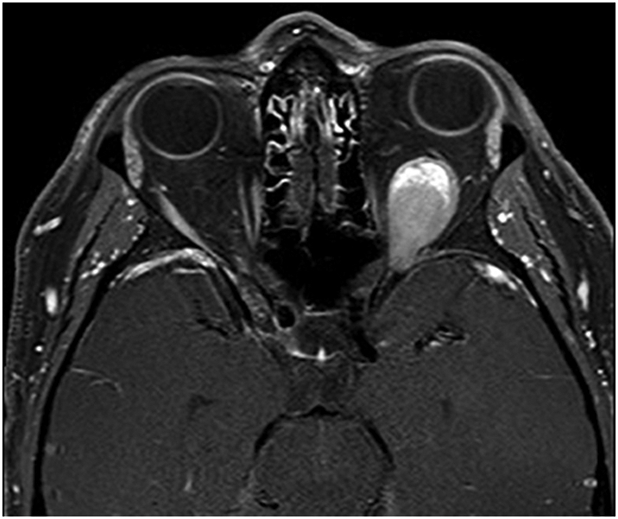
T1-weighted contrast-enhanced orbital MRI scan at presentation shows a left intraconal enhancing mass, causing mild proptosis.
A biopsy was performed through a lateral orbitotomy, which revealed pigmented and friable tissue infiltrating the superior rectus muscle. Histologic examination showed soft tissue infiltrated by polygonal blue cells with rare pigment. Immunohistochemistry staining revealed tumor cells positive for SOX-10 and HMB-45, with retention of BAP-1 expression and no BRAF V600E mutation. A diagnosis of malignant melanoma was made (Figure 2). Systemic evaluation, including positron emission tomography, total body skin exam, and ocular B-scan ultrasonography, failed to reveal other sites of melanoma involvement. The decision was made to initiate combined immunotherapy with ipilimumab and nivolumab. The patient underwent two cycles of immunotherapy, but immunotherapy was subsequently stopped after he developed immune-related aseptic meningitis and autoimmune hepatitis. MRI showed a stable left orbital mass without evidence of regression. Using next-generation sequencing, tumor mutational status was assessed. Sequencing was performed using the Illumina HiSeq 2500 platform, and analysis was performed using a customized bioinformatics pipeline, Halo_V1.3. The panel revealed a GNAQ Gln209Pro mutation as well as a splice-site variant in exon 1 of EIF1AX, suggesting a uveal melanoma-like genetic profile. Based on the tumor genetic profile and extensive workup, the mass was diagnosed as primary orbital melanoma.
Figure 2.
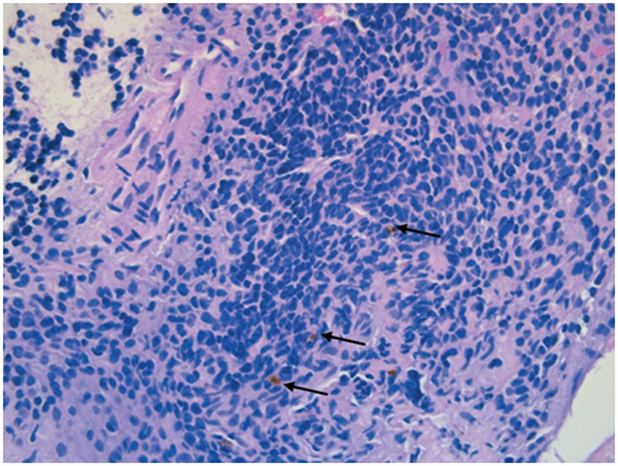
Hematoxylin and eosin-stained section of orbital lesion. Malignant cells show minimal atypia with sparse extra- cellular pigment (black arrows) (original magnification, ×40).
At this point, the decision was made to proceed with surgery and adjuvant proton beam radiation therapy (PBT). He underwent left eyelid-sparing orbital exenteration with free flap and skin graft construction. The resected mass was 25 mm in largest dimension, and there was bony involvement of the posterior orbital roof and positive margins in the deep orbital muscle and orbital apex intracranial nerve. His final pathology review reconfirmed the diagnosis of malignant melanoma. Based on the eighth edition of the American Joint Committee on Cancer Staging, the patient’s disease stage was determined to be TxN0M0.14 Four weeks after exenteration, he underwent adjuvant PBT to a total dose of 70 Gy in 30 fractions. The patient is currently doing well with no residual disease 5 months after initial diagnosis.
Materials and methods
A comprehensive review of the literature using PubMed was performed on February 14, 2020 to identify all relevant articles on primary orbital melanoma. Articles published between January 1, 1980 and February 14, 2020 were screened for eligibility. Articles were selected that met the following inclusion criteria: published in English and reported primary orbital melanoma in humans. This study was Health Insurance Portability and Accountability Act compliant and adhered to the principles outlined in the Declaration of Helsinki as amended in 2013. Institutional review board approval was obtained for the review of patient data at the University of Pennsylvania. Patient authorizations for release of images and protected health information were obtained. Data collected included patient demographics, presenting ocular symptoms, diagnostic imaging, histology, management, and outcomes.
All statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). One-sample tests of proportion were used to analyze gender and laterality. Survival estimates were calculated using the Kaplan–Meier method for case studies with documented follow-up times after diagnosis. Cox proportional hazards regression was used to determine the hazard ratio (HR) and its 95% confidence interval for treatment comparisons. Fisher’s exact test was used to determine the associations between treatment and outcomes of recurrence or survival for the subset of patients without documented follow-up times after diagnosis. A p-value <0.05 was considered statistically significant.
Results
A total of 254 articles were identified. After screening for case series and case reports meeting inclusion criteria, 32 papers describing a cumulative total of 88 patients with primary orbital melanoma, including the case described above, were included in this review. Demographic and clinical information is presented in Table 1. The mean age at presentation was 45.1 years (range 5–91, median 45). Males were affected more often than females (58% and 42%, respectively), although this difference was not statistically significant. Most individuals were of white European descent (95%). There was no significant difference in laterality of the tumor (58% left-sided, 42% right-sided). Ocular melanosis or nevus of Ota was present in 24% of cases.
Table 1.
Summary of previously reported cases of primary orbital melanoma in the literature.
| Author(s) (yr) | Number of cases |
Gender | Age of onset | Laterality | Treatment | Follow-up duration |
|
|---|---|---|---|---|---|---|---|
| Mudhar et al. (2019)13 | 6 | 4 M, 2 F | Mean age = 66 | 4 L, 2 R | Ocular melanocytosis (1/6) | Exenteration ± RT | Mean 39 months |
| Rose et al. (2017a, 2017b)11,12 | 13 | 5 M, 8 F | Mean age = 55.5 | 8 L, 5 R | Ocular melanosis (3/13) | Debulking (11) or exenteration (2) ± RT | Mean 44 months |
| Bains et al. (2016)15 | 1 | F | 28 | R | Exenteration + RT/chemotherapy | 21 months | |
| El-Sawy et al. (2014)7 | 3 | F | 9 | R | Blue eyelid nevus | Exenteration + PBT | 36 months |
| F | 54 | L | Orbital nevus | Exenteration, intensity-modulated RT | 24 months | ||
| F | 44 | R | Orbital nevus | Exenteration + RT | 24 months | ||
| Radhadevi et al. (2013)16 | 1 | M | 45 | L | Nevus of Ota | Excision + RT, Exenteration + chemotherapy | 168 months |
| De Potter et al. (2006)17 | 1 | M | 59 | L | Incisional biopsy + plaque RT/chemotherapy | 66 months | |
| Delaney et al. (2004)10 | 1 | M | 40 | - | Exenteration + interferon | 7 weeks | |
| Krishnakumar et al. (2003a, 2003b)18,19 | 3 | M | 43 | - | Orbital nevus | Exenteration + RT | 18 months |
| F | 33 | - | Orbital nevus | Exenteration | 12 months | ||
| F | 45 | - | Orbital nevus | Palliative chemotherapy | 2 months | ||
| Rice et al. (1990)20 | 1 | F | 17 | R | Orbital nevus | Excision | 29 months |
| Shields et al. (2004)3 | 10 | 61 | - | Blue nevus (4/10) | Unknown | Unknown | |
| Ferreira et al. (2019)21 | 1 | M | 59 | R | Unknown | Unknown | |
| Tellado et al. (1996)5 | 21 | 16 M, 5 F | Mean age = 42 | - | Congenital melanosis (10/19), Orbital nevus (19/21) | Exenteration (14) or excision (7) ± RT/chemotherapy | Mean 54 months |
| Ke et al. (2014)22 | 1 | F | 8 | R | Giant divided nevus | Exenteration + RT/chemotherapy | 10 months |
| Ranjit et al. (2016)23 | 1 | F | 87 | R | Nevus of Ota | Exenteration | Unknown |
| Alsuhaibani and Alhumayed (2011)24 | 1 | M | 60 | L | Poliosis | Exenteration | 9 months |
| Shields et al. (1993)25 | 1 | M | 76 | R | Excision | 22 months | |
| Elibol et al. (1995)26 | 1 | M | 79 | L | Excision | 11 months | |
| Mandeville et al. (2004)27 | 1 | F | 36 | R | Episcleral nevus | Exenteration + RT | 24 months |
| Korányi et al. (2000)6 | 1 | M | 29 | L | Ocular melanosis | Excision + interferon | 36 months |
| Patrocínio et al. (2006)28 | 1 | F | 64 | R | Exenteration + RT | 18 months | |
| Mahoney et al. (2008)29 | 1 | M | 50 | R | Orbital nevus | Exenteration + adjuvant therapy | Unknown |
| Polito et al. (1995)30 | 2 | M | 46 | L | Excision | 24 months | |
| M | 59 | R | Excision + chemotherapy/, interferon | 25 months | |||
| Lee et al. (2002)31 | 2 | F | 49 | L | Ocular melanosis | Excision + RT | 24 months |
| M | 30 | L | Excision + RT/chemotherapy | 48 months | |||
| Ijiri et al. (2000)32 | 1 | F | 5 | L | Excision + chemotherapy | 7 months | |
| Haskins et al. (2018)33 | 1 | F | 53 | L | Exenteration + intensity-modulated RT | 42 months | |
| Mukherjee et al. (2014)34 | 1 | M | 34 | L | Exenteration | Unknown | |
| Löffler and Witschel (1989)35 | 1 | M | 27 | R | Blue nevus | Exenteration | Unknown |
| Odashiro et al. (2005)36 | 1 | F | 43 | R | Blue nevus | Exenteration + RT | 19 months |
| Friedrich et al. (2007)37f | 1 | M | 22 | R | Excision | 14 months | |
| Hussain et al. (2017)38 | 1 | M | 59 | L | Blue nevus | Debulking + oncologic treatment | Unknown |
| Figueira et al. (2018)39 | 4 | 2 M, 2 F | Mean age = 43 | 3 L, 1 R | Blue nevus (2/4), orbital nevus (1/4), ocular melanosis (1/4) | Exenteration + RT | Mean 73 months |
| Adetunji et al. (2020)* | 2 | M | 38 | L | Exenteration + PBT | 5 months | |
| F | 65 | R | Exenteration + PBT | 51 months |
RT, radiotherapy; PBT, proton beam therapy.
Cases currently presented in this article.
The most common presenting symptoms and signs were unilateral proptosis (73%), decreased visual acuity (32%), periorbital pain (14%), diplopia (15%), and palpable mass (9%), but also included ptosis, eyelid swelling, visual disturbances, relative afferent pupillary defect, and poliosis. Imaging (CT or MRI) typically showed a well-circumscribed enhancing lesion, frequently resembling a benign tumor or arteriovenous malformation. A histologic diagnosis was performed in all cases via surgical biopsy or excision. Most tumors consisted of a variable mixture of spindled and epithelioid melanoma cells with some degree of pigmentation that were positive for melanocytic markers HMB-45, S-100, and Melan-A. Orbital blue nevus, a benign melanocytic precursor lesion, was present histologically in 42% of cases.
Treatment modality was reported in 77 of 88 cases. The majority of patients underwent orbital exenteration (57%) or excision/debulking (38%). The remaining patients were treated with systemic palliative support (4%) or a combination of radiotherapy and chemotherapy (1%). Thirty-six patients received orbital radiotherapy (47%) and 11 received chemotherapy (14%). Radiotherapy modalities included external beam radiotherapy (83%), proton beam radiotherapy (8%), intensity-modulated radiotherapy (6%), and plaque radiotherapy (3%). There was one case treated with adjuvant immunotherapy (1%).
Fifty-one of 88 cases reported follow-up after diagnosis of an average of 3 years (range 0.12–15 years). Of all published cases reporting patient outcomes (72/88), 26 patients developed metastases (36%), 11 developed local recurrence (15%), and 23 died of metastatic disease (32%). Three patients who developed metastases were in remission at last follow-up. Thirty-eight patients were alive without recurrence at last follow-up. The median event times for survival and metastasis were 174 and 168 months, respectively.
Among the 46 cases with reported follow-up duration who underwent exenteration or excision, adjuvant radiotherapy was associated with a lower hazard rate (HR) for death than no radiotherapy (HR 0.2, 95%CI 0.06–0.69, p = .01) (Figure 3). After adjustment for age and gender, surgery with radiotherapy remained associated with a lower HR for mortality (p = .013 and p = .014, respectively). The HR for recurrence did not differ between those who underwent surgery with radiotherapy compared with surgery without radiotherapy. There was no significant difference in the HR for mortality or recurrence between those who underwent exenteration compared with excision (Table 2). Twenty-one cases from a case series by Tellado et al did not include follow-up duration for each separate case, and these were analyzed separately (Table 3). The mean mortality rate in this series was 38%, with a mean follow-up duration of 4.5 years (range 0.2–13 years). One patient developed local recurrence after undergoing tumor excision.5 Among these cases, there was no significant difference in survival or recurrence between those who underwent exenteration compared with excision. In addition, in this case series of 21 patients, the presence of adjuvant radiotherapy was not associated with the rate of survival or recurrence.
Figure 3.
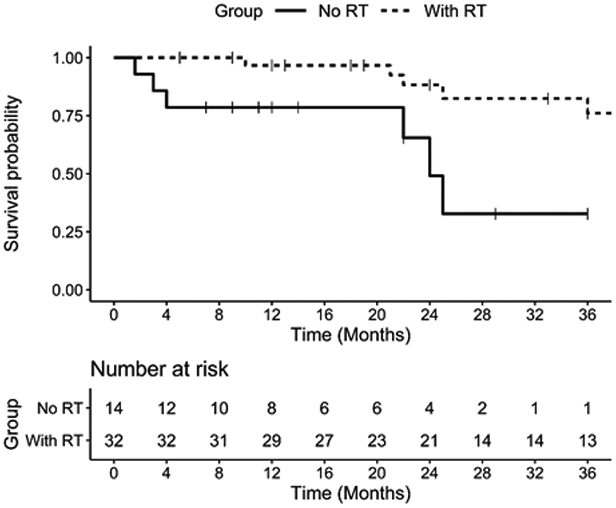
Kaplan-Meier curve for probability of survival for patients who underwent surgery with adjuvant radiotherapy (RT) compared with surgery with no RT. Among patients who underwent adjuvant RT, 6 had follow-up through 7 years without death, and 4 had follow-up to approximately 14 years with 1 death.
Table 2.
Death and recurrence among patients with recorded follow-up times, by treatment.
| Treatment | N | Deaths N (%) |
HR (95% CI) |
p-Value | Recurrence N (%) |
HR (95% CI) |
p-Value |
|---|---|---|---|---|---|---|---|
| Surgery type | |||||||
| Exenteration | 24 | 5 (21%) | 0.69 (0.21–2.27) | 0.54 | 2 (8%) | 0.32 (0.06–1.57) | 0.16 |
| Excision* | |||||||
| Radiotherapy with surgery† | 22 | 7 (32%) | Reference | - | 7 (32%) | Reference | - |
| Yes | 32 | 6 (19%) | 0.2 (0.06–0.69) | 0.01 | 6 (19%) | 0.25 (0.05–1.26) | 0.09 |
| No | 14 | 6 (43%) | Reference | - | 3 (21%) | Reference | - |
HR, hazard ratio; CI, confidence interval.
Excision also includes debulking surgery.
Surgery includes exenteration and excision/debulking procedures.
Table 3.
Death and recurrence among patients without recorded follow-up times, by treatment.
| Deaths | Recurrence | ||||
|---|---|---|---|---|---|
| Treatment | N | N (%) | p-Value | N (%) | p-Value |
| Surgery type | |||||
| Exenteration | 14 | 7 (50%) | 0.11 | 0 | 0.24 |
| Excision* | 7 | 1 (14%) | - | 1 (14%) | - |
| Radiotherapy with surgery† | |||||
| Yes | 4 | 2 (50%) | 0.62 | 0 | 1.0 |
| No | 17 | 6 (35%) | - | 1 (6%) | - |
Excision also includes debulking surgery.
Surgery includes exenteration and excision/debulking procedures.
Discussion
Primary orbital melanoma is a rare condition, comprising less than 1% of all orbital neoplasms.5 Over the past 40 years, there have been 22 case reports and a few small case series published on primary orbital melanoma. This article reviews all these cases in addition to presenting the two cases described above. The mean age at presentation was 45.1 (median 45), with the youngest reported case occurring in a 5-year-old girl and the oldest patient being 91 years old. Whites were predominantly affected, and the most common presentation was painless proptosis. Males were affected slightly more than females, although this difference was not statistically significant; interestingly, several studies on the incidence of uveal melanoma have also reported a slight male predominance.40,41 Predisposing pigmentary abnormalities such as nevus of Ota and ocular melanosis were present in a minority of cases (24%).
Imaging of patients frequently showed a well-circumscribed orbital mass with enhancement resembling a vascular lesion or benign tumor. Due to the rarity of primary orbital melanoma, benign radiographic characteristics and absence of ocular melanosis may lead to delays in diagnosis and treatment. Histologic diagnosis should be obtained by biopsy in cases where there is diagnostic uncertainty, with particular attention to differentiate primary orbital melanoma from other primary melanocytic neoplasms, such as orbital melanocytoma, which has a similar clinical and radiographic presentation.42,43
The most widely accepted treatment for primary orbital melanoma is surgery with adjuvant radiotherapy. Our study is the largest to date demonstrating differential patient outcomes based on treatment in primary orbital melanoma. In this review, although orbital exenteration was widely performed as primary treatment, it was not associated with a difference in survival or local recurrence. Orbital exenteration requires a second surgical procedure, necessitates significant cosmetic reconstruction, and may result in incomplete clearance of surgical margins.44 Additional factors, such as tumor characteristics and ability to achieve clear surgical margins, may have a greater influence on risk of local recurrence and metastasis than whether orbital exenteration is performed. This study also showed that surgery with adjuvant radiotherapy is associated with improved survival compared with surgery without radiotherapy for primary orbital melanoma. Correspondingly, previous studies of more common orbital malignancies have shown positive patient outcomes after treatment with surgery and radiotherapy.45,46 For instance, Hu et al46 reported effective local control in a study of patients with orbital malignancies treated with surgery and adjuvant carbon-ion or proton radiotherapy. Although external beam radiotherapy was the most common radiation modality used in this series, recently, there has been increasing interest in the use of proton beam radiotherapy (PBT) to treat orbital malignancies. PBT is a well-established treatment modality for uveal melanomas, resulting in high local control rates.47,48 It allows for close conformity to the tumor and uniform dose distribution, minimizing the total dose to normal tissues.48 Thus, high radiation doses can be delivered to tumors with decreased collateral damage to neighboring tissues.47 We report treatment of a case of primary orbital melanoma with orbital exenteration and adjuvant PBT. Currently, the patient remains without evidence of recurrence or metastasis 5 months after diagnosis. El-Sawy et al7 also described successful treatment of primary orbital melanoma with orbital exenteration and adjuvant PBT in a 9-year-old patient.
Despite recent advances in understanding the genetics of primary uveal and conjunctival melanoma, very few studies have analyzed the genetic alterations in primary orbital melanomas.12,13,49,50 A recent analysis of the genetic profiles of six cases of primary orbital melanoma revealed two potential genetic subgroups: a uveal-melanoma group and a conjunctival melanoma-like group.13 The uveal melanoma-like group had GNAQ, GNA11, and SF3B1 mutations, whereas the conjunctival melanoma-like group had NRAS12 and TERTp mutations. The authors speculated that proximity of the primary orbital melanoma to the conjunctiva in anteriorly located tumors may confer a conjunctiva-type genetic signature, possibly mediated by light exposure, while posteriorly located orbital melanomas have a uveal-like signature due to absence of exposure to light.13
Genetic changes are significant biomarkers of prognosis for uveal melanoma, but relatively less is known about the genetic alterations in conjunctival melanoma.13 For uveal melanoma, poor prognosis is indicated by the presence of monosomy 3, loss of 1p, and gain of 8q; in contrast, gain of 6p is associated with a better outcome.49,51 Loss of BAP-1 expression is associated with metastasis and with monosomy 3 in uveal melanoma, while mutations in SF3B1 and EIF1AX are indicators of better prognosis.49,52 Mutations in GNAQ and GNA11, frequently in codon 209, have been identified in up to 90% of uveal melanomas and lead to activation of the MAPK pathway.49,51 GNAQ/GNA11 mutations are not predictive of prognosis of uveal melanoma.49,51
A recent study by Rose et al12 found mutations in the SF3B1 gene present in 4 of 12 primary orbital melanomas and proposed that these mutations are associated with favorable outcomes in this type of tumor. The authors also reported a mutation in exon 1 of EIF1AX in one patient who had a favorable prognosis, suggesting that EIF1AX mutations may also correspond to better prognosis in primary orbital melanoma. In their study, loss of BAP-1 was not associated with worse outcomes, although only two cases had this variant.12 Interestingly, our case had a mutation in GNAQ (Gln209Pro, c.626A>C), indicating that his tumor had a uveal melanoma-like genetic profile. A splice-site variant in exon 1 of EIF1AX (c.17–1 G > T) was also present in this patient, which based on previous study, may indicate a better prognosis. Further studies of larger cohorts of primary orbital melanoma cases are necessary in order to determine biomarkers of prognosis based on genetic, molecular and histologic features.
Recently, advances in immunotherapy, particularly the development of immune checkpoint inhibitors, have shown notable success in treatment of advanced cutaneous melanoma. The combination of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor ipilimumab and programmed cell death 1 (PD-1) inhibitor nivolumab has led to a significant survival benefit in patients with metastatic cutaneous melanoma.53-55 The effect of immunotherapy in patients with ocular melanoma has been less well studied, although initial studies for uveal melanoma have shown limited success.56 Several small retrospective and prospective studies evaluating immune checkpoint inhibitors in metastatic uveal melanoma have demonstrated low response rates.56-58 Clinical trials investigating combination immune checkpoint inhibitors for metastatic uveal melanoma are ongoing.59,60 The efficacy of immunotherapy for primary orbital melanoma is currently unknown. This is the first report, to our knowledge, on the use of combined checkpoint inhibition with ipilimumab and nivolumab to treat primary orbital melanoma. Our case completed two cycles of ipilimumab and nivolumab, but therapy was discontinued due to immune-related toxicity and lack of tumor response. The lack of effectiveness of immunotherapy in this patient with primary orbital melanoma is perhaps unsurprising, given the low success rates in uveal melanoma and the genetic similarity between the two diseases. One proposed explanation for the limited efficacy of checkpoint inhibitors for uveal melanoma is the low mutational burden typically seen in uveal melanoma, leading to reduced recognition by immune cells.56 Interestingly, patients with metastatic uveal melanoma with atypically high mutational burdens have experienced more treatment success with checkpoint inhibitor therapy, lending support to this theory.56 This suggests that in patients with primary orbital melanoma, genetic testing may enable prediction of response to immunotherapy; further studies are needed to investigate this possible association.
This study has several limitations. As with any retrospective literature review, our findings were limited by the availability of data in published reports. Most notably, in this study, approximately one-fourth of cases with patient outcome data did not include follow-up duration, leading to exclusion of these cases from survival analyses. This review only included case reports and series, which are not controlled studies and may be subject to publication bias. Thus, the results of our quantitative analysis should be interpreted with caution. Prospective studies would provide stronger evidence for the efficacy of various treatments for primary orbital melanoma, but may not be feasible to conduct given the rarity of the clinical condition. Selection bias among those receiving more aggressive therapy (e.g., exenteration) due to tumor characteristics, as well as those not receiving adjuvant radiotherapy due to illness, may have influenced the observed associations between treatment modality and outcomes.
In conclusion, primary orbital melanoma is a rare malignancy which should be considered in patients with unilateral proptosis and a well-defined mass on imaging. Surgery remains the mainstay of treatment and adjuvant radiotherapy may improve patient survival. Postoperative PBT appears effective in providing local control of primary orbital melanoma. Genetic testing may provide insight into the prognosis and management of primary orbital melanoma, although further studies of larger cohorts are necessary.
Acknowledgments
Gui-Shuang Ying, PhDa.
References
- 1.Shields CL, Shields JA. Ocular melanoma: relatively rare but requiring respect. Clin Dermatol. 2009;27(1):122–133. doi: 10.1016/j.clindermatol.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Zografos L, Ducrey N, Beati D, Schalenbourg A, Spahn B, Balmer A, et al. Metastatic melanoma in the eye and orbit. Ophthalmology. 2003;110(11):2245–2256. doi: 10.1016/j.ophtha.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 montgomery lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela AA, Archibald CW, Fleming B, Ong L, O’Donnell B, Crompton JJ, et al. Orbital metastasis: clinical features, management and outcome. Orbit. 2009;28(2–3):153–159. doi: 10.1080/01676830902897470. [DOI] [PubMed] [Google Scholar]
- 5.Tellado M, Specht CS, McLean IW, Grossniklaus HE, Zimmerman LE. Primary orbital melanomas. Ophthalmology. 1996;103(6):929–932. doi: 10.1016/S0161-6420(96)30585-X. [DOI] [PubMed] [Google Scholar]
- 6.Koranyi K, Slowik F, Hajda M, Banfalvi T. Primary orbital melanoma associated with oculodermal melanocytosis. Orbit. 2000;19(1):21–30. doi: 10.1001/archopht.1990.01070100086040. [DOI] [PubMed] [Google Scholar]
- 7.El-Sawy T, Bakhoum MF, Tetzlaff M, Nasser QJ, Prieto VG, Ivan D. et al. Primary orbital melanoma in association with cellular blue nevus. Digit J Ophthalmol DJO. 2014;20:3. doi: 10.5693/djo.01.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan HHL, Kono T. Nevus of Ota: clinical aspects and management. Skinmed. 2003;2(2):89–96. doi: 10.1111/j.1540-9740.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- 9.Rootman J. Diseases of the Orbit: A Multidisciplinary Approach. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 10.Delaney YM, Hague S, McDonald B. Aggressive primary orbital melanoma in a young white man with no predisposing ocular features. Arch Ophthalmol. 2004;122(1):118–121. doi: 10.1001/archopht.122.1.118. [DOI] [PubMed] [Google Scholar]
- 11.Rose AM, Luthert PJ, Jayasena CN, Verity DH, Rose GE. Primary orbital melanoma: presentation, treatment, and long-term outcomes for 13 patients. Front Oncol. 2017;7:316. doi: 10.3389/fonc.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose AM, Luo R, Radia UK, Kalirai H, Thornton S, Luthert PJ, et al. Detection of mutations in SF3B1, EIF1AX and GNAQ in primary orbital melanoma by candidate gene analysis. BMC Cancer. 2018;18(1):1262. doi: 10.1186/s12885-018-5190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudhar HS, Doherty RE, Salvi SM, Currie ZI, Tan JH, Sisley K. Genetic profiling of primary orbital melanoma: an analysis of 6 cases with clinicopathologic correlation. Ophthalmology. 2019;126(7):1045–1052. doi: 10.1016/j.ophtha.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Chicago, IL: Springer; 2017. doi: 10.1001/jama.2010.1525. [DOI] [Google Scholar]
- 15.Bains S, Kim U, Shanti R. Orbital melanoma with calcification: a diagnostic dilemma. Indian J Ophthalmol. 2016;64(12):932–934. doi: 10.4103/0301-4738.198849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radhadevi CV, Charles KS, Lathika VK. Orbital malignant melanoma associated with nevus of Ota. Indian J Ophthalmol. 2013;61(6):306–309. doi: 10.4103/0301-4738.109526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Potter P, Levecq L, Godfraind C, Renard L. Primary orbital melanoma treated with iodine-125 plaque radiotherapy. Am J Ophthalmol. 2006;142(5):864–866. doi: 10.1016/j.ajo.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Krishnakumar S, Lakshmi SA, Abhyankar D, Biswas J. Loss of antigen-processing molecules in primary orbital melanoma. Orbit. 2003;22(4):265–270. doi: 10.1076/orbi.22.4.265.17249. [DOI] [PubMed] [Google Scholar]
- 19.Krishnakumar S, Lakshmi SA, Abhyankar D, Biswas J. Expression of HLA class I, β2-microglobulin and HLA class II antigens in primary orbital melanoma. Orbit. 2003;22(4):257–263. doi: 10.1076/orbi.22.4.257.17242. [DOI] [PubMed] [Google Scholar]
- 20.Rice CD, Brown HH. Primary orbital melanoma associated with orbital melanocytosis. Arch Ophthalmol. 1990;108(8):1130–1134. doi: 10.1001/archopht.1990.01070100086040. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira A, De F, Filho LG, Da S, Dias EL. Primary orbital melanoma in an anophthalmic socket. Radiol Bras. 2019;52(5):347–348. doi: 10.1590/0100-3984.2017.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke Y, Ren X, Zhu L, Hao R, Song W, Liu X, et al. Primary orbital melanoma combined with giant divided nevus of the eyelid. J Craniofac Surg. 2014;25(1):e4–7. doi: 10.1097/SCS.0b013e3182a32e89. [DOI] [PubMed] [Google Scholar]
- 23.Ranjit RU, Leyngold IM, Margo CE. Melanoma-associated spongiform scleropathy in oculodermal melanocytosis with primary orbital melanoma. Ocul Oncol Pathol. 2016;2(4):276–279. doi: 10.1159/000447595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsuhaibani AH, Alhumayed M. Primary orbital melanoma with poliosis and a palpable mass. Arch Ophthalmol. 2011;129(10):1382–1383. doi: 10.1001/archophthalmol.2011.302. [DOI] [PubMed] [Google Scholar]
- 25.Shields JA, Shields CL, Eagle RC, De Potter P, Oliver GL. Necrotic orbital melanoma arising de novo. Br J Ophthalmol. 1993;77(3):187–189. doi: 10.1136/bjo.77.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elibol O, Yuksel N, Egilmez HR, Arici S, Mizrak B. A case of primary orbital melanoma treated by local excision. Br J Ophthalmol. 1995;79(12):1146–1148. doi: 10.1136/bjo.79.12.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandeville JTH, Grove AS, Dadras SS, Zembowicz AM. Primary orbital melanoma associated with an occult episcleral nevus. Arch Ophthalmol. 2004;122(2):287–290. doi: 10.1001/archopht.122.2.287. [DOI] [PubMed] [Google Scholar]
- 28.Patrocínio LG, Lourenço C, Silva CDP, Barra DB, Patrocinio JA. Melanoma maligno primário da órbita. Rev Bras Otorrinolaringol. 2006;72(5):716. doi: 10.1590/s0034-72992006000500023. [DOI] [Google Scholar]
- 29.Mahoney NR, Engleman T, Morgenstern KE. Primary malignant melanoma of the orbit in an African-American man. Ophthal Plast Reconstr Surg. 2008;24(6):475–477. doi: 10.1097/IOP.0b013e31818d1ded. [DOI] [PubMed] [Google Scholar]
- 30.Polito E, Leccisotti A. Primary and secondary orbital melanomas: a clinical and prognostic study. Ophthal Plast Reconstr Surg. 1995;11(3):169–181. doi: 10.1097/00002341-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lee V, Sandy C, Rose GE, Moseley IM, Cree I, Hungerford JL. Primary orbital melanoma masquerading as vascular anomalies. Eye. 2002;16(1):16–20. doi: 10.1038/sj.eye.6700025. [DOI] [PubMed] [Google Scholar]
- 32.Ijiri R, Tanaka Y, Kato K, Sekido K, Sato H, Ito D. Primary orbital melanoma in a child. Med Pediatr Oncol. 2000;35(2):142–143. doi:. [DOI] [PubMed] [Google Scholar]
- 33.Haskins CP, Nurkic S, Fredenburg KM, Dziegielewski PT, Mendenhall WM. Primary orbital melanoma treated with orbital exenteration and postoperative radiotherapy: a case report and review of the literature. Head Neck. 2018;40(3):E17–E20. doi: 10.1002/hed.24983. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee B, Adulkar N, Krishnakumar S, Biswas J. Orbital melanoma: recurrence versus primary: A diagnostic dilemma. Indian J Cancer. 2014;51(3):379–380. doi: 10.4103/0019-509X.146733. [DOI] [PubMed] [Google Scholar]
- 35.Löffler KU, Witschel H. Primary malignant melanoma of the orbit arising in a cellular blue naevus. Br J Ophthalmol. 1989;73(5):388–393. doi: 10.1136/bjo.73.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odashiro AN, Arthurs B, Pereira PR, Filho JPS, Belfort E, Burnier MN. Primary orbital melanoma associated with a blue nevus. Ophthalmic Plast Reconstr Surg. 2005;21(3):247–248. doi: 10.1097/01.IOP.0000161716.46032.90. [DOI] [PubMed] [Google Scholar]
- 37.Friedrich RE, Grzyska U, Schäfer H, Li L. Navigation-assisted resection of a primary extraocular melanoma of the orbit. Anticancer Res. 2007;27:1799–1803. [PubMed] [Google Scholar]
- 38.Hussain A, Sidiropoulos M, Das S, Munoz DG, Nijhawan N. Orbital cellular blue nevus complicated by malignant melanoma. Can J Ophthalmol. 2017;52(3):e111–e113. doi: 10.1016/j.jcjo.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Figueira E, Rajak S, McKelvie P, Kalantzis G, Ismail A, Gonzales M, et al. Primary orbital melanoma: a case series and literature review. Orbit (London). 2018;37(5):352–357. doi: 10.1080/01676830.2017.1423354. [DOI] [PubMed] [Google Scholar]
- 40.Aronow ME, Topham AK, Singh AD. Uveal melanoma: 5-year update on incidence, treatment, and survival. Ocul Oncol Pathol. 2018;4(3):145–151. doi: 10.1159/000480640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology. 2003;110(5):956–961. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 42.Tregnago AC, Furlan MV, Bezerra SM, Porto GCLM, Mendes GG, Henklain JVR, et al. Orbital melanocytoma completely resected with conservative surgery in association with ipsilateral nevus of Ota: report of a case and review of the literature. Chen A, ed. Head Neck. 2015;37(4):E49–E55. doi: 10.1002/hed.23828. [DOI] [PubMed] [Google Scholar]
- 43.Mathai AM, Naik R, Pai MR, Kini JR, Kumar S, Ballal CK. Orbital melanocytoma. Orbit. 2008;27(5):383–387. doi: 10.1080/01676830802333626. [DOI] [PubMed] [Google Scholar]
- 44.Rahman I, Cook AE, Leatherbarrow B. Orbital exenteration: A 13 year Manchester experience. Br J Ophthalmol. 2005;89(10):1335–1340. doi: 10.1136/bjo.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holliday EB, Esmaeli B, Pinckard J, Garden AS, Rosenthal DI, Morrison WH, et al. A Multidisciplinary orbit-sparing treatment approach that includes proton therapy for epithelial tumors of the orbit and ocular adnexa. Int J Radiat Oncol Biol Phys. 2016;95(1):344–352. doi: 10.1016/j.ijrobp.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu W, Hu J, Gao J, Yang J, Qiu X, Kong L, et al. Outcomes of orbital malignancies treated with eye-sparing surgery and adjuvant particle radiotherapy: a retrospective study. BMC Cancer. 2019;19(1):776. doi: 10.1186/s12885-019-5964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra KK, Daftari IK. Proton therapy for the management of uveal melanoma and other ocular tumors. Chinese Clin Oncol. 2016;5(4):50. doi: 10.21037/cco.2016.07.06. [DOI] [PubMed] [Google Scholar]
- 48.Stannard C, Sauerwein W, Maree G, Lecuona K. Radiotherapy for ocular tumours. Eye. 2013;27(2):119–127. doi: 10.1038/eye.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helgadottir H, Höiom V. The genetics of uveal melanoma: current insights. Appl Clin Genet. 2016;9:147–155. doi: 10.2147/TACG.S69210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griewank KG, Westekemper H, Murali R, Mach M, Schilling B, Wiesner T, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19(12):3143–3152. doi: 10.1158/1078-0432.CCR-13-0163. [DOI] [PubMed] [Google Scholar]
- 51.Francis RE, Mohammed D, Jessica A, Lijka-Jones B, Sisley K. Genetics of Uveal Melanoma. In: Scott JF, Gerstenblith MR, eds. Noncutaneous Melanoma. Brisbane, Australia: Codon Publications; 2018: 19–35. [PubMed] [Google Scholar]
- 52.Staby KM, Gravdal K, Mørk SJ, Heegaard S, Vintermyr OK, Krohn J. Prognostic impact of chromosomal aberrations and GNAQ, GNA11 and BAP1 mutations in uveal melanoma. Acta Ophthalmol. 2018;96(1):31–38. doi: 10.1111/aos.13452. [DOI] [PubMed] [Google Scholar]
- 53.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;9(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 54.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob -J-J, Rutkowski P, Lao CD. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/nejmoa1910836. [DOI] [PubMed] [Google Scholar]
- 56.Schank TE, Hassel JC. Immunotherapies for the treatment of uveal melanoma—history and future. Cancers (Basel). 2019;11(8). doi: 10.3390/cancers11081048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heppt MV, Heinzerling L, Kähler KC, Forschner A, Kirchberger MC, Loquai C. et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer. 2017;82:56–65. doi: 10.1016/j.ejca.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 59.Pelster M, Gruschkus SK, Bassett R, Gombos DS, Shephard M, Posada L, et al. Phase II study of ipilimumab and nivolumab (ipi/nivo) in metastatic uveal melanoma (UM). J Clin Oncol. 2019;37(e):9522. doi: 10.1200/jco.2019.37.15_suppl.9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piulats Rodriguez JM, De La Cruz Merino L, Espinosa E, Alonso Carrión L, Martin Algarra S, López-Castro R. et al. Phase II multicenter, single arm, open label study of nivolumab in combination with ipilimumab in untreated patients with metastatic uveal melanoma (GEM1402.NCT02626962). Ann Oncol. 2018;29:viii442–viii466. doi: 10.1093/annonc/mdy289.003. [DOI] [Google Scholar]


