Abstract
Background:
Up to 20% of patients with chronic immune-mediated sensorimotor neuropathies (CIN) do not respond adequately to first-line therapies. However, studies on further treatment are scarce.
Methods:
We analyzed retrospectively 200 CIN patients regarding disease characteristics and response to therapy with cyclophosphamide (CYP), rituximab (RTX), and bortezomib (BTZ). Treatment response was defined as improvement or stabilization of inflammatory neuropathy cause and treatment overall disability score (INCAT-ODSS).
Results:
A total of 48 of 181 patients (26.5%) received therapy with CYP, RTX, or BTZ. The most frequently and first used therapy was CYP (69%). More than 40% of patients needed a second or third treatment. Overall, 71 treatments were applied in 48 patients. The combination of up to all three treatments enhanced the response-rate to 90%. Treatment within 24 months after initial diagnosis resulted in significantly higher response rate than late treatment (79% versus 50 %, p = 0.04, χ2-test, n = 46) and in lower disability in long-term follow up (INCAT-ODSS 3.8 versus 5.8, p = 0.02, t-test, n = 48). Patients with Lewis-Sumner syndrome (n = 9) and autoantibody mediated neuropathies (n = 13) had excellent response rates after treatment with RTX (90–100%). In contrast, typical chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) showed a response rate of 64% in CYP, 64% in RTX, and 75% in BTZ.
Conclusion:
Treatment with CYP, RTX, or BTZ was effective in this cohort of CIN refractory to first-line treatment. Our data increase evidence for an early use of these therapies. High efficacy of RTX in Lewis-Sumner syndrome in contrast to typical CIDP suggests a distinct pathophysiology.
Keywords: bortezomib, chronic inflammatory demyelinating polyneuropathy, CIDP, cyclophosphamide, efficacy, rituximab, safety, treatment
Introduction
Pathogenesis and clinical characteristics of chronic immune-mediated sensorimotor neuropathies (CIN) are complex.1 In addition to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), clinical subgroups like the multifocal acquired demyelinating sensory and motor neuropathy (MADSAM), also named Lewis-Sumner syndrome and autoantibody-mediated variants have been characterized in recent years.2–8 Accordingly, treatment of CIN is challenging. Up to 20% of patients do not adequately respond to first-line therapy with steroids (STE), immunoglobulins (IVIg), and plasmapheresis (PE) and require further immunotherapy.9–13 Studies on these treatments are limited.14 Two drugs used commonly in patients refractory to first-line treatment or with severe disease course are rituximab (RTX) and cyclophosphamide (CYP). RTX is a monoclonal anti-CD20 antibody selectively depleting premature B-cells. A total of 60 patients with CIDP with a response rate of 78% to RTX were reported in different case series.14–18 CYP has also been reported to be a sufficient immunosuppressant therapy. In a case series of 51 CIDP patients, 35 (69%) benefited from CYP therapy.14 A third novel treatment in refractory CIDP is bortezomib (BTZ) – a proteasome inhibitor. In a previous case series, our group described the efficacy of BTZ in 10 CIDP patients.19 Further reports about the effectiveness of these therapies in CIN are necessary. The aim of this study was to retrospectively analyze clinical response to treatment with CYP, RTX, and BTZ in a cohort of 200 CIN patients.
Methods
Patients
In a single-center retrospective observational study (St. Josef-Hospital, University Hospital Bochum, Germany), 200 patients with CIN (whole cohort) were analyzed regarding demographic, clinical, and treatment data during January 2005 and June 2019. Patients with typical CIDP (tCIDP) were diagnosed in accordance with the diagnostic criteria of the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS).20 Atypical CIDP (aCIDP) was diagnosed in accordance with EFNS/PNS criteria and criteria from Doneddu et al.,21 including distal acquired demyelinating symmetric neuropathy (DADS) and MADSAM. Paraproteinemic demyelinating neuropathy (PDN) was diagnosed according to EFNS/PNS criteria as demyelinating neuropathy with clinical phenotype of distal or distal and proximal symmetric neuropathy and IgM monoclonal gammopathy of undetermined significance (MGUS) with or without myelin-associated glycoprotein (MAG) antibodies.22 Paraprotein was searched by serum protein electrophoresis and immunofixation. If available, patients were stratified for the autoantibody status [anti-MAG, anti-ganglioside, Neurofascin-155 (NF155), Contactin1].
Drugs, dosage and duration
Treatments were applied in the following schemata 14,20,23:
1. First-line:
- IVIg: 2 g/kg divided over 2–4 days, maintained by 1–2 g/kg divided over 1–2 days every 4–8 weeks, attempting individual dose reduction after stabilization;
- subcutaneous immunoglobulins (scIg) 0.2–0.4 g/kg per week with individual dosing after stabilization;
- STE: 1 mg/kg per day orally, or intermittent high dose therapy with 500–1000 mg methylprednisolone for 3–5 days intravenous and repetition every 12 weeks;
- PE: 5–7 cycles of PE during 2 weeks.
2. Other immunotherapies (in or without combination to first-line):
- Azathioprine (AZA): 150–250 mg daily with a target lymphocyte count of 700–1200/μl;
- Mycophenolate mofetil/mycophenolic acid (MPA): 1000–2000 mg daily with a target trough level of 1–2 mg/l;
- Cyclosporin (CsA): 3–5 mg/kg daily with a target trough level of 70–150 ng/ml;
- Methotrexate (MTX): 15 mg per week orally or subcutaneous.
3. Therapy with CYP, RTX, and BTZ:
- CYP: induction with 350 mg/m2 daily for 3 days, then 600 mg/m2 every 4–8 weeks adjusted depending on leucocyte nadir;
- RTX: 500–1000 mg every 6–12 months depending on CD19 cell depletion in peripheral blood;
- BTZ: initiation with one or two cycles, one cycle consisting of four subcutaneous injections of BTZ (1.3 mg/m2) within 2 weeks, at days 1, 4, 8, and 11. Repetition after 6–12 months depending on the clinical response.19
In case of combination of CYP, RTX, and BTZ, these were used consecutively and not simultaneously.
Outcome analyses
Clinical disability was evaluated using the 12-point inflammatory neuropathy cause and treatment overall disability score (INCAT-ODSS), Medical Research Council Sum Score (MRC) and modified Rankin scale (mRS) at initial diagnosis, at timepoint of treatment with CYP, RTX, and BTZ, as well as 18–6 months prior to, and 3–18 months after, treatment.24–26 Long-term outcome was defined using last available INCAT-ODSS.
Response to every single treatment with CYP, RTX, and BTZ was defined as: (a) improvement by at least one INCAT-ODSS point, or (b) sustained stabilization of INCAT-ODSS for at least 6 months after prior worsening of at least one point in the previous 3 months.
Some patients received more than one, in some cases up to three, therapies consecutively (CYP, RTX, and BTZ). In these cases, the ‘overall response’ described the status at the end of all three applied therapies.
Non-responders were defined as: (a) failure to stabilize disease progression after a worsening of at least one INCAT-ODSS point in the previous 3 months, (b) failure to improve INCAT-ODSS by at least one point in case of prior stable INCAT-ODSS, (c) adverse side effects that do not allow continuation of therapy, (d) death due to progressive disease course.
Statistics
Statistical analysis was performed using IBM® SPSS Statistics (version 26.0.0.0). Demographics and clinical characteristics were compared between groups using Student’s t test for numerical normally distributed variables or chi-squared (χ2-test) for nominal variables. Analysis of variance (ANOVA) was used to analyze the outcome parameter INCAT-ODSS in dependence of the treatments. χ2-test was used to evaluate whether the therapy was associated with clinical response (response rate). For all analyses, the statistically significant threshold was set at p value < 0.05.
Results
Characteristics of the whole cohort
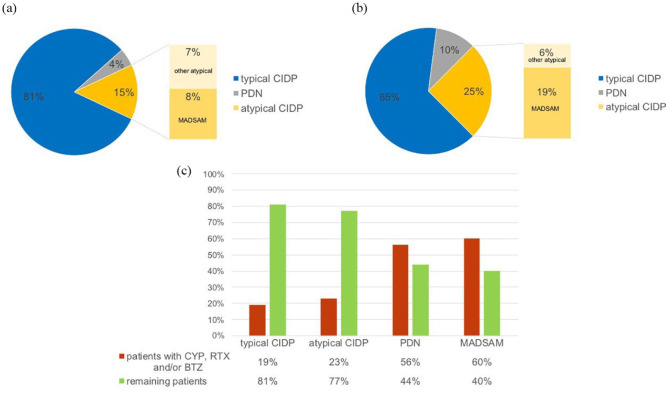
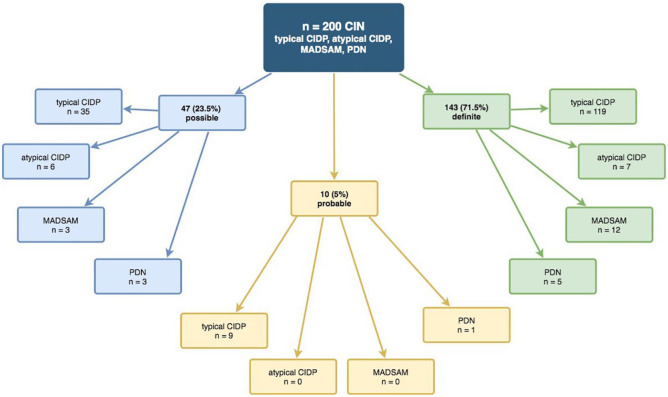
The whole cohort consisted of 200 patients, 138 male (69%) and 62 female (31%); 163 had tCIDP (81.5%), 13 had aCIDP (6.5%), 15 had MADSAM (7.5%), and 9 had PDN (4.5%, Figure 1a). The distribution of electrophysiological EFNS/PNS criteria in ‘possible,’ ‘probable,’ and ‘definite’ is shown in Figure 2 and Supplemental Table S1.
Figure 1.
(a) Distribution of types of CIN in the whole cohort. (b) Distribution of types of CIN in the patients receiving CYP, RTX, and/or BTZ. (c) Proportion of patients receiving CYP, RTX, and/or BTZ per group.
BTZ, bortezomib; CIDP, chronic inflammatory demyelination polyneuropathy; CIN, chronic immune-mediated sensorimotor neuropathies; CYP, cyclophosphamide; MADSAM, Lewis-Sumner syndrome/multifocal acquired demyelinating sensory and motor neuropathy; PDN, paraproteinemic demyelinating neuropathy; RTX, rituximab.
Figure 2.
Fulfillment of electrophysiological EFNS/PNS criteria in the whole cohort.20
CIDP, chronic inflammatory demyelination polyneuropathy; CIN, chronic immune-mediated sensorimotor neuropathies; EFNS/PNS, European Federation of Neurological Societies/Peripheral Nerve Society; MADSAM, Lewis-Sumner syndrome/multifocal acquired demyelinating sensory and motor neuropathy; PDN, paraproteinemic demyelinating neuropathy.
At the time point of diagnosis, mean age was 57 ± 13.8 years, MRC score was 56.5 ± 5.0 (n = 107) and mRS was 1.68 ± 0.99 (n = 165). Mean follow-up time from diagnosis until last follow up was 6.1 ± 5.8 years. During this time, mean INCAT-ODSS of the whole cohort deteriorated by 35% (0.8 points, Table 1).
Table 1.
Patient characteristics of whole cohort, patients treated with CYP, RTX, and/or BTZ and remaining patients.
| Whole cohort | Patients treated with CYP, RTX, and/or BTZ | Remaining patients | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | ||||||||
| Gender, male/female | 138/62 | 200 | 35/13 | 48 | 103/49 | 153 | ||||
| Age at first diagnosis, mean ± SD | 57.0 | 13.8 | 186 | 55.2 | 12.0 | 48 | 57.7 | 14.4 | 139 | 0.27 |
| INCAT-ODSS at initial diagnosis, mean ± SD | 2.3 | 1.9 | 169 | 3.1 | 2.5 | 48 | 2.0 | 1.6 | 121 | <0.01 |
| MRC sum score at initial diagnosis (max. 60), mean ± SD | 56.5 | 5.0 | 107 | 55.6 | 4.6 | 24 | 56.7 | 5.2 | 83 | 0.35 |
| mRS at initial diagnosis, mean ± SD | 1.7 | 1.0 | 165 | 2.0 | 1.1 | 42 | 1.6 | 0.9 | 123 | 0.04 |
| Time between manifestation and diagnosis (months), mean ± SD | 37.1 | 42.6 | 184 | 34.7 | 42.7 | 47 | 38.0 | 42.8 | 137 | 0.65 |
| Disease duration before treatment with CYP, RTX, and/or BTZ (months), mean ± SD | 39.7 | 48.9 | 48 | |||||||
| Follow-up time (years), mean ± SD | 6.1 | 5.8 | 186 | 7.5 | 6.1 | 48 | 5.6 | 5.7 | 138 | 0.04 |
| Age at the end of follow up, mean ± SD | 63.4 | 13.4 | 200 | 63.0 | 12.4 | 48 | 63.5 | 13.7 | 152 | 0.81 |
| INCAT-ODSS at the end of follow up, mean ± SD | 3.1 | 2.4 | 194 | 4.8 | 2.9 | 48 | 2.5 | 1.8 | 146 | <0.001 |
BTZ, bortezomib; CYP, cyclophosphamide; INCAT-ODSS, inflammatory neuropathy cause and treatment overall disability score; MRC, Medical Research Council; mRS, modified Rankin scale; RTX, rituximab; SD, standard deviation.
Treatment of the whole cohort
Therapy data were available for 181 (90.5%) patients. IVIg was the first-line therapy used most frequently (84.5%), followed by STE (73.5%). PE was rarely used (12.2%). Each patient received at least one attempt of first-line treatment. Of second-line therapies, AZA (36.3%) and mycophenolate (20.9%) were the treatments used most frequently. A total of 66 (36.5%) patients received first-line therapy only, while 67 (37.0%) received second-line drugs other than CYP, RTX, and/or BTZ; 48 patients (26.5%) received a therapy with CYP, RTX, and/or BTZ. Details of first-line and other therapies are given in Table 2.
Table 2.
Overview of first- and second-line drugs used in the whole cohort.
| n | % | |
|---|---|---|
| First-line drugs | ||
| Intravenous immunoglobulins | 153 | 85 |
| Steroids | 133 | 74 |
| Plasma exchange | 22 | 12 |
| Subcutaneous immunoglobulins | 15 | 8 |
| Second-line drugs | ||
| Azathioprine | 66 | 36 |
| Mycophenolate mofetil | 38 | 21 |
| Steroids (orally) | 30 | 17 |
| Ciclosporin A | 24 | 13 |
| Methotrexate | 6 | 3 |
| Therapy regimens of 181 patients (90.5%) were available. | ||
Characteristics of patients treated with CYP, RTX, and/or BTZ
A therapy with CYP, RTX, and/or BTZ was performed in 48 patients refractory to first-line treatments with IVIg, STE, or PE (26.5% of 181 patients). Characteristics of these patients are given in Table 1. The diagnoses PDN and MADSAM were more frequent in this group, while typical CIDP was more frequent in the group not receiving CYP, RTX, and/or BTZ (Figure 1). In particular, 60% of MADSAM and 55% of PDN received a therapy with CYP, RTX, and/or BTZ. In contrast, patients with tCIDP received these treatments in 19% of cases; 33.3% of MADSAM patients also had a MGUS (IgM n = 1, IgG n = 3). Patients treated with CYP, RTX, and/or BTZ did not differ from the remaining patients in age, sex, and time from first manifestation to diagnosis (Table 1). Those patients needing potent immunotherapy with CYP, RTX, and/or BTZ had a worse INCAT-ODSS at the time of diagnosis as well as at the end of follow up. The MRC sum score at timepoint of diagnosis did not differ, but was available in only 50% of patients. The mean duration from diagnosis to first treatment with CYP, RTX, or BTZ was 39.7 ± 48.9 months, median was 26.5 [interquartile range (IQR) 48, range 0–272]. The follow-up time was significantly longer in the patients treated with CYP, RTX, or BTZ (7.5 years) than in the remaining patients (5.6 years, Table 1).
Supplemental Table S2 shows the individual features in patients treated with CYP, RTX, or BTZ. A total of 29 (60%) patients were treated with a monotherapy of CYP, RTX, or BTZ, most frequently CYP (n = 18). RTX monotherapy was performed in nine patients, and two received a monotherapy with BTZ. Still 40% of the patients did not respond to the first therapy and needed further treatment. Of these, 19 patients (40%) received a combination of treatments in their disease courses. In total 48 patients were treated with 71 regimens of CYP, RTX, or BTZ (1.48 treatments/patient). Of these 71 regimens, CYP was the treatment used most frequently (n = 34, 47.9%), followed by RTX (n = 25, 35.2%) and BTZ (n = 12, 16.9%).
Distribution of the applied treatment regimen was strongly dependent on the type of CIN (Table 3): 87% of patients with tCIDP were treated with CYP. In contrast, 89% of patients with MADSAM and most patients with aCIDP and PDN were treated with RTX. BTZ was used in all groups similarly.
Table 3.
Distribution of therapy regimens dependent on type of CIN (n = 48).
| Type of CIN | Typical CIDP (n = 31 patients) | MADSAM (n = 9 patients) | Atypical CIDP (n = 3 patients) | PDN (n = 5 patients) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Therapy regimen | Regimens/type of CIN* % | Regimens/type of CIN* % | Regimens/type of CIN* % | Regimens/type of CIN* % | |||||
| CYP | 27 | 87 | 5 | 56 | 1 | 33 | 1 | 20 | <0.01 |
| RTX | 11 | 36 | 8 | 89 | 2 | 67 | 4 | 80 | 0.02 |
| BTZ | 8 | 26 | 2 | 22 | 1 | 33 | 1 | 20 | 0.97 |
Number of therapy regimens used per number of patients in each type of CIN, indicating inhomogenous use of drugs in different types of CIN.
BTZ, bortezomib; CIDP, chronic inflammatory demyelinating polyneuropathy; CIN, chronic immune-mediated sensorimotor neuropathies; CYP, cyclophosphamide; MADSAM, Lewis-Sumner syndrome/multifocal acquired demyelinating sensory and motor neuropathy; PDN, paraproteinemic demyelinating neuropathy; RTX, rituximab.
Treatment response and outcome of patients treated with CYP, RTX, and/or BTZ
As described in Methods, treatment response was defined as: (a) improvement by at least one INCAT-ODSS point or (b) sustained stabilization of INCAT-ODSS for at least 6 months after prior worsening of at least one point in the previous 3 months.
The overall response-rate was 85.4% in patients refractory to first-line treatments who were treated with CYP, RTX, and/or BTZ. Response-rates to first, second, and third therapy with CYP, RTX, and/or BTZ and response rates between the three different drugs were equal.
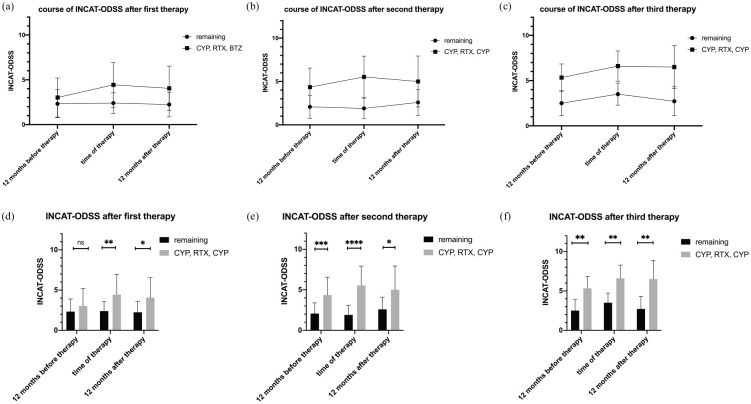
The INCAT-ODSS in Figure 3 illustrates the courses of disease after first, second, and third therapy with CYP, RTX, and/or BTZ in comparison with patients not receiving treatment with CYP, RTX, and/or BTZ (remaining patients). The INCAT-ODSS course in Figure 3 shows the therapeutic response to CYP, RTX, and/or BTZ at 12 months after treatment.
Figure 3.
INCAT-ODSS before and after first (n = 48), second (n = 18), and third therapy (n = 6) with CYP, RTX, and/or BTZ for patients refractory to first-line treatment. Mean duration until first treatment with CYP, RTX, and/or BTZ was 39.7 months, until second treatment 67.1 months, and until third treatment 119.8 months. For comparison, INCAT-ODSS at 40, 67, and 120 months after initial diagnosis of patients not treated with CYP, RTX, and/or is shown in the figure (remaining patients). At 12 months before first therapy, the INCAT-ODSS did not differ significantly between both groups but was higher in patients treated with CYP, RTX, and/or BTZ (3.0 versus 2.3, t test, p = 0.09). At the time of first treatment with CYP, RTX, and/or BTZ, the score increased to 4.4 points in patients treated with CYP, RTX, and/or BTZ and stayed at 2.4 points in the remaining patients (t test, p < 0.01). At 12 months after first therapy with CYP, RTX, and/or BTZ, the INCAT-ODSS decreased, showing the therapeutic response. However, patients treated with CYP, RT,X and/or BTZ were still more disabled than the remaining patients (INCAT-ODSS 4.0 versus 2.2 at 12 months after therapy; p < 0.01). Similar results were found for second and third therapy. The INCAT-ODSS in patients receiving a third therapy increased to up to 6.5 points, whereas the remaining patients stayed between 2.5 and 3.5 points (p < 0.01).
*p = 0.05, **p = 0.01,***p = 0.001,****p < 0.001.
BTZ, bortezomib; CYP, cyclophosphamide; INCAT-ODSS, inflammatory neuropathy cause and treatment overall disability score; RTX, rituximab.
The subgroup of patients receiving treatment with BTZ was clinically more affected than patients who were treated with CYP or RTX only. Patients treated with BTZ suffered from the disease 6.1 years longer than patients treated with CYP or RTX (6.0 ± 4.7 versus 12.1 ± 8.0, t test p < 0.01) and showed the worst outcome in INCAT-ODSS (4.1 ± 2.3 versus 7.7 ± 3.1, t test p < 0.01), but did not differ at time of diagnosis.
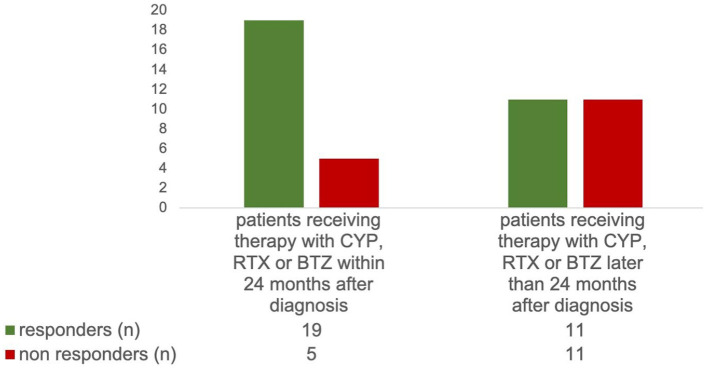
Patients who received therapy with CYP, RTX, or BTZ within 24 months after diagnosis had a significantly higher response rate after first therapy (79.2% versus 50.0%, χ2-test p = 0.034) and a better INCAT-ODSS 12 months after first therapy (3.3 versus 4.8, t test p = 0.03) than those who were treated later (Figure 4). At initial diagnosis and at time point of therapy with CYP, RTX, or BTZ, the INCAT-ODSS was equal in patients treated early and late.
Figure 4.
Patients who received therapy with CYP, RTX, or BTZ within 24 months after diagnosis had a significantly higher therapeutic response rate after first therapy than those who were treated later (79.2% versus 50.0%, χ2-test, p = 0.034).
BTZ, bortezomib; CYP, cyclophosphamide; RTX, rituximab.
Regarding the outcome of the different types of CIN refractory to first-line treatment, we found a significantly better response rate for the nine MADSAM patients to RTX than to CYP and BTZ (n = 15 regimens, response rate of RTX 100% (8/8), CYP 40% (2/5), BTZ 0% (0/2); p < 0.01, χ2-test). However, CYP was used most frequently as first drug out of CYP, RTX, and BTZ in MADSAM (55.6%), resulting in low response rates of MADSAM after CYP (44.4%).
For the 13 patients with autoantibodies of any kind, we found a better response rate after using RTX than CYP (response rate of RTX 90%, CYP 50%, p = 0.1005, χ2 test). One patient with anti-NF155 and one with anti-Contactin were initially treated with RTX, and both responded to treatment. Three of four cases with anti-MAG antibodies responded excellently to RTX (80%), whereas one further patient with a follow-up time of only 6 months did not (yet) respond. PDN showed an overall therapeutic response rate of 80%.
Patients with typical CIDP (n = 31) had an overall response rate to therapy with CYP, RTX, and/or BTZ of 90%. However, in 50% of the patients, this good response did not result from one individual therapy but from a consecutive combination of two (n = 11, i.e., CYP plus RTX) or three (n = 4, CYP plus RTX plus BTZ) drugs. The response rate of the individual drugs was 64% for CYP (n = 25), 63.6% for RTX (n = 11), and 75% for BTZ (n = 8). Typical CIDP was the only subgroup in which all three therapies (CYP plus RTX plus BTZ) were applied consecutively.
Side effects
CYP led to more adverse effects than RTX and BTZ. One patient receiving CYP developed a pancreatitis, one developed a leucopenia, and one severe nausea and vomiting. These events led to discontinuation of therapy. Two cancers occurred after CYP and at least one seemed to be associated with CYP. One patient developed urothelial carcinoma despite treatment with Mesna (cumulative dosage of CYP: 4110 mg/m2, carcinoma 18 months after last therapy with CYP); another had a colon carcinoma (cumulative dosage of CYP: 4340 mg/m2, carcinoma 22 months after last therapy with CYP). During RTX treatment, one patient described a mild itching, no severe reactions occurred in our cohort. Regarding BTZ, no undesirable effects were observed in our cohort.
Discussion
Treatment with CYP, RTX, or BTZ was effective in this cohort of CIN refractory to first-line treatment. The strength of this study is that we explored a large cohort of CIN patients. All patients analyzed in this study were refractory to first- and second-line treatments; 85% of these CIN patients showed therapeutic response to CYP, RTX, and BTZ. Early treatment with CYP, RTX, and BTZ improved the long-term outcomes compared with treatment later than 24 months after diagnosis.
Our whole cohort is comparable with other international CIN cohorts.21,27,28 Only disease duration was somewhat shorter in our cohort than in the Italian cohorts.21 Our ratio of 1.48 therapy regimens per patient is similar to the study of Cocito et al.29 However, our cohort is unique in their form. There are cohorts that were also treated with RTX and CYP in combination, but none that included BTZ.
An important aspect of our cohort is that RTX was used in a low-dose treatment regimen (500–1000 mg every 6–12 months depending on CD19 cell depletion), different to what is used in rheumatological conditions. Dosing of RTX is discussed frequently, and some studies show that lower dosing of RTX depending on CD19 depletion results in similar effectiveness with fewer side effects like infections.30–32
In our cohort, 26.5% of patients needed a therapy beyond the usual immunosuppressants. As expected, the patients receiving therapy with CYP, RTX, and/or BTZ were severely affected. However, it is noteworthy that these patients already had a more severe disability, measured as worse INCAT-ODSS and mRS values when initially diagnosed. Interestingly, patients receiving therapy with CYP, RTX, and/or BTZ more often had aCIDP or MADSAM than the remaining patients.
Due to the rapid onset of action and its broad immunological spectrum, we suppose CYP is the most common and first used drug. RTX as first drug was used mainly for patients with atypical CIDP and with suggested autoantibody or B-cell-driven disease like paranodopathies. BTZ served as third therapy in patients that did not respond to CYP and RTX. The combination of up to all three treatments enhanced the overall response rate to 85%, which we consider very good. The high response rate compared with some other cohorts is due to the fact that, with the INCAT-ODSS, we used a specific disability score for immune mediated polyneuropathies.29,33 In addition, stabilization of the disease after previous deterioration was already defined as response in our analysis. No drug appeared superior in the overall response. This supports the thesis that CYP, RTX, and BTZ address different pathophysiological means of generating independent respond rates.1
We should point out that the therapeutic response to RTX in MADSAM was excellent. Previous studies have shown that MADSAM is difficult to treat. In most studies, the therapeutic response rate is below that of typica CIDP.21,34 Doneddu et al. showed a low overall treatment response rate in MADSAM patients (67%).21
Cocito et al. also described a significantly lower response rate to therapies in patients with MGUS.29 PDN showed the worst overall response rate in our cohort. The response to CYP was good, but a strong limitation is the small number of PDN in our cohort. Other studies showed a good therapeutic response of MGUS patients after treatment with RTX.15,16
Ten patients were positive for any kind of autoantibodies (anti-MAG, anti-ganglioside, NF155, Contactin1) and had excellent response to RTX, although these have distinct disease entities and pathophysiology. This good response to RTX is in accordance with the literature.6,8,35–40 Therefore, RTX should be considered for patients with autoantibody related disease.
Typical CIDP was very well treatable, with an overall response of 90%. However, the response rate of the single drugs was low. Therapy of these patients still means trial and error. Further research into biomarkers and clinical patterns is urgently necessary in order to better understand the underlying pathophysiology of this group.
Importantly, our results do not imply that RTX or CYP are the best drugs for all refractory CIN patients, but only for certain groups.
We use lower dosage of CYP for treatment of CIN than the dose used for oncological diseases. This results in fewer side effects. However, combining the three drugs (CYP, RTX, and BTZ) has some potential risks, as side-effects and long-term effects in CIDP are still unknown. Detailed explanation of these aspects, informed consent of patients, and experience of the treating physicians are mandatory. The optimal time point of adding the next treatment is still unknown. In our experience, addition of BTZ to RTX could be performed quite safely after a few weeks, while we would prefer a longer interval of at least 3–6 months when adding RTX to CYP or vice versa, as treatment-failure of one drug needs at least some months of follow up to be determined.
Our study has some limitations. Firstly, the data were retrospective and obtained from a single center without a matched control group. Recommendations for combining CYP, RTX, and BTZ need confirmation in further, ideally randomized and controlled, studies to reach satisfactory evidence. Second, the group size was small for some subgroup analyses and the follow-up time was different in some comparisons. Moreover, only INCAT-ODSS was available for longitudinal assessment of clinical disability. We consider longitudinal use of INCAT-ODSS a good tool to detect treatment-refractory patients. A change of one point in INCAT-ODSS reflects relevant change of disability and an adjusted form, the “INCAT disability score”, was used as primary outcome measure in the large randomized ICE-study.41 Therefore, INCAT-ODSS can be considered as one standard tool to define treatment response. A limitation of INCAT-ODSS is that it does not detect minor clinical changes. The Rasch-built overall disability scale (R-ODS) is more suitable for this and is especially useful to detect activity and social participation impairment.42 A change of R-ODS of ⩾4 points was used to define treatment refractory patients in the PATH study.43 Grip strength is another good tool to detect improvement or worsening of motor functions in CIDP under therapy.44 Longitudinal data on grip strength and R-ODS were not available in our cohort. Moreover, the change from 0 to 1 point in the arm score in INCAT-ODSS is not considered as a relevant change in disability for treatment decisions according to the regulatory agencies of the ICE-study.41 It might be preferable to adopt these for our analysis but unfortunately only INCAT-ODSS sum score, and no data on separate arm and leg scores, were available in our cohort. Moreover, not all patients were tested for (para)nodal antibodies, as this manuscript includes data from 2005.
Nevertheless, the results from this study are valuable because they provide a foundation for future prospective treatment studies.
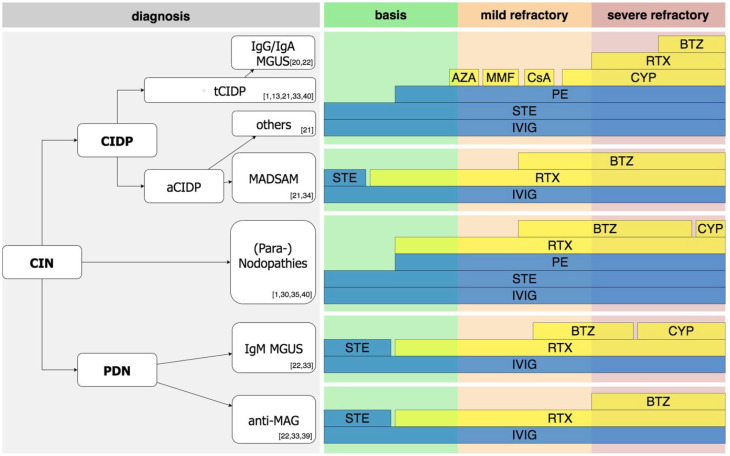
The main message of this study is that therapy with CYP, RTX, and BTZ is effective in a substantial proportion of refractory CIN patients. Early application improves long-term outcomes. MADSAM and autoantibody mediated neuropathies respond especially well to RTX. BTZ should be considered as a therapy option in CIN refractory to RTX. Severe side effects are rare if treatments are applied in the stated dosage. Our results suggest that therapy protocols need a strong pathophysiological driven algorithm in the context of personalized medicine. A treatment algorithm based on the findings of this study is proposed in Figure 5.
Figure 5.
Recommendation on therapeutic approaches in CIN. Note that the suggested algorithm is of evidence level III according to US Preventive Services Task Force. Due to the low number of patients in some subgroups, not only our own data from this manuscript but also recommendations from the literature are presented. Most important references are given in the figure. First, correct diagnosis according to the current diagnostic criteria needs to be made. As initial therapy, first-line therapy with STE, IVIG, or PE is recommended. If patients are refractory to these, early use of RTX is recommended in patients with MADSAM, (Para-) nodopathies, IgM MGUS, anti-MAG. In contrast, use of CYP prior to RTX is recommended in typical CIDP, patients with IgG or IgA MGUS, as well as atypical CIDP other than MADSAM and (Para-) nodopathies. In these, if course is rather mildly refractory, alternative immunosuppression, i.e., with AZA, MMF, or CsA is our recommendation. If RTX fails, BTZ should be considered. In rapid, severe refractory diseases, BTZ should be considered as add-on treatment after the first cycle of RTX.
aCIDP, atypical chronic inflammatory demyelination polyneuropathy; AZA, azathioprine; BTZ, Bortezomib; CIN, chronic immune-mediated sensorimotor neuropathies; CsA, cyclosporine A; CYP, Cyclophosphamide; IVIG, intravenous Immunoglobulins; MADSAM, Lewis-Sumner syndrome/multifocal acquired demyelinating sensory and motor; MAG, myelin-associated glycoprotein antibodies; MGUS, monoclonal gammopathy of undetermined significance; MMF, mycophenolate mofetil; PDN, paraproteinemic demyelinating neuropathy; PE, plasma exchange; RTX, Rituximab; STE, steroids; tCIDP, typical chronic inflammatory demyelination polyneuropathy; US, United States.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_1756286421999631 for Treatment response to cyclophosphamide, rituximab, and bortezomib in chronic immune-mediated sensorimotor neuropathies: a retrospective cohort study by Jeremias Motte, Anna Lena Fisse, Nuray Köse, Thomas Grüter, Hannah Mork, Diamantis Athanasopoulos, Miriam Fels, Susanne Otto, Ines Siglienti, Christiane Schneider-Gold, Kerstin Hellwig, Min-Suk Yoon, Ralf Gold and Kalliopi Pitarokoili in Therapeutic Advances in Neurological Disorders
Footnotes
Contributorship statement: JM: Data collection, statistical analysis, drafting, and revising the manuscript.
ALF: Data collection, statistical analysis, and revising the manuscript.
NK: Data collection.
TG: Data collection and revising the manuscript.
HM: Data collection.
DA: Data collection, statistical analysis, revising the manuscript.
MF: Critical comments during manuscript revision and drafting figures, language style editing.
SO: Data collection and revising the manuscript.
IS: Data collection.
CSG: Data collection and revising the manuscript.
KH: Data collection and revising the manuscript.
RG: Critical comments during data collection, drafting, and manuscript revision.
KP: Basic idea, critical comments during data collection, drafting, and manuscript revision.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Jeremias Motte: received travel grants from Biogen idec, Novartis AG, Teva, and Eisai GmbH, his research is funded by Klaus Tschira Foundation and Ruhr-University, Bochum (FoRUM-program); none related to this work.
Anna Lena Fisse: received research funding by Georgius Agricola Stiftung Ruhr and Ruhr-University, Bochum (FoRUM-program), received honoraria and travel grants from Novartis AG, Sanofi, and Eisai GmbH, none related to this work. Owns shares of Fresenius SE & Co., Gilead Sciences, Medtronic PLC, and Novartis AG.
Nuray Köse: none
Thomas Grüter: received travel reimbursement from Sanofi Genzyme and Biogen Idec, none related to this manuscript.
Hannah Mork: none
Diamantis Athanasopoulos: none
Miriam Fels: none
Susanne Otto: none
Ines Siglienti: none
Christiane Schneider-Gold: has received a FoRUM grant (F 701-2010) from the Ruhr-University of Bochum and consulting and speaker’s honoraria from Alexion Pharmaceuticals, Amicus Therapeutics, Bayer Schering, CSL Behring, Grünenthal, Lupin Pharmaceuticals, and TEVA.
Kerstin Hellwig: Received speaker honoraria, consultancy fees and research support from Bayer Healthcare, Biogen, Novartis, Merck, Teva, and Roche.
Min-Suk Yoon has received speaker honoraria from CSL Behring and Grifols, a scientific grant from CSL Behring, none related to this manuscript.
Ralf Gold: Serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, and Novartis; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Novartis; serves as editor for Therapeutic Advances in Neurological Diseases and on the editorial boards of Experimental Neurology and the Journal of Neuroimmunology; and receives research support from Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis, none related to this manuscript. Because this author is the Editor-in-Chief of Therapeutic Advances in Neurological Disorders, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Kalliopi Pitarokoili: received travel funding and speaker honoraria from Biogen Idec, Novartis and Bayer Schering Pharma and funding from the Ruhr-University, Bochum (FORUM-Program), none related to this work.
Ethics statement: All procedures performed in studies involving human participants were in accordance with the ethical standard of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendment or comparable ethical standards. The Ethics Committee of the Ruhr University Bochum approved our study [retrospective CIDP-database (vote-no. 18-6407), prospective Immunmediated Neuropathies Biobank (INHIBIT; vote-no. 18-6534-BR)]. The patients gave spoken and written consent for participation and publication of the study.
ORCID iDs: Jeremias Motte  https://orcid.org/0000-0002-6624-8565
https://orcid.org/0000-0002-6624-8565
Anna Lena Fisse  https://orcid.org/0000-0003-0493-8656
https://orcid.org/0000-0003-0493-8656
Kerstin Hellwig  https://orcid.org/0000-0003-4467-9011
https://orcid.org/0000-0003-4467-9011
Ralf Gold  https://orcid.org/0000-0002-7223-3052
https://orcid.org/0000-0002-7223-3052
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jeremias Motte, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Gudrunstrasse 56, Bochum, 44791, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Anna Lena Fisse, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Nuray Köse, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Thomas Grüter, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Hannah Mork, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Diamantis Athanasopoulos, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Miriam Fels, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Susanne Otto, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Ines Siglienti, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Christiane Schneider-Gold, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Kerstin Hellwig, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Min-Suk Yoon, Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany; Department of Neurology, Evangelisches Krankenhaus Hattingen, Hattingen, Germany.
Ralf Gold, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
Kalliopi Pitarokoili, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany; Immunmediated Neuropathies Biobank (INHIBIT), Ruhr-University Bochum, Bochum, Germany.
References
- 1. Fisse AL, Motte J, Grüter T, et al. Comprehensive approaches for diagnosis, monitoring and treatment of chronic inflammatory demyelinating polyneuropathy. Neurological Res Pract 2020; 2: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doppler K, Schuster Y, Appeltshauser L, et al. Anti-CNTN1 IgG3 induces acute conduction block and motor deficits in a passive transfer rat model. J Neuroinflammation 2019; 16: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vural A, Doppler K, Meinl E. Autoantibodies against the node of ranvier in seropositive chronic inflammatory demyelinating polyneuropathy: diagnostic, pathogenic, and therapeutic relevance. Front Immunol 2018; 9: 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appeltshauser L, Weishaupt A, Sommer C, et al. Complement deposition induced by binding of anti-contactin-1 auto-antibodies is modified by immunoglobulins. Exp Neurol 2017; 287: 84–90. [DOI] [PubMed] [Google Scholar]
- 5. Doppler K, Appeltshauser L, Wilhelmi K, et al. Destruction of paranodal architecture in inflammatory neuropathy with anti-contactin-1 autoantibodies. J Neurol Neurosurg Psychiatry 2015; 86: 720–728. [DOI] [PubMed] [Google Scholar]
- 6. Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 2013; 73: 370–380. [DOI] [PubMed] [Google Scholar]
- 7. Querol L, Siles AM, Alba-Rovira R, et al. Antibodies against peripheral nerve antigens in chronic inflammatory demyelinating polyradiculoneuropathy. Sci Rep 2017; 7: 14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Querol L, Devaux J, Rojas-Garcia R, et al. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol 2017; 13: 533–547. [DOI] [PubMed] [Google Scholar]
- 9. Dyck PJ, O’Brien PC, Oviatt KF, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol 1982; 11: 136–141. [DOI] [PubMed] [Google Scholar]
- 10. Lopate G, Pestronk A, Al-Lozi M. Treatment of chronic inflammatory demyelinating polyneuropathy with high-dose intermittent intravenous methylprednisolone. Arch Neurol 2005; 62: 249–254. [DOI] [PubMed] [Google Scholar]
- 11. Muley SA, Kelkar P, Parry GJ. Treatment of chronic inflammatory demyelinating polyneuropathy with pulsed oral steroids. Arch Neurol 2008; 65: 1460–1464. [DOI] [PubMed] [Google Scholar]
- 12. Lozeron P, Denier C, Lacroix C, et al. Long-term course of demyelinating neuropathies occurring during tumor necrosis factor-α–blocker therapy. Arch Neurol 2009; 66: 490–497. [DOI] [PubMed] [Google Scholar]
- 13. Yoon M-S, Chan A, Gold R. Standard and escalating treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Ther Adv Neurol Disord 2011; 4: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahdi-Rogers M, Brassington R, Gunn AA, et al. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 2017; 35: 1173–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muley SA, Jacobsen B, Parry G, et al. Rituximab in refractory chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. Epub ahead of print 21 January 2020. DOI: 10.1002/mus.26804. [DOI] [PubMed] [Google Scholar]
- 16. Roux T, Debs R, Maisonobe T, et al. Rituximab in chronic inflammatory demyelinating polyradiculoneuropathy with associated diseases. J Peripher Nerv Syst 2018; 23: 235–240. [DOI] [PubMed] [Google Scholar]
- 17. Velardo D, Riva N, Carro UD, et al. Rituximab in refractory chronic inflammatory demyelinating polyradiculoneuropathy: report of four cases. J Neurol 2017; 264: 1011–1014. [DOI] [PubMed] [Google Scholar]
- 18. Benedetti L, Briani C, Franciotta D, et al. Rituximab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a report of 13 cases and review of the literature. J Neurol Neurosurg Psychiatry 2011; 82: 306–308. [DOI] [PubMed] [Google Scholar]
- 19. Pitarokoili K, Yoon M-S, Kröger I, et al. Severe refractory CIDP: a case series of 10 patients treated with bortezomib. J Neurol 2017; 264: 2010–2020. [DOI] [PubMed] [Google Scholar]
- 20. Bergh PYKV den, Hadden RDM, Bouche P, et al. European federation of neurological societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European federation of neurological societies and the peripheral nerve society — first revision. Eur J Neurol 2010; 17: 356–363. [DOI] [PubMed] [Google Scholar]
- 21. Doneddu PE, Cocito D, Manganelli F, et al. Atypical CIDP: diagnostic criteria, progression and treatment response. Data from the Italian CIDP database. J Neurol Neurosurg Psychiatry 2019; 90: 125–132. [DOI] [PubMed] [Google Scholar]
- 22. Hadden RD, Nobile-Orazio E, Sommer C, et al. European federation of neurological societies/peripheral nerve society guideline* on management of paraproteinemic demyelinating neuropathies. Report of a joint task force of the European federation of neurological societies and the peripheral nerve society. J Peripher Nerv Syst 2006; 11: 9–19. [DOI] [PubMed] [Google Scholar]
- 23. Oaklander AL, Lunn MP, Hughes RA, et al. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev 2017; 12: CD010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merkies IS, Schmitz PI, van der Meché FG, et al. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry 2002; 72: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991; 14: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 26. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 27. Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol 2013; 21: 28–33. [DOI] [PubMed] [Google Scholar]
- 28. Rajabally YA, Simpson BS, Beri S, et al. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: study of a UK population. Muscle Nerve 2009; 39: 432–438. [DOI] [PubMed] [Google Scholar]
- 29. Cocito D, Grimaldi S, Paolasso I, et al. Immunosuppressive treatment in refractory chronic inflammatory demyelinating polyradiculoneuropathy. A nationwide retrospective analysis. Eur J Neurol 2011; 18: 1417–1421. [DOI] [PubMed] [Google Scholar]
- 30. Jiao L, Xiang Y, Li S, et al. Efficacy of low dose rituximab in treatment-resistant CIDP with antibodies against NF-155. J Neuroimmunol 2020; 345: 577280. [DOI] [PubMed] [Google Scholar]
- 31. Disanto G, Ripellino P, Riccitelli GC, et al. De-escalating rituximab dose results in stability of clinical, radiological, and serum neurofilament levels in multiple sclerosis. Mult Scler. Epub ahead of print 25 August 2020. DOI: 10.1177/1352458520952036. [DOI] [PubMed] [Google Scholar]
- 32. Maxted AP, Dalrymple RA, Chisholm D, et al. Low-dose rituximab is no less effective for nephrotic syndrome measured by 12-month outcome. Pediatr Nephrol 2019; 34: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cocito D, Paolasso I, Antonini G, et al. A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol 2010; 17: 289–294. [DOI] [PubMed] [Google Scholar]
- 34. Martinez-Thompson JM, Snyder MR, Ettore M, et al. Composite ganglioside autoantibodies and immune treatment response in MMN and MADSAM. Muscle Nerve 2018; 57: 1000–1005. [DOI] [PubMed] [Google Scholar]
- 35. Querol L, Rojas-García R, Diaz-Manera J, et al. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm 2015; 2: e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doppler K, Appeltshauser L, Villmann C, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain 2016; 139: 2617–2630. [DOI] [PubMed] [Google Scholar]
- 37. Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 2016; 86: 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pascual-Goñi E, Martín-Aguilar L, Lleixà C, et al. Clinical and laboratory features of anti-MAG neuropathy without monoclonal gammopathy. Sci Rep 2019; 9: 6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lunn MP, Nobile-Orazio E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst Rev 2016; 10: CD002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Athanasopoulos D, Motte J, Fisse AL, et al. Longitudinal study on nerve ultrasound and corneal confocal microscopy in NF155 paranodopathy. Ann Clin Transl Neurol. Epub ahead of print 20 May 2020. DOI: 10.1002/acn3.51061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008; 7: 136–144. [DOI] [PubMed] [Google Scholar]
- 42. van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 2011; 76: 337–345. [DOI] [PubMed] [Google Scholar]
- 43. van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2018; 17: 35–46. [DOI] [PubMed] [Google Scholar]
- 44. Vanhoutte EK, Latov N, Deng C, et al. Vigorimeter grip strength in CIDP: a responsive tool that rapidly measures the effect of IVIG – the ICE study. Eur J Neurol 2013; 20: 748–755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_1756286421999631 for Treatment response to cyclophosphamide, rituximab, and bortezomib in chronic immune-mediated sensorimotor neuropathies: a retrospective cohort study by Jeremias Motte, Anna Lena Fisse, Nuray Köse, Thomas Grüter, Hannah Mork, Diamantis Athanasopoulos, Miriam Fels, Susanne Otto, Ines Siglienti, Christiane Schneider-Gold, Kerstin Hellwig, Min-Suk Yoon, Ralf Gold and Kalliopi Pitarokoili in Therapeutic Advances in Neurological Disorders