Structured Abstract
Background:
Take-home naloxone (THN) kits have been designed to provide community members (including people who use drugs, their families and/or significant others) with the necessary resources to address out-of-hospital opioid overdose events. Kits typically include two doses of naloxone. This 'twin-pack' format means that lay responders need information on how to use each dose. Advice given tends to be based on dosage algorithms used by medical personnel. However, little is currently known about how and why people who use drugs, acting as lay responders, decide to administer the second dose contained within single THN kits. The aim of this article is to explore this issue.
Methods:
Data were generated from a qualitative semi-structured interview study that was embedded within a randomised controlled trial examining the risks and benefits of Overdose Education and Naloxone Distribution (OEND) training in New York City (NYC). Analysis for this article focuses upon the experiences of 22 people who use(d) opioids and who provided repeat naloxone administrations (RNA) during 24 separate overdose events. The framework method of analysis was used to compare the time participants believed had passed between each naloxone dose administered (‘subjective response interval’) with the ‘recommended response interval’ (2-4 minutes) given during OEND training. Framework analysis also charted the various reasons and rationale for providing RNA during overdose interventions.
Results:
When participants’ subjective response intervals were compared with the recommended response interval for naloxone dosing, three different time periods were reported for the 24 overdose events: i. ‘two doses administered in under 2 minutes’ (n=10); ii. ‘two doses administered within 2-4 minutes’ (n=7), and iii. ‘two doses administered more than 4 minutes apart’ (n=7). A variety of reasons were identified for providing RNA within each of the three categories of response interval. Collectively, reasons for RNA included panic, recognition of urgency, delays in retrieving naloxone kit, perceptions of recipients’ responsiveness/non-responsiveness to naloxone, and avoidance of Emergency Response Teams (ERT).
Conclusion:
Findings suggest that decision-making processes by people who use opioids regarding how and when to provide RNA are influenced by factors that relate to the emergency event. In addition, the majority of RNA (17/24) occurred outside of the recommended response interval taught during OEND training. These findings are discussed in terms of evidence-based intervention and ‘evidence-making intervention’ with suggestions for how RNA guidance may be developed and included within future/existing models of OEND training.
Keywords: Take Home Naloxone, Repeat Naloxone Administrations, Framework Analysis, People Who Use Opioids, Naloxone Response Intervals, Harm Reduction, New York City
Introduction
The operational approach of ‘take home naloxone’ (THN) has, as a key objective, the ‘technology transfer’ of an urgent medical procedure into community settings where the emergency is more likely to occur, and where the people administering naloxone are likely to be non-medically trained laypersons. This approach therefore requires training of a new 'intervention workforce' (e.g. peer groups, family members, general public, etc.) and the supply and distribution of the essentials for the emergency medical procedure prior to the event (in this example, the opioid-receptor antagonist, naloxone). As such the objective of THN is similar to earlier initiatives of technology transfer such as adrenaline/epinephrine (aka EpiPen®, for severe allergic reactions), glucagon (for diabetes), snakebite anti-venin, and cardiac defibrillation (Campbell 2020, De Santiago-Cardenas et al 2015, Kuowenhoven 1969, Strang et al 2006).
THN has gradually been introduced in many countries over the last two decades, and with increasing rapidity over the last ten years (McDonald et al 2017, Strang et al 2019). This new approach has been especially promoted and implemented in the US over the last five years, prompted by the growing awareness of need for overdose harm-reduction during the continuing opioid crisis (UNGASS 2016, Volkow and Collius 2017). Regardless of geographic setting, guidelines now exist to inform this development at local, national and international levels (Strang and McDonald 2016, World Health Organisation 2014) particularly about the range of naloxone technology (‘kits’) currently available in different countries.
From the onset of their implementation, THN kits were produced to provide naloxone and the necessary resources (e.g. needle and syringe) to the relevant people administering naloxone. Informal consensus emerged during their design that such kits should contain an initial naloxone dose to achieve reversal of opioid overdose, and a second dose, if needed (Clarke et al 2005, Clarke and Dargan 2002, World Health Organization 2014). This 'twin-pack' format of THN requires consideration of the clinical guidance that should be given to a non-medical administrator with regard to what dose to provide (from the ‘twin-pack’ kit) and how to make judgements about whether (or not) to give the second dose. For example, in dosage protocols provided to paramedics working in emergency ambulance (pre-hospital) situations, pharmacological guidance exists to give an initial dose of intramuscular (IM) naloxone and then, if there is not sufficient response after two or three minutes, a second dose should be considered (e.g. Clarke et al 2005, Clarke and Dargan 2002).
Despite these algorithms for naloxone dosage, there are also widely different opinions and practices around the world with regard to suitable and appropriate dosing. For example, the original dosing regimens for emergency naloxone in different jurisdictions were generally, according to the manufacturers and also consensus among emergency physicians, for an initial dose of 0.4mg by injection which could be increased by the clinician, as indicated, up to an upper dose limit of 2mg (Bardsley, 2019; Clarke et al 2005; Moe et al., 2020; Somerville et al., 2017. There is also an associated uncertainty about the overall approach to the naloxone reversal. For example, one view is that the objective of the emergency naloxone is to purposely ensure rapid reversals without regard to precipitating severe withdrawal symptoms in the person who overdosed. An alternative view is that resuscitation should involve a series of lower doses that produce slower recoveries (socalled ‘sleeping beauty’ reversals: Li et al 2018) in which the naloxone dosage ‘wakes them gently’ (Wanger et al 1998, Horowitz 1998).
These contrasting views on naloxone dosage may be noted in recent publications from North American settings. For example, Cozzolino et al (2019) recommend that the ‘ideal dose of naloxone is the one that improves breathing without inducing withdrawal, but an excessive dose is better than too low a dose’. Whereas Moss and Carlo (2019) conclude that due to prevalence of the potent synthetic opioids, fentanyl and its analogues, in North American settings, ‘administering higher doses of naloxone, particularly for self or layperson administration, may be a simple countermeasure that can be initiated rapidly in an attempt to lower the morbidity and mortality in the new opioid era.’ Elsewhere, the contrasting (and conflicting) positions on naloxone dosing has raised concern about the potential for harm to arise from excessive doses. Neale and Strang (2016) refer to this as ‘over-antagonism’, especially if/when the dosage triggers ‘behavioural toxicity’ such as self-discharge from ongoing medical care and/or further drug-seeking and drug use to purposely attenuate naloxone-precipitated withdrawal (Parkin et al 2020, Strang, Neale, McDonald, and Kalk, 2017)..
An opportunity to study repeat naloxone administrations by people who use opioids
Dosage algorithms for use by medical personnel form the basis of advice during Overdose Education and Naloxone Distribution (OEND) training given to people who use opioids and other laypersons that respond to overdoses within community settings. This also creates research opportunities to explore the influences on people’s decision-making processes during emergency responses to overdose. At present, there is a paucity of data regarding the reasons why people who use drugs (or their significant others) decide to provide both doses of naloxone contained within THN kits during an opioid overdose event. In recognition of the latter, the term ‘repeat naloxone administrations’ (RNA) is used throughout this article to describe the administration of both doses from assorted ‘twin-pack’ THN kits by people who use opioids during overdose intervention. In addition, the term RNA is a deliberate distinction from ‘multiple naloxone administrations’ (MNA) (cf: Faul et al 2017), in which the latter is seemingly more associated with overdose intervention that may involve several titrated naloxone doses (provided by clinicians, Emergency Response Teams, and law enforcement officers etc.). In this article, RNA therefore relates exclusively to the use of an available second dose by people who administer naloxone in community settings.
This opportunity to compare, contrast and explain RNA exists as all participants interviewed in this research were provided with a THN kit based on a ‘twin-pack’ dose format. Even though three different naloxone kits were used during the study (described below), each of these THN kits contained an initial naloxone dose, as well as a second dose if needed. As a result, it is possible to consider the application of recommended response intervals associated with RNA during opioid overdose events within community settings and the decision-making processes regarding RNA during these events by people who use opioids (the participants) from an entirely qualitative perspective. Such opportunities are typically less frequent within quantitative datasets regarding the administration of naloxone by people who use opioids.
The Study
This qualitative study was part of a 5-year randomised trial (National Institute of Health [NIH]/National Institute on Drug Abuse [NIDA] [removed for review]), conducted in the New York City (NYC) metropolitan area. The wider study evaluated two different OEND training models in various populations of people who use opioids (see Jones et al., 2017). Inclusion criteria for the study were any sex or gender, aged 21-65 who had met DSM-IV (American Psychiatric Association 2013) criteria for opioid use disorder within the past 6 months at the time of recruitment. Prospective participants were excluded for any active psychiatric disorder that may have affected their ability to provide informed consent or make participation hazardous. To ensure that participants without prior overdose knowledge were recruited, the study also excluded individuals who had received formal training in overdose prevention, cardiopulmonary resuscitation, or Basic Cardiac Life Support within the past 2 years.
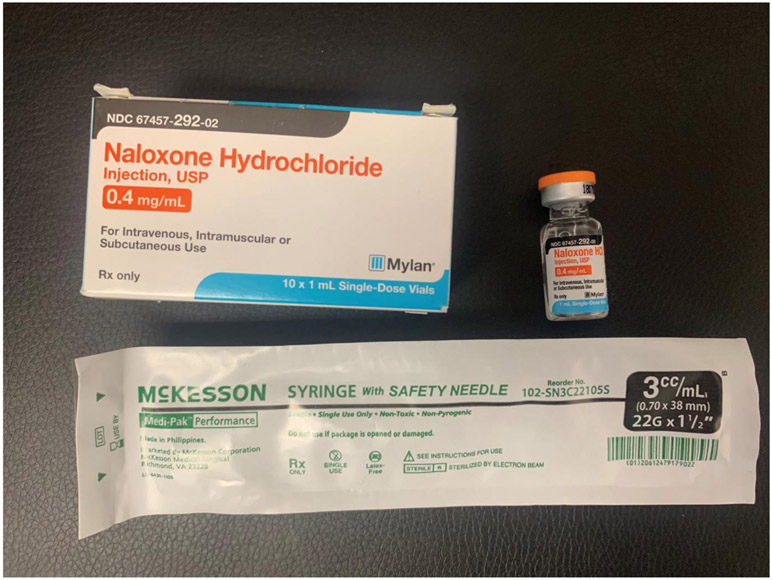
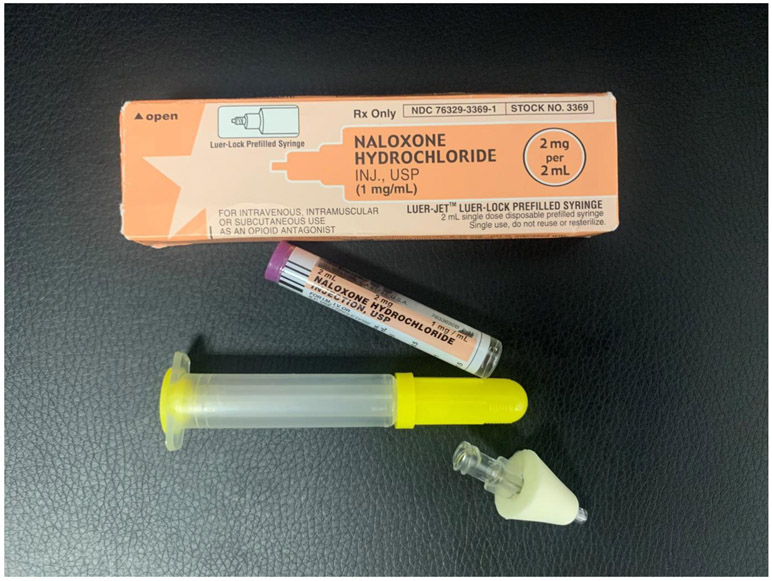
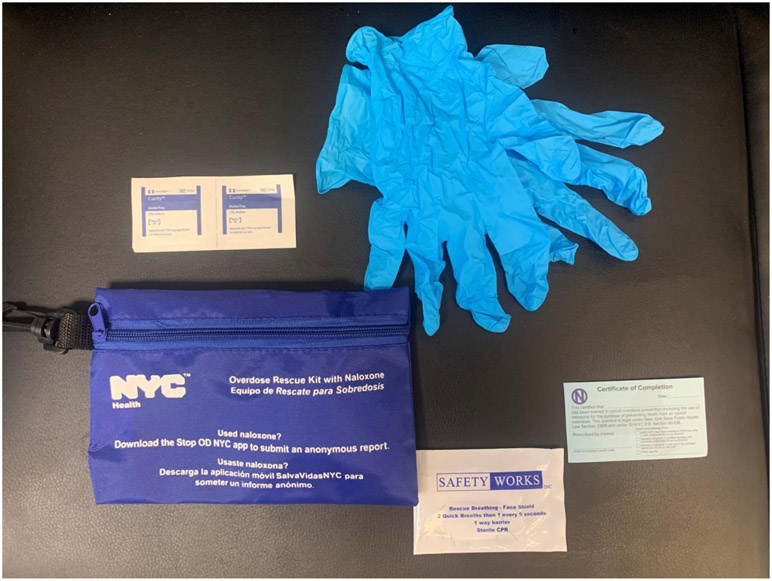
During 2014-2019, 403 participants received overdose prevention training as a part of the trial and 321 continued in a year-long follow-up period. All participants who received overdose prevention training were provided with a THN kit and given the option of a kit containing either an intramuscular (IM) or intranasal (IN) formulation of naloxone. Initially, the choice of THN kits were (1) an IM kit with two 0.4mg naloxone vials (concentration: 0.4mg/ml) i.e. a first dose and a second back-up vial for IM injection with two standard syringes (see Image 1)1; or (2) an ‘improvised nasal spray’ kit containing two 2mg vials i.e. providing two 2mg doses or naloxone (concentration 2mg/2ml per dose) with two Mucosal Atomisation Devices (M.A.D.) (see Image 2). (3) Later in the trial, the ‘improvised’ IN formulation was replaced with the purpose developed Narcan® Nasal spray product so that the THN kit contained two Narcan® nasal sprays, each containing 4mg/0.1ml. Thus, all three THN kits comprised two doses of naloxone, and the training to participants was that, after administering the first dose, they should wait 2-4 minutes (OEND training slides, n.d.) and then consider whether they needed to give the second dose (here termed the ‘recommended response interval’): this instruction was the same regardless of naloxone formulation. Participants were also provided with latex gloves, a face shield for rescue breathing, alcohol prep pads, and an instructional handout (see Image 4).
Image 1:
some content of the intramuscular naloxone kit
Image 2:
some content of the 'improvised nasal spray' naloxone kit
Image 4:
Additional content provided with naloxone as part of the take home kits
Image 3:
some content of the Narcan nasal spray (naloxone) kit
Qualitative Data Generation
All qualitative research linked to the trial received ‘human subjects’ ethical approval from the Institutional Review Board (IRB #6723)..
The objective of the qualitative study (embedded within the five-year trial) was to obtain and analyse data pertaining to first-hand accounts of attending opioid overdose events and assisting in any associated reversal (using THN). The qualitative inquiry sought to understand and inform the effectiveness of OEND practices throughout NYC. Inclusion criteria for the qualitative component were enrolment in the larger trial (above), having witnessed an opioid overdose and direct/indirect participation in an opioid overdose reversal using THN.
Semi-structured interviews were the method of data generation. Participation in the interview was optional and did not affect involvement in the larger trial. All interviews were conducted at New York State Psychiatric Institute by three researchers trained in qualitative research methods. All interviews were audio-recorded and followed a topic guide that included areas such as: ‘substance use and treatment’, ‘pre-trial overdoses (experienced and witnessed)’, and ‘last overdose witnessed’. Interviews varied in length (from 25-60 minutes), were transcribed verbatim for analysis and all participants were compensated US$40 for completing an interview. Participants were invited to interview as soon as possible (within 3 months) after witnessing/intervening in an overdose event in order to maximise recall of the event during interview. A more thorough account of the qualitative component of this study can be found in Neale et al (2019) and Parkin et al (2020)..
The following article compares the ‘subjective response intervals’ reported by participants with the ‘recommended response interval’ (regarding the time to provide a second dose of naloxone) as taught during OEND training sessions (see above). In this regard, the term ‘subjective response interval’ reflects the estimated time to administer both doses during stressful emergency situations as reported by participants during an interview that typically took place after the overdose event within a 3-month period. This term therefore acknowledges that the time reported between doses will not be completely accurate and instead provides an indication of time perceived to have lapsed by all participants at the time of their interview (i.e. based upon event recall)2. Furthermore, this article also examines the decision-making processes underpinning RNA by people who use opioids including the reasons for the range of timings involved in RNA during emergency situations.
Qualitative Data Analysis
Qualitative data analysis took place at the National Addiction Centre, King's College London, (UK)(UK) and focused upon participants’ experiences of administering naloxone at the ‘last overdose witnessed after study enrolment’. This involved analysis of a qualitative database containing accounts of 56 overdose events that were reversed by 46 participants, using one of the three formulations of naloxone described above. Of those 56 overdose events (see also Parkin et al 2020), 28 were reversed using a single dose of naloxone from the relevant ‘twin-pack’ kits used by 21 participants (n.b. several participants provided more than one account of overdose intervention at different periods throughout the study). As these 28 reversals did not involve RNA, they have been excluded from analysis for this article. A further 4 overdoses reported by 3 participants were also excluded from analysis due to missing data relating to the subjective response interval between RNA. The analysis is therefore informed by 24 overdoses that were managed by 22 participants (two people described 2 RNA events) and who each administered more than one dose of naloxone to achieve reversals of potentially fatal opioid overdose.
All interview data for this study were managed using software specifically designed for qualitative data analysis (MaxQDA Version 2018). All 24 accounts of overdose reversals involving RNA were subject to the principles and processes of framework analysis (Ritchie and Lewis 2003). Framework analysis (FA) is an approach that is concerned with the systematic organisation of qualitative data into a matrix (i.e. a table consisting of rows and columns). This tabular design permits key information obtained from individual research participants to be ‘charted’ (Gale et al 2013, Parkinson et al 2016) as concise data summaries that seek to reduce large tracts of transcript data within each categorical label (or theme) of a qualitative coding frame. Data summaries populate the matrix in a format that corresponds with individual participants and research theme (e.g. ‘reason for first naloxone dose’). As noted by Gale et al (ibid) the framework method maintains the subjectivity of the research participants (e.g. their lived experience of an issue), whilst simultaneously providing opportunities for interpreting in-depth descriptions of patterns, anomalies and deviations throughout the dataset. For these reasons, the analytical method is not associated with any epistemological or theoretical approach and may be adapted for either inductive or deductive analysis (Gale et al 2013).
Analysis commenced with a comparison of each participant’s subjective response interval between RNA to the recommended response interval between doses (2-4 minutes) as taught during the OEND training provided within the trial. This process logically organised the 24 overdose/RNA events into categories of time that were either within the recommended response interval of 2-4 minutes, or above/below the recommended 2-4 minutes. Identification of these time categories within the interview data then permitted the construction of framework tables to chart data pertaining to the social, physical and environmental circumstances surrounding each event as well as the various reasons why individuals delivered RNA. These assorted circumstances and subjective response intervals formed the columns of the matrix, and the anonymised identity code for each research participant formed the rows (see Matrix 1 for an illustration of three RNA events). A final row/column was added to the matrix to provide analytical space for inserting interpretative summaries of the charted data, as is required for any project utilising the framework method of analysis.
Matrix 1:
Edited illustration of the framework attached to analysis of repeat naloxone administrations (RNA)
| Participant | Relationship | Drugs thought to have been used |
Place | 911 | Kit | Doses given |
Why first dose? |
What happened? |
Why second dose? |
Response interval |
Reason for RNA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | Stranger | Heroin | Street | Called before naloxone given | IN2mg | 2 | Recognised symptoms of overdose as serious | After first dose was partially responsive so gave second after ‘about 2 mins’ | Only partially responding to first dose | 2 doses under 2 minutes | Recognised that person not fully conscious or responsive |
| 19 | Friend | Heroin with/or fentanyl | Inpatient program (IP) | Taken to hospital by ambulance | IN2mg | 3 (1 by IP staff plus 2 by Part.19) | Believed IP staff gave first dose incorrectly | Part 19 follows protocol after first dose. Times 4 mins on smartwatch and then gives a second dose. | Second dose given because no response after first dose. After second dose, OD comes around after 60 seconds | 2 doses (by Part 19) 2-4 minutes apart | Incorrect administration by IP staff and continued non-responsiveness in person OD’d. Part19 also thought fentanyl may have been responsible for OD as ‘3 jars of Narcan’ used during reversal. |
| 18 | Shelter friend | Heroin | Shelter accommodations | 911 called by another | IM | 2 | Recognised overdose symptoms and is nonresponsive. First dose follows physical checks | After first dose continues with resuscitation attempts including ‘wet-heading.’ Waits 8-12 minutes before second dose (was not sure how long to wait) | Still nonresponsive after wet-heading and sternum rub so gives second dose | 2 doses over 4 mins apart | Second dose given after trying to resuscitate using wet-heading and sternum rub. Decision based on non-response of person, but some uncertainty about response interval |
‘Two doses administered in under 2 minutes’
Of all 24 overdoses in this study of RNA, 10/24 were reversed using two doses of naloxone that were both administered in under 2 minutes. Reasons given for providing two doses of naloxone in under 2 minutes related to panic, a perceived sense of urgency (such as unexpectedly encountering an overdosed person in an outdoor location), the physicality of the person who overdosed (body position, pallor, breathing pattern) or as a means to compensate for the time delay in retrieving naloxone from another nearby location (as in 2/10 cases). In all these circumstances, participants considered the need for RNA as a means of urgently and rapidly addressing non-responsiveness in the person who had overdosed and/or who may have been unconscious for an uncertain/unknown period. This course of urgent, rapid action is typified in the following account, in which Participant 20 recognised that an overdose was occurring during a chance encounter in the bathroom of a homeless shelter. Following a verbal exchange to confirm that the person having difficulties was agreeable to receiving naloxone, Participant 20 ran to his room to retrieve his intranasal kit (see Image 2). When he returned to the bathroom (6-7 minutes later), the overdose had progressed with the person on the floor, head slumped, drooling, shallow breathing and had a ‘purple’ pallor. In recognition of these overdose symptoms, Participant 20 reacted immediately. In the following summary of intervention, RNA take place as a result of the time delay caused by retrieving the naloxone kit and by difficulties associated with assembling the improvised atomiser. In this regard, panic (and eventual relief) is inferred throughout the participant’s recognition of a need to provide an urgent and rapid response to the person concerned. Namely:
Respondent: And then I remember I have a little trouble putting the thing … cause I forgot how to put the thing ….
Interviewer: So you had a little trouble putting it together?
Respondent: Yeah cause I forgot how it was. But I do it. I did it. And I push half in half.
Interviewer: So right away, how long after you got back to the bathroom before you actually gave him the naloxone? How long did it take you to think he’s bad …?
Respondent: 6 or 7 minutes.
Interviewer: And after you gave him the one dose in one nostril, did you give him the other dose in the other nostril right away or did you wait?
Respondent: Right way. Right away. And I wait. So like in 3 or 4 minutes … the saliva that he have, he spit (and) he started, I don’t know. Like breathing a little more and I just talk to him. ‘yo, yo, wake up. You okay? Yo, you okay?’ He started to look at me like he don’t know where he at and stuff like that and look at me like weird you know? I tried to explain, ‘yo, you remember me? I was the one that was with you a couple minutes ago.’ So in the beginning he was disoriented. But then he started to like remember stuff. And I tell him what happened and what I did, you know? So he wake up, and say ‘that taste bad.’
(Participant 20 male, providing two doses of IN2mg naloxone to male in bathroom of homeless shelter).
In addition, 5/10 accounts of ‘two doses under two minutes’ involved the administration of two doses together, in which the second dose immediately followed the first (that is, simultaneously without hesitation or delay). The 5 participants who administered ‘simultaneous’ (back-to-back) doses of naloxone typically described this act as a decision that was both considered and intentional. Reasons given for administering naloxone in this way reflected the aforementioned ‘urgency’ attached to the event, but also partially related to the participants’ own presence at the overdose scene – and possible consequences of this co-presence (relating to place, relationships and emotional responses). For example, participants provided accounts of ‘panic’ associated with the overdose of a significant other (friend, relative), avoiding a fatality in a particular place (e.g. apartment) and avoiding disclosure of drug use to people in authority who may subsequently attend the overdose scene. The situated nature of such circumstances and the response to ‘panic’ may be noted in the following account.
Interviewer: (It was) just the two of you (there)?
Respondent: Yeah, just the two of us. So, I knew then she’s overdosed. … I tried waking her up. She wasn’t responding. At one point she stopped breathing.
Interviewer: What did you do to try to wake her up?
Respondent: I put her in the recovery position. I put her in my bed and put her in the recovery position. I was checking her breathing. Her pulse is super low at this point. She probably maybe stopped breathing for almost 30 seconds at this point, 40 seconds. I reacted super-quick. Once that little gargling noise went away, that’s when I knew she stopped breathing.
Interviewer: So, you could hear her?
Respondent: You could hear the breathing going down to the point you couldn’t hear it anymore. I was like that’s how I knew. I put her in the recovery position. I gave her CPR. She wasn’t responding. And then I remembered I still had the Narcan kit you guys gave me, but I didn’t know where it was cause I had just moved all my stuff. I was like, ‘shit, which bag do I have …’ - so I’m just ripping up all my stuff and finally found it. Gave her one shot. She was responding and then I gave her the other shot and then she just like, ‘oh, what happened?’ She just came to.
Interviewer: Just came to.
Respondent: Yeah, she came to. … I give the biggest sigh of relief, cause I didn’t know what to do. My landlord is upstairs. I just moved in here. This girl is probably going to die in my room right now. You what I’m saying? What do I do? I don’t need this fucking heat. I just came out of jail. I don’t need this shit. I got all these drugs in my house. So, you know, like this is crazy. So, all this shit is running through my head. I was nervous. … I’m not going to lie, I was really, really scared. … I did panic a little bit.
(Participant 46, male, providing double-dose IN4mg to female friend in his recently acquired apartment. Emphasis of panic added.)
Another reason given for providing RNA in less than two minutes was that the participant had been ‘told’ to do so by a person who held a position of authority at the location of the overdose event. In this situation, Participant 32 appears to provide two doses in quick succession following an instruction to avoid drug-related death within an employment setting. Panic and anxiety are inferred throughout the account, in which Participant 32 describes assorted actions within a chaotic and confused environment. Namely:
Interviewer: So you checked his pulse?
Respondent: I didn’t check his pulse - the worker - one of the staff members checked his pulse. So we moved him out the bathroom, put him on the floor, and then, he started - doing - giving?
Interviewer: CPR?
Respondent: Yeah, and …, he was telling me to check his pulse. I’m like ‘I don’t feel nothing’. He wasn’t breathing. I took his shirt, looked up like this and his stomach wasn’t moving … I just said ‘get the naloxone’. Then I had some in my bag, and … one of the staff guys, at first I wasn’t gonna do it, cause I was gonna let the staff do it, cause I was a little nervous. I’m not gonna lie. I was a little nervous and I gave it to (the staff member). He wouldn’t do it.
Interviewer: Why not?
Respondent: He was scared.
Interviewer: Really?
Respondent: Yeah, and I was telling him. I said ‘put it up his nose and half up the other nose’, and then I said ‘give me the thing’. And, he said, ‘if something happens to him it’s on your hands’ and I said ‘well I’ll take the weight for it’ and I did it. And then like a few - I say about 20-30 seconds he started going …. like that.
Interviewer: Really, after one dose?
Respondent: Yeah one dose. And then the Director came and said ‘give him some more’, and turned him on his side. I don’t know why I did that. I did the other one, did the same thing. Then he started coming around. Then the fireman and ambulance people came, they gave him another shot. (Participant 32, male, providing IN2mg to unknown male in workplace prior to a third dose delivered by emergency services)
‘Two doses administered within 2-4 minutes’
Overdose intervention in this category was characterised by the administration of a second separate dose of naloxone 2 minutes after the first, but within 4 minutes of the first. There were 7/24 overdoses reversed within this response interval. This was also the recommended response interval provided during the OEND training of the main study.
Reasons given for RNA within 2-4 minutes related to the aforementioned state of urgency, in which participants typically recognised the symptoms of opioid overdose that required immediate intervention in those they assisted. However, RNA within 2-4 minutes also typically included participants’ recognition that the naloxone recipient was not fully responding (if at all) to the first dose administered. In this regard, the overall rationale related to perceptions of clinical non-responsiveness and/or understandings of opioid overdose. These reasons are noted in the following examples. In the first account, Participant 19 refers to his awareness of overdose training ‘kicking in’ prior to the administration of both doses of naloxone. It is also noteworthy that he describes his use of a smartwatch to accurately measure the recommended response interval between doses that he delivered to his friend whilst they were both part of an inpatient drug treatment facility.
Respondent: …. but when I went to (my friend in the toilet cubicle) I seen the bags of dope on the floor. When I looked up, he was slumped like this, his hands were touching, and this was like a funny color grey, blue -
Interviewer: On his fingers?
Respondent: Yeah, like black and blue, and I stood up on the thing and said ‘oh shit’, I seen the fucking syringe, the whole movie scene. So, I (alerted staff) and I said go get (staff member). They came up with the Narcan … and put him in the recovery position. I didn’t want to intervene at first, but when they shot him with the first vial, it did absolutely nothing.
Interviewer: So, the staff did the first injection?
Respondent: Yes. It did absolutely nothing. … he was in the wrong position …
Interviewer: So, he was still sitting up when they gave the injection?
Respondent: Yes.
Interviewer: Was it the intramuscular?
Respondent: No, I think it was the nasal. The one I use is a nasal too. So, I ran to my room and got my kit. I told (staff member), ‘lay him down’. I laid him down and the training kicked in, like adjusting the neck, making sure the airway was open. I checked the pulse was faint. The breathing had already ceased. God knows how long he was without air. I locked it up, hooked it up and I had him at the whole jaw on one side. I had a smartwatch at the time so I put the timer on countdown. Waited a little under four minutes or a little over four minutes, because I didn’t want to lose him, because I know six minutes without air or five minutes you’ll go brain dead. Then I loaded up the second jar and I hit him. And about less than 60 seconds later he went like “whooo”, like decompressed. I think he was able to breathe in but not breathe out, because that’s what happened to me.
(Participant 19, male, providing two doses of IN2mg naloxone after thinking staff within the inpatient program had administered an initial dose incorrectly)
In the second example, Participant 30 describes the provision of RNA as part of an urgent response to discovering a friend who had overdosed, and due to the lack of response to a first dose of naloxone. The description of mixed messages associated with the recommended response interval for a second dose is also noteworthy. However, on this occasion, Participant 30 administered the second dose after a 3-minute response interval.
Respondent: When he didn’t open the door, … I was pretty sure he was home, because I know his schedule, but when he didn’t open the door and I couldn’t reach him on the phone, I went downstairs to ask his friend if we could go … see if he was at home. … So he said ‘okay’, we went upstairs, and he was on the bed, … passed out, not breathing. His legs were extremely swollen and blue.
Interviewer: His legs?
Respondent: His legs.
Interviewer: Was he naked?
Respondent: No. He had … clothes but no shoes, … you could see his lips were blue, and, and his legs, his feet were swollen up. So, I first checked to see if he was breathing and he wasn’t. I tried to … wake him up. I at that point knew exactly what it was. There was … drugs around, there was an open beer that was still pretty cold - I felt it. Uh, there was a couple of bags of dope on the table, and there was some pills, … so when he didn’t respond, I yelled his name very loud, and then I gave him the sternal grind, alright, to try to wake him. Because I didn’t want to give him naloxone if I didn’t have to wake him up, but I didn’t know how long he’d been like that, and I saw his lips were blue so when I gave him the sternal grind and he didn’t wake up, I immediately gave him the first vial of naloxone. I had an intranasal kit which I believe I got from you guys here.
Interviewer: You gave him the first vial?
Respondent: And he didn’t wake up.
Interviewer: Like, nothing happened or …?
Respondent: Nothing happened. So I was …, I remember in the training they said it’s varied. Some - up to 5 minutes. Some say 2 to 3 minutes before you give them the second vial, some say up to 5 minutes. So I started to do rescue breathing on him. He didn’t wake up after 3 minutes; I gave him the other one. He woke up in about 30 seconds after I gave him that.
(Participant 30, male, providing two doses of IN2mg naloxone 3 minutes apart after discovering friend non-responsive in the latter’s apartment)
‘Two doses administered more than 4 minutes apart’
A third category of RNA involved the administration of two doses of naloxone that were given separately and more than 4 minutes apart. This form of RNA occurred on 7/24 occasions, which were each described as deliberate and conscious acts by all relevant participants. The range of subjective response intervals reported was 5-20 minutes (4/7 were under 7 minutes, 3/7 over 8 minutes, with one of the latter involving a 20-minute interval between first and second dose).
In all 7 events, each person described the reason for the extended response interval between doses as a period of observing the person who had overdosed and noting responses (or not) to the first dose of naloxone. In some circumstances, these response intervals may have been due to not knowing how long somebody had been unconscious, a result of forgetting the recommended response interval of 2-4 minutes, or even due to not knowing ‘what to expect’ when using naloxone for the first time. These different explanations may be noted in the following examples. In the first illustration, Participant 28 describes an overdose taking place upon her arrival at the location concerned. Prior to her arrival, those present had attempted to resuscitate the person by placing her under a running shower. It is at this stage of the event that Participant 28 intervenes, and the anxiety and panic within the environment is perhaps noticeable in the following extract.
Respondent: … They said, ‘I think she ODed.’ That’s what they said. So I’m like, ‘well damn, where she at?’ He said in the bathroom. So I went in the bathroom, the guy was in there. And he had her in the water and everything like that.
Interviewer: He had her in the water?
Respondent: Yeah, he had her in the shower already, running the water and everything on her. And you know, he said, she was in the bathroom, and he heard the noise, and he went in there, she had the needle still stuck in her arm and she had hit the floor. So he tried to wake her up. You know, that’s what the word is on the street. That if somebody overdoses to ice them and showers and stuff like that. That’s what they tell you.
Interviewer: That’s the list of things to do, right?
Respondent: Yeah, list of things to do. And so he was doing it, and I was like … ‘you sure?’ I’m like, ‘Oh my God, do I have my kit with me?’ Because it could’ve very well been back in New York because I was in New Jersey. Because I go to New Jersey to do that. And they was like, ‘what?’ I was like, ‘remember I told you.’ I’m talking to them but I’m looking for it. And then I did find it. I said, ‘I took this class, to help somebody that did an overdose.’ And so I was, I wasn’t like, scared to do it. It’s just that I was anxious about trying to do it, to get out to see if it would work for her, to help it. So, I told her to bring her out the shower, (and lay) her on the floor and everything. I put the thing, the stuff together, and I put half in one nostril, half in the other nostril and she didn’t respond and everything. So I was like, ‘what is it? 3 minutes, 5 minutes?’ I’m calculating everything in my head. I’m going over everything (in) my head. And so I did the other one. And after I did the other she did. And I was like, ‘oh my God.’ (Confirmation of response interval follows later in Interview) … (I) Just gave it to her. Because again I didn’t know how long she had been that way. I know you have a grace period too with that.
Interviewer: And in-between the first and second dose?
Respondent: About 5 minutes?
Interviewer: About 5 minutes?
Respondent: Yeah.
(Participant 28, female, providing two doses of IN2mg naloxone)
Finally, Participant 18 describes his one-on-one intervention with a friend who had overdosed in a toilet cubicle within a hostel/shelter. In this situation, Participant 18 describes his uncertainty regarding the time required for the first dose of naloxone to have any antagonist effect. This uncertainty, coupled with anxiety and information obtained during training, leads to some hesitation in administering a second dose.
Respondent: … I took my phone and I put it under the nose to see if there was any kind of (breathing). It was very very faint. I could barely see … So I’m just telling you what (name removed) had told me. So boom, I did the sterno rub; no response. So … I was kind of really nervous you know what I’m saying? Because I didn’t know what to do; and I didn’t know once I give it to him what’s going to happen. You know what I’m saying? (Name removed) tells me something we played through, but I really didn’t know what to do. So I’m nervous, I’m not going to lie to you. So once I got him on the floor, I checked with my phone; I give him a sterno rub; no response. So then I checked for his pulse in his arm; very very faint, I could barely feel it. So I told the dude that came in the bathroom - I didn’t even know the individual. Some dude came in to use the bathroom. I said ‘listen, go downstairs and get security and tell them to call 911 right now.’ So he runs out the bathroom; so I take out the kit; hook it up. So I check for his pulse again; I smacked him a little more; nothing. So ‘boom.’ I administered in the thigh; I gave him the first one. And nothing. And (OEND trainer) telling me it’s supposed to be automatic; nothing. So I’m getting nervous. But I was like panicking in a way because I was expecting for him to wake up; and he didn’t wake up. So I went for the second one. I administered the second one. As soon as I pushed it in, I don’t want to say jumped up but he came awoke.
Interviewer: How long did you wait between the first and the second dose do you think?
Respondent: Maybe eight, 12 minutes; wasn’t that long. Maybe eight. I wouldn’t even want to say 15. I know it wasn’t 15 minutes; but I assume around eight to 12 minutes. As soon as I administered the second one he jumps up, and my response was to restrain him, to push him back down. So I said lay back down. (Confirmation of response interval follows later in the interview) … not even after I gave him the first one there was no response. Because I waited like ten to 12 minutes after I gave him the first one to administer the second one, because I wasn’t sure how long it took. Even though what (he) had told me it was supposed to be immediately, this was the first time me ever doing it. So I didn’t know what to expect time length or otherwise.
(Participant 18, male, providing two doses of IM naloxone)
Discussion
Findings from this study of RNA highlight how participation in overdose intervention is an extremely stressful event that requires people administering naloxone to make difficult decisions quickly regarding (i) whether or not to provide a second dose from a ‘twin-pack’ THN kit and (ii) the potential mortality of those who experience an opioid overdose. This is evident in the various interview extracts included above which (implicitly and explicitly) illustrate fear, panic, nervousness, anxiety and uncertainty amongst those providing RNA. In addition, it is necessary to emphasise that all 24 overdoses involved in this analysis (plus 32 excluded from analysis) concluded with a lifesaving reversal. Accordingly, this study further indicates that people who use opioids can make effective decisions during emergency overdoses (see also, Neale et al 2019, Wagner et al 2014, Webster 2017), including when and why to provide RNA.
However, the specific focus of this study concerned a qualitative comparative analysis of participants’ subjective response intervals to the recommended OEND response interval (of 2-4 minutes between RNA), and to identify the various reasons why RNA may have been given. In this regard, 3 types of subjective response interval were reported. Namely, RNA under 2-minutes, RNA that were 2-4 minutes apart, and RNA that were over 4-minutes apart. Perhaps most noteworthy, was the finding that the majority (17/24; 71%) of RNA occurred outside the recommended inter-dose response interval of 2-4 minutes. Yet, it should be further noted that most RNA occurring outside the recommended response was within what could be considered a reasonable time frame, as opposed to only 2 cases in which the person administering naloxone waited over 10 minutes. Prior to discussing these findings further, it is important to note some limitations of the data in order to avoid inadvertently drawing misleading conclusions regarding the efficacy (or not) of OEND training.
First, the aforementioned stress, panic and urgency (that was noted across all three response intervals identified in the findings section) may have affected the participants’ decision-making throughout the emergency events. As such, all overdose responses (cognitive and physical) may have been affected by the social environment in which each overdose occurred. This finding is consistent with previous studies that have identified similar emotional stresses attached to naloxone administration (McAauley et al 2018, Worthington et al 2006) and suggests that the ‘panic’ experienced by naloxone administrators during overdose intervention is a widespread phenomenon rather than one that is unique to participants in this NYC-based study. Accordingly, the role of panic/stress during overdose intervention by people administering naloxone should not be understated during any OEND training.
Second, and related to the latter, is the caveat that all timings used in this analysis are premised upon participants’ subjective response intervals (based upon recall of events in an interview that was detached from the scene of the overdose – and may have occurred up to 12 weeks after the event). For these reasons, the self-report timings used throughout analysis will not be completely accurate. Nevertheless, and equally, (as with any self-report data obtained from qualitative research interviews), those timings should not be dismissed because they are not completely precise. Instead, one may accept that such timings provide a clinical indication of the various responses by those who have administered naloxone.
Third, perhaps conjoining the previous two issues, is the wider matter of ‘time-perception’ as reported during emergency events. Various studies have considered the way in which perceptions of time may become distorted during stressful, life-threatening events (including medical emergencies) in which time appears to pass in ‘slow motion’, or more slowly than normally (Eisen 2009, Nunes et al 2016, Taylor 2014). For example, Stetson et al.’s (2007) study of such events suggests that those enduring a life-threatening incident appear to experience time 36% slower than those in the same environment (who are not affected by the same life-threatening situation). These studies variously suggest that the distortion of time in such a way is a psychological ‘trick’ (Taylor 2014) that aids survival during emergency events and provides time to respond to the imminent threat. This psychological ‘trick’ may be more commonly understood as a ‘fight or flight response’ to danger, in which individuals choose to respond to, or retreat from, a threatening situation. The ‘fight’ response involves the neurological distortion of time-lapse, that theoretically provides time to cognitively prepare and physically respond to the ‘threat’ situation whether that is a medical emergency or a serious accident (Arstilla 2012, Haguri et al 2012, Kimiecik and Stein 1992, van Wassenhove et al 2008, Wittman et al 2010). In the context of participation in a life-threatening situation involving the reversal of an opioid overdose, Stetson et al.’s study would imply that the subjective response intervals reported in this article are possibly under-estimates of the actual RNA response intervals that took place in real-time3. This, in turn, would have implications for the way in which these data have been presented throughout this article. Until further research can somehow include observational (ethnographic) attachment to overdose intervention in community settings, the self-report and subjective response intervals provided by participants during semi-structured interviews will (and can only) suffice.
Despite these limitations, the recommended response interval provided during OEND training is important. Adherence (where/when possible) to this response interval by people who administer naloxone during overdose intervention is equally important. In a successful response to the emergency, the effects of naloxone are expected to be discernible after a couple of minutes and clearly evident within 4 minutes, assuming the unconsciousness is caused by opioid overdose and that the naloxone administration has been of sufficient dose and effectively given. These timings relate to the speed and the efficiency of absorption of naloxone following either injectable or intranasal administration and can be seen from studies of the pharmacokinetics of naloxone following intramuscular or intranasal administration (Krieter et al, 2016; McDonald et al, 2017). There will, of course, be significant differences in the response to naloxone by different individuals according to inter-individual variation in absorption patterns as well as resulting from different levels of physiological dependence, drugs consumed, and the extent of the overdose itself. Nevertheless, the 2-4-minute period gives the non-medical responder a clear indication of the period over which to expect a recovery to occur. Accordingly, this recommended response interval is used widely in training sessions for lay persons being instructed in overdose management, and hence it becomes an appropriate timeframe to study regarding adherence to, or departure from, the instructions given during training. That said, adherence to and departure from these instructions may be viewed and understood differently from the methodological perspectives of evidence-based intervention and evidence-making intervention.
Evidence-based intervention vs Evidence-making intervention
As noted earlier, this study sought to inform the effectiveness of OEND practices throughout NYC. As such, from the perspective of evidence-based intervention (EBI), the findings relating to an overall lack of adherence to the recommended response interval protocol regarding RNA may raise questions about the ‘implementation fidelity’ (Carroll et al 2007) of the OEND training. Implementation fidelity as a component of implementation science refers to the ‘degree to which an intervention or program is delivered as intended’ and is considered critical in the measurement of evidence-based practice in community/real world settings (Breitenstein et al 2010, Carroll et al 2007). In addition, implementation fidelity includes the measurement of adherence to, and competence in delivering, the intervention and the quality of intervention delivery in applied settings. Barriers to maintaining implementation fidelity in community settings include ‘local adaptations of interventions, individual variations in practitioner adherence and competence, and competing demands for the practitioners' time that can diminish their commitment or effectiveness’. Furthermore, such barriers may weaken evidence of outcome and lead to inaccurate conclusions concerning effectiveness (Breitenstein et al 2010).
Alternatively, it is equally possible to view ‘local adaptations of intervention’ as micro-level applications of situated learning (Lave and Wenger 1991) that occur during emergency situations as part of ‘evidence-making intervention’ (EMI). According to Rhodes and Lancaster (2019, p1), EMI is a conceptual framework that ‘attends to health, evidence and intervention as matters of local knowledge-making practice’, ‘emphasises performativity’ and considers how people engage with interventions from their own knowledge or lived-experience as ‘matters of practice’. For these reasons, an EMI approach prioritises the way in which interventions may be ‘made’ (for example, through embodied practice or learning through practising) rather than how they may be ‘evidenced’ (for example, through clinical knowledge). In the context of this study, most RNA occurred outside the OEND recommended response interval of 2-4 minutes. Whereas this finding may be problematic from an EBI perspective (in terms of implementation fidelity), it may also be re-considered from an EMI point of view. That is, the administering of repeat doses of naloxone would instead focus upon the performativity, context and situated knowledge of people who use opioids during their individual episodes of overdose intervention. More simply, an EMI approach would focus upon the various situated reasons why the 2-4 minute guideline was not initiated, and why the recommended response interval was overlooked, ignored or over-ruled by the people who administered naloxone in this study.
For example, the emotional and stressful circumstances associated with overdose intervention may have provided competing demands for time in which the situated nature of uncertainty, panic, and anxiety may have resulted in rushed or prolonged RNA. In addition, overdose intervention included the use of ice, cold water, and restraint by those co-present at the scene of the overdose (but who may not have completed the OEND training) whilst naloxone was being administered. Elsewhere, another individual took the decision to administer naloxone to a friend after assuming that an initial dose had been given ‘incorrectly’ by a staff member of a drug treatment facility where the overdose occurred. Each of these unique, individualised circumstances (focused around intervention, implementation and social/environmental context) led to ‘local knowledge making’ (Rhodes and Lancaster, 2019, 1) that were not necessarily consistent with the OEND recommended response interval by people who use opioids regarding how and when to repeat naloxone administrations.
Despite the different methodological perspectives that may be applied to these data, it is perhaps necessary to emphasise that all 24 cases of RNA in this study concluded with lifesaving reversals. This outcome arose from actions that were conducted by people who use opioids who, overall, did not appear to fully follow (or, at least, ‘adapted’) the 2-4 minute guidance provided during OEND. Accordingly, it is possible to hypothesise that both tacit and explicit knowledge (Hildreth and Kimble 2002, Leonard and Sensiper 1998, Nonaka, 1991, Polanyi 1967) coexisted during episodes of RNA reported in this study, in which people who administered naloxone applied both ‘embodied’ and ‘learned’ knowledge whilst attending to overdose intervention and administering naloxone. That is, explicit knowledge (in the form of OEND guidelines) may have been acknowledged, but partially overlooked, as a result of the urgency attached to the situation, in which a need to provide repeated naloxone administrations was considered more appropriate (via tacit knowledge and recognising potential/actual drug-related harm) than following recommended response intervals.
Implications
Future (and existing) OEND training could be developed for remembering the recommended response intervals between RNA. This could take the form of a simple harm-reduction mnemonic for remembering essential information during an overdose, and feature on apparel (IM / IN naloxone kit-bags, pins, labels, stickers, stationery, t-shirts) associated with the OEND training. Whilst dosage algorithms may vary for different THN kits, opioids, and/or medical personnel, the OEND training in this study advocated subsequent doses of 2-4 minutes. As such, a slogan such as 2-2-4-B4-More (representing ‘wait 2 to 4 minutes before administering more’) could feature on any associated apparel as a visual reminder of the recommended response interval for MNA, especially if featured on the actual naloxone kit (carry-bag, vial, atomiser, etc.). However, such instruction would also need to be flexible enough in order to incorporate any local knowledge of the relevant context (relationships, set, setting, drugs used etc) housing the overdose/intervention, especially with regard to RNA reversals of fentanyl-based overdoses when using ‘twin-pack’ design THN kits.
The latter point is noteworthy given that the evolving nature of the opioid epidemic in North America may call for ongoing revision to the advisory dosage protocols and associated response intervals for RNA. As mentioned in the introduction, fentanyl adulteration of the illicit drug market has contributed to a ‘triple wave’ (Ciccarone 2019) in the opioid epidemic. This wave has been defined by the U.S. Center for Disease Control as the cause of significant increases in overdose deaths involving illicitly manufactured fentanyl and its analogues (Gladden et al., 2019; O’Donnell et al., 2017). Depending on the specific analogue, these opioids can be between 100-10,000 times more potent than morphine (Armenian et al., 2018). Some investigators have suggested that non-opioid mechanisms may contribute to the ineffectiveness of naloxone in some fentanyl-related overdoses (Torralva and Janowsky, 2019) and reports from overdose harm reduction providers suggest that more naloxone is needed to reverse an overdose event in which fentanyl is a contributing drug (Fairbairn et al., 2017). As such, harm reduction practitioners may need to constantly re-evaluate recommended naloxone dosage protocols (i.e. initial dose, response intervals, and the number of doses administered) with each type of overdose response kit. Associated with such re-evaluations should be ongoing training and advice relating to appropriate repeat naloxone administrations, whilst also avoiding overburdening naloxone responders with excessive demands and expectations (relating to titration, dosage, implementation fidelity, etc.) during a stressful situation. The primary objective of THN provision as a harm reduction practice is the prevention of opioid overdose death, but avoiding inadvertent negative outcomes is also important in order to prevent compromising the benefit achieved.
ACKNOWLEDGEMENTS
This study was supported by the National Institute on Drug Abuse Grant R01DA035207 to Dr. Sandra Comer (Principal Investigator). J.N. is part-funded by, and J.S. is supported by, the NIHR (National Institute for Health Research) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. J.N., S.P. and J.S acknowledge the Pilgrim Trust for supporting their involvement in the research. L.B. is funded by an Erwin Schroedinger Fellowship by the Austrian Science Fund (ASF).
The authors would like to thank the National Institute on Drug Abuse (NIDA) for supporting this research (R01DA035207; Comer – Principal Investigator); all study participants for agreeing to be interviewed; and Verena Metz, Gregory Cortorreal, Richard Eisenberg, Rebecca Abbott, Benjamin Foote, Claudia Tindall, and Janet Murray for their technical assistance.
Acknowledgements: to add post-review
Footnotes
DECLARATIONS OF INTEREST
In the last 3 years, J.N. has received, through her University, research funding from Mundipharma Research Ltd and Camurus AB. J.S. is a researcher and clinician who has advocated for wider preprovision of take-home naloxone, using several types of naloxone. He has also worked with pharmaceutical companies to seek to identify new or improved treatments (including forms of naloxone) from whom he and his employer (King’s College London) have received honoraria, travel costs and/or consultancy payments. This includes work with, during past 3 years, Indivior, MundiPharma, Braeburn/Camurus and trial medication supply from iGen and from Camurus. His employer (King’s College London) has registered intellectual property on a novel buccal naloxone formulation and he has also been named in a patent registration by a Pharma company regarding a concentrated nasal naloxone spray. For a fuller account, see J.S.’s web-page at http://www.kcl.ac.uk/ioppn/depts/addictions/people/hod.aspx.
Within the past three years, S.D.C. has received research funding from Alkermes, BioXcel, Braeburn Pharmaceuticals, Cerecor Inc., Corbus, Go Medical, Intra-cellular Therapies, and Lyndra. In addition, Dr. Comer has also consulted for: Alkermes, Charleston Labs, Clinilabs, Collegium, Daiichi Sankyo, Depomed, Egalet, Endo, Epiodyne, Inspirion Delivery Sciences, Janssen, KemPharm, Mallinckrodt, Nektar, Neurolixis, Newron, Opiant, Otsuka, Pfizer, and Sun Pharma. She also has received honoraria from the World Health Organization. Dr. Jones has received compensation - in the form of partial salary support - from a study partially supported by Cerecor Inc., and BioXcel is the recipient of an investigator-initiated grant from Merck Pharmaceuticals, and has worked as a consultant for Alkermes.
S.P., C.B., L.B., A.N.C.C., and F.C. have no disclosures or conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All photographs were taken by JDJ. (NB: all photographs used here do not feature the full contents of each THN kit in their entirety).
For example, if a participant reported that a second dose was administered after ‘about two minutes’, analysis assumes that this occurred within two minutes rather than over two minutes (as the participant chose to cite the nmnber two in their response). Similarly, if participants stated ‘about three and half minutes’, ‘ninety seconds’ or similar, then these subjective response intervals were rounded up to the nearest whole nmnber.
The following interview extract provides a clear empirical demonstration of this neurological distortion of time. Here, Participant 12 describes the way in which panic, danger and a life-threatening event had the effect of slowing down time from an actual 20-minute period to a perceived 2-minute period.
…. I had given some (heroin/fentanyl) to my girlfriend and her sister took some (but) her sister doesn’t do it. She took it; just a little bit; didn’t even take that much and she started bugging out and she started seeing black and blue. Started crying … and I kind of got scared and everybody was yelling. And I gave her the (naloxone) and I called the ambulance while I was giving her the (naloxone). And they came; and it was about 15-20 minutes until the ambulance came and after that (they) left. We spoke (and) she’s never done (heroin/fentanyl). She almost died supposedly. … I told the EMT drivers I gave her the naloxone and that was about it. It was only a 15-minute thing, really quick; especially since I thought she was dying. It felt like two minutes.
(Participant 12 describing panic, fear, urgency and time distortion associated with the reversal of a fentanyl-related overdose. Emphasis added).
References
- Armenian P, Vo KT, Barr-Walker J, Lynch KL. (2018). Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacol 134:121–132. [DOI] [PubMed] [Google Scholar]
- Arstila V (2012). Time slows down during accidents. Frontiers in Psychology, June 2012, 3, Article 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avetiana GK, Fiutyb P, Mazellac S, Koppab D, Heyeb V, and Hebbard P (2018). Use of naloxone nasal spray 4mg in the community setting: a survey of use by community organizations. Current Medical Research and Opinion 34, 4, 573–576 [DOI] [PubMed] [Google Scholar]
- Bardsley R (2019). Higher naloxone dosing may be required for opioid overdose. Am J Health-Syst Pharm., 76, 1835–1837 [DOI] [PubMed] [Google Scholar]
- Bell A, Bennet AS, Jones TS, Simkins M-D, Williams LD, (2019). Amount of naloxone used to reverse opioid overdoses outside of medical practice in a city with increasing illicitly manufactured fentanyl in illicit drug supply, Substance Abuse, 40, 1, 52–55 [DOI] [PubMed] [Google Scholar]
- Breitenstein SM, Gross D, Garvey C, Hill C, Fogg L, Resnick B, (2010). Implementation Fidelity in Community-Based Interventions. Res Nurs Health 33, 2, 164–173. doi: 10.1002/nur.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ND, (2019). Naloxone as a technology of solidarity: history of opioid overdose prevention. CMAJ, 191, 34, E945–E946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ND, (2020). OD: Naloxone and the politics of overdose. Cambridge, Mass., MIT Press. [Google Scholar]
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S, (2007). A conceptual framework for implementation fidelity. Implementation Science, 2, 40 10.1186/1748-5908-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D (2019). The Triple Wave Epidemic: Supply and Demand Drivers of the US Opioid Overdose Crisis. Int J Drug Policy, 71, 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S and Dargan P (2002). Intravenous or intramuscular/subcutaneous naloxone in opioid overdose. Emerg. Med. Jnl 19, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S, Dargan P, and Jones A (2005). Naloxone in opioid poisoning: walking the tightrope. Emerg. Med. Jnl 22, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino E, D’Egidio PF, De Facci R, Leonardi C, Nava F, Stella L, Maremmani I (2019) Improving response capacities in opioid overdose management. Heroin Addiction and Related Clinical Problems, 21, 1, 5–14 [Google Scholar]
- DeSantiago-Cardenas L, Rivkina V, Whyte SA, Harvey-Gintoft BC, Bunning BJ, Gupta RS, (2015). Emergency epinephrine use for food allergy reactions in Chicago Public Schools. Am J Prev Med. 48, 170–173 [DOI] [PubMed] [Google Scholar]
- Eisen LA, (2009) Time Perception Is Distorted During Responses to Medical Emergencies. Med Hypotheses 72, (6), 626–8. [DOI] [PubMed] [Google Scholar]
- Fairbairn N Coffin PO, Walley AY, (2017). Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: challenges and innovations responding to a dynamic epidemic. Int Jnl Drug Policy 46, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Lurie P, Kinsman JM, Dailey MW, Crabaugh C, and Sasser SM, (2017) Multiple naloxone administrations among Emergency Medical Service providers is increasing. Prehospital Emergency Care, 2, 4, 411–419, DOI: 10.1080/10903127.2017.1315203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden M, O’Donnell J, Mattson C, Seth P. (2019). Changes in Opioid-Involved Overdose Deaths by Opioid Type and Presence of Benzodiazepines, Cocaine, and Methamphetamine – 25 States, July-December 2017 to January-June 2018. MMWR Morb Mortal Wkly Rep 68, (34), 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NK, Heath G, Cameron E, Rashid S and Redwood S, (2013). Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology 13, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagura N, Kanai R, Orgs G, Haggard P (2012). Ready steady slow: action preparation slows the subjective passage of time. Proc. R. Soc. B 279, 4399–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth P, and Kimble C (2002) The Duality of Knowledge. Information Research: an international electronic journal, 8, 1, paper 142. [Google Scholar]
- Kimiecik JC and Stein GL, (1992) Examining flow experiences in sport contexts: Conceptual issues and methodological concerns. Journal of Applied Sport Psychology, 4:2,144–160 [Google Scholar]
- Kouwenhoven WB (1969). The development of the defibrillator. Ann Intern Med. 71, 3,449–58. [DOI] [PubMed] [Google Scholar]
- Krieter P, Chiang N, Gyaw S, Skolnick P, Crystal R, Keegan F, Aker J, Beck M, and Harris J (2016). Pharmacokinetic Properties and Human Use Characteristics of an FDA-Approved Intranasal Naloxone Product for the Treatment of Opioid Overdose. Journal of Clinical Pharmacology 56, 10, 1243–53 [DOI] [PubMed] [Google Scholar]
- Lave J and Wenger E (1991). Situated learning. Legitimate peripheral participation. Cambridge: Cambridge University Press [Google Scholar]
- Leonard DA, and Sensiper S, (1998). The Role of Tacit Knowledge in Group Innovation. California Management Review, 40, 3, 112–132 [Google Scholar]
- McAuley A, Munro A, and Taylor A (2018). “Once I’d done it once it was like writing your name”: Lived experience of take-home naloxone administration by people who inject drugs. International Journal of Drug Policy, 58, 46–54. [DOI] [PubMed] [Google Scholar]
- McDonald R, Campbell ND, and Strang J (2017). Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids - conception and maturation. Drug and Alcohol Dependence, 178, 176–187 [DOI] [PubMed] [Google Scholar]
- Moe J, Godwin J, Purssell R, O’Sullivan F, Hau JP, Purssell E, Curran J, Doyle-Waters MM, Brasher PMA, Buxton JA, and Hohl CM, (2020). Naloxone dosing in the era of ultra-potent opioid overdoses: a systematic review. CJEM – JCMU, 1–9. [DOI] [PubMed] [Google Scholar]
- Moss RB and Carlo DJ, (2019). Higher doses of naloxone are needed in the synthetic opioid era. Substance Abuse Treatment, Prevention, and Policy, 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale J, Brown C, Campbell AN, Jones JD, Metz VE, Strang J, and Comer SD (2019). How competent are people who use opioids at responding to overdoses? Qualitative analyses of actions and decisions taken during overdose emergencies. Addiction, 114, 4,708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale J, and Strang J (2015). Naloxone - does over-antagonism matter? Evidence of iatrogenic harm after emergency treatment of heroin/opioid overdose. Addiction. 110, 10, 1644–1652 [DOI] [PubMed] [Google Scholar]
- Nonaka I (1991) The knowledge creating company. Harvard Business Review, 69, (Nov-Dec), 96–104 [Google Scholar]
- Nunes M, Martins e Silva D, Bastos VH, Dias G, Silva Jnr F, and Teixeira S, (2016). Distortions of Time Perception during Critical Situations. International Journal of Emergency Mental Health and Human Resilience, 18, 2, 742 [Google Scholar]
- O’Donnell JK, Gladden RM, Seth P. (2017). Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region—United States, 2006–2015. MMWR MorbMortal Wkly Rep. 66, 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson S, Eatough V, Holmes J, Stapley E, and Midgley N (2016). Framework analysis: a worked example of a study exploring young people’s experiences of depression. Qualitative Research in Psychology, 13, 2, 109–129. [Google Scholar]
- Polanyi M (1967). The tacit dimension. London: Routledge and Kegan Paul [Google Scholar]
- Rhodes T, and Lancaster K, (2019). Evidence-making interventions in health: A conceptual framing. Social Science and Medicine, 238, October 2019, 112488. [DOI] [PubMed] [Google Scholar]
- Ritchie J, and Lewis J, (2003) Qualitative research practice: a guide for social science students and researchers. London, Sage. [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, Ruiz S, Babakhanlou-Chase H, Chan M, Callis BP, Kuramoto-Crawford J, Nields HM, Walley AY, (2017). Characteristics of Fentanyl Overdose — Massachusetts, 2014–2016. Morbidity and Mortality Weekly Report 66, 14, 382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson C, Fiesta MP, Eagleman DM, (2007). Does Time Really Slow Down During a Frightening Event? PLoS ONE 2 (12): e1295. doi: 10.1371/journal.pone.0001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Kelleher M, Best D, Mayet S and Manning V (2006). Emergency naloxone for heroin overdose. Should it be available over the counter? BMJ 2006, 333, 614–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J and McDonald R (2016). Preventing opioid overdose deaths with take-home naloxone. European Monitoring Centre for Drugs and Drug Addiction, Luxembourg. [Google Scholar]
- Strang J, McDonald R, Campbell G, Degenhardt L, Nielsen S, Ritter A, and Dale O (2019). Take-Home Naloxone for the Emergency Interim Management of Opioid Overdose: The Public Health Application of an Emergency Medicine. Drugs, 79, 13, 1395–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Neale J, McDonald R, and Kalk N (2017). Toxicity: exploring and expanding the concept. Addiction, 113,4, 592–594 [DOI] [PubMed] [Google Scholar]
- Taylor S (2014) When Seconds Turn Into Minutes. Why do accidents and emergencies make time slow down so radically? Psychology Today, 18 January 2014 https://www.psychologytoday.com/us/blog/out-the-darkness/201401/when-seconds-turnminutes [Google Scholar]
- Torralva R and Janowsky A, (2019). Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J Pharmacol Exp Ther 371, 453–475, November 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGASS (2016). Informal Scientific Network Statement: United Nations General Assembly Special Session on Drugs (UNGASS). United Nations Office on Drugs and Crime. [Google Scholar]
- van Wassenhove V, Buonomano DV, Shimojo S, and Shams L, (2008) Distortions of Subjective Time Perception Within and Across Senses. PLoS ONE 3, 1, e1437. doi: 10.1371/journal.pone.0001437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, and Collins FS (2017). The Role of Science in Addressing the Opioid Crisis. New Engl Jnl of Medicine 377, 4, 391–394 [DOI] [PubMed] [Google Scholar]
- Wagner KD, Davidson PJ, Iverson E, Washburn R, Burke E, Kral AH (2014). ‘I felt like a superhero’: the experience of responding to drug overdose among individuals trained in overdose prevention. Int J Drug Policy, 25, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R (2017). User-led Interventions: an Expanding Resource? Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction [Google Scholar]
- Wittmann M, van Wassenhove V, Craig AD and Paulus MP (2010). The neural substrates of subjective time dilation. Frontiers in Human Neuroscience, February 2010, 4, Article 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014). Community management of opioid overdose. Geneva, World Health Organization. [PubMed] [Google Scholar]
- Worthington N, Piper TM, Galea S, and Rosenthal D (2006). Opiate users' knowledge about overdose prevention and naloxone in New York City: a focus group study. Harm Reduction Journal, 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]