Abstract
Background:
Poor clearance of apoptotic cells has been suggested to contribute to severe asthma, but whether uptake of apoptotic cells by lung phagocytes might dampen House Dust Mite (HDM)-induced lung inflammation has not been shown.
Objective:
We investigated if apoptotic cell engulfment in the murine lung impacts the development of allergen-induced asthmatic airway inflammation and which immune modulating mechanisms were activated.
Methods:
Apoptotic cells were infused into the lungs of mice challenged with HDM allergen and lung inflammation, expression of suppressive molecules, and induction of Treg were monitored. Additionally, an adenosine receptor agonist was tested to study the mechanism of suppression elicited by apoptotic cells.
Results:
Apoptotic cell uptake by lung alveolar macrophages suppressed HDM-driven allergic asthma. This was associated with promoting the Treg-inducing molecule retinoic acid, inhibiting inflammatory cytokine production, and making macrophages more susceptible to receiving suppressive signals from adenosine. Correspondingly, adenosine receptor agonist treatment also limited HDM-driven allergic airway inflammation through an action on alveolar macrophages.
Conclusion:
These data provide insight into the mechanisms by which lung macrophages dampen allergen-induced airway inflammation. They suggest that targeting lung macrophages to increase their phagocytic capacity, enhance their ability to make retinoic acid, dampen their capacity to make inflammatory cytokines, and increase their responsiveness to adenosine, could be useful to suppress allergic responses.
Keywords: Apoptotic cell clearance, Asthma, Alveolar macrophage, Regulatory T cell, Adenosine
Capsule summary
Apoptotic cell clearance by lung alveolar macrophages limits allergic asthma induced by House Dust Mite (HDM) through promoting regulatory T cell-inducing molecules, inhibiting inflammatory cytokine production, and via receiving suppressive signals from adenosine.
Introduction
House dust mite (HDM) is one of the most common aeroallergens, inducing type 2 cytokine responses which promote eosinophil recruitment to the airways. Allergenicity is associated with both mite antigenic proteins and proteases, and with ligands derived from mite-associated bacterial and fungal products (1). Understanding mechanisms to suppress these insults in the lungs could then provide insights into how to treat allergic asthma.
We previously reported that macrophages in the mouse lung can drive the generation of Foxp3+ regulatory T cells (Treg) that promote airway tolerance (2), suggesting that enhancing macrophage suppressive activity could limit allergenicity of house dust mites. How macrophages differentiate into this regulatory state or how it can be augmented is still largely unclear (3). Impaired apoptotic cell clearance has been associated with lung disease (4–6), and reduced efferocytosis of macrophages from asthmatics has been noted (7, 8). Moreover, DNA release from secondary necrosis of apoptotic cells can directly accelerate Th2 responses (9, 10). In line with this, mice deficient in Mer, a molecule important for clearing apoptotic cells by macrophages, exhibited delayed resolution of Th2 lung inflammation induced by ovalbumin (11). Therefore, as macrophages are thought to be the primary lung phagocyte, their regulatory ability might be related to uptake of apoptotic cells. We investigated this by intratracheally infusing apoptotic cells into the lungs of mice to determine their impact on macrophage activity and HDM-induced airway inflammation. We report that apoptotic cell clearance by alveolar macrophages can limit HDM allergic asthma, through promoting molecules that induce peripheral Treg, as well as inhibiting inflammatory cytokine production, and making macrophages more susceptible to adenosine-derived suppressive signals.
Methods
For detailed methods, please see the Methods section in this article’s Online Repository at www.jacionline.org.
Results and Discussion
Mice given apoptotic thymocytes intratracheally, compared to viable cells, showed decreased eosinophil recruitment, Th2 cytokine production, and overall reduced histologic signs of lung inflammation, upon exposure to HDM allergen (Fig. 1A–C). Infusion of apoptotic Jurkat cells also inhibited development of this asthmatic phenotype (Fig. 1D). Moreover, after sensitization with allergen, apoptotic cell infusion still reduced airway inflammation upon rechallenge with allergen (Fig. 1E). Through adoptive transfer of naïve Foxp3-negative OVA-reactive CD4 T cells and challenge with OVA, we correlated this suppression with a significant increase in the number of antigen-specific Foxp3+ Treg generated in vivo (Fig. 1F). TGF-β and retinoic acid are two primary factors that promote the development of Foxp3+ Treg. In accordance, after apoptotic cell injection, lung tissue mRNA for RALDH1 and RALDH2, enzymes that drive retinoic acid production, was significantly up-regulated, and TGF-β was high, albeit unchanged (Fig. 1G). These results show that apoptotic cells can suppress HDM-driven lung inflammation and suggested that modifying antigen-presenting cells (APC) to induce Treg could be central to the action of engulfing apoptotic cells.
Fig. 1. Apoptotic cells suppress HDM-driven airway inflammation.
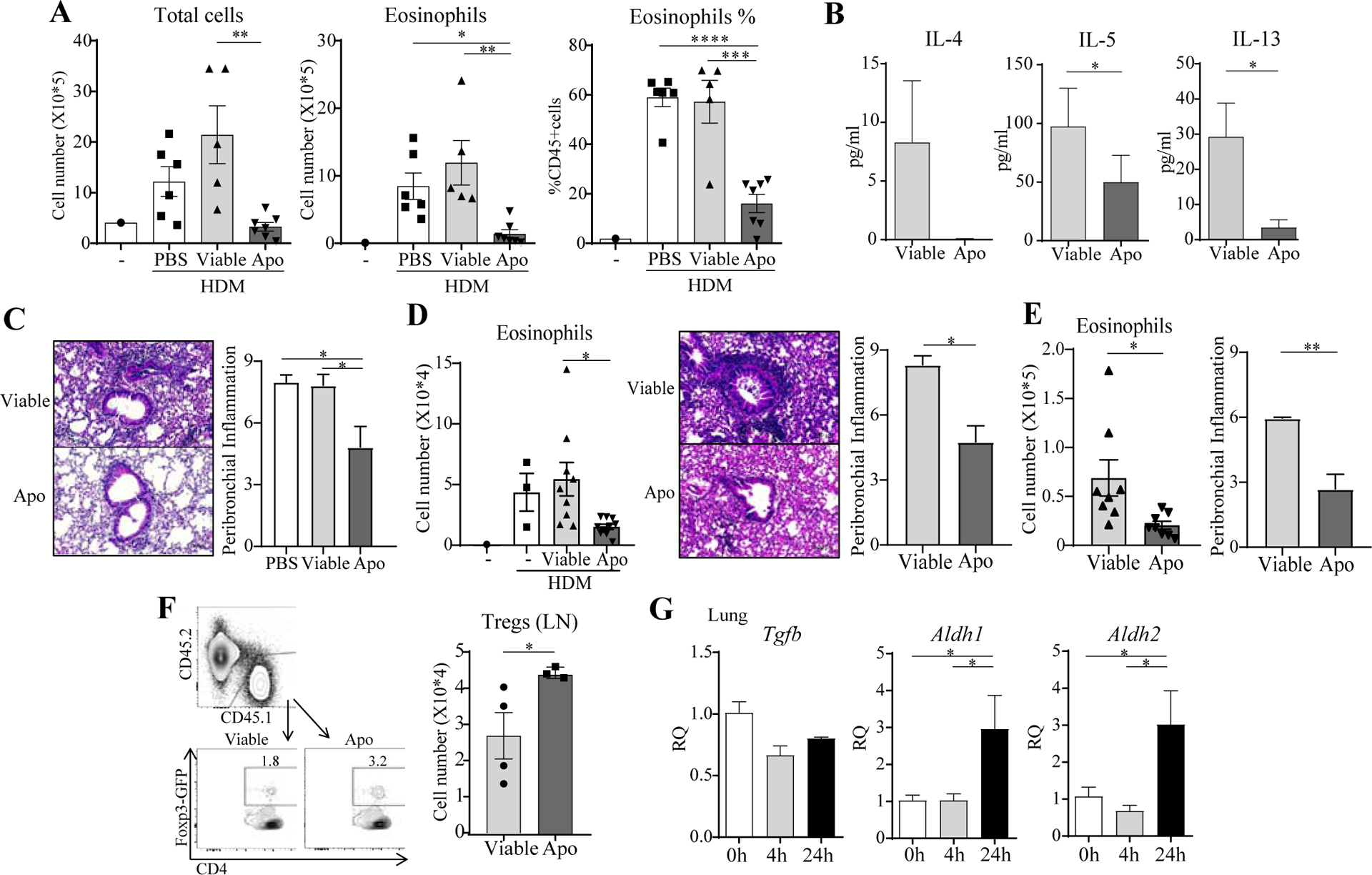
A-D, Viable or apoptotic (Apo) thymocytes (A-C) or Jurkat cells (D) were given i.t. to mice prior to HDM sensitization and challenge. BAL total cells, eosinophils, Th2 cytokines. Lung H&E and inflammation score. n = 3–9 mice/group. E, Viable or apoptotic thymocytes were given to mice on day 14 after sensitization followed by HDM challenge. BAL eosinophils. Lung inflammation score. n = 8 mice/group. F, CD45.1 Foxp3− OT-II T cells were transferred into CD45.2 recipients subsequently exposed to OVA with viable or apoptotic thymocytes. Foxp3+ OT-II T cell accumulation in the lung draining LN. n = 3–4 mice/group. G, Total lung mRNA after apoptotic cell transfer. n = 3 mice/group. All data representative of 3 experiments.
Alveolar macrophages display a strong capacity to phagocytose apoptotic cells compared to macrophages found in the lung interstitium, spleen, or liver (12). CFSE-labeling showed that intratracheally injected apoptotic cells were taken up by ~70% of BAL and ~30% of lung tissue alveolar macrophages (CD11b−SiglecFhighCD11c+) (Fig. 2A). This was not modified significantly by intranasal HDM (data not shown). How uptake of apoptotic cells by alveolar macrophages might control airway homeostasis is not understood (3, 13), but one report linked this with impaired signaling through TLR9 (12). We analyzed sorted alveolar macrophages for Treg-inducing molecules after in vivo engulfment of CFSE-labeled apoptotic cells. They expressed mRNA for TGF-β and RALDH2 at high, albeit similar levels, compared to macrophages from mice not injected with apoptotic cells, but showed significant up-regulation of RALDH1 and also IL-10 (Fig. 2B). Correspondingly, the in vitro co-culture of alveolar macrophages with apoptotic cells also showed up-regulation of RALDH1 with a trend to greater IL-10 (Fig. 2C). We then sorted CFSE-labeled vs. unlabeled lung macrophages and co-cultured them with OVA and naïve OVA-reactive CD4 T cells, and found they were significantly better at driving the upregulation of Foxp3 in vitro (Fig. 2D). Therefore, uptake of apoptotic cells by alveolar macrophages is associated with inhibition of allergic lung inflammation and a greater propensity for these APC to promote formation of regulatory T cells. Analysis of M1 or M2 markers found that the alveolar macrophages overall had a more M2 phenotype (Arg1, Ym1, Fizz1) which was unchanged with uptake of apoptotic cells (data not shown).
Fig. 2. Apoptotic cells trigger Treg-associated activity in alveolar macrophages.
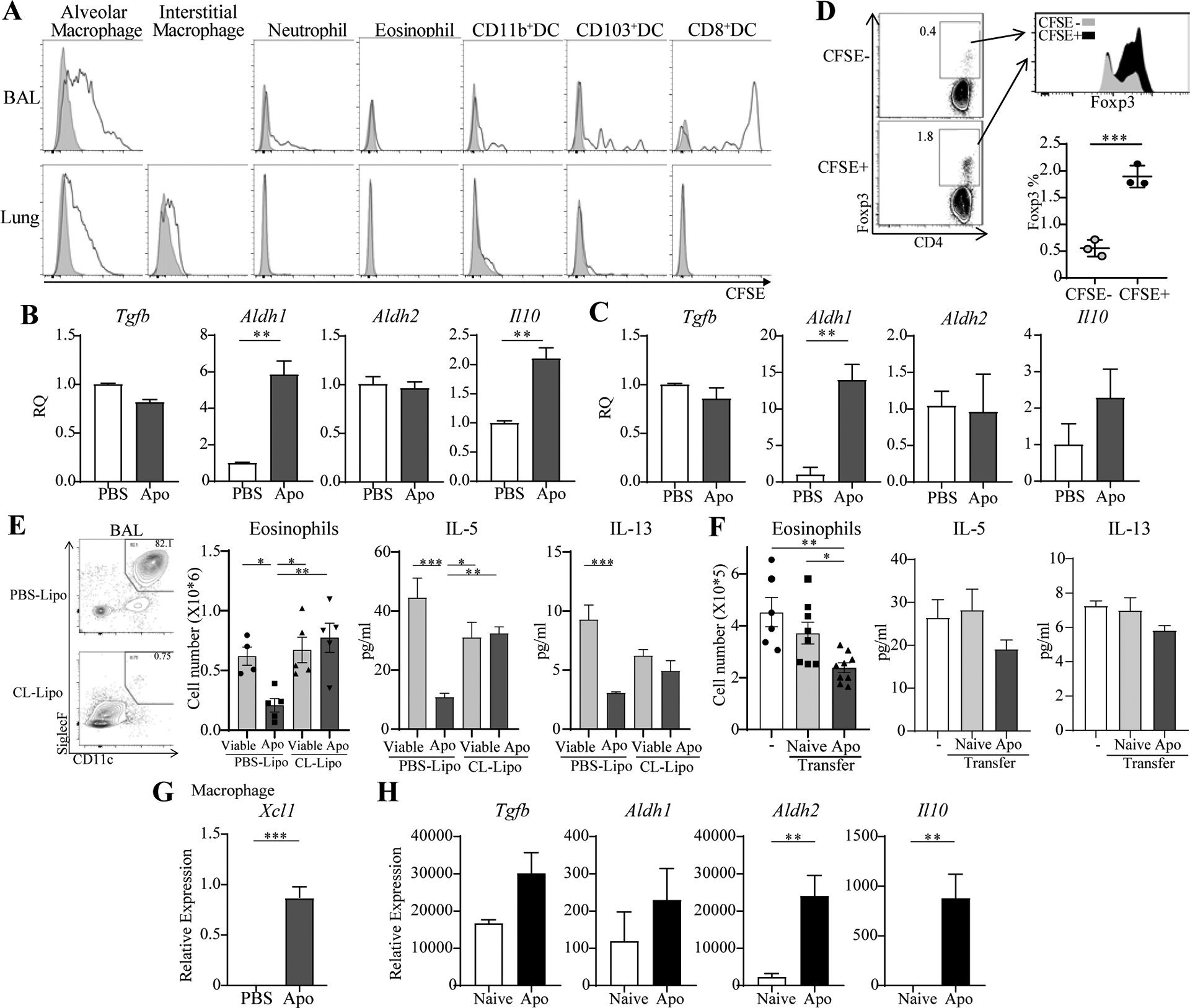
A, CFSE+ apoptotic thymocytes were given i.t. BAL and lung cells analyzed for uptake (black lines). B, mRNA in CFSE+ macrophages from BAL and lung 4hr after transfer of apoptotic cells. C, mRNA in alveolar macrophages co-cultured with apoptotic cells in vitro. D, Foxp3− OT-II T cells were cultured in vitro with OVA and sorted CFSE+ or CFSE− macrophages. Level and % Foxp3+ T cells. E, Alveolar macrophages were depleted with clodronate liposomes (left) before sensitization with HDM. BAL eosinophils and Th2 cytokines. n = 4–5 mice/group. F, Naïve or CFSE+ alveolar macrophages (Apo) were given to recipients prior to sensitization with HDM. BAL eosinophils and Th2 cytokines. n = 6–9 mice/group. G, Xcl1 mRNA expression in CFSE+ macrophages. H, mRNA in CFSE+ vs. naive CD8+ DC. All data representative of 3 experiments.
We next depleted alveolar macrophages by i.t. injection of clodronate liposomes. In these mice, suppression of HDM-driven lung inflammation by apoptotic cells was not observed (Fig. 2E). In addition, we sorted lung macrophages that engulfed apoptotic cells in vivo, and found that they suppressed eosinophilia when transferred into the lungs of HDM-challenged mice (Fig. 2F). Similar trends were seen when analyzing BAL Th2 cytokines. This strongly suggests that apoptotic cell uptake by alveolar macrophages was required for reducing the inflammation and Th2 responses caused by the allergen.
Previously, it was reported that airway epithelial cells could phagocytose apoptotic cells (14), but in our model, we did not find any significant number of CD45 negative lung cells that engulfed CFSE-labeled apoptotic cells (data not shown). However, a small population of CD11b−CD8+ DC took up apoptotic cells (Fig. 2A). Similar splenic CD8+ DC are thought to be specialized to capture dying cells and induce Treg (15). The CD8+ DC were undetectable in the naïve lung, and appeared in the lungs ~24 hours after apoptotic cell infusion (data not shown), likely recruited by signals from the lung macrophages that phagocytosed apoptotic cells. In support of this, the macrophages showed strong induction of XCL1, the chemokine ligand for XCR1 which is expressed on CD8+ DC (Fig. 2G). Moreover, the lung CD8+ DC showed higher expression of RALDH2 and IL-10 suggesting they may also reinforce suppression and Treg induction downstream of the alveolar macrophages (Fig. 2H).
In addition to upregulation of RALDH1, the in vitro co-culture of sorted alveolar macrophages and apoptotic cells also led to reduced TNF and IL-6 expression when macrophages were stimulated with HDM (Fig. 3A). Furthermore, CFSE+ lung macrophages that engulfed apoptotic cells in vivo exhibited significantly lower levels of mRNA for TNF and IL-6 (Fig. 3B). These cytokines can promote multiple inflammatory activities, and can also antagonize Treg induction, with their neutralization restoring the Treg-promoting activity of differentiated lung macrophages (2). This then represents a complementary way in which apoptotic cells may lead to suppression of allergen-induced inflammation. Expanding this notion, we found that mRNAs for the suppressor of cytokine signaling molecule, SOCS3, and adenosine A2A and A2B receptors, Adora2a and Adora2b, were significantly up-regulated in CFSE-labeled macrophages (Fig. 3C). SOCS3 expression and adenosine signaling in macrophages both can limit inflammatory cytokines, and have been associated with enhanced generation of Treg (3, 16–18).
Fig. 3. Uptake of apoptotic by alveolar macrophages results in anti-inflammatory activities.
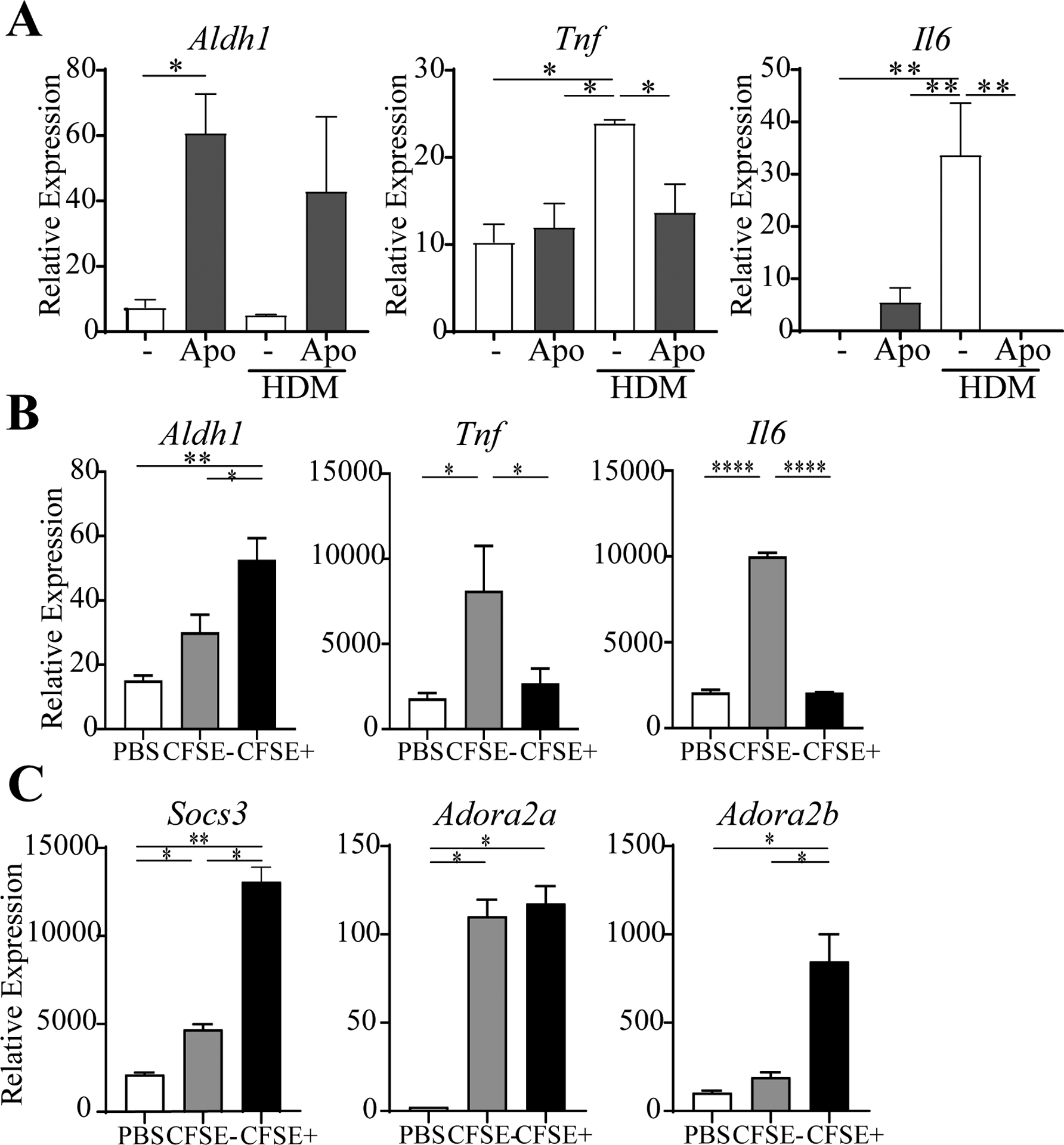
A, mRNA in naive alveolar macrophages co-cultured with apoptotic cells in vitro with or without HDM. B-C, mRNA in CFSE− or CFSE+ macrophages sorted from BAL and lung, compared to macrophages from mice given PBS. n = 6 mice/group. All data representative of 2–3 experiments.
Further reinforcing the idea that enhancing adenosine receptor expression could be of significance to suppression, we treated macrophages that engulfed apoptotic cells (CFSE+) with an A2A receptor agonist (ATL), and found that this significantly increased their ability to generate Treg in vitro (Fig. 4A). This correlated with an increase in mRNA for RALDH1 (Fig. 4B). Moreover, ATL administered intranasally to mice reduced airway inflammation induced by HDM (Fig. 4C–E). Lastly, when alveolar macrophages were depleted in vivo by chlodronate liposome treatment, we found that suppression of airway inflammation by ATL was abrogated (Fig. 4F). These data are in line with our previous report that adenosine agonist treatment attenuated neutrophilic lung inflammation induced by OVA and LPS, and increased Treg numbers, via an action on lung macrophages (19).
Fig. 4. Adenosine receptor signaling promotes suppressive activity in alveolar macrophages.
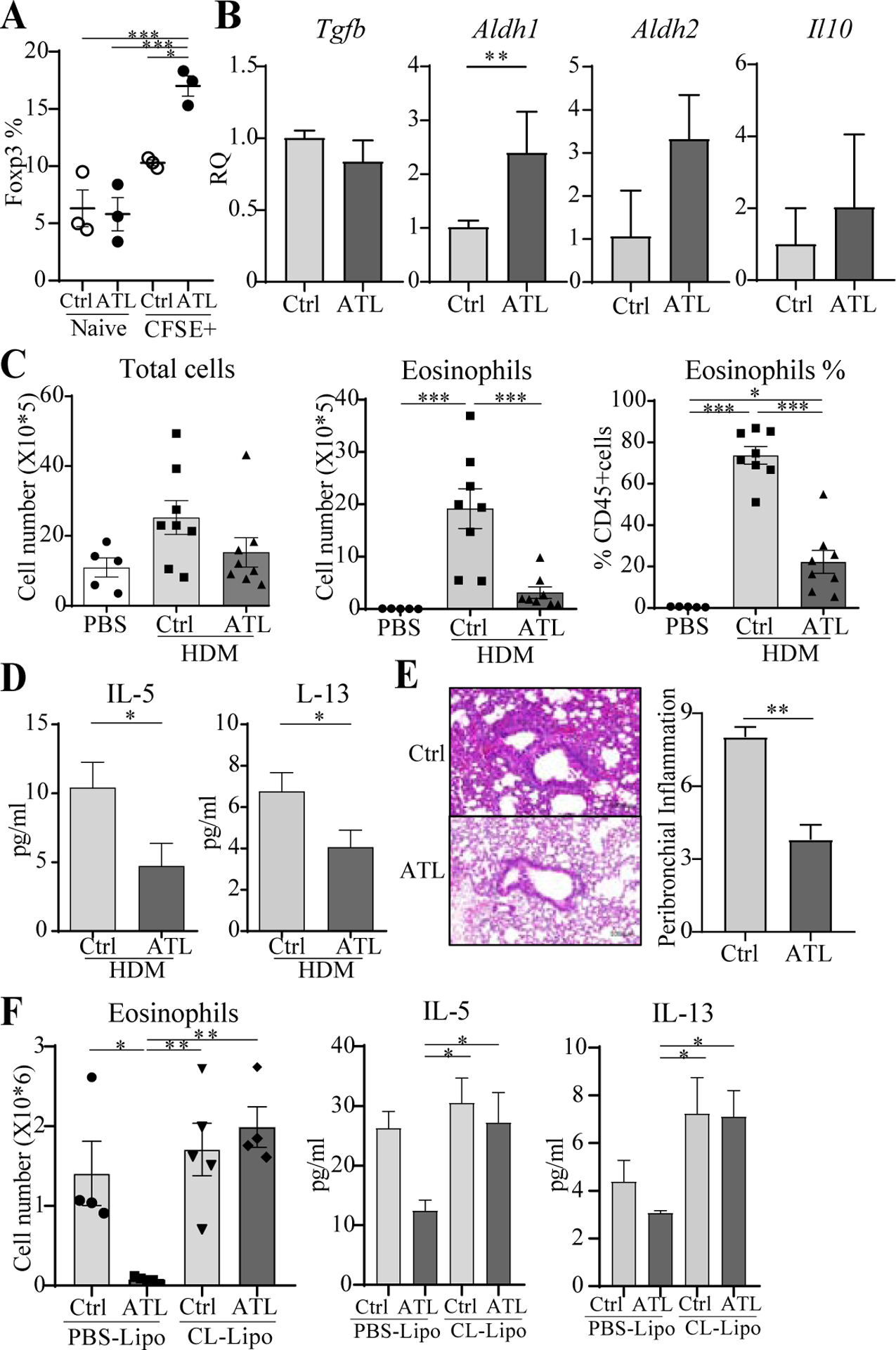
A, Foxp3− OT-II T cells were cultured with OVA and naïve or CFSE+ macrophages sorted from BAL and lung, in the presence or absence of ATL. % Foxp3+ T cells. B, mRNA in alveolar macrophages cultured with ATL in vitro. C-E, Mice were given ATL and sensitized and challenged as in Fig. 1 with HDM. BAL total cells, eosinophils, Th2 cytokines, lung H&E, and inflammation score. n = 6–9 mice/group. F, Alveolar macrophages were depleted with clodronate liposomes before treatment with ATL and sensitization with HDM. BAL eosinophils and Th2 cytokines. n = 5 mice/group. All data representative of 2–3 experiments.
In summary, our results indicate that apoptotic cell clearance by lung alveolar macrophages can limit airway inflammation to HDM, and is associated with enhanced adenosine activity, suppressed inflammatory cytokine production, and the generation of Treg. While a therapeutic approach with apoptotic cell infusion is being considered for inflammatory diseases, and can also prevent inflammation in experimental models as diverse as GvHD, sepsis, Type1 diabetes, arthritis, EAE, and CHS (20), understanding the mechanistic basis for how apoptotic cells induce suppression may be more important for formulating practical therapies. Targeting lung alveolar macrophages in severe asthmatic patients to increase their phagocytosis of apoptotic cells and increase their responsiveness to adenosine could be useful to suppress allergic responses.
Supplementary Material
Clinical Implications/Key Messages.
Targeting lung macrophages in asthmatic patients to increase their ability to promote Treg and increase their responsiveness to adenosine could be useful to suppress allergic responses.
Acknowledgments
Supported by NIH grant AI103021 to M.C. H.M. was supported by a JSPS Overseas Research Fellowship.
Abbreviations
- HDM
House Dust Mite
- Treg
regulatory T cells
- OVA
Ovalbumin
- DC
Dendritic cells
- BALF
Bronchoalveolar lavage fluid
- Apo
Apoptotic cells
- CL
Clodronate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare that they have no relevant conflicts of interest.
References
- 1.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends in immunology. 2011;32(9):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. The Journal of experimental medicine. 2013;210(4):775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabiec AM, Hussell T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Seminars in immunopathology. 2016;38(4):409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest. 2013;143(6):1750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szondy Z, Garabuczi E, Joos G, Tsay GJ, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Frontiers in immunology. 2014;5:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nature reviews Immunology. 2014;14(3):166–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erriah M, Pabreja K, Fricker M, Baines KJ, Donnelly LE, Bylund J, et al. Galectin-3 enhances monocyte-derived macrophage efferocytosis of apoptotic granulocytes in asthma. Respiratory research. 2019;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Boyanapalli R, Goleva E, Kolakowski C, Min E, Day B, Leung DY, et al. Obesity impairs apoptotic cell clearance in asthma. The Journal of allergy and clinical immunology. 2013;131(4):1041–7, 7 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imanishi T, Ishihara C, Badr Mel S, Hashimoto-Tane A, Kimura Y, Kawai T, et al. Nucleic acid sensing by T cells initiates Th2 cell differentiation. Nature communications. 2014;5:3566. [DOI] [PubMed] [Google Scholar]
- 10.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nature medicine. 2011;17(8):996–1002. [DOI] [PubMed] [Google Scholar]
- 11.Felton JM, Lucas CD, Dorward DA, Duffin R, Kipari T, Vermeren S, et al. Mer-mediated eosinophil efferocytosis regulates resolution of allergic airway inflammation. The Journal of allergy and clinical immunology. 2018;142(6):1884–93 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity. 2017;47(5):913–27 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draijer C, Peters-Golden M. Alveolar Macrophages in Allergic Asthma: the Forgotten Cell Awakes. Current allergy and asthma reports. 2017;17(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493(7433):547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. Journal of immunology. 2008;181(10):6923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Frontiers in immunology. 2014;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nature reviews Immunology. 2014;14(2):81–93. [DOI] [PubMed] [Google Scholar]
- 18.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature reviews Drug discovery. 2008;7(9):759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei H, Linden J. Adenosine influences myeloid cells to inhibit aeroallergen sensitization. American journal of physiology Lung cellular and molecular physiology. 2016;310(10):L985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saas P, Daguindau E, Perruche S. Concise Review: Apoptotic Cell-Based Therapies-Rationale, Preclinical Results and Future Clinical Developments. Stem cells (Dayton, Ohio). 2016;34(6):1464–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


