Abstract
Background.
In 2002, breast cancer patients with supraclavicular nodal metastases (cN3c) were downstaged from AJCC stage IV to IIIc, prompting management with locoregional treatment. We sought to estimate the impact of multimodal therapy on overall survival (OS) in a contemporary cohort of cN3c patients.
Methods.
Women ≥ 18 years with cT1-T4c/cN3c invasive breast cancer who underwent systemic therapy were identified from the 2004–2016 National Cancer Database. We compared three patient cohorts: (a) cN3c + multimodal therapy (systemic therapy, surgery, and radiation); (b) cN3c + non-standard therapy; and, (c) cM1. Logistic regression identified factors associated with receipt of multimodal therapy and Kaplan–Meier was used to estimate unadjusted OS. The Cox proportional hazards model estimated effects of diagnosis and treatment on OS after adjustment.
Results.
Overall, 1827 (3.7%) patients with cN3c disease and 46,919 (96.3%) cM1 patients were identified. Of cN3c patients, 74.5% (n = 1362) received multimodal therapy and 25.5% (n = 465) received non-standard therapy; receipt of multimodal therapy was associated with improved 5-year OS (multimodal: 59% vs. M1: 28% vs. non-standard: 28%, log-rank p < 0.001). Adjusting for covariates, non-standard therapy was associated with an increased risk of death compared with receipt of multimodal therapy (HR 2.20, 95% CI 1.71–2.83, p < 0.001). Private insurance was the only patient characteristic associated with a greater likelihood of receiving multimodal therapy (OR 2.81; 95% CI, 1.64–4.82; p < 0.001).
Conclusion.
Women with cN3c breast cancer who received multimodal therapy demonstrated improved overall survival when compared with patients undergoing non-standard therapy and those with metastatic (M1) disease. Although selection bias may contribute to worse overall survival among cN3c patients undergoing non-standard therapy, national guidelines should encourage locoregional treatment in carefully selected patients.
Contemporary staging guidelines for breast cancer are used to categorize women for the purpose of prognostication and to guide oncologic therapy; however, in the clinical setting, patients present on a spectrum from early stage to metastatic disease.1–3 Extensive ipsilateral nodal involvement without evidence of distant metastases presents a particular treatment challenge, as the value of locoregional therapy in these patients remains uncertain. In 2002, studies demonstrating an improved survival with receipt of comprehensive multimodal therapy prompted the American Joint Commission on Cancer (AJCC) to downstage breast cancer with supraclavicular nodal metastases (cN3c) from stage IV to IIIc disease.4–6 Reflecting this change, the National Comprehensive Cancer Network (NCCN) guidelines recommended these individuals be considered for locoregional therapy, including “total mastectomy…or lumpectomy with level I/II axillary lymph node dissection” and adjuvant irradiation to the chest wall/breast, supraclavicular lymph nodes ± internal mammary lymph nodes due to the “sufficient risk of local recurrence7.
Since reclassification, single institution studies have demonstrated that multimodal therapy improves survival in cN3c breast cancer, with 5-year overall survival rates ranging from 33 to 47%.8–11 However, to date, little is known about the national uptake of these guidelines into clinical practice, and the resulting impact on overall survival in this rare population. We sought to compare the overall survival of cN3c breast cancer patients treated with multimodal versus non-standard therapy to patients with de novo metastatic disease, and to identify predictors of receiving guideline-concordant care.
METHODS
Following institutional review board approval, adult female patients diagnosed with clinical T1–T4c, cN3c breast cancer between 2004 and 2016 were identified from the National Cancer Database (NCDB). The NCDB captures approximately 70% of all cancer diagnoses in the United States, including clinical and demographic data from more than 30 million patients in more than 1500 cancer registries.12 Women were categorized into three comparison treatment groups: (a) cN3c multimodal therapy: cN3c patients who received comprehensive multimodal therapy, including chemotherapy and/or endocrine therapy + axillary lymph node dissection (ALND) + radiation; (b) cN3c non-standard therapy: cN3c patients who received chemotherapy and/or endocrine therapy without recommended locoregional therapy (ALND + radiation); and (c) M1: patients with de novo M1 metastatic breast cancer who underwent treatment with chemotherapy and/or endocrine therapy ± surgery or radiation (Table 1). For the purpose of our study, we defined “locoregional treatment” in cN3c patients according to NCCN guidelines.7 This included receipt of both axillary lymph node dissection and radiation. Thus, in all cN3c patients, we excluded individuals who underwent axillary lymph node dissection or radiation alone. The cN3c non-standard treatment group included patients who underwent systemic therapy without radiation or surgery. The NCDB defines cN3c breast cancer as either biopsy-proven nodal metastases or clinically or radiographically detected lymph nodes. The method of axillary surgical staging was not captured by the NCDB throughout the study period; thus, we defined axillary lymph node dissection (ALND) as the retrieval of 10 or more lymph nodes or as lymphadenectomy included within the breast surgery codes (e.g., modified radical mastectomy).
TABLE 1.
Treatments included within the defined cohorts of patients
| cN3c multimodal | cN3c non-standard | cM1 | |
|---|---|---|---|
| Axillary lymph node dissection | Yes | No | Yes or No |
| Radiation | Yes | No | Yes or No |
| Chemotherapy and/or endocrine therapy | Yes | Yes | Yes |
Patients with non-invasive breast cancer and those with missing survival information were excluded. As required by NCDB guidelines, survival information is masked for patients diagnosed in the last reporting year, as such, all patients diagnosed in 2016 were excluded. Hospital volume was categorized as low, intermediate, or high using a similar methodology to define hospital volume from a prior study.14 Patient baseline demographic, clinical, and treatment characteristics are summarized in Table 1. Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables by treatment group. Chi square tests or Fisher’s exact tests were used to compare categorical variables, and Wilcoxon rank sum tests or t-tests were used to compare continuous variables, as appropriate. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Kaplan–Meier curves were used to visualize unadjusted OS, and the log-rank test was used to test for a difference among groups. Cox proportional hazards regression analysis was used to estimate the association of treatment group and OS after adjustment for covariates, including: estrogen receptor (ER)/progesterone receptor (PR)/HER2 status, age, race, Charlson/Deyo comorbidity score, insurance status, income, breast surgery, endocrine therapy, chemotherapy, histologic type (ductal/lobular), hospital type and location, hospital volume, histologic grade, and tumor size. We expected that chemotherapy and endocrine therapy as covariates would be associated with our main comparison group, resulting in collinearity. Accordingly, we used the rule of variance inflation factor (VIF) < 10 to address this issue. As a result, no collinearity was found in the multivariate model.13
Due to the recent addition of HER2 status to the NCDB, inclusion of HER2 status was limited in this model to patients diagnosed in or after 2010. A sensitivity analysis that included all patients and excluded HER2 as a covariate was also conducted. Because no major differences were present (data not shown), only the adjusted model including HER2 status is reported here. All survival models included a robust sandwich covariance estimator to account for the correlation of patients treated at the same hospital.
A logistic regression model was used to identify factors associated with receipt of multimodal therapy among cN3c patients. This model was built in the generalized estimating equations framework and included an exchangeable correlation structure to account for patients treated at the same hospital. No adjustments were made for multiple comparisons. Only patients with available data are included in each model and sample sizes are reported for each Table/Fig. We report hazard ratios (HRs) or odds ratios (ORs), 95% confidence intervals (CIs), and p values significant at < 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographic, Clinical, and Treatment Characteristics of cN3c Patients
The overall cohort included 48,746 patients, of which 1827 had cN3cM0 disease (3.7%) and 96.3% had de novo M1 disease. Among the cN3c patients, 74.5% (n = 1362) received multimodal therapy and 25.5% (n = 465) received non-standard therapy (Fig. 1). The median age was 60 years (IQR 50–69). Compared with cN3c patients treated with non-standard therapy, multimodal therapy was associated with private insurance (58.7% vs. 32.3%; p < 0.001), treatment at comprehensive cancer centers (36.8% vs. 30.8%; p < 0.001), and clinical T stage 2 or 3 disease (55.2% vs. 41.7%; p < 0.001). Notably, women in the multimodal therapy group were also more likely to undergo comprehensive nodal radiation compared with breast/chest wall alone (72.9% vs. 23%, p < 0.001) and mastectomy versus partial mastectomy (85.9% vs. 13.9%; p < 0.001). Among individuals with cN3c breast cancer, we selected receipt of ALND as the primary surgery of interest; the overwhelming majority of cN3c patients also underwent surgery of their primary breast cancer. Only n = 3 (0.2%) of patients underwent ALND without breast surgery (Table 2).
FIG. 1.
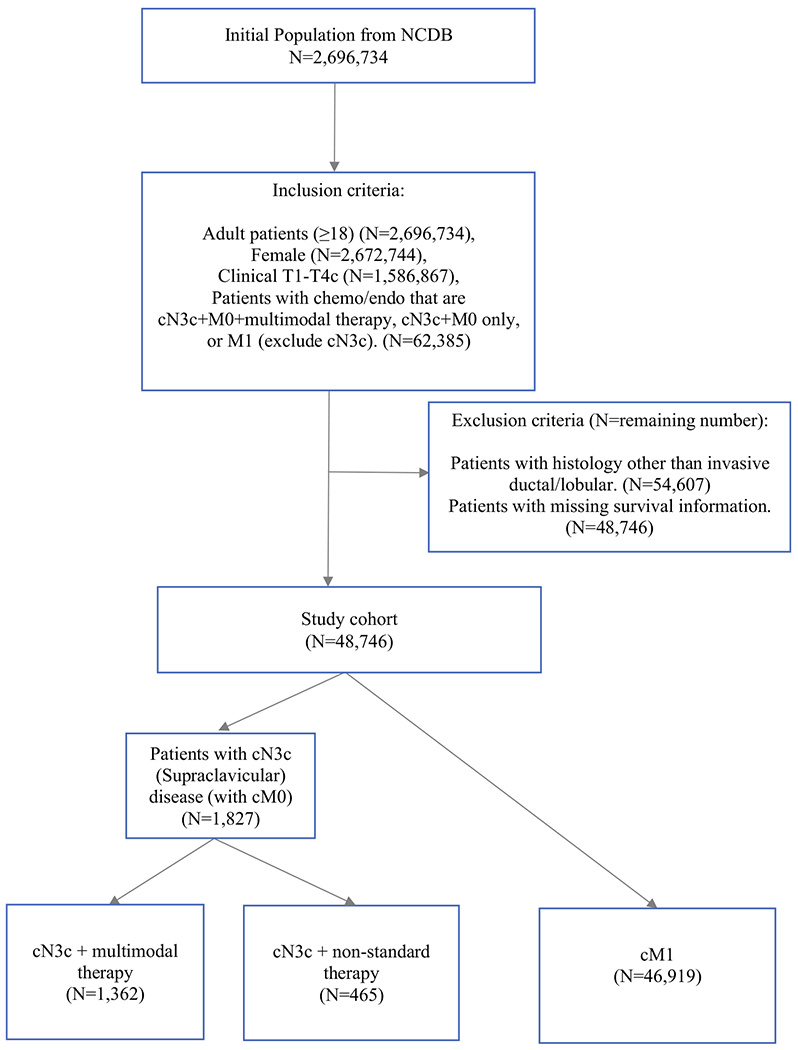
Cohort selection diagram
TABLE 2.
Patient demographic, clinical, and treatment characteristics, National Cancer Data Base 2004–2016 (N = 48,746)
| Study cohort (N = 48,746) | All patients N = 48,746 | cM1 N = 46,919 (96.2%) | cN3c + non-standard therapy N = 465 (0.9%) | cN3c + multimodal therapy N = 1362 (2.8%) | p value |
|---|---|---|---|---|---|
| Age—median (IQR) | 60 (50–69) | 60 (51–70) | 59 (49–70) | 54 (45–62) | < 0.001 |
| ER status | < 0.001 | ||||
| Negative | 11,316 (23.2%) | 10,460 (22.3%) | 202 (43.4%) | 654 (48%) | |
| Positive | 36,142 (74.1%) | 35,215 (75.1%) | 236 (50.8%) | 691 (50.7%) | |
| PR status | < 0.001 | ||||
| Negative | 17,803 (36.5%) | 16,758 (35.7%) | 250 (53.8%) | 795 (58.4%) | |
| Positive | 29,365 (60.2%) | 28,634 (61%) | 179 (38.5%) | 552 (40.5%) | |
| HER2 status | < 0.001 | ||||
| Negative | 22,186 (45.5%) | 21,383 (45.6%) | 205 (44.1%) | 598 (43.9%) | |
| Positive | 7680 (15.8%) | 7311 (15.6%) | 77 (16.6%) | 292 (21.4%) | |
| Clinical T stage | < 0.001 | ||||
| 1 | 7567 (15.5%) | 7346 (15.7%) | 50 (10.8%) | 171 (12.6%) | |
| 2 | 15,949 (32.7%) | 15,369 (32.8%) | 122 (26.2%) | 458 (33.6%) | |
| 3 | 8359 (17.1%) | 7993 (17%) | 72 (15.5%) | 294 (21.6%) | |
| 4 | 16,871 (34.6%) | 16,211 (34.6%) | 221 (47.5%) | 439 (32.2%) | |
| LNs examined- median (IQR) | 9 (2–15) | 8 (2–15) | 1 (1–3) | 14 (10–19) | < 0.001 |
| Breast surgery | < 0.001 | ||||
| Lumpectomy | 5188 (10.6%) | 4962 (10.6%) | 37 (8%) | 189 (13.9%) | |
| Mastectomy | 12,947 (26.6%) | 11,712 (25%) | 65 (14%) | 1170 (85.9%) | |
| Chemotherapy type | < 0.001 | ||||
| Adjuvant | 4316 (8.9%) | 4125 (8.8%) | 17 (3.7%) | 174 (12.8%) | |
| Neoadjuvant | 9025 (18.5%) | 7833 (16.7%) | 74 (15.9%) | 1118 (82.1%) | |
| Radiation field | < 0.0001 | ||||
| Breast/chest wall/regional nodal basins | 4945 (10.1%) | 3952 (8.4%) | 0 (0%) | 993 (72.9%) | |
| Breast/chest wall | 3117 (6.4%) | 2804 (6%) | 0 (0%) | 313 (23%) | |
| None | 29,686 (60.9%) | 29,227 (62.3%) | 459 (98.7%) | 0 (0%) | |
| Facility type | < 0.001 | ||||
| Academic | 15,188 (31.2%) | 14,572 (31.1%) | 206 (44.3%) | 410 (30.1%) | |
| Community | 4614 (9.5%) | 4495 (9.6%) | 36 (7.7%) | 83 (6.1%) | |
| Comprehensive | 18,963 (38.9%) | 18,319 (39%) | 143 (30.8%) | 501 (36.8%) | |
| Integrated network | 6303 (12.9%) | 6085 (13%) | 46 (9.9%) | 172 (12.6%) | |
| Hospital volume | < 0.001 | ||||
| High-volume | 16,840 (34.5%) | 16,093 (34.3%) | 214 (46%) | 533 (39.1%) | |
| Moderate-volume | 16,844 (34.6%) | 16,229 (34.6%) | 136 (29.2%) | 479 (35.2%) | |
| Low-volume | 15,062 (30.9%) | 14,597 (31.1%) | 115 (24.7%) | 350 (25.7%) | |
| Insurance status | < 0.001 | ||||
| Government | 23,674 (48.6%) | 23,000 (49%) | 214 (46%) | 460 (33.8%) | |
| Not insured | 2652 (5.4%) | 2539 (5.4%) | 36 (7.7%) | 77 (5.7%) | |
| Private | 21,364 (43.8%) | 20,414 (43.5%) | 150 (32.3%) | 800 (58.7%) |
IQR interquartile range, LN lymph node, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2
On multivariable analysis (Table 3), there were no statistically significant differences in the likelihood of receiving multimodal therapy versus non-standard therapy based on hospital volume, age, hormone receptor status, comorbidity score, or tumor histology. However, women with private insurance had a higher likelihood of receiving multimodal therapy compared with the uninsured (OR 2.81; 95% CI, 1.64–4.82; p < 0.001).
TABLE 3.
Logistic regression identifying factors associated with receipt of multimodal therapy versus non-standard therapy among cN3c patients, National Cancer Data Base 2004–2014 (N = 1480; events = 1122)
| OR (95% CI) | p value | Overall p value | |
|---|---|---|---|
| Age | 0.96 (0.95–0.97) | < 0.001 | < 0.001 |
| CDCC score | |||
| 0 | -REF- | 0.13 | |
| 1 | 0.73 (0.53–1.01) | 0.06 | |
| ≥ 2 | 0.70 (0.37–1.35) | 0.29 | |
| ER | |||
| Positive | -REF- | 0.39 | |
| Negative | 1.16 (0.82–1.65) | 0.39 | |
| PR | |||
| Positive | -REF- | 0.71 | |
| Negative | 0.93 (0.65–1.34) | 0.71 | |
| Histology | |||
| Invasive lobular | -REF- | 0.17 | |
| Invasive ductal | 0.77 (0.53–1.13) | 0.18 | |
| Facility type | |||
| Academic | -REF- | 0.03 | |
| Community | 0.97 (0.55–1.71) | 0.92 | |
| Comprehensive | 1.53 (1.11–2.10) | 0.009 | |
| Integrated network | 1.53 (0.97–2.40) | 0.07 | |
| Facility location | |||
| West | -REF- | 0.20 | |
| Midwest | 1.24 (0.85–1.83) | 0.27 | |
| Northeast | 0.85 (0.56–1.29) | 0.45 | |
| South | 0.94 (0.64–1.37) | 0.74 | |
| Hospital volume | |||
| Low-volume | -REF- | 0.77 | |
| Moderate-volume | 0.99 (0.68–1.43) | 0.95 | |
| High-volume | 1.11 (0.75–1.64) | 0.62 | |
| Income | |||
| ≥ 35,000 | -REF- | 0.06 | |
| < 35,000 | 0.77 (0.59–1.01) | 0.06 | |
| Insurance | |||
| Not insured | -REF- | < 0.001 | |
| Government | 1.83 (1.04–3.24) | 0.04 | |
| Private | 2.81 (1.64–4.82) | < 0.001 |
The model has accounted for the correlation of patients treated at the same hospital
Survival Analysis
In the unadjusted analysis, women with M1 disease and cN3c patients treated with non-standard therapy had similar 5-year OS rates at 28%. cN3c patients treated with multimodal therapy had the highest 5-year OS rates of all three groups (59% vs. 28% vs. 28%, log rank p < 0.001). Unadjusted survival curves are shown in Fig. 2. After adjustment for known covariates, receipt of non-standard therapy was associated with an increased risk of death in cN3c patients when compared with use of multimodal therapy (HR 2.20, 95% CI 1.71–2.83, p < 0.001) (Table 4). Factors associated with a worse OS were ER negative (ER−) tumors (HR 1.15; 95% CI 1.06–1.24, p < 0.001), PR negative (PR−) tumors (HR 1.33; 95% CI 1.26–1.40, p < 0.001), and HER2 negative (HER2−) tumors (HR 1.55; 95% CI 1.46–1.63, p < 0.001). Private insurance was associated with an improved survival (HR 0.76; 95% CI 0.70–0.83, p < 0.001) compared with no insurance.
FIG. 2.
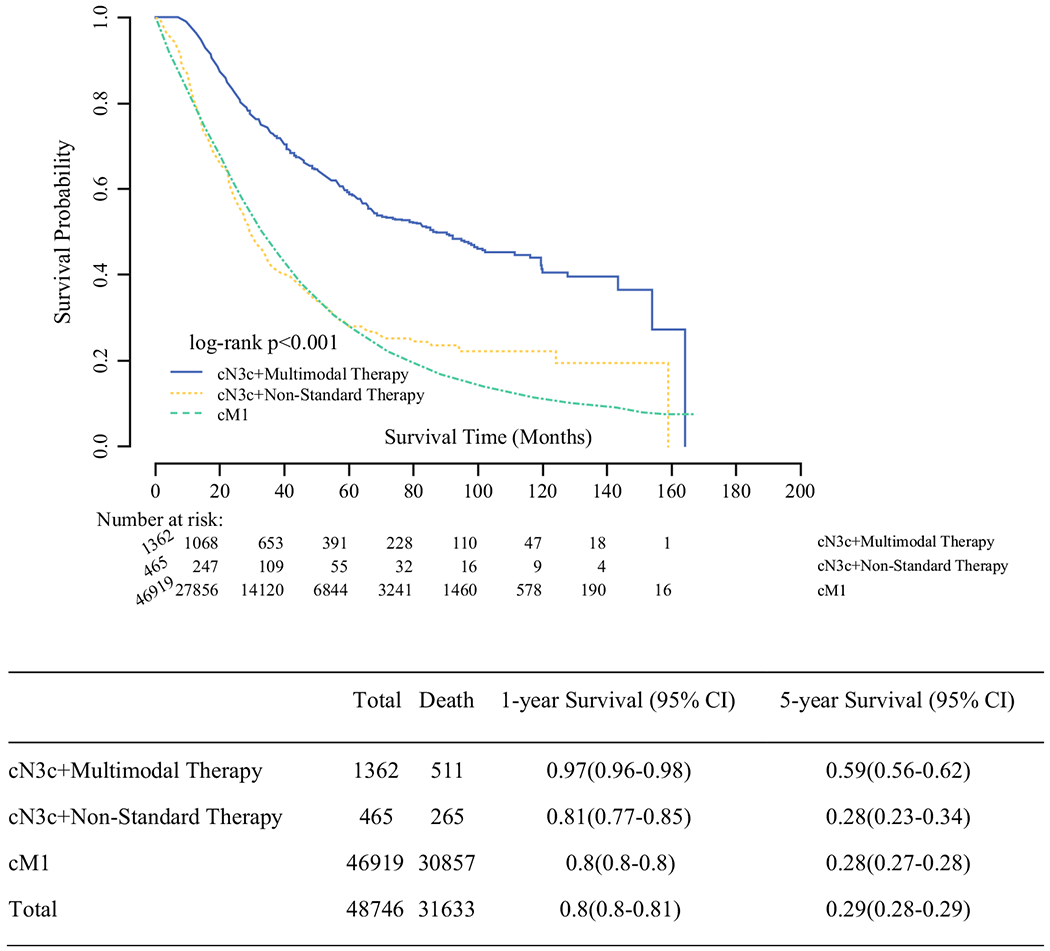
Kaplan-Meier curve for unadjusted overall survival of cM1 patients treated with systemic therapy alone (chemotherapy and/or endocrine therapy), cN3c patients treated with multimodal treatment, and cN3c patients treated with non-standard therapy, National Cancer Database 2004–2016 (N = 48,746)
TABLE 4.
Adjusted overall survival, cN3c + multimodal therapy versus cN3c + non-standard therapy versus cM1, National Cancer Data Base, 2010–2016 (N = 23,317/31,803, events = 13,018)
| HR (95% CI) | p value | Overall p value | |
|---|---|---|---|
| Group | |||
| cN3c + multimodal therapy | -REF- | < 0.001 | |
| cN3c + non-standard therapy | 2.20 (1.71–2.83) | < 0.001 | |
| cM1 | 2.68 (2.33–3.09) | < 0.001 | |
| Age (years) | 1.02 (1.01–1.02) | < 0.001 | < 0.001 |
| Charlson/Deyo comorbidity score | < 0.001 | ||
| 0 | -REF- | ||
| 1 | 1.22 (1.16–1.29) | < 0.001 | |
| ≥ 2 | 1.53 (1.40–1.66) | < 0.001 | |
| ER status | < 0.001 | ||
| Positive | -REF- | ||
| Negative | 1.15 (1.06–1.24) | < 0.001 | |
| Facility location | 0.11 | ||
| West | -REF- | ||
| South | 1.05 (0.97–1.13) | 0.22 | |
| Northeast | 1.07 (0.98–1.16) | 0.14 | |
| Midwest | 1.10 (1.02–1.19) | 0.02 | |
| Facility type | 0.25 | ||
| Academic | -REF- | ||
| Community | 1.03 (0.93–1.13) | 0.57 | |
| Integrated Network | 1.04 (0.95–1.14) | 0.39 | |
| Comprehensive | 1.06 (1.00–1.13) | 0.05 | |
| Histology | 0.75 | ||
| Invasive lobular | -REF- | ||
| Invasive ductal | 0.99 (0.95–1.04) | 0.75 | |
| Insurance | < 0.001 | ||
| Not insured | -REF- | ||
| Private | 0.76 (0.70–0.83) | < 0.001 | |
| Government | 0.90 (0.83–0.99) | 0.03 | |
| PR Status | < 0.001 | ||
| Positive | -REF- | ||
| Negative | 1.33 (1.26–1.40) | < 0.001 | |
| Endocrine therapy | < 0.001 | ||
| No | -REF- | ||
| Yes | 0.61 (0.57–0.65) | < 0.001 | |
| Chemotherapy | < 0.001 | ||
| No | -REF- | ||
| Yes | 0.83 (0.79–0.87) | < 0.001 | |
| HER2 status | < 0.001 | ||
| Positive | -REF- | ||
| Negative | 1.55 (1.46–1.63) | < 0.001 | |
| Income level | 0.002 | ||
| ≥ 35,000 | -REF- | ||
| < 35,000 | 1.07 (1.02–1.11) | 0.002 | |
| Race | < 0.001 | ||
| White | -REF- | ||
| Black | 1.13 (1.08–1.20) | < 0.001 | |
| Other | 0.86 (0.77–0.96) | 0.007 | |
| Tumor size | 1.00 (1.001–1.002) | < 0.001 | < 0.001 |
| Hospital volume | 0.03 | ||
| Low-volume | -REF- | ||
| Moderate-volume | 1.00 (0.94–1.06) | 0.89 | |
| High-volume | 0.92 (0.85–0.99) | 0.03 |
The model has accounted for the correlation of patients treated at the same hospital
DISCUSSION
To our knowledge, this study is the largest contemporary analysis of treatment patterns and survival in the rare population of women with cN3c invasive breast cancer. We found that women with breast cancer and supraclavicular nodal metastases have improved survival when compared with those with M1 disease, supporting the re-classification of cN3c disease as distinct from M1 disease. In alignment with national guidelines, we found that receipt of multimodal therapy, including surgery and radiation, was associated with an improved overall survival for these patients when compared with non-standard therapy. As advances in modern imaging continue to detect subclinical advanced nodal disease, receipt of multimodal therapy—including chemotherapy, axillary lymphadenectomy, and radiation—was associated with improved 5-year survival when compared with non-standard therapy. Notably, survival in cN3c patients that received non-standard therapy more closely reflected that of women with metastatic disease.
Similar to our findings, on review of 70 patients from 1974 to 1991, Brito et al. demonstrated a 5-year overall survival of 41% in cN3c patients treated with neoadjuvant therapy followed by total or partial mastectomy plus axillary lymph node dissection (ALND) with irradiation. Notably, there was no difference in overall survival among these patients when compared with individuals with stage IIIB disease.5 Among cN3c patients included in our cohort, 25.5% received non-standard therapy while 74.5% received multimodal treatment, which was not associated with patient characteristics.
Other authors have also shown similar improvements in survival with the use of multimodal breast cancer treatment among women with cN3c disease. In a single institution study evaluating cN3c patients treated with multimodal therapy, Huang et al. reported a 5-year survival of 47% and locoregional control of 77%. Of note, this study included patients treated from 1992 to 2000 and excluded those who did not complete multimodal therapy due to interval development of distant metastatic disease.10 In 2007, Olivotto et al. reported a 5-year overall survival of 33.3% in 51 patients with cN3c M0 breast cancer treated with multimodal therapy.6 These data, as well as our own, collectively support the reclassification of these women from stage IV to stage IIIc disease and the inclusion of locoregional treatment in their care.
No studies have evaluated surgical excision of involved supraclavicular lymph nodes in cN3c patients, and the benefit of this approach is unknown. However, Huang et al. reported a higher risk of locoregional recurrence with clinical or radiographic evidence of residual disease in the supraclavicular nodal basin. In clinical practice at MD Anderson, ultrasound is routinely performed for re-staging of these patients after neoadjuvant chemotherapy.10 While this practice is not incorporated into guideline recommendations for management, it is an approach that can assist physicians with prognostication or further management when faced with this subset of patients. Despite the NCCN guideline recommendations, we found that approximately 1 in 4 women with cN3c invasive breast cancer did not receive multimodal therapy and that chemotherapy was not given in the neoadjuvant setting in approximately 20% of patients. We were unable to determine the rationale behind non-standard care, discordant from national guidelines; however, disease progression, postoperative upstaging, and the slow uptake of evidence into clinical practice may explain these findings. Women with progression during neoadjuvant chemotherapy are more likely to have higher stage disease, including stage IIIB/IIIC breast cancer, when compared with responders to treatment (30.5% vs. 21%).15 Our study identified that only 74.5% of patients with cN3c breast cancer received guideline-concordant care. It is important to acknowledge that we cannot determine the reasons behind treatment recommendations; for example, omission of locoregional treatment might be indicated in these individual patients with extensive progression of disease portending a poor prognosis and limited life expectancy. Conversely, among cN3c patients and in certain cases of patients with metastatic breast cancer, surgery and radiation are more likely to have been offered to individuals with favorable baseline characteristics. Notably, historic downstaging of cN3 patients was based on improved survival and advances in systemic therapies. This heterogeneity of disease likely exists among contemporary breast cancer patients with metastatic disease and may warrant reclassification of stage IV patients.16 National guidelines for the management of cN3c patients changed in 2002. During the study period (2004–2015), 75% of patients received guideline-concordant care, which is unexpectedly high considering that clinicians are slower to adopt guideline-concordant care in other aspects of breast cancer treatment. By comparison, adoption rates of hypofractionated radiation for breast cancer were only 13.6% in SEER/Medicare data and only 26.1% of surgeons embraced Z0011 within the first 5 years.17, 18 In our logistic regression, hospital volume did not predict receipt of multimodal guideline-concordant care, suggesting that other factors, which we were unable to account for, including provider-level variation, may have contributed to these patterns of care.
Patient factors, including race, socioeconomic status, and insurance coverage, also influence breast cancer treatment patterns. Freedman et al. found that uninsured women and Medicare enrollees had lower odds of undergoing definitive locoregional therapy and adjuvant chemotherapy.19 We observed a similar finding in lower rates of guideline-concordant care seen among uninsured cN3c patients, which was the only patient factor associated with the receipt of multimodal therapy. The gaps in care among uninsured patients are multifactorial and provide targets for intervention to improve rates of appropriate breast cancer care.19, 20 Chen et al. demonstrated that use of patient navigators in uninsured breast cancer patients improved adherence to the American Society of Clinical Oncology’s quality indicators from 69 to 86%.20 Women with locally advanced breast cancer requiring intensive multimodal treatment may benefit from similar interventions, which have the potential to improve guideline-adherence, resulting in improved survival in cN3 patients.
Advances in diagnostic imaging have led to earlier detection of subclinical disease, and we suspect that oncologists will increasingly encounter radiographic evidence of supraclavicular nodal metastases in years to come. Jung et al. reported a 64.2% 5-year survival in 111 patients with pathologically proven cN3c disease by fine needle aspiration,21 compared with a 5-year survival of 78% among individuals diagnosed by PET scan alone without biopsy.22 These data likely reflect the over-staging that results from highly sensitive imaging. The NCDB defines cN3c breast cancer as either biopsy-proven nodal metastasis or clinically or radiographically detected lymph nodes. Therefore, the cohort in this study may have also been over-staged and potentially over-treated. Importantly, in these patients with subclinical nodal metastasis, the risk of local recurrence must be weighed against the risk of overtreatment and death from metastatic disease. This balance can be achieved in cN3c patients with support from a multidisciplinary team and accurate staging at the time of diagnosis.
Limitations
There are several limitations in our study that should be addressed. First, a certain degree of selection bias may contribute to our findings. Individuals who completed multimodal therapy likely had evidence of response to neoadjuvant chemotherapy. We were unable to account for patients who progressed during treatment, in whom omission of multimodal therapy might have been appropriate. Second, we included all de novo stage IV (cM1) breast cancer patients who underwent chemotherapy and/or endocrine therapy, a heterogeneous population including patients with improved prognosis (e.g., bone-only metastases) compared with patients with already limited survival at diagnosis (e.g., widespread visceral disease). We found that comorbidity was not statistically significantly associated with receipt of multimodal therapy; however, unmeasured variables likely resulted in a residual selection bias that cannot be accounted for. Although we attempted to minimize patient selection bias with an adjusted analysis, it is likely that these additional unmeasured variables contributed to our findings to an unknown degree. Furthermore, cN3c patients who had a limited life expectancy based on greater extent of disease may have been less likely to undergo multimodal therapy due to inoperability (treatment bias); notably, 47% of cN3c patients treated with non-standard therapy had T4 disease which may have correlated with limited overall survival. Breast cancer specific survival is not available in the NCDB; yet in this rare population of breast cancer patients, overall survival likely mirrors disease-specific survival based on the advanced nature of supraclavicular nodal disease. Even among the multimodal patients, we cannot account for differences in delivery of radiation. While 23% were reported to have chest wall/breast radiation without nodal radiation, this may have not been captured accurately by the NCDB. Furthermore, this study did not address the extent of locoregional treatment and outcomes. We could not compare survival based on a pathologic complete response to treatment because many of the cN3c patients who received non-standard therapy did not have pathology for review.
CONCLUSION
In conclusion, women with cN3c invasive breast cancer have improved survival when compared with women with distant metastatic disease, with further benefits seen upon receipt of multimodal therapy.
ACKNOWLEDGEMENT
Portions of this manuscript were presented at the Society of Surgical Oncology Annual Meeting on March 23, 2018. The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
FUNDING Dr. O. Fayanju was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 5KL2TR001115 (PI: Boulware). Dr. R. Greenup was supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work was also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DISCLOSURES Samantha Thomas - Abbvie, Inc: consulting on biosimilar and bioequivalence work unrelated to this work. Jeremy Force - Consulting: Genomic Health.
REFERENCES
- 1.Owusu C, Lash TL, Silliman RA. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res Treat. 2007;102(2):227–36. [DOI] [PubMed] [Google Scholar]
- 2.Poorvu PD et al. Variation in guideline-concordant care for elderly patients with metastatic breast cancer in the United States. Breast Cancer Res Treat. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Mann JM et al. The State of Surgical Axillary Management and Adjuvant Radiotherapy for Early-stage Invasive Breast Cancer in the Modern Era. Clin Breast Cancer. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singletary SE et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–36. [DOI] [PubMed] [Google Scholar]
- 5.Brito RA et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol. 200119(3):628–33. [DOI] [PubMed] [Google Scholar]
- 6.Olivotto IA et al. Long-term survival of patients with supraclavicular metastases at diagnosis of breast cancer. J Clin Oncol. 2003;21(5):851–4. [DOI] [PubMed] [Google Scholar]
- 7.https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 15 Mar 2020.
- 8.Fan Y et al. A retrospective study of metachronous and synchronous ipsilateral supraclavicular lymph node metastases in breast cancer patients. Breast. 2010;19(5):365–9. [DOI] [PubMed] [Google Scholar]
- 9.Shenkier T et al. Clinical practice guidelines for the care and treatment of breast cancer: 15. Treatment for women with stage III or locally advanced breast cancer. CMAJ. 2004;170(6): 983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang EH et al. Locoregional treatment outcomes for breast cancer patients with ipsilateral supraclavicular metastases at diagnosis. Int J Radiat Oncol Biol Phys. 2007;67(2):490–6. [DOI] [PubMed] [Google Scholar]
- 11.Chen SC et al. Prognosis of breast cancer after supraclavicular lymph node metastasis: not a distant metastasis. Ann Surg Oncol. 2006;13(11):1457–65. [DOI] [PubMed] [Google Scholar]
- 12.Surgeons ACO. American College of Surgeons: National Cancer Data Base [cited 2015 June 19th]; Available from: https://www.facs.org/quality-programs/cancer/ncdb. Accessed 1 Aug 2016.
- 13.Neter J, Kutner M, Wasserman W, Nachtsheim C. Applied linear statistical models. Vol 4. Chicago: Irwin; 1996. [Google Scholar]
- 14.Greenup RA et al. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018;267(2):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caudle AS et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(11): 1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plichta J et al. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann Surg. 2020;271(1): 169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagsi R et al. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys. 2014;90(5):1001–9. [DOI] [PubMed] [Google Scholar]
- 18.Ong CT et al. Patient age and tumor subtype predict the extent of axillary surgery among breast cancer patients eligible for the american college of surgeons oncology group trial Z0011. Ann Surg Oncol. 2017;24(12):3559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman RA et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–9. [DOI] [PubMed] [Google Scholar]
- 20.Chen F et al. Improving breast cancer quality of care with the use of patient navigators. Am Surg. 2010;76(10):1043–6. [PubMed] [Google Scholar]
- 21.Jung J et al. Treatment outcome of breast cancer with pathologyically proven synchronous ipsilateral supraclavicular lymph node metastases. J Breast Cancer. 2015;18(2):167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ et al. Outcomes of positron emission tomography-staged clinical N3 breast cancer treated with neoadjuvant chemotherapy, surgery, and radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(5):e689–95. [DOI] [PubMed] [Google Scholar]


