Abstract
Aims:
Immune-mediated beta cell destruction is known to cause hyperglycemia in patients receiving immune checkpoint inhibitor (ICI) cancer therapy. However, it is uncommon, and little is known about the full spectrum of hyperglycemia in patients receiving ICIs. We aimed to characterize the prevalence and factors associated with hyperglycemia in patients treated with ICIs.
Methods:
We retrospectively analyzed patients receiving ICIs at an NCI-designated Cancer Center. We assessed the proportion of patients with new onset hyperglycemia (random glucose >11.1 mmol/L) after starting ICIs and used logistic regression to determine hyperglycemia predictors in patients without known diabetes.
Results:
Of 411 patients, 385 had post-ICI glucose data. 105 (27%) had hyperglycemia. Of this group, 29 (28%) had new onset hyperglycemia, 19 of whom had glucocorticoid-associated hyperglycemia. The remaining 10 had unexplained hyperglycemia and none had known autoimmune diabetes. Among patients without known diabetes, race/ethnicity, obesity, and pre-ICI hyperglycemia were significantly associated with hyperglycemia after starting ICIs.
Conclusions:
We found that new hyperglycemia in patients receiving ICIs was most commonly related to glucocorticoids. A small patient subset had new unexplained hyperglycemia, suggesting ICIs might have a role in promoting hyperglycemia. Recognizing factors associated with hyperglycemia in this population is crucial for appropriate management.
Keywords: hyperglycemia, diabetes, immunotherapy
1. Introduction
Immune checkpoint inhibitors (ICIs) have increasingly become the standard of care for many types of advanced malignancies. These monoclonal antibodies (mAbs) treat cancer by targeting the cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed cell death ligand protein 1 (PD-L1) pathways. Blocking these pathways results in T-cell activation, which thereby augments immune responses against cancer cells [1]. In addition to activating the immune system against cancer cells, ICIs also can induce an immune response to host cells, leading to immune-mediated adverse events [2]. Autoimmune β cell destruction leading to insulin deficient diabetes is a known ICI-mediated adverse event that has been reported in 0.2-1.9% of patients on ICIs [3–5],
Two meta-analyses of 40 ICI clinical trials found that compared to controls, patients treated with ICIs had an increased risk of new onset hyperglycemic events [6, 7]. One study showed a 0.15-0.20% increase in hemoglobin A1C (HbA1c) in patients with metastatic melanoma who received ICI therapy for an average of 3 months [8]. The reasons for hyperglycemia in cancer patients treated with ICIs are likely multifactorial. As both the incidence of a number of cancers and the prevalence of diabetes increase with advancing age, uncontrolled diabetes may be more prevalent in the ICI-treated population. Cancer patients on ICIs are at high risk of steroid-induced hyperglycemia because approximately one third of this population receive glucocorticoid treatment for immune-mediated adverse events, or to manage symptomatic neurologic metastases [9, 10]. Malignancy-related illness and stress may also increase the risk of hyperglycemia in patients with cancer[11]. Additionally, ICI-related inflammatory and autoimmune mechanisms may be involved in some cases of hyperglycemia. Recent case reports have described that ICIs can lead to hyperglycemia by causing adipose tissue inflammation and lipodystrophy, associated with insulin resistance, hypertriglyceridemia, and non-alcoholic fatty liver disease [12–14]. ICI-induced pancreatitis is also a well-recognized complication of ICI therapies, reportedly occurring in 4% of patients, and associated with hyperglycemia [15].
Characterizing glycemic responses and addressing the different mechanisms for hyperglycemia in patients on ICIs is important, as hyperglycemia is associated with decreased survival in patients with solid tumors, as well as increased morbidity, such as infection [16, 17]. To our knowledge, there have been no publications assessing the prevalence and clinical features of ICI-associated hyperglycemia beyond autoimmune diabetes and little is known regarding this topic. Therefore, in this study, we aimed to characterize hyperglycemia in a real-world cohort of patients receiving ICI therapy to understand the prevalence and causes of hyperglycemia in this population.
2. Materials and methods
We analyzed data from a consecutive cohort of cancer patients (solid and hematological malignancies) who received ICIs (nivolumab, pembrolizumab, atezolizumab, ipilimumab, and tremilimumab) between 2011 and 2017 at a NCI-designated Cancer Center. We included patients who had ≥3 post-ICI glucose measurements to capture a patient population with laboratory follow-up while on ICIs.
Glucose data were obtained from the electronic medical record (EMR) and were collected in the course of oncologic treatment (e.g. metabolic panel drawn before cancer treatment, upon the development of adverse events, or during hospitalizations). The primary outcome of the study was hyperglycemia after initiating ICI therapy, defined as a random glucose >11.1 mmol/L (200 mg/dL) from the time of ICI initiation up to 6 months after cessation. Glucocorticoid-associated hyperglycemia was defined as hyperglycemia on laboratory samples collected while a patient was prescribed glucocorticoids until at least 3 days after completing their glucocorticoid course (in order to account for the effect of long-acting glucocorticoids such as dexamethasone). Hyperglycemia after starting ICIs was classified as new onset if it occurred in patients with no recorded pre-existing hyperglycemia and no pre-existing diagnosis of diabetes. We also recorded the time from ICI initiation to onset of hyperglycemia. Preexisting diabetes was defined as an established diagnosis of diabetes mellitus prior to ICI initiation that was documented either by diagnosis code listed in the EMR or if the diagnosis was listed in clinical progress notes. Pre-ICI glucose values were documented only if there were ≥3 pre-ICI glucose measurements and pre-ICI hyperglycemia was defined as a random glucose >11.1 mmol/L (200 mg/dL) prior to ICI initiation whether or not a patient had an established diabetes diagnosis.
Demographic data including age, sex, race, and ethnicity were obtained from the EMR. Primary tumor location, cancer stage (localized, regionally advanced, distant metastasis), and type of immune checkpoint inhibitor (anti-CTLA4 mAb, anti-PD-1 mAb, anti-PD-L1 mAb) were recorded. Height and weight were also collected from the EMR and obesity was defined as a body mass index (BMI)> 30 kg/m2. We obtained information regarding pre-existing diabetes from the EMR. We recorded glucocorticoid exposure as documented in the EMR; supraphysiological glucocorticoid doses were defined as greater than an equivalent of 5mg of prednisone.
The study was approved by our Institutional Review Board (IRB-17-01894). All procedures performed in studies involving human participants were in accordance with the ethical standards of our Institutional Review Board (IRB # 17-01894) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Waiver of consent was obtained based on Code for Federal Regulations Title 45 Part 46.116.
2.1. Statistical Analyses
We evaluated the proportion of patients with hyperglycemia after starting ICI therapy who had prior diabetes, documented hyperglycemia prior to initiating ICIs, and post-ICI glucocorticoid use, as well as new onset hyperglycemia after receiving ICIs. Descriptive analyses were performed for patients with and without pre-existing diabetes regarding the development of post-ICI hyperglycemia (including proportions, means and medians, and variability with standard deviation (SD). We also compared patients receiving ICI therapy who developed hyperglycemia versus those who did not, stratified by diabetes diagnosis, using the Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables. We conducted a logistic regression analysis to determine the association between risk factors and the development of hyperglycemia after initiating ICI therapy. Statistical analyses were conducted with Stata 14.2; (Statacorp, 2018); p-values < 0.05 were considered statistically significant.
3. Results
3.1. Characterization of hyperglycemia
Of 411 cancer patients in our cohort, 385 met inclusion criteria. Of these patients, 86 (22%) had preexisting diabetes and 299 (78%) did not (Figure 1). Overall, 105 (27%) patients experienced hyperglycemia after initiating ICI therapy, 57 with preexisting diabetes and 48 without preexisting diabetes. Timing of onset of hyperglycemia after ICI initiation ranged from 0.4 to 107 weeks, with a median of 9.7 weeks. Patients with pre-existing diabetes had a median time to onset of hyperglycemia of 7 weeks (range 0.9-100) after ICI initiation and patients without a pre-existing diagnosis of diabetes had a median timing of onset of 12 weeks (range 0.4-108) after ICI initiation (p<0.01).
Figure 1:
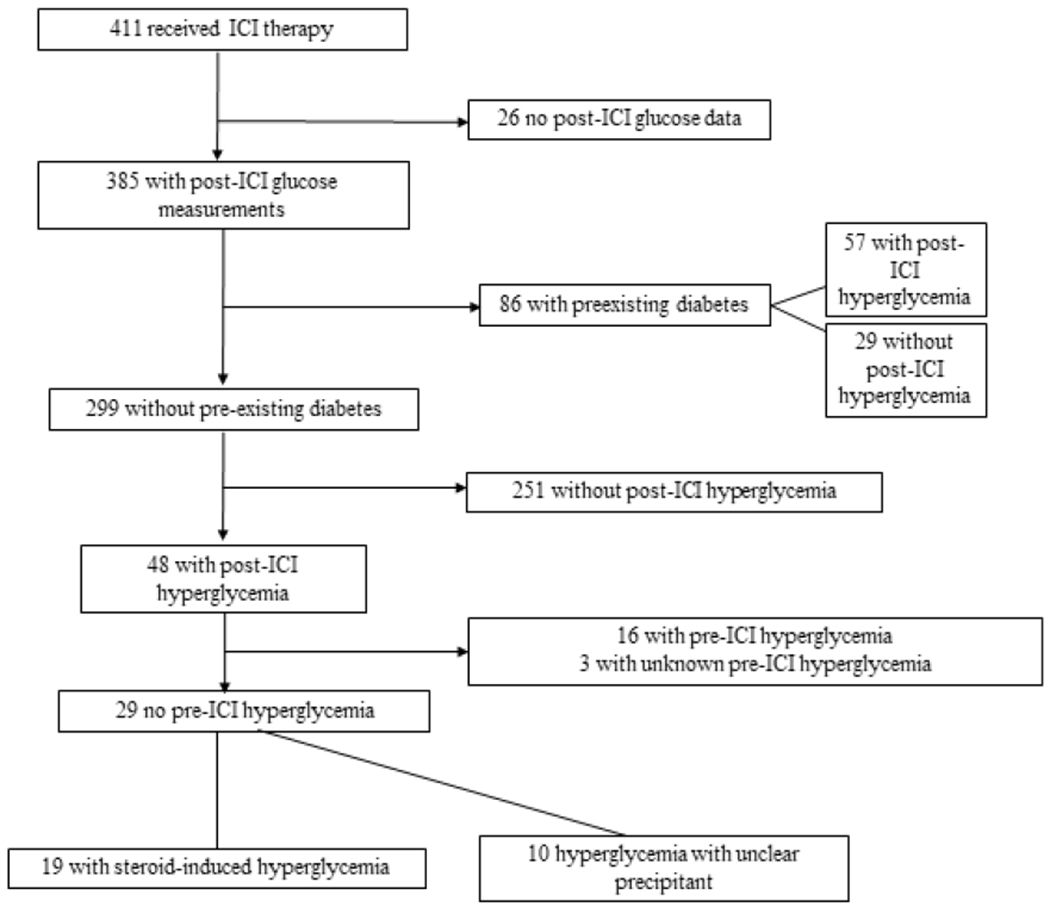
Flowchart describing hyperglycemia in patient cohort
ICI= immune checkpoint inhibitor;
Of the 48 patients without preexisting diabetes with hyperglycemia after initiating ICIs, 16 had hyperglycemia prior to ICI initiation, 3 did not have pre-ICI glucose measurements, and 29 had new-onset hyperglycemia. Of these 29 patients, 19 had steroid-related hyperglycemia and 10 had hyperglycemia with no clear precipitant. Of the 19 patients with steroid-related hyperglycemia, only one had C-peptide levels checked, which was in normal range. Hyperglycemia resolved without anti-hyperglycemic medications in 17 patients when glucocorticoids were stopped or doses were decreased and 2 patients were subsequently diagnosed with type 2 diabetes later in their treatment course. No obvious precipitant of hyperglycemia was detected in the remaining 10 patients and no patients with new onset hyperglycemia had antibody-confirmed autoimmune diabetes or ICI-mediated pancreatitis. Hyperglycemia in 5 of these patients resolved without intervention, 2 were lost to follow-up after developing hyperglycemia, 2 continued to have hyperglycemia and were being monitored for resolution, and 1 was started on insulin therapy with improvement of hyperglycemia (Table 1). None of these patients had C-peptide tested.
Table 1:
Description of patients with new-onset hyperglycemia after starting immune checkpoint inhibitors
| Age at ICI initiation | Malignancy type and stage | ICI | Weeks after initial treatment HG occurred | Peak glucose Mg/dL (mmol/L) | Setting of HG | Admission diagnosis | Hyperglycemia course |
|---|---|---|---|---|---|---|---|
| 70 | Colorectal cancer stage IV | Nivolumab | 32 | 418 (23.2) | Outpatient | N/A | HG improved with basal insulin and metformin treatment |
| 62 | HCC stage IV | Nivolumab | 108 | 230 (12.8) | Outpatient | N/A | Lost to follow up after HG |
| 81 | Melanoma stage IV | Ipilimumab | 11 | 309 (17.1) | Inpatient and outpatient | Debility from POD | Continued intermittent HG, insulin started |
| 57 | NSCLC stage IV | Atezolizumab | 18 | 204 (11.3) | Outpatient | N/A | No further HG without intervention |
| 50 | HCC stage IV | Nivolumab | 12 | 277 (15.4) | Inpatient | GI bleed | Received insulin dose, then no further HG without intervention, death 2 days later |
| 36 | HCC stage IV | Nivolumab | 5 | 250 (13.9) | Inpatient | Pneumonia and kidney injury | No further HG without intervention, death 2 days later |
| 87 | Melanoma Stage IV | Pembrolizumab | 55 | 233 (12.9) | Outpatient | N/A | No further HG without intervention |
| 69 | Metastatic adenocarcinoma of unknown primary | Nivolumab | 10 | 226 (12.5) | Inpatient | Dyspnea, POD | Glucose not checked after HG, death 6 days later |
| 47 | HCC stage IV | Nivolumab | 9 | 210 (11.7) | Inpatient | Empyema | Continued HG that then resolved without intervention |
| 59 | Melanoma stage IV | Ipilimumab | 4 | 500 (27.8) | Emergency Department | Migraine | Lost to follow-up after HG |
GI= gastrointestinal; HCC= hepatocellular carcinoma; HG= hyperglycemia; ICI= immune checkpoint inhibitor; N/A= not applicable; NSCLC= non-small cell lung cancer; POD= progression of disease
In this cohort, one patient with pre-existing type 2 diabetes controlled on oral medications developed autoimmune diabetes. This patient developed worsening hyperglycemia to >300 mg/dL (>16.7mmol/L) without diabetic ketoacidosis (DKA), and had undetectable C-peptide and positive anti-glutamic acid decarboxylase 65 (GAD65) antibodies. The patient did not have GAD65 antibodies tested prior to treatment. After starting ICI therapy, two patients developed DKA. One patient had preexisting type 1 diabetes (undetectable C-peptide) with steroid-induced hyperglycemia precipitating DKA. The other patient had preexisting type 2 diabetes, and developed DKA four months after initiation of ICI therapy. The patient did not have C-peptide or antibody testing.
3.2. Glucocorticoid-related hyperglycemia
In total, 259 patients (67%) received supraphysiologic doses of glucocorticoids after starting ICIs. In patients with hyperglycemia, 46 (12% of total cohort) had glucocorticoid-related hyperglycemia, while 59 (15% of total cohort) had hyperglycemia that was not glucocorticoid-related. The most common reasons for glucocorticoid administration were: adjunct treatment to chemotherapy (80 patients); management of adverse effects of ICIs (61 patients); management of neurologic metastases (49 patients), and palliation of symptoms related to cancer (45 patients). 228 (88%) of these patients received doses that were greater than or equal to a prednisone dose equivalent of 20 mg.
3.4. Comparison of patients with and without hyperglycemia
We compared individuals with and without post-ICI hyperglycemia from the group without pre-existing diabetes (Table 2A). Patients with diabetes who developed hyperglycemia on ICIs were more likely to have been exposed to supraphysiologic doses of glucocorticoids (65% vs 83%, p<0.01) and to have had pre-existing hyperglycemia (14% vs 33%, p<0.01). In patients without preexisting diabetes, the highest rates of hyperglycemia after starting ICIs were in Asian and Hispanic patients (p<0.01), and in those with hepatocellular carcinoma (HCC) (p=0.05).
Table 2:
Characteristics of patients with hyperglycemia after starting ICI therapy versus those without hyperglycemia stratified by diabetes diagnosis
| A. Patients without Diabetes (N=299) | ||||
|---|---|---|---|---|
| Total N=299 N(%) |
Hyperglycemia after starting ICIs N=48 (16.1%) N (%) |
No hyperglycemia after starting ICIs N=251 (83.9%) N (%) |
P-Value* | |
| Age (years) at treatment initiation (mean, SD) | 65.6 (21-93) | 63.9 (31-87) | 65.6 (21-93) | 0.87 |
| Gender (male) | 181 (60.5) | 33 (68.8) | 148 (59.0) | 0.26 |
| Race | <0.01 | |||
| White | 163 (54.5) | 16 (33.3) | 147 (58.6) | |
| Black | 28 (9.4) | 4 (8.3) | 24 (9.6) | |
| Hispanic | 29 (9.7) | 9 (18.8) | 20 (7.8) | |
| Asian | 22 (7.4) | 8 (16.7) | 14 (5.6) | |
| Other/Unknown | 57 (19.1) | 11 (22.9) | 46 (18.3) | |
| Supraphysiologic steroids with ICI | 203 (67.9) | 40 (83.3) | 163 (64.9) | 0.01 |
| Obesity (BMI≥30) | 52 (17.4) | 11 (22.9) | 41 (16.3) | 0.30 |
| Hyperglycemia prior to ICI | <0.01 | |||
| No | 214 (71.6) | 29 (60.4) | 185 (73.7) | |
| Yes | 51 (17.1) | 16 (33.3) | 35 (13.9) | |
| Unknown | 34 (11.4) | 3 (6.3) | 31 (12.4) | |
| Malignancy type | 0.05 | |||
| Melanoma | 68 (22.7) | 6 (12.5) | 62 (24.7) | |
| Non-small cell lung cancer | 68 (22.7) | 12 (25.0) | 56 (22.3) | |
| Urothelial cell carcinoma | 31 (10.4) | 4 (8.3) | 27 (10.8) | |
| Hepatocellular carcinoma | 41 (13.7) | 13 (27.1) | 28 (11.2) | |
| Renal cell carcinoma | 14 (4.7) | 12 (4.8) | 2 (4.2) | |
| Squamous cell carcinoma of the head and neck | 21 (7.0) | 17 (6.8) | 4 (8.3) | |
| Multiple Myeloma | 15 (5.0) | 4 (8.3) | 11 (4.4) | |
| Other | 76 (25.4) | 3 (6.3) | 38 (15.1) | |
| Clinical stage | 0.88 | |||
| Clinically localized | 26 (8.7) | 3 (6.3) | 23 (9.2) | |
| Regionally advanced | 28 (9.4) | 5 (10.4) | 23 (9.2) | |
| Distant metastasis | 230 (76.9) | 37 (77.1) | 193 (76.9) | |
| Other | 15 (5.0) | 3 (6.3) | 12 (4.8) | |
| Immune checkpoint type | 0.36 | |||
| CTLA-4 | 37 (12.9) | 6 (13.3) | 31 (12.8) | |
| PD1/PDL1 | 220 (76.4) | 34 (75.6) | 186 (76.5) | |
| Combination CTLA4 and PD1/PDL1 | 11 (3.8) | 0 | 11 (4.5) | |
| Sequential therapy | 20 (6.9) | 5 (11.1) | 15 (6.2) | |
| B. Patients with Diabetes (N=86) | ||||
| Total N= 86 N(%) |
Hyperglycemia after starting ICIs N=57 (66.3%) N (%) |
No hyperglycemia after starting ICIs N=29 (33.7%) N (%) |
P-Value* | |
| Age(years) at treatment initiation (median, range) | 68.9 (42-96) | 68.9 (50-87) | 68.8 (42-96) | 0.62 |
| Gender (male) | 58 (67.4) | 41 (71.9) | 17 (58.6) | 0.23 |
| Race | 0.88 | |||
| White | 39 (45.4) | 26 (45.6) | 13 (44.8) | |
| Black | 13 (15.1) | 7 (12.3) | 6 (20.7) | |
| Hispanic | 12 (14.0) | 8 (14.0) | 4 (13.8) | |
| Asian | 8 (9.3) | 6 (10.5) | 2 (6.9) | |
| Other/Unknown | 14 (16.3) | 10 (17.5) | 4 (13.8) | |
| Supraphysiologic steroids with ICI | 56 (65.1) | 43 (75.4) | 13 (44.8) | <0.01 |
| Obesity (BMI≥30) | 30 (34.9) | 21 (36.8) | 9 (31.0) | 0.64 |
| Hyperglycemia prior to ICI | 0.12 | |||
| No | 16 (18.6) | 7 (12.3) | 9 (31.0) | |
| Yes | 61 (70.9) | 43 (75.4) | 18 (62.1) | |
| Unknown | 9 (10.5) | 7 (12.3) | 2 (6.9) | |
| Malignancy type | 0.82 | |||
| Melanoma | 15 (17.4) | 7 (24.1) | 8 (14.0) | |
| Non-small cell lung cancer | 21 (24.4) | 13 (22.8) | 8 (27.6) | |
| Urothelial cell carcinoma | 13 (15.1) | 10 (17.5) | 3 (10.3) | |
| Hepatocellular carcinoma | 16 (18.6) | 12 (21.1) | 4 (13.8) | |
| Renal cell carcinoma | 4 (4.7) | 2 (6.9) | 2 (3.5) | |
| Squamous cell carcinoma of the head and neck | 3 (3.5) | 1 (3.5) | 2 (3.5) | |
| Multiple Myeloma | 5 (5.8) | 3 (5.3) | 2 (6.9) | |
| Other | 9 (10.5) | 11 (19.3) | 5 (17.2) | |
| Clinical stage | 0.72 | |||
| Clinically localized | 8 (9.3) | 6 (10.5) | 2 (6.9) | |
| Regionally advanced | 7 (8.1) | 6 (10.5) | 1 (3.5) | |
| Distant metastasis | 66 (76.7) | 42 (73.7) | 24 (82.8) | |
| Other | 5 (5.8) | 3 (5.3) | 2 (6.9) | |
| Immune checkpoint type | 0.09 | |||
| CTLA-4 | 11 (13.6) | 6 (11.5) | 5 (17.2) | |
| PD1/PDL1 | 60 (74.1) | 36 (69.2) | 24 (82.8) | |
| Combination CTLA4 and PD1/PDL1 | 5 (6.2) | 5 (9.6) | 0 | |
| Sequential therapy | 5 (6.2) | 5 (9.6) | 0 | |
BMI- body mass index; CTLA-4- cytotoxic T-lymphocyte associated protein 4; PD-1- programmed cell death protein 1; PD-L1-programmed death-ligand 1; ICI- immune checkpoint inhibitor; SD- standard deviation
Fisher’s exact test for categorical variables and Kruskal Wallis test for continuous variables
We next compared individuals with pre-existing diabetes who did and did not develop hyperglycemia after starting ICI therapy (Table 2B). Patients with hyperglycemia were more likely to have supraphysiologic glucocorticoid exposure (45% vs 75%, p<0.01). Race, obesity, and malignancy type were not significantly associated with hyperglycemia after receiving ICIs in this group.
3.5. Predictors of post-ICI hyperglycemia
In a multiple logistic regression analysis of patients without pre-existing diabetes (Table 3). Hispanic ethnicity (odds ratio [OR]: 4.0, 95% confidence interval [CI]: 1.3-11.6) and Asian race (OR: 4.9, 95% CI: 1.4-17.7) were associated with hyperglycemia after receiving ICI therapy when compared to white race. Obese BMI (≥ 30 kg/m2) (OR: 2.4, 95% CI: 1.0-5.8) was associated with hyperglycemia after ICI therapy initiation compared to non-obese BMI (< 30 kg/m2). Pre-ICI hyperglycemia (OR: 3.2, 95% CI: 1.4-7.0) was significantly associated with hyperglycemia after receiving ICI therapy compared to not having pre-ICI hyperglycemia.
Table 3:
Logistic Regression: Predictors of hyperglycemia in patients receiving ICI therapy without diabetes
| Variables | Odds Ratio | Confidence Interval | P-value (comparison to reference)* | P-value (across variables)* |
|---|---|---|---|---|
| Age (years) | 1.0 | 0.90-1.05 | 0.32 | 0.32 |
| Male gender (vs. female) | 1.2 | 0.656-2.50 | 0.66 | 0.66 |
| Race/Ethnicity | <0.01 | |||
| White | Ref | 0.03 | ||
| Black | 1.3 | 0.34-4.65 | 0.72 | |
| Hispanic | 4.0 | 1.34-11.6 | 0.01 | |
| Asian | 4.9 | 1.38-17.72 | 0.01 | |
| Unknown/other | 2.4 | 0.94-5.97 | 0.07 | |
| Obesity (BMI ≥30 vs BMI <30) | 2.4 | 1.02-5.77 | 0.05 | 0.05 |
| Supraphysiologic Steroid Use (yes vs. no) | 2.3 | 0.97-5.57 | 0.06 | 0.01 |
| Documented hyperglycemia prior to ICI use | <0.01 | |||
| No | Ref | |||
| Yes | 3.2 | 1.43-7.04 | <0.01 | |
| Unknown | 0.60 | 0.16-2.32 | 0.46 | |
| Malignancy type | 0.37 | |||
| Melanoma | Ref | |||
| Non-small cell lung carcinoma | 1.1 | 0.33-3.53 | 0.88 | |
| Urothelial carcinoma | 0.9 | 0.22-3.78 | 0.90 | |
| Hepatocellular carcinoma | 2.0 | 0.56-7.04 | 0.29 | |
| Renal cell carcinoma | 1.1 | 0.18-6.64 | 0.91 | |
| Squamous cell carcinoma of the head and neck | 2.0 | 0.43-8.94 | 0.39 | |
| Multiple myeloma | 1.0 | 0.90-1.05 | 0.32 | |
| Other | 1.2 | 0.656-2.50 | 0.66 |
BMI- body mass index; CTLA-4- cytotoxic T-lymphocyte associated protein 4; PD-1- programmed cell death protein 1; PD-L1-programmed death-ligand 1; ICI- immune checkpoint inhibitor; SD- standard deviation
N=299, 48 with post-ICI hyperglycemi and 251 without post-ICI hyperglycemia
Discussion
In this study of patients with cancer on ICI therapy, more than a quarter of patients developed hyperglycemia after initiation of immunotherapy, the majority were related to glucocorticoid use. A small subset of patients had new hyperglycemia that could not be explained by pre-existing diabetes, pre-existing hyperglycemia, or glucocorticoid exposure suggesting that ICIs could have a role in promoting de novo hyperglycemia in these patients. This study highlights that a large proportion of patients receiving ICIs experience hyperglycemia, largely associated with known risk factors with hyperglycemia. Recognition and treatment of diabetes and hyperglycemia is important in patients receiving ICIs and other cancer treatments, as hyperglycemia has been shown to negatively impact cancer morbidity and mortality [16, 17]. In pre-clinical settings, hyperglycemia has been shown to make cancer cells more chemotherapy-resistant [18, 19]. The effect of hyperglycemia on cancer outcomes in patients receiving ICIs has yet to be evaluated.
To our knowledge, this is the first description of hyperglycemia in a real-world cohort of patients receiving ICIs beyond reporting autoimmune diabetes. In two meta-analyses of ICI clinical trials, the incidence of all-grade hyperglycemia was 1.9-2.26% [6, 7], which likely accounts for hyperglycemia attributed to the study drug (and not hyperglycemia related to other causes like glucocorticoids). This differs from our less selected patient population in which 59 (15%) experienced post-ICI hyperglycemia that was not related to supraphysiologic glucocorticoid use. Clinical trial patients tend to be younger and healthier than those receiving ICIs in routine clinical practice. For example, meta-analyses of the major ICI clinical trials for metastatic melanoma and non-small cell lung cancer, the largest patient groups in our study, had median ages of 58 years and 63 years, respectively, which is lower than the median age of 65 years in our cohort [20, 21]. Additionally, assessing the rate of glucocorticoid-related hyperglycemia in patients receiving ICIs is important for real-world clinical management. Patients are less likely to receive glucocorticoids in clinical trials, which typically excluded patients on glucocorticoid treatment and/or who had brain metastases. Thus, our results are relevant to clinicians providing care for less selected cancer patients as part of their clinical practices.
Many targeted cancer treatments have known associations with hyperglycemia, such as phosphatidylinositol 3-kinase (PI3K) inhibitors with reported rates of all-grade hyperglycemia up to 86% and rates of high-grade hyperglycemia up to 41% [22]. Additionally, glucocorticoids in the setting of cancer treatment are also associated with hyperglycemia. The prevalence of hyperglycemia was reported as 11% (blood glucose >16.65 mmol/L (300 mg/dl)) in a population of patients with solid tumors receiving supraphysiologic glucocorticoids with chemotherapy and 18.9% (blood glucose >11.1 mmol/L (200 mg/dl)) in patients with lymphoma and primary brain tumors or brain metastases receiving glucocorticoids [23, 24]. These rates are similar to our study population of patients receiving post-ICI glucocorticoids, of whom 18% had glucocorticoid-associated hyperglycemia (glucose>11.1 mmol/L (200 mg/dL)). Further data regarding the prevalence of hyperglycemia (not mediated by glucocorticoids) in real-world patient populations receiving cancer treatment are limited. In patients with cervical cancer receiving neoadjuvant chemotherapy, hyperglycemia (defined as fasting blood glucose >5.5 mmol/L (100 mg/dL)) prevalence was 20.7% [25]. Hyperglycemia prevalence was 22.6% (fasting blood glucose > 7.5 mmol/L (135 mg/dl) in advanced breast cancer patients receiving palliative chemotherapy[26]. Our study highlights that hyperglycemia is a common problem among patients with cancer in general and those receiving ICI therapy in particular and that patients on ICIs should be monitored closely for hyperglycemia.
We identified a small subset of patients with new unexplained hyperglycemia that we hypothesize may be related to ICI therapy. While in some patients, this may be partially explained by stress-related hyperglycemia in the setting of acute illness, ICIs may also play a role in promoting hyperglycemia. Inflammation and immune cell activation have a well-established association with hyperglycemia and type 2 diabetes, particularly in obesity [27]. T-cell activation, induced by ICIs, results in an inflammatory cascade that facilitates the release of cytokines, such as TNF-α, IL-6, and IL-1, which have previously been found to lead to increased gluconeogenesis and insulin resistance [28]. Future studies should evaluate the pathways underlying the relationship of ICIs with hyperglycemia in order to identify potential interventions to reduce this unwanted side effect.
Our study has strengths and limitations. We collected a consecutive cohort of cancer patients treated with ICIs in routine practice and included a diverse patient population relative to clinical trials. However, we retrospectively reviewed data collected as part as routine care and thus glucose measures were unstandardized and not recorded at specific time intervals. This limited our ability to trend glucose data in detail, as well as monitor other metrics such as HbA1c, which may underestimate hyperglycemia incidence and overestimate time to hyperglycemia. We included a relatively heterogeneous group of patients with different types of malignancies and variable treatment courses preceding ICI therapy. However, we adjusted for some of these factors in our model attenuating the potential impact of these factors.
In summary, we found that more than a quarter of patients with cancer experienced hyperglycemia after initiation of ICI therapy. Obesity and pre-existing hyperglycemia were associated with the development of hyperglycemia after starting ICI therapy in patients without a diabetes diagnosis. The majority of new onset hyperglycemia was related to glucocorticoid use. However, there was a subset of patients who had new onset hyperglycemia after ICI therapy without a clear precipitant, suggesting that ICIs may contribute to hyperglycemia by immune-related insulin resistance or β cell dysfunction, and should be further explored. Assessing how ICIs impact blood glucose is crucial for appropriately treating patients with hyperglycemia and understanding its effect on ICI treatment response.
Acknowledgments
Funding: The Tisch Cancer Institute is supported by NCI Cancer Center Support Grant P30CA196521. AL is supported by NCI/NIH T32CA225617. EJG is supported by NCI/NIH K08CA190770. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of Data and materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests: AL, EC, DB, JBS, and EE have nothing to disclose. JPW reports consulting honoraria from GSK, Sanofi, and Banook, and a research grant from Sanofi. MG reports ownership interests in Rappta Therapeutics, research funding from Janssen Oncology, Dendreon, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, Genentech/Roche and consultancy/advisory roles for BioMotiv, Merck, Dendreon, Janssen, GlaxoSmithKline, Lilly, Estellas Pharma, Genentech, Bristol-Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Inovio Pharmaceuticals, NuMab. EJG reports funding from Alkeon Capital Management and an advisory role for Novartis, and consulting for Seattle Genetics unrelated to the current work.
References
- [1].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2017;28:2377–85. [DOI] [PubMed] [Google Scholar]
- [3].Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA oncology. 2018;4:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsang VHM, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, et al. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct From Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:5499–506. [DOI] [PubMed] [Google Scholar]
- [5].Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes. 2018;67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lu J, Yang J, Liang Y, Meng H, Zhao J, Zhang X. Incidence of Immune Checkpoint Inhibitor-Associated Diabetes: A Meta-Analysis of Randomized Controlled Studies. Front Pharmacol. 2019;10:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Monami M, Naletto L, Nreu B, Dicembrini I, Sesti G, Mannucci E. Immune checkpoints inhibitors and hyperglycemia: A Meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2020;162:108115. [DOI] [PubMed] [Google Scholar]
- [8].Gauci ML, Boudou P, Squara PA, Delyon J, Allayous C, Mourah S, et al. Checkpoint inhibitor treatment induces an increase in HbA1c in nondiabetic patients. Melanoma Res. 2019;29:328–32. [DOI] [PubMed] [Google Scholar]
- [9].Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: A systematic review. Critical reviews in oncology/hematology. 2017;120:86–92. [DOI] [PubMed] [Google Scholar]
- [11].Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014;2014:461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haddad N, Vidal-Trecan T, Baroudjian B, Zagdanski AM, Arangalage D, Battistella M, et al. Acquired generalized lipodystrophy under immune checkpoint inhibition. Br J Dermatol. 2020;182:477–80. [DOI] [PubMed] [Google Scholar]
- [13].Jehl A, Cugnet-Anceau C, Vigouroux C, Legeay AL, Dalle S, Harou O, et al. Acquired Generalized Lipodystrophy: A New Cause of Anti-PD-1 Immune-Related Diabetes. Diabetes care. 2019;42:2008–10. [DOI] [PubMed] [Google Scholar]
- [14].Eigentler T, Lomberg D, Machann J, Stefan N. Lipodystrophic Nonalcoholic Fatty Liver Disease Induced by Immune Checkpoint Blockade. Annals of internal medicine. 2020. [DOI] [PubMed] [Google Scholar]
- [15].Abu-Sbeih H, Tang T, Lu Y, Thirumurthi S, Altan M, Jazaeri AA, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. Journal for immunotherapy of cancer. 2019;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barua R, Templeton AJ, Seruga B, Ocana A, Amir E, Ethier JL. Hyperglycaemia and Survival in Solid Tumours: A Systematic Review and Meta-analysis. Clin Oncol (R Coll Radiol). 2018;30:215–24. [DOI] [PubMed] [Google Scholar]
- [17].Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–82. [DOI] [PubMed] [Google Scholar]
- [18].Gerards MC, van der Velden DL, Baars JW, Brandjes DPM, Hoekstra JBL, Vriesendorp TM, et al. Impact of hyperglycemia on the efficacy of chemotherapy-A systematic review of preclinical studies. Critical reviews in oncology/hematology. 2017;113:235–41. [DOI] [PubMed] [Google Scholar]
- [19].Vishvakarma NK, Kumar A, Singh V, Singh SM. Hyperglycemia of tumor microenvironment modulates stage-dependent tumor progression and multidrug resistance: implication of cell survival regulatory molecules and altered glucose transport. Mol Carcinog. 2013;52:932–45. [DOI] [PubMed] [Google Scholar]
- [20].Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2:Cd011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Califano R, Gomes F, Ackermann CJ, Rafee S, Tsakonas G, Ekman S. Immune checkpoint blockade for non-small cell lung cancer: What is the role in the special populations? European journal of cancer (Oxford, England : 1990). 2020;125:1–11. [DOI] [PubMed] [Google Scholar]
- [22].Curigliano G, Shah RR. Safety and Tolerability of Phosphatidylinositol-3-Kinase (PI3K) Inhibitors in Oncology. Drug Saf. 2019;42:247–62. [DOI] [PubMed] [Google Scholar]
- [23].Zylla D, Gilmore G, Eklund J, Richter S, Carlson A. Impact of diabetes and hyperglycemia on health care utilization, infection risk, and survival in patients with cancer receiving glucocorticoids with chemotherapy. J Diabetes Complications. 2019;33:335–9. [DOI] [PubMed] [Google Scholar]
- [24].Harris D, Barts A, Connors J, Dahl M, Elliott T, Kong J, et al. Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: an observational cohort study. Curr Oncol. 2013;20:e532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li J, Wu MF, Lu HW, Zhang BZ, Wang LJ, Lin ZQ. Impact of Hyperglycemia on Outcomes among Patients Receiving Neoadjuvant Chemotherapy for Bulky Early Stage Cervical Cancer. PloS one. 2016;11:e0166612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, Bacon L, Rivera D, Urzua L, et al. Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res. 2012;2012:732027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhong J, Gong Q, Mima A. Inflammatory Regulation in Diabetes and Metabolic Dysfunction. J Diabetes Res. 2017;2017:5165268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xia C, Rao X, Zhong J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J Diabetes Res. 2017;2017:6494795. [DOI] [PMC free article] [PubMed] [Google Scholar]


