Abstract
Many species have experienced dramatic changes in both geographic range and population sizes in recent history. Increases in the geographic range or population size of disease vectors have public health relevance as these increases often precipitate the emergence of infectious diseases in human populations. Accurately identifying environmental factors affecting the biogeographic patterns of vector species is a long-standing analytical challenge, stemming from a paucity of data capturing periods of rapid changes in vector demographics. We systematically investigated the occurrence and abundance of nymphal Ixodes scapularis ticks at 532 sampling locations throughout New York State (NY), USA, between 2008 and 2018, a time frame that encompasses the emergence of diseases vectored by these ticks. Analyses of these field-collected data demonstrated a range expansion into northern and western NY during the last decade. Nymphal abundances increased in newly colonized areas, while remaining stable in areas with long-standing populations over the last decade. These trends in the geographic range and abundance of nymphs correspond to both the geographic expansion of human Lyme disease cases and increases in incidence rates. Analytic models fitted to these data incorporating time, space, and environmental factors, accurately identified drivers of the observed changes in nymphal occurrence and abundance. These models accounted for the spatial and temporal variation in the occurrence and abundance of nymphs and can accurately predict nymphal population patterns in future years. Forecasting disease risk at fine spatial scales prior to the transmission season can influence both public health mitigation strategies and individual behaviors, potentially impacting tick-borne disease risk and subsequently human disease incidence.
Keywords: Ticks, Lyme disease, Environmental change, Predictive models, Distribution
1. Introduction
Zoonotic pathogens, those transmitted naturally among wildlife that can infect humans, represent a resurgent threat to human health (Taylor et al., 2001; Jones et al., 2008). The recent emergence and re-emergence of diseases caused by zoonotic pathogens has been associated with alterations in human land-use patterns and global climate change, although the mechanistic links are often obscure (Lashley, 2004; Aguirre and Tabor, 2008; Suzan et al., 2008; Aluwong and Bello, 2010; Chaves and Koenraadt, 2010). One proposed mechanistic driver precipitating zoonotic disease outbreaks is the impact of environmental features on the geographical distribution and abundance of arthropod vectors of diseases (Sleeman et al., 2009; Fouque and Reeder, 2019; Petrosillo, 2019). Thus, establishing which environmental features promote geographic range or population size changes of vectors can identify ecological processes that are exacerbating disease risks from many zoonotic pathogens. Further, empirically validated biogeographic models of the vectors can result in accurate predictions of future range or population size expansions which can be critical components in the design of effective mitigation strategies (e.g. Guerra et al., 2002; Gage et al., 2008; Patz et al., 2008; Diuk-Wasser et al., 2010; Kaplan et al., 2010; Khatchikian et al., 2011). Here, we use fine-scale spatio-temporal data collected over a decade and across a large geographic expanse to build and validate predictive biogeographic models of a medically-relevant tick vector, Ixodes scapularis. We focus on nymphs, the tick life stage that most commonly infects humans with pathogens including those responsible for Lyme disease, babesiosis, and anaplasmosis (https://www.cdc.gov/lyme/datasurveillance/maps-recent.html access 21 May 2020; https://www.cdc.gov/parasites/babesiosis/epi.html access 21 May 2020; https://www.cdc.gov/lyme/transmission/index.html accessed 21 May 2020).
Scientific and anecdotal evidence suggest that the population density and geographic range of I. scapularis have increased in the northeastern United States (US) over recent decades, following several hundred years at undetectable levels (Humphrey et al., 2010; Ginsberg et al., 2014; Khatchikian et al., 2015; Van Zee et al., 2015; Eisen et al., 2016; Sonenshine, 2018). These changes in the occurrence and abundance of I. scapularis correlate with changes in the spatial distribution and incidence of human diseases caused by pathogens vectored by this tick (Persing et al., 1990; Marshall et al., 1994; Hoen et al., 2009; https://www.cdc.gov/lyme/datasurveillance/maps-recent.html accessed 21 May 2020) (Fig. 1). While several of the potential environmental drivers of the re-emergence of I. scapularis in the northeastern US have changed little over recent time, the changes in I. scapularis population demography and human disease incidence have been very heterogeneous (Higgins, 2004; Khasnis and Nettleman, 2005; Ogden et al., 2008a, 2008b). For example, Westchester County, New York State (NY) (southern Hudson Valley) has experienced consistently high human Lyme disease incidence for more than 20 years while in Albany County, NY (northern Hudson Valley), incidence has steadily increased from around 0 cases in 1997 to 215 per 100,000 persons in 2009 (New York State Department of Health, 2019). Linking environmental features to current tick spatial patterns is challenging, as the observed patterns reflect the integration of multiple ecological processes over a long history, and ecological processes differ across geographic space due to environmental heterogeneity (Lemey et al., 2009; Chikhi et al., 2010). Analyzing data collected during the dynamic phase of population growth and geographic expansion, together with environmental metadata at similar spatial and temporal scales, can avert this statistical challenge (Lemey et al., 2009; Pybus and Rambaut, 2009). Remotely sensed environmental data, combined with a sufficiently large dataset of the occurrence and abundance of nymphal ticks, allow for high-resolution spatio-temporal modeling of these data with respect to the natural environmental heterogeneity and ecological processes (Drummond and Rambaut, 2007; Epps et al., 2007; Gasbarra et al., 2007; Hey and Nielsen, 2007; Balkenhol et al., 2009; Cushman and Landguth, 2010; Hey, 2010; Lacey Knowles and Alvarado-Serrano, 2010; Shirk et al., 2010; Spear et al., 2010).
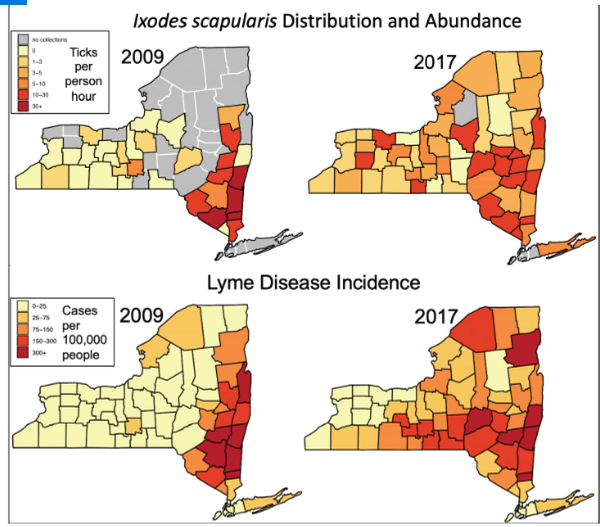
Fig. 1.
Changes in nymphal tick and Lyme disease incidence across New York State, USA. The geographic range of nymphs expanded across New York State between 2009 and 2017. Although nymphal sampling effort was geographically less expansive prior to 2013, data from all years suggest that few counties west of the Hudson River Valley had no or few detectable populations of nymphal Ixodes scapularis. By 2017, tick populations had expanded into most of the sampled counties (nymphs were detected in 50 of the 55 sampled counties in 2017 (90.9%) while nymphs were detected in only 18 of 33 (54.5%) counties in 2009). Further, nymphal abundances were relatively high across most counties in New York State in 2017, whereas high nymphal abundance was detected in only the eastern edge of the state and one other county in 2009. The increasing trends of both the occurrence and abundance of nymphs are apparent in the data collected across sampling (2008–2017). Human Lyme disease incidence is correlated with the geographic and demographic expansion of nymphs at the county level. That is, counties with high nymphal abundance tend to have higher human Lyme disease incidence rates. Further, human Lyme disease cases expanded westward between 2009 and 2017, with human cases appearing 1–3 years after nymphs were first detected in these counties. County-level abundance was calculated from surveillance data as total nymphs collected per total effort for each county.
The power to accurately identify and quantify the effect sizes of environmental features on population dynamics increases with the analysis of empirical data collected during changes in population size or geographic range within appropriate statistical frameworks (Bernatchez and Wilson, 1998; Fisher and Owens, 2004; Rowe et al., 2006; Carstens and Richards, 2007; Hickerson et al., 2010; Dawson, 2014). Data collected during the dynamic phases of range expansion and population growth are ideal for developing biogeographic models that explicitly incorporate time, space, and environmental features (Daniels et al., 1991; Fraser et al., 1997; Austin, 2002; Dolan et al., 2004; Guisan and Thuiller, 2005; Carstens and Richards, 2007; Elith and Leathwick, 2009; Pontius and Neeti, 2010; Leighton et al., 2012). We have amassed data on the occurrence and abundances of nymphal ticks during annual field collections (2008–2018) from locations distributed throughout NY, a large geographic area that is representative of much of the natural environment that I. scapularis may encounter in the northeastern US, which includes rapid and recent changes in climate and landscape features.
Determining how environmental features correlate with the spatial and temporal heterogeneity of I. scapularis in a natural ecosystem enables a mechanistic understanding of how the environmental factors in a real ecosystem influence the realized geographic range and population sizes of this important disease vector. We developed spatio-temporal biogeographic models to assess the potential impact of hundreds of environmental features, derived from remotely-sensed climate and landscape data, on the occurrence and abundance of I. scapularis nymphs. Understanding these general biological and ecological principles will ultimately lead to novel ecological control strategies such as landscape management or urban planning that may be broadly applicable. Further, models that accurately assess the correlation between environmental features and nymphal demography can be used to predict the risk of human contact with the disease vector and potentially predict future human disease incidence.
2. Materials and methods
2.1. Data
The occurrence and density of host-seeking nymphs at publicly accessible forested locations across NY were investigated between April and early December from 2008 to 2018. The sampling protocol at all sites consisted of standardized dragging, flagging, and walking surveys using 1 m2 of white flannel or canvas as previously described (Prusinski et al., 2014). A total of 532 unique locations were sampled at least once between 2008 and 2018 with varying number of sites per county. Some locations were sampled annually while others were sampled on a rotational basis every 2–5 years such that locations were visited in 2.5 different years on average. The variation among sites in the number sampling visits and years sampled was independent of tick collection success during prior sampling. Sites were visited an average of 4.7 times throughout our collection time period.
Local environmental conditions, including ambient air temperature and relative humidity measured by sling psychrometer (model 0012–7043, Bacharach, New Kensington, PA, USA) or digital hygro-thermometer, estimated wind speed (mph), general weather observations, and start and stop times of sampling were recorded at each sampling visit. Estimates of I. scapularis nymph population size, as represented by Fig. 1, were calculated by dividing the total number of nymphs collected by the total person-hours of sampling effort for each county.
Environmental features (Supplementary Table S1) were curated based on a priori hypotheses about how specific biotic and abiotic factors may influence the I. scapularis lifecycle. Environmental features can be classified into several general categories.
Local environmental features such as relative humidity, local temperature, and weather conditions that are specific to a location at the specific date and time of sampling.
Climate measures such as monthly temperature, precipitation, and humidity values. Cumulative degree days include degree days above zero degrees Celsius during different seasons and tick life stages – larval (Jan-Aug) and nymphal (Jan-May) – as well as days below zero degrees Celsius in the winter (Dec-Feb). These climate features were also calculated for one and 2 years prior to nymphal collection for each location. Climatic anomalies, the difference between a climate measure and the 30-year average of that climate variable from 1981–2010, were also calculated and used as a predictor in regression models.
Biodiversity indices: biodiversity scores were obtained from a predictive model that evaluates the terrestrial biodiversity of both lands and waters in NY. Aquatic biodiversity is based on watershed score.
Vertebrate populations: annual white-tailed deer harvest data and human population data were obtained for each county.
Landscape data includes elevation and proportions of urban, forest or shrub landcover.
Geographic data such as distance to roads and hydrological features, the nearest road classification, designation as a critical environmental area, and ecological zone classification.
2.2. Data accessibility
The tick collection data and the analytical code used in this paper are available at MendeleyData (doi:10.17632/rtd52gnbyy.1). The environmental and human disease data are available in public databases (Supplementary Table S1). Human Lyme disease incidence data was obtained from the NY Department of Health’s communicable disease annual reports (New York State Department of Health, 2019; https://www.health.ny.gov/statistics/diseases/communicable/). Environmental data were collected from multiple state and federal databases. Climatic gridded data were obtained from PRISM Climate Group (Prism Climate Group, 2018; https://prism.oregonstate.edu/); biodiversity indices and hydrography data were obtained from the NY Geographical Information Systems (GIS) Clearinghouse (NY, USA; https://gis.ny.gov/gisdata/); white-tailed deer data were acquired from the NY Department of Environmental Conservation (Department of Environmental Conservation, 2018; https://www.dec.ny.gov/outdoor/42232.html); landscape data were calculated from the USGS [United States Geological Survey] National Land Cover Database (https://www.usgs.gov/centers/eros/science/national-land-cover-database?qt-science_center_objects=0#qt-science_center_objects); and elevation data was from the USGS National Elevation Dataset (https://www.usgs.gov/core-science-systems/national-geospatial-program/national-map). Road data and human population data were obtained from the US Census Bureau (https://www.census.gov/en.html).
2.3. Statistical models
Independent models were built to investigate the presence and the abundance of nymphal ticks as distinct environmental features are likely to influence the biological processes affecting tick occurrence versus tick abundance. Fitting two separate models to the data also increases the interpretability of the model and model parameters. The occurrence of nymphal I. scapularis at each sampled location was related to our environmental features database using a logistic regression model with a binary outcome and binomial error distribution. The best fitting model according to Akaike’s information criterion (AIC) was selected using a forward stepwise algorithm and k-fold cross-validation. Briefly, k-fold cross-validation is used to evaluate the performance of machine learning models by training multiple models on different subsets of the data and testing prediction accuracy on the data subsets not used for model training. Our data subsets were chosen to be each collection year in order to limit overfitting the model to a single or aberrant year. Thus, k =10 forward stepwise feature selection algorithms were performed using training datasets consisting of nine of the 10 years between 2008 to 2017 with each year held out of the model training in one of the 10 models. Features included in more than eight of the 10 cross-validation models were included in our final predictive model. The statistical significance of the environmental features in the final model were determined using Wald tests. The out-of-sample predictive accuracy of this model was evaluated by predicting the 2018 collected tick data, which was not used in any of the model fitting described above. The 2018 dataset consists of sites that were sampled prior to 2018 (144 sites) and used to train the models as well as independent sites which were not sampled before 2018 (16 sites). We assessed predictive accuracy by (i) sensitivity, or the true positive rate – the ability of the model to correctly identify locations sampled in 2018 with ticks – and (ii) specificity, or the true negative rate – the ability of the model to correctly identify locations sampled in 2018 without ticks.
Abundance of nymphal I. scapularis at each site was related to our environmental features database using a linear regression model. Only sites with tick presence, and thus a smaller dataset than used for the occurrence model, were used for variable selection and building the nymphal abundance model. The number of nymphs collected at each site was log-transformed and modeled as a linear function of each environmental feature. This model assumes the presence of nymphs at all sites as the log of zero is undefined. The best fitting model according to AIC was selected using a similar forward stepwise algorithm and k-fold cross-validation as described above. The statistical significance of environmental features included in the final model were determined using t-tests. To assess the predictive accuracy of the model, actual and predicted abundances were categorized. Actual nymphal abundance was divided into similar sized categories of low (1–4 nymphs), medium (7–35 nymphs), and high (36+ nymphs), and was compared with nymphal abundance predictions that were categorized into accurate, under- and over-predictions. Model predictions were considered accurate if the size of the prediction error was within one natural log unit (e≈2.718) of average prediction error. The model prediction for a site was considered overpredicted if the prediction was greater than the actual value by more than the average prediction error plus one natural log unit. The model prediction was considered underpredicted if the prediction was less than the actual value by more than the average prediction error plus one natural log unit.
3. Results
3.1. Nymphal tick collections
Host-seeking nymph collections yielded over 22,000 nymphs from over 2,000 visits to 532 sites conducted between 2008 and 2018. Over this time frame, the geographic range of nymphal ticks expanded from 33% of the counties in NY in 2008 to more than 90% of the counties in 2017 (Fig. 1). Stable nymph populations have been detected in southeastern NY and the Hudson River Valley prior to 2008 and remained present throughout our collection period (Khatchikian et al., 2012). More recently, tick populations spread westward and likely northward (Fig. 1). The geographic range expansion did not appear to proceed uniformly as new populations were detected in areas that are geographically distant from all other known populations. Nymphal abundances also varied over the collection period with the largest changes occurring in areas where nymphs were recently detected (Burtis et al., 2016). That is, nymphal abundances in eastern NY remained relatively high throughout the sampling period while many areas in northern and western NY increased from undetectable to abundances approaching those in eastern NY.
The spatial and temporal variation in the county-level abundance of nymphal ticks corresponds with reported Lyme disease incidence (Fig. 1) (New York State Department of Health, 2019). Notably, Lyme disease incidence tended to be greater in counties with greater nymphal abundances within each year. Lyme disease incidence increased primarily in the western and northern NY counties between 2008 and 2017, similar to the observed distribution of nymphal ticks. However, nymphal I. scapularis populations were often detected 1– 3 years before Lyme disease cases were reported from a given county or NY region.
3.2. Model accuracy
We estimated separate regression models for the occurrence and abundance of the nymphal ticks in our collection data (Table 1). The occurrence model with the greatest predictive accuracy correctly identified 80.6% of the sites, with 87.4% of sites where nymphal ticks were collected and 85.5% of sites where ticks were not detected within the training dataset (2008–2017). Importantly, this model predicted a similar proportion of sites with and without ticks (80.6% and 80.7%) in the out-of-sample dataset collected in 2018, data not used to train the model. Additionally, the predictions were equally accurate for sites that had not been visited prior to 2018 as for those that had been previously sampled. The diagnostic accuracy of the occurrence model is summarized by a receiving operating characteristic (ROC) curve (Supplementary Fig. S1) demonstrating excellent discriminatory power in classifying sites by tick occurrence. These results indicate that this model incorporates environmental features that can explain the interannual and spatial heterogeneity in the data and can accurately predict nymphal tick distributions in future years (Fig. 2).
Table 1.
Ecological factors with predictive power of tick occurrence and abundance
| Occurrence Model | P value | Abundance Model | P value |
|---|---|---|---|
| 1. Physical and ecological habitat | |||
| Latitude | a (−) | Latitude | a (−) |
| Longitude | a (+) | ||
| Eelevation | a (−) | Elevation d | a |
| Forest | b (−) | ||
| Distance to nearest hydrography feature | c (−) | ||
| Road type of nearest road e, Interstate | a (+) | ||
| Road type of nearest road e, State/Other | c (+) | ||
| Distance to nearest road | (−) | ||
| Indicator of critical zone | (−) | ||
| 2. Nymph activity period | |||
| The spring/summer of the two years prior to collection | |||
| Degree days above 0 C (spring) | a (−) | ||
| 3. Adult activity period | |||
| The fall of the two years prior to collection | |||
| Maximum vapor deficit pressure anomalies (Oct) | b (+) | ||
| Minimum vapor deficit pressure anomalies d (Oct) | b (−) | ||
| 4. Overwinter success of adult ticks & eggs | |||
| The winter in the year before collection | |||
| Minimum vapor deficit pressure (Jan) | a (−) | ||
| Precipitation (Jan) | a (−) | ||
| Degree days below 0 C (winter) | a (+) | ||
| 5. Egg to larvae transition | |||
| The spring in the year before collection | |||
| Degree days above 0 C (spring-summer) | a (+) | Degree days above 0 C (spring-summer) | a (+) |
| 6. Year of collection | |||
| Annual deer harvest | a (−) | Annual deer harvest | c (+) |
| 7. Day of collection | |||
| Person-hours collecting | a (+) | Person-hours collecting | a (+) |
| Local temperature | b (+) | ||
| Month e | (+) | Month e, June | a (+) |
| Wet | (−) | ||
Wald test
≤ 0.001
≤ 0.01
≤ 0.05; (−) = negative relationship; (+) = positive relationship
Orthogonal polynomial variable (squared)
Categorical variable
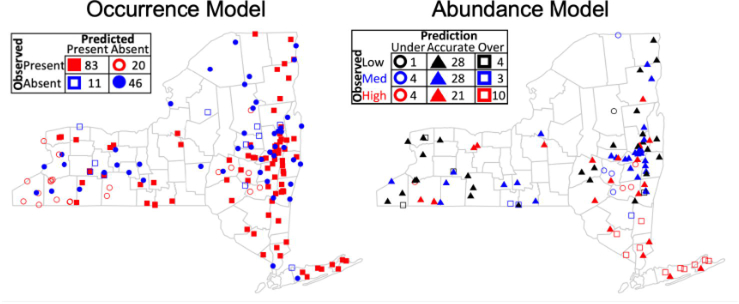
Fig. 2.
Predictive accuracy of occurrence and abundance models. The occurrence and abundance models trained with nymph collection data from 2008–2017 accurately predicted both the collection sites visited in 2018 where nymphs were present as well as the abundance of nymphs at those locations. The occurrence model accurately predicted the presence or absence of nymphs collected in 129 of the 160 (80.6%) sites sampled in 2018. The majority of inaccurate predictions occurred either at locations at which no ticks were collected in prior sampling years (three of the 20 false negatives) or sites in which ticks had been collected in 2017 but were not detected in 2018 (seven of the 11 false positives), likely due to local conditions in 2018 hindering collection as opposed to local extinction of tick populations. The observed abundance of nymphs was accurately predicted at 77 of the 103 (74.8%) sites where ticks were detected in 2018. Nearly half of the inaccurate predictions (11 of 26) occurred at very high (10) and very low (one) observed abundances where the model predicted high (or low) abundances, but not as high (or as low) as observed in the sample collection. The accuracy of predictions by both the occurrence and abundance models were nearly identical for the sites that were sampled for the first time in 2018 and for sites that were sampled in prior years, suggesting that the environmental features in these models are correlated with the occurrence and abundance of nymphs.
The abundance model with the greatest predictive accuracy estimated nymphal abundances at 83.5% sites in the training dataset. Model predictions were not biased as 8.2% of the sites were overpredicted and 8.2% underpredicted. Similar to the occurrence model, the accuracy of the abundance model predictions in the 2018 dataset were only slightly lower than in the training data (74.8%), indicating that the environmental features incorporated in this model capture the interannual and spatial heterogeneity needed to accurately predict tick abundances in future years (Fig. 2). Further, the predictions of the abundance model were equally accurate for sites that had not been visited prior to 2018 as for those that were visited, demonstrating that the environmental factors included in the model have high predictive power across the region.
3.3. Selected environmental features
Both statistical models include geographic, temporal, seasonal, environmental, climatic, and landscape features as regression covariates (Table 1). However, the models differed in the number (16 covariates in the occurrence model versus 11 in the abundance model) and identity of the environmental features selected for the final models. Only six covariates were shared between the best performing models, suggesting that the ecological processes that determine nymphal occurrence differ from those that determine nymphal abundance. All of the covariates included in the model predicting nymphal abundances were statistically significant as were 12 of the covariates included in the model predicting nymphal occurrence; four environmental features improved the accuracy of nymphal occurrence predictions but were not statistically significant (P>0.05). Several competing models identified during feature selection of the occurrence or abundance models exhibited predictive accuracy similar to that of the final best-fitting models. Each of these competing models differed from the best-fitting model only by replacing one environmental feature in the best-fitting models with a similar, highly correlated feature. The coefficients estimated for each of these correlated features were nearly identical among these models, suggesting robustness in the cross-validated features selected. The ecological mechanisms through which some of the environmental features in the models impact the occurrence or abundance of nymphs can be surmised by the timing of their occurrence in the tick life cycle (Fig. 3). That is, several environmental features in the models occur at specific times during specific years relative to the date of sampling (i.e. precipitation in January of the winter prior to sampling) and thus impact identifiable life stages.
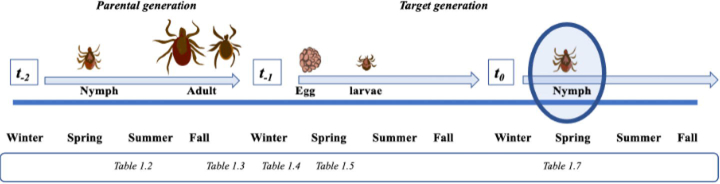
Fig. 3.
Lifecycle of Ixodes scapularis. Environmental features are important at different times during the I. scapularis life cycle. The timing at which some of the environmental features included in the occurrence and abundance models (Table 1) occur during the tick life cycle indicates the ecological impact of these features on where nymphs are present and their abundance at those locations. Nymphal activity period of the prior generation (see Table 1.2): the presence and abundance of nymphs at each location at the time of collection depends upon the survival at preceding life stages such as the nymphal stage of the parental generation. The occurrence of nymphs at the time of collection is impacted by a warm spring 2 years prior, during the activity period of the nymphs of the parental generation. Interestingly, both the occurrence and abundance of nymphs at the time of collection are also affected by warmer spring and summer days during the egg to larva transition (see Table 1.5) stage of the current generation, suggesting warmer spring and summer temperatures may impact survival rates. Adult activity period of the prior generation (see Table 1.3): the survival and questing activity of unfed I. scapularis adults of the parental generation are related to vapor pressure deficit, resulting in changes in feeding and mating success that directly impact population sizes in the following generation. Overwinter success of adults or eggs (see Table 1.4): the rate of overwinter survival of adults from the parental generation, or their eggs laid prior to winter onset, is correlated with temperature, precipitation, and vapor pressure deficit in the winter. Survival rate at this stage appears to have a large effect on the occurrence of the nymphs that hatch from these eggs. Local conditions on the day of collection (see Table 1.7): an accurate assessment of the occurrence and abundance of nymphs is influenced by the local conditions on the day of collection, such as temperature and rain, which influence both questing behavior and dragging success.
3.4. Out-of-sample predictions
The accuracy of model predictions out-of-sample both temporally (data from 2018 were not used to train the models) and spatially (sites sampled for the first time in 2018) suggest that these models can predict the occurrence and abundance of I. scapularis nymphs at previously unsampled locations across NY (Fig. 4). The predicted occurrence of nymphal ticks varied across sampling years (Supplementary Fig. S2A), similar to the data observed from our field collections. Three broad regions associated with higher elevation in mountainous areas are predicted to have a low probability of nymphal tick populations throughout all sampling years including 2018, although the size of the geographic expanse of the broad areas with low probabilities of nymphs is variable among years. Predictions with limited confidence occurred geographically at interfaces between confident presence and absence predictions, adding confidence to the accuracy of model predictions (Fig. 4). Predictions at fine scales revealed fine-scale heterogeneity in the probability of occurrence within broad regions with generally high expected nymph probabilities. This heterogeneity in nymph occurrence is caused by fine-scale heterogeneity in environmental features (i.e. nymphs are unlikely to be found on roads or in urban areas).
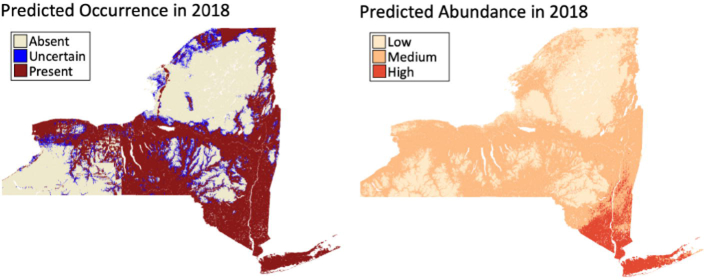
Fig. 4.
Abundance and occurrence predictions for ticks across New York State, USA for 2018. The occurrence and abundance of nymphal ticks can be predicted to future years at very fine spatial scales (500 m). The shared environmental features among the models has caused some similarities in predictions such that most areas predicted to have low nymphal abundance are also predicted to have no detectable populations. However, some locations predicted to be devoid of nymphs are also predicted to have moderate nymphal densities as the abundance model utilizes a different set of environmental features and predicts abundances under the assumption that nymphs are present. The risk of encountering a questing Ixodes scapularis nymph at any location is the composite of these models. Any potential confusion from discrepancies between the models can be calculated as the product of the predictions from these models.
The predicted abundance of nymphal ticks (Supplementary Fig. S2B) also varied among years, although to a lesser degree than the predicted occurrence (Supplementary Fig. S2A). These predicted results are corroborated by the field collection data, showing dramatic temporal changes in nymphal distribution and relatively steady abundances in areas with long-standing tick populations (Fig. 1). The nymphal abundance model, which predicts the number of nymphs assuming that nymphs are present, suggests that nymphal abundances generally vary gradually over space, with large predicted differences between proximal locations caused by abrupt changes in environmental features (i.e. river edges). The residuals from the abundance model showed no departure from normality and no evidence of autocorrelation, indicating that the spatial autocorrelation inherent in the dataset was accounted for by the covariates in the models. Similar to the predictions from the occurrence model, the abundance model predicted lower nymphal abundances in three broad higher elevation regions across all years, likely due to shared environmental features between these models.
4. Discussion
The occurrence and abundance of I. scapularis nymphs are determined by interactions with environmental features that vary over space and time. Data collected during the dynamic phases of population growth and range expansion are ideal for developing population dynamic models that explicitly incorporate space, time, and environmental factors (Daniels et al., 1991; Fraser et al., 1997; Dolan et al., 2004; Carstens and Richards, 2007; Leighton et al., 2012). Such models cope with the inherent heterogeneity of dynamic populations, thus enabling accurate assessments of the environmental factors affecting changes in occurrence and abundances while simultaneously limiting biases (Austin, 2002; Guisan and Thuiller, 2005; Elith and Leathwick, 2009; Pontius and Neeti, 2010). Analyses of our field collection dataset, which was collected systematically over 11 years (2008–2018) across NY, reveals an active range expansion of I. scapularis into northern and western NY that occurred during the last decade. Nymphal abundances increased rapidly in areas where ticks were only recently detected while nymphal abundances remained consistently high throughout the sampling period in areas with long-standing tick populations. The spatial and temporal variation in nymphal tick occurrence and abundance can be explained by corresponding spatio-temporal variation in the environmental features with which nymphs interact. Statistical models that incorporate environmental features as covariates have high predictive accuracy to future years; accurate predictions of future spread and abundance of nymphal tick populations will be a useful public health tool to combat the rapid increases in tick-borne diseases by aiding in resource allocation decisions and the targeting of interventional strategies.
The predicted occurrence of nymphal ticks was highly accurate in far-eastern NY, where active tick surveillance sampling has occurred for over a decade. Predictive accuracy was nearly as high in northeastern NY, despite a shorter sampling history, highlighting the robustness in model predictions across climatic regions. Model inaccuracy occurred primarily in southwestern NY, due exclusively to locations where nymphs were only recently detected. No nymphal ticks were detected in this region prior to 2008, suggesting either that the environmental conditions only recently supported populations of detectable sizes or that migration to this area was limited prior to 2008 (Diuk-Wasser et al., 2006; Prusinski et al., 2006). Regardless, the environmental features accurately predicted the occurrence of nymphs in more than 80% in sites in 2018 (data not included in model training), where field collections had not been attempted prior to 2018. This accuracy suggests that the environmental features in the model directly or indirectly impact the distribution of nymphal ticks in NY.
The predicted abundance of nymphal ticks was also highly accurate across NY. For nearly half of our inaccurate predictions, our model predicted in the correct direction but underestimated the magnitude of the observed value (i.e. predicted high for an observed value that was extremely high). This is an example of the common “regression to the mean” problem that prevents accurate characterizations of extreme values observed in datasets. Most of the inaccurate predictions occurred in southwestern NY, similar to the area with the greatest error in the occurrence model. This is likely due to the year-to-year variation in observed nymphal abundances in this region, the rate of which varied both temporally and spatially. The elevated prediction accuracy to the sites in eastern NY was aided by the relatively limited year-to-year variation. Importantly, only one of the 18 sites that had not been visited prior to 2018 was inaccurately predicted. A careful point of consideration is that our objective was to estimate the abundance of nymphs sustainable by the local environment at each location, which is distinct from the likelihood of collecting the supportable number questing nymphs on the day of collection. That is, the number of nymphs collected is impacted by variable ‘day-of conditions such as local temperature, weather, and hours of surveillance, which are more difficult to assess statistically. Therefore, sites that were visited multiple times presented a more accurate assessment of nymph abundances. Thus, the models presented analyzed the maximum number nymphs collected within each year as most representative of underlying nymphal abundance at each site.
Our prediction models are based on environmental features that can be categorized into those that are static (Table 1) and those that vary within and across years (Table 1). Of the six features selected in both models, four impacted nymphal tick occurrence and abundance in the same direction. For example, ticks were more likely to be present and at greater abundance at lower latitudes (Clow et al., 2017), higher degree days above 0°C into the summer (Ogden et al., 2006), with greater collection effort, and in some months of the year. These factors all suggest that nymphal ticks are expected to expand in range and population size as the climate warms. In contrast, deer population size decreased the probability that nymphs would be present, but assuming they are present, nymphal abundance was weakly and positively correlated with deer population size. Empirical data suggest that deer abundance is weakly correlated with I. scapularis population sizes, but is complicated by interactions with multiple ecological factors and other animal species (Rand et al., 2003; Kilpatrick et al., 2014; Kugeler et al., 2016). The impact of elevation follows a similar pattern as elevation delineates the limit of habitat suitability for tick occurrence (Rand et al., 2003) but, within that limit, elevation does not have a clear directional influence on tick abundance. Several additional features are unique to each model, the timing of which can indicate the stage of the I. scapularis life cycle impacted by these environmental variables. For example, the observed abundance of nymphs is impacted by vapor deficit pressure anomalies in the October 2 years prior to sampling, during the adult tick activity period of the previous generation. That is, these results suggest that vapor pressure deficit impacted the survival and reproductive success of adult ticks, the offspring of which were collected 2 years later as questing nymphs. Temporal changes in the occurrence or abundance of nymphs could be explained by coordinated temporal changes in specific environmental features. For example, sites that shifted in the probability of absence to presence between 2009 and 2017 had concomitant decreases in winter minimum vapor deficit pressure and winter precipitation of the year prior to sampling, compared with sites where the probability of occurrence of nymphs was unaltered. Similarly, sites in which tick abundances increased had large increases in forest fragmentation, maximum vapor pressure deficit in October 2 years before collection, and spring-summer temperatures. With projections of significant climate change – including temperature, humidity, and precipitation – this region is likely to experience future growth of tick populations and human Lyme disease risk (Stocker et al., 2013).
Models that accurately predict the occurrence and abundance of nymphal ticks in years beyond the temporal frame of the training data at sites that had not been previously sampled are ideal for predicting the potential risk of human encounters with nymphal ticks across unsurveyed regions of NY (Fig. 4). Predicted occurrence across NY presents regions of uncertainty at the interface between regions of predicted presence and absence, denoting regions where nymphal occurrence is in flux. Similar regions vary annually in the predicted abundances of nymphs. The nymphal abundance model, however, should be interpreted carefully as this model assumes that nymphs are present. This results in different environmental features explaining the occurrence and the abundance of ticks that can lead to some disparity between the predictions of the two models. Thus, a composite of these models is needed to estimate the risk of encountering nymphal ticks at locations across NY. Multiplying the predictions of the abundance model with those of the occurrence model, which are binary, results in an accurate representation of the composite model estimates of the underlying tick population size. However, caution should be exercised in locations with greater uncertainty in the occurrence model predictions. Utilizing these models for public health objectives should conservatively assume the presence of tick populations in locations with greater uncertainty to encourage preventative behavior.
Within the US, there are currently no vaccines available to prevent tick-borne diseases such as Lyme disease. NY has one of the highest burdens of Lyme disease in the country and the number of reported cases increases annually (CDC, 2019c; New York State Department of Health, 2019). Personal protective measures, tick-borne disease prevention education, and habitat modification all have the potential to effectively reduce disease risk, the targeting and implementation of which can be greatly aided by fine-scale knowledge of entomological risks. Use of environmental features to accurately predict future nymphal I. scapularis occurrence and abundance across the region at fine spatial scales provides a useful tool to communicate potential risk of tick encounters 1 year in advance, granting opportunities to implement timely and targeted management and education strategies to reduce the incidence of tick-borne disease.
Supplementary Material
Supplementary Fig. S1. Receiver Operating Characteristic (ROC) curve of the logistic model. ROC was performed for n = 314 collections and the vertical bars represents a 90% confidence interval. The Area under the ROC Curve (AUC), a measure of how well the model distinguishes sites with tick presence from tick absence, is 93%.
Supplementary Fig. S2. Variation in predictions of tick population dynamics parallel Lyme disease incidence patterns in New York State, USA. The predicted occurrence of nymphs increased across New York State relatively consistently from 2009 through 2017. The predicted abundance of ticks also increased across the state during this time period, although less dramatically. These trends corroborate the data from the field collections. Further, these increases in the geographic range and abundance of nymphs correspond to similar increases in human Lyme disease cases as well as increases in incidence rates. The predicted areas of nymphal occurrence and nymphal abundances in 2018 were both considerably smaller across New York State than in 2017. Similarly, Lyme disease incidence in 2018 was much lower in New York State than in prior years, suggesting that a composite of these models can be predictive of the future entomological risk of Lyme disease.
Acknowledgements
The authors would like to express their gratitude to the New York State Department of Environmental Conservation, USA, the New York State Department of Parks, Recreation and Historic Preservation, USA and various county, town and village park managers for granting us use of lands to conduct this research. We extend thanks to the following individuals for their assistance in collection, identification, and/or molecular testing of ticks: the students of Paul Smith’s College, USA, Jake Sporn and the boat launch stewards of the Adirondack Watershed Institute, USA, Brian Leydet, Samantha Lanthier, New York Department of Health, USA (NYSDOH) employees and student interns: Lauren Rose, Alexis Russell, Collin O’Connor, Anna Perry, Joshua Rosansky, Konrad Fondrie, Kaitlin Driesse, Michael Suatoni, Margaret Mahoney, Michelle Wemette, Sandra Beebe, Kayla Mehigan, Emily Haner, Franz Soiro, Katherine Guilbo, Samantha Sandwick, Morgan Thorne, Kate Turcotte, Jacob Miller, Joseph Prusinski Jr., Jennifer DeLorenzo, Lauren DeLorenzo, James Sherwood, John Howard, Rachel Reichel, Ariel Robinson, Marly Katz, Adam Rowe, Jean Stella, Donald Campbell, Daniella Giardina, Melissa Stone, Thomas Mistretta, R.C. Rizzitello, Emily Gicewicz, Christopher Murphy, David Rice, Nicholas Piedmonte, Melissa Fierke and associates with the State University of New York (SUNY) College of Environmental Science and Forestry, USA, Colgate University, USA students and faculty, Claire Hartl and others from SUNY Brockport, Niagara County DOH, USA, Erie County DOH, USA, Cornell Cooperative Extension of Onondaga County, USA, Scott Campbell, Michael Santoriello, Christopher Romano and Suffolk County DOH, USA, and Ilia Rochlin and Moses Cucura and Suffolk County Vector Control, USA. This work was supported by the NYSDOH, the National Institutes of Health, USA (grants R01-AI097137, R01-AI142572, R21-AI137433, T32-AI55400-15), and the Burroughs Wellcome Fund, USA (1012376). Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number F31-AI133871. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre AA, Tabor GM, 2008. Global Factors Driving Emerging Infectious Diseases. Ann. N.Y. Acad. Sci 1149, 1–3. 10.1196/annals.1428.052 [DOI] [PubMed] [Google Scholar]
- Aluwong T, Bello M, 2010. Emerging diseases and implications for Millennium Development Goals in Africa by 2015 – an overview. Vet. Ital 46, 9. [PubMed] [Google Scholar]
- Austin MP, 2002. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Model. 157, 101–118. 10.1016/S0304-3800(02)00205-3 [DOI] [Google Scholar]
- Balkenhol N, Waits LP, Dezzani RJ, 2009. Statistical approaches in landscape genetics: An evaluation of methods for linking landscape and genetic data. Ecography 32, 818–830. 10.1111/j.1600-0587.2009.05807.x [DOI] [Google Scholar]
- Bernatchez L, Wilson CC, 1998. Comparative phylogeography of Nearctic and Palearctic fishes. Mol. Ecol 7, 431–452. 10.1046/j.1365-294x.1998.00319.x [DOI] [Google Scholar]
- Burtis JC, Sullivan P, Levi T, Oggenfuss K, Fahey TJ, Ostfeld RS, 2016. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasit Vectors 9, 606. 10.1186/s13071-016-1894-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens BC, Richards CL, 2007. Integrating coalescent and ecological niche modeling in comparative phylogeography. Evolution 61, 1439–1454. 10.1111/j.1558-5646.2007.00117.x [DOI] [PubMed] [Google Scholar]
- Chaves LF, Koenraadt CJM, 2010. Climate change and highland malaria: Fresh air for a hot debate. Q. Rev. Biol 85, 27–55. 10.1086/650284 [DOI] [PubMed] [Google Scholar]
- Chikhi L, Sousa VC, Luisi P, Goossens B, Beaumont MA, 2010. The confounding effects of population structure, genetic diversity and the sampling scheme on the detection and quantification of population size changes. Genetics 186, 983–995. 10.1534/genetics.110.118661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, Jardine CM, 2017. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One 12. 10.1371/journal.pone.0189393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SA, Landguth EL, 2010. Spurious correlations and inference in landscape genetics. Mol. Ecol 19, 3592–3602. 10.1111/j.1365-294X.2010.04656.x [DOI] [PubMed] [Google Scholar]
- Daniels TJ, Fish D, Falco RC, 1991. Evaluation of host-targeted acaricide for reducing risk of Lyme disease in southern New York state. J. Med. Entomol 28, 537–543. 10.1093/jmedent/28A537 [DOI] [PubMed] [Google Scholar]
- Dawson MN, 2014. Natural experiments and meta-analyses in comparative phylogeography. J. Biogeogr 41, 52–65. 10.1111/jbi.12190 [DOI] [Google Scholar]
- Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, Hickling G, Brownstein JS, Walker E, Piesman J, Fish D, 2006. Spatiotemporal Patterns of Host-Seeking Ixodes scapularis Nymphs (Acari: Ixodidae) in the United States. J. Med. Entomol. 43, 166–176. 10.1603/0022-2585(2006)043[0166:spohis]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vourc’h G, Cislo P, Hoen AG, Melton F, Hamer SA, Rowland M, Cortinas R, Hickling GJ, Tsao JI, Barbour AG, Kitron U, Piesman J, Fish D, 2010. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob. Ecol. Biogeogr 19, 504–514. 10.1111/j.1466-8238.2010.00526.x [DOI] [Google Scholar]
- Dolan MC, Maupin GO, Schneider BS, Denatale C, Hamon N, Cole C, Zeidner NS, Stafford KC, 2004. Control of Immature Ixodes scapularis (Acari: Ixodidae) on Rodent Reservoirs of Borrelia burgdorferi in a Residential Community of Southeastern Connecticut. J. Med. Entomol 41, 1043–1054. 10.1603/0022-2585-41.6.1043 [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A, 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol 7, 214. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016. County-scale distribution of Ixodes scapularis and Ixodespacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol 53, 349–386. 10.1093/jme/tjv237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J, Leathwick JR, 2009. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst 40, 677. 10.1146/annurev.ecolsys.110308.120159 [DOI] [Google Scholar]
- Epps CW, Wehausen JD, Bleich VC, Torres SG, Brashares JS, 2007. Optimizing dispersal and corridor models using landscape genetics. J. Appl. Ecol 44, 714–724. https://doi.Org/10.1111/j.1365-2664.2007.01325.x [Google Scholar]
- Fisher DO, Owens IPFF, 2004. The comparative method in conservation biology. Trends in Ecol. Evol. 19, 391–398. 10.1016/jTree.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Fouque F, Reeder JC, 2019. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: A look at the evidence. Infect. Dis. Poverty 8, 51. 10.1186/s40249-019-0565-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, Van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC, 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586. 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- Gage KL, Burkot TR, Eisen RJ, Hayes EB, 2008. Climate and Vectorborne Diseases. Am J Prev Med 35, 436–450. 10.1016/j.amepre.2008.08.030 [DOI] [PubMed] [Google Scholar]
- Gasbarra D, Pirinen M, Sillanpaa MJ, Salmela E, Arjas E, 2007. Estimating genealogies from unlinked marker data: A Bayesian approach. Theor. Popul. Biol 72, 305–322. 10.1016/jTpb.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Rulison EL, Azevedo A, Pang GC, Kuczaj IM, Tsao JI, Lebrun RA, 2014. Comparison of survival patterns of northern and southern genotypes of the North American tick Ixodes scapularis (Acari: Ixodidae) under northern and southern conditions. Parasit Vectors 7, 394. 10.1186/1756-3305-7-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra M, Walker E, Jones C, Paskewitz S, Cortinas MR, Stancil A, Beck L, Bobo M, Kitron U, 2002. Predicting the Risk of Lyme Disease: Habitat Suitability for Ixodes scapularis in the North Central United States. Emerg. Infect. Dis 8, 289–297. 10.3201/eid0803.010166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A, Thuiller W, 2005. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett 8, 993–1009. 10.1111/j.1461-0248.2005.00792.x [DOI] [PubMed] [Google Scholar]
- Hey J, 2010. Isolation with migration models for more than two populations. Mol. Biol. Evol 27, 905–920. 10.1093/molbev/msp296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey J, Nielsen R, 2007. Integration within the Felsenstein equation for improved Markov chain Monte Carlo methods in population genetics. Proc. Natl. Acad. Sci. U S A 104, 2785–2790. 10.1073/pnas.0611164104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickerson MJ, Carstens BC, Cavender-bares J, Crandall KA, Graham CH, Johnson JB, Rissler L, Victoriano PF, Yoder AD, 2010. Phylogeography’s past, present, and future: 10 years after Avise, 2000. Mol. Phylogenet. Evol 54, 291–301. 10.1016/j.ympev.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Higgins R, 2004. Emerging or re-emerging bacterial zoonotic diseases: bartonellosis, leptospirosis, Lyme borreliosis, plague. Rev. Sci. Tech 23, 569–581. 10.20506/rst.23.2.1503 [DOI] [PubMed] [Google Scholar]
- Hoen AG, Margos G, Bent SJ, Diuk-Wasser MA, Barbour A, Kurtenbach K, Fish D, 2009. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc. Natl. Acad. Sci. U S A 106, 15013–15018. 10.1073/pnas.0903810106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PT, Caporale DA, Brisson D, 2010. Uncoordinated phylogeography of Borrelia burgdorferi and its tick vector, Ixodes scapularis. Evolution 64, 2653–2663. 10.1111/j.1558-5646.2010.01001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P, 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L, Kendell D, Robertson D, Livdahl T, Khatchikian C, 2010. Aedes aegypti and Aedes albopictus in Bermuda: extinction, invasion, invasion and extinction. Biol. Invasions 12, 3277–3288. 10.1007/s10530-010-9721-z [DOI] [Google Scholar]
- Khasnis AA, Nettleman MD, 2005. Global warming and infectious disease. Arch. Med. Res 36, 689–696. 10.1016/j.arcmed.2005.03.041 [DOI] [PubMed] [Google Scholar]
- Khatchikian C, Sangermano F, Kendell D, Livdahl T, 2011. Evaluation of species distribution model algorithms for fine-scale container-breeding mosquito risk prediction. Med. Vet. Entomol 25, 268–275. 10.1111/j.1365-2915.2010.00935.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatchikian CE, Prusinski M, Stone M, Backenson PB, Wang I-N, Levy MZ, Brisson D, 2012. Geographical and environmental factors driving the increase in the Lyme disease vector Ixodes scapularis. Ecosphere 3, 1–18. 10.1890/ES12-00134.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatchikian CE, Prusinski MA, Stone M, Backenson PB, Wang IN, Foley E, Seifert SN, Levy MZ, Brisson D, 2015. Recent and rapid population growth and range expansion of the Lyme disease tick vector, Ixodes scapularis, in North America. Evolution 69, 1678–1689. 10.1111/evo.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick HJ, Labonte AM, Stafford KC, 2014. The Relationship Between Deer Density, Tick Abundance, and Human Cases of Lyme Disease in a Residential Community. J. Med. Entomol 51, 777–784. 10.1603/me13232 [DOI] [PubMed] [Google Scholar]
- Kugeler KJ, Jordan RA, Schulze TL, Griffith KS, Mead PS, 2016. Will Culling White-Tailed Deer Prevent Lyme Disease? Zoonoses Public Health 63, 337–345. 10.1111/zph.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey Knowles L, Alvarado-Serrano DF, 2010. Exploring the population genetic consequences of the colonization process with spatio-temporally explicit models: Insights from coupled ecological, demographic and genetic models in montane grasshoppers. Mol. Ecol 19, 3727–3745. 10.1111/j.1365-294X.2010.04702.x [DOI] [PubMed] [Google Scholar]
- Lashley FR, 2004. Emerging infectious disease: Vulnerabilities, contributing factors and approaches. Expert Rev. Anti. Infect. Ther. 2, 299–316. 10.1586/14787210.2.2.299 [DOI] [PubMed] [Google Scholar]
- Leighton PA, Koffi JK, Pelcat Y, Lindsay LR, Ogden NH, 2012. Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 49, 457–464. 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- Lemey P, Rambaut A, Drummond AJ, Suchard MA, 2009. Bayesian phylogeography finds its roots. PLoS Comput. Biol. 5. 10.1371/journal.pcbi.1000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Telford SR, Rys PN, Rutledge BJ, Mathiesen D, Malawista SE, Spielman A, Persing DH, 1994. Detection of Borrelia burgdorferi dna in museum specimens of Peromyscus. J. Infect. Dis 170, 1027–1032. 10.1093/infdis/170A1027 [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Hanincová K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O’Callaghan CJ, Schwartz I, Thompson RA, 2008a. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasmaphagocytophilum in Canada. Appl. Environ. Microbiol 74, 1780–1790. 10.1128/AEM.01982-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Maarouf A, Barker IK, Bigras-Poulin M, Lindsay LR, Morshed MG, O’Callaghan CJ, Ramay F, Waltner-Toews D, Charron DF, 2006. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol 36, 63–70. 10.1016/j.ijpara.2005.08.016 [DOI] [PubMed] [Google Scholar]
- Ogden NH, St.-Onge L, Barker IK, Brazeau S, Bigras-Poulin M, Charron DF, Francis CM, Heagy A, Lindsay LR, Maarouf A, Michel P, Milord F, O’Callaghan CJ, Trudel L, Thompson RA, 2008b. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health. Geogr. 7, 24. 10.1186/1476-072X-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Olson SH, Uejio CK, Gibbs HK, 2008. Disease Emergence from Global Climate and Land Use Change. Med. Clin 92, 1473–1491. 10.1016/j.mcna.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Persing DH, Telford SR, Rys PN, Dodge DE, White TJ, Malawista SE, Spielman A, 1990. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science 249, 1420–1423. 10.1126/science.2402635 [DOI] [PubMed] [Google Scholar]
- Petrosillo N, 2019. Emerging Infections and Future Threats. Erciyes Med. J 41, 130–135. [Google Scholar]
- Pontius RGJ, Neeti N, 2010. Uncertainty in the difference between maps of future land change scenarios. Sustain. 5, 39–50. [Google Scholar]
- Prusinski MA, Chen H, Drobnack JM, Kogut SJ, Means RG, Howard JJ, Oliver J, Lukacik G, Backenson PB, White DJ, 2006. Habitat Structure Associated with Borrelia burgdorferi Prevalence in Small Mammals in New York State. Environ. Entomol. 35, 308–319. 10.1603/0046-225x-35.2.308 [DOI] [Google Scholar]
- Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB, 2014. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) Collected From Recreational Lands in the Hudson Valley region, New York State. J. Med. Entomol 51, 226–236. 10.1603/me13101 [DOI] [PubMed] [Google Scholar]
- Pybus OG, Rambaut A, 2009. Evolutionary analysis of the dynamics of viral infectious disease. Nat. Rev. Genet 10, 540–550. 10.1038/nrg2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand PW, Lubelczyk C, Lavigne GR, Elias S, Holman MS, Lacombe EH, Smith RP, 2003. Deer Density and the Abundance of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 40, 179–184. 10.1603/0022-2585-40.2.179 [DOI] [PubMed] [Google Scholar]
- Rowe KC, Heske EJ, Paige KN, 2006. Comparative phylogeography of eastern chipmunks and white-footed mice in relation to the individualistic nature of species. Mol. Ecol 15, 4003–4020. 10.1111/j.1365-294X.2006.03063.x [DOI] [PubMed] [Google Scholar]
- Shirk AJ, Wallin DO, Cushman SA, Rice CG, Warheit KI, 2010. Inferring landscape effects on gene flow: A new model selection framework. Mol. Ecol 19, 3603–3619. 10.1111/j.1365-294X.2010.04745.x [DOI] [PubMed] [Google Scholar]
- Sleeman JM, Howell JE, Matthew Knox W, Stenger PJ, 2009. Incidence of Hemorrhagic Disease in White-Tailed Deer Is Associated with Winter and Summer Climatic Conditions. EcoHealth 6, 11. 10.1007/s10393-009-0220-6 [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, 2018. Range expansion of tick disease vectors in north america: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 15, 478. 10.3390/ijerph15030478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear SF, Balkenhol N, Fortin MJ, McRae BH, Scribner K, 2010. Use of resistance surfaces for landscape genetic studies: Considerations for parameterization and analysis. Mol. Ecol 19, 3576–3591. 10.1111/j.1365-294X.2010.04657.x [DOI] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner GK, Tignor MM, Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM, 2014. Climate Change 2013: The Physical Science Basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change 1535. 10.1017/CBO9781107415324 [DOI] [Google Scholar]
- Suzán G, Marcé E, Giermakowski JT, Armien B, Pascale J, Mills J, Ceballos G, Gómez A, Aguirre AA, Salazar-Bravo J, Armién A, Parmenter R, Yates T, 2008. The Effect of Habitat Fragmentation and Species Diversity Loss on Hantavirus Prevalence in Panama. Ann. N.Y. Acad. Sci 1149, 80–83. 10.1196/annals.1428.063 [DOI] [PubMed] [Google Scholar]
- Taylor LH, Latham SM, Woolhouse ME, 2001. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci 356, 983–9. 10.1098/rstb.2001.0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zee J, Piesman JF, Hojgaard A, Black IV WC, 2015. Nuclear Markers Reveal Predominantly North to South Gene Flow in Ixodes scapularis, the Tick Vector of the Lyme Disease Spirochete. PLoS One 10. 10.1371/journal.pone.0139630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Receiver Operating Characteristic (ROC) curve of the logistic model. ROC was performed for n = 314 collections and the vertical bars represents a 90% confidence interval. The Area under the ROC Curve (AUC), a measure of how well the model distinguishes sites with tick presence from tick absence, is 93%.
Supplementary Fig. S2. Variation in predictions of tick population dynamics parallel Lyme disease incidence patterns in New York State, USA. The predicted occurrence of nymphs increased across New York State relatively consistently from 2009 through 2017. The predicted abundance of ticks also increased across the state during this time period, although less dramatically. These trends corroborate the data from the field collections. Further, these increases in the geographic range and abundance of nymphs correspond to similar increases in human Lyme disease cases as well as increases in incidence rates. The predicted areas of nymphal occurrence and nymphal abundances in 2018 were both considerably smaller across New York State than in 2017. Similarly, Lyme disease incidence in 2018 was much lower in New York State than in prior years, suggesting that a composite of these models can be predictive of the future entomological risk of Lyme disease.
Data Availability Statement
The tick collection data and the analytical code used in this paper are available at MendeleyData (doi:10.17632/rtd52gnbyy.1). The environmental and human disease data are available in public databases (Supplementary Table S1). Human Lyme disease incidence data was obtained from the NY Department of Health’s communicable disease annual reports (New York State Department of Health, 2019; https://www.health.ny.gov/statistics/diseases/communicable/). Environmental data were collected from multiple state and federal databases. Climatic gridded data were obtained from PRISM Climate Group (Prism Climate Group, 2018; https://prism.oregonstate.edu/); biodiversity indices and hydrography data were obtained from the NY Geographical Information Systems (GIS) Clearinghouse (NY, USA; https://gis.ny.gov/gisdata/); white-tailed deer data were acquired from the NY Department of Environmental Conservation (Department of Environmental Conservation, 2018; https://www.dec.ny.gov/outdoor/42232.html); landscape data were calculated from the USGS [United States Geological Survey] National Land Cover Database (https://www.usgs.gov/centers/eros/science/national-land-cover-database?qt-science_center_objects=0#qt-science_center_objects); and elevation data was from the USGS National Elevation Dataset (https://www.usgs.gov/core-science-systems/national-geospatial-program/national-map). Road data and human population data were obtained from the US Census Bureau (https://www.census.gov/en.html).