Abstract
Background:
Gestational diabetes (GD) leads to earlier onset and heightened risk of type 2 diabetes, a strong risk factor for cardiovascular disease (CVD). However, it is unclear whether attaining normoglycemia can ameliorate the excess CVD risk associated with GD history. This study sought to evaluate GD history and glucose tolerance after pregnancy associated with coronary artery calcification (CAC) in women, a manifestation of atherosclerotic CVD (ASCVD), and predictor of CVD clinical events.
Methods:
Data from the Coronary Artery Risk Development in Young Adults Study, a U.S. multicenter, community-based prospective cohort of young Black (50%) and White adults aged 18–30 years at baseline (1985–1986). The sample included 1,133 women without diabetes at baseline, who had one or more singleton births (n=2,066) during follow up, glucose tolerance testing at baseline and up to 5 times during 25 years (1986–2011), GD status, and CAC measurements at follow up exam at years 15, 20, and/or 25 (2001–2011). CAC was measured by non-contrast cardiac computed tomography; dichotomized as Any CAC (score>0) or No CAC (score=0). Complementary log-log models for interval-censored data estimated adjusted hazard ratios of CAC and 95% confidence intervals for GD history and subsequent glucose tolerance groups (normoglycemia, prediabetes, or incident diabetes) on average 14.7 years after the last birth adjusted for pre-pregnancy and follow up covariates.
Results:
Of 1,133 women, 139 (12.3%) reported GD and were aged 47.6 years (4.8 SD) at follow up. CAC was present in 25% (34/139) of women with GD and 15% (149/994) of women with no GD. Compared to no GD/normoglycemia, adjusted hazard ratios (95% confidence intervals) were 1.54 (1.06, 2.24) for no GD/prediabetes and 2.17 (1.30, 3.62) for no GD/incident diabetes, and 2.34 (1.34, 4.09), 2.13 (1.09, 4.17) and 2.02 (0.98, 4.19) for GD/normoglycemia, GD/prediabetes, and GD/incident diabetes, respectively (overall p-value=0.003).
Conclusions:
Women without previous GD showed a graded increase in the risk of CAC associated with worsening glucose tolerance. Women with a history of GD had a 2-fold higher risk of CAC across all subsequent levels of glucose tolerance. Mid-life ASCVD risk among women with previous GD is not diminished by attaining normoglycemia.
Keywords: cardiovascular, imaging, glucose tolerance, coronary artery calcium, gestational diabetes, type 2 diabetes, prediabetes, atherosclerosis, cardiovascular disease, women, prospective cohort, African American, hyperglycemia
Journal Subject Heads: Gestational diabetes mellitus, Type 2 Diabetes Mellitus, Atherosclerotic Cardiovascular Disease
Introduction
Gestational diabetes (GD), glucose intolerance first recognized during pregnancy, affects 8–9% (n~250,000) of U.S. pregnancies,1, 2 and up to 17–20% worldwide.3 Before pregnancy, women who develop GD may have impaired glucose tolerance (i.e., prediabetes) and/or dyslipidemia.4 After pregnancy, they are 4 to 7 times more likely to develop type 2 diabetes (T2D).5–7 T2D is a contributing factor to the 1.7- to 3-fold higher risk of cardiovascular disease (CVD) and/or coronary artery disease (CAD) in women with a history of GD.8–12 Yet, evidence is mixed about whether GD history increases CVD risk independent of subsequent T2D; with relative risks ranging from a null association among older European women,13 to a 1.25- to 2-fold higher risk among younger women.12, 14 Women with a history of GD who do not develop T2D have a 30% 15, 16 to 56% higher CVD risk based on a pooled-risk estimate from a meta-analysis.12 Yet, current evidence, based on retrospective study designs utilizing self-report or administrative hospital data sources to ascertain new onset diabetes after pregnancy may underestimate the risks. The misclassification of T2D, particularly among young women and minority groups, is likely because routine diabetes testing is not recommended in adults under age 45 years, except with obesity and other risk factors (e.g., history of GD). Further, previous studies could not distinguish prediabetes from normoglycemia, which represents the lowest risk group with highest relevance as the referent group for younger populations. Thus, what is the risk of CVD in women with a history of GD in the context of sustained postpartum normoglycemia?
Prediabetes is a strong predictor of T2D,17 and a risk factor for coronary heart disease (CHD), especially in women.18–20 About 24% of U.S. adults aged 18 to 44 years have prediabetes,21 but almost 75% are unaware they have the condition.22 Prediabetes is more common in women with a history of GD, affecting about 40%.23 Yet, the low uptake of post-delivery diabetes testing,24, 25 and lack of routine screening for CVD risk factors in young women are barriers to detection.26 Prediabetes is rarely available by self-report, or from administrative and hospital databases. Thus, our understanding of transitions in clinical glucose tolerance related to the development CVD outcomes after GD pregnancy is incomplete.
This study sought to evaluate the relationship of GD history and subsequent transitions in glucose tolerance across the reproductive years to the presence of coronary artery calcium (CAC) in women during mid-life, a strong predictor of atherosclerotic CVD (ASCVD).27, 28 We hypothesized that worsening glucose tolerance, including prediabetes, after pregnancy will increase the risk of CAC independent of other CVD risk factors, and a history of GD will be associated with higher risk of CAC, even among subsequently normoglycemic women. This research fills a major gap in the evidence base for clinical practice recommendations regarding traditional CVD risk factor screening among young women in general as well as those with a history of GD.
RESEARCH DESIGN AND METHODS
The CARDIA Study is a U.S. multi-center, longitudinal observational study examining the determinants of coronary heart disease risk factors in young black and white men and women. In 1985–1986 (baseline), 5,115 participants (2,787 women) aged 18–30 years (52% black) were recruited from four U.S. geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. Retention at exams in follow-up years 15, 20, 25 was 74%, 72% and 72% of the surviving cohort. Written informed consent was obtained at enrollment and each subsequent exam. Institutional review boards at each study site granted study approvals.
Anonymized data have been made publicly available at the [BioLINCC] and can be accessed at [https://biolincc.nhlbi.nih.gov/home/]. Access to biospecimen samples may be requested from the CARDIA Study with information available at [https://www.cardia.dopm.uab.edu/].
Sample Selection
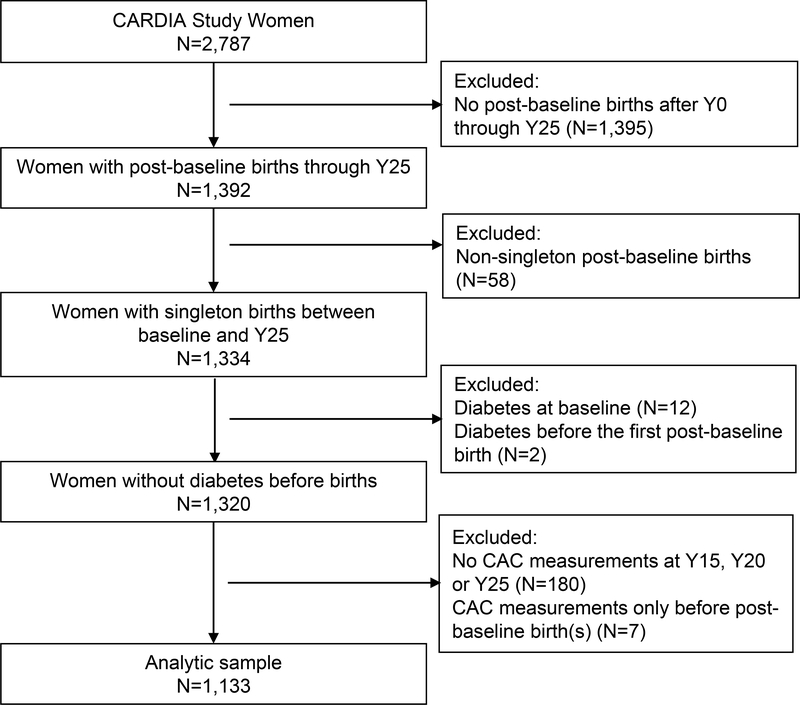
Among 2,787 women at baseline (1985–1986), we included 1,392 who had ≥1 post-baseline birth(s). We excluded women with (n=58) multi-fetal gestations, (n=12), overt diabetes at baseline, (n=2) diabetes before a first post-baseline birth, (n=180) with no CAC measurements, or (n=7) CAC measured only before post-baseline births. The analytic sample included 1,133 parous women who had 2,066 births after baseline, and metabolic risk factors measured before the first pregnancy (Figure 1). Parous women excluded were slightly younger at the first post-baseline birth, more likely to be Black race and to have educational level at high school or lower and had higher level of pre-pregnancy HOMA-IR than those included (Supplemental Table I).
Figure 1.
Selection Flow Chart: CARDIA Women who reported one or more post-baseline births and had Coronary Artery Calcification (CAC) measurements at exams in years 15, 20 and/or 25 since baseline (Y0); (1985–1986 through 2010–2011).
Blood Pressure, Anthropometry, and Biochemical Measurements
Trained and certified staff assessed medical and clinical attributes, sociodemographic factors and lifestyle behaviors at in-person exams using standardized methodologies, anthropometry using calibrated equipment, and interviewer and self-administered questionnaires. Research protocol for blood pressure (BP) measurements, venipuncture, laboratory quality control, and biochemical assays are detailed elsewhere.29,28 Hypertension was defined as systolic BP (SBP) ≥140 and/or diastolic BP (DBP) ≥90 mmHg, and/or self-report of antihypertensive medication using criteria during the study period. Body mass index (BMI) was calculated as weight divided by height (kg/m2). Blood was drawn after an overnight fast to measure plasma total cholesterol and triglycerides, high-density lipoprotein cholesterol (HDL-C),30 and low-density lipoprotein cholesterol (LDL-C) calculated using the Friedewald equation.31, 32 Serum glucose and insulin were measured at fasting in years 0, 7, 10, 15, 20, 25, and 2 hours after a 75 g oral glucose load (2-h OGTT) in years 10, 20, 25. Glycosylated hemoglobin (HbA1C) was measured in years 20 and 25. The homeostasis model assessment index (HOMA-IR) was calculated as fasting glucose (mmol/l) multiplied by fasting insulin (mU/l) and divided by 22.5 to estimate insulin resistance.33 High sensitivity C-reactive protein (hs-CRP) was measured at exam years 15 and 20 using a nephelometry-based high throughput assay (BNII nephelometer) and at exam year 25 using a Roche latex-particle enhanced immunoturbidimetric assay kit.34, 35 The metabolic syndrome was diagnosed for having any three of five factors using the National Cholesterol Education Program/Adult Treatment Panel III (NCEP-ATP III) criteria: (1) waist circumference >88 cm; (2) triglycerides ≥150 mg/dL; (3) HDL-C <50 mg/dL; (4) SBP ≥130 and/or DBP ≥85 mmHg, and/or self-reported use of antihypertensive medication; and (5) fasting glucose ≥100 mg/dL and/or self-reported use of diabetes medication.36
Measurement of Coronary Artery Calcium by Computed Tomography
Non-contrast cardiac computed tomography (CT) was performed using standard protocol at years 15 (2000–01), 20 (2005–06), and 25 (2010–11) following the baseline exam. At years 15 and 20, CAC scores were averaged from two sequential scans. At year 25 a single scan was used based on the reproducibility of the CAC score observed for prior exams and to reduce radiation exposure. Technical details of the CT systems, protocol, and observed radiation exposure are previously reported.27 CAC was estimated using the FDA approved calcium scoring software (Aquarius Workstation, TeraRecon, Foster City, CA) and reported as the Agatston score, corrected for slice thickness with a minimum lesion size of 4 adjacent pixels (minimum calcification area of 1.87 mm2) and attenuation threshold of ≥130 Hounsfield units.28 The year 15 CAC scores were reanalyzed to ensure longitudinal standardization. A physician adjudicated the CAC scores under the following conditions: discordant for CAC presence within paired scans at years 15 and 20, a score change of >200, a change in CAC status from positive to negative, or a potential surgical intervention identified by the analyst. CAC was categorized as Any CAC (Score >0; range 0.93 to 4428) or No CAC (Score=0). The first exam (years 15, 20, or 25) with CAC>0, or the last exam with CAC=0 after the last birth was defined as the end-of-follow-up for each participant.
Glucose Tolerance Categories (Normoglycemia, Prediabetes and Diabetes)
Biochemical testing of glucose tolerance was performed at baseline and at subsequent exam years (7, 10, 15, 20, and 25). We classified women into glucose tolerance categories (normoglycemia, prediabetes, and incident diabetes) based on fasting glucose at exam years 0, 7, 10, 15, 20, and 25; 2-hour 75-gram post-load glucose in years 10, 20, and 25; and/or glycosylated hemoglobin (HbA1C) in years 20 and 25 using the American Diabetes Association diagnostic criteria.37 Diabetes was classified by fasting glucose ≥126 mg/dL, 2-hour glucose ≥ 200 mg/dL, or HbA1C ≥ 6.5%, and/or self-report of diabetes and diabetes medication use. Prediabetes was classified by fasting glucose 100 to 125 mg/dL, 2-hour glucose 140 to 199 mg/dL, or HbA1C 5.7% to 6.4%.37 Diabetes could also be classified based on self-report of medications prescribed to treat diabetes.
Pregnancies and Gestational Diabetes Status:
At each exam, women reported current pregnancy status, number of pregnancies, and births (≥20 weeks’ gestation), dates of deliveries, and perinatal outcomes [e.g., gestational diabetes (GD) hypertensive disorders of pregnancy (HDP)]. We calculated the age at the first birth after baseline (post-baseline) and at the last post-baseline birth for each woman based on the delivery dates. GD status based on report of “diabetes only during pregnancy”, and no diabetes prior to pregnancy based on biochemical testing, or self-report of diabetes as described above.6 We validated self-report of GD using the 3-hr 100 g OGTT results abstracted from prenatal medical records among 165 CARDIA women who delivered 200 births. GD classification had a sensitivity of 100% (20/20), and specificity of 92% (134/145).6 Women delivered pregnancies between consecutive exams. Parity (total number of births) and GD status were updated at each exam, but women remained in the GD category once classified.
Women were classified into time-varying GD status and glucose tolerance categories from the first post-baseline birth through the end of follow up as: 1) No GD/normoglycemia (referent group), 2) No GD/prediabetes, 3) No GD/incident diabetes, 4) GD/normoglycemia, 5) GD/prediabetes, or 6) GD/incident diabetes. Women transitioned across the six groups as their GD and glucose tolerance status was updated at exam years 15, 20 and/or 25 through the end of follow up that corresponded to the same exam years for available CAC measurements. Once a woman transitioned into a higher glucose intolerance and/or the GD group, the classification was maintained through the end of follow up (i.e., normoglycemia to incident prediabetes or incident diabetes, or prediabetes to incident diabetes).
Covariates:
Structured questionnaires at up to 8 exams assessed socio-demographics, reproductive history, medical history, medication use (e.g., anti-hypertensive, diabetes, and lipid-lowering medications, oral contraceptives, and other hormones) and lifestyle behaviors (tobacco use, alcohol consumption, dietary intake and physical activity). Menopausal status and menopausal hormone therapy were assessed at years 15, 20 and 25. The Diet History questionnaire assessed dietary intake at baseline (year 0) and years 7 and 20.38 We calculated the a priori diet quality score (the average of years 0, 7 and 20), as an index of plant-based food patterns detailed elsewhere.39 The CARDIA Physical Activity History questionnaire estimated a physical activity score (race-specific quartiles), which is correlated with the symptom-limited graded treadmill exercise test duration.40 Family history of diabetes and heart disease was classified by report of diseases for primary relatives.
Statistical Analysis
Pre-pregnancy and the end of follow up characteristics were assessed among glucose tolerance groups and by CAC status using chi-square statistics, analysis of variance methods, and the Kruskal-Wallis test of the equality of medians for variables with skewed distributions. CAC status for each woman was classified for up to three exams at years 15, 20 and/or 25. The association between the proportion of women with Any CAC (score>0) at the end of follow up was evaluated among worsening glucose tolerance within the GD and no GD groups using the Cochran–Armitage test for trend (p-value trend).
We estimated the hazard ratio (HR) and 95% confidence interval (95%CI) of CAC associated with GD status, and by GD status stratified by Incident Diabetes (yes or no), and then by GD status and the subsequent glucose tolerance categories (normoglycemia, prediabetes, or incident diabetes). We used the complementary log-log model,41, 42 an analogue to the Cox proportional hazards model to handle interval-censored time to event (CAC>0) and time-dependent covariates. Examination years (time) and an indicator variable for the length of each interval were included in all models to account for the unequal intervals between the consecutive exams. Participants entered the analysis at the first post-baseline birth and exited at the first presence of CAC>0, or the last follow up exam in years 15, 20 or 25 (censoring). We adjusted for age at first post-baseline birth to account for differences in time at entry into the analysis.
Potential confounders were evaluated based on a priori hypotheses and statistical significance (p-value <0.05) for the association with main effects and/or risk of CAC. These included race, lifestyle behaviors, pre-pregnancy (closest exam preceding the first post-baseline birth) systolic BP, BMI, and blood lipids, HDP, and other CVD risk factors. We also evaluated the average a priori diet quality score, and time-varying covariates during follow up (i.e., cigarette smoking habit (pack-years), hypertension, parity, lipid-lowering medication, hormone use, and change in BMI). All p-values are for two-sided tests with statistical significance <0.05. All analyses used Statistical Analysis Software (SAS) for Windows 9.4 (SAS Institute Inc, Cary, NC, USA).
Multivariate models evaluated the covariates’ impact on HRs of CAC in a stepwise manner. Model 1 included race, age at first birth, and pre-pregnancy Systolic BP. Model 2 (fully adjusted) included Model 1 covariates plus pre-pregnancy BMI, and time-varying smoking in pack-years. Models 3 and 4 sequentially added time-varying hypertension status and BMI change, respectively, as intervening risk factors during follow up. Addition of CVD risk factors (i.e., pre-pregnancy LDL-C, HDL-C, HOMA-IR, physical activity score, a priori diet quality score, HDP, parity, lipid-lowering medication, hormonal contraception, menopausal hormone therapy, and change in BMI) had minimal impact on HRs (data not shown). Sensitivity analyses excluded women with history of HDP (analysis of n=891 women with no reported HDP), and women parous at baseline (analysis of n=782 nulliparas).
We also estimated the probability of a woman being free of CAC as a survival curve for each of the six GD status and glucose tolerance groups based on results from the unadjusted model. For calculation of the survival curves, the average length of time interval between the first post-baseline birth and the year 15 exam is 9.4 years, and each of the later intervals are 5.0 years.
RESULTS
The sample of 1,133 parous women (49% Black, 51% White) had 2,066 births after baseline and up through year 25. Of these, 92% of births occurred before year 15, 6.6% between years 15 and 20, and 1.4% between years 20 and 25. There were 139 women (12.3%) who reported GD during pregnancy (6.7 per 100 pregnancies). The mean (SD) age at first post-baseline birth was 30.1 (4.9) years, and at end of follow up was 47.6 (4.8) years (range 33–56). The mean (SD) time since the last birth to end of follow up was 14.7 (5.9) years.
Women with previous GD were more likely to develop prediabetes or incident diabetes than maintain normoglycemia after pregnancy (36%, 25.9%, or 38.1%) compared to women with No GD (35%, 9%, or 56%); overall p-value<0.001. Of the 125 incident diabetes cases, women with previous GD had earlier onset compared to the no GD group; 16.7% and 10.1% had onset before year 15 (p-value=0.36), and 69.5% and 47.2% had onset between years 15 and 20 (p-value=0.009), respectively.
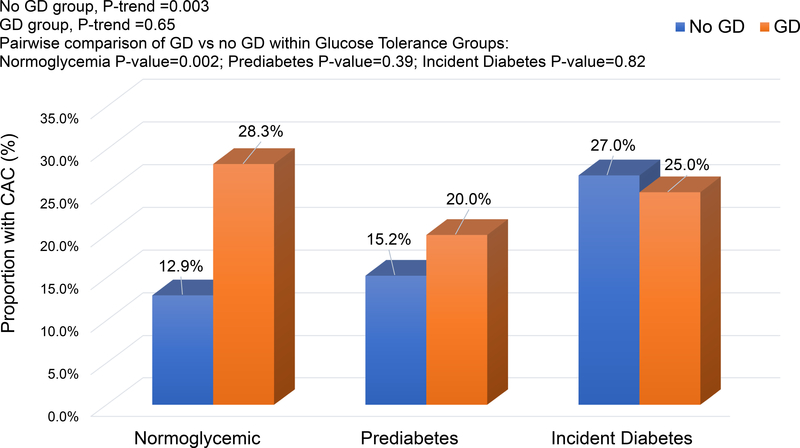
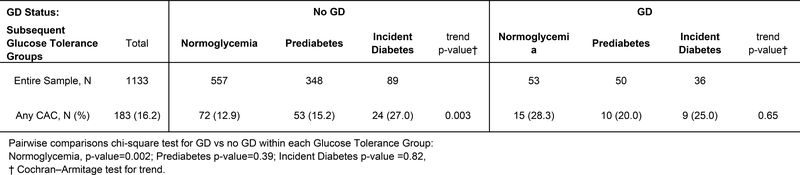
Overall, CAC (score >0) was present in 16.2% (183/1133) of women. About 24.5% (34/139) with previous GD had CAC compared to 15.0% (149/994) with No GD (p-value=0.005). The proportion with CAC (Figure 2) did not vary by glucose tolerance categories among women with GD (p-value=0.65), but increased with worsening glucose intolerance among women with No GD (p-value=0.003). Among women who were normoglycemic at end of follow up, 12.9% (72/557) and 28.3% (15/53), respectively, were classified with CAC among no GD and GD groups; p-value=0.002. Within the prediabetes and incident diabetes groups, there were no significant differences in CAC prevalence by GD status.
Figure 2.
No.(%) of Women with Any CAC At the End of Follow Up (Years 15, 20 or 25) by GD Status and Subsequent Glucose Tolerance Groups.
Higher levels of CAC scores were associated with worsening glucose tolerance among women with no GD (p-value=0.003), but women with GD showed more similar proportions across the glucose tolerance groups (Table 1). The overall CAC levels increased over time, with higher proportions having scores above 10 at exam year 25 than years 15 and 20. The distribution of overall CAC scores of >0 to 10, >10 to 50, >50 to 99, and ≥100 were 8.3, 4.4, 2.0, and 1.4 percent of women, respectively, as expected for their young age (Supplemental Table II).
Table 1.
Distribution of CAC Scores at End of Follow Up Among Women by GD Status and Subsequent Glucose Tolerance Groups After Pregnancy.
| No GD (N=994) | GD (N=139) | |||||||
|---|---|---|---|---|---|---|---|---|
| Categories of CAC score | Glucose Tolerance After Pregnancy | Glucose Tolerance After Pregnancy | ||||||
| N (%) | Normoglycemia | Prediabetes | Incident Diabetes | p-value* | Normoglycemia | Prediabetes | Incident Diabetes | p-value* |
| (N=557) | (N=348) | (N=89) | (N=53) | (N=50) | (N=36) | |||
| End of follow up | 0.003 | 0.051 | ||||||
| CAC = 0 | 485 (87.1) | 295 (84.8) | 65 (73.0) | 38 (71.7) | 40 (80.0) | 27 (75.0) | ||
| CAC >0 to 10 | 42 (7.5) | 25 (7.2) | 9 (10.1) | 10 (18.9) | 3 (6.0) | 5 (13.9) | ||
| CAC >10 to 50 | 18 (3.2) | 16 (4.6) | 6 (6.7) | 1 (1.9) | 7 (14.0) | 2 (5.6) | ||
| CAC >50 | 12 (2.2) | 12 (3.5) | 9 (10.1) | 4 (7.6) | 0 | 2 (5.6) | ||
Based on the chi-square test.
Glucose intolerance at follow up was associated with higher pre-pregnancy BMI, waist circumference, fasting glucose, insulin and HOMA-IR, and lower HDL-C, as well as characteristics at follow up, including higher BMI, waist circumference, fasting triglycerides, glucose, and insulin, hs-CRP, and HOMA-IR, higher percentages of women with obesity, the metabolic syndrome, hypertension, and lipid-lowering medication use, as well as longer time since last birth to end of follow up, regardless of GD status; all p-values<0.01 (Tables 2 and 3). Among women with No GD, worse glucose tolerance after pregnancy was associated with lower HDL-C and physical activity, higher pre-pregnancy BP, fasting triglycerides, and waist girth, higher percentages with post-menopausal status, family history of diabetes and heart disease, and higher weight change and HOMA-IR at follow up, as well as lower average a priori dietary quality score (all p-values <0.01). Among women with GD, HOMA-IR increased among all glucose tolerance groups (p-value=0.07), including those with normoglycemia, despite no differences in weight change (p-value=0.68). Among women with normoglycemia at follow up (Table 3), median hs-CRP was higher for women with previous GD compared to women with no GD (p-value=0.05). There was no significant difference in mean HOMA-IR change between these groups.
Table 2.
Pre-pregnancy Characteristics (1985–2006) by GD Status and Subsequent Glucose Tolerance Groups After Pregnancy (2000–2011).
| Pre-pregnancy Characteristics | No GD (N=994) | GD (N=139) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glucose Tolerance After Pregnancy | Glucose Tolerance After Pregnancy | |||||||
| Mean (SD) † or N (%) | Normoglycemia | Prediabetes | Incident Diabetes | p-value | Normoglycemia | Prediabetes | Incident Diabetes | p-value |
| (N=557) | (N=348) | (N=89) | (N=53) | (N=50) | (N=36) | |||
| Age at first post-baseline birth,* y | 30.2 (4.7) | 30.1 (5.2) | 29.2 (4.6) | 0.25 | 30.1 (4.9) | 29.7 (5.2) | 30.7 (4.4) | 0.68 |
| Black, n (%) | 218 (39) | 204 (59) | 66 (74) | <.001 | 23 (43) | 18 (36) | 22 (61) | 0.07 |
| Nulliparous, n (%) | 405 (73) | 235 (68) | 49 (55) | 0.002 | 36 (68) | 33 (66) | 24 (67) | 0.98 |
| Education, n (%) | 0.001 | 0.43 | ||||||
| High school or less | 136 (24) | 94 (27) | 30 (34) | 14 (26) | 11 (22) | 4 (11) | ||
| College education | 280 (50) | 202 (58) | 46 (52) | 31 (58) | 28 (56) | 23 (64) | ||
| Graduate/professional degree | 141 (25) | 52 (15) | 13 (15) | 8 (15) | 11 (22) | 9 (25) | ||
| Weight status, n (%) | <.001 | <.001 | ||||||
| Normal (BMI <25) | 408 (73) | 207 (59) | 30 (34) | 36 (68) | 27 (54) | 10 (28) | ||
| Overweight (BMI: 25–29.9) | 95 (17) | 75 (22) | 21 (24) | 14 (26) | 11 (22) | 5 (14) | ||
| Obese (BMI ≥30) | 54 (10) | 66 (19) | 38 (43) | 3 (6) | 12 (24) | 21 (58) | ||
| BMI, kg/m2 | 23.7 (4.7) | 25.1 (5.5) | 29.1 (7.1) | <.001 | 23.8 (4.5) | 26.0 (6.2) | 30.9 (7.7) | <.001 |
| Waist circumference, cm | 72.6 (9.6) | 76.2 (11.6) | 84.5 (15.1) | <.001 | 72.9 (9.3) | 77.5 (13.3) | 87.9 (16.1) | <.001 |
| Systolic BP, mm Hg | 103 (9) | 105 (10) | 109 (12) | <.001 | 104 (9) | 104 (10) | 105 (9) | 0.88 |
| Diastolic BP, mm Hg | 65 (9) | 66 (10) | 69 (12) | <.001 | 66 (7) | 65 (13) | 67 (9) | 0.67 |
| Total cholesterol, mg/dL | 179 (35) | 180 (36) | 182 (39) | 0.69 | 184 (36) | 183 (39) | 178 (25) | 0.69 |
| LDL-cholesterol, mg/dL | 105 (30) | 108 (33) | 111 (35) | 0.17 | 110 (29) | 109 (37) | 109 (24) | 0.98 |
| HDL-cholesterol, mg/dL | 60 (14) | 57 (13) | 55 (16) | 0.007 | 59 (12) | 59 (15) | 52 (13) | 0.01 |
| Fasting Triglycerides, mg/dL † | 57 (41, 78) | 60 (45, 84) | 68 (54, 97) | <.001 | 61 (46, 86) | 63 (50, 89) | 76 (57, 105) | 0.08 |
| Fasting glucose, mg/dL | 79 (7) | 82 (8) | 83 (10) | <.001 | 79 (8) | 82 (9) | 88 (13) | <.001 |
| Fasting insulin, μU/mL † | 8.6 (6.1, 12.0) | 10.0 (6.8, 14.0) | 14.5 (9.2, 19.0) | <.001 | 8.3 (6.6, 14.3) | 10.0 (6.3, 17.0) | 13.9 (9.4, 22.0) | 0.008 |
| HOMA-IR † | 1.6 (1.2, 2.3) | 2.0 (1.3, 2.8) | 3.1 (1.7, 4.1) | <.001 | 1.7 (1.3, 2.7) | 2.1 (1.4, 3.7) | 3.1 (1.8, 5.2) | <.001 |
| Prediabetes, n (%) | 0 | 12 (3) | 8 (9) | <.001 | 0 | 4 (8) | 5 (14) | 0.03 |
| Physical activity score † | 276 (133, 459) | 255 (139, 442) | 191 (84, 278) | <.001 | 236 (146, 406) | 269 (148, 378) | 329 (148, 558) | 0.51 |
| Smoking, lifetime pack-years | 1.9 (4.1) | 2.4 (4.9) | 2.4 (4.1) | 0.20 | 2.2 (3.8) | 2.8 (5.8) | 2.0 (4.3) | 0.68 |
| A priori diet quality score*, Year 0 | 65 (14) | 64 (13) | 60 (12) | 0.001 | 65 (13) | 66 (12) | 65 (14) | 0.95 |
Abbreviations: BMI, body mass index; BP, blood pressure; GD, gestational diabetes; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; SD, standard deviation.
Pre-pregnancy measurements from the closest exam before the first post-baseline birth,
a priori diet quality score was obtained from the CARDIA baseline exam (Year 0; 1985–86).
Age at first post-baseline birth is calculated from the date of the delivery of first pregnancy >20 weeks gestation after CARDIA baseline (>1985 to 2009).
Median (25th, 75th percentiles)
Table 3.
Characteristics at the End of Follow Up by GD Status and Subsequent Glucose Tolerance Groups After Pregnancy (2000–2011).
| End of Follow Up Characteristics* | No GD (N=994) | GD (N=139) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glucose Tolerance After Pregnancy | Glucose Tolerance After Pregnancy | |||||||
| Mean (SD) † or N (%) | Normoglycemia | Prediabetes | Incident Diabetes | p-value | Normoglycemia | Prediabetes | Incident Diabetes | p-value |
| (N=557) | (N=348) | (N=89) | (N=53) | (N=50) | (N=36) | |||
| Age, years | 47.0 (4.9) | 48.4 (4.3) | 48.7 (4.1) | <.001 | 46.5 (6.0) | 47.7 (5.1) | 48.2 (4.9) | 0.31 |
| Weight status, n (%) | <.001 | <.001 | ||||||
| Normal (BMI <25) | 226 (41) | 82 (24) | 6 (7) | 18 (34) | 13 (26) | 4 (11) | ||
| Overweight (BMI: 25–29.9) | 161 (29) | 83 (24) | 17 (19) | 22 (42) | 11 (22) | 4 (11) | ||
| Obese (BMI ≥30) | 170 (31) | 183 (53) | 66 (74) | 13 (25) | 26 (52) | 28 (78) | ||
| BMI, kg/m2 | 27.6 (6.3) | 31.4 (7.6) | 36.1 (8.4) | <.001 | 28.0 (5.9) | 31.1 (7.4) | 35.0 (7.8) | <.001 |
| Waist circumference, cm | 83.6 (13.0) | 92.7 (15.3) | 103.7 (17.8) | <.001 | 85.2 (12.5) | 91.4 (15.5) | 102.2 (17.6) | <.001 |
| Systolic BP, mm Hg | 113 (16) | 118 (16) | 123 (21) | <.001 | 121 (19) | 115 (18) | 116 (14) | 0.22 |
| Diastolic BP, mm Hg | 72 (12) | 75 (11) | 78 (11) | <.001 | 76 (13) | 73 (12) | 74 (9) | 0.46 |
| Total cholesterol, mg/dL | 192 (32) | 191 (36) | 190 (42) | 0.95 | 191 (34) | 195 (41) | 183 (35) | 0.35 |
| LDL-cholesterol, mg/dL | 109 (28) | 112 (31) | 110 (37) | 0.36 | 113 (27) | 111 (38) | 101 (30) | 0.21 |
| HDL-cholesterol, mg/dL | 65 (18) | 59 (16) | 53 (16) | <.001 | 61 (17) | 61 (21) | 58 (25) | 0.74 |
| Fasting Triglycerides, mg/dL † | 74 (56, 100) | 89 (67, 123) | 118 (84, 163) | <.001 | 72 (53, 102) | 91 (73, 138) | 98 (74, 133) | 0.01 |
| Fasting glucose, mg/dL | 86 (7) | 95 (9) | 130 (55) | <.001 | 86 (7) | 97 (10) | 122 (39) | <.001 |
| Fasting insulin, μU/mL † | 8.0 (5.0, 11.0) | 11.3 (7.0, 16.9) | 15.7 (9.3, 23.8) | <.001 | 9.8 (5.8, 15.0) | 10.9 (7.2, 15.7) | 14.0 (8.2, 26.7) | 0.05 |
| HOMA-IR † | 1.7 (1.0, 2.4) | 2.7 (1.6, 4.0) | 4.8 (2.6, 8.6) | <.001 | 1.8 (1.2, 3.1) | 2.5 (1.8, 4.0) | 4.0 (2.9, 7.0) | <.001 |
| Metabolic syndrome, n (%) | 59 (11) | 138 (40) | 69 (78) | <.001 | 5 (9) | 19 (38) | 28 (78) | <.001 |
| hs-CRP, μg/mL † | 1.0 (0.4, 2.8) | 2.2 (0.7, 5.1) | 4.8 (1.6, 8.7) | <.001 | 1.6 (0.6, 4.3) | 1.9 (0.7, 5.8) | 4.0 (1.1, 8.8) | 0.03 |
| hs-CRP (>3 μg/mL), n (%) | 123 (23) | 151 (44) | 53 (60) | <.001 | 15 (28) | 17 (34) | 20 (56) | 0.03 |
| Hypertension, n (%) | 99 (18) | 120 (34) | 61 (69) | <.001 | 13 (25) | 14 (28) | 20 (56) | 0.006 |
| Lipid-lowering medication, n (%) | 29 (5) | 29 (8) | 30 (34) | <.001 | 4 (8) | 5 (10) | 14 (39) | <.001 |
| Physical activity score † | 244 (111, 450) | 214 (95, 383) | 147 (62, 294) | <.001 | 256 (144, 504) | 210 (80, 344) | 231 (61, 406) | 0.25 |
| Smoking, lifetime pack-years | 3.2 (6.7) | 4.3 (9.1) | 4.6 (7.6) | 0.07 | 3.8 (6.7) | 3.9 (6.9) | 3.3 (6.8) | 0.93 |
| Average a priori diet quality score (Years 0, 7 and 20) | 69 (12) | 66 (11) | 64 (9) | <.001 | 67 (11) | 69 (11) | 68 (11) | 0.77 |
| Oral contraceptive use, n (%) | 521 (94) | 335 (96) | 86 (97) | 0.14 | 50 (94) | 48 (96) | 33 (92) | 0.70 |
| Post-menopausal status, n (%) | 157 (28) | 139 (40) | 41 (46) | <.001 | 17 (32) | 18 (36) | 18 (50) | 0.22 |
| Menopausal hormone therapy, n (%) | 32 (6) | 22 (6) | 6 (7) | 0.90 | 4 (8) | 5 (10) | 4 (11) | 0.84 |
| Family history of diabetes, n (%) | 186 (34) | 180 (52) | 66 (74) | <.001 | 27 (51) | 27 (54) | 19 (53) | 0.95 |
| Family history of heart disease, n (%) | 273 (49) | 209 (60) | 57 (64) | 0.001 | 26 (49) | 30 (60) | 19 (53) | 0.53 |
| Weight change, kg | 10.6 (11.9) | 17.1 (14.1) | 19.4 (17.7) | <.001 | 11.4 (11.1) | 13.5 (11.2) | 11.5 (17.9) | 0.68 |
| HOMA-IR change | 0.0 (1.4) | 0.9 (2.7) | 2.8 (4.3) | <.001 | 0.3 (1.7) | 0.5 (2.4) | 2.7 (8.7) | 0.07 |
| Time from first birth to CAC, y | 10.7 (4.9) | 11.2 (5.0) | 12.2 (5.0) | 0.02 | 10.9 (4.5) | 11.9 (5.8) | 10.7 (4.0) | 0.47 |
| Time from last birth to EFU, y | 13.8 (5.9) | 15.7 (5.8) | 17.5 (4.5) | <.001 | 12.7 (6.0) | 14.3 (5.6) | 15.8 (5.5) | 0.04 |
Abbreviations: BMI, body mass index; BP, blood pressure; GD, gestational diabetes; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein; SD, standard deviation.
“End of follow up” (EFU) is defined as the first exam with CAC >0, or the last exam with CAC=0 measurement at exam years 15, 20 or 25.
Change in weight and HOMA-IR from the nearest exam preceding the first birth to the end of follow up.
Based on measurements from the last exam, or the first exam when the women had CAC>0.
Median (25th, 75th percentiles)
Note: n=15 women were missing hs-CRP in exam years 10, 15 and 20.
Characteristics associated with CAC included older age, smoking, the metabolic syndrome, and hypertension, as well as higher pre-pregnancy BMI, waist circumference, SBP, DBP, fasting glucose, insulin, HOMA-IR, triglycerides, total cholesterol and LDL-C (Supplemental Table III). During follow up, CAC was associated with incident diabetes, the metabolic syndrome, hypertension, pregnancy complications (GD and hypertensive disorders), and lipid-lowering medication use. Any CAC was also associated with higher BMI, fasting insulin, HOMA-IR, hs-CRP, LDL-C, and triglycerides, and lower HDL-C at follow up.
In multivariate models, GD status was associated with higher risk of CAC; HR (95%CI) of 1.85 (1.28, 2.69) adjusted for age, race and pre-pregnancy SBP (Model 1) (Table 4). Addition of pre-pregnancy BMI, and time-varying smoking in pack-years (Model 2) slightly attenuated the HR to 1.73 (1.18, 2.52), but hypertension during follow-up (Model 3) had minimal impact. In models stratified by diabetes status after pregnancy, GD was not associated with CAC in women with incident diabetes, but GD was associated with a twofold higher risk of CAC in women with No diabetes; adjusted HR (95%CI) of 2.02 (1.31, 3.11) from Model 1 that was attenuated to 1.95 (1.27, 3.01) with the addition of other covariates; pre-pregnancy BMI, time-varying smoking (Model 2), and time-varying hypertension during follow up (Model 3).
Table 4.
Unadjusted and Adjusted Hazard Ratios (HR) and (95%CI) of CAC Associated with GD Status and Subsequent Glucose Tolerance Status (normoglycemia, prediabetes or incident diabetes) in Women During Mid-life (1986–2011).
| GD and Glucose Tolerance Groups | % Any CAC | Model 1 HR | Model 2 HR | Model 3 HR | Model 4 HR | ||||
|---|---|---|---|---|---|---|---|---|---|
| (n/N)† | (95%CI) | (95%CI) | (95%CI) | (95%CI) | |||||
| GD Status | |||||||||
| No GD | 15.0 (149/994) | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| GD | 24.5 (34/139) | 1.85 | (1.28, 2.69) | 1.73 | (1.18, 2.52) | 1.66 | (1.13, 2.43) | 1.66 | (1.13, 2.42) |
| GD Status Stratified by Incident Diabetes | |||||||||
| Incident diabetes | |||||||||
| No GD | 27.0 (24/89) | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| GD | 25.0 (9/36) | 1.10 | (0.50, 2.42) | 0.97 | (0.43, 2.19) | 1.03 | (0.45, 2.33) | 1.06 | (0.46, 2.44) |
| No diabetes (prediabetes & normoglycemia) | |||||||||
| No GD | 13.8 (125/905) | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| GD | 24.3 (25/103) | 2.02 | (1.31, 3.11) | 2.01 | (1.30, 3.09) | 1.95 | (1.27, 3.01) | 1.96 | (1.27, 3.02) |
| GD and Subsequent Glucose Tolerance | |||||||||
| No GD/Normoglycemia | 12.9 (72/557) | 1 | Referent | 1 | Referent | 1 | Referent | 1 | Referent |
| No GD/Prediabetes | 15.2 (53/348) | 1.69 | (1.17, 2.46) | 1.54 | (1.06, 2.24) | 1.50 | (1.03, 2.17) | 1.52 | (1.04, 2.22) |
| No GD/Incident diabetes | 27.0 (24/89) | 2.68 | (1.62, 4.44) | 2.17 | (1.30, 3.62) | 1.79 | (1.06, 3.01) | 1.82 | (1.08, 3.09) |
| GD/Normoglycemia | 28.3 (15/53) | 2.30 | (1.32, 4.02) | 2.34 | (1.34, 4.09) | 2.24 | (1.28, 3.92) | 2.25 | (1.29, 3.94) |
| GD/Prediabetes | 20.0 (10/50) | 2.46 | (1.26, 4.78) | 2.13 | (1.09, 4.17) | 2.08 | (1.06, 4.07) | 2.11 | (1.07, 4.14) |
| GD/Incident diabetes | 25.0 (9/36) | 2.65 | (1.31, 5.37) | 2.02 | (0.98, 4.19) | 1.76 | (0.85, 3.67) | 1.76 | (0.84, 3.66) |
Model 1: adjusted for race, age at the first birth and pre-pregnancy systolic BP.
Model 2: Model 1 + adjusted for pre-pregnancy BMI and time-varying lifetime smoking exposure (pack-years). (Fully adjusted model)
Model 3: Model 2 + adjusted for time-varying hypertension during follow up (intervening variable).
Model 4: Model 3 + adjusted for time-varying BMI change during follow up (intervening variable).
n = number of women with Any CAC, and N = number of women within the group strata.
At the end of follow up
For GD status and subsequent glucose tolerance groups (Table 4), HRs (95%CIs) of CAC adjusted for race, age at first birth and pre-pregnancy SBP (Model 1) were 1.69 (1.17, 2.46) and 2.68 (1.62, 4.44) for prediabetes, and incident diabetes among women with No GD, respectively, and were 2.30 (1.32, 4.02), 2.46 (1.26, 4.78), and 2.65 (1.31, 5.37) among women with GD and normoglycemia, prediabetes, and incident diabetes, respectively, compared to women with No GD and normoglycemia. In Model 2 (fully adjusted), addition of pre-pregnancy BMI and time-varying smoking in pack-years covariates slightly attenuated the HRs that remained statistically significant, except for the GD/incident diabetes group [2.02 (0.98, 4.19)]. In Models 3 and 4, addition of time-varying hypertension during follow up and time-varying BMI change (intervening risk factors), respectively, resulted in modest attenuation of the HRs that remained statistically significant with the exception of the GD/Incident diabetes group. Inclusion of other lifestyle behaviors, lipid-lowering medication use, time-varying hs-CRP, or change in HOMA-IR had minimal impact on model estimates.
The sensitivity analyses using stage 1 cut-points (SBP ≥130 and/or DBP ≥80 mm Hg) to define hypertension, or defining Any CAC as scores >10 yielded similar results. Other sensitivity analyses limiting the sample to nulliparas at baseline, or women with no previous HDP showed consistent or stronger associations for GD and glucose tolerance groups with risk of CAC (Supplemental Tables IV and V). We show curves for the probability of being free of CAC among GD status and glucose tolerance groups at follow up. The probability is lower for women with GD subgroups, including normoglycemic group, and for incident diabetes among women no GD (Figure 3).
Figure 3.
Probability of Being CAC Free by GD Status and Subsequent Glucose Tolerance Groups During Follow Up.
Note: Estimates are based on the models unadjusted for covariates. On average, the length of the time interval between the first post-baseline birth and Year 15 is 9.4 years, and the length of each of the following two intervals is 5.0 years. Births after baseline occurred between 1986 and 2010.
DISCUSSION
Importantly, the findings show that even sustained normoglycemia after pregnancy was associated with increased risk of CAC among women with a history of GD. Compared to women without GD and with normoglycemia, the risk of CAC was about two times higher for women with a history of GD across all levels of glucose tolerance, independent of sociodemographic, clinical and lifestyle behavioral risk factors. The risk associations were not confounded by use of lipid-lowering medications in our study. This indicates that GD history may adversely affect CVD risk apart from glucose tolerance. To our knowledge, ours is the first study to differentiate normoglycemia from prediabetes as well as overt diabetes in estimating the association of GD history and subsequent glucose tolerance with the risk of subclinical coronary artery disease. In contrast, others found much weaker relative risks of 1.30 to 1.56 for future CVD outcomes among women with GD and no T2D.12, 15, 16 Previous estimates may be biased toward the null from a higher prevalence of prediabetes and/or undiagnosed overt diabetes in the referent group of women without prior GD due to a lack of routine testing in clinical population settings (i.e., detection bias).15, 16 As expected, the risk of CAC increased with worsening glucose tolerance (prediabetes, or incident diabetes) compared to normoglycemia among women with no previous GD.
In CARDIA, a history of GD was associated with larger carotid artery intima media thickness (cIMT), representing a 6-year increase in vascular aging, among women without T2D or the metabolic syndrome.32 Other studies found a 26% higher risk of hypertension associated with GD history independent of pregnancy hypertensive disorders, and T2D.43 Thus, a history of GD may entail underlying vascular changes or other mechanisms that adversely affect cardiovascular health independent of hyperglycemia.44 The pathways could include common metabolic derangements that women with GD experience including heightened insulin resistance, delayed insulin secretion, endothelial dysfunction, systemic inflammation, dyslipidemia, and/or other vascular changes such as hypertension.43, 45, 46 In a longitudinal study of women with previous GD, women with normal glucose tolerance and no obesity also exhibited both insulin resistance and reduced insulin secretion.47 Insulin resistance is an independent predictor of cardiovascular disease and coronary artery atherosclerotic plaque progression in adults, regardless of dysglycemia.48 Evidence from Hannukainen et al. showed these same metabolic features in patients with ischemic coronary artery disease; i.e., enhanced glucose oxidation with lower insulin sensitivity, and a blunting of insulin secretion in response to a glucose load in the absence of hyperglycemia. The study findings were independent of traditional cardiometabolic risk factors (cholesterol, blood pressure, age, race, and BMI).49 Thus, impaired insulin secretion and insulin resistance, the hallmarks of GD dysmetabolism, may explain the increased coronary artery atherogenesis in the absence of glucose intolerance. Further, in our study, traditional risk factors (i.e., total and LDL-C, and smoking) for atherosclerosis did not vary by glucose tolerance status among the GD groups. Of note, average weight gain was inversely correlated with glucose tolerance in women with no GD, but not in women with previous GD. Thus, a history of GD may confer additional underlying risk for ASCVD through obesity-related cardiometabolic pathways without apparent clinical manifestations. Our findings add to evidence that women with previous GD may need additional screening beyond the testing of glycemia, and that a history of GD may need incorporation into women’s CVD risk calculations.50
Strengths of this study are the systematic biochemical testing of glucose tolerance across the childbearing years to distinguish all levels of glucose tolerance including normoglycemia from prediabetes and incident diabetes, and measurements of CVD risk factors (BMI, blood lipid profiles, and blood pressure) up to 6 times during 25 years with high retention (72%).6 All previous studies relied on self-report or administrative data to dichotomize glucose tolerance as T2D versus no T2D, which combines prediabetes and normoglycemia into a single referent group. Thus, previous estimates may underestimate excess CVD risks in young to middle aged women,14 especially for Black women who are seldom tested, and for women with GD, in whom uptake of post-delivery testing is low.25, 51 Most importantly, our study estimated relative risks of CAC compared to a normoglycemic group without GD. Also, CARDIA women delivered pregnancies from 1986–2011 during a period of universal screening for GD. The 6.7 GD pregnancies per 100 deliveries in CARDIA is comparable to overall rates in the U.S.2, 52
There were also some limitations, including no CAC measurements before pregnancy to establish whether higher CAC preceded the onset of GD or overt diabetes before year 15. This concern is mitigated by the extremely low prevalence of CAC prior to age 35 years in women. We used a surrogate measure, CAC score, since the young age at follow-up in our parous sample limited our ability to evaluate clinical CHD or CVD events (n=26). However, CAC measured in younger to middle aged adults is a strong risk factor for subsequent CVD outcomes. Using CAC measurements at ages 32 to 56 years, CARDIA previously found a 3-fold increase in CVD events (n=108) and 5-fold increase in CHD events (n=56), both fatal and nonfatal, with 12.5 years of follow-up in the sample of 3043 men and women.28 CAC scores of 1–19, 20–99, and ≥100 were associated with increased risk of premature CHD (HRs =2.6, 5.8, and 9.8), and CAC scores ≥100 were associated with early death (HR=22.4).28 Thus, even low CAC scores were associated with later CHD and CVD events, and CAC increased exponentially during 10 years men and women through mid-life.
Heart disease is the leading cause of death for women worldwide. In 2018, the American Heart Association Guidelines specified history of GD as an important ASCVD risk-enhancing factor for women.8, 53 Yet, the evidence basis to screen younger women for CVD risk has been starkly lacking. For example, few CVD risk prediction models have evaluated pregnancy complications because of limited data sources, particularly for young women.54 A study of northern European women found a borderline significant 26% higher Framingham CVD risk score with a history of GD, but GD prevalence in the sample was extremely low (0.5%) compared to contemporary cohorts (8%).55 Another study evaluated reproductive risk factors, including breastfeeding, age at first birth, and stillbirths, and found slight improvements in CVD risk prediction for women,56 but they did not evaluate any pregnancy complications (e.g., GD history, and preeclampsia). Other studies included hypertensive disorders, size at birth and preterm birth in CVD risk prediction models with none, or only minimal improvement in risk prediction, but they did not evaluate GD in any models.57–59
In summary, development of coronary calcified plaque as measured by CAC is present in some women in midlife. In our study, relative risk of such coronary plaque was about two times higher in women with previous GD for all subsequent glucose tolerance levels, including normoglycemia, compared to women without GD and normoglycemia. Thus, GD history may represent a constellation of risk factors (e.g., dyslipidemia, cumulative BP increases, mounting insulin resistance, endothelial dysfunction or inflammatory responses),60 that promote development of atherosclerotic plaque in the absence of hyperglycemia. Insulin resistance and possibly higher inflammation (hs-CRP) among women with prior gestational diabetes who remained normoglycemic at follow up in our study is consistent with this hypothesis. Gestational diabetes61 may be an especially vulnerable condition of dysmetabolism leading to initiation and/or propagation of coronary atherogenesis32 from early lesions to the advanced calcified coronary plaque in younger women.
Higher ASCVD risk among women with GD history has been primarily attributed to their younger age at T2D onset, and several fold higher risk of progression to T2D.10, 14 It is well-known that onset of T2D under age 40 increases (3.6 to 6.2-fold higher) cardiovascular-related mortality and outcomes in women.62 Our findings represent a shift in this paradigm by showing that normoglycemia after GD pregnancy was still related to higher CAC risk. The risk did not further increase with transition to prediabetes and T2D. By contrast, women with no prior GD who subsequently developed prediabetes, or overt diabetes had a 1.5-fold and 2.1-fold higher risk of CAC, respectively, compared to those with normoglycemia. The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD risk factor testing (i.e., blood pressure, dyslipidemia, hyperinsulinemia), and perhaps incorporation of GD into risk calculators to improve CVD risk stratification and prevention.63
Better characterization of the GD phenotypes is also needed to assess CVD risk, because GD diagnostic criteria differ between the U.S. and other countries.64 In CARDIA, 25.9% of women with GD progressed to diabetes on average 15 years later, which is similar to the 16% to 29% cumulative incidence after 10 to 20 years of follow up in contemporary meta-analyses,65, 66 and the U.S. population.2
Life course epidemiologic studies are challenging to undertake in population-based clinical settings because of extended time between pregnancy complications and onset of CVD events. A major limitation of this research in general is the lack of routine biochemical testing for diabetes or CVD risk factors among young women of childbearing age. The importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risk during the first year postpartum (i.e., lactation, and sleep) merit increased attention.67, 68 Furthermore, more accurate clinical prediction tools are needed for women that take into account a history of GD as well as other pregnancy complications. Finally, this study adds to the mounting evidence that enhanced CVD risk factor screening among women with a history of GD is needed to better risk stratify women for early ASCVD prevention.
Supplementary Material
Clinical Perspective.
What is new?
Among black and white women with no history of gestational diabetes, progression to impaired glucose tolerance, or overt diabetes within 15 years after pregnancy was associated with a graded increase in the relative risk (1.5 to 2.2-fold) of coronary artery calcification in mid-life compared to women who maintained normoglycemia.
Among black and white women with a history of gestational diabetes, the relative risk of coronary artery calcification in mid-life was twofold higher for those with normoglycemia, impaired glucose tolerance (prediabetes), or overt diabetes within 15 years after pregnancy compared to women with no history of gestational diabetes who maintained normoglycemia.
What are the clinical implications?
Sustained normoglycemia among women with previous gestational diabetes may not diminish future atherosclerotic cardiovascular disease risk in women during mid-life.
A history of gestational diabetes may entail underlying vascular changes, and/or adversely affect development of cardiovascular disease through pathways such as insulin resistance and impaired insulin secretion that promote atherogenic plaques independent of dysglycemia.
These findings add to the mounting evidence that enhanced cardiovascular disease risk factor screening among women with a history of gestational diabetes is needed to better risk stratify women for early atherosclerotic cardiovascular disease prevention.
Acknowledgments:
Dr. Gunderson is the guarantor of the manuscript and takes full responsibility for the work as a whole, including (if applicable) the study design, access to data, and the decision to submit and publish the manuscript. The authors thank the participants of the CARDIA study for their long-term commitment and important contributions to the study.
Funding Sources: The analyses were supported by grants from R01 DK106201 (Gunderson, PI), R01 DK090047 (Gunderson, PI) and K01 DK059944 (Gunderson, PI) from the National Institute of Diabetes, Digestive and Kidney Diseases. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I).
Study Sponsors: The study sponsor provided funding. The National Heart, Lung, and Blood Institute program official served on the study steering committee and publications subcommittee with one vote on each. The sponsor did not otherwise influence the analyses or decisions to publish this manuscript.
Disclosures:
Funding unrelated to the current research project from Janssen Pharmaceuticals, Inc., in 06/01017-12/31/2018 (Gunderson, EP)
Non-standard Abbreviations and Acronyms
- 2-h OGTT
Two-hour oral glucose tolerance test
- 95%CI
95% confidence interval
- ASCVD
Atherosclerotic cardiovascular disease
- BMI
Body mass index
- BP
Blood pressure
- CAC
Coronary artery calcification/calcium
- CAD
Coronary artery disease
- CARDIA
The Coronary Artery Risk Development in Young Adults
- CHD
Coronary heart disease
- cIMT
Carotid artery intima media thickness
- CT
Computed tomography
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- GD
Gestational diabetes
- HbA1C
Glycosylated hemoglobin
- HDL-C
High-density lipoprotein cholesterol
- HDP
Hypertensive Disorders of Pregnancy
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- HR
Hazard ratio
- hs-CRP
high sensitivity C-reactive protein
- LDL-C
Low-density lipoprotein cholesterol
- NCEP-ATP III
National Cholesterol Education Program/Adult Treatment Panel III
- SAS
Statistical Analysis Software
- SBP
Systolic blood pressure
- T2D
Type 2 diabetes
Footnotes
Contributors: Dr. Gunderson had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Gunderson designed the study, developed that analytic plan, directed the data analysis, and led the ancillary studies for additional data collection and evaluation in CARDIA women. Ms. Baiyang Sun was responsible for the configuration of the variables, and conducted the statistical analysis. Dr. Catov contributed to the data analysis, and provided critical review of the manuscript content. Drs. Lewis, Sidney, and Carr were responsible for the data collection, and provided critical review of the manuscript content. Drs. Rana, Hou, Wellons, and Allen critically reviewed the manuscript content and/or statistical methodology. This manuscript has been reviewed and approved by the CARDIA Study for scientific content, including the statistical power and analysis.
Institutional Review Boards and the Study Approval numbers
CARDIA Field Centers:
University of Alabama at Birmingham Institutional Review Board. # IRB0000726
Northwestern University Biomedical Institutional Review Board, #STU00024971-CR0001
University of Minnesota Institutional Review Board, Human Subjects #8304M00575
Kaiser Permanente Northern California Institutional Review Board #CN-98SSidn-03-H
Pending patent unrelated to the current research project on Metabolite Biomarkers Predicting Type 2 Diabetes. (Gunderson, EP).
Supplemental Materials
References
- 1.DeSisto CL, Kim SY and Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014;11:130415. DOI: 10.5888/pcd11.130415 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casagrande SS, Linder B and Cowie CC. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res Clin Pract 2018;141:200–208. [DOI] [PubMed] [Google Scholar]
- 3.Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, Minkoff HL, Poindexter B, Prosser LA, Sawaya GF, Scott JR, Silver RM, Smith L, Thomas A and Tita AT. NIH Consensus Development Conference: Diagnosing Gestational Diabetes Mellitus. NIH Consens State Sci Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 4.Gunderson EP, Quesenberry CP Jr., Jacobs DR Jr., Feng J, Lewis CE and Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. Am J Epidemiol 2010;172:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD and Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson EP, Lewis CE, Tsai AL, Chiang V, Carnethon M, Quesenberry CP Jr. and Sidney S. A 20-Year Prospective Study of Childbearing and Incidence of Diabetes Mellitus in Young Women Controlling for Glycemia before Conception: The Coronary Artery Risk Development in Young Adults Study. Diabetes. 2007;56:2990–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson EP, Jacobs DR Jr., Chiang V, Lewis CE, Tsai A, Quesenberry CP Jr. and Sidney S. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol 2009;201:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr., Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V and Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−−2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah BR, Retnakaran R and Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31:1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, Shofer JB, Heckbert SR, Boyko EJ, Fujimoto WY and Kahn SE. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29:2078–2083. [DOI] [PubMed] [Google Scholar]
- 11.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K and Platt RW. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation. 2019;139:1069–1079. [DOI] [PubMed] [Google Scholar]
- 12.Kramer CK, Campbell S and Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. [DOI] [PubMed] [Google Scholar]
- 13.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, Oudijk MA, Bots ML and van der Schouw YT. Earlier Age of Onset of Chronic Hypertension and Type 2 Diabetes Mellitus After a Hypertensive Disorder of Pregnancy or Gestational Diabetes Mellitus. Hypertension. 2015;66:1116–1122. [DOI] [PubMed] [Google Scholar]
- 14.Goueslard K, Cottenet J, Mariet A-S, Giroud M, Cottin Y, Petit J-M and Quantin C. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovascular Diabetology. 2016;15:15. doi: 10.1186/s12933-016-0338-0 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, Hu FB, Manson JE and Zhang C. Association of History of Gestational Diabetes With Long-term Cardiovascular Disease Risk in a Large Prospective Cohort of US Women. JAMA Intern Med 2017;177:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Retnakaran R and Shah BR. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women With Previous Gestational Diabetes Mellitus. Diabetes Care. 2017;40:101–108. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, Haffner SM, Califf RM and Holman RR. Prediabetes and the risk of diabetes. Lancet. 2012;380:1225–1226. [DOI] [PubMed] [Google Scholar]
- 18.Defronzo RA and Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol 2011;108:3B–24B. [DOI] [PubMed] [Google Scholar]
- 19.Levitzky YS, Pencina MJ, D’Agostino RB, Meigs JB, Murabito JM, Vasan RS and Fox CS. Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol 2008;51:264–270. [DOI] [PubMed] [Google Scholar]
- 20.Faeh D, William J, Yerly P, Paccaud F and Bovet P. Diabetes and pre-diabetes are associated with cardiovascular risk factors and carotid/femoral intima-media thickness independently of markers of insulin resistance and adiposity. Cardiovasc Diabetol 2007;6:32. 10.1186/1475-2840-6-32 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albright AL and Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med 2013;44:S346–S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiss LS, James C, Gregg EW, Albright A, Williamson DF and Cowie CC. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am J Prev Med 2010;38:403–409. [DOI] [PubMed] [Google Scholar]
- 23.Prados M, Flores-Le Roux JA, Benaiges D, Llauradó G, Chillarón JJ, Paya A and Pedro-Botet J. Previous Gestational Diabetes Increases Atherogenic Dyslipidemia in Subsequent Pregnancy and Postpartum. Lipids. 2018;53:387–392. [DOI] [PubMed] [Google Scholar]
- 24.Tovar A, Chasan-Taber L, Eggleston E and Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis 2011;8:A124. http://www.cdc.gov/pcd/issues/2011/nov/11_0031.htm. (Accessed 08/01/2020) [PMC free article] [PubMed] [Google Scholar]
- 25.Dietz PM, Vesco KK, Callaghan WM, Bachman DJ, Bruce FC, Berg CJ, England LJ and Hornbrook MC. Postpartum screening for diabetes after a gestational diabetes mellitus-affected pregnancy. Obstet Gynecol 2008;112:868–874. [DOI] [PubMed] [Google Scholar]
- 26.Robbins CL, Dietz PM, Bombard JM, Gibbs F, Ko JY and Valderrama AL. Blood pressure and cholesterol screening prevalence among U.S. women of reproductive age opportunities to improve screening. Am J Prev Med 2011;41:588–595. [DOI] [PubMed] [Google Scholar]
- 27.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr., Sidney S, Bild DE, Williams OD and Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 28.Carr JJ, Jacobs DR Jr., Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP and Goff DC Jr. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol 2017;2:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Manolio TA. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. [DOI] [PubMed] [Google Scholar]
- 30.Pisani T, Gebski CP, Leary ET, Warnick GR and Ollington JF. Accurate direct determination of low-density lipoprotein cholesterol using an immunoseparation reagent and enzymatic cholesterol assay. Arch Pathol Lab Med 1995;119:1127–1135. [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI and Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S and Lewis CE. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc 2014;3:e000490. doi: 10.1161/JAHA.113.000490. (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF and Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 34.Rifai N, Tracy RP and Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem 1999;45:2136–2141. [PubMed] [Google Scholar]
- 35.Lolekha PH, Chittamma A, Roberts WL, Sritara P, Cheepudomwit S and Suriyawongpaisal P. Comparative study of two automated high-sensitivity C-reactive protein methods in a large population. Clin Biochem 2005;38:31–35. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA and Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 2005;13:322–327. [PubMed] [Google Scholar]
- 37.American Diabetes Association. Standards of medical care in diabetes - 2010. 2010;33:S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D Jr., Liu K, Hubert H and Gernhofer N. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc 1991;91:1104–1112. [PubMed] [Google Scholar]
- 39.Sijtsma FP, Meyer KA, Steffen LM, Van HL, Shikany JM, Odegaard AO, Gross MD, Kromhout D and Jacobs DR Jr. Diet quality and markers of endothelial function: the CARDIA study. Nutr Metab Cardiovasc Dis 2014;24:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderssen N, Jacobs DR Jr., Sidney S, Bild DE, Sternfeld B, Slattery ML and Hannan P. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA). Am J Epidemiol 1996;143:351–362. [DOI] [PubMed] [Google Scholar]
- 41.Prentice R and Gloeckler L. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 42.Allison PD. Discrete-Time Methods for the Analysis of Event Histories. In: Leinhardt S, ed. Sociological Methods and Research San Francisco: Jossey-Bass; 1982: 61–98. [Google Scholar]
- 43.Tobias DK, Hu FB, Forman JP, Chavarro J and Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34:1582–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murthy VL, Abbasi SA, Siddique J, Colangelo LA, Reis J, Venkatesh BA, Carr JJ, Terry JG, Camhi SM, Jerosch-Herold M, de Ferranti S, Das S, Freedman J, Carnethon MR, Lewis CE, Lima JA and Shah RV. Transitions in Metabolic Risk and Long-Term Cardiovascular Health: Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc 2016;5:(10):e003934. doi: 10.1161/JAHA.116.003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuller LH and Catov J. Invited Commentary: Gestational Hypertension and Diabetes-A Major Public Health Concern. Am J Epidemiol 2017;186:1125–1128. [DOI] [PubMed] [Google Scholar]
- 46.Lai M, Al Rijjal D, Rost HL, Dai FF, Gunderson EP and Wheeler MB. Underlying dyslipidemia postpartum in women with a recent GDM pregnancy who develop type 2 diabetes. Elife. 2020;9:e59153. 10.7554/eLife.59153 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damm P, Kuhl C, Hornnes P and Molsted-Pedersen L. A longitudinal study of plasma insulin and glucagon in women with previous gestational diabetes. Diabetes Care. 1995;18:654–665. [DOI] [PubMed] [Google Scholar]
- 48.An X, Yu D, Zhang R, Zhu J, Du R, Shi Y and Xiong X. Insulin resistance predicts progression of de novo atherosclerotic plaques in patients with coronary heart disease: a one-year follow-up study. Cardiovasc Diabetol 2012;11:71. 10.1186/1475-2840-11-71 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannukainen JC, Lautamaki R, Mari A, Parkka JP, Bucci M, Guzzardi MA, Kajander S, Tuokkola T, Knuuti J and Iozzo P. Elevated Glucose Oxidation, Reduced Insulin Secretion, and a Fatty Heart May Be Protective Adaptions in Ischemic CAD. J Clin Endocrinol Metab 2016;101:2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwala A, Michos ED, Samad Z, Ballantyne CM and Virani SS. The Use of Sex-Specific Factors in the Assessment of Women’s Cardiovascular Risk. Circulation. 2020;141:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah BR, Lipscombe LL, Feig DS and Lowe JM. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study. BJOG. 2011;118:1484–1490. [DOI] [PubMed] [Google Scholar]
- 52.Lavery JA, Friedman AM, Keyes KM, Wright JD and Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124:804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS and Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 54.Baart SJ, Dam V, Scheres LJJ, Damen JAAG, Spijker R, Schuit E, Debray TPA, Fauser BCJM, Boersma E, Moons KGM, van der Schouw YT and CREW consortium. Cardiovascular risk prediction models for women in the general population: A systematic review. PloS one. 2019;14:e0210329. 10.1371/journal.pone.0210329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N and Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas-Sun E, Ryckman KK, Waring ME, Schenken RS, Johnson KC, Edstedt-Bonamy AK, Allison MA and Howard BV. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation. 2016;133:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuart JJ. Identifying Women With a History of a Hypertensive Disorder of Pregnancy: Values, Challenges, and Opportunities. Mayo Clinic proceedings. 2018;93:1695–1697. [DOI] [PubMed] [Google Scholar]
- 58.Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, Tanz LJ, Haug EB, Fraser A, Timpka S, Klykken B, Dalen H, Romundstad PR, Rich-Edwards JW and Asvold BO. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J 2019;40:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timpka S, Fraser A, Schyman T, Stuart JJ, Asvold BO, Mogren I, Franks PW and Rich-Edwards JW. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol 2018;33:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N and Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab 2005;90:3983–3988. [DOI] [PubMed] [Google Scholar]
- 61.Di Benedetto A, Russo GT, Corrado F, Di Cesare E, Alessi E, Nicocia G, D’Anna R and Cucinotta D. Inflammatory markers in women with a recent history of gestational diabetes mellitus. J Endocrinol Invest 2005;28:34–38. [DOI] [PubMed] [Google Scholar]
- 62.Sattar N, Rawshani A, Franzén S, Rawshani A, Svensson AM, Rosengren A, McGuire DK, Eliasson B and Gudbjörnsdottir S. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations With Cardiovascular and Mortality Risks. Circulation. 2019;139:2228–2237. [DOI] [PubMed] [Google Scholar]
- 63.Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, Margolis KL, O’Connor P, Magid DJ and Bibbins-Domingo K. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension. 2011;57:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30 Suppl 2:S246–S250. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Cheng Y, Wang D, Chen H, Chen H, Ming WK and Wang Z. Incidence Rate of Type 2 Diabetes Mellitus after Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of 170,139 Women. J Diabetes Res 2020; April 27;2020:3076463. 10.1155/2020/3076463 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ and Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. 10.1136/bmj.m1361 (Accessed 08/01/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunderson EP. Prospective evidence that lactation protects against cardiovascular disease in women. Am J Obstet Gynecol 2009;200:119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunderson EP and Jaffe MG. Pregnancy and Subsequent Glucose Intolerance in Women of Childbearing Age: Heeding the Early Warning Signs for Primary Prevention of Cardiovascular Disease in Women. JAMA Intern Med 2017;177:1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.