Abstract
Background
Current hypertension guidelines vary substantially in their definition of who should be offered blood-pressure-lowering medications. Understanding the impact of guideline choice on the proportion of adults who require treatment will be crucial for planning and scaling up hypertension care in low- and middle-income countries (LMICs).
Methods
We extracted cross-sectional data on age, sex, blood pressure, hypertension treatment and diagnosis status, smoking, and body mass index for adults ages 30–70 from nationally representative surveys in 50 LMICs (N = 1,037,215). Our main objective was to determine the impact of hypertension guideline choice on the proportion of adults in need of blood-pressure-lowering medications. We considered four hypertension guidelines: the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline, the commonly used 140/90 mmHg threshold, the 2016 World Health Organization HEARTS guideline (WHO), and the 2019 United Kingdom National Institute for Health and Care Excellence (NICE) guideline.
Results
The proportion of adults in need of blood-pressure-lowering medications was highest under the ACC/AHA followed by the 140/90, NICE, and WHO guidelines (ACC/AHA: women, 27.7% [95% CI: 27.2%, 28.2%], men, 35.0% [34.4%, 35.7%]; 140/90: women: 26.1% [25.5%, 26.6%], men, 31.2% [30.6%, 31.9%]; NICE: women, 11.8% [11.4%, 12.1%]; men, 15.7% [15.3%, 16.2%]; WHO: women, 9.2% [8.9%, 9.5%], men, 11.0% [10.6%,11.4%]). Individuals who were unaware that they have hypertension were the primary contributor to differences in the proportion needing treatment under different guideline criteria. Differences in the proportion needing blood-pressure-lowering medications were largest in the oldest, 65–69, age group (ACC/AHA: women, 60.2% [58.8%, 61.6%], men, 70.1% [68.8%, 71.3%]; WHO: women, 20.1% [18.8%, 21.3%], men, 24.1.0% [22.3%, 25.9%]). For both women and men and across all guidelines, countries in the European and Eastern Mediterranean regions had the highest proportion of adults in need of blood-pressure-lowering medicines while the South and Central Americas had the lowest.
Conclusions
There was substantial variation in the proportion of adults in need of blood-pressure-lowering medications depending on which hypertension guideline was used. Given the great implications of this choice for health system capacity, policymakers will need to carefully consider which guideline they should adopt when scaling up hypertension care in their country.
Keywords: hypertension, treatment, health policy, population science, international comparison
Introduction
Preventing and controlling hypertension is a major global public health strategy for reducing premature mortality from cardiovascular and cerebrovascular diseases (CVD).11 Hypertension control is especially important in low- and middle-income countries (LMICs) where CVD has already become the leading cause of both mortality and disability-adjusted life years lost,2,3 the prevalence of hypertension is high,4 and both the mortality burden from CVD and hypertension prevalence are expected to increase dramatically over the coming decades due to population aging.5,6 However, despite its importance, rates of hypertension control in LMICs are generally low: in a recent study,4 on average, only 10% of individuals with hypertension across 44 LMICs had controlled blood pressure. Improving blood pressure (BP) control in LMICs, thus, has tremendous potential to improve population health outcomes. Importantly, it is feasible to realize this potential as measuring BP does not require extensive training or costly equipment, and BP-lowering medications are both highly effective and low-cost.7,8
LMICs have adopted ambitious goals to scale up hypertension care.9,10 A necessary step for scaling up BP-lowering medications in LMICs is to clearly define which individuals are in need of treatment. Available and widely used hypertension treatment guidelines differ considerably in how they define the population who would benefit and should be initiated on treatment.1,11–13 Deciding who should receive BP-lowering medications is important for balancing the benefits of treatment with potential side effects and, due to the differences in the proportion of the population who would need treatment across guidelines, for helping policy makers in LMICs decide which guidelines can be realistically operationalized.
In this study, we estimate how the proportion of adults aged 30–70 years in need of BP-lowering medications varies across four major hypertension guidelines in 50 LMICs that collectively account for 55% of the global population and 66% of the global LMIC population. To inform the targeting of scale-up efforts for hypertension care, we categorize the proportion of individuals who are in need of BP-lowering medicines into three groups based on the hypertension care cascade stage: (i) the proportion who is unaware that they have hypertension; (ii) the proportion who is diagnosed but not initiated on treatment; and (iii) the proportion who is diagnosed and receives BP-lowering medications but still has uncontrolled BP.
Methods
The data, analytic methods, and study materials will be made available in the Harvard Dataverse.
Data sources
We conducted a systematic search to identify household survey datasets with the following characteristics: (i) conducted in a country that was an LMIC at the time of data collection (according to the World Bank income classification14), (ii) carried out during or after 2005, (iii) conducted in at least two ten-year age groups above the age of 15 years, (iv) nationally representative, (v) response rate ≥50%, and (vi) took at least two BP measurements during the survey interview. We judged a survey to be nationally representative if the official survey documentation explicitly stated that the survey was nationally representative and was based on a probability sample designed to produce national-level estimates. Based on these criteria, we identified and included 50 datasets. The only two exceptions we included were the Indonesian Family Life Survey, which is representative of 83% of the Indonesian population (more remote islands and areas under conflict at the time of the baseline survey were excluded from the sampling frame),15 and the China Health and Nutrition Survey, which has broad coverage from several geographic regions in China but is not explicitly nationally representative.16 We provide an overview of each of the datasets in Table 1; more detailed information on the data identification process has been published previously.4
Table 1.
Descriptive characteristics of the countries and samples used in the analysis
| Country | World Bank Income Group* | Population Aged 30–70 (1000s) ** | Survey | Survey Year | Response rate | Proportion Missing | Sample Size*** | Proportion Female**** | Median Age**** |
|---|---|---|---|---|---|---|---|---|---|
| Africa | |||||||||
| Algeria | UM | 16425 | STEPS | 2016 | 93% | 0.054 | 5185 | 0.55 | 45 |
| Benin | L | 2918 | STEPS | 2015 | 99% | 0.042 | 3344 | 0.51 | 42 |
| Botswana | UM | 746 | STEPS | 2014 | 64% | 0.039 | 2408 | 0.69 | 43 |
| Burkina Faso | L | 4634 | STEPS | 2013 | 98% | 0.173 | 2838 | 0.51 | 41 |
| Comoros | L | 196 | STEPS | 2011 | 97% | 0.075 | 4020 | 0.69 | 42 |
| Eswatini | LM | 313 | STEPS | 2014 | 82% | 0.136 | 1807 | 0.67 | 45 |
| Ghana | L | 7618 | SAGE | 2008–09 | 79% | 0.127 | 3394 | 0.44 | 55 |
| Kenya | LM | 13689 | STEPS | 2015 | 95% | 0.043 | 2896 | 0.58 | 42 |
| Lesotho | LM | 593 | STEPS | 2012 | 80% | 0.066 | 1773 | 0.66 | 46 |
| Liberia | L | 1100 | STEPS | 2011 | 87% | 0.069 | 1225 | 0.53 | 39 |
| Mozambique | L | 5352 | STEPS | 2005 | 98% | 0.1 | 2303 | 0.56 | 42 |
| Namibia | UM | 711 | Demographic and Health Surveys | 2013 | 97% | 0.226 | 3410 | 0.58 | 46 |
| Seychelles | UM | 47 | National Survey of Noncommunicable Diseases | 2013 | 73% | 0.002 | 1123 | 0.57 | 49 |
| South Africa | UM | 22086 | National Income Dynamics Study | 2017 | 69% | 0.186 | 10935 | 0.61 | 44 |
| Tanzania | L | 11578 | STEPS | 2012 | 95% | 0.038 | 4483 | 0.52 | 43 |
| Togo | L | 1797 | STEPS | 2010 | 91% | 0.069 | 2395 | 0.49 | 41 |
| Uganda | L | 8790 | STEPS | 2014 | 99% | 0.056 | 2237 | 0.59 | 41 |
| Zambia | LM | 3900 | STEPS | 2017 | 78% | 0.063 | 2513 | 0.61 | 43 |
| South and Central Americas | |||||||||
| Belize | UM | 86 | CAMDI | 2005–06 | 93% | 0.149 | 1336 | 0.57 | 45 |
| Brazil | UM | 96623 | Pesquisa Nacional de Saude | 2013 | 86% | 0.077 | 38847 | 0.57 | 45 |
| Chile | UM | 8014 | National Health Survey | 2009–10 | 85% | 0.085 | 3127 | 0.6 | 48 |
| Costa Rica | UM | 2002 | STEPS | 2010 | 88% | 0.154 | 2218 | 0.74 | 47 |
| Ecuador | UM | 5682 | Encuesta Nacional de Salud y Nutrición | 2012 | 82% | 0.003† | 9926 | 0.32 | 40 |
| Grenada | UM | 44 | STEPS | 2010–11 | 65% | 0.042 | 902 | 0.6 | 47 |
| Guyana | UM | 308 | STEPS | 2016 | 67% | 0.018 | 1926 | 0.59 | 46 |
| Mexico | UM | 45285 | Mexican Family Life Survey | 2009–12 | 90% | 0.245 | 9159 | 0.59 | 46 |
| St. Vincent and the Grenadines | UM | 50 | STEPS | 2013 | 68% | 0.018 | 2661 | 0.54 | 47 |
| Eastern Mediterranean | |||||||||
| Iran | UM | 35799 | STEPS | 2016 | 99% | 0.04 | 20798 | 0.52 | 45 |
| Iraq | UM | 10735 | STEPS | 2015 | 94% | 0.165 | 2350 | 0.62 | 45 |
| Lebanon | UM | 2747 | STEPS | 2017 | 70% | 0.091 | 1491 | 0.58 | 50 |
| Morocco | LM | 14661 | STEPS | 2017 | 89% | 0.024 | 3835 | 0.65 | 47 |
| Sudan | LM | 11272 | STEPS | 2015 | 88% | 0.051 | 5039 | 0.61 | 44 |
| Europe | |||||||||
| Azerbaijan | UM | 4491 | STEPS | 2017 | 97% | 0.042 | 2224 | 0.59 | 50 |
| Belarus | UM | 5152 | STEPS | 2016 | 87% | 0.006 | 4296 | 0.59 | 51 |
| Georgia | LM | 2040 | STEPS | 2016 | 76% | 0.057 | 3391 | 0.71 | 53 |
| Kazakhstan | UM | 7043 | Kazakhstan Household Health Survey | 2012 | 93% | 0.158 | 6780 | 0.57 | 47 |
| Kyrgyzstan | LM | 2246 | STEPS | 2013 | 97% | 0.024 | 2179 | 0.63 | 47 |
| Moldova | LM | 2150 | STEPS | 2013 | 84% | 0.07 | 3682 | 0.62 | 52 |
| Tajikistan | LM | 2776 | STEPS | 2016 | 94% | 0.017 | 1937 | 0.6 | 45 |
| South-East Asia | |||||||||
| Bhutan | LM | 273 | STEPS | 2014 | 97% | 0.019 | 2114 | 0.59 | 44 |
| India | LM | 537312 | Annual Health Survey / District Level Household Survey | 2013–14 | 89%‡ | 0.281 | 790848 | 0.53 | 45 |
| Indonesia | LM | 114835 | Indonesian Family Life Survey | 2014 | 83% | 0.027 | 19743 | 0.52 | 42 |
| Myanmar | LM | 22540 | STEPS | 2014 | 90% | 0.045 | 7141 | 0.65 | 47 |
| Nepal | L | 9227 | STEPS | 2013 | 98% | 0.01 | 3139 | 0.67 | 45 |
| Sri Lanka | LM | 10021 | STEPS | 2014 | 72% | 0.083 | 4021 | 0.6 | 47 |
| Timor-Leste | LM | 344 | STEPS | 2014 | 96% | 0.047 | 1845 | 0.55 | 45 |
| Western Pacific | |||||||||
| Cambodia | L | 4514 | STEPS | 2010 | 96% | 0.035 | 4445 | 0.65 | 46 |
| China | UM | 757572 | China Health and Nutrition Survey | 2015 | 88% | 0.114 | 8836 | 0.54 | 52 |
| Mongolia | LM | 1071 | STEPS | 2009 | 95% | 0.015 | 3777 | 0.41 | 42 |
| Vanuatu | LM | 75 | STEPS | 2011 | 94% | 0.044 | 3641 | 0.49 | 43 |
| Across-survey median | 4502 | 90% | 0.055 | 3242 | 0.59 | 45 | |||
L: Low, LM: Lower middle, UM: Upper middle; Classifications are for the survey year.
Population size is from the United Nations World Population Prospects, 2019 revision, data for the nearest year to the survey year.
Sample size after excluding individuals with missing data.
These estimates are unweighted.
This is an approximate estimate of the share missing as we were not able to exactly determine which individuals in the survey were eligible for the blood pressure measurements.
The Indian data were collected as two separate surveys; this response rate is for the District Level Household Survey 4, which covered the majority of the Indian states. The second survey, the Annual Health Survey, did not publish a response rate.
We also extracted data on population counts for each country by sex and five-year age group between 30 and 70 years from the 2019 United Nations World Population Prospects (UN WPP).17 For each country, we chose the UN WPP year that was closest to the country survey year.
Inclusion criteria
Our population of interest is adults aged 30 to 70 years. We use 30 years as our lower age threshold since hypertension guidelines generally recommend screening from around this age onward.11 We restrict our upper threshold to 70 years since that is the maximum age for which we have measurements of BP for the majority of countries in our data. This was a complete case analysis. We excluded individuals who were missing any information on age, sex, systolic blood pressure, diastolic blood pressure, hypertension diagnosis and treatment information, body mass index, and current smoking status.
Hypertension treatment guidelines
We consider four separate hypertension guidelines to determine which individuals are in need of BP-lowering medications: the commonly used “140/90” threshold,13,18–21 the 2016 World Health Organization HEARTS / Package of Essential Noncommunicable disease interventions (WHO HEARTS) guidelines,1 the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline,12 and the United Kingdom’s National Institute for Health and Care Excellence (NICE) 2019 guideline.11 We included the 140/90 threshold because it is widely used across LMICs, including in major countries such as China, India, Brazil, and South Africa,18–22 across Europe for individuals below age 80 in the form of the 2018 European Society of Cardiology/European Society of Hypertension guidelines,13 and recently as the 2020 International Society of Hypertension guidelines (for patients deemed low risk of a cardiovascular disease endpoint in settings with limited availability of medicines, the threshold to determine treatment is moved to 160/100);23 the WHO HEARTS guideline as it is officially recommended by the WHO for all LMICs; and the ACC/AHA and NICE guidelines because they are the two other main guidelines currently used in high-income countries. Importantly, while the ACC/AHA guideline was developed for the United States, several prominent papers have raised the question of whether it should also be used in LMICs;24–28 we thus included this guideline to provide further evidence to inform this decision across LMICs. We show the thresholds for each guideline in Table I in the Supplement.
Cardiovascular disease risk
Three of the guidelines (ACC/AHA, NICE, and WHO HEARTS) base their guidance on both BP and predicted 10-year risk of a CVD event (from here forward, “CVD risk”). Importantly, each guideline recommends using a distinct CVD risk calculator; however, while there are many CVD risk scores,29–33 most have not been calibrated for LMIC populations. For this reason, we use the WHO and International Society of Hypertension (WHO/ISH) risk score for all three guidelines since it is widely used across LMICs and is calibrated for different world regions.32 Using a common CVD risk calculator also allowed us to assess the influence of different thresholds and guidelines net of the influence of differences in CVD risk prediction. Specifically, we use the “non-laboratory” score that uses information on body mass index (BMI) in place of total cholesterol and diabetes status since blood glucose and cholesterol measurements were not collected for the majority of our surveys. As a sensitivity analysis, we present the main results using the Globorisk CVD scores instead of the WHO/ISH score because Globorisk is the only other score, to our knowledge, that has been calibrated for all LMICs (Figures I–III in the Supplement).29
Measurement of blood pressure
The surveys in 40 countries measured BP using a digital upper arm meter, 1 using a digital wrist meter, and 2 using a manual mercury sphygmomanometer (Table II in the Supplement). We were unable to find documentation on the BP measurement device that was used in seven surveys (Algeria, Azerbaijan, Botswana, Kyrgyzstan, Sri Lanka, Sudan, and Tajikistan). Forty-three countries measured BP at least three times, three two times with a third measurement if the first two differed by a pre-defined margin, and four two times. For participants with three or more BP measurements, we used the mean of the last two measurements; for participants with only two BP measurements, we averaged both available measurements. In case the second or third BP measurement was missed in a participant, we used the single measurement provided.
Statistical analyses
Our analysis had four main steps. First, we determined which individuals in the data are in need of BP-lowering medications under each of the four guidelines based on their measured systolic and diastolic blood pressure, CVD risk, and the thresholds specified in Table I in the Supplement. Among those individuals, we further classified them into three mutually exclusive categories (separately for each guideline): those who are unaware that they have hypertension, those who have been diagnosed but are not currently on treatment, and those who have been diagnosed and are on treatment but are in need of further care (i.e. to improve adherence) or treatment (i.e. additional or a higher dose of medicines) to achieve BP control.
Second, we used a multinomial logistic regression model to estimate the proportion of individuals in each of these three treatment categories for each five-year age group between 30 and 70 (separately for each guideline and by sex and country). We used a regression model to smoothly estimate these proportions rather than estimate them directly to reduce sampling error. Importantly, 14 of the 50 countries did not contain information on individuals between ages 65 and 69, and 2 (Ecuador and Namibia) additionally did not contain information for ages 60–64. For these countries, we used the estimated regression models to extrapolate the age-pattern of treatment needs into the missing age groups. These models were weighted by the survey-specific sample weights. We present the fit of the models for each country in as Figures IV–LIII in the Supplement.
Third, to determine the number of individuals in need of BP-lowering medications by levels of the treatment cascade within each age group, we multiplied the estimated age-specific proportions of individuals in each treatment category by the age-specific population counts for each country.
Fourth, we estimated the overall proportion of adults between the ages of 30 and 70 in need of BP-lowering medications by simply dividing the total number of individuals in each treatment category across ages by the total population between ages 30 and 70. We present our results across LMICs globally and separately by country, age, and sex, and WHO World Regions. Using the UN WPP data ensured that aggregated estimates (across ages, countries, and regions) were weighted proportionately to each contributing country’s population size.
Institutional Review Board (IRB) Approval
Our study was exempt from IRB review since it uses publicly available and de-identified secondary data.
Results
Descriptive characteristics of the sample
Our analytical sample consisted of 1,037,215 individuals with an across-survey median response rate of 90%. Among the 50 countries included in our data, 11 were classified as low-income, 18 as lower-middle-income, and 21 as upper-middle-income countries (Table 1). The countries represented population sizes ranging from 44,000 in Grenada to 757,572,000 in China with an across-country median 30–70 population of 4.5 million individuals. The proportion of individuals excluded due to missingness varied from 28% in India to less than 1% in Belarus, with an across-country median of 5.5%.
Proportion of individuals in need of BP-lowering medications by guideline
There was substantial variation in the proportion of adults in need of BP-lowering medications across the four guidelines (Figure 1, 95% CIs are shown in Table III in the Supplement). The ACC/AHA guideline placed the greatest proportion of adults in need of BP-lowering medications (women: 27.7% [95% CI: 27.2%, 28.2%]; men: 35.0% [34.4%, 35.7%]) followed by the 140/90 threshold (women: 26.1% [25.5%, 26.6%]; men: 31.2% [30.6%, 31.9%]), the NICE guideline (women: 11.8% [11.4%, 12.1%]; men: 15.7% [15.3%, 16.2%]), and lastly the WHO HEARTS guideline (women: 9.2% [8.9%, 9.5%]; men: 11.0% [10.6%,11.4%]).
Figure 1.
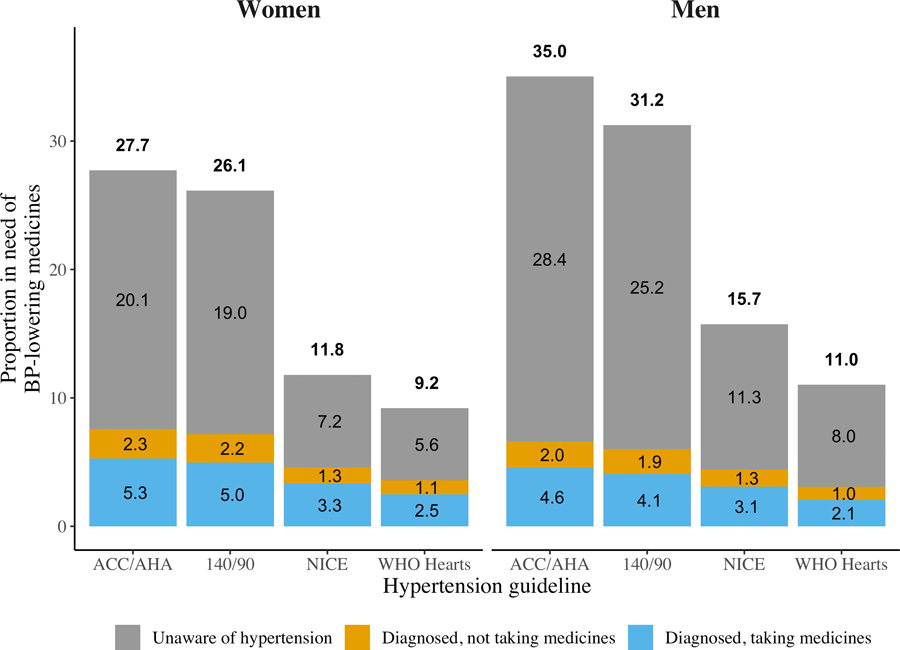
BP treatment needs by guidelines across 50 LMICs. Proportion (%) of adults ages 30–70 from 50 low- and middle-income countries in need of BP-lowering medicines by hypertension treatment guidelines, stages of hypertension care, and sex. Countries are weighted by proportionate size of their 30–70 population. We show the 95% CIs for each estimate in Supplemental Table 3.
Individuals who were unaware that they have hypertension were the primary contributor to differences in the total proportion in need of BP-lowering medications across guidelines. For example, this proportion was 20.1% (95% CI: 19.7%, 20.6%) for women and 28.4% (95% CI: 27.7%, 29.1%) under the ACC/AHA guideline compared to just 5.6% (95% CI: 5.4%, 5.9%) and 8.0% (95% CI: 7.6%, 8.3%) under the WHO HEARTS guideline for women and men, respectively. In contrast, there were only small differences in the proportion of individuals who were diagnosed and in need of BP-lowering medications across guidelines.
Differences in the proportion of individuals in need of BP-lowering medications by age
The proportion of individuals in need of BP-lowering medications increased substantially with age across all four guidelines (Figure 2). Beyond this overall age trend, there were three distinct points of divergence and convergence. Between ages 30 and 50 for women and 30 and 45 for men, the primary differences were between the ACA/AHA and 140/90 guidelines on the one hand and the NICE and WHO HEARTS guidelines on the other hand. After ages 45–50, the four guidelines diverged, with the ACC/AHA having the highest proportions, followed by the 140/90, NICE, and WHO HEARTS. By age 70, the ACC/AHA guideline had much higher proportions while differences between the 140/90 threshold and NICE guidelines nearly disappeared, and the WHO guideline continued to have the lowest proportions.
Figure 2.
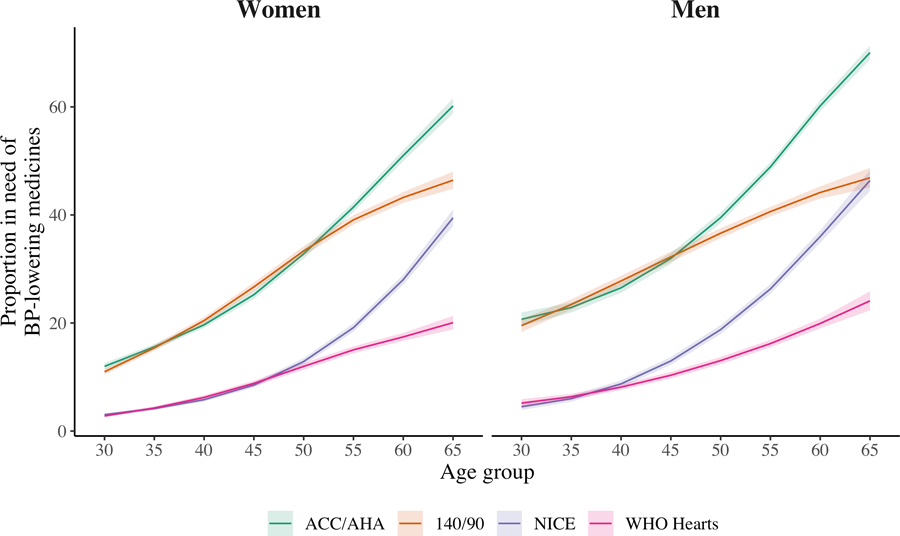
BP treatment needs by guidelines and age. Proportion of the population in need of BP-lowering medicines by guideline across age groups and sex. Shaded areas are 95% confidence intervals.
Differences in the proportion of individuals in need of BP-lowering medications by country
Across all four guidelines, Belarus, Moldova, Kyrgyzstan, and Tajikistan had the highest proportions in need of BP-lowering medications while Belize, Costa Rica, and Cambodia had the lowest (Figure 3, 95% CIs are shown in Table IV in the Supplement). For example, under the ACC/AHA guidelines the proportion in need of treatment in Belarus was 57.9% (95% CI: 56.4%, 59.3%) and 14.4% (13.2%, 15.6%) in Cambodia. These differences, however, became smaller under the more conservative guidelines: under the WHO HEARTS guideline, the proportion in need of treatment became 25.6% (24.2%, 26.9%) and 3.9% (3.1%, 3.8%) in Belarus and Cambodia respectively.
Figure 3.
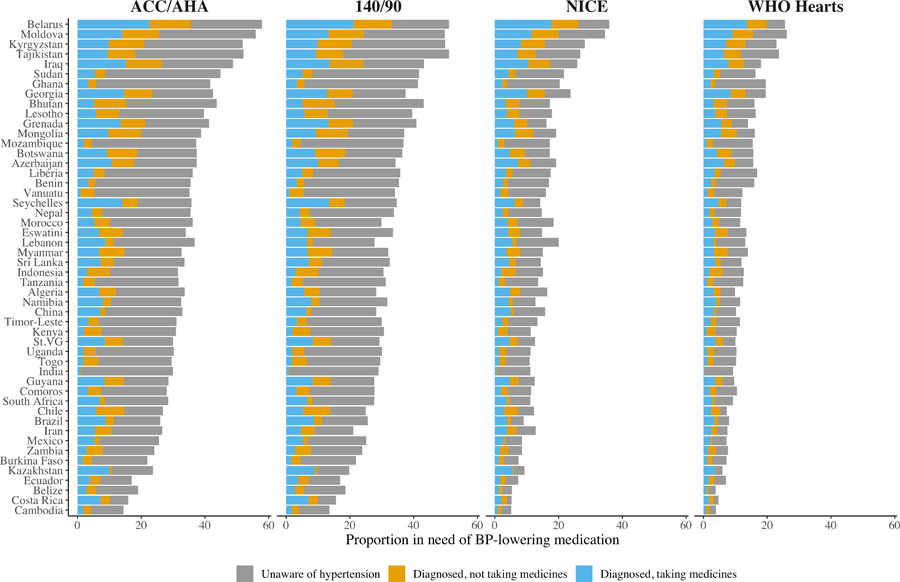
BP treatment needs by guidelines and country. Proportion of adults ages 30–70 from 50 low- and middle-income countries in need of BP-lowering medicines by hypertension treatment guideline and stages of the hypertension care. We show the numerical estimates with 95% CIs in Supplemental Table 5.
Similar to the overall results, the largest contributor to the variance in treatment needs across countries and guidelines were country differences in the proportion of individuals unaware that they have hypertension. However, the proportion of individuals who were diagnosed but not taking treatment and who were diagnosed and taking treatment but still had uncontrolled BP were increasingly important among countries with larger overall treatment gaps.
Regional results
In Table V and Figures LIV and LV in the Supplement, we present differences in the proportion of individuals in need of BP-lowering medications across guidelines by WHO regions and decompose the difference across regions. Broadly, the European region had the highest proportion of adults in need of BP-lowering medicines with the lowest proportion in the South and Central Americas. The ordering of needs across guidelines was identical for all regions and followed the pattern seen for the overall results. Higher age-specific CVD risks in Europe explained nearly all the difference in treatment needs compared to the South and Central Americas, Asia, and the Western Pacific, while the older age-distribution of Europe was more important for differences compared to Africa and the Eastern Mediterranean regions.
Discussion
We found that across 50 LMICs, there were strikingly large differences in the proportion of adults in need of BP-lowering medicines depending on which of the major hypertension treatment guidelines was used to identify individuals for treatment. Within every country, for both sexes, and across ages, the 2017 ACC/AHA guideline placed the largest proportion of adults in need of BP-lowering medicines followed closely by the widely used “140/90” threshold.18–21 In contrast, the NICE and WHO HEARTS guidelines classified less than half as many adults as being in need of BP-lowering medicines. Importantly, these differences were most pronounced among older individuals and primarily driven by differences across guidelines in the proportion of individuals in need of BP-lowering medicines who were not diagnosed as having hypertension. This suggests that the choice of guideline to scale up BP-medicine coverage will primarily affect how many new, and likely older, individuals will need to be brought into the care continuum and consequently the health system capacity that is needed to provide care to these new hypertension patients.
In addition to the health system care burden, policymakers will need to weigh the expected health benefits and long-term cost savings (e.g., from reducing the incidence of CVD events) from placing a larger proportion of the population onto BP-lowering medicines against the potential side effects of providing treatment to larger shares of the population. Unfortunately, despite a large body of evidence on the effects of hypertension treatment,34 there is no conclusive evidence on which risk groups (in terms of BP levels, CVD risk, and age) benefit the most from BP treatment.13 This uncertainty is heightened for LMIC populations where there have been few longitudinal studies to ascertain individuals’ long-term benefits from BP treatment. Therefore, an important open question is how guidelines based on high-income country clinical-trial populations should be translated to LMIC populations who may benefit differently from BP-lowering medicines and have different CVD risks. Randomized controlled clinical trials or cohort studies of BP treatment in LMIC settings will be essential for resolving these debates.
The local health economic context is an important, but often overlooked, component of the content of hypertension care guidelines. To our knowledge, only the recent ISH 2020 guideline explicitly accounts for these factors in the guideline recommendations, making distinctions, for example, between BP treatment thresholds when medicines are and are not easily available.23 More broadly, other guidelines need to be aware of the availability and costs of anti-hypertensive medicines. In contexts with a limited availability of anti-hypertensive medications, guidelines that place a larger share of the population on treatment may be unfeasible to realize and may even prevent individuals at higher BPs -- for whom BP reductions are more pressing -- from having sufficient medication supply to adequately control their BP. Such guidelines would also place a greater financial burden on populations, as not only more individuals would require medicines but also because individuals on treatment may require more medications to achieve control. This is especially important in poorer communities where aggressive hypertension guidelines may result in a high financial burden for families with individuals in need of treatment.
Closing the large treatment gaps across countries requires focusing resources at the largest bottlenecks in the hypertension care continuum. This is especially important in the Eastern European and Central Asian countries, where treatment gaps are much larger than in the other countries we considered. Our results reveal that the largest share of those in need of treatment are individuals who are unaware that they have hypertension. A major barrier to improving awareness and consequently diagnosis is that individuals in LMICs do not commonly seek preventive care, such as for hypertension screening.35 Therefore, closing this gap will require either significantly expanding opportunistic screening of individuals at health facilities or home- and community-based hypertension screening and diagnosis campaigns.36,37 The effectiveness of community-based and opportunistic screening, however, will depend on communities’ access to health facilities -- in countries and regions with a limited supply of high-quality health facilities, closing hypertension gaps will first require addressing these health-systems shortages.
The next largest gap following awareness is treatment among diagnosed individuals. Although reasons for this gap are less explored in LMICs, emerging evidence suggests that beliefs that individuals have towards treatment may be a significant barrier to long-term treatment adherence. For example, studies of hypertension and of other conditions requiring repeat treatment find that individuals often do not understand that they have to continuously take medicines even after they feel better,38 or in the case of hypertension, after their BP reduces to a controlled level.39 Improving knowledge on how to correctly use BP medications will be essential for closing this second gap.
There are several limitations that are important to the interpretation of our results. First, in most clinical care settings, BP medicines are given based on the average of BP measurements taken on at least two consecutive occasions rather than based on measurements taken in one sitting such as we used here. Several studies have found that rates of hypertension are exaggerated when estimated based on measurements from one point in time. This is an important limitation and implies that our approach likely overestimates the number of individuals with persistently raised BP. Our estimates of the proportion in need of BP-lowering medication may be additionally overstated for Comoros, India, Mexico, and South Africa since they are based on only 2 BP measurements rather than the average of the last 2 of 3 readings as was the case for the other 46 countries. We are unfortunately unaware of any nationally representative data sources from LMICs that collect BP in a manner similar to clinical care; collecting such data or examining differences across guidelines in clinical data sources with BP measured on multiple occasions will be essential for future work in this area. Second, an across-survey median of 5.5% of age-eligible individuals were missing data and thus dropped from the sample. Our results may incorrectly represent the proportion of individuals in need of BP-lowering medicines if those who were missing data were more likely to require treatment. Third, the procedure used to measure BP and the questions used to ascertain CVD risk factors varied somewhat between certain countries. Therefore, differences in our study between countries may be partially driven by these measurement differences. However, this limitation does not affect our analysis of differences between guidelines within countries, which was the main objective of our study. Fourth, the surveys used in this study were collected in different years. Therefore, our findings should be interpreted as relating to each country-year pair rather than providing estimates for a common year across countries. Fifth, we had to extrapolate the proportion of individuals in need of BP-lowering medicines for one age group (65–69) for 14 countries and two age groups (60–64 and 65–69) for two countries based on the relationship between age and BP treatment needs in the ages with data. Given the regularity of the age-BP treatment needs relationship in ages with data for countries that have data all the way to age 70 (Figures IV–LIII in the Supplement), we believe any bias from the extrapolation is unlikely to change our main conclusions. A similar limitation is that we used the non-laboratory CVD risk equations rather than the equations based on measured lipids and glucose. However, given the high cost of these laboratory measurements, the non-laboratory scores are more likely to be used for efforts to scale up BP treatment in LMICs. Lastly, we did not distinguish between “natural” BP and the BP of individuals on medication; however, the guidelines considered here do not call for differential treatment for those who are already on medicines but rather are based on measured levels of BP and CVD risk. However, our results are likely conservative because they miss the small proportion of individuals in these countries who are on BP medicines but have their BP under control and thus do not get identified as in need of BP-lowering medicines under the guidelines.
This study of nationally representative data for a set of countries that collectively represent two-thirds of the global LMIC population highlights that the decision of which hypertension guideline is adopted has immense implications for the proportion of the adult population who are in need of BP-lowering medicines. Ultimately, the results of this study call attention to an important unresolved discussion on which individuals should actually receive BP treatment in LMICs. Developing guidelines that acknowledge and are built for the unique conditions in each country will be critical for health service planning as LMICs prepare to scale up hypertension care over the coming years.
Supplementary Material
Supplemental Tables I-V
Supplemental Figures I-LV
Clinical Perspective.
What is new?
The choice of a hypertension treatment guideline has a substantial influence on the number of adults who require blood pressure lowing medications across low- and middle-income countries.
The primary contributors to differences in treatment needs across guidelines are the number of older individuals and individuals who are unaware that they have hypertension and are in need of treatment.
What are the clinical implications?
As the clinical care burden of hypertension is strongly influenced by which guideline is used, countries need to carefully decide which treatment guidelines can be realistically scaled up without overburdening clinical care or creating shortages in the supply of blood pressure lowering medications.
In addition to clinical care burdens, physicians and health policy experts will need to decide whether guidelines that place substantial numbers of older individuals on treatment can be justified in terms of their expected benefits relative to side effects within their specific country contexts.
Acknowledgements
NS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. NS and PG developed the initial idea and methodology; NS conducted the formal data analysis and wrote the initial draft; all authors were involved in developing the study idea and critically reviewed and edited the main manuscript.
Funding Sources
This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 850896). NS is supported by the Alexander von Humboldt Foundation. PG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR003143. The funders had no role in the manuscript.
Non-standard Abbreviations and Acronyms
- CVD
Cardiovascular and cerebrovascular diseases
- LMICs
Low- and middle-income countries
- BP
Blood pressure
- UN WPP
2019 United Nations World Population Prospects
- WHO HEARTS
2016 World Health Organization HEARTS / Package of Essential Noncommunicable disease interventions guideline
- ACC/AHA
2017 American College of Cardiology/American Heart Association guideline
- NICE
United Kingdom’s National Institute for Health and Care Excellence 2019 guideline
- WHO/ISH
World Health Organization and International Society of Hypertension
- BMI
Body mass index
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.World Health Organization. HEARTS: Technical package for cardiovascular disease management in primary health care. Geneva, Switzerland: 2016. [Google Scholar]
- 2.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geldsetzer P, Manne-Goehler J, Marcus M-E, Ebert C, Zhumadilov Z, Wesseh CS, Tsabedze L, Supiyev A, Sturua L, Bahendeka SK, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1· 1 million adults. Lancet 2019;394:652–662. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JE, Stevens GA, Mathers CD, Bonita R, Rehm J, Kruk ME, Riley LM, Dain K, Kengne AP, Chalkidou K, et al. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018;392:1072–1088. [DOI] [PubMed] [Google Scholar]
- 6.Sudharsanan N, Geldsetzer P. Impact of Coming Demographic Changes on the Number of Adults in Need of Care for Hypertension in Brazil, China, India, Indonesia, Mexico, and South Africa: A Modeling Study. Hypertension. 2019;73:770–776. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakaran D, Anand S, Gaziano TA, Mbanya J-C, Nugent R. Disease Control Priorities, (Volume 5): Cardiovascular, Respiratory, and Related Disorders. World Bank Publications; 2017. [PubMed] [Google Scholar]
- 8.Kontis V, Cobb LK, Mathers CD, Frieden TR, Ezzati M, Danaei G. Three Public Health Interventions Could Save 94 Million Lives in 25 Years: Global Impact Assessment Analysis. Circulation. 2019;140:715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020 [Internet]. Geneva, Switzerland: World Health Organization; 2013. [accessed 2020 Nov 11]. Available from: https://www.who.int/publications/i/item/9789241506236 [Google Scholar]
- 10.Hogan DR, Stevens GA, Hosseinpoor AR, Boerma T. Monitoring universal health coverage within the Sustainable Development Goals: development and baseline data for an index of essential health services. Lancet Glob Health. 2018;6:e152–e168. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management, NICE Guideline [NG136] [Internet]. 2019. [accessed 2020 Nov 11];Available from: https://www.nice.org.uk/guidance/ng136
- 12.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 13.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, De Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 14.The World Bank Group. World Bank Country and Lending Groups [Internet]. [accessed 2020 Nov 11];Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 15.Frankenberg E, Karoly LA, Gertler P, Achmad S, Agung IGN, Hatmadji SH, Sudharto P. The 1993 Indonesian Family Life Survey: Overview and Field Report [Internet]. Santa Monica, CA: RAND Corporation; 1995. [accessed 2020 Nov 11]. Available from: https://www.rand.org/pubs/drafts/DRU1195z1.html. [Google Scholar]
- 16.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol 2010;39:1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2019 Revision [Internet]. 2019. [accessed 2020 Nov 11]. Available from: https://population.un.org/wpp/Download/Standard/Population/
- 18.Liu S 2018 Chinese Guidelines for Prevention and Treatment of Hypertension--A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. Journal of Geriatric Cardiology. 2019;16:182–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malachias M, Paulo César Veiga Jardim P, Almeida F, Lima Júnior E, Feitosa G. 7th Brazilian guideline of arterial hypertension: chapter 7-pharmacological treatment. Arquivos brasileiros de cardiologia. 2016;107:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health and Family Welfare. Screening, Diagnosis, Assessment, and Management of Primary Hypertension in Adults in India [Internet]. New Delhi: Government of India; 2016. [accessed 2020 Nov 11]. Available from: https://nhm.gov.in/images/pdf/guidelines/nrhm-guidelines/stg/Hypertension_full.pdf [Google Scholar]
- 21.Seedat Y, Rayner B, Veriava Y. South African hypertension practice guideline 2014. Cardiovasc J Afr 2014;25:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahdavi M, Parsaeian M, Mohajer B, Modirian M, Ahmadi N, Yoosefi M, Mehdipour P, Djalalinia S, Rezaei N, Haghshenas R, et al. Insight into blood pressure targets for universal coverage of hypertension services in Iran: the 2017 ACC/AHA versus JNC 8 hypertension guidelines. BMC Public Health. 2020;20:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension global hypertension practice guidelines: J Hypertens 2020;38:982–1004. [DOI] [PubMed] [Google Scholar]
- 24.Rayner B, Jones E, Veriava Y, Seedat Y. South African Hypertension Society commentary on the American College of Cardiology/American Heart Association hypertension guidelines. Cardiovasc J Afr 2019;30:184–187. [DOI] [PubMed] [Google Scholar]
- 25.Poulter NR, Castillo R, Charchar FJ, Schlaich MP, Schutte AE, Tomaszewski M, Touyz RM, Wang J-G. Are the American Heart Association/American College of Cardiology High Blood Pressure Guidelines Fit for Global Purpose?: Thoughts From the International Society of Hypertension. Hypertension. 2018;72:260–262. [DOI] [PubMed] [Google Scholar]
- 26.Venkateshmurthy NS, Geldsetzer P, Jaacks LM, Prabhakaran D. Implications of the New American College of Cardiology guidelines for hypertension prevalence in India. JAMA Intern Med 2018;178:1416–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wander GS, Ram CVS. Global impact of 2017 American Heart Association/American College of Cardiology hypertension guidelines: a perspective from India. Circulation. 2018;137:549–550. [DOI] [PubMed] [Google Scholar]
- 28.Khera R, Lu Y, Lu J, Saxena A, Nasir K, Jiang L, Krumholz HM. Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and China: nationally representative cross sectional study. BMJ 2018;362:k2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas CA, Azizi F, Cifkova R, Di Cesare M, Eriksen L, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol 2015;3:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol 2004;94:20–24. [DOI] [PubMed] [Google Scholar]
- 31.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, Brindle P. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, Stevens G, Riley LM, Savin S, Khan T, Altay S, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7:e1332–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muntner P, Colantonio LD, Cushman M, Goff DC, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 35.Dupas P, Miguel E. Impacts and determinants of health levels in low-income countries. In: Handbook of economic field experiments. Elsevier; 2017. pages 3–93. [Google Scholar]
- 36.Sudharsanan N, Chen S, Garber M, Bärnighausen T, Geldsetzer P. The Effect Of Home-Based Hypertension Screening On Blood Pressure Change Over Time In South Africa. Health Aff (Millwood). 2020;39:124–132. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Sudharsanan N, Huang F, Liu Y, Geldsetzer P, Bärnighausen T. Impact of community based screening for hypertension on blood pressure after two years: regression discontinuity analysis in a national cohort of older adults in China. BMJ 2019;366:l4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osamor PE, Owumi BE. Factors associated with treatment compliance in hypertension in southwest Nigeria. Journal H Popul Nutr 2011;29:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables I-V
Supplemental Figures I-LV


