Abstract
Liver stiffness measurement (LSM) by transient elastography (TE) is a non-invasive assessment for diagnosing and staging liver fibrosis in non-alcoholic fatty liver disease (NAFLD). Evidence on its role as a longitudinal monitoring tool is lacking. This study aims to evaluate the role of TE in monitoring NAFLD improvement following bariatric surgery. This study prospectively recruited 101 morbidly obese patients undergoing laparoscopic bariatric surgery for intraoperative liver biopsy. Thirty-seven patients of the cohort received perioperative TE. Postoperative anthropometric, biochemical and LSM data were collected annually for 5 years. In 101 patients receiving liver biopsy (mean age 40.0 ± 10.3 years, mean body-mass-index (BMI) 40.0 ± 5.7 kg/m2), NASH and liver fibrosis were diagnosed in 42 (41.6%) and 48 (47.5%) patients respectively. There were 29 (28.7%) stage 1, 11 (10.9%) stage 2, 7 (6.9%) stage 3, and 1 (1.0%) stage 4 fibrosis. In 37 patients receiving TE (mean age 38.9 ± 10.8 years, mean BMI 41.1 ± 5.6 kg/m2), the percentages of total weight loss were 21.1 ± 7.6% at 1 year, 19.7 ± 8.3% at 3 years, and 17.1 ± 7.0% at 5 years after surgery. The mean LSM reduced significantly from 9.8 ± 4.6 kPa at baseline to 6.9 ± 3.4 kPa at 1 year, 7.3 ± 3.0 kPa at 3 years, and 6.8 ± 2.6 kPa at 5 years (P = 0.002). Using pre-defined LSM cut-offs, the rates of significant fibrosis, advanced fibrosis and cirrhosis being ruled out at 5 years improved from baseline values of 43.7 to 87.5% (P < 0.001), 56.8 to 91.7% (P < 0.001), and 64.9 to 91.7% (P < 0.001), respectively. TE was a useful monitoring tool in demonstrating the improvement of liver fibrosis following bariatric surgery.
Subject terms: Liver, Liver diseases, Obesity
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of chronic liver diseases characterized by liver steatosis, steatohepatitis (NASH) and liver fibrosis due to over-nutrition and its associated metabolic syndrome1. With the rising global epidemic of obesity, the prevalence of NAFLD has increased rapidly over the last two decades2. Bariatric surgery is currently recommended as a standard treatment for obese type 2 diabetes mellitus (T2DM)3. Because NAFLD represents the hepatic manifestation of metabolic syndrome, bariatric surgery is increasingly advocated as a treatment for NAFLD4–6. Despite the lack of prospective randomized trials comparing the effects of bariatric surgery with other interventions on NAFLD, recent systematic reviews and meta-analyses have demonstrated the benefits of bariatric surgery in normalizing liver biochemistry and reversing histological markers of NAFLD7–10.
To evaluate the efficacy of bariatric surgery in treating NAFLD, an optimal assessment tool has not yet been established. While most studies rely on liver biochemistry as surrogate markers of NAFLD, normal liver enzyme levels cannot exclude the disease7–10. To date, histological assessment by liver biopsy is regarded as the gold standard in diagnosing different degrees of NAFLD and confirming the improvement of NAFLD in response to interventions1,4–6,11,12. Being an invasive assessment with concerns on complications, high execution cost, sampling error and inter-observer variability, repeated liver biopsies cannot be recommended to every patient after bariatric surgery13. Hence, other non-invasive modalities, such as imaging techniques, are warranted for follow-up monitoring of NAFLD. Ultrasonography and magnetic resonance imaging (MRI) are commonly employed for the initial diagnosis and subsequent monitoring of NAFLD in an attempt to reduce the need of liver biopsy. Although ultrasonography offers the advantages of wide availability and low cost, its sensitivity in detecting liver steatosis in morbidly obese patients is disappointing14. While MRI is a sensitive tool in quantifying liver steatosis even in morbidly obese patients and magnetic resonance elastography (MRE) is highly accurate in detecting liver fibrosis, both techniques are largely limited by high costs and low accessibility14.
Vibration-controlled transient elastography (TE) is one of the available non-invasive assessment tools for NAFLD. It works by generating vibrations of mild amplitude and low frequency to induce elastic shear waves that propagate through liver tissues for stiffness measurement15. Newer models currently allow simultaneous quantification of liver fibrosis by liver stiffness measurement (LSM) and liver steatosis by controlled attenuation parameter (CAP)16. TE provides multiple advantages of low cost, short procedure time, immediate result availability, good reproducibility and the ability to be performed in an outpatient setting. Its roles in diagnosing NAFLD and assessing the severity of NAFLD have been extensively investigated in cross-sectional studies15,17–20. However, evidences on its role as a monitoring tool in longitudinal studies are largely lacking21–25. Whether TE can serve as a follow-up assessment tool after bariatric surgery or other interventions remains to be elucidated. This study aims to evaluate the role of TE as a monitoring tool for the improvement of NAFLD in patients undergoing bariatric surgery.
Material and methods
Patients
This was a prospective longitudinal observational study on consecutive adults who received laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG), and laparoscopic Roux-en-Y gastric bypass (RYGB) for the treatment of morbid obesity between January 2011 and February 2013. Following the International Federation for the Surgery of Obesity and Metabolic Disorders—Asian Pacific Chapter consensus statement 2011, patients with a body-mass-index (BMI) ≥ 35 kg/m2 regardless of the existence of comorbidities or BMI ≥ 30 kg/m2 with inadequately controlled T2DM or metabolic syndrome were eligible for surgery26. We excluded patients who had (i) excessive alcohol consumption > 140 g/week in men and > 70 g/week in women; (ii) positive hepatitis B surface antigen or anti-hepatitis C virus antibody; (iii) secondary causes of liver steatosis including autoimmune or metabolic causes; (iv) been using drugs that could induce liver steatosis or insulin sensitization, such as corticosteroids, methotrexate, and tamoxifen; (iv) prior hepatocellular carcinoma or liver resection; (v) any malignancy in the preceding 5 years, and (vi) the operation performed as a revisional procedure from other types of bariatric surgery. The study protocol was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee and was conducted in accordance with the ethical standards of the Helsinki Declaration of 1964 and its later versions. Written informed consent was obtained from all patients for study enrolment.
Operative technique
The decision on the type of surgery was made by the patients after a detailed counselling on the risks and benefits of different procedures by the bariatric surgeons. All procedures were performed by two experienced bariatric surgeons. Our techniques of LAGB, LSG and RYGB were previously described27–29.
Laparoscopic liver biopsy
Transperitoneal liver biopsy was performed on all patients during laparoscopic bariatric surgery. A liver sample of ≥ 15 mm long was taken from left hepatic lobe using 16-gauge Temno™ II semi-automatic biopsy needle (Cardinal Health, Dublin, Ohio, United States). It was evaluated by one experienced histopathologist after preparation with hematoxylin & eosin stain and Masson’s trichrome stain. Histopathological findings were reported using Non-Alcoholic Steatohepatitis Clinical Research Network Scoring System30. The NAFLD activity score (NAS) was the sum of scores for hepatic steatosis (score S0–3), lobular inflammation (score L0–3) and hepatocyte ballooning (score B0–2). In this study, NASH was defined using the histopathological algorithm for morbidly obese patients proposed by Bedossa et al.31 NASH was defined as present when the three scores for steatosis, lobular inflammation and hepatocellular ballooning were all ≥ 1. Fibrosis was staged from 0 to 4: stage F0, no fibrosis; F1, peri-sinusoidal or peri-portal fibrosis; F2, peri-sinusoidal and portal/peri-portal fibrosis; F3, bridging fibrosis; and F4, cirrhosis5. Stage F2 or above was defined as significant fibrosis (SF) and stage F3 or above was defined as advanced fibrosis (AF).
Transient elastography
Our study protocol was revised in the latter third of the study to add perioperative TE for LSM in the recruited patients. TE by FIBROSCAN (Echosens, Paris, France) was performed within 4 weeks prior to bariatric surgery and then annually for 5 years after surgery. All examinations were performed by a single officially-trained operator who had independently performed over 50 examinations. After prior fasting for 6 h, LSM was conducted using M and XL probes being placed between intercostal spaces over right hepatic lobe when patients were lying in dorsal decubitus position with their right arms in maximal abduction. For every patient, ten valid acquisitions were obtained. The median value was used to represent the liver elastic modulus in kilopascal (kPa). The interquartile range (IQR) of LSM was taken as the interval containing 50% of valid measurements between 25 and 75th percentiles. The success rate was calculated by dividing the number of valid acquisitions with the total number of acquisitions. LSM was considered reliable if ≥ 10 valid acquisitions were obtained and the IQR-to-median ratio of LSM was ≤ 0.3. At baseline, both M and XL probes were used. During subsequent follow-up assessments, M probe was used for patients with BMI < 30 kg/m2 and XL probe was used for those having BMI ≥ 30 kg/m2. If reliable results could not be obtained from M probe, additional examination using XL probe was conducted. Because one patient might have reliable TE obtained by both M and XL probes, an overall LSM was chosen from reliable results by XL probe in patients having BMI ≥ 30 kg/m2 or from reliable results by M probe in those having BMI < 30 kg/m2.
Follow-up assessment
All patients recruited for TE were prospectively assessed at baseline and then followed annually for 5 years after surgery. During each assessment, anthropometric measurement of body weight, BMI, percentage of body fat, and waist circumference was conducted. Body fat percentage was estimated by an impedance body composition analyser (X-Scan Plus, Accuniq, Seoul, Korea). The percentage of total weight loss (%TWL) and the percentage of excess weight loss (%EWL) were used to represent the extent of weight reduction. %EWL was calculated using a BMI value of 25 kg/m2 as the ideal body weight. Biochemical parameters including total bilirubin, alkaline phosphatase (ALP), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), fasting blood glucose (FBG), glycosylated hemoglobin (A1c), total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), C reactive protein (CRP), creatinine, albumin, platelet count and mean platelet volume were measured after overnight fasting.
Primary outcome
The primary outcome was the changes in the proportions of patients having significant fibrosis ruled out, advanced fibrosis ruled out, and cirrhosis ruled out before and after bariatric surgery as measured by LSM cut-off. Because different LSM cut-off values were being reported for NAFLD with different sensitivities and specificities in different population cohorts, this study adopted the cut-off values as previously reported by our group32,33. These same cut-off values were adopted and recommended by the World Federation for Ultrasound in Medicine and Biology Guidelines34. For M probe, the cut-off values used to rule out significant fibrosis, advanced fibrosis and cirrhosis were < 9.0 kPa, < 9.6 kPa, and < 11.5 kPa, respectively32. For XL probe, the cut-off values used to rule out significant fibrosis, advanced fibrosis and cirrhosis were < 8.2 kPa, < 9.3 kPa, and < 11.0 kPa, respectively33.
Secondary outcomes
Several other measures were assessed as secondary outcomes. The baseline prevalence of NASH and liver fibrosis in the whole cohort receiving intraoperative liver biopsy was estimated. In patients receiving TE, the postoperative changes in weight parameters, LSM, and liver biochemistry were assessed. The rates of patients having LSM improvement during follow-up were also estimated. LSM improvement was defined as having ≥ 30% reduction from baseline in the overall LSM measured. Comparison of the changes in the clinical and biochemical parameters between patients with and without LSM improvement at 1 year and 5 years after bariatric surgery was also conducted.
Statistical analysis
Statistical analysis was performed using IBM SPSS STATISTICS version 25 (IBM, New York, United States). Changes in postoperative weight loss outcomes and biochemical parameters were compared by Wilcoxon signed rank test and Friedman test. Chi-square test or Fisher exact test was used for comparison of nominal and categorical variables. The area under the curve (AUC) of a receiver operating characteristics (ROC) curve was used to assess the performance of LSM as a diagnostic test for liver fibrosis. A two-sided P value of < 0.05 was regarded as significant. Follow-up data of those with lost-to-follow-up were not included in the analyses.
Results
Baseline characteristics
In this study, 101 ethnic Chinese morbidly obese patients (38 males and 63 females) received intraoperative liver biopsy during laparoscopic bariatric surgery (39 LAGB, 57 LSG and 5 RYGB). A total of 37 patients (17 males and 20 females) consented for LSM and received TE before and after surgery (9 LAGB, 25 LSG and 3 RYGB). The baseline characteristics and biochemical variables of the cohort with LSM (n = 37) are summarized in Table 1.
Table 1.
Baseline characteristics of the patient cohorts.
| Patients receiving intraoperative liver biopsy and perioperative LSM (N = 37) | |
|---|---|
| Age (years) | 38.9 ± 10.8 |
| Gender (male : female)a | 17 (45.9) : 20 (54.1) |
| Body weight (kg) | 108.8 ± 17.9 |
| Body mass index (kg/m2) | 41.1 ± 5.6 |
| Body fat percentage (%) | 48.5 ± 11.3 |
| Waist circumference (cm) | 123.5 ± 13.5 |
| Metabolic syndromea | 16 (43.2) |
| Type 2 diabetes mellitusa | 18 (48.6) |
| Pre-diabetesa | 7 (18.9) |
| Hypertensiona | 19 (51.4) |
| Dyslipidemiaa | 16 (43.2) |
| Osteoarthritisa | 20 (54.1) |
| Obstructive sleep apneaa | 21 (56.8) |
| Gastroesophageal reflux diseasea | 6 (16.2) |
| Polycystic ovarian syndromea | 6/20 (30.0) |
| Bilirubin (umol/L) | 11.5 ± 4.1 |
| Alkaline phosphatase (IU/L) | 69.9 ± 16.6 |
| Alanine transferase (IU/L) | 45.9 ± 25.4 |
| Gamma glutamyl transferase (U/L) | 54.3 ± 37.2 |
| Fasting blood glucose (mmol/L) | 6.9 ± 2.9 |
| Glycosylated hemoglobin (%) | 7.3 ± 1.8 |
| Total cholesterol (mmol/L) | 4.9 ± 1.1 |
| Triglyceride (mmol/L) | 1.7 ± 0.7 |
| High density lipoprotein cholesterol (mmol/L) | 1.2 ± 0.3 |
| Low density lipoprotein cholesterol (mmol/L) | 3.0 ± 1.0 |
| C reactive protein (ug/ml)b | 3.9 (2.5–7.0) |
| Creatinine (umol/L) | 66.8 ± 16.9 |
| Albumin (g/L) | 43.5 ± 2.7 |
| Platelet count (× 109/L) | 270.4 ± 58.6 |
| Mean platelet volume (fL) | 8.7 ± 0.8 |
| Liver biopsy findingsa | |
| Steatosisc | |
| S0 | 1 (2.7%) |
| S1 | 11 (29.7%) |
| S2 | 14 (37.8%) |
| S3 | 11 (29.7%) |
| Lobular inflammationc | |
| L0 | 6 (16.2%) |
| L1 | 27 (73.0%) |
| L2 | 4 (10.8%) |
| L3 | 0 (0%) |
| Hepatocellular ballooningc | |
| B0 | 14 (37.8%) |
| B1 | 21 (56.8%) |
| B2 | 2 (5.4%) |
| NAFLD activity scorec | |
| 0 | 1 (2.7%) |
| 1 | 3 (8.1%) |
| 2 | 5 (13.5%) |
| 3 | 8 (21.6%) |
| 4 | 8 (21.6%) |
| 5 | 9 (24.3%) |
| 6 | 3 (8.1%) |
| 7 | 0 (0%) |
| 8 | 0 (0%) |
| Fibrosis staged | |
| F0 | 13 (35.1%) |
| F1 | 14 (37.8%) |
| F2 | 6 (16.2%) |
| F3 | 3 (8.1%) |
| F4 | 1 (2.7%) |
| NASHe | 23 (62.2%) |
Data are mean ± standard deviations unless otherwise specified. LSM, liver stiffness measurement; NASH, non-alcoholic steatohepatitis.
aCount (percentage).
bMedian (interquartile range).
cHepatic steatosis was graded by the percentage of parenchymal involvement with steatosis under low- to medium-power evaluation: score S0, steatosis < 5%; score S1, steatosis 5–33%; score S2, steatosis 33–66%; and score S3, steatosis > 66%. Lobular inflammation was assessed by the number of inflammatory foci per 200-power field: score L0, no foci; score L1, < 2 foci; score L2, 2–4 foci; and score L3, > 4 foci. Hepatocellular ballooning was graded as B0 for no ballooning, B1 for few balloon cells, and B2 for many cells or prominent ballooning. NAFLD activity score was the sum of scores for hepatic steatosis (score S0–3), lobular inflammation (score L0–3) and hepatocyte ballooning (score B0–2).
dNASH was defined as steatosis score ≥ 1 plus lobular inflammation score ≥ 1 plus hepatocellular ballooning score ≥ 1.
eFibrosis was staged from 0 to 4: stage F0, no fibrosis; F1, peri-sinusoidal or peri-portal fibrosis; F2, peri-sinusoidal and portal/peri-portal fibrosis; F3, bridging fibrosis; and F4, cirrhosis.
Baseline prevalence of NAFLD in whole cohort
Liver biopsy was successfully performed without perioperative complications in all 101 patients. Forty-two (41.6%) patients were diagnosed to have NASH. A total of 62 (61.4%) patients and 24 (23.8%) patients had NAS ≥ 3 and NAS ≥ 5 respectively. Forty-eight (47.5%) patients had different degrees of liver fibrosis. The prevalence of SF, AF and cirrhosis was 18.8% (n = 19), 7.9% (n = 8) and 1.0% (n = 1) respectively.
Baseline performance of pre-defined LSM cut-offs
At baseline, all 37 patients successfully completed LSM by TE. The degree of liver fibrosis upon liver biopsy was significantly correlated with the baseline LSM results (r = 0.415, P = 0.015). Using pre-defined LSM cut-off values, 16 (43.2%) patients had SF ruled out, 21 (56.8%) patients had AF ruled out, and 24 (64.9%) patients had cirrhosis ruled out. Using liver biopsy, SF was absent in 27 (73.0%) patients, AF was absent in 33 (89.2%) patients, and cirrhosis was absent in 36 (97.3%) patients. For the diagnostic sensitivity of the pre-defined cut-off values, it was 51.9% for ruling out SF, 57.6% for ruling out AF, and 66.7% for ruling out cirrhosis. As for the diagnostic specificity, it was 80.0% for ruling out SF, 75.0% for ruling out AF, and 100% for ruling out cirrhosis. LSM was a fair diagnostic tool for ruling out SF (AUC = 0.715, 95%C.O. 0.517–0.913, P = 0.047) but not for ruling out AF (AUC = 0.663, 95%C.I. 0.390–0.936, P = 0.293).
Postoperative rates of different fibrosis degrees using pre-defined LSM cut-offs
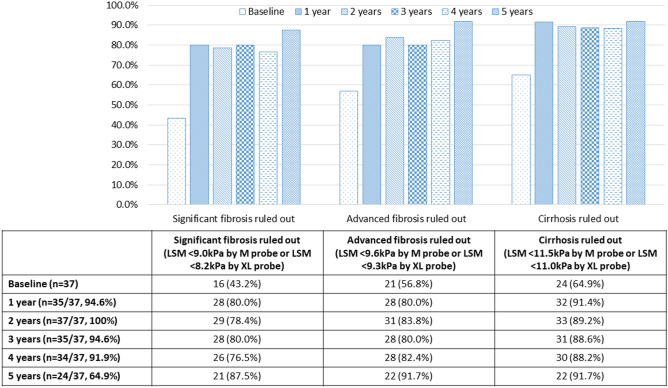
Among 37 patients receiving TE, the rates of follow-up after surgery were 94.6% (n = 35/37) at 1 year, 100% (n = 37/37) at 2 years, 94.6% (n = 35/37) at 3 years, 91.9% (n = 34/37) at 4 years, and 64.9% (n = 24/37) at 5 years. The postoperative rates of ruling out SF, AF and cirrhosis by the overall LSM results are presented in Fig. 1. The rates of patients having SF ruled out by LSM improved from 43.2% at baseline to 80.0% at 1 year (P = 0.001), 80.0% at 3 years (P = 0.001), and 87.5% at 5 years (P < 0.001). Similarly, the rates of patients having AF ruled out by LSM increased from 56.8% at baseline to 80.0% at 1 year (P = 0.001), 80.0% at 3 years (P = 0.001), and 91.7% at 5 years (P < 0.001). The rates of patients having cirrhosis ruled out by LSM also improved significantly from 64.9% at baseline to 91.4% at 1 year (P < 0.001), 88.6% at 3 years (P < 0.001), and 91.7% at 5 years (P < 0.001).
Figure 1.
Postoperative rates of significant fibrosis ruled out, advanced fibrosis ruled out and cirrhosis ruled out using the pre-defined LSM cut-off.
Postoperative LSM improvement
There was significant reduction in the overall LSM results from 9.8 ± 4.6 kPa at baseline to 6.9 ± 3.4 kPa at 1 year, 7.3 ± 3.0 kPa at 3 years, and 6.8 ± 2.6 kPa at 5 years (P = 0.002) (Table 2). The rates of patients having LSM improvement (≥ 30% reduction in LSM level) were 54.3% (19/35) at 1 year, 48.6% (17/35) at 3 years, and 45.8% (11/24) at 5 years. After surgery, the rates of reliable LSM results obtained by M probe increased significantly from 32.4% at baseline to 84.0% at 1 year, 96.6% at 3 years, and 100% at 5 years (P < 0.001). While there was a decrease in the number of patients being eligible to use XL probe after surgery due to BMI reduction, the rates of reliable results achieved were similar throughout the five postoperative years.
Table 2.
LSM results of transient elastography by XL probe and M probe after bariatric surgery.
| Baseline (n = 37) | 1 year (n = 35/37, 94.6%) | 2 years (n = 37/37, 100%) | 3 years (n = 35/37, 94.6%) | 4 years (n = 34/37, 91.9%) | 5 years (n = 24/37, 64.9%) | |
|---|---|---|---|---|---|---|
| XL probe | ||||||
| Patients receiving test | 37 | 22 | 14 | 8 | 9 | 6 |
| Patients with reliable test | 35 (94.6%) | 20 (90.9%) | 12 (85.7%) | 8 (100%) | 9 (100%) | 6 (100%) |
| LSM (kPa)a | 9.7 ± 4.4 | 7.3 ± 4.0 | 8.8 ± 6.2 | 7.7 ± 4.4 | 8.0 ± 3.0 | 7.2 ± 4.0 |
| M probe | ||||||
| Patients receiving test | 37 | 25 | 28 | 29 | 25 | 18 |
| Patients with reliable test | 12 (32.4%) | 21 (84.0%) | 24 (85.7%) | 28 (96.6%) | 25 (100%) | 18 (100%) |
| LSM (kPa)a | 10.7 ± 5.4 | 6.5 ± 2.0 | 6.8 ± 2.7 | 7.0 ± 2.5 | 6.7 ± 2.9 | 6.7 ± 2.2 |
| Overallb | ||||||
| Patients receiving test | 37 | 35 | 37 | 35 | 34 | 24 |
| Patients with reliable results | 37 (100%) | 35 (100%) | 35 (94.6%) | 35 (100%) | 34 (100%) | 24 (100%) |
| LSM (kPa)a | 9.8 ± 4.6 | 6.9 ± 3.4 | 7.4 ± 4.3 | 7.3 ± 3.0 | 7.0 ± 3.0 | 6.8 ± 2.6 |
| LSM improvement (≥ 30% reduction from baseline) | – | 19 (54.3%) | 17 (48.6%) | 17 (48.5%) | 12 (35.3%) | 11 (45.8%) |
Data are counts (percent) unless otherwise specified. LSM, liver stiffness measurement.
aLSM results (mean ± standard deviation) from reliable tests.
bOverall results from tests using XL probe for patients with BMI ≥ 30 kg/m2 or from tests using M probe for patients with BMI < 30 kg/m2.
Postoperative weight and biochemical improvement
As demonstrated in Table 3, there was significant reduction in body weight, BMI, percentage of body fat and waist circumference after surgery (all P < 0.001). The %EWL was 58.6 ± 22.8% at 1 year, 53.9 ± 23.1% at 3 years, and 47.1 ± 21.4% at 5 years. The %TWL was 21.1 ± 7.6% at 1 year, 19.7 ± 8.3% at 3 years, and 17.1 ± 7.0% at 5 years. Both %EWL and %TWL were maintained without significant reduction till 5 years (both P = 0.149). Regarding liver biochemistry, there was significant reduction in ALT (P < 0.001) and GGT (P < 0.001) while serum bilirubin and ALP remained unchanged. For metabolic parameters, significant improvement was observed in FBG (P = 0.002), A1c (P < 0.001), TG (P < 0.001), HDL-C (P = 0.005), and CRP (P < 0.001) levels. Although serum creatinine levels showed statistically significant change (P = 0.002), all the postoperative creatinine results were within normal range. There was no change in the postoperative results of serum albumin, platelet count and mean platelet volume.
Table 3.
Changes in anthropometric and biochemical outcomes after bariatric surgery.
| Baseline (n = 37) | 1 year (n = 35/37, 94.6%) | 2 years (n = 37/37, 100%) | 3 years (n = 35/37, 94.6%) | 4 years (n = 34/37, 91.9%) | 5 years (n = 24/37, 64.9%) | P valueb | |
|---|---|---|---|---|---|---|---|
| Body weight (kg) | 108.8 ± 17.9 | 84.2 ± 15.0 | 86.6 ± 19.0 | 86.1 ± 17.1 | 87.7 ± 18.1 | 90.3 ± 18.0 | < 0.001 |
| Body mass index (kg/m2) | 41.1 ± 5.6 | 31.9 ± 4.7 | 32.7 ± 6.1 | 32.7 ± 5.4 | 33.3 ± 5.7 | 33.8 ± 5.3 | < 0.001 |
| Percentage of excess weight loss (%) | 0 | 58.6 ± 22.8 | 56.1 ± 27.1 | 53.9 ± 23.1 | 51.4 ± 22.6 | 47.1 ± 21.4 | – |
| Percentage of total weight loss (%) | 0 | 21.1 ± 7.6 | 20.5 ± 9.5 | 19.7 ± 8.3 | 19.1 ± 8.3 | 17.1 ± 7.0 | – |
| Body fat percentage (%) | 48.5 ± 11.3 | 35.9 ± 9.7 | 35.8 ± 10.9 | 33.5 ± 6.8 | 32.2 ± 7.6 | 32.7 ± 7.8 | < 0.001 |
| Waist circumference (cm) | 123.5 ± 13.5 | 105.3 ± 13.4 | 104.4 ± 15.9 | 103.6 ± 14.1 | 105.6 ± 14.7 | 107.8 ± 14.2 | < 0.001 |
| Bilirubin (umol/L) | 11.5 ± 4.1 | 13.7 ± 9.6 | 13.3 ± 7.0 | 12.6 ± 5.6 | 12.5 ± 6.9 | 11.5 ± 4.4 | 0.869 |
| Alkaline phosphatase (IU/L) | 69.9 ± 16.6 | 68.6 ± 18.0 | 64.8 ± 14.0 | 65.7 ± 16.4 | 67.8 ± 15.5 | 69.2 ± 19.6 | 0.339 |
| Alanine transferase (IU/L) | 45.9 ± 25.4 | 22.4 ± 10.7 | 22.3 ± 14.1 | 23.4 ± 13.0 | 23.4 ± 12.0 | 24.5 ± 11.8 | < 0.001 |
| Gamma glutamyl transferase (U/L) | 54.3 ± 37.2 | 30.8 ± 33.4 | 25.9 ± 15.7 | 27.3 ± 15.5 | 27.6 ± 17.9 | 26.7 ± 14.5 | < 0.001 |
| Fasting blood glucose (mmol/L) | 6.9 ± 2.9 | 5.2 ± 1.2 | 5.4 ± 1.6 | 6.2 ± 2.6 | 5.8 ± 2.1 | 5.7 ± 1.1 | 0.002 |
| Glycosylated hemoglobin (%) | 7.3 ± 1.8 | 5.9 ± 0.7 | 6.1 ± 1.1 | 6.2 ± 1.2 | 6.4 ± 1.4 | 6.4 ± 1.3 | < 0.001 |
| Total cholesterol (mmol/L) | 4.9 ± 1.1 | 4.8 ± 1.0 | 4.7 ± 0.9 | 4.5 ± 1.2 | 4.6 ± 1.1 | 5.0 ± 1.1 | 0.748 |
| Triglyceride (mmol/L) | 1.7 ± 0.7 | 1.1 ± 0.5 | 1.2 ± 0.6 | 1.4 ± 0.9 | 1.4 ± 1.0 | 1.4 ± 0.8 | < 0.001 |
| High density lipoprotein cholesterol (mmol/L) | 1.2 ± 0.3 | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.5 | 0.005 |
| Low density lipoprotein cholesterol (mmol/L) | 3.0 ± 1.0 | 2.7 ± 0.7 | 2.7 ± 0.8 | 2.6 ± 0.8 | 2.7 ± 0.8 | 2.9 ± 0.8 | 0.778 |
| C reactive protein (ug/ml)a | 3.9 (2.5–7.0) | 0.9 (0.6–2.1) | 0.9 (0.6–2.2) | 1.4 (0.6–2.7) | 1.3 (0.6–2.7) | 1.9 (0.8–2.7) | < 0.001 |
| Creatinine (umol/L) | 66.8 ± 16.9 | 66.8 ± 17.6 | 65.9 ± 16.6 | 69.8 ± 20.5 | 72.3 ± 21.6 | 71.1 ± 24.2 | 0.002 |
| Albumin (g/L) | 43.5 ± 2.7 | 43.7 ± 2.7 | 43.2 ± 2.6 | 43.0 ± 2.6 | 42.7 ± 2.9 | 41.4 ± 4.4 | 0.350 |
| Platelet count (× 109/L) | 270.4 ± 58.5 | 253.0 ± 58.6 | 251.0 ± 58.3 | 254.6 ± 63.0 | 249.1 ± 59.4 | 251.2 ± 51.4 | 0.056 |
| Mean platelet volume (fL) | 8.7 ± 0.8 | 8.6 ± 0.8 | 8.6 ± 0.7 | 8.6 ± 0.7 | 8.6 ± 0.8 | 8.6 ± 0.7 | 0.221 |
Data are mean ± standard deviations unless otherwise specified.
aMedian (interquartile range).
bStatistical analysis by Friedman test.
Comparison between patients with and without LSM improvement
As shown in Table 4, postoperative LSM improvement at 1 year was significantly associated with higher percentages of FBG improvement (median 26.9% vs 8.7%, P = 0.020) and A1c improvement (median 19.4% vs 7.7%, P = 0.020). There was no association with weight loss, body fat reduction, changes in the biochemical variables or other components of metabolic syndrome. At 1 year, LSM improvement was detected in 70.6% (N = 12/17) of T2DM patients, 50.0% (N = 3/6) of pre-diabetic patients, and 33.3% (N = 4/12) of non-diabetic patients (P = 0.132). At 5 years, there was no significant association between LSM improvement and all measured clinical and biochemical variables.
Table 4.
Postoperative changes in clinical and biochemical factors associated with LSM improvement at 1 year and 5 years.
| 1 year | 5 years | |||||
|---|---|---|---|---|---|---|
| No LSM improvement N = 16 (45.7%) | LSM improvement N = 19 (54.3%) | P value | No LSM improvement N = 13 (54.2%) |
LSM improvement N = 11 (45.8%) |
P value | |
| Percentage of excess weight loss (%) | 60.9 ± 27.6 | 56.7 ± 18.4 | 0.567 | 50.7 + 24.5 | 42.9 = 17.1 | 0.459 |
| Percentage of total weight loss (%) | 20.6 ± 8.0 | 21.6 ± 7.4 | 0.756 | 16.7 = 7.2 | 17.5 = 7.0 | 0.820 |
| Body fat percent reduction (%) | 22.6 ± 17.7 | 26.2 ± 13.5 | 0.528 | − 47.8 (− 78.9 to − 25.0) | − 44.7 (− 75.9 to − 38.8) | 1.000 |
| Waist circumference reduction (%) | 13.8 ± 10.2 | 13.7 ± 8.0 | 0.935 | 13.2 = 7.0 | 12.8 = 8.3 | 0.740 |
| Total cholesterol decreased (%)a | 4.8 (− 9.9 to 16.3) | 0 (− 22.5to 20.0) | 0.502 | 0 (− 9.3 to 23.2) | − 7.6 (− 19.2 to 0.1) | 0.088 |
| Triglyceride decrease (%)a | 28.3 (15.1 to 40.0) | 27.3 (6.3 to 52.4) | 0.987 | 16.7 (− 16.5 to 41.7) | 8.3 (− 18.8 to 25.0) | 0.494 |
| High density lipoprotein cholesterol increase (%)a | 29.2 (1.4 to 54.2) | 15.4 (7.7 to 42.9) | 0.987 | 30.8 (0 to 43.9) | 27.3 (− 10.0 to 57.1) | 0.910 |
| Low density lipoprotein cholesterol decrease (%)a | 12.4 (− 3.3 to 26.5) | 5.9 (− 35.7 to 19.4) | 0.350 | 7.1 (− 7.2 to 24.8) | − 5.9 (− 46.7 to 4.7) | 0.072 |
| Fasting blood glucose decrease (%)a | 8.7 (− 4.2 to 16.1) | 26.9 (2.1 to 38.7) | 0.020 | 2.1 (− 4.3 to 23.4) | 25.8 (3.9 to 37.5) | 0.148 |
| Glycosylated hemoglobin decrease (%)a | 7.7 (1.7 to 14.6) | 19.4 (9.5 to 32.4) | 0.020 | 10.8 (4.4 to 18.1) | 12.2 (7.9 to 20.5) | 0.531 |
| Systolic blood pressure decrease (%)a | 5.1 (− 1.2 to 13.4) | 10.9 (− 1.8 to 14.6) | 0.441 | 4.5 (3.0 to 12.0) | 14.2 (2.6 to 21.5) | 0.197 |
| Diastolic blood pressure decrease (%)a | 8.0 (0.8 to 13.9) | 5.3 (− 9.4 to 27.2) | 0.612 | − 2.3 (− 10.8 to 4.1) | 1.7 (− 5.9 to 10.3) | 0.223 |
| Bilirubin decrease (%)a | − 12.5 (− 45.0 to 11.5) | − 14.3 (− 28.6 to 20.0) | 0.589 | − 8.3 (− 49.2 to 11.3) | − 16.7 (− 30.0 to 18.2) | 0.733 |
| Alkaline phosphatase decrease (%)a | − 2.4 (− 10.5 to 7.3) | 7.4 (1.3 to 13.8) | 0.067 | 4.9 (− 8.9 to 14.2) | 3.3 (− 17.1 to 12.5) | 0.776 |
| Alanine transferase decrease (%)a | 40.3 (7.6 to 63.2) | 53.1 (31.0 to 71.2) | 0.422 | 13.0 (4.4 to 47.9) | 52.1 (16.4 to 69.0) | 0.096 |
| Gamma glutamyl transferase decrease (%)a | 45.2 (31.4 to 57.3) | 51.1 (33.6 to 67.3) | 0.502 | 38.1 (24.0 to 54.8) | 48.0 (25.0 to 65.5) | 0.459 |
| C reactive protein decrease (%)a | 32.2 (16.7 to 79.3) | 73.1 (47.4 to 85.7) | 0.117 | 36.9 (25.2 to 67.1) | 37.5 (− 10.3 to 68.1) | 0.651 |
| Creatinine decrease (%)a | 0.6 (− 12.9 to 5.8) | 4.5 (− 14.9 to 11.9) | 0.502 | 2.3 (− 8.1 to 12.1) | 12.8 (1.7 to 21.7) | 0.167 |
| Albumin increase (%)a | 0 (− 2.3 to 4.1) | 0 (− 4.5 to 7.1) | 0.683 | 0 (− 7.8 to 5.9) | 4.3 (− 9.8 to 10.5) | 0.691 |
| Platelet count decrease (%)a | 5.7 (4.6 to 20.2) | 6.3 (2.5 to 10.8) | 0.523 | 11.0 (0.3 to 15.7) | 3.2 (7.3 to 13.2) | 0.277 |
| Mean platelet volume decrease (%)a | 3.2 (− 7.8 to 7.1) | 2.1 (1.1 to 3.8) | 0.949 | 0.5 (− 8.0 to 4.3) | 1.7 (− 8.4 to 7.0) | 0.683 |
Data are percentages of improvement of the parameters at 1 year and 5 years presented as mean ± standard deviation.
aPercentages of change of the parameters in median (interquartile range).
LSM, liver stiffness measurement.
Discussion
To evaluate the effectiveness of bariatric surgery in treating NAFLD, measuring the improvement in liver fibrosis is crucial for predicting prognosis35. Liver biopsy is recommended as the gold standard in determining treatment responses or disease progression during NAFLD management30. However, when liver biopsy is used to monitor responses to bariatric surgery, the long-term compliance rate is often disappointing. In two longitudinal studies investigating the impact of bariatric surgery on NAFLD improvement, the compliance rates to repeat liver biopsy at 1 to 5 years were 47.2 to 68.9% only36,37. Because of these pitfalls, non-invasive assessment for liver fibrosis improvement is more preferred. This can be accomplished by either serological or physical tests1,38. Despite the advantages of wide availability, high applicability and good reproducibility, none of the available serological predictive models are liver specific or independent of confounding factors38. Hence, serological tests are not commonly used in bariatric surgical literature39–41. Physical tests of liver fibrosis consist of ultrasound-based elastography and magnetic resonance-based elastography38. Although MRE is highly accurate with low failure rate, it is not justified as a monitoring tool for the concerns of high cost and low availability17. Thus, a more readily-available point-of-care tool with lower cost, like TE, should better suit the purpose of follow-up monitoring. TE is a simple and fast examination with immediate result availability, good reproducibility, and low intra-observer and inter-observer variability38. Our study demonstrated that TE could be adopted as a longitudinal monitoring tool for the reversal of liver fibrosis after bariatric surgery. Despite a slightly lower compliance rate at 5 years due to lost-of-follow-up, TE had high acceptance among our patients with over 90% compliance rates in the first 4 years.
To the best of our knowledge, our study was the first study to evaluate the long-term treatment responses of NAFLD to bariatric surgery using TE as a monitoring tool. In the literature, TE had been tested on the roles of monitoring disease progression and response to treatment of NAFLD21–25. Suzuki et al. reported the changes of LSM over 4 years in 36 patients without paired liver biopsies for monitoring disease progression of NAFLD21. Fibrosis progression as reflected by LSM was observed in 25% of patients over 4 years. The changes in LSM were shown to have correlation with the changes in non-invasive fibrosis markers. However, histopathological correlation with paired liver biopsies was not available. Nogami et al. evaluated the disease progression of NAFLD in terms of fibrosis stage using LSM in 34 patients over 10 years22. Based on LSM, 32.4% of patients had progression in fibrosis stage and 17.6% had improvement. In 14 out of 34 patients, paired liver biopsies were available and the changes in LSM were shown to have correlation with the change in histological fibrosis stage. In a large scale study of 611 diabetic patients conducted by Lee et al., monitoring by TE could identify fibrosis progression (from LSM < 10 kPa to LSM ≥ 10 kPa) and steatosis progression (from CAP < 248 dB/m to CAP ≥ 248 dB/m) in 4.3% and 52.0% of patients, respectively, over 3 years23. As for the longitudinal monitoring of response to treatment for NAFLD, TE had been adopted as a monitoring tool in a few prospective studies24,25. Handzlik et al. evaluated the role of metformin in treating 42 patients with NAFLD using CAP and LSM without liver biopsy24. Significant reductions in CAP and LSM as surrogate markers for hepatic steatosis and fibrosis were observed in patients receiving metformin over 5 months. Similarly, Shimizu et al. investigated the role of dapagliflozin in treating 57 diabetic patients with NAFLD25. Significant improvement in CAP and LSM was observed in patients receiving dapagliflozin over 24 weeks. For the longitudinal response to bariatric surgery, TE had only been evaluated in one short-term follow-up study of 42 patients which showed a significant reduction in median LSM from 8.6 kPa preoperatively to 6.0 kPa at 1 year42. Our study similarly demonstrated a significant reduction in LSM at 1 year. Further to this, our study showed that TE was useful in defining sustainable LSM improvement till 5 years after bariatric surgery.
Looking at the factors associated with LSM improvement at 1 year, the effect of bariatric surgery in treating NAFLD was not attributed to weight loss or changes in other body composition parameters. Instead, LSM improvement was only associated with glycemic improvement after bariatric surgery. Both insulin resistance and hyperinsulinemia play pivotal roles in the pathophysiology of NAFLD. Insulin resistance increases adipocyte lipolysis to promote hepatic uptake of circulating free fatty acids. It also augments hepatic de novo lipogenesis by reducing hepatic glycogen storage, increasing gluconeogenesis, and inducing hyperinsulinemia. All these can result in hepatic lipid accumulation and lipotoxicity that further impair insulin signaling, induce oxidative damage, promote inflammation and cause fibrosis within the liver43. Bariatric surgery is effective in improving insulin resistance, altering lipid metabolism and reversing inflammatory pathways related to NAFLD44. Hence, better improvement in glycemic control after bariatric surgery was associated with LSM improvement. Achieving better postoperative glycemic control was thus the key to treat NAFLD by bariatric surgery.
Earlier studies suggested that the failure rate of TE was lower in obese patients with the use of XL probe45. A study by Wong et al. suggested that the median LSM obtained by M and XL probes were nearly identical at each fibrosis stage when M probe was used in patients with BMI < 30 kg/m2 and XL probe was used in those with BMI ≥ 30 kg/m246. Hence, probe selection during postoperative TE in our study was based on BMI results. While the latest model of TE provides an automated M or XL probe selection based on skin-to-liver capsule distance, our experience suggested that M probe could be readily adopted in providing reliable results in at least 84–100% of patients after bariatric surgery.
We recognize several limitations in this study. First, our patient sample of 37 patients was not large enough to allow direct comparison among different bariatric procedures for the rates of resolution of liver fibrosis. With the additional concerns of loss-to-follow-up from a small sample size, our study also failed to identify any significant association between potential clinical or biochemical variables and long-term LSM improvement at 5 years. Second, this study was conducted with earlier models of TE that did not provide CAP measurement to estimate liver steatosis. Hence, this study was not able to evaluate the rates of resolution of liver steatosis after bariatric surgery. In addition, repeated liver biopsy was not conducted during postoperative follow-up. It was therefore impossible to assess the correlation between LSM improvement and liver biopsy findings. Lastly, our study did not measure the plasma insulin and C-peptide levels for non-diabetic patients. Thus, evaluation of the postoperative changes in insulin resistance (e.g. homeostasis model assessment of insulin resistance) was not possible.
In conclusion, LSM by TE was a useful monitoring tool in assessing the improvement of NAFLD following bariatric surgery. Using LSM for follow-up assessment, bariatric surgery was effective in improving liver fibrosis in a sustainable manner during long-term follow-up.
Acknowledgements
None.
Author contributions
S.Y.L. was responsible for data collection, data analysis, data interpretation, literature search, writing of the manuscript, and generation of figures. V.W.W. was responsible for study design, data collection, data interpretation, and writing of the manuscript. S.K.W. was responsible for study design, data collection, data interpretation, and writing of the manuscript. G.L.W. was responsible for study design, data collection and data interpretation. C.M.L. was responsible for data analysis, data interpretation and generation of figures. C.C.L. was responsible for data collection, data analysis, data interpretation, and generation of figures. S.S.S. was responsible for study design, data collection, and data interpretation. H.L.C. was responsible for study design, data collection, data interpretation, and writing of the manuscript. E.K.N. was responsible for study design, data interpretation, literature search and writing of the manuscript. All authors reviewed the manuscript.
Funding
This study did not receive any funding, grant or support in its conduction, data analyses and manuscript writing.
Competing interests
VW Wong has served as a consultant or advisory board member for 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Hanmi Pharmaceutical, Intercept, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET-NASH, and Terns; and a speaker for AbbVie, Bristol-Myers Squibb, Echosens, and Gilead Sciences. He has also received an unrestricted grant from Gilead Sciences for fatty liver research. GL Wong has served as an advisory committee member for Gilead Sciences and Janssen, and as a speaker for Abbott, Abbvie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen and Roche. She has also received an unrestricted grant from Gilead Sciences for hepatitis research. HL Chan has served as an advisor and speaker for Gilead and Roche. All other authors do not have personal interests to disclose. There is no financial conflict of interests involved in this manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018;33(1):70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 3.Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–877. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol.64(6), 1388–1402 (2016). [DOI] [PubMed]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 6.Chitturi S, Wong VW, Chan WK, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: management and special groups. J. Gastroenterol. Hepatol. 2018;33(1):86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 7.Chavez-Tapia, N. C. et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst. Rev. (1), CD007340 (2010). [DOI] [PMC free article] [PubMed]
- 8.Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019;15(3):502–511. doi: 10.1016/j.soard.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar-Olivos NE, Almeda-Valdes P, Aguilar-Salinas CA, Uribe M, Méndez-Sánchez N. The role of bariatric surgery in the management of nonalcoholic fatty liver disease and metabolic syndrome. Metabolism. 2016;65(8):1196–1207. doi: 10.1016/j.metabol.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Perysinakis I, Pappis HC, Margaris E. Current controversies in metabolic surgery for nonalcoholic fatty liver disease. Obes. Surg. 2019;29(3):1058–1067. doi: 10.1007/s11695-019-03705-x. [DOI] [PubMed] [Google Scholar]
- 11.Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig. Liver Dis.49(5), 471–483 (2017). [DOI] [PubMed]
- 12.Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J. Gastroenterol. 2018;24(30):3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American association for the study of liver diseases. Liver biopsy. Hepatology. 2009;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 14.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Wong GL, Wong VW. Application of transient elastography in nonalcoholic fatty disease [published online ahead of print, 2019 Nov 8] Clin. Mol. Hepatol. 2019 doi: 10.3350/cmh.2019.0001n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lédinghen V, Wong GL, Vergniol J, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016;31(4):848–855. doi: 10.1111/jgh.13219. [DOI] [PubMed] [Google Scholar]
- 17.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 18.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598–607.e2. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–637.e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease–the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol. Ther. 2014;39(3):254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, Yoneda M, Imajo K, et al. Transient elastography for monitoring the fibrosis of non-alcoholic fatty liver disease for 4 years. Hepatol. Res. 2013;43(9):979–983. doi: 10.1111/hepr.12039. [DOI] [PubMed] [Google Scholar]
- 22.Nogami A, Yoneda M, Kobayashi T, et al. Assessment of 10-year changes in liver stiffness using vibration-controlled transient elastography in non-alcoholic fatty liver disease. Hepatol. Res. 2019;49(8):872–880. doi: 10.1111/hepr.13349. [DOI] [PubMed] [Google Scholar]
- 23.Lee HW, Wong GL, Kwok R, et al. Serial transient elastography examinations to monitor patients with type 2 diabetes: a prospective cohort study [published online ahead of print, 2020 Jan 28] Hepatology. 2020 doi: 10.1002/hep.31142. [DOI] [PubMed] [Google Scholar]
- 24.Handzlik G, Holecki M, Kozaczka J, et al. Evaluation of metformin therapy using controlled attenuation parameter and transient elastography in patients with non-alcoholic fatty liver disease. Pharmacol. Rep. 2019;71(2):183–188. doi: 10.1016/j.pharep.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019;21(2):285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 26.Kasama K, Mui W, Lee WJ, et al. IFSO-APC consensus statements 2011. Obes. Surg. 2012;22(5):677–684. doi: 10.1007/s11695-012-0610-7. [DOI] [PubMed] [Google Scholar]
- 27.Wong SK, So WY, Yau PY, et al. Laparoscopic adjustable gastric banding for the treatment of morbidly obese patients: early outcome in a Chinese cohort. Hong Kong Med. J. 2005;11(1):20–29. [PubMed] [Google Scholar]
- 28.Liu SY, Wong SK, Lam CC, Yung MY, Kong AP, Ng EK. Long-term results on weight loss and diabetes remission after laparoscopic sleeve gastrectomy for a morbidly obese Chinese population. Obes. Surg. 2015;25(10):1901–1908. doi: 10.1007/s11695-015-1628-4. [DOI] [PubMed] [Google Scholar]
- 29.Liu SY, Wong SK, Lam CC, Ng EK. Bariatric surgery for Prader-Willi syndrome was ineffective in producing sustainable weight loss: long term results for up to 10 years. Pediatr Obes. 2020;15(1):e12575. doi: 10.1111/ijpo.12575. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 32.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 33.Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2012;107(12):1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 34.Ferraioli G, Wong VW, Castera L, et al. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med. Biol. 2018;44(12):2419–2440. doi: 10.1016/j.ultrasmedbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 36.Caiazzo R, Lassailly G, Leteurtre E, et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann. Surg. 2014;260(5):893–899. doi: 10.1097/SLA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 37.Salman MA, Mikhail HMS, Nafea MA, et al. Impact of laparoscopic sleeve gastrectomy on fibrosis stage in patients with child-A NASH-related cirrhosis [published online ahead of print, 2020 Mar 9] Surg. Endosc. 2020;20:20. doi: 10.1007/s00464-020-07498-4. [DOI] [PubMed] [Google Scholar]
- 38.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281.e4. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan CH, Al-Kalifah N, Ser KH, Lee YC, Chen JC, Lee WJ. Long-term effect of bariatric surgery on resolution of nonalcoholic steatohepatitis (NASH): an external validation and application of a clinical NASH score. Surg. Obes. Relat. Dis. 2018;14(10):1600–1606. doi: 10.1016/j.soard.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Koh ZJ, Salgaonkar HP, Lee WJ, et al. Improvement in non-alcoholic fatty liver disease score correlates with weight loss in obese patients undergoing laparoscopic sleeve gastrectomy: a two-centre study from an Asian cohort. Obes. Surg. 2019;29(3):862–868. doi: 10.1007/s11695-018-3581-5. [DOI] [PubMed] [Google Scholar]
- 41.Ooi GJ, Burton PR, Doyle L, et al. Modified thresholds for fibrosis risk scores in nonalcoholic fatty liver disease are necessary in the obese. Obes. Surg. 2017;27(1):115–125. doi: 10.1007/s11695-016-2246-5. [DOI] [PubMed] [Google Scholar]
- 42.Garg H, et al. Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg. Obes. Relat. Dis. 2018;14(1):81–91. doi: 10.1016/j.soard.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol. Clin. North Am. 2016;45(4):639–652. doi: 10.1016/j.gtc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laursen TL, Hagemann CA, Wei C, et al. Bariatric surgery in patients with non-alcoholic fatty liver disease—from pathophysiology to clinical effects. World J. Hepatol. 2019;11(2):138–149. doi: 10.4254/wjh.v11.i2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia B, Wang F, Friedrich-Rust M, et al. Feasibility and efficacy of transient elastography using the XL probe to diagnose liver fibrosis and cirrhosis: a meta-analysis. Medicine (Baltimore) 2018;97(39):e11816. doi: 10.1097/MD.0000000000011816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong VW, Irles M, Wong GL, et al. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut. 2019;68(11):2057–2064. doi: 10.1136/gutjnl-2018-317334. [DOI] [PubMed] [Google Scholar]