Abstract
Our aim was to analyse body core temperature and peripheral vascular microcirculation at skin hypothenar eminence of the hands and its relationship to symptoms in fibromyalgia syndrome (FMS). A total of 80 FMS women and 80 healthy women, matched on weight, were enrolled in this case–control study. Thermography and infrared thermometer were used for evaluating the hypothenar regions and core body temperature, respectively. The main outcome measures were pain pressure thresholds (PPTs) and clinical questionnaires. Significant associations were observed between overall impact [β = 0.033; 95% confidence interval (95%CI) = 0.003, 0.062; p = 0.030], daytime dysfunction (β = 0.203; 95%CI = 0.011, 0.395; p = 0.039) and reduced activity (β = 0.045; 95%CI = 0.005, 0.085; p = 0.029) and core body temperature in FMS women. PPTs including greater trochanter dominant (β = 0.254; 95%CI = 0.003, 0.504; p = 0.047), greater trochanter non-dominant (β = 0.650; 95%CI = 0.141, 1.159; p = 0.013), as well as sleeping medication (β = −0.242; 95%CI = −0.471, −0.013; p = 0.039) were also associated with hypothenar eminence temperature. Data highlighted that FMS women showed correlations among body core temperature and hand temperature with the clinical symptoms.
Keywords: central sensitization, core body temperature, fibromyalgia, peripheral nervous system, pain pressure threshold, symptoms, thermography
Introduction
Fibromyalgia syndrome (FMS) is defined as a chronic widespread musculoskeletal pain on the left and right side of the human body accompanied by a complex set of symptoms such as hyperalgesia, tenderness on pressure threshold, persistent fatigue, stiffness, sleep problems and psychiatric disorders such as anxiety.1 The prevalence of the FMS within the European population is 4.7%, and has higher prevalence in women than men (4.2% versus 0.2% respectively).2
Although the pathogenesis of FMS remains unknown, disorders of the central nervous system (CNS) regarding pain modulation may explain the diffuse musculoskeletal pain. Several studies have reported that patients with FMS present hyperactivity and hyper-excitability of their CNS which suggest it is supported by continued nociceptive peripheral inputs.3 However, new findings indicate the existence of blood microcirculation alterations by changes in the innervation to the arterio–venous anastomoses (AVAs) and peripheral skin disorders in patients with FMS as an underlying mechanism in order to understand this syndrome.4,5 AVAs are direct connections between small arteries and veins located in the depth of the dermis of the glabrous skin, especially in the hypothenar regions of the hands.4,6 The principal role of AVAs is to transport the heat from the body core to the surface areas, and they are innervated by sympathetic and sensory branches.4,6 Albrecht et al.4 examined hypothenar skin biopsies of FMS patients to study the innervation of AVAs and their connection with the peripheral nervous system (PNS). They found an altered neural vasoregulation of the AVAs characterized by an over-representation of peptidergic sensory innervation over the noradrenergic sympathetic function. Likewise, this peptidergic sensory innervation of AVAs expressed hyper-responsiveness of the alpha-2-adrenergic receptor, which produces a sympathetic release of noradrenaline/norepinephrine that constricts the smooth muscles of the tunica media of blood vessels.4 Conceivably, this increase of the sensory innervation of hand AVAs in women diagnosed with FMS causes vasodilatation due to that the stimulation of peptidergic sensory fibres at the skin can activate the local ‘axon reflex’ and liberate a large amount of substance P and calcitonin-gene-related peptide (CGRP) at blood flow.4 These molecules are potent vasodilators and inflammatory mediators able to change the sensory mechanisms that operate among capillaries and precapillary arterioles, thereby influencing the peripheral blood circulation.7,8 So, on the basis of this information, the neurovascular pathology of the AVAs would lead a dysregulation of the core body temperature and could cause a lack of blood flow, nutrition and optimal oxygenation to the musculoskeletal deep tissues, favouring the deposit of metabolites and lactic acid that would explain the deep pain and generalized fatigue characteristic in patients diagnosed with FMS. Moreover, it could affect the circadian blood flow and therefore the quality of sleep and the cognition health of these patients.9,10
Regarding the core body temperature, Scholander et al.11 introduced the notion of thermoneutral zone as ‘the basal metabolism in a resting mammal when the ambient temperature is between 37°C of the core body temperature and a lower temperature depending on the insulation of the animal body’. So, the thermogenesis is defined as heat production related to the physiological process necessary for maintaining general body thermoregulation and basal metabolism.11 The scientific literature shows that a dysfunction in the autonomic nervous system (ANS) of FMS patients could affect the sweating functions and the regulation of body heat loss, altering the processes of global body human thermoregulation and explaining, in this way, the perfusion deficits of blood flow at musculoskeletal level, deep pain, exercise intolerance, and ‘brain fog’ symptoms of this syndrome.12 In contrast, it has been reported that FMS patients present an increase of their core body temperature (which contradicts the lack of blood flow in deep tissues) due to ANS disorders,12,13 but the results on this aspect are still controversial due to the discrepancies in the use of different devices for determining the body temperature.14
On the other hand, infrared thermography (IT) reports information about cutaneous temperature and skin thermal functionality due to circulation and the peripheral blood flow of the cutaneous tissues.15,16 Thermography is related to the ‘heat transfer theory’, indicating that a major blood flow in a part of the body reflects heat regions. By contrast, smaller blood flow in a part of the body reflects cold regions.17
To the best of our knowledge, no studies have measured the peripheral blood flow of the glabrous skin of the hands throughout thermography, core body temperature and their relationship with pain intensity, tenderness and general symptoms in FMS patients. Besides, the scientific literature shows contradictions about the lack of blood flow in deep tissues and the increase of the body core temperature in FMS patients. In light of this background, the purpose of this study was to analyse the core body temperature and the peripheral temperature at the skin surface of the hypothenar eminence of the hands and to examine the association of these outcomes with the pain intensity, pain pressure threshold (PPT), fatigue, sleep quality, anxiety and quality of life in a population of women diagnosed with FMS and healthy controls.
Material and methods
Design and participants
An observational case–control study was conducted on patients with FMS from two fibromyalgia associations (AGRAFIM and AFIXA, Spain). We recruited 80 female patients with FMS and 80 healthy women matched for weight, aged between 30 and 70, from relatives or friends of the patients with FMS and volunteers by local advertisement from the Faculty of Health Sciences (University of Granada) to participate in the study. FMS women had been previously diagnosed by a professional rheumatologist of the Public Health System of Andalucía (Spain) and met the 1990 American College of Rheumatology (ACR) criteria for FMS.18 The exclusion criteria were male sex, presence of cardiac, renal or hepatic insufficiency, severe physical disability, fever after infection in the previous 2 weeks, hypotension/hypertension, psychiatric illness, neurological disorders, cancer, previous history of surgery, treatment with vasoactive drugs or anticoagulants or drug history, and skin alterations.
Each participant signed informed consent and completed some structured questionnaires regarding the medical history, medications, menopause status and clinical symptoms. The study was approved by the ethics committee of the University Hospital of Granada (Approval Number 1797-N-17) and was conducted between January 2019 and June 2019. This research was performed in strict compliance with the international code of medical ethics established by the World Medical Association and the Declaration of Helsinki (modified in 2013).
Clinical variables
Patient-reported outcomes were evaluated in the following order: (a) self-administered questionnaires: Visual Analogue Scale (VAS), Revised Fibromyalgia Impact Questionnaire (FIQ-R), Central Sensitization Inventory (CSI), Pittsburgh Quality of Sleep Questionnaire Index (PSQI), Multidimensional Fatigue Inventory (MFI) and Beck Anxiety Inventory (BAI); (b) peripheral vascular response of the hands through IT; (c) core body temperature through IT; and (d) PPT with a digital pressure algometer, which was included at the end of the protocol, since it may influence the results of the other outcomes.
Peripheral blood flow
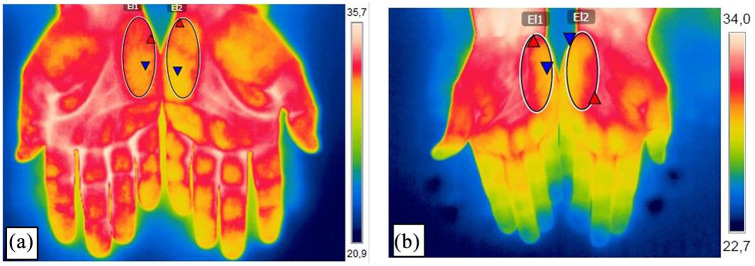
Peripheral blood flow was recorded with a digital thermal camera (FLIR B335, FLIR Systems AB, Täby, Sweden). Atmospheric temperature of the camera was set at 20°C and spectral emissivity was set at 0.98, since human skin behaves as a blackbody with an emissivity of 0.96–0.99.19,20 It has demonstrated a sensitivity of 90% and a specificity of 86%.21 All thermograms were conducted with compliance to the recommendations proposed by the European Association of Thermology (EAT)22 and performed by the same specialist. Before participation in the study, patients were instructed to wear comfortable clothing and avoid wearing watches, bracelets and rings on the hands. The patients stayed in a sitting position in a quiet room at a constant room temperature of 20°C following an acclimatization period of 20 min. During thermal image acquisition, the patients had their hands positioned with splayed fingers resting on a table. The distance between the camera and the skin of the hypothenar eminence of both hands was 60 cm.23 After, the mean temperature of the hypothenar eminence regions of both hands was calculated using the software FLIR Tools software (Figure 1). Taking into consideration the changes in the circadian rhythm, thermography was performed in the afternoon for the FMS and control groups.24
Figure 1.
Thermography image of the hypothenar eminence of both hands of a woman diagnosed with fibromyalgia and a healthy control.
(a) Image of the palms of the hands from a fibromyalgia patient; (b) image of the palms of the hands from a healthy control patient. The analysis of the temperature of the skin surface was conducted through a circle at the hypothenar eminence of each hand (diameter 31 × 75 mm).
Core body temperature
The core body temperature was evaluated in the external auditory canal through an infrared thermometer (Infrared Dermal Thermometers, Exergen, Watertown, Massachusetts 02472 U.S.A. This method reflects an accurate measurement of core temperature due to the association of the tympanic artery with the hypothalamus, which is the thermoregulatory centre in mammals.25 Infrared thermometry has demonstrated a sensitivity of 91% and a specificity of 90%.25,26
Pain pressure threshold
A digital pressure algometer (Wagner Instruments, Greenwich, CT, USA) was used to measure PPT. PPT was assessed bilaterally over the 18 tender points considered by the ACR for FMS diagnosis.18 The mean of three trials was calculated and it used for the main analysis. A 30 s resting period was allowed between each recording. The reliability of pressure algometry has been found to be high the same day (intraclass correlation coefficient: 0.91).27
Visual analogue scale
To assess the subjective intensity of pain, we used a VAS of pain consisting of a 100 mm line from 0 (no pain) to 100 (the worst pain imaginable). The VAS was shown to be an important instrument in pain evaluation, being sensitive and specific in the assessment of pain in FMS.28
Revised Fibromyalgia Impact Questionnaire
The Spanish version of the FIQ-R was used to assess the degree of FMS symptoms and potential limitations. This self-reported questionnaire consisted of 21 items assessing physical impairment, number of days feeling good, work missed, ability to do work, pain, fatigue, restfulness, stiffness, anxiety and depressive symptoms. The total score came from the sum of all subscales (activity level, overall impact and intensity of symptoms), where higher scores indicated negative impact (0–100).29 The degree of FMS symptoms according to FIQ-R is established by the following cut-off points: FIQ-R ⩽30 reflects no severity, FIQ-R >30 and ⩽45 reflects mild severity, FIQ-R >46 and ⩽65 reflects moderate severity, and FIQ-R >65 reflects high severity.30 The test–retest reliability analysis showed a correlation of 0.82.29
Central sensitization
The characteristic symptoms of patients with central sensitization syndrome (CSS) in conjunction with FMS were assessed using the Spanish version of the CSI.31 The questionnaire consisted of 25 items with current symptoms related to the CSS. Each item is scored from 0 (never) to 4 points (always). The total score is 100 points, where higher scores reflected a major severity of symptoms. The test–retest reliability analysis showed a correlation of 0.91 for the CSI in Spanish.31
Quality of sleep
To assess the quality of sleep, we used the Spanish version of the PSQI.32 This questionnaire had seven subscales: subjective quality, sleep latency, sleep duration, habitual sleep efficacy, sleep perturbations, use of night medication, and daily dysfunction. Each subscale was scored from 0 (no problems) to 3 points (severe problems). The total score varies in a range from 0 to 21 points, where higher scores reflected worse quality of sleep. The test–retest reliability analysis showed a correlation of 0.80.32
Impact of fatigue
We used the Spanish version of the MFI to assess the impact of fatigue of the FMS patients.33 The questionnaire contained five dimensions: general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue. The scores per dimension run from 1 to 5 points, where higher scores indicated more fatigue. The test–retest reliability analysis showed a correlation of 0.64 from 0.91.33
Anxiety
The symptoms of anxiety and the psychological health were assessed using the BAI.34 This questionnaire contained 21 items that evaluated the degree of anxiety. Each item is scored from 0 points (nothing anxiety) to 3 points (a lot of anxiety). The total scores run from 0 to 63 points, where higher scores indicate increased anxiety. The test–retest reliability analysis showed a correlation of 0.75.34
Statistical analysis
The data were analysed with the SPSS version 24.0 for Windows (IBM Corporation, Armonk, NY, USA). To verify whether the variables had a normal distribution, the Kolmogorov–Smirnov test was used (p > 0.05). To compare the differences of means between fibromyalgia/healthy women groups, we used unpaired Student’s t test with 95% confidence interval (95%CI; α-value = 0.05) for continuous data. To analyse the differences in clinical variables between core body temperature and peripheral blood flow at the skin surface of the hypothenar eminence of the hands of women diagnosed with FMS and healthy women, first, we divided both populations according to their medians. Therefore, the FMS population was categorized in the low core body temperature group: median <36.05°C; high core body temperature group: median ⩾36.05°C; low hypothenar eminence temperature group: median <32.87°C; high hypothenar eminence temperature group: median ⩾32.87°C. In the same way, the medians for core body temperature (low temperature group: median <35.80°C; high temperature group: median ⩾35.80°C) and the hypothenar eminence temperature (low temperature group: median <31.00°C; high temperature group: median ⩾31.00°C) were also obtained and categorized for healthy women. Second, a two-way analysis of covariance after adjustment for age and body mass index (BMI) was performed. Finally, linear regression analyses were conducted to evaluate the associations among core body temperature and peripheral blood-flow findings and PPT, VAS, FIQ-R, central sensitization, sleep, fatigue, and anxiety, after adjustment for age, BMI and menopause status.
According to the previous study of Brusselmans et al.,13 a sample size of 40 FMS patients and 40 healthy controls was estimated in order to provide a 95%CI, a power of 80%, and α-value = 0.05. Finally, sample size was increased until 225 subjects, taking account of an expected percentage of losses around 60.39% for women diagnosed with FMS and around 67.74% for healthy controls. Sample size calculation was performed by using NCSS-PASS software (http://www.ncss.com/).
Results
Demographic and physical characteristics
Table 1 lists the demographic and physical characteristics of 80 women diagnosed with FMS (mean age 56.11 ± 7.93 years) and 80 healthy controls (mean age of 57.34 ± 9.72 years). Significant differences were observed between FMS women and healthy controls regarding to hypothenar eminence temperature (p < 0.001). However, no significant differences were found between FMS women and healthy controls for age (p = 0.384), height (p = 0.401), weight (p = 0.198), BMI (p = 0.200) and tympanic temperature (p = 0.071). On the other hand, the mean and standard deviation of VAS, central sensitization, sleep quality, fatigue and anxiety were significantly higher in FMS women than in the controls (p < 0.001). All PPTs were significantly lower in FMS women than in the controls (p < 0.001). Note that the mean total score of FIQ-R was 70.95 ± 16.65 in FMS women, which reflects high severity.
Table 1.
Demographic and physical characteristics of women with fibromyalgia and healthy women.
| Cases (n = 80) | Controls (n = 80) | p value | ||
|---|---|---|---|---|
| Age (years) | 56.11 ± 7.93 | 57.34 ± 9.72 | 0.384 | |
| Height (cm) | 159.14 ± 5.64 | 158.34 ± 6.35 | 0.401 | |
| Weight (kg) | 71.28 ± 11.06 | 69.18 ± 9.40 | 0.198 | |
| BMI (kg/m2) | 28.69 ± 5.91 | 27.66 ± 3.95 | 0.200 | |
| VAS (mm) | 73.25 ± 18.54 | 19.49 ± 26.06 | <0.001* | |
| FIQ-R | ||||
| FIQ-R.1 | 19.92 ± 5.71 | – | – | |
| FIQ-R.2 | 13.69 ± 5.11 | – | – | |
| FIQ-R.3 | 37.35 ± 8.89 | – | – | |
| Total score | 70.95 ± 16.65 | – | – | |
| CSS | 67.73 ± 11.58 | 27.99 ± 15.45 | <0.001* | |
| Sleep Questionnaire Index global score | 15.21 ± 3.82 | 7.06 ± 4.79 | <0.001* | |
| Fatigue | ||||
| MFI general fatigue | 18.16 ± 2.36 | 10.10 ± 4.64 | <0.001* | |
| MFI physical fatigue | 16.69 ± 2.81 | 9.71 ± 4.61 | <0.001* | |
| MFI mental fatigue | 15.16 ± 1.72 | 11.94 ± 3.14 | <0.001* | |
| MFI reduced activity | 15.29 ± 3.72 | 8.15 ± 4.24 | <0.001* | |
| MFI reduced motivation | 14.31 ± 3.34 | 7.61 ± 3.54 | <0.001* | |
| Anxiety | 32.14 ± 9.86 | 11.96 ± 10.99 | <0.001* | |
| Hypothenar eminence temperature (°C) | 32.71 ± 1.33 | 30.95 ± 1.78 | <0.001* | |
| Tympanic temperature (°C) | 35.96 ± 0.66 | 35.77 ± 0.67 | 0.071 | |
| Pain pressure thresholds | ||||
| Occipital | D | 0.96 ± 0.72 | 3.28 ± 1.38 | <0.001* |
| ND | 0.89 ± 0.68 | 3.09 ± 1.28 | <0.001* | |
| Cervical Low | D | 1.11 ± 0.76 | 3.07 ± 1.82 | <0.001* |
| ND | 1.06 ± 0.77 | 3.11 ± 2.06 | <0.001* | |
| Trapezium | D | 1.03 ± 0.72 | 3.09 ± 1.41 | <0.001* |
| ND | 0.99 ± 0.62 | 3.32 ± 1.73 | <0.001* | |
| Supraspinatus | D | 1.27 ± 0.80 | 3.61 ± 1.93 | <0.001* |
| ND | 1.27 ± 0.73 | 3.57 ± 1.89 | <0.001* | |
| Second rib | D | 0.91 ± 0.48 | 2.40 ± 1.28 | <0.001* |
| ND | 0.87 ± 0.47 | 2.49 ± 1.16 | <0.001* | |
| Epicondyle | D | 0.97 ± 0.60 | 3.18 ± 1.46 | <0.001* |
| ND | 0.99 ± 0.58 | 3.35 ± 1.50 | <0.001* | |
| Second metacarpal | D | 1.23 ± 0.79 | 3.16 ± 1.47 | <0.001* |
| ND | 1.18 ± 0.66 | 3.22 ± 1.79 | <0.001* | |
| Gluteal muscle | D | 1.99 ± 1.57 | 6.20 ± 2.59 | <0.001* |
| ND | 1.89 ± 1.27 | 6.13 ± 2.48 | <0.001* | |
| Greater trochanter | D | 2.09 ± 1.19 | 5.91 ± 2.40 | <0.001* |
| ND | 2.09 ± 1.12 | 5.48 ± 2.39 | <0.001* | |
| Knee | D | 1.70 ± 1.24 | 5.41 ± 2.31 | <0.001* |
| ND | 1.86 ± 1.22 | 5.70 ± 2.49 | <0.001* | |
| Anterior tibial | D | 1.86 ± 1.40 | 5.09 ± 2.47 | <0.001* |
| ND | 1.82 ± 1.18 | 5.04 ± 2.37 | <0.001* | |
Data are expressed as mean ± SD.
Statistically significant.
Significance level p < 0.05.
BMI, body mass index; CSS, central sensitization syndrome; D, dominant; FIQ-R, revised Fibromyalgia Impact Questionnaire; FIQ-R.1, activity level of the FIQ; FIQ-R.2, overall impact of the FIQ-R; FIQ-R.3, intensity of symptoms of the FIQ-R; MFI, Multidimensional Fatigue Inventory; ND, non-dominant; SD, standard deviation; VAS, visual analogue scale.
Comparison of clinical variables between higher core body temperature and lower core body temperature in women diagnosed with FMS and healthy women
The comparison of clinical variables between the higher core body temperature and the lower core body temperature is shown in Table 2. For the FMS group (higher core temperature and lower core temperature), statistically significant differences in daytime dysfunction assessed by the PSQI (F = 4.562; p = 0.036) and mental fatigue using the MFI (F = 5.209; p = 0.025) were observed. For the healthy controls, no significant differences were found.
Table 2.
Comparison of clinical data between higher core body temperature and lower core body temperature in women with fibromyalgia (cases) and healthy women (controls).
| Cases (n = 80) |
Controls (n = 80) |
||||||
|---|---|---|---|---|---|---|---|
| Low core body temperature °C (n = 40) | High core body temperature °C (n = 40) | MD between groups | Low core body temperature °C (n = 45) | High core body temperature °C (n = 35) | MD between groups | ||
| VAS (mm) | 72.00 ± 21.27 (65.20, 78.80) | 74.50 ± 15.52 (69.54, 79.46) | −2.50 (−10.78, 5.79) | 15.33 ± 23.32 (8.33, 22.34) | 25.00 ± 28.74 (14.97, 35.03) | −9.667 (−21.33, 1.99) | |
| FIQ-R total score | 68.77 ± 17.00 (63.34, 74.22) | 73.13 ± 16.21 (67.95, 78.32) | −4.358 (−11.75, 3.04) | – | – | – | |
| CSS | 66.33 ± 11.1 (62.71, 69.94) | 69.13 ± 11.81 (65.35, 72.90) | −2.800 (−7.95, 2.35) | 27.89 ± 13.60 (23.80, 31.98) | 28.11 ± 17.75 (22.02, 34.21) | −0.225 (−7.20, 6.75) | |
| Sleep Questionnaire Index | |||||||
| Sleep quality | 2.15 ± 0.71 (1.92, 2.38) | 2.25 ± 0.87 (1.97, 2.53) | −0.096 (−0.45, 0.26) | 1.22 ± 0.97 (0.93, 1.51) | 1.29 ± 0.79 (1.01, 1.56) | −0.063 (−0.47, 0.34) | |
| Sleep latency | 2.34 ± 0.78 (2.09, 2.60) | 2.33 ± 0.92 (2.03, 2.62) | 0.017 (−0.37, 0.40) | 1.40 ± 1.16 (1.05, 1.75) | 1.20 ± 1.05 (0.84, 1.56) | 0.200 (−0.30, 0.70) | |
| Sleep duration | 2.00 ± 0.89 (1.70, 2.30) | 2.13 ± 0.98 (1.81, 2.45) | −0.128 (−0.56, 0.30) | 0.98 ± 1.12 (0.64, 1.31) | 0.71 ± 1.05 (0.36, 1.07) | 0.263 (−0.22, 0.75) | |
| Habitual sleep efficiency | 2.11 ± 1.09 (1.74, 2.48) | 1.86 ± 1.16 (1.48, 2.25) | 0.246 (−0.28, 0.77) | 0.93 ± 1.23 (0.56, 1.30) | 0.51 ± 1.07 (0.15, 0.88) | 0.419 (−0.10, 0.94) | |
| Sleep disturbances | 2.21 ± 0.47 (2.05, 2.37) | 2.33 ± 0.92 (2.16, 2.49) | −0.114 (−0.34, 0.11) | 1.38 ± 0.61 (1.19, 1.56) | 1.51 ± 0.56 (1.32, 1.71) | −0.137 (−0.40, 0.13) | |
| Sleeping medication | 2.05 ± 1.32 (1.62, 2.48) | 2.10 ± 1.30 (1.69, 2.51) | −0.049 (−0.63, 0.54) | 0.64 ± 1.17 (0.29, 1.00) | 0.80 ± 1.18 (0.39, 1.21) | −0.156 (−0.68, 0.37) | |
| Daytime dysfunction | 2.00 ± 0.89 (1.71, 2.29) | 2.40 ± 0.63 (2.20, 2.60) | −0.400* (−0.75, 0.06) | 0.80 ± 0.87 (0.54, 1.06) | 0.66 ± 0.84 (0.37, 0.95) | 0.143 (−0.24, 0.53) | |
| Global score | 15.06 ± 3.40 (13.90, 16.21) | 15.35 ± 4.22 (13.94, 16.76) | −0.296 (−2.01, 1.50) | 7.36 ± 4.76 (5.92, 8.79) | 6.69 ± 4.87 (5.01, 8.36) | 0.670 (−1.49, 2.83) | |
| Fatigue | |||||||
| MFI general fatigue | 18.08 ± 2.40 (17.31, 18.84) | 18.25 ± 2.34 (17.50, 19.00) | −0.175 (−1.2, 0.88) | 10.24 ± 5.06 (8.73, 11.76) | 9.91 ± 4.11 (8.50, 11.33) | 0.330 (−1.76, 2.42) | |
| MFI physical fatigue | 16.53 ± 2.78 (15.64, 17.41) | 16.85 ± 2.86 (15.94, 17.76) | −0.325 (−1.58, 0.93) | 9.73 ± 4.54 (8.37, 11.10) | 9.69 ± 4.76 (8.05, 11.32) | 0.048 (−2.03, 2.13) | |
| MFI mental fatigue | 14.75 ± 1.86 (14.15, 15.35) | 15.58 ± 1.48 (15.10, 16.05) | −0.825* (−1.58, 0.08) | 11.91 ± 3.20 (10.95, 12.87) | 11.97 ± 3.10 (10.91, 13.04) | −0.060 (−1.48, 1.35) | |
| MFI reduced activity | 14.60 ± 3.61 (13.45, 15.75) | 15.98 ± 3.74 (14.78, 17.17) | −1.375 (−3.01, 0.26) | 8.71 ± 4.32 (7.41, 10.01) | 7.43 ± 4.09 (6.02, 8.83) | 1.283 (−0.61, 3.18) | |
| MFI reduced motivation | 14.08 ± 3.56 (12.94, 15.21) | 14.55 ± 3.13 (13.55, 15.55) | −0.475 (−1.97, 1.02) | 8.16 ± 3.70 (7.05, 9.26) | 6.91 ± 3.25 (5.80, 8.03) | 1.241 (−0.33, 2.81) | |
| Anxiety | 30.43 ± 10.98 (26.91, 33.94) | 33.85 ± 8.38 (31.17, 36.53) | −3.425 (−7.77, 0.93) | 12.78 ± 11.63 (9.28, 16.27) | 10.88 ± 10.15 (7.34, 14.42) | 1.895 (−3.09, 6.88) | |
| Pain pressure thresholds | |||||||
| Occipital | D | 0.92 ± 0.71 (0.69, 1.15) | 0.99 ± 0.74 (0.76, 1.23) | −0.075 (−0.40, 0.25) | 3.13 ± 0.93 (2.84, 3.40) | 3.49 ± 1.80 (2.88, 4.12) | −0.371 (−0.99, 0.25) |
| ND | 0.87 ± 0.67 (0.66, 1.09) | 0.91 ± 0.70 (0.68, 1.13) | −0.036 (−0.35, 0.27) | 3.00 ± 1.04 (2.68, 3.32) | 3.21 ± 1.53 (2.68, 3.73) | −0.204 (−0.78, 0.37) | |
| Pain pressure thresholds | |||||||
| Cervical Low | D | 1.15 ± 0.70 (0.92, 1.37) | 1.06 ± 0.83 (0.80, 1.33) | 0.084 (−0.26, 0.43) | 2.91 ± 1.58 (2.43, 3.39) | 3.27 ± 2.09 (2.56, 3.99) | −0.367 (−1.19, 0.46) |
| ND | 1.10 ± 0.81 (0.84, 1.35) | 1.04 ± 0.73 (0.80, 1.27) | 0.061 (−0.28, 0.41) | 2.94 ± 1.76 (2.40, 3.47) | 3.33 ± 2.39 (2.51, 4.16) | −0.396 (−1.33, 0.54) | |
| Trapezium | D | 1.00 ± 0.70 (0.78, 1.23) | 1.06 ± 0.75 (0.82, 1.30) | −0.052 (−0.37, 0.27) | 2.93 ± 1.21 (2.57, 3.30) | 3.29 ± 1.63 (2.73, 3.86) | −0.358 (−0.99, 0.27) |
| ND | 1.00 ± 0.66 (0.79, 1.21) | 0.98 ± 0.60 (0.78, 1.17) | 0.026 (−0.25, 0.31) | 3.28 ± 1.69 (2.76, 3.79) | 3.38 ± 1.80 (2.76, 4.00) | −0.105 (−0.89, 0.68) | |
| Supraspinatus | D | 1.21 ± 0.72 (0.98, 1.43) | 1.33 ± 0.89 (1.05, 1.62) | −0.131 (−0.49, 0.23) | 3.36 ± 1.81 (2.80, 3.91) | 3.92 ± 2.06 (3.22, 4.63) | −0.567 (−1.43, 0.31) |
| ND | 1.29 ± 0.79 (1.04, 1.54) | 1.25 ± 0.68 (1.04, 1.47) | 0.034 (−0.29, 0.36) | 3.40 ± 2.00 (2.79, 4.00) | 3.79 ± 1.76 (3.18, 4.40) | −0.390 (−1.25, 0.47) | |
| Second rib | D | 0.86 ± 0.44 (0.72, 1.00) | 0.95 ± 0.53 (0.78, 1.12) | −0.090 (−0.31, 1.26) | 2.35 ± 1.09 (2.02, 2.67) | 2.48 ± 1.50 (1.97, 3.00) | −0.139 (−0.72, 0.44) |
| ND | 0.86 ± 0.44 (0.70, 1.01) | 0.89 ± 0.45 (0.75, 1.03) | −0.034 (−0.24, 0.17) | 2.46 ± 1.11 (2.12, 2.79) | 2.54 ± 1.24 (2.11, 2.96) | −0.078 (−0.61, 0.45) | |
| Epicondyle | D | 0.91 ± 0.53 (0.74, 1.08) | 1.03 ± 0.58 (0.85, 1.22) | −0.124 (−0.37, 0.12) | 3.17 ± 1.32 (2.77, 3.58) | 3.18 ± 1.65 (2.62, 3.75) | −0.007 (−0.67, 0.66) |
| ND | 0.99 ± 0.66 (0.78, 1.21) | 0.98 ± 0.49 (0.82, 1.13) | 0.022 (−0.24, 0.28) | 3.31 ± 1.55 (2.84, 3.78) | 3.38 ± 1.47 (2.88, 3.89) | −0.07 (−0.76, 0.61) | |
| Second metacarpal | D | 1.12 ± 0.65 (0.92, 1.34) | 1.33 ± 0.92 (1.03, 1.63) | −0.202 (−0.58, 0.15) | 2.97 ± 1.23 (2.60, 3.34) | 3.40 ± 1.72 (2.80, 3.98) | −0.426 (−1.08, 0.24) |
| ND | 1.05 ± 0.58 (0.87, 1.24) | 1.18 ± 0.72 (0.95, 1.41) | −0.129 (−0.42, 0.16) | 2.95 ± 1.43 (2.51, 3.38) | 3.56 ± 2.14 (2.83, 4.30) | −0.614 (−1.41, 0.19) | |
| Gluteal muscle | D | 1.89 ± 1.46 (1.42, 2.36) | 2.09 ± 1.68 (1.55, 2.64) | −0.205 (−0.91, 0.49) | 6.12 ± 2.55 (5.34, 6.89) | 6.30 ± 2.69 (5.38, 7.23) | −0.188 (−1.36, 0.99) |
| ND | 1.88 ± 1.27 (1.48, 2.30) | 1.89 ± 1.28 (1.48, 2.30) | −0.004 (−0.57, 0.56) | 6.08 ± 2.42 (5.34, 6.81) | 6.21 ± 2.61 (5.32, 7.10) | −0.136 (−1.26, 0.99) | |
| Greater trochanter | D | 2.12 ± 1.25 (1.72, 2.52) | 2.07 ± 1.14 (1.71, 2.44) | 0.043 (−0.48, 0.57) | 5.73 ± 2.43 (4.99, 6.47) | 6.15 ± 2.38 (5.33, 6.96) | −0.418 (−1.51, 0.68) |
| ND | 2.03 ± 1.13 (1.66, 2.38) | 2.16 ± 1.11 (1.80, 2.52) | −0.135 (−0.63, 0.37) | 5.31 ± 2.26 (4.63, 5.99) | 5.71 ± 2.56 (4.83, 6.58) | −0.392 (−1.48, 0.69) | |
| Knee | D | 1.73 ± 1.14 (1.36, 2.10) | 1.67 ± 1.33 (1.25, 2.10) | 0.058 (−0.49, 0.61) | 5.33 ± 2.03 (4.71, 5.95) | 5.51 ± 2.66 (4.60, 6.42) | −0.179 (−1.23, 0.87) |
| ND | 2.04 ± 1.38 (1.60, 2.49) | 1.68 ± 1.03 (1.35, 2.01) | 0.363 (−0.18, 0.91) | 5.76 ± 2.18 (5.10, 6.43) | 5.61 ± 2.87 (4.63, 6.60) | 0.150 (−0.98, 1.28) | |
| Anterior tibial | D | 1.81 ± 1.35 (1.38, 2.25) | 1.91 ± 1.45 (1.45, 2.38) | −0.099 (−0.72, 0.53) | 4.75 ± 2.16 (4.09, 5.40) | 5.51 ± 2.79 (4.55, 6.47) | −0.766 (−1.87, 0.34) |
| ND | 1.86 ± 1.18 (1.49, 2.24) | 1.77 ± 1.19 (1.39, 2.15) | 0.095 (−0.43, 0.62) | 4.87 ± 2.05 (4.25, 5.49) | 5.24 ± 2.73 (4.31, 6.18) | −0.372 (−1.44, 0.70) | |
Data are expressed as mean ± standard deviation (95% confidence interval).
Significant differences between cases and controls (two-way analysis of covariance).
CSS, central sensitization syndrome; D, dominant; FIQ-R, revised Fibromyalgia Impact Questionnaire; FIQ-R.1, activity level of the FIQ; FIQ-R.2, overall impact of the FIQ-R; FIQ-R.3, intensity of symptoms of the FIQ-R; MD, difference in means; MFI, Multidimensional Fatigue Inventory; ND, non-dominant; VAS, Visual Analogue Scale.
Comparison
of clinical variables between higher hypothenar temperature and lower hypothenar temperature in women diagnosed with FMS and healthy women
Table 3 shows the comparison of clinical variables between the higher and the lower hypothenar eminence temperature of the hands for the FMS women and the healthy controls. For the FMS group (higher hypothenar temperature and lower hypothenar temperature), statistically significant differences in the subjective intensity of pain (F = 4.426; p = 0.039) only, were observed. For the healthy controls, no significant differences were found.
Table 3.
Comparison of clinical data between higher hypothenar eminence temperature and lower hypothenar eminence temperature in women with fibromyalgia (cases) and healthy women (controls).
| Cases (n = 80) |
Controls (n = 80) |
||||||
|---|---|---|---|---|---|---|---|
| Low thenar temperature °C (n = 40) | High thenar temperature °C (n = 40) | MD between groups | Low thenar temperature °C (n = 40) | High thenar temperature °C (n = 40) | MD between groups | ||
| VAS (mm) | 69.00 ± 22.28 (61.87, 76.13) | 77.50 ± 12.76 (73.42, 81.58) | −8.500* (−16.62, 0.38) | 16.00 ± 23.51 (8.48, 23.52) | 23.08 ± 28.29 (13.90, 32.25) | −7.077 (−18.72, 4.56) | |
| FIQ-R total score | 71.77 ± 18.42 (65.87, 77.65) | 70.14 ± 14.86 (65.39, 74.89) | 1.625 (−5.83, 9.07) | – | – | – | |
| CSS | 67.50 ± 12.51 (63.50, 71.50) | 67.95 ± 10.72 (64.52, 71.38) | −0.450 (−5.64, 4.74) | 27.43 ± 15.70 (22.40, 32.45) | 28.55 ± 15.37 (23.63, 33.47) | −1.125 (−8.04, 5.79) | |
| Sleep Questionnaire Index | |||||||
| Sleep quality | 2.28 ± 0.72 (2.05, 2.50) | 2.13 ± 0.86 (1.85, 2.41) | 0.147 (−0.21, 0.50) | 1.20 ± 0.91 (0.91, 1.49) | 1.30 ± 0.88 (1.02, 1.58) | −0.100 (−0.50, 0.30) | |
| Sleep latency | 2.40 ± 0.78 (2.15, 2.65) | 2.26 ± 0.92 (1.96, 2.57) | 0.137 (−0.25, 0.52) | 1.23 ± 1.16 (0.85, 1.60) | 1.40 ± 1.06 (1.0, 1.74) | −0.175 (−0.67, 0.32) | |
| Sleep duration | 2.13 ± 0.96 (1.81, 2.45) | 2.00 ± 0.91 (1.70, 2.30) | 0.132 (−0.30, 0.56) | 0.83 ± 1.03 (0.49, 1.16) | 0.90 ± 1.15 (0.53, 1.27) | −0.075 (−0.56, 0.41) | |
| Habitual sleep efficiency | 1.95 ± 1.13 (1.57, 2.32) | 2.03 ± 1.13 (1.64, 2.41) | −0.082 (−0.61, 0.47) | 0.65 ± 1.10 (0.30, 1.00) | 0.85 ± 1.25 (0.45, 1.25) | −0.200 (−0.72, 0.32) | |
| Sleep disturbances | 2.30 ± 0.52 (2.13, 2.47) | 2.24 ± 0.49 (2.08, 2.40) | 0.063 (−0.16, 0.29) | 1.43 ± 0.59 (1.23, 1.62) | 1.45 ± 0.60 (1.26, 1.64) | −0.025 (−0.29, 0.24) | |
| Sleeping medication | 2.33 ± 1.16 (1.95, 2.70) | 1.82 ± 1.39 (1.37, 2.27) | 0.504 (−0.07, 1.08) | 0.65 ± 1.14 (0.28, 1.02) | 0.78 ± 1.21 (0.39, 1.16) | −0.125 (−0.65, 0.40) | |
| Daytime dysfunction | 2.28 ± 0.78 (2.02, 2.53) | 2.13 ± 0.80 (1.87, 2.39) | 0.147 (−0.21, 0.50) | 0.70 ± 0.72 (0.47, 0.93) | 0.78 ± 0.97 (0.46, 1.09) | −0.075 (−0.46, 0.31) | |
| Global score | 15.57 ± 3.55 (14.38, 1675) | 14.83 ± 4.08 (13.45, 16.22) | 0.734 (−1.05, 2.52) | 6.68 ± 4.47 (5.24, 8.11) | 7.45 ± 5.12 (5.81, 9.09) | −0.775 (−2.91, 1.36) | |
| Fatigue | |||||||
| MFI general fatigue | 18.05 ± 2.64 (17.21, 18.89) | 18.28 ± 2.06 (17.62, 18.93) | −0.225 (−1.28, 0.83) | 10.53 ± 4.93 (8.95, 12.10) | 9.68 ± 4.35 (8.28, 11.07) | 0.850 (−1.22, 2.92) | |
| MFI physical fatigue | 16.70 ± 2.87 (15.78, 17.62) | 16.68 ± 2.77 (15.79, 17.56) | 0.025 (−1.23, 1.28) | 10.13 ± 4.88 (8.56, 11.69) | 9.30 ± 4.35 (7.91, 10.69) | 0.825 (−1.23, 2.88) | |
| MFI mental fatigue | 15.18 ± 1.89 (14.57, 15.78) | 15.15 ± 1.56 (14.65, 15.65) | 0.025 (−0.75, 0.79) | 12.08 ± 3.12 (11.08, 13.07) | 11.80 ± 3.19 (10.78, 12.82) | 0.275 (−1.13, 1.68) | |
| MFI reduced activity | 15.65 ± 4.01 (14.37, 16.93) | 14.93 ± 3.42 (13.83, 16.02) | 0.725 (−0.93, 2.83) | 8.65 ± 4.54 (7.20, 10.10) | 7.65 ± 3.91 (6.40, 8.90) | 1.000 (−0.88, 2.88) | |
| MFI reduced motivation | 14.28 ± 3.34 (13.21, 15.34) | 14.35 ± 3.38 (13.27, 15.43) | −0.075 (−1.57, 1.42) | 7.98 ± 3.59 (6.83, 9.12) | 7.25 ± 3.50 (6.13, 8.37) | 0.725 (−0.85, 2.30) | |
| Anxiety | 32.40 ± 11.23 (28.81, 35.99) | 31.88 ± 8.42 (29.18, 34.57) | 0.525 (−3.89, 4.94) | 12.78 ± 11.97 (8.95, 16.60) | 11.13 ± 9.97 (7.89, 14.36) | 1.647 (−3.30, 6.58) | |
| Pain pressure thresholds | |||||||
| Occipital | D | 1.06 ± 0.84 (0.78, 1.33) | 0.86 ± 0.57 (0.67, 1.03) | 0.199 (−0.12, 0.52) | 3.39 ± 1.54 (2.91, 3.88) | 3.18 ± 1.23 (2.78, 3.57) | 0.213 (−0.41, 0.84) |
| ND | 0.93 ± 0.78 (0.68, 1.18) | 0.85 ± 0.57 (0.66, 1.03) | 0.085 (−0.22, 0.39) | 3.13 ± 1.46 (2.66, 3.60) | 3.06 ± 1.08 (2.70, 3.41) | 0.068 (−0.51, 0.65) | |
| Pain pressure thresholds | |||||||
| Cervical Low | D | 1.14 ± 0.83 (0.87, 1.41) | 1.07 ± 0.71 (0.84, 1.30) | 0.072 (−0.27, 0.42) | 3.13 ± 1.98 (2.49, 3.76) | 3.02 ± 1.66 (2.48, 3.55) | 0.108 (−0.71, 0.93) |
| ND | 1.04 ± 0.69 (0.82, 1.267) | 1.09 ± 0.84 (0.82, 1.35) | −0.045 (−0.38, 0.30) | 3.23 ± 2.22 (2.51, 3.93) | 2.99 ± 1.90 (2.38, 3.61) | 0.226 (−0.69, 1.16) | |
| Trapezium | D | 1.11 ± 0.77 (0.86, 1.36) | 0.95 ± 0.66 (0.74, 1.16) | 0.155 (−0.16, 0.48) | 3.01 ± 1.12 (2.65, 3.37) | 3.17 ± 1.67 (2.63, 3.72) | −0.166 (−0.80, 0.47) |
| ND | 1.02 ± 0.67 (0.80, 1.24) | 0.96 ± 0.57 (0.77, 1.14) | 0.062 (−0.22, 0.34) | 3.13 ± 1.75 (2.49, 3.76) | 3.33 ± 1.73 (2.77, 3.89) | −0.022 (−0.81, 0.47) | |
| Supraspinatus | D | 1.28 ± 0.86 (1.01, 1.23) | 1.26 ± 0.75 (1.02, 1.49) | 0.020 (−0.34, 0.38) | 3.55 ± 2.00 (2.90, 4.19) | 3.67 ± 1.88 (3.06, 4.27) | −0.124 (−0.99, 0.75) |
| ND | 1.26 ± 0.77 (1.01, 1.56) | 1.28 ± 0.70 (1.06, 1.51) | −0.032 (−0.36, 0.29) | 3.76 ± 2.11 (3.08, 4.43) | 3.39 ± 1.66 (2.85, 3.93) | 0.368 (−0.48, 1.22) | |
| Second rib | D | 0.94 ± 0.51 (0.77, 1.10) | 0.87 ± 0.47 (0.72, 1.02) | 0.063 (−0.16, 0.28) | 2.52 ± 1.33 (2.09, 2.94) | 2.29 ± 1.23 (1.90, 2.69) | 0.216 (−0.36, 0.79) |
| ND | 0.95 ± 0.51 (0.77, 1.10) | 0.81 ± 0.42 (0.67, 0.94) | 0.134 (−0.07, 0.34) | 2.57 ± 1.23 (2.17, 2.96) | 2.42 ± 1.11 (2.06, 2.77) | 0.150 (−0.37, 0.67) | |
| Epicondyle | D | 0.96 ± 0.55 (0.79, 1.14) | 0.97 ± 0.57 (0.78, 1.15) | −0.002 (−0.25, 0.25) | 3.17 ± 1.37 (2.73, 3.60) | 3.19 ± 1.58 (2.67, 3.69) | −0.020 (−0.68, 0.64) |
| ND | 1.01 ± 0.65 (0.81, 1.22) | 0.96 ± 0.52 (0.79, 1.12) | 0.053 (−0.21, 0.32) | 3.52 ± 1.57 (3.02, 4.03) | 3.16 ± 1.43 (2.70, 3.63) | 0.357 (−0.32, 1.03) | |
| Second metacarpal | D | 1.23 ± 0.93 (0.93, 1.53) | 1.23 ± 0.66 (1.02, 1.44) | 0.006 (−0.35, 0.36) | 2.90 ± 1.46 (2.44, 3.37) | 3.42 ± 1.46 (2.95, 3.90) | −0.516 (−1.17, 0.14) |
| ND | 1.12 ± 0.75 (0.88, 1.36) | 1.11 ± 0.54 (0.93, 1.28) | 0.012 (−0.28, 0.31) | 3.26 ± 1.93 (2.65, 3.88) | 3.18 ± 1.66 (2.64, 3.72) | 0.092 (−0.71, 0.89) | |
| Gluteal muscle | D | 2.02 ± 1.74 (1.46, 2.57) | 1.97 ± 1.41 (1.52, 2.42) | 0.038 (−0.66, 0.74) | 6.20 ± 2.74 (5.33, 7.07) | 6.19 ± 2.47 (5.39, 6.70) | 0.006 (−1.16, 1.17) |
| ND | 1.85 ± 1.28 (1.44, 2.26) | 1.93 ± 1.27 (1.52, 2.34) | −0.085 (−0.65, 0.48) | 6.23 ± 2.68 (5.37, 7.08) | 6.03 ± 2.31 (5.29, 6.78) | 0.196 (−0.92, 1.32) | |
| Greater trochanter | D | 1.93 ± 1.11 (1.57, 2.28) | 2.26 ± 1.26 (1.85, 2.66) | −0.330 (−0.86, 0.20) | 5.88 ± 2.44 (5.10, 6.66) | 5.94 ± 2.39 (5.17, 6.72) | −0.061 (−1.14, 1.02) |
| ND | 1.93 ± 1.06 (1.59, 2.27) | 2.26 ± 1.16 (1.88, 2.62) | −0.319 (−0.81, 0.17) | 5.45 ± 2.38 (4.68, 6.21) | 5.52 ± 2.43 (4.73, 6.31) | −0.072 (−1.15, 1.01) | |
| Knee | D | 1.72 ± 1.29 (1.31, 2.14) | 1.68 ± 1.19 (1.29, 2.06) | 0.045 (−0.51, 0.59) | 5.65 ± 2.53 (4.84, 6.46) | 5.16 ± 2.07 (4.48, 5.83) | 0.500 (−0.53, 1.54) |
| ND | 1.81 ± 1.14 (1.4, 2.17) | 1.92 ± 1.32 (1.49, 2.33) | −0.108 (−0.65, 0.44) | 6.07 ± 2.69 (5.21, 6.93) | 5.32 ± 2.24 (4.59, 6.05) | 0.755 (−0.35, 1.87) | |
| Anterior tibial | D | 1.88 ± 1.43 (1.43, 2.33) | 1.85 ± 1.38 (1.40, 2.28) | 0.038 (−0.59, 0.66) | 5.05 ± 2.47 (4.26, 5.84) | 5.12 ± 2.50 (4.31, 5.93) | −0.073 (−1.18, 1.04) |
| ND | 1.83 ± 1.25 (1.43, 2.23) | 1.81 ± 1.12 (1.44, 2.16) | 0.030 (−0.50, 0.56) | 5.02 ± 2.26 (4.29, 5.74) | 5.05 ± 2.50 (4.24, 5.86) | −0.032 (−1.09, 1.03) | |
Data are expressed as mean ± standard deviation (95% confidence interval).
Significant differences between cases and controls (two-way analysis of covariance).
CSS, central sensitization syndrome; D, dominant; FIQ-R, revised Fibromyalgia Impact Questionnaire; FIQ-R.1, activity level of the FIQ; FIQ-R.2, overall impact of the FIQ-R; FIQ-R.3, intensity of symptoms of the FIQ-R; MD, difference in means; MFI, Multidimensional Fatigue Inventory; ND, non-dominant; VAS, Visual Analogue Scale.
Core
body temperature and temperature at skin surface of the hypothenar eminence of the hands and PPT, VAS, FIQ-R, central sensitization, sleep, fatigue and anxiety in FMS women and healthy women
Beta estimates and 95%CI for core body temperature and hypothenar temperature of the hands and clinical symptoms in the FMS women and the healthy controls are summarized in Table 4. Linear analysis regression revealed significant associations among FIQ-R.2 (β = 0.033; 95%CI = 0.003, 0.062; p = 0.030), daytime dysfunction assessed by the PSQI (β = 0.203; 95%CI = 0.011, 0.395; p = 0.039), and reduced activity using the MFI (β = 0.045; 95%CI = 0.005, 0.085; p = 0.029), and core body temperature after adjustment for age, menopause status and BMI in FMS women. No significant associations were observed between core body temperature and clinical variables in healthy controls. On the other hand, linear analysis regression identified significant correlations among greater-trochanter-dominant PPT (β = 0.254; 95%CI = 0.003, 0.504; p = 0.047), greater-trochanter non-dominant PPT (β = 0.650; 95%CI = 0.141, 1.159; p = 0.013), and sleep medication assessed by PSQI (β = −0.242; 95%CI = −0.471, −0.013; p = 0.039), and temperature at skin surface of the hypothenar eminence of the hands after adjustment for age, menopause status and BMI in FMS women. No significant associations were observed between temperature at skin surface of the hypothenar eminence of the hands and clinical variables in healthy controls.
Table 4.
Beta estimates and confidence intervals for the association between core body temperature and hypothenar temperature and PPT, VAS, FIQ-R, CSS, sleep, fatigue, and anxiety among women with fibromyalgia (cases) and healthy women (controls).
| Core body temperature °C |
Hypothenar temperature °C |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 80) |
Controls (n = 80) |
Cases (n = 80) |
Controls (n = 80) |
||||||||||
| β | 95%CI | p value | β | 95%CI | p value | β | 95%CI | p value | β | 95%CI | p value | ||
| VAS (mm) | 0.006 | (−0.002, 0.014) | 0.164 | 0.003 | (−0.003, 0.009) | 0.354 | 0.009 | (−0.008, 0.026) | 0.283 | 0.006 | (−0.010, 0.022) | 0.477 | |
| FIQ-R | |||||||||||||
| FIQ-R.1 | 0.017 | (−0.010, 0.043) | 0.210 | – | – | – | −0.045 | (−0.097, 0.007) | 0.086 | – | – | – | |
| FIQ-R.2 | 0.033 | (0.003, 0.062) | 0.030* | – | – | – | −0.013 | (−0.073, 0.047) | 0.676 | – | – | – | |
| FIQ-R.3 | 0.013 | (−0.005, 0.030) | 0.153 | – | – | – | 0.000 | (−0.001, 0.001) | 0.269 | – | – | – | |
| Total score | 0.009 | (0.001, 0.018) | 0.061 | – | – | – | −0.012 | (−0.030, 0.007) | 0.213 | – | – | – | |
| CSS | 0.001 | (−0.014, 0.014) | 0.971 | −0.003 | (−0.013, 0.007) | 0.499 | −0.009 | (−0.036, 0.019) | 0.518 | −0.014 | (−0.041, 0.012) | 0.282 | |
| Sleep Questionnaire Index | |||||||||||||
| Sleep quality | 0.050 | (−0.144, 0.244) | 0.607 | 0.002 | (−0.173, 0.176) | 0.985 | −0.134 | (−0.514, 0.247) | 0.486 | −0.210 | (−0.674, 0.253) | 0.368 | |
| Sleep latency | −0.048 | (−0.229, 0.133) | 0.602 | −0.021 | (−0.164, 0.123) | 0.776 | −0.031 | (−0.385, 0.324) | 0.863 | −0.094 | (−0.475, 0.287) | 0.623 | |
| Sleep duration | 0.026 | (−0.150, 0.201) | 0.771 | −0.038 | (−0.185, 0.110) | 0.612 | −0.035 | (−0.371, 0.301) | 0.835 | −0.193 | (−0.584, 0.197) | 0.327 | |
| Habitual sleep efficiency | −0.066 | (−0.214, 0.081) | 0.374 | −0.061 | (−0.202, 0.080) | 0.395 | 0.069 | (−0.214, 0.351) | 0.629 | −0.156 | (−0.531, 0.220) | 0.412 | |
| Sleep disturbances | 0.165 | (−0.144, 0.473) | 0.291 | 0.205 | (−0.054, 0.464) | 0.119 | −0.321 | (−0.925, 0.282) | 0.292 | −0.252 | (−0.951, 0.447) | 0.475 | |
| Sleeping medication | −0.021 | (−0.141, 0.099) | 0.726 | 0.044 | (−0.094, 0.182) | 0.531 | −0.242 | (−0.471, −0.01) | 0.039* | −0.212 | (−0.578, 0.154) | 0.252 | |
| Daytime dysfunction | 0.203 | (0.011, 0.395) | 0.039* | −0.161 | (−0.341, 0.019) | 0.079 | −0.012 | (−0.402, 0.377) | 0.950 | −0.163 | (−0.651, 0.325) | 0.508 | |
| Global score | −0.001 | (−0.044, 0.042) | 0.960 | −0.006 | (−0.040, 0.028) | 0.723 | −0.018 | (−0.100, 0.064) | 0.656 | 0.001 | (−0.109, 0.111) | 0.983 | |
| Fatigue | |||||||||||||
| MFI general fatigue | 0.058 | (−0.066, 0.066) | 0.998 | −0.010 | (−0.043, 0.023) | 0.547 | 0.011 | (−0.119, 0.141) | 0.872 | 0.008 | (−0.101, 0.117) | 0.885 | |
| MFI physical fatigue | −0.014 | (−0.068, 0.040) | 0.603 | −0.017 | (−0.051, 0.016) | 0.298 | −0.048 | (−0.154, 0.058) | 0.372 | −0.038 | (−0.127, 0.051) | 0.397 | |
| MFI mental fatigue | 0.069 | (−0.020, 0.157) | 0.125 | −0.031 | (−0.082, 0.019) | 0.215 | 0.052 | (−0.125, 0.228) | 0.562 | −0.041 | (−0.176, 0.093) | 0.541 | |
| MFI reduced activity | 0.045 | (0.005, 0.085) | 0.029* | −0.007 | (−0.048, 0.034) | 0.729 | −0.039 | (−0.121, 0.042) | 0.337 | −0.081 | (−0.177, 0.015) | 0.097 | |
| MFI reduced motivation | 0.001 | (−0.044, 0.046) | 0.953 | −0.031 | (−0.075, 0.012) | 0.151 | −0.022 | (−0.111, 0.067) | 0.623 | −0.083 | (−0.198, 0.032) | 0.156 | |
| Anxiety | 0.012 | (−0.003, 0.028) | 0.116 | −0.008 | (−0.022, 0.007) | 0.293 | −0.022 | (−0.053, 0.009) | 0.156 | −0.016 | (−0.053, 0.022) | 0.400 | |
| Pain pressure thresholds | |||||||||||||
| Occipital | D | −0.047 | (−0.269, 0.176) | 0.678 | 0.034 | (−0.077, 0.144) | 0.543 | −0.189 | (−0.627, 0.248) | 0.391 | −0.091 | (−0.389, 0.208) | 0.547 |
| ND | −0.056 | (−0.279, 0.168) | 0.621 | 0.030 | (−0.091, 0.151) | 0.622 | −0.150 | (−0.590, 0.291) | 0.500 | −0.015 | (−0.341, 0.311) | 0.927 | |
| Pain pressure thresholds | |||||||||||||
| Cervical Low | D | −0.060 | (−0.256, 0.135) | 0.541 | 0.016 | (−0.073, 0.105) | 0.719 | −0.167 | (−0.551, 0.218) | 0.91 | −0.068 | (−0.307, 0.171) | 0.572 |
| ND | −0.022 | (−0.219, 0.175) | 0.823 | 0.022 | (−0.057, 0.101) | 0.575 | 0.060 | (−0.328, 0.449) | 0.757 | −0.072 | (−0.286, 0.141) | 0.500 | |
| Trapezium | D | 0.024 | (−0.196, 0.244) | 0.827 | 0.064 | (−0.046, 0.174) | 0.253 | −0.092 | (−0.256, 0.343) | 0.676 | 0.056 | (−0.243, 0.356) | 0.709 |
| ND | −0.002 | (−0.257, 0.254) | 0.988 | 0.024 | (−0.066, 0.114) | 0.600 | 0.023 | (−0.481, 0.528) | 0.926 | −0.045 | (−0.288, 0.198) | 0.715 | |
| Supraspinatus | D | 0.072 | (−0.119, 0.264) | 0.453 | 0.034 | (−0.045, 0.112) | 0.400 | 0.034 | (−0.345, 0.413) | 0.858 | −0.015 | (−0.229, 0.199) | 0.888 |
| ND | −0.048 | (−0.256, 0.161) | 0.651 | 0.009 | (−0.033, 0.052) | 0.659 | −0.001 | (−0.413, 0.411) | 0.996 | −0.099 | (−0.319, 0.121) | 0.372 | |
| Second rib | D | −0.010 | (−0.331, 0.311) | 0.952 | 0.012 | (−0.032, 0.055) | 0.598 | −0.060 | (−0.693, 0.573) | 0.850 | −0.078 | (−0.417, 0.260) | 0.645 |
| ND | −0.073 | (−0.402, 0.255) | 0.659 | 0.018 | (−0.119, 0.155) | 0.794 | −0.279 | (−0.925, 0.367) | 0.392 | −0.027 | (−0.397, 0.343) | 0.886 | |
| Epicondyle | D | 0.049 | (−0.228, 0.325) | 0.726 | 0.030 | (−0.078, 0.138) | 0.585 | 0.070 | (−0.476, 0.616) | 0.799 | −0.036 | (−0.328, 0.256) | 0.806 |
| ND | −0.141 | (−0.406, 0.124) | 0.291 | 0.029 | (−0.076, 0.134) | 0.579 | 0.010 | (−0.517, 0.537) | 0.970 | −0.103 | (−0.386, 0.180) | 0.471 | |
| Second metacarpal | D | −0.030 | (−0.224, 0.163) | 0.757 | 0.071 | (−0.033, 0.174) | 0.178 | −0.010 | (−0.393, 0.372) | 0.957 | 0.075 | (−0.207, 0.358) | 0.596 |
| ND | −0.039 | (−0.271, 0.194) | 0.740 | 0.072 | (−0.014, 0.157) | 0.098 | 0.005 | (−0.454, 0.464) | 0.982 | 0.004 | (−0.230, 0.239) | 0.971 | |
| Gluteal muscle | D | 0.009 | (−0.090, 0.107) | 0.861 | 0.002 | (−0.058, 0.063) | 0.941 | 0.058 | (−0.137, 0.252) | 0.556 | 0.016 | (−0.148, 0.180) | 0.848 |
| ND | −0.025 | (−0.146, 0.095) | 0.678 | −0.005 | (−0.068, 0.057) | 0.864 | 0.117 | (−0.120, 0.354) | 0.327 | −0.003 | (−0.172, 0.165) | 0.968 | |
| Greater trochanter | D | −0.029 | (−0.159, 0.101) | 0.660 | 0.008 | (−0.057, 0.073) | 0.802 | 0.254 | (0.003, 0.504) | 0.047* | 0.001 | (−0.115, 0.115) | 0.995 |
| ND | −0.006 | (−0.143, 0.131) | 0.931 | 0.007 | (−0.059, 0.073) | 0.836 | 0.650 | (0.141, 1.159) | 0.013* | 0.226 | (−0.753, 1.205) | 0.647 | |
| Knee | D | −0.003 | (−0.128, 0.122) | 0.963 | −0.011 | (−0.081, 0.059) | 0.753 | 0.107 | (−0.139, 0.353) | 0.390 | −0.068 | (−0.255, 0.120) | 0.475 |
| ND | −0.101 | (−0.223, 0.021) | 0.102 | −0.037 | (−0.099, 0.025) | 0.239 | 0.089 | (−0.154, 0.332) | 0.469 | −0.090 | (−0.257, 0.078) | 0.291 | |
| Anterior tibial | D | 0.048 | (−0.061, 0.156) | 0.384 | 0.014 | (−0.028, 0.057) | 0.504 | 0.062 | (−0.152, 0.277) | 0.564 | 0.050 | (−0.123, 0.223) | 0.568 |
| ND | −0.020 | (−0.147, 0.108) | 0.761 | 0.033 | (−0.033, 0.100) | 0.320 | 0.035 | (−0.217, 0.287) | 0.784 | 0.026 | (−0.154, 0.207) | 0.772 | |
Beta (β) represents the regression coefficient. Adjusted for age, menopause status and body mass index.
95%CI, 95% confidence interval; CSS, central sensitization syndrome; D, dominant; FIQ-R, revised Fibromyalgia Impact Questionnaire; FIQ-R.1, activity level of the FIQ; FIQ-R.2, overall impact of the FIQ-R; FIQ-R.3, intensity of symptoms of the FIQ-R; MFI, Multidimensional Fatigue Inventory; ND, non-dominant; VAS, Visual Analogue Scale.
Discussion
The lack of definitive pathogenesis of FMS makes it very difficult to diagnose and manage this illness. The results of this study showed that women diagnosed with FMS present worse basal levels of pain intensity, disease severity, central sensitization, quality of sleep, morning stiffness, anxiety, alterations in mood, and decreased pressure pain on musculoskeletal tissue in comparison with healthy controls. We found significant associations between core body temperature and daytime sleep, fatigue, and impact of symptoms in women diagnosed with FMS. Moreover, we also found significant associations between hypothenar eminence temperature and dominant and non-dominant greater trochanter. We found no significant association between clinical variables and temperature measures in healthy women.
Relationship among core body temperature and clinical symptoms in FMS women
Research has demonstrated an imbalance of the ANS relating to increase of body temperature in FMS patients that may contribute to central sensitization and maintaining the diverse FMS manifestations of fatigue and sleep disorders.12,13,35 The ANS provides information about the cerebrospinal fluid concentrations of pro-nociceptive substances such as substance P and CGRP.36 Several studies have showed an excessive concentration of these molecules at blood flow in FMS patients,37 or in other pathologies as psoriatic illness.38 So, the increase of these substances and their vasoactive and inflammatory capacities might relate to the elevated core body temperature, and with the association on overall impact of symptoms assessed by the FIQ-R, the daytime disturbances and the reduced of the activity that we have recorded in our women FMS population. Taking into account these preliminary findings, and that no previous publishes have explored the association of these outcomes about the core temperature with the symptomatology in FMS patients and healthy controls, further studies are needed to clarify our data.
Relationship among peripheral blood microcirculation and clinical symptoms in FMS women
Our data revealed significant correlations of temperature at skin surface of the hypothenar eminence of the hands with PPTs of some tender points of the musculoskeletal system (dominant and non-dominant greater trochanter) and with the sleeping medication in women with FMS. The peripheral microvasculature of the hands and feet play an important role in thermogenesis, since the peripheral cutaneous nerves and small capillary vessels have a function regulating heat production of the human body.6,39 This control is performed inside vascular structures which lie deep in the dermis-denominated AVAs.6 Facing cold environments, the adrenergic nerves of the AVAs are activated, detecting a lack of peripheral skin temperature. Afterwards, they transmit this information to the CNS; stimulating sympathetic vasoconstrictor nerves like this provides blood to the deep venous system, thereby preserving metabolism viability.40 However, the phenomenon ‘cold-induced vasodilation’ could happen in this anatomic region if there is persistent exposure to cold, thus increasing blood flow to the peripheral cutaneous system.6,39 In line with cold stress, FMS patients share similar characteristics to those associated with Raynaud’s phenomenon, such as the peripheral disorders linked to excessive vasoconstriction, like cyanosis or vasospasm of the palms of the hands.41 Albrecht and colleagues4 demonstrated excessive sympathetic and sensory innervation of AVAs exacerbated by cold weather.4 Therefore, this excessive innervation could result in insufficient arterial-blood-flow shunt to the venous deep plexus system, explaining the central pain, tenderness, fatigue and clinical features in this population.4,18 These findings are in line with the association between hypothenar eminence temperature of the hands and several symptoms relative to decrease on pressure pain and the non-restorative sleep that we have found in our population of FMS women in comparison with healthy women.
This study has some limitations. First, due to its cross-sectional design, no causal conclusions can be drawn. Further studies are needed to investigate the mechanisms by which core body temperature and peripheral blood flow of the hands could determine severity of clinical symptoms, the sleepiness during daytime activities, the decreases on PPT, and the anxiety in FMS patients. Second, we decided to include only women because the higher frequency of FMS among women has been attributed to the fact that they feel intensity of pain more than men.42 In addition, the menstrual cycle can affect the vascular response and we did not control for this aspect.43 Finally, we know that an intra-subject variability exists in peripheral blood-flow thermographic measurement.44 Knowing this, we tried to minimize this effect by incorporating an acclimation period and asking women to avoid consuming vasoactive substances before testing.45 Furthermore, our study sample consisted of a well-characterized population of FMS women and, therefore, our data might not be generalizable to other populations. Despite its limitations, the current study presents one strength. To the best of our knowledge, this is the first study to investigate the associations between core body temperature and peripheral blood flow of the hands and pain intensity, hypersensitivity and the main symptoms in FMS. Additionally, the pressure algometry method has been reported to have good discriminating power in FMS and provides objective and direct measures of PPTs.28
Conclusion
We have found that FMS women present an increase of their tympanic core temperature and hypothenar eminence temperature of the hands in comparison with healthy women. Besides, our findings revealed that exist associations between core body temperature and hand temperature with the severity of clinical symptoms assessed by the FIQ-R, the sleepiness in the daytime activities, the decreases on PPT, and the anxiety in FMS patients. Findings herein provide a new perspective on the possible influence of neurovascular skin disorders and thermogenesis processes in the symptoms of patients diagnosed with FMS. Therefore, future scientific studies should investigate the relationship between these altered hand thermographic patterns and the core body temperature of FMS patients with the characteristics and clinical manifestations to gain further information on the knowledge, diagnosis, and treatment of this chronic condition.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Antonio Casas-Barragán  https://orcid.org/0000-0002-6761-4811
https://orcid.org/0000-0002-6761-4811
María Correa-Rodríguez  https://orcid.org/0000-0001-9165-4349
https://orcid.org/0000-0001-9165-4349
Contributor Information
Antonio Casas-Barragán, Department of Physical Therapy, Faculty of Health Sciences, Instituto de Investigación Biosanitaria ibs.GRANADA, University of Granada, Granada, Spain.
Francisco Molina, Department of Health Sciences, University of Jaén, Jaén, Spain.
Rosa María Tapia-Haro, Department of Physical Therapy, Faculty of Health Sciences, Instituto de Investigación Biosanitaria ibs.GRANADA, University of Granada, Granada, Spain.
María Carmen García-Ríos, Department of Physical Therapy, Faculty of Health Sciences, Instituto de Investigación Biosanitaria ibs.GRANADA, University of Granada, Granada, Spain.
María Correa-Rodríguez, Department of Nursing, Faculty of Health Sciences, Instituto de Investigación Biosanitaria ibs. GRANADA, University of Granada, Ave. de la Ilustración, 60, Granada 18016, Spain; Department of Nursing, Faculty of Health Sciences, Instituto de Investigación Biosanitaria ibs.GRANADA, University of Granada, Granada, Spain.
María Encarnación Aguilar-Ferrándiz, Department of Physical Therapy, Faculty of Health Sciences, Instituto de Investigación Biosanitaria ibs.GRANADA, University of Granada, Granada, Spain.
References
- 1. Häuser W, Ablin J, Fitzcharles M-A, et al. Fibromyalgia. Nat Rev Dis Primers 2015; 1: 15022. [DOI] [PubMed] [Google Scholar]
- 2. Cabo-Meseguer A, Cerdá-Olmedo G, Trillo-Mata JL. Fibromyalgia: prevalence, epidemiologic profiles and economic costs. Med Clin (Barc) 2017; 149: 441–448. [DOI] [PubMed] [Google Scholar]
- 3. Russell IJ, Larson AA. Neurophysiopathogenesis of fibromyalgia syndrome: a unified hypothesis. Rheum Dis Clin North Am 2009; 35: 421–435. [DOI] [PubMed] [Google Scholar]
- 4. Albrecht PJ, Hou Q, Argoff CE, et al. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med 2013; 14: 895–915. [DOI] [PubMed] [Google Scholar]
- 5. Choi D-H, Kim H-S. Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J Intern Med 2015; 30: 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature (Austin) 2016; 3: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg 1994; 47: 527–543. [DOI] [PubMed] [Google Scholar]
- 8. Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 1998; 30: 5–11. [DOI] [PubMed] [Google Scholar]
- 9. Kasikcioglu E, Dinler M, Berker E. Reduced tolerance of exercise in fibromyalgia may be a consequence of impaired microcirculation initiated by deficient action of nitric oxide. Med Hypotheses 2006; 66: 950–952. [DOI] [PubMed] [Google Scholar]
- 10. Katz DL, Greene L, Ali A, et al. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69: 517–525. [DOI] [PubMed] [Google Scholar]
- 11. Scholander PF, Hock R, Walters V, et al. Heat regulation in some arctic and tropical mammals and birds. Biol Bull 1950; 99: 237–258. [DOI] [PubMed] [Google Scholar]
- 12. Elmas O, Yildiz S, Bilgin S, et al. Physiological parameters as a tool in the diagnosis of fibromyalgia syndrome in females: a preliminary study. Life Sci 2016; 145: 51–56. [DOI] [PubMed] [Google Scholar]
- 13. Brusselmans G, Nogueira H, De Schamphelaere E, et al. Skin temperature during cold pressor test in fibromyalgia: an evaluation of the autonomic nervous system? Acta Anaesthesiol Belg 2015; 66: 19–27. [PubMed] [Google Scholar]
- 14. Abdi A, Asadian S, Khatony A, et al. Accuracy and precision of four common peripheral temperature measurement methods in intensive care patients. Med Devices (Auckl) 2016; 9: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujimasa I, Chinzei T, Saito I. Converting far infrared image information to other physiological data. IEEE Eng Med Biol Mag 2000; 19: 71–76. [DOI] [PubMed] [Google Scholar]
- 16. Sagaidachnyi AA, Skripal AV, Fomin AV, et al. Determination of the amplitude and phase relationships between oscillations in skin temperature and photoplethysmography-measured blood flow in fingertips. Physiol Meas 2014; 35: 153–166. [DOI] [PubMed] [Google Scholar]
- 17. Sagaidachnyi AA, Fomin AV, Usanov DA, et al. Thermography-based blood flow imaging in human skin of the hands and feet: a spectral filtering approach. Physiol Meas 2017; 38: 272–288. [DOI] [PubMed] [Google Scholar]
- 18. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016; 46: 319–329. [DOI] [PubMed] [Google Scholar]
- 19. Ring EFJ, Ammer K. Infrared thermal imaging in medicine. Physiol Meas 2012; 33: R33–R46. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez-Marin FJ, Calixto-Carrera S, Villaseñor-Mora C. Novel approach to assess the emissivity of the human skin. J Biomed Opt 2009; 14: 024006. [DOI] [PubMed] [Google Scholar]
- 21. Mirbod SM, Sugiura H. A non-invasive technique for the evaluation of peripheral circulatory functions in female subjects with Raynaud’s phenomenon. Ind Health 2017; 55: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ring F. Thermal imaging technique – protocol and sources of terror in thermal imaging. In: Ring F, Jung A, Żuber J. (eds) A case book of infrared imaging in clinical medicine. Warszawa: Medpress, 2003. [Google Scholar]
- 23. Lim MJ, Kwon SR, Jung K-H, et al. Digital thermography of the fingers and toes in Raynaud’s phenomenon. J Korean Med Sci 2014; 29: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salamunes ACC, AMW Stadnik, EB. Neves. The effect of body fat percentage and body fat distribution on skin surface temperature with infrared thermography. J Therm Biol 2017; 66: 1–9. [DOI] [PubMed] [Google Scholar]
- 25. Gasim GI, Musa IR, Abdien MT, et al. Accuracy of tympanic temperature measurement using an infrared tympanic membrane thermometer. BMC Res Notes 2013; 6: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bijur PE, Shah PD, Esses D. Temperature measurement in the adult emergency department: oral, tympanic membrane and temporal artery temperatures versus rectal temperature. Emerg Med J 2016; 33: 843–847. [DOI] [PubMed] [Google Scholar]
- 27. Chesterton LS, Sim J, Wright CC, et al. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain 2007; 23: 760–766. [DOI] [PubMed] [Google Scholar]
- 28. Marques AP, Assumpção A, Matsutani LA, et al. Pain in fibromyalgia and discrimination power of the instruments: visual analog scale, dolorimetry and the McGill pain questionnaire. Acta Reumatol Port 2008; 33: 345–351. [PubMed] [Google Scholar]
- 29. Salgueiro M, García-Leiva JM, Ballesteros J, et al. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual Life Outcomes 2013; 11: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salaffi F, Di Carlo M, Arcà S, et al. Categorisation of disease severity states in fibromyalgia: a first step to support decision-making in health care policy. Clin Exp Rheumatol 2018; 36: 1074–1081. [PubMed] [Google Scholar]
- 31. Cuesta-Vargas AI, Roldan-Jimenez C, Neblett R, et al. Cross-cultural adaptation and validity of the Spanish central sensitization inventory. Springerplus 2016; 5: 1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hita-Contreras F, Martínez-López E, Latorre-Román PA, et al. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol Int 2014; 34: 929–936. [DOI] [PubMed] [Google Scholar]
- 33. Munguía-Izquierdo D, Segura-Jiménez V, Camiletti-Moirón D, et al. Multidimensional fatigue inventory: Spanish adaptation and psychometric properties for fibromyalgia patients. The Al-Andalus study. Clin Exp Rheumatol 2012; 30(Suppl. 74): 94–102. [PubMed] [Google Scholar]
- 34. Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56: 893–897. [DOI] [PubMed] [Google Scholar]
- 35. Kulshreshtha P, Gupta R, Yadav RK. et al. A comprehensive study of autonomic dysfunction in the fibromyalgia patients. Clin Auton Res 2012; 22: 117–122. [DOI] [PubMed] [Google Scholar]
- 36. Clauw DJ, Arnold LM, McCarberg BH.; FibroCollaborative. The science of fibromyalgia. Mayo Clin Proc 2011; 86: 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsilioni I, Russell IJ, Stewart JM, et al. Neuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J Pharmacol Exp Ther 2016; 356: 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A 2010; 107: 4448–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung SS. Responses of the hands and feet to cold exposure. Temperature (Austin) 2015; 2: 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benzinger TH. Peripheral cold- and central warm-reception, main origins of human thermal discomfort. Proc Natl Acad Sci U S A 1963; 49: 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scolnik M, Vasta B, Hart DJ, et al. Symptoms of Raynaud’s phenomenon (RP) in fibromyalgia syndrome are similar to those reported in primary RP despite differences in objective assessment of digital microvascular function and morphology. Rheumatol Int 2016; 36: 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buskila D, Neumann L, Alhoashle A, et al. Fibromyalgia syndrome in men. Semin Arthritis Rheum 2000; 30: 47–51. [DOI] [PubMed] [Google Scholar]
- 43. Lafferty K, De Trafford JC, Potter C, et al. Reflex vascular responses in the finger to contralateral thermal stimuli during the normal menstrual cycle: a hormonal basis to Raynaud’s phenomenon? Clin Sci (Lond) 1985; 68: 639–645. [DOI] [PubMed] [Google Scholar]
- 44. Clark S, Hollis S, Campbell F, et al. The “distal-dorsal difference” as a possible predictor of secondary Raynaud’s phenomenon. J Rheumatol 1999; 26: 1125–1128. [PubMed] [Google Scholar]
- 45. Mariotti A, Grossi G, Amerio P. et al. Finger thermoregulatory model assessing functional impairment in Raynaud’s phenomenon. Ann Biomed Eng 2009; 37: 2631–2639. [DOI] [PubMed] [Google Scholar]