Abstract
Background:
Nusinersen was the first approved disease-modifying therapy for all 5q-spinal muscular atrophy (SMA) patients regardless of age or disease severity. Its efficacy in adults has recently been demonstrated in a large cohort by motor outcome measures, which were only partially suitable to detect changes in very mildly or severely affected patients. Patient-reported outcome measures (PROs) have been suggested as a valuable addition. Here, we aimed to assess treatment satisfaction and investigate whether it may be a useful PRO to monitor SMA patients.
Methods:
We enrolled 91 mainly adult 5q-SMA patients treated with nusinersen in a national, multicenter, cross-sectional observational study. 21 patients underwent longitudinal follow up. Patients’ satisfaction with treatment in four dimensions (global, effectiveness, convenience, side effects) was assessed by the Treatment Satisfaction Questionnaire for Medication German version 1.4 (TSQM-1.4©) and related to clinical parameters, motor scores, and treatment duration.
Results:
More than 90% of SMA patients were consistently satisfied over a median treatment duration of 10 months. Highest mean scores were observed in the dimensions ‘side effects,’ ‘global satisfaction,’ and ‘effectiveness’ (93.5 ± 14.8 versus 73.1 ± 21.0 and 64.8 ± 20.6, respectively). Patients’ satisfaction with the convenience of treatment was considerably lower (43.6 ± 20.2). Interestingly, satisfaction with the effectiveness was higher in ambulatory (p = 0.014) compared with non-ambulatory patients and directly correlated to motor outcome measures. Five non-ambulatory patients withdrew from therapy. All of them presented with a deterioration of motor outcome measures and reported dissatisfaction with treatment effectiveness and convenience.
Conclusion:
Most patients were satisfied with nusinersen treatment effectiveness. Less severely affected patients indicated higher satisfaction. The TSQM-1.4© helped to identify therapy non-responders, who mainly addressed dissatisfaction with effectiveness and convenience. We suggest introducing the TSQM-1.4© as an additional PRO in SMA into clinical practice.
Keywords: antisense oligonucleotide, nusinersen, patient-reported outcome measures, spinal muscular atrophy, treatment satisfaction, TSQM-1.4©
Introduction
Spinal muscular atrophy (SMA) caused by mutations in the survival of motor neuron (SMN) 1 gene on chromosome 5q is the most common childhood-onset inherited motor neuron disease.1 The antisense oligonucleotide nusinersen obtained US Food and Drug Administration (FDA) approval in 2016 for all 5q-SMA patients based on two phase III trials in infants and children.2,3 Phenotypes of adolescent and adult SMA patients comprise a continuum from mildly affected and ambulatory late-onset cases to fully wheelchair-bound patients suffering from severe scoliosis, contractures, and respiratory insufficiency.4,5 Nusinersen treatment comes with many challenges: it is only offered by specialized neuromuscular centers so that patients often need to travel long distances, administration can be difficult and there is substantial uncertainty about the benefits and the risk of potential long-term side effects. Nusinersen must be administered repeatedly via lumbar puncture,6 and often image-guided in patients who have severe scoliosis.7–9 Treatment effectiveness is mainly monitored by motor-function scores, which are only partially suitable for adult patients, due to floor and ceiling effects in very mildly or severely affected patients, and lacks validation.10,11 Motor-function changes in severely affected patients may be small but are individually significant within their daily lives. For example, an improvement in thumb mobility could imply maintenance of (electrically supported) self-mobility and communication skills but is not captured by commonly administered motor-function scores. Alternative measures are therefore needed to evaluate long-term treatment outcomes and adherence. Patient-reported outcome measures (PROs) may provide the necessary information regardless of disease status.12,13 Their advantage is the ability to reflect the patient’s perspective directly and to assess the impact of the disease on activities of daily living, psychological well-being, or the patients’ perspective toward the disease or the treatment.14 PROs have already been shown to have an important role in the evaluation of care in various chronic diseases.12,13,15
One widely used PRO is the patients’ treatment satisfaction, which was shown to be suitable in evaluating the effectiveness of medical treatments and healthcare delivery systems.16,17 Further, it was shown to be strongly decisive for therapy adherence.18 Patient satisfaction with treatment depends on multiple aspects, including effectiveness, handling/burden, side effects, and individual impact on daily life.19 Other (e.g. motor) outcome measures cannot be replaced but can reasonably be complemented by the use of PRO, but knowledge of the association between motor improvement and treatment satisfaction in neuromuscular diseases remains sparse.
Therefore, this study’s primary objective was to explore SMA patients’ treatment satisfaction under nusinersen therapy and to identify patient features associated with treatment satisfaction. Secondly, we assessed treatment satisfaction development during the treatment course and evaluated whether treatment satisfaction could serve as a substantial complementary PRO during therapy follow up, in addition to motor-function outcome measures.
Methods
Multicenter cross-sectional study design
We conducted a multicenter cross-sectional observational study, in which treatment satisfaction of SMA patients under nusinersen therapy (at random therapy duration) was assessed mainly during routine therapy visits at one single time point by use of the Treatment Satisfaction Questionnaire for Medication Version 1.4© (TSQM-1.4©), in addition to motor-function outcomes between January 2019 and September 2019. Participating sites were Hannover (n = 24), Essen (n = 22), Ulm (n = 21), Dresden (n = 13), and Munich (n = 11). Study approval was obtained from the local ethics committees of all participating sites (Hannover: 6269, Essen: 18-8285-BO, Ulm: 19/12, Dresden: EK393122012, Munich: 16/14) within the German Motor Neuron Disease Network. Written informed consent was obtained from all study participants.
This study was reported following the guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).20
Monocenter longitudinal substudy
Additionally to the cross-sectional approach, 21 nusinersen-treated patients at the Hannover site were enrolled from January 2018 and individually followed up until September 2019. Treatment satisfaction was first assessed after 2 months of nusinersen treatment (end of loading-dose period) and followed up at least once again during the treatment course in this subgroup. Data collected at the latest time point was included in the cross-sectional analyses.
Study participants and setting
Inclusion criteria comprised SMA patients with genetically confirmed SMN1 gene mutations on chromosome 5q under nusinersen treatment (independent of treatment duration). Subjects <10 years and those not able to answer the study questionnaire (e.g. because of a language barrier) were not considered (exclusion criterion). Nusinersen was intrathecally administered (12 mg, 5 ml) according to the approved regimen on days 0, 14, 28 (month 1) and 63 (month 2) with subsequent maintenance administrations every 4 months, designated as months 6, 10, 14, 18, 22. The data obtained at different treatment time points were compared, to evaluate the effect of treatment duration on treatment satisfaction in a representative cohort. To reduce bias, patients were informed in advance that study participation would not influence future treatment decisions. Moreover, all patients without exception who met the inclusion criteria were enrolled at the study sites during the mentioned recruitment periods to avoid a selection bias.
Demographic and disease characteristics
To identify factors that were associated with treatment satisfaction and to compare patients in different degrees of clinical severity, information on demographic characteristics (age, sex), disease characteristics (SMA type, SMN2 copy number, age at symptom onset, ambulatory state, ventilator use) and the duration of nusinersen treatment were recorded. Furthermore, motor-function impairment at nusinersen initiation (baseline), or, if baseline scores were not available, the first motor-function scores collected during therapy, and motor scores at the time point of study enrollment were captured. Patients were defined as ambulatory if they were able to walk at least 10 m without assistance or use of a device, such as a cane or a walker.21 All patients were classified into the well-known SMA subtypes 1–4 based on age at symptom onset and the best-ever reached motor milestone.4
Individual motor-function impairment was assessed by trained raters using two common motor-function outcome measures: the Revised Upper Limb Module (RULM),22 and the Hammersmith Functional Motor Scale Expanded (HFMSE).23 The RULM comprises 20 items with a maximum of 37 points, focusing on upper limb function. The HFMSE was validated for the assessment of gross motor function in type 2 and type 3 SMA.24 It consists of 33 items, which concern an individual’s capacity to perform actions such as sitting, rolling over, and getting up. Each item is scored on a scale from 0 to 2, with a total of up to 66 points. Higher scores indicate a better motor function.
To clarify the suitability of TSQM-1.4© for treatment monitoring, its results were correlated to changes in motor-function outcome measures. The change of motor function was calculated as the difference between motor function at TSQM-1.4© assessment and motor function at nusinersen treatment initiation (or, if missing, the first available score during treatment). As participants started nusinersen therapy sequentially, individual treatment duration varied in this comparison.
Treatment Satisfaction Questionnaire for Medication German version 1.4, TSQM-1.4©
The TSQM-1.4© in a German linguistically validated version is a reliable and psychometrically robust instrument to measure patients’ satisfaction with medication. The TSQM-1.4© is not disease specific but has been developed from comparisons across multiple indications, such as depression, migraine, and type 1 diabetes.19 It has not yet been routinely used in neuromuscular diseases (NMDs). Here, SMA patients were asked to self-report satisfaction with nusinersen therapy since the last drug administration by paper- or digital-based data collection of the TSQM-1.4©. The questionnaire comprises 14 items that can be categorized into four key dimensions of treatment satisfaction: ‘effectiveness’ (three items), ‘side effects’ (five items), ‘convenience’ (three items), and ‘global satisfaction’ (three items).19
The domain ‘effectiveness’ is composed of items 1–3, all of which carry the prefix ‘How satisfied or dissatisfied are you with’ followed by: ‘the ability of the medication to prevent or treat your condition?’ [Question (Q) 1]; ‘the way the medication relieves your symptoms?’ (Q2); and ‘the amount of time it takes the medication to start working?’ (Q3).
Next, satisfaction with the medication ‘side effects’ is asked in Q4 (dichotomous answer yes/no): ‘As a result of taking this medication, do you currently experience any side effects at all?’ If yes, further specifications have to be made in the following four questions: Q5 ‘How bothersome are the side effects?’; Q6 ‘To what extent do the side effects interfere with your physical health and ability to function (i.e. strength, energy levels, etc.)?’; Q7 ‘To what extent do the side effects interfere with your mental function (i.e. ability to think clearly, stay awake, etc.)?’; and Q8 ‘To what degree have medication side effects affected your overall satisfaction with the medication?’
The domain ‘convenience’ is analyzed by three items: Q9 ‘How easy or difficult is it to use the medication in its current form?’; Q10 ‘How easy or difficult is it to plan when you will use the medication each time?’; and Q11 ‘How convenient or inconvenient is it to take the medication as instructed?’
Lastly, ‘global’ treatment satisfaction is composed of the final three items (12–14). Q12 ‘Overall, how confident are you that taking this medication is a good thing for you?’; Q13 ‘How certain are you that the good things about your medication outweigh the bad things?’; and Q14 ‘Taking all things into account, how satisfied or dissatisfied are you with this medication?’
Except for the dichotomous question 4 (presence of side effects: yes or no), answers can be provided on a five- or seven-point scale from 1 (extremely dissatisfied) to 5 or 7 (extremely satisfied).
Transformation of scale scores results in scores for each dimension from 0 to 100, with higher scores representing a higher satisfaction.19,25 Dimension scores are calculated according to the user manual, which is available on request from www.iqvia.com/TSQM. In case of one missing answer within a subdomain, the calculation was adjusted as proposed by the manual; if two or more answers were omitted, subdomains were not determined. In addition, the four subdomains (maximum 100 each) were added to a sum score with a maximum of 400 points, with 400 representing the maximal satisfaction. The use of cut-off values is not common within the current literature.19 Incomplete datasets were excluded for the sum score analysis; and this also applied if the calculation of one or more subdomains was not possible due to missing answers.
Cohort description
A total of 91 adolescent (n = 6, <18 years) and adult (n = 85, ⩾18 years) SMA patients aged 10–65 (mean 35.4) years were enrolled at five German neuromuscular centers (Hannover n = 24, Essen n = 22, Ulm n = 21, Dresden n = 13, Munich n = 11) for cross-sectional analysis. In the cross-sectional study, mean nusinersen treatment duration at the time of enrollment was 11.8 months (median 10 months) and was distributed as follows: month 1 n = 1, month 2 n = 7, month 6 n = 18, month 10 n = 21, month 14 n = 18, month 18 n = 21, and month 22 n = 5. The last follow up of 21 Hannover site patients was at month 6 n = 9, month 10 n = 5, month 14 n = 5, and month 18 n = 2. Information on demographic and disease characteristics are summarized in Table 1.
Table 1.
Characteristics of enrolled SMA patients under nusinersen therapy.
| SMA all (n = 91) |
Ambulatory (n = 26) |
Non-ambulatory (n = 65) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Median (range) | n (%) | Mean (SD) | Median (range) | n (%) | Mean (SD) | Median (range) | |
| Female | 33 (36.3) | 11 (42.3) | 22 (33.8) | ||||||
| Age (years) | 35.4 (13.0) | 34 (10–65) | 37.1 (13) | 35.5 (19–65) | 34.7 (12.9) | 33 (10–65) | |||
| Symptom onset (years) | 4.7 (6.8) | 2 (0–47) | 11.4 (8.9) | 12 (1–47) | 2.0 (3.0) | 1 (0–15) | |||
| Disease duration (years) | 30.7 (13.0) | 30 (2–63) | 25.6 (14.4) | 24 (2–58) | 32.7 (12.0) | 31 (9–63) | |||
| SMN2 gene copy number | |||||||||
| 2 | 4 (4.4) | 0 (0.0) | 4 (6.2) | ||||||
| 3 | 44 (48.4) | 4 (15.4) | 40 (61.5) | ||||||
| 4 | 27 (29.7) | 16 (61.5) | 11 (16.9) | ||||||
| 5 | 1 (1.1) | 1 (3.9) | 0 (0.0) | ||||||
| 6 | 3 (3.3) | 2 (7.7) | 1 (1.5) | ||||||
| Unknown | 12 (13.2) | 3 (11.5) | 9 (13.8) | ||||||
| SMA type | |||||||||
| Type 1 | 3 (3.3) | 0 (0.0) | 3 (4.6) | ||||||
| Type 2 | 33 (36.3) | 0 (0.0) | 33 (50.8) | ||||||
| Type 3 | 54 (59.3) | 25 (96.2) | 29 (44.6) | ||||||
| Type 4 | 1 (1.1) | 1 (3.9) | 0 (0.0) | ||||||
| Ventilation | 23 (25.3) | 1 (3.9) | 22 (33.8) | ||||||
| Feeding tube | 4 (4.4) | 0 (0.0) | 4 (6.2) | ||||||
| Scoliosis | 51 (56.0) | 1 (3.9) | 50 (76.9) | ||||||
| CT-guided LP | 44 (48.4) | 1 (3.9) | 43 (66.2) | ||||||
| Motor scores at nusinersen initiation | |||||||||
| RULM (max. 37) | 78 | 17.7 (13.2) | 16.5 (0.0–37.0) | 22 | 34.5 (4.7) | 37.0 (21.0–37.0) | 56 | 11.1 (8.8) | 10.5 (0.0–37.0) |
| HFMSE (max. 66) | 78 | 17.4 (21.3) | 6.5 (0.0–66.0) | 22 | 48.5 (11.9) | 48.5 (30.0–66.0) | 56 | 5.3 (6.8) | 3.0 (0.0–30.0) |
| Treatment period at enrollment (months) | |||||||||
| 1 | 1 (1.1) | 0 (0.0) | 1 (1.5) | ||||||
| 2 | 7 (7.7) | 1 (3.9) | 6 (9.2) | ||||||
| 6 | 18 (19.8) | 5 (19.2) | 13 (20.0) | ||||||
| 10 | 21 (23.1) | 4 (15.4) | 17 (26.2) | ||||||
| 14 | 18 (19.8) | 4 (15.4) | 14 (21.5) | ||||||
| 18 | 21 (23.1) | 10 (38.5) | 11 (16.9) | ||||||
| 22 | 5 (5.5) | 2 (7.7) | 3 (4.6) | ||||||
| Motor scores at enrollment | |||||||||
| RULM (max. 37) | 91 | 18.6 (13.1) | 18 (0.0–37.0) | 26 | 35.5 (3.2) | 37 (27.0–37.0) | 65 | 11.8 (8.6) | 12 (0.0–37.0) |
| HFMSE (max. 66) | 91 | 18.2 (22.3) | 6 (0.0–66.0) | 26 | 51.2 (11.0) | 50 (30.0–66.0) | 65 | 5 (6.0) | 4 (0.0–26.0) |
CT, computed tomography; HFMSE, Hammersmith Functional Motor Scale expanded; LP, lumbar puncture; max., maximum; RULM, revised upper limb module; SD, standard deviation; SMA, spinal muscular atrophy; SMN2, survival of motor neuron 2.
In brief, 36.3% of the participants were female. The majority were classified as type 3 SMA (59.3%), followed by SMA type 2 (36.3%). Scoliosis was (anamnestically or clinically) present in 56.0% of enrolled patients, and 48.4% of the patients required computed tomography (CT)-guided nusinersen administration; 28.9% of enrolled patients were ambulatory. These patients showed a shorter disease duration (mean 25.6 years versus 32.7 years for non-ambulatory) and a less severe clinical phenotype, for example, regarding the need of ventilator support, feeding tube or CT-guided drug application. This subgroup consisted of SMA type 3 and 4 patients with a mean of 4 SMN2 copies and a mean symptom onset at 11.4 years of age. Motor-function scores (RULM and HFMSE) were available for 78 patients at nusinersen therapy initiation (Table 1).
Five patients in the reported study cohort terminated nusinersen treatment after 6 (n = 1), 14 (n = 2), or 18 (n = 2) months. All of them were non-ambulatory (one SMA type 2 patient and four SMA type 3 patients) and had a mean disease duration of 39 years (range 31–50 years). Individual reasons for treatment discontinuation were (a) disease progression (n = 2), (b) high individual efforts compared with little clinical benefit (n = 2), and (c) severe procedure-related side effects (n = 1, spinal bleeding).
Statistical analysis
Statistical analysis was performed using IBM statistical product and service solutions 24® (SPSS, Chicago, IL, USA). Descriptive statistics were calculated and listed as percentage, mean and standard deviation (SD), or/and median and range. Internal consistency within a specific domain of the TSQM-1.4© was determined by using Cronbach’s alpha coefficient.
We performed linear regression analyses to examine the contribution of demographic and clinical features on treatment satisfaction with nusinersen. First, in univariate analyses, each TSQM-1.4© dimension and the sum score were selected as the dependent variables while demographic and clinical features such as age at onset, disease duration, SMA type, SMN2 copy number, ambulatory state, the procedure of nusinersen administration (CT-guided versus conventional), and motor function measures (RULM and HFMSE) at treatment initiation, as well as confounding factors (age, sex), were analyzed as independent variables. In a second step, relevant variables (p < 0.2) were analyzed in a multivariate regression analysis.26 By using backward selection, significant variables were detected as defined by p < 0.05. Additionally, another linear regression analysis was used to investigate the impact of the change in motor-function measures (ΔRULM and ΔHFMSE; independent variables) on the subdomains ‘global satisfaction’ and satisfaction with ‘effectiveness’ (dependent variables).
Direct comparison of individual TSQM-1.4© questions, TSQM-1.4© dimensions and the TSQM-1.4© sum score between dichotomized variables was performed by the Mann–Whitney U test. Dichotomous variables were: ambulatory versus non-ambulatory patients; CT-guided versus conventional drug administration; and treatment duration (<10 months versus ⩾10 months; according to the median in this cohort). Analysis of motor-function scores (HFMSE and RULM) of ambulatory versus non-ambulatory patients and disease duration of dropout patients versus those still on treatment was also performed by the Mann-Whitney-U-test. The frequency of dropouts in non-ambulatory patients versus ambulatory patients was analyzed by the two-sided Fisher’s exact test. Correlation between TSQM-1.4© subdomains and the association between motor-function outcome changes and TSQM-1.4© results were further analyzed by Spearman’s correlation coefficient.
The non-parametric Kruskal–Wallis test was performed to detect differences in treatment satisfaction between five different treatment time points in 85 SMA patients [months (m)2 n = 7, m6 n = 18, m10 n = 21, m14 n = 18, and m18 n = 21] and between patients with improved versus unchanged versus decreased RULM scores. Patients who were enrolled at a treatment duration of either 1 month (m1 n = 1) or 22 months (m22 n = 5) were excluded from the analysis due to the small number of patients for these time points (totally, n = 6). To analyze longitudinal changes in individual treatment satisfaction, the earliest and latest treatment satisfaction measures collected in the Hannover site subgroup [minimum 2 months, maximum 18 months, follow up was either after 4 (n = 16) or 8 (n = 5) months] were compared by the Wilcoxon signed-rank test for non-parametric dependent paired variables.
For motor-function outcome changes, first assessed RULM and HFMSE scores were used (n = 91). In 13 patients, motor outcome measures were not available at baseline but obtained latest at month 6 of nusinersen treatment. Otherwise, patient numbers were reduced by the missing datasets, such as missing individual answers for the TSQM-1.4© questions or dimensions or the TSQM-1.4© sum score (remaining n = 84). SMN2 copy numbers were missing in n = 12 patients and motor-function outcome measures at treatment initiation in n = 13. All p values were two tailed; a p value of ⩽0.05 was considered statistically significant (significance levels: *⩽0.05, **⩽0.01).
Results
Dimensions of treatment satisfaction and relevant influencing factors
‘Global satisfaction’ with nusinersen treatment was rated at a mean of 73.1 (range 7–100). Satisfaction with ‘side effects’ was highest (mean 93.5, 38–100), followed by treatment ‘effectiveness’ (mean 64.8, 0–100), and ‘convenience’ (mean 43.6, 0–89). All results of the TSQM-1.4©, including individual questions and different dimensions, are presented in Table 2. The study participants answered at least 97% of mandatory questions in the subdomains ‘effectiveness,’ ‘convenience,’ and ‘global satisfaction.’ Omitted questions were mainly Q2, left out by five patients, followed by Q3 and Q9 (n = 5 and n = 4, respectively, missing). In the case of four patients, who received nusinersen treatment for 1 (n = 1), 2 (n = 1), 6 (n = 1), or 10 (n = 1) months, the domain ‘effectiveness’ was not determined due to missing answers of Q1, Q2, and Q3. An excellent internal consistency for the TSQM-1.4© domains ‘effectiveness’ and ‘global satisfaction’ (both Cronbach’s alpha = 0.87) was detected, comparable with previous reports from Atkinson et al.19 Further good results were found for the dimensions ‘convenience’ and ‘side effects,’ with a Cronbach’s alpha of 0.78 and 0.76, respectively.
Table 2.
TSQM-1.4© scores divided into questions, dimensions, and subgroups.
| SMA all n = 91 |
Ambulatory n = 26 |
Non-ambulatory n = 65 |
p value | |
|---|---|---|---|---|
| Mean (SD, range) | Mean (SD, range) | Mean (SD, range) | ||
| Effectiveness (max. 100) | 64.8 (20.6, 0–100) | 72.9 (18.2, 28–100) | 61.3 (20.7, 0–100) | 0.014 |
| n = 87 | n = 26 | n = 61 | ||
| Q1: Ability to prevent or treat condition (scale 1–7) | 5.2 (1.3, 1–7) | 5.7 (1.1, 3–7) | 4.9 (1.4, 1–7) | 0.015 |
| n = 90 | n = 26 | n = 64 | ||
| Q2: Way medication relieves symptoms (scale 1–7) | 4.7 (1.4, 1–7) | 5.3 (1.4, 2–7) | 4.4 (1.2, 1–7) | 0.004 |
| n = 86 | n = 26 | n = 60 | ||
| Q3: Time it takes medication to start working (scale 1–7) | 4.8 (1.47, 1–7) | 5.1 (1.3, 2–7) | 4.6 (1.5, 1–7) | 0.141 |
| n = 87 | n = 26 | n = 61 | ||
| Side effects (max. 100) | 93.5 (14.76, 38–100) | 91.1 (16.4, 44–100) | 94.4 (14.1, 38–100) | 0.212 |
| n = 91 | n = 26 | n = 65 | ||
| % who reported any side effects (yes/no) | 23.1 | 30.8 | 20.0 | 0.283 |
| n = 91 | n = 26 | n = 65 | ||
| Q5: Bothersomeness of side effects (scale 1–5) | 3.6 (1.1, 1–5) | 3.7 (1, 2–5) | 3.5 (1.2, 1–5) | 0.687 |
| n = 22 | n = 9 | n = 13 | ||
| Q6: Side effects interfere with physical function (scale 1–5) | 3.9 (0.99, 2–5) | 3.9 (1.1, 2–5) | 3.9 (1, 2–5) | 0.889 |
| n = 22 | n = 9 | n = 13 | ||
| Q7: Side effects interfere with mental function (scale 1–5) | 4.6 (0.73, 3–5) | 4.7 (0.7, 3–5) | 4.6 (0.8, 3–5) | 0.927 |
| n = 22 | n = 9 | n = 13 | ||
| Q8: Side effects impact overall satisfaction (scale 1–5) | 3.7 (1.11, 2–5) | 3.7 (1, 3–5) | 3.7 (1.2, 2–5) | 0.920 |
| n = 23 | n = 9 | n = 14 | ||
| Convenience (max. 100) | 43.6 (20.21, 0–89) | 54.1 (18.7, 6–89) | 39.8 (19.5, 0–78) | 0.003 |
| n = 88 | n = 23 | n = 65 | ||
| Q9: Ease/difficulty of use (scale 1–7) | 2.9 (1.3, 1–6) | 3.7 (1.3, 1–6) | 2.7 (1.2, 1–5) | 0.002 |
| n = 86 | n = 22 | n = 64 | ||
| Q10: Ease/difficulty of planning (scale 1–7) | 4.8 (1.54, 1–7) | 5.1 (1.3, 2–7) | 4.7 (1.6, 1–7) | 0.360 |
| n = 88 | n = 23 | n = 65 | ||
| Q11: Convenience to take as instructed (scale 1–7) | 3.1 (1.41, 1–6) | 3.8 (1.3, 1–6) | 2.8 (1.4, 1–6) | 0.005 |
| n = 88 | n = 23 | n = 65 | ||
| Global satisfaction (max. 100) | 73.1 (21, 7–100) | 78.9 (18.7, 21–100) | 70.7 (21.5, 7–100) | 0.060 |
| n = 91 | n = 26 | n = 65 | ||
| Q12: Confidence that taking medication is good (scale 1–5) | 4.1 (0.97, 1–5) | 4.5 (0.8, 2–5) | 3.9 (1, 1–5) | 0.010 |
| n = 88 | n = 23 | n = 65 | ||
| Q13: Certainty that good things about medication outweigh the bad things (scale 1–5) | 3.9 (1.02, 1–5) | 4.1 (1, 2–5) | 3.9 (1, 1–5) | 0.212 |
| n = 91 | n = 26 | n = 65 | ||
| Q14: Satisfaction with medication (scale 1–7) | 5.3 (1.27, 1–7) | 5.6 (1.2, 2–7) | 5.1 (1.3, 1–7) | 0.102 |
| n = 88 | n = 26 | n = 62 | ||
| TSQM-1.4© sum score (max. 400) | 275.8 (54.2, 66.9–372.2) | 302.7 (42.4, 199.2–365.1) | 265.7 (55, 66.9–372.2) | 0.003 |
| n = 84 | n = 23 | n = 61 |
Bolded numerals indicate statistical significance.
max., maximum; Q, question; SD, standard deviation; TSQM-1.4©, Treatment Satisfaction Questionnaire for Medication (version 1.4, German): reduced numbers for specific questions, dimensions, and the sum score result from omitted questions.
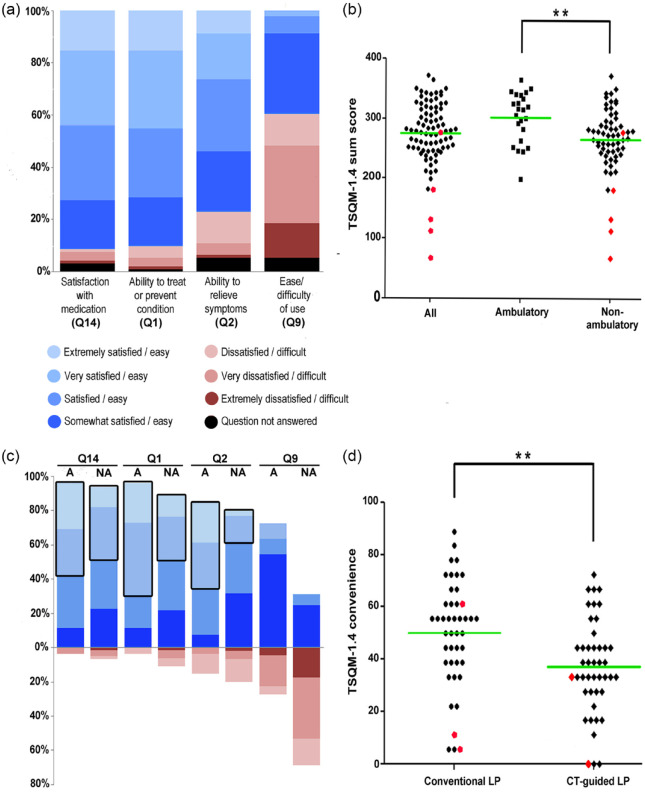
Selected TSQM-1.4© questions regarding ‘global satisfaction,’ satisfaction with ‘effectiveness,’ and ‘convenience’ are highlighted in Figure 1. About 91% stated to be at least ‘somewhat satisfied’ with the medication (Q14). Nearly half of the patients (44%) declared to be either ‘very’ or ‘extremely’ satisfied. Only 5.7% (n = 5) stated dissatisfaction with medication. Accordingly, more than 90% of study participants expressed satisfaction with the ability of nusinersen to prevent or treat SMA (Q1) and about 80% were satisfied with the way nusinersen relieved their symptoms (Q2). In contrast, much fewer patients confirmed that nusinersen was easy to use [Figure 1(a)]. The TSQM-1.4© sum score revealed a mean score of 275.8 [Figure 1(b)].
Figure 1.
TSQM-1.4© scores during nusinersen therapy in 5q-SMA patients and clinical subgroups.
(a) Displayed is the distribution of SMA patients’ responses to selected TSQM-1.4© questionnaire items (Q; in %), representing three of the four TSQM-1.4© subdomains. Answers ranged on a seven-point scale from extremely dissatisfied or difficult (in red) to extremely satisfied or easy (in blue). Q14 (n = 88) represents the subdomain ‘global satisfaction,’ Q1 (n = 90) and Q2 (n = 86) represent the dimension satisfaction with ‘effectiveness,’ and Q9 (n = 86) represents satisfaction with ‘convenience.’ Most patients indicated being at least somewhat satisfied with the therapy. (b) The mean TSQM-1.4© sum score (maximum 400) was 275.8 in n = 84 SMA patients. By comparing ambulatory (n = 23) with non-ambulatory patients (n = 61), significantly higher scores were detected in ambulatory patients (**p ⩽ 0.01). Red data points indicate patients who withdrew from treatment (n = 5). (c) Moreover, by comparison of ambulatory (n = 22) with non-ambulatory (n = 64) patients, differences in the convenience question Q9 appeared. Also, ambulatory patients reported being more frequently ‘extremely’ or ‘very’ satisfied compared with non-ambulatory patients, particularly in questions addressing ‘effectiveness’ Q1 and Q2 and ‘global’ satisfaction Q14, indicated by a black frame. (d) SMA patients treated by computed tomography (CT)-guided lumbar puncture (LP) for nusinersen administration (n = 44) were significantly less satisfied with the ‘convenience’ than patients who received nusinersen by conventional LP (n = 44; **p ⩽ 0.01). Red data points indicate patients who withdrew from treatment (n = 5).
A, ambulatory; NA, non-ambulatory; SMA, 5q-spinal muscular atrophy; TSQM-1.4©, Treatment Satisfaction Questionnaire for Medication German version 1.4©.
To investigate whether patient characteristics or disease-related conditions may influence treatment satisfaction with nusinersen, we analyzed their association with the different dimensions and the sum score of the TSQM-1.4©. Demographic and disease-specific characteristics of ambulatory versus non-ambulatory patients are shown in Table 1. All analyzed variables and results of the univariate linear regression analysis are listed in Supplementary Table 1. In the multivariate regression analyses, the ability to walk, as the only variable, showed a significant association with satisfaction with ‘effectiveness’ [β = 13.23, 95% confidence interval (CI; 3.33–21.12), p = 0.009]. Moreover, ambulatory state was the main influencer (regarding the β coefficients) on the TSQM-1.4© sum score [β = 126.05, 95% CI (56.66–195.44), p = 0.009] as well as satisfaction with ‘convenience’ [β = 28.31, 95% CI (5.67–50.95), p = 0.015]. The latter was also statistically significantly influenced by the procedure used for nusinersen administration [CT-guided β = −19.64, 95% CI (−29.95 to −9.33), p < 0.001], and mildly by the HFMSE score [β = −0.68, 95% CI (−1.19 to −0.18), p = 0.009] and the disease duration [β = −0.43, 95% CI (−0.81 to −0.06), p = 0.024]. The TSQM-1.4© sum score was additionally influenced by the HFMSE score [β = −2.64, 95% CI (−4.27 to −1.01), p = 0.002] and age at disease onset [β = 2.39, 95% CI (−0.12 to 4.66), p = 0.039]. Of note, the effect of the variables ambulatory state, HFMSE score, and disease duration on the ‘convenience’ domain and the TSQM-1.4© sum score appears contradictory. However, the impact (measured by the β coefficients) of the HFMSE score and/or the disease duration on the TSQM-1.4© scores were only very mild and barely relevant compared with those measured for the variable ambulatory state [e.g. for TSQM-1.4© sum score (0–400) HFMSE β = −2.64 versus ambulatory β = 126.05]. In summary, regarding patient characteristics or disease-related conditions, the ambulatory state was identified to have the main impact on treatment satisfaction. The dimension ‘side effects’ was mildly dependent of the HFMSE [β = −0.22, 95% CI (−0.40 to −0.04), p = 0.017]. In contrast, for the subdomain ‘global satisfaction,’ no significant influencing factor was identified.
Next, we compared TSQM-1.4© scores in ambulatory and non-ambulatory nusinersen-treated patients, as the ability to walk was the primary disease-related variable with an important impact on outcomes in our study. A significant difference between these subgroups in treatment satisfaction with ‘effectiveness’ (72.9 versus 61.3, p = 0.014), ‘convenience’ (54.1 versus 39.8, p = 0.003), and the TSQM-1.4© sum score (302.7 versus 265.7, p = 0.003) was identified [Figure 1(b), Table 2].
As expected, performance in motor-function scores (RULM and HFMSE) at the time point of nusinersen treatment initiation was significantly better in the ambulatory patients (HFMSE and RULM p < 0.0001). About 23% of ambulatory patients were extremely satisfied with the way nusinersen relieved their symptoms (Q2), as opposed to 3.1% of non-ambulatory patients. Nevertheless, 73.8% of non-ambulatory patients were at least ‘somewhat satisfied’ with symptom relief [Figure 1(c)]. In contrast, two thirds of non-ambulatory (versus 23.1% in ambulatory) patients, of which 60% required CT-guided nusinersen administration, stated that nusinersen is difficult to use [Q9; p = 0.002; Figure 1(c)].
In line with this finding, satisfaction with ‘convenience’ was significantly lower in patients who needed CT-guided drug administration (n = 44) compared with those patients who underwent conventional lumbar puncture [n = 44 (37.1 versus 50.0, p ⩽ 0.001) Figure 1(d)]. In contrast, no statistically significant differences were observed in the dimensions ‘global satisfaction’ and satisfaction with ‘effectiveness’ and ‘side effects’ while comparing CT-guided versus conventional lumbar puncture, but there was a trend towards greater satisfaction concerning side effects (96.0 versus 91.1 p = 0.145) in patients who had CT-guided drug administration.
Inter-dimensional correlation analysis of the TSQM-1.4© items to investigate associations between dimensions revealed striking associations of satisfaction with ‘convenience’ to all subdomains (‘global satisfaction’ r = 0.215, p = 0.044, ‘effectiveness’ r = 0.227, p = 0.038, ‘side effects’ r = 0.252, p = 0.018). However, the correlation of ‘global satisfaction’ to satisfaction with ‘effectiveness’ was identified as being most striking (r = 0.693, p < 0.0001). In contrast, satisfaction with ‘effectiveness’ and ‘side effects’ did not significantly correlate to each other (r = 0.021, p = 0.845).
Treatment satisfaction at different treatment time points
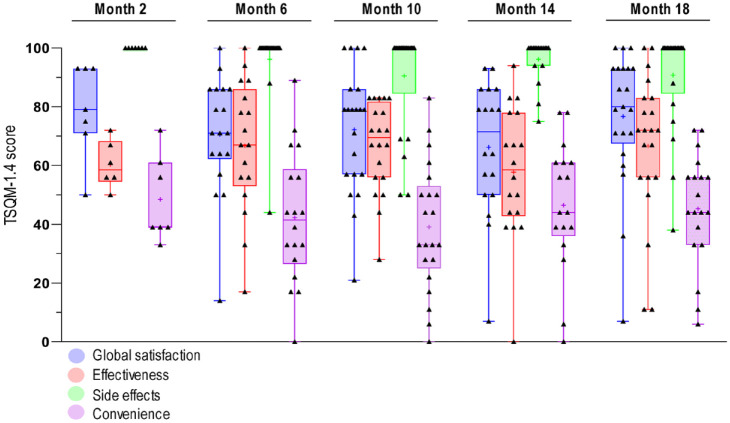
In the cross-sectional analysis of treatment satisfaction (n = 85 patients at five nusinersen treatment time points (m2 n = 7; m6 n = 18; m10 n = 21; m14 n = 18 and m18 n = 21), no significant differences in any of the dimensions of treatment satisfaction (‘global satisfaction’ p = 0.387, ‘effectiveness’ p = 0.404, ‘side effects’ p = 0.408, ‘convenience’ p = 0.659) were observed (Figure 2). ‘Global’ treatment satisfaction in TSQM-1.4© remained stable at a high level (mean m2 = 79.1, m6 = 70.6, m10 = 72.1, m14 = 66.1, m18 = 76.7) during treatment continuation. The analysis of treatment duration (<m10 versus ⩾m10) as an influencing factor revealed no significant differences of treatment satisfaction in any dimension (‘global satisfaction’ p = 0.835, ‘effectiveness’ p = 0.664, ‘side effects’ p = 0.236, ‘convenience’ p = 0.233). Consistently, individual follow up of 21 patients with a maximum treatment duration of 18 months showed no significant changes of TSQM-1.4© results over time (‘global satisfaction’ p = 0.568, ‘effectiveness’ p = 0.612, ‘side effects’ p = 0.310, ‘convenience’ p = 0.827).
Figure 2.
Cross-sectional TSQM-1.4© dimension outcomes during nusinersen treatment at five treatment time points.
TSQM-1.4© scores of the dimensions ‘global satisfaction’ (blue), ‘effectiveness’ (red), ‘side effects’ (green) and ‘convenience’ (violet) corresponding to the specific time point of data collection during nusinersen treatment course (months m2 n = 7, m6 n = 18, m10 n = 21, m14 n = 18, and m18 n = 21) are depicted in a boxplot chart. The median is represented by a horizontal bar, the mean by a ‘+’. The overlapping scatter plot and the error bars present the range of TSQM-1.4© scores in the specific subdomain at the analyzed time point.
TSQM-1.4© versus motor-function outcome measures
To further evaluate TSQM-1.4© as a PRO, results were compared with changes (Δ) in motor-function outcome scores during therapy. ΔRULM, stronger than ΔHFMSE, positively correlated to patients’ satisfaction measured by TSQM-1.4© (satisfaction with ‘effectiveness’ versus ΔRULM r = 0.233, p = 0.030; versus ΔHFMSE r = 0.221, p = 0.040; TSQM-1.4© sum score versus ΔRULM r = 0.305, p = 0.005; versus ΔHFMSE r = 0.204, p = 0.062, ‘global satisfaction’ versus ΔRULM r = 0.257, p = 0.014; versus ΔHFMSE r = 0.148, p = 0.161 and ‘side effects’ ΔRULM r = 0.229, p = 0.029 versus ΔHFMSE r = 0.154, p = 0.146). No correlation of satisfaction with ‘convenience’ and ΔRULM r = 0.171, p = 0.112, ΔHFMSE r = 0.160, p = 0.136 was observed. A linear regression analysis showed evidence of significantly higher satisfaction with ‘effectiveness’ and ‘global satisfaction’ in patients with increasing RULM [β = 2.34, 95% CI (−0.43 to 4.26), p = 0.017 and β = 3.029, 95% CI (1.14–4.91), p = 0.002] and HFMSE scores [β = 1.30, 95% CI (−0.19 to 2.41), p = 0.023 and β = 1.30, 95% CI (−0.17 to 2.43), p = 0.025]. The influence of ΔRULM on ‘global’ treatment satisfaction was identified as most powerful [Figure 3(a)].
Figure 3.
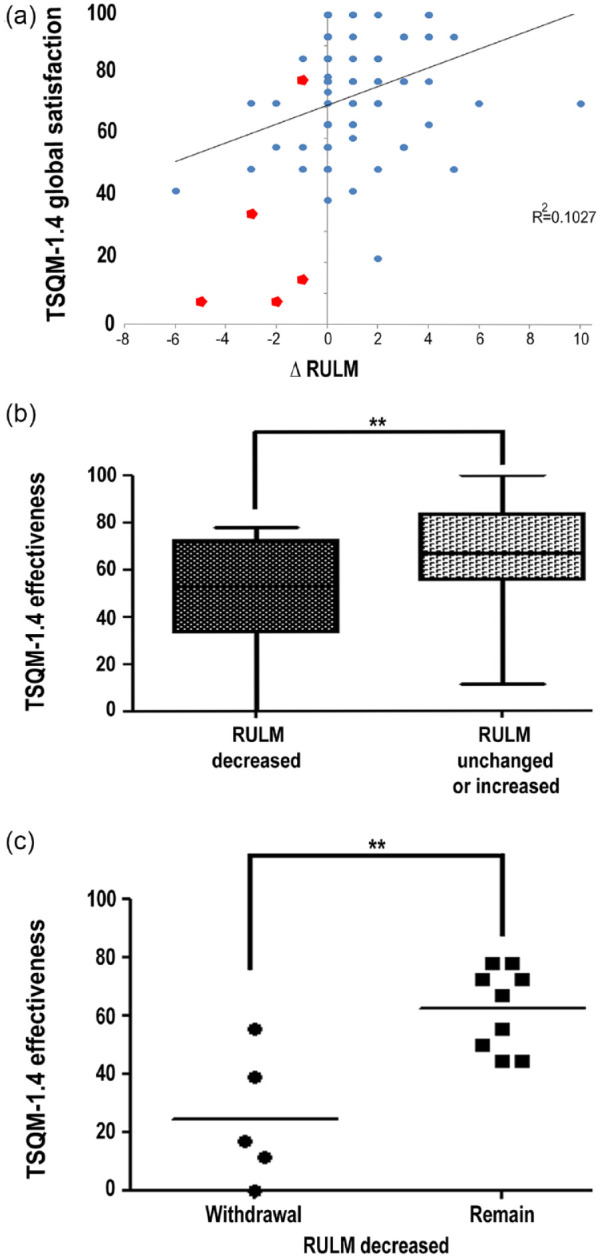
Treatment satisfaction compared with motor-function outcome measures.
(a) Changes in the motor-function outcome measure revised upper limb module (∆RULM) during nusinersen therapy directly correlated with ‘global satisfaction’ (r = 0.257, p = 0.014) in 91 SMA patients. Patients with a better motor-function outcome under nusinersen reported a higher treatment satisfaction. Red dots indicate patients who withdrew from treatment (n = 5). For clarity reasons, overlapping data points were not shown. (b) Comparison of patients with unchanged or improved ∆RULM to patients with deterioration in RULM during nusinersen treatment (median of 10 months). Satisfaction with effectiveness was significantly lower in patients with a deterioration in RULM scores under therapy (**p ⩽ 0.01). Error bars indicate the range of scores. (c) Five patients, all non-ambulatory, terminated nusinersen treatment after six (n = 1), 14 (n = 2), or 18 (n = 2) months. Satisfaction with effectiveness was significantly lower in these patients (withdrawal) compared with patients with a decline in motor-function measured by the RULM, who, in contrast, remained on therapy (n = 9; remain; **p ⩽ 0.01).
RULM, revised upper limb module; SMA, spinal muscular atrophy; TSQM-1.4©, Treatment Satisfaction Questionnaire for Medication German version 1.4; Δ, change.
We next compared treatment satisfaction of patients with different ΔRULM courses under therapy: increased (n = 38) versus unchanged (n = 39) versus decreased (n = 14) RULM scores. By applying the Kruskal–Wallis test, a significant difference in treatment satisfaction with ‘effectiveness’ (p = 0.028) and ‘global satisfaction’ (p = 0.028) was identified. Interestingly, when looking at patients with increased RULM and unchanged RULM, no significant differences in treatment satisfaction with ‘effectiveness’ (mean 68.7 versus 68.7, p = 0.811) or ‘global satisfaction’ (mean 76.6 versus 77.7 p = 0.813) were identified. However, significant differences in treatment satisfaction were found while comparing patients with decreased RULM scores with those with increased ones (‘global’ mean 50.5 versus 76.6, p = 0.001, ‘effectiveness’ mean 48.8 versus 68.7, p = 0.012), as well as to those with unchanged RULM scores (‘global’ mean 50.5 versus 77.7, p < 0.001, ‘effectiveness’ mean 48.8 versus 67.0, p = 0.016).
These data suggested that motor-function improvement and stabilization were satisfying treatment responses, so we divided our study cohort into two outcome groups. Patients with an increased or unchanged RULM score [mean +1.30 (SD 1.84; n = 77)] were defined as motor responders as previously described,27 and those with a decrease in RULM scores [mean −2.29 (SD 1.59); n = 14] as motor non-responders. The motor-responder group consisted of all ambulatory patients (n = 26) and 51 non-ambulatory patients. The motor non-responder group consisted of non-ambulatory patients only. Higher satisfaction with the ‘effectiveness’ of nusinersen [67.8 versus 48.8, p = 0.008; Figure 3(b)] and a higher ‘global satisfaction’ (77.2 versus 50.5, p ⩽ 0.001) were identified in motor responders. However, this did not apply to satisfaction with ‘side effects’ (95.1 versus 84.4, p = 0.093) and ‘convenience’ (44.7 versus 37.3, p = 0.330).
Treatment discontinuation
Interestingly, 35.7% (n = 5) of the motor non-responder group versus none in the motor-responder group terminated nusinersen treatment [red dots in Figures 1(b), (d), and 3(a)]. All of these patients were non-ambulatory. A frequency of 7.7% of dropouts in non-ambulatory patients versus 0% in ambulatory patients (two-sided Fishers exact test, p = 0.316) did not reach statistical significance. The total RULM score by the time of enrollment was, on average, lower in patients who discontinued nusinersen therapy compared with patients still undergoing treatment (10.0 versus 19.1, p = 0.150). A trend toward a longer disease duration in those five patients compared with patients still on treatment (mean 39.0 versus 30.2 years, p = 0.096) was identified. Correspondingly, dropout patients were older, on average (42 versus 35 years, p = 0.203).
In the cross-sectional analysis, four out of five dropout patients filled in the TSQM-1.4© questionnaire at their last nusinersen treatment that took place. One patient received one further application after the TSQM-1.4© was filled in and reached month 18 of treatment.
A mean decrease of −2.40 (SD 1.67) points in the RULM score during a median of 14 months of treatment was recorded in the group of patients who discontinued treatment. Correspondingly, low satisfaction with ‘effectiveness’ and ‘global’ satisfaction were apparent in this subgroup (mean ‘effectiveness’ 24.4, ‘global’ 28.6). Treatment satisfaction was even significantly lower (‘effectiveness’ 24.4 versus 62.4, p = 0.007, and ‘global’ 28.6 versus 62.7, p = 0.044) compared with motor non-responders who still opted for continuation of therapy despite a comparable motor function decrease [RULM −2.22 (SD 1.64); Figure 3(c)]. While treatment satisfaction regarding ‘side effects’ (78.8 versus 87.5, p = 0.587) did not significantly differ between patients who withdrew and patients who continued treatment, a trend towards a lower rating of treatment ‘convenience’ was observed in dropout patients (22.2 versus 45.7, p = 0.071).
In terms of treatment satisfaction trajectories before dropout, the TSQM-1.4© at the treatment visit previous to (4 months before) the last nusinersen administration was available in three of five patients who discontinued treatment by including results of the longitudinal substudy.TSQM-1.4© scores in two patients (S16 and S18, part of longitudinal substudy in Hannover) were clearly below average of the total cohort except for the satisfaction with ‘side effects’ (patient S16 ‘global’ 14.3, ‘effectiveness’ 33.3, ‘side effects’ 100.0, ‘convenience’ 27.8 and patient S18 ‘global’ 42.9, ‘effectiveness’ 33.3, ‘side effects’ 100.0, ‘convenience’ 27.8). In one further patient, mainly average or even above the average TSQM-1.4© scores in most dimensions (‘global’ 78.6, ‘side effects’ 100.0, ‘convenience’ 61.1) were identified when compared with the mean results of the total cohort (‘global’ 73.1, ‘side effects’ 93.5, ‘convenience’ 43.6). Nevertheless, this patient scored considerably lower regarding satisfaction with ‘effectiveness’ (38.9 versus 64.8 in the total cohort).
Discussion
To our best knowledge, this is the first multicenter study that analyzed treatment satisfaction in the four essential dimensions ‘effectiveness,’ ‘side effects,’ ‘convenience,’ and ‘global satisfaction’ in adolescent and adult 5q-SMA patients under nusinersen therapy. The majority of the investigated patients were satisfied with treatment both globally and in terms of effectiveness.
According to TSQM-1.4© scores in the investigated patients, the highest satisfaction was shown with ‘side effects,’ although side effects were reported by 20% of them. Treatment satisfaction with ‘convenience’ was lowest, mainly due to the need for repeated lumbar punctures. Patients who underwent CT-guided nusinersen administrations rated treatment ‘convenience’ even lower. CT-guided lumbar punctures are well known to be challenging in SMA.7,8 In our study, two thirds of the non-ambulatory patients stated that nusinersen was difficult to use. Of those, 60% required a CT-guided administration. Whether differences between intervertebral and transforaminal administration do exist, still need to be further evaluated. Satisfaction with ‘convenience’ positively correlated to all other domains, like ‘effectiveness,’ ‘side effects,’ and ‘global satisfaction.’ Therefore, it might be expected that an improvement in medication delivery would consequently improve patients’ satisfaction in other dimensions. Other, less invasive, and more convenient admission routes, as recently approved by the FDA, could address this problem.28–30
In our study, patients’ satisfaction remained nearly stable over the treatment course. Neither cross-sectional comparison between different time points nor longitudinal follow up of a subgroup of patients revealed any significant time-dependent differences. These results seem to contradict recent reports on long-term results from the phase I/II study in later-onset SMA, which showed improvement in motor function over time that was not yet present at 2 months of treatment.31 However, this study focused on gross motor-function changes measured by the HFMSE. The TSQM-1.4© captures additional aspects, such as small improvements in finger movements or non-motor functions. Besides improvement in muscle strength, SMA patients considered mobility, endurance, and disease stabilization as relevant treatment outcomes in a previous study.32 Early high treatment satisfaction could therefore already arise from the interruption of further disease progression. As TSQM-1.4© lacks qualitative analyses, the exact causes underlying patients’ satisfaction with effectiveness remain to be further elucidated.
In non-ambulatory patients, at least moderate satisfaction with nusinersen effectiveness was found, which is in line with recent studies that also showed nusinersen efficacy in more severely affected patients.10,27,33
Significantly higher treatment satisfaction was seen in ambulatory patients. ‘Walkers’ were significantly more satisfied with ‘effectiveness’ and ‘convenience’ besides higher TSQM-1.4© sum scores compared with non-ambulatory patients. Early treatment start produced the best response to therapy in SMA mouse models.34 Subgroup analyzes of nusinersen studies in infantile-onset (ENDEAR)2 and later-onset (CHERISH)3 SMA indicated greater motor-function improvements in patients with shorter disease duration at treatment initiation. This suggests that earlier treatment in functionally less impaired patients may lead to a better clinical outcome. Similarly, interim results of the open-label NURTURE phase II study in presymptomatic infants revealed treatment benefits exceeding those observed in the ENDEAR study, where treatment was initiated in a symptomatic period.35 The underlying hypothesis is that more severely affected patients with longer disease duration have less remaining motor neurons and functional capacity, and therefore show less benefit from SMN replacement.34 Stabilization of disease progression in non-ambulatory patients might be rated less enthusiastically compared with actual gross motor-function improvement in ambulatory patients. In addition, a trend toward longer disease duration in patients who discontinued treatment, all of whom were non-ambulatory, was observed, indicating a higher proportion of treatment non-responders in this group. Another important aspect is not to be missed: non-ambulatory patients often require substantial organizational and personal effort to obtain treatment, including the assistance of a caregiver, special transportation solutions to treatment centers, and a bothersome application under radiation exposure.36–38
Along with growing real-world experience in SMA treatment, challenges for the evaluation of specific phenotypes arise. Primarily, minor clinical changes are not captured sensitively enough and non-motor-function improvements are completely disregarded by currently used outcome measures. This applies especially to very severe or only mildly affected patients due to floor and ceiling effects. Moreover, despite widespread use in children, motor scores are often not validated for adult SMA patients. Lastly, a thorough motor-function assessment requires trained personnel and time, both of which are usually not reimbursed in the clinic setting. Therefore, the use of PROs for the evaluation of nusinersen treatment in adult SMA patients in clinical practice has already been suggested.39,40
PROs are entirely based on individual patient experience.40,41 They are used to evaluate the impact of the disease and treatment on patients’ daily needed functions, well-being, and health-related quality of life. Primarily, they were developed for clinical trials, but nowadays can be part of the standard clinical assessment, such as the revised Amyotrophic Lateral Sclerosis Functional Rating Scale in amyotrophic lateral sclerosis.42
Treatment satisfaction is already in use as a PRO in other neurological conditions.43,44 It is particularly useful for evaluating the patient’s perspective on their current treatment. Treatment satisfaction represents an important outcome, as it has been shown to affect treatment-related decisions such as compliance and adherence.19,45 In SMA, direct feedback of patients on their health and quality of life benefits may enable earlier identification of potential dropouts. This may be particularly useful in the future for the transition to new disease-modifying therapies (DMTs).28,29
This study showed that results of the most widely used scores, HFMSE and RULM, correlated with patients’ treatment satisfaction measured by the TSQM-1.4©. Satisfaction with treatment effectiveness in patients with improved motor-function scores (here RULM) did not significantly differ from those with no motor-function changes during treatment. However, both these patient groups reported significantly higher TSQM-1.4© ‘effectiveness’ scores than patients with a deterioration in the RULM score. These data suggest both motor function stabilization and improvement as a satisfying treatment response.
Of note, ‘no change,’ or a decrease in motor-function scores, is not to be equated with no treatment response at all. Our data identified patients with decreased motor-function scores still on treatment and satisfied with treatment effectiveness as much as those with improved motor scores. We suggest that alleviation or improvement of factors apart from gross motor function is of significant clinical relevance for these patients. These factors may include fatigue, endurance, quality of life, ventilation, swallowing and many more, that are not captured by motor-function scores.10,32,46,47
However, five patients discontinued the therapy. All experienced a reduction in RULM scores during the treatment course. These patients indicated significantly lower satisfaction with ‘effectiveness’ than patients with similar decreases in RULM scores (classified as motor non-responders) but wishing to continue nusinersen treatment. Interestingly, satisfaction with side effects seemed not to play a role in the decision to stop the therapy. However, a trend toward lower satisfaction with ‘convenience’ could be observed that presumably only missed significance due to small patient numbers. The TSQM-1.4© results were not discussed with patients. Thus, the decision-making process of discontinuation was not actively interfered with by the TSQM-1.4©. The questionnaire was completed mainly during the treatment visits or shortly thereafter. Filling out the TSQM-1.4©, however, may have supported the patients’ decision to stop treatment through reflecting on their treatment satisfaction.
In three of the five patients, who discontinued treatment, TSQM-1.4© results of the visit prior to (4 months before) the last nusinersen administration were available. By then, the patients’ satisfaction with treatment ‘effectiveness’ was significantly below average already. Atkinson et al.19 measured the likelihood of discontinuing treatment in chronic diseases by using the TSQM-1.4© and questions specifically regarding ‘the likelihood that they would continue to take the medication given its current level of effectiveness and side effects’ generating a composite variable. Through this, ‘global’ treatment satisfaction was identified as a key factor for discontinuation next to ‘effectiveness’ and ‘side effects’; satisfaction with ‘convenience’ as a relevant contributor was dismissed. In our study, the composite variable could not be generated due to the missing additional questions. However, focusing on the TSQM-1.4© scores at the time of discontinuation, treatment satisfaction with ‘side effects’ was not crucial, whereas treatment satisfaction with ‘convenience’ seemed to be an important source of dissatisfaction. Therefore, the results of Atkinson et al. regarding the main subdomains (‘side effects,’ ‘global,’ and ‘effectiveness’) in the TSQM-1.4© relevant for discontinuation may not be transferred one-to-one to the SMA treatment. A larger number of treatment dropouts would be needed to confirm these findings.
In some aspects, evaluation of patient-reported treatment satisfaction therefore seems to be superior to commonly used motor-function outcome measures. This is primarily due to its potential to evaluate treatment effects in general, including non-motor improvements such as ventilation and swallowing, which are missed by the HFMSE and RULM scores.10 Second, it is easier and faster to use in daily routine and does not require any trained personnel or equipment. Besides, it appears to be an early indicator of impending therapy withdrawal, even if a reliable cut-off value to distinguish those who stopped treatment could not be established in this study due to the small number of patients and the lack of additional questions as suggested by Atkinson et al.19
However, the TSQM-1.4© needs to be accompanied by outcome scores that measure or indicate disease-specific symptoms. Adding the TSQM-1.4© to commonly used outcome measures can be particularly useful in light of well-established concerns regarding motor-function measurements in severely or mildly affected SMA patients, as discussed earlier.
We appreciate that this study has some limitations. The first one is the missing availability of other treatment options for SMA for comparison of respective treatment satisfaction at the time of investigation. A potential placebo effect and prior individual expectations may have an impact on treatment satisfaction regarding effectiveness. Second, the unequal size of patient subgroups (in particular regarding ambulatory state and age) assessed at different time points may reduce statistical power. A larger and more evenly distributed patient cohort followed up during a longer observation period is needed to validate our findings. However, SMA is a rare disease and nusinersen treatment is expensive and has only been available since the middle of 2017 in a few specific centers. Therefore, larger datasets are sparse. Furthermore, as SMA is mainly a disease with onset in early childhood, patients who have reached adulthood are more likely to have lost the ability to walk due to the natural disease history.11 As phenotypes will change under nusinersen treatment, the prevalence of ambulatory adolescent and adult patients (‘walkers’) might increase within the next years.48 Third, while previous studies demonstrated a correlation between treatment satisfaction and depression,49 individual mental status was not taken into account in our study so that emotional aspects of satisfaction were not specifically addressed. This includes a potential secondary gain of illness due to increased care and attention by medical staff and caregivers with the introduction of nusinersen therapy. Moreover, a response bias toward ‘better’ responses may have occurred, given the hope of having a novel DMT for the first time after decades of living with a neurodegenerative disease. Furthermore, the longitudinal part of this study needs to be interpreted with caution as the majority of patients were followed up only in between two nusinersen administrations. An extended study with longer follow-up periods would provide more insight into individual long-term treatment satisfaction with nusinersen.
Lastly, the TSQM-1.4© has been designed as a general questionnaire to assess patients’ satisfaction with medication. It has been validated across a wide range of diseases,13,19 but not in NMD. Therefore, it does not incorporate SMA or NMD-specific items and, on the other hand, comprises items not entirely suited for this group of patients, which leads to omitted answers. For example, question two (Q2) addresses the ability of the medication to relieve symptoms. As SMA patients rather hope for an increase of muscle function instead of symptom relief, a different wording would better appeal to them. Also, the questionnaire does not distinguish precisely between procedure and treatment-associated side effects, which may cause confusion and limit definitive conclusions.
To sum up, this is the first study demonstrating patients’ satisfaction with nusinersen treatment. Intrathecal injections are well tolerated but rated as rather inconvenient. Interestingly, ambulatory patients are more satisfied with treatment effectiveness. None of the 26 ambulatory patients withdrew from therapy. More severely affected patients seem to be at a higher risk of therapy dropout, corresponding to low TSQM-1.4© scores and a decrease in motor function. However, besides only mild or missing measurable motor-function changes, the majority of non-ambulatory patients, reported satisfaction with the treatment, assuming that their goal of disease stabilization was reached or non-motor symptoms were relieved.
In conclusion, the patients’ perspective supports a therapeutic benefit of nusinersen in SMA. Improvement as well as a stabilization of motor function is associated with satisfaction with treatment effectiveness. More convenient ways of drug delivery could further improve patients’ satisfaction. The evaluation of treatment satisfaction appears to be a promising PRO in adult SMA patients in different disease stages and may help in the decision-making process of discontinuing or switching treatment. Whether the same applies to children with SMA needs investigating in future studies.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_1756286421998902 for Treatment satisfaction in 5q-spinal muscular atrophy under nusinersen therapy by Alma Osmanovic, Gresa Ranxha, Mareike Kumpe, Claudia D. Wurster, Benjamin Stolte, Isabell Cordts, René Günther, Maren Freigang, Lars H. Müschen, Camilla Binz, Andreas Hermann, Marcus Deschauer, Paul Lingor, Albert C. Ludolph, Tim Hagenacker, Olivia Schreiber-Katz and Susanne Petri in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors gratefully thank all study participants, as well as the local raters who supported the assessment of motor scores.
We further thank James A. Mohajer for proofreading the manuscript.
Individual centers received funding from SMArtCare and Biogen, who both had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Author contributions: AO: designed and conceptualized the study; contributed patient clinical data; analyzed and interpreted the data; carried out statistics; drafted and revised the manuscript and figures; GR: major role in the acquisition of data; contributed patient clinical data; analyzed the data; contributed to manuscript draft and figures; MK, CDW, BS, IC, RG, MF, LHM, CB, AH, MD, PL, ACL, TH: contributed patient clinical data; revised the manuscript for intellectual content; OSK, SP: designed and conceptualized the study; contributed patient clinical data; analyzed and interpreted the data; drafted and revised the manuscript for intellectual content.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: no targeted funding. AO is supported by PRACTIS, Clinician Scientist Program of Hannover Medical School, funded by the German Research Foundation (DFG, ME 3696/3-1, 2019–22). OSK receives academic research support from the Hannover Medical School Young Faculty Program, 2018–20. AH is supported by the Hermann and Lilly Schilling-Stiftung für medizinische Forschung im Stifterverband.
Conflict of interest statement: AO received honoraria as a speaker/consultant from the German Neuromuscular Society ‘Deutsche Gesellschaft fuer Muskelkranke’ (DGM e.V.), and Biogen GmbH. GR and MK received travel cost compensations from Biogen GmbH. CDW received honoraria and travel expenses from Biogen as an advisory board member and for lectures and as a consultant from Roche. BS received travel reimbursement and speaker honoraria from Biogen GmbH. IC received honoraria as speaker/consultant from Biogen GmbH. RG received honoraria as speaker/consultant from Biogen GmbH and Roche, and received research support from Biogen GmbH. MF reports non-financial support from Biogen outside the submitted work. LHM and CB report no disclosures. AH received personal fees and non-financial support from Biogen GmbH and from Destin, during the conduct of the study; grants from Helmholtz Foundation, BMBF, Innovationsausschuss des G-BA, DGM e.V., Schilling-Stiftung, outside the submitted work. MD received honoraria for advisory activities, talks and travel fees from Biogen GmbH, Roche and DGM e.V. PL received support for symposium organization from Biogen GmbH during the conduct of the study; and speaker honoraria from Desitin, BIAL, and AbbVie, and fees for advisory activities from Novartis, outside of the submitted work. ACL reports no disclosures. TH received honoraria as speaker/consultant from Biogen GmbH, Roche, Novartis, CSL Behring, Pfizer, Akcea, Sanofi-Aventis, and research support from Biogen GmbH and Sanofi-Aventis. OSK received honoraria as a speaker/consultant and/or funding for travel expenses from DGM e.V., Novartis, Biogen GmbH, the Jain Foundation and Biermann Verlag GmbH, and research support from the DGM e.V. SP received honoraria as speaker/consultant from Biogen GmbH, Roche, Novartis, Teva, Cytokinetics Inc., Desitin; and grants from DGM e.V, Federal Ministry of Education and Research, German Israeli Foundation for Scientific Research and Development, EU Joint Program for Neurodegenerative Disease Research.
ORCID iDs: Alma Osmanovic  https://orcid.org/0000-0002-6012-8423
https://orcid.org/0000-0002-6012-8423
Isabell Cordts  https://orcid.org/0000-0002-2078-1997
https://orcid.org/0000-0002-2078-1997
Tim Hagenacker  https://orcid.org/0000-0002-3631-3450
https://orcid.org/0000-0002-3631-3450
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alma Osmanovic, Department of Neurology, Hannover Medical School, Carl-Neuberg-Strasse 1, Hannover 30625, Germany.
Gresa Ranxha, Department of Neurology, Hannover Medical School, Hannover, Germany.
Mareike Kumpe, Department of Neurology, Hannover Medical School, Hannover, Germany.
Claudia D. Wurster, Department of Neurology, Ulm University, Ulm, Germany
Benjamin Stolte, Department of Neurology, University Hospital Essen, Essen, Germany.
Isabell Cordts, Department of Neurology, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany.
René Günther, Department of Neurology, Technische Universität Dresden, Dresden, Sachsen, Germany; German Center for Neurodegenerative Diseases (DZNE) Dresden, Dresden, Germany.
Maren Freigang, Department of Neurology, Technische Universität Dresden, Dresden, Sachsen, Germany.
Lars H. Müschen, Department of Neurology, Hannover Medical School, Hannover, Germany
Camilla Binz, Department of Neurology, Hannover Medical School, Hannover, Germany.
Andreas Hermann, Translational Neurodegeneration Section ‘Albrecht-Kossel,’ Department of Neurology, University Medical Center Rostock, University of Rostock, Rostock, Germany; German Center for Neurodegenerative Diseases (DZNE) Rostock, Rostock, Germany.
Marcus Deschauer, Department of Neurology, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany.
Paul Lingor, Department of Neurology, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany.
Albert C. Ludolph, Department of Neurology, Ulm University, Ulm, Germany German Center for Neurodegenerative Diseases (DZNE) Ulm, Ulm, Germany.
Tim Hagenacker, Department of Neurology, University Hospital Essen, Essen, Germany.
Olivia Schreiber-Katz, Department of Neurology, Hannover Medical School, Hannover, Germany.
Susanne Petri, Department of Neurology, Hannover Medical School, Hannover, Germany.
References
- 1. Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008; 371: 2120–2133. [DOI] [PubMed] [Google Scholar]
- 2. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017; 377: 1723–1732. [DOI] [PubMed] [Google Scholar]
- 3. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med 2018; 378: 625–635. [DOI] [PubMed] [Google Scholar]
- 4. Darras BT. Spinal muscular atrophies. Pediatr Clin North Am 2015; 62: 743–766. [DOI] [PubMed] [Google Scholar]
- 5. Wirth B, Brichta L, Schrank B, et al. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum Genet 2006; 119: 422–428. [DOI] [PubMed] [Google Scholar]
- 6. Bennett CF, Baker BF, Pham N, et al. Pharmacology of antisense drugs. Annu Rev Pharmacol Toxicol 2017; 57: 81–105. [DOI] [PubMed] [Google Scholar]
- 7. Bortolani S, Stura G, Ventilii G, et al. Intrathecal administration of nusinersen in adult and adolescent patients with spinal muscular atrophy and scoliosis: transforaminal versus conventional approach. Neuromuscul Disord 2019; 29: 742–746. [DOI] [PubMed] [Google Scholar]
- 8. Wurster CD, Winter B, Wollinsky K, et al. Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. J Neurol 2019; 266: 183–194. [DOI] [PubMed] [Google Scholar]
- 9. Oldenburg D, Guberina N, Stolte B, et al. Radiation exposure of image-guided intrathecal administration of nusinersen to adult patients with spinal muscular atrophy. Neuroradiology 2019; 61: 565–574. [DOI] [PubMed] [Google Scholar]
- 10. Hagenacker T, Wurster CD, Gunther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol 2020; 19: 317–325. [DOI] [PubMed] [Google Scholar]
- 11. Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology 2012; 79: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monnery D, Webb E, Richardson L, et al. Targeted palliative care day therapy interventions using modified MYMOP2 tool can improve outcomes for patients with non-malignant diseases. Int J Palliat Nurs 2018; 24: 92–95. [DOI] [PubMed] [Google Scholar]
- 13. Glanz BI, Musallam A, Rintell DJ, et al. Treatment satisfaction in multiple sclerosis. Int J MS Care 2014; 16: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande PR, Rajan S, Sudeepthi BL, et al. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res 2011; 2: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golin CE, DiMatteo MR, Gelberg L. The role of patient participation in the doctor visit. Implications for adherence to diabetes care. Diabetes Care 1996; 19: 1153–1164. [DOI] [PubMed] [Google Scholar]
- 16. Turnbull JE, Luther KM. Patient satisfaction report paves way to improved care. QRC Advis 1996; 13: 1–7. [PubMed] [Google Scholar]
- 17. Wright JG. Evaluating the outcome of treatment. Shouldn’t we be asking patients if they are better? J Clin Epidemiol 2000; 53: 549–553. [DOI] [PubMed] [Google Scholar]
- 18. Revicki DA. Patient assessment of treatment satisfaction: methods and practical issues. Gut 2004; 53(Suppl. 4): iv40–iv44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 21. Montes J, McDermott MP, Mirek E, et al. Ambulatory function in spinal muscular atrophy: age-related patterns of progression. PLoS One 2018; 13: e0199657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazzone ES, Mayhew A, Montes J, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve 2017; 55: 869–874. [DOI] [PubMed] [Google Scholar]
- 23. Pera MC, Coratti G, Forcina N, et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol 2017; 17: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glanzman AM, O’Hagen JM, McDermott MP, et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol 2011; 26: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 2005; 8(Suppl. 1): S9–S24. [DOI] [PubMed] [Google Scholar]
- 26. Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3—a prospective observational study. J Neuromuscul Dis 2019; 6: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratni H, Ebeling M, Baird J, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA). J Med Chem 2018; 61: 6501–6517. [DOI] [PubMed] [Google Scholar]
- 29. Cheung AK, Hurley B, Kerrigan R, et al. Discovery of small molecule splicing modulators of survival motor neuron-2 (SMN2) for the treatment of spinal muscular atrophy (SMA). J Med Chem 2018; 61: 11021–11036. [DOI] [PubMed] [Google Scholar]
- 30. Hoy SM. Onasemnogene abeparvovec: first global approval. Drugs 2019; 79: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 31. Darras BT, Chiriboga CA, Iannaccone ST, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology 2019; 92: e2492–e2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osmanovic A, Ranxha G, Kumpe M, et al. Treatment expectations and patient-reported outcomes of nusinersen therapy in adult spinal muscular atrophy. J Neurol 2020; 267: 2398–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jochmann E, Steinbach R, Jochmann T, et al. Experiences from treating seven adult 5q spinal muscular atrophy patients with nusinersen. Ther Adv Neurol Disord 2020; 13: 1756286420907803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farrar MA, Park SB, Vucic S, et al. Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol 2017; 81: 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord 2019; 29: 842–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sansone VA, Albamonte E, Salmin F, et al. Intrathecal nusinersen treatment for SMA in a dedicated neuromuscular clinic: an example of multidisciplinary and integrated care. Neurol Sci 2019; 40: 327–332. [DOI] [PubMed] [Google Scholar]
- 37. Cordts I, Deschauer M, Lingor P, et al. Radiation dose reduction for CT-guided intrathecal nusinersen administration in adult patients with spinal muscular atrophy. Sci Rep 2020; 10: 3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cordts I, Lingor P, Friedrich B, et al. Intrathecal nusinersen administration in adult spinal muscular atrophy patients with complex spinal anatomy. Ther Adv Neurol Disord 2020; 13: 1756286419887616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pechmann A, Konig K, Bernert G, et al. SMArtCARE: a platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J Rare Dis 2019; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmed S, Berzon RA, Revicki DA, et al. The use of patient-reported outcomes (PRO) within comparative effectiveness research: implications for clinical practice and health care policy. Med Care 2012; 50: 1060–1070. [DOI] [PubMed] [Google Scholar]
- 41. Wiering B, De Boer D, Delnoij D. Patient involvement in the development of patient-reported outcome measures: a scoping review. Health Expect 2017; 20: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J Neurol Sci 1999; 169: 13–21. [DOI] [PubMed] [Google Scholar]
- 43. Meyer T, Funke A, Munch C, et al. Real world experience of patients with amyotrophic lateral sclerosis (ALS) in the treatment of spasticity using tetrahydrocannabinol:cannabidiol (THC:CBD). BMC Neurol 2019; 19: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vermersch P, Hobart J, Dive-Pouletty C, et al. Measuring treatment satisfaction in MS: is the Treatment Satisfaction Questionnaire for Medication fit for purpose? Mult Scler 2017; 23: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rio J, Porcel J, Tellez N, et al. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler 2005; 11: 306–309. [DOI] [PubMed] [Google Scholar]
- 46. Landfeldt E, Edstrom J, Sejersen T, et al. Quality of life of patients with spinal muscular atrophy: a systematic review. Eur J Paediatr Neurol 2019; 23: 347–356. [DOI] [PubMed] [Google Scholar]
- 47. Binz C, Schreiber-Katz O, Kumpe M, et al. An observational cohort study on impact, dimensions and outcome of perceived fatigue in adult 5q-spinal muscular atrophy patients receiving nusinersen treatment. J Neurol. Epub ahead of print 7 October 2020. DOI: 10.1007/s00415-020-10227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy—new phenotypes, new challenges, new implications for care. J Neuromuscul Dis 2020; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marshall GN, Hays RD, Mazel R. Health status and satisfaction with health care: results from the medical outcomes study. J Consult Clin Psychol 1996; 64: 380–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_1756286421998902 for Treatment satisfaction in 5q-spinal muscular atrophy under nusinersen therapy by Alma Osmanovic, Gresa Ranxha, Mareike Kumpe, Claudia D. Wurster, Benjamin Stolte, Isabell Cordts, René Günther, Maren Freigang, Lars H. Müschen, Camilla Binz, Andreas Hermann, Marcus Deschauer, Paul Lingor, Albert C. Ludolph, Tim Hagenacker, Olivia Schreiber-Katz and Susanne Petri in Therapeutic Advances in Neurological Disorders