Abstract
Aims:
Xpert® Bladder Cancer Monitor is a urinary marker based on the evaluation of five target mRNAs overexpressed in patients with bladder cancer (BC). The aim of our study is to update our results regarding the diagnostic accuracy of the Xpert® Bladder Cancer Monitor test in the follow-up of patients with non-muscle invasive bladder cancer (NMIBC).
Methods:
We conducted a prospective study on 1015 samples of 416 patients (mean age 72.2 ± 10.3 years) under follow-up for NMIBC. Patients underwent voided urinary cytology, the Xpert® Bladder Cancer Monitor test and cystoscopy and, if positive, a transurethral resection of the bladder. Xpert® Bladder Cancer Monitor was reported as negative or positive: cut-off total Linear Discriminant Analysis (LDA) = 0.5.
Results:
We identified 168 recurrent tumours: 126 (75%) were low-grade (LG) and 42 (25%) high-grade (HG). Overall sensitivity was 17.9% for cytology, 52.4% for Xpert® Bladder Cancer Monitor and 54.2% for the two tests combined. The sensitivity of cytology increased from 6.3% in LG to 52.4% in HG tumours whereas Xpert® Bladder Cancer Monitor showed a sensitivity ranging from 42.9% in LG to 80.9% in HG tumours. Combined cytology and Xpert® Bladder Cancer Monitor yielded an overall sensitivity of 45.2% for LG and 80.9% for HG tumours. Overall specificity was 98.5% for cytology and 78.4% for Xpert® Bladder Cancer Monitor and 78.2% for the two tests combined. The area under the curve (AUC) for Xpert® Bladder Cancer Monitor was 0.71; stratifying the patients according to the European Association of Urology risk groups, the AUC was 0.69, 0.67 and 0.85 for low, intermediate and high risk, respectively (p = 0.0003).
Conclusion:
Our data confirm a significantly higher sensitivity of Xpert® Bladder Cancer Monitor than for cytology in a larger patient cohort. The test performed very well in terms of specificity but could not reach the high value of cytology. Along with voided urinary cytology the test could allow to reduce cystoscopies in follow-up patients, reducing discomfort to the patients and costs.
Keywords: bladder cancer, cystoscopy, follow-up, urinary markers
Introduction
In 2018, worldwide there were 549,393 new cases of bladder cancer (BC), with 197,105 new cases in Europe.1 BC requires a life-long follow-up with cytology and cystoscopy, resulting in a significant psychological burden for the patients2 as well as an economic burden for society.3
At the moment, no urinary marker for the follow-up of non-muscle invasive BC (NMIBC) patients which aims at replacing cystoscopy can be found in the European Association of Urology (EAU) Guidelines, on the one hand due to the risk of missing tumours in high-risk patients, on the other hand due to the low sensitivity in low grade recurrences.4
Xpert® Bladder Cancer Monitor is a new mRNA-based BC marker test based on the evaluation of five target mRNAs [ABL Proto-oncogene 1 (ABL1), Uroplakin 1B (UPK1B), Corticotropin Releasing Hormone (CRH), Annexin 10 (ANXA10), Insulin-like Growth Factor 2 (IGF2)], which are overexpressed in patients with BC.5
In a previous cohort of 230 patients under follow-up for NMIBC at our institution, the test showed an overall sensitivity of 46.2%, a specificity of 77% and a fair diagnostic efficacy, with an area under the curve (AUC) of 0.65.6 In other studies, Xpert® Bladder Cancer Monitor showed excellent performances with higher sensitivity and specificity rates and with higher AUC values.7,8
The aim of our study was to further evaluate the diagnostic value of Xpert® Bladder Cancer Monitor in a larger cohort of patients followed for NMIBC, including those already analysed in the previous study.6
Material and methods
After approval of the local institutional ethics committee (Ethic Committee of General Hospital of Bolzano, study registration number: 47-2017) and after written informed consent was obtained from the patients, 416 patients under follow-up for NMIBC in our outpatient department were enrolled in the present study.
Patients were routinely evaluated by voided urinary cytology, by the Xpert® Bladder Cancer Monitor test and by cystoscopy. Any cystoscopically suspicious lesion was biopsied or removed trans-urethrally and specimens were evaluated according to the 2017 TNM classification of urinary BC and graded according to both the 1973 and the 2004 World Health Organization grade classification, as recommended by the European Association of Urology (EAU) Guidelines for NMIBC.4,9,10
Of the voided urine of every patient admitted to the outpatient clinic for a routine follow-up control, 4.5 ml were added to the Xpert Urine Transport Reagent Kit, a RNA stabilizing reagent, and inverted three times in order to mix it properly. The residual urine was added to 15 ml Cytolyt fixation liquid (Hologic, Inc., Marlborough, MA, USA) in a Falcon tube and sent to the laboratory along with the stabilized urine for the Xpert® Bladder Cancer Monitor test.
Cytology
The tubes were centrifuged for 10 min at 2000 rev/min. The resulting cell pellets were resuspended in ThinPrep vials that contained PreservCyt solution and processed by the TP 5000 System (Hologic, Marlborough, MA, USA). Cytological evaluation was performed using the Papanicolaou staining procedure and the Paris System for Reporting Urinary Cytology.11 The latter classifies the cytological specimens accordingly into negative for high grade urothelial carcinoma (NHGUC), atypical urothelial cells, suspicious for high grade urothelial carcinoma (SHGUC), high grade urothelial carcinoma (HGUC), low grade intraepithelial neoplasia (LGUN) and not diagnostic. For the statistical analysis NHGUC and atypical urothelial cells were grouped as negative, SHGUC, HGUC and LGUN as positive.
Xpert® Bladder Cancer Monitor
According to the manufacturer, the Xpert® Bladder Cancer Monitor, performed on the Cepheid GeneXpert Instrument System, is a qualitative in vitro diagnostic test created to monitor the recurrence of BC in patients previously diagnosed with BC.
The test measures the level of five target mRNAs, which are overexpressed in BC: ABL1, UPK1B, CRH, ANXA10, IGF2.
ABL1 is a protein-tyrosine kinase, which is found overexpressed in the progression of several solid tumours. An increased ABL1 expression was found in urine specimens of patients with BC, probably due to increased urothelial cellularity in these subjects. UPK1B is a structural protein of urothelial cells and can be high in BC patients. CRH is secreted from the hypothalamus and regulates biological and psychological response to stress, playing a role in regulation of several human cancers’ development; ANXA10 is a member of calcium-dependent phospholipid-binding protein family and plays a role in the regulation of cellular growth, IGF2 mRNA is often upregulated in BC and the level of IGF2 protein is high in urine samples of BC patients;12 CRH, ANXA10 and IGF2 were already identified as BC markers in a prospective study of gene expression pattern in urine samples of BC patients.13
Xpert® Bladder Cancer Monitor detects these five mRNA targets, by means of real-time, reverse transcription-polymerase chain reaction (RT-PCR). The results are interpreted by the GeneXpert Instrument System from measured fluorescent signals and embedded calculation algorithms; Test Result, LDA totals and Analyte Results are shown on the Test Report. A cut-off is set at a LDA of ⩾0.5.
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the Xpert® score have been evaluated and area under the receiver operating characteristic curve and its confidence interval were calculated. In addition to the Xpert® score the predictiveness of demographic characteristics (such as age and sex) were evaluated with respect to cystoscopy using classification and regression trees.14
Results
One thousand and fifteen samples were obtained from 416 patients (89 women and 327 men). Mean age of the patients included in this prospective study was 72.2 ± 10.3 years. An average of two urine samples (from one to eight) for each patient were analysed over a period of 3–48 months after diagnosis. Thirty-one samples (3%) had to be excluded due to a not diagnostic Xpert® Bladder Cancer Monitor test and/or cytology.
Of the 1015 samples, 168 (16.5%) were related to tumour recurrence; 126 (75%) were low grade (LG) and 42 (25%) high grade (HG) tumours. The cases were stratified according to the EAU risk tables in low, intermediate and high risk (25.4%, 37.4% and % 37.2% of the cases, respectively) (Table 1).
Table 1.
Clinicodemographic characteristics.
| % | ||
|---|---|---|
| Median age | 72.2 ± 10 | |
| Male | 327 | 78.6 |
| Female | 89 | 21.4 |
| Total of samples | 1015 | |
| Excluded due to invalid/error Xpert® Bladder Cancer Monitor | 31 | 3 |
| EAU recurrence risk classification | ||
| Low risk | 258 | 25.4 |
| Intermediate risk | 379 | 37.4 |
| High risk | 378 | 37.2 |
| Tumour recurrence: | 168 | 16.4 |
| Low grade | 126 | 75 |
| High grade | 42 | 25 |
EAU, European Association of Urology.
The sensitivity of cytology increased from 6.3% in LG to 52.4% in HG tumours whereas Xpert® Bladder Cancer Monitor showed a sensitivity ranging from 42.9% in LG to 80.9% in HG tumours (Table 2). Combined, cytology and Xpert® Bladder Cancer Monitor yielded an overall sensitivity of 45.2% for LG and 80.9% for HG tumours. In the subgroup of HG recurrence, using cytology alone we missed 20 positive cases (47.6%) and eight (19.1%) by using only Xpert® Bladder Cancer Monitor. Using both tests together we missed only seven cases of HG recurrence (16.5%).
Table 2.
Performance of cytology, Xpert® Bladder Cancer Monitor and combination of the two tests in low- and high-grade cases.
| Sensitivity | Cytology (%) | Xpert® (%) | Both (%) |
|---|---|---|---|
| Low grade | 6.35 | 42.86 | 45.24 |
| High grade | 52.38 | 80.95 | 80.95 |
Overall specificity was 98.5% for cytology and 78.4% for Xpert® Bladder Cancer Monitor and 78.2% for the two tests combined. PPV for cytology was 69.8% and for Xpert® Bladder Cancer Monitor 32.5%; NPV was 85.8% for cytology versus 89.2% for Xpert® Bladder Cancer Monitor (Table 3).
Table 3.
Performance of cytology, Xpert® Bladder Cancer Monitor and combination of the two tests.
| Cytology (%) | Xpert® (%) | Both (%) | |
|---|---|---|---|
| Sensitivity | 17.86 | 52.38 | 54.17 |
| Specificity | 98.47 | 78.39 | 78.16 |
| PPV | 69.77 | 32.47 | 32.97 |
| NPV | 85.80 | 89.25 | 89.58 |
NPV, negative predictive value; PPV positive predictive value.
After treatment with BCG (30/294; 10.2%), Xpert® Bladder Cancer Monitor performed well with a sensitivity of 66.6% (47.1–82.7) and specificity of 74.62% (68.9–79.6), respectively.
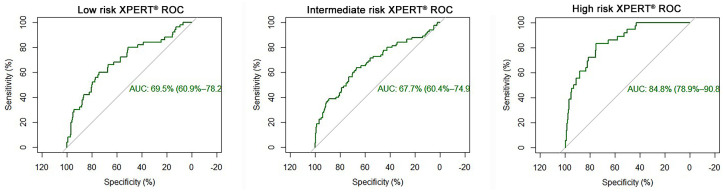
The diagnostic efficacy of Xpert® Bladder Cancer Monitor was good, with an AUC of 0.71 (95% confidence interval 0.66–0.76) (Figure 1); stratifying the patients according to the EAU risk categories,4 AUC was 0.69, 0.67 and 0.84 for low, intermediate and high risk, respectively (p = 0.0003) (Table 4 and Figure 2).
Figure 1.
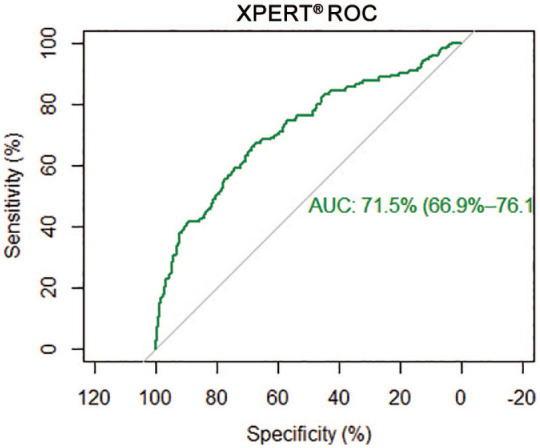
Area under the curve of Xpert® Bladder Cancer Monitor.
AUC, area under the curve; ROC, receiver operating characteristic.
Table 4.
Performance of Xpert® Bladder Cancer Monitor in low-, intermediate- and high-risk cases.
| Xpert® | LR (%) | IR (%) | HR (%) |
|---|---|---|---|
| Sensitivity | 39.22 | 49.38 | 77.78 |
| Specificity | 86.47 | 75.84 | 75.73 |
| PPV | 41.67 | 35.71 | 25.23 |
| NPV | 85.24 | 84.64 | 97.00 |
HR, high-risk; IR, intermediate risk; LR, low risk; NPV, negative predictive value; PPV positive predictive value.
Figure 2.
Area under the curve of Xpert® Bladder Cancer Monitor in low-risk, intermediate-risk and high-risk cases.
AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
The Xpert® Bladder Cancer Monitor is a test presented in October 2016. It evaluates the level of five target mRNAs in voided urine, by using a RT-PCR and thereby identifies patients with NMIBC recurrence.
Our study, which is based on 1015 analyses of patients under follow-up for NMIBC, showed an overall sensitivity of 17.9% for cytology, 52.4% for Xpert® Bladder Cancer Monitor and 54.2% for the two tests combined. The specificity of cytology was excellent with 98.5%, conforming with our previous study,6 whereas it was 78.4% for Xpert® Bladder Cancer and 78.2% for the two tests combined.
Compared with the data in our validation study on 230 patients, in which the test showed a sensitivity of 46.2% for Xpert® Bladder Cancer Monitor and 48.1% for the combination of cytology and Xpert® Bladder Cancer Monitor, it slightly increased to 52.4% and 54.2%, respectively, in this larger series. Our data confirmed the sensitivity of 42.9% in LG and 80.9% in HG tumours.
The sensitivity could, however, not reach the results published by Pichler et al.7 on 140 patients with an overall sensitivity of 84% for Xpert® Bladder Cancer Monitor test versus 52.4% in our series. The lower sensitivity in our series could be explained by the fact that Pichler et al.7 evaluated the performance of the test on bladder washings in contrast to our study on voided urinary cytology.
We could also not reach the overall sensitivity of 74% reported in the multicentric study conducted by Van Valenberg et al.,8 while the sensitivity of 80.9% was good in HG tumours in our series (versus 83%) and the specificity was almost the same (78.4% versus 80%).
PPV in the present study was significantly lower in comparison with those of Pichler and Van Valenberg (respectively 32.5%, 80% and 44%), while NPV was good for all studies (89.2%, 93% and 93%), confirming and ameliorating the data of our initial series. In fact, NPV was significantly higher than in our previous study (89.2% versus 83%). Although sensitivity and specificity are the most commonly used outcome measures, NPV and PPV, even if influenced by the prevalence of the disease,15 could be of help in ruling out a recurrence.
A possible explanation for the different outcomes between the single studies could be the recurrence distribution. In a study published by Trenti et al.,16 comparing two urinary markers, Xpert® Bladder Cancer Monitor versus Bladder Epicheck, the performance of Xpert® Bladder Cancer Monitor was significantly higher, with a sensitivity of 66.3% versus 52.4% in the present study; the HG recurrence prevalence in that study was significantly higher than in the present (43.5% versus 25%). The number of HG recurrences could so far influence the performance of the test.
In the present study the cases were stratified according to the EAU risk tables in low, intermediate and high risk (respectively, 25.4%, 37.4% and 37.2% of the cases).
The AUCs of Xpert® Bladder Cancer Monitor in these three groups are respectively 0.69, 0.67 and 0.84 (Figure 2), showing the significantly higher performance of the test in high-risk patients (p = 0.0003), suggesting a possible tailored use of this urinary marker in a specific subgroup of patients. Currently, no other analysis of the performance of this urinary marker has been conducted in a specific group of patients.
As reported by Seklehner et al.,2 cystoscopy represents an important source of stress for the patients. Avoiding unnecessary cystoscopies could ameliorate the quality of life of the patients and reduce the cost of follow-up of patients with NMIBC. Mossanen et al.17 estimated that the cumulative costs of care over a 5-year period of NMIBC follow-up are US$52,125 for low-risk, US$146,250 for intermediate-risk, and US$366,143 for high-risk NMIBC patients. According to the data of Svatek et al.,18 the cost of an office cystoscopy ranges from €163 to €520, so far reducing the number of cystoscopies could be an economical advantage.
Although urinary cytology remains the marker with the highest specificity, it seems obvious that the use of the Xpert® Bladder Cancer Monitor test improves sensitivity in contrast to cytology alone; even though the combined use of cytology and Xpert® Bladder Cancer Monitor does not seem much better than each of the two alone, by analysing the subgroups they show to be complementary and allow to increase the diagnostic accuracy. Therefore, cytology and Xpert® Bladder Cancer Monitor should be used together to increase the interval between cystoscopies in the follow-up, if both are negative.
However, further prospective studies with a larger number of patients and longer follow-up are needed to validate the prognostic value of Xpert® Bladder Cancer Monitor and its potential role in risk stratification of BC patients in terms of predictor of a recurrence or of response to BCG therapy.
Conclusions
Our data confirm a significantly higher sensitivity of Xpert® Bladder Cancer Monitor than of cytology in a larger patient cohort. The test performed very well in terms of specificity but could not reach the high value of cytology, while PPV and NPV performed approximately the same for both tests. Along with voided urinary cytology the test could allow to reduce cystoscopies in follow-up patients, thus reducing discomfort to the patients and costs.
Significant conclusions
Our data confirm a significantly higher sensitivity of Xpert® Bladder Cancer Monitor than of cytology in a larger patient cohort. The test performed very well in terms of specificity but could not reach the high value of cytology.
Supplemental Material
Supplemental material, sj-pdf-1-tau-10.1177_1756287221997183 for Diagnostic value of Xpert® Bladder Cancer Monitor in the follow-up of patients affected by non-muscle invasive bladder cancer: an update by Carolina D’Elia, Decio M. Folchini, Christine Mian, Esther Hanspeter, Christine Schwienbacher, Giorgio Alfredo Spedicato, Stefan Pycha, Egils Vjaters, Stephan Degener, Mona Kafka, Armin Pycha and Emanuela Trenti in Therapeutic Advances in Urology
Acknowledgments
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the position of the organization of which they are part.
Footnotes
Author contributions: Carolina D’Elia: conceptualization, writing, formal analysis, review and editing
Decio M. Folchini: review and editing
Christine Mian: conceptualization, data acquisition, data curation, review and editing
Esther Hanspeter: review and editing
Christine Schwienbacher: review and editing
Giorgio Alfredo Spedicato: formal analysis, review and editing
Stefan Pycha: review and editing
Egils Vjaters: review and editing
Stephan Degener: data acquisition, review and editing
Mona Kafka: review and editing
Armin Pycha: conceptualization, review and editing, project administration
Emanuela Trenti: conceptualization, review and editing, project administration
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Carolina D’Elia  https://orcid.org/0000-0002-8227-9978
https://orcid.org/0000-0002-8227-9978
Giorgio Alfredo Spedicato  https://orcid.org/0000-0002-0315-8888
https://orcid.org/0000-0002-0315-8888
Emanuela Trenti  https://orcid.org/0000-0003-3034-531X
https://orcid.org/0000-0003-3034-531X
Contributor Information
Carolina D’Elia, Department of Urology, Hospital of Bolzano, Bolzano, Italy.
Decio M. Folchini, Department of Urology, Hospital of Bolzano, Bolzano, Italy
Christine Mian, Department of Pathology, Hospital of Bolzano, Bolzano, Italy.
Esther Hanspeter, Department of Pathology, Hospital of Bolzano, Bolzano, Italy.
Christine Schwienbacher, Department of Pathology, Hospital of Bolzano, Bolzano, Italy.
Giorgio Alfredo Spedicato, Data Science Management, Unipol Group, Bologna, Italy.
Stefan Pycha, Department of Urology, Riga Stradins University Hospital, Riga, Latvia.
Egils Vjaters, Department of Urology, Riga Stradins University Hospital, Riga, Latvia.
Stephan Degener, Department of Urology, Helios-Klinikum Wuppertal, Witten Herdecke University, Wuppertal, Germany.
Mona Kafka, Department of Urology, Medical University of Innsbruck, Innsbruck, Austria.
Armin Pycha, Department of Urology, Hospital of Bolzano, Bolzano, Italy; Sigmund Freud Private University, Medical School, Vienna, Austria.
Emanuela Trenti, Department of Urology, Bolzano General Hospital, Lorenz Böhler St 5, Bolzano, 39100, Italy.
References
- 1. International Agency for Research on Cancer. Bladder, https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf 2018
- 2. Seklehner S, Engelhardt PF, Remzi M, et al. Anxiety and depression analyses of patients undergoing diagnostic cystoscopy. Qual Life Res 2016; 25: 2307–2314. [DOI] [PubMed] [Google Scholar]
- 3. Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol 2014; 24: 487–491. [DOI] [PubMed] [Google Scholar]
- 4. Babjuk M, Burger M, Compérat E, et al. Non-muscle-invasive urothelial carcinoma of the bladder: EAU guidelines. 2019. https://uroweb.org [DOI] [PubMed] [Google Scholar]
- 5. Wallace E, Higuchi R, Satya M, et al. Development of a 90-Minute Integrated Noninvasive Urinary Assay for Bladder Cancer Detection. J Urol 2018. Mar; 199: 655–662. doi 10.1016/jjuro.2017.09.141 [DOI] [PubMed]
- 6. D Elia C, Pycha A, Folchini DM, et al. Diagnostic predictive value of Xpert bladder cancer monitor in the follow-up of patients affected by non-muscle invasive bladder cancer. J Clin Pathol 2019; 72: 140–144. [DOI] [PubMed] [Google Scholar]
- 7. Pichler R, Fritz J, Tulchiner G, et al. Increased accuracy of a novel mRNA-based urine test for bladder cancer surveillance. BJU Int 2018; 121: 29–37. [DOI] [PubMed] [Google Scholar]
- 8. Van Valenberg FJP, Hiar AM, Wallace E, et al. Prospective validation of an mRNA-based urine test for surveillance of patients with bladder cancer. Eur Urol 2019; 75: 853–860. [DOI] [PubMed] [Google Scholar]
- 9. Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998; 22: 1435–1448. [DOI] [PubMed] [Google Scholar]
- 10. Brierley JD, Gospodarowicz M, Wittekind C. UICC international union against cancer. In: TNM classification of malignant tumors. 8th ed. Hoboken, NJ: Wiley-Blackwell, 2017. [Google Scholar]
- 11. Rosenthal DL, Wojcik EM, Kurtycz DFI. The Paris system for reporting urinary cytology. Switzerland: Springer International Publishing, 2016. [Google Scholar]
- 12. Wallace E, Higuchi R, Satya M, et al. Development of a 90-minute integrated non-invasive urinary assay for bladder cancer detection. J Urol 2018; 199: 655–662. [DOI] [PubMed] [Google Scholar]
- 13. Mengual L, Burset M, Ribal MJ, et al. Gene expression signature in urine for and assessing aggressiveness of bladder urothelial carcinoma. Clin Cancer Res 2010; 16: 2624–2633. [DOI] [PubMed] [Google Scholar]
- 14. Kuhn M, Weston S, Culp M, et al. C50: C5.0 decision trees and rule-based models. R Package Version 0.8.0. https://cran.r-project.org/web/packages/C50/index.html 2015. [Google Scholar]
- 15. Ranganathan P, Aggarwal R. Common pitfalls in statistical analysis: understanding the properties of diagnostic tests – part 1. Perspect Clin Res 2018; 9: 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trenti E, Pycha S, Mian C, et al. Comparison of 2 new real-time polymerase chain reaction-based urinary markers in the follow-up of patients with non-muscle-invasive bladder cancer. Cancer Cytopathol 2020; 128: 341–347. [DOI] [PubMed] [Google Scholar]
- 17. Mossanen M, Wang Y, Szymaniak J, et al. Evaluating the cost of surveillance for non-muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol 208; 37: 2059–2065. [DOI] [PubMed] [Google Scholar]
- 18. Svatek RS, Hollenbeck BK, Holmäng S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014; 66: 253–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tau-10.1177_1756287221997183 for Diagnostic value of Xpert® Bladder Cancer Monitor in the follow-up of patients affected by non-muscle invasive bladder cancer: an update by Carolina D’Elia, Decio M. Folchini, Christine Mian, Esther Hanspeter, Christine Schwienbacher, Giorgio Alfredo Spedicato, Stefan Pycha, Egils Vjaters, Stephan Degener, Mona Kafka, Armin Pycha and Emanuela Trenti in Therapeutic Advances in Urology