Abstract
Background:
High morbidity has been reported regarding Achilles tendon (AT) injuries, and the upward trend has accelerated since the mid-1990s. A chronic Achilles tendon rupture usually results from a neglected or misdiagnosed acute rupture, and about one-fifth of acute AT ruptures are missed and lead to chronic AT rupture. Although many techniques have been described, there is no gold standard in the treatment of chronic AT ruptures.
Hypothesis:
Endoscopically assisted, minimally invasive reconstruction for chronic AT rupture using a double-bundle flexor hallucis longus (FHL) tendon would result in improvement of the overall function, with a low rate of wound complications.
Study Design:
Case series; Level of evidence, 4.
Methods:
Between May 2015 and November 2016, a total of 19 consecutive patients were enrolled and treated using endoscopically assisted, minimally invasive reconstruction for chronic AT rupture using a double-bundle FHL. The operative assessment comprised the Achilles Tendon Total Rupture Score, the American Orthopaedic Foot & Ankle Society score, the Victorian Institute of Sports Assessment–Achilles score, and a postoperative questionnaire. All postoperative complications were recorded.
Results:
The mean follow-up time for all patients was 31 months (range, 20-42 months). According to the postoperative questionnaire, the result of surgery was excellent in 8 (42%) of 19 patients, good in 10 (53%), and fair in 1 (5%). All clinical outcome scores (mean ± SD) improved significantly after surgery: Achilles Tendon Total Rupture Score, 23.3 ± 10.3 vs 98.3 ± 9.2 (postoperatively vs preoperatively); American Orthopaedic Foot & Ankle Society, 52.1 ± 12.4 vs 97.5 ± 18.9; and Victorian Institute of Sports Assessment–Achilles, 23.4 ± 11.2 vs 95.7 ± 17.1 (P < .05). No complications with regard to wound healing or infection were noted. Twelve relatively young patients returned to preinjury activity levels, such as playing basketball or badminton, and the older patients were able to meet their daily needs, such as walking up stairs and jogging.
Conclusion:
Chronic AT ruptures were successfully treated via minimally invasive reconstruction using a double-bundle FHL, which provided excellent functional improvement. It is best suited for patients with complex requirements who are at high risk for wound complications.
Keywords: endoscopically assisted, minimally invasive reconstruction, chronic ATR, double-bundle FHL
The Achilles tendon (AT) is the strongest ankle plantar flexor and an important component of the normal gait cycle. High morbidity has been reported regarding AT injuries in Scandinavian countries (31-55 per 100,000 per year).9 Furthermore, the upward trend has accelerated since the mid-1990s.4 A chronic AT rupture usually results from a neglected or misdiagnosed acute rupture. Although no exact incidence has been reported for chronic AT ruptures, up to 25% of acute AT ruptures are missed and lead to chronic AT ruptures.16 Because of reduced plantarflexion strength, some daily activities, such as walking uphill or upstairs, can be limited.10 Patients frequently report persistent swelling and recurrent pain of the ankle.
Even if acute AT ruptures are untreated, a scar tissue bridge will form spontaneously between the 2 ends, which is the body’s natural attempt at repair.19 Nevertheless, the new tissue is not as strong as the original AT, and it elongates slowly,14,19 which increases the potential for rerupture. Additionally, the force arm of the gastrocsoleus complex is lengthened, leading to weakened heel-rise strength. Furthermore, as a result of the retracted proximal stump, the gastrocsoleus complex becomes atrophic, which exacerbates plantarflexion strength loss on the affected side. The untreated injury affects activities of daily living, and in professional athletes, this can end sports careers. Thus, most surgeons prefer operative management for chronic AT ruptures as compared with acute AT ruptures.11
Many procedures, such as V-Y myotendinous lengthening, turndown flaps, tendon transfers (peroneus brevis, flexor digitorum longus, gracilis, and flexor hallucis longus [FHL]), and allografts/synthetic grafts, have been described to treat chronic AT ruptures.1,17,20,22,28 Generally, after the scar tissue is resected, direct end-to-end repair is impossible. Therefore, the key surgical consideration is the length of the gap between the 2 ends, as described by Myerson.19 Tendon transfers are used widely in the treatment of chronic AT ruptures. FHL is the strongest muscle among the transfer candidates. Additionally, it has a durable and long tendon (10-12 cm) that is suitable to bridge a large gap. Most important, the axis of muscle contraction is comparable with that of the AT, and the transferred tendon does not disturb the mechanical balance of the ankle (both muscles are plantar flexors).15,19
Because of the tightened transferred tendon, tension-free skin closure is difficult after open surgery. Wound healing is challenging, especially for high-risk patients. In 1 study, tobacco use, steroid use, and female sex were identified as significant risk factors for wound infection.5 Moreover, age >50 years, repair time >4 weeks, and body mass index >30 were considered important disadvantages, despite not being associated with significant differences.5 The percutaneous technique was developed to solve the wound problem, but sural nerve lesions are not rare.29
Accordingly, some investigators have reported the results of using endoscopic FHL tendon transfers to treat chronic AT ruptures.2,12 However, they all performed a short harvest of the FHL and single-bundle transfer to the calcaneus. To obtain maximal strength and prevent wound complications, we perform endoscopically assisted, minimally invasive reconstruction for chronic AT ruptures using a double-bundle FHL tendon. The purpose of this study was to assess the clinical outcomes of minimally invasive reconstruction of chronic AT ruptures using a double-bundle FHL.
Methods
Patients
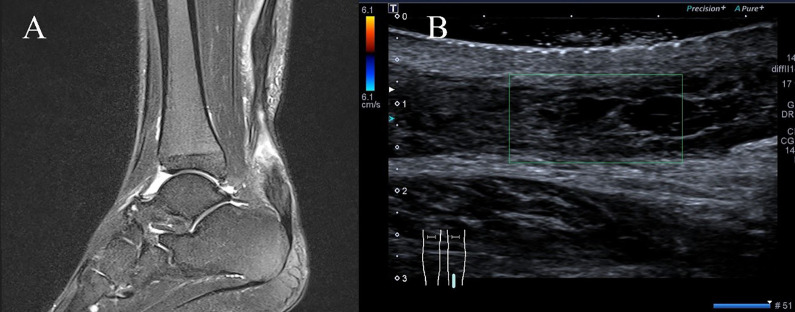
The present study was approved by our institutional ethics review board, and all participants provided informed consent. Between May 2015 and November 2016, a total of 19 consecutive patients were enrolled and treated using endoscopically assisted, minimally invasive reconstruction for chronic AT rupture using a double-bundle FHL. A chronic AT rupture was defined as a rupture occurring >6 weeks before the study.8 All patients described a sudden kick from behind and a subsequent sharp pain in the calf. They either ignored this after a rest or were misdiagnosed. Ultimately, all patients experienced chronic pain and recurrent swelling and had some trouble in daily life with activities, such as walking uphill or climbing upstairs. On inspection, a gap could be palpated at the rupture site in some patients. Although the single-leg heel-rise test result was abnormal in all patients, active plantarflexion of the ankle was possible because of the compensatory effects of the tibialis posterior, FHL, flexor digitorum longus, and peroneal muscles. The calf muscles were atrophied in all patients. All patients were evaluated by the same experienced foot and ankle surgeon (X.L.). A combination of the Thompson calf squeeze test25 and the Matles knee flexion test18 was used to confirm the diagnosis. Regarding imaging examinations, 15 patients preferred magnetic resonance imaging, and 4 preferred ultrasound (Figure 1).
Figure 1.
Preoperative imaging showing chronic Achilles tendon rupture: (A) magnetic resonance imaging and (B) ultrasound. The green box shows the Achilles tendon adhered to the surrounding soft tissues, the proximal stump contracted, the gap was about 60mm, the internal echo was irregular, the distal stump did not move with the proximal.
Surgical Technique
Surgery was performed with patients under spinal and peripheral nerve block and positioned prone and with a small support placed under their lower leg, making it possible to move the ankle freely. A tourniquet was applied on the thigh and inflated to 300 mm Hg. A single dose of prophylactic antibiotics was administered 30 minutes before the surgery.
Long FHL Harvest Technique
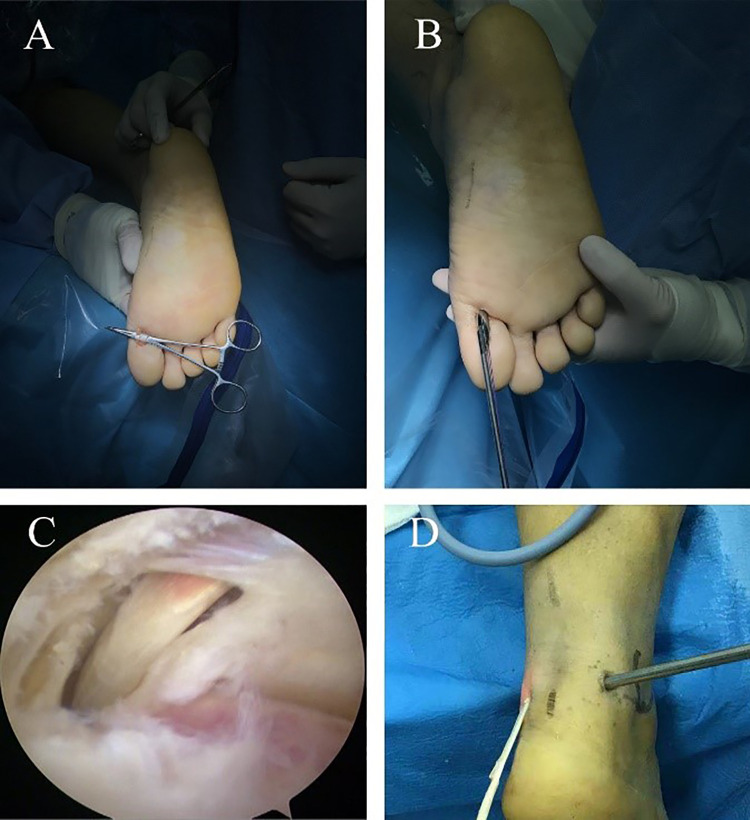
To perform the long FHL harvest technique, a small longitudinal incision was made on the plantar surface of the first metatarsophalangeal joint, and the FHL was identified carefully (Figure 2A). After a stable knitting stay stitch was placed, the tendon was cut at the distal side of the stitch. The tendon was tightened manually using the suture, and a tendon stripper was then inserted into the fibrous tendon sheath to release the interconnection between the FHL and flexor digitorum longus (Figure 2B). The surgeon pushed proximally, slowly and smoothly, to a barrier where the Henry knot was located and then rotated the stripper until a sudden breakthrough was felt. After that, the stripper was pushed gently as far as possible and then pulled out.
Figure 2.
(A) A small incision is made on the first metatarsophalangeal joint, and the flexor hallucis longus (FHL) tendon is identified carefully. (B) The FHL tendon is tightened manually using the suture, and a long tendon stripper is inserted into the fibrous tendon sheath to release it. (C) The FHL tendon is identified endoscopically. (D) The FHL tendon is pulled out through the posteromedial incision.
Endoscopic Technique
A standard posterior ankle endoscopy was performed using posterolateral and posteromedial portals, as described by van Dijk.26 A 4.0-mm 30° arthroscope was utilized, as routinely used for posterior ankle endoscopy. The endoscope was introduced through a posterolateral viewing portal, and a shaver was introduced through the posteromedial portal for debridement. The FHL tendon was identified laterally. The extra-articular fatty tissue and joint capsule were removed using a few turns of the shaver to reach the subtalar joint. Blunt-tip straight forceps were used to cut the flexor retinaculum at the level of the subtalar joint medially. After that, the FHL was identified and released (Figure 2C). With the ankle and hallux positioned in maximum plantarflexion, straight grasping forceps were used to pull the FHL out through the posteromedial incision (Figure 2D).
AT Reconstruction
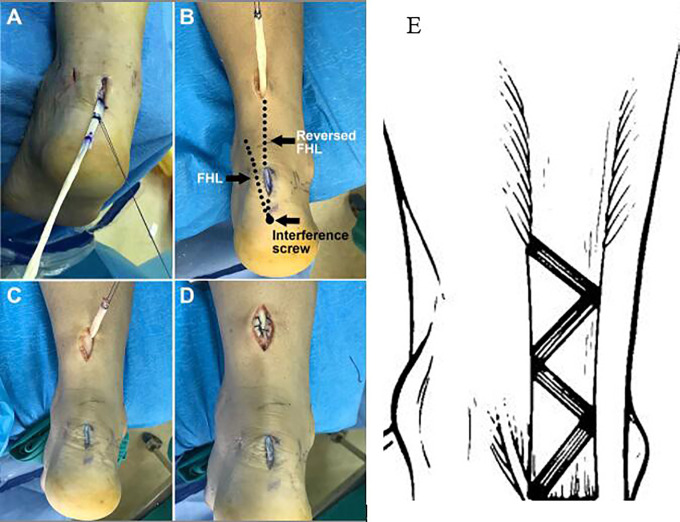
After FHL harvest, a posterior midline approach was made at the end point of the AT using a 1.5- to 2-cm incision to achieve visualization of the AT insertion, and the FHL was pulled into the incision using a PDS-2 suture loop (Depuy Ethicon) through a tunnel made using 4.0-mm Kirschner wire (Depuy) (Figure 3A). Subsequently, the calcaneal tunnel was created obliquely just deep to the AT insertion for stabilization through a guide pin using a cannulated drill. The diameter of the tunnel depended on the tendon width. It should be approximately 2 mm larger than double the tendon width. A needle with a tail hole was used to draw the overlapped tendon into the bone tunnel using a PDS-2 suture loop. With the ankle positioned and maintained in plantarflexion (about 10°), the tendon was fastened to appropriate tension using the suture loop. The FHL was fixed carefully using a bioabsorbable interference screw, and the residual tendon segment was reversed proximally.
Figure 3.
(A) The flexor hallucis longus (FHL) tendon is pulled into the midline incision using a PDS-2 suture loop (Depuy Ethicon). (B) The reversed FHL tendon is passed through the tunnel to the distal end of the proximal incision. The left dotted line indicates the original FHL tendon, and the right dotted line indicates the reversed FHL tendon. (C, D) The FHL tendon is woven through the proximal stump for augmentation. (E) The FHL tendon is woven into the proximal stump in a Z-shaped tunnel.
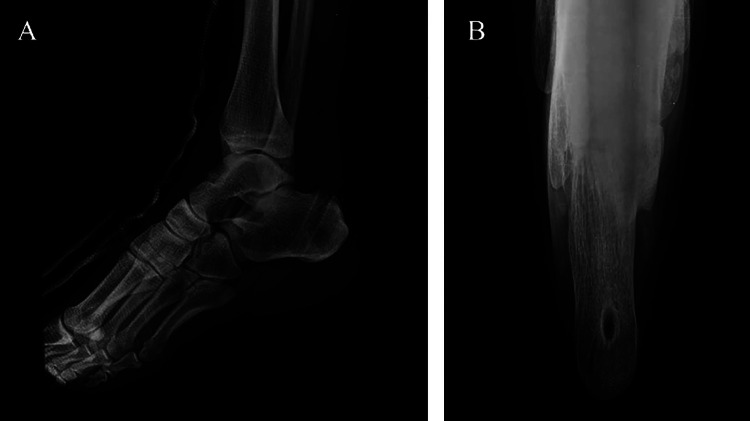
To prepare for the following weave, a 3-cm longitudinal incision was made on the proximal stump. A tunnel was created using 4.0-mm Kirschner wire, and a straight clamp was placed from the AT insertion incision to the proximal incision through the distal AT stump, scar tissue, and proximal stump in sequence. Guided by a tail-holed needle, the reversed FHL was passed through the tunnel to the distal end of the proximal incision (Figure 3B). A Z-shaped tunnel was created in the proximal stump for the FHL, as described previously.28 The FHL was woven through the proximal stump for augmentation (Figure 3, C-E). The weave process was repeated to utilize the full length of the incision. After the portals and wounds were closed, a below-knee synthetic cast was applied with the ankle in plantarflexion. The calcaneal bone tunnel was displayed on a postoperative radiograph (Figure 4).
Figure 4.
The calcaneal bone tunnel is displayed on postoperative radiographs: (A) lateral view and (B) axial view.
Postoperative Care and Rehabilitation
Active toe exercises were encouraged postoperatively as tolerated. All patients were nonweightbearing with a cast for 2 weeks, but they were allowed to walk with the use of elbow crutches with the affected leg elevated. A walking boot with wedges was applied after cast removal for 2 weeks postoperatively. Full weightbearing walking was permitted with the boot, and 1 wedge was removed every 2 weeks. All patients regained a normal plantigrade foot after boot removal at 8 weeks postoperatively.
Follow-up and Outcomes
The follow-up times were 2 and 8 weeks after the surgery, when the cast and boot, respectively, were removed. Patients were informed that they should return for a follow-up visit every 3 months in the first postoperative year. After the first postoperative year, the patients were advised to restore the patients’ previous sports and life without any restrictions. The operative clinical functional assessment contained the Achilles Tendon Total Rupture Score (ATRS),21 the American Orthopaedic Foot & Ankle Society (AOFAS) score,13 and Victorian Institute of Sports Assessment–Achilles (VISA-A) score.23 These functional scores were assessed pre- and postoperatively. To assess the outcome of the surgery, patients were asked to complete a questionnaire first described by Boyden et al,3 which graded pain, level of activity, footwear restrictions, and satisfaction on the basis of 4 domain levels each (eg, none, mild/occasional, moderate, and severe for pain).
Statistical Analysis
The pre- and postoperative clinical functional outcomes were compared using the Wilcoxon signed rank test. SPSS Version 23.0 (IBM Corp) was used for all data analyses. The alpha level was set at P < .05.
Results
A total of 19 patients with chronic AT rupture were recruited for the study, and all patients attended their follow-up visits. The mean follow-up time was 31 months (range, 20-42 months). Patient characteristics are reported in Table 1. The results of the postoperative questionnaire by Boyden et al3 are reported in Table 2. The result of surgery was excellent in 8 (42%) of 19 patients, good in 10 (53%), and fair in 1 (5%), in accordance with the grading standard.3 No poor results were recorded. As shown in Table 3, all clinical outcome scores (ATRS, AOFAS, and VISA-A) improved significantly after surgery.
Table 1.
Characteristics of the Study Patientsa
| Variable | Value |
|---|---|
| Age, y | |
| Mean ± SD | 46.7 ± 10.8 |
| Range | 30-57 |
| Sex, No. | |
| Male | 17 |
| Female | 2 |
| Side, No. | |
| Left | 9 |
| Right | 10 |
| Injury time, wk | |
| Mean ± SD | 14.7 ± 3.9 |
| Range | 6-24 |
| BMI, mean ± SD | 26.5 ± 8.4 |
| Imaging examinations, No. | |
| MRI | 15 |
| Ultrasound | 4 |
| Gap length, cm | |
| Mean ± SD | 5.5 ± 1.1 |
| Range | 5-8 |
aBMI, body mass index; MRI, magnetic resonance imaging.
Table 2.
Postoperative Questionnaire for Evaluating the Surgical Outcomea
| Item | No. of Patients |
|---|---|
| Pain | |
| None | 16 |
| Mild/occasional | 2 |
| Moderate | 1 |
| Severe | 0 |
| Activity limitation | |
| None | 16 |
| Limited recreational but not daily activities | 3 |
| Limited recreational and daily activities | 0 |
| Footwear restriction | |
| None, mild (most shoes tolerated) | 18 |
| Moderate (unable to tolerate fashionable shoes, with or without insert) | 1 |
| Severe (only modified shoes tolerated or brace) | 0 |
| Satisfaction | |
| Satisfied | 18 |
| Satisfied, with minor reservations | 1 |
| Satisfied, with major reservations | 0 |
| Dissatisfied | 0 |
aAs described by Boyden et al.3
Table 3.
Clinical Scores of Participants Preoperatively and at Final Postoperative Follow-upa
| ATRS | AOFAS | VISA-A | |
|---|---|---|---|
| Preoperative (n = 19) | 23.3 ± 10.3 | 52.1 ± 12.4 | 23.4 ± 11.2 |
| Postoperative (n = 19) | 98.3 ± 9.2 | 97.5 ± 18.9 | 95.7 ± 17.1 |
| P value | <.01 | <.01 | <.01 |
aAOFAS, American Orthopaedic Foot & Ankle Society; ATRS, Achilles Tendon Total Rupture Score; VISA-A, Victorian Institute of Sports Assessment–Achilles. Data are given as mean ± SD.
No complications with regard to wound healing or infection were noted. However, 2 patients reported mild muscle aches when the weather changed. Mostly better function and increased plantarflexion power were reported by patients 8 months postoperatively. All patients could perform a single-leg heel raise on the affected limb at the final follow-up. Hallux weakness in daily life was not reported during the follow-up. Overall, 12 relatively young patients returned to preinjury activity levels: 5 to basketball, 3 to badminton, 3 to running, and 1 to swimming. Note that Chinese people play sports erratically and seldom have the habit of keeping exercise, so the reported sport level could be inaccurate. The older patients were able to meet their daily needs, such as walking up stairs and jogging.
Discussion
In this study, we assessed the clinical outcomes of minimally invasive reconstruction for chronic AT ruptures using a double-bundle FHL at a mean follow-up of 31 months. Nineteen consecutive patients were enrolled, and clinical outcome measures including the ATRS, AOFAS, VISA-A, and a postoperative questionnaire by Boyden et al were evaluated.3
Our results showed a significant increase in ATRS, AOFAS, and VISA-A scores after surgery. This means that the AT function of the patients improved postoperatively. In addition, the result of surgery was excellent or good in 18 (95%) of 19 patients, and only 1 (5%) result was fair. No wound-healing problems, infections, or sural nerve lesions were reported in our series. Thus, the efficacy and safety of the surgery were validated.
To our knowledge, there is no gold standard in the treatment of chronic AT ruptures. Many techniques, such as V-Y myotendinous lengthening, turndown flaps, tendon transfers (peroneus brevis, flexor digitorum longus, gracilis, and FHL), and allografts/synthetic grafts, have been described. Generally, tendon transfers are used to handle large gaps (>5 cm). Peroneus brevis and FHL are the 2 most commonly transferred tendons, with similar mechanical properties.24 Although no significant functional deficits have been reported with either tendon, surgeons tend to prefer using the FHL. Because the loss of the peroneus brevis, which provides 28% of the eversion capacity of the hindfoot, would affect the muscle strength balance of the foot and ankle, using the FHL is considered more advantageous.6
With traditional FHL tendon transfer reconstructions, the 2-incision and single-incision techniques are open surgical methods. During the operation, the soft tissue is dissected extensively, and the scarred AT portion is excised. The peritendon is also stripped, resulting in an impaired blood supply. The reported wound complication rates of the open surgery are 4% to 36%.2,7 Thus, some investigators have used the hindfoot endoscopic FHL transfer technique to minimize wound risk.2,12 The endoscopic technique is attractive and has a low rate of soft tissue complication, especially for those with diabetes, older patients, and some patients with poor skin conditions.
Several investigators have reported no wound complications after endoscopic FHL transfer2,27; however, postoperative hyperesthesia and reduced sensation of the heel have still been reported, although the symptoms disappeared by 12 months after surgery.12 Despite the 2 additional minimal incisions, there were no wound problems in our patients.
Four clinical outcomes measures were used to evaluate the effects of surgery: the ATRS, AOFAS, VISA-A, and postoperative questionnaire by Boyden et al.3 The clinical scores were all significantly improved after surgery, in accordance with other studies.2,12,27 As we know, the FHL tendon is not as strong as the AT. Furthermore, the muscle strength of the tendon decreases after transposition. Theoretically, a single-bundle transferred FHL is not sufficient to provide plantarflexion. However, good function was described after a single-bundle FHL transfer. The reason may be the compensatory hyperplasia after tendon remodeling. Even so, the recovery time may be lengthened. Satisfactory function was predictable at 12 months after endoscopic transfer of a single-bundle FHL.12 However, the planterflexion power was still reduced when compared with that of the nonaffected side.12 So far, there have been no reports on minimally invasive AT reconstruction using a double-bundle FHL for chronic AT rupture. Anatomic double-bundle anterior crucial ligament reconstruction has been reported to have better anterior and rotational stability and a higher objective International Knee Documentation Committee score than has anatomic single-bundle reconstruction.30 Similarly, we argue that double-bundle could be superior to single-bundle since it has been seen to provide more powerful strength and stability. Although no statistical analysis was performed, our results show that the function and plantarflexion of the double bundle was encouraging 8 months postoperatively. In our opinion, the participation of the triceps muscles would shorten the recovery time. In spite of the theoretical advantage, further studies are needed to validate this.
The endoscopy is technically demanding, meaning a longer learning curve. Surgeons must pay attention to perioperative details. First, the FHL should be identified carefully via the endoscope, and the tibial neurovascular bundle should be avoided. Second, the screw insertion on the end of the AT should be located, and the tunnel should be drilled obliquely. Third, and most importantly, the tension of the transferred FHL should be modulated. In our opinion, the FHL should be pulled out so that the FHL belly is close to the bone tunnel and the reversed portion is fastened as tight as possible.
This study did have some limitations. First, there was no control group that received single-bundle reconstruction. Thus, there were no comparison results to determine the optimal treatment method. Second, the sample size was small. Because of the infrequent morbidity, a multicenter study is needed. Third, some surgical details, such as the tension of the transferred FHL and the optimal angulation of the bone tunnel, were not quantified.
Conclusion
Chronic AT ruptures were successfully treated using minimally invasive reconstruction using a double-bundle FHL, which provided excellent functional improvement. This procedure had the advantages of strong connection, fast recovery, and soft tissue benefits. All patients returned to preoperative activities within about 31 months postoperatively. It is best suited for patients with complex requirements who are at high risk for wound complications, such as patients with diabetes, those with rheumatoid arthritis controlled by steroid, and older people with thin skin.
Footnotes
Final revision submitted June 15, 2020; accepted July 6, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Medical Ethics Committee of Foshan Hospital of Traditional Chinese Medicine.
References
- 1. Abraham E, Pankovich AM. Neglected rupture of the Achilles tendon: treatment by V-Y tendinous flap. J Bone Joint Surg Am. 1975;57(2):253–255. [PubMed] [Google Scholar]
- 2. Baumfeld D, Baumfeld T, Figueiredo AR, et al. Endoscopic flexor halluces longus transfer for chronic Achilles tendon rupture—technique description and early post-operative results. Muscles Ligaments Tendons J. 2017;7(2):341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyden EM, Kitaoka HB, Cahalan TD, An KN. Late versus early repair of Achilles tendon rupture: clinical and biomechanical evaluation. Clin Orthop Relat Res. 1995;317:150–158. [PubMed] [Google Scholar]
- 4. Brorsson A, Grävare Silbernagel K, Olsson N, Nilsson Helander K. Calf muscle performance deficits remain 7 years after an Achilles tendon rupture. Am J Sports Med. 2018;46(2):470–477. [DOI] [PubMed] [Google Scholar]
- 5. Bruggeman NB, Turner NS, Dahm DL, et al. Wound complications after open Achilles tendon repair: an analysis of risk factors. Clin Orthop Relat Res. 2004;427:63–66. [DOI] [PubMed] [Google Scholar]
- 6. Clarke HD, Kitaoka HB, Ehman RL. Peroneal tendon injuries. Foot Ankle Int. 1998;19(5):280–288. [DOI] [PubMed] [Google Scholar]
- 7. Dalton GP, Wapner KL, Hecht PJ. Complications of Achilles and posterior tibial tendon surgeries. Clin Orthop Relat Res. 2001;391:133–139. [DOI] [PubMed] [Google Scholar]
- 8. Gabel S, Manoli A, II. Neglected rupture of the Achilles tendon. Foot Ankle Int. 1994;15(9):512–517. [DOI] [PubMed] [Google Scholar]
- 9. Ganestam A, Kallemose T, Troelsen A, Barfod KW. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013: a nationwide registry study of 33,160 patients. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3730–3737. [DOI] [PubMed] [Google Scholar]
- 10. Gossage W, Kohls-Gatzoulis J, Solan M. Endoscopic assisted repair of chronic Achilles tendon rupture with flexor hallucis longus augmentation. Foot Ankle Int. 2010;31(4):343–347. [DOI] [PubMed] [Google Scholar]
- 11. Hadi M, Young J, Cooper L, Costa M, Maffulli N. Surgical management of chronic ruptures of the Achilles tendon remains unclear: a systematic review of the management options. Br Med Bull. 2013;108:95–114. [DOI] [PubMed] [Google Scholar]
- 12. Husebye EE, Molund M, Hvaal KH, Stødle AH. Endoscopic transfer of flexor hallucis longus tendon for chronic Achilles tendon rupture: technical aspects and short-time experiences. Foot Ankle Spec. 2018;11(5):461–466. [DOI] [PubMed] [Google Scholar]
- 13. Ibrahim T, Beiri A, Azzabi M, Best AJ, Taylor GJ, Menon DK. Reliability and validity of the subjective component of the American Orthopaedic Foot and Ankle Society clinical rating scales. J Foot Ankle Surg. 2007;46(2):65–74. [DOI] [PubMed] [Google Scholar]
- 14. Kissel CG, Blacklidge DK, Crowley DL. Repair of neglected Achilles tendon ruptures—procedure and functional results. J Foot Ankle Surg. 1994;33(1):46–52. [PubMed] [Google Scholar]
- 15. Leslie HDH, Edwards WHB. Neglected ruptures of the Achilles tendon. Foot Ankle Clin N Am. 2005;10(2):357–370. [DOI] [PubMed] [Google Scholar]
- 16. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2011;1(4):133–136. [PMC free article] [PubMed] [Google Scholar]
- 17. Maffulli N, Leadbetter WB. Free gracilis tendon graft in neglected tears of the Achilles tendon. Clin J Sport Med. 2005;15(2):56–61. [DOI] [PubMed] [Google Scholar]
- 18. Matles AL. Rupture of the tendo Achilles: another diagnostic sign. Bull Hosp Jt Dis. 1975;36(1):48–51. [PubMed] [Google Scholar]
- 19. Myerson MS. Achilles tendon ruptures. Instr Course Lect. 1999;48:219–230. [PubMed] [Google Scholar]
- 20. Nellas ZJ, Loder BG, Wertheimer SJ. Reconstruction of an Achilles tendon defect utilizing an Achilles tendon allograft. J Foot Ankle Surg. 1996;35(2):144–148. [DOI] [PubMed] [Google Scholar]
- 21. Nilsson-Helander K, Thomeé R, Silbernagel KG, et al. The Achilles Tendon Total Rupture Score (ATRS): development and validation. Am J Sports Med. 2007;35(3):421–426. [DOI] [PubMed] [Google Scholar]
- 22. Parsons JR, Weiss AB, Schenk RS, Alexander H, Pavlisko F. Long-term follow-up of Achilles tendon repair with an absorbable polymer carbon fiber composite. Foot Ankle. 1989;9(4):179–184. [DOI] [PubMed] [Google Scholar]
- 23. Robinson JM, Cook JL, Purdam C; et al. Victorian Institute of Sport Tendon Study Group. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sebastian H, Datta B, Maffulli N, Neil M, Walsh WR. Mechanical properties of reconstructed Achilles tendon with transfer of peroneus brevis or flexor hallucis longus tendon. J Foot Ankle Surg. 2007;46(6):424–428. [DOI] [PubMed] [Google Scholar]
- 25. Thompson TC, Doherty JH. Spontaneous rupture of tendon of Achilles: a new clinical diagnostic test. J Trauma. 1962;2:126–129. [DOI] [PubMed] [Google Scholar]
- 26. van Dijk CN. Hindfoot endoscopy. Foot Ankle Clin. 2006;11(2):391–414. [DOI] [PubMed] [Google Scholar]
- 27. Vega J, Vilá J, Batista J, Malagelada F, Dalmau-Pastor M. Endoscopic flexor hallucis longus transfer for chronic noninsertional Achilles tendon rupture. Foot Ankle Int. 2018;39(12):1464–1472. [DOI] [PubMed] [Google Scholar]
- 28. Wapner KL, Pavlock GS, Hecht PJ, Naselli F, Walther R. Repair of chronic Achilles tendon rupture with flexor hallucis longus tendon transfer. Foot Ankle. 1993;14(8):443–449. [DOI] [PubMed] [Google Scholar]
- 29. Yang B, Liu Y, Kan S, et al. Outcomes and complications of percutaneous versus open repair of acute Achilles tendon rupture: a meta-analysis. Int J Surg. 2017;40:178–186. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Xu C, Dong S, Shen P, Su W, Zhao J. Systemic review of anatomic single- versus double-bundle anterior cruciate ligament reconstruction: does femoral tunnel drilling technique matter? Arthroscopy. 2016;32(9):1887–1904. [DOI] [PubMed] [Google Scholar]