Abstract
Acquired aplastic anemia (AA) is characterized by a reduced stem cell reserve. Several preclinical studies have confirmed the beneficial effect of thrombopoietin (TPO) on the expansion and maintenance of hematopoietic stem cells (HSCs). Thus, TPO receptor agonists seem to be an ideal therapeutic agent for AA to augment marrow function. First studies with eltrombopag as a single agent at 150 mg/day showed an overall response rate of 40–50% in patients with refractory severe AA (rSAA). Subsequent studies examined the first-line use of eltrombopag together with horse antithymocyte globulin and cyclosporine, reaching response rates up to 94%. Although used at high doses, known adverse events in the form of skin, gastrointestinal, or hepatic impairment are feasible in AA, however first data show a relatively high rate of clonal evolution in the form of karyotypic aberrations in patients with rAA. Nonetheless, there is a strong rationale that eltrombopag can contribute to restoring hematopoiesis in SAA by stimulating HSCs. Further studies are needed to decide if eltrombopag is clearly superior to current established treatments and to determine optimal treatment duration, dosage, and long-term effects.
Keywords: aplastic anemia, eltrombopag
Introduction
The term ‘aplastic anemia’ (AA) comprises a heterogeneous spectrum of rare hematological disorders caused by an impaired bone marrow function. It can be either acquired (acquired AA) or inherited (inherited bone marrow failures) and is clinically characterized by cytopenias of one or more blood cell lines (anemia, neutropenia, and/or thrombocytopenia). The most frequent mechanism leading to acquired AA, which is the focus of this review, is an immune-mediated destruction of hematopoietic stem cells (HSCs) and progenitor cells.1 Together with a hypocellular bone marrow the acquired form is further classified by the extent of cytopenias into moderate (MAA), severe (SAA), and very severe AA (vSAA). Very different pathomechanisms apply in inherited forms, mainly due to mutations in genes resulting in faulty DNA repair (Fanconi anemia), telomere elongation (dyskeratosis congenita), or ribosomal assmbly (Shwachman–Diamond syndrome). Possible effects of thrombopoietin (TPO) analogues in these conditions need to be examined separately. Similarly, there is an indistinct border between AA and hypoplastic myelodysplastic syndrome (MDS), a clearly clonal and malignant disorder, where immune-mediated mechanisms also play a role. Tranfusion requirements for red cells and platelets, life-threatening bleeding, and infection are the clinical consequences and need for therapy. For many decades, the therapy of SAA primarily focused on the immune pathogenesis of the disease, treating patients with either intensive immunosuppression therapy (IST) and/or hematopoietic stem cell transplantation (HSCT). Many efforts were made by intensifying the classic IST consisting of antithymocyte globulin (ATG) and cyclosporine (CSA) with other lymphotoxic agents (e.g. cyclophosphamide, alemutuzumab, mycophenolate) without a significant increase in response rate.2 Consequently it was suggested that an insufficient marrow unable to recover from a severe stem cell loss was the reason for treatment failure. Unfortunately, attempts to restore the stem cell compartment with growth factors, such as erythropoietin, granulocyte colony-stimulating factor (G-CSF), stem cell factor and interleukins were unsuccessful.3 Ultimately, TPO receptor agonists were established in the setting of immune thrombocytopenia (ITP),4 and are increasingly studied in AA. This article gives an overview of the current evidence and the emerging role of the TPO receptor agonist eltrombopag in SAA.
Current standard treatment of SAA
The current standard treatment of AA results from patient-specific factors (age, general condition, comorbidities, and donor availability) and disease-specific factors (severity and cause of the disease). Primarily, it is important to differentiate inherited from acquired forms, since inherited AA is treated differently and is not focus of this review. The decision for treatment of acquired AA is mainly driven by the severity of the disease and expert groups have released treatment recommendations based on various studies, which have changed little in the past decades.1,5 Since the subgroup of patients with SAA is characterized by severe neutropenia (absolute neutrophil count <500/µl), thrombocytopenia (platelet count <20 × 109/L), and/or anemia (absolute reticulocyte count <60 × 109/L), these patients are at high risk for life-threatening complications and should be treated as soon as the diagnosis is established. It is prognostically highly relevant that the interval from presentation to initiation of therapy is kept as short as possible.6 HSCT is one of the most important therapy modalities for acquired AA since it can cure the disease, but is generally only recommended as a first-line treatment for younger patients with an HLA -identical family donor, or, in some centers, also with unrelated donors in children. It is also performed as second-line therapy after failure of at least one cycle of IST, where unrelated or other alternative donors are used more frequently. If the treatment is carried out promptly with an HLA-identical family donor, long-term survival is excellent (≈ 90% young children, ≈ 80% adolescents), but decreases with increasing age (only approximately 50% for patients >40 years of age), and still bears some major toxicties (e.g. graft-versus-host disease) and late effects (e.g. quality of life impairment, secondary malignancies).
IST is the second main treatment for SAA, which targets the immune-mediated pathogenesis. It is recommended for first-line therapy of SAA in older adults and all those without an HLA-identical family donor, and also serves as second-line therapy for relapse after a first cycle of IST. Horse ATG and CSA are considered the standard IST. The latter is given orally for at least 6–12 months and is tapered carefully. Response to this treatment is slow, and it takes at least 4–12 weeks, sometimes even longer, until blood counts improve. Consequently, patients initially are still heavily transfusion dependent and at risk for severe bleeding and opportunistic infections, making this treatment difficult to tackle in the beginning. Overall, hematological response can be seen in approximately two-thirds of patients, but responding patients may relapse to about 30–40%.2 Retreatment of relapse with IST, usually only re-institution of CSA but occasionally repeating lymphocyte depletion with ATG or alemtuzumab, can be successful.7 Patients might be on CSA for years as they show a clear dependence of blood count on the drug.8 Finally, a small subgroup of patients do not respond to the initial treatment of IST and cytopenias persist (rAA), which can result in long-term transfusion dependence and repeating complications.
TPO and hematopoiesis
TPO is a glycoprotein class one cytokine that binds to the receptor c-mpl and is synthesized in the liver.9 At first, TPO was associated with megakaryocyte stimulation and platelet production.10 However, in vitro studies also suggested that TPO plays a key role in the proliferation and maintenance of HSCs. All HSCs express the c-mpl receptor11 and with the support of TPO stem cells proliferate when other cytokines are added.12 In TPO knockout mice models HSCs were also significantly reduced aside from megakaryocytopenia and thrombocytopenia.13 In humans, mutations in the TPO receptor (c-mpl) resulted in multilineage cytopenias.14 Autosomal-recessive AA was also linked to a mutation in the gene coding TPO.15
Rationale for the use of eltrombopag in AA
AA is characterized by a reduced HSC reserve mainly due to immune-mediated destruction of the bone marrow. Several preclinical studies have confirmed the beneficial effect of TPO and the TPO-receptor on the expansion of HSCs and hematopoietic progenitor cells,11,16,17 thereby TPO receptor agonists seemed to be ideal therapeutic agent for AA.
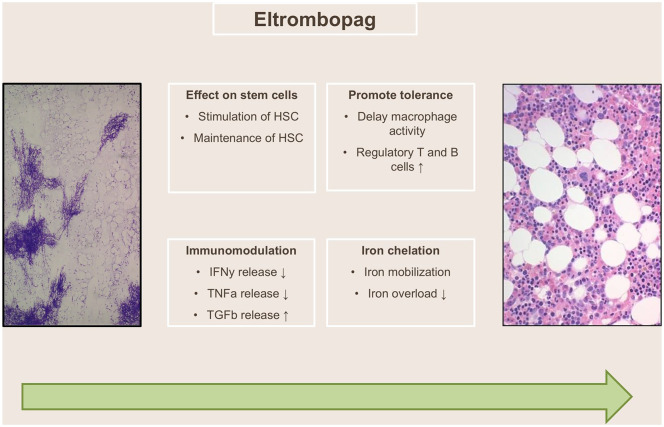
The TPO receptor agonist eltrombopag is a synthetic, nonpeptide TPO mimetic, which selectively binds to c-mpl at the transmembrane and juxtamembrane domains of the TPO receptor, at sites distinct from the binding site of TPO. Eltrombopag thus does not compete for binding with the endogenous cytokine.9 Considering the effect of TPO on megakaryopoiesis, eltrombopag was first studied successfully for the treatment of ITP.4 However, TPO agonists were also suggested to augment marrow function in AA based on the positive effect on HSC proliferation and maintenance (see above) (Figure 1). The high serum level of TPO measured in AA was thought to hinder the effect of TPO agonists,18 but the National Institutes of Health (NIH) proved with a dose-escalating ramp-up study that the elevated endogenous TPO serum levels can be overcome by using a high-dose of the oral TPO agonist eltrombopag.19 The compelling results on hematologic response by this landmark study lead to further studies in AA.
Figure 1.
Proposed mechanism of action of eltrombopag in aplastic anemia. (Adapted from Scheinberg, P. Activity of eltrombopag in severe aplastic anemia. Blood Adv 2018; 2: 3054–3062.)
HSC, human stem cell; IFNɣ, interferon gamma; TGFβ, transforming growth factor beta; TNFɑ, tumor necrosis factor alpha.
The clinical evidence of eltrombopag in SAA
First attempts with eltrombopag were performed in 43 patients with refractory SAA (rSAA) in a nonrandomized phase II dose-escalating study from the NIH.19 Patients had failed at least one course of IST, given at least 6 months previously. Starting at a dose of 50 mg, dose escalation was performed every 2 weeks by 25 mg to a maximum of 150 mg. Around 40% of patients reached the primary endpoint of hematologic response at 3–4 months, including several patients with multilineage response. Patients remaining on eltrombopag could further improve their blood counts in the majority of cases. Despite the doses being higher than administered in ITP, eltrombopag was well tolerated. The known adverse events in the form of skin, gastrointestinal, or hepatic impairment did not occur at a higher frequency than that known from ITP. Increased reticulin staining, which was discussed intensively in previous ITP studies, was not seen. This promising data led to a nonrandomized, phase II trial at the NIH for treatment-naïve patients with SAA using eltrombopag first-line in addition to horse ATG and CSA. Based on the results of the dose-escalation trial, a starting dose of 150 mg was selected in combination with the standard IST (ATG/CSA) for a study duration of 6 months.20 Initially, eltrombopag was started on day 14 (cohort 1) to avoid further liver toxicity, a well-known side effect of ATG/CSA. Subsequent patients were only treated for 3 months instead of 6 months (cohort 2) due to concerns about clonal evolution. This shorter treatment period resulted in a decrease in the complete response rate (from 33% to 26%). This lead to treatment of an additional series of patients from day 1 until 6 months (cohort 3), which showed the best results with an overall hematological response rate of 95% (complete response 58% at 6 months). Comparing these results with historical data (overall hematological response 66%, complete response 10%), the response rates were increased by the addition of eltrombopag to standard IST. Next to the improved hematological response rate, quality of life was also improved and increased bone marrow progenitor cells were observed.
Interestingly, another single-center phase II trial from the MD Anderson Cancer Center could not confirm the benefit of adding eltrombopag to standard IST with G-CSF.21 Initially this trial was designed to study the combination of IST with G-CSF alone, but study investigators decided to add eltrombopag after the encouraging results from the previous NIH trial. This led to a slight increase in the overall response rate (from 71% to 76%), but was not statistically significant. A comparison between these two studies is difficult to make since the trial design differed substantially, as Assi et al. started eltrombopag at a later timepoint, continued eltrombopag for more than 6 months, and administered CSA only shortly for at least 6 months. 21 In the end, the response rate was assessed during the complete follow up of a median 21 months, whereas the NIH investigated response after 6 months. Lastly, there is a general drawback to the currently available trials on eltrombopag, which is that they used very different definitions of response and timepoints of response assessment (Table 1).
Table 1.
Previous and ongoing landmark trials evaluating eltrombopag as a single agent or in combination in SAA and MAA as first- or second-line therapy.
| Study (institution/country) | Indication | N | Eltrombopag dose (duration) | Treatment regimen | Response rate |
|---|---|---|---|---|---|
| First-line therapy | |||||
| Phase I/II, single-arm, open- label, nonrandomized, prospective study of eltrombopag + horse ATG + CSA. (NCT01623167) NIH/USA |
SAA/vSAA | Cohort 1: 30 Cohort 2: 31 Cohort 3: 31 |
150 mg/day | Eltrombopag/ATG/CSA | Overall response (CR + PR) at 6 months: Cohort 1: 80% Cohort 2: 87% Cohort 3: 94% |
| Phase III, horse ATG + CSA + eltrombopag; prospective, randomized, open label (RACE). (NCT02099747) Europe |
SAA/vSAA | 197 | 150 mg/day day 14 until 6 months (or 3 months if CR) | Eltrombopag/ATG/CSA | CR response at 3 months: 76% |
| Phase II, eltrombopag for moderate AA. (NCT01328587) NIH/USA |
MAA, unilineage cytopenia | 34 | Dose escalation from 50 mg to 300 mg for 16–20 weeks | Eltrombopag | Hematologic response* at 16–20 weeks: 50% |
| Efficacy and safety of eltrombopag in combination with CSA in moderate AA: prospective, randomized, multicenter study. (NCT02773225) Europe |
MAA | – | 150 mg/day until 12 months (or stopped after 6 months if not in PR/CR) | Eltrombopag/CSA | Ongoing |
| Second-line therapy | |||||
| Phase II, eltrombopag; nonrandomized, single arm, open label. (NCT00922883) NIH |
AA refractory to standard IST | 25 | Dose escalation from 50 mg/day to 150 mg/day for a total of 12 weeks | Eltrombopag | Hematologic response$ at 12 weeks: 44% |
| Phase II, eltrombopag; nonrandomized, single arm, open label. Extended fixed dosing (150 mg) in SAA. (NCT01891994) NIH |
rSAA/vSAA | 39 | 150 mg/day for 6 months (possible to continue if response) | Eltrombopag | Hematologic response$ at 3–4 months: 49% |
Hematologic response in patients eligible for a platelet response was defined as an increase in platelets of >20 × 109/L from baseline platelet nadirs and transfusion independence; for patients eligible for a RBC response, an increase in hemoglobin of >15 g/L from baseline for those not transfusion dependent, or a reduction of RBC transfusions by >50% over an 8-week period compared with the 8 weeks before study entry for transfusion-dependent patients. As all patients had an ANC >0.5 × 109/L at study entry and were therefore not at risk for serious infections related to neutropenia, increases in ANC were not considered for response assessment.
Hematologic response was defined as uni- or multilineage recovery by one or more of the following criteria: (a) platelet response (increase to 20 × 103/ml above baseline or stable platelet counts with transfusion independence for a minimum of 8 weeks in those who were transfusion dependent on entry into the protocol); (b) erythroid response (when pretreatment hemoglobin was 90 g/L, defined as an increase in hemoglobin by 15 g/L or, in transfused patients, a reduction in the units of packed RBC transfusions by an absolute number of at least four transfusions for 8 consecutive weeks, compared with the pretreatment transfusion number in the previous 8 weeks); (c) neutrophil response (when pretreatment ANC of 0.5 × 103/ml as at least a 100% increase in ANC, or an ANC increase 0.5 × 103/ml, and the toxicity profile as measured using Common Terminology Criteria for Adverse Events).
AA, aplastic anemia; ANC, absolute neutrophil count; ATG, antithymocyte globulin; CR, complete response; CSA, cyclosporine; IST, immunosuppression therapy; MAA, moderate aplastic anemia; NIH, National Institute of Health; PR, partial response; RBC, red blood cell; SAA, severe aplastic anemia; rSAA, refractory severe aplastic anemia; vSAA, very severe aplastic anemia.
To further clarify the effectiveness and to perform a direct controlled comparison, the European Group for Blood and Bone Marrow Transplantion (EBMT) initiated a prospective, randomized, multicenter phase III trial comparing standard IST with and without eltrombopag (RACE study, NCT02099747), which was recently completed. In this study eltrombopag was given from day 14 to 3–6 months based on the NIH data from cohorts 1 and 2, consequently this study cannot answer if eltrombopag from day 1 can give the best results, but the head-to-head comparison can ultimately confirm if eltrombopag is superior to standard IST alone. Longer follow up will be essential to define the durability of response, possible cure of the disease, and long-term effects. Trial results have been presented in abstract form but have not been published so far, overall response rates (complete response + partial response) at 6 months were 50% and 76%, without or with added eltrombopag to standard IST.22
So far, the available data show a significant improvement in marrow function in combination with standard IST, thereby suggesting a translation into event-free and possibly also survival benefits, although long-term data are still lacking. The FDA has already approved the use of eltrombopag for the treatment of refractory patients and for first-line treatment in addition to standard IST on the basis of the NIH results.23 The EMA refused to extend the indication to first-line treatment in combination with IST as a robust comparison against established treatment has not yet been published.24
Although not the topic of our review, we want to comment on the potential effect of eltrombopag in MAA. This subgroup of patients is particularly interesting since they have a larger and more heterogeneous stem cell reserve which may ensure a better response to eltrombopag. Consequently, the NIH performed a dose-escalating study in MAA and unilineage cytopenic syndromes.25 Eltrombopag was given at doses from 50 mg up to 300 mg and hematologic response was assessed after 14–20 weeks. Half of the study population responded in at least one previously affected lineage and the treatment could be stopped in the majority of the responding patients. However, most of these patients required a re-introduction of their treatment. The ongoing study from the EBMT (EMAA) is evaluating the use of eltrombopag in addition to CSA and might give further answers on the role of eltrombopag in this subgroup.
In addition to the effect of eltrombopag on hematopoiesis, other potential benefits of eltrombopag in AA merit consideration. Patients with AA are frequently iron overloaded as a result of chronic red blood cell transfusions. In vitro studies suggested that eltrombopag can mobilize intracellular iron via direct chelation,26 consequently plasma iron and ferritin levels of patients with rAA or MAA (NCT01891994, NCT01328587) enrolled in the NIH trial were analysed.27 The data confirmed the iron-mobilizing properties of eltrombopag, particularly with prolonged therapy (>3–6 months).
Adverse effects of eltrombopag in SAA
The main concern around the use of eltrombopag in AA is whether the known long-term risk for clonal evolution (inherent in all marrow failure syndromes) and relapse28 is increased.
Clonality in AA has been discussed for decades, since Dameshek29 first asked the question in 1967 “What do AA, PNH and hypoplastic leukemia have in common?”. Since then many groups have investigated the development of clonal evolution in AA, including paroxysmal nocturnal hemoglobinuria and MDS/acute myeloid leukemia (AML), which occurs in about 5–15% of patients within 5–12 years of disease course.30–33 More recently sequencing technologies have revolutionized the field, uncovering frequent somatic mutations in AA, which are also commonly seen in myeloid malignancies. The emergence of multiple independent clones with different mutations in the same gene suggests that they arise as a result of selection processes and not by genetic drift from a reduced pool of surviving HSCs. In some cases, certain genetic events (PIGA mutation, uniparenteral disomy for the short arm of chromosome)6 are linked with immune escape.34 Nonetheless, clonal evolution does not necessary mean the development of cancer since it still can be compatible with normal hematopoiesis. Interestingly, the incidence of clonal evolution significantly increases in patients after treatment with IST, although it has already been documented before the widespread introduction of IST. Eltrombopag also has been discussed to potentially increase clonal evolution since HSC proliferation is stimulated by this growth factor, leading to accelerated telomere shortening. Winkler et al.35 first studied the risk of clonal progression under eltrombopag with long-term data of patients with rSAA enrolled in the two NIH studies.19,36 The rate of clonal evolution in the form of cytogenic abnormalities (i.e. chromosome 7) and/or transformation to MDS/AML was relatively high (18%) in both studies, with clonal events occurring most often early (87% occurred within the first 6 months of treatment start). This phenomenon could be explained by the loss of the sterile alpha-domain 9 and sterile alpha-domain 9-like genes located on chromosome 7, which confers hypersensitivity to eltrombopag, as shown in mice with TPO.37 Otherwise, the early occurrence of cytogenetic abnormalities suggests that eltrombopag may stimulate dormant clones with an aberrant karyotype at baseline, as postulated by Winkler et al.35 In contrast, other experimental studies suggest a protective role of eltrombopag as it can lead to a rapid cell death of leukemic cells by reducing reactive oxygen species levels.38 Other data show that eltrombopag can inhibit leukemia proliferation by reduction of intracellular iron and induction of differentiation.26 Different to cytogenetic evolution, the prevalence of somatic mutations in myeloid cancer genes (45% of patients) was similar in patients with newly diagnosed AA .39 Overall, the clonal progression in patients with rSAA may not apply to treatment lines since the incidence of clonal evolution in treatment-naïve patients with SAA receiving ATG/CSA/eltrombopag was comparable with historical cohorts treated with IST alone.28 Giving the available data, whether eltrombopag increases the risk of cytogenetic progression, clonal evolution (somatic mutations), and/or transformation to MDS/AML cannot be fully answered, requiring longer follow up, larger patient numbers, and further experimental studies to investigate potential underlying mechanisms.
The answers to the question as to whether eltrombopag induces higher relapse rates are also not clear. Per se, relapse occurs in one-third of responding IST patients,7 which often can be rescued with a second course of IST. Currently, we know from the first published results of the NIH study with treatment-naïve patients receiving ATG/CSA/eltrombopag that a higher relapse rate (54%) after 6 months was documented in cohort 1 and part of 220 than that reported in historical trials (30–40%).2 This lead to an amendment halfway with cohort 2 that allowed for continuation of low-dose CSA after 6 months, resulting in a significantly reduced relapse rate of 15%. This re-emphasizes that long-term CSA maintenance therapy is highly relevant in preventing relapse as we already know from Frickhofen et al.,8 in turn the role of eltrombopag for relapse is not understood. Recently, the NIH presented longer follow-up data (median time of 2 years) of this study at the European Hematology Association Conference, showing that relapse rates remained similar to those previously reported with IST alone.40 In consequence, eltrombopag used in its current form (short term within the first months of first-line treatment) is possibly not preventing relapse long term, suggesting that further treatment strategies are needed to tackle relapse.
For now, longer follow up, larger patient numbers, and randomized controlled studies are needed to confirm conclusions regarding the effect of eltrombopag on the risk of clonal evolution and relapse.
Discussion: practice recommendations for TPO mimetics in SAA and open questions
Overall, the current data repeatedly confirmed the effectiveness of eltrombopag for treatment-naïve but also rAA patients in the short term. Nonetheless, there is a need for further studies as direct head-to-head comparisons with established treatments and data on long-term efficacy are missing. Also the following open questions about the optimal use of eltrombopag in AA and cost-effectiveness emerged.
Foremost, it is not clear for how long eltrombopag should be given and if eltrombopag can be discontinued without a risk of relapse. In the initial NIH study with patients with rSAA, one patient had to stop the treatment at week 10 due to a cataract misdiagnosis. Interestingly, the patient still showed a trilineage response in the further course, which lead to an amendment of the study protocol allowing eltrombopag to be stopped in case of trilineage response and transfusion independency. By this, further patients could be withdrawn from the treatment and continuously showed stable blood counts. In consequence, one could hypothesize that patients able to regain a critical mass of HSCs do not need to maintain treatment with eltrombopag. However, studies also suggested that some patients benefit from a prolonged administration of eltrombopag. In a extension study carried out by the NIH, eltrombopag at a dose of 150 mg given for 24 weeks rescued some patients who showed no response after 12 weeks.35 Recently, the NIH presented their data from longer follow up (median 2 years) of the trial using eltrombopag in treatment-naïve patients showing that overall relapse rates remained similar to those previously reported with IST alone.40 In consequence, eltrombopag used in its current form (only within the first months of first-line treatment) is possibly not preventing relapse long term, suggesting that further treatment strategies are needed to tackle relapse. For instance, the reduction of eltrombopag in a step-wise manner, as already routinely applied with CSA, is possibly more effective for durable responses instead of stopping from one day to the other. Despite these uncertainties, a major advantage of adding eltrombopag to current standard IST is the effect on response time, as patients show a more rapid reconstitution of hematopoiesis with eltrombopag, potentially resulting in fewer complications (bleeding, infections) and reduced transfusion burden in this early vulnerable time period.
It is also not known whether there are synergistic effects of eltrombopag with other agents. This is highly relevant since eltrombopag is used as monotherapy in current hematology practices, sometimes even in treatment-naïve patients, thereby potentially missing out on the beneficial effects of other agents in AA. For now, the combination of eltrombopag with immunosuppressive agents (CSA, ATG) seems to be beneficial in comparison with historical controls, but further studies in combination with other agents are needed to better understand potential interactions. Interestingly, Hosokawa et al.41 recently showed in patients refractory to eltrombopag that the addition of high-dose romiplostim accelerates hematologic recovery.
Ultimately, the optimal dosage is also not clearly defined. Based on the initial dose-escalation study by the NIH, further studies administered 150 mg/day in AA (except a 50% dose reduction in patients of East Asian heritage and pediatric patients). However, it is not known if this dosage fits all and can be reduced over time.
The previous open questions are also highly important in the context of cost-effectiveness. In fact, the use of eltrombopag for first-line treatment has been calculated to increase costs in the USA by about US$50 million over 3 years, despite costs savings of US$19 million due to reduced rescue medication use and mortality. This calculation was based on a model in which patients receive IST or eltrombopag 150 mg/day on top of IST for 6 months. Upon achieving a complete response, patients would continue with low-dose CSA for the remainder of the year, whereas nonresponders would additionally receive eltrombopag for another 6 months. Based on this data, a treatment with eltrombopag longer than 6 months, as already observed as daily routine at some centers and within the study by Assi et al.,21 would most likely increase costs further. On the other hand, synergistic effects with other agents might result in dose reductions and consequently cost reductions. Currently, eltrombopag is about to be added to the first-line treatment for SAA. Only studies such as the RACE study will provide a precise estimate of effect size thus establishing the basis on which to calculate cost–benefit ratios.
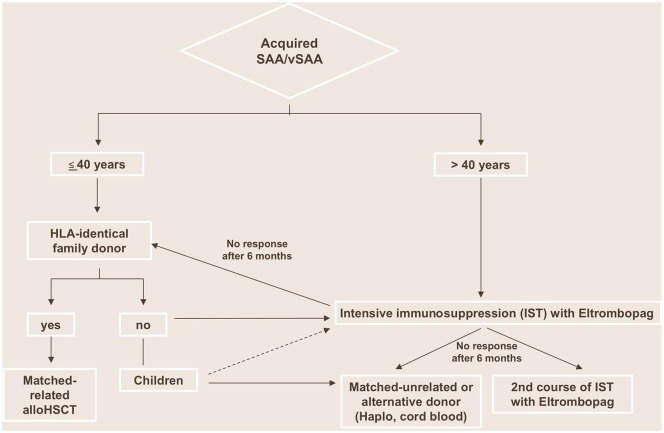
Irrespective of these open questions and budget impact, eltrombopag has already found its way into current treatment practice recommendations. The NIH, the leading institution in this field after performing the landmark studies with eltrombopag in AA, has clearly endorsed the use of eltrombopag for treatment-naïve patients with rAA (Figure 2).1 The EMA also sees great potential for this agent in AA, but is demanding a direct head-to-head comparison with established therapy to clearly confirm its therapeutic effect. In this respect, the published results of the RACE trial, comparing IST with eltrombopag to IST alone in the first-line treatment of SAA, are eagerly awaited. Regarding adverse events, the risk of clonal evolution and relapse is not clearly determined. Considering that patients with rAA showed rapid cytogenetic evolution after the initiation of eltrombopag,35 for now we recommend the careful follow up of all patients under and after discontinuation of eltrombopag with regular marrow examinations, and cytogenetic and molecular genetic analysis.
Figure 2.
Proposed treatment algorithm for first-line treatment of acquired SAA/vSAA.
HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; IST, immunosuppression therapy; SAA, severe aplastic anemia; vSAA, very severe aplastic anemia.
Conclusion
For many decades the suppresion (IST) or replacement of immune cells (HSCT) was the only treatment strategy for SAA. Now there is a strong rationale that TPO agonist eltrombopag can contribute to restoring hematopoiesis in SAA by stimulating HSCs. Further studies are needed to decide if eltrombopag is clearly superior to current established treatments and to determine the optimal treatment duration, dosage, and long-term effects.
Footnotes
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: BD received funding from a junior research grant of the University of Basel, a grant from the ProPatient foundation and an unrestricted grant from Novartis.
ORCID iD: Beatrice Drexler  https://orcid.org/0000-0002-7046-0825
https://orcid.org/0000-0002-7046-0825
Contributor Information
Beatrice Drexler, Division of Hematology, University Hospital Basel, Petersgraben 4, Basel, 4031, Switzerland.
Jakob Passweg, Division of Hematology, University Hospital Basel, Basel, Switzerland.
References
- 1. Young NS. Aplastic anemia. N Engl J Med 2018; 379: 1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 2006; 108: 2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marsh JC. Hematopoietic growth factors in the pathogenesis and for the treatment of aplastic anemia. Semin Hematol 2000; 37: 81–90. [DOI] [PubMed] [Google Scholar]
- 4. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 2007; 357: 2237–2247. [DOI] [PubMed] [Google Scholar]
- 5. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol 2016; 172: 187–207. [DOI] [PubMed] [Google Scholar]
- 6. Bacigalupo A, Socie G, Schrezenmeier H, et al. Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica 2012; 97: 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tichelli A, Passweg J, Nissen C, et al. Repeated treatment with horse antilymphocyte globulin for severe aplastic anaemia. Br J Haematol 1998; 100: 393–400. [DOI] [PubMed] [Google Scholar]
- 8. Frickhofen N, Heimpel H, Kaltwasser JP; et al. German Aplastic Anemia Study Group. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood 2003; 101: 1236–1242. [DOI] [PubMed] [Google Scholar]
- 9. Garnock-Jones KP, Keam SJ. Eltrombopag. Drugs 2009; 69: 567–576. [DOI] [PubMed] [Google Scholar]
- 10. Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci U S A 1994; 91: 11104–11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeigler FC, de Sauvage F, Widmer HR, et al. In vitro megakaryocytopoietic and thrombopoietic activity of c-mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood 1994; 84: 4045–4052. [PubMed] [Google Scholar]
- 12. Ku H, Yonemura Y, Kaushansky K, et al. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood 1996; 87: 4544–4551. [PubMed] [Google Scholar]
- 13. Alexander WS, Roberts AW, Nicola NA, et al. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 1996; 87: 2162–2170. [PubMed] [Google Scholar]
- 14. Ihara K, Ishii E, Eguchi M, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci U S A 1999; 96: 3132–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dasouki MJ, Rafi SK, Olm-Shipman AJ, et al. Exome sequencing reveals a thrombopoietin ligand mutation in a Micronesian family with autosomal recessive aplastic anemia. Blood 2013; 122: 3440–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander WS, Roberts AW, Maurer AB, et al. Studies of the c-Mpl thrombopoietin receptor through gene disruption and activation. Stem Cells 1996; 14(Suppl. 1): 124–132. [DOI] [PubMed] [Google Scholar]
- 17. Kimura S, Roberts AW, Metcalf D, et al. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A 1998; 95: 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng X, Scheinberg P, Wu CO, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica 2011; 96: 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med 2012; 367: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med 2017; 376: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of Eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer 2018; 124: 4192–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peffault de Latour R, Marsh J, Iacobelli S, et al. Results of the EBMT saawp phase III prospective randomized multicenter race study of horse ATG and ciclosporin with or without Eeltrombopag in naïve SAA patients. Presented at the 46th Annual Meeting of the EBMT, 22–25 March 2020, Madrid, Spain. [Google Scholar]
- 23. Novartis. FDA approves Novartis drug Promacta® for first-line SAA and grants breakthrough therapy designation for additional new indication, https://www.novartis.com/news/media-releases/fda-approves-novartis-drug-promacta-first-line-saa-and-grants-breakthrough-therapy-designation-additional-new-indication (accessed 22 February 2021)
- 24. European Medicines Agency. Refusal of a change to the marketing authorisation for Revolade (eltrombopag), https://www.ema.europa.eu/en/documents/smop/questions-answers-refusal-change-marketing-authorisation-revolade-eltrombopag_en.pdf (2019) (accessed 22 February 2021).
- 25. Fan X, Desmond R, Winkler T, et al. Eltrombopag for patients with moderate aplastic anemia or uni-lineage cytopenias. Blood Adv 2020; 4: 1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood 2012; 120: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao Z, Sun Q, Sokoll LJ, et al. Eltrombopag mobilizes iron in patients with aplastic anemia. Blood 2018; 131: 2399–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenfeld S, Follmann D, Nunez O, et al. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA 2003; 289: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 29. Dameshek W. Riddle: what do aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH) and “hypoplastic” leukemia have in common? Blood 1967; 30: 251–254. [PubMed] [Google Scholar]
- 30. Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leuk Lymphoma 2004; 45: 433–440. [DOI] [PubMed] [Google Scholar]
- 31. Socie G, Rosenfeld S, Frickhofen N, et al. Late clonal diseases of treated aplastic anemia. Semin Hematol 2000; 37: 91–101. [PubMed] [Google Scholar]
- 32. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood 2016; 128: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Socie G, Henry-Amar M, Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia. European bone marrow transplantation-severe aplastic anaemia working party. N Engl J Med 1993; 329: 1152–1157. [DOI] [PubMed] [Google Scholar]
- 34. Ferrando AA, Lopez-Otin C. Clonal evolution in leukemia. Nat Med 2017; 23: 1135–1145. [DOI] [PubMed] [Google Scholar]
- 35. Winkler T, Fan X, Cooper J, et al. Treatment optimization and genomic outcomes in refractory severe aplastic anemia treated with Eltrombopag. Blood 2019; 133: 2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood 2014; 123: 1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagamachi A, Matsui H, Asou H, et al. Haploinsufficiency of SAMD9L, an endosome fusion facilitator, causes myeloid malignancies in mice mimicking human diseases with monosomy 7. Cancer Cell 2013; 24: 305–317. [DOI] [PubMed] [Google Scholar]
- 38. Kalota A, Selak MA, Garcia-Cid LA, et al. Eltrombopag modulates reactive oxygen species and decreases acute myeloid leukemia cell survival. PLoS One 2015; 10: e0126691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med 2015; 373: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel B, Groarke E, Lotter J, et al. Relapse and clonal evolution in severe aplastic anemia patients treated with immunosuppression and Eltrombopag. Paper presented at European Hematology Association Congress, Oral presentation, 2020. Abstract S193. [Google Scholar]
- 41. Hosokawa K, Yamazaki H, Tanabe M, et al. High-dose romiplostim accelerates hematologic recovery in patients with aplastic anemia refractory to Eltrombopag. Leukemia. Epub ahead of print 3 July 2020. DOI: 10.1038/s41375-020-0950-6. [DOI] [PubMed] [Google Scholar]