Abstract
Background and aims:
Various attempts have been made to support recovery following optic neuritis (ON), but the respective trials have mostly been negative. The aim of this study was to determine whether disease-modifying treatment (DMT) following ON as first manifestation of relapsing-remitting multiple sclerosis influences long-term outcomes.
Methods:
A total of 79 patients with ON were identified and evaluated at relapse, DMT induction, and 12 months following treatment induction with either glatiramer acetate (GLAT), interferon-beta (IFN), or teriflunomide (TRF). Low-contrast letter acuity (LCLA) and full-field visual-evoked potentials (FF-VEP) were compared between treatment groups using multivariable regression models. The impact of TRF treatment induction compared with IFN or GLAT following relapses outside the optic nerves was evaluated in an independent cohort of 122 patients. Magnetic resonance imaging (MRI) outcomes and rates of confirmed improvement of relapse-related disability were evaluated.
Results:
TRF-treated patients exhibited higher LCLA and lower relative P100 latencies normalized to the fellow-eye. Findings were significant following covariate-adjustment by multivariable analyses. Cranial MRI lesion load as well as disability progression rates were not significantly different between groups. The cohort of patients following relapses other than ON showed no differences in confirmed improvement of disability.
Conclusion:
TRF treatment is associated with favorable outcomes regarding functional optic nerve recovery following ON in early multiple sclerosis.
Keywords: disease-modifying treatment, multiple sclerosis, optic neuritis, teriflunomide
Introduction
Optic neuritis (ON) is the most abundant first clinical manifestation of relapsing-remitting multiple sclerosis (RRMS). It is characterized by a varying combination of visual loss, color desaturation, field defects, and orbital pain.1
Determined by the extent of optic nerve demyelination and axonal damage, outcomes vary from only minor recovery to full restitution. However, electrophysiological examination usually identifies life-long persistence of prolonged conduction latencies of the affected optic nerve indicating myelin sheath damage.2 Optic coherence tomography studies also identify retinal ganglion cell layer degeneration as surrogate marker for axonal damage in patients following ON.3
Although there is good potential of clinical recovery compared with other relapse manifestations in RRMS patients, persistent visual impairment can have a major impact on patient’s quality of life. Additionally, around 5% of patients develop at least one further episode of ON of the affected eye within their life, further increasing the risk of persistent, severe visual impairment.4
Although various attempts have been made to enhance recovery of the optic nerve following acute ON in early RRMS, the impact of different disease-modifying treatments (DMTs) used in multiple sclerosis remains poorly evaluated. Hence, we evaluated a local cohort of patients with ON and subsequent RRMS diagnosis to determine whether early DMT initiation is associated with favorable long-term outcomes in early RRMS patients.
Methods
Patients with acute relapses and subsequent diagnosis of RRMS (according to 2017 revised McDonald criteria) between January 2014 and June 2019, and treated with glatiramer acetate (GLAT), an interferon-beta formulation (IFN), or teriflunomide (TRF), were identified from our local database. Patients with acute ON were considered as the main study cohort for analysis. A secondary cohort, independent of the ON cohort, was defined by patients with acute relapses affecting localizations other than the optic nerves.
Diagnosis of acute ON was re-evaluated depending on presence of at least two of the following symptoms: (a) decrease of visual acuity, (b) presence of a relative afferent pupillary deficit, (c) visual field defects, (d) color desaturation, (e) orbital pain, and (f) swelling of the optic disc.
In the ON cohort patients with further episodes of ON of either eye during follow up, fellow eye abnormalities at baseline, or drug discontinuation during follow up were excluded. We defined relapse onset as baseline for our study as full-field visual-evoked potentials (FF-VEP) have been first assessed here. Pattern reversal FF-VEP were conducted at baseline (mean: 5.3 ± 1.9 days after symptom onset) and 12 months following treatment induction using a Multiliner® recording system (Viasys Healthcare, Hoechberg, Germany) using monocular black and white checkerboard stimulation. Check sizes were 12 mm/40’ and the pattern reversal rates 1.5–2/s. At least two trials were performed for each VEP recording, averaging >150 responses using electrodes positioned at Oz (active) and Fz (reference). Differences were calculated by subtracting the P100 latency value of the fellow eye from the respective P100 latency of the affected eye similar to previous studies and resulting differences were used for analysis.3
Low-contrast letter acuity (LCLA) was assessed at baseline, at 1-month follow up (DMT induction), and 12 months following treatment induction. A 2.5% low-contrast Sloan chart (Precision Vision, LaSalle, IL, USA) was used for determination since LCLA appeared as superior outcome parameter in ON patients compared with high-contrast acuity.5 LCLA is reported in decimals and was determined at our local eye clinic as part of the clinical routine guaranteeing a standardized examination setting as well as rater-independence. Patients were placed at a distance of 2 m in front of a retro-illuminated cabinet providing a constant lighting level of 85 cd/m2 on the non-letter proportion of the chart. Eyes were assessed separately by subsequent covering of the other eye. Best visual correction was applied and was determined by previous testing on high-contrast visual acuity.
Magnetic resonance imaging (MRI) data obtained within clinical routine were independently re-evaluated in a blinded-fashion for presence of T2-hyperintense and contrast-enhancing T1-hyperintense lesions.
In patients with acute relapses affecting localizations other than the optic nerves, EDSS rating was performed independently. For determination of confirmed improvement of disability, EDSS scores at 6- and 12-month follow up were analyzed and improvement was accepted relevant in all patients with a decrease of at least 1.0 points confirmed at both timepoints.
Baseline characteristics were analyzed by descriptive statistics such as mean/median and standard deviation (SD)/interquartile range (IQR) or absolute and relative frequencies, and were compared between DMT groups using one-way analysis-of-variance (ANOVA), the Kruskal–Wallis or Fisher’s exact test, where appropriate.
The covariate-adjusted effect of DMT on FF-VEP and LCLA measurements in ON patients was assessed by multivariable linear mixed model analysis for longitudinal data. Fixed effects were the main effects of measurement time (since ON onset), DMT group, sex, age, duration since MS onset and number of T2-hyperintense MRI lesions at baseline, as well as the interaction between DMT and time. The models included a random intercept to account for dependencies between measurements on the same patient. Pairwise group comparisons resulting from the models were based on a Tukey correction to adjust for multiple testing. A multivariable logistic regression analysis was performed to compare the probability to develop new/enlarging T2-hyperintense MRI lesions in ON patients and the probability for confirmed improvement of disability in patients with other relapses at follow up between DMT groups, adjusting for sex, age, and duration since MS onset at baseline. Inferential statistics were intended to be exploratory. Statistical analysis was carried out using SPSS 26 and R, version 3.6.0; p values ⩽ 0.05 were considered statistically significant.
Results
A total of 108 patients with acute ON and subsequent RRMS diagnosis were identified. Data on FF-VEP and/or LCLA were missing in 17 patients and 4 patients had fellow eye abnormalities. At follow up, seven patients had discontinued treatment (TRF: three patients; IFN/GLAT: two patients each) and one patient experienced another episode of acute ON in the eye that had initially been affected.
Accordingly, 79 patients were included in our main analysis of ON patient outcomes. In the ON cohort, baseline data were balanced evenly between DMT groups. Patients were generally young and early in their disease course, which was corroborated by a low T2-hyperintense MRI lesion-load at baseline (Table 1). None of the patients exhibited a multifocal MS relapse although half of the patients presented with contrast-enhancing lesions at localizations other than the optic nerves at baseline. MRI findings of the affected optic nerves were mostly also suggestive of ON showing both edema and contrast-enhancement in variable combinations.
Table 1.
Baseline characteristics of the ON cohort; p values were obtained using ANOVA (+), the Kruskal–Wallis test (*) or Fisher’s exact test (#).
| TRF | IFN | GLAT | p | |
|---|---|---|---|---|
| Patients, n | 27 | 24 | 28 | n/a |
| Age, years, mean (SD) | 29.1 (5.6) | 27.1 (4.8) | 28.00 (5.7) | 0.410+ |
| Male patients, n (%) | 9 (33.3) | 8 (33.3) | 11 (39.3) | 0.100# |
| Patients with previous demyelinating events, n (%) | 16 (59.3) | 14 (58.3) | 14 (50.0) | 0.780# |
| Duration since first demyelinating event, months, median (IQR) | 9 (7–11) | 9 (7–11) | 8 (6–10) | 0.111* |
| Previous relapses, n (%) | 0.411# | |||
| 0 | 16 (59.3) | 14 (58.3) | 14 (50) | |
| 1 | 9 (33.3) | 10 (41.7) | 14 (50) | |
| 2 | 2 (7.4) | 0 (0) | 0 (0) | |
| Right eye affected, n (%) | 14 (51.9) | 10 (41.7) | 19 (67.9) | 0.053# |
| Criteria for ON diagnosis, n (%) | ||||
| Decreased visual acuity | 27 (100) | 24 (100) | 28 (100) | n/a |
| Relative afferent pupillary deficit | 19 (70.4) | 18 (75.0) | 18 (64.3) | 0.715# |
| Visual field defect | 14 (51.9) | 13 (54.2) | 1 (57.1) | 0.099# |
| Color desaturation | 20 (74.1) | 19 (79.2) | 22 (78.6) | 0.117# |
| Ocular pain | 25 (92.6) | 20 (83.3) | 22 (78.6) | 0.054# |
| Swollen optic disc | 17 (63) | 13 (54.2) | 16 (57.1) | 0.099# |
| LCLA in the affected eye at baseline, mean (SD) | 0.6 (0.2) | 0.6 (0.2) | 0.6 (0.2) | 0.655+ |
| FF-VEP latency in the affected eye at baseline, ms, mean (SD) | 127.0 (3.9) | 126.3 (5.6) | 127.9 (7.3) | 0.598+ |
| FF-VEP latency in the fellow eye at baseline, ms, mean (SD) | 101.4 (2.0) | 100.9 (1.9) | 101.4 (2.1) | 0.617+ |
| FF-VEP conduction block in the affected eye at baseline, n (%) | 1 (3.7) | 2 (8.3) | 2 (7.1) | 0.128# |
| Patients with MRI T2-hyperintensity/swelling of the affected optic nerve, n (%) | 23 (85.2) | 19 (79.2) | 22 (78.6) | 0.820# |
| Patients with MRI contrast-enhancement of the affected optic nerve, n (%) | 10 (48.1) | 10 (41.7) | 14 (50.0) | 0.848# |
| Number of MRI T2 lesions at baseline, median (IQR) | 6 (5–8) | 6 (3–8.8) | 5 (3.3–7.8) | 0.455* |
| Patients with contrast-enhancing lesions (apart from optic nerves) at baseline, n (%) | 15 (55.6) | 13 (54.2) | 16 (57.1) | 0.106# |
| Number of contrast-enhancing lesions at baseline, n (%) | 0.981# | |||
| 0 | 12 (44.4) | 11 (45.8) | 12 (42.9) | |
| 1 | 10 (37.0) | 7 (29.2) | 8 (28.6) | |
| 2 | 4 (14.8) | 4 (16.7) | 6 (21.4) | |
| 3 | 1 (3.7) | 2 (8.3) | 2 (7.1) | |
| Time to initiation of IVMPS since relapse onset, days, mean (SD) | 5.2 (1.7) | 5.7 (1.9) | 5.1 (2.0) | 0.528+ |
| Time to initiation of DMT from discharge, days, mean (SD) | 33.5 (5.4) | 32.0 (3.8) | 32.9 (3.5) | 0.453+ |
ANOVA, analysis of variance; DMT, disease-modifying treatment; FF-VEP, full-field visual-evoked potentials; GLAT, glatiramer acetate; IFN, interferon-beta; IQR, interquartile range; IVMPS, intravenous methyl prednisolone; LCLA, low-contrast letter acuity; MRI, magnetic resonance imaging; ON, optic neuritis; SD, standard deviation; TRF, teriflunomide.
Visual impairment was present in all patients and LCLA at relapse peak was comparable between groups (mean ± SD: TRF: 0.6 ± 0.2; IFN: 0.6 ± 0.2; GLAT: 0.6 ± 0.2). A variable combination of further symptoms was present as listed in Table 1.
All patients received intravenous methyl prednisolone (IVMPS; 1000 mg for 5 consecutive days) as inpatients. Following IVMPS treatment all patients reported (beginning) resolution of symptoms depicted by increasing LCLA, resolution of orbital pain, and amelioration of color perception and visual fields. None of the patients required a second course of IVMPS or even plasma exchange for symptom control. Patients were transferred to our outpatient clinic for DMT induction about 1 month from discharge. None of the patients received any immunosuppressive or immunomodulatory treatment in the meantime.
At DMT induction, mean LCLA widely recovered and was comparable between treatment groups (mean ± SD: TRF: 0.7 ± 0.1; IFN: 0.7 ± 0.2; GLAT: 0.7 ± 0.1). At this time, re-deterioration of symptoms was not observed and none of the patients had developed further symptoms suggestive of an acute relapse of multiple sclerosis. FF-VEP was not assessed here.
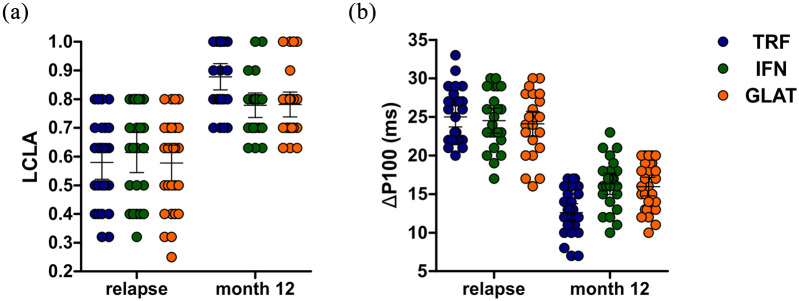
At follow up, TRF-treated patients showed higher mean LCLA (mean ± SD: TRF: 0.9 ± 0.1; IFN: 0.8 ± 0.1; GLAT: 0.8 ± 0.1; see Figure 1a). Findings were statistically significant following covariate-adjustment by multivariable linear mixed model analysis (covariate-adjusted pairwise mean LCLA difference at follow-up: 0.106 and 0.101 with p = 0.017 and p = 0.019 for TRF versus IFN and TRF versus GLAT, respectively; see Table 2a).
Figure 1.
Optic nerve function parameters at relapse, DMT induction, and follow up (month 12). (a) LCLA. (b) P100 latency of the affected eye at relapse and follow-up normalized to values obtained from fellow eye at baseline. Error bars indicate the 95% CIs of means (horizontal line).
CI, confidence interval; DMT, disease-modifying treatment; GLAT, glatiramer acetate; IFN, interferon-beta; LCLA, low-contrast letter acuity; TRF, teriflunomide.
Table 2.
Multivariable regression analyses for optic-nerve outcomes. (a) Parameter estimates from the multivariable linear mixed regression analysis for LCLA and covariate-adjusted pairwise mean VA differences of DMT groups at relapse, DMT induction, and follow up (month 12 from treatment induction), resulting from the multivariable model. (b) Parameter estimates from the multivariable linear mixed regression analysis for relative P100 latency (FF-VEP) and covariate-adjusted pairwise mean FF-VEP differences of DMT groups at relapse and follow up, resulting from the multivariable model.
| (a) | Covariate | Estimate (95% CI) | p |
|---|---|---|---|
| Main effects | Time (DMT induction versus relapse) | 0.149 (0.107; 0.192) | <0.001* |
| Time (follow-up versus relapse) | 0.298 (0.256; 0.341) | ||
| Group (IFN versus TRF) | 0.028 (−0.044; 0.100) | 0.333 | |
| Group (GLAT versus TRF) | −0.007 (−0.077; 0.062) | ||
| Sex (male versus female) | 0.033 (−0.019; 0.086) | 0.235 | |
| Age | −0.004 (−0.009; 0.001) | 0.144 | |
| Duration since RRMS onset | −0.005 (−0.010; 0.001) | 0.142 | |
| Number of MRI T2 lesions | 0.003 (−0.008; 0.015) | 0.574 | |
| Interaction | DMT induction × IFN | −0.049 (−0.111; 0.013) | 0.001* |
| Follow up × IFN | −0.134 (−0.195; −0.072) | ||
| DMT induction × GLAT | −0.025 (−0.085; 0.034) | ||
| Follow up × GLAT | −0.094 (−0.153; −0.034) | ||
| Pairwise group comparisons | Estimate (95% CI) | p | |
| At relapse | TRF versus IFN | −0.028 (−0.118 to 0.062) | 0.743 |
| TRF versus GLAT | 0.007 (−0.080 to 0.094) | 0.979 | |
| IFN versus GLAT | 0.035 (−0.054 to 0.124) | 0.621 | |
| At DMT induction | TRF versus IFN | 0.021 (−0.069 to 0.111) | 0.846 |
| TRF versus GLAT | 0.033 (−0.055 to 0.120) | 0.650 | |
| IFN versus GLAT | 0.012 (−0.078 to 0.101) | 0.949 | |
| At follow up | TRF versus IFN | 0.106 (0.016 to 0.196) | 0.017* |
| TRF versus GLAT | 0.101 (0.014 to 0.188) | 0.019* | |
| IFN versus GLAT | −0.005 (−0.094 to 0.085) | 0.992 | |
| (b) | Covariate | Estimate (95% CI) | p |
| Main effects | Time (follow up versus relapse) | −13.037 (−15.552; −10.522) | <0.001* |
| Group (IFN versus TRF) | −0.348 (−3.036; 2.340) | 0.111 | |
| Group (GLAT versus TRF) | 0.825 (−1.768; 3.418) | ||
| Sex (male versus female) | 0.626 (−1.047; 2.299) | 0.481 | |
| Age | −0.038 (−0.190; 0.113) | 0.633 | |
| Duration since RRMS onset | 0.081 (−0.101; 0.263) | 0.403 | |
| Number of MRI T2 lesions | −0.036 (−0.400; 0.327) | 0.850 | |
| Interaction | follow up × IFN | 3.995 (0.330; 7.661) | 0.105 |
| follow up × GLAT | 2.466 (−1.059; 5.990) | ||
| Pairwise group comparisons | Estimate (95% CI) | p | |
| At relapse | TRF versus IFN | 0.348 (−2.991; 3.687) | 0.967 |
| TRF versus GLAT | −0.825 (−4.047; 2.397) | 0.817 | |
| IFN versus GLAT | −1.173 (−4.484; 2.138) | 0.680 | |
| At follow up | TRF versus IFN | −3.647 (−6.987; −0.308) | 0.029* |
| TRF versus GLAT | −3.291 (−6.513; −0.068) | 0.044* | |
| IFN versus GLAT | 0.357 (−2.954; 3.668) | 0.965 |
Significant (p⩽0.05).
CI, confidence interval; DMT, disease-modifying treatment; FF-VEP, full-field visual-evoked potentials; GLAT, glatiramer acetate; IFN, interferon-beta; LCLA, low-contrast letter acuity; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple sclerosis; TRF, teriflunomide.
Findings were corroborated by FF-VEP analysis, which indicated lower P100 latency differences at month 12 in TRF-treated patients as compared with other DMT groups (TRF: 12.6; IFN: 16.3; GLAT: 16.0; see Figure 1b). Again, findings were statistically significant following covariate-adjustment by multivariable linear mixed model analysis (covariate-adjusted pairwise mean FF-VEP difference at follow-up: −3.647 and −3.291 with p = 0.029 and p = 0.044 for TRF versus IFN and TRF versus GLAT, respectively; see Table 2b).
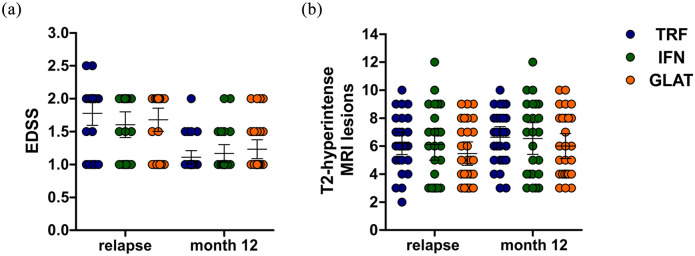
Besides optic nerve recovery, disease courses appeared similar between groups. During the follow up period of 12 months, we observed two MS relapses in our group including one case of hemihypesthesia (GLAT) and one case of acute neurogenic bladder dysfunction (IFN). Both cases completely resolved following intravenous methyl prednisolone. None of our patients experienced relevant EDSS progression during follow up, resulting in an overall low disability burden at follow up. We therefore refrained from regression analysis here (Figure 2a).
Figure 2.
MRI outcomes and disability burden at relapse and follow up (month 12). (a) EDSS scores at relapse and follow up. (b) T2-hyperintense MRI lesions at relapse and follow up. Error bars indicate the 95% CIs of means (horizontal line).
CI, confidence interval; EDSS, expanded disability status scale; GLAT, glatiramer acetate; IFN, interferon-beta; MRI, magnetic resonance imaging; TRF, teriflunomide.
Follow-up MRI after 12 months identified 29 patients with new/enlarging T2-hyperintense MRI lesions (TRF: 10 patients; IFN: 9 patients; GLAT: 10 patients). Nine patients showed contrast-enhancing lesions at follow up (TRF: two patients; IFN: three patients, GLAT: four patients; Figure 2b). In the multivariable logistic regression analysis, only duration since RRMS onset was associated significantly with development of new/enlarging T2-hyperintense MRI lesions at follow up [odds ratio (OR) 1.170 per month increase; 95% confidence interval (CI): 1.048–1.318; p = 0.005] whereas the respective DMT had no relevant impact (p = 0.984; see Table 3).
Table 3.
Multivariable regression analysis for MRI outcome in optic neuritis patients. Parameter estimates from the multivariable logistic regression model for the probability of developing new/enlarging T2-hyperintense MRI lesions at month 12.
| Covariate | Odds ratio (95%-CI) | p | |
|---|---|---|---|
| Main effects | Group (IFN versus TRF) | 0.931 (0.264; 3.241) | 0.984 |
| Group (GLAT versus TRF) | 1.093 (0.337; 3.578) | ||
| Sex (male versus female) | 2.082 (0.751; 5.991) | 0.257 | |
| Age | 0.972 (0.886; 1.065) | 0.346 | |
| Duration since RRMS onset | 1.170 (1.048; 1.318) | 0.005* |
Significant (p⩽0.05).
CI, confidence interval; GLAT, glatiramer acetate; IFN, interferon-beta; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple sclerosis; TRF, teriflunomide.
To further evaluate whether the observed effects were specific to the optic nerves, we additionally evaluated the outcomes of patients with relapses affecting function systems other than the optic nerve. Since such events were – as described above – not regularly present in the ON cohort, we evaluated our local cohort of patients with first demyelinating events and subsequent diagnosis of RRMS according to 2017 revised McDonald criteria. Here, we identified 147 patients who were diagnosed with RRMS following their first documented relapse and subsequently started treatment with TRF, GLAT, or IFN. Of those, 10 patients experienced further relapses within the 1-year follow up, 8 patients had incomplete data on the six- or 12-month follow up and 7 patients discontinued their treatment or were switched to other substances. Excluding these patients, 122 patients were evaluated. These patients appeared again evenly balanced in terms of their baseline criteria (shown in Table 4) and moreover had baseline characteristics comparable with those of the ON cohort. We evaluated EDSS scores, which were independently rated within follow up according to standardized rating sheets in our hospital during clinical routine. The proportion of patients with confirmed improvement of disability following DMT induction was deemed the best surrogate for relapse recovery. Notably, we found no difference regarding this parameter between DMT groups (TRF: 42.4%; IFN: 37.5%; GLAT: 36.6%; p = 0.892). A multivariable logistic regression analysis of disability improvement showed no advantage of TRF over IFN or GLAT (Table 5). Furthermore, we again did not find a relevant difference of new or enlarging T2-hyperintense MRI lesions among groups (TRF: 12 patients; IFN: 13 patients; GLAT: 18 patients; p = 0.887). In the multivariable regression model, we also identified disease duration since onset to be associated with development of new or enlarging T2-hyperintense MRI lesions (OR 1.137 per month increase; 95% CI: 1.044–1.245; p = 0.003), with the DMT group again showing no impact (Table 6).
Table 4.
Baseline characteristics of our control cohort; p values were obtained using ANOVA (+), the Kruskal–Wallis test (*) or Fisher’s exact test (#).
| TRF | IFN | GLAT | p | |
|---|---|---|---|---|
| Patients, n | 33 | 41 | 48 | n/a |
| Age, years, mean (SD) | 28.8 (5.1) | 27.2 (5.3) | 26.3 (5.2) | 0.111+ |
| Male patients, n (%) | 14 (42.4) | 14 (34.1) | 13 (27.1) | 0.343# |
| Previous relapses, n (%) | 0.919# | |||
| 0 | 19 (57.6) | 25 (61.0) | 30 (62.5) | |
| 1 | 14 (42.4) | 16 (39.0) | 18 (37.5) | |
| Duration since first demyelinating event, months, median (IQR) | 8 (6–11) | 7 (4–9) | 9 (7–11) | 0.332* |
| Affected function system, n (%) | 0.585# | |||
| Pyramidal | 20 (60.6) | 20 (48.8) | 21 (43.8) | |
| Sensory | 5 (15.2) | 11 (26.8) | 17 (35.4) | |
| Cerebellar | 5 (15.2) | 5 (12.2) | 6 (12.5) | |
| Brainstem | 3 (9) | 5 (12.2) | 4 (8.3) | |
| EDSS at relapse peak, median (IQR) | 2.5 (2–3) | 2 (2–3) | 2 (2–3) | 0.798* |
| Number of MRI T2 lesions at baseline, median (IQR) | 7 (5–9) | 7 (5–9) | 7 (5–9) | 0.754* |
| Number of contrast-enhancing lesions at baseline, n (%) | 0.917# | |||
| 0 | 10 (30.3) | 9 (22.0) | 13 (27.1) | |
| 1 | 13 (39.4) | 22 (53.7) | 21 (43.8) | |
| 2 | 7 (21.2) | 8 (19.5) | 11 (22.9) | |
| 3 | 3 (9.1) | 2 (4.9) | 3 (6.3) | |
| Time to initiation of IVMPS since relapse onset, days, mean (SD) | 4.4 (2.3) | 4.8 (2.6) | 4.7 (2.5) | 0.779+ |
| Time to initiation of DMT from discharge, days, mean (SD) | 32.1 (4.9) | 30.2 (3.6) | 30.8 (4.2) | 0.156+ |
| Patients with confirmed improvement of disability at month 12, n (%) | 14 (42.4) | 15 (37.5) | 18 (36.6) | 0.892# |
ANOVA, analysis of variance; DMT, disease-modifying treatment; EDSS, expanded disability status scale; GLAT, glatiramer acetate; IFN, interferon-beta; IQR, interquartile range; IVMPS, intravenous methyl prednisolone; MRI, magnetic resonance imaging; SD, standard deviation; TRF, teriflunomide.
Table 5.
Multivariable regression analysis for confirmed improvement of disability. Parameter estimates from the multivariable logistic regression model for the probability of experiencing confirmed improvement of disability.
| Covariate | OR (95% CI) | p | |
|---|---|---|---|
| Main effects | Group (IFN versus TRF) | 0.903 (0.343; 2.389) | 0.862 |
| Group (GLAT versus TRF) | 0.992 (0.348; 2.598) | ||
| Sex (male versus female) | 1.096 (0.490; 2.431) | 0.980 | |
| Age | 1.044 (0.972; 1.125) | 0.201 | |
| Duration since RRMS onset | 1.062 (0.978; 1.155) | 0.153 |
CI, confidence interval; GLAT, glatiramer acetate; IFN, interferon-beta; OR, odds ratio; RRMS, relapsing-remitting multiple sclerosis; TRF, teriflunomide.
Table 6.
Multivariable regression analysis for MRI outcome in patients with non-ON-relapses. Parameter estimates from the multivariable logistic regression model for probability of developing new/enlarging T2-hyperintense MRI lesions at month 12.
| Covariate | Odds ratio (95% CI) | p | |
|---|---|---|---|
| Main effects | Group (IFN versus TRF) | 1.029 (0.369; 2.912) | 0.838 |
| Group (GLAT versus TRF) | 1.446 (0.537; 4.041) | ||
| Sex (male versus female) | 1.247 (0.538; 2.869) | 0.810 | |
| Age | 1.040 (0.964; 1.124) | 0.224 | |
| Duration since RRMS onset | 1.137 (1.044; 1.245) | 0.003* |
Significant (p⩽0.05).
CI, confidence interval; GLAT, glatiramer acetate; IFN, interferon-beta; MRI, magnetic resonance imaging; ON, optic neuritis; RRMS, relapsing-remitting multiple sclerosis; TRF, teriflunomide.
Discussion
After ON related to multiple sclerosis, residual deficits often remain detectable by prolonged FF-VEP latencies or impaired LCLA, both indicating persistent structural damage to the optic nerve.6 Of note, even moderate residual deficits can interfere with daily function and were shown to significantly impact health-related quality of life.7
However, treatment trials for RRMS DMT often do not specifically focus on patients with early ON and current real-world analyses comparing approved DMT for RRMS did not investigate this subgroup either.8 Previous studies indicated no beneficial effect of either glatiramer acetate [ClinicalTrials.gov identifier: NCT00856635] or beta-interferon on ON outcomes compared with placebo, data on “newer” substances are mostly absent.9
We evaluated here the impact of DMT in a cohort of young patients with ON as first manifestation in RRMS. Compared with treatment with IFN or GLAT, TRF treatment was associated with beneficial long-term outcomes involving an increase of LCLA and decreased P100 FF-VEP latencies. Apart from recovery from ON, patient courses were comparable in our ON cohort, including absence of differences in relapse or MRI progression rates. Furthermore, a control cohort including patients with relapses other than ON showed no differences in abundance of MRI progression or in recovery from relapse-related disability over a 12-month period among different DMT groups. These findings appear in line with results from the TENERE study, a randomized clinical trial that compared TRF and IFN in RRMS yet involved patients with a longer disease course.10
Beneficial properties of TRF beyond pure peripheral immunomodulation have been hypothesized before. Preclinical data already indicated beneficial properties of TRF on the central nervous system (CNS). Generally, its capability to cross the blood–brain barrier appears a prerequisite and this feature distinguishes it from injectable substances such as GLAT and IFN.11 Within the CNS, potential mechanisms of action involve promotion of oligodendrocyte function and induction of myelin formation,11 modulation of microglia function,12 and halting of axonal perturbation.13 Interestingly, its involvement in cellular metabolism also appears to underlie its anti-inflammatory effect on lymphocytes.14
The preclinical findings regarding TRF have been supported by clinical observations of beneficial long-term effects of TRF. First, the brain atrophy rate was slowed in RRMS following TRF treatment.15 Furthermore, patients receiving TRF appeared to have favorable relapse outcomes in general as they experienced lesser relapse-driven disability progression and required less hospitalization days compared with placebo in a post hoc analysis of the TEMSO trial.16 Surprisingly, we were unable to reproduce the findings from the TEMSO trial regarding relapse-recovery in general here but it remains unclear whether this is due to differences in patient populations or the fact that patients in the TEMSO trial experienced their relapses whilst already having been on treatment, whereas our patients started treatment afterwards. Unfortunately, nothing is known about the subset of ON patients in the abovementioned post hoc analysis.
Similar combinations of the abovementioned effects were also observed for fingolimod, which had beneficial effects following ON in a smaller study, and, besides its profound anti-inflammatory effects, exerts a plethora of direct effects on CNS cells by S1P receptor modulation.17,18 Furthermore, preclinical data also suggested protective effects for 4-aminopyridine in ON, which was potentiated by a combination of immunomodulation and explained by direct effects on the optic nerve.19
Several substances have demonstrated subtle or borderline significant beneficial effects on structural or functional outcomes in controlled clinical trials on acute ON or chronic optic nerve demyelination. This involved substances like phenytoin (modulates optic nerve metabolism),20 opicinumab (anti-LINGO1 antibody),3 or clemastine (promote differentiation of oligodendrocyte-precursor cells).21
Comparing our analysis with data obtained from the opicinumab trial, it appears somehow confusing that, on the one hand, a significant proportion of retinal ganglion cell damage has already taken place 4 weeks following ON. This was suggested as major confounder for the failure of opicinumab to reach significance in the intention-to-treat population.3 On the other hand, our patients started their treatment around this time, and we were still able to demonstrate beneficial long-term outcomes. There are various potential explanations. First of all, our patients were mostly prone to milder forms of ON compared with patients from RENEW as peak vision impairment and ganglion cell loss was previously correlated with ON severity.22 Unfortunately, we were unable to validate this since optical coherence tomography was not conducted in our patients and MRI scans did not regularly include double-inversion-recovery sequences that could have been sensitive to optic nerve axonal loss in previous studies.23
The data presented are of course exploratory and include some limitations, including retrospective data analysis, absence of randomization, and lack of specialized techniques such as MF-VEP that have not entered clinical routine yet. Consequently, results should be preferably be interpreted as “hypothesis-generating” rather than “treatment-defining”.
However, our data involve platform therapies as active comparator and standardized follow-up involving FF-VEP, determination of LCLA, and cranial MRI in all patients, and a second cohort of patients was introduced for evaluation of our finding’s specificity to the optic nerve.
Taken together, our data indicate particular advantages for TRF treatment compared with injectable therapies in ON as first presentation of RRMS. Further evaluation of our findings in larger cohorts appears warranted, yet TRF represents a favorable therapeutic option following ON. Generally, future studies with deeper characterization of early RRMS patients are warranted in order to select the optimal treatment.
Author contributions: Steffen Pfeuffer: study concept and design, acquisition of data, analysis of data, writing of the manuscript;
Laura Kerschke: analysis of data, critical revision of the manuscript for intellectual content;
Tobias Ruck: critical revision of the manuscript for intellectual content;
Leoni Rolfes: critical revision of the manuscript for intellectual content;
Marc Pawlitzki: critical revision of the manuscript for intellectual content;
Philipp Albrecht: critical revision of the manuscript for intellectual content;
Heinz Wiendl: critical revision of the manuscript for intellectual content;
Sven G. Meuth: study concept and design, critical revision of the manuscript for intellectual content.
All authors have read and agreed to the submitted version of the manuscript. The current manuscript is not submitted to or under revision at another journal. The submitting author hereby declares that he takes responsibility for conduction of the study and analysis of the data and that he had full access to all study data. The submitting author furthermore declares that there are no competing interests concerning these data and that the authors have all rights to publish the data. The submitted manuscript does not contain data that have been published in any other journal. The authors have no related articles under submission.
Conflict of interest statement: Steffen Pfeuffer: received travel grants from Sanofi Genzyme and Merck Serono, lecturing honoraria from Sanofi Genzyme, Mylan Healthcare, and Biogen, and research support from Diamed, Merck Serono, and the German Multiple Sclerosis Society Northrhine-Westphalia.
Laura Kerschke: declares no conflicts of interest
Tobias Ruck: received travel grants and financial research support from Genzyme and Novartis and received honoraria for lecturing from Roche, Merck, Genzyme, Biogen, and Teva.
Leoni Rolfes: received travel grants from Merck Serono and Sanofi-Genzyme.
Marc Pawlitzki: received speaker honoraria and travel reimbursements from Novartis.
Philipp Albrecht: received compensation for serving on Scientific Advisory Boards for Allergan, Biogen, Celgene, Ipsen, Merck Serono, Merz Pharmaceuticals, Novartis, and Roche. He received speaker honoraria and travel support from Allergan, Bayer Vital GmbH, Biogen, Celgene, Ipsen, Merck Serono, Merz Pharmaceuticals, Novartis, Roche. Philipp Albrecht received research support from Allergan, Biogen, Celgene, Ipsen, Merck Serono, Merz Pharmaceuticals, Novartis, and Roche.
Heinz Wiendl: received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Sanofi Genzyme, Merck Serono, and Novartis. He received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, Merck Serono, Omniamed, Novartis, and Sanofi Aventis. He received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, Roche, and Sanofi-Genzyme. Heinz Wiendl also received research support from Bayer Healthcare, Bayer Vital, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Sanofi US, and Teva.
Sven G. Meuth: received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva.
The current work was conducted outside of third-party funding.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: The Ethical Committee of the Medical Council Northrhine-Westphalia and the University of Muenster approved the study (2020-475-f-S). All patients provided written informed consent. The study data will be shared with qualified investigators upon a reasonable request. The study was conducted without any third-party funding.
ORCID iDs: Steffen Pfeuffer  https://orcid.org/0000-0001-5171-4845
https://orcid.org/0000-0001-5171-4845
Philipp Albrecht  https://orcid.org/0000-0001-7987-658X
https://orcid.org/0000-0001-7987-658X
Heinz Wiendl  https://orcid.org/0000-0003-4310-3432
https://orcid.org/0000-0003-4310-3432
Contributor Information
Steffen Pfeuffer, Department of Neurology and Institute of Translational Neurology, University Hospital Muenster, Albert-Schweitzer-Campus 1, Muenster, 48149, Germany.
Laura Kerschke, Institute of Biostatistics and Clinical Research, University of Muenster, Muenster, Germany.
Tobias Ruck, Department of Neurology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany.
Leoni Rolfes, Department of Neurology with Institute of Translational Neurology, University Hospital Muenster, Muenster, Germany.
Marc Pawlitzki, Department of Neurology with Institute of Translational Neurology, University Hospital Muenster, Muenster, Germany.
Philipp Albrecht, Department of Neurology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, University Hospital Muenster, Muenster, Germany.
Sven G. Meuth, Department of Neurology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany
References
- 1. Kale N. Optic neuritis as an early sign of multiple sclerosis. Eye Brain 2016; 8: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the optic neuritis treatment trial. Ophthalmology 2008; 115: 1079–1082.e5. [DOI] [PubMed] [Google Scholar]
- 3. Cadavid D, Balcer L, Galetta S, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2017; 16: 189–199. [DOI] [PubMed] [Google Scholar]
- 4. Burman J, Raininko R, Fagius J. Bilateral and recurrent optic neuritis in multiple sclerosis. Acta Neurol Scand 2011; 123: 207–210. [DOI] [PubMed] [Google Scholar]
- 5. Balcer LJ, Raynowska J, Nolan R, et al. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler 2017; 23: 734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pihl-Jensen G, Schmidt MF, Frederiksen JL. Multifocal visual evoked potentials in optic neuritis and multiple sclerosis: a review. Clin Neurophysiol 2017; 128: 1234–1245. [DOI] [PubMed] [Google Scholar]
- 7. Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015; 2: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buron MD, Chalmer TA, Sellebjerg F, et al. Comparative effectiveness of teriflunomide and dimethyl fumarate: a nationwide cohort study. Neurology 2019; 92: e1811–e1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suhs KW, Hein K, Pehlke JR, et al. Retinal nerve fibre layer thinning in patients with clinically isolated optic neuritis and early treatment with interferon-beta. PLoS One 2012; 7: e51645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014; 20: 705–716. [DOI] [PubMed] [Google Scholar]
- 11. Gottle P, Manousi A, Kremer D, et al. Teriflunomide promotes oligodendroglial differentiation and myelination. J Neuroinflammation 2018; 15: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wostradowski T, Prajeeth CK, Gudi V, et al. In vitro evaluation of physiologically relevant concentrations of teriflunomide on activation and proliferation of primary rodent microglia. J Neuroinflammation 2016; 13: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groh J, Horner M, Martini R. Teriflunomide attenuates neuroinflammation-related neural damage in mice carrying human PLP1 mutations. J Neuroinflammation 2018; 15: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klotz L, Eschborn M, Lindner M, et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci Transl Med 2019; 11: eaao5563. [DOI] [PubMed] [Google Scholar]
- 15. Radue EW, Sprenger T, Gaetano L, et al. Teriflunomide slows BVL in relapsing MS: a reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflamm 2017; 4: e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Connor PW, Lublin FD, Wolinsky JS, et al. Teriflunomide reduces relapse-related neurological sequelae, hospitalizations and steroid use. J Neurol 2013; 260: 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albert C, Mikolajczak J, Liekfeld A, et al. Fingolimod after a first unilateral episode of acute optic neuritis (MOVING) - preliminary results from a randomized, rater-blind, active-controlled, phase 2 trial. BMC Neurol 2020; 20: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs 2016; 30: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dietrich M, Koska V, Hecker C, et al. Protective effects of 4-aminopyridine in experimental optic neuritis and multiple sclerosis. Brain 2020; 143: 1127–1142. [DOI] [PubMed] [Google Scholar]
- 20. Raftopoulos R, Hickman SJ, Toosy A, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 259–269. [DOI] [PubMed] [Google Scholar]
- 21. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 2017; 390: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 22. Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012; 119: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hadhoum N, Hodel J, Defoort-Dhellemmes S, et al. Length of optic nerve double inversion recovery hypersignal is associated with retinal axonal loss. Mult Scler 2016; 22: 649–658. [DOI] [PubMed] [Google Scholar]