Abstract
Purpose
Postoperative stereotactic radiosurgery (SRS) is associated with up to 30% risk of subsequent leptomeningeal disease (LMD). Radiographic patterns of LMD (classical sugarcoating [cLMD] vs. nodular [nLMD]) in this setting has been shown to be prognostic. However, the association of these findings with neurologic death (ND) is not well described.
Methods and Materials
The records for patients with brain metastases who underwent surgical resection and adjunctive SRS to 1 lesion (SRS to other intact lesions was allowed) and subsequently developed LMD were combined from 7 tertiary care centers. Salvage radiation therapy (RT) for LMD was categorized according to use of whole-brain versus focal cranial RT.
Results
The study cohort included 125 patients with known cause of death. The ND rate in these patients was 79%, and the rate in patients who underwent LMD salvage treatment (n = 107) was 76%. Univariate logistic regression demonstrated radiographic pattern of LMD (cLMD vs. nLMD, odds ratio: 2.9; P = .04) and second LMD failure after salvage treatment (odds ratio: 3.9; P = .02) as significantly associated with ND. The ND rate was 86% for cLMD versus 68% for nLMD. Whole-brain RT was used in 95% of patients with cLMD and 52% with nLMD. In the nLMD cohort (n = 58), there was no difference in ND rate based on type of salvage RT (whole-brain RT: 67% vs. focal cranial RT: 68%, P = .92).
Conclusions
LMD after surgery and SRS for brain metastases is a clinically significant event with high rates of ND. Classical LMD pattern (vs. nodular) and second LMD failure after salvage treatment were significantly associated with a higher risk of ND. Patients with nLMD treated with salvage focal cranial RT did not have higher ND rates compared with WBRT. Methods to decrease LMD and the subsequent high risk of ND in this setting warrant further investigation.
Introduction
Brain metastases from solid cancer occur in up to 30% of patients and is a substantial source of morbidity and mortality.1 Postoperative stereotactic radiosurgery (SRS) has been shown in 2 recent phase 3 trials to significantly reduce cavity local recurrence compared with gross total resection alone, and also significantly improved neurocognitive preservation compared with adjuvant whole-brain radiation therapy (WBRT), establishing postoperative SRS as a standard of care.2,3
However, resection followed by postoperative SRS has been increasingly associated with leptomeningeal disease (LMD) failure rates of up to 30% to 45% at 1 year.2,4,5 Two distinct radiographic patterns of LMD in this setting have been reported: Classic sugarcoating (cLMD) and nodular (nLMD), which has been recently described and is more specific to the postoperative setting.6,7 The radiographic pattern of LMD has been previously demonstrated to be prognostic, with cLMD having significantly worse overall survival (OS) compared with nLMD.5
A single-institution study reported the neurologic death (ND) rate after nodular LMD failure to be 72%.7 Whether there is an association between radiographic pattern of LMD, type of salvage RT for LMD, and neurologic death in this setting is not known.
Methods and Materials
The records of patients with brain metastases who underwent surgical resection and adjunctive SRS to 1 lesion and subsequently developed LMD were collected. SRS to other synchronous intact brain metastases was allowed. Patient information was gathered from 7 tertiary care centers under separate institutional review board–approved protocols from either prospectively or retrospectively maintained institutional databases. Patients with nonsolid or classic radiosensitive tumors (eg, lymphoma, small cell cancer, germinoma), >1 brain metastasis resected, or prior or planned WBRT were excluded.
ND was defined as neurologic decline or dysfunction attributable clinically or radiographically to brain metastases or related therapy preceding death without life-threatening systemic symptoms or progression.7 Additionally, patients with severe neurologic dysfunction attributable to brain metastases or related therapy who died of intercurrent illness were also deemed to have a ND.8 This definition of ND is somewhat stricter than used in previous trials of cranial radiation therapy (RT) because we intentionally did not include patients with both neurologic decline and symptomatic rapidly progressive systemic disease as ND because of our inability to accurately determine the cause of death in that setting.8,9 Cause of death was determined based on a review of clinical and radiologic medical records. Salvage cranial RT for LMD was categorized according to use of WBRT (either alone or as part of a WBRT-containing regimen, such as craniospinal RT) versus focal cranial RT (SRS or partial-brain RT). The details of the initial surgical and SRS treatments for the resected index lesions and patient radiographic and clinical follow up have been previously described.5 Treatment of subsequent LMD was not standardized and was determined by the treating physicians, of which details have also been previously reported.5,10
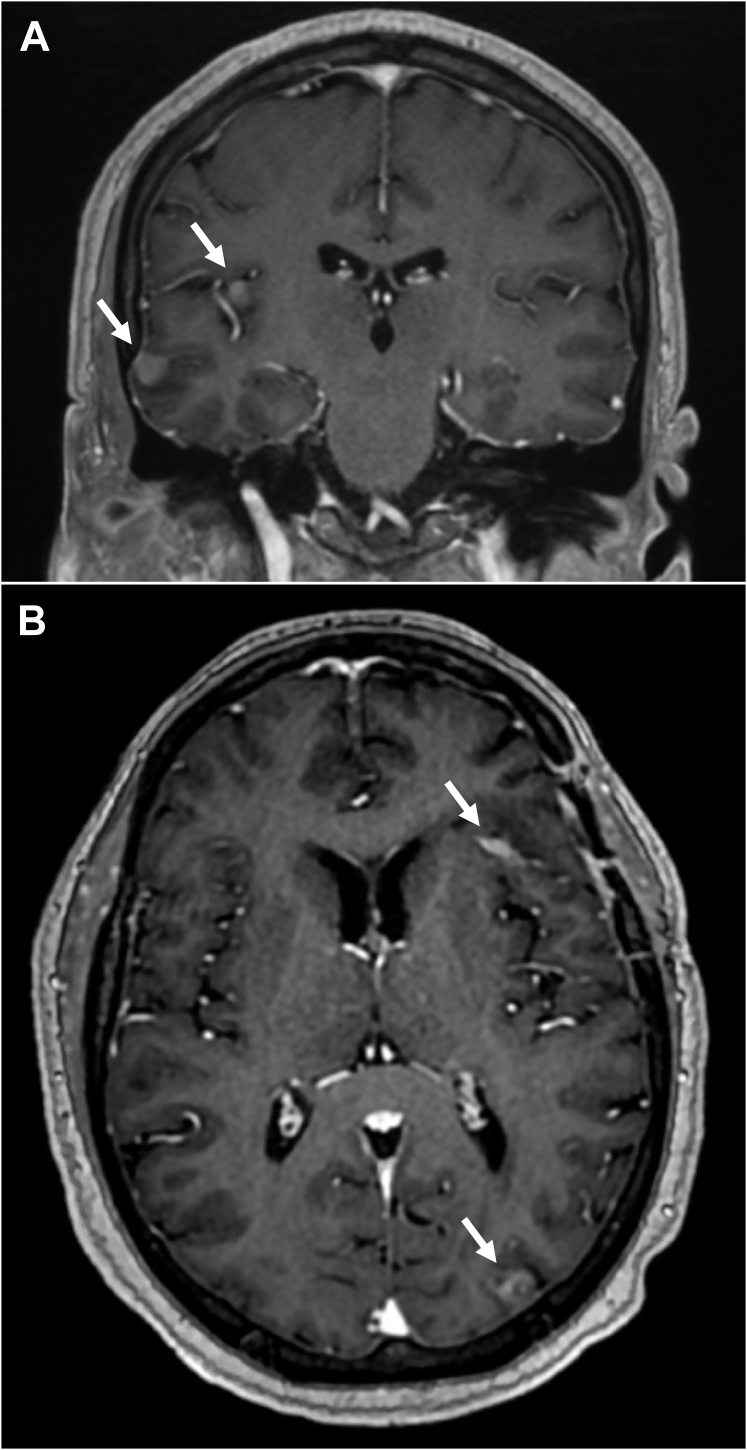
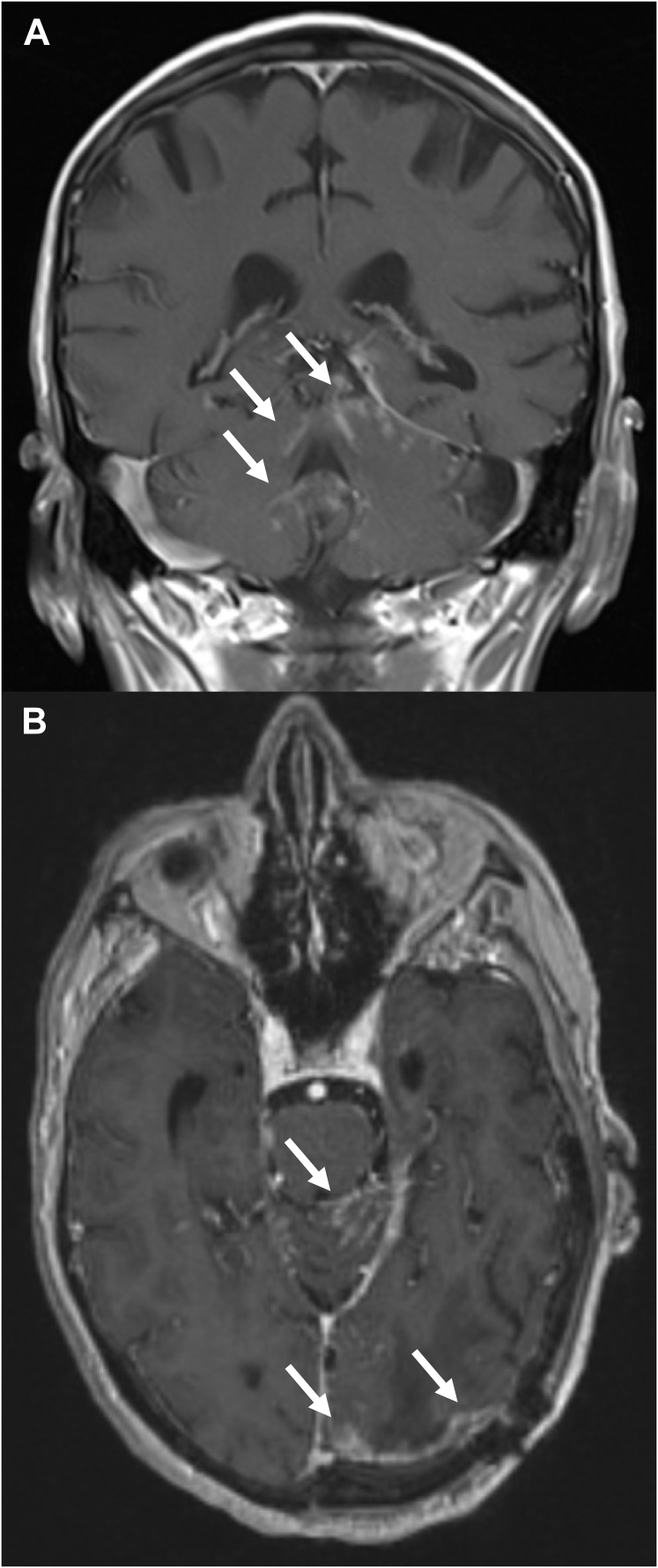
The definition of radiographic LMD is as previously described.5,6 LMD was classified as either classic or nodular based on magnetic resonance imaging (MRI) appearance according to a guide developed by 4 of the study authors (B.E.T., R.S.P., S.H.B., S.G.S.) for the purposes of standardization.6 The categorization of radiographic LMD was performed at each participating institution by an experienced radiation oncologist or radiologist. nLMD was defined as new, focal, extra-axial, distinct, nodular-enhancing lesions on the leptomeninges or ependyma (Fig 1).6 cLMD or sugarcoating enhancement was defined as new linear or curvilinear enhancement of the leptomeninges involving the sulci of the cerebral hemispheres, cranial nerves, brain stem, cerebellar folia, or ependyma (Fig 2).11
Figure 1.
Magnetic resonance image example of nodular pattern of leptomeningeal disease in (A) coronal and (B) axial orientations. White arrows point to areas of nodular extra-axial enhancement consistent with leptomeningeal disease.
Figure 2.
Magnetic resonance image example of classical (sugarcoating) pattern of leptomeningeal disease in (A) coronal and (B) axial orientations. White arrows point to areas of abnormal linear cerebellar folia and brain surface enhancement consistent with leptomeningeal disease.
ND rate was defined as the proportion of NDs relative to the number of total deaths. A univariate logistic regression was used to assess the association of variables with ND outcome. OS was estimated using the Kaplan–Meier method from the time of initial LMD, with patients censored at the time they were last known to be alive. Statistical significance was indicated by P < .05. Statistics were performed using SPSS, version 25 (IBM, Armonk, NY).
Results
The study cohort initially consisted of 147 patients, of whom 125 (85%) died with known cause. Of these 125 patients, 107 (86%) also received LMD salvage treatment and 82 (66%) also had cranial MRI follow up. ND occurred in 99 of 125 patients (79%) who died with a known cause.
The 107 patients with a known cause of death and who received LMD salvage therapy form the primary cohort for this study. Additionally, the 82 patients who also had a cranial MRI follow up constitute the subset used to evaluate the association between second LMD failure and ND. Non-small cell lung cancer was the most common primary disease (39%), followed by breast (23%), melanoma (16%), and gastrointestinal (12%) cancers. Most patients (65%) had a single brain metastasis at the time of presentation. The radiographic LMD pattern was nLMD for 59% of patients and cLMD for 41%. The median interval from initial SRS to LMD was 5 months (interquartile range, 3–12 months). Patient, tumor, and treatment characteristics by cause of death (nonneurologic vs. neurologic) are presented in Table 1. There were no significant differences between the groups, except for radiographic pattern of LMD and recurrence of LMD after salvage treatment. ND occurred in 81 of 107 patients (76%). All 18 patients (100%) who did not receive salvage treatment for LMD experienced ND, which is significantly higher than the 76% ND rate in patients who received salvage therapy (P = .02). The median OS with salvage therapy after initial LMD was 5 months (95% confidence interval, 3.6–6.5 months) compared with only 0.9 months (95% confidence interval, 0.7–1.1 months; P < .001) for the 18 patients who did not receive salvage treatment for LMD.
Table 1.
Patient, tumor, and treatment characteristics in the primary cohort (n = 107) by cause of death (nonneurologic vs neurologic)
| Variable | Nonneurologic death no. or median (% or IQR) | Neurologic death no. or median (% or IQR) | P value |
|---|---|---|---|
| Patients who received LMD salvage therapy | 26 (100) | 81 (100) | |
| Sex: | .71 | ||
| Male | 11 (42) | 31 (38) | |
| Female | 15 (58) | 50 (62) | |
| Primary site: | .3 | ||
| NSCLC | 10 (39) | 32 (40) | |
| Breast | 8 (31) | 17 (21) | |
| Melanoma | 2 (8) | 15 (19) | |
| Renal cell | 1 (4) | 2 (3) | |
| Gastrointestinal | 5 (19) | 8 (10) | |
| Other | 0 (0) | 7 (9) | |
| If NSCLC, histology: | .67 | ||
| Squamous cell | 2 (20) | 4 (13) | |
| Adenocarcinoma | 8 (80) | 25 (78) | |
| Large cell NOS | 0 (0) | 3 (9) | |
| Unknown | 0 (0) | 0 (0) | |
| Breast cancer receptor status: | |||
| Estrogen receptor positive | 5 (63) | 5 (29) | .12 |
| HER-2 positive | 6 (75) | 9 (53) | .29 |
| Melanoma molecular status: | .64 | ||
| BRAF mutated | 1 (50) | 5 (33) | |
| BRAF unknown | 1 (50) | 10 (67) | |
| RPA class at time of SRS: | .41 | ||
| 1 | 6 (23) | 30 (37) | |
| 2 | 19 (73) | 49 (61) | |
| 3 | 1 (4) | 2 (3) | |
| Extent of index lesion resection: | .66 | ||
| Gross total | 19 (73) | 64 (79) | |
| Subtotal | 7 (27) | 15 (19) | |
| Unknown | 0 (0) | 2 (3) | |
| Type of surgery: | .54 | ||
| Piecemeal | 15 (58) | 43 (53) | |
| En bloc | 6 (23) | 14 (17) | |
| Unknown | 5 (19) | 24 (30) | |
| Timing of SRS: | .82 | ||
| Postoperative | 25 (96) | 77 (95) | |
| Preoperative | 1 (4) | 4 (5) | |
| Index brain metastasis location: | .65 | ||
| Frontal | 7 (27) | 19 (24) | |
| Parietal | 2 (8) | 15 (19) | |
| Temporal | 5 (19) | 14 (17) | |
| Occipital | 4 (15) | 7 (9) | |
| Cerebellum | 8 (31) | 26 (32) | |
| Other | 0 (0) | 0 (0) | |
| Total number of brain metastases treated in initial SRS session: | .4 | ||
| 1 | 18 (69) | 51 (63) | |
| 2 | 5 (19) | 19 (23) | |
| 3 | 1 (4) | 7 (9) | |
| 4 | 1 (4) | 0 (0) | |
| ≥5 | 1 (4) | 4 (5) | |
| Radiographic pattern of LMD: | .03 | ||
| Classical | 6 (23) | 38 (47) | |
| Nodular | 20 (77) | 43 (53) | |
| LMD treatment type: | .41 | ||
| Craniospinal RT | 1 (4) | 10 (12) | |
| SRS | 9 (35) | 18 (22) | |
| Spine only RT | 0 (0) | 2 (2) | |
| WBRT | 14 (54) | 40 (49) | |
| Surgery | 1 (4) | 0 (0) | |
| Partial brain RT | 0 (0) | 2 (2) | |
| Chemotherapy only | 0 (0) | 7 (9) | |
| Surgery + SRS | 0 (0) | 1 (1) | |
| WBRT + SRS | 1 (4) | 1 (1) | |
| Cranial RT type for LMD salvage (of 97 patients who received salvage cranial RT): | .52 | ||
| WBRT | 16 (64) | 51 (71) | |
| Focal cranial RT | 9 (36) | 21 (29) | |
| Year of LMD diagnosis/treatment: | .87 | ||
| 2006–2010 | 5 (19) | 14 (17) | |
| 2011–2014 | 12 (46) | 41 (51) | |
| 2015–2017 | 9 (35) | 26 (32) | |
| Recurrence of LMD after salvage therapy (of 82 patients with MRI follow up): | .01 | ||
| Yes | 5 (23) | 32 (53) | |
| No | 17 (77) | 28 (47) | |
| Age (years) | 57 (49–65) | 58 (49–64) | .53 |
| GPA score | 2.5 (2–3) | 2.5 (2–3) | .81 |
| Index GTV (cc) | 7.8 (6.5–14.2) | 12.2 (7.5–20.7) | .1 |
| Interval from initial SRS to initial LMD (months) | 7.4 (3.4–13.9) | 4.9 (2.9–10.7) | .24 |
Abbreviations: GPA = graded prognostic index; GTV = gross tumor volume; HER-2 = human epidermal growth factor receptor 2; IQR = interquartile range; LMD = leptomeningeal disease; MRI = magnetic resonance imaging; NSCLC = non-small cell lung cancer; NOS = not otherwise specified; RPA = recursive partitioning analysis; RT = radiation therapy; SRS = stereotactic radiosurgery; WBRT = whole-brain radiation therapy.
GTV is defined as the treated cavity for postoperative SRS and the intact brain metastasis for preoperative SRS.
Percentages may not add up to 100% as a result of rounding.
Univariate logistic regression demonstrated that radiographic pattern of LMD (cLMD vs. nLMD, odds ratio: 2.9; P = .04) as significantly associated with ND. The ND rate was 86% for cLMD versus 68% for the nLMD pattern. Of the 82 patients with a cranial MRI follow up, 37 (45%) experienced a second LMD failure. Second LMD failure after salvage treatment (vs. not) was also associated with an increased risk of ND in this patient subset (odds ratio: 3.9; P = .02). The ND rate for patients with a second LMD failure was 87% versus 62% for those without.
WBRT was used as salvage therapy in 95% of patients with cLMD versus 52% of patients with nLMD. In the nLMD cohort treated with salvage cranial RT (n = 58), there was no difference in ND rate based on the type of salvage RT used (WBRT [67%] vs. focal cranial RT [68%; P = .92]). Second LMD failure (vs. not) after salvage therapy was associated with a higher ND rate within the nLMD cohort (77% vs. 52%; P = .02). Of the 26 patients with nLMD who experienced a second LMD failure, 7 had classical second LMD, of whom 100% experienced ND and 19 had nodular second LMD, of whom 68% experienced ND (P = .09).
Discussion
This study demonstrates that radiographic LMD occurrence after surgery and SRS for brain metastases is a clinically significant event with high rates of ND. The overall rate of ND in the primary cohort analysis was 76%, which is notably higher than the 14% to 48% rate of ND in randomized trials of local therapy (SRS or surgery) ± WBRT for 1 to 4 brain metastases.2,8,9,12,13 nLMD pattern has been previously demonstrated to be associated with improved OS compared with cLMD in this setting (median OS after LMD: 8.2 vs. 3.3 months, respectively; P < .001),5 but the association between these LMD patterns and ND has not been well described.
The ND rate of 68% in our cohort with nLMD is quite similar to the 72% rate reported in a single-institution study by Cagney et al. for patients with pachymeningeal seeding, which is functionally equivalent to nLMD by their definition.7 In the current study, we additionally demonstrated that the radiographic pattern of LMD is associated with ND, and specifically that cLMD confers a higher risk of ND than nLMD despite more use of WBRT. However, type of salvage cranial RT (WBRT or focal RT) did not influence the rate of ND within the nLMD cohort. We previously demonstrated that there was no OS detriment associated with the use of focal cranial RT compared with WBRT-containing regimens for nLMD.5 These 2 findings of no increase in ND rates and no detriment in OS with focal cranial RT compared with WBRT indicate that focal cranial RT (and omission of WBRT) may be a therapy option for patients with nLMD. We also demonstrated that patients who did not receive salvage therapy for LMD experienced a 100% ND rate with a very short median OS.
The strengths of this study include its relatively large patient population, multi-institutional design, and uniform protocol definition of LMD and radiographic LMD subtypes. The limitations of this study include those inherent to retrospective studies, including potential selection bias for type of LMD salvage therapy owing to lack of randomization, misclassification bias for cause of death attribution (ND vs. other), and lack of central review for radiographic pattern of LMD classification. Additionally, patient subgroups were relatively small, precluding the ability to perform multivariable analyses.
Conclusions
LMD after surgery and SRS for brain metastases is a clinically significant event with high rates of ND. Classical LMD pattern (vs. nodular) and second LMD failure after initial LMD salvage treatment were significantly associated with a higher risk of ND. Patients with nLMD treated with salvage focal cranial RT did not have an increased risk of ND compared with those treated with WBRT, indicating that focal cranial RT (and omission of WBRT) may be a therapy option for nLMD. Methods to decrease LMD and the subsequent high risk of ND in this setting warrant further investigation.
Footnotes
Sources of support: No specific funding was received for this study.
Disclosures: No conflicts of interest related to the study or manuscript exists for any of the authors.
References
- 1.Nayak L., Lee E.Q., Wen P.Y. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan A., Ahmed S., McAleer M.F. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcrom S.R., Foreman P.M., Colvin T.B. Focal management of large brain metastases and risk of leptomeningeal disease. Adv Radiat Oncol. 2020;5:34–42. doi: 10.1016/j.adro.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhu R.S., Turner B.E., Asher A.L. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol. 2019;21:1049–1059. doi: 10.1093/neuonc/noz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner B.E., Prabhu R.S., Burri S.H. Nodular leptomeningeal disease-a distinct pattern of recurrence after postresection stereotactic radiosurgery for brain metastases: A multi-institutional study of interobserver reliability. Int J Radiat Oncol Biol Phys. 2020;106:579–586. doi: 10.1016/j.ijrobp.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cagney D.N., Lamba N., Sinha S. Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019;5:703–709. doi: 10.1001/jamaoncol.2018.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patchell R.A., Tibbs P.A., Regine W.F. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu R.S., Turner B.E., Asher A. A multi-institutional analysis of patterns of salvage therapy for leptomeningeal disease after surgical resection and radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2019;105:E86–E87. [Google Scholar]
- 11.Le Rhun E., Weller M., Brandsma D. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28:iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 12.Chang E.L., Wefel J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 13.Hong A.M., Fogarty G.B., Dolven-Jacobsen K. Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: A multicenter, randomized phase III trial. J Clin Oncol. 2019;37:3132–3141. doi: 10.1200/JCO.19.01414. [DOI] [PubMed] [Google Scholar]