Abstract
Purpose
As age increases, oxidative stress increases, sperm motility decreases, and DNA fragmentation increases. To date, reports of age-related effects on semen have focused on reactive oxygen species (ROS) or total antioxidant capacity (TAC) as indicators of oxidative stress. However, assessments of ROS and TAC must be considered within a more comprehensive context in order to correctly evaluate oxidative stress and interpret findings. In this regard, the purpose of this study was to investigate the relationship between the static oxidation reduction potential (sORP) and paternal age with the goal of using the sORP as an indicator of semen oxidative stress.
Materials and Methods
Semen samples from 173 men were analyzed for the following parameters: volume, motility, and beat cross frequency (BCF). The sORP was measured by using the MiOXSYS™ system. The correlation between semen parameters and the sORP level was analyzed as a function of age. The rate of sORP positivity was compared between men <34 and ≥34 years of age, with a positive sORP defined as a level ≥1.38.
Results
Volume, motility, and BCF were negatively correlated with age (p<0.001). The semen sORP level was positively correlated with age (p<0.05). The rate of sORP positivity was significantly increased in men ≥34 years of age compared with that in men <34 years of age (33% compared with 12%, respectively; p<0.01).
Conclusions
The sORP may play a role in age-related decreases in semen parameters (volume, motility, and BCF). The rate of sORP positivity increased significantly after 34 years of age.
Keywords: Aging, Oxidation reduction, Oxidative stress, Semen, Sperm
Graphical Abstract
INTRODUCTION
As women age, their fertility declines owing to normal, age-related changes that occur in the ovaries. Unlike a man who continues to produce sperm throughout his life, a woman is born with all the egg-containing follicles in her ovaries that she will ever have. The number of oocytes reaches its peak during the fetal period. Therefore, stored eggs age, naturally, as a function of maternal aging. Oocyte aging affects fertility, pregnancy, and abortion [1]. Therefore, the age of a woman is one of the determinants of pregnancy, with a decline in birth rate as women age, particularly after 35 years of age [2]. By comparison, spermatozoa are considered to be less susceptible to aging than oocytes, as they continue to be produced after sexual maturation. However, in recent studies, it has been reported that certain parameters of semen (namely volume, total sperm count, motility, and morphology) degrade as a function of age [3,4]. Furthermore, men after the age of 40 years have a significantly reduced fertility rate [5].
An increase in oxidative stress is one of the causes of the age-related deterioration in semen parameters. The balance between reactive oxygen species (ROS), such as hydroxyl radicals, superoxide anions, and hydrogen peroxide, and antioxidants, such as superoxide dismutase and vitamin E, is normally actively maintained in vivo. As such, any imbalance between ROS and antioxidants can lead to oxidative stress, which adversely affects cells [6]. Oxidative stress causes a decrease in sperm motility [7,8] and an increase in DNA damage [9,10]. Studies have reported that aging results in an increase in oxidative stress in blood [11,12], as well as an increase in ROS in semen [13]. In fact, the total antioxidant capacity (TAC) of semen decreases with aging [14]. Overall, these findings confirm an increase in oxidative stress with aging. However, in order to evaluate the state of oxidative stress more accurately, an index that considers both oxidation and antioxidants is necessary. The static oxidation reduction potential (sORP) comprehensively evaluates the balance between oxidation and antioxidants, with a correlation between the sORP index and the oxidative stress status of serum [15] and semen [16] having previously been reported. Our aim in this study was to investigate the relationship between the sORP of semen, measured by using the MiOXSYS™ system (Aytu BioScience, Inc., Englewood, CO, USA), and paternal age. The MiOXSYS™ system quantifies the overall redox state more quickly than conventional methods by measuring the movement of electrons in a small sample of semen. It is in this capacity that the system is used in fertility clinics. In our study, we used the MiOXSYS™ system to evaluate the relationship between semen sORP and age; if a strong relationship can be confirmed, then semen sORP could be a useful indicator of male aging, as it relates to fertility.
MATERIALS AND METHODS
1. Semen samples and parameter assessment
This study included 173 men who visited our hospital between November 2017 and May 2020 (mean age, 36.6±5.9 years; mean body mass index [BMI], 23.1±2.9 kg/m2; mean sexual abstinence period, 3.6±1.6 days; mean semen collection to examination time, 63±47 min). Only patients who had not undergone fertility treatment were included. The study group, ultimately, did not include patients with diabetes, but did include 38 smokers and 135 nonsmokers. Semen samples were evaluated by using a sperm motility analysis system (SMAS™; DITECT Ltd., Tokyo, Japan), after liquefaction of semen at room temperature. After semen volume (mL) was determined, sperm motility (%) and beat cross frequency (BCF; Hz) were calculated.
2. Measurement of semen static oxidation reduction potential
The measurement of semen sORP was performed almost simultaneously with the measurement of semen parameters. To measure the semen sORP, 30 µL of liquefied semen was placed in a disposable MiOXSYS sensor, which was then loaded onto the sample port of the MiOXSYS analyzer, serving as the reference electrode to complete the electrochemical circuit. In this way, the sORP values (mV) obtained are indicative of the number of electrons transferred from the reductant (i.e., the antioxidant) to the oxidant. These data were then normalized to sperm concentration to control for differences in cell numbers, with the sORP data reported in units of mV/concentration/mL. A positive sORP indicates that oxidation is stronger than reduction, with a negative sORP indicating that reduction is stronger than oxidation. The larger the sORP, the greater the imbalance between oxidation and reduction.
3. Statistical analysis
The correlation between semen parameters and sORP with age was evaluated by using Pearson's correlation coefficient. Welch's t-test or chi-square tests were used for comparisons between the two groups. A positive rate of sORP was defined by an sORP value ≥1.38, which can differentiate normal from abnormal semen samples [17]. A p-value <0.05 was significant for all statistical tests. These analyses were performed by using Statcel 4 (OMS publishing Inc., Saitama, Japan). A receiver operating characteristic (ROC) curve was used to identify the paternal age criterion (cutoff, sensitivity, specificity, and area under curve [AUC]) that best predicted the sORP positivity. These analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [18], which is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.
4. Ethics statement
Our study protocol was approved by the ethics committee of the Denentoshi Ladies Clinic (Yokohama, Kanagawa, Japan) (approval number: 17101004). All 173 patients provided informed consent.
RESULTS
1. Correlation between age and sperm parameters
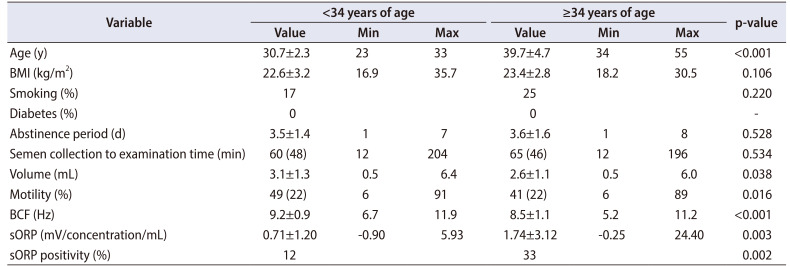
The relationship between various semen parameters and age, evaluated by using Pearson's correlation coefficient, is shown in Fig. 1. The following semen parameters were negatively correlated with age: volume (R=−0.277, p<0.001), motility (R=−0.258, p<0.001), and BCF (R=−0.415, p<0.001).
Fig. 1. Correlation between age and semen parameters. Scatter plots and linear regression lines showing the relationship between age and each of the semen parameters measured: (A) volume, (B) motility, and (C) beat cross frequency (BCF).
2. Relationship between age and semen static oxidation reduction potential
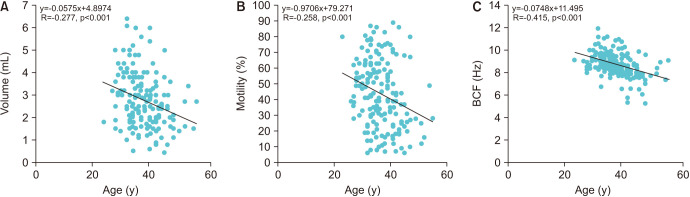
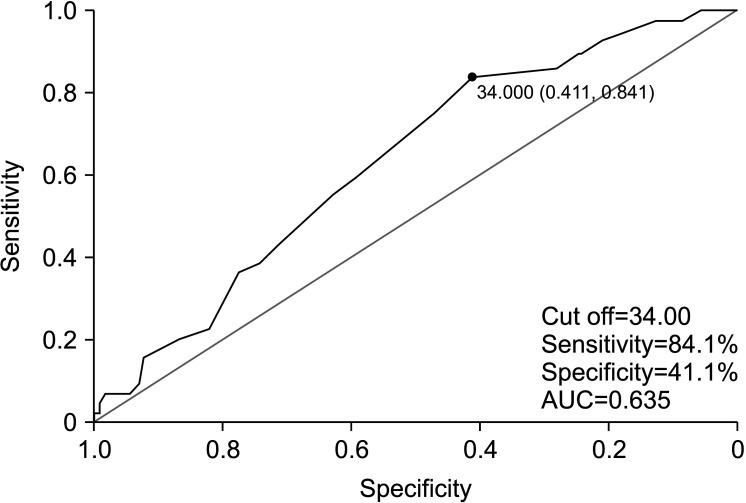
Semen sORP was positively correlated with age (R=0.192, p<0.05; Fig. 2). An ROC curve was used to identify the paternal age criterion (cutoff, sensitivity, specificity, and AUC) that best predicted the sORP positivity. The age at which the sum of sensitivity and specificity was a maximum was 34 years; therefore, the cutoff age of 34 years was predictive of sORP positivity, with a sensitivity of 84.1%, a specificity of 41.1%, and an AUC of 0.635 (Fig. 3). The positive semen sORP rate was significantly greater among men in the age group of ≥34 years (33%) than in the age group of <34 years (12%; p<0.01; Fig. 4). There were no significant differences in BMI, smoking rate, prevalence of diabetes, abstinence period, or time between semen collection and examination between the two groups (Table 1).
Fig. 2. Correlation between age and the static oxidation reduction potential (sORP) level in semen. Scatter plot and linear regression line showing the relationship between age and the sORP in semen.
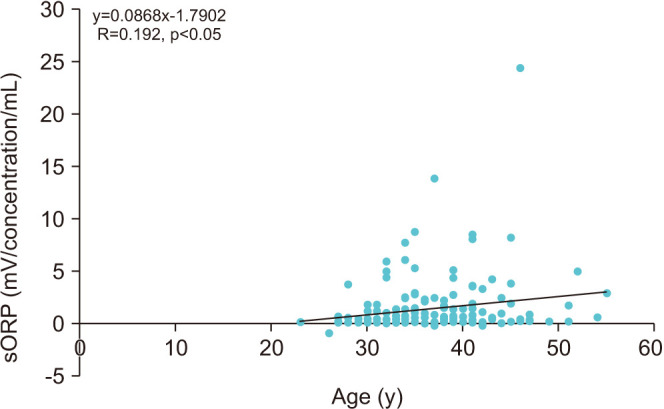
Fig. 3. Receiver operating characteristic (ROC) curves and paternal age cutoff. A ROC curve was used to identify the paternal age criterion (cutoff, sensitivity, specificity, and area under curve [AUC]) that best predicted static oxidation reduction potential positivity.
Fig. 4. Comparisons of the rate of static oxidation reduction potential (sORP) positivity between men aged <34 and ≥34 years. The vertical axis shows the rate of positive sORP, with the horizontal axis showing age. sORP ≥1.38 was defined as a positive level. **p<0.01.
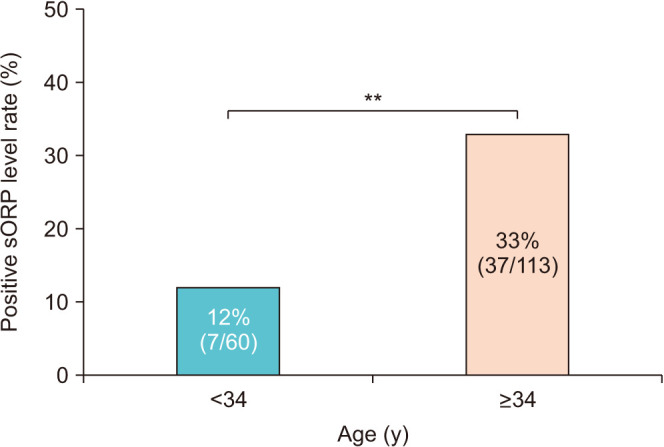
Table 1. Characteristics and semen parameters of male patients <34 and ≥34 years of age.
| Variable | <34 years of age | ≥34 years of age | p-value | ||||
|---|---|---|---|---|---|---|---|
| Value | Min | Max | Value | Min | Max | Max | |
| Age (y) | 30.7±2.3 | 23 | 33 | 39.7±4.7 | 34 | 55 | <0.001 |
| BMI (kg/m2) | 22.6±3.2 | 16.9 | 35.7 | 23.4±2.8 | 18.2 | 30.5 | 0.106 |
| Smoking (%) | 17 | 25 | 0.220 | ||||
| Diabetes (%) | 0 | 0 | - | ||||
| Abstinence period (d) | 3.5±1.4 | 1 | 7 | 3.6±1.6 | 1 | 8 | 0.528 |
| Semen collection to examination time (min) | 60 (48) | 12 | 204 | 65 (46) | 12 | 196 | 0.534 |
| Volume (mL) | 3.1±1.3 | 0.5 | 6.4 | 2.6±1.1 | 0.5 | 6.0 | 0.038 |
| Motility (%) | 49 (22) | 6 | 91 | 41 (22) | 6 | 89 | 0.016 |
| BCF (Hz) | 9.2±0.9 | 6.7 | 11.9 | 8.5±1.1 | 5.2 | 11.2 | <0.001 |
| sORP (mV/concentration/mL) | 0.71±1.20 | −0.90 | 5.93 | 1.74±3.12 | −0.25 | 24.40 | 0.003 |
| sORP positivity (%) | 12 | 33 | 0.002 | ||||
Values are presented as mean±standard deviation, number only, or number (%).
Min, minimum; Max, maximum; BMI, body mass index; BCF, beat cross frequency; sORP, static oxidation reduction potential; -, not available.
DISCUSSION
In Japan, the number of couples addressing issues of infertility is increasing as a function of people getting married at a later age. This has resulted in a yearly increase in the rate of in vitro fertilization. This effect of aging on fertility is attributed to age-related effects on fertility in both women and men. Therefore, there is a need to address the effects of aging not only on oocytes but on sperm as well. Previous studies have reported age-related decreases in semen parameters [3,4] and DNA [9,10]. Oxidative stress is one of the causes of decreased sperm motility and sperm DNA damage [19]. Spermatozoa are very susceptible to peroxidative damage because the spermatozoal membrane is rich in the polyunsaturated fatty acids that are necessary to maintain the fluidity required for membrane fusion during fertilization. In addition, since sperm cells are very small, they are relatively weak against oxidative stress because they have few antioxidant enzymes [20]. Moreover, the sperm itself does not have sufficient antioxidant capacity, leaving sperm vulnerable to the external environment. Therefore, the oxidative stress status of semen is an important criterion for determining the quality of spermatozoa.
Previous studies have measured ROS as an indicator of oxidative stress [21,22]. However, measurement of ROS alone does not take into account the presence of antioxidants and, therefore, does not reflect the overall redox state. As such, the ROS-TAC score is a better predictor of the redox status than either the ROS or TAC alone [23]. However, obtaining the ROS-TAC score still has drawbacks, requiring statistical modeling. Additionally, the tests required to measure ROS-TAC are cumbersome, requiring sophisticated instruments, a large semen sample volume, and time, needing approximately 45 minutes to complete. Therefore, a prompt and straight-forward method for measuring the overall redox status of semen would be helpful to investigate the relationship between sperm function and oxidative stress. It is for these reasons that we used the MiOXSYS™ system to analyze the relationship between sORP and age-related spermatozoal parameters.
In this study, we further analyzed how the sORP value in semen and sperm parameters change with aging, with our main findings being as follows. First, semen volume, motility, and BCF decreased with age. Second, semen sORP levels increased with age, with the prevalence rate of a positive semen sORP value being significantly higher among individuals aged ≥34 years. Our findings are in agreement with a previous study that reported an age-related decline in semen volume and motility [24]. Other studies have also reported age-delated decreases in sperm morphology [3,4,14], viability [25], and swimming speed (motility) [26].
Overall, various sperm parameters decrease with aging, with oxidative stress being one of the contributing factors. Oxidative stress in various parts of the body [27], including the serum and semen, increases with aging. However, there is no correlation between the antioxidant capacity of the serum and semen [28,29]. Moreover, the semen antioxidant capacity is more strongly correlated with semen than with the serum parameters [29]. Our results also show that sORP positivity was significantly higher in the aging group, thus reflecting the effects of aging. More accurate oxidative stress status can be confirmed by sORP than by ROS or TAC assessment. These results suggest that semen sORP may serve as an indicator of the effects of aging on sperm parameters.
Although we did not specifically address the following issue, we do note that oxidative stress increases the DNA fragmentation of spermatozoa. As such, higher sORP values with age may be related to an increase in DNA fragmentation, which would reduce the fertility-capacity of the sperm. Considering the increasing age at which men conceive and the increase in age-related oxidative stress, male infertility will become a more serious problem. Not all infertility can be treated with antioxidant therapy, but for some infertility patients, appropriate antioxidant therapy is an important treatment to consider. The semen sORP may also help to confirm the effectiveness of antioxidant therapy.
Limitations
It was not possible to completely investigate the patients' lifestyle and diseases. Factors such as varicocele, hormonal status, and alcohol consumption can affect male fertility, and more research is needed to take these factors into account to fully clarify the effects of aging on semen. In addition, our study included only 173 men (with 44 sORP-positive cases); therefore, a greater sample size is needed to confirm our findings.
CONCLUSIONS
The findings of our study indicate a decrease in certain parameters of semen, namely, volume, sperm motility, and BCF, with age. These age-related effects appear to be related to an increase in the sORP level in semen, with the effect increasing significantly after the age of 34 years.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Mitsuru Nago, Toshihiro Kawamura, and Yasushi Yumura.
- Data acquisition: Mitsuru Nago, Akane Arichi, Naoki Omura, and Yuka Iwashita.
- Statistical analysis: Mitsuru Nago and Yuka Iwashita.
- Data analysis and interpretation: Mitsuru Nago, Toshihiro Kawamura, and Yasushi Yumura.
- Drafting of the manuscript: Mitsuru Nago and Yasushi Yumura.
- Critical revision of the manuscript: Mitsuru Nago, Akane Arichi, Naoki Omura, Yuka Iwashita, Toshihiro Kawamura, and Yasushi Yumura.
- Obtaining funding: Toshihiro Kawamura.
- Administrative, technical, or material support: Toshihiro Kawamura and Yasushi Yumura.
- Supervision: Mitsuru Nago, Toshihiro Kawamura, and Yasushi Yumura.
- Approval of the final manuscript: Mitsuru Nago, Akane Arichi, Naoki Omura, Yuka Iwashita, Toshihiro Kawamura, and Yasushi Yumura.
References
- 1.Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Fetal Diagn Ther. 2011;29:263–273. doi: 10.1159/000323142. [DOI] [PubMed] [Google Scholar]
- 2.Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod. 2008;23:538–542. doi: 10.1093/humrep/dem431. [DOI] [PubMed] [Google Scholar]
- 3.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–248. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 4.Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95:1–8. doi: 10.1016/j.fertnstert.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood) Hum Reprod. 2000;15:1703–1708. doi: 10.1093/humrep/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 6.Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992;57:409–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–346. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 9.Desai N, Sharma R, Makker K, Sabanegh E, Agarwal A. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril. 2009;92:1626–1631. doi: 10.1016/j.fertnstert.2008.08.109. [DOI] [PubMed] [Google Scholar]
- 10.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza-Núñez VM, Ruiz-Ramos M, Sánchez-Rodríguez MA, Retana-Ugalde R, Muñoz-Sánchez JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213:261–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- 12.Pandey KB, Mehdi MM, Maurya PK, Rizvi SI. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis Markers. 2010;29:31–36. doi: 10.3233/DMA-2010-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocuzza M, Athayde KS, Agarwal A, Sharma R, Pagani R, Lucon AM, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology. 2008;71:490–494. doi: 10.1016/j.urology.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Omran HM, Bakhiet M, Dashti MG. Evaluation of age effects on semen parameters of infertile males. Andrology. 2013;2:106 [Google Scholar]
- 15.Rael LT, Bar-Or R, Salottolo K, Mains CW, Slone DS, Offner PJ, et al. Injury severity and serum amyloid A correlate with plasma oxidation-reduction potential in multi-trauma patients: a retrospective analysis. Scand J Trauma Resusc Emerg Med. 2009;17:57. doi: 10.1186/1757-7241-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homa ST, Vassiliou AM, Stone J, Killeen AP, Dawkins A, Xie J, et al. A comparison between two assays for measuring seminal oxidative stress and their relationship with sperm DNA fragmentation and semen parameters. Genes (Basel) 2019;10:236. doi: 10.3390/genes10030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arafa M, Agarwal A, Al Said S, Majzoub A, Sharma R, Bjugstad KB, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia. 2018;50:e12881. doi: 10.1111/and.12881. [DOI] [PubMed] [Google Scholar]
- 18.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 20.Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28:1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]
- 21.Lenzi A, Lombardo F, Gandini L, Alfano P, Dondero F. Computer assisted sperm motility analysis at the moment of induced pregnancy during gonadotropin treatment for hypogonadotropic hypogonadism. J Endocrinol Invest. 1993;16:683–686. doi: 10.1007/BF03348911. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal A, Ikemoto I, Loughlin KR. Relationship of sperm parameters with levels of reactive oxygen species in semen specimens. J Urol. 1994;152:107–110. doi: 10.1016/s0022-5347(17)32829-x. [DOI] [PubMed] [Google Scholar]
- 23.Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–2807. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 24.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Stone BA, Alex A, Werlin LB, Marrs RP. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100:952–958. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 26.Sloter E, Schmid TE, Marchetti F, Eskenazi B, Nath J, Wyrobek AJ. Quantitative effects of male age on sperm motion. Hum Reprod. 2006;21:2868–2875. doi: 10.1093/humrep/del250. [DOI] [PubMed] [Google Scholar]
- 27.Ortuño-Sahagún D, Pallàs M, Rojas-Mayorquín AE. Oxidative stress in aging: advances in proteomic approaches. Oxid Med Cell Longev. 2014;2014:573208. doi: 10.1155/2014/573208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guz J, Gackowski D, Foksinski M, Rozalski R, Zarakowska E, Siomek A, et al. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS One. 2013;8:e68490. doi: 10.1371/journal.pone.0068490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eroglu M, Sahin S, Durukan B, Ozakpinar OB, Erdinc N, Turkgeldi L, et al. Blood serum and seminal plasma selenium, total antioxidant capacity and coenzyme q10 levels in relation to semen parameters in men with idiopathic infertility. Biol Trace Elem Res. 2014;159:46–51. doi: 10.1007/s12011-014-9978-7. [DOI] [PubMed] [Google Scholar]