Abstract
Background
Most lung cancer patients are diagnosed at an advanced stage with metastases. There was no population-based data on metastases in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutation. This study focused on the metastases in NSCLC patients with EGFR mutation.
Methods
In our research, we retrospectively studied 365 NSCLC patients with EGFR mutation (EGFR positive-mutant group) were not resistant to first-generation EGFR TKIs and 316 NSCLC patients with T790M mutation (T790M-mutant group) who were resistant to first-generation EGFR TKIs. In the study, we also investigated sex, smoking status, age at diagnosis, histology, T, N, and M stage, and mutation status. In addition, we analyzed metastatic sites in stage IV patients.
Results
Among the EGFR positive-mutant group, 248 (67.95%) patients were stage IV disease. Among them, 41 patients had brain metastases, 86 patients had bone metastases, 16 patients had liver metastases, 168 patients had intrapulmonary metastases, and 39 patients had metastases in other sites. Among the T790M-mutant group, 277 (87.66%) patients were stage IV disease. Among them, 158 patients had brain metastases, 82 patients had bone metastases, 241 patients had liver metastases, 53 patients had intrapulmonary metastases, and 229 patients had metastases in other sites. We also found that lung cancer patients in the T790M-mutant group had higher incidences of the brain (P<0.001), bone (P<0.001), liver (P=0.001), and intrapulmonary metastases (P<0.001). Moreover, wherever the metastatic site was, the metastasis time all centrally distributed in the first two months after diagnosis.
Conclusions
For patients with metastatic lung cancer, most metastases happened before diagnosis, which indicated that metastases related to driving mutations, such as EGFR positive mutation or T790M mutation, but not to the survival time. Lung cancer patients with T790M mutation were more likely to metastasize before the diagnosis.
Keywords: Non-small cell lung cancer (NSCLC), metastases, epidermal growth factor receptor (EGFR), T790M
Introduction
The mortality rate of lung cancer increases year by year (1,2). The annual diagnosis rate of new cases is approximately 1.6 million all over the world (3,4). About 85% of lung cancer patients are diagnosed as non-small cell lung cancer (NSCLC) (5). A total of 47.3% of NSCLC patients presented with metastases at the time of diagnosis (6). Distant metastasis is the leading cause of most cancer deaths. NSCLC metastasizes to the brain (47%), bone (36%), liver (22%), adrenal glands (15%), thoracic cavity (11%), distant lymph nodes (10%), and other organs (less than 5%), which leads to shorter survival (7,8). Metastasis seems to be a random process, which has not well qualified. Some researchers put forward an opinion that the oncogenic drivers, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS1 proto-oncogene receptor tyrosine kinase, may induce the metastases (9-12). EGFR tyrosine kinase inhibitors (TKIs) are effective in treating NSCLC with EGFR mutation. However, some patients may have the mutation that substitutes methionine for threonine at amino acid position 790 (T790M) after being treated with first-generation TKI (13). T790M mutation inhibits first-generation TKI to its binding site, and the resistance to first-generation TKI arises. Some reviews have illustrated the relationship between T790M and the development of resistance to first-generation TKI (14,15). Whether patients with T790M mutation are more likely to have metastases remains unknown. In this study, we analyzed the time distribution of lung cancer distant metastases and the correlation between EGFR mutation, T790M mutation and lung cancer metastases.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/atm-20-2925).
Methods
We collected the data retrospectively from the clinical records of patients with lung cancer and metastases diagnosed at the Oncology Department of Shanghai Pulmonary Hospital. After admission, performed systemic bone image, brain MRI, abdominal MRI, or color Doppler ultrasonography, and chest computed tomography (CT) on patients diagnosed with lung cancer every six to eight weeks in case of metastasis. We enrolled a total of 681 lung cancer patients with EGFR mutant who had provided their written consent in the study. Among them, 316 patients from Feb 2001 to Dec 2016 were enrolled. After treating with first-generation EGFR TKIs, such as erlotinib and gefitinib, we observed T790M mutation in these patients. We also enrolled another 365 patients diagnosed from June 2018 to May 2019 who were all treated with the first-generation EGFR TKIs. However, unlike the last 316 patients, these patients did not get resistance to the first-generation EGFR TKIs when we analyzed the data. Their metastatic sites included the brain, liver, bone, contralateral lung, pleural, pleural effusion, adrenal gland, pericardium, abdominal cavity, subcutaneous tissue, and lymph nodes in the cervical, retroperitoneal, and inguinal regions. For analysis, besides the brain, the liver, and the bone, we divided the rest of the sites into two parts: intrapulmonary metastases (contralateral lung, pleural, pleural effusion) and other sites metastases (adrenal gland, pericardium, abdominal cavity, subcutaneous tissue, and lymph nodes). Some patients suffered from two or more metastases and were respectively analyzed in each metastatic site. We collected information from the diagnostic imaging, pathology reports, physician notes, and treatment information to identify the confirmed diagnosis date and the metastasis date. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by Ethics Committee of Shanghai Pulmonary Hospital. The ethics reference number was NO K18-203.
Statistical analysis
We tableted data according to the information and used a histogram to visualize the distribution of the time between the confirmed diagnosis date and the metastasis date (metastasis time). Statistical analysis performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Enumeration data expressed as number were analyzed by Chi-square test. Statistical significance was considered as P value less than 0.05.
Results
In the T790M-mutant group, most patients were adenocarcinoma (88.6%), and 119 (37.7%) patients had brain metastases, 195 (61.7%) patients had bone metastases, 36 (11.4%) patients had liver metastases, 224 (70.9%) patients had intrapulmonary metastases, and 48 (15.2%) patients had other metastatic sites.
In the EGFR positive-mutant group, 54.2% of them were adenocarcinoma, and 41 (11.2%) patients had brain metastases, 86 (23.6%) patients had bone metastases, 16 (4.4%) patients had liver metastases, 169 (46.3%) patients had intrapulmonary metastases, and 39 (10.7%) patients had other sites metastases. The pathological results of 23.8% patients only suggested NSCLC, since some patients were just tested by cytology rather than immunohistochemistry. Patient characteristics were summarized in Table 1 and Table 2. (The sum of patients with different metastatic sites exceeds the total number of patients because patients with different metastases were counted for each site independently.)
Table 1. Patient Characteristics of T790M mutant group.
| Patient characteristics | Total | Metastases | Brain metastases | Bone metastases | Liver metastases | Intrapulmonary metastases | Other sites metastases | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | |||||||
| Total | 316 | 277 (87.7) | 39 (12.3) | 119 (37.7) | 197 (62.3) | 121 (38.3) | 121 (38.3) | 36 (11.4) | 280 (88.6) | 224 (70.9) | 92 (29.1) | 48 (15.2) | 268 (84.8) | |||||||||||
| Age | 0.002 | 0.004 | 0.022 | 0.011 | 0.435 | 0.013 | ||||||||||||||||||
| <70 | 246 | 223 (90.7) | 23 (9.3) | 103 (41.9) | 143 (58.1) | 86 (35.0) | 86 (35.0) | 34 (13.8) | 212 (86.2) | 177 (72.0) | 69 (28.0) | 44 (17.9) | 202 (82.1) | |||||||||||
| ≥70 | 70 | 54 (77.1) | 16 (22.9) | 16 (22.9) | 54 (77.1) | 35 (50.0) | 35 (50.0) | 2 (2.9) | 68 (97.1) | 47 (67.1) | 23 (32.9) | 4 (5.7) | 66 (94.3) | |||||||||||
| Sex | 0.128 | 0.583 | 0.17 | 0.077 | 0.141 | 0.726 | ||||||||||||||||||
| Male | 131 | 116 (88.5) | 15 (11.5) | 47 (35.9) | 84 (64.1) | 56 (42.7) | 56 (42.7) | 10 (7.6) | 121 (92.4) | 87 (66.4) | 44 (33.6) | 21 (16.0) | 110 (84.0) | |||||||||||
| Female | 185 | 161 (87.0) | 24 (13.0) | 72 (38.9) | 113 (61.1) | 65 (35.1) | 65 (35.1) | 26 (14.1) | 159 (85.9) | 137 (74.1) | 48 (25.9) | 27 (14.6) | 158 (85.4) | |||||||||||
| Smoking | 0.000 | 0.457 | 0.002 | 0.251 | 0.082 | 0.136 | ||||||||||||||||||
| Smoked | 57 | 42 (73.7) | 15 (26.3) | 19 (33.3) | 38 (66.7) | 32 (56.1) | 32 (56.1) | 4 (7.0) | 53 (93.0) | 35 (61.4) | 22 (38.6) | 5 (8.8) | 52 (91.2) | |||||||||||
| Non-smoked | 259 | 235 (90.7) | 24 (9.3) | 100 (38.6) | 159 (61.4) | 89 (34.4) | 89 (34.4) | 32 (12.4) | 227 (87.6) | 189 (73.0) | 70 (27.0) | 43 (16.6) | 216 (83.4) | |||||||||||
| Pathology | 0.611 | 0.032 | 0.696 | 0.327 | 0.315 | 0.348 | ||||||||||||||||||
| Squamous | 7 | 6 (85.7) | 1 (14.3) | 7 (100.0) | 3 (42.9) | 3 (42.9) | 7 (100.0) | 4 (57.1) | 3 (42.9) | 2 (28.6) | 5 (71.4) | |||||||||||||
| Non-squamous | 309 | 271 (91.2) | 26 (8.8) | 119 (40.1) | 178 (59.9) | 106 (35.7) | 106 (35.7) | 36 (12.1) | 261 (87.9) | 220 (74.1) | 77 (25.9) | 46 (15.5) | 251 (84.5) | |||||||||||
| T | 0.988 | 0.344 | 0.305 | 0.315 | 0.294 | 0.847 | ||||||||||||||||||
| 1 | 20 | 19 (95.0) | 1 (5.0) | 6 (30.0) | 14 (70.0) | 9 (45.0) | 9 (45.0) | 1 (5.0) | 19 (95.0) | 13 (65.0) | 7 (35.0) | 3 (15.0) | 17 (85.0) | |||||||||||
| 2−4 | 270 | 249 (92.2) | 21 (7.8) | 110 (40.7) | 160 (59.3) | 91 (33.7) | 91 (33.7) | 34 (12.6) | 236 (87.4) | 204 (75.6) | 66 (24.4) | 45 (16.7) | 225 (83.3) | |||||||||||
| N | 0.007 | 0.009 | 0.000 | 0.764 | 0.243 | 0.14 | ||||||||||||||||||
| 0 | 29 | 23 (79.3) | 6 (20.7) | 5 (17.2) | 24 (82.8) | 19 (65.5) | 19 (65.5) | 3 (10.3) | 26 (89.7) | 19 (65.5) | 10 (34.5) | 2 (6.9) | 27 (93.1) | |||||||||||
| 1−3 | 261 | 244 (93.5) | 17 (6.5) | 110 (42.1) | 151 (57.9) | 82 (31.4) | 82 (31.4) | 32 (12.3) | 229 (87.7) | 197 (75.5) | 64 (24.5) | 46 (17.6) | 215 (82.4) | |||||||||||
| Stage | 0.000 | 0.158 | 0.018 | 0.52 | 0.003 | 0.447 | ||||||||||||||||||
| I−II | 3 | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | ||||||||||||||||
| III−V | 297 | 277 (93.3) | 20 (6.7) | 119 (40.1) | 178 (59.9) | 102 (34.3) | 102 (34.3) | 36 (12.1) | 261 (87.9) | 224 (75.4) | 73 (24.6) | 48 (16.2) | 249 (83.8) | |||||||||||
| EGFR 19 DEL | 0.036 | 0.927 | 0.565 | 0.108 | 0.489 | 0.027 | ||||||||||||||||||
| Negative | 145 | 121 (83.4) | 24 (16.6) | 55 (37.9) | 90 (62.1) | 58 (40.0) | 58 (40.0) | 12 (8.3) | 133 (91.7) | 100 (69.0) | 45 (31.0) | 15 (10.3) | 130 (89.7) | |||||||||||
| Positive | 171 | 156 (91.2) | 15 (8.8) | 64 (37.4) | 107 (62.6) | 63 (36.8) | 63 (36.8) | 24 (14.0) | 147 (86.0) | 124 (72.5) | 47 (27.5) | 33 (19.3) | 138 (80.7) | |||||||||||
| EGFR L858R | 0.224 | 0.273 | 0.215 | 0.471 | 0.928 | 0.055 | ||||||||||||||||||
| Negative | 198 | 177 (89.4) | 21 (10.6) | 70 (35.4) | 128 (64.6) | 117 (59.1) | 81 (40.9) | 25 (12.6) | 173 (87.4) | 140 (70.7) | 58 (29.3) | 36 (18.2) | 162 (81.8) | |||||||||||
| Positive | 118 | 100 (84.7) | 18 (15.3) | 49 (41.5) | 69 (58.5) | 78 (66.1) | 40 (33.9) | 11 (9.3) | 107 (90.7) | 84 (71.2) | 34 (28.8) | 12 (10.2) | 106 (89.8) | |||||||||||
| EGFR T790M | ||||||||||||||||||||||||
| Negative | 0 | |||||||||||||||||||||||
| Positive | 316 | 277 (87.7) | 39 (12.3) | 119 (37.7) | 197 (62.3) | 121 (38.3) | 121 (38.3) | 36 (11.4) | 280 (88.6) | 224 (70.9) | 92 (29.1) | 48 (15.2) | 268 (84.8) | |||||||||||
EGFR, epidermal growth factor receptor; T790M, a mutation that substitutes methionine for threonine at amino acid position 790; 19 DEL, exon 19 deletion; L858R, substitutions of leucine for arginine in exon 21.
Table 2. Patient Characteristics of EGFR positive mutant group.
| Patient characteristics | Total | Metastases | Brain metastases | Bone metastases | Liver metastases | Intrapulmonary metastases | Other sites metastases | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | Yes, n (%) | No, n (%) | P | |||||||
| Total | 365 | 248 (69.5) | 109 (30.5) | 41 (11.2) | 324 (88.8) | 86 (23.6) | 279 (76.4) | 16 (4.4) | 349 (95.6) | 169 (46.3) | 196 (53.7) | 39 (10.7) | 326 (89.3) | |||||||||||
| Age | 0.l56 | 0.104 | 0.005 | 0.354 | 0.725 | 0.86 | ||||||||||||||||||
| <70 | 229 | 165 (72.1) | 64 (27.9) | 31 (13.5) | 198 (86.5) | 66 (28.8) | 163 (71.2) | 12 (5.2) | 217 (94.8) | 110 (48.0) | 119 (52.0) | 26 (11.4) | 203 (88.6) | |||||||||||
| ≥70 | 128 | 83 (64.8) | 45 (35.2) | 10 (7.8) | 118 (92.2) | 20 (15.6) | 108 (84.4) | 4 (3.1) | 124 (96.9) | 59 (46.1) | 69 (53.9) | 13 (10.2) | 115 (89.8) | |||||||||||
| Sex | 0.019 | 0.436 | 0.017 | 0.05 | 0.066 | 0.14 | ||||||||||||||||||
| Male | 237 | 155 (65.4) | 82 (34.6) | 25 (10.5) | 212 (89.5) | 48 (20.3) | 189 (79.7) | 7 (3.0) | 230 (97) | 104 (43.9) | 133 (56.1) | 30 (12.7) | 207 (87.3) | |||||||||||
| Female | 120 | 93 (77.5) | 27 (22.5) | 16 (13.3) | 104 (86.7) | 38 (31.7) | 82 (68.3) | 9 (7.5) | 111 (92.5) | 65 (54.2) | 55 (45.8) | 9 (7.5) | 111 (92.5) | |||||||||||
| Smoking | 0.098 | 0.481 | 0.008 | 0.454 | 0.478 | 0.259 | ||||||||||||||||||
| Smoked | 166 | 108 (65.1) | 58 (34.9) | 17 (10.2) | 149 (89.8) | 29 (17.5) | 137 (82.5) | 6 (3.6) | 160 (96.4) | 75 (45.2) | 91 (54.8) | 21 (12.7) | 145 (87.3) | |||||||||||
| Non-smoked | 190 | 139 (73.2) | 51 (26.8) | 24 (12.6) | 166 (87.4) | 56 (29.5) | 134 (70.5) | 10 (5.3) | 180 (94.7) | 93 (48.9) | 97 (51.1) | 17 (8.9) | 173 (91.1) | |||||||||||
| Pathology | 0.000 | 0.128 | 0.002 | 0.033 | 0.292 | 0.335 | ||||||||||||||||||
| Squamous | 78 | 41 (53.2) | 36 (46.8) | 5 (6.4) | 73 (93.6) | 8 (10.3) | 70 (89.7) | 78 (100.0) | 32 (41.0) | 46 (59.0) | 6 (7.7) | 72 (92.3) | ||||||||||||
| Non- squamous | 287 | 207 (73.9) | 73 (26.1) | 36 (12.5) | 251 (87.5) | 78 (27.2) | 209 (72.8) | 16 (5.6) | 271 (94.4) | 137 (47.7) | 150 (52.3) | 33 (11.5) | 254 (88.5) | |||||||||||
| T | 0.000 | 0.103 | 0.462 | 0.959 | 0.000 | 0.124 | ||||||||||||||||||
| 1 | 46 | 21 (45.7) | 25 (54.3) | 2 (4.3) | 44 (95.7) | 9 (19.6) | 37 (80.4) | 2 (4.3) | 44 (95.7) | 10 (21.7) | 36 (78.3) | 2 (4.3) | 44 (95.7) | |||||||||||
| 2−4 | 310 | 226 (72.9) | 84 (27.1) | 39 (12.6) | 271 (87.4) | 76 (24.5) | 234 (75.5) | 14 (4.5) | 296 (95.5) | 159 (51.3) | 151 (48.7) | 37 (11.9) | 273 (88.1) | |||||||||||
| N | 0.001 | 0.237 | 0.002 | 0.11 | 0.469 | 0.038 | ||||||||||||||||||
| 0 | 47 | 23 (48.9) | 24 (51.1) | 3 (6.4) | 44 (93.6) | 3 (6.4) | 44 (93.6) | 47 (100.0) | 20 (42.6) | 27 (57.4) | 1 (2.1) | 46 (97.9) | ||||||||||||
| 1−3 | 309 | 225 (72.8) | 84 (27.2) | 38 (12.3) | 271 (87.7) | 83 (26.9) | 226 (73.1) | 16 (5.2) | 293 (94.8) | 149 (48.2) | 160 (51.8) | 38 (12.3) | 271 (87.7) | |||||||||||
| Stage | 0.000 | 0.039 | 0.001 | 0.214 | 0.000 | 0.045 | ||||||||||||||||||
| I−II | 30 | 1 (3.3) | 29 (96.7) | 30 (100.0) | 30 (100.0) | 30 (100.0) | 1 (3.3) | 29 (96.7) | 30 (100.0) | |||||||||||||||
| III−IV | 326 | 247 (75.8) | 79 (24.2) | 41 (12.6) | 285 (87.4) | 86 (26.4) | 240 (73.6) | 16 (4.9) | 310 (95.1) | 168 (51.5) | 158 (48.5) | 39 (12.0) | 287 (88.0) | |||||||||||
| EGFR 19 DEL | 0.000 | 0.019 | 0.179 | 0.536 | 0.000 | 0.713 | ||||||||||||||||||
| Negative | 298 | 188 (64.8) | 102 (35.2) | 28 (9.4) | 270 (90.6) | 66 (22.1) | 232 (77.9) | 14 (4.7) | 284 (95.3) | 124 (41.6) | 174 (58.4) | 31 (10.4) | 267 (89.6) | |||||||||||
| Positive | 67 | 60 (89.6) | 7 (10.4) | 13 (19.4) | 54 (80.6) | 20 (29.9) | 47 (70.1) | 2 (3.0) | 65 (97.0) | 45 (67.2) | 22 (32.8) | 8 (11.9) | 59 (88.1) | |||||||||||
| EGFR T790M | − | |||||||||||||||||||||||
| Negative | 365 | 248 (69.5) | 109 (30.5) | 41 (11.2) | 324 (88.8) | 86 (23.6) | 279 (76.4) | 16 (4.4) | 349 (95.6) | 169 (46.3) | 196 (53.7) | 39 (10.7) | 326 (89.3) | |||||||||||
| Positive | 0 | |||||||||||||||||||||||
EGFR, epidermal growth factor receptor; T790M, a mutation that substitutes methionine for threonine at amino acid position 790; 19 DEL, exon 19 deletion; L858R, substitutions of leucine for arginine in exon 21.
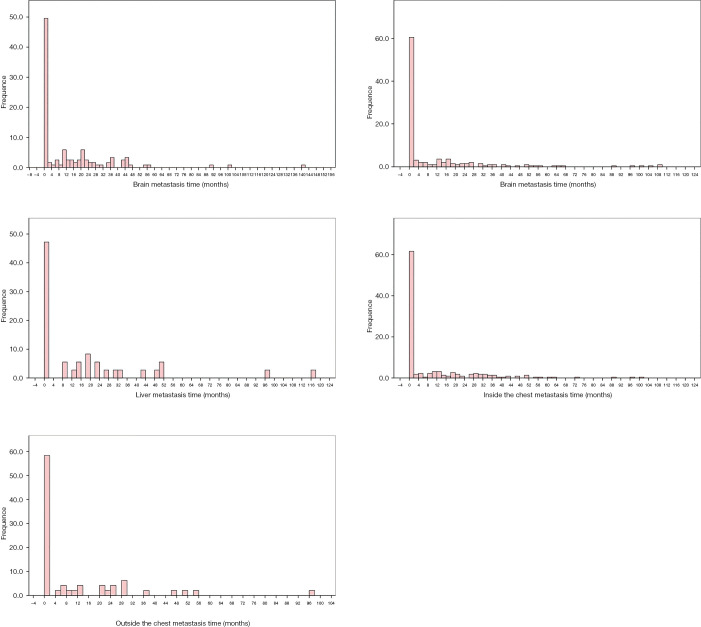
We obtained the metastasis time in months of the T790M-mutant group by calculating the length of time between the confirmed diagnosis date and the metastasis date. We made histograms to analyze better the distribution of metastasis time of different sites (Figure 1). In patients with metastases, no matter the metastatic site was brain, bone, liver, intrapulmonary, or other sites, the metastases more likely happened before the diagnosis, suggesting that in patients with metastatic lung cancer, most metastases were detected at first diagnosis.
Figure 1.
The distribution of metastasis time of different sites in T790M group. The metastases most happened before the diagnosis, suggesting that in patients with metastatic lung cancer, most metastases were detected at first diagnosis.
Nearly 49.6% of the patients with brain metastasis were found to have metastasis before or in the first two months after diagnosis, far more than the number of patients whose metastasis time was distributed at another periods. For bone, liver, inside the chest and outside the chest, the proportion of patients whose metastasis time spread before or in the first two months after diagnosis was 60.5%, 42.2%, 61.6%, and 58.3%, respectively, which was far more than those at another period.
To find whether T790M is related to lung cancer metastases, we used the chi-square test to determine whether there was a difference between the T790M-mutant group and EGFR positive-mutant group.
There were 87.7% of patients in the T790M-mutant group and 69.7% of patients in the EGFR positive-mutant group with metastasis. There was a statistically significant difference between the two groups in metastasis (P<0.001) (Table 3).
Table 3. Analysis of the association between metastases and T790M.
| T790M mutant group | EGFR positive mutant group | χ2 | p | |
|---|---|---|---|---|
| No metastases, n (%) | 39 (12.3) | 108 (30.3) | 31.721 | <0.001 |
| With metastases, n (%) | 277 (87.7) | 248 (69.7) | ||
| No brain metastases, n (%) | 197 (62.3) | 324 (88.8) | 65.798 | <0.001 |
| With brain metastases, n (%) | 119 (37.7) | 41 (11.2) | ||
| No bone metastases, n (%) | 121 (38.3) | 279 (76.4) | 101.692 | <0.001 |
| With bone metastases, n (%) | 195 (61.7) | 86 (23.6) | ||
| No liver metastases, n (%) | 280 (88.6) | 349 (95.6) | 11.797 | 0.001 |
| With liver metastases, n (%) | 36 (11.4) | 16 (4.4) | ||
| No intrapulmonary metastases, n (%) | 92 (29.1) | 196 (53.7) | 41.944 | <0.001 |
| With intrapulmonary metastases, n (%) | 224 (70.9) | 169 (46.3) | ||
| No other sites metastases, n (%) | 268 (84.8) | 326 (89.3) | 3.085 | 0.079 |
| With other sites metastases, n (%) | 48 (15.2) | 39 (10.7) |
EGFR, epidermal growth factor receptor; T790M, a mutation that substitutes methionine for threonine at amino acid position 790.
There were 37.7% of patients in the T790M-mutant group and 11.2% of patients in the EGFR positive-mutant group with brain metastasis. There was a statistically significant difference between the two groups in brain metastasis (P<0.001; Table 3).
There were 61.7% of patients in the T790M-mutant group and 31.6% of patients in the EGFR positive-mutant group with bone metastasis. There was a statistically significant difference between the two groups in bone metastasis (P<0.001) (Table 3).
There were 11.4% of patients in the T790M-mutant group and 4.4% of patients in the EGFR positive-mutant group with liver metastasis. There was a statistically significant difference between the two groups in liver metastasis (P=0.001; Table 3).
There were 70.9% of patients in the T790M-mutant group and 46.3% of patients in the EGFR positive-mutant group with intrapulmonary metastasis. There was a statistically significant difference between the two groups in intrapulmonary metastasis (P<0.001; Table 3).
Discussion
The metastasis and the resistance against treatment make lung cancer the leading cause of cancer-related death. EGFR is one of the most essential driver genes in both pathological and cancerous processes (2,16). When EGFR binds to a ligand, the downstream signal is activated, mediating proliferation, migration, invasion and suppression of apoptosis (17). The overactivation and mutation of EGFR signaling were proved to be related to poor prognosis in lung cancer (18,19).
Patients with different characteristics are prone to different metastatic sites. Carcinoembryonic antigen (20), size of the tumor, nodal stage, adenocarcinoma (21), presence of bone metastases (22), and EGFR mutation (23), might be the predictive factors for brain metastases. Besides, high serum level of hepatoma-derived growth factor (HDGF) might correlate to bone metastasis (24). Researchers showed that histology, age at diagnosis, and sex influenced on the pattern of metastasis. Women, younger patients, and SCLC patients were more likely to have metastases. Patients with liver or bone metastases had a shorter survival time (7). It was reported that lung adenocarcinoma patients with EGFR mutations were more likely to have distant metastases (25). T790M mutation is a common EGFR mutation in patients with resistance to first-generation EGFR-TKIs. However, the correlation between T790M and metastases remains unclear. Our research found that lung cancer patients with T790M mutation were more likely to have metastases, especially brain metastases, bone metastases, liver metastases, and intrapulmonary metastases. However, the mechanism was still unclear, and further investigation was indispensable, which might be necessary for finding new methods to restrict the development of metastases.
EGFR exon 19 deletion and L858R are driver mutations in NSCLC. EGFR TKIs are effective in treating EGFR positive lung cancer. Comparing with conventional cytotoxic chemotherapy, target therapy improved the overall survival (OS) and reduced the side effects of treatments in lung cancer patients with EFGR mutation (26). The median OS of advanced NSCLC patients treated with combination chemotherapy was 8 to 12 months, and the median progression-free survival (PFS) was 5 to 6 months (27-29). While treated with target therapy, the median OS was 20 to 30 months, and the median PFS was 10 to 14 months (10,30-34). Unfortunately, within a median period of 10–14 months, acquired resistance to first- and second-generation EGFR TKIs happened (35). The occurrence of EGFR T790M mutation in exon 20 was the most common mechanism of EGFR TKI resistance (36-38) Third-generation EGFR TKIs showed significant efficacy in preclinical studies for patients with T790M mutation (39-42). The use of target therapy had extended lung cancer patient life. The metastasis was an essential factor leading to poor prognosis of lung cancer patients with metastasis. Did the metastasis of lung cancer result from the longer lifetime of patients or the drive mutation? The results of our research might answer. From our results, wherever cancer metastasized, the time of metastasis largely concentrated before diagnosis or in the first two months after diagnosis. Even patient lives were prolonged, the metastases were happened before diagnosis or in the first two months after diagnosis. Although these patients all accepted the targeted therapy, the metastasis time seemed not to be related to the longer lifetime. Based on our research, we found that NSCLC patients with T790M had a higher incidence of metastases.
Despite these significant findings, there were also limitations in our study. First, our data were not large enough. Second, this was a retrospective study. Third, the mechanism of the correlation between T790M and metastases still needs more in-depth exploration.
Conclusions
For T790M-mutant patients with metastatic lung cancer, most metastases were detected before diagnosis or in the first two months after diagnosis, which certified that the metastases not related to the prolonged lifetime of patients or the use of target therapy. Moreover, NSCLC patients with T790M mutation had a higher incidence of metastases. We should conduct further studies to explore the mechanism of the correlation between T790M and metastases.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported in part by a grant of young talents in Shanghai, National Natural Science Foundation of China (81802255), Young Talents in Shanghai (2019 QNBJ), ‘Dream Tutor’ Outstanding Young Talents Program (fkyq1901), Clinical Research Project of Shanghai Pulmonary Hospital (fk18005), Key Discipline in 2019 (oncology), Project of Shanghai Municipal Science and Technology Commission (Project of Municipal Science and Technology Commission), Scientific research project of Shanghai Pulmonary Hospital (fkcx1903), Shanghai Municipal Commission of Health and Family Planning (2017YQ050), Innovation Training Project of SITP of Tongji University, and key projects of leading talent (19411950300).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shanghai Pulmonary Hospital (NO.: NO K18-203). Written informed consents were obtained from the patients for publication of this study and any accompanying images.
Footnotes
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/atm-20-2925
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2925
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-2925
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2925). The authors have no conflicts of interest to declare.
References
- 1.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin 2015;65:30-54. 10.3322/caac.21261 [DOI] [PubMed] [Google Scholar]
- 2.Dopeso H, Jiao HK, Cuesta AM, et al. PHD3 Controls Lung Cancer Metastasis and Resistance to EGFR Inhibitors through TGFα. Cancer Res 2018;78:1805-19. 10.1158/0008-5472.CAN-17-1346 [DOI] [PubMed] [Google Scholar]
- 3.Evans M. Lung cancer: needs assessment, treatment and therapies. Br J Nurs 2013;22:S15-22. 10.12968/bjon.2013.22.Sup17.S15 [DOI] [PubMed] [Google Scholar]
- 4.Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ 2015;18:312-22. 10.3111/13696998.2014.1003644 [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Wang P, Zhang C, et al. Epidermal growth factor receptor (EGFR): A rising star in the era of precision medicine of lung cancer. Oncotarget 2017;8:50209-20. 10.18632/oncotarget.16854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol 2015;3:217-21. 10.3892/mco.2014.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 8.Niu FY, Zhou Q, Yang JJ, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer 2016;16:149. 10.1186/s12885-016-2169-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.In G, Mason J, Lin S, Newton PK, et al. Development of metastatic brain disease involves progression through lung metastases in EGFR mutated non-small cell lung cancer. Converg Sci Phys Oncol 2017;3:034001. 10.1088/2057-1739/aa7a8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 12.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. 10.1200/JCO.2011.35.6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Z, Cheng Y, Chen Y, et al. EGFR T790M ctDNA testing platforms and their role as companion diagnostics: Correlation with clinical outcomes to EGFR-TKIs. Cancer Lett 2017;403:186-94. 10.1016/j.canlet.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Nagano T, Tachihara M, Nishimura Y. Mechanism of Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors and a Potential Treatment Strategy. Cells 2018;7:212. 10.3390/cells7110212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SM, Syn NL, Cho BC, et al. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev 2018;65:1-10. 10.1016/j.ctrv.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Che TF, Lin CW, Wu YY, et al. Mitochondrial translocation of EGFR regulates mitochondria dynamics and promotes metastasis in NSCLC. Oncotarget 2015;6:37349-66. 10.18632/oncotarget.5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge M, Zhuang Y, Zhou X, et al. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol 2017;135:413-8. 10.1007/s11060-017-2590-x [DOI] [PubMed] [Google Scholar]
- 18.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001;37 Suppl 4:S9-15. 10.1016/S0959-8049(01)00231-3 [DOI] [PubMed] [Google Scholar]
- 19.Suda K, Mitsudomi T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol 2015;89:1227-40. 10.1007/s00204-015-1524-7 [DOI] [PubMed] [Google Scholar]
- 20.Lee DS, Kim YS, Jung SL, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumour Biol 2012;33:1065-73. 10.1007/s13277-012-0344-0 [DOI] [PubMed] [Google Scholar]
- 21.Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882-8. 10.1148/radiol.2423051707 [DOI] [PubMed] [Google Scholar]
- 22.Na II, Lee TH, Choe DH, et al. A diagnostic model to detect silent brain metastases in patients with non-small cell lung cancer. Eur J Cancer 2008;44:2411-7. 10.1016/j.ejca.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. 10.1097/JTO.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Liu Z, Chen Y, et al. High Serum HDGF Levels Are Predictive of Bone Metastasis and Unfavorable Prognosis in Non-Small Cell Lung Cancer. Tohoku J Exp Med 2017;242:101-8. 10.1620/tjem.242.101 [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto D, Ueda H, Shimizu R, et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: importance of bone metastasis. Clin Exp Metastasis 2014;31:543-51. 10.1007/s10585-014-9648-3 [DOI] [PubMed] [Google Scholar]
- 26.Mayekar MK, Bivona TG. Current Landscape of Targeted Therapy in Lung Cancer. Clin Pharmacol Ther 2017;102:757-64. 10.1002/cpt.810 [DOI] [PubMed] [Google Scholar]
- 27.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 28.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 29.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 30.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 31.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 32.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 33.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 34.Lin JJ, Cardarella S, Lydon CA, et al. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol 2016;11:556-65. 10.1016/j.jtho.2015.12.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao X, Le X, Costa DB. The safety and efficacy of osimertinib for the treatment of EGFR T790M mutation positive non-small-cell lung cancer. Expert Rev Anticancer Ther 2016;16:383-90. 10.1586/14737140.2016.1162103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji W, Choi CM, Rho JK, et al. Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer 2013;13:606. 10.1186/1471-2407-13-606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. 10.1158/2159-8290.CD-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. 10.1056/NEJMoa1411817 [DOI] [PubMed] [Google Scholar]
- 41.Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. 10.1056/NEJMoa1413654 [DOI] [PubMed] [Google Scholar]
- 42.Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov 2013;3:1404-15. 10.1158/2159-8290.CD-13-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as