Abstract
Background
Gestational diabetes mellitus (GDM) is among the most common metabolic diseases during pregnancy and inevitably leads to maternal and fetal complications. Hyperglycemia results in injury to vascular endothelial cells, including monocyte-endothelial adhesion, which is considered to be the initiating factor of vascular endothelial cell injury. Connexin 43 (Cx43) plays a key role in this adhesion process. Therefore, this study aimed to explore the effects of Cx43 on monocyte-endothelial adhesion in GDM-induced injury of vascular endothelial cells.
Methods
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords from pregnant women with and without GDM. THP-1 cells (a human leukemia monocytic cell line) adhering to HUVECs, related molecules [intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1)], and the activity of the phosphoinositide 3-kinase/protein kinase B/Nuclear factor- kappa B (PI3K/AKT/NF-κB) signaling pathway were compared between the normal and GDM-HUVECs. Oleamide and specific small interfering ribonucleic acids (siRNAs) were used to inhibit Cx43 expression in GDM-HUVECs to observe the effects of Cx43 on the adhesion of THP-1 cells and HUVECs.
Results
A much higher number of THP-1 cells adhered to GDM-HUVECs than to normal HUVECs. This was accompanied by an increased expression of Cx43, ICAM-1, and VCAM-1, as well as activation of the PI3K/AKT/NF-κB signaling pathway. After the inhibition of Cx43 expression in GDM-HUVECs with oleamide and specific siRNA, THP-1-HUVEC adhesion, ICAM-1 and VCAM-1 expression, and activation of PI3K/AKT/NF-κB signaling pathway were all attenuated. Hyperglycemia was able to increase expression of Cx43 in HUVECs.
Conclusions
For the first time, Cx43 expression was found to be substantially higher in GDM-HUVECs than in normal HUVECs. Hyperglycemia caused the overexpression of Cx43 in HUVECs, which resulted in the activation of the PI3K/AKT/NF-κB signaling pathway and the increase of its downstream adhesion molecules, including ICAM-1 and VCAM-1, ultimately leading to increased monocyte-endothelial adhesion.
Keywords: Connexin 43 (Cx43), hyperglycemia, gestational diabetes mellitus (GDM), monocyte-endothelial adhesion
Introduction
Gestational diabetes mellitus (GDM) is defined as “diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes prior to gestation” (1-3). It is among the most common metabolic disease during pregnancy. The risks of severe complications, including cesarean delivery, shoulder dystocia, macrosomia, and even long-term negative effects on glucose tolerance in the offspring, are increased for pregnant women with GDM (4-6). Notably, women with GDM are also more likely to suffer from endothelial dysfunction, vascular injury, and even cardiovascular diseases (CVD), such as atherosclerosis (7). Therefore, a deeper understanding of the underlying mechanisms of endothelial dysfunction in GDM is critical.
Previous studies have confirmed that long-term hyperglycemia not only leads to the overexpression of reactive oxygen species (ROS) and inhibition of nitric oxide (NO) activity, but also to the activation of signaling pathways including the protein kinase C (PKC) and nuclear factor-kappa B (NF-κB) pathways, which can regulate the expression of related genes and cause further vascular endothelial cell failure and even atherosclerosis (8,9). This hyperglycemia-induced change may even lead to increased monocyte-endothelial cell adhesion; an important indicator of vascular endothelial cell injury. Increased monocyte-endothelial adhesion causes adherent monocytes to release a large number of chemokines and inflammatory factors, resulting in self-reinforcing cell adhesion and further damage to vascular endothelial cells, which can even initiate atherosclerosis. In summary, if monocyte-endothelial adhesion is not well controlled, endothelial cell damage will deteriorate, especially in cases of hyperglycemia. Therefore, the discovery of appropriate ways to prevent cell adhesion is of great clinical significance for protecting against vascular endothelial cell injury, which could help to reduce complications for both pregnant women with GDM and patients with diabetes.
Connexins are structurally related transmembrane proteins that assemble to form expression gap junctions and are named according to their molecular weight (e.g., Cx32, Cx40, and Cx43). Connexin 43 (Cx43) is expressed in almost every human organ and tissue, especially in the cardiovascular system (10), and is involved in various cardiovascular processes, including atherosclerosis (11,12). In our previous study, we preliminarily explained the importance of Cx43 in monocyte-endothelial adhesion (13-15). However, the mechanism of elevated Cx43 expression has yet to be elucidated. In the present study, we extracted human umbilical vein endothelial cells (HUVECs) from pregnant women with GDM for the first time, and found that Cx43 expression in GDM-HUVECs was substantially higher than that in normal HUVECs, which had an obvious effect monocyte-endothelial adhesion. More importantly, hyperglycemia was found to induce the overexpression of Cx43 in HUVECs. Therefore, we believe that hyperglycemia-induced overexpression of Cx43 is an important risk factor for vascular endothelial cell injury.
Cx43 acts as both a channel protein and as a regulatory protein. The long cytosolic C-terminus of Cx43 contains multiple domains involved in protein interactions, which allows Cx43 to interact with regulatory proteins such as proto-oncogene tyrosine-protein kinase Src (Src), protein kinase A (PKA), and protein kinase C (PKC) (16). PKA and PKC are critical in the phosphorylation of some gene regulatory proteins and the activation of specific gene transcription, such as the PI3K/protein kinase B (AKT) and NF-κB signaling pathways (15,17,18). From other aspects, both the PI3K/AKT and NF-κB signaling pathways can be activated by hyperglycemia (9,19,20). Thus, we speculated that Cx43 might play an important role in this process; namely that hyperglycemia results in an inflammatory reaction, including activation of the PI3K/AKT and NF-κB signaling pathways, the expression of its downstream adhesion-related molecules, and even monocyte-endothelial adhesion, all of which are the main research focuses in the present study (3).
In the current investigation, HUVECs were obtained from pregnant women with GDM to illustrate the effects of hyperglycemia on Cx43 expression and monocyte-endothelial adhesion. This report is the first to confirm that Cx43 expression in GDM-HUVECs is considerably higher than that in normal HUVECs, and that overexpression of Cx43 can result in increased monocyte-endothelial adhesion. It is crucial that the possible mechanisms of vascular endothelial cell injury in pregnant women with GDM are clarified. Furthermore, hyperglycemia-induced overexpression of Cx43 and increased monocyte-endothelial adhesion may also provide new evidence of endothelial cell injury in diabetic patients. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-19-4738).
Methods
Cell line and cell culture
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Chinese Clinical Trial Registry (No. ChiCTR1900025456, registered 26 August 2019, http://www.chictr.org.cn/showproj.aspx?proj=42067). Informed consent was obtained from all patients. HUVECs were extracted from 30 pregnant women including 20 women who had a normal pregnancy and 10 women who had GDM. The patients ranged in age from 25 to 35 years old. The umbilical cord was collected from each patient after written informed consent had been obtained. The patients were screened in strict accordance with GDM diagnostic criteria (2). None of the patients had tumors, blood diseases, essential diabetes, other secretory diseases, contagious diseases, or severe liver or kidney diseases.
Primary HUVECs (normal HUVECs or GDM-HUVECs) were isolated from the human umbilical vein as described below and used between three and five passages (13,21). Sterile placentas and umbilical cords were collected after cesarean section and stored in phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA, USA) at 4 °C within 2 h. The umbilical vein was cannulated on a super clean bench with a blunt 14-gauge needle clamped with hemostatic forceps. The vein was rinsed and drained, and then infused with 1% collagenase I (Sigma-Aldrich, St. Louis, MO, USA). Next, the full umbilical vein was placed in a water bath containing sterile double-distilled water (ddH2O) and incubated at 37 °C for 20 min. After incubation, the collagenase was collected, and the umbilical vein was washed twice with Media 199 Serum-Free Media (M199 SFM, Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen). Subsequently, the collected fluid was sedimented at 2,000 ×g at 37 °C for 10 min and then resuspended in M199 SFM (Invitrogen), containing 20% FBS (Invitrogen), 30 ng/mL recombinant human vascular endothelial growth factor (VEGF) (PeproTech, Rocky Hill, New Jersey, USA), and 100 U/mL penicillin-streptomycin (Invitrogen).
THP-1 cells (a human leukemia monocytic cell line; accession number: ATCC® TIB-202™, organism: homo sapiens, human, cell type: monocyte, tissue: peripheral blood) were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640 medium (Invitrogen) supplemented with 20% FBS (Invitrogen) and 100 U/mL penicillin-streptomycin (Invitrogen).
Both HUVECs and THP-1 cells were cultured at 37 °C in a 5% carbon dioxide (CO2) incubator at 90% humidity (Thermo Fisher Scientific, Waltham, MA, USA).
Cell treatments
In different experiments, HUVECs were pretreated with different concentrations of glucose. For instance, normal HUVECs (N) were pretreated with high glucose medium (30 mM for 7 days) (the N + G group), and GDM-HUVECs (GDM) were pretreated with reduced glucose medium (5.6 mM for 7 days) (the GDM-G group). Moreover, in different experiments, HUVECs were also pretreated with oleamide (50 µM for 24 h, Sigma-Aldrich), retinoic acid (RA, 10 µM for 24 h, Sigma-Aldrich), LY294002 (LY, inhibiting PI3K/AKT, 50 µM, for 24 h, Sigma-Aldrich), and Bay11-7082 (BAY, inhibiting NF-κB, 1 µM, for 24 h, Sigma-Aldrich). Each time, the corresponding solvent was dimethyl sulfoxide (DMSO, Sigma-Aldrich).
THP-1-HUVEC adhesion assays
THP-1 cells were labeled with 5 µmol/L calcein-acetoxymethyl ester (Invitrogen) and cultured at 37 °C in a 5% CO2 incubator at 90% humidity for 30 min. The labeled THP-1 cells were washed twice with PBS (Invitrogen) and resuspended in M199 medium (Invitrogen). The HUVEC medium was removed and the suspended THP-1 cells (HUVECs: THP-1 cells =30:1) were added to the confluent monolayer. Then, the plates were put back into the incubator. After 3 h, the supernatant was removed, and the plates were washed with PBS and then carefully dried with filter paper. The adherent calcein-acetoxymethyl ester labeled THP-1 cells were counted using a fluorescence microscope (Thermo Fisher Scientific). Each dish was divided into eight quadrants. The middle position (200× visual field) of each quadrant was selected for counting, and the average was taken as the number of adherent THP-1 cells from each quadrant.
Protein detection
Cx43 was tested with Western blotting and immunofluorescence. In Western blotting, Cx43 expression was tested with anti-Cx43 (1:1,000, rabbit polyclonal Cx43 antibody raised against human; #3512; Cell Signaling Technology, Danvers, MA, USA) and horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000, goat polyclonal antibody raised against rabbit IgG; #7074; Cell Signaling Technology). In immunofluorescence, the dilution of anti-Cx43 was 1:100 (rabbit polyclonal Cx43 antibody raised against human; #3512; Cell Signaling Technology).
The expression levels of VCAM-1, ICAM-1, Akt, inhibitor of nuclear factor kappa B (IκBα), transcription factor p65, phosphorylated-Akt (p-Akt), phosphorylated-IκBα (p-IκBα), and phosphorylated-p65 (p-p65) were analyzed with Western blotting. Both anti-VCAM-1 and ICAM-1 (rabbit monoclonal antibody raised against human; VCAM-1: #13662; ICAM-1: #67836; Cell Signaling Technology) were at a 1:1,000 dilution and HRP-conjugated secondary antibodies were at a 1:5,000 dilution (goat polyclonal antibody raised against rabbit IgG; #7074; Cell Signaling Technology). All of the anti-Akt, IκBα, p65, p-Akt, p-IκBα, and p-p65 were used at a 1:1,000 dilution (anti-p-Akt: rabbit monoclonal antibody raised against human, #4060, pSer473, Cell Signaling Technology; anti-Akt: rabbit monoclonal antibody raised against human, ab179463, Abcam, Cambridge, UK; anti-p-IκBα: rabbit monoclonal antibody raised against human, #2859; Cell Signaling Technology; anti-IκBα: mouse monoclonal antibody raised against human, #4814; Cell Signaling Technology; anti-p-p65: rabbit monoclonal antibody raised against human, #3033; Cell Signaling Technology; anti-p65: rabbit polyclonal antibody raised against human, ab16502, Abcam), and HRP-conjugated secondary antibodies (goat polyclonal antibody raised against rabbit IgG; #7074, Cell Signaling Technology; horse polyclonal antibody raised against mouse IgG) were at a 1:5,000 dilution. Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (rabbit monoclonal antibody raised against human; #5174; Cell Signaling Technology) was at a 1:2,000 dilution, and HRP-conjugated secondary antibody (goat polyclonal antibody raised against rabbit IgG; #7074, Cell Signaling Technology) was at a 1:5,000 dilution. All protein bands were detected using chemiluminescence (Tanon, Shanghai, CN). The sizes of protein bands were analyzed using Image J software, and the data were expressed as the value normalized to GAPDH and the control. All experiments were repeated at least three times.
Cx43 knock-down
Cx43 small interfering ribonucleic acid (siRNA) targeting the human gene (CAGUCUGCCUUUCGUUGUA) and nonspecific control siRNA (siNC) were transfected into HUVECs in the experiments. SiRNA transfection was carried out using a transfection kit (RIBOBIO, Guangzhou, CN), and the transfection efficiency was measured with Western blotting.
Detailed methods of electrophoretic mobility shift assay (EMSA) are shown in Appendix 1.
Statistical analysis
All experiments were repeated at least five times in biological replicates. Statistical analysis was performed using GraphPad Prism 7.0 software (2,365 Northside Dr. Suite 560 San Diego, CA 92108). The data were analyzed using t-test and one-way analysis of variance (ANOVA), followed by Tukey’s test for post-hoc comparison. Differences were considered significant when the two-tailed P value was <0.05.
Results
Cell adhesion and Cx43 expression were both increased in HUVECs isolated from pregnant women with GDM
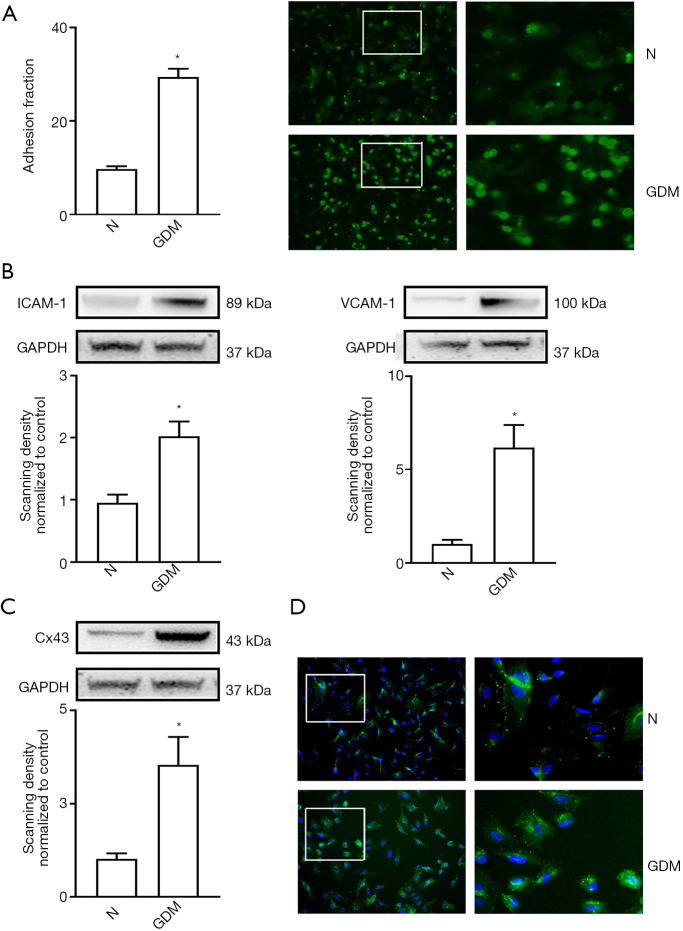
A higher number of THP-1 cells adhered to GDM-HUVECs than to normal HUVECs (Figure 1A, P<0.05). Also, the expression levels of ICAM-1 and VCAM-1 were up-regulated in GDM-HUVECs (Figure 1B, P<0.05). Two methods, Western blotting, and immunofluorescence, were used to detect the changes of Cx43 expression in HUVECs. As shown in Figure 1C,D, Cx43 expression in GDM-HUVECs was notably higher than that in normal HUVECs, indicating that the increase in GDM-induced cell adhesion might have been related to the changes of Cx43 expression in HUVECs.
Figure 1.
Cell adhesion and Cx43 expression were both increased in HUVECs isolated from pregnant women with GDM. (A) Cell adhesion was detected by THP-1 cells (green). (B) ICAM-1 and VCAM-1 protein expression in GDM-HUVECs (GDM) and normal HUVECs (N). (C,D) Cx43 protein expression in GDM-HUVECs and normal HUVECs. In all experiments, data are presented as mean ± SEM; n=9 per group, *, P<0.05 vs. normal. Scale bar in A and D: ×200 and ×400. Cx43, Connexin 43; HUVECs, human umbilical vein endothelial cells; GDM, gestational diabetes mellitus; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion protein 1.
Hyperglycemia affected Cx43 expression in HUVECs and cell adhesion.
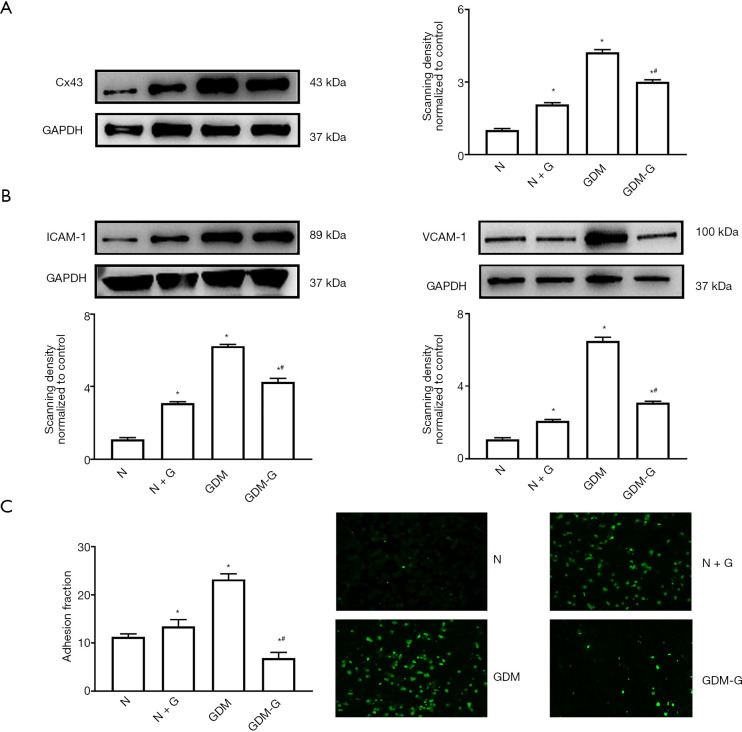
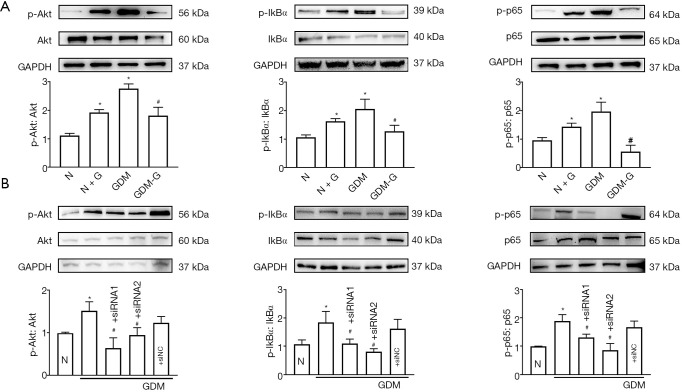
To confirm the effects of hyperglycemia on Cx43 expression and cell adhesion, the levels of glucose in the medium were changed. Exogenous glucose was used to simulate hyperglycemia. Figure 2A,B show that, when normal HUVECs were pretreated with high glucose (30 mM, 7 days), the expression levels of Cx43 and the adhesion-related molecules ICAM-1 and VCAM-1 were increased. In contrast, when GDM-HUVECs were pretreated with normal glucose (5.6 mM, 7 days), the levels of Cx43, ICAM-1, and VCAM-1 were decreased. Figure 2A,B also indicate that hyperglycemia could increase Cx43 expression in HUVECs. Furthermore, the tendency of THP-1-HUVEC adhesion was also consistent with the changes of Cx43, ICAM-1, and VCAM-1 expression in HUVECs (Figure 2C, P<0.05).
Figure 2.
Hyperglycemia affected Cx43 expression in HUVECs and cell adhesion. Normal HUVECs (N) were pretreated with high glucose (30 mM, 7 days) (N + G). Correspondingly, GDM-HUVECs (GDM) were pretreated with reduced glucose (5.6 mM, 7 days) (GDM-G). (A,B) Expression levels of Cx43, ICAM-1, and VCAM-1 proteins in HUVECs under hyperglycemia (N + G and GDM groups) and normal glucose (N and GDM-G groups). (C) Cell adhesion in the N, N + G, GDM, and GDM-G groups. In all experiments, data are presented as mean ± SEM; n=9, *, P<0.05 vs. N; #, P<0.05 vs. GDM. Scale bar in C: ×200 and ×400. Cx43, Connexin 43; HUVECs, human umbilical vein endothelial cells; GDM, gestational diabetes mellitus; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion protein 1.
Changes of Cx43 expression on GDM-HUVECs affected hyperglycemia-induced cell adhesion and ICAM-1/VCAM-1 expression.
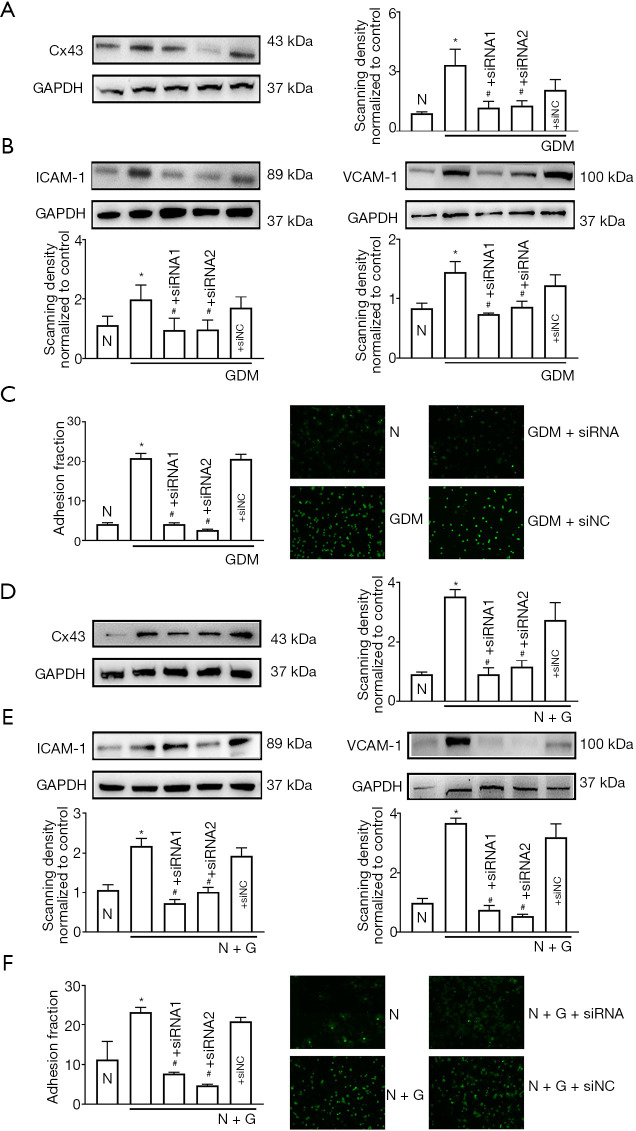
Two specific siRNAs (siRNA1 and siRNA2) targeting Cx43 were designed to knock down Cx43 expression in GDM-HUVECs. The results show that as Cx43 was knocked down specifically, hyperglycemia-induced cell adhesion, and ICAM-1 and VCAM-1 expression were markedly reduced (Figure 3A,B,C, P<0.05). Also, Figure 3D shows that these two siRNAs could also attenuate Cx43 expression in HUVECs induced by high exogenous glucose (30 mM, 7 days). More importantly, the increases in cell adhesion and ICAM-1 and VCAM-1 expression that were stimulated by exogenous high glucose (30 mM, 7 days) were observed to be decreased with the down-regulation of Cx43 (Figure 3D,E,F, P<0.05).
Figure 3.
Cx43 inhibition ameliorated hyperglycemia-induced cell adhesion and ICAM-1/VCAM-1 expression. (A) Cx43; (B) ICAM-1, VCAM-1 protein expression; (C) cell adhesion. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. N; #, P<0.05 vs. GDM. (D) Cx43; (E) ICAM-1, VCAM-1 protein expression; (F) cell adhesion. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. N; #, P<0.05 vs. N + G. Scale bar in C and F: ×200 and ×400. Cx43, Connexin 43; GDM, gestational diabetes mellitus; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion protein 1; NC: negative control.
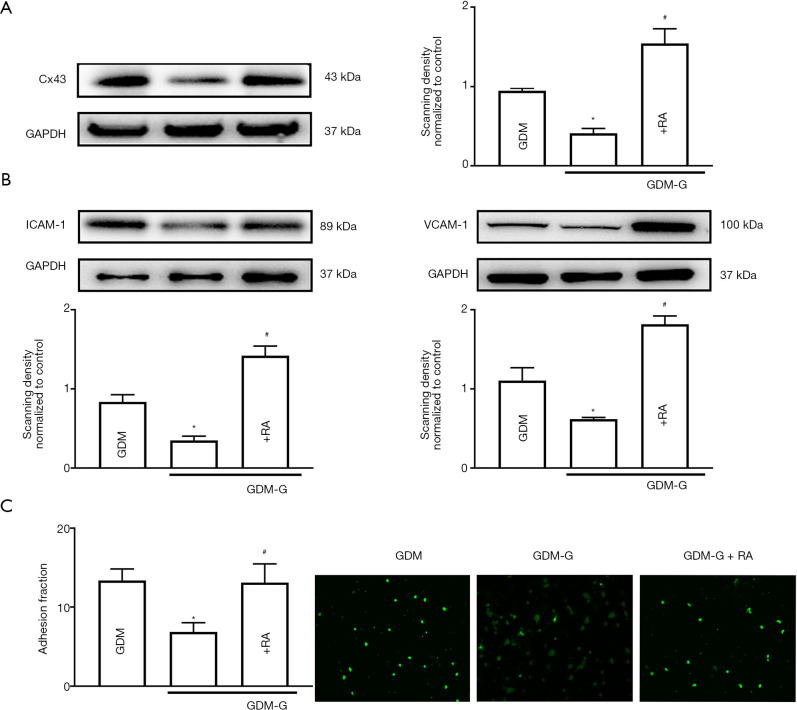
On the other hand, the Cx43 enhancer, RA, was used to up-regulate Cx43 expression in HUVECs. Figure 4A shows that when GDM-HUVECs were pretreated with normal glucose (5.6 mM, 7 days), Cx43 expression was down-regulated. However, exposure to RA could reverse the down-regulation of Cx43 expression in GDM-HUVECs. Figure 4B,C show that when GDM-HUVECs were pretreated with normal glucose (5.6 mM, 7 days), the decreases in cell adhesion and ICAM-1 and VCAM-1 expression that were induced by normal glucose (5.6 mM, 7 days) culture could be reversed by RA exposure, because Cx43 expression in GDM-HUVECs was re-upregulated.
Figure 4.
Overexpression of Cx43 reversed the protective effect of normal glucose in GDM-HUVECs. (A) Cx43; (B) ICAM-1, VCAM-1 protein expression; (C) cell adhesion. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. GDM, #, P<0.05 vs. GDM-G. Scale bar in C: ×200 and ×400. Cx43, Connexin 43; GDM, gestational diabetes mellitus; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion protein 1; RA, retinoic acid (Cx43 protein expression fortifier).
The results above (Figures 3,4) indicate that hyperglycemia could increase cell adhesion via the regulation of Cx43 expression in HUVECs.
Hyperglycemia activated the PI3K/AKT/NF-κB signaling pathway on GDM-HUVECs via regulation of Cx43
To explore the underlying mechanism of Cx43 affected ICAM-1/VCAM-1 expression, we focused primarily on the activity changes of the PI3K/AKT/NF-κB signaling pathway, which is considered to play an important role in monocyte-endothelial adhesion. As shown in Figure 5A, the key molecules of the PI3K/AKT/NF-κB signaling pathway, including p-AKT, p-IKBα, and p-p65, changed with the varying levels of glucose. When normal HUVECs were cultured with high glucose (30 mM, 7 days), the PI3K/AKT/NF-κB signaling pathway was significantly activated, which manifested as the up-regulation of p-AKT, p-IKBα, and p-p65. In contrast, when GDM-HUVECs were cultured with normal glucose (5.6 mM, 7 days), the activation of the PI3K/AKT/NF-κB signaling pathway was inhibited, which manifested as the down-regulation of p-AKT, p-IKBα, and p-p65.
Figure 5.
Glucose affected cell adhesion by activation of the PI3K/AKT/NF-κB signaling pathway on GDM-HUVECs via Cx43. (A) Expression levels of P-Akt, p-IκBα, and p-p65 protein expression changed according to the alteration of glucose levels. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. N; #, P<0.05 vs. GDM. (B) P-Akt, p-IκBα, and p-p65 protein expression were also attenuated when specific Cx43-siRNA (50 nM, 48 h) was added in GDM-HUVECs. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. control; #, P<0.05 vs. GDM. The corresponding proteins were not affected. Histograms were made according to the ratio of phosphorylated protein/total protein. Cx43, Connexin43; HUVECs, human umbilical vein endothelial cells; GDM, gestational diabetes mellitus; NC, negative control.
An EMSA using a biotinylated probe was performed to detect the transcription level of NF-κB. The results in Figure S1 show that hyperglycemia increased the combinative level between NF-κB and the promotor of ICAM-1 and VCAM-1. With the downregulation of Cx43 in GDM-HUVECs, the hyperglycemia-induced activation of the PI3K/AKT/NF-κB signaling pathway was reversed and the expression levels of p-AKT, p-IKBα, and p-p65 expression were reduced (Figure 5B). Coupled with the finding that hyperglycemia could up-regulate Cx43 expression (Figures 1,2), it can be concluded that hyperglycemia could influence the activation of the PI3K/AKT/NF-κB signaling pathway in GDM-HUVECs via Cx43.
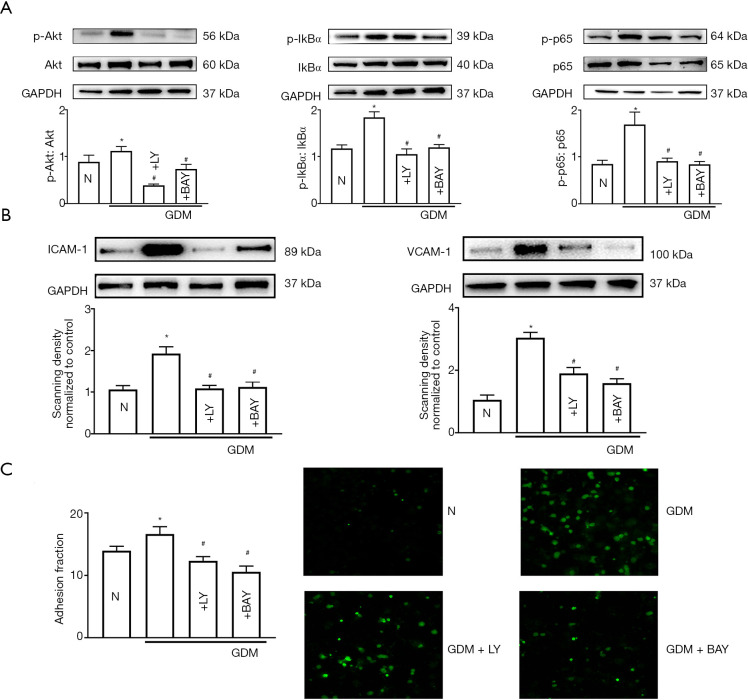
LY294002 and Bay11-7082 attenuated hyperglycemia-induced cell adhesion and ICAM-1/VCAM-1 protein
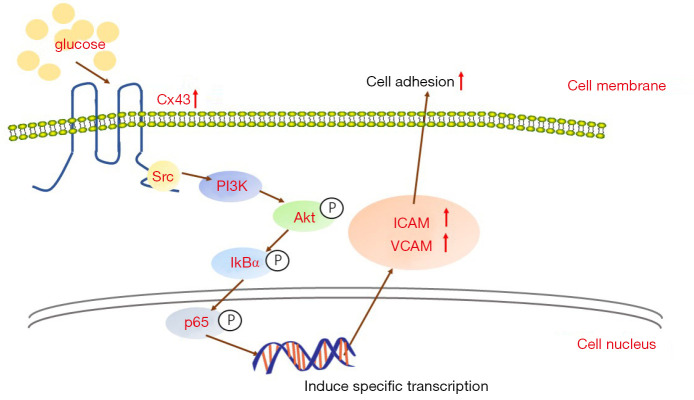
LY (inhibiting PI3K/AKT, 50 µM, for 24 h) and BAY (inhibiting NF-κB, 1 µM, for 24 h) were used to confirm the effects of the PI3K/AKT/NF-κB signaling pathway in this process. As shown in Figure 5C, the PI3K/AKT/NF-κB signaling pathway was significantly activated in GDM-HUVECs. LY and BAY could inhibit the activation of the PI3K/AKT/NF-κB signaling pathway. More importantly, with the inhibition of the PI3K/AKT/NF-κB signaling pathway using LY AND BAY, cell adhesion and the expression levels of ICAM-1 and VCAM-1 were decreased (Figure 6A,B). Therefore, it can be concluded that hyperglycemia up-regulates Cx43 protein expression, leading to the overexpression of adhesion-related molecules through the activation of the PI3K/AKT/NF-κB signaling pathway, and ultimately resulting in increased monocyte-endothelial adhesion (Figure 7).
Figure 6.
LY294002 and Bay11-7082 attenuated cell adhesion and ICAM-1/VCAM-1 protein provoked by hyperglycemia. GDM-HUVECs were pretreated with LY294002 (LY, 50 µM, 24 h) and Bay11-7082 (BAY, 1 µM, 24 h). (A) P-Akt, p-IκBα, and p-p65 protein expression. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. N; #, P<0.05 vs. GDM. Histograms were made according to the ratio of phosphorylated protein/total protein. (B) ICAM-1, VCAM-1 protein expression. (C) Cell adhesion. Data are presented as mean ± SEM; n=5, *, P<0.05 vs. N; #, P<0.05 vs. GDM. Scale bar in C: ×200 and ×400. HUVECs, human umbilical vein endothelial cells; GDM, gestational diabetes mellitus; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion protein 1.
Figure 7.
The mechanism of hyperglycemia-induced cell adhesion. The effect of hyperglycemia-induced Cx43 on cell adhesion is related to the modulation of PI3K/AKT/NF-κB signaling pathway activity and the regulation of its downstream ICAM-1 and VCAM-1 protein expression. Cx43, Connexin43; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion protein 1.
Discussion
GDM is one of the most common metabolic diseases during pregnancy and poses significant health risks (22). Although the traditional complications that can affect mothers and fetuses have been studied extensively, the effects of GDM on monocyte-endothelial adhesion have not been explored and, therefore, were the key focus the present study.
Vascular inflammation and damage caused by hyperglycemia create favorable conditions for monocyte-endothelial adhesion (14). Adhered monocytes can lead to the activation of inflammation-related signaling pathways and the mass release of cytokines, which causes further deterioration of vascular injuries and the development of related complications, such as gestational hypertension, preeclampsia, thrombus formation, and fetal hypoxia (23,24). Therefore, we speculated that if monocyte-endothelial adhesion is inhibited, then vascular injury and further complications in GDM may be prevented. In our present study, we report for the first time that hyperglycemia-induced Cx43 expression in GDM-HUVECs is substantially higher than that in normal HUVECs. Cx43 overexpression can result in the activation of the PI3K/AKT/NF-κB signaling pathway and the increase of its downstream adhesion molecules, including ICAM-1 and VCAM-1, ultimately increasing monocyte-endothelial adhesion.
GDM, diabetes and its related long-term complications, including diabetic nephropathy, foot, and cardiovascular complications, are typically accompanied by hyperglycemia and severe vascular damage. Adhered monocytes can result in the continuous aggravation of vascular damage via activation of inflammation-related signaling pathways or the release of cytokines (25). Therefore, we speculate that monocyte-endothelial adhesion plays an important role in these processes. In our present study, we also focused on the effects of hyperglycemia on monocyte-endothelial adhesion and confirmed that hyperglycemia can enhance monocyte-endothelial adhesion via regulation of Cx43 expression in HUVECs. Based on these results, we propose a novel perspective regarding the initiation of hyperglycemia-induced vascular endothelial injury, and offer a new target for the prevention and treatment of GDM and diabetes, and their related complications (26).
Currently, the understanding of Cx43 in hyperglycemia or diabetes is extremely limited; however, in recent years, it has attracted increasing attention. Mugisho et al. demonstrated that the inhibition of Cx43 could improve diabetic retinopathy. Furthermore, malfunctioning cardiac Cx43 signaling was found to play an important role in myocardial dysfunction in diabetic subjects, and the up-regulation of Cx43 in glomerular mesangial cells and diabetic mice was shown to ameliorate experimental diabetes-induced renal oxidative stress and fibronectin (27,28). For the first time, our research team clarified that Cx43 in hyperglycemia could induce monocyte-endothelial adhesion. Therefore, Cx43 might be a new potential target for the treatment of hyperglycemia and its related complications.
Like a cell membrane reactive protein, the carboxyl-terminal domain of Cx43 not only contains some phosphorylation sites itself but also interacts with some special elements of cellular signaling pathways, such as NF-κB, Wingless/Integrated (Wnt), and Src, which has been demonstrated that Cx43 expression alternation could influence its downstream signaling pathways (15). Cx43 expression on membranes mediates the effective signal transduction from extracellular to intracellular. Numerous studies have reported that the NF-κB signaling pathway involves a variety of inflammatory processes that can interact with Cx43 (29,30). Moreover, the NF-κB signaling pathway is also reported to play a key role in regulating the expression of adhesion-related molecules, including ICAM-1 and VCAM-1. Besides the activity of NF-κB can be manipulated through the PI3K/AKT signaling pathway (31,32), we explored the interaction between Cx43 and the PI3K/AKT/NF-κB signaling pathway in monocyte-endothelial adhesion and the effects of hyperglycemia in this process. Our results show that hyperglycemia can result in the activation of the PI3K/AKT and NF-κB signaling pathways via the regulation of Cx43 expression in HUVECs. With the activation of the PI3K/AKT and NF-κB signaling pathways, the adhesion-related molecules ICAM-1 and VCAM-1 become up-regulated, resulting in increased monocyte-endothelial adhesion.
GDM and diabetes mellitus have been widely studied, and one of the most important pathological features is vascular injury (5). Monocyte-endothelial adhesion results in the deterioration of vascular damage, and is sometimes considered to initiate vascular damage (14). Thus, in this study, HUVECs were obtained from pregnant women with and without GDM to evaluate the effects of hyperglycemia on monocyte-endothelial adhesion. Based on our results, we conclude that Cx43 expression in HUVECs is significantly induced by hyperglycemia. This triggers the activation of intracellular PI3K/AKT/NF-κB signaling pathways, up-regulating the expression of ICAM-1 and VCAM-1, and ultimately resulting in greater monocyte-endothelial adhesion. Through our current investigation of Cx43, we not only provide a novel idea for the hyperglycemia-induced initiation of vascular endothelial injury, but also offer a new perspective regarding vascular damage in GDM.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Guangdong Basic and Applied Basic Research Foundation (grant number, 2019A1515010093), the National Natural Science Foundation of China (grant number, 81871597, 82072216), the Guangdong Medical Research Foundation (grant number, A2017042) and the Natural Science Foundation of Guangdong Province, China (grant number, 2017A030313492).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Chinese Clinical Trial Registry (No. ChiCTR1900025456, registered 26 August 2019, http://www.chictr.org.cn/showproj.aspx?proj=42067). Written informed consent was obtained from the patients for publication of this study and any accompanying images.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-19-4738
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-19-4738
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-19-4738). The authors have no conflicts of interest to declare.
(English Language Editors: A. Kassem and J. Reynolds)
References
- 1.Chiefari E, Arcidiacono B, Foti D, et al. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest 2017;40:899-909. 10.1007/s40618-016-0607-5 [DOI] [PubMed] [Google Scholar]
- 2.Classification and Diagnosis of Diabetes . Diabetes Care 2019;42:S13-28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 3.Bai R, Cui Z, Ma Y, et al. The NF-κB-modulated miR-19a-3p enhances malignancy of human ovarian cancer cells through inhibition of IGFBP-3 expression. Mol Carcinog 2019;58:2254-65. 10.1002/mc.23113 [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773-9. 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 5.Sallam NA, Palmgren VAC, Singh RD, et al. Programming of Vascular Dysfunction in the Intrauterine Milieu of Diabetic Pregnancies. Int J Mol Sci 2018;19:3665. 10.3390/ijms19113665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe WL, Scholtens DM, Kuang A, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019;42:372-80. 10.2337/dc18-1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj HS, Ye C, Hanley AJ, et al. Biomarkers of vascular injury and endothelial dysfunction after recent glucose intolerance in pregnancy. Diab Vasc Dis Res 2018;15:449-57. 10.1177/1479164118779924 [DOI] [PubMed] [Google Scholar]
- 8.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351-66. 10.1210/er.2007-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813-20. 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- 10.Dbouk HA, Mroue RM, El-Sabban ME, et al. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal 2009;7:4. 10.1186/1478-811X-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel S, Chanson M, Nguyen TD, et al. Titration of the gap junction protein Connexin43 reduces atherogenesis. Thromb Haemost 2014;112:390-401. 10.1160/TH13-09-0773 [DOI] [PubMed] [Google Scholar]
- 12.Boengler K, Schulz R. Connexin 43 and Mitochondria in Cardiovascular Health and Disease. Adv Exp Med Biol 2017;982:227-46. 10.1007/978-3-319-55330-6_12 [DOI] [PubMed] [Google Scholar]
- 13.Yuan D, Wang Q, Wu D, et al. Monocyte-endothelial adhesion is modulated by Cx43-stimulated ATP release from monocytes. Biochem Biophys Res Commun 2012;420:536-41. 10.1016/j.bbrc.2012.03.027 [DOI] [PubMed] [Google Scholar]
- 14.Yuan D, Sun G, Zhang R, et al. Connexin 43 expressed in endothelial cells modulates monocyte endothelial adhesion by regulating cell adhesion proteins. Mol Med Rep 2015;12:7146-52. 10.3892/mmr.2015.4273 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhang Q, Zhang R, et al. Down-regulation of Cx43 expression on PIH-HUVEC cells attenuates monocyte-endothelial adhesion. Thromb Res 2019;179:104-13. 10.1016/j.thromres.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 16.Leithe E, Mesnil M, Aasen T. The connexin 43 C-terminus: A tail of many tales. Biochim Biophys Acta Biomembr 2018;1860:48-64. 10.1016/j.bbamem.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Fan Y, Fan W, et al. RNASET2 impairs the sperm motility via PKA/PI3K/calcium signal pathways. Reproduction 2018;155:383-92. 10.1530/REP-17-0746 [DOI] [PubMed] [Google Scholar]
- 18.Law NC, White MF, Hunzicker-Dunn ME. G protein-coupled receptors (GPCRs) That Signal via Protein Kinase A (PKA) Cross-talk at Insulin Receptor Substrate 1 (IRS1) to Activate the phosphatidylinositol 3-kinase (PI3K)/AKT Pathway. J Biol Chem 2016;291:27160-9. 10.1074/jbc.M116.763235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott C, Jacobs K, Haucke E, et al. Role of advanced glycation end products in cellular signaling. Redox Biol 2014;2:411-29. 10.1016/j.redox.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Liu G, Guo J, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 2018;14:1483-96. 10.7150/ijbs.27173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azcutia V, Abu-Taha M, Romacho T, et al. Inflammation determines the pro-adhesive properties of high extracellular d-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS One 2010;5:e10091. 10.1371/journal.pone.0010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47. 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- 23.Li S, Zhang Y, Sun Y, et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes 2019;9:28. 10.1038/s41387-019-0095-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khambule L, George JA. The Role of Inflammation in the Development of GDM and the Use of Markers of Inflammation in GDM Screening. Adv Exp Med Biol 2019;1134:217-42. 10.1007/978-3-030-12668-1_12 [DOI] [PubMed] [Google Scholar]
- 25.Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab 2017;102:4343-410. 10.1210/jc.2017-01922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu H, Sun H, Wang X. Neogenin-1 Promotes Cell Proliferation, Motility, and Adhesion by Up-Regulation of Zinc Finger E-Box Binding Homeobox 1 Via Activating the Rac1/PI3K/AKT Pathway in Gastric Cancer Cells. Cell Physiol Biochem 2018;48:1457-67. 10.1159/000492255 [DOI] [PubMed] [Google Scholar]
- 27.Mugisho OO, Green CR, Zhang J, et al. Connexin43 hemichannels: A potential drug target for the treatment of diabetic retinopathy. Drug Discov Today 2019;24:1627-36. 10.1016/j.drudis.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 28.Leffler KE, Abdel-Rahman AA. Estrogen-Dependent Disruption of Adiponectin-Connexin43 Signaling Underlies Exacerbated Myocardial Dysfunction in Diabetic Female Rats. J Pharmacol Exp Ther 2019;368:208-17. 10.1124/jpet.118.254029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei J, Fu Y, Zhuang Y, et al. miR-382-3p suppressed IL-1β induced inflammatory response of chondrocytes via the TLR4/MyD88/NF-κB signaling pathway by directly targeting CX43. J Cell Physiol 2019;234:23160-8. 10.1002/jcp.28882 [DOI] [PubMed] [Google Scholar]
- 30.Pakuła M, Witucka A, Uruski P, et al. Senescence-related deterioration of intercellular junctions in the peritoneal mesothelium promotes the transmesothelial invasion of ovarian cancer cells. Sci Rep 2019;9:7587. 10.1038/s41598-019-44123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CW, Lee TL, Chen YC, et al. PM-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part Fibre Toxicol 2018;15:4. 10.1186/s12989-018-0240-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin MA, Haas CS, Zhu K, et al. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood 2006;107:2252-61. 10.1182/blood-2005-05-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as