Abstract
Background
Immunological disease-related chronic cerebrospinal venous insufficiency (CCSVI) is rarely reported. This study aimed to analyze clinical characteristics, inflammation, and coagulation status in patients with immunological disease-related CCSVI.
Methods
Patients with CCSVI were enrolled from 2017 to 2019 and divided into three cohorts based on their immunological disease backgrounds, including groups with confirmed autoimmune disease, with suspected/subclinical autoimmune disease, and with non-immunological etiology. Immunological, inflammatory, and thrombophilia biomarker assay in blood samples were obtained. Mann-Whitney U test or Fisher’s exact test was used to compare continuous variables or categorical variables between the CCSVI patients with or without the immunological etiology. Spearman’s correlation analysis was conducted among age, baseline neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), interleukin-6 (IL-6), C-reactive protein (CRP), and neuron-specific enolase (NSE) in the three groups.
Results
A total of 255 consecutive patients with CCSVI were enrolled, including three subgroups: CCSVI with confirmed autoimmune disease (n=41), CCSVI with suspected/subclinical autoimmune disease (n=116) and CCSVI with non-immunological etiology (n=98). In the first subgroup, a series of 41 cases was confirmed with eight different autoimmune diseases including antiphospholipid syndrome (n=18), Sjögren’s syndrome (n=8), immunoglobulin G4-related disease (n=7), Behçet’s disease (n=2), autoimmune hepatitis (n=2), Wegener's granulomatosis (n=2), systemic sclerosis (n=1) and AQP4 antibody-positive neuromyelitis optica spectrum disorder (n=1). Groups with immunological etiology did not show a higher incidence of thrombophilia or increased pro-inflammatory biomarkers (e.g., neutrophil, IL-6). However, patients with non-immunological etiology had a higher baseline level of CRP. Additionally, baseline PLR was moderately correlated to NLR and CRP in CCSVI patients with non-immunological etiology and suspected/subclinical autoimmune disease.
Conclusions
The formation of CCSVI may be based on the inflammatory process, facilitated by multiple risk factors, among which medical history of immunological diseases may play a significant role due to the intricate relationship between inflammation and coagulation. Moreover, CCSVI may also cause an independent inflammatory injury in venous walls, leading to focal stenosis or thrombus, without attacks from autoimmune antibodies.
Keywords: Chronic cerebrospinal venous insufficiency (CCSVI), cerebral venous sinus stenosis (CVSS), internal jugular vein stenosis (IJVS), inflammatory biomarkers, autoimmune disease
Introduction
Chronic cerebrospinal venous insufficiency (CCSVI), as a state of impaired intracranial or extracranial venous drainage, has been heatedly discussed over its role in the pathogenesis of multiple sclerosis (MS) in the last decades (1-4). However, with the increasing evidence of CCSVI not unique to MS (5-7), the relationship between CCSVI and other autoimmune diseases has emerged. Given that autoimmune diseases are associated with hypercoagulation state due to elevated autoimmune antibodies, venous thromboembolism (VTE) is one of the most common complications. Nevertheless, studies on autoimmune disease-mediated cerebral venous sinus thrombosis (CVST) are rather rare. Only a few cohort studies of patients with systemic lupus erythematosus (SLE) (8-11) or Behçet’s disease (BD) (12,13) were reported to have CCSVI as well. Moreover, several case series presented patients with the antiphospholipid-antibody syndrome (APS) (14,15), inflammatory bowel disease (IBD) (16), or Wegener's granulomatosis (17,18) with the coexistence of CVST.
The clinical features of autoimmune disease-mediated CCSVI are still unclear. Moreover, we also found a number of patients with CCSVI had positive tests of immunological biomarkers, such as decreased complement 3 (C3) or complement 4 (C4), increased erythrocyte sedimentation rate (ESR), immunoglobulin G (IgG), or immunoglobulin E (IgE), despite negative findings of antinuclear bodies or antineutrophil cytoplasmic antibodies. Thus, we aimed to enroll CCSVI patients with confirmed autoimmune disease or suspected/subclinical autoimmune disease and provided comprehensive clinical features of CCSVI with or without immunological etiology. Furthermore, thanks to the intricate relationship between coagulation, inflammation (adaptive immune system), and complement pathway (innate immune system), we evaluated relevant biomarkers in this study. The following article is presented in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4201).
Methods
Population
A total of 255 consecutive patients with confirmed CCSVI were enrolled, with admission in the Department of Neurology, Xuanwu Hospital, Capital Medical University, from 2017 to 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Xuanwu Hospital, Capital Medical University (2019-006), and informed consent was taken from all individual participants.
Inclusion criteria were as follows: (I) patients with CCSVI, including internal jugular vein stenosis (IJVS), cerebral venous sinus stenosis (CVSS), or CVSS combined with IJVS were confirmed by contrast-enhanced magnetic resonance venography (CE-MRV) or digital subtraction angiography (DSA); (II) patients did not have parenchymal lesions due to CCSVI; (III) the course of the disease was at a subacute or chronic stage, defined as an interval of more than 4 weeks; (IV) patients with autoimmune diseases were confirmed by the Department of Rheumatology, Xuanwu Hospital, Capital Medical University.
We excluded patients with definite acute or chronic infection; use of anti-inflammatory medication within 4 weeks prior to blood collection; intracranial hypertension (IH) resulting from other reasons: (I) drug-induced IH; (II) cerebrospinal fluid shunt history; (III) intracranial mass occupation; (IV) arteriovenous malformations.
Clinical and demographic data
Age, gender, course of the disease (from onset to admission), treatments, and presumable risk factors known before hospitalization or discovered during hospitalization were recorded. The common risk factors included hypertension (use of antihypertensive medications or systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg before hospitalization diastolic blood pressure >90 mmHg before hospitalization), diabetes mellitus (use of antidiabetic therapies or fasting blood glucose >7 mmol/L on two occasions during hospitalization), hypercholesterolemia (use of lipid-lowering medications or low-density lipoprotein cholesterol >1 g/L), a history of myocardial infarction or angina, overweight (body mass index >25 kg/m2), anemia (hemoglobin <12.5 g/dL), hepatitis B virus (HBV) infection (use of anti-HBV therapies or positive hepatitis B core antibody/antigen or hepatitis B e antibody/antigen), hyperhomocysteinemia (>15 mmol/L), hyperuricemia (>416 µmol/L), chronic rhinosinusitis, history of otitis media/mastoiditis, suspected thyroid disorders (including either abnormal thyroid ultrasound results or abnormal thyroid function results), autoimmune disease, thrombophilia (including protein S deficiency, protein C deficiency, antithrombin-III deficiency, hyperfibrinogenemia, primary thrombocythemia or increased D-dimer level), and history of ischemic or hemorrhagic stroke. We also collected clinical signs, such as papilledema and IH. The severity of papilledema was evaluated by the Frisen papilledema grade criteria. Intracranial pressure (ICP) was detected by lumbar puncture, and IH was defined as ICP more than 200 mmH2O.
Subgroup analysis was conducted based on etiology. CCSVI patients with immunological etiology were defined as the coexistence of confirmed autoimmune disease or suspected/subclinical autoimmune disease, while CCSVI patients with non-immunological etiology included CVST-related CCSVI or bone/vessel/lymph node-compression-related CCSVI.
Immunological, inflammatory, and thrombophilia biomarkers assay in the blood sample
Immunological biomarker assay included autoimmune antibody tests in serum samples, including antinuclear antibodies (ANAs), anti-neutrophil cytoplasmic antibodies (ANCAs), and antiphospholipid antibodies (APLAs), as well as other immunological markers, such as C3, C4, IgG, IgE, ESR, and rheumatic factor (RF).
Inflammatory biomarker assay consisted of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in plasma EDTA samples, as well as interleukin-6 (IL-6), C-reactive protein (CRP), and neuron-specific enolase (NSE) in serum samples. Baseline levels were measured on admission. NLR was computed using the absolute neutrophil count divided by the absolute lymphocyte count. PLR was calculated using the absolute platelet count divided by the absolute lymphocyte count. Baseline inflammatory markers were considered as continuous variables.
Thrombophilia biomarker assay evaluated both antigens, including platelet, fibrinogen, d-dimer, antithrombin-III, protein C, and protein S, as well as activity, such as thrombin time, partial thromboplastin time (PTT) and activated PTT (aPTT) in plasma sodium-citrate sample without platelet depleted. Cutoff values were based on the referential interval in the Laboratory of Xuanwu Hospital, Capital Medical University.
All blood samples were collected in VACUETTE® Blood Collection Tubes (Greiner Bio-One, Kremsmünster, Austria). Detailed information on coagulation and inflammatory kits and instruments is presented in Table S1.
Statistical analysis
Bartlett’s test for equal variances and the Shapiro-Wilk normality test for distribution were conducted for each continuous variable. We then used the Mann-Whitney U test or Fisher’s exact test to compare continuous variables or categorical variables between patients with immunological etiology CCSVI and non-immunological etiology CCSVI. Difference between levels of baseline inflammatory markers [NLR, PLR, and red blood cell distribution width (RDW)] and that at discharge was tested by Wilcoxon signed-rank test. Correlation coefficients were calculated with Spearman’s test among age, baseline NLR, PLR, RDW, IL-6, CRP, and NSE. Quantitative variables with a normal distribution were specified as mean ± standard deviation, and those with abnormal distribution were expressed as median with interquartile range (IQR). Differences were considered significant at a two-sided P<0.05 level. Analyses were performed with Stata software (version 15.0 SE, Stata Corp., LP, Texas, USA) and R software [version 3.6.2 (2019-12-12)].
Results
Baseline clinical features
A total of 255 patients (104 males and 151 females) with CCSVI were enrolled in this retrospective study, of which more than 95% had a disease course over 1 month (chronic stage), and followed up with 18.13±5.58 months. Patients most likely presented with sleep disturbances (60.4%), eye discomfort (58.4%), head noise (53.7%), tinnitus (51.8%), headache (45.1%), and hearing loss (32.2%). Combined risk factors were commonly seen, for instance, thrombophilia state, overweight, hyperlipidemia, hypertension, anemia, and suspected thyroid disorders. Based on locations of CCSVI, IJVS, CVSS, and CVSS combined with IJVS were found in 68.2%, 16.9%, and 14.9% of enrolled patients, respectively. Treatments for CCSVI patients were antiplatelet drugs (60.0%), anticoagulants (32.2%), and endovascular therapy (12.5%). Details were displayed in Table 1.
Table 1. Demographic and basic clinical features.
| Variables | All (n=255) | Immunological etiology (n=157) | Non-immunological etiology (n=98) | P value | |
|---|---|---|---|---|---|
| Confirmed autoimmune disease (n=41) | Suspected/subclinical autoimmune disease (n=116) | ||||
| Personal data | |||||
| Age, years | 53.47±15.04 | 50.88±18.77 | 56.42±14.14* | 51.05±13.81 | 0.017 |
| Gender (M:F) | 104:151 | 14:27 | 54:62 | 36:62 | 0.223 |
| Course of disease | 0.543 | ||||
| Subacute (within 1 month) | 11 (4.3) | 3 (7.3) | 4 (3.4) | 4 (4.1) | |
| Chronic (more than 1 month) | 244 (95.7) | 38 (92.7) | 112 (96.6) | 94 (95.9) | |
| Follow-up time, months^ | 18.13±5.58 | 18.27±5.52 | 18.46±5.31 | 17.69±5.94 | 0.656 |
| mRS on admission | 0.43±0.68 | 0.60±0.60 | 0.42±0.61 | 0.39±0.77 | 0.110 |
| Symptoms and signs | |||||
| Sleep disturbances | 154 (60.4) | 22 (53.7) | 79 (68.1) | 53 (54.1) | 0.078 |
| Eye discomfort | 149 (58.4) | 23 (56.1) | 71 (61.2) | 55 (56.1) | 0.716 |
| Papilledema | 49 (19.2) | 16 (39.0) | 17 (14.7) | 16 (16.3) | 0.004 |
| Frisen scale | 1.08±1.31 | 1.56±1.47 | 0.82±1.23 | 1.03±1.21 | 0.139 |
| Head noises | 137 (53.7) | 18 (43.9) | 72 (62.1) | 47 (48.0) | 0.045 |
| Tinnitus | 132 (51.8) | 21 (51.2) | 61 (52.6) | 50 (51.0) | 0.985 |
| Headache | 115 (45.1) | 20 (48.8) | 43 (37.1) | 52 (53.1) | 0.055 |
| Neck discomfort | 77 (30.2) | 9 (22.0) | 42 (36.2) | 26 (26.5) | 0.152 |
| Hearing loss | 82 (32.2) | 11 (26.8) | 39 (33.6) | 32 (32.7) | 0.738 |
| Anxiety | 45 (17.6) | 4 (9.8) | 20 (17.2) | 21 (21.4) | 0.266 |
| Nausea/vomiting | 47 (18.4) | 4 (9.8) | 19 (16.4) | 24 (24.5) | 0.097 |
| Memory loss | 21 (8.2) | 0 (0.0) | 11 (9.5) | 10 (10.2) | 0.078 |
| IH | 44 (17.3) | 8 (19.5) | 21 18.1) | 15 (15.3) | 0.368 |
| Presumable risk factors | |||||
| Obesity | 93 (36.5) | 13 (31.7) | 35 (30.2) | 43 (43.9) | 0.172 |
| Type 2 diabetes mellitus | 22 (8.6) | 4 (9.8) | 11 (9.5) | 7 (7.1) | 0.794 |
| HBP | 83 (32.5) | 15 (36.6) | 35 (30.2) | 33 (33.7) | 0.727 |
| Hyperlipidemia | 90 (35.3) | 11 (26.8) | 47 (40.5) | 32 (32.7) | 0.236 |
| Anemia | 57 (22.4) | 13 (31.7) | 26 (22.4) | 18 (18.4) | 0.234 |
| Stroke | 20 (7.8) | 2 (4.9) | 10 (8.6) | 8 (8.2) | 0.821 |
| Hemorrhage | 6 (2.4) | 0 (0.0) | 3 (2.6) | 3 (3.1) | 0.747 |
| Hyperuricemia | 18 (7.1) | 5 (12.2) | 4 (3.4) | 9 (9.2) | 0.075 |
| Hyperhomocysteinemia | 20 (7.8) | 7 (17.1) | 7 (6.0) | 6 (6.1) | 0.082 |
| CAD | 27 (10.6) | 7 (17.1) | 12 (10.3) | 8 (8.2) | 0.264 |
| Previous otitis media/mastoiditis | 7 (2.7) | 3 (7.3) | 2 (1.7) | 2 (2.0) | 0.175 |
| Chronic rhinosinusitis | 14 (5.5) | 4 (9.8) | 6 (5.2) | 4 (4.1) | 0.431 |
| HBV infection | 47 (18.4) | 9 (22.0) | 24 (20.7) | 14 (14.3) | 0.383 |
| Suspected thyroid disorders | |||||
| Abnormal thyroid ultrasound | 34 (13.3) | 7 (17.1) | 15 (12.9) | 12 (12.2) | 0.707 |
| Abnormal thyroid function test | 66 (25.9) | 12 (29.3) | 17 (14.7) | 27 (27.6) | 0.681 |
| Pregnancy/postpartum | 2 (0.8) | 1 (2.4) | 0 (0.0) | 1 (1.0) | 0.149 |
| Thrombophilia | |||||
| Protein S deficiency | 65 (25.5) | 11 (26.8) | 36 (31.0) | 18 (18.4) | 0.385 |
| Protein C deficiency | 25 (9.8) | 5 (12.2) | 11 (9.5) | 9 (9.2) | 0.191 |
| Antithrombin III deficiency | 27 (10.6) | 8 (19.5) | 13 (11.2) | 6 (6.1) | 0.213 |
| Increased D-dimer level | 22 (8.6) | 7 (17.1) | 7 (6.0) | 8 (8.2) | 0.192 |
| Hyperfibrinogenemia | 27 (10.6) | 6 (14.6) | 10 (8.7) | 11 (11.2) | 0.498 |
| Primary thrombocythemia | 25 (9.8) | 5 (12.2) | 11 (9.5) | 9 (9.2) | 0.100 |
| Autoimmune disease | |||||
| APS | 18 (7.1) | 18 (43.9) | NA | NA | NA |
| Sjögren’s syndrome | 8 (3.1) | 8 (19.5) | NA | NA | NA |
| IgG4-related disease | 7 (2.7) | 7 (17.1) | NA | NA | NA |
| Behcet’s disease | 2 (0.8) | 2 (4.9) | NA | NA | NA |
| Autoimmune hepatitis | 2 (0.8) | 2 (4.9) | NA | NA | NA |
| Wegener’s granulomatosis | 2 (0.8) | 2 (4.9) | NA | NA | NA |
| Others | 2 (0.8) | 2 (4.9) | NA | NA | NA |
| Suspected autoimmune disease | |||||
| Increased IgE | 5 (2.0) | NA | 5 (4.3) | NA | NA |
| Increased IgG | 10 (3.9) | NA | 10 (8.6) | NA | NA |
| Decreased C3 | 76 (29.8) | NA | 76 (65.5) | NA | NA |
| Decreased C4 | 38 (14.9) | NA | 38 (32.8) | NA | NA |
| Positive RF | 4 (1.6) | NA | 4 (3.4) | NA | NA |
| Increased ESR | 21 (8.2) | NA | 21 (18.1) | NA | NA |
| Inflammatory markers | |||||
| NLR on admission& | 1.81±0.77 | 1.78±0.70 | 1.82±0.80 | 1.79±0.75 | 0.961 |
| NLR at discharge | 2.91±2.56# | 2.16±1.09 | 2.53±1.29# | 3.88±4.03 | 0.581 |
| Delta-NLR | 1.12±2.15 | 0.66±0.97 | 0.81±1.16 | 1.83±3.38 | 0.912 |
| PLR on admission | 124.13±46.94 | 120.31±41.89 | 122.47±41.78 | 127.54±54.31 | 0.878 |
| PLR at discharge& | 151.32±100.88 | 125.39±33.03 | 132.91±46.55 | 192.51±160.72 | 0.592 |
| Delta-PLR | 26.75±103.07 | 25.07±33.10 | 4.09±75.83 | 59.84±151.17 | 0.580 |
| RDW on admission (%) | 13.14±1.43 | 13.25±1.16 | 13.10±1.63 | 13.14±1.26 | 0.685 |
| RDW at discharge (%)& | 13.49±2.28 | 12.77±0.43 | 13.52±2.37 | 13.92±2.84 | 0.637 |
| Delta-RDW (%) | 0.44±2.37 | 0.28±0.95 | 0.42±2.09 | 0.95±3.30 | 0.462 |
| IL-6 (pg/mL) | 4.70±5.71 | 6.36±7.51 | 4.65±5.91 | 4.12±4.48 | 0.391 |
| CRP (mg/L) | 2.80±3.69 | 2.44±1.07 | 2.38±1.89* | 3.45±5.50 | 0.015 |
| NSE (ng/mL) | 12.95±2.72 | 13.56±3.33 | 12.82±2.26 | 12.88±2.95 | 0.734 |
| Localization of CVSS/IJVS | 0.026 | ||||
| CVSS | 43 (16.9) | 7 (17.1) | 13 (11.2) | 23 (23.5) | |
| SSS | 15 (5.9) | 2 (4.9) | 5 (4.3) | 8 (8.2) | 0.523 |
| LTS | 34 (13.3) | 7 (17.1) | 9 (7.8) | 21 (21.4) | 0.013 |
| RTS | 36 (14.1) | 10 (24.4) | 11 (9.5) | 15 (15.3) | 0.063 |
| SS | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 0.445 |
| LSigS | 22 (8.6) | 6 (14.6) | 5 (4.3) | 11 (11.2) | 0.058 |
| RSigS | 19 (7.5) | 8 (19.5) | 5 (4.3) | 6 (6.1) | 0.011 |
| IJVS | 174 (68.2) | 24 (58.5) | 90 (77.6) | 60 (61.2) | |
| LIJV-J1 segment | 16 (6.3) | 5 (12.2) | 7 (6.0) | 4 (4.1) | 0.218 |
| RIJV-J1 segment | 11 (4.3) | 4 (9.8) | 4 (3.4) | 3 (3.1) | 0.195 |
| LIJV-J2 segment | 26 (10.2) | 5 (12.2) | 13 (11.2) | 8 (8.2) | 0.667 |
| RIJV-J2 segment | 12 (4.7) | 2 (4.9) | 5 (4.3) | 5 (5.1) | 1.000 |
| LIJV-J3 segment | 144 (56.5) | 21 (51.2) | 71 (61.2) | 52 (53.1) | 0.530 |
| RIJV-J3 segment | 122 (47.8) | 18 (43.9) | 61 (52.6) | 43 (43.9) | 0.397 |
| CVSS combined with IJVS | 38 (14.9) | 10 (24.4) | 13 (11.2) | 15 (15.3) | |
| Treatment | |||||
| Antiplatelet drugs | 153 (60.0) | 22 (53.7) | 77 (66.4) | 54 (55.1) | 0.133 |
| Anticoagulants | 82 (32.2) | 14 (34.1) | 33 (28.4) | 35 (35.7) | 0.526 |
| Endovascular therapies | 32 (12.5) | 8 (19.5) | 10 (8.6) | 14 (14.3) | 0.138 |
| Stenting | 25 (9.8) | 8 (19.5) | 9 (7.8) | 8 (8.2) | 0.096 |
| Balloon dilation | 5 (2.0) | 0 (0.0) | 2 (1.7) | 3 (3.1) | 0.592 |
| Intrasinus thrombolysis | 4 (1.6) | 0 (0.0) | 0 (0.0) | 4 (4.1) | 0.035 |
| ONSD | 8 (3.1) | 4 (9.8) | 1 (0.9) | 3 (3.1) | 0.026 |
Data were presented as mean ± standard deviation or n (%). *, compared with group of non-immunological etiology group, statistically significant at P<0.05; &, the number of patients who had complete blood count (CBC) test at discharge (n=36); ^, time from discharge to follow-up (months); #, compared with group of NLR tested on admission, statistically significant at P<0.05. P=0.001 in general groups (n=255), P=0.008 in group with suspected/subclinical autoimmune disease (n=116). mRS, modified Rankin scale; HBP, high blood pressure; CAD, coronary artery disease; HBV, hepatic type B virus; APS, antiphospholipid syndrome; C3, complement 3; C4, complement 4; RF, rheumatic factor; ESR, erythrocyte sedimentation rate; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; RDW, red blood cell distribution width; IL-6, interleukin-6; CRP, C-reactive protein; NSE, Neuron-specific enolase; CVSS, cerebral venous sinus stenosis; IJVS, internal jugular vein stenosis; SSS, superior sagittal sinus; TS, transverse sinus; LTS, left transverse sinus; RTS, right transverse sinus; SigS, sigmoid sinus; LSigS, left sigmoid sinus; RSigS, right sigmoid sinus; ONSD, optic nerve sheath decompression; NA, not applicable.
Difference between CCSVI with or without immunological etiology
Immunological disease-related CCSVI was defined as confirmed autoimmune disease or suspected/subclinical autoimmune disease. The prevalence of autoimmune disease-related CCSVI was relatively low as 16.1% (n=41), while CCSVI was rather common in suspected/subclinical autoimmune disease (45.5%, n=116). A total of 41 cases with confirmed autoimmune disease-related CCSVI (14 males and 27 females, mean age 50.88±18.77 years) were collected, among which, APS (n=18), Sjögren’s syndrome (SS) (n=8), and IgG4-related disease (IgG4-RD) (n=7) were highly prevalent in our cohort. Apart from specific elevated autoantibodies, such as ANAs or ANCAs or APLAs, abnormal levels of nonspecific immunological markers were also coexisted within the CCSVI cohort of confirmed autoimmune disease, for instance, a decreased level of C3 (n=16) or C4 (n=9) as well as an increased level of ESR (n=9) or IgG (n=8). Table 2 presented the case series of 41 cases with confirmed autoimmune disease-related CCSVI in detail.
Table 2. Case series of autoimmune disease-related CCSVI (n=41).
| Variables | Number | Age (years)/gender | Symptoms and signs | Course of disease | Presumable risk factors | Localization of CCSVI | Abnormal lab test | Treatment |
|---|---|---|---|---|---|---|---|---|
| APS (n=18) | Case 1 | 55/M | Eye discomfort, anxiety and sleep disorder | 7 months | Obesity, hyperlipidemia, hyperuricemia and previous HBV infection, and AT-III deficiency | LIJV-J3 | AT-III, C3, C4↓; anti-β2GP1 Ab↑ (113 RU/mL) | Anti-PLT |
| Case 2 | 75/F | Hearing loss, Head noises, tinnitus, papilledema [1]*, neck discomfort, anxiety and sleep disorder | 30 years | Type 2 DM, HBP, and anemia | RIJV-J3 | IgE↑, C3↓; anti-β2GP1 Ab↑ (47 RU/mL) | None | |
| Case 3 | 29/M | Headache, papilledema [1]*, head noises and sleep disorder | 2 months | Current HBV infection, and anemia | LIJV-J3, RIJV-J1/J3 | C4, C3↓; anti-β2GP1 Ab↑ (40 RU/mL) | Anti-PLT | |
| Case 4 | 30/F | Hearing loss, tinnitus, headache, neck discomfort, papilledema [3]* and IH | 1.5 years | Obesity, hyperhomocysteinemia, and anemia | RTS, RSigS | DD, ESR↑; anti-β2GP1 Ab↑ (50 RU/mL) | Anti-PLT, anti-coagulation, ONSD | |
| Case 5 | 33/M | Headache and papilledema [5]* | 3 months | Obesity and current HBV infection | RTS | Anti-β2GP1 Ab↑ (60 RU/mL) | Anti-PLT, anti-coagulation, stenting, ONSD | |
| Case 6 | 75/F | Dizziness and tinnitus | 2 years | Obesity, hyperlipidemia, HBP, Hashimoto’s thyroiditis, and previous otitis media | Bilateral IJV-J1 | Anti-β2GP1 Ab↑ (65 RU/mL) | Anti-PLT | |
| Case 7 | 62/F | Head noises, neck discomfort, blurry vision and sleep disorder | 2 months | Obesity, type 2 DM, hyperlipidemia, HBP, and CAD | LIJV-J1 | aCL↑ (40 RU/mL) | Anti-PLT | |
| Case 8 | 32/F | Fever, headache, papilledema [3]* and IH | 1 month | Postpartum, anemia | Bilateral TS, SigS, RIJV-J2/J3, LIJV-J3 | WBC, hs-CRP, IL-6, PLT↑; anti-β2GP1 Ab↑ (61 RU/mL) | Corticosteroids | |
| Case 9 | 54/F | Tinnitus, eye discomfort and sleep disorder | 4 years | Obesity, hyperlipidemia, Hashimoto’s thyroiditis, and CAD | RTS, LIJV-J3, RIJV-J3 | ESR↑; anti-β2GP1 Ab↑ (151 RU/mL) | Anti-PLT, anti-coagulation and stenting | |
| Case 10 | 63/F | Headache and head noises | 2 years | HBP | RTS, LIJV-J3 | DD↑; anti-β2GP1 Ab↑ (59 RU/mL) | Anti-PLT, anti-coagulation and stenting | |
| Case 11 | 33/F | Headache, nausea/vomiting, neck discomfort, sleep disorder, and papilledema [4]* | 2 weeks | Hashimoto’s thyroiditis | RTS, RSigS, LIJV-J3 | DD↑; IgG ↑; C4, C3↓; anti-β2GP1 Ab↑ (50 RU/mL) | Anti-coagulation and stenting | |
| Case 12 | 56/F | Tinnitus, hearing loss and sleep disorder | 5 months | HBP, CAD | RIJV-J3 | aCL↑ (42 RU/mL) | Anti-PLT | |
| Case 13 | 19/M | Headache, nausea/vomiting, and eye discomfort | 3 weeks | Obesity | LIJV-J3, RIJV-J3 | AT-III↓; anti-β2GP1 Ab↑ (108 RU/mL) | None | |
| Case 14 | 60/M | Tinnitus, and hearing loss | 1.5 years | None | LIJV-J3 | PS, PC, C3↓; anti-β2GP1 Ab↑ (43.4 RU/mL) | Anti-coagulation and stenting | |
| Case 15 | 69/F | Tinnitus, head noises, eye discomfort and sleep disorder | 15 years | Hyperuricemia, CAD, and Hashimoto’s thyroiditis | LIJV-J2/J3 | DD↑; anti-β2GP1 Ab↑ (135 RU/mL) | Anti-PLT | |
| Case 16 | 39/F | Headache | 20 years | Hashimoto’s thyroiditis | RIJV-J2/J3 | Anti-β2GP1 Ab↑ (53 RU/mL) | None | |
| Case 17 | 62/M | Tinnitus, head noises, neck discomfort, hearing loss, and sleep disorder | 20 years | Hyperuricemia and anemia | LIJV-J3 | PS, PC, C3↓; anti-β2GP1 Ab↑ (81 RU/mL) | Anti-PLT | |
| Case 18 | 61/F | Headache, tinnitus, and sleep disorder | 4 months | Anemia and Hashimoto’s thyroiditis | LTS, LIJV-J3, RIJV-J3 | Fig↑, C3↓, ESR↑; anti-β2GP1 Ab↑ (52 RU/mL) | None | |
| SS (n=8) | Case 19 | 74/M | Headache, head noises, tinnitus, and papilledema [1]* | 3 years | Anemia, Hashimoto’s thyroiditis, and previous ischemic stroke | RIJV-J1 | PS↓; anti-Ro-52 (+++), anti-CENP-B (+++) | Anti-PLT |
| Case 20 | 63/F | Headache, head noises, sleep disorder and papilledema [1]* | 2 years | HBP and Hashimoto’s thyroiditis | LTS, LIJV-J3, RIJV-J3 | C3, C4↓, RF↑; anti-SS-A (+++), anti-Ro-52 (+++) | Anti-coagulation | |
| Case 21 | 61/F | Headache, head noises, tinnitus and hearing loss | 4 months | HBP, CAD, anemia, and previous HBV infection | RTS, RSigS, LIJV-J2/J3, RIJV-J3 | Fig, IgG ↑, PS↓; anti-SS-A (+++), anti-SS-B (+), anti-Ro-52 (+++) | Anti-PLT | |
| Case 22 | 72/M | Head noises, sleep disorder and IH | 3.5 months | Type 2 DM, hyperlipidemia, HBP, CAD, and previous HBV infection | LIJV-J3, RIJV-J3 | PS, C3↓; anti-SS-A (++), anti-Ro-70 (++) | Anti-PLT | |
| Case 23 | 68/M | Tinnitus, hearing loss, and sleep disorder | 10 years | Obesity, hyperhomocysteinemia, previous ischemic stroke, Hashimoto’s thyroiditis | LIJV-J1/J3 | AT-III, PS, PC↓; anti-SS-A, anti-Ro-52, anti-β2GP1 Ab↑ (40 RU/mL) | None | |
| Case 24 | 56/F | Head noises and eye discomfort | 2 years | Hyperlipidemia, HBP, and anemia | RIJV-J3 | IgG, ESR, DD↑; anti-SS-A (+++), anti-SS-B (+), anti-Ro-52 (+++), | Anti-coagulation | |
| Case 25 | 44/F | Headache, papilledema [3]* and IH | 10 years | Obesity and anemia | Bilateral TS, SigS | Anti-SS-A (++), anti-Ro-70 (++) | Optic nerve decompression surgery | |
| Case 26 | 56/F | Eye discomfort, neck discomfort, and sleep disorder | 1 month | Hyperlipidemia, HBP, and previous HBV infection | LSigS | Fig, DD↑, AT-III↑; C3, C4↓, RF, IgG↑; anti-SS-A (++), anti-Ro-52 (++) | Anti-coagulation | |
| IgG4-related disease (n=7) | Case 27 | 53/F | Tinnitus, head noises, hearing loss, sleep disorder and IH | 4 years | HBP and Hashimoto’s thyroiditis | LIJV-J3 | C3, C4↓, RF↑; anti-PNMA2 (CSF) (+) | Anti-PLT |
| Case 28 | 15/F | Headache, nausea/vomiting, and papilledema [2]* | 1.5 months | Obesity, hyperhomocysteinemia, and hyperuricemia | RTS, SSS | Fig↑, PS, PC↓, IgE↑; IgG4 (serum) ↑ (1,760 mg/L) | Anti-coagulation and ONSD | |
| Case 29 | 23/F | Headache, tinnitus, head noises, nausea/vomiting, sleep disorder, papilledema [3]* and IH | 4 months | Hashimoto’s thyroiditis | RTS, RSigS | TBil, DBil, IBILI, ALP↑; IL-6, C3↓; IgG4 (serum)↑ (1,660 mg/L); | Anti-coagulation and ONSD | |
| Case 30 | 78/F | Headache, tinnitus, head noises, hearing loss, sleep disorder, anxiety and IH | 4 years | HBP | LIJV-J3, RIJV-J3 | DD↑, AT-III↓; C3, C4↓; anti-RNP/Sm (+) | Anti-PLT | |
| Case 31 | 25/M | Headache, neck discomfort, sleep disorder, papilledema [2]*, and IH | 2 years | Hyperlipidemia, hyperhomocysteinemia, and previous mastoiditis | SSS, LTS, LSigS, LIJV-J1, RIJV-J2/J3 | IgG4 (CSF) (2,020 mg/L) | Anti-coagulation | |
| Case 32 | 36/F | Tinnitus, papilledema [3], and IH | 1.5 years | None | Bilateral TS, LIJVS-J1 | C3↓; IgG (serum)↑ (1,180 mg/L); IgG4 (serum) ↑ (363 mg/L) | Anti-PLT, anti-coagulation, stenting and ONSD | |
| Case 33 | 32/M | Dizziness, eye discomfort, papilledema [2]*, and IH | 12 years | Chronic nasal sinusitis, Hashimoto’s thyroiditis | Bilateral TS, SigS | ESR↑, IgG4 (serum) (2,550 mg/L) | Anti-PLT, anti-coagulation, stenting | |
| Behcet’s disease (n=2) | Case 34 | 28/M | Headache, head noises, eye discomfort and sleep disorder | 20 years | Hyperhomocysteinemia, and anemia | LIJV | PS, PC, C3, C4↓; IgE↑ | Anti-PLT |
| Case 35 | 23/F | Headache, tinnitus, papilledema [2]* and IH | 3 years | Anemia and Hashimoto’s thyroiditis | Bilateral TS, SigS | PS, C4↓ | Immunomodulatory drugs (corticosteroids+ mycophenolate mofetil) and anticoagulation | |
| Autoimmune hepatitis (n=2) | Case 36 | 74/F | Head noises, tinnitus, eye discomfort, and sleep disorder | 5 years | Hyperlipidemia, chronic nasal sinusitis, Hashimoto’s thyroiditis and previous HBV infection | LIJV-J3 | ALT, AST, LDH, ALP↑; Fig, DD↑, AT-III, PS↓, IgG, IgM, CRP, Hs-CRP, RF, ESR↑; anti-Ro-52(+++), anti-CENP (+++) | Anti-PLT |
| Case 37 | 51/M | Tinnitus and eye discomfort | 2 months | Obesity, hyperlipidemia, HBP, CAD, and previous HBV infection | RIJV-J1 | C3↓; TBil, DBil, IBILI, ALP↑; IgG (CSF), IgG (serum)↑ | Anti-PLT | |
| Wegener’s granulomatosis (n=2) | Case 38 | 67/F | Headache, tinnitus, head noises, hearing loss, eye discomfort, and sleep disorder | 20 years | Obesity, chronic nasal sinusitis, previous mastoiditis, Hashimoto’s thyroiditis, and previous HBV infection | RIJV-J3 | AT-III, C3↓; anti-PR3↑ | Anti-PLT |
| Case 39 | 55/M | Tinnitus, head noises, and neck discomfort | 5 years | Obesity | LIJV-J3, RIJV-J3 | C3, C4↓; anti-PR3 (120 RU/mL) | None | |
| Systemic sclerosis (n=1) | Case 40 | 83/M | Headache | 2 months | Obesity, HBP, type 2 DM, Hashimoto’s thyroiditis | RTS, RSigS, LIJV-J1, RIJV-J2 | Anti-CENP-B (+++) | Anti-PLT and stenting |
| AQP4+ NMOSD (n=1) | Case 41 | 42/M | Progressive blurry vision, vision defect, papilledema [2]*, hyposmia | 1 year | Chronic nasal sinusitis, splenomegaly | LTS, LSigS, LIJV-J2/J3 | WBC (CSF) ↑, AQP4 (serum) (+) | Corticosteroids |
*, the severity of papilledema was evaluated by Frisen scale, presented as papilledema [Frisen scale]. DM, diabetes mellitus; CCSVI, chronic cerebrospinal venous insufficiency; APS, antiphospholipid syndrome; AT-III, anti-thrombin III; RIJV, right internal jugular vein; LIJV, left internal jugular vein; C3, complement 3; C4, complement 4; PLT, platelet; anti-β2GP1 Ab, anti-beta-2 glycoproteins antibodies; HBV, hepatic type B virus; IH, intracranial hypertension; TS, transverse sinus; LTS, left transverse sinus; RTS, right transverse sinus; LSigS, left sigmoid sinus; RSigS, right sigmoid sinus; DD, di-dimer; ESR, erythrocyte sedimentation rate; ONSD, optic nerve sheath decompression; HBP, high blood pressure; DM, diabetes mellitus; CAD, coronary artery disease; WBC, white blood cell; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; aCL, anti-cardiolipin antibodies; SigS, sigmoid sinus; PS, protein S; PC, protein C; anti-Ro-52, anti-Ro52 antibodies; anti-Ro-70, anti-Ro70 antibodies; anti-CENP-B, anti-centromere protein B antibodies; Fig, fibrinogen; anti-SS-A, anti-Sjögren’s-syndrome-related antigen A; anti-SS-B, anti-Sjögren’s-syndrome-related antigen B; RF, rheumatic factor; SSS, superior sagittal sinus; CSF, cerebrospinal fluid; TBil, total bilirubin; DBil, direct bilirubin; IBILI, indirect bilirubin; ALP, alkaline phosphatase; anti-PR3, anti-proteinase 3 antibodies; anti-PLT, antiplatelet drugs; AQP, aquaporin-4; AQP4 antibody-positive neuromyelitis optica spectrum disorder.
There was a significant difference between CCSVI with or without immunological etiology in terms of symptoms and signs (Table 1). CCSVI patients with suspected/subclinical autoimmune disease presented at an older age on admission (P=0.017) and had a higher prevalence of head noises (P=0.045), while those with confirmed autoimmune disease were more prone to have papilledema (P=0.004). Intriguingly, the groups with immunological etiology did not show a higher incidence of thrombophilia or increased pro-inflammatory factors (e.g., neutrophil, IL-6) as we previously expected than the group with non-immunological etiology. However, patients with non-immunological etiology had a higher baseline level of CRP (P=0.015), which further predicted that an independent inflammatory process might involve in the pathogenesis of CCSVI.
Subgroup analysis in CCSVI with immunological etiology
We further conducted a subgroup analysis between CCSVI with confirmed autoimmune diseases and suspected/subclinical autoimmune diseases, particularly focusing on the difference of inflammatory biomarkers between these two subgroups (Table 3). CCSVI patients with APS and IgG4-RD had a relatively younger age than that with SS and a higher CRP level than that with decreased C3. The gender difference was also remarkable. The group of CCSVI with confirmed autoimmune diseases was female-dominated while that of CCSVI with suspected autoimmune diseases (e.g., group of increased ESR and IgG) was male-dominated.
Table 3. Inflammatory biomarkers in subgroup analysis of autoimmune diseases and suspected autoimmune diseases-related CCSVI.
| Variables | APS (n=18) | SS (n=8) | IgG4-related disease (n=7) | Decreased C3 (n=76) | Decreased C4 (n=38) | Increased ESR (n=21) | Increased IgG (n=10) | Increased IgE (n=5) | Positive RF (n=4) | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 49.61±3.75^ | 61.75±3.48 | 43.00±9.73^ | 57.17±1.49#^ | 53.71±2.75^ | 58.61±2.65^ | 58.90±5.62 | 47.00±6.17^ | 70.75±1.75 | 0.009 |
| Gender (M:F) | 8:10 | 3:5 | 1:6 | 40:36 | 23:15 | 4:17 | 2:8 | 4:1 | 2:2 | 0.021 |
| NLR on admission& | 1.81±0.16 | 2.20±0.36 | 1.48±0.12 | 1.80±0.09 | 1.76±0.12 | 1.97±0.24 | 1.74±0.17 | 1.81±0.45 | 1.70±0.16 | 0.865 |
| NLR at discharge | NR | 1.98±0.79 | 1.90±0.45 | 2.59±0.49 | 2.76±0.49 | 2.41±0.38 | NR | NR | NR | 0.350 |
| PLR on admission | 112.13±9.27 | 148.78±16.02 | 104.96±13.76 | 121.59±5.36 | 117.43± 7.57 | 123.45±6.41 | 108.23±8.73 | 139.56±25.72 | 116.68±8.80 | 0.472 |
| PLR at discharge& | NR | 143.48±21.89 | 99.46±4.44 | 126.27±16.43 | 151.60±12.82 | 121.41±29.79 | NR | NR | NR | 0.695 |
| RDW on admission (%) | 13.15±0.34 | 13.2±0.23 | 12.83±0.14 | 13.06±0.17 | 13.0±0.17 | 13.35±0.53 | 15.3±1.44 | 13.2±0.14 | 13.08±0.33 | 0.513 |
| RDW at discharge (%)& | NR | 12.77±0.27 | 12.90±0.29 | 12.69±0.13 | 12.62±0.12 | 15.52±1.72 | NR | NR | NR | 0.373 |
| IL-6 (pg/mL) | 4.66±1.26 | 8.72±5.42 | 5.80±2.13 | 4.82±0.90 | 4.99±1.67 | 4.75±1.02 | 5.30±1.29 | 2.66±0.66 | 3.57±0.81 | 0.291 |
| CRP (mg/L) | 2.44±0.22§ | 2.29±0.43 | 2.46±0.65§ | 1.78±0.10 | 1.89±0.16 | 4.39±0.76 | 3.30±0.85§ | 2.66±0.69 | 2.08±0.33 | <0.001 |
| NSE (ng/mL) | 12.70±0.47 | 14.75±1.58 | 14.97±2.35 | 12.57±0.79 | 11.58±0.61 | 12.82±0.37 | 11.58±0.61 | 12.57±0.79 | 13.39±1.01 | 0.536 |
&, the number of patients who had complete blood count (CBC) test at discharge (n=36); #, compared with group of APS, statistically significant at P<0.05; ^, compared with group of SS, statistically significant at P<0.05; §, compared with group of decreased C3, statistically significant at P<0.05. CCSVI, chronic cerebrospinal venous insufficiency; APS, antiphospholipid syndrome; SS, Sjögren’s syndrome; C3, complement 3; C4, complement 4; ESR, erythrocyte sedimentation rate; RF, rheumatic factor; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; RDW, red blood cell distribution width; IL-6, interleukin-6; CRP, C-reactive protein; NSE, neuron-specific enolase.
Correlations between inflammatory cells and inflammatory cytokines
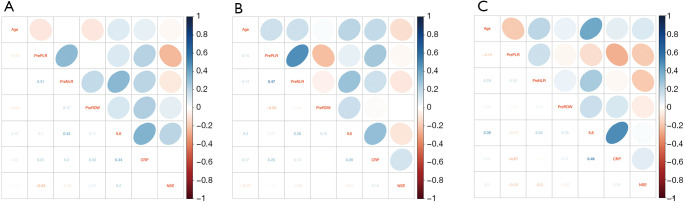
Correlation coefficients were calculated with Spearman’s test among age, baseline NLR, PLR, RDW, IL-6, CRP, and NSE in groups with non-immunological etiology (Figure 1A), with suspected/subclinical autoimmune disease (Figure 1B), and with confirmed autoimmune disease (Figure 1C). As shown in Table 4, baseline PLR level was moderately correlated to NLR and CRP in the group of CCSVI patients with non-immunological etiology and suspected/subclinical autoimmune disease, indicating CCSVI itself may relate to the inflammatory process (Table 4, a and b). However, the level of IL-6 was only positively associated with CRP and age in the group of CCSVI patients with confirmed autoimmune disease (Table 4, c).
Figure 1.
Spearman’s correlations between age and inflammatory biomarkers in CCSVI with non-immunological etiology (A), with suspected/subclinical autoimmune disease (B), and with confirmed autoimmune disease (C). CCSVI, chronic cerebrospinal venous insufficiency.
Table 4. Spearman correlations among inflammatory markers and age.
| Variables | Age | PrePLR | PreNLR | PreRDW | IL-6 | CRP |
|---|---|---|---|---|---|---|
| (a) CCSVI with non-immunological etiology (n=98) | ||||||
| PrePLR | −0.094 | |||||
| PreNLR | −0.008 | 0.306* | ||||
| PreRDW | −0.091 | −0.005 | 0.175 | |||
| IL-6 | 0.090 | 0.105 | 0.324* | 0.107 | ||
| CRP | 0.062 | 0.213* | 0.196 | 0.224* | 0.339* | |
| NSE | −0.028 | −0.245* | −0.084 | 0.070 | 0.201 | 0.004 |
| (b) CCSVI with suspected/subclinical autoimmune disease (n=116) | ||||||
| PrePLR | 0.139 | |||||
| PreNLR | 0.137 | 0.465* | ||||
| PreRDW | 0.008 | −0.231* | −0.045 | |||
| IL-6 | 0.202 | 0.061 | 0.275* | 0.161 | ||
| CRP | 0.166 | 0.249* | 0.144 | −0.019 | 0.291* | |
| NSE | −0.124 | −0.029 | −0.090 | −0.002 | −0.101 | 0.118 |
| (c) CCSVI with confirmed autoimmune disease (n=41) | ||||||
| PrePLR | −0.194 | |||||
| PreNLR | 0.188 | 0.148 | ||||
| PreRDW | 0.049 | −0.045 | 0.055 | |||
| IL-6 | 0.388* | −0.117 | 0.241 | 0.163 | ||
| CRP | 0.069 | −0.268 | −0.031 | 0.108 | 0.477* | |
| NSE | 0.095 | −0.188 | −0.197 | −0.065 | 0.005 | 0.083 |
*, statistically significant at P<0.05. PreNLR, neutrophil to lymphocyte ratio on admission; PrePLR, platelet to lymphocyte ratio on admission; PreRDW, red blood cell distribution width on admission; CRP, C-reactive protein; NSE, neuron-specific enolase; IL-6, interleukin-6.
Discussion
To our knowledge, this is the first study evaluating the comprehensive clinical features of CCSVI with immunological disease background. In this study, a case series with 41 patients with confirmed autoimmune disease-related CCSVI was presented. Eight different immune-complex diseases, including APS, SS, IgG4-RD, BD, autoimmune hepatitis, Wegener’s granulomatosis, systemic sclerosis, and AQP4-antibody (AQP4-IgG)-positive neuromyelitis optica spectrum disorder (AQP4+ NMOSD) were analyzed. CCSVI cases combined with IgG4-RD, SS, and AQP4+ NMOSD-related CCSVI were never reported before. Furthermore, we explored the inflammatory and coagulation status in CCSVI patients with immunological etiology.
Groups with immunological etiology (including CCSVI patients with confirmed autoimmune disease and suspected/subclinical autoimmune disease) did not show a higher incidence of thrombophilia or increased pro-inflammatory factors (e.g., neutrophil, IL-6). However, patients with non-immunological etiology had a higher baseline level of CRP. Besides, baseline PLR level was moderately correlated to NLR and CRP in CCSVI patients with non-immunological etiology and suspected/subclinical autoimmune disease. Due to these findings, we postulated that an independent inflammatory process might involve in the pathogenesis of CCSVI, facilitated by multiple risk factors, among which autoimmune disease background could play a major role. In the subgroup analysis of CCSVI patients with immunological etiology, patients with confirmed autoimmune disease had a higher prevalence of thyroid dysfunction and HBV infection. Apart from having elevated autoimmune antibodies, they were also correlated with decreased C3 or C4 and increased ESR or IgG. These results were consistent with former clinical studies (19-21). Moreover, groups with APS and IgG4-RD presented in a relatively younger population than that with SS and had a higher CRP level than that with decreased C3.
The intricate relationship between inflammation (adaptive immune system) and complement pathway (innate immune system) and hemostasis (coagulation and thrombolysis) in immune-complex-mediated autoimmune diseases was reviewed in several studies (22-24). Based on their common points, we preferred to explain the mechanism from two perspectives: on a physiological level, the coexistence of hemostatic and inflammatory mediators is served as the first line to protect the body from self-antigens and non-self antigens, also termed as “immunothrombosis” or “thromboinflammation” in recent years (22,24). The common structural characteristic of consisting serine proteinases with trypsin-like activity contributes to the precise interplay between the complement system, the coagulation, and fibrinolytic cascade (25). Certain coagulation factors (FXa, thrombin, plasmin) have C3 and C5 convertase activity, contributing to an additional pathway of complement activation (21,26). Complement-derived inflammatory mediators (anaphylatoxin), such as C3a, C4a, and C5a, could increase vascular permeability, activate neutrophils, promote the release of tumor necrosis factor (TNF) from monocyte, upregulate tissue factor (TF), then initiating extrinsic coagulation pathway (27). Platelet can also be activated by the deposition of C4d split fragments, resulting in the facilitation of the coagulation process (20). While on the pathological level, hypocomplementemia is frequently prevalent in patients with APS and SLE, which predominantly relates to the chronic inflammatory process with the basis of the pathophysiology of immune complex-mediated diseases. The overly activated immune system causes the production of self-antigens with unbalanced consumption of complements. With the inverse relationship between complements and their derivatives, anaphylatoxin (C3a, C4a, and C5a) would further cause hypercoagulation state and even thrombotic events. Meanwhile, complement regulatory factors also decrease due to either attack from autoimmune antibodies, or increased consumption or lower expression of relevant genes (28,29). For example, during the pathogenesis of APS, beta-2 glycoprotein-I (β2-GPI) undergoes a conformational change from a circular form to an elongated form that can bind C3; then, C3 exposes its binding sites, which is more susceptible to degradation by complement factor H (CFH) and factor I (30). Moreover, β2-GPI shares structural similarity to CFH so that antibodies could also combine with CFH (31). With the growing appreciation of complement activation and thrombosis in immune-complex-mediated autoimmune diseases, novel therapies would be fostered, including antiplatelet, anticoagulants as immunomodulators, and targeted molecular therapy toward complements (25,26).
There are several limitations in our study. This is a real-world case-control study of patients with well-defined CCSVI. Patients with a history of autoimmune diseases usually underwent long-term standardized immunomodulation treatment prior to their enrollment, so that the inflammatory biomarker assay tended to be normal despite positive findings of autoimmune antibodies. Therefore, further studies on acute thrombotic events of autoimmune disease are needed. Moreover, our findings indicated the difference in the inflammatory activity among immunological diseases. Based on the complementary relationship between inflammation and thrombosis, we further raised a question on whether the severity of immunological diseases was correlated with increased inflammatory activity and elevated risk of thrombotic events.
Conclusions
Groups with immunological etiology did not show a higher incidence of thrombophilia or increased pro-inflammatory biomarkers. However, patients with non-immunological etiology had a higher baseline level of CRP. Besides, baseline PLR was moderately correlated to NLR and CRP in CCSVI patients with non-immunological etiology and suspected/subclinical autoimmune disease. Therefore, an independent inflammatory process may involve in the pathogenesis of CCSVI. For those with immunological etiology, autoimmune antibodies-mediated vessel wall damage and hypercoagulation state may also play a major role.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank Prof. Yuchuan Ding for his help in polishing the language of our paper.
Funding: This study was sponsored by the National Key R&D Program of China (2017YFC1308400), the National Natural Science Foundation (81371289), and the Project of Beijing Municipal Top Talent for Healthy Work of China (2014-2-015).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Xuanwu Hospital, Capital Medical University (2019-006), and informed consent was taken from all individual participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4201
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4201
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4201). The authors have no conflicts of interest to declare.
References
- 1.Albayram S, Kantarci F. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011;77:e126. [PubMed] [Google Scholar]
- 2.Simka M, Kazibudzki M. Chronic cerebrospinal venous insufficiency and multiple sclerosis: A commentary. Ann Neurol 2010;68:562-3; author reply 563-4. 10.1002/ana.22141 [DOI] [PubMed] [Google Scholar]
- 3.Zivadinov R, Ramanathan M, Dolic K, et al. Chronic cerebrospinal venous insufficiency in multiple sclerosis: diagnostic, pathogenetic, clinical and treatment perspectives. Expert Rev Neurother 2011;11:1277-94. 10.1586/ern.11.117 [DOI] [PubMed] [Google Scholar]
- 4.Laupacis A, Lillie E, Dueck A, et al. Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ 2011;183:E1203-E1212. 10.1503/cmaj.111074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traboulsee AL, Knox KB, Machan L, et al. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study. Lancet 2014;383:138-45. 10.1016/S0140-6736(13)61747-X [DOI] [PubMed] [Google Scholar]
- 6.Sundstrom P, Wahlin A, Ambarki K, et al. Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study. Ann Neurol 2010;68:255-9. 10.1002/ana.22132 [DOI] [PubMed] [Google Scholar]
- 7.Zivadinov R, Marr K, Cutter G, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011;77:138-44. 10.1212/WNL.0b013e318212a901 [DOI] [PubMed] [Google Scholar]
- 8.Singh RK, Bhoi SK, Kalita J, et al. Cerebral Venous Sinus Thrombosis Presenting Feature of Systemic Lupus Erythematosus. J Stroke Cerebrovasc Dis 2017;26:518-22. 10.1016/j.jstrokecerebrovasdis.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Steinlin MI, Blaser SI, Gilday DL, et al. Neurologic manifestations of pediatric systemic lupus erythematosus. Pediatr Neurol 1995;13:191-7. 10.1016/0887-8994(95)00110-2 [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Chen H, Zhang Y, et al. Clinical Characteristics of Cerebral Venous Sinus Thrombosis in Patients with Systemic Lupus Erythematosus: A Single-Centre Experience in China. J Immunol Res 2015;2015:540738. 10.1155/2015/540738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman T, Demirci S, Uluduz D, et al. Cerebral Venous Sinus Thrombosis as a Rare Complication of Systemic Lupus Erythematosus: Subgroup Analysis of the VENOST Study. J Stroke Cerebrovasc Dis 2019;28:104372. 10.1016/j.jstrokecerebrovasdis.2019.104372 [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Huang X, Li G, et al. Cerebral venous sinus thrombosis in Behcet's disease: a retrospective case-control study. Clin Rheumatol 2018;37:51-7. 10.1007/s10067-017-3718-2 [DOI] [PubMed] [Google Scholar]
- 13.Sorgun MH, Rzayev S, Kural MA, et al. Cerebral Venous Thrombosis in Behcet's Disease Patients Compared to Other Causes of Cerebral Venous Thrombosis: a Retrospective Study. Arch Rheumatol 2016;31:248-53. 10.5606/ArchRheumatol.2016.5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlebak A. Antiphospholipid syndrome presenting as cerebral venous sinus thrombosis: a case series and a review. J Clin Pathol 2016;69:337-43. 10.1136/jclinpath-2015-203077 [DOI] [PubMed] [Google Scholar]
- 15.Sareen D, Jain A, Paljor P. Pseudotumor syndrome associated with antiphospholipid antibodies and cerebral venous sinus thrombosis. J Assoc Physicians India 2002;50:603-5. [PubMed] [Google Scholar]
- 16.Standridge S, de los Reyes E. Inflammatory bowel disease and cerebrovascular arterial and venous thromboembolic events in 4 pediatric patients: a case series and review of the literature. J Child Neurol 2008;23:59-66. 10.1177/0883073807308706 [DOI] [PubMed] [Google Scholar]
- 17.Gilyarov MY, Belikova LP, Shchukin IA, et al. Neurological disorders in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Zh Nevrol Psikhiatr Im S S Korsakova 2016;116:93-102. 10.17116/jnevro201611610193-102 [DOI] [PubMed] [Google Scholar]
- 18.Shuleshova NV, Skoromets AA, Matskevich OR, et al. Acute cerebral sinus-thrombosis due to polyangiitis overlap syndrome with granulomatosis with polyangiitis (Wegener's granulomatosis) and eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Zh Nevrol Psikhiatr Im S S Korsakova 2016;116:30-5. 10.17116/jnevro201611612230-35 [DOI] [PubMed] [Google Scholar]
- 19.Scambi C, Ugolini S, Tonello M, et al. Complement activation in the plasma and placentas of women with different subsets of antiphospholipid syndrome. Am J Reprod Immunol 2019;82:e13185. 10.1111/aji.13185 [DOI] [PubMed] [Google Scholar]
- 20.Petri MA, Conklin J, O'Malley T, et al. Platelet-bound C4d, low C3 and lupus anticoagulant associate with thrombosis in SLE. Lupus Sci Med 2019;6:e000318. 10.1136/lupus-2019-000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foley JH, Walton BL, Aleman MM, et al. Complement Activation in Arterial and Venous Thrombosis is Mediated by Plasmin. EBioMedicine 2016;5:175-82. 10.1016/j.ebiom.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaertner F, Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol 2016;28:561-9. 10.1016/j.smim.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 23.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13:34-45. 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 24.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019;133:906-18. 10.1182/blood-2018-11-882993 [DOI] [PubMed] [Google Scholar]
- 25.Oikonomopoulou K, Ricklin D, Ward PA, et al. Interactions between coagulation and complement--their role in inflammation. Semin Immunopathol 2012;34:151-65. 10.1007/s00281-011-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keragala CB, Draxler DF, McQuilten ZK, et al. Haemostasis and innate immunity - a complementary relationship: A review of the intricate relationship between coagulation and complement pathways. Br J Haematol 2018;180:782-98. 10.1111/bjh.15062 [DOI] [PubMed] [Google Scholar]
- 27.Oku K, Nakamura H, Kono M, et al. Complement and thrombosis in the antiphospholipid syndrome. Autoimmun Rev 2016;15:1001-4. 10.1016/j.autrev.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi S, Brodsky RA, McCrae KR. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front Immunol 2019;10:449. 10.3389/fimmu.2019.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaturvedi S, Braunstein EM, Yuan X, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood 2020;135:239-51. 10.1182/blood.2019003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gropp K, Weber N, Reuter M, et al. beta(2)-glycoprotein I, the major target in antiphospholipid syndrome, is a special human complement regulator. Blood 2011;118:2774-83. 10.1182/blood-2011-02-339564 [DOI] [PubMed] [Google Scholar]
- 31.Ferluga J, Kishore U, Sim RB. A potential anti-coagulant role of complement factor H. Mol Immunol 2014;59:188-93. 10.1016/j.molimm.2014.02.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as