Summary
Matrin3 (MATR3) is a nuclear RNA/DNA-binding protein that plays pleiotropic roles in gene expression regulation by directly stabilizing target RNAs and supporting the activity of transcription factors by modulating chromatin architecture. MATR3 is involved in the differentiation of neural cells, and, here, we elucidate its critical functions in regulating pluripotent circuits in human induced pluripotent stem cells (hiPSCs). MATR3 downregulation affects hiPSCs' differentiation potential by altering key pluripotency regulators' expression levels, including OCT4, NANOG, and LIN28A by pleiotropic mechanisms. MATR3 binds to the OCT4 and YTHDF1 promoters favoring their expression. YTHDF1, in turn, binds the m6A-modified OCT4 mRNA. Furthermore, MATR3 is recruited on ribosomes and controls pluripotency regulating the translation of specific transcripts, including NANOG and LIN28A, by direct binding and favoring their stabilization. These results show that MATR3 orchestrates the pluripotency circuitry by regulating the transcription, translational efficiency, and epitranscriptome of specific transcripts.
Subject areas: molecular biology, cellular neuroscience, stem cells research
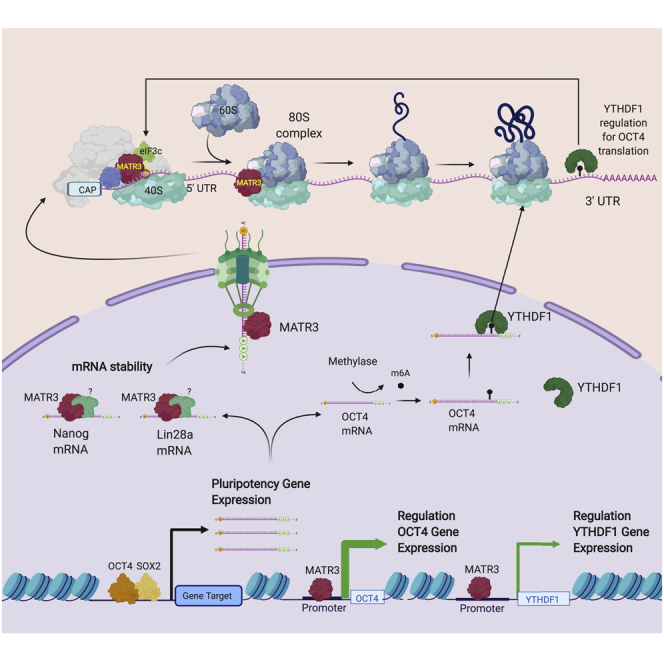
Graphical abstract
Highlights
-
•
MATR3 orchestrates the pluripotency circuitry in hiPSCs
-
•
MATR3 binds to the OCT4 and YTHDF1 promoters and favors their expression
-
•
YTHDF1 binds the m6A-modified OCT4 mRNA
-
•
MATR3 regulates the translation of specific transcripts including NANOG and LIN28A
Molecular biology; cellular neuroscience; stem cells research
Introduction
MATR3 is a highly conserved 125-kDa protein, abundantly expressed in the inner nuclear matrix (Zeitz et al., 2009). It has a multi-domain structure composed of a nuclear localization signal (NLS), two C2H2-type zinc finger DNA-binding domains, and two RNA recognition motif domains (Belgraders et al., 1991; Hisada-Ishii et al., 2007). MATR3 plays pleiotropic roles by binding both DNA and RNA, and it is involved in RNA metabolism, regulating the levels of long noncoding RNAs (Banerjee et al., 2017) and controlling nuclear mRNA export (Boehringer et al., 2017; Zhang and Carmichael, 2001) and mRNA stabilization (Salton et al., 2011). RNA-mediated MATR3 interaction with DHX9 and HNRNPK indicates that MATR3 is involved in the assembly of multiprotein complexes to exert its functions in RNA metabolism (Salton et al., 2011). MATR3 was initially identified as a nuclear scaffold protein involved in the assembly of the internal fibrogranular network by binding DNA sites known as matrix/scaffold attachment regions (Hisada-Ishii et al., 2007). The binding of the POU-homeodomain transcription factor Pit1 (Pou1f1) to its transcriptional enhancers is dependent on the presence of a MATR3-rich network: MATR3-dependent physical organization of the chromatin allows the transcriptional assembly of the Pit1-β-catenin-SatB1 complex (Skowronska-Krawczyk et al., 2014). MATR3 genetic variants are associated with autosomal dominant distal myopathy, pharyngeal and vocal cord dysfunction, as well as familial and sporadic cases of amyotrophic lateral sclerosis (ALS) (Johnson et al., 2014; Leblond et al., 2016; Lin et al., 2015; Marangi et al., 2017; Origone et al., 2015). MATR3 has also been found in neuronal cytoplasmic inclusions alone or associated with TDP-43 (Tada et al., 2018), C9orf72 hexanucleotide repeat expansion Di-Peptide Repeats (Johnson et al., 2014), or ectopic ALS-linked FUS mutant (Yamaguchi and Takanashi, 2016) in cells derived from patients with ALS. MATR3 is a key element for neural stem cell (NSC) differentiation, and its role is regulated by ATM-mediated phosphorylation (Niimori-kita et al., 2018), but, to date, its role in pluripotency maintenance and cell fate commitment has not been elucidated. Pluripotent stem cell maintenance and differentiation involves numerous and coordinated changes that require transcription factors, including OCT4, NANOG, SOX2, KLF4 (Takahashi and Yamanaka, 2016), as well as several RNA-binding proteins (RBPs) (Guallar and Wang, 2014). Among the RBPs, the most notable example is LIN28A, a factor that can be used to reprogram human somatic cells to pluripotent stem cells (Yu et al., 2007). The main function of LIN28A is to regulate mRNAs involved in embryonic development by interacting directly with RNAs, such as OCT4 (Qiu et al., 2009), also in collaboration with other RBPs such as L1TD1 (Närväa et al., 2012), and by disrupting the maturation of certain miRNAs, as the LET7 family (Qiu et al., 2009; Rybak et al., 2008). Other notable examples of RBPs are PTBP1, which is essential for embryonic stem cells (ESC) proliferation (Shibayama et al., 2009), and, more recently, the readers of the N6-methyladenosine (m6A) RNA modification (Deng et al., 2018), including the YTH domain-containing proteins and the IGF2BP proteins, which bind and regulate the stability or translation of target genes (Huang et al., 2018; Wu et al., 2019). Indeed, transcripts encoding core pluripotency transcription factors are m6A methylated and the decrease of the global RNA methylation status reduces the ESCs' ability to properly differentiate (Batista et al., 2014; Geula et al., 2015). Therefore, the m6A modification plays pivotal physiological functions in regulating RNA metabolism in pluripotency, leading to changes in the expression of key factors such as OCT4, SOX2, LIN28A, and NANOG (Chen et al., 2015a).
We investigated the role of MATR3 in maintaining pluripotency and generated human iPSCs in which MATR3 was stably silenced. We found that MATR3 plays a critical role in regulating the hiPSCs' single-cell growth, colony formation, and neural differentiation potential, by modulating the expression level of critical regulators of pluripotency including NANOG, LIN28A, and OCT4. MATR3 interactome investigation revealed the role of the protein in the translation process, supported by direct binding and stabilization of NANOG and LIN28A transcripts and thus favoring their expression. In addition, MATR3 chromatin immunoprecipitation (ChIP) revealed a dual level of OCT4 regulation. MATR3 binds the promoter regions of OCT4 and YTHDF1 and regulates their transcription. YTHDF1, an m6A reader, binds and regulates the levels of the m6A OCT4 and LIN28A transcripts. In conclusion, MATR3 contributes, both directly and indirectly, to the maintenance of the pluripotency in hiPSCs, fine-tuning the expression of key pluripotency regulators.
Results
MATR3 is essential for the clonogenic ability of human iPSCs
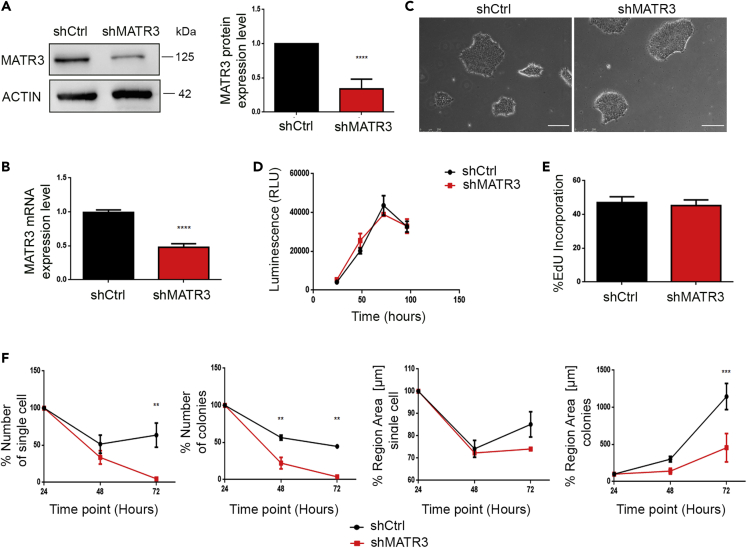
To understand the role of MATR3 in pluripotency maintenance and differentiation, we generated hiPSCs in which MATR3 was stably silenced by short hairpin RNA (shRNA) strategy. Commercial hiPSCs were infected with lentiviral particles loaded with shRNA sequence targeting human MATR3 (shMATR3), and stably silenced cells were selected. In the polyclonal shMATR3-iPSC population, MATR3 protein levels were reduced by 60% when compared with control hiPSCs (shCtrl) (Figures 1A, 1B, and S1A). We obtained a homogeneous population in which 55% of the cells showed a decrease of MATR3 (Figures S1A and S1B). shCtrl and shMATR3 cells formed round and compact colonies with no appreciable morphological differences (Figure 1C) and did not show any cell proliferation or viability defects (Figures 1D and 1E). Interestingly, shMATR3 cells exhibited a significant growth deficit in sub-optimal conditions, i.e., following single-cell dissociation. Indeed, shMATR3 cells plated in these conditions showed reduced growth and colony formation ability compared with shCtrl cells (Figures 1F and S1C). The same result was obtained by a limiting dilution assay. Control cells showed no defect in clonogenic capability, whereas only cells with at least 50% of MATR3 expression level were able to form colonies. In addition, the level of pluripotency markers decreased in accordance with the MATR3 level (Figures S1D and S1E). These results suggest that MATR3 downregulation does not impact cell growth in standard culturing conditions, but it hampers single-cell growth and colony formation of hiPSCs.
Figure 1.
MATR3 is essential for single-cell growth of hiPSCs
(A) MATR3 protein expression levels were analyzed by western blot (WB) in hiPSCs stably infected with shMATR3 and shCtrl lentiparticles. β-ACTIN was used as housekeeping (left panel). MATR3 levels were quantified by densitometry analysis. The bar plots show the mean values of three independent experiments (right panel). Graph represents a mean of three biological replicates ± SEM. p value was calculated by t test (∗∗∗∗p < 0.0001).
(B) MATR3 RNA expression levels were measured by real-time qPCR in hiPSC line stably infected with shMATR3 and shCtrl lentiparticles. Data are presented as mean of three biological replicates ± SEM. p value was calculated by t test (∗∗∗∗p < 0.0001).
(C) Pictures of shCtrl and shMATR3 hiPSCs (scale bar, 250 μm).
(D) Growth curve of shCtrl and shMATR3 hiPSCs were performed using OZBlue assay. Data are presented as the mean ± SEM of three biological replicates; two-way ANOVA (not significant, p>0.05).
(E) EdU proliferation assay for shMATR3 and shCtrl hiPSCs. Percentage of EdU-incorporating cells was measured by flow cytometry. Data are presented as means ± SEM (n = 2 biological replicates, 6 technical replicates); t test not significant, p>0.05 .
(F) Analyses of shMATR3 and shCtrl hiPSCs following single-cell seeding. Cells were incubated with AP Live Stain at each time point (time point, 24, 48, and 72 h). Images were acquired with the Operetta High Content Screening System using 2× objective 0.08NA. The region areas [μm] for the single cells and for the colonies are reported for each time point. The comparison is between shCtrl and shMATR3 cells. Two-way ANOVA (∗∗ p<0.01; ∗∗∗ p< 0.001).
MATR3 is required for hiPSCs neuronal differentiation
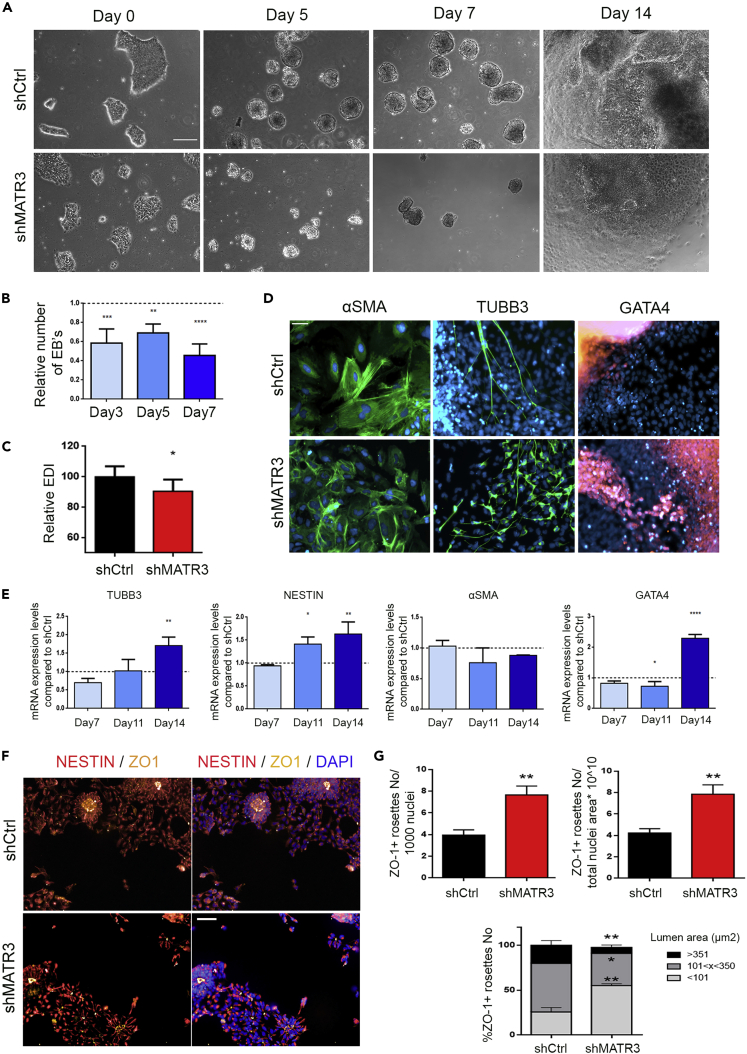
As MATR3 downregulation impacts hiPSCs self-renewal, we explored the possible occurrence of defects during the trilineage commitment of shMATR3 cells by performing an embryoid bodies (EBs) assay (Lin et al., 2014) (Figures 2A and S2A). MATR3 silencing impaired EB formation ability of hiPSCS, as shown by the substantial reduction in EB number (Day 7, 47% reduction; Figure 2B) and by the marked, altered morphology of surviving floating EBs (Figure 2A), as shown by an increased elongation distortion index (EDI) (Figure 2C). Following the plating in adhesion and culturing for one additional week, EBs spontaneously differentiated toward cell types representative of all the three germ layers (Figure 2D). Fourteen-day-old MATR3-silenced cultures showed the expression of all three lineage markers and an up-regulation of mRNA expression levels of neuroectodermal (TUBB3 and NESTIN) (Niimori-kita et al., 2018) and endodermal (GATA4) markers (Vallier et al., 2009). Notably, no alteration in mesodermal marker (αSMA) expression was detected (Figure 2E). To better elucidate MATR3 role during in vitro neuralization, we exposed shMATR3 (and shCtrl) hiPSCs to monolayer neural induction conditions to generate neural precursors cells (NPCs) (Jha et al., 2015). At Day 12 of the neuralization protocol, cultures were enriched in NPCs as assessed by FABP7 live imaging assay (Leong et al., 2013; Yun et al., 2012) (Figure S2B). NPCs were organized in the neural rosette, i.e., radially ordered structures in which NPCs show apical tight junction marker ZO-1 in the central lumen (Curchoe et al., 2012) (Figure 2F). Quantitative analysis of rosette formation capacity on neural-oriented cultures revealed that shMATR cells had a 2-fold increased capability to generate ZO-1-positive neural rosettes in culture with respect to control cells (Figures 2F and 2G). Moreover, morphometric analyses showed that shMATR3 cultures were characterized by a 2-fold increase in the percentage of rosettes characterized by a small lumen (<101 μm2) and by a 1.5-fold decrement of medium (101 < area <350 μm2) and 3-fold decrement of large (>350 μm2) rosettes (Figure 2G). Differentiation of NPC cultures toward the neuronal lineage showed that shMATR3 cells were characterized by a reduction of neurite length and arborization (Figure S2C). Collectively, these data suggest that MATR3 plays a pivotal role in the polarization process leading to the neural rosette cytoarchitecture and that its absence affects the subsequent terminal neuronal maturation progression.
Figure 2.
MATR3 is required for hiPSCs' neural differentiation and neuronal maturation
(A) Phase contrast pictures of shCtrl and shMATR3 embryoid body (EB) cultures at specific time points (scale bar, 250 μm).
(B) EBs count analysis performed with Harmony 4.1 software (PerkinElmer) on images acquired with Operetta High Content Screening System (PerkinElmer) using 2× 0.08NA objective. Data are presented as the mean ± SEM. p value was calculated using one-way ANOVA (∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001).
(C) Analysis of the elongation distortion index (EDI), a derivative of circularity (EDI = 1/Circularity − 1). Data are presented as the mean ± SEM. p value was calculated using t test (∗p < 0.05).
(D) Immunofluorescence staining for germ layer markers performed on 14 days of EB cultures. EBs were stained for α-SMA (Mesoderm), TUBB3 (Ectoderm), and GATA-4 (Endoderm). Cell nuclei were stained with DAPI (blue). Scale bar, 50 μm.
(E) qRT-PCR analysis performed on 14 days of EB cultures. Values are normalized on the internal housekeeping (β-ACTIN) and reported in comparison with shCtrl samples (reported at value 1 in the graph). Results for NESTIN and TUBB3, α-SMA, and GATA-4 transcripts are presented as mean ± SEM. p value was calculated by one-way ANOVA (∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001).
(F) Immunofluorescent pictures of Day 12 neuralized shCtrl- and ShMATR3-hiPSC cultures. Neural rosettes' lumens are immunoreactive for ZO-1, and NPCs are stained for Nestin. Scale bar, 50 μm.
(G) (Top) Quantification of ZO-1+ rosettes number (on 1,000 nuclei or on total nuclei area (∗1010)). (Bottom) The percentage of the number of ZO-1+ rosettes having lumen area (x) >351, 101<x<350 or <100 μm2. Quantification was done using Columbus software. Statistical analysis was presented as mean ± SEM of three biological replicates. p value was calculated by t test (∗p < 0.05; ∗∗p < 0.01).
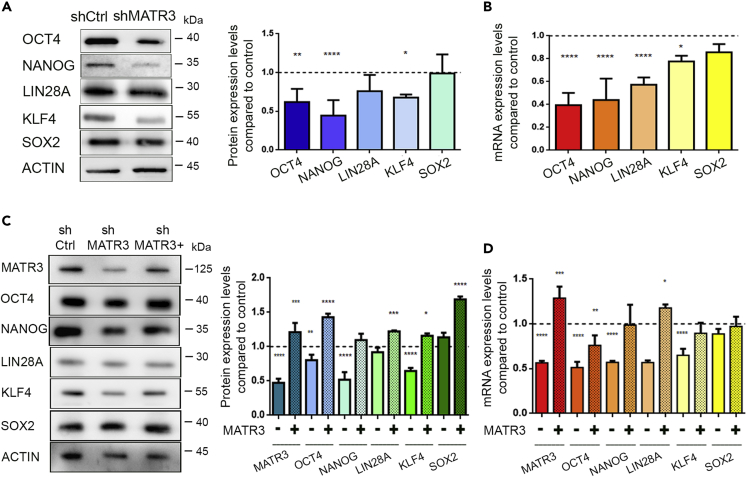
MATR3 downregulation decreases the expression level of OCT4, NANOG, KLF4, and LIN28A
As we observed that MATR3 downregulation impairs single-cell growth and alters lineage commitment during EBs differentiation and neuralization, we reasoned that this might be the consequence of altered pluripotency circuitries. Analysis of the transcript and protein expression levels for several pluripotency determinants revealed that MATR3 downregulation in hiPSCs leads to decreased levels of OCT4, NANOG, and KLF4 (Figures 3A, 3B, S3A, and S3B), but not of SOX2. We also observed a significant decrease in LIN28A mRNA levels and only a faint reduction of the protein amount (Figures 3A, 3B, S3A, and S3B). Noteworthy, restoration of MATR3 levels in shMATR3 hiPSCs ultimately rescued the expression of the pluripotency markers (Figures 3C and 3D) and the ability of shMATR3 cells to grow in sub-optimal conditions (Figures S3C and S3D), suggesting a specific role for MATR3 in their regulation. These data indicate that MATR3 contributes to safeguarding the expression levels of several pluripotency regulators, likely by modulating distinctive molecular mechanisms.
Figure 3.
MATR3 downregulation affects the expression levels of pluripotency determinants
(A and B) WB and qRT-PCR analyses for OCT4, NANOG, KLF4, and LIN28A performed on shMATR3-and shCtrl-hiPSC cultures. WB and RT-qPCR results were normalized on the internal housekeeping (β-ACTIN) and reported in comparison to the shCtrl samples (reported as value 1 in the graph). Both analyses were presented as the mean ± SEM of three biological replicates. p value was calculated by one-way ANOVA (∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001).
(C and D) WB and RT-qPCR analyses for OCT4, NANOG, KLF4, and LIN28A performed on shCtrl-, shMATR3- (−), shMATR3- and on MATR3 rescued shMATR3- (+) cultures. WB and qRT-qPCR analyses were normalized on the internal housekeeping (β-ACTIN) and reported in comparison to the shCtrl values (reported as value 1 in the graph). Values as reported as mean ± SEM. p value was calculated by one-way ANOVA (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001).
MATR3 is associated with the translation machinery and RNA processing
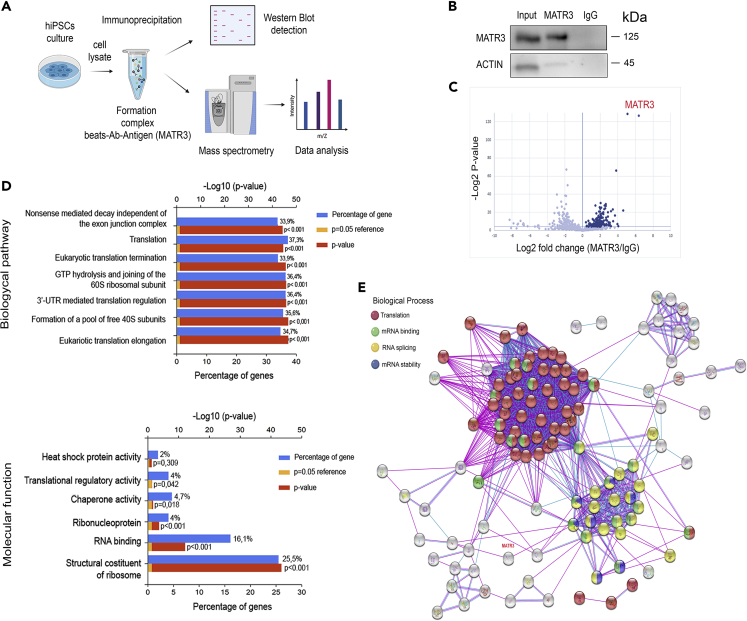
To investigate how MATR3 could fine-tune the pluripotency circuitry, we explored total cell MATR3 interactome by evaluating its binding partners in parental hiPSCs. Immunoprecipitation of MATR3 and subsequent tandem mass spectrometry (IP-MS) led to the identification of MATR3-associated proteins in hiPSCs (Figure 4A). Immunoblot analysis of the immunoprecipitated fractions confirmed MATR3 enrichment. In contrast, no MATR3 was detected in the IgG immunoprecipitated lysates, confirming the specificity of the assay (Figure 4B). We found 151 proteins that were at least 1.5-fold enriched over IgG (p ≤ 0.05) (Figure 4C, Table S1, Figure S4). Among them, we uncovered several proteins involved in RNA metabolic processes, including alternative splicing, mRNA stability, transcription, and RNA translocation (Table S1, Figures S4A and S4B). The dataset included some known MATR3-interacting RBPs including RALY (Tenzer et al., 2013); PTBP1, which plays a role in alternative splicing (Coelho et al., 2015); DHX9; and HNRNPK, which have been proposed to interact with MATR3 and regulate RNA processing (Salton et al., 2011). We also observed a protein-protein interaction with nucleopore proteins (NUP-205, NUP-93) that can suggest the mechanism by which MATR3 shuttles from the nucleus. In addition, several proteins involved in the translational machinery were found to be enriched, including EIF4A1, EIF4A3, EIF3CL, EIF3D, and EIF3F (Table S1), along with RBPs involved in the process of translation such as L1TD1 (Närväa et al., 2012) and IGF2BP1, one of the readers of m6A modification (Deng et al., 2018). Indeed, a functional enrichment analysis of Gene Ontology categories within this dataset showed significant enrichment in terms related to translation, mRNA surveillance, and ribosome components (Figure 4D and Table S2). Accordingly, protein-protein interaction network analysis revealed that MATR3 interactors group into a distinct Cytoplasmic/Translation cluster, which includes ribosomal subunits and translation initiation factors (Figure 4E and Table S3). Overall, MATR3 protein interactome suggests a pleiotropic role of this protein in hiPSC nuclear RNA metabolism and a previously unrecognized role in the cytoplasm, specifically at the translation apparatus.
Figure 4.
MATR3 is associated to the translational machinery and RNA processing
(A) Schematic representation of the strategy used for the identification of MATR3 interactors. (B) WB performed on parental hiPSC cell lysates co-immunoprecipitated with MATR3 antibody (ab) and IgG as control. Inputs are also shown (Input). The β-ACTIN signal is present only in the input sample confirming the specificity of MATR3 antibody.
(C) Volcano plot representation of MS analysis results. The ScatterPlot showed 151 proteins significantly enriched in MATR3 (dark blue dots, right of the graph) versus IgG pulldown (left part of the graph), with a t test p value < 0.05 (or > 4.32 when −log2 transformed).
(D) Functional enrichment analysis of MATR3 interactors conducted by FunRich software. Two different GO biological processes analyses, Biological pathway and Molecular function, were used. Significance of the interactions is represented by a red histogram, p < 0.001 (orange with minimum p < 0.05), and the percentage of overrepresentation of the genes is represented by a blue bar.
(E) Protein-protein interactions calculated by STRING interaction database. Only high confidence interactions (interaction score >0.7), as determined by the STRING database, were accepted. Each node represents a protein, and edges represent protein-protein associations. In the graph, proteins involved in translation (red plot), mRNA processing (green plot), RNA splicing (yellow plot), and mRNA stabilization (blue plot) are reported.
MATR3 regulates the translation efficiency of LIN28A, NANOG, and SOX2 mRNAs
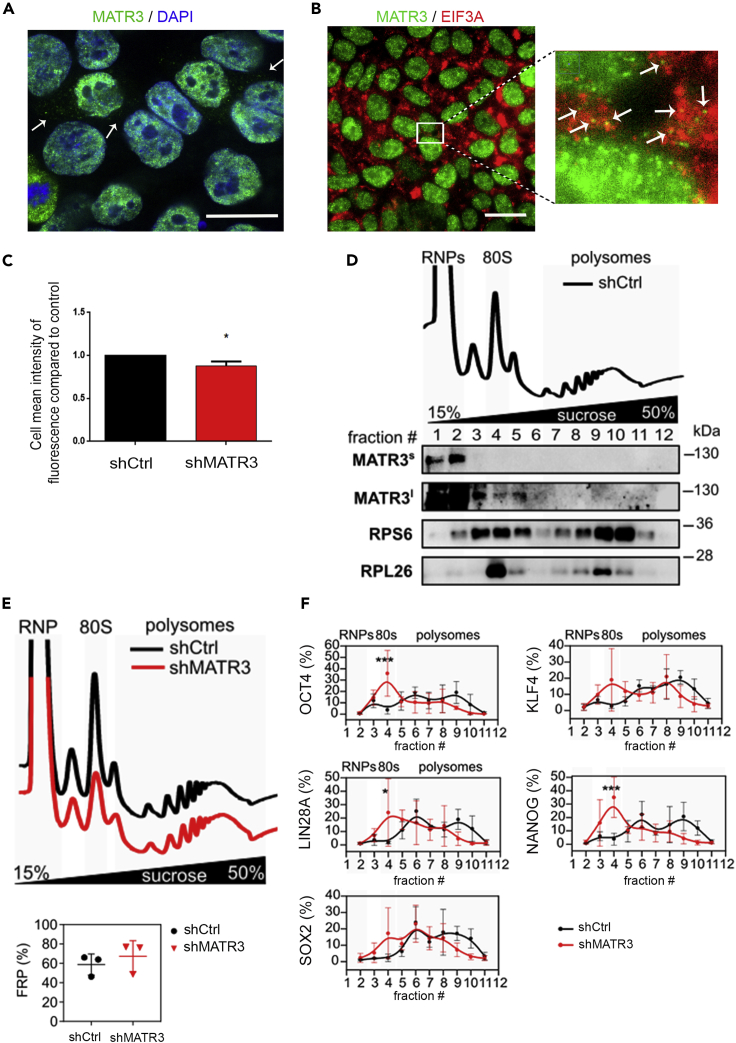
IP-MS results support a putative new role for MATR3 in the control of protein synthesis in the cytoplasm. To better investigate this issue, we performed a confocal imaging analysis that revealed a partial localization of MATR3 in the cytoplasm (Figure 5A). Quantitative analysis showed that 25% of total MATR3 immunoreactive spots are in the cytoplasm (Figure S5A). However, this result is overestimating the amount of cytoplasmic MATR3 as the real number of nuclear MATR3 spots is hardly detectable, due to the high presence of MATR3 in the nucleus. The quantification of MATR3 fluorescence intensity in the cytosol and in the nucleus revealed that about 17% of the entire MATR3 fluorescence intensity derived from cytoplasmic MATR3 ( Figure S5B). Importantly, this analysis shows that MATR3 maintained a comparable cell distribution in shCtrl and shMATR3 cell lines (Figures S5C and S5D) suggesting that its downregulation did not affect specifically either nuclear or cytoplasmic functions.
Figure 5.
MATR3 regulates the translation efficiency of OCT4, LIN28A, and NANOG mRNAs
(A) Immunofluorescence pictures to detect the subcellular localization of MATR3 in hiPSCs. Scale bar, 20 μm.
(B) Pictures of hiPSCs stained for MATR3 (green signal) and eIF3a (red signal). Nuclei were stained with DAPI (blue signal). Scale bar, 20 μm. Zoom highlights an overlight between MATR3-EIF3A (orange spots).
(C) Evaluation of de novo protein synthesis measured by O-propargyl-puromycin (OPP) Alexa Fluor 488 incorporations. Quantification performed by Operetta High Content Screening System. Data presented as mean ± SEM of three biological replicates. shMATR3 values are compared with shCtrl (reported as value 1). The statistical significance was calculated by t test, 95% of confidence.
(D) Polysome profiling performed on shCtrl hiPSC and co-sedimentation profiles of MATR3 and ribosome markers RPS6 and RPL26. The signal of MATR3 along the profile is shown for short (MATR3s) and long (MATR3l) exposure times of acquisition. A single biological replicate is shown.
(E) Polysomal profiles of shCtrl and shMATR3 hiPSC and comparison between FRP in shCtrl and shMATR3 (shCtrl: n = 3, shMATR3: n = 3). No significant changes identified with paired two-tailed t test (p = 0.1075).
(F) qRT-PCR analysis for SOX2, OCT4, LIN28A, NANOG, and KLF4 performed on mRNA from fractions obtained from the polysome profiling. Statistical analysis was performed on the mean of three biological replicates, two-way ANOVA (∗p < 0.05; ∗∗∗p < 0.001).
To validate IP-MS experiment and evaluate the presence of MATR3 with the translational machinery, we performed confocal images of MATR3 and EIF3A and EIF3C, components of the eIF3 complex that is required for the initiation of protein synthesis (Masutani et al., 2007) (Figures 5B and 5E). Co-localization analysis performed on 3D (x,y,z) revealed that about 20% of cytoplasmic MATR3 signal co-localizes with the eIF3 proteins (Figure S5F). As the high concentration of the ElF3 proteins in the cytoplasm makes the co-localization uncertain, we used, β-ACTIN, another highly abundant cytoplasmic protein, as negative control. In this case, only a few spots were double-positive (Figures S5G and S5H) suggesting that our previous co-localizations were EIF3 specific. Next, by evaluating de novo protein synthesis, we investigated the role of MATR3 in translation and whether its downregulation affected global translation. O-propargyl-puromycin (OPP) incorporation assays revealed a marginal reduction of de novo protein synthesis in shMATR3 cells (Figure 5C). To explore the potential interaction of MATR3 with the components of the translational machinery, we performed polysome profiling after sucrose gradient fractionation of shCtrl cytoplasmic lysate (Panda et al., 2017). In agreement with our proteomics analysis of MATR3 interactors, we observed that a subpopulation of MATR3 co-sediments with ribosomes and polysomes in control cells (Figure 5D). We ruled out the possibility that MATR3 signal is caused by nuclear contamination of the cytoplasmic lysates, performing a control co-sedimentation analysis of Histone 3 (H3) (Figure S5I). Comparing the polysome profiles in shCtrl and shMATR3 conditions (Figure 5E), we observed an increase of the 80S upon MATR3 silencing. We quantified this effect, calculating the fraction of ribosomes in polysomes in both conditions, finding that when MATR3 is downregulated, this value increased. Although this change is limited and not statistically significant, the trend suggests that reduced MATR3 expression has a translational effect, as also observed in the reduction of de novo protein synthesis observed in OPP assays of shMATR3 iPS cells (Figure 5C). To further investigate if MATR3 may play a translational role in regulating specific genes as the pluripotency factors at the translation level, we performed a co-sedimentation analysis of OCT4, KLF4, LIN28A, NANOG, and SOX2 mRNAs in shCtrl and shMATR3 iPS cells. Our results showed that, in shMATR3 cells, OCT4, LIN28A, and NANOG mRNAs shift from heavier to lighter fractions along the profile, suggesting a possible defect in the recruitment of these mRNAs on polysomes (Figure 5F). Taken together, these data suggest that a portion of cytoplasmic MATR3 protein is associated with the translational apparatus and that its absence affects global translation only marginally. However, MATR3 downregulation induced a decrease in the loading on polysomes of mRNAs encoding for OCT4, LIN28A, and NANOG pluripotency factors. Taken together, these data suggest that the presence of MATR3 on the translation apparatus is necessary to favor the polysomal loading of a specific subset of mRNAs.
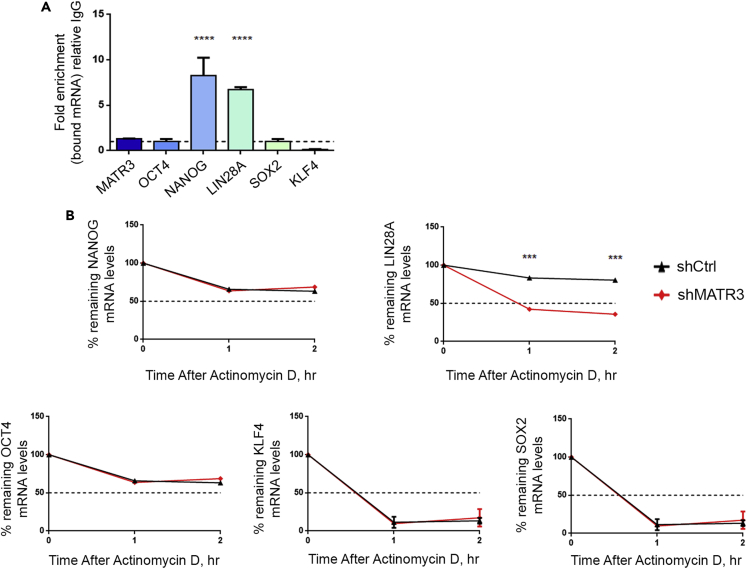
MATR3 stabilizes NANOG and LIN28A mRNAs
To understand whether translation efficiency was linked to MATR3 RNA-binding ability, we investigated which of the pluripotency regulators transcripts among OCT4, NANOG, LIN28A, SOX2, and KLF4 were directly bound by MATR3. RNA immunoprecipitation (RIP) of endogenous MATR3 in parental hiPSCs and analysis of the abundance of transcripts by qPCR revealed that MATR3 significantly binds to NANOG and LIN28A mRNAs (Figure 6A). Interestingly, the same result was not seen for OCT4, SOX2, and KLF4, suggesting that changes in their transcript levels, observed during MATR3 downregulation, are likely dependent on indirect molecular mechanisms. MATR3 binds hundreds of transcripts contributing to the stabilization of some of them, including HTLF (FOXN2) and HNT (RREB1) (Salton et al., 2011). We measured the mRNA half-lives of pluripotency regulators by arresting their de novo RNA synthesis in shCtrl- and shMATR3-hiPSCs (Figure 6B). MATR3 silencing led to significant reductions in NANOG (p<0.05 at 1 h time point) and LIN28A (p<0.05 at 1 h) mRNA half-lives, but did not affect OCT4, SOX2, and KLF4 transcripts half-lives (Figures 6B and S6). These results indicate that MATR3 regulates the mRNA levels of NANOG and LIN28A by increasing their stability via direct binding, thus protecting these transcripts from degradation. Coherently, half-lives of the OCT4, SOX2, and KLF4 transcripts, which are not bound by MATR3, are not affected by MATR3 downregulation.
Figure 6.
MATR3 stabilizes NANOG and LIN28A mRNAs
(A) MATR3 RNA immunoprecipitation (RIP) performed on parental hiPSCs followed by qRT-PCR analysis. Fold enrichment was relative to IgG. Values are presented graphically as mean ± SEM from three biological replicates. One-way ANOVA (∗∗∗∗p < 0.0001).
(B) qRT-PCR analysis for the evaluation of mRNA half-lives in shCtrl and shMATR3 hiPSCs performed by actinomycin-D assay. qRT-PCR values are normalized to their internal housekeeping (β-ACTIN). Results are expressed as percentages of mRNA abundance relative to Time 0. Statistical analysis was performed on the mean of three biological replicates, two-way ANOVA (∗∗∗p < 0.001).
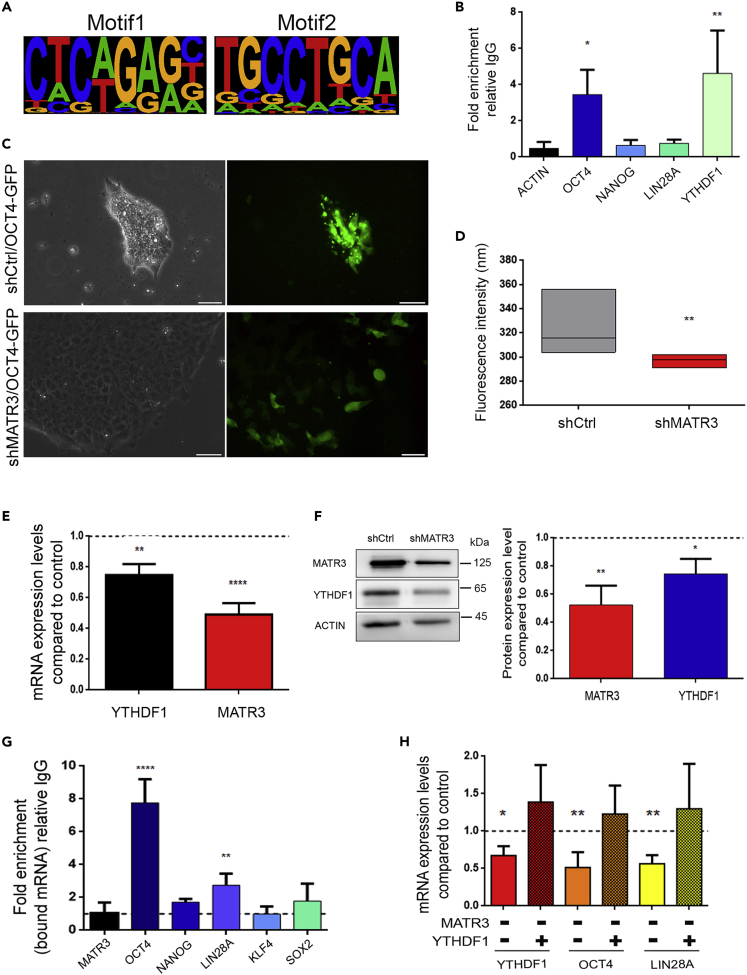
MATR3 sustains OCT4 expression by regulating YTHDF1 expression
We then sought to understand whether MATR3 could regulate these factors at the transcriptional level (Skowronska-Krawczyk et al., 2014). In rat pituitary cells (GC cells), ChIP sequencing (ChIP-seq) using a matrin-3 antibody, revealed that matrin-3 binds to DNA regulatory sequences, by physically interacting with the Pou1f1 transcription factor and contributes to its activity (Skowronska-Krawczyk et al., 2014). Inspection of Chip-seq peaks revealed that their genomic positions corresponded mainly to distal regions from the transcription start sites (Table S4). Notably, a fraction was directly associated with promoter regions, including the binding sequence of the Pou family, as described in the original (Skowronska-krawczyk et al., 2015). Functional analysis performed on members of this list revealed that MATR3 was mainly present in promoter regions of genes involved in the development, stemness, and stem cell proliferation processes (Figures S7A and S7B and Table S4). Indeed, we found notable examples of genes regulating embryo development including Shh (sonic hedgehog signaling molecule) (Blaess et al., 2015; Haraguchi et al., 2001; Tickle et al., 2017; Weed et al., 1997), Sox factors (Sox4, Sox5, Sox11) (Bowles et al., 2000; Potzner et al., 2010; Sarkar and Hochedlinger, 2014; She and Yang, 2015), and Wnt signalling (Sfrp2, Wnt7a) (Hao et al., 2006; Kele et al., 2012; Miao et al., 2018; Ren et al., 2018). We then shortlisted the first most significant 500 ChIP-seq peak sequences, ordered according to peak score, and obtained the two best MATR3 DNA-binding motifs by using HOMER (Figure 7A). Comparing these motifs to available databases we could not find any obvious matching (Table S4). We examined the LIN28A, NANOG, SOX2, KLF4, and OCT4 transcriptional start site (TSS) upstream regions and checked for the presence of the MATR3 DNA-binding consensus sequence. We did not find any consensus sequence on these genes, but on OCT4 (Table S5, 114 significant putative binding sequences). Experimental validation by ChIP assay in hiPSCs using the anti-MATR3 antibody confirms the enrichment of MATR3 on the OCT4 promoter, but we did not report any enrichment of MATR3 in the NANOG and LIN28A upstream TSS regions (Figure 7B). To confirm MATR3 role in OCT4 transcription, we used a stable hiPSC line expressing GFP under the control of OCT4 promoter (hiPSCs OCT4-GFP). Indeed, stable MATR3 silencing (shMATR3/OCT4-GFP cell line) (Figure 7C) showed strong loss of GFP fluorescence when compared with shCtrl/OCT4-GFP, highlighting the importance of MATR3 in regulating OCT4 transcription (Figures 7C and 7D).
Figure 7.
MATR3 regulates OCT4 expression by binding to its promoter and by regulating YTHDF1 transcription
(A) Highest scoring motifs analysis (best motif on the left, and second-best on the right) on the 500 DNA sequences most significantly bound by MATR3 according to ChIP-seq assay (Skowronska-Krawczyk et al., 2014), obtained with HOMER (Heinz et al., 2011).
(B) Chromatin immunoprecipitation (ChIP) on ACTIN, OCT4, NANOG, LIN28A, and YTHDF1 promoters performed using antibodies against MATR3 and IgG as the negative control. Data were normalized to non-immunoprecipitated samples (INPUT) and compared with the IgG sample. OCT4 and YTHDF1 promoters showed a region predicted to contain the MATR3 DNA consensus sequence; reported as mean ± SEM. p value values were calculated by one-way ANOVA (∗p < 0.05; ∗∗p < 0.01).
(C) Generation of stable hiPSC line expressing green fluorescent protein (GFP) under control of the OCT4 promoter. hiPSCs OCT4-GFP stably infected with shMATR3 and shCtrl lentiviral vectors. Scale bar, 50 μm.
(D) shMATR3/OCT4-GFP fluorescence intensity was quantified and compared with shCtrl/OCT4-GFP. The quantification of immunofluorescence intensity was performed using the ImageJ mean fluorescence intensity measurement. Data are reported as mean ± SEM for three biological replicates; p value was calculated using a t test (∗∗p < 0.01).
(E) qRT-PCR analyses of YTHDF1 in shMATR3 hiPSCs compared with shCtrl cells. Values are normalized on internal housekeeping (β-ACTIN) and reported in comparison to shCtrl values (reported as value 1 in the graph). The mean ± SEM of three biological replicates are reported. p value was calculated by one-way ANOVA (∗∗p < 0.01; ∗∗∗∗p< 0.0001).
(F) Left: Representative WB of MATR3 and YTHDF1 in shMATR3 hiPSCs compared with shCtrl cells. Right: densitometric analysis of three biological replicates. Values are normalized on internal housekeeping (β-ACTIN) and reported in comparison to shCtrl values (reported as value 1 in the graph). The mean of three biological replicates are reported. p value was calculated by one-way ANOVA (∗p < 0.05; ∗∗p < 0.01).
(G) RIP using the YTHDF1 antibody in the Gibco cell line (A18945), followed by RT-qPCR. Fold enrichment was relative IgG. Represented graphically as mean ± SEM of three biological replicates. One-way ANOVA (∗∗∗p < 0.001; ∗∗∗∗p < 0.0001).
(H) RT-qPCR analyses of YTHDF1, OCT4, and LIN28A, on shMATR3(−) cells rescued with overexpression of YTHDF1 (YTHDF1 +) cultures. RT-qPCR analyses were normalized on internal housekeeping (β-ACTIN) and reported in comparison to shCtrl values (reported as value 1 in the graph). Values are reported as mean ± SEM. p value was calculated by One-way ANOVA (∗p < 0.05, ∗∗p < 0.01).
Transcript levels of the stemness regulators, including OCT4, LIN28A, SOX2, and NANOG mRNAs, can also regulated by m6A methylation (Chen et al., 2015b). Thus, by RIP using anti-m6A antibody (MeRIP), OCT4 and LIN28A transcripts show a more than 20- and 6-fold enrichment in methylation over the IgG background, respectively (Figure S7C). We searched for the MATR3-DNA-binding consensus in promoter regions of genes involved in the m6A machinery and found enrichment in the promoters of all the genes involved in m6A regulation (Table S5). We focused our attention on YTHDF1 that was the gene with the highest presence of predicted MATR3-binding elements in its promoter (123 significant binding sequences). Indeed, experimental validation of computational predictions by ChIP indicated that MATR3 bound to the YTHDF1 promoter region (Figure 7B). In accordance with a transcriptional-mediated regulation of YTHDF1, we found both YTHDF1 mRNA and protein expression levels decreased in shMATR3 hiPSCs (Figures 7E and 7F). In addition, YTHDF1 bound to OCT4 and LIN28A methylated transcripts but not to SOX2, KLF4, and NANOG, by RIP (Figure 7G), suggesting that these two transcripts are under the direct regulation of YTHDF1. Indeed, this RNA-binding protein binds to m6A-methylated RNAs and regulates their metabolism, generally promoting their translation (Wang et al., 2015). Noteworthy, restoration of YTHDF1 levels in shMATR3 hiPSCs rescued both OCT4 and LIN28A transcript levels (Figure 7H), suggesting the presence of a regulatory loop by which MATR3 triggers the expression of YTHDF1 that, in turn, regulates the metabolism of the OCT4 and LIN28A transcripts.
Discussion
In this work, we spotted a crucial role of MATR3 in maintaining the pluripotency circuitry by reporting novel and pleiotropic functions. We found that MATR3 fine-tunes pluripotency regulators' expression at the post-transcriptional level by regulating their translational efficiency (NANOG, LIN28A, and SOX2) and mRNA stabilization (NANOG and LIN28A). In addition, we found that MATR3 binds to OCT4 and YTHDF1 promoter regions regulating their mRNAs transcription, and, finally, YTHDF1 maintains the expression level of m6A OCT4 and LIN28A mRNAs. The relevance of MATR3 in pluripotency was observed in stressful conditions, as its absence impaired single cell growth and colony formation. Indeed, we observed altered phenotypes in shMATR3 hiPSCs during pluripotency exit and lineage commitment as assessed by the EB formation assay, showing both abnormal EB size and number and impaired expression of trilineage markers. These effects could be ascribed to the reduced capability of shMATR3 cells to survive and efficiently aggregate following dissociation to form regular EBs. Accordingly, shMATR3 EBs exhibited an irregular structure that could be responsible for altered axis formation in EBs, resulting in an impaired germ layer specification (Fuchs and Pasteiner, 2012). Altered phenotypes were also observed in specific neural induction conditions, where MATR3-silenced cultures showed an increase in the number of neural rosettes and decreased lumen's size. Proper neural rosette's cytoarchitecture and size have been shown to be the direct consequence of a precise balance between self-renewal and differentiation and are mandatory for the correct neuronal differentiation/maturation (Curchoe et al., 2012; Temple, 2001). Coherently, morphometric analyses showed a reduced number of neurites and neurite arborization, in shMATR3 hiPSC-derived neurons. These results are consistent with a recent report describing that MATR3 knockdown results in disordered in vitro differentiation of mouse NSCs into neurons, causing the collapse of the developing mouse cerebral cortical layer structure in vivo (Niimori-kita et al., 2018).
Although MATR3 is predominantly a nuclear protein, our proteomic investigation suggested a role of MATR3 in the cytoplasm. The binding to nucleopore proteins may indicate the mechanism by which MATR3 conveys its RNA cargo from the nucleus to the cytoplasm, supporting defects of mRNA transport, possibly, to the translation machinery, observed with ALS-related mutated MATR3 in NSC-34 cells (Boehringer et al., 2017). Indeed, the binding to the eukaryotic initiation factors of the multiprotein eIF3, required for the binding of mRNAs to the 40S ribosomal subunit to form the 43S preinitiation complex (Hershey et al., 2012; (Marchione et al., 2013)), underlined a possible role in the translation. In addition, besides detecting previously reported interactors including DHX9, which forms a complex with HNRNPK acting in RNA splicing and translation (Salton et al., 2011), we also found novel interactors specifically involved in the translation of genes regulating stemness competence. Among them, we found L1TD1, an RBP that is expressed in iPSCs, whose promoter is bound by OCT4, NANOG, and SOX2 and that regulates pluripotency maintenance via direct binding to LIN28A (Emani et al., 2015; Närväa et al., 2012). Indeed, polysome profiling results indicate MATR3 main association with the monosome fraction, and that MATR3 silencing produces a decrease in the monosome peak. These data may suggest a possible role of MATR3 in the assembly of the 80S complex, which requires further investigation. The mild reduction of de novo protein synthesis and the decrease of translational efficiency of specific genes, following MATR3 silencing, suggest that MATR3 is not involved in global transcriptome translation, but rather it specifically regulates the translation of a relevant subset of transcripts. Coherently, mRNAs directly bound by MATR3, such as NANOG and LIN28A, showed a decrease in transcript stability that can be explained by a MATR3-dependent reduction in ribosome recruitment and polysomal loading. However, we found that transcripts not directly bound by MATR3 also underwent a decrease in polysomal loading, thus suggesting additional indirect effects on translation by MATR3. For example, SOX2 expression level was not dependent on MATR3, and its transcript was not bound by MATR3. Nevertheless, SOX2 mRNA exhibited a translation efficiency impairment. On the other side, KLF4 whose expression level is affected by MATR3 silencing, but was not bound by MATR3, did not show change in its translation efficiency. A different scenario emerged for OCT4, a core pluripotency factor (Shi and Jin, 2010). OCT4 expression was dependent on MATR3 in various manners. MATR3 was found on the OCT4 promoter suggesting a direct control of the OCT4 transcript in hiPSCs. Moreover, an indirect regulation by MATR3 was also observed. To this end, we identified a new gene regulatory network, in which MATR3 is essential to sustain YTHDF1 expression, likely via a transcription-mediated mechanism and, the encoded protein binds to m6A-methylated OCT4 and LIN28A transcripts. YTHDF1 has been shown to play a crucial role in mediating m6A-RNA translation by interacting with eIF3 and facilitating translation initiation (Wang et al., 2015; Zhuang et al., 2019). Noteworthy, an important role for YTHDF1 in the maintenance of PSCs' pluripotency by binding to m6A JAK2 mRNA has also been reported (Wu et al., 2019). Accordingly, we found that the restoration of YTHDF1 expression in shMATR3-hiPSCs completely rescued the expression level of OCT4 and LIN28A. In summary, our findings reveal the multifaceted role of MATR3 in maintaining the pluripotency circuitry and introduce translation as a new layer of regulation, functionally ruled by this pleiotropic protein.
Limitations of the study
This study has been performed in hiPSCs, therefore a validation in human ESCs is required to generalize the proposed model.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Alessandro Provenzani (alessandro.provenzani@unitn.it).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
The published article includes all datasets generated or analyzed during this study. Supplementary Tables are available at https://data.mendeley.com/drafts/xwx6p86dzj?folder=fed972f6-5de9-4bd4-9155-8804a3279708.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
This work was supported by grants from Fondazione Cassa Di Risparmio Di Trento E Rovereto (Drug repositioning project #40102838) to A. Provenzani and A.Q.; Fondazione ARISLA (project TARGET RAN # 40103385 to A. Provenzani and A. Poletti and project MLOpathy to A. Poletti); Fondazione Cariplo, Italy (n. 2014-0686, # 40102636) to A. Poletti; Fondazione Telethon, Italy (GGP19128 to A. Poletti); Italian Association for Cancer Research (AIRC) grants IG 12869, IG 18985, and IG 21548; Kennedy's Disease Association (2018 grant to R.C.); Italian Ministry of University and Research (MIUR); PRIN - Progetti di ricerca di interesse nazionale (n. 2017F2A2C5 to A. Poletti); Agenzia Italiana del Farmaco (AIFA) (Co_ALS to A. Poletti); and Fondazione Regionale per la Ricerca Biomedica (FRRB) (Regione Lombardia, TRANS_ALS, project nr. 2015-0023, to A. Poletti). The authors wish to thank the Facilities of High Throughput Screening (HTS) and Advanced Imaging for their support in performing the experiments.
Author contributions
Conceptualization, A. Provenzani, L.C., D. Pollini, A. Poletti, G.V., and A.Q.; methodology, D. Pollini, R.L., M.C., G.V., and A. Provenzani; investigation, D. Peroni, R.L., F.M., A.R., M.M., I.B., W.T., N.V.L., V.C., and E.D.; software, E.D.; data curation, D. Pollini and E.D; writing – original draft, D. Peroni and A. Provenzani; writing – review & editing, all authors; visualization, D. Pollini; funding acquisition, A. Provenzani, A.Q., and A. Poletti; resources, L.C., G.V., and M.C.; supervision and project administration, A. Provenzani.
Declaration of interests
The authors declare no competing interest.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102197.
Supplemental information
References
- Banerjee A., Vest K.E., Pavlath G.K., Corbett A.H. Nuclear poly ( A ) binding protein 1 ( PABPN1 ) and Matrin3 interact in muscle cells and regulate RNA processing. Nucleic Acids Res. 2017;45:10706–10725. doi: 10.1093/nar/gkx786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista P.J., Molinie B., Wang J., Qu K., Zhang J., Li L., Bouley D.M., Lujan E., Haddad B., Daneshvar K. M6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgraders P., Dey R., Berezneyg R. Molecular cloning of matrin 3. J. Biol. Chem. 1991;266:9893–9899. [PubMed] [Google Scholar]
- Blaess S., Szabó N., Haddad-tóvolli R., Zhou X., Álvarez-bolado G. Sonic hedgehog signaling in the development of the mouse hypothalamus. Front. Neuroanat. 2015;8:1–6. doi: 10.3389/fnana.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer A., Garcia-Mansfield K., Singh G., Bakkar N., Pirrotte P., Bowser R. ALS associated Mutations in matrin 3 alter protein-protein interactions and Impede mRNA nuclear export. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-14924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J., Schepers G., Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Chen T., Hao Y., Zhang Y., Li M., Wang M., Han W., Wu Y. m 6 A RNA methylation is regulated by MicroRNAs and promotes reprogramming to pluripotency article m 6 A RNA methylation is regulated by MicroRNAs and promotes reprogramming to pluripotency. Stem Cell. 2015;16:289–301. doi: 10.1016/j.stem.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Chen T., Hao Y.J., Zhang Y., Li M.M., Wang M., Han W., Wu Y., Lv Y., Hao J., Wang L. M6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16:289–301. doi: 10.1016/j.stem.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Coelho M.B., Attig J., Bellora N., Konig J., Hallegger M., Kayikci M., Eyras E., Ule J., Smith C.W. Nuclear matrix protein Matrin3 regulates alternative splicing and forms overlapping regulatory networks with PTB. EMBO J. 2015;34:653–668. doi: 10.15252/embj.201489852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curchoe C.L., Russo J., Terskikh A.V. HESC derived neuro-epithelial rosettes recapitulate early mammalian neurulation events; an in vitro model. Stem Cell Res. 2012;8:239–246. doi: 10.1016/j.scr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Deng X., Su R., Weng H., Huang H., Li Z., Chen J. RNA N 6 -methyladenosine modification in cancers: Current status and perspectives. Cell Res. 2018 doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emani M.R., Närvä E., Stubb A., Chakroborty D., Viitala M., Rokka A., Rahkonen N., Moulder R., Denessiouk K., Trokovic R. The L1TD1 protein interactome reveals the importance of post-transcriptional regulation in human pluripotency. Stem Cell Reports. 2015;4:519–528. doi: 10.1016/j.stemcr.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C., Pasteiner W. Self-organization phenomena in embryonic stem cell-derived embryoid Bodies: Axis formation and breaking of symmetry during Cardiomyogenesis. Cell Tissues Organs. 2012:377–391. doi: 10.1159/000328712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula S., Moshitch-Moshkovitz S., Dominissini D., Mansour A.A., Kol N., Salmon-Divon M., Hershkovitz V., Peer E., Mor N., Manor Y.S. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:0–5. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- Guallar D., Wang J. RNA-binding proteins in pluripotency, differentiation, and reprogramming. Front. Biol. (Beijing) 2014 doi: 10.1007/s11515-014-1326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Li T., Qi X., Zhao D., Zhao G. WNT/h -catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Haraguchi R., Mo R., Hui C., Motoyama J., Makino S., Shiroishi T., Gaffield W., Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Hershey J.W.B., Sonenberg N., Mathews M.B. Principles of translational control: an overview. Cold Spring Harb. Perspect. Biol. 2012;4:a011528. doi: 10.1101/cshperspect.a011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada-Ishii S., Ebihara M., Kobayashi N., Kitagawa Y. Bipartite nuclear localization signal of matrin 3 is essential for vertebrate cells. Biochem. Biophys. Res. Commun. 2007;354:72–76. doi: 10.1016/j.bbrc.2006.12.191. [DOI] [PubMed] [Google Scholar]
- Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., Zhao B.S., Mesquita A., Liu C., Yuan C.L. Recognition of RNA N 6 -methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha B.S., Rao M., Malik N. Motor neuron differentiation from pluripotent stem cells and other Intermediate proliferative Precursors that can be discriminated by lineage specific reporters. Stem Cell Rev. Rep. 2015;11:194–204. doi: 10.1007/s12015-014-9541-0. [DOI] [PubMed] [Google Scholar]
- Johnson J.O., Pioro E.P., Boehringer A., Chia R., Feit H., Renton A.E., Pliner H.A., Abramzon Y., Marangi G., Winborn B.J. Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat. Neurosci. 2014;17:664–666. doi: 10.1038/nn.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kele J., Andersson E.R., Villaescusa J.C., Cajanek L., Parish C.L., Bonilla S., Toledo E.M., Bryja V., Rubin J.S., Shimono A., Arenas E. SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cells. 2012;30:865–875. doi: 10.1002/stem.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond C.S., Gan-Or Z., Spiegelman D., Laurent S.B., Szuto A., Hodgkinson A., Dionne-Laporte A., Provencher P., de Carvalho M., Orrù S. Replication study of MATR3 in familial and sporadic amyotrophic lateral sclerosis. Neurobiol. Aging. 2016;37:209.e17–209.e21. doi: 10.1016/j.neurobiolaging.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Leong C., Zhai D., Kim B., Yun S., Chang Y. ScienceDirect Neural stem cell isolation from the whole mouse brain using the novel FABP7-binding fluorescent dye, Cdr3. Stem Cell Res. 2013;11:1314–1322. doi: 10.1016/j.scr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Lin Y., Chen G., Engineering G., Facility C. Harvard Stem Cell Institute; 2014. Embryoid Body Formation from Human Pluripotent Stem Cells in Chemically Defined E8 Media; pp. 1–4. [PubMed] [Google Scholar]
- Lin K., Tsai P., Liao Y., Chen W., Tsai C., Soong B., Lee Y. Neurobiology of Aging Mutational analysis of MATR3 in Taiwanese patients with amyotrophic lateral sclerosis. Neurobiol. Aging. 2015;36:2005.e1–2005.e4. doi: 10.1016/j.neurobiolaging.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Marangi G., Lattante S., Doronzio P.N., Conte A., Tasca G., Monforte M., Patanella A.K., Bisogni G., Meleo E., La Spada S. Matrin 3 variants are frequent in Italian ALS patients. Neurobiol. Aging. 2017;49:218. doi: 10.1016/j.neurobiolaging.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Marchione R., Leibovitch S.A., Lenormand J. The translational factor eIF3f: the ambivalent eIF3 subunit. Cell Mol. Life Sci. 2013;70:3603–3616. doi: 10.1007/s00018-013-1263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani M., Sonenberg N., Yokoyama S. Reconstitution reveals the functional core of mammalian eIF3. EMBO J. 2007;26:3373–3383. doi: 10.1038/sj.emboj.7601765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao N., Bian S., Lee T., Mubarak T., Huang S., Wen Z. Opposite roles of Wnt7a and Sfrp1 in modulating proper development of neural progenitors in the mouse cerebral Cortex. Front. Mol. Neurosci. 2018;11:1–14. doi: 10.3389/fnmol.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Närväa E., Rahkonena Nelly, Maheswara Reddy Emania R.L., Huha-Pekka P., Nästia J., Autioa R., Rasoola O., Denessiouka K., Harri L., Raod A., Lahesmaaa R. RNA-binding protein L1TD1 interacts with LIN28 via RNA and is required for human embryonic stem cell self-renewal and Cancer cell proliferation. Stem Cells. 2012;30:452–460. doi: 10.1002/stem.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimori-kita K., Tamamaki N., Koizumi D., Niimori D. Matrin-3 is essential for fibroblast growth factor 2-dependent maintenance of neural stem cells. Sci. Rep. 2018;8:13412. doi: 10.1038/s41598-018-31597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origone P., Verdiani S., Bandettini M., Poggio D., Vignolo M., Caponnetto C., Mandich P., Origone P., Verdiani S., Bandettini M. A novel Arg147Trp MATR3 missense mutation in a slowly progressive ALS Italian patient A novel Arg147Trp MATR3 missense mutation in a slowly progressive ALS. Ital. Patient. 2015;8421:3–5. [Google Scholar]
- Panda A.C., Martindale J.L., Gorospe M. Polysome fractionation to analyze mRNA distribution profiles amaresh. Bio Protoc. 2017;7:e2126. doi: 10.21769/BioProtoc.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potzner M.R., Tsarovina K., Binder E., Penzo-Méndez A., Lefebvre V., Rohrer H., Wegner M., Sock E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;137:775–784. doi: 10.1242/dev.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Ma Y., Wang J., Peng S., Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2009;38:1240–1248. doi: 10.1093/nar/gkp1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.I.E., Jian F., Jiang H., Sun Y., Pan S., Gu C. Decreased expression of SFRP2 promotes development of the pituitary corticotroph adenoma by upregulating Wnt signaling. Int. J. Oncol. 2018;52:1934–1946. doi: 10.3892/ijo.2018.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E.E., Nitsch R., Wulczyn F.G. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- Salton M., Elkon R., Borodina T., Davydov A., Yaspo M.-L., Halperin E., Shiloh Y. Matrin 3 binds and stabilizes mRNA. PLoS One. 2011;6:e23882. doi: 10.1371/journal.pone.0023882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Hochedlinger K. The Sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2014;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Z.-Y., Yang W.-X. SOX family transcription factors involved in diverse cellular events during development. Eur. J. Cell Biol. 2015;94:547–563. doi: 10.1016/j.ejcb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Shi G., Jin Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. e Ther. 2010:1–9. doi: 10.1186/scrt39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama M., Ohno S., Osaka T., Sakamoto R., Tokunaga A., Nakatake Y., Sato M., Yoshida N. Polypyrimidine tract-binding protein is essential for early mouse development and embryonic stem cell proliferation. FEBS J. 2009;276:6658–6668. doi: 10.1111/j.1742-4658.2009.07380.x. [DOI] [PubMed] [Google Scholar]
- Skowronska-krawczyk D., Ma Q., Schwartz M., Scully K., Li W., Kohwi Y., Kohwi-shigematsu T., Rosenfeld M.G. HHS public access. Nature. 2015;514:257–261. [Google Scholar]
- Skowronska-Krawczyk D., Ma Q., Schwartz M., Scully K., Li W., Liu Z., Taylor H., Tollkuhn J., Ohgi K.A., Notani D. Required enhancer-matrin-3 network interactions for a homeodomain transcription program. Nature. 2014;514:257–261. doi: 10.1038/nature13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Doi H., Koyano S., Kubota S., Fukai R., Hashiguchi S., Hayashi N., Kawamoto Y., Kunii M., Tanaka K. Matrin 3 is a component of neuronal cytoplasmic inclusions of motor neurons in sporadic amyotrophic lateral sclerosis. Am. J. Pathol. 2018;188:507–514. doi: 10.1016/j.ajpath.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016 doi: 10.1038/nrm.2016.8. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001 doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tenzer S., Moro A., Kuharev J., Francis A.C., Vidalino L., Provenzani A., Macchi P. Proteome-wide characterization of the RNA-binding protein RALY-interactome using the in vivo-biotinylation-pulldown-quant (iBioPQ) approach. J. Proteome Res. 2013;12:2869–2884. doi: 10.1021/pr400193j. [DOI] [PubMed] [Google Scholar]
- Tickle C., Towers M., Davey M. Sonic hedgehog signaling in limb development. Front. Cell Dev. Biol. 2017;5:1–19. doi: 10.3389/fcell.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L., Touboul T., Chng Z., Brimpari M., Hannan N., Millan E., Smithers L.E., Trotter M., Rugg-Gunn P., Weber A., Pedersen R.A. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One. 2009;4:e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhao B.S., Roundtree I.A., Lu Z., Han D., Ma H., Weng X., Chen K., Shi H., He C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed M., Mundlos S., Olsen B.R. The role of sonic hedgehog in vertebrate development. Matrix Biol. 1997;16:53–58. doi: 10.1016/s0945-053x(97)90072-x. [DOI] [PubMed] [Google Scholar]
- Wu R., Liu Y., Zhao Y., Bi Z., Yao Y., Liu Q., Wang F., Wang Y., Wang X. m 6 A methylation controls pluripotency of porcine induced pluripotent stem cells by targeting SOCS3/JAK2/STAT3 pathway in a YTHDF1/YTHDF2-orchestrated manner. Cell Death Dis. 2019;10:171. doi: 10.1038/s41419-019-1417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Takanashi K. FUS interacts with nuclear matrix-associated protein SAFB1 as well as Matrin3 to regulate splicing and ligand-mediated transcription. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep35195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yun S., Leong C., Zhai D., Ling Y., Lim L., Bi X., Lee J., Jo H. Neural stem cell specific fluorescent chemical probe binding to FABP7. PNAS. 2012;109:10214–10217. doi: 10.1073/pnas.1200817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz M.J., Malyavantham K.S., Seifert B., Berezney R. Matrin 3: Chromosomal distribution and protein interactions. Cell. Biochem. 2009;133:125–133. doi: 10.1002/jcb.22234. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Carmichael G.G. The fate of dsRNA in the Nucleus: a p54nrb-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. doi: 10.1016/s0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]
- Zhuang M., Li X., Zhu J., Zhang J., Niu F., Liang F., Chen M., Li D., Han P., Ji S. The m 6 A reader YTHDF1 regulates axon guidance through translational control of Robo3 . 1 expression. Nucleid Acids Res. 2019;47:4765–4777. doi: 10.1093/nar/gkz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets generated or analyzed during this study. Supplementary Tables are available at https://data.mendeley.com/drafts/xwx6p86dzj?folder=fed972f6-5de9-4bd4-9155-8804a3279708.