Abstract
Cardiovascular diseases (CVDs) are the most common cause of death in patients with nonalcoholic fatty liver disease (NAFLD) and dyslipidemia is considered at least partially responsible for the increased CVD risk in NAFLD patients. The aim of the present study is to understand how hepatic de novo lipogenesis influences hepatic cholesterol content as well as its effects on the plasma lipid levels. Hepatic lipogenesis was induced in mice by feeding a fat-free/high-sucrose (FF/HS) diet and the metabolic pathways associated with cholesterol were then analyzed. Both liver triglyceride and cholesterol contents were significantly increased in mice fed an FF/HS diet. Activation of fatty acid synthesis driven by the activation of sterol regulatory element binding protein (SREBP)-1c resulted in the increased liver triglycerides. The augmented cholesterol content in the liver could not be explained by an increased cholesterol synthesis, which was decreased by the FF/HS diet. HMG-CoA reductase protein level was decreased in mice fed an FF/HS diet. We found that the liver retained more cholesterol through a reduced excretion of bile acids, a reduced fecal cholesterol excretion, and an increased cholesterol uptake from plasma lipoproteins. Very low-density lipoprotein-triglyceride and -cholesterol secretion were increased in mice fed an FF/HS diet, which led to hypertriglyceridemia and hypercholesterolemia in Ldlr-/- mice, a model that exhibits a more human like lipoprotein profile. These findings suggest that dietary cholesterol intake and cholesterol synthesis rates cannot only explain the hypercholesterolemia associated with NAFLD, and that the control of fatty acid synthesis should be considered for the management of dyslipidemia.
Keywords: cholesterol, dyslipidemia, fatty liver, lipogenesis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common liver condition with a prevalence of ~33% among the adult population in the US (Browning et al., 2004). NAFLD is associated with an increased incidence of cardiovascular diseases (CVDs) (Anstee et al., 2013; Targher et al., 2010) and patients with NAFLD are more likely to die from CVD than liver disease (Chatrath et al., 2012; Cohen and Fisher, 2013). Atherogenic dyslipidemia, which is characterized by an increased level of plasma triglycerides, decreased level of high-density lipoprotein (HDL), and the presence of small and dense low-density lipoprotein (LDL) particles, is considered at least partially responsible for the increased CVD risk in NAFLD patients (Cohen and Fisher, 2013). NAFLD is strongly associated with obesity and insulin resistance, which are also known risk factors for dyslipidemia (Angulo, 2002). However, hepatic steatosis was independently associated with atherogenic dyslipidemia after adjustment for obesity, physical activity, hyperglycemia, and systemic inflammation (Makadia et al., 2013). The pathogenesis of dyslipidemia in NAFLD is not completely understood, but hepatic overproduction of very low-density lipoprotein (VLDL), alteration in lipoprotein lipase activity, and dysregulated clearance of lipoproteins are considered major contributors (Chatrath et al., 2012; Howard, 1987; Semenkovich, 2006). Understanding the pathophysiology of the dyslipidemia associated with NAFLD would guide better strategies to reduce morbidity and mortality in patients with NAFLD.
In the liver, triglycerides come from the diet, de novo fatty acid synthesis, and free fatty acids released from the adipose tissue (Cohen et al., 2011). Sterol regulatory element binding protein (SREBP)-1c is the principle transcription factor that activates genes required for de novo fatty acid synthesis and the first step of triglyceride synthesis (Horton et al., 2002). In hepatocytes, cholesterol exists as either free cholesterol or cholesteryl ester. Cholesterol is an essential component of cellular membranes and a precursor for bile acids and steroid hormones (Ikonen, 2008). Although most cells synthesize cholesterol, the liver plays a central role in cholesterol synthesis and homeostasis. Cholesterol is synthesized from acetyl-CoA through a cascade of reactions performed by enzymes that are regulated by SREBP-2. SREBP-2 also activates the genes encoding the LDL receptor (LDLR) and protein convertase subtilisin/kexin type 9 (PCSK9), a secreted protein that degrades hepatic LDLRs, thereby reducing cholesterol uptake (Horton et al., 2002; 2003; 2009).
The role of SREBP-1c activation in the development of hypertriglyceridemia has been studied in different animal models (Horton et al., 1999; Moon et al., 2012; Okazaki et al., 2010). In hamsters, activation of SREBP-1 by a high sucrose diet induced hypertriglyceridemia (Moon et al., 2012). They also exhibited hypercholesterolemia, characterized by a substantial increase in VLDL-cholesterol levels. In a mouse model with a Ldlr -/- background that overexpressed the nuclear form of SREBP-1a in the liver (Tg-SREBP1a; Ldlr -/-), the expression of both fatty acid and cholesterol synthesis pathway-related genes was highly elevated because SREBP-1a can induce genes associated with both pathways (Horton and Shimomura, 1999). The hypercholesterolemia observed in the Tg-SREBP1a; Ldlr -/- mice could be at least partly due to the elevated cholesterol synthesis in the liver (Horton et al., 1999). Ldlr -/- mice treated with an LXR activator (T0901317), which induces SREBP-1c expression, also developed dyslipidemia, similar to that of Tg-SREBP1a; Ldlr -/- mice (Okazaki et al., 2010). Massive hypercholesterolemia and hypertriglyceridemia with chylomicron-sized VLDL developed, which were nearly abolished when Srebp-1c was deleted. While SREBP-1c activation was the primary reason for hypertriglyceridemia, the exact mechanisms for hypercholesterolemia were not fully investigated.
In the present study, we determined how hepatic de novo lipogenesis influences hepatic cholesterol content and metabolic pathways, as well as its effects on plasma lipid levels. Hepatic lipogenesis was induced in mice by feeding an FF/HS diet. Hepatic cholesterol synthesis and the associated metabolic pathways were then analyzed. We show that liver cholesterol content was elevated in the liver not by increased cholesterol synthesis, but by a decrease in bile acids and fecal cholesterol excretion, and an increase in cholesterol uptake from lipoproteins. Increased hepatic fat resulted in hypercholesterolemia with hypertriglyceridemia in Ldlr -/- mice.
MATERIALS AND METHODS
Animals and diet
Males C57BL/6J wild-type and Ldlr-/- mice were purchased from The Jackson Laboratories (USA). Mice were housed in colony cages with free access to food and water under a 12-h light/12-h dark cycle in a temperature-controlled environment. Mice were fed a chow diet obtained from Harlan Teklad Premier Laboratory Diets (USA) (Teklad Mouse/Rat Diet 2018) or a fat-free/high-sucrose (FF/HS) diet containing 60% sucrose (w/w) (Fat Free, Modified, Cat No. 960238; MP Biomedicals, USA) for 20 days. Mice were fed only during the dark cycle (7:00 P.M. to 7:00 A.M.) for the last 3 days prior to the experiments. All experiments were conducted using 10- to 12-week-old male mice. All animal studies were approved by the Institutional Animal Care & Use Committee at the University of Texas Southwestern Medical Center (APN#2015-101135).
Metabolic parameters
Blood was obtained from the tail vein of restrained animals in heparin-coated tubes and plasma was separated and stored at –80°C. Plasma cholesterol and triglyceride concentrations were determined using Infinity Cholesterol Liquid Stable Reagent and Infinity Triglycerides Liquid Stable Reagent (Thermo Fisher Scientific, USA), respectively. Plasma lipoprotein profiles were obtained for each group by pooling equal amounts of plasma from each mouse. After filtration (Costar; Corning, USA), 20 μl of each plasma sample was pooled to a total of 200 μl per group, and the pooled plasma sample was injected into the fast protein liquid chromatography (FPLC) system. FPLC analysis was performed at 4°C using a Superose 6 10/300 GL exclusion column at a flow rate of 0.5 ml/min. Cholesterol and triglyceride concentrations in the collected fractions were then determined. Plasma Apo-CIII concentration was measured by the APOC3 (mouse) ELISA Kit (Abnova, USA). Bile was collected from the gallbladder after 3 h of fasting, and bile acid concentrations were measured using the Colorimetric Total Bile Acid Assay Kit from Diazyme Laboratories (USA).
Radiolabeling of human lipoproteins
Human LDL (1.019-1.063 g/ml) and HDL (1.125-1.215 g/ml) were prepared as described previously (Goldstein et al., 1983). The purity of each lipoprotein was validated by FPLC. Human LDL or HDL was labeled with cholesteryl oleate [cholesteryl-1,2-3H(N)] (Perkin Elmer, USA) as described previously (May et al., 2013).
Lipid uptake and plasma clearance
Mice were anesthetized using pentobarbital (80 mg/kg of body weight) and 3H-cholesteryl oleate-labeled LDL or HDL (106 cpm/mouse) was injected via the penile vein. Blood was collected before injection (0 min) and at 2, 15, 30, and 60 min after injection. Plasma was separated, and triglyceride and cholesterol concentrations were measured as described above. Radioactivity in 20 μl of the plasma was measured. One hour after injection, mice were euthanized with isoflurane and perfused with saline. The liver was collected, washed with saline, and the whole liver was dissolved in 3 ml of 50% KOH in ethanol. The 3H-labelled cholesterol content of the liver was measured.
In vivo VLDL secretion
Tyloxapol was purchased from Sigma-Aldrich (USA); 10% tyloxapol solution was prepared using saline. Mice were fasted for 6 h and injected with tyloxapol (500 mg/kg) via the penile vein. Blood was collected from the tail vein before injection (0 min) and at 15, 30, 60, 90, 120, and 180 min after injection. Plasma was separated, and triglyceride and cholesterol concentrations were determined. Triglyceride and cholesterol secretion rates were calculated from the linear regression analysis of time versus concentration.
Fatty acid and cholesterol synthesis in vivo
The synthesis rates of sterol and fatty acids in the liver were determined in mice using 3H-labeled water as described previously (Shimano et al., 1996).
Preparation of primary hepatocytes and measurement of lipid synthesis rates
Hepatocytes were isolated from C57BL/6J wild-type mice and cultured as described previously (Kim et al., 2020; Matsuda et al., 2001). After a 3-h attachment period, the cells were incubated with 0.5 mM 14C-sodium acetate for 4 h. Lipids were extracted, and then, fatty acids, cholesterol, cholesteryl ester, and triglycerides were separated by TLC as described previously (Matsuda et al., 2001; Moon et al., 2009). Incorporation of 14C-acetate into newly synthesized lipids was then determined.
Fecal cholesterol excretion
The amount of cholesterol in feces was measured as described previously, with slight modifications (Turley et al., 1996). Mice were housed in individual cages a week before the experiment, and feces were collected every 24 h for three days. Feces were dried completely, ground, and resuspended in water (100 μg/2 ml). Lipids were extracted in chloroform/methanol (2:1) and resuspended in isopropanol. Cholesterol concentrations in the extracts were measured using Infinity Cholesterol Liquid Stable Reagent (Thermo Fisher Scientific).
Live cell imaging
Hepatocytes isolated from C57BL/6J wild-type mice were seeded at a density of 1 × 106 cells in a 60-mm dish. After a 2-h attachment period, the cells were washed and incubated with 1 μg/ml of BODIPY 493/503 (Thermo Fisher Scientific) and 15 μg/ml of dehydroergosterol (DHE; Sigma-Aldrich) in either the presence or absence of 20 μM oleic acid for 24 h. After incubation, the cells were imaged with a ZEISS LSM 780 confocal microscope (Zeiss, USA).
Immunoblot analysis
Membrane and nuclear proteins were prepared individually from frozen livers, and equal amounts of protein from each mouse of the same group were pooled as previously described (Engelking et al., 2004). Aliquots of pooled proteins were subjected to SDS-PAGE on 8% gels and transferred to a nitrocellulose membrane (Bio-Rad, USA). Immunoblot analyses were performed using polyclonal anti-mouse SREBP-1, SREBP-2, and HMG-CoA reductase antibodies as previously described (Rong et al., 2017). Anti-Scavenger receptor class B type 1 (SR-BI) antibody was obtained from Abcam (USA) and anti-cluster of differentiation 36 (CD36) antibody was obtained from R&D Systems (USA). Anti-mouse cAMP response element binding protein (CREB; Invitrogen, USA) and anti-dog calnexin (Enzo Life Science, USA) antibodies were used as loading controls for nuclear and membrane proteins, respectively. Signals were detected using the SuperSignal West Pico Chemiluminescent Substrate System (Thermo Fisher Scientific) and visualized with Odyssey CLx Imaging system and LI-COR Image StudioTM software (LI-COR, USA).
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was prepared from mouse livers with an RNA STAT-60 kit (Tel-Test, USA), and qPCR was performed as previously described (Rong et al., 2017). All reactions were performed in triplicate, and the relative amount of all mRNAs was calculated using the comparative threshold cycle (CT) method. Mouse ApoE was used as the invariant control. The Sequences of the primers for qPCR are shown in Supplementary Table S1.
Statistics
All results are reported as the mean ± SE. Statistical significance was analyzed using a Student’s t-test (Prism 9; GraphPad Software, USA). P values < 0.05 were considered statistically significant.
RESULTS
FF/HS diet increases liver cholesterol content despite reduced cholesterol synthesis
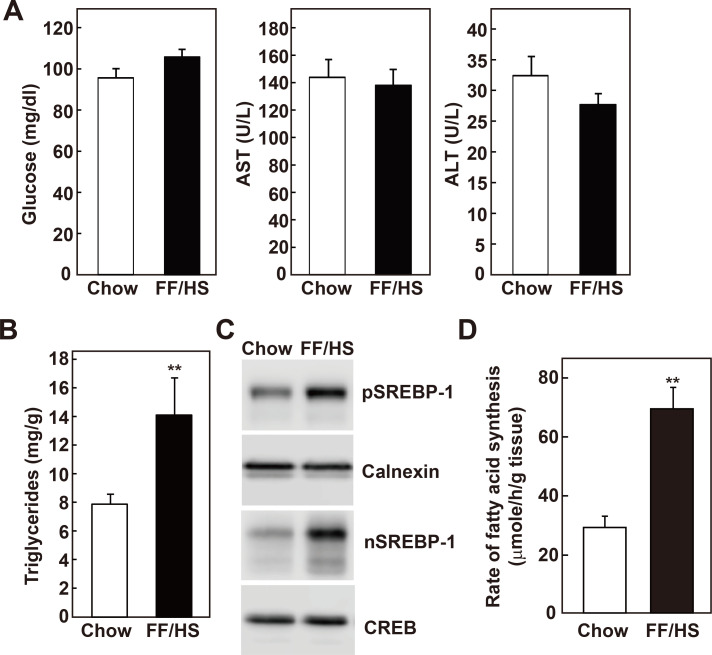
FF/HS diets have been previously used to induce hepatic de novo lipogenesis (Iritani et al., 1992; Kim and Freake, 1996; Moon et al., 2012; Weigand et al., 1980). Here, the effects of de novo lipogenesis on overall lipid metabolism in C57BL/6J wild-type mice fed an FF/HS diet for 20 days were compared with those in mice fed a chow diet. Plasma glucose, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were not different between the groups (Fig. 1A). As previously reported (Moon et al., 2012), liver triglyceride content significantly increased in the mice fed an FF/HS diet (Fig. 1B). SREBP-1c is a major transcription factor that regulates the expression of lipogenic genes. The expression of both the precursor and the nuclear form of SREBP-1 was elevated in the livers of the mice fed an FF/HS diet (Fig. 1C). Consistent with the SREBP-1 protein levels, the mRNA expression of SREBP-1c and its known target genes, such as ATP-citrate lyase (Acl), acetyl-CoA carboxylase-α (Acaca), fatty acid synthase (Fasn), elongation of long chain fatty acids 6 (Elovl6), and stearoyl-CoA desaturase-1 (Scd-1), showed a 1.6- to 4.6-fold elevation (Supplementary Fig. S1A). Fatty acid synthesis rates were measured in vivo in these mice. 3H-labelled water was injected into the mice, their hepatic lipids were extracted, and the incorporation of 3H-water into the newly synthesized fatty acids was measured. Consistent with the level of the nuclear form of SREBP-1 and the expression levels of its target genes, the rate of fatty acid synthesis was elevated in the liver of the mice fed an FF/HS diet (Fig. 1D). These results confirmed that the FF/HS diet activated SREBP-1c, thereby inducing the expression of genes involved in fatty acid synthesis, which resulted in an increase in fatty acid synthesis and an accumulation of triglycerides in the liver.
Fig. 1. Hepatic triglyceride content and synthesis rates in mice fed a chow or an FF/HS diet.
(A) Plasma glucose, AST, ALT levels were measured in mice fed a chow or an FF/HS diet for 20 days. (B) Hepatic triglyceride contents were measured. Data represents the mean ± SE of 10 mice. (C) Membrane and nuclear proteins were prepared individually from livers. Equal amounts of proteins from 10 mice of the same group were pooled and immunoblot analysis of SREBP-1 was performed. Calnexin and CREB were used as loading controls for membrane and nuclear proteins, respectively. (D) In vivo synthesis rates of fatty acids were measured in livers of five mice fed with chow or an FF/HS diet. Mice were injected intraperitoneally with 3H-labeled water. One hour after the injection, incorporation of 3H-labeled water into the newly synthesized fatty acids was measured in the liver. The data represents the mean ± SE of 5 mice. **P < 0.01 by Student’s t-test.
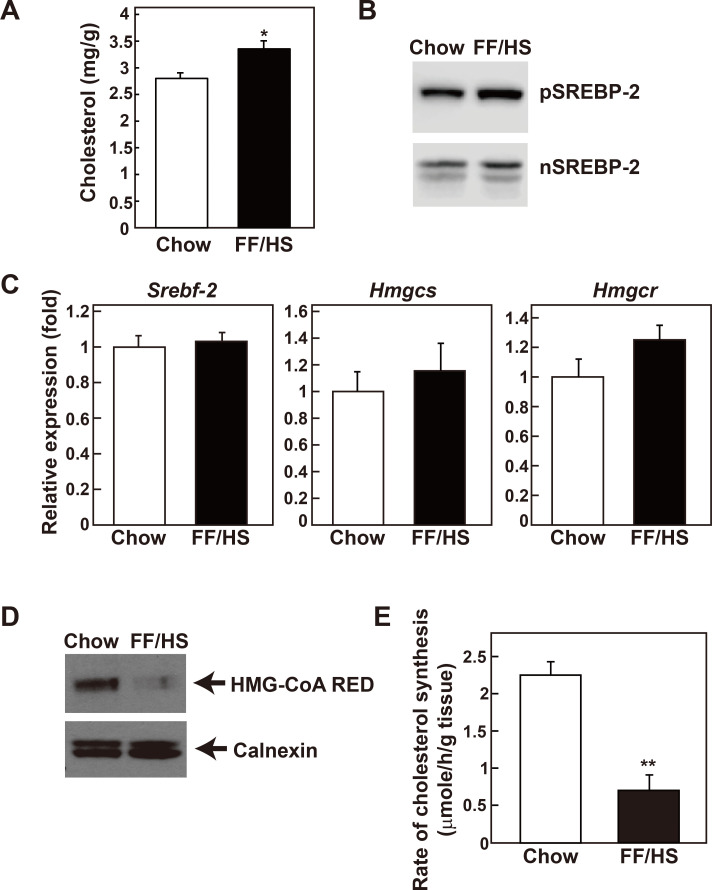
Cholesterol content was also significantly increased in the livers of mice fed an FF/HS diet (Fig. 2A). All genes encoding enzymes responsible for cholesterol synthesis are regulated by SREBP-2 (Horton et al., 2002); therefore, SREBP-2 and its target genes were analyzed. While the precursor form of SREBP-2 appeared slightly increased, the amount of the transcriptionally active nuclear form was not altered by the FF/HS diet (Fig. 2B). The mRNA expression levels of neither SREBP-2 (Srebf-2) nor the SREBP-2 target genes such as HMG-CoA synthase (Hmgcs) and HMG-CoA reductase (Hmgcr) were changed by the FF/HS diet (Fig. 2C). HMG-CoA reductase is the rate limiting enzyme in the cholesterol synthesis pathway and is regulated at the transcriptional, post-transcriptional, and post-translational levels (DeBose-Boyd, 2008). Despite no change in the mRNA level, we observed that the expression of the HMG-CoA reductase protein was significantly reduced in the liver of the mice fed an FF/HS diet (Fig. 2D), indicating a post-transcriptional regulation of the enzyme, such as ER-associated degradation (Johnson and DeBose-Boyd, 2018). Next, in vivo cholesterol synthesis rates were measured by determining the incorporation of 3H-water to the newly synthesized hepatic sterols. Consistent with the HMG-CoA reductase protein level, sterol synthesis rates were significantly reduced in the livers of the mice fed an FF/HS diet (Fig. 2E). These results suggest that following the administration of the FF/HS diet, cholesterol contents were increased. This could not be explained because of an increased cholesterol synthesis which was decreased due to the reduction of HMG-CoA reductase protein expression. As a result of the increased hepatic cholesterol content induced by the FF/HS diet, the post-transcriptional regulation of HMG-CoA reductase might be involved.
Fig. 2. Hepatic cholesterol content and synthesis rates in mice fed a chow or an FF/HS diet.
(A) Cholesterol contents were measured in the liver of mice fed a chow or an FF/HS diet for 20 days. Data represents the mean ± SE of 10 mice. (B) Membrane and nuclear proteins presented in Fig. 1 were used for immunoblot analysis of SREBP-2. (C) mRNA expression of genes involved in cholesterol synthesis were determined. Values represent the mean ± SE of 10 mice relative to mice fed a chow diet, which is defined as 1. Apoe was used as the invariant control. (D) Equal amounts of membrane proteins prepared individually from 6 mice of the same group were pooled and immunoblot analysis of HMG-CoA reductase was performed. Calnexin was used as a loading control. (E) In vivo synthesis rates of cholesterol were measured in livers of mice fed with a chow or an FF/HS diet. Mice were injected intraperitoneally with 3H-labeled water. One hour after the injection, the incorporation of 3H-labeled water into newly synthesized cholesterol was measured in the liver. Each bar represents the mean ± SE of the values from 5 mice. *P < 0.05, **P < 0.01 by Student’s t-test.
Cholesterol excretion was reduced in the mice fed an FF/HS diet
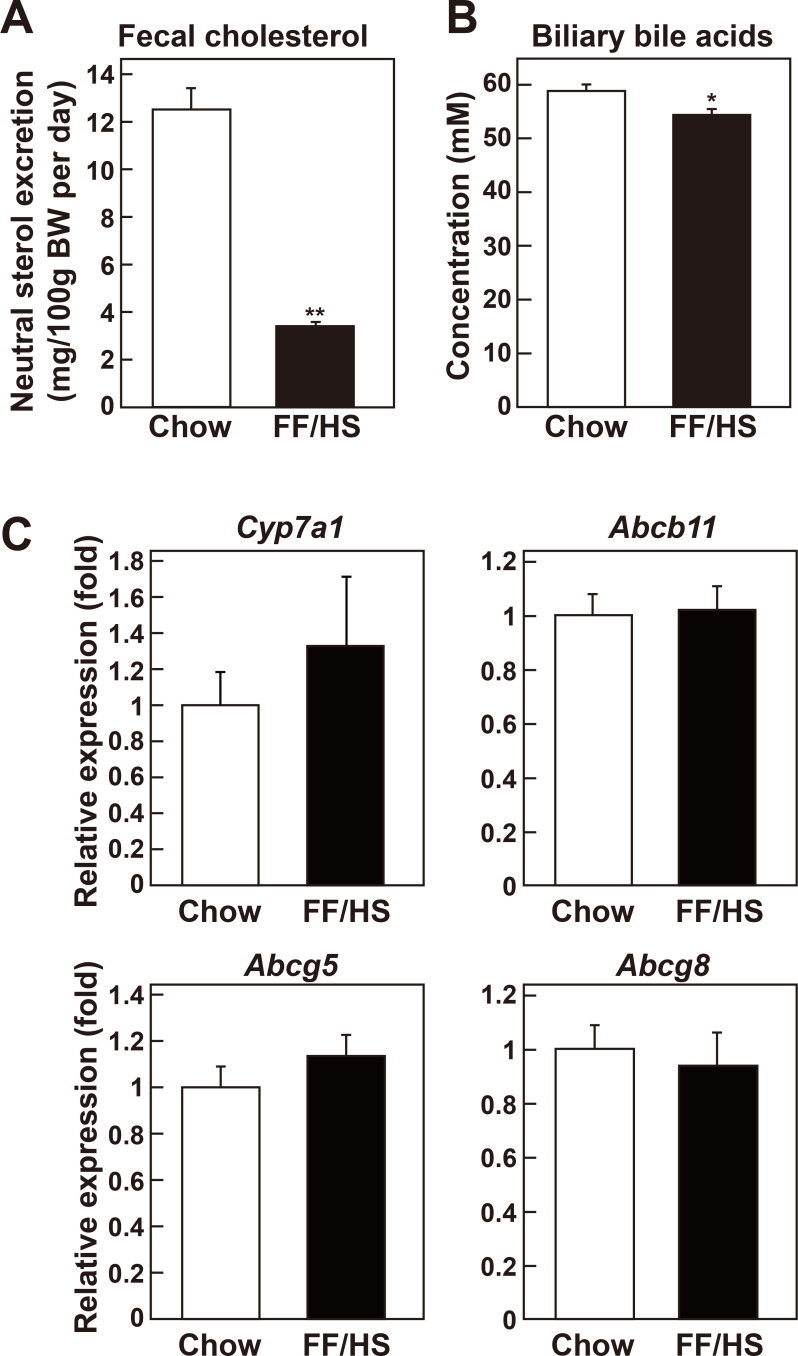
To determine why the liver cholesterol content was increased despite the reduced cholesterol synthesis in the mice fed an FF/HS diet, additional metabolic pathways involved in cholesterol homeostasis were examined. In the liver, cholesterol can be excreted into the bile directly as free cholesterol or after its conversion into bile acids. Feces contains cholesterol from bile, diet, and transintestinal cholesterol excretion. Both diets used in this study are cholesterol-free; therefore, the cholesterol found in the feces must be endogenous. Feces of mice fed a chow or an FF/HS diet were collected for 3 days at the end of the diet feeding period, and fecal cholesterol contents were measured. Fecal cholesterol content of mice fed an FF/HS diet was reduced by 73% compared to mice fed a chow diet (Fig. 3A). Bile acid concentrations were also significantly reduced in the bile of mice fed the FF/HS diet, compared to the mice fed the chow (Fig. 3B). These results suggest that cholesterol excretion was reduced in the mice fed an FF/HS diet and may partly explain why the liver retained more cholesterol despite a reduced hepatic cholesterol synthesis. mRNA expression of genes involved in bile acid synthesis (Cyp7a1), bile acid excretion (Abcb11) (Gerloff et al., 1998), and cholesterol excretion (Abcg5 and Abcg8) (Yu et al., 2002) was measured in liver. These were not significantly different between the two groups of mice suggesting that post-transcriptional regulation might be involved in bile acid excretion (Fig. 3C).
Fig. 3. Fecal cholesterol content and biliary bile acid concentration in mice fed a chow or an FF/HS diet.
(A) Mice were fed a chow or an FF/HS diet for 20 days. For the last 3 days of the experiment, feces were collected from each mouse, and cholesterol concentrations were measured. Values represent the mean ± SE of 10 mice. (B) Bile acid concentrations were measured in each mouse following a 4-h fast. Values represent the mean ± SE of 10 mice. (C) mRNA expression of genes involved in bile acid synthesis (Cyp7a1), bile acid excretion (Abcb11), and cholesterol excretion (Abcg5 and Abcg8) were measured in the livers of mice fed a chow or an FF/HS diet. Values represent mean ± SE of 10 mice, relative to those of mice fed a chow diet, which are defined as 1. Apoe was used as the invariant control. *P < 0.05, **P < 0.01 by Student’s t-test.
Hepatic uptake of cholesterol from plasma lipoproteins was increased in mice fed an FF/HS diet
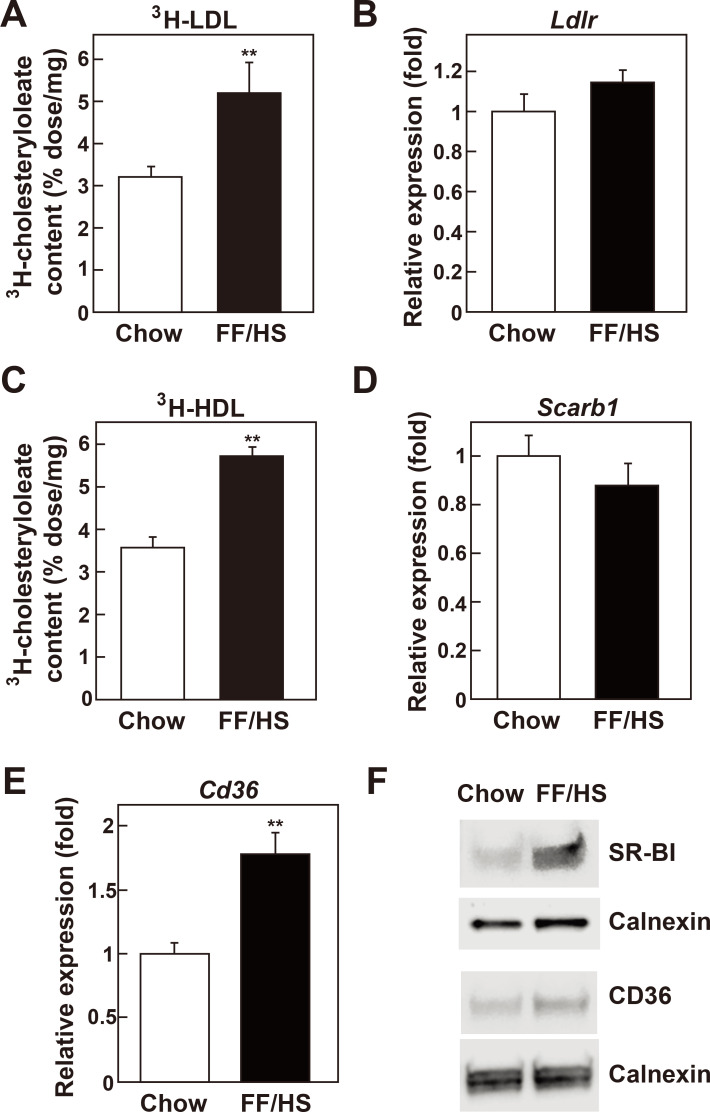
Hepatocytes can acquire cholesterol from the uptake of plasma LDL and HDL. Therefore, we examined whether hepatic cholesterol uptake was altered in the liver of mice fed an FF/HS diet. Mice were injected with LDL labeled with 3H-cholesteryl oleate, then hepatic content of 3H-cholesteryl oleate was measured 1 h after injection. As shown in Fig. 4A, the hepatic content of 3H-cholesterol from LDL was significantly increased in mice fed the FF/HS diet. mRNA expression of the LDL receptor was not changed compared to the mice fed a chow (Fig. 4B).
Fig. 4. In vivo hepatic uptake of LDL and HDL cholesterol in mice fed a chow or an FF/HS diet.
Mice fed a chow or an FF/HS diet for 20 days were injected with 3H-cholesteryl oleate-labeled LDL (A) or HDL (C). Uptake of 3H-cholesteryl oleate from LDL (A) and HDL (C) was determined. Values represent the mean ± SE of 10 mice. mRNA expression of Ldlr (B), Scarb1 (D), and Cd36 (E) in the livers was determined by qPCR. Values represent the mean ± SE of 10 mice, relative to mice fed a chow diet, which is defined as 1. Apoe was used as the invariant control. The protein levels of SR-BI and CD36 were determined by immunoblotting. Equal amounts of membrane proteins individually prepared from 6 mice of the same group were pooled and used. Calnexin was used as a loading control (F). **P < 0.01 by Student’s t-test.
Next, the hepatic uptake of HDL-cholesterol was analyzed by injecting HDL labeled with 3H-cholesteryl oleate. We observed that the hepatic content of 3H-cholesteryl oleate from HDL was significantly increased in the mice fed an FF/HS diet (Fig. 4C). SR-BI is one of the major proteins responsible for the uptake of cholesterol from plasma HDL into the liver (Acton et al., 1996; Connelly and Williams, 2004). Liver mRNA expression level of SR-BI (Scarb1) was determined; it showed no change compared to the control group. However, SR-BI protein level was increased 3-fold in the mice fed with FF/HS diet compared to that in mice fed with a chow diet (Fig. 4F). CD36, also known as fatty acid translocase, is an integral membrane protein present on the surface of the cells. It binds various ligands including long-chain fatty acids, naive and oxidized LDL, and HDL (Brundert et al., 2011; Endemann et al., 1993; Febbraio and Silverstein, 2007). Both the mRNA expression and the protein level of CD36 were increased respectively by 1.8- and 1.6-fold in the liver of mice fed the FF/HS diet compared to mice fed a chow diet (Figs. 4E and 4F). These results show that the hepatic uptake of plasma LDL- and HDL- cholesterol was increased in mice fed the FF/HS diet, which may further explain the increased hepatic cholesterol contents in these mice.
Higher fatty acid availability in hepatocytes facilitates cholesteryl ester formation and its incorporation into lipid droplets
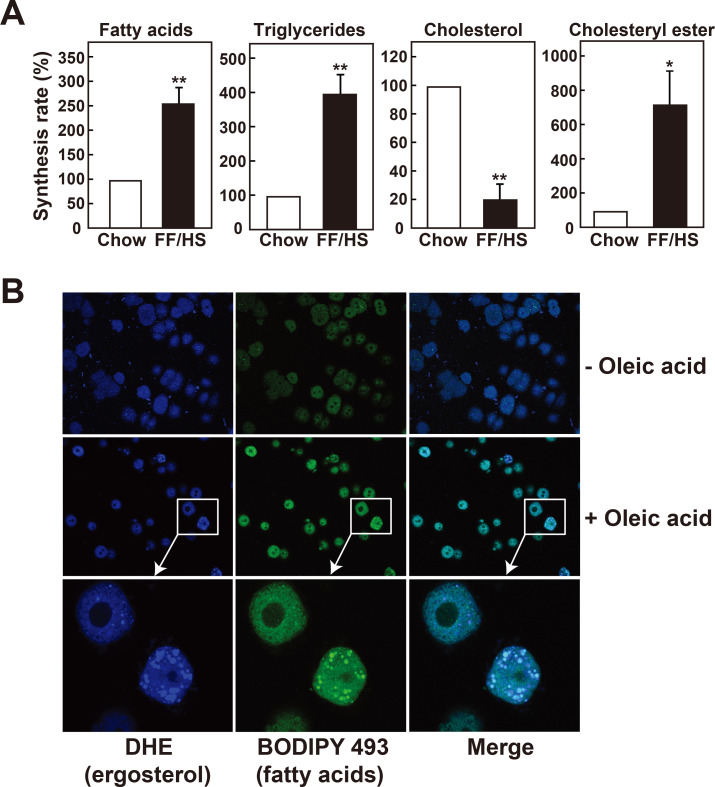
Formation of cholesteryl esters, the storage form of cholesterol, was compared using primary hepatocytes isolated from mice fed a chow or an FF/HS diet. Hepatocytes were incubated with 14C-acetate, and its incorporation into fatty acids, triglycerides, cholesterol, and cholesteryl esters was measured (Fig. 5A). Consistent with the in vivo synthesis results, hepatocytes isolated from mice fed an FF/HS diet exhibit higher synthesis rates of fatty acids and triglycerides (Fig. 5A). Although cholesterol synthesis was lower in hepatocytes of the FF/HS-fed group, cholesteryl ester formation was higher compared to the chow-fed group (Fig. 5A). These results suggest that the conversion of cholesterol to cholesteryl esters were elevated in hepatocytes isolated from the FF/HS fed mice. This process may be driven by higher fatty acid availability from the upregulated de novo synthesis of fatty acids within the cells.
Fig. 5. Synthesis rates of cholesteryl esters and incorporation of cholesterol into lipid droplets in mouse primary hepatocytes.
(A) Primary hepatocytes were isolated from mice fed a chow or an FF/HS diet. Cells were labeled with 14C-acetate for 4 h, and the incorporation of 14C-acetate into fatty acids, triglycerides, cholesterol, and cholesteryl esters was measured. The synthesis rates in hepatocytes isolated from mice fed a chow diet are defined as 100% and those in hepatocytes isolated from mice fed the FF/HS diet were compared. The values represent the mean ± SE of 3 experiments. *P < 0.05, **P < 0.01 by Student’s t-test. (B) Primary hepatocytes isolated from mice fed a chow diet were incubated with DHE in the absence (–) or presence (+) of 20 μM oleic acid. DHE (blue) and triglycerides (BODIPY 493/503, green) were detected by confocal microscopy.
To examine whether fatty acids in hepatocytes can affect the localization of cholesterol in lipid droplets, primary hepatocytes were isolated from C57BL/6J mice and incubated with fluorescence-labeled DHE, a tracer of cholesterol, in either the presence or absence of oleic acid. BODIPY 493/503 was used to stain neutral lipid droplets (Fig. 5B). When the cells were incubated with DHE in the presence of oleic acid, a strong fluorescence from DHE was detected within the cytosolic lipid droplets, while fluorescence from DHE was dispersed and weak in the absence of oleic acid (Fig. 5B). Results in Fig. 5 suggest that increased fatty acids in hepatocytes facilitates the incorporation of cholesterol into lipid droplets, mainly as cholesteryl esters. Increased cholesteryl ester formation and facilitated incorporation into lipid droplets may further explain the increased hepatic cholesterol content in mice with high de novo lipogenesis.
Hepatic VLDL secretion was increased in mice fed an FF/HS diet
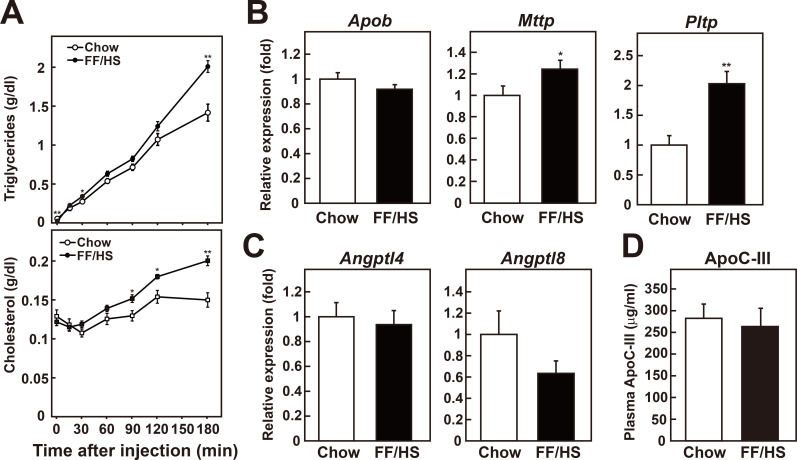
Lipids stored in cytoplasmic lipid droplets are mobilized through lipid droplet-associated proteins for the lipidation of apoB to form VLDL (Ye et al., 2009; Zhang et al., 2017). To determine whether fatty liver induced by the FF/HS diet secretes more VLDL-triglyceride and -cholesterol, plasma triglyceride and cholesterol concentrations were measured at various time points after injection of tyloxapol, an inhibitor of lipoprotein lipase. Mice fed an FF/HS diet secreted more VLDL-triglyceride and -cholesterol into the plasma compared to the chow-fed group (Fig. 6A). The expression levels of genes that are essential for VLDL assembly, such as Apob, microsomal triglyceride transfer protein (Mttp), and phospholipid transfer protein (Pltp), were measured (Fig. 6B). The gene expression of ApoB, a key component of VLDL particles, was not significantly changed, while the expression of Mttp, a key player in the lipoprotein assembly, and Pltp, a protein involved in the lipidation and the secretion of VLDL (Okazaki et al., 2010; Yazdanyar and Jiang, 2012), were increased in mice fed an FF/HS diet (Fig. 6B). The expression of genes encoding proteins that affect VLDL-triglyceride hydrolysis through inhibition of lipoprotein lipase activity, such as Angptl4 (Sukonina et al., 2006), Angptl8 (Zhang, 2012), and Apoc3 (Yao and Wang, 2012), were determined. The mRNA expression levels of Angptl4 and Angptl8 did not differ between the groups (Fig. 6C). Apoc3 mRNA expression was slightly elevated in the liver of the mice fed the FF/HS diet (Supplementary Fig. S1), whereas plasma APO-CIII protein level showed no difference between groups (Fig. 6D). Even though more lipids were secreted as VLDL into the plasma of the mice fed an FF/HS diet, we did not observe an increase in plasma VLDL- or LDL-triglycerides and cholesterol most likely because of an intact LDL receptor function in C57BL/6J mice (Supplementary Fig. S2) (Horton et al., 1999).
Fig. 6. In vivo VLDL secretion from livers of mice fed a chow diet or an FF/HS diet.
(A) Mice were fed a chow or an FF/HS diet for 20 days and were then injected with tyloxapol. Blood was collected at the indicated time points, and concentrations of triglycerides and cholesterol were measured. Values represent the mean ± SE of 8 mice. mRNA expression of genes related to VLDL secretion (B) and triglyceride lipolysis (C) was measured by qPCR. (D) Plasma ApoC-III level was determined by ELISA. Each bar represents the mean ± SE of 10 mice. *P < 0.05, **P < 0.01 by Student’s t-test.
Hepatic steatosis induced by FF/HS diet leads to severe hypercholesterolemia in Ldlr -/- mice
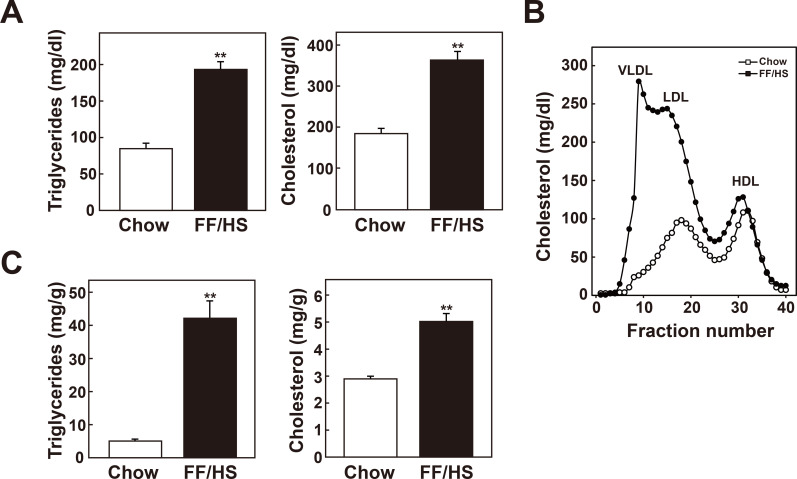
To determine the effects of an FF/HS diet on dyslipidemia, Ldlr -/- mice that exhibit a more human like lipoprotein profile as well as impaired uptake of ApoB-containing lipoproteins were used. Plasma concentrations of triglycerides and cholesterol and lipoprotein profiles were analyzed. Chow fed Ldlr -/- mice developed hypercholesterolemia with a moderate elevation of LDL cholesterol (Figs. 7A and 7B). Ldlr -/- mice fed an FF/HS diet showed increased hepatic triglyceride and cholesterol content (Fig. 7C) and exhibited higher concentrations of plasma cholesterol and triglycerides (Fig. 7A). Moreover, Ldlr -/- mice fed the FF/HS diet developed a dramatic increase in plasma VLDL- and LDL-cholesterol concentrations accompanied with higher VLDL-triglyceride levels (Fig. 7B). These results suggest that when the LDL receptor function is impaired, hepatic steatosis induced by FF/HS diet leads to severe dyslipidemia, characterized by hypercholesterolemia as well as hypertriglyceridemia.
Fig. 7. Development of hypertriglyceridemia and hypercholesterolemia in Ldlr-/- mice fed an FF/HS diet.
Male Ldlr-/- mice were fed a chow or an FF/HS diet for 20 days. Plasma concentrations of triglycerides and cholesterol (A) and lipoprotein profiles were determined (B). (C) Hepatic contents of triglycerides and cholesterol were measured. The data represent the mean ± SE of 8 mice. **P < 0.01 by Student’s t-test.
DISCUSSION
SREBP-1c activation leads to hepatic triglyceride accumulation and hypertriglyceridemia in different animal models including hamsters fed a high sucrose diet (Moon et al., 2012), Tg-SREBP1a; Ldlr -/- mice (Horton et al., 1999), and Ldlr -/- mice treated with an LXR activator (T0901317) (Okazaki et al., 2010). These animal models also exhibit an elevation of hepatic cholesterol content and hypercholesterolemia, characterized by a substantial increase in VLDL-cholesterol levels. While SREBP-1c activation was the primary reason for hypertriglyceridemia, the exact mechanisms for hypercholesterolemia have not been fully investigated. In the present study, we determined how hepatic de novo lipogenesis influences hepatic cholesterol content and cholesterol metabolic pathways. Hepatic lipogenesis was induced in mice by feeding an FF/HS diet. In these mice, SREBP-1c was activated, which induced the expression of genes involved in fatty acid and triglyceride synthesis, and as a result, hepatic triglyceride concentrations increased. Cholesterol content was also elevated in the liver, although the mechanism was different from that of triglyceride accumulation. In contrast to fatty acid synthesis, cholesterol synthesis was suppressed. However, no significant change was observed in SREBP-2 activity or the expression of its target genes required for cholesterol synthesis. Nevertheless, the liver retained more cholesterol as a result of reduced excretion of bile acids and reduced excretion of cholesterol into feces. Concomitantly, the liver acquired more cholesterol from plasma LDL and HDL. The liver secreted more VLDL-TG and -cholesterol, which resulted in hypertriglyceridemia and hypercholesterolemia in Ldlr -/- mice.
The chow diet and the FF/HS diet used in this study were of different nature and it has been shown that dietary fiber contents might influence the rate of cholesterol excretion into the feces. We used diets with similar total dietary fiber contents for this study as the chow diet contains 17% fiber and the FF/HS diet contains 16.5% fiber. However, there is still a possibility that the difference in the nature of the diets might affect the cholesterol retention in the body.
The availability of fatty acids facilitates the conversion of cholesterol to cholesteryl esters (Xie et al., 2002), which could be readily incorporated into lipid droplets in hepatocytes, as presented in Fig. 5. Fatty acids not only drove cholesterol esterification, but also enhanced secretion of cholesteryl esters into the plasma (Xie et al., 2002). Acyl-coenzyme A: cholesterol acyltransferase 2 (ACAT2), the enzyme responsible for cholesteryl ester formation in the liver, prefers oleic acid as a substrate, and its activity is primarily regulated by the availability of fatty acid substrates (Xie et al., 2002). Upon activation of fatty acid synthesis by SREBP-1c in the liver, oleic acid (C18:1, n-9) is the fatty acid that is the most increased (Moon et al., 2014; Shimomura et al., 1998). Although ACAT2 mRNA expression levels were not significantly different between mice fed a chow and an FF/HS diet (Supplementary Fig. S1C), our findings suggest that the increased fatty acid availability from de novo synthesis in mice fed an FF/HS diet might drive cholesteryl ester formation (Fig. 5). This induced cholesterol incorporation into lipid droplets along with triglycerides accumulation in the hepatocytes.
Triglycerides and cholesteryl esters are packed into VLDL in hepatocytes. SREBP-1c increases PLTP expression as well as genes required for fatty acid synthesis. PLTP plays a major role in the incorporation of phospholipids to growing VLDLs to expand the particle and its lipidation, resulting in bigger VLDL particles rich in triglycerides (Okazaki et al., 2010; Yazdanyar and Jiang, 2012). The FF/HS diet induced PLTP expression as well as other targets of SREBP-1c, leading to an increased VLDL-triglyceride and -cholesterol secretion and subsequent development of hypercholesterolemia and hypertriglyceridemia in Ldlr -/- mice.
In humans, while fatty acids released from adipose tissue are the major source of fat in livers of individuals with NAFLD (Donnelly et al., 2005), de novo lipogenesis is also increased, and inhibition of lipogenesis through the inhibition of ACC markedly reduced hepatic fat accumulation (Kim et al., 2017; Lambert et al., 2014). High carbohydrate diets stimulate de novo lipogenesis and induce hypertriglyceridemia in humans (Chong et al., 2007; Hellerstein and Parks, 2000; Hudgins et al., 1996; Sanders et al., 2018). Findings in this study suggest that dietary cholesterol intake and cholesterol synthesis rates are only partially responsible for the hypercholesterolemia associated with NAFLD and that the control of fatty acid resources and fatty acid synthesis rates should also be considered for the management of NAFLD-associated hypercholesterolemia.
Supplemental Materials
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation of Korea funded by the Korean government (2018R1A2B6007576), the National Institutes of Health (HL-20948), and the Leducq Foundation (5200829301).
The authors thank Sijeong Bae (Department of Molecular Medicine, Inha University College of Medicine) Angel Loza Valdes, Ajit Kumar Koduri, Tuyet Dang, Judith Sanchez, Norma Anderson, and Lisa Beatty (Department of Molecular Genetics, UT Southwestern Medical Center), and Abhijit Bugde (the Live Cell Imaging Core Facility, UT Southwestern Medical Center) for their technical assistance. The authors also thank Dr. Youngah Jo for providing the anti-HMG CoA-R antibody and Dr. Jay Horton for scientific advice.
Footnotes
AUTHOR CONTRIBUTIONS
J.M.B. and Y.A.M. conceived and performed experiments, analyzed the data, and wrote the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Acton S., Rigotti A., Landschulz K.T., Xu S., Hobbs H.H., Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., Grundy S.M., Hobbs H.H. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Brundert M., Heeren J., Merkel M., Carambia A., Herkel J., Groitl P., Dobner T., Ramakrishnan R., Moore K.J., Rinninger F. Scavenger receptor CD36 mediates uptake of high density lipoproteins in mice and by cultured cells. J. Lipid Res. 2011;52:745–758. doi: 10.1194/jlr.M011981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrath H., Vuppalanchi R., Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin. Liver Dis. 2012;32:22–29. doi: 10.1055/s-0032-1306423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M.F.F., Fielding B.A., Frayn K.N. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc. Nutr. Soc. 2007;66:52–59. doi: 10.1017/S0029665107005290. [DOI] [PubMed] [Google Scholar]
- Cohen D.E., Fisher E.A. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin. Liver Dis. 2013;33:380–388. doi: 10.1055/s-0033-1358519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M.A., Williams D.L. Scavenger receptor BI: a scavenger receptor with a mission to transport high density lipoprotein lipids. Curr. Opin. Lipidol. 2004;15:287–295. doi: 10.1097/00041433-200406000-00008. [DOI] [PubMed] [Google Scholar]
- DeBose-Boyd R.A. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18:609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G., Stanton L.W., Madden K.S., Bryant C.M., White R.T., Protter A.A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Engelking L.J., Kuriyama H., Hammer R.E., Horton J.D., Brown M.S., Goldstein J.L., Liang G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J. Clin. Invest. 2004;113:1168–1175. doi: 10.1172/JCI20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M., Silverstein R.L. CD36: implications in cardiovascular disease. Int. J. Biochem. Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff T., Stieger B., Hagenbuch B., Madon J., Landmann L., Roth J., Hofmann A.F., Meier P.J. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Basu S.K., Brown M.S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Hellerstein M.K., Parks E.J. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 2000;71:412–433. doi: 10.1093/ajcn/71.2.412. [DOI] [PubMed] [Google Scholar]
- Horton J.D., Cohen J.C., Hobbs H.H. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 2009;50(Suppl):S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.D., Goldstein J.L., Brown M.S. SREBPs:activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.D., Shah N.A., Warrington J.A., Anderson N.N., Park S.W., Brown M.S., Goldstein J.L. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.D., Shimano H., Hamilton R.L., Brown M.S., Goldstein J.L. Disruption of LDL receptor gene in transgenic SREBP-1a mice unmasks hyperlipidemia resulting from production of lipid-rich VLDL. J. Clin. Invest. 1999;103:1067–1076. doi: 10.1172/JCI6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.D., Shimomura I. SREBPs: activators of cholesterol and fatty acid biosynthesis. Curr. Opin. Lipidol. 1999;10:143–150. doi: 10.1097/00041433-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Howard B.V. Lipoprotein metabolism in diabetes mellitus. J. Lipid Res. 1987;28:613–628. [PubMed] [Google Scholar]
- Hudgins L.C., Hellerstein M., Seidman C., Neese R., Diakun J., Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Iritani N., Nishimoto N., Katsurada A., Fukuda H. Regulation of hepatic lipogenic enzyme gene expression by diet quantity in rats fed a fat-free, high carbohydrate diet. J. Nutr. 1992;122:28–36. doi: 10.1093/jn/122.1.28. [DOI] [PubMed] [Google Scholar]
- Johnson B.M., DeBose-Boyd R.A. Underlying mechanisms for sterol-induced ubiquitination and ER-associated degradation of HMG-CoA reductase. Semin. Cell Dev. Biol. 2018;81:121–128. doi: 10.1016/j.semcdb.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., Burgess S.C., Li C., Ruddy M., Chakravarthy M., et al. Acetyl-CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:394–406.e396. doi: 10.1016/j.cmet.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Choi W.G., Ahn K.J., Chae I.G., Yu R., Back S.H. Reduced EGFR level in eIF2 phosphorylation-deficient hepatocytes is responsible for susceptibility to oxidative stress. Mol. Cells. 2020;43:264–275. doi: 10.14348/molcells.2020.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.S., Freake H.C. High carbohydrate diet and starvation regulate lipogenic mRNA in rats in a tissue-specific manner. J. Nutr. 1996;126:611–617. doi: 10.1093/jn/126.3.611. [DOI] [PubMed] [Google Scholar]
- Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia S.S., Blaha M., Keenan T., Ndumele C., Jones S., DeFilippis A., Martin S., Kohli P., Conceicao R., Carvalho J., et al. Relation of hepatic steatosis to atherogenic dyslipidemia. Am. J. Cardiol. 2013;112:1599–1604. doi: 10.1016/j.amjcard.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Korn B.S., Hammer R.E., Moon Y.A., Komuro R., Horton J.D., Goldstein J.L., Brown M.S., Shimomura I. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 2001;15:1206–1216. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C.L., Berger J.M., Lespine A., Pillot B., Prieur X., Letessier E., Hussain M.M., Collet X., Cariou B., Costet P. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 2013;33:1484–1493. doi: 10.1161/ATVBAHA.112.300263. [DOI] [PubMed] [Google Scholar]
- Moon Y.A., Hammer R.E., Horton J.D. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J. Lipid Res. 2009;50:412–423. doi: 10.1194/jlr.M800383-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y.A., Liang G., Xie X., Frank-Kamenetsky M., Fitzgerald K., Koteliansky V., Brown M.S., Goldstein J.L., Horton J.D. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y.A., Ochoa C.R., Mitsche M.A., Hammer R.E., Horton J.D. Deletion of ELOVL6 blocks the synthesis of oleic acid but does not prevent the development of fatty liver or insulin resistance. J. Lipid Res. 2014;55:2597–2605. doi: 10.1194/jlr.M054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki H., Goldstein J.L., Brown M.S., Liang G. LXR-SREBP-1c-phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size. J. Biol. Chem. 2010;285:6801–6810. doi: 10.1074/jbc.M109.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Cortés V.A., Rashid S., Anderson N.N., McDonald J.G., Liang G., Moon Y.A., Hammer R.E., Horton J.D. Expression of SREBP-1c requires SREBP-2-mediated generation of a sterol ligand for LXR in livers of mice. Life. 2017;6:e25015. doi: 10.7554/eLife.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders F.W.B., Acharjee A., Walker C., Marney L., Roberts L.D., Imamura F., Jenkins B., Case J., Ray S., Virtue S., et al. Hepatic steatosis risk is partly driven by increased de novo lipogenesis following carbohydrate consumption. Genome Biol. 2018;19:79. doi: 10.1186/s13059-018-1439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenkovich C.F. Insulin resistance and atherosclerosis. J. Clin. Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H., Horton J.D., Hammer R.E., Shimomura I., Brown M.S., Goldstein J.L. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I., Shimano H., Korn B.S., Bashmakov Y., Horton J.D. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J. Biol. Chem. 1998;273:35299–35306. doi: 10.1074/jbc.273.52.35299. [DOI] [PubMed] [Google Scholar]
- Sukonina V., Lookene A., Olivecrona T., Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- Turley S.D., Daggy B.P., Dietschy J.M. Effect of feeding psyllium and cholestyramine in combination on low density lipoprotein metabolism and fecal bile acid excretion in hamsters with dietary-induced hypercholesterolemia. J. Cardiovasc. Pharmacol. 1996;27:71–79. doi: 10.1097/00005344-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Weigand W., Hannappel E., Brand K. Effect of starvation and refeeding a high-protein or high-carbohydrate diet on lipid composition and glycogen content of rat livers in relation to age. J. Nutr. 1980;110:669–674. doi: 10.1093/jn/110.4.669. [DOI] [PubMed] [Google Scholar]
- Xie C., Woollett L.A., Turley S.D., Dietschy J.M. Fatty acids differentially regulate hepatic cholesteryl ester formation and incorporation into lipoproteins in the liver of the mouse. J. Lipid Res. 2002;43:1508–1519. doi: 10.1194/jlr.m200146-jlr200. [DOI] [PubMed] [Google Scholar]
- Yao Z., Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr. Opin. Lipidol. 2012;23:206–212. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]
- Yazdanyar A., Jiang X.C. Liver phospholipid transfer protein (PLTP) expression with a PLTP-null background promotes very low-density lipoprotein production in mice. Hepatology. 2012;56:576–584. doi: 10.1002/hep.25648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Li J.Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–190. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Yu L., Hammer R.E., Li-Hawkins J., von Bergmann K., Lutjohann D., Cohen J.C., Hobbs H.H. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zamani M., Thiele C., Taher J., Alipour M.A., Yao Z., Adeli K. AUP1 (ancient ubiquitous protein 1) is a key determinant of hepatic very-low density lipoprotein assembly and secretion. Arterioscler. Thromb. Vasc. Biol. 2017;37:633–642. doi: 10.1161/ATVBAHA.117.309000. [DOI] [PubMed] [Google Scholar]
- Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012;424:786–792. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.