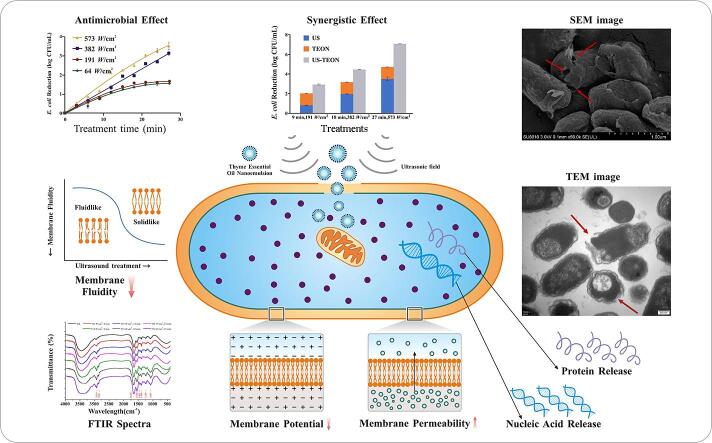
Graphical abstract
Keywords: Ultrasonic field, Antimicrobial mechanism, Escherichia coli O157:H7, Membrane permeability
Highlights
-
•
The antibacterial effect of US was in a time- and intensity-dependent manner.
-
•
US remarkably increased the sensitivity of E. coli cells to TEON.
-
•
US significantly increased the outer and inner membrane permeability.
-
•
US resulted in membrane fluidity reduction and membrane depolarization.
-
•
US caused conformational and compositional changes in some membrane components.
Abstract
This study was aimed at providing new insights on the response of bacterial cell membranes to ultrasound exposure. Escherichia coli (E. coli) O157:H7 cells were exposed to different ultrasound treatments (power intensities of 64, 191, 372, and 573 W/cm2, frequency of 20 kHz, pulsed mode of 2 sec: 2 sec) and the dynamic changes in cell viability within 27 min were assessed. With an increase in ultrasonic intensity and prolonged duration, a 0.76–3.52 log CFU/mL reduction in E. coli populations was attained. The alterations in the sensitivity of ultrasound-treated cells to antimicrobial compounds were evaluated by exposure to thyme essential oil nanoemulsion (TEON). The treatment reduced the E. coli population by 2.16–7.10 log CFU/mL, indicating the effects of ultrasonic field on facilitating the antibacterial efficacy of TEON. Ultrasonic-treated E. coli cells also displayed remarkable morphological and ultrastructural damages with destroyed membrane integrity and misshaped cell structures, which was observed by electron microscopy analysis. Significant increase in outer and inner membrane permeability, along with the cytoplasmic leakage and membrane depolarization were assessed utilizing spectrophotometry. For the first time, significant reduction in the membrane fluidity in response to ultrasound exposure were investigated. Additional efforts in exploring the effect of ultrasonic field on some bacterial membrane compositions were performed with infrared spectroscopy. In this study, multiple lines of evidence effectively served to elucidate the alterations on cellular membrane structure and property during exposure to sonication that could extend our understanding of the antimicrobial molecular mechanisms of ultrasound.
1. Introduction
Ultrasound is generally considered to be an ecofriendly non-thermal processing technology and has been widely adopted in the field of food disinfection [1], [2], [3]. It has been acknowledged that the antimicrobial action of ultrasound is attributed to the large number of cavitation bubbles generated when ultrasound treatment is applied to a liquid medium [4], [5]. However, numerous existing data revealed that ultrasound treatment alone may not exert sufficient antimicrobial activity, failing to guarantee the microbial safety of food items [6]. The combination of ultrasound and biocide (chlorine) has been of considerable interest since the 1990′s when the work in Mason’s group was first published [7]. In recent years, some studies have also highlighted the combination of ultrasound with other preservation technologies or compounds with inherent biocidal action aiming at exhibiting an inactivating synergistic effect [8], [9]. Previous studies conducted in our lab also demonstrated an extraordinary synergistic effect of ultrasound and thyme essential oil nanoemulsion (TEON) against E. coil O157:H7. We hypothesized that ultrasound treatment might allow for larger amounts of TEON to enter cells, substantially enhancing its inherent biocidal effect and ultimately leading to cell death. This was preliminarily demonstrated by microscopy images showing the disintegration of the structure of the cell membrane [10], [11]. However, there is little information regarding to the response of E. coli cell membranes in the ultrasonic field.
The outer membrane (OM) is a fundamental organelle of Gram-negative bacterial cells [12]. It acts as a robust and selective permeability barrier that protects these cells against harsh environments or noxious compounds [13]. It has been reported that the mechanical integrity and strength of Escherichia coli cells were largely conferred by the outer membrane [12]. Li et al. (2016) conducted a study by flow cytometry analysis to observe the alterations in membrane permeability in the ultrasonic field. They proposed that the outer membrane was likely to be the primary target upon ultrasound treatment, and the inner membrane might start to be destabilized as the processing time increased [14]. However, ultrasound-induced alterations in other membrane properties that are closely related to the viability of bacterial cells have not been deeply explored. Thereinto, membrane fluidity affects a number of cellular functions, such as carrier-mediated transport, the properties of certain membrane-bound enzymes and cell growth. Membrane potential is also closely correlated with membrane integrity and the physiological state of bacteria [15]. The correlation between the alterations in membrane fluidity and inactivation effect under pulse electric field against Salmonella typhimurium were elucidated in the previous studies [16]. Furthermore, Fourier transform infrared (FTIR) spectroscopy is known to be of a great value in characterizing the cell metabolism and biochemical properties of some cell membrane components, such as lipids, proteins, and nucleic acid, etc. [17]. Given all the functional groups of these organic molecules absorb infrared light specifically, the structural and physiological alterations on the bacterial cells upon different stress could be studied by FTIR analysis [18]. This technique has been applied to explore changes at cellular level of E. coli and Staphylococcus aureus upon plasma treatment [19] and the physiological state of Bacillus subtilis spores subjected to high pressure treatment [17]. However, to date, very few studies have reported the changes of cell membrane fluidity and potential under ultrasonic field. In previous study, the cell envelop was taken as an integral part, while the ultrasound-induced changes in the permeability of outer membrane and inner membrane have not been clarified separately. Meanwhile, the conformational and compositional changes of E. coli cell membrane compositions after ultrasound exposure were not explored yet.
In this study, we focused on investigating the antimicrobial activity of ultrasound treatment, and analyzing ultrasound-induced alterations in the cellular membrane. Especially, more in-depth insights into the inactivation behaviors of the ultrasonic field against bacterial membrane, involving the membrane morphology, ultrastructure, and properties were provided. The antimicrobial efficacy of ultrasound at different processing intensities and durations was evaluated; the sensitivity of ultrasound-treated cells to TEON was assessed; moreover, the changes in the morphological appearance and ultrastructure of the cells were observed. Also, the membrane properties such as outer and inner membrane permeability, membrane fluidity and membrane potential were determined. To better understand the cellular membrane destruction response to ultrasound exposure, the intramolecular chemical bonding of the bacterial cells was investigated with FTIR spectra.
2. Materials and methods
2.1. Bacterial strains and culture conditions
E. coli O157:H7 ATCC 35150 was purchased from Guangdong Culture Collection Center (Guangzhou, China). Frozen stock cultures of the strain were incubated in nutrient broth (NB) medium and cultured on eosin methylene blue (EMB) plate. A single colony was transferred into 200 mL NB medium. It was incubated at 37°C with a reciprocal shaker (TS-2102C, Tensuc, Shanghai, China) for 17 h. Cell suspensions were harvested by centrifugation at 4000 × g for 10 min, washed twice by resuspension in a 0.85% sterile saline solution. The initial bacterial population was 8.93 ± 0.11 log CFU/mL.
2.2. Ultrasound treatments
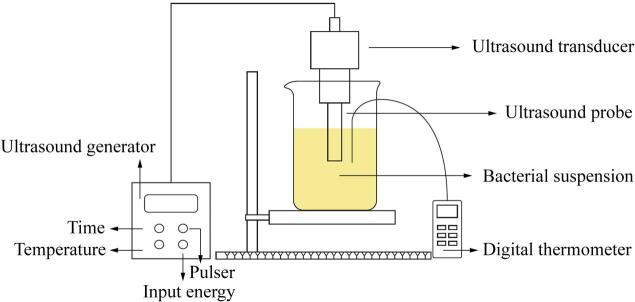
The sketch diagram of experimental set-up was displayed in Fig. 1. Ultrasound treatments were performed using a probe system (Scientz-II D, Ningbo Scientz, Ningbo, China), which operated at a frequency of 20 kHz. 30 mL of each bacterial suspensions was filled into a 75-mL columniform glass vial. Suspensions were sonicated by submerging a 10-mm-diameter probe below the surface of the suspension (operation immersion depth, 20 mm). The acoustic densities applied were calculated using the reported methods [5] with the equation:
where UI is the applied ultrasonic power intensity (W/cm2), P is the input ultrasonic power we set, and r is the radius (cm) at the probe tip. The input power levels, which adjusted to 6%, 17%, 33%, and 50% of the total input power (900 W), corresponded to ultrasonic power of 50, 150, 300, and 450 W, respectively. Accordingly, the calculated power intensities were 64, 191, 372, and 573 W/cm2. The ultrasound processes were conducted applying these power intensities every 3 min within 27 min (3, 6, 9, 12, 15, 18, 21, 24, and 27 min) on a pulsed mode (2 sec on: 2 sec off). Bacterial suspensions of ultrasound-treated and control samples were serially diluted and plated. The inactivation of E. coli was assessed by the calculation of log10 reduction in the treated samples compared to the control group.
Fig. 1.
Sketch diagram of ultrasound equipment.
2.3. Sensitivity analysis of ultrasound-treated E. coli to TEON
The thyme essential oil nanoemulsion was formulated according to the method of Guo et al. (2020) [10]. The concentration of the thyme essential oil in the nanoemulsion formulated by ultrasonication was 10 mg/mL. After the ultrasonic inactivation treatments mentioned in Section 2.2, 0.75 mL of TEON solution was immediately added to each of the 30-mL suspensions immediately (US-TEON). The final concentration of thyme essential oil in the cell suspensions was 0.25 mg/mL, i.e. its MIC value. The mixture was vortexed thoroughly and gently shaken at a speed of 200 rpm in a 20℃ incubator for 9 min. Thereafter, microbial numbers of each sample were analyzed by spread-plating the appropriate serial dilutions onto Plate Count Agar (PCA) media, and the colonies were enumerated after the incubation for 17 h at 37°C. The synergistic antimicrobial effect (log CFU/mL) was estimated by applying the following equation:
where RUS-TEON represents the microbial reduction resulting from the application of US-TEON; RUS and RTEON represents the microbial reduction resulting from the exclusive application of US or TEON, respectively.
2.4. Electron microscopy observation
2.4.1. Scanning electron microscopy
The morphological alterations in the membranes of E. coli cells subject to ultrasound treatments were revealed by scanning electron microscopy (SEM). Cells exposed to ultrasound at various power intensities (191 W/cm2 and 573 W/cm2) and duration times (9, 18 and 27 min) were selected. The cell suspensions were harvested by centrifugation at 8000 × g for 10 min. The precipitated cells were immersed in a 2.5% (v/v) glutaraldehyde fixative at 4 overnight and this was followed by a post-fixation in 1% (v/v) osmium tetroxide at room temperature for 1 h. The pellets were then rinsed thoroughly three times with 0.1 M phosphate buffer saline (pH 7.0) for 15 min separately before being successively dehydrated by a graded ethanol series (30, 50, 70, 80, 90 and 95%, v/v) for 15 min each and treated twice with absolute ethanol for 30 min. Subsequently, the specimens were dried in a critical point drying system, attached to the SEM stubs, and sputter coated with a layer of gold prior to observation. Samples were visualized and photographed using a field emission scanning electron microscope (SU-8010, Hitachi Ltd., Tokyo, Japan).
2.4.2. Transmission electron microscopy
The intracellular changes induced by ultrasound treatments were revealed by transmission electron microscopy (TEM). After using the same fixation and dehydration process as SEM analysis, the specimens were embedded in Spurr’s resins, incubated at room temperature for 4 h, and then placed in an oven for 24 h at 65℃ to polymerize. Each hardened resin block was cut into approximately 90 nm ultrathin-sections using a Leica EM UC7 Ultramicrotome, and stained on grids with uranyl acetate and alkaline lead citrate. The specimens were observed under a transmission electron microscope (JEM-1230, JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 80 kV.
2.5. Permeability of outer membrane
Outer membrane (OM) permeabilization was examined by the N-Phenyl-1-naphthylamine (NPN) assay described by Qin et al., (2020) with minor modifications [20]. Overnight cultured E. coli cells were collected, rinsed and resuspended in sterile saline solution. Bacterial cells undergone different ultrasound treatments (power intensities of 64, 191, 372, and 573 W/cm2, processing duration of 9, 18, and 27 min) were collected. Cell suspensions were adjusted to an absorbance at 600 nm of 0.5, and then mixed with NPN (Macklin Biochemical Co., Ltd, Shanghai, China) solution to reach a final concentration of 40 µM. The excitation wavelengths of NPN was set at 350 nm and the fluorescence emission at 420 nm. Fluorescence intensity of each mixture was monitored until no further increase was observed, and the maximum values were recorded using the Cary Eclipse Fluorescence Spectrophotometer (Varian Inc., Palo Alto, CA, USA).
2.6. Permeability of inner membrane
β-galactosidase, which is an induced endoenzyme in E. coli, undergoes progressive outward release from the cytoplasm when the bacterial inner membrane is destroyed. When o-nitrophenol-β-D-galactopyranoside (ONPG) is hydrolyzed by β-galctosidase, o-nitrophenol (ONP), a yellow compound, is released. ONP absorbs light at 420 nm. β-galactosidase activity is monitored at this absorbance value [21], [22]. Hence, the alteration of inner membrane permeability was evaluated by monitoring the ONP production under different sonication treatments. E. coli slurries cultured in Luria broth medium containing 2% lactose were collected, rinsed, and resuspended in sterile saline solution. Bacterial cells undergone different ultrasound treatments (power intensities of 64, 191, 372, and 573 W/cm2, processing duration of 9, 18, and 27 min) were collected. Subsequently, the optical density at 420 nm of the ultrasound-treated cells were adjusted to 1.0. Samples (200 µL) containing 190 µL of E. coli suspensions and 10 µL of 30 mM ONPG (Beyotime Biotechnology, Shanghai, China) solution were mixed thoroughly and incubated at 37℃ for 30 min. Absorption values at 420 nm for each mixed solution were read using a Multiskan GO microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Cytoplasm leakage analysis
The leakage of intracellular protein and nucleic acid was determined using a previously described method [10]. Ultrasound-treated and non-treated (control) cell suspensions were centrifuged (8000 × g, 10 min, 4℃) for the collection of supernatants. The supernatants were then transferred into aseptic Eppendorf tubes prior to conducting the cytoplasm leakage assays.
The content of nucleic acid release was measured by reading absorbance of the supernatant utilizing a UV–Vis spectrophotometer (UV-2600, Shimadzu, Tokyo, Japan). The results were presented in term of optical density values at 260 nm (OD260 nm). Simultaneously, the amount of released proteins was calculated using absorbance values at 280 nm and a standard curve generated using bovine serum albumin (BSA) standard solutions.
2.8. Membrane fluidity analysis
Bacterial membrane fluidity of E. coli and its response to ultrasound treatment were assessed by fluorescence anisotropy analysis using the fluorescent probe 1,6-dephenyl-1,3,5-hexatriene (DPH) (Sigma-Aldrich, St. Louis, MO, USA), which exhibited strong fluorescence upon incorporation into the hydrophobic regions of the phospholipid bilayer. The DPH working solution was prepared by diluting a 2.0 mM stock solution (dissolved in tetrahydrofuran) with PBS solution to a final concentration of 2 µM. After the ultrasound treatments, E. coli cell pellets were collected by centrifugation (8000 × g, 10 min, 4℃), and the final cell suspensions were adjusted to an optical density at 600 nm (OD600 nm) of 0.5. Subsequently, 4 mL of DPH working solution was added to 5 mL harvested cell pellets and incubated for 45 min at 37℃ under dark conditions. Excessive DPH were removed from the suspensions by centrifugation (8000 × g, 10 min, 4℃), double rinses and resuspension with 4 mL PBS buffer. Afterward, the labeled bacterial suspensions were transferred to a black 96-well plate for detection. For the measurement of fluorescence anisotropy, a SpectraMax M5 multi-mode microplate reader (Molecular Devices, LLC., San Jose, CA, USA) was employed at the excitation and emission wavelength of 360 nm and 430 nm (5.0/5.0 nm slit widths), respectively. Fluorescence polarization (P), fluorescence anisotropy (r), and micro-viscosity (η) were calculated according to the following equations:
where IVH and IVV denote fluorescence intensities of the emitted beam obtained from the horizontal and vertical analyzer when the excitation polarizer is oriented vertically, respectively. G represents the instrumental grating factor.
2.9. Membrane potential measurement
A membrane potential sensitive fluorescence probe, 3,3′-diethyloxacarbocyanine iodide (DiOC2(3)), was used to monitor changes in membrane potential [24]. DiOC2(3) (Aladdin Bio-Chem Technology Co., Ltd, Shanghai, China) was dissolved in dimethylsulfoxide (DMSO) to prepare a 30 mM stock solution, and further diluted at a ratio of 1:10 with PBS to get a working probe solution. Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China), a chemiosmotic inhibitor contributing to the disruption of membrane potential, was employed as the depolarized control. Specifically, after being treated with ultrasound, E. coli cell pellets were harvested by further centrifugation (8000 × g, 10 min, 4℃) and re-suspended to an absorbance at 600 nm of 0.5. Then, 1 mL of suspension was labeled with the fluorescent probe by the addition of 10 µL of DiOC2(3) working solution (3 mM). For the control samples, 10 µL of 500 µM CCCP was mixed with the sample prior to addition of DiOC2(3). Stained samples were incubated at 25℃ for 30 min under dark conditions. Afterward, samples were immediately centrifuged, washed twice, re-suspended in 1 mL sterile saline solution, and then transferred into a black 96-well plate. Fluorescence spectroscopy was performed on a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA). Samples were excited at 460 nm with the emission spectrum recorded over the range of 490 to 700 nm.
2.10. FTIR analysis
FTIR spectroscopy was intensively applied to investigate the action mode of antimicrobial techniques and to monitor the structural changes in bacterial cell membranes under different stress [24], [25]. After different treatments, bacterial suspensions were obtained by centrifugation (8000 × g, 10 min, 4℃), double rinses, and resuspension with aseptic PBS buffer. Samples were subjected successively to overnight refrigeration at −20℃, two days of freezing at −80℃, vacuum freeze-drying, and then stored in desiccator until use. The FTIR analyses were performed by the Nicolet iN10 FT-IR spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The absorption spectra were recorded within the range of 4000–400 cm−1. Background obtained from the pure potassium bromide (KBr) disc was automatically subtracted from each sample spectrum, since the embedding of dried samples into the KBr pastille prior to analysis. For every single sample, average spectrum was acquired from three independent replicates, followed by the smoothing, normalization, and baseline-correction processing.
2.11. Statistical analysis
Data were reported as mean value ± standard deviation (SD) of three independent measurements (n = 3). Results were analyzed with SPSS software (SPSS 25.0 for MAC; IBM Co., USA). Levels of differences were determined by one-way ANOVA followed by Duncan’s test and statistical significance was defined at P < 0.05.
3. Results and discussion
3.1. E. coli inactivation by ultrasound treatment
The inactivation efficacy under various ultrasound process parameters is given in Table 1. An increase in exposure time resulted in a significantly higher reduction in E. coli O157:H7 populations. Ultrasound treatment at 64 W/cm2 reduced E. coli O157:H7 populations by 0.24 ± 0.02 to 1.54 ± 0.11 log CFU/mL over 27 min treatment. Li et al. (2016) investigated the sterilization effect of E. coli and S. aureus using a fixed power intensity (60 W/cm2) during sonication from 0 to 20 min. In the present study, a series of ultrasonic power intensities (64, 191, 372, and 573 W/cm2) were systematically studied to explore the ultrasonic inactivation effect.
Table 1.
Reduction of E. coli O157:H7 populations after various ultrasound treatments for different power intensity and treatment time.
| Time (min) | Ultrasonic intensity |
|||
|---|---|---|---|---|
| 64 W/cm2 | 191 W/cm2 | 382 W/cm2 | 573 W/cm2 | |
| 3 min | 0.24 ± 0.02Fc | 0.26 ± 0.00Fb | 0.40 ± 0.02Fb | 0.51 ± 0.02Ia |
| 6 min | 0.38 ± 0.09Ec | 0.58 ± 0.04Eb | 0.66 ± 0.06Eb | 0.87 ± 0.07Ha |
| 9 min | 0.76 ± 0.09Db | 0.82 ± 0.01Db | 0.81 ± 0.04Eb | 1.15 ± 0.01Ga |
| 12 min | 1.09 ± 0.02Cc | 1.10 ± 0.01Cc | 1.36 ± 0.12Db | 2.15 ± 0.03Fa |
| 15 min | 1.29 ± 0.09Bc | 1.40 ± 0.07Bc | 1.94 ± 0.05Cb | 2.26 ± 0.05Ea |
| 18 min | 1.38 ± 0.06Bd | 1.59 ± 0.10Ac | 1.96 ± 0.05Cb | 2.50 ± 0.03Da |
| 21 min | 1.51 ± 0.04Ac | 1.61 ± 0.01Ac | 2.59 ± 0.09Bb | 3.05 ± 0.04Ca |
| 24 min | 1.53 ± 0.07Ac | 1.60 ± 0.05Ac | 2.69 ± 0.07Bb | 3.27 ± 0.10Ba |
| 27 min | 1.54 ± 0.11Ac | 1.66 ± 0.01Ac | 3.13 ± 0.12Ab | 3.52 ± 0.19Aa |
Note: Initial bacterial population was approximately 8.93 ± 0.11 log CFU/mL.
Values are the mean of triplicate measurements ± standard deviation; values with different lowercase letters in the same row and uppercase letters in the same column showed a significant difference at P < 0.05.
3.2. Sensitivity of ultrasound-treated E. coli to TEON
For all processing conditions, a remarkable increase in reducing the number of microbes was observed, indicating the enhanced inactivation effects of US-TEON treatment than that of TEON as a standalone treatment (Table 2). Additionally, a significant increase (P < 0.05) in the synergistic effect of the US-TEON treatment was also detected when cells were subjected to ultrasound-pretreatment for a longer duration, where treatment for 27 min at 573 W/cm2 reduced E. coli populations by 7.10 ± 0.02 log CFU/mL. It was observed that pre-sonication of E. coli substantially enhanced the antimicrobial efficiency of TEON against E. coli and increased the susceptibility of the bacteria to TEON stimulation compared to those which were not exposed to ultrasound.
Table 2.
Reduction of US and US-TEON treatment and the synergistic effect (log CFU/mL) against E. coli O157:H7 at different processing intensities and durations.
| Treatments | 64 W/cm2 |
191 W/cm2 |
382 W/cm2 |
573 W/cm2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 min | 18 min | 27 min | 9 min | 18 min | 27 min | 9 min | 18 min | 27 min | 9 min | 18 min | 27 min | |
| US | 0.76 ± 0.09h | 1.38 ± 0.06f | 1.54 ± 0.11ef | 0.82 ± 0.01h | 1.59 ± 0.10e | 1.66 ± 0.01e | 0.81 ± 0.04h | 1.96 ± 0.05d | 3.13 ± 0.12b | 1.15 ± 0.01g | 2.50 ± 0.03c | 3.52 ± 0.19a |
| US-TEON | 2.16 ± 0.06i | 2.87 ± 0.08h | 4.46 ± 0.11d | 2.93 ± 0.07h | 3.71 ± 0.14e | 4.97 ± 0.11c | 3.37 ± 0.06f | 4.48 ± 0.10d | 6.49 ± 0.14b | 3.63 ± 0.02e | 5.02 ± 0.05c | 7.10 ± 0.02a |
| Synergistic effect | 0.19 ± 0.07f | 0.28 ± 0.10f | 1.71 ± 0.13c | 0.90 ± 0.07e | 0.91 ± 0.13e | 2.10 ± 0.03b | 1.29 ± 0.04d | 1.30 ± 0.03d | 2.15 ± 0.17b | 1.27 ± 0.01d | 1.31 ± 0.04d | 2.37 ± 0.02a |
Note:
The microbial reduction by TEON was 1.21 ± 0.03 log CFU/mL.
Synergistic effect (log CFU/mL) was estimated by applying the following equation: Synergistic effect = RUS-TEON - (RUS + RTEON). Where, RUS-TEON represents the microbial reduction resulting from the application of US-TEON; RUS and RTEON represents the microbial reduction resulting from the exclusive application of US or TEON, respectively.
The experiments were carried out three times. Error bars indicate the standard deviation of three technical replicates.
Values with different letters in the same row showed a significant difference at P < 0.05.
The limited antimicrobial activity of ultrasound treatment alone highlights the need to combine ultrasound with other hurdle sterilization technologies to guarantee the microbial safety of food items without compromising their nutritional value and quality [6], [1]. Mason et al., (2015) have demonstrated that ultrasound substantially improved the effect of biocide such as chlorine or ozone against pathogens in food products [26]. Moreover, several studies have also reported the great versatility of ultrasound when it was combined with other antimicrobial agents such as peroxyacetic acid [27], fumaric acid [28], sodium hypochlorite [29], lysozyme [30], and plant-derived essential oils [31], etc. Our previous study initially reported an integrated technology utilizing both ultrasound and TEON simultaneously, which showed significantly enhanced antimicrobial effects than either the ultrasound or TEON used alone [10], [11]. These combinations exhibited synergistic effects, allowing for the optimization of pathogen inactivation without diminishing the physicochemical properties of foods. In the present work, the sensitivity of bacterial cells towards TEON was elevated once it was previously subject to ultrasonic treatment, implying the altered membrane properties or functions and the consequently decreased membrane resistance. Wu et al. (2017) studied the synergistic effects of ultrasound and antimicrobial peptide melittin against Listeria monocytogenes [32]. Bastarrachea et al. (2017) also found that the inactivation rate of Listeria innocua was substantially elevated through the implementation of a sequential treatment in which L. innocua cells were subject to ultrasound treatment for four minutes followed by exposure to 25 µM Erythrosin B [33]. The sensitivity of Listeria innocua cells to the intrinsic antimicrobial effect of Erythrosin B was boosted by more than 4 log CFU/mL. However, literature about ultrasound treatment enhancing the antimicrobial activity of TEON against E. coli is still limited. In order to determine how ultrasound treatment enhanced inactivation effect of TEON, the following assays with regard to the changes in membrane morphologies and properties were performed.
3.3. SEM
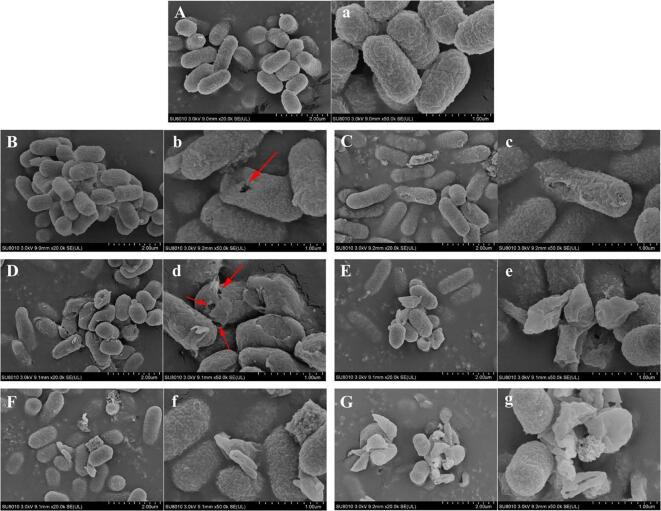
SEM analysis was performed to record the alterations in surface morphology and cell membrane integrity of E. coli cells upon ultrasound treatment. The images shown in Fig. 2 illustrate that the level of morphological damage induced by ultrasound was highly dependent on the intensity of ultrasound treatment and duration time. Untreated E. coli cells displayed a typical rod-like shape with smooth and intact cell surfaces (Fig. 2A-a). After sonication processing at 191 W/cm2 for 9 min, most of the cells retained their original shape. Only a few of them exhibited visible partial pore formation (red arrows) and blurry cell envelopes. Prolonging the sonication time to 18 min led to the formation of more pores on the membrane and a considerably higher number of ruptured cells (Fig. 2D-d). In Fig. 2F-f, bacterial cells with profound shrinkage, significant deformation, and mass formation of cell debris were observed when the treatment time was extended to 27 min. E. coli cells subject to treatment at a higher intensity (573 W/cm2) displayed more remarkable structural changes compared to those subjected to treatment at a lower intensity (191 W/cm2) under the same processing time. High-intensity ultrasound treatment also induced morphological damage on cellular surfaces in a time-dependent manner.
Fig. 2.
SEM images of E. coli O157:H7 upon different ultrasound treatments. Note: A, a—Control; B, b—191 W/cm2, 9 min; D,d—191 W/cm2, 18 min; F, f—191 W/cm2, 27 min; C,c—573 W/cm2, 9 min; E, e—573 W/cm2, 18 min; G, g—573 W/cm2, 27 min; Magnification = 20,000 ×, bar marker = 2 µm; Magnification = 50,000 ×, bar marker = 1 µm; Red arrows indicate the occurrence of perforations.
Ultrasound has been shown to elevate the membrane permeability via the sonoporation, namely the phenomenon of transient pore formation on the cellular membrane resulting from acoustic cavitation [34]. This may stem from the fact that large number of cavitation bubbles generated when ultrasound treatment is applied to a liquid medium. These bubbles can expand and collapse in very short periods of time, and shock waves are released when this happens. Areas of extreme pressure and temperature can also develop in the liquid medium. These effects are strong enough to shear and break the structure of the cell membrane, allowing for the formation of temporal cell permeabilization [35]. Such perforation was comprehensively studied as a tool for non-invasive transfer and internalization of macromolecular substances which are normally non-permeable to the cell membrane for therapeutic application [36], [37]. There are very few published studies on the sterilization mechanism of sonoporation [34]. In our present work, the perforations of E. coli membranes were systematically studied under ultrasound exposure at various treatment parameters. It was noticed that E. coli cells responded effectively to the sonication through acting in varying degrees of destruction in cell morphology and ultrastructure. This is supported by Wu et al. (2019), who described the large pores on the Microcystis aeruginosa cells produced by low-frequency sonication, pointing out the mechanism of microbial inactivation that was attributed to the harsh damage on the cell envelope via acoustic shear force [5]. The current results of electron microscopy observation correlated well with the inactivation efficacy mentioned above, supporting our assumptions that the generation of pores on the membranes conduced cell permeabilization, thereby increasing the susceptibility of E. coli cells to the subsequent TEON treatment.
3.4. TEM
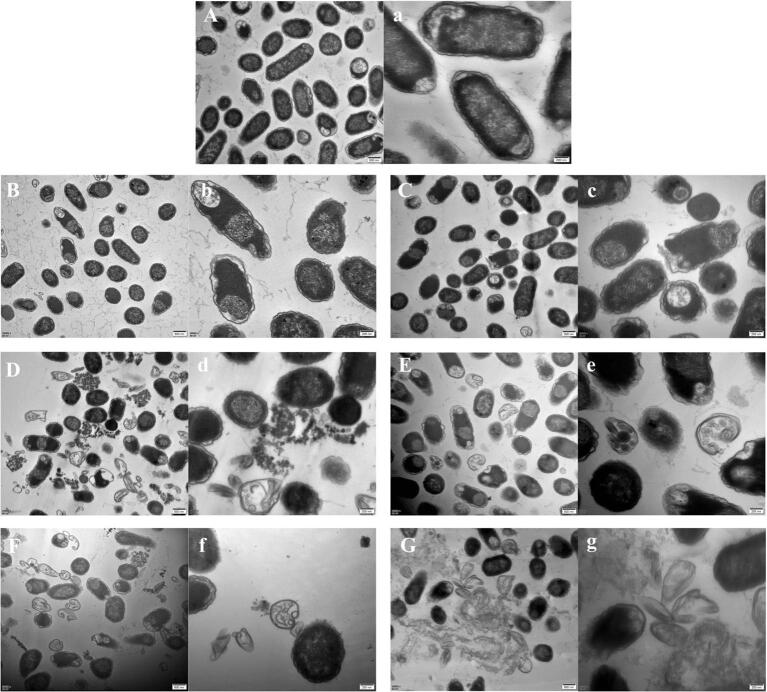
The effect of ultrasound irradiation on the microstructure of E. coli cells was observed under TEM (Fig. 3). These images show that untreated E. coli cells had an intact cell membrane and homogenous electron density in the cytoplasm (Fig. 3A-a). As depicted in Fig. 3B-b, cells which were subjected to ultrasound treatment at 191 W/cm2 ultrasound for 9 min displayed a slightly dislocated and shrunken cytoplasmic membrane. The E. coli cells which were subjected to ultrasound treatment at a higher intensity ultrasound (573 W/cm2) exhibited a relatively larger extent of damage with discontinuities on the cell walls (indicated with red arrow in Fig. 3C-c). Increase in treatment time led to destruction of the integrity of the cell membrane, misshapen cell structures, leakage of the internal cellular constituents, and destruction of the cells. Specifically, in Fig. 3C-c and 3D-d, fragments of cell wall debris could be viewed along with the partially disrupted cells and the cytoplasm appeared to be extruded via the destroyed membrane boundary. Increasing the treatment time to 27 min caused more severe alterations in the cell structures. The images shown in Fig. 3E-e and 3F-f show irreversible cell distortion, huge vacuoles, uniformly distributed cytoplasmic content, and heterogenous electron density. At this extreme point, a pile of cellular debris observed with the lysed cells was due to the forceful flow out of cell inclusions.
Fig. 3.
TEM images of E. coli O157:H7 upon different ultrasound treatments. Note: A, a—Control; B, b—191 W/cm2, 9 min; D,d—191 W/cm2, 18 min; F, f—191 W/cm2, 27 min; C,c—573 W/cm2, 9 min; E, e—573 W/cm2, 18 min; G, g—573 W/cm2, 27 min; Magnification = 20,000 ×, bar marker = 500 nm; Magnification = 50,000 ×, bar marker = 200 nm.
Gao et al. (2014) demonstrated the multiple destructive mechanisms of low frequency ultrasound treatment and the changes it causes in the ultrastructure of E. aerogenes and B. subtilis cells [38]. Li et al., (2016) further stated the significance of the outer membrane of Gram-negative bacteria as the primary target of ultrasound treatment through TEM images [14]. In the current study, TEM analysis visually revealed that cells underwent different levels of destruction in a time- and intensity- dependent manner. These observations clearly confirmed that sonication introduced severe physical damage to the E. coli cells through the irregular separation of the cytoplasmic membrane from cell walls, the disruption of cell walls, and decomposition of the inner structures of the cell, which led to the release of cellular contents.
3.5. Permeability of outer membrane
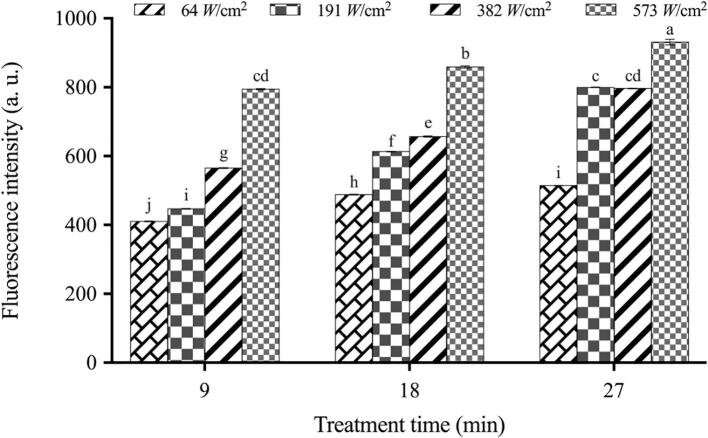
NPN uptake by ultrasound-treated E. coli was illustrated in Fig. 4. The results demonstrated that ultrasound treatment could directly contribute to outer membrane permeabilization in a time- and intensity- dependent manner. In this regard, the outer membrane of ultrasound-treated cells appeared to suffer more significant damage (P < 0.05) compared to that of the control groups as the treatment time was prolonged. Zhang et al. (2017) also reported that ultrasound treatment enhanced permeabilization of the bacterial outer membrane [36].
Fig. 4.
Cell outer membrane permeability of E. coli O157:H7 under different ultrasound treatments. Note: The fluorescence intensity of NPN in control group was 410.31 ± 1.38k.
NPN, a hydrophobic probe, is largely excluded by the intact outer membrane of the Gram-negative bacteria, and exhibits weak fluorescence in the aqueous medium. Once it penetrated into bacterial cells whose outer membranes were compromised and functionally invalid, NPN incorporated into the glycerophospholipid milieu, resulting in increased quantum yield and prominent fluorescence [21], [39]. Accordingly, the degree of outer membrane permeability was reflected by the fluorescence intensity values. Based on the literature, bacterial cells with higher outer membrane permeability usually exhibit diminished resistance to antimicrobial substances [23]. For instance, Qin et al. (2020) demonstrated one of the mutant strains of S. enteritidis, which possessed increased outer membrane permeability compared with that of wild-type, showed higher sensitivity to lysozyme [21]. It is worth noting that in this work, there was generally good consistency between the outer membrane permeability and the synergistic effect of ultrasound combined with TEON. This phenomenon further confirmed the findings in our prior study [11], where E. coli exhibited higher permeability upon ultrasound treatment, allowing for increased penetration of TEON into the bacterial cell wall and eventually resulting in cell death. Zhang et al. (2017) also demonstrated that ultrasound treatment significantly increased the permeability of the outer membrane, allowing for increased penetration of ZnO nanoparticles into E. coli bacterial cells and enhancing its antimicrobial effect [36]. The current results correlated well with electron microscope observation mentioned above, supporting our assumptions that the generation of pores on the membranes conduced cell permeabilization, thereby increasing the susceptibility of E. coli cells to the subsequent TEON treatment. Furthermore, in our previous study, where the cellular proteome profiling of E. coli response to sonication was identified using a TMT-based proteomic analysis, some membrane lipid homeostasis-related proteins were analyzed to be differentially expressed upon treatment, such as phospholipase A (PldA), intermembrane phospholipid transport system lipoprotein MlaA and MlaD, outer membrane protein C (OmpC), etc. (data not shown). This might disrupt the membrane lipid metabolism and further destabilize the outer membrane, leading to a more dramatic defect on the membrane permeability. The findings might also be a credible elucidation for the synergistic effect of ultrasound and TEON, viz., ultrasound-induced overproduction of these membrane permeability-related proteins are responsible for the reduced lipid homeostasis, which exacerbates the defects in the permeability of the cell membranes, and ultimately promotes the uptake of TEON.
3.6. Permeability of inner membrane
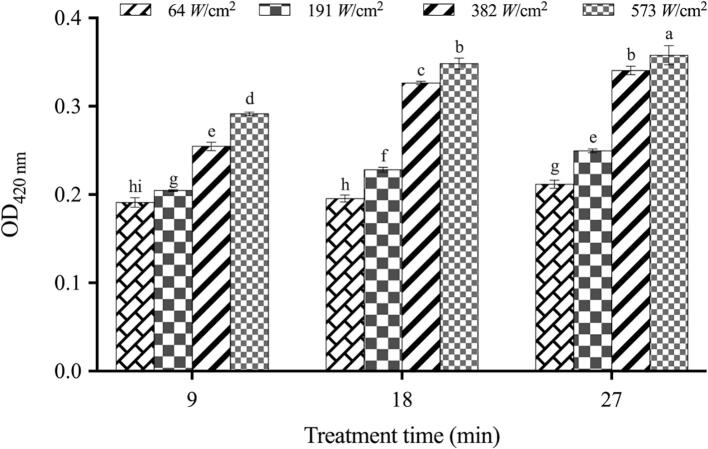
As seen in Fig. 4, bacterial cells presented a significant increase (P < 0.05) in inner membrane permeability, and this was influenced by treatment time and the intensity of the ultrasound treatment. It was noticed that at 64 W/cm2 sonication, there was no significant increase (P greater than 0.05) in inner membrane permeability within the first 9 min of treatment. Afterward, the permeability started to increase, suggesting that the alterations on the inner membrane gradually became severe. For high-intensity treatment, the occurrence of permeabilization on both the outer and inner membrane appeared to be synchronized over time (Fig. 4, Fig. 5). Li et al. (2016) proposed that the outer membrane was the only target during the first 8 min-treatment under certain ultrasonic processing conditions (20 kHz, 60 W/cm2), while the destabilization of inner membrane occurred and intensified as treatment time increased [14]. In the present work, we provided more information concerning the modification of permeability in both the outer and inner membrane under ultrasound irradiation with different intensities, whereas published studies only addressed the corresponding changes upon low-intensity ultrasound treatment.
Fig. 5.
Cell inner membrane permeability of E. coli O157:H7 under different ultrasound treatments. Note: The optical density of ONPG at 420 nm in control group was 0.185 ± 0.003i.
Moreover, identical trends were also seen between the changes in inner membrane permeability states and the response of ultrasound-treated E. coli to TEON, indicating that the permeabilizing phenomenon probably sensitized the bacteria to TEON. Taken together, the synergistic mechanism could be described in detail as the ability of sonication to disrupt the permeability barrier of the outer membrane of E. coli, which progressively opens up the inner membrane and consequently facilitates the access of TEON to the cytoplasm.
3.7. Cytoplasm leakage
The leakage of intracellular contents from bacterial cells was regarded as an indicator representing the reduction in membrane integrity upon various sterilization techniques [40], [41]. The OD260 nm values reflecting the amount of nucleic acids release during ultrasound treatment were shown in Table 3. Cytoplasm leakage in the control samples was presumably attributed to the death of some bacterial cells within their normal life cycle. Higher power intensity and longer duration of ultrasound treatment significantly (P < 0.05) enhanced the release of the nucleic acids, indicating the progressive deterioration of membrane integrity.
Table 3.
Determination of nucleic acids release by measuring the absorbance of the aqueous solutions surrounding the bacteria at 260 nm (OD260 nm) upon different ultrasound treatments.
| Time (min) | OD260 nm |
|||
|---|---|---|---|---|
| 64 W/cm2 | 191 W/cm2 | 382 W/cm2 | 573 W/cm2 | |
| 9 | 2.136 ± 0.027J | 2.554 ± 0.030G | 2.856 ± 0.014F | 3.226 ± 0.008D |
| 18 | 2.282 ± 0.023I | 2.827 ± 0.023F | 3.307 ± 0.007C | 3.391 ± 0.016B |
| 27 | 2.423 ± 0.010H | 2.966 ± 0.017E | 3.382 ± 0.010B | 3.637 ± 0.013A |
Note: The release of nucleic acids in control groups were 0.317 ± 0.013K.
Different letters indicate a significant difference of nucleic acids release from bacteria with different treatments (P < 0.05).
The presence of protein released to the extracellular space, as a primary signal of membrane damage and cell lysis, was also monitored based on the absorbance at 280 nm [42]. Significant increases in the extracellular concentration of protein were observed as the power intensity and duration time increased, whereas the untreated samples exhibited no outflow of cell constituents (Table 4). After 27 min of ultrasound treatment at 573 W/cm2, the amount of protein release reached 3.546 ± 0.021 mg/mL, which was around a 16-fold increase as compared to control. The trends in variation trends of protein release were in agreement with those of the nucleic acids, which further supported the alteration or loss of membrane integrity upon ultrasound treatment. These results correlated well with the altered cellular morphology and ultrastructure and decreased cell membrane permeability illustrated above, further confirming the rupture of bacterial cellular membranes triggered by the sonication treatment.
Table 4.
Measurements of protein release from bacteria after different ultrasound treatments.
| Time (min) | OD280 nm |
Protein concentration (mg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| 64 W/cm2 | 191 W/cm2 | 382 W/cm2 | 573 W/cm2 | 64 W/cm2 | 191 W/cm2 | 382 W/cm2 | 573 W/cm2 | |
| 9 min | 1.262 ± 0.016k | 1.657 ± 0.011 h | 1.947 ± 0.023f | 2.214 ± 0.008d | 1.750 ± 0.024K | 2.329 ± 0.016H | 2.753 ± 0.034F | 3.144 ± 0.012D |
| 18 min | 1.346 ± 0.014j | 1.902 ± 0.016g | 2.250 ± 0.004c | 2.317 ± 0.015b | 1.873 ± 0.020J | 2.687 ± 0.024G | 3.197 ± 0.006C | 3.295 ± 0.022B |
| 27 min | 1.433 ± 0.006i | 1.991 ± 0.013e | 2.272 ± 0.014c | 2.488 ± 0.014a | 2.000 ± 0.009I | 2.817 ± 0.019E | 3.229 ± 0.020B | 3.546 ± 0.021A |
Note: The release of protein determined by OD280 nm was 0.214 ± 0.010 in control groups; The concentration of released protein in control groups was 0.215 ± 0.015 mg/mL.
Different uppercase letters indicate a significant difference of protein concentrations from bacteria with different treatments (P < 0.05);
Different lowercase letters indicate a significant difference of protein release from bacteria with different treatments (P < 0.05).
3.8. Membrane fluidity
To illustrate how E. coli responded to ultrasound treatments, the fluorescence polarization was monitored since its values (P values) were inversely proportional to membrane fluidity. The results in Fig. 6a showed that the P values increased in a time- and intensity-dependent manner. The increase in fluorescence polarization of DPH indicated the corresponding decreased membrane fluidity of E. coli in response to ultrasound exposure. Similar to the polarization results, as depicted in Fig. 6b, ultrasound irradiation significantly contributed to an increase in fluorescence anisotropy (r) of DPH compared to the control. Specifically, among all the duration times studied, the r values increased in a time- and intensity-dependent manner. The micro-viscosity (η) of the cell membrane, whose values were positively associated with the membrane rigidity, was also monitored post ultrasound exposure (Fig. 6c). As the intensity of ultrasound treatment increased, the η values significantly increased, indicating higher rigidity of the membrane. Compared to untreated E. coli cells, an approximate 2-fold increase in η values was obtained in bacteria treated with ultrasound at 573 W/cm2. Additionally, the membrane rigidity increased gradually as treatment time prolonged, as reflected by the increase in the η values.
Fig. 6.
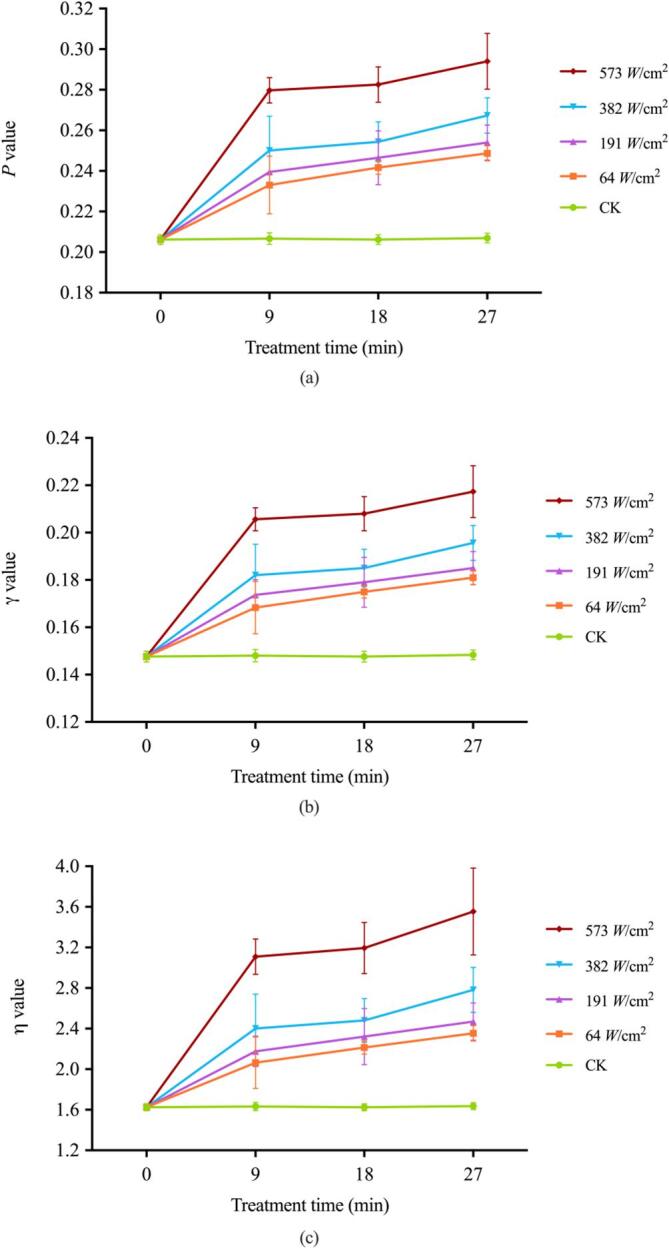
Fluorescence polarization (a), fluorescence anisotropy (b), and microviscosity (η) values (c) of E. coli O157:H7 membrane under different ultrasound treatment.
Taken together, these results indicated that E. coli responded effectively to ultrasound irradiation by modifying the fluidity and rigidity of cell membrane. The reduction in membrane fluidity denoted the solidification of the membrane surface layer, suggesting a more motion-restricted state of the bacterial cells upon treatment [43], [44]. It should be noted that maintenance of proper membrane fluidity is crucial for microorganisms due to its involvement in a variety of cellular functions including nutrient transport, cellular morphology and protection from external detrimental situations, which directly affects the biological and physiological status of the cells [45], [46]. Ewe et al., (2012) reported that lower membrane fluidity, which they attributed to the decreased extent of acyl chain motion within the phospholipid bilayer, eventually decreased cellular membrane permeability [47]. In our present study, higher power intensity and longer duration time apparently diminished the fluid state of E. coli cell membranes, corresponding to the increased membrane permeability and cell lethality demonstrated above. Jia et al (2015) exposed breast cancer cells to ultrasound at various intensities and also found that treatment at higher intensities decreased the membrane fluidity of these cells [48]. There are no published studies in the literature which focus on the alterations in membrane fluidity in response to ultrasonic irradiation treatment. Hence, we hypothesized that the remarkable alterations in membrane fluidity and rigidity induced by ultrasound may disrupt the membrane functions, consequently contributing to the cell malfunctioning and even to death. Thus, the results of fluorescence polarization analysis were in agreement with the above-mentioned severe loss of cell viability. In addition, the membrane fluidity to be closely related to alterations in the membrane fatty acid profile. The decrease in membrane fluidity could be deemed as the detecting ‘input signal’ that initiated the increased biosynthesis of unsaturated fatty acids accompanied by the decreased production of saturated fatty acids in E. coli [44]. These results were in accordance with the results of proteomic analysis, where the ‘biosynthesis of unsaturated fatty acids’ and ‘fatty acids metabolism’ were found as main enriched pathways of the up-regulated proteins (data not shown). Based on this, we inferred that the disruption of membrane lipid metabolism caused by ultrasonic field might alter the fatty acids composition, which led to the decrease of membrane fluidity.
3.9. Modification in membrane potential
Fig. 7 illustrated the alterations in E. coli membrane potential in response to ultrasound treatment by spectrofluorometry analysis. The fluorescence intensity of DiOC2(3) was altered accordingly along with the variations in power intensity and processing time of ultrasound treatment (Fig. 7a). In general, all treated samples showed higher green fluorescence intensity at 550 nm in comparison with control. This phenomenon might be partly attributed to the increase in membrane permeability that facilitated the uptake of the lipophilic dye DiOC2(3) [49]. The color shifts from green to red at around 600 nm, which corresponded to the polarized cells, were observed in the normal cells and cells treated with low-intensity ultrasound (191 W/cm2). For this study, the results obtained with ratiometric calculation of red/green fluorescence was directly correlated with the magnitude of membrane potential; the higher fluorescence ratio reflected the hyperpolarized membrane; whereas the decrease of those values characterized the depolarization of the cell membrane. Upon sonication at 191 W/cm2, there was a slight increase in membrane potential and the maximum value was reached at 18 min. There was a slight decrease in this value after treatment for 27 min. Li et al. (2018) reported that a similar decrease in cell membrane potential appeared after increasing to a maximum value with the elongation of ultrasound treatment (20 kHz, 252 W/cm2, 25 min) [50]. This phenomenon might be related to the activation of transmembrane ion channels, which resulted in an excessive influx of Ca2+ into cells [50], [51]. Further, the fluorescence ratio decreased significantly (P < 0.05) at a higher intensity of ultrasound (573 W/cm2) from 9 min to 27 min, indicating the decrease in membrane potential in a time-dependent manner. Thereby, the depolarized membrane potential gradually occurred as a response to high-intensity sonication treatment for long periods of time.
Fig. 7.
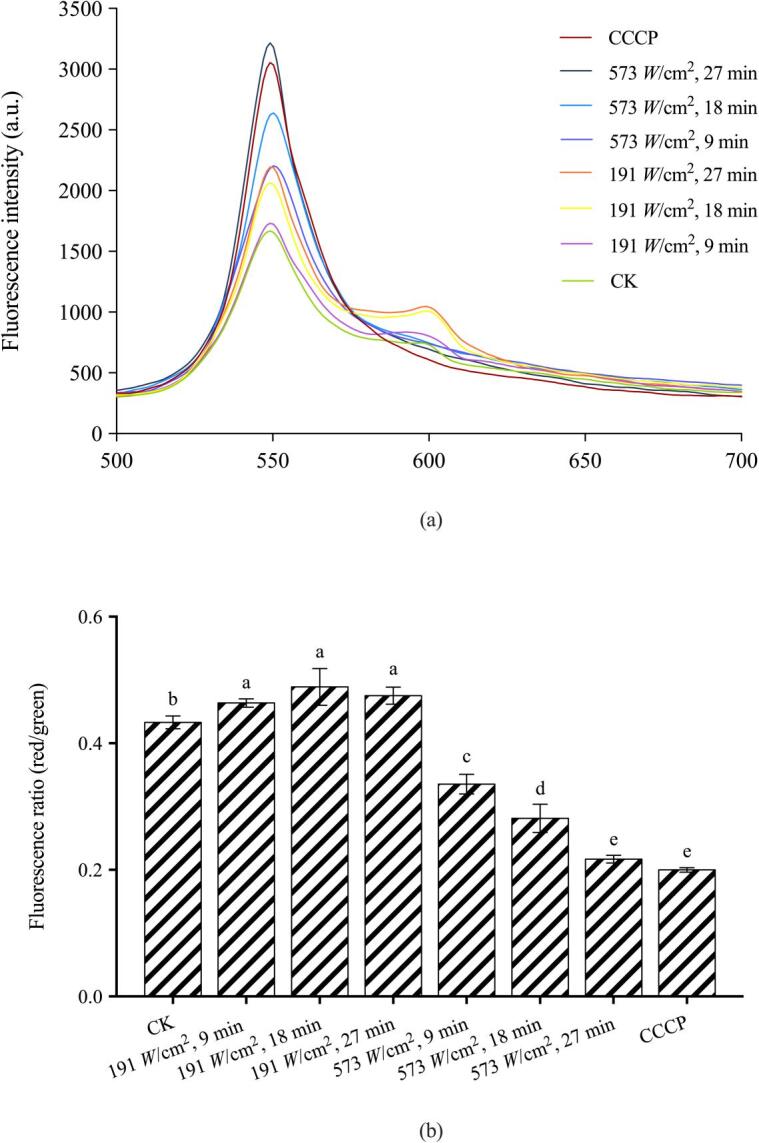
Membrane potential changes of E. coli O157:H7 upon different ultrasound treatments expressed as the fluorescence spectra of DiOC2(3) in E. coli O157:H7 membrane (a) and ratio of red and green fluorescence intensity (b). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Membrane potential analysis was employed to illustrate the mechanism of antimicrobial action from another perspective since it is well recognized as a critical factor involved in bacterial physiological processes such as membrane transport, energy production, and metabolic-related activity [52], [53]. Cheach et al. (2018) and Ma et al. (2017) associated the potential loss of membrane potential with membrane permeabilization and a loss in cell viability and stated the biological alterations invoked by the disturbance of membrane potential were partly responsible for the antibacterial effect [15], [54]. Another perspective was put forward by Qin et al. (2014), who conducted membrane potential evaluations after sonication treatment on HeLa cells and ascribed the depolarization of membrane to the onset of ultrasound-induced sonoporation [55]. According to the literature, the greatly dissipated membrane potential was found to be synchronized with the anomalous ion fluxes, which were elicited in a sonoporation episode. In our investigation, levels of perturbation on the membrane potential were in correlation with the disruption of membrane permeability, loss of cell viability and onset of sonoporation, therefore, providing more information for microbial behaviors upon exposure to different intensities of ultrasound.
3.10. FTIR analysis
The ultrasound-induced changes in membrane composition of E. coli cells were investigated by the FTIR spectra (Fig. 8). The spectral features of ultrasound-treated cells were different at 191 W/cm2 and 573 W/cm2 and corresponded to the processing duration. In general, increases in the spectral intensity of the dominant peaks were observed at 2919, 2851, 1650, 1550, 1463, 1390, 1220, and 1085 cm−1 as the ultrasonic power intensity increased and treatment time prolonged. Especially, the noticeable increases occurred in a region of fatty acids in the cell membrane, where the spectrum was dominated by two absorbance bands at about 2919 cm−1 and 2851 cm−1 (attributed to the CH2 antisymmetric stretching and CH2 symmetric stretching of lipid, respectively). These increases suggested a rise in the amounts of saturated lipid concentration of membranes, which were significant for the determination of membrane fluidity levels [25], [56]. The results were also supported by the enhancement of peak intensity of another fatty acid-associated band (CH2 bending) at around 1463 cm−1. The increment in saturated lipid concentration were often interpreted as the increase of rigidity and permeability of the bacterial cell membranes, which further confirmed the results illustrated above. Another substantial change was recorded in the protein region (1700–1500 cm−1) dominated by the amide I (1650 cm−1) and amide II (1550 cm−1) bands [19], [57]. Peak intensities increased in gradually with the ultrasound process prolonged, reflecting an increase in the protein concentration upon the treatment. A probable cause of this increase could be the induction of the synthesis of several membrane-related proteins. The presented results were in consistent with our data obtained from proteomics analysis, where the overexpression of membrane biosynthesis-related proteins or enzymes were detected, such as outer membrane protein C (OmpC), cardiolipin synthase A (ClsA), glycerol-3-phosphate acyltransferase (PlsB), phosphatidylserine decarboxylase proenzyme (Psd), 3-ketoacyl-CoA thiolase FadA, etc. (data not shown). Furthermore, it was also observed that ultrasound treatment caused changes in bands at 1390, 1220, and 1085 cm−1 corresponding to chemical bonds forming the phospholipids and fatty acids, which are mainly present in the bacterial cell membranes [25]. There changes were generally reflected the ultrasound-induced cellular membrane damage or chemical bonds changes of cell membrane components.
Fig. 8.
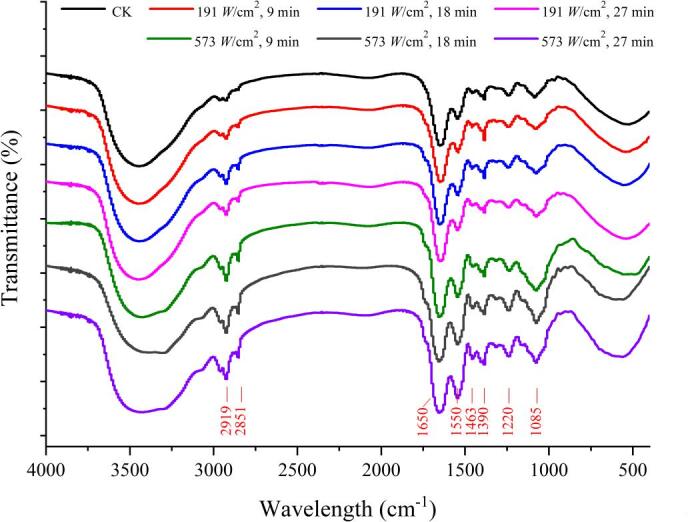
FTIR spectra of E. coli O157:H7 upon different ultrasound treatments.
4. Conclusion
In the present work, the antimicrobial behavior under ultrasound treatment showed its high correlation with ultrasonic intensity and duration. The remarkable synergistic inactivation effects of ultrasound treatment and TEON implied that ultrasound increased sensitivity of cells to antibacterial compounds. Related changes in the cell membrane induced by ultrasound treatment were addressed, with a special emphasis on the alterations in the membrane structure and characteristics. First, SEM and TEM images showed the detrimental effects of ultrasound on cell morphology and ultrastructure with regards to pore formation, leakage of the internal cellular contents, and cell fragmentation. In line with our expectation, ultrasonic field led to outer and inner membrane permeabilization, membrane fluidity reduction, and membrane potential depolarization. Furthermore, the conformational and compositional changes of some cell membrane components were also determined, showing significant changes in lipids, proteins and phospholipids. These results could be considered as a complementary set of antimicrobial behaviors associated with the comprehensively studied inactivation mechanism of ultrasound. To our best knowledge, for the first time, the antimicrobial mechanism of ultrasound against foodborne bacteria was investigated targeting bacterial cell membranes. Hence, the current work provided a more in-depth theoretical basis for the antibacterial mechanism of ultrasonic physical field and its synergistic effects with antibacterial compounds. Moreover, we inferred that not only the physical damage but also the disruption of membrane lipid homeostasis accounted for membrane disintegration during ultrasound treatment. Further studies are required to uncover the role of membrane lipid homeostasis-related genes and proteins upon ultrasound treatment and focus specifically on the regulations and functions of the lipid metabolism pathways involved.
CRediT authorship contribution statement
Qiao He: Methodology, Software, Writing - original draft. Donghong Liu: Conceptualization, Supervision. Muthupandian Ashokkumar: Writing - review & editing. Xingqian Ye: Supervision. Tony Z. Jin: Visualization, Writing - review & editing. Mingming Guo: Conceptualization, Funding acquisition, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study received the support of the National Natural Science Foundation of China (32001799) and the Natural Science Foundation of Zhejiang Province, China (LQ20C200014). The authors wish to thank Anita Parameswaran (USDA-ARS-ERRC) for her thoughtful review of this manuscript.
References
- 1.Barba F.J., Koubaa M., do Prado-Silva L., Orlien V., Sant’Ana A.d.S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Tech. 2017;66:20–35. doi: 10.1016/j.tifs.2017.05.011. [DOI] [Google Scholar]
- 2.Bevilacqua A., Petruzzi L., Perricone M., Speranza B., Campaniello D., Sinigaglia M., Corbo M.R. Nonthermal technologies for fruit and vegetable juices and beverages: Overview and advances. Compr. Rev. Food Sci. Food Saf. 2018;17(1):2–62. doi: 10.1111/crf3.2018.17.issue-110.1111/1541-4337.12299. [DOI] [PubMed] [Google Scholar]
- 3.Huang G., Chen S., Dai C., Sun L., Sun W., Yingxiu T., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Andrés J.M., Charoux C.M.G., Cullen P.J., Tiwari B.K. Chemical modifications of lipids and proteins by nonthermal food processing technologies. J. Agric. Food Chem. 2018;66(20):5041–5054. doi: 10.1021/acs.jafc.7b06055. [DOI] [PubMed] [Google Scholar]
- 5.Wu X., Liu J., Zhu J.J. Sono-Fenton hybrid process on the inactivation of Microcystis aeruginosa: Extracellular and intracellular oxidation. Ultrason. Sonochem. 2019;53:68–76. doi: 10.1016/j.ultsonch.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Deng L.-Z., Mujumdar A.S., Pan Z., Vidyarthi S.K., Xu J., Zielinska M., Xiao H.-W. Emerging chemical and physical disinfection technologies of fruits and vegetables: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2020;60(15):2481–2508. doi: 10.1080/10408398.2019.1649633. [DOI] [PubMed] [Google Scholar]
- 7.Phull S.S., Newman A.P., Lorimer J.P., Pollet B., Mason T.J. The development and evaluation of ultrasound in the biocidal treatment of water. Ultrason. Sonochem. 1997;4(2):157–164. doi: 10.1016/S1350-4177(97)00029-1. [DOI] [PubMed] [Google Scholar]
- 8.Lattwein K.R., Shekhar H., Kouijzer J.J.P., van Wamel W.J.B., Holland C.K., Kooiman K. Sonobactericide: An emerging treatment strategy for bacterial infections. Ultrasound Med. Biol. 2020;46(2):193–215. doi: 10.1016/j.ultrasmedbio.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolau-Lapeña I., Lafarga T., Viñas I., Abadias M., Bobo G., Aguiló-Aguayo I. Ultrasound processing alone or in combination with other chemical or physical treatments as a safety and quality preservation strategy of fresh and processed fruits and vegetables: a review. Food Bioprocess Technol. 2019;12(9):1452–1471. doi: 10.1007/s11947-019-02313-y. [DOI] [Google Scholar]
- 10.Guo M., Zhang L., He Q., Arabi S.A., Zhao H., Chen W., Ye X., Liu D. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrason. Sonochem. 2020;66:104988. doi: 10.1016/j.ultsonch.2020.104988. [DOI] [PubMed] [Google Scholar]
- 11.He Q., Guo M., Jin T.Z., Arabi S.A., Liu D. Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 2021;337:108936. doi: 10.1016/j.ijfoodmicro.2020.108936. [DOI] [PubMed] [Google Scholar]
- 12.Rojas E.R., Billings G., Odermatt P.D., Auer G.K., Zhu L., Miguel A., Chang F., Weibel D.B., Theriot J.A., Huang K.C. The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature. 2018;559(7715):617–621. doi: 10.1038/s41586-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67(4):593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Ahn J., Liu D., Chen S., Ye X., Ding T., Elkins C.A. Evaluation of ultrasound-induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Appl. Environ. Microbiol. 2016;82(6):1828–1837. doi: 10.1128/AEM.03080-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleach J., Watier D., Fur B.L., Brauge T., Duflos G., Grard T., Lencel P. Use of ratiometric probes with a spectrofluorometer for bacterial viability measurement. J. Microbiol. Biotechnol. 2018;28(11):1782–1790. doi: 10.4014/jmb.1804.04048. [DOI] [PubMed] [Google Scholar]
- 16.Yun O., Liu Z.W., Zeng X.A., Han Z. Salmonella typhimurium resistance on pulsed electric fields associated with membrane fluidity and gene regulation. Innovative Food Sci. Emerging Technol. 2016;36:252–259. doi: 10.1016/j.ifset.2016.06.013. [DOI] [Google Scholar]
- 17.Modugno C., Peltier C., Simonin H., Dujourdy L., Capitani F., Sandt C., Perrier-Cornet J.-M. Understanding the effects of high pressure on bacterial spores using synchrotron infrared spectroscopy. Front. Microbiol. 2020;10 doi: 10.3389/fmicb.2019.03122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.N. Jin, K.T. Semple, L. Jiang, C. Luo, D. Zhang, F.L. Martin, Spectrochemical analyses of growth phase-related bacterial responses to low (environmentally-relevant) concentrations of tetracycline and nanoparticulate silver, Analyst 143 (2018) 768-776. http://doi.org/10.1039.C7AN01800B. [DOI] [PubMed]
- 19.Tantala J., Thumanu K., Rachtanapun C. An assessment of antibacterial mode of action of chitosan on Listeria innocua cells using real-time HATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2019;135:386–393. doi: 10.1016/j.ijbiomac.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Qin X., Dong R., He S., Zhou X., Zhang Z., Cui Y., Shi C., Liu Y., Shi X. Characterization of the role of ybgC in lysozyme resistance of Salmonella Enteritidis. Food Control. 2020;109:106732. doi: 10.1016/j.foodcont.2019.106732. [DOI] [Google Scholar]
- 21.Gravel J., Paradis-Bleau C., Schmitzer A.R. Adaptation of a bacterial membrane permeabilization assay for quantitative evaluation of benzalkonium chloride as a membrane-disrupting agent. MedChemComm. 2017;8(7):1408–1413. doi: 10.1039/C7MD00113D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y.Q., Sun X.X., Feng J.L., Mo H.Z. Antibacterial activities and membrane permeability actions of glycinin basic peptide against Escherichia coli. Innovative Food Sci. Emerging Technol. 2015;31:170–176. doi: 10.1016/j.ifset.2015.07.009. [DOI] [Google Scholar]
- 23.Kirchhoff C., Cypionka H. Boosted membrane potential as bioenergetic response to anoxia in Dinoroseobacter shibae. Front. Microbiol. 2017;8:695. doi: 10.3389/fmicb.2017.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas D., Tiwari M., Tiwari V., Tripathi T. Molecular mechanism of antimicrobial activity of chlorhexidine against carbapenem-resistant Acinetobacter baumannii. PLoS ONE. 2019;14(10):e0224107. doi: 10.1371/journal.pone.0224107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji S.H., Ki S.H., Ahn J.H., Shin J.H., Hong E.J., Kim Y.J., Choi E.H. Inactivation of Escherichia coli and Staphylococcus aureus on contaminated perilla leaves by Dielectric Barrier Discharge (DBD) plasma treatment. Arch. Biochem. Biophys. 2018;643:32–41. doi: 10.1016/j.abb.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Mason T.J., Chemat F., Ashokkumar M. Power ultrasonics for food processing. In: Gallego-Juarez J.A., Graff K.F., editors. Power Ultrasonics. Woodhead Publishing; Cambridge: 2015. pp. 816–843. [Google Scholar]
- 27.Joo H.-J., Mizan M.F.R., Hossain M.I., Lee D.-U., Ha S.-D. Enhanced elimination of Salmonella Typhimurium and Campylobacter jejuni on chicken skin by sequential exposure to ultrasound and peroxyacetic acid. J. Food Saf. 2020;40(4) doi: 10.1111/jfs.v40.410.1111/jfs.12803. [DOI] [Google Scholar]
- 28.Park J.-S., Ha J.-W. Ultrasound treatment combined with fumaric acid for inactivating food-borne pathogens in apple juice and its mechanisms. Food Microbiol. 2019;84:103277. doi: 10.1016/j.fm.2019.103277. [DOI] [PubMed] [Google Scholar]
- 29.Guo L., Sun Y., Zhu Y., Wang B., Xu L., Huang M., Li Y., Sun J. The antibacterial mechanism of ultrasound in combination with sodium hypochlorite in the control of Escherichia coli. Food Res. Int. 2020;129:108887. doi: 10.1016/j.foodres.2019.108887. [DOI] [PubMed] [Google Scholar]
- 30.Bi X., Wang X., Chen Y., Chen L., Xing Y., Che Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020;60:104763. doi: 10.1016/j.ultsonch.2019.104763. [DOI] [PubMed] [Google Scholar]
- 31.Millan-Sango D., McElhatton A., Valdramidis V.P. Determination of the efficacy of ultrasound in combination with essential oil of oregano for the decontamination of Escherichia coli on inoculated lettuce leaves. Food Res. Int. 2015;67:145–154. doi: 10.1016/j.foodres.2014.11.001. [DOI] [Google Scholar]
- 32.Wu X., Narsimhan G. Synergistic effect of low power ultrasonication on antimicrobial activity of melittin against Listeria monocytogenes. LWT - Food Sci. Technol. 2017;75:578–581. doi: 10.1016/j.lwt.2016.10.008. [DOI] [Google Scholar]
- 33.Bastarrachea L.J., Walsh M., Wrenn S.P., Tikekar R.V. Enhanced antimicrobial effect of ultrasound by the food colorant Erythrosin B. Food Res. Int. 2017;100:344–351. doi: 10.1016/j.foodres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Matafonova G., Batoev V. Review on low- and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 2019;166:115085. doi: 10.1016/j.watres.2019.115085. [DOI] [PubMed] [Google Scholar]
- 35.Lentacker I., De Cock I., Deckers R., De Smedt S.C., Moonen C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Qi H., Yan Z., Gu Y., Sun W., Zewde A.A. Sonophotocatalytic inactivation of E. coli using ZnO nanofluids and its mechanism. Ultrason. Sonochem. 2017;34:232–238. doi: 10.1016/j.ultsonch.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 37.Guo X., Cai C., Xu G., Yang Y., Tu J., Huang P., Zhang D. Interaction between cavitation microbubble and cell: A simulation of sonoporation using boundary element method (BEM) Ultrason. Sonochem. 2017;39:863–871. doi: 10.1016/j.ultsonch.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014;21(1):446–453. doi: 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Mellegård H., Strand S.P., Christensen B.E., Granum P.E., Hardy S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011;148(1):48–54. doi: 10.1016/j.ijfoodmicro.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Dong S., Yang X., Zhao L., Zhang F., Hou Z., Xue P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020;149:112350. doi: 10.1016/j.indcrop.2020.112350. [DOI] [Google Scholar]
- 41.Khan I., Bahuguna A., Shukla S., Aziz F., Chauhan A.K., Ansari M.B., Bajpai V.K., Huh Y.S., Kang S.C. Antimicrobial potential of the food-grade additive carvacrol against uropathogenic E. coli based on membrane depolarization, reactive oxygen species generation, and molecular docking analysis. Microb. Pathog. 2020;142:104046. doi: 10.1016/j.micpath.2020.104046. [DOI] [PubMed] [Google Scholar]
- 42.Moghimi R., Aliahmadi A., McClements D.J., Rafati H. Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT - Food Sci. Technol. 2016;71:69–76. doi: 10.1016/j.lwt.2016.03.018. [DOI] [Google Scholar]
- 43.Pan Y., Zhang Y., Cheng J.-H., Sun D.-W. Inactivation of Listeria Monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT - Food Sci. Technol. 2020;118:108635. doi: 10.1016/j.lwt.2019.108635. [DOI] [Google Scholar]
- 44.Wang L.-H., Zeng X.-A., Wang M.-S., Brennan C.S., Gong D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. Biochim. Biophys. Acta, Biomembr. 2018;1860(2):481–490. doi: 10.1016/j.bbamem.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Yoon Y., Lee H., Lee S., Kim S., Choi K.H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015;72:25–36. doi: 10.1016/j.foodres.2015.03.016. [DOI] [Google Scholar]
- 46.F. Zhao, Y. Wang, H. An, Y. Hao, X. Hu, X. Liao, New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2, mBio 7 (2016) e00961-00916. Doi: 10.1128/mBio.00961-16. [DOI] [PMC free article] [PubMed]
- 47.Ewe J.-A., Wan Abdullah W.-N., Bhat R., Karim A.A., Liong M.-T. Enhanced growth of lactobacilli and bioconversion of isoflavones in biotin-supplemented soymilk upon ultrasound-treatment. Ultrason. Sonochem. 2012;19(1):160–173. doi: 10.1016/j.ultsonch.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Jia Y., Yuan W., Zhang K., Wang J., Wang P., Liu Q., Wang X. Comparison of cell membrane damage induced by the therapeutic ultrasound on human breast cancer MCF-7 and MCF-7/ADR cells. Ultrason. Sonochem. 2015;26:128–135. doi: 10.1016/j.ultsonch.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Khater M., Khater S.S., Gholap H., Patil R., Kulkarni G. Comparative studies on measurement of membrane potential of bacterial cells treated with ZnO nanoparticles by spectrofluorometry, fluorescence microscopy and flowcytometry. J. Microbiol. Methods. 2020;173:105920. doi: 10.1016/j.mimet.2020.105920. [DOI] [PubMed] [Google Scholar]
- 50.Li J., Ma L., Liao X., Liu D., Lu X., Chen S., Ye X., Ding T. Ultrasound-induced Escherichia coli O157:H7 cell death exhibits physical disruption and biochemical apoptosis. Front. Microbiol. 2018;9:2486. doi: 10.3389/fmicb.2018.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao X., Li J., Suo Y., Chen S., Ye X., Liu D., Ding T. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Hum. Well. 2018;7(1):102–109. doi: 10.1016/j.fshw.2018.01.002. [DOI] [Google Scholar]
- 52.Benarroch J.M., Asally M. The microbiologist's guide to membrane potential dynamics. Trends Microbiol. 2020;28(4):304–314. doi: 10.1016/j.tim.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y., Liu X., Wang Y., Jiang P., Quek S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 54.Ma W., Zhang D., Li G., Liu J., He G., Zhang P., Yang L., Zhu H., Xu N., Liang S. Antibacterial mechanism of daptomycin antibiotic against Staphylococcus aureus based on a quantitative bacterial proteome analysis. J. Proteomics. 2017;150:242–251. doi: 10.1016/j.jprot.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Qin P., Han T., Yu A.C.H., Xu L. Mechanistic understanding the bioeffects of ultrasound-driven microbubbles to enhance macromolecule delivery. J. Controlled Release. 2018;272:169–181. doi: 10.1016/j.jconrel.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Kepenek E.S., Gozen A.G., Severcan F. Molecular characterization of acutely and gradually heavy metal acclimated aquatic bacteria by FTIR spectraoscopy. J. Biophotonics. 2019;12 doi: 10.1002/jbio.201800301. [DOI] [PubMed] [Google Scholar]
- 57.Moghimi R., Aliahmadi A., Rafati H. Ultrasonic nanoemulsification of food grade trans-cinnamaldehyde: 1,8-Cineol and investigation of the mechanism of antibacterial activity. Ultrason. Sonochem. 2017;35:415–421. doi: 10.1016/j.ultsonch.2016.10.020. [DOI] [PubMed] [Google Scholar]