Abstract
β-Glucosidases are enzymes present in all living organisms, playing a pivotal role in diverse biological processes. These enzymes cleave β-glycosidic bonds between carbohydrates, or between a carbohydrate and a non-carbohydrate moiety, which may result in the liberation of volatile aglycones. Released compounds execute diverse physiological roles, while the industry takes advantage of exogenously added β-glucosidases for aroma enrichment during food and beverage production. β-Glucosidase enzymatic activity has been reported in human saliva and given the fact that these enzymes are involved in aroma release, we investigated here the correlation between β-glucosidase activity in human saliva and the occurrence of halitosis. Measurement of salivary enzyme activity of 48 volunteers was performed using p-nitrophenyl-β-d-glucopyranoside as substrate. Each volunteer was clinically evaluated by a dental surgeon and clinical and laboratorial data were statistically analyzed. Gas-chromatography of saliva headspace allowed the analysis of the direct role of exogenous β-glucosidase on aromatic /volatile profile of saliva samples. The data demonstrated a positive correlation between halitosis and enzymatic activity, suggesting that the enzyme exerts a direct role in the occurrence of bad breath. Gas-chromatography analysis demonstrated that exogenously added enzyme led to the alteration of volatile organic content, confirming a direct contribution of β-glucosidase activity on saliva volatile compounds release. Although halitosis is a multifactorial condition, the complete understanding of all governing factors may allow the development of more effective treatment strategies. Such studies may pave the way to the use of β-glucosidase inhibitors for halitosis clinical management.
Keywords: Biofilm, Halitosis, Oral health, Saliva, β-Glucosidase
Highlights
-
•
β-Glucosidases are capable of altering the aromatic profile of saliva.
-
•
Increased salivary β-glucosidase is associated with halitosis.
-
•
Increased salivary β-glucosidase is associated with dental biofilm.
-
•
Salivary β-glucosidases are produced by oral microrganisms.
1. Introduction
β-Glucosidases are enzymes present in all living organisms being involved in several essential biological processes. These enzymes cleave β-glycosidic bonds between two carbohydrates, or between a carbohydrate and a non-carbohydrate moiety via different catalytic mechanisms [1]. In bacteria and fungi, they mainly act on polysaccharide degradation for energy harvesting, and such enzymes are part of the cellulolytic enzymatic complex, where hydrolysis of cellulose, cello-oligosaccharides and cellobiose ends with the production of fermentable sugars [[2], [3], [4]]. In plants, β-glucosidase activity is also involved in key developmental processes, such as growth, pathogen defense, and hormone hydrolysis [[1], [2], [3],5].
The hydrolysis of beta-D-glycosidic bonds is often required for the release of physiologically important compounds. For instance, the cleavage of defense glycosides, which are stored in their non-active form, is catalyzed by the enzymatic action of β-glucosidases [1,2,5,6]. Aromatic precursors in plants frequently exist as glycosides, which are composed of volatile compounds (terpenes, phenolic compounds, or cyclic alcohols) fused to a sugar moiety, where enzymatic action releases the volatile aglycone when necessary [1,[7], [8], [9]]. In fact, the food industry takes advantage of these facts, adding exogenous β-glucosidases during wine, juice or tea production for aroma enrichment [8,10,11].
Recent studies demonstrated that β-glucosidases from oral bacteria hydrolyze aroma precursors from grapes; releasing different types of wine odorant molecules, suggesting that oral microbiota is an important factor influencing aroma perception [12]. Recent studies demonstrated that β-galactosidase activity from oral bacteria is associated with an increased presence of volatile compounds in breath [13,14]. The study suggested that β-galactosidase-dependent deglycosylation followed by proteolysis of salivary glycoproteins leads to saliva putrefaction and the consequent production of bad oral odor [[14], [15], [16]]. Indeed, studies demonstrated that deglycosylation of salivary glycoproteins facilitates proteolysis accelerating volatile compounds production from amino acid metabolism [17]. Salivary glycoconjugates are the most significant carbon source for oral microorganisms in carbon-limited conditions. Therefore, carbohydrate-active enzymes such as glycoside hydrolase and glycosyltransferases play an important role in the adaptation of such microorganisms [18].
The dental biofilm colonize oral surfaces and embed a self-produced extracellular matrix of polysaccharides and DNA [19]. Streptococci are frequently the first colonizers, which initially adhere to the pellicle through binding to glycoproteins present in the oral surfaces. Subsequent colonization or co-colonization occurs by the aggregation of other microorganisms, gram + and gram -, metabolically active, which use substrates present in the saliva for structuring, maturing and protecting the dental biofilm [19].
The dental plaque development increases over time forming a multispecies community that synthesizes polymers (often polysaccharides and proteins) for biofilm matrix formation and stratification [20]. It is widely recognized that glycosyltransferases are essential for the extracellular polysaccharide synthesis [21]. Interestingly, many hydrolytic glycosidases can also catalyze transglycosylation reactions, through the transference of the glycosyl moiety to different acceptors depending on reaction conditions [22]. Therefore, a biofilm-rich oral cavity is also rich in glycosidases and glycosyltransferases that act not only on exopolysaccharides synthesis but in glycoside hydrolysis, perhaps directly contributing to volatile organic compounds (VOC) alteration and halitosis.
Taken together all these facts along with the recent reported detection of β-glucosidase activity in human saliva [23,24] we investigated here the correlation between β-glucosidase activity and the occurrence of halitosis. In our hypothesis, increased salivary β-glucosidase activity is directly involved in the hydrolysis of glycosidic substrates from human saliva, which can alter the volatile profile leading to the occurrence of halitosis. We selected 48 volunteers, examined their dental clinical conditions such as halitosis, tongue coating, caries, calculus, gingivitis, and periodontitis and correlated these clinical signs with salivary β-glucosidase activity measurements. To confirm our hypothesis, we examined, by gas chromatography, whether a direct action of exogenous β-glucosidase on human saliva causes the enrichment of volatile compounds, influencing the occurrence of halitosis. These studies demonstrate a new and unexplored role of these enzymes and may pave the way for a better understanding of halitosis, as well as the development of disease control mechanisms.
2. Materials and methods
2.1. Material
The chromogenic substrate P-nitrophenyl-β-d-glucopyranoside (pNPG), cellobiose and all reagents for enzymatic assays were purchased from Sigma Aldrich (St. Louis, Missouri, USA). The portable device Breath Checker Tanita HC - 212SF was purchased from Tanita Corporation of America (Arlington Heights, Illinois, USA).
2.2. Volunteer selection and saliva collection
This work has been reviewed and approved by the Human Research Ethics Committee of the State University of Santa Catarina (UDESC), Brazil. Forty-eight patients, older than 18 years of age, non-smokers and non-pregnant were randomly recruited as volunteers for this study. The volunteers were asked about the use of antibiotics in the last 30 days prior to the saliva collection and oral evaluation; volunteers not taking any antibiotics were selected for this study. All volunteers were interviewed about daily habits and were asked to follow specific instructions, which comprised of not to eat or do oral hygiene for 2h before saliva collection. The experiments were undertaken with the understanding and written consent of each participant. Mouthwashes and the consumption of alcoholic beverages were also interrupted for 48 h prior to collection.
Saliva was collected by mechanical stimulation, where the volunteer chewed a piece of sterilized rubber for 6 min, the first min for salivary trial collection (these samples were discarded) and another 5 min for saliva collection. The collected saliva was deposited in a 15 mL conical tube, kept on ice and immediately analyzed for enzymatic activity. Part of the samples was stored at -80 °C for further gas chromatography analysis.
2.3. Dental clinical examination
A dental surgeon performed an oral examination in each volunteer to determine the presence of halitosis and additional clinical conditions like visible dental biofilm (materia alba), caries, tongue coating, gingivitis and periodontitis. All clinical analyzes, including breath assessment, were performed by the same dental surgeon. Patients with gingivitis were those who had gingival bleeding on probing. The presence of periodontitis was determined in patients with probing depth greater than 4 mm and gingival bleeding at least one site of one or more teeth. There are several methods to evaluate the presence of tongue coating. In this study, this clinical condition was determined by visual examination in heavy, medium, light, or no tongue coating [25]. Volunteers who presented some degree of tongue coating were classified as positive for this clinical condition. The degree of halitosis was determined by the Organoleptic Test as well as using a portable monitor of sulfur compound detection through a semi - conductor gas sensor (Breath Checker Tanita HC - 212SF). The Organoleptic Test is based on the olfactory analysis of breath exhaled through the mouth, after breath holding for 30s, at approximately 20 cm of the examiner. The presence and severity of oral malodor are classified on a ‘0–5’ scale, also referred as the “Rosenberg scale” [26], where 0 means no odor; 1, barely noticeable odor; 2, slight odor; 3, moderate odor; 4, strong odor; and 5, extremely strong odor. The volunteers were also instructed to breathe through the nose with their mouth closed for 30s and then exhale towards the portable monitor of sulfur detection that indicated the presence of sulfur compounds. The Breathe Checker Tanita HC - 212SF uses a Semi-Conductor gas sensor that measures the amount of volatile sulfur compounds (VSCs) in breath and correlates to halitosis in a 0–5 scale, where: 0: no odor; 1: slight odor, 2: moderate odor , 3: heavy odor, 4: strong odor and 5:intense odor.
The volunteers who presented zero in both tests were classified without halitosis. The volunteers who presented scores ≥1 at least in one of the tests were classified with halitosis.
2.4. Enzyme activity measurements
Measurement of enzyme activity was performed using pNPG as substrate and fresh saliva samples. To measure the initial rates, enzyme assays are typically carried out while the reaction has progressed only a few percent towards total completion [27]. To assure the measurement of enzymatic initial rates, reactions in this study were performed under 10% completion. Briefly, reactions were performed in 20 mM sodium phosphate buffer, pH 7.2, in the presence of 0.075 mL of saliva (or bacterial isolates) and 1 mM pNPG in a 0.25 mL final reaction volume at 37 °C, during 60 min. After the incubation period, the reaction was quenched with 1 M Na2CO3 (0.25 mL) and the absorbance was measured in a Genesys 150 UV–visible (Thermo Fisher Scientific, Inc.) at 405 nm. Product concentration was calculated using p-nitrophenol extinction coefficient (18,000 M-1 cm-1). Heat-inactivated samples were used as control, where each saliva sample was incubated at 100 °C during 3 min for enzyme denaturation. Enzymatic rates were calculated as the amount of the product p-nitrophenol released per minute per g of saliva.
2.5. Identification of microorganisms
Fresh saliva samples (10 μL) from 22 volunteers were seeded on M9 minimal media agar-plates containing cellobiose (5 mg/mL) as the sole carbon source in order to select β-glucosidase producing organisms. Plates were incubated at 37 °C for 48h. Isolated microorganisms were then plated in Blood-Agar plates and incubated at 37 °C for 24 h under aerobic and anaerobic conditions. Colonies were submitted to Gram staining and subjected to biochemical tests to determine genus and species, according to the protocol of Oliveira & Vaz [28]. The microbiology tests were performed at the Laboratory of Bacteriology - Center of Diagnostic Animal Microbiology – Center of Agroveterinary Sciences of State University of Santa Catarina- UDESC. Isolated bacterial colonies were added to Luria Bertani (LB) liquid media and cultivated in an orbital shaker at 37 °C until reached an optical density of 0.500 at 600 nm. 1 mL of liquid LB media containing bacteria was centrifuged and bacterial pellet was suspended in 1 mL of ultrapure sterile water where 0.075 mL was used to test enzymatic activity using pNPG as substrate as described in section “Enzyme activity measurements”.
2.6. Sample analysis by gas chromatography
Saliva samples from only twelve patients contained enough volume to allow further gas chromatography (GC) analyses; therefore, such samples were stored at -80 °C for GC investigation. Samples were centrifuged 10.000×g, stored in glass vials with perforating caps and incubated overnight at 15 °C with 1.4U of Bacillus polymyxa β-glucosidase. One unit of enzyme is defined as the amount of the enzyme that catalyzes the conversion of 1 μmol of pNPG to pNP and glucose per minute (μmol/min) under standard reaction conditions. Control samples contained heat-inactivated enzyme, in which the enzyme was incubated at 100 °C during 3 min for enzyme denaturation. The headspace of each reaction was collected with a glass syringe after heating the samples at 60 °C for 20 min, followed by 0.02 mL gas injection into the Gas Chromatography - Mass Spectrometer Clarus 680 (Capillary GC-MS). The reading lasted 47 min using temperature range from 30 °C to 350 °C, Helium as drag gas and Elite 5MS-30 mm column as stationary phase. Identification of the sample VOC was performed by spectral matching with entries in the National Institute of Standards and Technology (NIST 14) reference library using the NIST Mass Spectral Search Program (Version 2.2) with a minimum requirement for 70% spectral match using -10 ion counts for peak filtering through the NIST14 library.
2.7. Statistical analysis
Statistical analysis was performed in three stages. In the first stage, data was subjected to a descriptive analysis where the variables caries and periodontitis were discarded due to the small number of positive cases. We, then, investigated the association among analyzed variables and the occurrence of halitosis. The variable presence/absence of calculus presented low degree of association with halitosis (Pearson's contingency coefficient equal to 0.002) and therefore was discarded of further analysis. Since the halitosis variable response is dichotomous, the presence/absence of halitosis was adjusted in a binary logistic regression model, taking as explanatory variables the presence/absence of visible dental biofilm, tongue coating or enzymatic activity. The quality of adjusted models was graphically analyzed by means of residuals deviance plot and normal plot with simulation envelopes. It was also assessed using the statistics of Hosmer and Lemeshow (QHL) statistics and the Akaike information criterion (AIC). In the confirmed analyzes, the minimum significance level of 5% was adopted. All analyzes were conducted using the R software [29].
3. Results
3.1. Dental clinical examination
In this study, clinical oral conditions such as caries, calculus, dental biofilm, tongue coating, gingivitis and periodontitis were investigated (Supplementary Table 2). Out of the 48 examined volunteers, 52.1% presented visible dental biofilm (materia alba), 37.5% presented tongue coating, 56.3% presented gingivitis and 29.2% presented calculus (Table 1). Due to the small number of caries and periodontitis positive cases (3 and 2 volunteers respectively), these variables were discarded from further analysis. Eight patients (16.7%) did not present any of the investigated oral conditions during the dental examination.
Table 1.
Analysis of evaluated clinical conditions. Forty-eight volunteers were examined to determine the presence of specific clinical conditions. Of these, only eight did not present any clinical condition. Of the remaining 40 volunteers, the majority presented an association of two or more clinical conditions.
| Clinical condition | % volunteer a | % Halitosis b | % Additional clinical conditions c | N |
|---|---|---|---|---|
| Visible dental biofilm | 52.1% | 80% | 88% | 25 |
| Halitosis | 66.7% | N/A | 96.9% | 32 |
| Calculus | 29.2% | 71.4% | 92.9% | 14 |
| Tongue Coating | 37.5% | 83.3% | 72.2% | 18 |
| Gingivitis | 56.3% | 81.5% | 96.3% | 27 |
| Caries | 6.3% | 66.7% | 100% | 3 |
| Periodontitis | 4.2% | 50% | 100% | 2 |
| No clinical condition |
16.7% |
12.5% |
N/A |
8 |
| Total of volunteers | 48 | |||
N total number of volunteers presenting clinical condition.
Percentage of the volunteers presenting the specified clinical condition.
Percentage of the volunteers with the specific condition that presented halitosis.
Percentage of volunteers that presented additional clinical conditions.
The presence of halitosis was determined using the Organoleptic Test (OT) as well as using the portable monitor for sulfur compound detection. A total of 32 volunteers presented different degrees of halitosis in OT (3 volunteers were considered moderate halitosis, 16 slight odor and 13 barely noticeable odor). Only 10 volunteers presented volatile sulfur compounds in the expelled air; all of them were also classified as halitosis-positive by the Organoleptic Test. In this study, all volunteers who presented a result greater than 0 in at least one of the tests were considered with halitosis. Only one out of 32 volunteers presenting halitosis did not present any additional oral conditions. The remaining 31 volunteers who presented halitosis also had tongue coating, gingivitis or visible dental biofilm and in most cases possessed more than one condition combined.
3.2. β-Glucosidase enzymatic activity profile
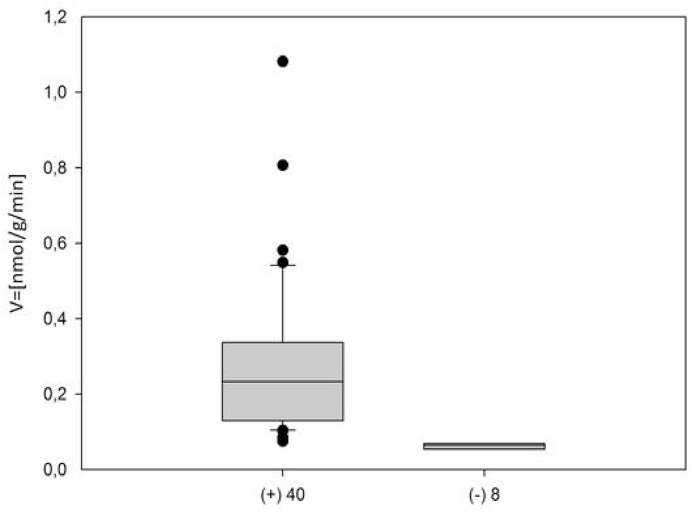
β-Glucosidase enzymatic activity was measured in saliva samples collected from 48 selected volunteers. Fig. 1 shows a boxplot with the average salivary β-glucosidase enzyme activity of volunteers.
Fig. 1.
Mean of β-glucosidase activity in fresh saliva samples. 40 (83.3%) of 48 volunteers showed positive β-glucosidase activity in fresh whole saliva samples. Positive activity was considered for samples with V > 0.07 nmol/g/min. The volunteers in this group had an average of β-glucosidase activity triplicates ranging from v = 0.075 nmol /g /min to 1.08 nmol /g /min.
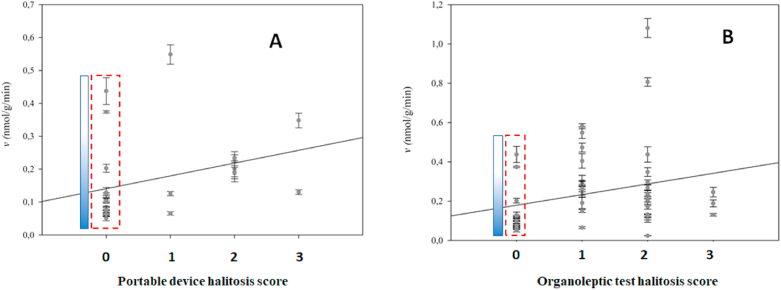
The mean of β-glucosidase enzymatic activity among patient groups is reported in Table 2. Interestingly, the mean of β-glucosidase activity among halitosis-patients was two-fold higher compared to β-glucosidase activity in halitosis-absent patients (0.294 ± 0.03 nmol/g/min and 0.133 ± 0.02 nmol/g/min respectively). Patients with visible dental biofilm presented an average enzymatic activity of 0.284 nmol/g/min as compared to 0.193 nmol/g/min in visible dental biofilm absent patients. A scattered plot of β-glucosidase activity versus halitosis score obtained from the use of the portable monitor of sulfur compounds, Breath Checker Tanita HC - 212SF, is shown in Fig. 2.
Table 2.
Mean of β-glucosidase activity in fresh saliva samples in the presence or absence of clinical conditions. Volunteers presenting halitosis, visible dental biofilm and gingivitis presented higher enzymatic activity in saliva compared to volunteers without this clinical condition. Interestingly the mean of β-glucosidase activity among halitosis-patients was two-fold higher compared to β-glucosidase activity in halitosis-absent patients.
| Clinical sign | N | Mean β-glucosidase activitya | p-value |
|---|---|---|---|
| Halitosis | |||
| + | 32 | 0.294 ± 0.03 nmol/g/min 0.133 ± 0.02 nmol/g/min |
0.0003 |
| – | 16 | ||
| Biofilm | |||
| + | 25 | 0.284 ± 0.04 nmol/g/min 0.193 ± 0.03 nmol/g/min |
0.0390 |
| – | 23 | ||
| Gingivitis | |||
| + | 27 | 0.281 ± 0.04 nmol/g/min 0.187 ± 0.02 nmol/g/min |
0.0585 |
| – | 21 | ||
| Tongue coating | |||
| + | 18 | 0.213 ± 0.02 nmol/g/min 0.250 ± 0.04 nmol/g/min |
0.6624 |
| – |
30 |
||
| Total of volunteers | 48 | ||
+ Positive cases for the specified clinical condition.
- Negative cases for the specified clinical condition.
N Total of volunteers with or without the clinical condition.
Mean of triplicates after enzymatic assays ± standard error.
Fig. 2.
Analysis of β-glycosidase activity versus halitosis score. Panel A: Halitosis score was determined based on the level of odor intensity (0,1, 2 and 3) determined by the portable sulfur monitor (Tanita Breath Checker) or by the organoleptic test (Panel B). The enzymatic activity of volunteers that did not present halitosis (score zero) are represented in the dashed red boxes. Symbols represent the mean of salivary enzymatic activity of each volunteer and error bars represent the standard deviation. Measurements were performed in triplicates. The black diagonal line is the regression line fit. Panel A R2 = 0.099, Panel B R2 = 0.065. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Microbial identification
The enzymatic activity was not observed in the supernatant of centrifuged saliva, suggesting a bacterial origin of the enzyme. Therefore, isolated bacterial colonies obtained by seeding saliva samples into cellobiose minimal media were subjected to identification by biochemical tests. The oral cavity comprises a highly complex microbiology enclosing more than 700 prokaryote species [30]. In the Human oral microbiome (HOMD) database, there are approximately 150 genera and 700 species in the oral cavity, which vary among individuals and according to different metabolic conditions. The complete identification of all genus and species of the isolated colonies are out of the scope of the current study, and the microbial identification served as a preliminary identification of β−glucosidase positive organisms present in human saliva. Therefore, saliva samples from the 22 volunteers were individually seeded into cellobiose minimal media in order to select for β-glucosidase producing organisms and isolated colonies were subjected to biochemical identification. The use biochemical tests performed in this study allowed the identification of some bacterial species, while other microorganisms were identified only to the genus level. Identified genera included Streptococcus, Arcanobacterium, Serratia, Pseudomonas and Neiseria, while bacterial species identified were Staphylococcus schleiferi, Staphylococcus aureus and Acinetobacter baumanni. Organisms were classified according to the List of Prokaryotic names with Standing in Nomenclature LPSN and are presented in Supplementary Table 1.
The organisms were viable and able to grow on Luria Bertani (LB) liquid media. Out of the 22 isolated colonies, ten were also tested for enzymatic activity, resulting in different levels of β-glucosidase activity detected. Although all tested organisms presented enzymatic activity, rates varied markedly not only among different organisms, but also among same organisms isolated from different volunteers. Therefore, the relative β-glucosidase activity in each microorganism needs to be further evaluated.
3.4. Statistical analyses
The selected model analyzed the main effects of plaque, tongue coating and enzymatic activity on the occurrence of halitosis. Statistical analyses of the collected data demonstrated that the effects of the variables, dental plaque (X1), tongue coating (X2) and β-glucosidase enzymatic activity (X3) are significant to the occurrence of halitosis (P <0.05). The parameters estimated by this model are shown in Table 3. Since halitosis is a multifactorial condition, we intended to rule out the possibility of the observed effect of β-glucosidase activity on halitosis being only be due to the presence of these enzymes in a biofilm-rich environment. It is known that tongue coating as well as dental biofilm exert effects on the occurrence of halitosis, therefore, the rationale in this study is to evaluate if β-glucosidase activity exerts a direct role on halitosis and it's not just a consequence of the presence of oral biofilms. Therefore, the X3|X1, X2 utilized model resulted in a p-value of 0.01134, which means that even after discounting the effects of biofilm (X1) and tongue coating (X2), the enzymatic activity (X3) still performs a significant effect on the occurrence of halitosis.
Table 3.
Statistical analysis. Residual deviances and AIC values of adjusted models. Only the main effects of visible dental biofilm, tongue coating and enzymatic activity were significant (P <0.05).
| Model | d.f. | Null deviances | Residual deviances | d.f.residual | p-value | AIC |
|---|---|---|---|---|---|---|
| Null | 45 | 59.44 | 1 | 61.4 | ||
| X1 | 44 | 55.06 | 4.38 | 1 | 0.036 * | 59.06 |
| X2|X1 | 43 | 50.56 | 4.50 | 1 | 0.034 * | 56.56 |
| X3|X1, X2 |
42 |
44.15 |
6.41 |
1 |
0.011 * |
52.15 |
| Model |
Estimates |
Standard error |
||||
| β0- Constant | -1.883 | 0.837 | ||||
| β1- X1 | 1.026 | 0.755 | ||||
| β2- X2 | 1.520 | 0.815 | ||||
| β3- X3 | 8.196 | 3.733 | ||||
AIC Akaike Information Criterion; d.f degree of freedom; X1presence /absence of visible dental biofilm; X2 presence /absence of tongue coating; X3salivary β-glucosidase activity.
Also, we performed Odds ratio (OR) analyses for dental plaque as variable, discounting the effects of tongue coating and enzymatic activity, being equal to exp (1.02568) = 2.79, which means that the chance of a patient with visible dental plaque experiencing halitosis is 2.79 times greater than that of a patient without plaque. Likewise, the calculated OR for tongue coating, adjusted for dental plaque and enzymatic activity is exp (1.52199) = 4.58, where the chance of a patient with tongue coating having halitosis is 4.58 times greater than a patient without tongue coating. In turn, for enzymatic activity variable, the OR adjusted for plaque and tongue coating, is equal to exp (8.1962*(0.5815–0.0239)) = 96.56, that is, the chance of a patient with high enzymatic activity (~0.5815 nmol/g/min, one of the highest observed rates) having halitosis is 96.56 times higher than that of a patient with low enzymatic activity (~0.0239 nmol/g/min, one of the lowest observed values).
The goodness of fit of the statistical model can be verified using the QHL statistic, which presented a value equal to 8.71 (P = 0.367; gl = 8) indicating the adequacy of the adjusted model in this study. Still, it is observed that the “deviance” residues are located between the values -3 and 3 and inside the simulated envelope which translates into a favorable evidence for the adjusted model.
3.5. Volatile organic compound profile of saliva upon exogenously added β-glucosidase
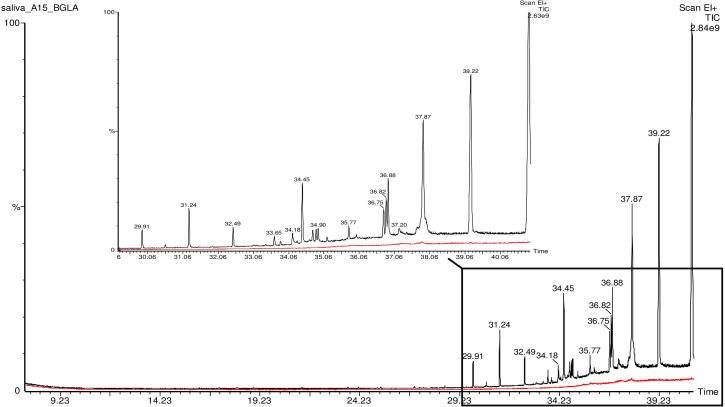
To investigate whether the direct action of β-glucosidase on saliva substrates leads to a modified VOC profile, we performed headspace gas chromatography of saliva samples upon addition of purified Bacillus polymyxa β-glucosidase. B. polymyxa is a Gram positive bacteria found in soil, plant roots, and marine sediments, and is known to produce biofilm on plant roots through the synthesis of exopolysaccharides, protecting the plants from pathogens [31]. VOC alteration on headspace chromatography was observed in 6 out of the 12 saliva samples tested. Upon addition of active β-glucosidase, additional peaks were observed in the chromatogram as compared to the control (heat-inactivated enzyme) (Fig. 3), indicating that the enzyme acts on saliva glycosides and release volatile aglycones. In total, seventeen different organic compounds were identified accounting all tested saliva samples that were submitted to enzymatic treatment. The list of all identified compounds and the correspondent saliva samples are presented in Table 4.
Fig. 3.
Headspace Gas Chromatography of saliva samples. The figure shows the overlapped VOC profile of β-glucosidase treated saliva sample (black trace) and control saliva sample (red trace) from one volunteer. The zoomed in region presents several VOC peaks in the treated sample, which are absent in the control sample. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 4.
Volatile compounds released upon action of exogenousβ-glucosidase in human saliva samples. Analysis of volatile headspace compounds of centrifuged saliva samples by gas-chomatography-Mass Spectometry (GC-MS) after overnight incubation with active β-glucosidase from Bacillus polymyxa. Analysis of salivary samples from six patients identified 17 different volatile compounds. Control: Samples containing heat inactivated β-glucosidases purified from B. polymyxa.
| Volatile compounds | Samplea |
|---|---|
| Trimethylsilyl | 4 |
| 2-(Methylthio)benzothiazole | 4 |
| Methyl((24-oxo-3-[trimethylsilyl)oxy] cholan-24yl) amino) acetate | 4 |
| 7,8-Epoxylanostan-11-ol, 3-acetoxy | 4 |
| 9-Desoxo-9-x-acetoxy-3,8,12-tri-O-acetylingol | 4, 12 |
| 3-pyridinecarboxylic acid | 12 |
| Lycoxanthin | 12 |
| Carbamic acid | 12, 10 |
| Fluoracetylene | 15 |
| 4-methyl-1-pentene | 15 |
| 2-aziridinylethyl, amine | 10 |
| Nitrous Oxide | 10 |
| Phenethylamine alfa dimethyl | 10 |
| 2-Bromobutyloxychalcone | 10 |
| Pterin-6-carboxylic acid | 11 |
| Quinoline1 2-dihydro-2 2 4-trimethyl | 11 |
| N-Methylnicotinimidate, O-trimethylsilyl | 8 |
Sample number.
4. Discussion
Halitosis is a major patient complaint and may be associated to systemic or local conditions and is considered a multifactorial alteration [[14], [15], [16]]. Poor hygiene, dental biofilm accumulation, tongue coating, periodontal diseases, caries and the presence of food are the main local factors associated with oral malodor [15,17]. Halitosis associated with oral conditions usually arise from microbial degradation of proteins, peptides and amino acids present in oral fluids or food retention between teeth [32]. Tongue coating is the most common clinical condition related to intraoral halitosis [33,34].
Deglycosylation of salivary glycoproteins is the first and important step in the production of oral malodor. Salivary mucins are highly glycosylated proteins, being a common source of metabolites associated to halitosis [33]. Glycoprotein degradation commences with the removal of carbohydrates by glycosyl-hydrolases commonly found in Gram-positive bacteria. The exposed protein is then subjected to the action of proteases and peptidases, which are particularly produced by Gram-negative bacteria [34].
The activity of β-galactosidase in human saliva has been previously associated to halitosis, salivary putrefaction and supragingival biofilm [14,15]. Authors suggested that bacterial β-galactosidase contributes to the production of sulfur compounds through the deglycosylation of salivary glycoproteins [14,15,18]. Indeed, most halitosis-related compounds are produced through bacterial amino acid metabolism [35]. According to current studies, β-galactosidases contribute to halitosis via removal of carbohydrate side chains from glycoproteins in saliva, leading to a facilitated proteolysis and consequent production of odoriferous amino acid metabolites [36]. However, there was no evidence supporting a direct role of glycosidases on halitosis as a direct release of volatile aglycones upon enzymatic hydrolysis of saliva substrates. As a multifactorial condition, the understanding of all governing factors is of fundamental importance for condition control.
Saliva is composed of inorganic as well as organic compounds, containing more than 700 microorganisms associated with systemic or oral health and diseases [37,38]. Earlier studies investigated the role of bacteria on grape aroma release, demonstrating that human saliva, as well as isolated tested bacteria, were able to hydrolyze white grape glycosides releasing different types of aromatic aglycones that might be associated with differences on taste perception among individuals [12].
Stradwick et al. confirmed β-glucosidase activity in the supernatant of human saliva after centrifugation. The activity was detected in 19 out of the 20 tested patients, but no correlation with clinical data was performed and the authors suggest that individual taste perception and food preference may be associated with β-glucosidase activity in saliva [23]. Our data showed no β-glucosidase activity in saliva supernatant after centrifugation, in agreement with other studies [18] however, enzymatic activity was observed in the salivary pellet after centrifugation and in whole saliva samples, suggesting that these enzymes are produced by oral bacteria,. Some species of the identified bacterial groups, such as Streptococcus salivarius and Pseudomonas aeruginosa are potentially related to intraoral halitosis [34]. P. aeruginosa is also involved in human infections as pneumonia and chronical opportunistic infections related to diabetes. Interestingly, some iminosugars are capable of reducing the biofilm formation and the authors suggest that the inhibition of glycosyltransferases and other enzymes, related to polysaccharides synthesis, can interfere directly on P. aeruginosa bacterial biofilm [39].
Teeth, gums and tongue are oral surfaces prone to accumulation of microorganisms, forming the oral biofilm or dental plaque. When the accumulation of the biofilm is persistent, maturation occurs, a situation that involves modification of the local microbiota and adhesion of pathogenic bacteria [40,41]. In fact, according to Kiliç et al. who studied gene regulation of Streptococcus gordonii, a dental plaque associated microorganism, demonstrated that β-glucosidase gene influence the cell adhesiveness of these oral bacteria [42].
In the present study, few volunteers presented periodontitis clinically, which impaired proper analysis. Earlier studies demonstrated a positive relation between periodontal diseases and β-glucosidase activity. For example, Nakamura & Slots evaluated diverse enzyme activities in human saliva and the relationship with periodontal diseases [43]. The results showed that increased β-glucosidase activity was found in the saliva of periodontal compromised patients, while reduction of enzymatic activity was observed after adequate dental treatment [44].
Obtained data demonstrated that the mean β-glucosidase activity among patients presenting halitosis was two-fold higher as compared to patients not presenting halitosis, suggesting that β-glucosidase activity is increased in halitosis patients (Table 2). Statistical analysis data demonstrated that the presence of dental plaque, tongue coating and enzymatic activity affect the occurrence of halitosis (Table 3).
Although biofilm related microbiota (as means of tongue coating and dental plaque) is known to produce volatile sulfur compounds (VSCs) responsible for the generation of malodor [45], the correlation observed between β- glucosidase and halitosis presented in this study accounted for these effects, and the correlation among enzymatic activity and halitosis still persisted.
Most of the compounds associated with oral malodor are volatile sulphide compounds: hydrogen sulphide (H2S), methyl mercaptan (CH3SH) and dimethyl sulphide (CH3SCH3) and other compounds such as methylamine, dimethylamine, propionic acid, butyric acid, indole and cadaverine which are usually derived from bacterial metabolism in the oral cavity [16,17,46]. Bad breath caused by food impaction is predominantly due to bacterial activity, resulting in volatile compounds causing malodor. Foods like onions, garlic and dairy products have compounds that when metabolized by bacteria in the oral cavity result in volatile sulfur compounds [34].
To investigate whether occurs a direct action of β-glucosidase on the release of volatile compounds from saliva substrates, we have added exogenous β-glucosidases to saliva samples and analyzed the VOC profile by headspace gas chromatography. We observed the presence of additional volatile compounds on enzyme treated saliva as compared to the control (heat-inactivated enzyme) in 6 out of 12 analyzed saliva samples. Seventeen compounds were identified upon enzymatic hydrolysis (Table 4), among them are 2- (Methylthio) benzothiazole, a member of benzothiazoles and a methyl sulfide, as well as Dymethil sulfide (known to be associated with bad breath); and Pterin-6-carboxylic acid, an organooxygen compound derived from alpha-amino acids isolated from biological sources such as fish and soybean [47].
Although most identified compounds were not halitosis-associated in the enzyme treated samples, the utilized enzyme has a soil-bacteria origin, and substrate specificity indeed varies significantly among different enzymes, therefore, studies using oral bacteria β-glucosidases should be performed in the future. In addition, the observed different VOC profile changes upon enzyme addition among tested individuals is likely due to different glycoside substrates present in each saliva sample. Interestingly, in this study, each individual volatile compound was rarely detected in more than one saliva sample, our hypothesis is that the release of compounds can be directly associated with individual salivary glycosides. Therefore, different individuals might present different VOC alteration depending on glycoside profile present in saliva. To our knowledge, this is the first report that investigates the correlation among salivary β-glucosidase activity and the occurrence of halitosis.
Interestingly, previous studies demonstrated that the presence of glucose inhibits the production of fetid compounds in incubated saliva, and the authors earlier explained this finding based on pH modifications [48]. In addition, according to Sterer & Rosenmerg, putrefaction of saliva is inhibited in the presence of glucose, and the authors suggested that this sugar can interfere in the process of deglycosylation of salivary glycoproteins, reducing VSCs [49]. In fact, glucose is a well-known β-glucosidase inhibitor [3], and inhibited salivary β-glucosidase in the present study (Supplementary Fig. 1). Therefore, the lack of fetid compounds observed in earlier studies in the presence of glucose, can now be attributed to the product inhibition of salivary β-glucosidases, causing a diminished release of volatile aglycones.
While the portable device used in the present study is able to detect only sulphurous compounds, the organoleptic measurements are able to detect other volatile compounds such as alcohols, phenolic compounds, alkenes, ketones, polyamines and fatty acids [15]. In this study, 45.8% of the volunteers presenting halitosis detected by the organoleptic test were not positive for the presence of sulfur volatile compounds, which is in accordance with the fact that many volatile compounds associated with halitosis are not necessarily sulphurous compounds.
In conclusion, these results suggested that β-glucosidases are part of a range of enzymes capable of altering the aromatic profile of saliva, being a possible target of other studies in oral health. Interestingly, alike glucose, xylitol also inhibits β-glucosidase present in human saliva [24]. This polyol has been studied and can be used in several health areas with antibacterial and nonstick effect, assisting the bacterial biofilm control [50,51]. Its action on β-glucosidase enzymes may also assist to halitosis control treatments.
Declaration of competing interest
The authors declare no financial or commercial conflict of interest.
Acknowledgements
Authors would like to thank Dr. César Augusto Rodenbusch Poletto, dentist of the Center of Agroveterinary Sciences, State University of Santa Catarina, for assisting on the use of the dentist office to collect saliva samples. The authors thank the financial support from State University of Santa Catarina (UDESC), Fundação de Amparo à Pesquisa do Estado de Santa Catarina (FAPESC) and Financiadora de Estudos e Projetos (FINEP).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100965.
Contributor Information
Lucimari Teixeira Essenfelder, Email: lucimari_teixeira@hotmail.com.
Anderson Albino Gomes, Email: andersonalbino.g@gmail.com.
Jefferson Luis Meirelles Coimbra, Email: jefferson.coimbra@udesc.br.
Marcelo Alves Moreira, Email: marcelo.moreira@udesc.br.
Sandra Maria Ferraz, Email: sandra.ferraz@udesc.br.
David José Miquelluti, Email: david.miquelluti@udesc.br.
Gustavo Felippe da Silva, Email: gustavo.silva@udesc.br.
Maria de Lourdes Borba Magalhães, Email: maria.magalhaes@udesc.br.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cairns J.R.K., Esen A. β-Glucosidases. Cell. Mol. Life Sci. 2010;67(20):3389–3405. doi: 10.1007/s00018-010-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isorna P., Polaina J., Latorre-García L., Cañada F.J., González B., Sanz-Aparicio J. Crystal structures of paenibacillus polymyxa β-glucosidase B complexes reveal the molecular basis of substrate specificity and give new insights into the catalytic machinery of family I glycosidases. J. Mol. Biol. 2007;371(5):1204–1218. doi: 10.1016/j.jmb.2007.05.082. [DOI] [PubMed] [Google Scholar]
- 3.Krisch J., Takó M., Papp T., Vágvölgyi C. In Book: Current Research; Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Formatex Research Center; 2010. Characteristics and potential use of β-glucosidases from Zygomycetes; pp. 891–896. 2010. [Google Scholar]
- 4.Singh G., Verma A.K., Kumar V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech. 2016;6(1):3. doi: 10.1007/s13205-015-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleczkowski K., Schell J., Bandur R. Phytohormone conjugates: nature and function. Crit. Rev. Plant Sci. 1995;14(4):283–298. doi: 10.1080/07352689509382361. [DOI] [Google Scholar]
- 6.Jones P., Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213(2):164–174. doi: 10.1007/s004250000492. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia Y., Mishra S., Bisaria V.S. Microbial β-glucosidases: cloning, properties, and applications. Crit. Rev. Biotechnol. 2002;22(4):375–407. doi: 10.1080/07388550290789568. [DOI] [PubMed] [Google Scholar]
- 8.Gueguen Y., Chemardin P., Pien S., Arnaud A., Galzy P. Enhancement of aromatic quality of muscat wine by the use of immobilized β-glucosidase. J. Biotechnol. 1997;55(3):151–156. doi: 10.1016/S0168-1656(97)00069-2. [DOI] [Google Scholar]
- 9.Maicas S., Mateo J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl. Microbiol. Biotechnol. 2005;67(3):322–335. doi: 10.1007/s00253-004-1806-0. [DOI] [PubMed] [Google Scholar]
- 10.Su E., Xiaa T., Gaoa L., Daia Q., Zhanga Z. Immobilization of β-glucosidase and its aroma-increasing effect on tea beverage. Food Bioprod. Process. 2010;88 [Google Scholar]
- 11.Wang Y., Zhang C., Li J., Xu Y. Different influences of β-glucosidases on volatile compounds and anthocyanins of Cabernet Gernischt and possible reason. Food Chem. 2013;140(1–2):245–254. doi: 10.1016/j.foodchem.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz-González C., Cueva C., Ángeles Pozo-Bayón M., Victoria Moreno-Arribas M. Ability of human oral microbiota to produce wine odorant aglycones from odourless grape glycosidic aroma precursors. Food Chem. 2015;187:112–119. doi: 10.1016/j.foodchem.2015.04.068. [DOI] [PubMed] [Google Scholar]
- 13.Masuo Y., Suzuki N., Yoneda M., Naito T., Hirofuji T. Salivary β-galactosidase activity affects physiological oral malodour. Arch. Oral Biol. 2012;57(1):87–93. doi: 10.1016/j.archoralbio.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Sterer N., Greenstein R.B.N., Rosenberg M. β-galactosidase activity in saliva is associated with oral malodor. J. Dent. Res. 2002;81(3):182–185. doi: 10.1177/154405910208100308. [DOI] [PubMed] [Google Scholar]
- 15.Aylikci B., Çolak H. Halitosis: from diagnosis to management. J. Nat. Sci. Biol. Med. 2013;4(1):14. doi: 10.4103/0976-9668.107255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N., Yoneda M., Takeshita T., Hirofuji T., Hanioka T. Induction and inhibition of oral malodor. Mol. Oral Microbiol. 2019;34(3):85–96. doi: 10.1111/omi.12259. [DOI] [PubMed] [Google Scholar]
- 17.Takehara S., Yanagishita M., Podyma-Inoue K.A., Ueno M., Shinada K., Kawaguchi Y. Relationship between oral malodor and glycosylated salivary proteins. J. Med. Dent. Sci. 2010;57(1):25–33. doi: 10.11480/jmds.570104. [DOI] [PubMed] [Google Scholar]
- 18.Inui T., Walker L.C., Dodds M.W.J., Hanley A.B. Extracellular glycoside hydrolase activities in the human oral cavity. Appl. Environ. Microbiol. 2015;81(16):5471–5476. doi: 10.1128/AEM.01180-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen T., Fiehn N.-E. Dental biofilm infections - an update. APMIS. 2017;125(4):376–384. doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- 20.Marsh P., Do, Devine Oral biofilms: molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Invest. Dent. 2013;11 doi: 10.2147/CCIDE.S31005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowen W.H., Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson K.G.I. Enzymatic synthesis of oligosaccharides. Trends Biotechnol. 1988;6(10):256–264. doi: 10.1016/0167-7799(88)90058-3. [DOI] [Google Scholar]
- 23.Stradwick L., Inglis D., Kelly J., Pickering G. Development and application of assay for determining β-glucosidase activity in human saliva. Flavour. 2017;6(1):1–8. doi: 10.1186/s13411-017-0054-z. [DOI] [Google Scholar]
- 24.Teixeira Essenfelder L., Gomes A.A., Miquelutti D., da Silva G.F., Magalhães M.L.B. Effect of xylitol on salivary β ‐glucosidase in humans. Eur. J. Oral Sci. 2019;127(5):472–475. doi: 10.1111/eos.12649. [DOI] [PubMed] [Google Scholar]
- 25.Bosy A., Kulkarni G.V., Rosenberg M., McCulloch C.A.G. Relationship of oral malodor to periodontitis: evidence of independence in discrete subpopulations. J. Periodontol. 1994;65(1):37–46. doi: 10.1902/jop.1994.65.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Greenman J., Duffield J., Spencer P., Rosenberg M., Corry D., Saad S., Lenton P., Majerus G., Nachnani S., El-Maaytah M. Study on the organoleptic intensity scale for measuring oral malodor. J. Dent. Res. 2004;83(1):81–85. doi: 10.1177/154405910408300116. [DOI] [PubMed] [Google Scholar]
- 27.Gibson Q.H. [6] Rapid mixing: stopped flow. Methods Enzymol. 1969;16(C):187–228. doi: 10.1016/S0076-6879(69)16009-7. [DOI] [Google Scholar]
- 28.de Oliveira Sérgio J., Vaz Adil K. In: Guia Bacteriológico Prático: identificação, patogenicidade e imunidade. first ed. ULBRA, editor. 2018. [Google Scholar]
- 29.R Foundation for Statistical Computing . 2019. A Language and Environment for Statistical Computing. [Google Scholar]
- 30.Gao L., Xu T., Huang G., Jiang S., Gu Y., Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmusk S., Grantcharova N., Wagner E.G.H. Paenibacillus polymyxa invades plant roots and forms biofilms. Appl. Environ. Microbiol. 2005;71(11):7292–7300. doi: 10.1128/AEM.71.11.7292-7300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campisi G., Musciotto A., Di Fede O., Di Marco V., Craxì A. Halitosis: could it be more than mere bad breath? Inter. Emerg. Med. 2011;6(4):315–319. doi: 10.1007/s11739-010-0492-4. [DOI] [PubMed] [Google Scholar]
- 33.Seerangaiyan K., van Winkelhoff A.J., Harmsen H.J.M., Rossen J.W.A., Winkel E.G. The tongue microbiome in healthy subjects and patients with intra-oral halitosis. J. Breath Res. 2017;11(3) doi: 10.1088/1752-7163/aa7c24. [DOI] [PubMed] [Google Scholar]
- 34.Thoppay J.R., Filipp i A., Ciarrocca K., Greenman J., De Rossi S.S. Halitosis. In: Farah C., Balasubramaniam R., McCullough M., editors. Contemporary Oral Medicine. Springer; Cham: 2019. [DOI] [Google Scholar]
- 35.Suzuki N., Yoneda M., Hirofuji T. Relationship between oral malodor and oral microbiota. In: Virdi Mandeep Singh., editor. Oral Health Care - Prosthodontics, Periodontology, Biology, Research And Systemic Conditions (ISBN 978-953-51-0040-9) Chapter 9. In Tech; Croatia: 2012. pp. 121–130. 2012. [Google Scholar]
- 36.Sterer N., Shaharabany M., Rosenberg M. β-Galactosidase activity and H2S production in an experimental oral biofilm. J. Breath Res. 2009;3(1) doi: 10.1088/1752-7155/3/1/016006. [DOI] [PubMed] [Google Scholar]
- 37.Aas J.A., Paster B.J., Stokes L.N., Olsen I., Dewhirst F.E., Aas J.A., Paster B.J., Stokes L.N., Olsen I., Dewhirst F.E. Defining the normal bacterial flora of the oral cavity defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005;43(11) doi: 10.1128/JCM.43.11.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C.Z., Cheng X.Q., Li J.Y., Zhang P., Yi P., Xu X., Zhou X.D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016;8(3):133–137. doi: 10.1038/ijos.2016.38. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strus M., Mikołajczyk D., Machul A., Heczko P.B., Chronowska A., Stochel G., Gallienne E., Nicolas C., Martin O.R., Kyzioł A. Effects of the selected iminosugar derivatives on Pseudomonas aeruginosa biofilm formation. Microb. Drug Resist. 2016;22(8):638–645. doi: 10.1089/mdr.2015.0231. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons R.J., Houte J.V. Bacterial adherence in oral microbial ecology. Annu. Rev. Microbiol. 1975;29(1):19–42. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- 41.Kriebel K., Hieke C., Müller-Hilke B., Nakata M., Kreikemeyer B. Oral biofilms from symbiotic to pathogenic interactions and associated disease - connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front. Microbiol. 2018;9(JAN):1–14. doi: 10.3389/fmicb.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiliç A.O., Tao L., Zhang Y., Lei Y., Khammanivong A., Herzberg M.C. Involvement of Streptococcus gordonii beta-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J. Bacteriol. 2004;186(13):4246–4253. doi: 10.1128/JB.186.13.4246-4253.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura M., Slots J. Salivary enzymes: origin and relationship to periodontal disease. J. Periodontal. Res. 1983;18(6):559–569. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 44.Zambon J.J., Nakamura M., Slots J. Effect of periodontal therapy on salivary enzymatic activity. J. Periodontal. Res. 1985;20(6):652–659. doi: 10.1111/j.1600-0765.1985.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 45.Nakano Y., Yoshimura M., Koga T. Correlation between oral malodor and periodontal bacteria. Microb. Infect. 2002;4(6):679–683. doi: 10.1016/S1286-4579(02)01586-1. [DOI] [PubMed] [Google Scholar]
- 46.Bollen C.M.L., Beikler T. Halitosis: the multidisciplinary approach. Int. J. Oral Sci. 2012;4(Issue 2):55–63. doi: 10.1038/ijos.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., Zaslavsky L., Zhang J., Bolton E.E. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinberg I., Westbay G. Salivary and metabolic factors involved in oral malodor formation. J. Periodontol. 1992;63(9):768–775. doi: 10.1902/jop.1992.63.9.768. [DOI] [PubMed] [Google Scholar]
- 49.Sterer N., Rosenberg M. Effect of deglycosylation of salivary glycoproteins on oral malodour production. Int. Dent. J. 2002;52(S5P1):229–232. doi: 10.1002/j.1875-595X.2002.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 50.Delgado Arcaño Y., Valmaña García O.D., Mandelli D., Carvalho W.A., Magalhães Pontes L.A. Xylitol: a review on the progress and challenges of its production by chemical route. Catal. Today. 2020;344:2–14. doi: 10.1016/j.cattod.2018.07.060. [DOI] [Google Scholar]
- 51.Söderling E.M., Hietala-Lenkkeri A.M. Xylitol and erythritol decrease adherence of polysaccharide-producing oral streptococci. Curr. Microbiol. 2010;60(1):25–29. doi: 10.1007/s00284-009-9496-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.