Summary
Pain is a negative affective state arising from tissue damage or inflammation. Because pain is aversive and its relief is innately rewarding, animals may learn to avoid a context in which pain is experienced and prefer one where pain relief occurs. It is generally accepted that vertebrate animals experience pain; however, there is currently inconclusive evidence that the affective component of pain occurs in any invertebrate. Here, we show that octopuses, the most neurologically complex invertebrates, exhibit cognitive and spontaneous behaviors indicative of affective pain experience. In conditioned place preference assays, octopuses avoided contexts in which pain was experienced, preferred a location in which they experienced relief from pain, and showed no conditioned preference in the absence of pain. Injection site grooming occurred in all animals receiving acetic acid injections, but this was abolished by local anesthesia. Thus, octopuses are likely to experience the affective component of pain.
Subject areas: Biological Sciences, Ethology, Neuroscience
Graphical abstract
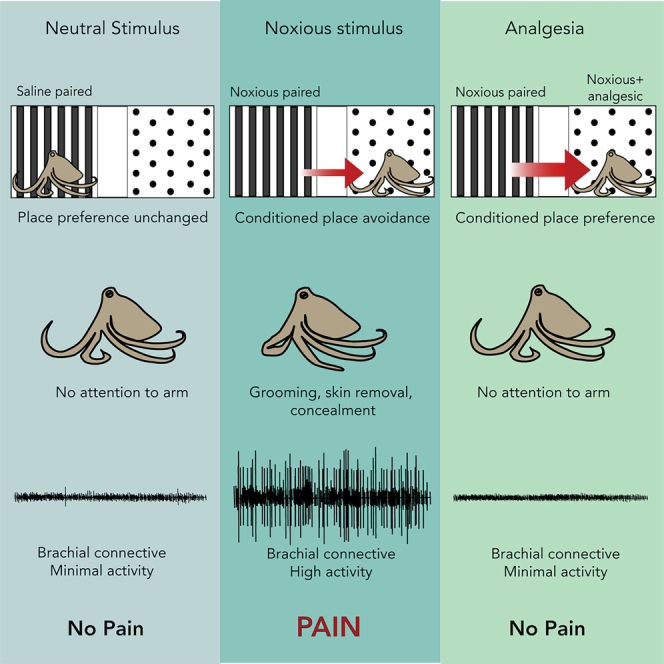
Highlights
-
•
Octopuses avoid a location after it is associated with a noxious stimulus
-
•
Injection of dilute acetic acid induces lasting, location-specific grooming
-
•
Nerve recordings show central processing of noxious sensory input
-
•
Octopuses are capable both of discriminative and affective pain experience
Biological Sciences; Ethology; Neuroscience
Introduction
Whether invertebrate animals are capable of experiencing pain is the subject of ongoing debate (Elwood, 2019a; Groening et al., 2017; King and Marino, 2019; Walters et al., 2019). Unlike nociception, which is a simple reflex response, pain is a complex emotional state encompassing distress and suffering and is generally considered to require a highly complex nervous system (Treede et al., 1999). Discrete pain circuits within the central brain produce two distinct aspects of pain experience: the “discriminative” component, encompassing the location, quality, and intensity of pain, and the “affective” component, encompassing the negative emotional state (Auvray et al., 2010). Pain is accepted to occur in all vertebrate animals, although pain experience that is persistent and ongoing (tonic pain) has to date only been demonstrated in mammals (King et al., 2009; Mogil, 2019). Although the evolutionary origins of pain remain unresolved, there are indications in several invertebrate taxa of at least some of the requirements for pain experience (Elwood, 2019b; Kavaliers, 1988; Key and Brown, 2018). It is generally accepted that there are specific requirements for a nervous system to be capable of producing a negative affective state (i.e., pain experience) in response to noxious sensory input, including the presence of nociceptive sensory neurons, which then must connect to integrative brain regions capable of complex processing (Sneddon, 2019). Nociceptors are present and well characterized in multiple invertebrate taxa, including cephalopods ((Crook et al., 2013), other molluscs (Illich and Walters, 1997), insects (Tracey, 2017), nematodes (Chatzigeorgiou et al., 2010), crustaceans (Barr et al., 2008). There are also accepted behavioral criteria that are used to suggest the presence of affective state going beyond simple nociceptive reflex, such as complex behavioral responses that can be modulated by analgesia, motivational trade-offs that balance potential pain against other, usually protective or positively valenced experiences, and associative learning about contexts that signal noxious sensations (Appel and Elwood, 2009; Elwood, 2019b; Walters, 2018). Behavioral studies showing evidence for these capabilities have been conducted most extensively in crustaceans (Elwood, 2019b). Interestingly, there is also accumulating evidence for the existence of positive affective state in some invertebrates as well (Perry et al., 2016), suggesting emotional processing of sensory experiences in invertebrates may be both complex and widespread.
A common argument against the possibility of affective state in invertebrates is that their brains are insufficiently complex to encompass circuits that produce emotional valence (Broom, 2007; Sømme, 2005). A similar argument has also been used to suggest that fish are incapable of pain experience (Derbyshire, 2016; Key, 2016; Rose, 2016), indicating the ongoing controversy over the question of pain in non-mammalian species. Cephalopod molluscs are extreme outliers in the realm of invertebrate brains; unlike all other invertebrates, their brain size, cognitive ability and behavioral flexibility surpass those of some smaller-brained vertebrates, including amphibians and reptiles (Hochner et al., 2006; Schnell et al., 2020). Their nervous system is organized fundamentally differently from that of vertebrates, with extensive peripheral control of sensing and movement which seems to occur largely independently of the central brain (Gutfreund et al., 2006, but see Gutnick et al., 2020; Hooper, 2020). Their large brains and complex behaviors have led to increasing concern for their welfare, and efforts to regulate invasive procedures performed on cephalopods in research laboratories are now established in many nations (Ponte et al., 2019). These rules are informed by the “precautionary principle” (Birch, 2017), which posits that that neural and cognitive complexity is sufficient to suggest that an animal can experience pain, even where no conclusive evidence exists.
Somewhat surprisingly, there have been few experimental studies focusing on potential for pain experience in cephalopod molluscs. In this study, a well-established assay for demonstrating the affective component of pain in mammals (Navratilova et al., 2013; Sufka, 1994) was applied to octopus, along with detailed measurements of spontaneous pain-associated behaviors and neural activity in centripetal pathways. All three lines of evidence indicate that octopuses are capable of experiencing pain.
Results
Octopuses experience the affective component of pain
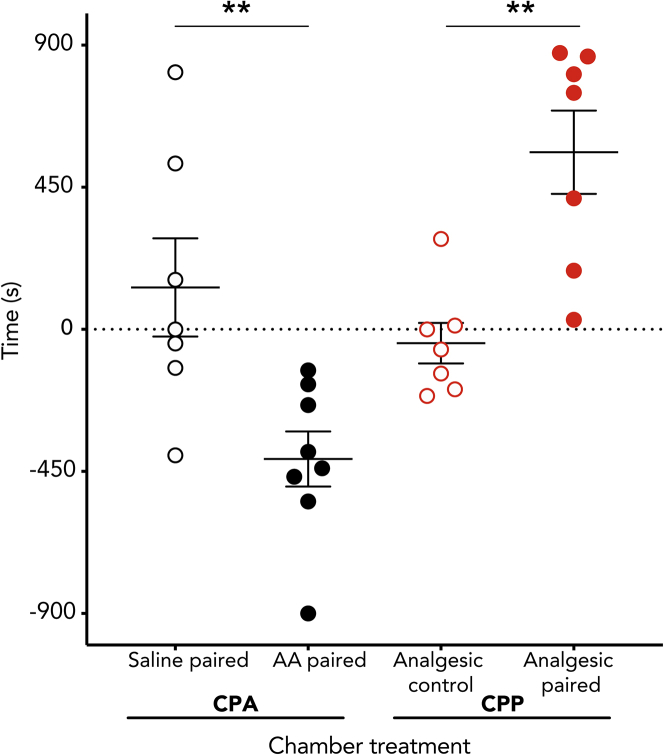
After a single training session in a three-chamber conditioned place preference (CPP) box (Figure 1), octopuses that received a subcutaneous injection of dilute (0.5%) acetic acid (AA) into one arm (n = 8) showed clear avoidance of their initially preferred chamber, in which they were confined after injection (Figure 2A, one-sample t test, p = 0.003). Saline-injected animals (n = 7) showed no change in their chamber preference before and after training trials (p = 0.19). The change in time spent in the initially preferred chamber also differed between the two groups (Bonferroni post-hoc test, p = 0.006, Figure 2).
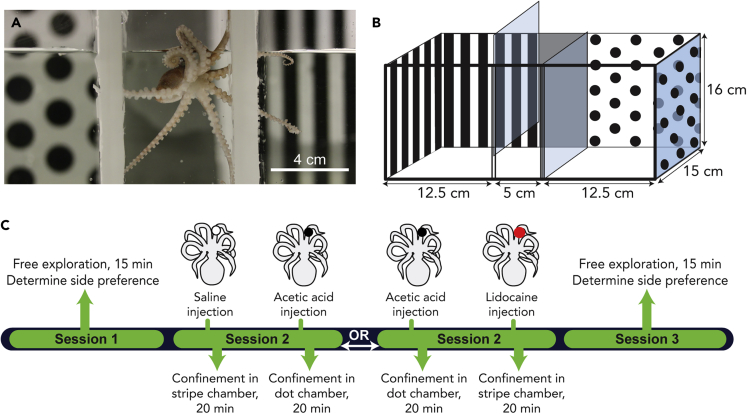
Figure 1.
CPP design and timeline
(A) Octopus bocki in the start chamber of the CPP box.
(B) Diagram of the apparatus, with pattern shown on the back and sides only for clarity. In experimental trials, visual cues covered all four walls.
(C) Timeline of an experiment showing sequences for CPA and CPP procedures. In this example, an octopus showed an initial preference in session 1 for the dot chamber and is thus trained against initial preference (i.e., the octopus is given AA injection prior to confinement in the dot chamber or lidocaine prior to the stripe chamber). Different animals were used to test each of the four conditions (see Figure 2). Control sequences (saline/saline and saline/lidocaine) not shown.
Figure 2.
Conditioned place avoidance (CPA) and conditioned place preference (CPP) assays reveal the affective component of pain in octopus
In trials where initially preferred chambers were paired with 0.5% acetic acid (AA) injection, octopuses spent less time in their initially preferred chamber in a post-training period of free exploration, compared with octopuses receiving saline. In trials where octopuses received lidocaine over an area of prior injection (either saline or AA), octopuses preferred the chamber paired with lidocaine only if they had previously been given AA injection. Points show individual change in time values for each subject. Different animals were used to test each condition. Central, wide bars show the mean and whiskers show standard error of the mean. Asterisks indicate significant between-group differences (Bonferroni post-hoc test, ∗∗p < 0.01).
Relief from tonic pain is rewarding, and thus, a drug that provides pain relief provides a strong training signal in the presence of tonic pain but no signal in its absence. CPP for a location associated with an analgesic is considered strong evidence for pain in vertebrate animals (Navratilova et al., 2013; Roughan et al., 2014). Here, octopuses with AA-induced tonic pain received a localized injection of lidocaine (Butler-Struben et al., 2018) immediately prior to being confined to the chamber they least preferred in initial preference tests. Lidocaine injection induced strong preference for that chamber in test trials for AA-injected animals (Figure 2A, one-sample t test, p = 0.005), but there was no preference for the lidocaine-paired chamber in animals that received saline injection instead of AA (p = 0.51), and chamber preference also differed between the two groups (Bonferroni post-hoc test, p = 0.003). This demonstrates that lidocaine injection was rewarding to animals only if they were experiencing ongoing pain and that lidocaine alone is not innately rewarding for octopuses.
Octopuses show discriminative pain experience
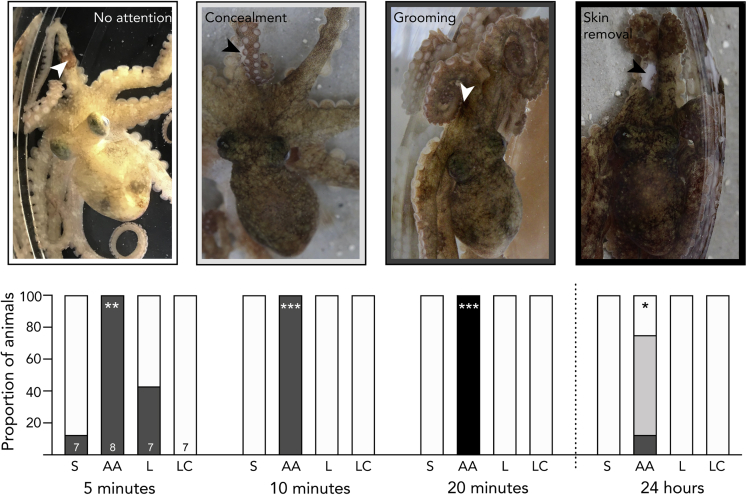
While CPP is useful for testing the affective component of pain, it does not necessarily reveal the discriminatory aspect, which includes awareness of the location, quality, and intensity of pain (Auvray et al., 2010; Treede et al., 1999). Point observations of potential pain-associated behaviors (grooming, guarding, and concealment) were made at 5-min intervals during conditioning trials (session 2) and again 24 h after conditioning trials. All octopuses injected with AA groomed the injection site with the beak for the full 20-min training trial (Figure 3A), but this behavior was either brief or completely absent in the other groups (Figure 3B). While wound-directed behavior has been reported previously in octopuses (Alupay et al., 2014) and other invertebrates (Elwood, 2011), the behaviors observed here appear to be specific to acid injection. In all animals receiving AA injection, beak grooming resulted in the removal of a small area of skin over the injection site, which was apparent at the conclusion of the 20-min conditioning trial that followed the injection. This behavior was never observed in animals receiving saline injection or after injection of lidocaine.
Figure 3.
Precise and specific wound-directed grooming behaviors show discriminative pain experience in octopus
Top panel shows examples of wound-directed behaviors, and colors surrounding each image correspond to shaded frequencies in stacked bars, below. Arrowheads indicate the location of AA injection on the arm. Behaviors were observed during training trials and 24 h later. AA-injected octopuses showed sustained wound attention and concealment that persisted for at least 24 hr after AA injection. Skin removal behavior was observed in all AA-injected animals, suggesting a specific representation of acid-induced pain that elicits a highly specific behavioral response. Bar acronyms: AA, acetic acid injection; S, saline injection; L, lidocaine injection after earlier AA injection; LC, lidocaine control (lidocaine injected after earlier saline injection). Numbers inside bars in first panel (5 min) are group sample sizes, which are the same for each subsequent interval. Asterisks indicate significant difference in proportions of animals performing each behavior, relative to saline-injected controls (fishers exact tests, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Noxious sensory information is processed in the central brain
Cephalopods are highly unusual in the degree to which higher-order sensory information processing occurs in the peripheral nervous system (Gutfreund et al., 2006; Sumbre et al., 2001). Ongoing pain in mammals is driven by sustained activity in primary nociceptors that then drives long-term changes within higher-order, central circuits (Davoody et al., 2011; King et al., 2011). Spontaneous nociceptor firing after tissue injury has also been shown in cephalopods, to date the only invertebrate taxon where this mammalian-like pattern has been recorded (Crook et al., 2013). Whether spontaneous activity in nociceptors drives ongoing excitation of central circuits in the cephalopod brain has not been clear, raising questions of if and how the central brain perceives noxious sensations in peripheral tissues.
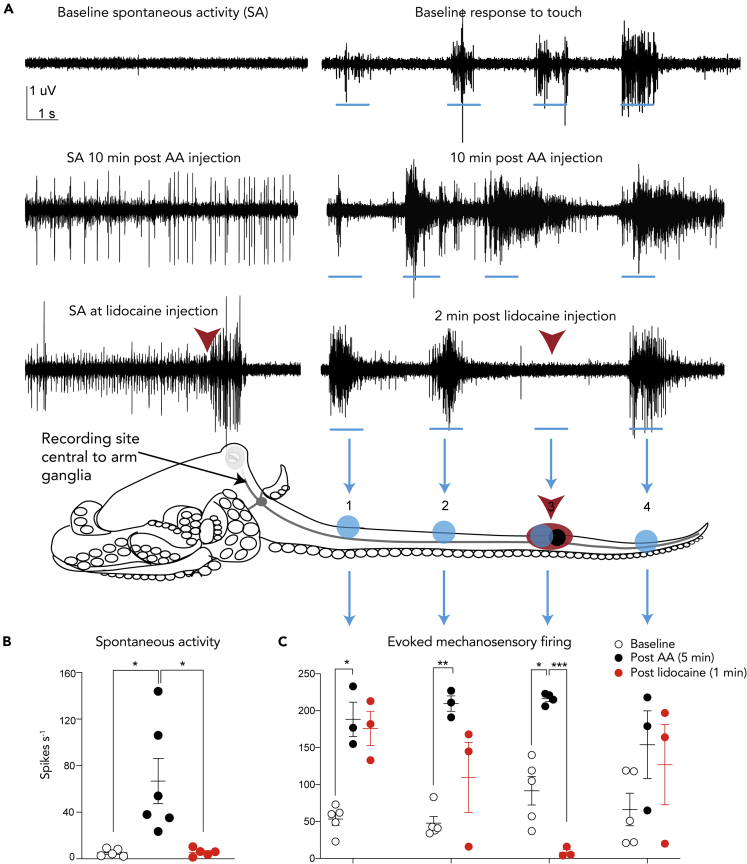
To assess what information the central brain receives about nociceptive stimuli in the arms, electrophysiological recordings were taken from the brachial connectives, which connect the arm nerve cords to the brain and are central to the major arm ganglia situated in the interbrachial commissure. In a reduced preparation where the connective were severed central to the interbrachial commissure and the Central Nervous System (CNS) removed, injection of a bolus of AA subcutaneously into one arm resulted in a prolonged period (>30 min) of ongoing activity in numerous recorded units, which was silenced rapidly by injection of 2% lidocaine overlying the site of AA injection (Figures 4A and 4B, baseline vs. post-AA injection, p = 0.046, post-AA vs. post-lidocaine, p = 0.045). This activity generated within the area of AA infiltration likely provides information to the brain about the location of the painful stimulus. Lidocaine injection into the infiltration site also reversed the sensitization of afferent activity evoked by strong mechanical stimulation at and proximal to the injection site (baseline vs. post-AA, p = 0.02, post-AA vs. post-lidocaine, p = 0.001, Figure 4C).
Figure 4.
Examples of electrophysiology recordings and summary data showing that nociceptive signal from the arms is available to the octopus CNS
(A) Examples of spontaneous (ongoing) and evoked activity in the brachial connective before and after injection of acetic acid (AA, shown as a black circle at arm stimulation position 3) and at the point where lidocaine is injected locally over the region of prior AA injection (shown as a red overlay of the black circle on the arm at position 3). Note the almost immediate cessation of ongoing activity after lidocaine injection, and the complete suppression of evoked activity in the region where lidocaine was injected at position 3 on the arm of the octopus.
(B) Ongoing, spontaneous firing in the brachial connective is increased after AA injection and blocked by injection of lidocaine into the same position on the arm.
(C) Summary data showing responses to touch on the arm at four locations (indicated by shaded blue circles on the octopus body outline). There is clear enhancement of evoked activity after injection that is suppressed by injection of a local anesthetic. Points show individual values for each subject. Central, wide bars show the mean and whiskers show S.E.M.
Asterisks above linking bars indicate significant differences between groups (paired, Holm-Bonferroni corrected t-tests, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
Together, these data provide strong support for the existence of a lasting, negative affective state in octopuses: the first evidence for pain experience in this neurologically complex invertebrate clade. Although a number of previous studies in cephalopods and other invertebrates have shown avoidance learning of a context in which a brief noxious stimulus was delivered (Budelmann and Young, 1987; Elwood and Appel, 2009; Magee and Elwood, 2013; Sanders, 1970), here, octopuses were able to learn to avoid a visually specific location that was explicitly unlinked both in time and space from the injection procedure that initiated nociceptor activation. Thus, the most plausible explanation for the strong place avoidance behavior observed here is that octopuses experience a state of ongoing (tonic) pain and negative affect after AA injection. Tonic pain has to date been demonstrated only in mammals (Davoody et al., 2011; King et al., 2009; Uhelski and Fuchs, 2010), and this study provides the first example of probable ongoing pain in any non-mammalian animal. While there is now considerable evidence for transient pain experience in crustaceans (Elwood, 2019b) and both positive and negative affective states in other invertebrates (Baracchi et al., 2017; Bateson et al., 2011; Gibson et al., 2015; Perry et al., 2016), the presence of lasting pain experience in cephalopods (and possibly other invertebrate species) as a result of tissue injury raises both significant concerns for their welfare and interesting new questions about the evolutionary origins of pain experience.
Pain experience in cephalopods has long been considered likely based on their exceptional neural and behavioral complexity, and this probability has informed various regulatory efforts aimed at protecting their welfare in research and other settings (Fiorito et al., 2015). With this study, we not only provide the first strong evidence supporting the existence of a pain state in cephalopods but also suggest that CPP may be an effective screening tool for assessing analgesic drug candidates and other welfare-promoting interventions.
In addition to affective pain experience as demonstrated by conditioned place avoidance (CPA) and CPP, spontaneous pain-associated behaviors in octopuses reveal the presence also of discriminative pain experience. The nervous system of octopuses is unusual in its high degree of distributed processing, which has led to conjecture that the arms are at least partially autonomous and the central brain does not necessarily process all information gathered by the sense organs within them (Alupay et al., 2014; Fouke and Rhodes, 2020; Gutfreund et al., 2006; Gutnick et al, 2011, 2020; Rowell, 1966; Sumbre et al., 2001). In this study, we show clearly that the central brain receives location-specific information about noxious sensation in the arms and that the central brain coordinates location-specific beak grooming behavior. The specific quality of the pain also appears to be represented in the octopus CNS. In other studies of nociception in octopus, arm compression, skin pinch, and skin incision induced prolonged beak grooming and concealment but never skin removal (Alupay et al., 2014), suggesting that AA injection produced a central representation of pain that was quite different to other injury modalities. Noxious stings, which AA injection likely approximated, are likely encountered by octopuses as they hunt venomous prey (Brooks, 1988; Ross, 1971). It is plausible that skin stripping is an injury-induced behavior that has evolved to release injected venom from the skin. This distinct behavior suggests that the octopus central brain is capable of encoding not only the location but also the specifics of pain quality. In this study, central representation of intensity—the third component of discriminative pain experience—was not tested directly; however, previous research has shown that intensity is encoded and transmitted faithfully in the peripheral nerves and central projections from the mantle of cephalopods (Crook et al., 2013; Howard et al., 2019); thus, it is likely that intensity is also an aspect of pain experience in octopus.
Reversal of pain-associated spontaneous and cognitive behaviors by analgesics is considered to be strong evidence for the presence of pain. Among invertebrates, the use of analgesics to demonstrate potential pain experience has had mixed results (Barr et al., 2008; Barr and Elwood, 2011; Groening et al., 2017; Puri and Faulkes, 2010). Analgesia-induced place preference in mammals is widely accepted to signal pain experience; however, a common criticism of such studies is that the chosen analgesic drug is innately rewarding, and its hedonic quality is sufficient to create place preference even in animals in neutral affective states (Sufka, 1994; Tzschentke, 2007). The use of lidocaine in this experiment precludes this alternative explanation; lidocaine had no central effect when injected locally and produced no place preference in octopuses who had not previously received AA injection (and thus were not in pain).
The functionality of pain experience—rather than nociception alone—in cephalopods is not yet clear. In evolutionary terms, pain has been hypothesized to be adaptive primarily among social species where injured individuals can recruit help from in-group members while ongoing pain reinforces resting and recuperative behaviors (Walters et al., 2019). Additionally, the strong negative affect produced by injury is cited as an adaptive mechanism for reinforcing contextual memory of danger that lasts throughout life. Although the octopus is often described as being “vertebrate like” in cognitive ability and intelligence, its asocial habits, short life span, and severe nutritional costs of recuperative inactivity (Wells and Clarke, 1996) argue against the prevailing evolutionary hypotheses cited for the evolution of pain in vertebrates and instead suggest a wider, more ecological perspective on the evolution of pain and affective state. Indeed, the evolution of exceptional neural complexity in cephalopods is typically attributed to their ecological association with complex habitats, niche competition with fish, and their reliance on complex camouflage and signaling behaviors (Birch et al., 2020; Hanlon and Messenger, 2018; Mather et al., 2014; O'Dor and Webber, 1986). How and why pain experience has evolved in cephalopods remains to be understood, and further, careful investigations of the molecular, genetic, and anatomical bases of pain in cephalopods will be necessary to shed light on the extraordinary parallel evolution of pain experience in this unique invertebrate clade.
Limitations of the study
Reversal of place preference by lidocaine was variable, which may have been due to incomplete or off-target lidocaine infiltration of the acetic-acid-affected region. More generally, there is ongoing debate about the relationship between sentience, consciousness, and affect that can complicate links between behavioral experimental readout and internal state. If an animal must necessarily be conscious and sentient to experience negative affect, it must therefore be necessary for octopuses to be conscious to experience pain; a controversial proposition, but once which has received considerable attention (Birch et al., 2020; Low et al., 2012; Mikhalevich and Powell, 2020; Turnbull and Bär, 2020). Even in the absence of proof on conscious awareness or sentience in cephalopods, it remains clear that the responses demonstrated by octopuses in this study are so similar to those that would be expressed by mammals experiencing pain that a reasonable, cautionary argument can be made that internal state of these disparate species is likely also similar.
Resource availability
Lead contact
Robyn J Crook, rcrook@sfsu.edu.
Material availability
Raw video electrophysiological files are available upon reasonable request from the lead contact.
Data and code availability
Data associated with each figure are available for download from Open Science Forum under the Project Name “Conditioned Place Preference reveals tonic pain in octopus”, https://osf.io/3fmuy/.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This study was supported in part by a CSUPERB New Investigator grant to R.J.C. Lisa Abbo, DVM provided feedback on the design and conception of the study, and Edgar Walters provided feedback on an initial draft of the manuscript.
Author contributions
This study was conceived, designed, conducted and written by R.J.C.
Declarations of interests
The author declared no competing interests.
Published: March 19, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2021.102229.
Supplemental information
References
- Alupay J.S., Hadjisolomou S.P., Crook R.J. Arm injury produces long-term behavioral and neural hypersensitivity in octopus. Neurosci. Lett. 2014;558:137–142. doi: 10.1016/j.neulet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Appel M., Elwood R.W. Motivational trade-offs and potential pain experience in hermit crabs. Appl. Anim. Behav. Sci. 2009;119:120–124. [Google Scholar]
- Auvray M., Myin E., Spence C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci. Biobehav. Rev. 2010;34:214–223. doi: 10.1016/j.neubiorev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Baracchi D., Lihoreau M., Giurfa M. Do insects have emotions? Some insights from bumble bees. Front. Behav. Neurosci. 2017;11:157. doi: 10.3389/fnbeh.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr S., Elwood R.W. No evidence of morphine analgesia to noxious shock in the shore crab, Carcinus maenas. Behav. Process. 2011;86:340–344. doi: 10.1016/j.beproc.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Barr S., Laming P.R., Dick J.T.A., Elwood R.W. Nociception or pain in a decapod crustacean? Anim. Behav. 2008;75:745–751. [Google Scholar]
- Bateson M., Desire S., Gartside S.E., Wright G.A. Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 2011;21:1070–1073. doi: 10.1016/j.cub.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J. Animal sentience and the precautionary principle. Anim. Sentience. 2017;2:1. [Google Scholar]
- Birch J., Schnell A.K., Clayton N.S. Dimensions of animal consciousness. Trends Cogn. Sci. 2020;24:789–801. doi: 10.1016/j.tics.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks W.R. The influence of the location and abundance of the sea anemone Calliactis tricolor (Le Sueur) in protecting hermit crabs from octopus predators. J. Exp. Mar. Bio. Ecol. 1988;116:15–21. [Google Scholar]
- Broom D.M. Cognitive ability and sentience: which aquatic animals should be protected? Dis. Aquat. Organ. 2007;75:99–108. doi: 10.3354/dao075099. [DOI] [PubMed] [Google Scholar]
- Budelmann B., Young J. Brain pathways of the brachial nerves of sepia and Loligo. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1987;315:345–352. [Google Scholar]
- Butler-Struben H.M., Brophy S.M., Johnson N.A., Crook R.J. In vivo recording of neural and behavioral correlates of anesthesia induction, reversal, and euthanasia in cephalopod molluscs. Front. Physiol. 2018;9:109. doi: 10.3389/fphys.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M., Yoo S., Watson J.D., Lee W.H., Spencer W.C., Kindt K.S., Hwang S.W., Miller D.M., Treinin M., Driscoll M., Schafer W.R. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook R.J., Hanlon R.T., Walters E.T. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J. Neurosci. 2013;33:10021–10026. doi: 10.1523/JNEUROSCI.0646-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoody L., Quiton R.L., Lucas J.M., Ji Y., Keller A., Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J. Pain. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire S.W.G. Fish lack the brains and the psychology for pain. Anim. Sentience. 2016;025:1–4. [Google Scholar]
- Elwood R.W. Assessing the potential for pain in Crustaceans and other invertebrates. In: Carere C., Mather J., editors. The Welfare of Invertebrate Animals. Springer; 2019. pp. 147–177. [Google Scholar]
- Elwood R.W. Discrimination between nociceptive reflexes and more complex responses consistent with pain in crustaceans. Philos Trans R Soc Lond B Biol Sci.. 2019;374:20190368. doi: 10.1098/rstb.2019.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood R.W. Pain and suffering in invertebrates? ILAR J. 2011;52:175–184. doi: 10.1093/ilar.52.2.175. [DOI] [PubMed] [Google Scholar]
- Elwood R.W., Appel M. Pain experience in hermit crabs? Anim. Behav. 2009;77:1243–1246. [Google Scholar]
- Fiorito G., Affuso A., Basil J., Cole A., de Girolamo P., D’Angelo L., Dickel L., Gestal C., Grasso F., Kuba M. Guidelines for the care and welfare of cephalopods in research –A consensus based on an initiative by CephRes, FELASA and the boyd group. Lab. Anim. 2015;49:1–90. doi: 10.1177/0023677215580006. [DOI] [PubMed] [Google Scholar]
- Fouke K.E., Rhodes H.J. Electrophysiological and motor responses to chemosensory stimuli in isolated cephalopod arms. Biol. Bull. 2020;238:1–11. doi: 10.1086/707837. [DOI] [PubMed] [Google Scholar]
- Gibson W.T., Gonzalez C.R., Fernandez C., Ramasamy L., Tabachnik T., Du R.R., Felsen P.D., Maire M.R., Perona P., Anderson D.J. Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr Biol. 2015;25:1401–1405. doi: 10.1016/j.cub.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groening J., Venini D., Srinivasan M.V. In search of evidence for the experience of pain in honeybees: a self-Administration study. Sci. Rep. 2017;7:45825. doi: 10.1038/srep45825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund Y., Matzner H., Flash T., Hochner B. Patterns of motor activity in the isolated nerve cord of the octopus arm. Biol. Bull. 2006;211:212–222. doi: 10.2307/4134544. [DOI] [PubMed] [Google Scholar]
- Gutnick T., Byrne R.A., Hochner B., Kuba M. Octopus vulgaris uses visual information to determine the location of its arm. Curr. Biol. 2011;21:460–462. doi: 10.1016/j.cub.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Gutnick T., Zullo L., Hochner B., Kuba M.J. Use of peripheral sensory information for central nervous control of arm movement by Octopus vulgaris. Curr. Biol. 2020;30:4322–4327.e3. doi: 10.1016/j.cub.2020.08.037. [DOI] [PubMed] [Google Scholar]
- Hanlon R., Messenger J. Cambridge University Press; 2018. Cephalopod Behaviour. [Google Scholar]
- Hochner B., Shomrat T., Fiorito G. The octopus: a model for a comparative analysis of the evolution of learning and memory mechanisms. Biol. Bull. 2006;210:308–317. doi: 10.2307/4134567. [DOI] [PubMed] [Google Scholar]
- Hooper S.L. Operant learning: Octopus arms need brains to learn their way. Curr. Biol. 2020;30:R1301–R1304. doi: 10.1016/j.cub.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Howard R.B., Lopes L.N., Lardie C.R., Perez P.P., Crook R.J. Early-life injury produces lifelong neural hyperexcitability, cognitive deficit and altered defensive behaviour in the squid Euprymna scolopes. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20190281. doi: 10.1098/rstb.2019.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illich P.A., Walters E.T. Mechanosensory neurons innervating Aplysia siphon encode noxious stimuli and display nociceptive sensitization. J. Neurosci. 1997;17:459–469. doi: 10.1523/JNEUROSCI.17-01-00459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M. Evolutionary and comparative aspects of nociception. Brain Res. Bull. 1988;21:923–931. doi: 10.1016/0361-9230(88)90030-5. [DOI] [PubMed] [Google Scholar]
- Key B. Why fish do not feel pain. Anim. Sentience. 2016;3:1–33. [Google Scholar]
- Key B., Brown D. Designing brains for pain: human to mollusc. Front. Physiol. 2018;9:1027. doi: 10.3389/fphys.2018.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B.J., Marino L. Octopus minds must lead to octopus ethics. Anim. Sentience. 2019;263:1–4. [Google Scholar]
- King T., Qu C., Okun A., Mercado R., Ren J., Brion T., Lai J., Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T., Vera-Portocarrero L., Gutierrez T., Vanderah T.W., Dussor G., Lai J., Fields H.L., Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P., Panksepp J., Reiss D., Edelman D., Van Swinderen B., Koch C. The cambridge declaration on consciousness. Fr. Crick Meml. Conf. Conscious. Hum. Non Human Anim. 2012:1–2. [Google Scholar]
- Magee B., Elwood R.W. Shock avoidance by discrimination learning in the shore crab (Carcinus maenas) is consistent with a key criterion for pain. J. Exp. Biol. 2013;216:353–358. doi: 10.1242/jeb.072041. [DOI] [PubMed] [Google Scholar]
- Mather J.A., Leite T.S., Anderson R.C., Wood J.B. Foraging and cognitive competence in octopuses. In: Mather J., editor. Cephalopod Cognition. Cambridge University Press; 2014. pp. 125–149. [Google Scholar]
- Mikhalevich I., Powell R. Minds without spines: evolutionarily inclusive animal ethics. Anim. Sentience. 2020;329:1–26. [Google Scholar]
- Mogil J.S. The translatability of pain across species. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20190286. doi: 10.1098/rstb.2019.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E., Xie J.Y., King T., Porreca F. Evaluation of reward from pain relief. Ann. N. Y. Acad. Sci. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dor R.K., Webber D.M. The constraints on cephalopods: why squid aren’t fish. Can. J. Zool. 1986;64:1591–1605. [Google Scholar]
- Perry C.J., Baciadonna L., Chittka L. Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumblebees. Science. 2016;353:1529–1531. doi: 10.1126/science.aaf4454. [DOI] [PubMed] [Google Scholar]
- Ponte G., Andrews P., Galligioni V., Pereira J., Fiorito G. Springer; 2019. Cephalopod Welfare, Biological and Regulatory Aspects: An EU Experience; pp. 209–228. [Google Scholar]
- Puri S., Faulkes Z. Do decapod crustaceans have nociceptors for extreme pH? PLoS One. 2010;5:e10244. doi: 10.1371/journal.pone.0010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.D. Pain in fish: weighing the evidence. Anim. Sentience. 2016;032:1–3. [Google Scholar]
- Ross D.M. Protection of hermit crabs (Dardanus spp.) from octopus by commensal sea anemones (Calliactis spp.) Nature. 1971;230:401–402. doi: 10.1038/230401a0. [DOI] [PubMed] [Google Scholar]
- Roughan J.V., Coulter C.A., Flecknell P.A., Thomas H.D., Sufka K.J. The conditioned place preference test for assessing welfare consequences and potential refinements in a mouse bladder cancer model. PLoS One. 2014;9:e103362. doi: 10.1371/journal.pone.0103362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell C.H.F. Activity of interneurones in the arm of Octopus in response to tactile stimulation. J. Exp. Biol. 1966;44:589–605. doi: 10.1242/jeb.44.3.589. [DOI] [PubMed] [Google Scholar]
- Sanders G.D. Long-term memory of a tactile discrimination in Octopus vulgaris and the effect of vertical lobe removal. Brain Res. 1970;20:59–73. doi: 10.1016/0006-8993(70)90154-x. [DOI] [PubMed] [Google Scholar]
- Schnell A.K., Amodio P., Boeckle M., Clayton N.S. How intelligent is a cephalopod? Lessons from comparative cognition. Biol. Rev. 2020 doi: 10.1111/brv.12651. [DOI] [PubMed] [Google Scholar]
- Sneddon L.U. Evolution of nociception and pain: evidence from fish models. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20190290. doi: 10.1098/rstb.2019.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sømme L.S. Sentience and pain in invertebrates. Rep. Nor. Sci. Comm. Food Safety. Nor. Univ. Life Sci. 2005 https://www.semanticscholar.org/paper/SENTIENCE-AND-PAIN-IN-INVERTEBRATES-Report-to-for-Somme/6b16c458c4eec3cc163af5f68835ceea1a0f7a10 [Google Scholar]
- Sufka K.J. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Sumbre G., Gutfreund Y., Fiorito G., Flash T., Hochner B. Control of Octopus arm extension by a peripheral motor program. Science. 2001;5536:1845–1848. doi: 10.1126/science.1060976. [DOI] [PubMed] [Google Scholar]
- Tracey W.D. Nociception. Curr. Biol. 2017;27:R129–R133. doi: 10.1016/j.cub.2017.01.037. [DOI] [PubMed] [Google Scholar]
- Treede R.D., Kenshalo D.R., Gracely R.H., Jones A.K.P. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Turnbull O.H., Bär A. Animal minds: the case for emotion, based on neuroscience. Neuropsychoanalysis. 2020:1–20. [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Uhelski M.L., Fuchs P.N. Maternal separation stress leads to enhanced emotional responses to noxious stimuli in adult rats. Behav. Brain Res. 2010;212:208–212. doi: 10.1016/j.bbr.2010.03.055. [DOI] [PubMed] [Google Scholar]
- Walters E. Defining pain and painful sentience in animals. Anim. Sentience. 2018;21 [Google Scholar]
- Walters E.T., de Williams A.C.C., De C Williams A.C. Evolution of mechanisms and behaviour important for pain. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20190275. doi: 10.1098/rstb.2019.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M.J., Clarke A. Energetics: the costs of living and reproducing for an individual cephalopod. Philos. Trans. R. Soc. B Biol. Sci. 1996;351:1083–1104. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with each figure are available for download from Open Science Forum under the Project Name “Conditioned Place Preference reveals tonic pain in octopus”, https://osf.io/3fmuy/.