Abstract
Objective
To determine the effectiveness of telemedicine in the delivery of diabetes care in low- and middle-income countries.
Methods
We searched seven databases up to July 2020 for randomized controlled trials investigating the effectiveness of telemedicine in the delivery of diabetes care in low- and middle-income countries. We extracted data on the study characteristics, primary end-points and effect sizes of outcomes. Using random effects analyses, we ran a series of meta-analyses for both biochemical outcomes and related patient properties.
Findings
We included 31 interventions in our meta-analysis. We observed significant standardized mean differences of −0.38 for glycated haemoglobin (95% confidence interval, CI: −0.52 to −0.23; I2 = 86.70%), −0.20 for fasting blood sugar (95% CI: −0.32 to −0.08; I2 = 64.28%), 0.81 for adherence to treatment (95% CI: 0.19 to 1.42; I2 = 93.75%), 0.55 for diabetes knowledge (95% CI: −0.10 to 1.20; I2 = 92.65%) and 1.68 for self-efficacy (95% CI: 1.06 to 2.30; I2 = 97.15%). We observed no significant treatment effects for other outcomes, with standardized mean differences of −0.04 for body mass index (95% CI: −0.13 to 0.05; I2 = 35.94%), −0.06 for total cholesterol (95% CI: −0.16 to 0.04; I2 = 59.93%) and −0.02 for triglycerides (95% CI: −0.12 to 0.09; I2 = 0%). Interventions via telephone and short message service yielded the highest treatment effects compared with services based on telemetry and smartphone applications.
Conclusion
Although we determined that telemedicine is effective in improving several diabetes-related outcomes, the certainty of evidence was very low due to substantial heterogeneity and risk of bias.
Résumé
Objectif
Mesurer l'efficacité de la télémédecine dans la prise en charge du diabète au sein des pays à faible et moyen revenu.
Méthodes
Nous avons examiné sept bases de données, la plus récente datant de juillet 2020, en quête d'essais cliniques randomisés s'intéressant à l'efficacité de la télémédecine dans les soins prodigués aux patients diabétiques résidant dans des pays à faible et moyen revenu. Nous y avons trouvé des informations sur les caractéristiques de l'étude, les principaux critères d'évaluation et l'ampleur des effets pour les résultats obtenus. Grâce à des modèles à effets aléatoires, nous avons mené une série de méta-analyses sur les résultats biochimiques et les propriétés des patients concernés.
Résultats
Notre méta-analyse a tenu compte de 31 interventions. Nous avons observé d'importantes différences moyennes standardisées: −0,38 pour l'hémoglobine glyquée (intervalle de confiance de 95%, IC: −0,52 à −0,23; I2 = 86,70%), −0,20 pour la glycémie à jeun (IC de 95%: −0,32 à −0,08; I2 = 64,28%), 0,81 pour l'adhésion au traitement (IC de 95%: 0,19 à 1,42; I2 = 93,75%), 0,55 pour la connaissance de la maladie (IC de 95%: 0,10 à 1,20; I2 = 92,65%) et 1,68 pour l'auto-efficacité (IC de 95%: 1,06 à 2,30; I2 = 97,15%). Nous n'avons constaté aucune répercussion significative du traitement sur d'autres résultats, avec des différences moyennes standardisées de −0,04 pour l'indice de masse corporelle (IC de 95%: −0,13 à 0,05; I2 = 35,94%), de −0,06 pour le cholestérol total (IC de 95%: −0,16 à 0,04; I2 = 59,93%) et de −0,02 pour les triglycérides (IC de 95%: −0,12 à 0,09; I2 = 0%). Ce sont les interventions par téléphone et par SMS qui ont eu le plus d'impact sur le traitement par rapport aux services recourant à la télémétrie et aux applications sur smartphone.
Conclusion
Bien que nous ayons établi l'efficacité de la télémédecine dans l'amélioration de nombreux résultats liés au diabète, le degré de certitude s'est révélé très bas en raison d'une grande hétérogénéité et du risque de biais.
Resumen
Objetivo
Determinar la eficacia de la telemedicina en la prestación de servicios de atención sanitaria de la diabetes en los países con ingresos bajos y pocos medios.
Métodos
Se realizaron búsquedas en siete bases de datos hasta julio de 2020 para encontrar ensayos controlados aleatorios que investigaran la eficacia de la telemedicina en la prestación de servicios de atención de la diabetes en países con ingresos bajos y pocos medios. Se extrajeron datos sobre las características del estudio, las principales variables de evaluación y los tamaños del efecto de los resultados. Utilizando el análisis de los efectos aleatorios, realizamos una serie de metaanálisis tanto para los resultados bioquímicos como para las propiedades relacionadas con los pacientes.
Resultados
Incluimos 31 intervenciones en nuestro metaanálisis. Se observaron diferencias medias estandarizadas significativas de -0,38 para la hemoglobina glicosilada (intervalo de confianza del 95%, IC: -0,52 a -0,23; I2 = 86,70%), -0,20 para la glucemia en ayunas (IC del 95%: -0,32 a -0,08; I2 = 64,28%), 0,81 para el cumplimiento del tratamiento (IC del 95%: 0,19 a 1,42; I2 = 93,75%), 0,55 para el conocimiento de la diabetes (IC del 95%: 0,10 a 1,20; I2 = 92,65%) y 1,68 para la autoeficacia (IC del 95%: 1,06 a 2,30; I2 = 97,15%). No se observaron efectos significativos del tratamiento para otros resultados, con diferencias medias estandarizadas de -0,04 para el índice de masa corporal (IC del 95%: -0,13 a 0,05; I2 = 35,94%), -0,06 para el colesterol total (IC del 95%: -0,16 a 0,04; I2 = 59,93%) y -0,02 para los triglicéridos (IC del 95%: -0,12 a 0,09; I2 = 0%). Las intervenciones telefónicas y el servicio de mensajes cortos de texto produjeron los efectos de tratamiento más altos en comparación con los servicios basados en la telemetría y las aplicaciones de los teléfonos inteligentes.
Conclusión
Aunque se determinó que la telemedicina es eficaz para mejorar varios resultados relacionados con la diabetes, la certeza de las pruebas fue muy baja debido a la considerable heterogeneidad y el riesgo de sesgo.
ملخص
الغرض تحديد مدى فعالية العلاج الطبي عن بعد في تقديم الرعاية لمرض السكري في البلدان منخفضة الدخل والبلدان متوسطة الدخل.
الطريقة قمنا بالبحث في سبع قواعد للبيانات حتى يوليو/تموز 2020، عن تجارب معشاة مضبطة بالشواهد للتحقق من فعالية العلاج الطبي عن بعد في تقديم الرعاية لمرض السكري في البلدان منخفضة الدخل والبلدان متوسطة الدخل. واستخلصنا البيانات حول خصائص الدراسة، ونقاط النهاية الأولية، وأحجام تأثير النتائج. وباستخدام تحليلات التأثيرات العشوائية، قمنا بإجراء سلسلة من التحليلات التلوية لكل من النتائج الحيوية الكيميائية، والخصائص المتعلقة بالمريض.
النتائج قمنا بتضمين 31 تدخلاً في تحليلنا التلوي. لاحظنا متوسطات فروق معيارية ملموسة بقيمة -0.38 في الهيموجلوبين السكري (فاصل الثقة 95%: -0.52 إلى -0.23؛ / 2 )، -0.20 لسكر الدم أثناء الصيام (فاصل الثقة 95%: -0.32 إلى -0.08؛ / 2 )، 0.81 للتمسك بالعلاج (فاصل الثقة 95%: 0.19 إلى 1.42؛ / 2 )، 0.55 للإلمام بمرض السكري (فاصل الثقة 95%: 0.10 إلى 1.20؛ / 2 )، و1.68 للفعالية الذاتية (فاصل الثقة 95%: 1.06 إلى 2.30؛ / 2 = 97.15%). لم نلحظ أي آثار ملموسة للعلاج بالنسبة للنتائج الأخرى، مع متوسطات فروق معيارية قدرها -0.04 لمؤشر كتلة الجسم (فاصل ثقة 95%: -0.13 إلى 0.05؛ / 2 = 35.94%)، -0.06 للكوليسترول الكلي (فاصل الثقة 95%: -0.16 إلى 0.04؛ / 2 = 59.93%)، -0.02 للدهون الثلاثية (فاصل الثقة 95%: -0.12 إلى 0.09؛ / 2 = 0%). حققت التدخلات عبر الهاتف وخدمة الرسائل القصيرة أعلى تأثيرات للعلاج مقارنة بالخدمات القائمة على تطبيقات القياس عن بعد والهواتف الذكية.
الاستنتاج على الرغم من أننا قد قررنا أن العلاج الطبي عن بعد فعال في تحسين العديد من النتائج المتعلقة بمرض السكري، إلا أن الثقة في الأدلة كانت منخفضة للغاية بسبب عدم التجانس الموضوعي وخطر التحيز.
摘要
目的
探讨远程医疗在中低收入国家糖尿病护理方面的应用效果。
方法
截至 2020 年 7 月,我们检索了 7 个数据库,开展随机对照试验以研究远程医疗在中低收入国家糖尿病护理方面的应用效果。我们提取了与研究特征、主要终点和结果效应值有关的数据。通过使用随机效应分析,我们针对生化结果和相关患者属性进行了一系列荟萃分析。
结果
我们将 31 项干预措施纳入了荟萃分析。我们观察到显著的标准化均数差,具体地,糖化血红蛋白的标准化均数差为0.38(95% 置信区间,CI:0.52 至 0.23;I2 = 86.70%),空腹血糖的标准化均数差为0.20 (95% 置信区间:0.32 至 0.08;I2= 64.28%),坚持治疗率的标准化均数差为 0.81(95% 置信区间:0.19 至 1.42;I2 = 93.75%),糖尿病知识普及的标准化均数差为 0.55(95% 置信区间:0.10 至 1.20;I2 = 92.65%)且自我效能感的标准化均数差为 1.68(95% 置信区间:1.06 至 2.30;I2 = 97.15%)。根据我们的观察,其他结果没有表现出明显的治疗效果,具体地,身体质量指数的标准化均数差为0.04(95% 置信区间:0.13 至 0.05;I2 = 35.94%),总胆固醇的标准化均数差为0.06(95% 置信区间:0.16 至 0.04;I2 = 59.93%)且甘油三酯的标准化均数差为0.02(95% 置信区间:0.12 至 0.09;I2= 0%)。与基于遥测技术和智能手机应用的服务相比,通过电话和短信服务进行干预所取得的治疗效果最佳。
结论
虽然我们确定远程医疗可以有效地改善一些糖尿病引起的症状,但由于存在严重的异质性和偏差风险,证据确定性非常低。
Резюме
Цель
Определить эффективность телемедицины для оказания медицинской помощи при диабете в странах с низким и средним уровнем доходов.
Методы
Авторы провели поиск по семи базам данных до июля 2020 года на предмет рандомизированных контролируемых исследований, посвященных эффективности телемедицины как способа оказания помощи при диабете в странах с низким и средним уровнем доходов. Были извлечены данные о характеристиках исследования, основных конечных точках и величинах эффекта в результатах. Используя анализ случайных эффектов, авторы выполнили серию метаанализов как биохимических результатов, так и связанных с ними свойств пациентов.
Результаты
В метаанализ был включен 31 случай вмешательства. Авторы наблюдали значительную стандартизованную разницу средних значений, составившую -0,38 для гликированного гемоглобина (95%-й ДИ: -0,52–-0,23; I2 = 86,70%), -0,20 для уровня сахара крови натощак (95%-й ДИ: -0,32–-0,08; I2 = 64,28%), 0,81 для соблюдения режима лечения (95%-й ДИ: 0,19–1,42; I2 = 93,75%), 0,55 для осведомленности о диабете (95%-й ДИ: 0,10–1,20; I2 = 92,65%) и 1,68 для уверенности в собственных силах (95%-й ДИ: 1,06–2,30; I2 = 97,15%). Не наблюдалось значительных эффектов лечения для других результатов со стандартизованной разностью средних значений: -0,04 для индекса массы тела (95%-й ДИ: -0,13–0,05; I2 = 35,94%), -0,06 для общего холестерина (95%-й ДИ: -0,16–0,04; I2 = 59,93%) и -0,02 для триглицерида (95%-й ДИ: -0,12–0,09; I2 = 0%). Вмешательства по телефону и посредством службы коротких сообщений дали наивысший лечебный эффект по сравнению с услугами, основанными на телеметрии и приложениях для смартфонов.
Вывод
Хотя было выявлено, что телемедицина эффективна для улучшения некоторых результатов, связанных с диабетом, достоверность доказательств была очень низкой из-за значительной неоднородности и риска систематической ошибки.
Introduction
Diabetes mellitus is one of the most prevalent chronic and preventable conditions affecting over 415 million people globally, and accounted for over 5 million deaths in 2015.1 Because the symptoms of diabetes affect both the micro- and macrovascular systems, the disease is associated with significant morbidity, mortality and a poor quality of life.2,3 By 2030, the estimated annual medical and related costs of both type 1 and type 2 diabetes in the United States of America alone will reach a staggering 622 billion United States dollars (US$).4 Over 75% of patients with diabetes live in low- and middle-income countries,1 where most patients obtain diabetes treatment only after making out-of-pocket payments. A study reported the costs of diabetes treatment as US$ 7 per visit for an outpatient, US$ 290 per year for an inpatient, and US$ 25 and US$ 177 per patient per year for laboratory and medication costs, respectively.5 In sub-Saharan Africa, recent studies estimate the financial burden related to diabetes as US$ 19.5 billion, about 1.2% of the cumulative regional gross domestic product.6
The prevalence of diabetes in low- and middle-income countries has risen faster than in high-income countries, with the highest rise observed in the Eastern Mediterranean Region. The prevalence of diabetes is highest in low- and middle-income countries (12.3%) followed by upper-middle-income countries (11.1%), and lowest in high-income countries (6.6%).7 This high prevalence in low- and middle-income countries, coupled with a lack of both quality health-care services and equity in health care, means that the long-term management of diabetes is a major global challenge.1,8 It is often opined that these inequalities in diabetes care, as well as inequities in health-care services and slow progress towards achievement of universal health coverage, could be addressed by employing telemedicine-based interventions.
Telemedicine is the practice by which telecommunication and information technology are used to provide clinical health care to distant patients.9 In a broader context, digital health interventions can help stakeholders to overcome several health systems challenges. These challenges include an insufficient supply of commodities, poor adherence to guidelines by health-care professionals, poor adherence to treatment by patients, a lack of access to information or data, and a loss of patients to follow-up. Combined with decision support systems, telemedicine can help by providing protocol checklists, providing prompts and alerts as per protocols, enhancing communication between health-care providers and patients, and compiling the schedules of health-care providers. Telemedicine can also aid in routine health-care data collection by increasing the use of electronic medical records and health management information systems.10
Several telemedicine interventions targeting diabetes have been implemented in low- and middle-income countries;11–18 however, evidence synthesis efforts in such settings are scarce. Although studies to evaluate the clinical efficacy or cost–effectiveness of telemedicine interventions for diabetes have previously been conducted,9,11–19 these reviews were performed over a global context and were dominated by evidence from high-income countries (mostly the USA). Such previous syntheses have little relevance in determining the effectiveness of telemedicine for diabetes treatment in low- and middle-income countries.
In this systematic review and meta-analysis, we aim to address the lack of evidence synthesis efforts in telemedicine for diabetes care in low- and middle-income countries. We designed our study to: (i) estimate the effectiveness of telemedicine in improving biochemical outcomes and patient characteristics such as adherence to treatment and self-efficacy; (ii) evaluate the implementation processes involved in telemedicine interventions; and (iii) determine the certainty of evidence for telemedicine-based interventions for diabetes care in low- and middle-income countries.
Methods
Database search
We conducted this systematic review and meta-analysis as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (data repository).20,21 We registered the review protocol before at the PROSPERO database for systematic reviews (CRD42019141271).22 Using a pre-tested search strategy (data repository),21 we searched Web of Science, PubMed®, MEDLINE®, Global Health Library and Cochrane Central Register of Controlled Trials from their inception to August 2019. We also searched New York Academy of Medicine and POPLINE databases for grey literature. We applied no restrictions in terms of participant age, year of publication or region of study at this stage.
We conducted an updated database search up to July 2020. We augmented the previously used search strategy with the keywords health informatics, wireless devices, text messaging, clinical decision support system, mobile app, blood glucose, diabetes mellitus type 2 and T2DM (Box 1; available at: http://www.who.int/bulletin/volumes/99/3/19-250068).
Box 1. PubMed search strings used in systematic review and meta-analysis of the effectiveness of telemedicine in the delivery of diabetes care, low- and middle-income countries, 2010–2020.
• Telemedicine: ((Telemedicine[Title/Abstract]) OR (“health informatics”[Title/Abstract]) OR (“wireless devices”[Title/Abstract]) OR (“text messag*”[Title/Abstract]) OR (“clinical decision support system”[Title/Abstract]) OR (“mobile app”[Title/Abstract]) OR (tele-health[Title/Abstract]) OR (telerehabilitation[Title/Abstract]) OR (telecommunication[Title/Abstract]) OR (“remote consultation”[Title/Abstract]) OR (“mobile health”[Title/Abstract]) OR (mHealth[Title/Abstract]) OR (eHealth[Title/Abstract]))
• Diabetes: ((Diabetes[Title]) OR (“blood glucose”[Title]) OR (“Diabetes mellitus type 2”[Title]) OR (T2DM[Title]) OR (“Diabetes mellitus”[Title]))
• Trial: ((RCT[Title/Abstract]) OR (trial*[Title/Abstract]) OR (“controlled trial”[Title/Abstract]) OR (“randomized controlled”[Title/Abstract]) OR (“cluster randomized controlled trial”[Title/Abstract]))
• Country: (“Middle income country” OR “Middle income countries” OR “low income countries” OR “low income country” OR LMIC OR “developing world” OR “developing country” OR “developing countries” OR Afghanistan OR “Kyrgyz Republic” OR Bangladesh OR Liberia OR Benin OR Madagascar OR “Burkina Faso” OR Malawi OR Burundi OR Mali OR Cambodia OR Mauritania OR “Central African Republic” OR Mozambique OR Chad OR Myanmar OR Comoros OR Nepal OR Congo OR Niger OR Eritrea OR Rwanda OR Ethiopia OR “Sierra Leone” OR Gambia OR Somalia OR Guinea OR Tajikistan OR Guinea-Bissau OR United Republic of Tanzania OR Haiti OR Togo OR Kenya OR Uganda OR Korea OR Zimbabwe OR Algeria OR Libyan Arab Jamahirya OR American-Samoa OR Lithuania OR Angola OR The former Yugoslav Republic of Macedonia OR Antigua OR Malaysia OR Argentina OR Maldives OR Azerbaijan OR Mauritius OR Belarus OR Mexico OR Bosnia OR Montenegro OR Botswana OR Namibia OR Brazil OR Palau OR Bulgaria OR Panama OR Chile OR Peru OR China OR Romania OR Colombia OR “Russian Federation” OR “Costa Rica” OR Serbia OR Cuba OR Seychelles OR Dominica OR “South Africa” OR “Dominican Republic” OR “St Lucia” OR Ecuador OR “St Vincent” OR “The Grenadines” OR Gabon OR Suriname OR Grenada OR Thailand OR “Iran Islamic Republic” OR Islamic Republic of Iran OR Tunisia OR Jamaica OR Turkey OR Jordan OR Turkmenistan OR Kazakhstan OR Tuvalu OR Latvia OR Uruguay OR Lebanon OR Venezuela OR Albania OR the Republic of Moldova OR Armenia OR Mongolia OR Belize OR Morocco OR Bhutan OR Nicaragua OR Bolivia OR Nigeria OR Cameroon OR Pakistan OR “Cape Verde” OR “Papua New Guinea” OR “Congo Republic” OR Paraguay OR “Cote d Ivoire” OR Philippines OR Djibouti OR Samoa OR “Egypt Arab Republic” OR Egypt OR “Sao Tome” OR “El Salvador” OR Senegal OR Fiji OR “Solomon Islands” OR Georgia OR “South Sudan” OR Ghana OR “Sri Lanka” OR Guatemala OR Sudan OR Guyana OR Swaziland OR Honduras OR “Syrian Arab Republic” OR India OR “Timor Leste” OR Indonesia OR Tonga OR Iraq OR Ukraine OR Kiribati OR Uzbekistan OR Kosovo OR Vanuatu OR “Lao PDR” OR Viet Nam OR Lesotho OR “West Bank” OR Gaza OR “Marshall Islands” OR “Yemen Republic” OR Federated States of Micronesia OR Zambia)
Note: Country names are reported here as in the authors’ search string, and have not been edited as per the naming standards of the World Health Organization. Quotation marks are required for multiword search items.
Inclusion and exclusion criteria
We included telemedicine interventions encompassing various modes of delivery including, but not limited to, short message service (SMS), smartphone applications (apps), telemetry (devices that allow remote monitoring of health data by automatic transmission from patients to clinicians), telephone and web-based systems. We only included randomized controlled trials (RCTs) and cluster RCTs that tested the effectiveness of telemedicine-based interventions in type 1, type 2 and gestational diabetes. We included studies conducted among participants aged ≥ 18 years resident in low- and middle-income countries. We considered all studies that reported serum glycated haemoglobin (HbA1c) levels as either their primary or secondary outcomes; however, to formulate a clinical recommendation for telemedicine interventions, we considered HbA1c levels as a primary outcome in our review.
We excluded studies employing semi-experimental designs, such as pre–post studies or those lacking control groups. In the case where two separate studies were based on an overlapping data set, we only included the study with the most complete information. We excluded short papers, brief reports, abstracts, conference papers, posters and letters to editors because these types of publications often lack important quantitative information. We also excluded studies published in languages other than English because of our lack of translational resources.
Outcome choice
We included biochemical parameters such as body mass index (BMI) and serum levels of fasting blood sugar, HbA1c, total cholesterol and triglycerides. We also included non-biochemical characteristics such as adherence to treatment, knowledge of diabetes and self-efficacy. However, our primary outcome was HbA1c levels reported post-intervention.
Screening process
Two independent reviewers performed the screening process to retrieve bibliographic records of eligible studies in two phases. First, the reviewers screened the titles and abstracts of all bibliographic records against the inclusion and exclusion criteria. Second, the reviewers thoroughly read the full texts of these eligible titles to ensure that all inclusion and exclusion criteria were met. Studies judged to be eligible at this stage were then included in the qualitative and quantitative synthesis where applicable. In the case of any uncertainty or difference in opinion between the reviewers, the reviewers and first author reached a final decision through discussion.
Data extraction
We extracted data related to the characteristics of the studies, primary end-points and effect sizes of outcomes. We also compiled data on intervention implementation processes, such as mode of delivery, theoretical orientation, rationale, materials, and development and training procedures. We closely examined the individual elements of the interventions according to the World Health Organization guidelines on digital interventions.10 We assessed the risk of bias in the RCTs using the Cochrane tool against randomization, allocation concealment, blinding of outcome assessors, attrition rate, selective reporting and any other bias matrices (e.g. a priori protocol registration and statistical power).23 It was not possible to assess the rigour of the blinding procedures of participants and personnel in this review because of the nature of telemedicine-based interventions. We assessed certainty of evidence for telemedicine-based interventions using the grading of recommendations, assessment, development and evaluations (GRADE) guidelines.24 We conducted the grading of evidence only for the HbA1c end-point as this was our primary outcome. We downgraded evidence by 1 or 2 points according to the presence and extent of flaws such as bias, inconsistency, publication bias, imprecision or indirectness related to patients, outcomes and interventions.24
For quantitative synthesis, we assessed the mean and the standard deviation (SD) of outcomes for both the intervention and active control groups.25 We used categorical data, such as frequency of events and sample size, for outcomes that lacked quantitative data in the form of mean and SD.25 We then used these raw data to calculate standardized mean differences and their SDs for each outcome reported in the included studies. Because of the expected methodological and statistical heterogeneity, we calculated pooled effect sizes by performing random effects analyses.25 We present these pooled effect sizes as forest plots depicting standardized mean differences and 95% confidence intervals (CIs). We ran a sensitivity analysis using the single-study knockout approach to assess the contribution of each study to the pooled effect size. We assessed publication bias by creating Begg funnel plots and performing Egger regression analyses (considered significant at P ≤ 0.1).25 For outcomes demonstrating significant publication bias, we calculated adjusted standardized mean differences using the Duval and Tweedie trim-and-fill method.25 For each outcome, we performed a series of subgroup analyses to quantify the specific difference in effect sizes for each mode of delivery.26
Results
Intervention characteristics
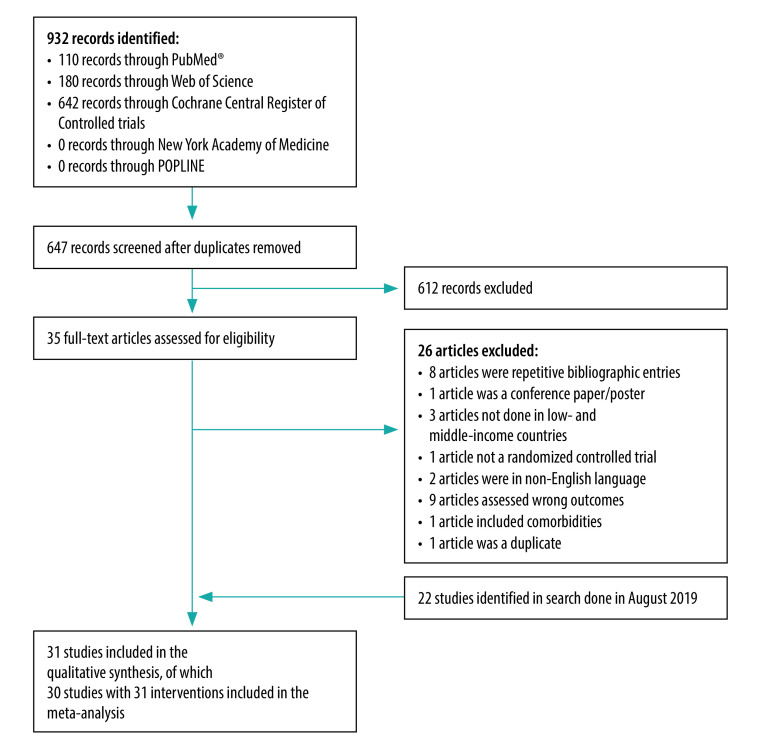
Out of 376 studies retrieved in our initial database search, we included a total of 22 studies describing 23 interventions in the qualitative analysis, and 21 studies describing 22 interventions in the quantitative synthesis (data repository).21 In our updated database search, we retrieved 647 non-duplicate bibliographic records; we included 31 of these studies describing 32 interventions (an increase of nine compared with our initial search) in our synthesis (Fig. 1). All included studies were published between 2010 and 2020. A breakdown of the studies by country revealed that the highest number of studies were conducted in China (eight studies), followed by India (five studies), the Islamic Republic of Iran (five studies), Malaysia (three studies), Turkey (two studies) and South Africa (two studies). A single RCT was conducted in each of Brazil, Egypt, Mexico, Mongolia and Pakistan. One of the studies described an intervention conducted at several sites in Cambodia, Congo and the Philippines. The minimum sample size of the RCTs ranged from 6015,27,28 to 3324.14 We list the properties of the 31 studies in Table 1, and the values for the outcomes considered that were extracted from the studies in the data repository.21
Fig. 1.
Flowchart of study selection process in the systematic review and meta-analysis of the effectiveness of telemedicine in the delivery of diabetes care, low- and middle-income countries, July 2020
Notes: The database Web of Science includes MEDLINE®. We did not include the Global Health Library, because it had been discontinued at the time of the search. The flowchart for the search done in August 2019 is available in the data repository.21
Table 1. Characteristics of included studies in the systematic review and meta-analysis of the effectiveness of telemedicine in the delivery of diabetes care, low- and middle-income countries, 2010–2020.
| Author, year | Country | Primary end-point for outcome | Mean age (years) | Mode of delivery of intervention | Outcomes analysed |
|---|---|---|---|---|---|
| Nesari et al., 201029 | Iran (Islamic Republic of) | 12 weeks | 51.9 | Telephone follow-ups | Adherence to treatment, HbA1c |
| Shetty et al., 201130 | India | 1 year | 50.1 | SMS reminders | BMI, HbA1c, total cholesterol, triglycerides |
| Zhou et al., 201431 | China | 3 months | NR | Web-based diabetes telemedicine system | BMI, fasting blood sugar, HbA1c, total cholesterol, triglycerides |
| Shahid et al., 201518 | Pakistan | 4 months | 48.95 | Telephone follow-ups | BMI, HbA1c |
| Anzaldo-Campos et al., 201632 | Mexico | 4 months | 51.5 | SMS, educational videos and online brochures | BMI, diabetes knowledge, HbA1c, total cholesterol, triglycerides |
| Aytekin Kanadli et al., 201633 | Turkey | 3 months | NR | Telephone follow-ups | BMI, HbA1c, self-efficacy, total cholesterol, triglycerides |
| Gopalan et al., 201634 | South Africa | NR | NR | Educational emails | Qualitatively analysed dietary-consumption-related outcomes |
| Kim et al., 201635 | China | 3 months | NR | Internet-based glucose monitoring system | BMI, fasting blood sugar, HbA1c, total cholesterol, triglycerides |
| Peimani et al., 201613 | Iran (Islamic Republic of) | 3 months | 49.78 | Educational SMS and telephone follow-ups | BMI, fasting blood sugar, HbA1c, self-efficacy, total cholesterol, triglycerides |
| Abaza & Marschollek, 201736 | Egypt | 3 months | 51.24 | SMS and reminder prompts | Adherence to treatment, diabetes knowledge, HbA1c, self-efficacy |
| Kleinman et al., 201737 | India | 3 months | 48.4 | Smartphone app and web portal | Adherence to treatment, BMI, fasting blood sugar, HbA1c |
| Lee et al., 201738 | Malaysia | NR | 53.24 | Smartphone app | BMI, diabetes knowledge, fasting blood sugar, HbA1c, self-efficacy |
| Hemmati Maslakpak et al., 201727 | Iran (Islamic Republic of) | 3 months | 49.46 | Educational telephone calls | Adherence to treatment, BMI, HbA1c, fasting blood sugar, self-efficacy, total cholesterol, triglycerides |
| Namjoo Nasab et al., 201715 | Iran (Islamic Republic of) | NR | NR | Telephone follow-ups | Adherence to treatment, BMI |
| Van Olmen et al., 201739 | Cambodia, Congo, Philippines | 1 year | NR | SMS and voice messages | Diabetes knowledge, HbA1c |
| Wang et al., 201740 | China | 3 months | 52.6 | U-Healthcare website, website messages, telephone prompt | BMI, fasting blood sugar, HbA1c, total cholesterol, triglycerides |
| de Vasconcelos et al., 201841 | Brazil | 24 weeks | 60.9 | Guidance and coaching via telephone calls from research nurse | BMI, HbA1c, high-density lipoproteins, total cholesterol, triglycerides |
| Fang & Deng, 201842 | China | 3 months | 57.69 | SMS | BMI, fasting blood sugar, HbA1c, total cholesterol, triglycerides |
| Ramadas et al., 201843 | Malaysia | 6 months | 49.6 | Web-delivered intervention program, log-in reminders via email and text message follow-ups | Diabetes knowledge, fasting blood sugar, HbA1c |
| Sarayani et al., 201844 | Iran (Islamic Republic of) | 3 months | 53.4 | Telephone-based consultation | Adherence to treatment, HbA1c, total cholesterol, triglycerides |
| Duruturk & Özköslü, 201945 | Turkey | 6 weeks | 52.82 | Video conference consultation | HbA1c |
| Goruntla et al., 201946 | India | 6 months | 58.8 | SMS and reminder prompts | Adherence to treatment, BMI, HbA1c, triglycerides |
| Guo et al., 201947 | China | 13 weeks | 31.2 | D-nurse app for monitoring of blood glucose levels and transmission of data to clinic | HbA1c |
| Prabhakaran et al., 201914 | India | 1 year | 56.7 | Android app and SMS reminders | BMI, fasting blood sugar HbA1c, total cholesterol |
| Sun et al., 201948 | China | 3 months | 67.9 | Web-based app, SMS reminders, telephonic reminders | BMI, fasting blood sugar, HbA1c, total cholesterol, triglycerides |
| Vinitha et al., 201949 | India | 24 months | 43.3 | SMS to reinforce healthy lifestyle practices and adherence to medication | BMI, HbA1c, high-density lipoproteins, fasting blood sugar, total cholesterol, triglycerides |
| Wang et al., 201950 | China | 6 months | 45.13 | Hand-held clinic, monitoring prompts, dietary recommendations and exercise guidance via app | Diabetes knowledge, fasting blood sugar, HbA1c, self-efficacy |
| Zhang et al., 201951 | China | 6 months | 53 | Smartphone app and online interactive management | BMI, fasting blood sugar, HbA1c |
| Lee et al., 202052 | Malaysia | 52 weeks | 53.3 | Telemetry | Diabetes knowledge, fasting blood sugar, HbA1c, high-density lipoproteins, self-efficacy, total cholesterol, triglycerides |
| Owolabi et al., 202053 | South Africa | 6 months | 60.64 | SMS for lifestyle advice and appointment reminders | Adherence to treatment |
| Wang et al., 202054 | Mongolia | 12 months | 55.4 | SMS for health awareness, diet control, physical activities, living habits and weight control | Fasting blood sugar |
app: application; BMI: body mass index; HbA1c: glycated haemoglobin; NR: not reported; SMS: short message service.
Risk of bias
Our assessment of the risk of bias revealed that 19 studies had a high risk of bias, while 12 studies had a low risk of bias. Within the studies with a low risk of bias, the highest number of individual matrices were found to have a low risk of bias across matrices of random sequence generation (24 matrices), followed by attrition bias (20 matrices), other risk of biases (10 matrices), allocation concealment (eight matrices) and blinding of outcome assessors (six matrices; data repository).21
Intervention strategies
Our included interventions varied in their strategies for the management of diabetes. We identified five modes of intervention delivery, through either smartphone apps (five studies), 14,37,47,50,51 SMS (nine studies), 13,30,36,39,42,46,49,53,54 telemetry (five studies),32,38,40,48,52 telephone (10 studies)13,15,18,27,29,33,40,41,44,48 and web-based systems including video conferencing (four studies).31,35,43,45 Most studies focused on a single mode of telemedicine delivery; however, one study considered both telephone and SMS and another study investigated the use of both telephone and telemetry.13,40 Major strategies included health record-keeping, follow-ups, reminders for follow-ups and logins, psychoeducation, glucose monitoring, monitoring prompts for serum glucose levels, pervasive alerts and online consultations (Table 1 and data repository).21 We did not observe any trend in the emergence of unique technologies; however, smartphone app-based interventions began to be tested from 2017. We provide details of individual interventions in the data repository.21
Meta-analysis
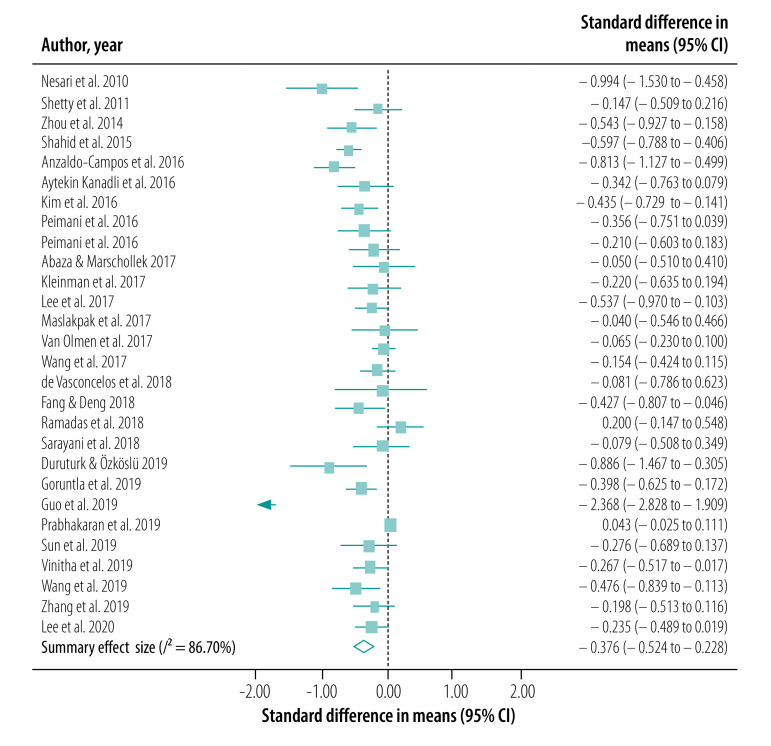
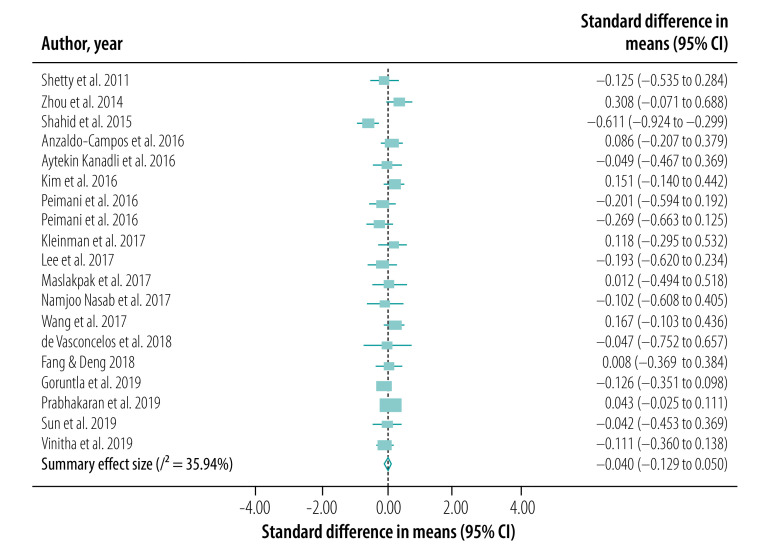
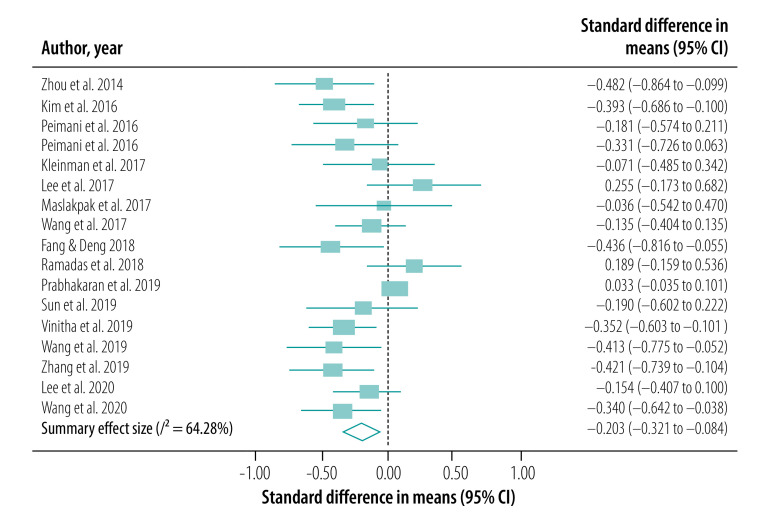
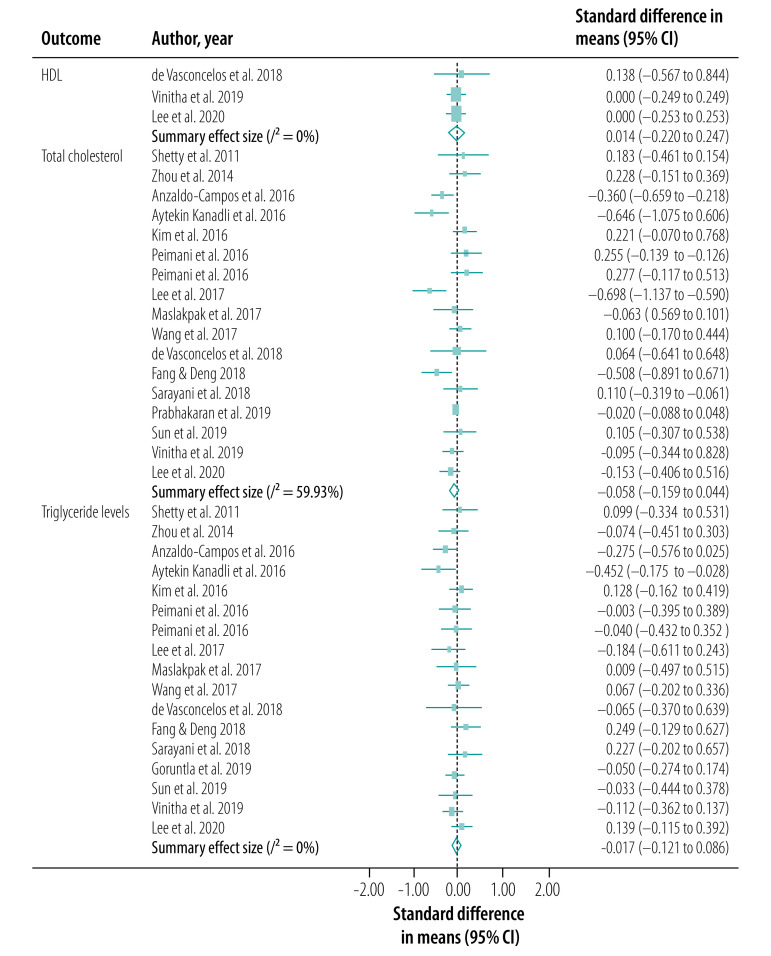
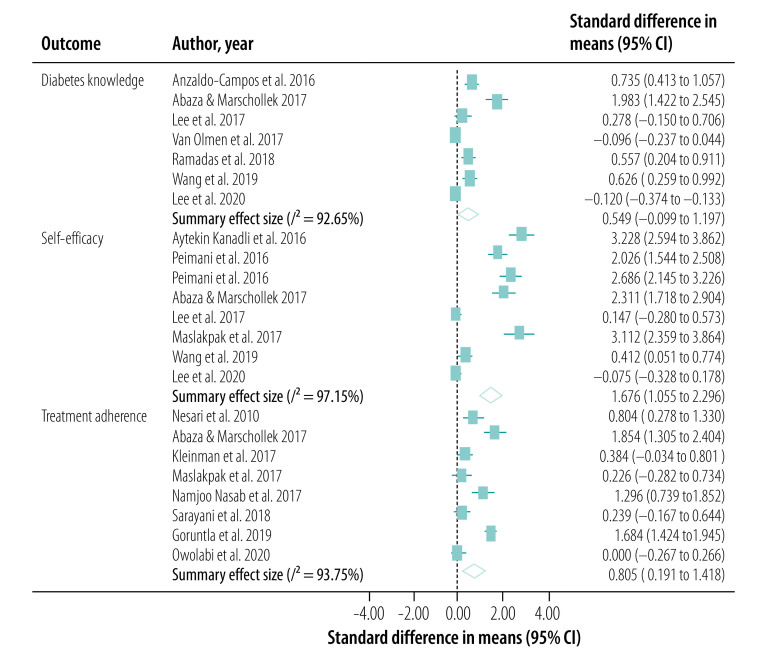
We conducted our meta-analysis of the 30 studies, including 31 interventions, across eight outcomes. Our primary outcome, HbA1c levels, was reported in a total of 27 studies (28 interventions; Fig. 2). BMI was reported in 18 studies (19 interventions; Fig. 3), serum levels of fasting blood sugar in 16 studies (17 interventions; Fig. 4), and total cholesterol and triglycerides in 16 studies (17 interventions; Fig. 5). Adherence to treatment was reported in eight studies (Fig. 6), knowledge of diabetes in seven studies (Fig. 6) and self-efficacy in seven studies (eight interventions; Fig. 6).
Fig. 2.
Forest plot showing effectiveness of telemedicine for diabetes treatment in improving serum HbA1c levels, low- and middle-income countries, 2010–2020
CI: confidence interval; HbA1c: glycated haemoglobin.
Fig. 3.
Forest plot showing effectiveness of telemedicine for diabetes treatment in improving body mass index, low- and middle-income countries, 2011–2019
CI: confidence interval.
Fig. 4.
Forest plot showing effectiveness of telemedicine for diabetes treatment in improving fasting blood sugar levels, low- and middle-income countries, 2014–2020
CI: confidence interval.
Fig. 5.
Forest plot showing effectiveness of telemedicine for diabetes treatment in improving serum lipid profile efficacy, low- and middle-income countries, 2011–2020
CI: confidence interval; HDL: high density lipoproteins.
Fig. 6.
Forest plot showing effectiveness of telemedicine for diabetes treatment in improving adherence to treatment, diabetes knowledge and self-efficacy, low- and middle-income countries, 2010–2020
CI: confidence interval.
We observed a significant treatment effect among several outcomes, with standardized mean differences of −0.38 for HbA1c (95% CI: −0.52 to −0.23; n = 7703; I2 = 86.70%), −0.20 for fasting blood sugar (95% CI: −0.32 to −0.08; n = 5524; I2 = 64.28%), 0.81 for adherence to treatment (95% CI: 0.19 to 1.42; n = 959; I2 = 93.75%), 0.55 for knowledge of diabetes (95% CI: −0.10 to 1.20; n = 1585; I2 = 92.65%) and 1.68 for self-efficacy (95% CI: 1.06 to 2.30; n = 866; I2 = 97.15%). We did not observe any significant treatment effect in other outcomes, with standardized mean differences of −0.04 for BMI (95% CI: −0.13 to 0.05; n = 5957; I2 = 35.94%), −0.06 for total cholesterol (95% CI: −0.16 to 0.04; n = 5381; I2 = 59.93%) and −0.02 for triglycerides (95% CI: −0.12 to 0.09; n = 2360; I2 = 0%).
Funnel plots and Egger regression analyses revealed that publication bias was significant for the outcomes of HbA1c, knowledge of diabetes, fasting blood sugar and self-efficacy (data repository).21 Publication bias was non-significant in outcomes including BMI, total cholesterol, triglycerides and adherence to treatment (data repository).21 Our sensitivity analysis did not reveal any significant changes in the effect sizes of these outcomes.
Our subgroup analysis based on mode of intervention delivery revealed that the only outcomes that yielded statistical significance were BMI and self-efficacy. Telephone- and SMS-based telemedicine interventions yielded the highest treatment effects when compared with telemetry and smartphone-based services for a range of outcomes (Table 2; available at: http://www.who.int/bulletin/volumes/99/3/19-250068).
Table 2. Subgroup analysis of intervention delivery mode in the systematic review and meta-analysis of the effectiveness of telemedicine in the delivery of diabetes care, low- and middle-income countries, 2010–2020.
| Outcome and type of intervention | Standardized mean difference (95% CI) | I2a | τ2a | Between-group difference, P |
|---|---|---|---|---|
| BMI | 0.002 | |||
| Smartphone app | 0.05 (−0.02 to 0.11) | 0.00 | 0.00 | |
| SMS | −0.13 (−0.26 to 0.00) | 0.00 | 0.00 | |
| Telemetry | 0.05 (−0.11 to 0.22) | 0.00 | 0.00 | |
| Telephone | −0.27 (−0.47 to −0.07) | 47.68 | 0.05 | |
| Web-based | 0.21 (−0.02 to 0.44) | 0.00 | 0.00 | |
| Self-efficacy | < 0.01 | |||
| Smartphone app | 0.41 (0.05 to 0.77) | 0.00 | 0.00 | |
| SMS | 2.32 (2.01 to 2.62) | 37.22 | 0.04 | |
| Telemetry | −0.02 (−0.24 to 0.20) | 0.00 | 0.00 | |
| Telephone | 3.18 (2.69 to 3.66) | 0.00 | 0.00 | |
| Adherence to treatment | 0.67 | |||
| Smartphone app | 0.38 (−1.42 to 2.19) | 0.00 | 0.00 | |
| SMS | 1.16 (0.13 to 2.20) | 97.77 | 1.21 | |
| Telephone | 0.64 (−0.28 to 1.55) | 74.15 | 0.18 | |
| Serum HbA1c levels | 0.66 | |||
| Smartphone app | −0.58 (−0.96 to −0.20) | 96.42 | 0.54 | |
| SMS | −0.24 (−0.54 to 0.06) | 14.16 | 0.00 | |
| Telemetry | −0.40 (−0.78 to −0.02) | 66.59 | 0.05 | |
| Telephone | −0.37 (−0.74 to 0.00) | 61.15 | 0.07 | |
| Web-based (video conference) | −0.89 (−1.85 to 0.08) | 0.00 | 0.00 | |
| Diabetes knowledge | 0.90 | |||
| Smartphone app | 0.63 (−1.02 to 2.27) | 0.00 | 0.00 | |
| SMS | 0.89 (−0.28 to 2.05) | 97.99 | 2.12 | |
| Telemetry | 0.30 (−0.65 to 1.24) | 88.11 | 0.21 | |
| Web-based | −0.26 (−0.75 to 0.23) | 80.33 | 0.12 | |
| Fasting blood sugar levels | 0.56 | |||
| Smartphone app | −0.17 (−0.39 to 0.04) | 76.54 | 0.06 | |
| SMS | −0.33 (−0.54 to −0.12) | 0.00 | 0.00 | |
| Telemetry | −0.08 (−0.31 to 0.15) | 2.10 | 0.00 | |
| Telephone | −0.04 (−0.64 to 0.56) | 0.00 | 0.00 | |
| Web-based | 0.56 (−1.08 to 2.20) | 0.00 | 0.00 | |
| Serum total cholesterol levels | 0.50 | |||
| Smartphone app | −0.02 (−0.48 to 0.44) | 0.00 | 0.00 | |
| SMS | 0.00 (−0.27 to 0.27) | 63.77 | 0.07 | |
| Telemetry | −0.18 (−0.44 to 0.07) | 68.37 | 0.06 | |
| Telephone | −0.16 (−0.50 to 0.18) | 56.98 | 0.09 | |
| Serum triglyceride levels | 0.90 | |||
| Smartphone app | 0.22 (−0.18 to 0.63) | 0.00 | 0.00 | |
| SMS | −0.01 (−0.14 to 0.12) | 0.00 | 0.00 | |
| Telemetry | −0.03 (−0.17 to 0.12) | 23.97 | 0.01 | |
| Telephone | −0.08 (−0.33 to 0.17) | 40.59 | 0.04 | |
| Web-based | 0.05 (−0.19 to 0.29) | 0.00 | 0.00 |
app: application; CI: confidence interval; HbA1c: glycated haemoglobin; SMS: short message service.
a Both I2 and τ2 are measures of heterogeneity in subgroup analyses.
Our meta-regression analysis did not reveal any association between effect size and either quality assessment score or year of study for the HbA1c outcome (P > 0.05; data repository).21 We could not run a meta-regression analysis based on sex, mean age and time at which the measurement of outcomes was reported because of missing data.
GRADE evidence
We assessed the certainty of evidence for the efficacy of telemedicine-based interventions for diabetes management in low- and middle-income countries, according to GRADE guidelines, for the critical outcome of HbA1c levels. We graded the certainty of evidence as very low because of substantial heterogeneity, publication bias and risk of bias (data repository).21
Discussion
Our meta-analysis showing that telemedicine-based interventions are effective in improving serum levels of HbA1c and fasting blood sugar, adherence to treatment and self-efficacy is consistent with several individual RCTs conducted in both high-income countries and in low- and middle-income countries.13,16,18,29,35,37,55 Our results are also in accordance with previously conducted meta-analyses; for example, in the 2014 global study of the clinical effectiveness of telemedicine in reducing serum levels of HbA1c, researchers reported a small but statistically significant decrease (standardized mean difference: −0.37).19 Our results are also corroborated by the 2017 global systematic review, which showed moderate reductions in HbA1c post-intervention with reduced effect sizes at follow-up.11 Telemedicine interventions were also found to be cost-effective for diabetes management; for example, for retinal screening alone, telemedicine interventions were reported to yield 113.48–3828.46 quality-adjusted life years.12 Although an outcome of interest, we could not find any cost–effectiveness data applicable to low- and middle-income countries.
Despite the encouraging effect sizes for a range of outcomes, we found no improvement in BMI or serum levels of total cholesterol or triglycerides. It was not possible to corroborate these results as our literature review did not yield any similar meta-analytic reviews exploring these indicators. The statistical non-significance or poor treatment effects of these interventions for these outcomes can be explained, however. First, most of these interventions were developed to target single outcomes, such as adherence to treatment, HbA1c serum levels or dietary behaviour.13,55,56 None of the interventions focused on the measurement of lipid profile or BMI, and none assessed knowledge of diabetes (telemetric or otherwise). Second, the sample size calculations of these trials were based on improvement in HbA1c levels. We recommend that future interventions should be developed as comprehensive packages providing sessions on diet, physical exercise, monitoring of HbA1c levels and adherence to treatment.
For several outcomes, including BMI, adherence to treatment and self-efficacy, we found telephone- and SMS-based interventions (i.e. low-tech services) to be more effective than telemetry, smartphone apps or other web-based interventions. However, previous studies did not compare outcomes for different telemedicine delivery modes, meaning that we could not find any corroboratory or contradictory evidence. We suggest that our observed increased effectiveness of telephone- and SMS-based interventions may be attributable to the improved relationship between health-care professional and patient obtainable through these media.57 Importantly, the fact that these delivery modes performed as well as or better than more advanced apps and software means that they can be adopted with confidence in low-resource settings.
Regarding other modes of delivery, smartphone apps developed in future studies should of course be user-friendly with a patient-centredness perspective, and considerate of the computer literacy levels of patients. Future researchers should consider conducting participatory approaches, including pilot surveys, design science, cost–effectiveness studies, and knowledge, attitude and practice surveys to explore levels of computer literacy among consumers.
Our review has several limitations. First, our search was limited to only five major academic databases and two minor grey-literature databases. Several additional databases, such as Embase and APA PsycINFO®, also warrant searching for potential articles. Although the databases Web of Science, PubMed® and Cochrane registry are highly inclusive, literature may have been missed. Our results are also limited by a high statistical and methodological heterogeneity across a few outcomes. For instance, outcomes such as self-efficacy and adherence to treatment were assessed using heterogeneous questionnaires. The heterogeneity in the outcomes could also be explained by different modes of intervention delivery, geographical regions and intervention contents.
Despite the above limitations, by synthesizing evidence from 31 studies conducted in low- and middle-income countries, we have provided more inclusive evidence in terms of number of articles than the previously published key reviews.11,19 Our study also benefited from the exploration of additional outcomes, including a variety of biochemical indicators.
Our meta-analysis revealed a very low certainty of evidence that telemedicine interventions were effective in improving diabetes management in low- and middle-income countries. Higher-quality RCTs are required before a solid recommendation for the use of telemedicine-based interventions can be made. We recommend that future interventions should be designed to address both the biological and socioeconomic determinants of diabetes. Studies that explore the evaluation, feasibility or acceptability of data are important in the scaling up of interventions.
To conclude, telemedicine-based services are frequently considered to be costly to the health system, but there should be more reviews addressing the cost–effectiveness of implementing and integrating such interventions within national health systems. Telemedicine would benefit from integration with the community-based health system with the support of community health workers. Despite barriers to this integration, telemedicine could improve the accessibility and quality of health-care services, improve personnel training and management processes, and optimize the use of epidemiological and clinical data.58
Competing interests:
None declared.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017. June;128:40–50. 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 2.Nisar MU, Asad A, Waqas A, Ali N, Nisar A, Qayyum MA, et al. Association of diabetic neuropathy with duration of type 2 diabetes and glycemic control. Cureus. 2015. August 12;7(8):e302. 10.7759/cureus.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019. January;62(1):3–16. 10.1007/s00125-018-4711-2 [DOI] [PubMed] [Google Scholar]
- 4.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017. February;20(1):6–12. 10.1089/pop.2015.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moucheraud C, Lenz C, Latkovic M, Wirtz VJ. The costs of diabetes treatment in low- and middle-income countries: a systematic review. BMJ Glob Health. 2019. February 27;4(1):e001258. 10.1136/bmjgh-2018-001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atun R, Davies JI, Gale EAM, Bärnighausen T, Beran D, Kengne AP, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017. August;5(8):622–67. 10.1016/S2213-8587(17)30181-X [DOI] [PubMed] [Google Scholar]
- 7.Dagenais GR, Gerstein HC, Zhang X, McQueen M, Lear S, Lopez-Jaramillo P, et al. Variations in diabetes prevalence in low-, middle-, and high-income countries: results from the prospective urban and rural epidemiological study. Diabetes Care. 2016. May;39(5):780–7. 10.2337/dc15-2338 [DOI] [PubMed] [Google Scholar]
- 8.Global report on diabetes. Geneva: World Health Organization; 2016. Available from: https://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf [cited 2020 Oct 20].
- 9.de la Torre-Díez I, López-Coronado M, Vaca C, Aguado JS, de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015. February;21(2):81–5. 10.1089/tmj.2014.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Classification of digital health interventions v1.0: a shared language to describe the uses of digital technology for health. Geneva: World Health Organization; 2018. Available from: https://apps.who.int/iris/handle/10665/260480 [cited 2020 Oct 20].
- 11.Faruque LI, Wiebe N, Ehteshami-Afshar A, Liu Y, Dianati-Maleki N, Hemmelgarn BR, et al. ; Alberta Kidney Disease Network. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017. March 6;189(9):E341–64. 10.1503/cmaj.150885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018. July;20(7):492–500. 10.1089/dia.2018.0098 [DOI] [PubMed] [Google Scholar]
- 13.Peimani M, Rambod C, Omidvar M, Larijani B, Ghodssi-Ghassemabadi R, Tootee A, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016. August;10(4):251–8. 10.1016/j.pcd.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Prabhakaran D, Jha D, Ajay VS, Roy A, Perel P. Response by Prabhakaran et al to letter regarding article, “Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare Cluster-Randomized Controlled Trial”. Circulation. 2019. June 11;139(24):e1039. 10.1161/CIRCULATIONAHA.119.040782 [DOI] [PubMed] [Google Scholar]
- 15.Namjoo Nasab M, Ghavam A, Yazdanpanah A, Jahangir F, Shokrpour N. Effects of self-management education through telephone follow-up in diabetic patients. Health Care Manag (Frederick). 2017. Jul-Sep;36(3):273–81. 10.1097/HCM.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 16.Saleh S, Farah A, Dimassi H, El Arnaout N, Constantin J, Osman M, et al. Using mobile health to enhance outcomes of noncommunicable diseases care in rural settings and refugee camps: randomized controlled trial. JMIR Mhealth Uhealth. 2018. July 13;6(7):e137. 10.2196/mhealth.8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limaye T, Kumaran K, Joglekar C, Bhat D, Kulkarni R, Nanivadekar A, et al. Efficacy of a virtual assistance-based lifestyle intervention in reducing risk factors for type 2 diabetes in young employees in the information technology industry in India: LIMIT, a randomized controlled trial. Diabet Med. 2017. April;34(4):563–8. 10.1111/dme.13258 [DOI] [PubMed] [Google Scholar]
- 18.Shahid M, Mahar SA, Shaikh S, Shaikh ZUD. Mobile phone intervention to improve diabetes care in rural areas of Pakistan: a randomized controlled trial. J Coll Physicians Surg Pak. 2015. March;25(3):166–71. [PubMed] [Google Scholar]
- 19.Zhai YK, Zhu WJ, Cai YL, Sun DX, Zhao J. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltimore). 2014. December;93(28):e312. 10.1097/MD.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009. October;62(10):e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 21.Correia J, Meraj H, Teoh SH, Waqas A, Ahmad M, Lapão LV, et al. Implementation and effectiveness of telemedicine-based interventions for the treatment of diabetes in low- and middle-income countries: a systematic review and meta-analysis. Supplemental file [data repository]. London: figshare; 2020. 10.6084/m9.figshare.13150841 10.6084/m9.figshare.13150841 [DOI]
- 22.Correia J, Meraj H, Waqas A. Implementation and effectiveness of tele-medicine based interventions for treatment of diabetes mellitus in low and middle income countries : a systematic review and meta- analysis. PROSPERO; 2019. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019141271 [cited 2020 Oct 20].
- 23.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011. October 18;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011. April;64(4):383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions, version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook [cited 20 Oct 27].
- 26.Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011. November;64(11):1187–97. 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 27.Hemmati Maslakpak M, Razmara S, Niazkhani Z. Effects of face-to-face and telephone-based family-oriented education on self-care behavior and patient outcomes in type 2 diabetes: a randomized controlled trial. J Diabetes Res. 2017;2017:8404328. 10.1155/2017/8404328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Jpn J Nurs Sci. 2010. December;7(2):121–8. 10.1111/j.1742-7924.2010.00146.x [DOI] [PubMed] [Google Scholar]
- 29.Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Jpn J Nurs Sci. 2010. December;7(2):121–8. 10.1111/j.1742-7924.2010.00146.x [DOI] [PubMed] [Google Scholar]
- 30.Shetty AS, Chamukuttan S, Nanditha A, Raj RK, Ramachandran A. Reinforcement of adherence to prescription recommendations in Asian Indian diabetes patients using short message service (SMS)–a pilot study. J Assoc Physicians India. 2011. November;59:711–4. [PubMed] [Google Scholar]
- 31.Zhou P, Xu L, Liu X, Huang J, Xu W, Chen W. Web-based telemedicine for management of type 2 diabetes through glucose uploads: a randomized controlled trial. Int J Clin Exp Pathol. 2014. December 1;7(12):8848–54. [PMC free article] [PubMed] [Google Scholar]
- 32.Anzaldo-Campos MC, Contreras S, Vargas-Ojeda A, Menchaca-Díaz R, Fortmann A, Philis-Tsimikas A. Dulce Wireless Tijuana: a randomized control trial evaluating the impact of project Dulce and short-term mobile technology on glycemic control in a family medicine clinic in Northern Mexico. Diabetes Technol Ther. 2016. April;18(4):240–51. 10.1089/dia.2015.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aytekin Kanadli K, Ovayolu N, Ovayolu Ö. Does telephone follow-up and education affect self-care and metabolic control in diabetic patients? Holist Nurs Pract. 2016. Mar-Apr;30(2):70–7. 10.1097/HNP.0000000000000137 [DOI] [PubMed] [Google Scholar]
- 34.Gopalan A, Paramanund J, Shaw PA, Patel D, Friedman J, Brophy C, et al. Randomised controlled trial of alternative messages to increase enrolment in a healthy food programme among individuals with diabetes. BMJ Open. 2016. November 30;6(11):e012009. 10.1136/bmjopen-2016-012009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Sun C, Yang SJ, Sun L, Li F, Choi IY, et al. Randomized, open-label, parallel group study to evaluate the effect of internet-based glucose management system on subjects with diabetes in China. Telemed J E Health. 2016. August;22(8):666–74. 10.1089/tmj.2015.0170 [DOI] [PubMed] [Google Scholar]
- 36.Abaza H, Marschollek M. SMS education for the promotion of diabetes self-management in low & middle income countries: a pilot randomized controlled trial in Egypt. BMC Public Health. 2017. December 19;17(1):962. 10.1186/s12889-017-4973-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V. Improved medication adherence and frequency of blood glucose self-testing using an m-Health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed J E Health. 2017. September;23(9):733–40. 10.1089/tmj.2016.0265 [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Wong CP, Tan CSS, Nasir NH, Lee SWH. Telemonitoring in fasting individuals with type 2 diabetes mellitus during Ramadan: a prospective, randomised controlled study. Sci Rep. 2017. August 31;7(1):10119. 10.1038/s41598-017-10564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Olmen J, Kegels G, Korachais C, de Man J, Van Acker K, Kalobu JC, et al. The effect of text message support on diabetes self-management in developing countries - a randomised trial. J Clin Transl Endocrinol. 2017. January 3;7:33–41. 10.1016/j.jcte.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G, Zhang Z, Feng Y, Sun L, Xiao X, Wang G, et al. Telemedicine in the management of type 2 diabetes mellitus. Am J Med Sci. 2017. January;353(1):1–5. 10.1016/j.amjms.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 41.de Vasconcelos HCA, Lira Neto JCG, de Araújo MFM, Carvalho GCN, de Souza Teixeira CR, de Freitas RWJF, et al. Telecoaching programme for type 2 diabetes control: a randomised clinical trial. Br J Nurs. 2018. October 18;27(19):1115–20. 10.12968/bjon.2018.27.19.1115 [DOI] [PubMed] [Google Scholar]
- 42.Fang R, Deng X. Electronic messaging intervention for management of cardiovascular risk factors in type 2 diabetes mellitus: a randomised controlled trial. J Clin Nurs. 2018. February;27(3-4):612–20. 10.1111/jocn.13962 [DOI] [PubMed] [Google Scholar]
- 43.Ramadas A, Chan CKY, Oldenburg B, Hussein Z, Quek KF. Randomised-controlled trial of a web-based dietary intervention for patients with type 2 diabetes: changes in health cognitions and glycemic control. BMC Public Health. 2018. June 8;18(1):716. 10.1186/s12889-018-5640-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarayani A, Mashayekhi M, Nosrati M, Jahangard-Rafsanjani Z, Javadi M, Saadat N, et al. Efficacy of a telephone-based intervention among patients with type-2 diabetes; a randomized controlled trial in pharmacy practice. Int J Clin Pharm. 2018. April;40(2):345–53. 10.1007/s11096-018-0593-0 [DOI] [PubMed] [Google Scholar]
- 45.Duruturk N, Özköslü MA. Effect of tele-rehabilitation on glucose control, exercise capacity, physical fitness, muscle strength and psychosocial status in patients with type 2 diabetes: a double blind randomized controlled trial. Prim Care Diabetes. 2019. December;13(6):542–8. 10.1016/j.pcd.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 46.Goruntla N, Mallela V, Nayakanti D. Impact of pharmacist-directed counseling and message reminder services on medication adherence and clinical outcomes in type 2 diabetes mellitus. J Pharm Bioallied Sci. 2019. Jan-Mar;11(1):69–76. 10.4103/JPBS.JPBS_211_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo H, Zhang Y, Li P, Zhou P, Chen LM, Li SY. Evaluating the effects of mobile health intervention on weight management, glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus. J Endocrinol Invest. 2019. June;42(6):709–14. 10.1007/s40618-018-0975-0 [DOI] [PubMed] [Google Scholar]
- 48.Sun C, Sun L, Xi S, Zhang H, Wang H, Feng Y, et al. Mobile phone-based telemedicine practice in older Chinese patients with type 2 diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2019. January 4;7(1):e10664. 10.2196/10664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinitha R, Nanditha A, Snehalatha C, Satheesh K, Susairaj P, Raghavan A, et al. Effectiveness of mobile phone text messaging in improving glycaemic control among persons with newly detected type 2 diabetes. Diabetes Res Clin Pract. 2019. December;158:107919. 10.1016/j.diabres.2019.107919 [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Li M, Zhao X, Pan X, Lu M, Lu J, et al. Effects of continuous care for patients with type 2 diabetes using mobile health application: a randomised controlled trial. Int J Health Plann Manage. 2019. July;34(3):1025–35. 10.1002/hpm.2872 [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, He X, Shen Y, Yu H, Pan J, Zhu W, et al. Effectiveness of smartphone app-based interactive management on glycemic control in Chinese patients with poorly controlled diabetes: randomized controlled trial. J Med Internet Res. 2019. December 9;21(12):e15401. 10.2196/15401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JY, Chan CKY, Chua SS, Ng CJ, Paraidathathu T, Lee KKC, et al. Telemonitoring and team-based management of glycemic control on people with type 2 diabetes: a cluster-randomized controlled trial. J Gen Intern Med. 2020. January;35(1):87–94. 10.1007/s11606-019-05316-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owolabi EO, Goon DT, Ajayi AI. Impact of mobile phone text messaging intervention on adherence among patients with diabetes in a rural setting: a randomized controlled trial. Medicine (Baltimore). 2020. March;99(12):e18953. 10.1097/MD.0000000000018953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Liu D, Du M, Hao R, Zheng H, Yan C. The role of text messaging intervention in Inner Mongolia among patients with type 2 diabetes mellitus: a randomized controlled trial. BMC Med Inform Decis Mak. 2020. May 14;20(1):90. 10.1186/s12911-020-01129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V. Impact of the Gather mHealth system on A1C: primary results of a multisite randomized clinical trial among people with type 2 diabetes in India. Diabetes Care. 2016. October;39(10):e169–70. 10.2337/dc16-0869 [DOI] [PubMed] [Google Scholar]
- 56.Fottrell E, Ahmed N, Morrison J, Kuddus A, Shaha SK, King C, et al. Community groups or mobile phone messaging to prevent and control type 2 diabetes and intermediate hyperglycaemia in Bangladesh (DMagic): a cluster-randomised controlled trial. Lancet Diabetes Endocrinol. 2019. March;7(3):200–12. 10.1016/S2213-8587(19)30001-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmad W, Krupat E, Asma Y, Fatima NE, Attique R, Mahmood U, et al. Attitudes of medical students in Lahore, Pakistan towards the doctor-patient relationship. PeerJ. 2015. June 30;3:e1050. 10.7717/peerj.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra SR, Lygidakis C, Neupane D, Gyawali B, Uwizihiwe JP, Virani SS, et al. Combating non-communicable diseases: potentials and challenges for community health workers in a digital age, a narrative review of the literature. Health Policy Plan. 2019. February 1;34(1):55–66. 10.1093/heapol/czy099 [DOI] [PubMed] [Google Scholar]