Abstract
Background
Digital health interventions might extend service provisions for youth with chronic medical conditions (CC) and comorbid mental health symptoms. We aimed to comprehensively evaluate the efficacy of Internet- and mobile-based interventions (IMIs) for different psychological and disease-related outcomes in children and adolescents with CC.
Method
Studies were identified by systematic searches in CENTRAL, Embase, MEDLINE/PubMed and PsycINFO, complemented by searches in reference lists of eligible studies and other reviews. We included studies, when they were randomized controlled trials (RCTs) comparing the efficacy of an IMI to control conditions in improving psychological and disease-related outcomes in youth (mean age ≤ 18 years) with CC. Study selection, data extraction and risk of bias assessment were conducted independently by two reviewers. Meta-analyses were performed within a random-effects model, and Hedges' g (with 95% confidence intervals) was calculated as effect size measure. Primary outcomes were comorbid mental health symptoms (i.e., depression, anxiety and stress), as well as quality of life and self-efficacy.
Results
A total of 19 randomized controlled trials (2410 patients) were included in this meta-analysis. IMIs were associated with improvements in self-efficacy (g = 0.38; 95% CI, 0.15 to 0.61; I2 = 0) and combined disease-related outcomes (g = −0.13; 95% CI, −0.25 to −0.01; I2 = 21). Meta-analyses on other outcomes were non-significant, and some pre-planned analyses were not feasible because of a shortage of studies.
Conclusion
The available evidence on IMIs for improving mental and health-related outcomes in youth with CC is limited. Our findings point to a rather small benefit and limited efficacy. Future research is needed, to comprehensively assess the potential of IMIs to extend collaborative care, and to identify factors contributing to improved user-centered interventions with better treatment outcomes.
Keywords: Adolescent mental health, Pediatrics, Psychotherapy, Internet- and mobile-based intervention, eHealth, mHealth
Highlights
-
•
We examined the efficacy of digital health interventions in youth with chronic medical conditions (CC).
-
•
We included 19 randomized controlled trials (2410 patients) in this meta-analysis.
-
•
Interventions were efficacious in improving self-efficacy and combined disease-related outcomes.
-
•
Meta-analyses on other outcomes, including depression severity, were non-significant.
-
•
The available evidence points to a limited benefit of digital health interventions in youth with CC.
1. Introduction
Chronic medical conditions (CC) in children and adolescents are highly prevalent (van der Lee et al., 2007), and are associated with substantial personal suffering and disease burden worldwide (WHO, 2018). These medical conditions comprise a range of different disorders, including diabetes, respiratory diseases or chronic pain (WHO, 2018), with prevalence rates between 13 and 27% in western countries like the US (van Cleave et al., 2010). Although there is no consensual definition of CC, these non-communicable diseases are typically characterized by a long duration (at least three months; van der Lee et al., 2007), functional impairment (van der Lee et al., 2007), absence of cure or presence of disease progression (Stanton et al., 2007), physical disability or pain (de Ridder et al., 2008), and the need for extensive (continuous) medical and psychological care (Mokkink et al., 2008).
Functional impairments in daily lives due to the CC are substantial, and include hampered school attendance, interference with positive relationships to peers or social isolation, as well as specific challenges associated with the medical therapy and adherence to treatment (Compas et al., 2012). Thus, CC interfere with normal psychosocial development and can lead to negative psychological outcomes in youth, like prolonged stress (Compas et al., 2012), reduced quality of life (QoL; Holmbeck et al., 2002) or symptoms of depression (Pinquart and Shen, 2011) and anxiety (Cobham et al., 2020). Although effective psychotherapeutic interventions for these mental health issues exist (Zhou et al., 2015; Zhou et al., 2019), the availability and uptake of evidence-based face-to-face treatments is limited (Gloff et al., 2015) due to different structural and individual barriers, involving shortage of service supplies with long waiting times (Andrade et al., 2014), high economic costs (Mojtabai, 2005), or stigma threat (Gulliver et al., 2010).
Digital health interventions, delivered via the Internet or mobile devices, might be a promising alternative to overcome these barriers and contribute to the extension of mental health care practices for children and adolescents with CC (Lunkenheimer et al., 2020). Possible advantages of Internet- and mobile-based interventions (IMIs) might be their delivery mode irrespective of the constraints of space and time, potential anonymity and flexibility in conduct, special attractiveness for youth, potential cost-effectiveness, as well as scalability on a larger scale on a steady basis (Andersson et al., 2019; Domhardt et al., 2018; Domhardt and Baumeister, 2018; Ebert et al., 2018). Obviously, some assets are especially important when the access to conventional mental health care services for youth with CC is even further reduced – as observed during the Covid-19 crisis (Torous and Wykes, 2020). Regardless of contemporary circumstances, IMIs can be implemented as stand-alone interventions, as part of blended-therapy approaches or within a framework of stepped and collaborative care (Andersson et al., 2019; Domhardt et al., 2018; Domhardt and Baumeister, 2018; Ebert et al., 2018; Yonek et al., 2020). Of note, interventions with accompanying human support evince consistently higher effect sizes than pure self-help interventions for depression and anxiety symptoms in adults (Baumeister et al., 2014; Domhardt et al., 2019), reaching even comparable effect sizes to face-to-face psychotherapies in some instances (Carlbring et al., 2018). Meta-analytic evidence also indicates that internet-based interventions are efficacious in treating symptoms of anxiety and depression in children and adolescents, with medium to large and small to medium effect sizes respectively (Domhardt et al., 2020b). There are also preliminary indications derived from single studies that internet-based interventions might be acceptable (Lenhard et al., 2017) and cost-effective in youth (Jolstedt et al., 2018).
However, the evidence-base in regard to the efficacy of IMIs specific for youth with CC and comorbid mental health problems is fragmented and constrained by several shortcomings. For example, Vigerland et al. (2016) found in subgroup analyses a medium overall effect (standardized mean difference, SMD, 0.85; 95% CI, 0.63 to 1.07) for internet-based cognitive behavior therapy (iCBT) for some somatic conditions (i.e., functional gastrointestinal disorder, insomnia and chronic pain), omitting mobile-based interventions and therapeutic approaches beyond CBT. Furthermore, Fedele et al. (2017) found a small effect for mobile-based interventions for various health outcomes in youth with or without CC (SMD, 0.22; 95% CI, 0.14 to 0.29), reporting only an overall estimate with high clinical heterogeneity and neglecting fine-grained analyses on specific outcomes and subgroups. Other meta-analyses were constrained by a small number of included trials (Low and Manias, 2019; Thabrew et al., 2018), focusing either on specific control conditions (i.e., treatment-as-usual; Low and Manias, 2019) or psychological outcomes (i.e., symptoms of depression and anxiety; Thabrew et al., 2018), evincing non-significant findings for QoL, self-efficacy, and self-management (Low and Manias, 2019), as well as depression and anxiety symptom severity (Thabrew et al., 2018). Altogether, important research questions in regard to the efficacy and effect-modifying factors of IMIs for youth with CC are unsettled to date.
Hence, this meta-analysis aims to extend the current evidence-base by comprehensively investigating the efficacy of IMIs for novel and (disorder-)specific outcomes, as well as by resolving methodological shortcomings of prior reviews, focusing on three overarching research questions: First, to assess the efficacy of IMIs in improving psychological outcomes (i.e., depression, anxiety and stress symptom severity, as well as QoL and self-efficacy) in youth with CC. Second, to assess the efficacy of IMIs in improving disease-related physical/somatic outcomes, self-management and treatment adherence in regard to the CC. Third, to identify moderators of intervention effects.
2. Method
This meta-analysis is reported in accordance to the PRISMA guidelines (Moher et al., 2009; Table S1 in the supplementary material) and was registered with Open Science Framework (https://osf.io/83cwt/).
2.1. Eligibility criteria
Only randomized controlled trials (RCTs) published in English language were eligible for inclusion. Participants were children and adolescents (0–18 years; mean age ≤ 18) diagnosed with a CC, with the diagnosis established by a qualified health care professional or confirmed by valid diagnostic instruments. All CC as defined by the WHO (2018) and van der Lee et al. (2007) were eligible for inclusion. Interventions had to be delivered digitally via Internet- or mobile-based communication technology (e.g., web-platform, mobile health app for smartphones or tablets) and had a psychotherapeutic or medical focus (i.e., provided health- and/or mental-health-related support, with or without human guidance in an individual setting; group-based interventions were not included). Comparison conditions eligible for inclusion were various active and passive control groups (e.g., wait-list, treatment-as-usual or active treatments). Outcomes were mental health symptoms, physical/somatic symptoms related to the CC, QoL, self-efficacy, self-management and treatment adherence in regard to the CC. Outcome measures eligible for inclusion were standardized questionnaires or structured interviews, relying on self-, and/or parent report, or clinician ratings. Studies that evaluated IMIs targeting parents or legal guardians of youth with CC were also included, as long as they were primarily intended to improve outcomes in children (≤12 years) and adolescents (13–18 years).
2.2. Literature search and study selection
The search strategy was twofold. First, systematic searches were conducted in CENTRAL, Embase, MEDLINE/PubMed and PsycINFO, with a predefined set of search strings specifically developed for each database in Ovid, from database inception to May 15, 2019 (Table S2 in the supplementary material/e-Component). Second, reference lists of all eligible studies and other relevant reviews (Fedele et al., 2017; Kew and Cates, 2016; Lancaster et al., 2018; Thabrew et al., 2018) were manually searched to identify further studies that met our inclusion criteria.
One reviewer (A.S.) screened all titles and abstracts and discarded obviously irrelevant articles. Afterwards, the full texts of all potentially relevant articles were screened in terms of the aforementioned eligibility criteria independently by two reviewers (A.S. and L.S.).
2.3. Data extraction
Two independent reviewers (A.S. and L.S.) extracted data from the included studies. Disagreements were solved by consultation of a third reviewer (M.D.).
2.4. Risk of bias assessment
The methodological quality of included studies was assessed independently by two reviewers (A.S. and L.S.) with the Cochrane risk of bias assessment tool (Higgins et al., 2011) on all seven criteria: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting and (7) other bias. Of note, although blinding of participants and personnel is hardly to be achieved in psychotherapy research (Munder and Barth, 2018), it might be principally possible in pure self-help IMIs (Domhardt et al., 2020a; Furukawa et al., 2019).
The included studies were rated as having “low”, “unclear” or “high risk of bias” on each domain. Inter-rater reliability was calculated using Cohen's Kappa.
2.5. Data analysis
Random effects meta-analyses were performed to compute estimates of intervention effects for each a priori defined primary and secondary outcomes, contrasted with either active, passive or combined (i.e., active and passive) control conditions. Standardized mean differences (SMD) with 95% confidence intervals (95% CI) were calculated for continuous outcomes. Hedges' g, which corrects for biases due to small sample sizes (Cuijpers, 2016), was deployed.
Statistical heterogeneity was assessed with Cochran's Q and the I2-statistic, which designates the level of heterogeneity in percentages (Cuijpers, 2016; Higgins et al., 2003). It is assumed that a percentage of 25% indicates low, 50% moderate and 75% high statistical heterogeneity (Higgins et al., 2003). Forest plots were used for a visual presentation of the presence and nature of statistical heterogeneity. The possible influence of publication bias was planned to be determined by Egger's regression test for funnel plot asymmetry (at least 10 trials per meta-analysis; Higgins and Green, 2011) and the trim-and-fill method (Duval and Tweedie, 2000).
In order to identify possible moderators, subgroup analyses were intended to be performed in case of three or more studies per subgroup for: (1) variations of IMIs (internet-based psychotherapy, guided self-help, unguided self-help), (2) type of delivery (internet- vs. mobile-based), (3) psychotherapeutic background (CBT vs. other approaches), (4) type of CC, (5) participants (children, adolescents, parents/caregivers or families), (6) type of outcome assessment (self-report or ratings by parents/caregivers or clinicians/personnel), (7) type of control condition and (8) study quality.
3. Results
3.1. Study selection
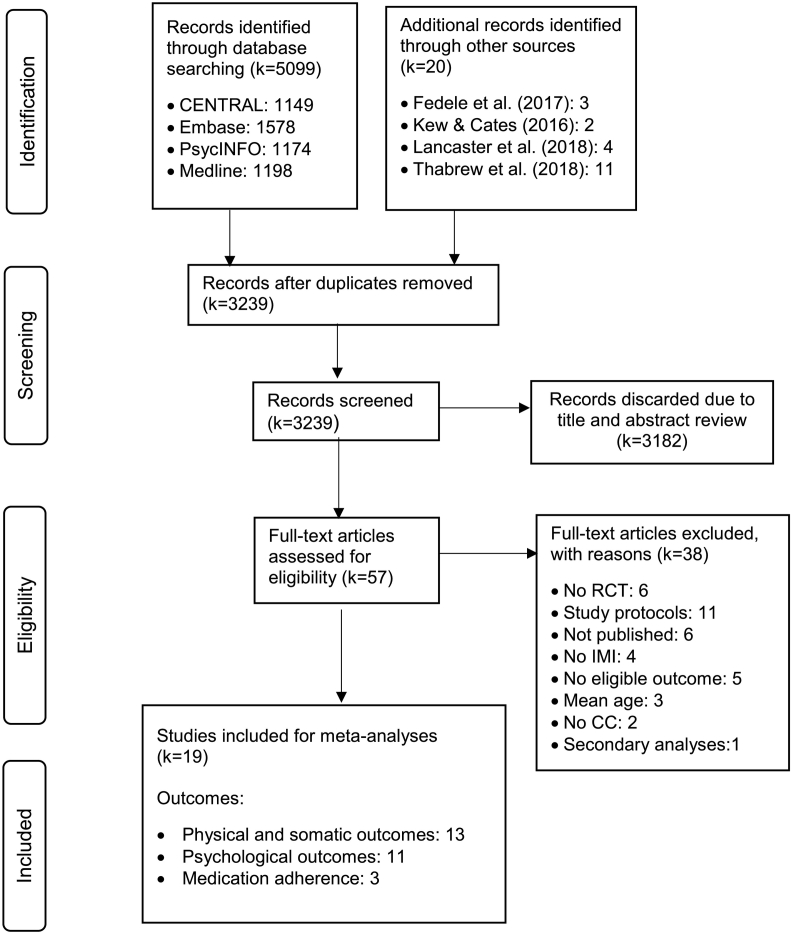
The systematic literature search identified a total of 5099 references. Finally, 19 studies (Berndt et al., 2014; Carlsen et al., 2017; Fiks et al., 2015; Franklin et al., 2006; Guendelman et al., 2002; Gustafson et al., 2012; Hanberger et al., 2013; Hicks et al., 2006; Jan et al., 2007; Johnson et al., 2016; Joseph et al., 2013; Law et al., 2015; Newcombe et al., 2012; Newton et al., 2009; Newton and Ashley, 2013; Nijhof et al., 2012; Palermo et al., 2009; Stinson et al., 2010; Stinson et al., 2016) representing 2410 participants fulfilled our eligibility criteria and were included in this meta-analysis. The detailed study selection process and reasons for exclusion are outlined in the PRISMA flow chart in Fig. 1.
Fig. 1.
PRISMA flow chart.
3.2. Study characteristics
The main characteristics of included studies are detailed in Table 1. Of the 19 eligible RCTs, five studies focused on diabetes (Franklin et al., 2006; Newton et al., 2009; Hanberger et al., 2013; Newton and Ashley, 2013; Berndt et al., 2014), seven on chronic respiratory conditions – among them six exclusively on asthma – (Fiks et al., 2015; Guendelman et al., 2002; Gustafson et al., 2012; Jan et al., 2007; Johnson et al., 2016; Joseph et al., 2013; Newcombe et al., 2012), three on pediatric pain (Hicks et al., 2006; Law et al., 2015; Palermo et al., 2009), two on juvenile idiopathic arthritis (Stinson et al., 2010; Stinson et al., 2016), and one study each on chronic fatigue syndrome (Nijhof et al., 2012) or inflammatory bowel disease (Carlsen et al., 2017). The vast majority of studies (94.7%) were conducted in western countries. Seven trials enrolled adolescents, five mixed samples of children and adolescents, five used families as entity for analysis and one trial either only children or parents. The mean sample size was 127 patients (SD = 129.88), with a mean age of 13.2 years (SD = 2.23), and 60.6% of participants were being females. Internet-based interventions were evaluated in 13 studies (68.4%) and mobile-based interventions were examined in three studies (15.8%); interventions combining web-based and mobile-based components were evaluated in three studies (15.8%). The three most frequently researched theoretical foundations and treatment approaches in IMIs were CBT (k = 4 studies; 21.0%), disorder-specific treatment guidelines (k = 4; 21.0%) and self-efficacy or self-management approaches (k = 4; 21.0%). Other theoretical orientations and treatment approaches evaluated in one study each (k = 1; 5.3%) were problem-solving therapy, skills-training, social cognitive theory, symptom-monitoring, decision-support-systems, behavior change and mixed approaches. Post-treatment assessment was 17 weeks (SD = 16.52) after base-line on average.
Table 1.
Main characteristics of included studies.
| Study | Chronic condition | Population (recruitment) | Age M (SD); range | Intervention (name) |
Theoretical foundation/treatment approach | Intervention components | Control group | Primary outcome | Post-treatment (weeks) |
Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Berndt et al. (2014)/GER | Type-1 diabetes | 68 children and adolescents (clinic) | 13.05 (2.45); 8–18 | TAU plus mobile or web-based disease monitoring applications (Mobil Diab) | German Diabetes Association guidelines | Telemonitoring: tracks health information, sharable with professionals and family members | TAU | Anthropometric data; metabolic control parameters; diabetes-related knowledge/children and adolescents |
4 | – |
| Carlsen et al. (2017)/DNK | Inflammatory bowel disease | 29 children and adolescents (clinic) | 14.86 (−); 10–17 |
Web-based disease monitoring | Patient-managed eHealth, with symptom-monitoring; Constant-care.com | Symptom monitoring with electronic traffic light system: schedules next treatment depending on cut off scores | TAU | Ability of the program (Individual treatment intervals)/children and adolescents | 4–12 | 24 (max.) |
| Fiks et al. (2015)/USA | Asthma | 60 parents (clinic) | 8.3 (1.9); 6–12 | Web-based support portal (MyAsthma) | Decision support system to both clinicians and parents; electronic health records | Identification of parents' concerns and goals for asthma treatment; tracking of symptoms, medication side effects & progress toward goals; asthma educational content; access to the asthma care plan | TAU plus decision support system | Acceptability of asthma care/parents and clinicians; satisfaction with the portal, experience using the portal/parents and clinicians | 26 | – |
| Franklin et al. (2006)/UK | Type-1 diabetes | 92 children and adolescents (clinic) | 13.17 (−);8–18 | TAU plus mobile-based support system (Sweet Talk) | Social cognitive theory | Automated, scheduled text messaging system: weekly reminder of goals set, daily tips to reinforce & optimize self-management and control | TAU | Glycemic control (HbA1c); Self-efficacy (SED)/children and adolescents; Diabetes knowledge (DKN)/children and adolescents; Social support (DSSI)/children and adolescents | 52 | – |
| Guendelman et al. (2002)/USA | Asthma | 134 children and adolescents (clinic) | 12.1 (2.6); 8–16 | Web-based self-management education program (Health Buddy) | The National Heart, Lung and Blood Institute recommendations | Interactive communication device: instant feedback about asthma symptoms, use of medication and health service, school attendance and activities | Asthma diary | Physical activity | 6 | 3 |
| Gustafson et al. (2012)/USA | Asthma | 301 families (managed care organizations) | 7.92 (2.52); 4–12 | Mobile-based support system (CHESS) plus case management | Self-efficacy theory | Psychoeducation, adherence strategies, decision-making tools, and support services; monthly calls to the parent; medication adherence; symptom-monitoring; psychosocial challenges and support | TAU plus asthma information | Symptom free days; adherence to Asthma controller medications (diary)/children | 12 | 6,9,12 |
| Hanberger et al. (2013)/SWE | Type 1 diabetes | 474 families (clinic) | 13.25 (3.7); 0–18 | Web-based network and education portal (Diabit) | Self-management; user-centred design process; web 2.0 portal | Self-management portal: diabetes-related information, social networking functions, enabling communication with peers and health-professionals; services for medical prescription renewal, appointments, etc. | TAU | Clinical variables (HbA1c, numbers of severe hypoglycemia) | 52 | – |
| Hicks et al. (2006)/CAN | Pediatric Pain | 47 families (physicians' offices and school) | 11.7 (2.1); 9–16 | Web-based CBT program | CBT | Relaxation techniques, cognitive strategies, psychoeducation, positive lifestyle choices (e.g. on diet, exercise, and social activity) | Wait-list | Pain (diary)/children and adolescents | 11 | 5 |
| Jan et al. (2007)/TWN | Asthma | 164 children (clinic) | 10.44 (2.83); 6–12 | Web-based educational and monitoring program (Blue Angel) | Global Initiative for Asthma program guidelines | Internet-based diary cards for symptom recording, peak expiratory flow rate, symptomatic support information; action plan; uploading and retrieving data for personal and physicians use | TAU plus asthma diary | PEF records/children; Asthma symptom (diary)/children; Pulmonary spirometric tests; Pediatric Asthma Quality of Life/children and caregivers; Childhood ACT/children and caregivers; Survey of asthma knowledge/caregivers; Treatment adherence (ACT)/children; Adherence to the diaries (survey on satisfaction)/children | 12 | – |
| Johnson et al. (2016)/USA | Asthma | 98 adolescents (academic medical center) | 14.05 (1.68); 12–17 | Web-based application medication monitoring and SMS dosing reminders (MMH) |
Behavior change theory | Tools to create and print a structured medication list, to attach a dosing schedule to each medication, to request a text-message reminder for each dose, and to visualize medication adherence performance for each medication | TAU and action list | Asthma management self-efficacy (Child Asthma Self-Efficacy Scale)/adolescents | 3 | – |
| Joseph et al. (2013)/USA | Asthma | 422 adolescents (school) | 15.6 (−); 15–19 | Web-based, tailored intervention (Puff City) | Health belief model, attribution theory, behavioral theory, motivational interviewing | Health messages and information on medication adherence, “inhaler nearby”, smoking reduction/cessation; tailored feedback; risk assessment reports; four submodules on (e.g., emotional support, motivation, etc.) | Generic asthma education | – | 52 | – |
| Law et al. (2015)/USA | Pediatric Headache | 83 families (clinic) | 14.5 (1.7); 11–17 | Web-based family CBT (Web-MAP) plus specialized headache treatment | CBT | Education; relaxation; cognitive strategies; sleep hygiene education; operant training; communication | Specialized head-ache treatment | Headache frequency (diary)/adolescents | 8–10 | 3 |
| Newcombe et al. (2012)/AUS | Chronic respiratory condition | 42 children and adolescents (clinic) | 13.58 (1.92); 10–17 | Web-based problem-solving program (Breathe Easier Online) | Problem solving therapy | Interactive online skills training that targeted problems in general in addition to illness-specific problems; (a)synchronous communication platform for peers; homework assignments | Wait-list | Depression (CES-DC)/children and adolescents; Social problem solving (SPSI-R:SF)/children and adolescents | 9 | – |
| Newton et al. (2009)/NZL | Type 1 diabetes | 78 adolescents (diabetes services) | 14.4 (2.37); 11–18 | Pedometer plus Mobile-based weekly text messages | American Diabetes Association recommendations | Pedometers and motivational text messages to increase physical activity | TAU | Physical activity (step count and self-report)/adolescents | 12 | – |
| Newton and Ashley (2013)/USA | Type-1 diabetes | 59 adolescents (Diabetes Center) | 14.5 (−); 13–18 | Web-based networking portal (Diabetes Teen Talk) | Bandura's self-efficacy theory | Blog, chat room and discussion forums with weekly topics (“Frustrations with diabetes”; “Benefits of good control”; “Family”; “Friends”; “Body image, exercise and diet”; “Community, school and sports”; “Worries about diabetes”) | TAU | HRQOL (DQOLY)/adolescents; Self-Efficacy (Diabetes Self-Management)/adolescents; Outcome Expectations (Diabetes Self-Management)/adolescents |
7 | – |
| Nijhof et al. (2012)/NLD | Chronic fatigue syndrome | 135 adolescents (clinic) | 15.85 (1.3); 12–18 | Web-based CBT (FITNET) | CBT | Guided and tailored iCBT with 21 modules and a comprehensive psychoeducation part (e.g., goals; sleep routine; cognition; fatigue specific interventions; physical activities and balance) | TAU | School attendance (percentage of the normal school schedule)/parents; Fatigue (CIS-20)/adolescents; Physical functioning (CHQ-CF87)/adolescents | 26 | – |
| Palermo et al. (2009)/USA | Pediatric Pain | 48 families (clinic) | 14.8 (2.0); 11–17 | Web-based family CBT (Web-MAP) | CBT and social learning frameworks | Child modules (psychoeducation; stress & negative emotions; deep breathing & relaxation; distraction; cognitive skills; sleep hygiene and lifestyle; staying active; relapse prevention) Parent modules (psychoeducation; stress & negative emotions; operant strategies; modeling; sleep hygiene & lifestyle; communication; relapse prevention) |
Wait-list | Activity limitations (CALI)/children and adolescents; Pain (diary)/children and adolescents | 8–10 | 3 |
| Stinson et al. (2010)/CAN | Juvenile idiopathic arthritis | 46 adolescents (care center) | 14.5 (1.48); 12–18 | Web-based self-management program (Teens Taking Charge) plus telephone support | Multicomponent treatment protocol based on self-management strategies, disease-specific information, and social support | Arthritis-specific information/psychoeducation; arthritis medications; managing symptoms, stress and negative thoughts; relaxation; distraction; other types of care (exercise, nutrition, splints); self-monitoring and supports; lifestyle issues; transitional care issues; two modules specifically for parents/caregivers | Tele-phone support | HRQOL (JAQQ)/adolescents and parents | 12 | – |
| Stinson et al. (2016)/CAN | Juvenile idiopathic arthritis | 30 adolescents (clinic) | 14.3 (1.7); 12–18 | Web-based mentoring program (iPeer2Peer) | Peer mentoring with a focus on skills training, self-management and social support strategies | Peer mentoring program with 10 sessions of 20–30 min Skype video calls; the training manual for peers provided suggested topics (e.g., coping strategies, lifestyle management, communication) | Wait-list | Feasibility (recruitment and withdrawal rates, program adherence, completed questionnaires, program engagement and satisfaction)/adolescents | 8 | – |
Abbreviations: ACQ, Asthma Control Questionnaire; ACT, asthma control test; ARCS, Adult Responses to Children's Symptoms; AUS, Australia; BMI, body mass index; CDI, Children's Depression Inventory; CALI, Child Activity Limitations Interview; CAN, Canada; CASES, Children's arthritis self-efficacy; CBT, cognitive behavior therapy; CES-DC, Center for Epidemiologic Studies Depression Scale for Children; CHQ-CF87, child health questionnaire physical functioning subscale; CIS, checklist individual strength; DISABKIDS, Quality of live questionnaires for children with chronic conditions; DKA, diabetic ketoacidosis; DKN, diabetes knowledge score; DNK, Denmark; DQOLY, quality of life for youth scale; DSSI, diabetes social support interview; ED, emergency department; GER, Germany; HRQOL, health related quality of life; IFX, infliximab; JAQQ, Juvenile Arthritis Quality of Life Questionnaire; M, mean; Max, maximal; MEPS, Medical issues, exercise, pain and social support questionnaire; MMH, MyMediHealth; NA, not applicable; NLD, Netherlands; NLZ, New Zealand; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; PedsQL, Pediatric Quality of Life Inventory; PEF, peak expiratory flows; PSQ, perceived severity of stress questionnaire; QPP, Patients' Perspective questionnaire; RCADS, Revised Child Anxiety and Depression Scale; RCMAS, Revised Children's Manifest Anxiety Scale; RPI, Recalled Pain Inventory; SD, standard deviation; SED, self-efficacy for diabetes; SPSI-R:SF, Social Problem-Solving Inventory–Revised Short Form; SQOL, subjective quality of life; SWE, Sweden; SWE-DES-SF, Swedish Diabetes Empowerment Scale, short version; TAU, Treatment as usual; TWN, Taiwan; UK, United Kingdom, VAS, visual analogue scale.
3.3. Risk of bias assessment
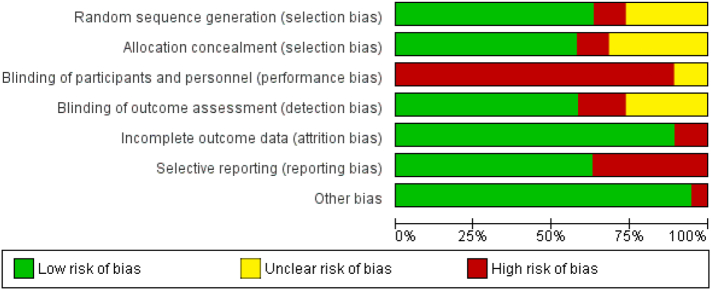
The results of the risk of bias assessments are detailed in Fig. 2, Fig. 3. The criterion least met was blinding of participants/personnel, as most studies were rated with a high risk of bias on this domain (89%). In the other domains, studies were predominantly rated with low risk of bias (random sequence generation: 63%; allocation concealment: 58%; blinding of outcome assessment: 58%; incomplete outcome data: 89%; selective reporting: 63%; other bias: 95%). The inter-rater reliability between reviewers was adequate (Cohen's Kappa = 0.78).
Fig. 2.
Risk of bias assessment presented as percentages across all included studies.
Fig. 3.
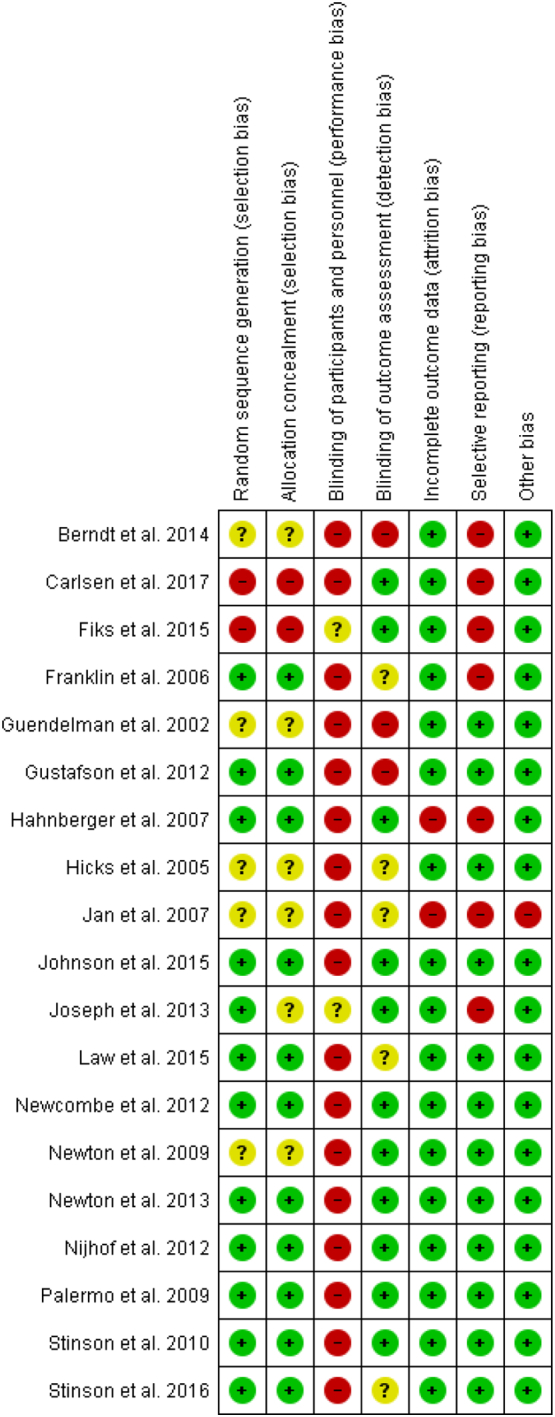
Risk of bias assessment: review authors' judgements about each risk of bias item for each included study.
3.4. Meta-analyses
Random-effects meta-analyses were performed on 16 comparisons altogether (Table 2). Studies that reported multiple outcome measures for the same outcome domain were aggregated into a single effect size for each outcome.
Table 2.
Meta-analytic comparisons.
| Outcome | k | Controls | Sample size (IG/CG) | Meta-analyses |
Heterogeneity |
||||
|---|---|---|---|---|---|---|---|---|---|
| g | P | 95% CI | Q | P | I2 (%) | ||||
| Primary outcomes | |||||||||
| All psychological outcomes | 11 | Any control condition | 308/290 | −0.14 | 0.09 | −0.30, 0.02 | 8.49 | 0.58 | 0 |
| Depression symptom severity | 3 | Any control condition | 69/65 | −0.29 | 0.1 | −0.63, 0.05 | 1.96 | 0.37 | 0 |
| QoL | 7 | Any control condition | 205/198 | −0.04 | 0.67 | −0.24, 0.15 | 2.4 | 0.88 | 0 |
| Self-efficacy | 5 | Non-active control condition | 156/143 | 0.38 | 0.001 | 0.15, 0.61 | 2.44 | 0.65 | 0 |
| Secondary outcomes | |||||||||
| All somatic outcomes | 13 | Any control condition | 851/825 | −0.13 | 0.03 | −0.25, −0.01 | 15.27 | 0.23 | 21 |
| Type-1 diabetes | |||||||||
| HbA1c | 4 | Non-active control condition | 349/331 | −0.03 | 0.67 | −0.18, 0.12 | 1.87 | 0.6 | 0 |
| Asthma | |||||||||
| Day symptoms | 3 | Active control condition | 312/316 | −0.13 | 0.25 | −0.35, 0.09 | 3.01 | 0.22 | 34 |
| Night symptoms | 3 | Active control condition | 312/316 | −0.05 | 0.56 | −0.20, 0.11 | 0.58 | 0.75 | 0 |
| Missed school days | 2 | Active control condition | 230/245 | −0.11 | 0.24 | −0.29, 0.07 | 0 | 0.99 | 0 |
| Health care utilization | |||||||||
| Care visits | 4 | Any control condition | 326/337 | −0.02 | 0.8 | −0.17, 0.13 | 2.52 | 0.47 | 0 |
| Care visits | 3 | Active control condition | 293/310 | −0.05 | 0.54 | −0.21, 0.11 | 0.98 | 0.61 | 0 |
| Hospitalization | 3 | Active control condition | 293/310 | −0.38 | 0.69 | −0.85, 0.1 | 16.42 | <0.001 | 82 |
| Pain | |||||||||
| Pain intensity | 5 | Any control condition | 127/113 | −0.33 | 0.06 | −0.67, 0.02 | 6.94 | 0.14 | 43 |
| Pain intensity | 4 | Non-active control condition | 87/76 | −0.5 | 0.002 | −0.81, 0.18 | 1.95 | 0.58 | 0 |
| Adherence | |||||||||
| Medication adherence | 3 | Active control condition | 206/206 | −0.15 | 0.5 | −0.58, 0.28 | 6.22 | 0.04 | 68 |
Abbreviations: CG, control group; IG, intervention group; IMI, internet- and mobile-based intervention; k = number of studies; QoL, quality of life.
An overall effect size was calculated for all psychological and disease-related outcomes combined, resulting in a non-significant Hedges` g of −0.08 (95% CI, −0.17 to 0.00; k = 19 studies; n = 2410 participants; I2 = 0%; Fig. S1).
3.5. Primary outcomes
One effect size was calculated for all psychological outcomes (studies that reported multiple outcomes were aggregated into a single effect size). IMIs showed no significant improvement in psychological outcomes at post-intervention when compared to controls (g = −0.14; 95% CI, −0.30 to 0.02; k = 11; n = 598; I2 = 0%; Fig. S2).
3.5.1. Depression symptom severity
For depression severity at post-intervention, IMIs evinced no significant differences compared to control conditions (g = −0.29; 95% CI, −0.63 to 0.05; k = 3; n = 134; I2 = 0%; Fig. S3).
3.5.2. Quality of life
IMIs showed no significant differences in the level of QoL at post-intervention compared to controls (g = −0.04; 95% CI, −0.24 to 0.15; k = 7; n = 403; I2 = 0%; Fig. S4).
3.5.3. Self-efficacy
A small effect was found in favor of IMIs compared to non-active control conditions for increasing the level of self-efficacy at post-intervention (g = 0.38; 95% CI, 0.15 to 0.61; k = 5; n = 300; I2 = 0%; Fig. S5).
3.6. Secondary outcomes
One effect size was calculated for all disease-related physical and somatic outcomes combined (multiple outcomes were aggregated into a single effect size). IMIs revealed a small significant effect in improving disease-related somatic outcomes at post-intervention compared to controls (g = −0.13; 95% CI, −0.25 to −0.01; k = 13; n = 1676; I2 = 21%; Fig. S6).
3.6.1. Diabetes related outcome
The aggregated effect size for changes in HbA1c in comparison of IMIs to non-active controls at post-intervention was not significant (g = −0.03; 95% CI, −0.18 to 0.12; k = 4; n = 680; I2 = 0%; Fig. S7).
3.6.2. Chronic respiratory diseases related outcomes
The efficacy for asthma symptoms was evaluated with random-effects meta-analyses specific for day and night symptoms. IMIs evinced no significant differences on day (g = −0.13; 95% CI, −0.35 to 0.09; k = 3; n = 628; I2 = 34%; Fig. S8) and night symptom severity (g = −0.05; 95% CI, −0.2 to 0.11; k = 3; n = 628; I2 = 0%; Fig. S9) at post-intervention when compared to active controls. Furthermore, the effect size on the number of missed school days in patients with asthma revealed no significant differences (g = −0.11; 95% CI, −0.29 to 0.07; k = 2; n = 475; I2 = 0%; Fig. S10).
3.6.3. Health service utilization
The two meta-analyses on the number of care visits revealed no advantages of IMIs, either when they were compared to combined control conditions (g = −0.02; 95% CI, −0.17 to 0.13; k = 4; n = 663; I2 = 0; Fig. S11) or compared to active controls only (g = −0.05; 95% CI, −0.21 to 0.11; k = 3; n = 603; I2 = 0; Fig. S12). Furthermore, no significant differences in the number of hospitalizations at post-intervention were found between IMIs and active controls (g = −0.38; 95% CI, −0.85 to 0.1; k = 3; n = 603; I2 = 82%; Fig. S13).
3.6.4. Pain intensity
The meta-analysis for pain intensity at post-intervention missed statistical significance, favoring IMIs compared to combined controls conditions (g = −0.33; 95% CI, −0.67 to 0.02; k = 5; n = 240; I2 = 43%; Fig. S14). When excluding the single study with an active comparison condition (Law et al., 2015), the meta-analysis revealed a moderate effect size in favor of IMIs compared to non-active controls in reducing pain intensity (g = −0.5; 95% CI, −0.81 to −0.18; k = 4; n = 163; Fig. S15), with reduced statistical heterogeneity (I2 = 0%).
3.6.5. Medication adherence
No significant differences were found for medication adherence at post-intervention between IMIs and active controls (g = −0.15; 95% CI, −0.58 to 0.28; k = 3; n = 412; I2 = 68%; Fig. S16).
3.7. Subgroup analyses and assessment of publication bias
Subgroup analyses were not possible, because of the limited number of trials per subgroup except for analyses on QoL. The analyses for studies focusing on type-1 diabetes (Berndt et al., 2014; Newton et al., 2009; Newton and Ashley, 2013) and studies investigating diseases other than diabetes (Hicks et al., 2006; Johnson et al., 2016; Stinson et al., 2010; Stinson et al., 2016) did not result in significant differences in the level of QoL at post-intervention (Fig. S17). Pre-planned analyses to determine the possibility of publication bias were not feasible, because of the small number of studies per comparison.
4. Discussion
This systematic review comprehensively evaluated research investigating the efficacy of digital health interventions in improving psychological and disease-related outcomes in youth with CC. Altogether, 19 RCTs representing 2410 patients were identified and included in this meta-analysis. Our results indicate that IMIs might be beneficial in improving levels of self-efficacy and some disease-related somatic outcomes; however, the effect sizes were small (to moderate) and most meta-analytic comparisons revealed no beneficial effects of IMIs over control conditions on a range of outcomes. These findings are in line with previous reviews (Kew and Cates, 2016; Lancaster et al., 2018; Thabrew et al., 2018), suggesting a limited benefit of IMIs in children and adolescents with CC. This empirical knowledge is of high relevance, since the digitalization of psychotherapeutic and psychosocial interventions is often considered as a powerful way – or even panacea – to overcome existing treatment gaps. Yet, the present study points to the need to look carefully on the specific benefits and limitations of IMIs in different populations and health care settings, especially when it comes to pediatric patients with comorbid mental health symptoms.
The ability to cope and psychologically adjust to CC is of great importance (de Ridder et al., 2008), and patients` self-efficacy beliefs (Bandura, 1978) and self-management (Ng et al., 2018) might be key to this endeavor. We found evidence that IMIs can increase the levels of self-efficacy in regard to the disease in children and adolescents with CC; a finding which is consistent with research by Lancaster et al. (2018) that has documented an increase in self-efficacy in patients through digital monitoring tools too. In their review of 13 RCTs, they also found promise that IMIs might increase the disease-related self-management in youth, which was not corroborated in our meta-analysis. Principally, the strength of IMIs to support the self-efficacy and self-management in juvenile patients seems not to be comparable to the magnitude of IMIs in adults with CC, consistently evincing larger effect sizes for a range of psychological outcomes (Bendig et al., 2018).
Furthermore, except for the meta-analyses on disease-related somatic outcomes and pain intensity (compared to non-active controls), our meta-analytic review revealed predominantly non-significant findings. These rather sobering results are in accordance with prior reviews (Kew and Cates, 2016; Lancaster et al., 2018; Thabrew et al., 2018), which equally could not find beneficial effects of IMIs in youth with CC for a range of psychological and disease-related outcomes, like depression and anxiety symptoms (Thabrew et al., 2018), QoL (Lancaster et al., 2018), disease status (Kew and Cates, 2016; Thabrew et al., 2018), health service utilization (Kew and Cates, 2016; Lancaster et al., 2018), and medication adherence (Lancaster et al., 2018). However, since we were able to include substantially more studies than former meta-analyses and evaluated novel outcomes additionally, the current findings are more robust and further underpin the assumption that IMIs might not be as powerful in pediatric patients with CC than in youth with mental disorders alone (Domhardt et al., 2020b; Vigerland et al., 2016) or in youth without clinically relevant pathology (Fedele et al., 2017). Nevertheless, since some comparisons only scantly missed significance, other reviews have revealed more encouraging findings for IMIs (Fedele et al., 2017; Hieftje et al., 2013), the question arises why the findings of the current meta-analysis are rather disenchanting. This is even more pressing, since the evidence-base for the effectiveness for psychological treatments delivered face-to-face for various mental health issues for youth without CC is well-ascertained (Zhou et al., 2015; Zhou et al., 2019), and there is some evidence that these interventions are also effective for several outcomes in youth with CC (Bennett et al., 2015; Kahana et al., 2008; Kibby et al., 1998; Sanders et al., 1994). For example, two systematic reviews revealed that face-to-face psychological interventions are effective for disease-management and emotional/behavioral problems (Kibby et al., 1998), as well as for symptoms of depression and anxiety (Bennett et al., 2015) in children and adolescents with CC, all with large effect sizes respectively. Similar to the theoretical foundations of IMIs found in our study, the majority of interventions in both reviews (Bennett et al., 2015; Kibby et al., 1998) were based on principles of CBT and related behavioral interventions.
The present predominantly non-significant findings in our meta-analysis might be due to several reasons. First, some IMIs had no clear psychotherapeutic foundation and were merely intended to assist the patients` self-management in one specific aspect (e.g., monitoring) and not to reduce mental health symptoms – while these outcomes were assessed in primary studies. As such, some interventions themselves might not have been suitably designed in order to achieve relevant effect sizes for youth with CC, lacking sophisticated psychotherapeutic components and strategies directly targeting comorbid mental health symptoms. Furthermore, a substantial number of IMIs (8 out of 19) are older than ten years, presumably not representative to the mobile technology and platforms available today. Second, although we were able to include substantially more studies than prior reviews (Low and Manias, 2019; Thabrew et al., 2018), single meta-analytic comparisons might be still underpowered to detect significant effects (Cuijpers et al., 2014; Harrer et al., 2019; Hedges and Pigott, 2001) – which might be in addition (very) small for single-component interventions, but clinically relevant for patients nonetheless (Domhardt et al., 2019). Third (and clinically most relevant), given the high strain and specific needs of youth with CC, it might be that this particular group of patients is not responsive to IMIs to the same extent than other patient populations (Andersson et al., 2019; Bendig et al., 2018; Domhardt et al., 2020b). Additionally, empirical knowledge on moderators, for example in regard to therapist involvement (Baumeister et al., 2014; Domhardt et al., 2020a; Reyes-Portillo et al., 2014), support by parents (Domhardt et al., 2020b; Fedele et al., 2017) or developmentally-related differences in age groups (Reins et al., 2020; Thabrew et al., 2018), is still largely pending, but is central to inform the allocation of individual patients to the most fitting psychological intervention, either delivered face-to-face or via digital technology. Next to these efforts toward precision medicine (Cohen and DeRubeis, 2018) and “precision digital health” (Domhardt et al., accepted), important future directions for forthcoming research are on how to best improve interventions and maximize treatment outcomes. At that, involving the patients` perspective into intervention development (Geirhos et al., submitted; Holmes et al., 2018; Low and Manias, 2019) and disentangling the active ingredients of interventions by means of component and mediation studies (Domhardt et al., 2020c) might be key avenues, to increase the potential benefits of digital health interventions for youth with CC and other underserved populations.
Besides several strengths (e.g., comprehensive scope, rigorous study selection and data extraction, low statistical heterogeneity across most meta-analyses and high overall quality of included studies), this review has also limitations. First, the results need to be interpreted with caution, as the number of eligible studies was rather small and some pre-planned analyses on specific outcomes were not possible. Second, distinctive conclusions are challenging, because of differences in interventions and treatment approaches (Table 1), as well as types of CC diseases and outcomes. Third, the generalizability of this meta-analytic review might be curtailed, since the vast majority of studies were conducted in western countries and resorted to predominantly older female adolescents. Hence, generalizations should only be applied to the CC and populations included in this meta-analysis. Lastly, the intended tests for publication bias, as well as pre-planned subgroup and moderator analyses were not feasible, given the lack of maturity of this specific research field.
5. Conclusion
The findings of this meta-analysis point to a limited benefit and efficacy of digital health interventions in children and adolescents with CC. Yet, we also found indications that IMIs improve self-efficacy and disease-related somatic outcomes in pediatric patients, warranting further investigations. Moreover, future high-quality research is urgently needed, to identify factors contributing to improved user-centered interventions with better treatment outcomes; as well as to comprehensively weigh the actual potential of IMIs to (cost-)effectively scale up and complement collaborative mental health care for youth with CC.
Role of the funder
The funding source BMBF had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit this paper for publication.
CRediT authorship contribution statement
Matthias Domhardt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Domhardt, Schröder, Baumeister.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Domhardt, Schröder.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Schröder, Domhardt.
Administrative, technical, or material support: Baumeister, Schröder, Domhardt.
Supervision: Baumeister, Domhardt.
Declaration of competing interest
Harald Baumeister is a consultant for different insurance companies, health care associations, and psychotherapy chambers and gives talks and workshops and conducts third party-funded projects on e-health interventions. All authors declare that they have no conflicts of interest.
Acknowledgment
This work is funded by the German Federal Ministry of Education and Research (BMBF; Grant Identification FKZ 01GL1740A and FKZ 01GL1740E) and realized within the project “Chronic Conditions in Adolescents: Implementation and Evaluation of Patient-centred Collaborative Healthcare (COACH)”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.invent.2021.100373.
Appendix A. Supplementary data
Supplementary material
References
- Andersson G., Titov N., Dear B.F., Rozental A., Carlbring P. Internet-delivered psychological treatments: from innovation to implementation. World Psychiatry. 2019;18(1):20–28. doi: 10.1002/wps.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L.H., Alonso J., Mneimneh Z., Wells J.E., Al-Hamzawi A., Borges G., Bromet E., Bruffaerts R., de Girolamo G., de Graaf R., Florescu S., Gureje O., Hinkov H.R., Hu C., Huang Y., Hwang I., Jin R., Karam E.G., Kovess-Masfety V.…Kessler R.C. Barriers to mental health treatment: results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2014;44(6):1303–1317. doi: 10.1017/S0033291713001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Adv. Behav. Res. Ther. 1978;1(4):139–161. doi: 10.1016/0146-6402(78)90002-4. [DOI] [PubMed] [Google Scholar]
- Baumeister H., Reichler L., Munzinger M., Lin J. The impact of guidance on internet-based mental health interventions — a systematic review. Internet Interv. 2014;1(4):205–215. doi: 10.1016/j.invent.2014.08.003. [DOI] [Google Scholar]
- Bendig E., Bauereiß N., Ebert D.D., Snoek F., Andersson G., Baumeister H. Internet-based interventions in chronic somatic disease. Deutsches Aerzteblatt Online. Advance online publication. 2018 doi: 10.3238/arztebl.2018.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S., Shafran R., Coughtrey A., Walker S., Heyman I. Psychological interventions for mental health disorders in children with chronic physical illness: a systematic review. Arch. Dis. Child. 2015;100(4):308–316. doi: 10.1136/archdischild-2014-307474. [DOI] [PubMed] [Google Scholar]
- Berndt R.-D., Takenga C., Preik P., Kuehn S., Berndt L., Mayer H., Kaps A., Schiel R. Impact of information technology on the therapy of type-1 diabetes: a case study of children and adolescents in Germany. Journal of Personalized Medicine. 2014;4(2):200–217. doi: 10.3390/jpm4020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlbring P., Andersson G., Cuijpers P., Riper H., Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn. Behav. Ther. 2018;47(1):1–18. doi: 10.1080/16506073.2017.1401115. [DOI] [PubMed] [Google Scholar]
- Carlsen K., Houen G., Jakobsen C., Kallemose T., Paerregaard A., Riis L.B., Munkholm P., Wewer V. Individualized infliximab treatment guided by patient-managed eHealth in children and adolescents with inflammatory bowel disease. Inflamm. Bowel Dis. 2017;23(9):1473–1482. doi: 10.1097/MIB.0000000000001170. [DOI] [PubMed] [Google Scholar]
- van Cleave J., Gortmaker S.L., Perrin J.M. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623–630. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- Cobham V.E., Hickling A., Kimball H., Thomas H.J., Scott J.G., Middeldorp C.M. Systematic review: anxiety in children and adolescents with chronic medical conditions. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(5):595–618. doi: 10.1016/j.jaac.2019.10.010. [DOI] [PubMed] [Google Scholar]
- Cohen Z.D., DeRubeis R.J. Treatment selection in depression. Annu. Rev. Clin. Psychol. 2018;14:209–236. doi: 10.1146/annurev-clinpsy-050817-084746. [DOI] [PubMed] [Google Scholar]
- Compas B.E., Jaser S.S., Dunn M.J., Rodriguez E.M. Coping with chronic illness in childhood and adolescence. Annu. Rev. Clin. Psychol. 2012;8:455–480. doi: 10.1146/annurev-clinpsy-032511-143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P. 2016. Meta-Analysis in Mental Health: A Practical Guide. (Colofon) [Google Scholar]
- Cuijpers P., Turner E.H., Koole S.L., van Dijke A., Smit F. What is the threshold for a clinically relevant effect? The case of major depressive disorders. Depress. Anxiety. 2014;31(5):374–378. doi: 10.1002/da.22249. [DOI] [PubMed] [Google Scholar]
- Domhardt M., Baumeister H. Psychotherapy of adjustment disorders: current state and future directions. tHE World Journal of Biological Psychiatry. 2018;19:S21–S35. doi: 10.1080/15622975.2018.1467041. sup1. [DOI] [PubMed] [Google Scholar]
- Domhardt M., Ebert D.D., Baumeister H. Internet- und mobilebasierte Interventionen. In: Kohlmann C.-W., Salewski C., Wirtz M.A., editors. Psychologie in der Gesundheitsförderung. 1st ed. Hogrefe; 2018. pp. 397–410. [Google Scholar]
- Domhardt M., Geßlein H., von Rezori R.E., Baumeister H. Internet- and mobile-based interventions for anxiety disorders: a meta-analytic review of intervention components. Depress. Anxiety. 2019;36(3):213–224. doi: 10.1002/da.22860. [DOI] [PubMed] [Google Scholar]
- Domhardt M., Letsch J., Kybelka J., Koenigbauer J., Doebler P., Baumeister H. Are internet- and mobile-based interventions effective in adults with diagnosed panic disorder and/or agoraphobia? A systematic review and meta-analysis. J. Affect. Disord. 2020;276:169–182. doi: 10.1016/j.jad.2020.06.059. [DOI] [PubMed] [Google Scholar]
- Domhardt M., Steubl L., Baumeister H. Internet- and Mobile-based interventions for mental and somatic conditions in children and adolescents: a systematic review of meta-analyses. Zeitschrift Für Kinder- Und Jugendpsychiatrie Und Psychotherapie. 2020;48(1):33–46. doi: 10.1024/1422-4917/a000625. [DOI] [PubMed] [Google Scholar]
- Domhardt M., Steubl L., Boettcher J., Buntrock C., Karyotaki E., Ebert D.D., Cuijpers P., Baumeister H. Mediators and mechanisms of change in internet- and mobile-based interventions for depression: a systematic review. Clin. Psychol. Rev. 2020:101953. doi: 10.1016/j.cpr.2020.101953. [DOI] [PubMed] [Google Scholar]
- Domhardt M., Cuijpers P., Ebert D.D., Baumeister H. More light? Opportunities and pitfalls in digitalised psychotherapy process research. Front. Psychol. 2021 doi: 10.3389/fpsyg.2021.544129. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Ebert D.D., van Daele T., Nordgreen T., Karekla M., Compare A., Zarbo C., Brugnera A., Øverland S., Trebbi G., Jensen K.L., Kaehlke F., Baumeister H. Internet- and Mobile-based psychological interventions: applications, efficacy, and potential for improving mental health. Eur. Psychol. 2018;23(2):167–187. doi: 10.1027/1016-9040/a000318. [DOI] [Google Scholar]
- Fedele D.A., Cushing C.C., Fritz A., Amaro C.M., Ortega A. Mobile health interventions for improving health outcomes in youth: a meta-analysis. JAMA Pediatr. 2017;171(5):461–469. doi: 10.1001/jamapediatrics.2017.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiks A.G., Mayne S.L., Karavite D.J., Suh A., O’Hara R., Localio A.R., Ross M., Grundmeier R.W., Adams Akinbami, Ammenwerth Andrews, Bell Bloom, Bourgeois Bukstein, Cabana Charles, Gustafson Harris, Hartman Hibbard, Jan Joseph, Joseph Laforest, Lorence Schmier, Shields Silvestre, Smith Stacey, Cleave Van, Viswanath Zorc. 135(4) 2015. Parent-Reported Outcomes of a Shared Decision-making Portal in Asthma: a Practice-based RCT; pp. e965–e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin V.L., Waller A., Pagliari C., Greene S.A. A randomized controlled trial of sweet talk, a text-messaging system to support young people with diabetes. Diabetic Medicine: a Journal of the British Diabetic Association. 2006;23(12):1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- Furukawa T.A., Karyotaki E., Suganuma A., Pompoli A., Ostinelli E.G., Cipriani A., Cuijpers P., Efthimiou O. Dismantling, personalising and optimising internet cognitive-behavioural therapy for depression: a study protocol for individual participant data component network meta-analysis. BMJ Open. 2019;8(11) doi: 10.1136/bmjopen-2018-026137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirhos A., Lunkenheimer F., Holl R.W., Minden K., Schmitt A., Temming S., Baumeister H., Domhardt M. Involving patients’ perspective in the development of an internet- and mobile-based CBT intervention for adolescents with chronic somatic conditions: findings from a qualitative study. Internet Interv. 2021 doi: 10.1016/j.invent.2021.100383. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloff N.E., LeNoue S.R., Novins D.K., Myers K. Telemental health for children and adolescents. International Review of Psychiatry (Abingdon, England) 2015;27(6):513–524. doi: 10.3109/09540261.2015.1086322. [DOI] [PubMed] [Google Scholar]
- Guendelman S., Meade K., Benson M., Chen Y.Q., Samuels S. 156(2) 2002. Improving Asthma Outcomes and Self-Management Behaviors of Inner-City Children; p. 114. [DOI] [PubMed] [Google Scholar]
- Gulliver A., Griffiths K.M., Christensen H. Perceived barriers and facilitators to mental health help-seeking in young people: a systematic review. BMC Psychiatry. 2010;10:113. doi: 10.1186/1471-244X-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D., Wise M., Bhattacharya A., Pulvermacher A., Shanovich K., Phillips B., Lehman E., Chinchilli V., Hawkins R., Kim J.S. The effects of combining web-based eHealth with telephone nurse case management for pediatric asthma control: a randomized controlled trial. J. Med. Internet Res. 2012;14(4) doi: 10.2196/jmir.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanberger L., Ludvigsson J., Nordfeldt S. Use of a Web 2.0 portal to improve education and communication in young patients with families: randomized controlled trial. J. Med. Internet Res. 2013;15(8):55–68. doi: 10.2196/jmir.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer M., Cuijpers P., Ebert D. Doing meta-analysis in R. Advance Online Publication. 2019 doi: 10.5281/zenodo.2551803. [DOI] [Google Scholar]
- Hedges L.V., Pigott T.D. The power of statistical tests in meta-analysis. Psychol. Methods. 2001;6(3):203–217. doi: 10.1037//1082-989X.6.3.203. [DOI] [PubMed] [Google Scholar]
- Hicks C.L., von Baeyer C.L., McGrath P.J. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. J. Pediatr. Psychol. 2006;31(7):724–736. doi: 10.1093/jpepsy/jsj065. [DOI] [PubMed] [Google Scholar]
- Hieftje K., Edelman E.J., Camenga D.R., Fiellin L.E. Electronic media-based health interventions promoting behavior change in youth: a systematic review. JAMA Pediatr. 2013;167(6):574–580. doi: 10.1001/jamapediatrics.2013.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Green S. John Wiley & Sons; 2011. Cochrane Handbook for Systematic Reviews of Interventions.https://handbook.cochrane.org Version 5.1.0. [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:1–9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G.N., Johnson S.Z., Wills K.E., McKernon W., Rose B., Erklin S., Kemper T. Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: the mediational role of behavioral autonomy. J. Consult. Clin. Psychol. 2002;70(1):96–110. doi: 10.1037/0022-006X.70.1.96. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Ghaderi A., Harmer C.J., Ramchandani P.G., Cuijpers P., Morrison A.P., Roiser J.P., Bockting C.L.H., O’Connor R.C., Shafran R., Moulds M.L., Craske M.G. The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry. 2018;5(3):237–286. doi: 10.1016/S2215-0366(17)30513-8. [DOI] [PubMed] [Google Scholar]
- Jan R.-L., Wang J.-Y., Huang M.-C., Tseng S.-M., Su H.-J., Liu L.-F. An internet-based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine Journal and E-Health: The Official Journal of the American Telemedicine Association. 2007;13(3):257–268. doi: 10.1089/tmj.2006.0053. [DOI] [PubMed] [Google Scholar]
- Johnson K.B., Patterson B.L., Ho Y.-X., Chen Q., Nian H., Davison C.L., Slagle J., Mulvaney S.A. The feasibility of text reminders to improve medication adherence in adolescents with asthma. Journal of the American Medical Informatics Association: JAMIA. 2016;23(3):449–455. doi: 10.1093/jamia/ocv158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolstedt M., Wahlund T., Lenhard F., Ljótsson B., Mataix-Cols D., Nord M., Öst L.-G., Högström J., Serlachius E., Vigerland S. Efficacy and cost-effectiveness of therapist-guided internet cognitive behavioural therapy for paediatric anxiety disorders: a single-Centre, single-blind, randomised controlled trial. The Lancet Child & Adolescent Health. 2018;2(11):792–801. doi: 10.1016/S2352-4642(18)30275-X. [DOI] [PubMed] [Google Scholar]
- Joseph C.L.M., Ownby D.R., Havstad S.L., Saltzgaber J., Considine S., Johnson D., Peterson E., Alexander G., Lu M., Gibson-Scipio W., Johnson C.C., Akinbami Bartholomew, Borrelli Boudreaux, Bussey-Smith Croft, Dahlem Dijkstra, Emmons Geller, Guendelman Janz, Joseph Joseph, Joseph Joseph, Kreuter Kreuter, Krishna Larimer, Lenhart Lucas, Marlatt Moorman, Nelson O’Connor, Resnicow Rhee, Richman Riera, Rosenstock Ryan, Sonnad Tyc, van der Meer Weiland. Evaluation of a web-based asthma management intervention program for urban teenagers. Reaching the Hard to Reach. 2013;52(4):419–426. doi: 10.1016/j.jadohealth.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana S., Drotar D., Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J. Pediatr. Psychol. 2008;33(6):590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- Kew K.M., Cates C.J. Home telemonitoring and remote feedback between clinic visits for asthma. The Cochrane Database of Systematic Reviews. 2016;8 doi: 10.1002/14651858.CD011714.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby M.Y., Tyc V.L., Mulhern R.K. Effectiveness of psychological intervention for children and adolescents with chronic medical illness: a META-analysis. Clin. Psychol. Rev. 1998;18(1):103–117. doi: 10.1016/S0272-7358(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Lancaster K., Abuzour A., Khaira M., Mathers A., Chan A., Bui V., Lok A., Thabane L., Dolovich L. The use and effects of electronic health tools for patient self-monitoring and reporting of outcomes following medication use: systematic review. J. Med. Internet Res. 2018;20(12) doi: 10.2196/jmir.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law E.F., Beals-Erickson S.E., Noel M., Claar R., Palermo T.M. Pilot randomized controlled trial of internet-delivered cognitive-behavioral treatment for pediatric headache. Headache. 2015;55(10):1410–1425. doi: 10.1111/head.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee J.H., Mokkink L.B., Grootenhuis M.A., Heymans H.S., Offringa M. Definitions and measurement of chronic health conditions in childhood: a systematic review. JAMA. 2007;297(24):2741–2751. doi: 10.1001/jama.297.24.2741. [DOI] [PubMed] [Google Scholar]
- Lenhard F., Andersson E., Mataix-Cols D., Rück C., Vigerland S., Högström J., Hillborg M., Brander G., Ljungström M., Ljótsson B., Serlachius E. Therapist-guided, internet-delivered cognitive-behavioral therapy for adolescents with obsessive-compulsive disorder: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(1):10–19.e2. doi: 10.1016/j.jaac.2016.09.515. [DOI] [PubMed] [Google Scholar]
- Low J.K., Manias E. Use of technology-based tools to support adolescents and young adults with chronic disease: systematic review and meta-analysis. JMIR MHealth and UHealth. 2019;7(7) doi: 10.2196/12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer F., Domhardt M., Geirhos A., Kilian R., Mueller-Stierlin A.S., Holl R.W., Meissner T., Minden K., Moshagen M., Ranz R., Sachser C., Staab D., Warschburger P., Baumeister H. Effectiveness and cost-effectiveness of guided internet- and mobile-based CBT for adolescents and young adults with chronic somatic conditions and comorbid depression and anxiety symptoms (youthCOACHCD): study protocol for a multicentre randomized controlled trial. Trials. 2020;21(1):253. doi: 10.1186/s13063-019-4041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R. Trends in contacts with mental health professionals and cost barriers to mental health care among adults with significant psychological distress in the United States: 1997-2002. Am. J. Public Health. 2005;95(11):2009–2014. doi: 10.2105/AJPH.2003.037630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokkink L.B., van der Lee J.H., Grootenhuis M.A., Offringa M., Heymans H.S.A. Defining chronic diseases and health conditions in childhood (0–18 years of age): national consensus in the Netherlands. Eur. J. Pediatr. 2008;167(12):1441–1447. doi: 10.1007/s00431-008-0697-y. [DOI] [PubMed] [Google Scholar]
- Munder T., Barth J. Cochrane’s risk of bias tool in the context of psychotherapy outcome research. Psychother. Res. 2018;28(3):347–355. doi: 10.1080/10503307.2017.1411628. [DOI] [PubMed] [Google Scholar]
- Newcombe P.A., Dunn T.L., Casey L.M., Sheffield J.K., Petsky H., Anderson-James S., Chang A.B. Breathe easier online: evaluation of a randomized controlled pilot trial of an internet-based intervention to improve well-being in children and adolescents with a chronic respiratory condition. J. Med. Internet Res. 2012;14(1) doi: 10.2196/jmir.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K.H., Wiltshire E.J., Elley C.R. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care. 2009;32(5):813–815. doi: 10.2337/dc08-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K.T., Ashley A. Pilot study of a web-based intervention for adolescents with type 1 diabetes. J. Telemed. Telecare. 2013;19(8):443–449. doi: 10.1177/1357633X13512069. [DOI] [PubMed] [Google Scholar]
- Ng C.Y., Thomas-Uribe M., Yang Y.A., Chu M.C., Liu S.-D., Pulendran U.P., Lin B.-J., Lerner D.S., King A.C., Wang C.J. Theory-based health behavior interventions for pediatric chronic disease management: a systematic review. JAMA Pediatr. 2018;172(12):1177–1186. doi: 10.1001/jamapediatrics.2018.3039. [DOI] [PubMed] [Google Scholar]
- Nijhof S.L., Bleijenberg G., Uiterwaal C.S., Kimpen J.L.L., van de Putte E.M. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet. 2012;379(9824):1412–1418. doi: 10.1016/S0140-6736(12)60025-7. [DOI] [PubMed] [Google Scholar]
- Palermo T.M., Wilson A.C., Peters M., Lewandowski A., Somhegyi H. Randomized controlled trial of an internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1–2):205–213. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M., Shen Y. Depressive symptoms in children and adolescents with chronic physical illness: an updated meta-analysis. J. Pediatr. Psychol. 2011;36(4):375–384. doi: 10.1093/jpepsy/jsq104. [DOI] [PubMed] [Google Scholar]
- Reins J.A., Buntrock C., Zimmermann J., Grund S., Harrer M., Lehr D., Baumeister H., Weisel K., Domhardt M., Imamura K., Kawakami N., Spek V., Nobis S., Snoek F., Cuijpers P., Klein J.P., Moritz S., Ebert D.D. Efficacy and moderators of internet-based interventions in adults with subthreshold depression: an individual participant data meta-analysis of randomized controlled trials. Psychother. Psychosom. 2020:1–13. doi: 10.1159/000507819. [DOI] [PubMed] [Google Scholar]
- Reyes-Portillo J.A., Mufson L., Greenhill L.L., Gould M.S., Fisher P.W., Tarlow N., Rynn M.A. Web-based interventions for youth internalizing problems: a systematic review. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(12):1254–1270.e5. doi: 10.1016/j.jaac.2014.09.005. [DOI] [PubMed] [Google Scholar]
- de Ridder D., Geenen R., Kuijer R., van Middendorp H. Psychological adjustment to chronic disease. Lancet (London, England) 2008;372(9634):246–255. doi: 10.1016/S0140-6736(08)61078-8. [DOI] [PubMed] [Google Scholar]
- Sanders M.R., Shepherd R.W., Cleghorn G., Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J. Consult. Clin. Psychol. 1994;62(2):306–314. doi: 10.1037/0022-006X.62.2.306. [DOI] [PubMed] [Google Scholar]
- Stanton A.L., Revenson T.A., Tennen H. Health psychology: psychological adjustment to chronic disease. Annu. Rev. Psychol. 2007;58:565–592. doi: 10.1146/annurev.psych.58.110405.085615. [DOI] [PubMed] [Google Scholar]
- Stinson J., Ahola K.S., Forgeron P., Amaria K., Bell M., Kaufman M., Luca N., Luca S., Harris L., Victor C., Spiegel L. 14(1) 2016. The iPeer2Peer Program: A Pilot Randomized Controlled Trial in Adolescents with Juvenile Idiopathic Arthritis; p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson J.N., McGrath P.J., Hodnett E.D., Feldman B.M., Duffy C.M., Huber A.M., Tucker L.B., Hetherington C.R., Tse S.M.L., Spiegel L.R., Campillo S., Gill N.K., White M.E. An internet-based self-management program with telephone support for adolescents with arthritis: a pilot randomized controlled trial. J. Rheumatol. 2010;37(9):1944–1952. doi: 10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- Thabrew H., Stasiak K., Hetrick S.E., Wong S., Huss J.H., Merry S.N. E-health interventions for anxiety and depression in children and adolescents with long-term physical conditions. Cochrane Database of Systematic Reviews. Advance Online Publication. 2018 doi: 10.1002/14651858.CD012489.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torous J., Wykes T. Opportunities from the coronavirus disease 2019 pandemic for transforming psychiatric care with Telehealth. JAMA Psychiatry. Advance online publication. 2020 doi: 10.1001/jamapsychiatry.2020.1640. [DOI] [PubMed] [Google Scholar]
- Vigerland S., Lenhard F., Bonnert M., Lalouni M., Hedman E., Ahlen J., Olén O., Serlachius E., Ljótsson B. Internet-delivered cognitive behavior therapy for children and adolescents: a systematic review and meta-analysis. Clin. Psychol. Rev. 2016;50:1–10. doi: 10.1016/j.cpr.2016.09.005. [DOI] [PubMed] [Google Scholar]
- WHO Noncommunicable Diseases. 2018. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- Yonek J., Lee C.-M., Harrison A., Mangurian C., Tolou-Shams M. JAMA Pediatrics. Advance online publication; 2020. Key components of effective pediatric integrated mental health care models: a systematic review. [DOI] [PMC free article] [PubMed]
- Zhou X., Hetrick S.E., Cuijpers P., Qin B., Barth J., Whittington C.J., Cohen D., Del Giovane C., Liu Y., Michael K.D., Zhang Y., Weisz J.R., Xie P. Comparative efficacy and acceptability of psychotherapies for depression in children and adolescents: a systematic review and network meta-analysis. World Psychiatry: Official Journal of the World Psychiatric Association (WPA) 2015;14(2):207–222. doi: 10.1002/wps.20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhang Y., Furukawa T.A., Cuijpers P., Pu J., Weisz J.R., Yang L., Hetrick S.E., Del Giovane C., Cohen D., James A.C., Yuan S., Whittington C., Jiang X., Teng T., Cipriani A., Xie P. Different types and acceptability of psychotherapies for acute anxiety disorders in children and adolescents: a network meta-analysis. JAMA Psychiatry. 2019;76(1):41–50. doi: 10.1001/jamapsychiatry.2018.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material