Abstract
Introduction
Brazilian guidelines for schistosomiasis elimination recommend regular search of infection carriers and their timely treatment. This study evaluates the effect of educational actions (EAs) among schoolchildren on adherence to diagnosis and treatment, as well as on knowledge of the disease.
Methods
In April/2013, a questionnaire was applied to 6th-to-8th-grade pupils of eight public schools to evaluate prior knowledge of disease and self-reported risk behavior. Baseline parasitological survey (PS) was done in May/2013, followed by selective treatment and cure assessment. The schools were then randomly allocated to experimental (EG) and control (CG) groups, with and without EAs, respectively. EAs were conducted for 3 months from August/2013. Questionnaire was reapplied in November/2013, April/2014, October/2014, and October/2015 to evaluate changes in knowledge about the disease and self-reported risk behavior. Two further annual PSs (May/2014 and May/2015), each followed by treatment of positives, allowed to evaluate between-group differences and intra-group changes in adherence to diagnosis and treatment, and to follow-up prevalence and intensity of infection.
Results
Adherence to diagnosis did not differ significantly between EG (84.1%) and CG (81.1%) at baseline but was significantly higher in EG in subsequent PSs. Overall, adherence to treatment was higher than 90% in all three PSs; cure was 98.4%, egg-reduction was 99.8% and reinfection, 2.8%. Prevalence fell significantly in EC (from 23.5% to 6.8%) and CG (from 21.8% to 2.4%), the same occurring with intensity (from 54.2 to 4.6 epg in EG and from 38.4 to 1.3 epg in CG). Disease knowledge increased significantly in EG and CG; knowledge about disease transmission increased significantly more in the EG. Self-reported risk behavior remained above 67% and did not differ significantly between EG and CG.
Conclusion
EAs increased adherence of schoolchildren and improved knowledge about the disease, confirming that EAs are an important tool to enhance schoolchildren participation in control campaigns.
Keywords: Schistosoma mansoni, Schoolchildren, Health education, Brazil
Highlights
-
•
Educational actions improved adherence to stool testing.
-
•
Disease knowledge also improved with educational actions.
-
•
Risk behavior stayed high despite educational actions.
1. Background
Schistosomiasis is one of the main neglected diseases perpetuated by poverty in the world (King, 2010). At present, World Health Organization recommends its control by large-scale periodical treatment of population groups under risk, access of these groups to potable water, sanitation and health education, and control of intermediate hosts (WHO, 2020). As none of these measures employed separately has shown to be sustainable and effective in the long run, the involvement and effort of various public sectors, and the adequacy of different control strategies to local realities is essential (Rollinson et al., 2013; Tchuem-Tchuenté et al., 2017; Bergquist et al., 2017; Hurlimann et al., 2018; Parisi et al., 2019).
It is consensus that treatment conjugated to sanitation and safe water supply are the most effective measures to eliminate schistosomiasis (Al Ghahtani and Amin, 2005; N'Diaye et al., 2016; Shiff, 2017; Bergquist and Gray, 2019). The sustainability of these measures may be enhanced if they are associated to educational health initiatives capable of considering local specificities (Schall, 1987; Massara and Schall, 2004; Ejike et al., 2017; Sun et al., 2017; Inobaya et al., 2018; Hailu et al., 2018; Appleby et al., 2019).
Children at school age are a particularly important target group in actions related to the control of schistosomiasis in endemic areas. First, because they concentrate relatively high prevalence and intensity rates of infection, associated to persistent risk factors, including behavioral (Gazzinelli et al., 2017); second, because this group's rates of infection may be used as a reference to determine the most appropriate treatment scheme in the communities under risk (WHO, 2006, WHO, 2011), as well as to evaluate the reach of morbidity control and elimination goals (WHO, 2013a). It is therefore, a group who has to be specially mobilized and sensitized not only to avoid risk behaviors (Munisi et al., 2017) but also to accept the offer of frequent treatments in high risk areas and have periodic follow-up stool tests, even in low-risk areas.
Low adherence rates in school-age children are common in areas subject to repeated test-and-treat cycles (Massara et al., 2006; Favre et al., 2015) as well as periodic mass drug administration (MDA), (Sady et al., 2013; Knopp et al., 2016; Inobaya et al., 2018), which may hamper WHO recommendations (WHO, 2011) not only of treatment coverage (75% or more of children in school-age under risk), as also of follow-up tests in sentinel schools to obtain minimally reliable prevalence estimates. The occurrence of low adherence in this key group can compromise the work of local health teams, hinder the satisfactory implementation of control actions and weaken the efforts to eliminate the disease as a public health problem (Cabello et al., 2016).
The present Brazilian Ministry of Health (MoH) guideline for most of the endemic areas involves active search followed by treatment of infection carriers, through periodic parasitological surveys of populations under risk; the minimum coverage recommended is 80% for diagnosis and treatment (Brasil, 2014; Favre et al., 2015). Although the MoH does not follow strictly WHO's recommendation of administering praziquantel annually to elementary and junior high school students independent of individual diagnosis (WHO, 2012), this guideline meets public health policies and the epidemiological specificities of the country, maintaining the commitment with WHO's (WHO, 2013a) goals of eliminating schistosomiasis.
As occurs in various schistosomiasis endemic countries (Price et al., 2015; Sacolo et al., 2018), in Brazil school has been considered a strategic space to construct, debate and spread issues related to this and other diseases that engage the community as a whole (Massara and Schall, 2004; Gazzinelli et al., 2006; Favre et al., 2009; Pereira et al., 2010; Gazzinelli et al., 2016). The school space is also especially advantageous for implementing strategies that involve diagnosis and treatment and a starting point to expand such actions for the community (Cabello et al., 2016).
Under this perspective, the present study developed an educational strategy directed and adequate to the reality of children from elementary schools of endemic municipalities, having schistosomiasis as inductor theme, the school as operational base and teachers as multipliers of knowledge of the disease. The study's primary goal was to evaluate if this strategy may contribute to obtain and/or maintain satisfactory levels of adherence to the diagnosis by stool testing and treatment with praziquantel. The secondary goal was to verify if such strategy is also capable of improving the knowledge of the disease.
2. Materials and methods
2.1. Study area and baseline information
The study was carried out in Malacacheta municipality, located in the schistosomiasis endemic area in Minas Gerais, Brazil. Cabello et al. (2016) described the municipality's demographic and socioeconomic characteristics and obtained baseline information of the disease in the elementary school population (1st to 9th grades). Thus, of 3102 schoolchildren enrolled in March 2013, 2519 (81.2%) adhered to the baseline parasitological survey using the Kato-Katz method (Katz et al., 1972), one stool sample with two slides, that identified 539 (21.3%) positives for S. mansoni. Of the positives, 503 (93.3%) adhered to treatment with praziquantel (60 mg/kg); however, only 363 (72.1%) of those who received treatment adhered to the follow-up survey 45 days after.
Cabello et al. (2016) also applied a closed-question questionnaire to schoolchildren from 6th to 8th grades to obtain information on their previous knowledge of schistosomiasis and risk behavior related to it. Thus, of a total of 924 schoolchildren who answered the questionnaire, 95% showed some knowledge of schistosomiasis, although 76.2% reported contact with untreated water.
2.2. Study design and target group
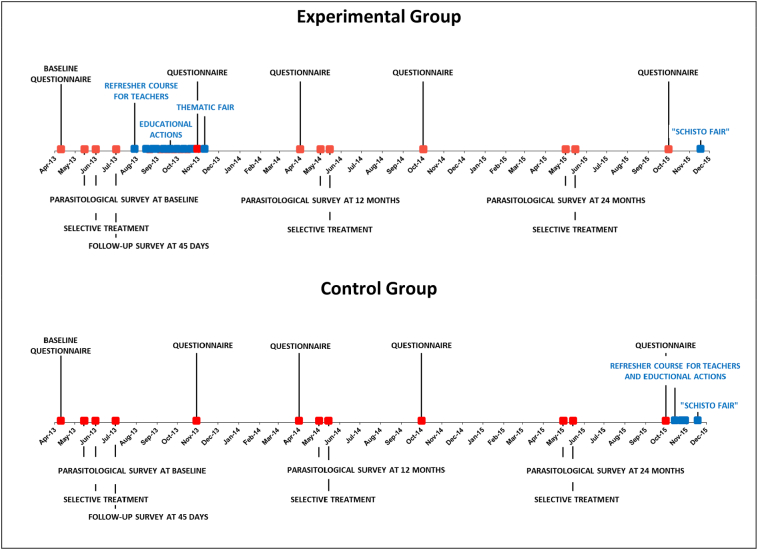
This was a longitudinal intervention study, carried out from March 2013 to October 2015, in which schoolchildren from 6th to 8th grades of the elementary schools were target of an educational strategy, having schistosomiasis as inducing theme. This strategy, applied in four schools chosen as Experimental Group, consisted of: (a) providing a refresher course in schistosomiasis for teachers of 6th to 8th grades in August 2013, (b) implementing EAs from August to October 2013 and (c) holding thematic fairs (one at each school) in November 2013; in four other schools, chosen as Control Group, this educational strategy was not implemented. Schoolchildren from both groups were presented to the following activities during the study (Fig. 1): (a) three annual parasitological surveys (May 2013, May 2014, May 2015), each followed by treatment of those positive, allowing to evaluate differences between groups and intragroup changes in adherence to diagnosis and treatment, and also to follow up the prevalence and intensity of infection during the period, and (b) questionnaire application on five occasions (April 2013, November 2013, April 2014, October 2014, October 2015), allowing to compare the knowledge and reported risk behavior between baseline (Pre-EAs) and each subsequent time point, as well as within groups.
Fig. 1.
Timeline of questionnaire applications, parasitological surveys, selective treatments (all in red) and educational actions (in blue) against schistosomiasis mansoni targeted at schoolchildren in the middle years of schooling (6th to 8th grades) from to 2013 to 2015 in eight elementary schools of Malacacheta, Minas Gerais, either subjected to the educational actions in 2013 (Experimental Group) or not (Control Group). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The impact of the educational strategy was evaluated comparing differences between the two groups (experimental and control) as for the proportion of schoolchildren that adhered to each diagnosis and treatment cycle among those who were attending school at the times of all three annual parasitological surveys. Changes in knowledge and self-reported risk behavior were followed up in schoolchildren of both groups that answered the questionnaire on all occasions (from April 2013 to October 2015).
2.3. Inclusion and exclusion criteria
This study included schoolchildren who, in April 2013, were enrolled in the 6th, 7th or 8th grades of eight schools that, from the 18 elementary schools in the municipality, were chosen to constitute the experimental and control groups. The choice of these schools was made by the following reasons: (a) the content “waterborne diseases”, previewed in the Science classes, would have been approached and therefore could enhance the research's educational actions, (b) inclusion of schoolchildren whose age group would have enough insight to understand and answer the questionnaire and (c) students from the middle years would remain at the same school during the 2 years of follow-up, reducing loss of subjects due to change of school caused by schooling progression.
2.4. Sample size
The minimum size of each group was calculated at 388 to detect two-tailed statistical significance at the 5% (0.05) level, power of 80% (Lwanga and Lemeshow, 1991). However, to compensate follow-up losses all students enrolled from the 6th to the 8th grade were invited to participate.
2.5. Data collection
2.5.1. Questionnaire
The questionnaire, described in the baseline study (Cabello et al., 2016), consisted of closed questions to access knowledge on schistosomiasis and self-reported risk behavior including three types of variable categories: (a) demographic: school, age, gender and area of residence (rural/urban); (b) knowledge of schistosomiasis: if they have heard of the disease (yes/no), if the theme was approached at school (yes/no), if they know people who have or had the disease (yes/no), if the schoolchildren had or have the disease (yes/no), if they know where transmission occurs (water/earth/air), if they know the transmitting agent (snail/mosquito/kissing bug), if they know where the transmission agent lives (water/earth/air), if they know what biological material is used for diagnosis (stool/urine/blood) and if they know if the disease can be treated (yes/no); and (c) risk behavior: if they have contact with freshwater bodies (yes/no) and the kind of contact (multiple answers): washing animals; washing vehicles/car/bike/clothes/utensils; swimming, fishing, collecting sand and bathing. For the analysis, occurrence of risk behavior was considered affirmative if the schoolchildren marked at least one of the options of contact with freshwater bodies.
The questionnaire was first applied before the beginning of the research, in April 2013, to access students' previous knowledge of the disease (Cabello et al., 2016). At that time neither the status of infection by S. mansoni nor the intervention group in which schools would be allocated were known. The questionnaire was reapplied after one (November/2013–1 M post-EAs), six (April/2014–6 M post-EAs), 12 (October/2014–12 M post-EAs) and 24 (October/2015–24 M post-EAs) months of EAs completion, the same procedure being adopted on all five occasions. Changes of knowledge and self-reported behavior were evaluated comparing answers provided in the pre-EAs application with each the four post-EAs applications. To avoid doubts and reduce chances of bias due to exchange of information between schoolchildren, a member from the research team applied the questionnaire, reading question by question, informing the rule for its filling out and clarifying doubts.
2.5.2. Parasitological information
The baseline parasitological survey was carried out in May 2013, right after the first application of the questionnaire, and included all schoolchildren that were attending school in April 2013 (Cabello et al., 2016). Diagnosis of schistosomiasis was performed by parasitological testing, using Kato-Katz quantitative method (Katz et al., 1972). The survey included distribution of containers and collection of samples the following day, preparation and reading of slides by experienced technicians. For quality control 10% of samples were read by an outside microscopist (WHO, 2013b).
The baseline survey allowed identify schoolchildren infected by S. mansoni, estimate the individual egg load and prevalence in the eight schools studied. Those tested positives were treated with 600 mg praziquantel tablets (Farmanguinhos/Fiocruz), administered in school environment under medical supervision, in a single 60 mg/kg dose as MoH's recommendation; those having soil-transmitted helminths (STH) received one tablet of albendazol (400 mg) to be taken at home under parental supervision (Brasil, 2014; Cabello et al., 2016). At 45 days after the first cycle of treatment, those positive to S. mansoni at baseline were invited to provide a new stool sample to evaluate treatment efficacy (WHO, 2013b). Two other annual surveys followed by treatment of the positives were carried out in May 2014 and May 2015 to follow up infection.
2.6. Paring and allocating schools in intervention groups
Six of the eight schools were paired according to the number of schoolchildren enrolled from the 6th to the 8th grades, the type of area they were located (rural/urban), and the percentages of adherence to the test and of positives for S. mansoni obtained in the baseline survey (Cabello et al., 2016). After pairing, these schools were allocated at random in the two intervention groups: Manoel, Mucuri and Stella in the Experimental Group, and Aristides, Coimbra and Monsenhor in the Control Group. Two other schools were allocated without pairing: Jaguaritira (rural) in the Experimental Group and Mestre (urban) in the Control Group.
2.7. Refresher course for teachers
In the beginning of August 2013, after schools had been allocated in the intervention groups, a refresher course of knowledge of schistosomiasis was offered to ten teachers from the 6th to the 8th grades of the four schools in the experimental group. Teacher participation was voluntary and approved by the direction of the four schools. The course took 5 days, full time, with the goal of informing, instrumenting and motivating teachers to act as knowledge multipliers on the disease in the classroom. It was developed to allow critical discussions on the “whys” of the disease's permanence in our country and in the municipality, orienting contents to the participant's reality.
The theoretic content available at classes was supplemented with laboratory and field practices, in order to make the process more dynamic and attractive by associating the theoretic content to real conditions where disease transmission occurs and emphasizing social and environmental aspects that determine the epidemiological reality of the municipality.
2.8. Selection of educational material
A workshop was carried out during the course to define the most adequate educational material for the target group and to standardize procedures to be adopted in the EAs to be implemented in the classroom by all multiplier teachers. Various educational materials were made available by the research group to approach the theme (booklets, mockups, videos, games, pieces in “cold porcelain”, showcase box with snail shells, microscopy slides of the developmental stages of S. mansoni, posters, folders). After ample discussion by the research team and multiplier teachers on the ludic nature of materials, their adequacy to the target age-range and coherence of themes to be approached, an “educational kit” to be used in the classroom by teachers was defined, consisting of the following material: (a) theoretical lesson, set up by multiplier teachers and revised by researchers, (b) the video “The X in ‘Xistose’” (https://www.youtube.com/watch?v=9xYRkS5Eq1U), a cartoon approaching the elements involved in the disease transmission cycle from a story of a pupil and his friends, (c) the video “Schistosomiasis: breaking the cycle” (https://www.youtube.com/watch?v=w7RXt8d1u6g), a documentary associating the disease cycle to narratives of residents of an endemic area in Northeastern Brazil, (d) booklets approaching the developmental stages of the parasite in hosts and the environment (Schall et al., 2007a, Schall et al., 2007b), (e) games to identify developmental stages of S. mansoni and assembly of the biological cycle and (f) showcase box for identifying shells of host snails and other freshwater snails. At the end of the course, all multiplier teachers received a certificate of participation issued by the Academic Secretariat of the René Rachou Institute - Fiocruz Minas.
2.9. Implementation of EAs
A second workshop was carried out with multiplier teachers before the beginning of EAs to discuss the best approach for each material, standardize the dynamics and basic procedures and define the time necessary for developing the EAs. After considering the other tasks of teachers at school and the school calendar, it was defined that the EAs would be implemented in two weekly classes, during the months of August, September and October/2013.
Two follow-up meetings were carried out with teachers during the implementation of EAs in the classroom to guide any possible adjustments, avoid discontinuity of actions and ensure that all educational kit material was addressed. A total of 19 classes and 520 schoolchildren participated in EAs and had access to the educational kit.
In December 2013, after the closing of EAs, science fairs on schistosomiasis were held at the four experimental schools. Materials and contents developed by schoolchildren and teachers during EAs (mockups, brochures, booklets, poems, dolls representing schistosomiasis carriers, parodies, songs and plays) approached aspects of local epidemiologic reality, disease cycle and risk situations were presented to the entire school community. The fairs were set up under the supervision of the research team, that furnished other material to enrich the exhibition, and they were shown during a whole day at each school, so that schoolchildren enrolled in all grades and teachers from all school shifts could have access to the material.
After applying the questionnaire 24 months post-EAs, in October 2015, the same schistosomiasis refreshing course was offered to all teachers of the four schools of the Control Group, and their schoolchildren had access to the same educational strategy implemented in the schools of the Experimental Group.
In November 2015, a fair called “’Xistose’ X-Day (Schisto Fair)” was set up in the main municipality square, in which educational material, songs and plays developed by schoolchildren and teachers of all eight schools were exhibited to the entire community.
2.10. Data management and analysis
Data were double entered into Microsoft Office Excel 2013, crosschecked for accuracy, and exported to Systat 13 for statistical analysis. Categorical variables such as adherence (yes/no) to testing and to praziquantel treatment, presence of parasite eggs (yes/no), gender (male/female), area of residence (rural/urban), classes of infection intensity (light/moderate/heavy) and the questionnaire answers (yes/no or correct/incorrect) were presented as frequencies and percentages (Cabello et al., 2016).
The following indices were calculated for S. mansoni infection (WHO, 2011; WHO, 2013b): (a) prevalence of infection was estimated as the proportion (%) of egg-positives among the tested subjects; (b) intensity of infection was expressed as the arithmetic mean of egg counts per gram of stool (epg) of the tested subjects; (c) the positive subjects were categorized into classes of egg counts: light (1–99 epg), moderate (100–399 epg) and heavy (≥ 400 epg); (d) parasitological cure rate was estimated as the proportion (%) of egg-positive subjects at baseline who became negative at 45 days after first treatment; (d) egg reduction rate (ERR) at 45 days after treatment was calculated by the formula: ERR (%) = 100 x (1- (arithmetic mean epg at follow up/arithmetic mean epg at baseline); (e) reinfection rate was determined by the proportion (%) of egg-negative subjects at 45 days after first treatment who became egg-positive at a subsequent parasitological survey. Adherence to stool testing was calculated as the percentage of subjects provided with collection containers who then returned stool samples. Adherence to treatment with praziquantel was calculated as the percentage of egg-positives who took the medication as prescribed.
The statistical analysis followed the guidelines of Sokal and Rohlf (1995). Univariate analysis of the adherence between the intervention groups (experimental/control), areas of residence (rural/urban) and gender (girls/boys) was performed by Pearson's chi-square (χ2) test at each time point (2013, 2014 and 2015 surveys). p-Values below 0.05 (5%) was assumed to indicate statistical significance. Binary logistic regression analysis was used to assess the independent effect of the variables shown to be statistically significant by univariate analysis. School clustering was accounted for and robust standard errors were used. Odds Ratio (OR) and 95% Confidence Intervals (CI) were used to evaluate significant effects of each variable; if the 95% CI did not contain unity, the effect was considered as significant. Significant differences (p < 0.05) in egg counts between experimental and control groups was determined by t-test of log10-transformed values, 1 being added to each egg-count value to include the logarithm of zero egg counts (negative results). The significance of differences in the percentages of assertive or correct responses on the knowledge of schistosomiasis and risk behaviors within each group (EG and CG), between baseline (Pre-EAs) and each subsequent time point (one, six, 12 and 24 months after the EAs) was evaluated by the McNemar test. The significance of the differences between the two groups (EG and CG) in each questionnaire application was evaluated by Pearson's chi-square (χ2) test.
3. Results
A total of 937 schoolchildren were enrolled in the study, as they were attending school at the times of all three annual parasitological surveys. Of the 767 adhering to stool testing in 2013, 395 (51.5%) were rural residents, 388 (50.6%) were females (Table 1), and their age was 12.7 ± 1.4 years (arithmetic mean and standard deviation). Adherence of participants to the yearly stool testing was higher in the 2013 survey than in the two subsequent ones and did not significantly differ between the groups later chosen as experimental and control. It decreased progressively in subsequent surveys in both intervention groups staying above 70% and significantly higher in the experimental group. In the three surveys, adherence to stool testing was significantly higher among schoolchildren residing in the rural area than those from the urban area and did not differ between boys and girls (Table 1). The logistic regression to evaluate the independent effect of the intervention group and area of residence in the adherence to stool testing showed that, at baseline, being resident in the rural area increased chances of adhering to testing; in the two subsequent surveys, both residing in the rural area and belonging to the experimental group, i.e. having access to EAs, significantly increased the chance of adhering to stool testing (Table 2).
Table 1.
Univariate analysis of the adherence to yearly stool testing from 2013 (baseline) to 2015 among schoolchildren in the middle years of schooling (6th to 8th year) in eight public schools of Fundamental Education in Malacacheta, Minas Gerais, either subjected to schistosomiasis-related educational actions in 2013 (Experimental Group) or not (Control Group).
| Parameter | 2013 |
2014 |
2015 |
||||
|---|---|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | ||
| Number adhering to stool testing (N = 937) | 767 (81.8) | 78.8–84.6 | 632 (67.4) | 63.9–70.8 | 557 (59.4) | 55.7–63.0 | |
| By intervention Group | Experimental (N = 414) | 343 (84.1) | 78.2–86.7 | 306 (73.9) | 68.7–78.6 | 307 (74.2) | 68.9–78.8 |
| Control (N = 523) | 424 (81.1) | 76.8–84.7 | 326 (62.3) | 57.3–67.0 | 250 (47.8) | 42.8–52.7 | |
| Test statistics | χ2 = 0.49, p = 0.48 | χ2 = 14.12, p = 0.00⁎ | χ2 = 66.57, p = 0.00⁎ | ||||
| By area of residence | Rural (N = 449) | 395 (88.0) | 84.0–91,1 | 358 (79.7) | 75.0–83.8 | 355 (79.1) | 74.3–83.2 |
| Urban (N = 488) | 372 (76.2) | 71.5–80.3 | 274 (56.2) | 50.9–61.2 | 202 (41.4) | 36.3–46.5 | |
| Test statistics | χ2 = 21.72, p = 0.00⁎ | χ2 = 69.28, p = 0.000⁎ | χ2 = 137.65, p = 0.00⁎ | ||||
| By gender | Female (N = 461) | 388 (84.2) | 80.0–87.7 | 314 (68.1) | 62.9–72.9 | 273 (59.2) | 53.9–64.3 |
| Male (N = 476) | 379 (79.6) | 75.1–83.6 | 318 (66.8) | 61.7–71.5 | 284 (59.7) | 54.4–64.7 | |
| Test statistics | χ2 = 3.26, p = 0.07 | χ2 = 0.18, p = 0.67 | χ2 = 0.02, p = 0.89 | ||||
Significance of differences in adherence between intervention groups, between areas of residence (rural/urban) and between genders (female/male) were evaluated by chi-square (χ2) test. p-Values below 0.05 indicate statistical significance. N, total number of children attending school at the times of the three annual parasitological surveys; n, number of adhering individuals; CI, confidence interval
Significant difference (p < 0.05).
Table 2.
Binary logistic regression analysis to assess the independent effect of the intervention group (experimental vs. control) and the area of residence (rural vs. urban) on the data of Table 1.
| Parameter | 2013 |
2014 |
2015 |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Group (Experimental) | 0.92 | 0.65–1.31 | 1.36 | 1.01–1.83⁎ | 2.44 | 1.81–3.28⁎ |
| Area (rural) | 2.33 | 1.61–3.36⁎ | 2.87 | 2.12–3.88⁎ | 4.61 | 3.43–6.20⁎ |
OR, odds-ratio; CI, confidence interval. Asterisks indicate a statistically significant effect, as the 95% CI did not contain unity.
Table 3 shows parasitological data relative to the schoolchildren who adhered to stool testing in each of the three annual surveys. At baseline, 64.2% of the egg-positives had light infection, and 35.8% eliminated more than 100 epg (moderate-to-high infection). In the 2014 and 2015 surveys there was a significant reduction in prevalence of infection as well as of moderate and intense infections that may be attributed to selective treatment, since adherence was above 94% in the three cycles and the parasitological cure after the first treatment was 98.4%. Of 125 schoolchildren who tested negative after treatment in 2013, three became egg-positive in the 2014 survey and none of them tested positive in 2015. In 2015, of the 27 positives, 24 (89%) had low infection and only three of them eliminated more than 100 epg.
Table 3.
Parasitological variables of schoolchildren in the middle years of schooling (6th to 8th year) who adhered to stool testing among those attending school at the times of the three annual parasitological surveys from to 2013 to 2015 in eight public schools of Fundamental Education in Malacacheta, Minas Gerais, Brazil.
| Variables | Year |
||
|---|---|---|---|
| 2013 (N = 767) | 2014 (N = 632) | 2015 (N = 557) | |
| Positives for S. mansoni (n, %, 95% CI) | 187 | 55 | 27 |
| 24.3 (20.9–28.0) | 8.7 (6.3–11.5) | 4.8 (3.0–7.3) | |
| Positives with light infection (1–99 epg) (n, %, 95% CI) | 120 | 42 | 24 |
| 64.2 (55.0–72.2) | 76.4 (59.0–88.0) | 88.9 (66.0–97.8) | |
| Positives with moderate infection (100–399 epg) (n, %, 95% CI) | 47 | 7 | 3 |
| 25.1 (17.7–33.3) | 12.7 (3.8–26.6) | 11.1 (1.2–31.0) | |
| Positives with heavy infection (≥ 400 epg) (n, %, 95% CI) | 20 | 6 | 0 |
| 10.7 (5.8–17.1) | 10.9 (2.8–24.3) | ||
| EPG (Arithmetic mean, 95% CI) | 45.5 | 11.3 | 3.1 |
| (29.0–62.0) | (5.1–17.4) | (1.2–5.1) | |
| Adherence to selective treatment (n, %, 95% CI) | 176 | 53 | 27 |
| 94.1 (88.7–97.2) | 96.4 (84.7–99.6) | 100 (85.1–100) | |
| Adherence to testing at 45 days after treatment (n, %, 95% CI) | 127 | – | – |
| 72.2 (63.7–79.3) | |||
| Egg –negatives at 45 days after treatment (n, %, 95% CI)a | 125 | – | – |
| 98.4 (93.1–99.8) | |||
| Egg reduction rate at 45 days after treatment (n, %, 95% CI) | 127 | – | – |
| 99.8 (99.4–100) | |||
| Re-infected after negativation at 45 days (n, %, 95% CI) | – | 3 | 0 |
| 2.8 (0.3–8.7) | |||
N, number of individuals adhering to stool testing. n, number of individuals in each variable; CI, confidence interval; epg, eggs per gram of stool; AM, arithmetic mean.
Parasitological cure.
At baseline, prevalence of infection was 30.3% (CI 95%: 23.5%–37.5%) in EG and 21.8% (CI 95%: 15.1% - 29.4%) in CG; intensity of infection was 54.2 (95% CI: 35.1–73.3) eggs per gram of stool (epg) for EG and 38,4 (95% CI: 12.9–64.0) epg for CG. Prevalence of infection fell to 12.7% (CI 95%: 8.7%–17.6%) in EG and 4.9% (95% CI: 2.5%–8.2%) in CG in 2014, and it fell further to 6.8% (95% CI: 3.9%–10.7%) in EG and 2.4% (95% CI: 0.7%–5.5%) in CG in 2015. Intensity of infection fell to 15.3 epg (95% CI: 6.0–24.6 epg) in EG and 7.5 epg (95% CI: −0.7–15.7 epg) in CG in 2014, and also fell further to 4.6 epg (95% CI: 1.3–7.9 epg) in EG and 1.3 epg (95% CI: −0.3 3.0 epg) in CG in 2015. The dataset of these results is available from the first author and the corresponding author upon request.
Table 4 shows the answers on knowledge and risk behavior of 279 schoolchildren who adhered to the stool testing in the three annual surveys and answered the questionnaire on the five occasions in which it was applied. At baseline, the percentages of assertive (yes) or correct answers did not differ significantly between EG and CG for topics 1, 2, 3, 4, 6 and 8; however, they were significantly higher in EG for topics 5 and 7, referring to the transmission agent (snail) and the biological material (stool) used for diagnosis, respectively. The lowest percentages were observed in topic 3, related to the individual disease history (16.7% in EG and 12.2% in CG). Percentages of reported contact with water bodies (topic 9 in Table 4) were 52% in EG and 59% in CG at baseline and did not differ significantly within or between the groups throughout the study. The percentages of reported risk behavior (topic 10 in Table 4), which were 85.3% in EG and 87.5% in CG at baseline, did not differ significantly between groups but decreased significantly within them along the study to 72.4% and 67.5%, respectively.
Table 4.
Percentage values of assertive (yes) or correct answers to topics relative to knowledge of schistosomiasis and risk behavior reported by 279 schoolchildren who adhered to the stool testing in the three annual surveys and answered the questionnaire on the five occasions in which it was applied.
| Topics | Groups | Statistics | Pre-EAs | 1 month post-EAs | 6 months post-EAs | 12 months post-EAs | 24 months post-EAs |
|---|---|---|---|---|---|---|---|
| 1) If already heard of schistosomiasis (yes/no) | CG | % (yes) | 87.0 | 95.9 | 99.2 | 99.2 | 100 |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| EG | % (yes) | 94.2 | 100 | 100 | 100 | 100 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 3.58/0.06 | 4.35/0.04 | 0.01/0.05 | 0.01/0.91 | 0.00/1.00 | |
| 2) If already heard of disease at school (yes/no) | CG | % (yes) | 56.1 | 80.5 | 92.7 | 97.6 | 93.5 |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| EG | % (yes) | 64.7 | 97.4 | 97.4 | 99.4 | 100 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 1.81/0.18 | 20.04/0.00 | 2.51/0.11 | 0.56/0.46 | 8.24/0.00 | |
| 3) If has or had schistosomiasis (yes/no) | CG | % (yes) | 12.2 | 28.5 | 27.6 | 29.3 | 30.1 |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| EG | % (yes) | 16.7 | 42.9 | 41.7 | 46.2 | 47.4 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 0.77/0.38 | 5.62/0.02 | 5.31/0.02 | 7.57/0.01 | 7.94/0.01 | |
| 4) Where transmission occurs (water/soil/air) | CG | % (water) | 82.9 | 91.9 | 93.5 | 95.1 | 96.7 |
| McNemar's p | – | 0.01 | 0.01 | 0.00 | 0.00 | ||
| EG | % (water) | 81.4 | 100 | 99.4 | 99.4 | 100 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 0.29/0.86 | 10.91/0.00 | 5.81/0.02 | 3.46/0.06 | 3.10/0.08 | |
| 5) What animal transmits schistosomiasis (snail/mosquito/kissing bug) | CG | % (snail) | 30.9 | 65.9 | 73.2 | 78.9 | 81.3 |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| EG | % (snail) | 43.5 | 98.7 | 96.8 | 98.7 | 100 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 4.18/0.04 | 53.47/0.00 | 30.65/0.00 | 27.87/0.00 | 29.39/0.00 | |
| 6) Where transmitter animal lives (water/soil/air) | CG | % (water) | 56.9 | 76.4 | 74.8 | 77.2 | 85.4 |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| EG | % (water) | 65.4 | 97.4 | 97.4 | 98.1 | 98.1 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 1.75/0.186 | 27.14/0.000 | 30.10/0.000 | 28.17/0.000 | 14.19/0.000 | |
| 7) What material is used for schistosomiasis diagnosis (stool/blood/urine/other) | CG | % (stool) | 43.1 | 89.4 | 92.7 | 95.1 | 96.0 |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| EG | % (stool) | 59.6 | 96.2 | 86.5 | 98.1 | 99.4 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 6.88/0.01 | 3.90/0.05 | 2.10/0.15 | 1.09/0.30 | 2.38/0.12 | |
| 8) Can schistosomiasis be treated (yes/no) | CG | % (yes) | 95.3 | 98.1 | 99.1 | 99.1 | 99.1 |
| McNemar's p | – | 0.08 | 0.21 | 0.21 | 0.21 | ||
| EG | % (yes) | 93.2 | 97.2 | 98.3 | 97.8 | 98.8 | |
| McNemar's p | – | 0.05 | 0.02 | 0.03 | 0.00 | ||
| CG vs EG | χ2/p | 2.77/0.09 | 0.51/0.48 | 0.00/1.00 | 0.07/0.79 | 0.00/1.00 | |
| 9) If has or had contact with water bodies (yes/no) | CG | % (yes) | 59.0 | 56.1 | 60.4 | 59.0 | 55.7 |
| McNemar's p | – | 0.47 | 0.71 | 1.00 | 0.41 | ||
| EG | % (yes) | 52.0 | 44.0 | 52.6 | 49.1 | 45.7 | |
| McNemar's p | – | 0.05 | 0.89 | 0.48 | 0.18 | ||
| CG vs EG | χ2/p | 0.00/0.96 | 0.01/0.91 | 1.32/0.25 | 0.31/0.58 | 0.00/0.97 | |
| 10) If contact with water bodies involved any risk behaviora (yes/no) | CG | % (yes) | 87.5 | 78.9 | 74 | 77.2 | 67.5 |
| McNemar's p | – | 0.03 | 0.00 | 0.02 | 0.00 | ||
| EG | % (yes) | 85.3 | 73.7 | 71.2 | 71.8 | 72.4 | |
| McNemar's p | – | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CG vs EG | χ2/p | 0.19/0.66 | 0.49/0.49 | 0.15/0.70 | 0.80/0.37 | 0.59/0.44 |
The Experimental Group (EG) had 156 children and the Control Group (CG), 123. Significant differences (p-values <0.05) between baseline (Pre-EAs) and each subsequent time point (1 month, 6 months, 12 months, and 24 months post-EAs) were evaluated by the McNemar test. Significant differences between the two groups in each questionnaire application was assessed by chi-square (χ2) test.
Washing animals/vehicles/clothes/utensils, swimming, fishing, collecting sand, bathing.
4. Discussion
Adherence (81.8%, 95% CI: 78.8%–84.6%) to the stool testing in the baseline survey was satisfactory, considering that the Technical Guidelines of the MoH recommend at least 80% of diagnosis coverage (Brasil, 2014). This high adherence may be attributed, in part, to the fact that the survey was carried out in the classroom, an environment that concentrates schoolchildren in a physical space where they socialize and tend to be more sensitive to the influence of teachers (Favre et al., 2009; Pereira et al., 2010). It may have been a consequence of the general mobilization triggered by the research team in the school environment before the first survey, through meetings with guardians and the school team where the importance of schistosomiasis in the local epidemiological context was stressed. The opportunity of knowing the individual infection status for both schistosomiasis and STH followed by prompt treatment of the infection carriers, after at least 4 years without any active-search campaign in the municipality, may have also contributed to the high adherence at baseline.
It is possible that embarrassment of schoolchildren as they grew older may have increased refusal to provide stool samples, as other studies have reported difficulties in obtaining adherence from teenagers (Massara et al., 2006; Sady et al., 2013; Favre et al., 2015). However, the significant difference in adherence to the testing between the two groups in the 2014 and 2015 surveys allows infer that EAs implemented by multiplier teachers, contextualized to the students' epidemiological reality, employing the educational kit adopted and reinforced by thematic fairs was a motivating strategy and contributed to maintain higher adherence to stool testing. Gazinelli et al. (2006) developed two educational strategies based on dialogues with schoolchildren of an endemic location in Minas Gerais and concluded that they encouraged the construction of concepts about the disease and influenced the adoption of positive attitudes toward their health, including improvement in adherence to testing. Studies carried out in China also promoted a higher adherence of children and teenagers to test and treatment after the implementation of EAs (Hu et al., 2005; Zhou et al., 2013). Evidence that EAs may contribute to increase adherence to stool tests is of special importance considering that MoH recommends regular test-and-treat cycles in low-risk areas. The difficulty of access of rural residents both to diagnosis and to treatment, may have contributed to a higher adherence of schoolchildren from this area (Table 2), where socioeconomic and environmental conditions tend to be more precarious and the risk of disease transmission higher if compared to urban areas.
Schoolchildren adherence to treatment in the three cycles was above the 80% percentage recommended for endemic areas by MoH (Brasil, 2014). The difficulty of ingestion of praziquantel by children due to the tablet size and bitterness, as well as the fear of adverse events (Coulibaly et al., 2018) were probably the main reasons for not achieving complete adherence in the two first cycles. In 2013, 11 infected schoolchildren did not adhere to treatment. Treatment by the local doctor proceeded without any serious adverse event and adherence reached 100% in the third treatment cycle.
The high percentage of treatment coverage and parasitological cure obtained here were particularly beneficial if we take in account that in 2013, 35.8% of schoolchildren infected by S. mansoni had moderate-to-intense infections, which are related to severe morbidity (McManus et al., 2018). Therefore, selective treatment was capable of zeroing or diminishing the egg load, decreasing the chance of serious sequelae in adult life (Savioli et al., 2004).
The following points may be important in reaching the high adherence to treatment in the 3 years of the study: (a) relevant information about the disease being provided by the research team to guardians and school staff, (b) easy access to infection status by delivering individual test results directly, (c) prompt treatment of the infection carriers overcoming the difficulty in obtaining praziquantel timely through the local health service. The difficulty of access to praziquantel has been reported in other municipalities of the endemic area in Minas Gerais (Reis et al., 2010; Quites et al., 2016).
Prevalence of S. mansoni obtained in the baseline survey was 24.3%, although the National Schistosomiasis Control Program has carried out successive control campaigns in this area since 1998. This worrying situation may be explained by the epidemiological history of the disease, the persistence of precarious socioeconomic and environmental conditions favorable to transmission, and the lack of any active-search campaign in the municipality in the previous 4 years (Cabello et al., 2016). A substantial reduction observed in both prevalence and intensity of infection after two annual stool surveys followed each by treatment of the positives. Studies carried out with schoolchildren resident in endemic localities in Brazil and in other countries also observed a similar impact of treatment for S. mansoni (Favre et al., 2009; Galvão et al., 2010; Olliaro et al., 2011; Reta and Erko, 2013; Favre et al., 2015). It is important to highlight that the 4.8% of positives registered in 2015 (Table 3) may reflect cases not previously detected by Kato-Katz and/or new cases represented by infected schoolchildren who, even attending school at the occasion of the two previous surveys did not deliver the stool sample to be tested.
The observed decrease in both prevalence and intensity of infection indicate an unquestionable impact of treatment, regardless of the educational actions implemented in EG, especially considering that a parasitological cure of 98% was detected before the beginning of EAs. The data show a desired and expressive reduction in infection, but they do not allow to attest the effectivity of EAs employed and affirm its direct contribution in reducing infection. Isolating the effect of EAs on infection is a methodologically complex task and ethically unworkable, as the MoH recommends that the identification of infection carriers must be followed by prompt treatment (Brasil, 2014). This difficulty was not exclusive to the present study. Other works designed to evaluate the impact of EAs on indicators of infection by S. mansoni were not capable of establishing a direct relation between Information, Education and Communication (IEC) actions and reduction of prevalence (Schall et al., 1993; Lima-e-Costa et al., 2002; Price et al., 2015). However, as pointed out by Asaolu and Ofoezie (2003), health education is a key measure for providing sustainability to results produced by treatment and sanitation. A meta-analysis carried out by Zhou et al. (2013) on the effects of health education in the prevalence of schistosomiasis japonicum showed a considerable impact in disease prevention, particularly if educational actions are implemented long term.
High percentages of schoolchildren from both groups reported having already heard of the disease at baseline (topics 1 and 2 in Table 4). It is not unlikely that control interventions, which ceased 4 years earlier, may have given some prior knowledge about the disease. However, the most plausible explanation is that previous knowledge may have been acquired when the theme “waterborne diseases” was presented as part of the curriculum content. However, it cannot be ruled out that baseline knowledge about the disease may have been influenced by the discussion triggered by the research team during the preliminary school meetings. In them, it was clear that the research's goal was to construct an educational proposal, adequate to schoolchildren reality, taking in account the previous knowledge they had of the disease, which would be accessed from answers in the Pre-EAs questionnaire. We emphasized that information exchanged during meetings should not be discussed at home or in the classroom. It is noteworthy that when the meeting was carried out, we did not know which schools would be target of the EAs. Thus, if there was leakage of information to schoolchildren, it was random and had a chance of occurring at any school.
Schoolchildren from both EG and CG showed a significant increase in most of the assertive (yes) or correct answers between baseline and each subsequent time point (Table 4), both in topics that reflect a more generic knowledge of the disease (topics 1, 2, 3) and those that involve a more specific level of information (topics 4, 5, 6, 7). The percentage of schoolchildren responding that the disease can be treated (topic 8) was greater than 90% in both groups at baseline and remained so in subsequent applications of the questionnaire; however, it increased significantly in EG but not in the CG.
Despite the overall improvement of schoolchildren knowledge of schistosomiasis, there were important differences between EG and CG (Table 4). At one-month post-EAs, the percentages of assertive (yes) or correct answers in topics 1 to 6 were significantly higher in EG. This indicates that information on schistosomiasis was satisfactorily addressed, allowing the schoolchildren from EG to enlarge knowledge of the disease and have better performance in the questionnaire soon after the implementation of EAs in the classroom. Six months after the EAs, children from EG maintained significantly higher percentages of correct answers in topics that denoted a more specific knowledge about transmission (where transmission occurs, what animal is the transmitter and where does it live), probably because they were shown snails and taught about the life cycle of schistosomes.
One of the practices implemented by multiplier teachers was the construction of a showcase box, an innovative activity in the school context and that allowed schoolchildren to identify the snail and get closer to local reality by creating their own material. As pointed out by Schall et al. (1987), the educational approach for the age group studied should make use of materials that help discrimination in learning and that makes schoolchildren experience science not only in school books or in readymade materials, but “setting their hands to work” and creating their own material, perceiving reality. As percentages of correct answers about the transmitter animal (topics 5, 6) remained significantly higher in EG up 2 years post-EAs, it is plausible to claim that the knowledge acquired through this innovative activity is more enduring.
Persistence of self-reported risk behaviors in both groups may be attributed to the lack of leisure options in the municipality, an issue not approached in the study, but pointed out by teachers, parents, and schoolchildren from both groups. Children, youth and even adults residing in the municipality adopt swimming, fishing, and bathing of animals as outdoor leisure. It is noteworthy that more than 50% of the schoolchildren who adhered to stool testing lived in the rural area (Table 1) where the supply of piped water is intermittent thus obliging families to frequently use freshwater bodies for leisure and domestic activities. Takeuchi et al. (2019) also observed high percentages (70% - 80%) of contact with unsafe water even after educational activities and suggested that the lack of leisure alternatives propitiates the maintenance of risk behaviors.
Other studies that developed educational programs in health also were not well succeeded in changing water-contact behavior (Palmeirim et al., 2018; Takeuchi et al., 2019). Uchoa et al. (2000), in a work developed in a small village in the north of Minas Gerais, Brazil, report disappointing results since the educational program was not effective in transforming information in preventive practices to avoid contact with transmission foci. Stothard et al. (2016), working on urinary schistosomiasis in teenage schoolchildren obtained unsatisfactory results when applying an educational strategy based on comic books and concluded that it is inadequate to think of reaching voluntary changes of behavior by using only educational material. However, an integrated health education module applying the Knowledge, Attitudes and Practices (KAP) approach may achieve improved preventive behavior in relation to schistosomiasis (Sacolo et al., 2018) and STH (Nath et al., 2020). Educational interventions to prevent schistosomiasis infection need to be coupled with alternative and safe water sources for occupational and recreational use to minimize infective water contact.
5. Conclusion
This study shows that EAs significantly improve adherence to stool surveys and knowledge about schistosomiasis among schoolchildren. The results confirm the importance of developing EAs in endemic communities associated to a broad mobilization of the target group in the school setting. This accomplishment may be attributed mainly to the involvement of teachers as multiplier agents of knowledge to ensure the attainment and sustainability of EAs in the classroom and promote reflections on the socioeconomic and cultural factors that determine disease, affect health of schoolchildren and exposes them to infection.
6. Study limitations
The main limitation of this study was a prior awareness about schistosomiasis elicited by the presence of the research team and by the diagnosis and treatment actions carried out at baseline in the 18 schools of the municipality, including the eight schools of this study. This may have made the impact of EAs less clear, as the number of children who reported having heard about the disease previously were high in both EG and CG. Other limitation of the study was the bias caused by interchange between EG and CG participants, which may have favored the diffusion of information and consequent improvement of knowledge among CG participants, since many of them attend common social spaces and even the same residence for being relatives. Although this situation may represent a bias, dissemination of relevant information on a health problem that disturbs the entire community is a desirable outcome. Further work is needed to validate the results, including in areas subjected to MDA, where individual diagnosis is not a precondition for treatment.
7. Research ethics
Before the study began, parents and guardians of the children at the 18 schools were invited to attend a meeting at their school, where they received clarifications about the study's goals and procedures and how it would benefit their children. Once informed and assured as to the terms of the study, they were asked to sign a declaration of free and informed consent to their child's inclusion in the study.
The research protocol was approved by the Research Ethics Committee of the René Rachou Institute/Fiocruz Minas (protocol n°18/2011). Research clarification procedures and signature of the Informed Consent Form for the students' participation in the research were the same as those adopted and described in the baseline study (Cabello et al., 2016).
Funding
This study was supported by the Oswaldo Cruz Foundation, Brazil (Papes VI - Fiocruz grant 407616/2012-8).
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors wish to thank Dr. Bárbara P. Gaspar for medical supervision, Marielly Gonçalves Abrantes and Wilson da Silva Guimarães for technical support, and the municipal health and education staff for providing facilities. Special thanks go to the school directors, teachers, schoolchildren, and families of Malacacheta.
Contributor Information
Tereza Cristina Favre, Email: tfavre@ioc.fiocruz.br.
Cristiano Lara Massara, Email: cristiano.massara@fiocruz.br.
Lilian Christina Nóbrega Holsbach Beck, Email: lbeck@ioc.fiocruz.br.
Otavio Sarmento Pieri, Email: opieri@ioc.fiocruz.br.
References
- Al Ghahtani A.G., Amin M.A. Progress achieved in the elimination of schistosomiasis from the Jazan region of Saudi Arabia. Ann. Trop. Med. Parasitol. 2005;99:483–490. doi: 10.1179/136485905X51292. [DOI] [PubMed] [Google Scholar]
- Appleby L.J., Tadesse G., Wuletawu Y., Dejene N.G., Grimes J.E.T., French M.D., Teklu A., Moreda B., Negussu N. Integrated delivery of school health interventions through the school platform: investing for the future. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaolu S.O., Ofoezie I.E. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86(2–3):283–294. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Bergquist R., Gray D.J. Schistosomiasis elimination: beginning of the end or a continued March on a trodden path. Trop. Med. Infect Dis. 2019;4 doi: 10.3390/tropicalmed4020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist R., Zhou X.N., Rollinson D., Reinhard-Rupp J., Klohe K. Elimination of schistosomiasis: the tools required. Infect. Dis. Poverty. 2017;6:158. doi: 10.1186/s40249-017-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil . 4th ed. MS/SVS/DVDT; Brasília: 2014. Vigilância da esquistossomose mansoni: diretrizestécnicas.http://bvsms.saude.gov.br/bvs/publicacoes/vigilanciaesquistossome mansoni diretrizes tecnicas.pdf [Google Scholar]
- Cabello R., Beck L., Massara C.L., Murta F.L.G., Guimaraes R., Pieri O.S., Schall V.T., Favre T.C. Schistosoma mansoni infection and related knowledge among schoolchildren in an endemic area of Minas Gerais, Brazil, prior to educational actions. Acta Trop. 2016;164:208–215. doi: 10.1016/j.actatropica.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Coulibaly J., Panic G., Yapi R., Kovač J., Barda B., N’Gbesso Y., Hattendorf J., Keiser J. Efficacy and safety of ascending doses of praziquantel against Schistosoma haematobium infection in preschool-aged and school-aged children: a single-blind randomised controlled trial. BMC Med. 2018:16. doi: 10.1186/s12916-018-1066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejike C.U., Oluwole A.S., Mogaji H.O., Adeniran A.A., Alabi O.M., Ekpo U.F. Development and testing of Schisto and ladders, an innovative health educational game for control of schistosomiasis in schoolchildren. BMC Res. Notes. 2017;10:236. doi: 10.1186/s13104-017-2545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre T.C., Pereira A.P., Galvao A.F., Zani L.C., Barbosa C.S., Pieri O.S. A rationale for schistosomiasis control in elementary schools of the rainforest zone of Pernambuco, Brazil. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre T.C., Pereira A.P., Beck L.C., Galvao A.F., Pieri O.S. School-based and community-based actions for scaling-up diagnosis and treatment of schistosomiasis toward its elimination in an endemic area of Brazil. Acta Trop. 2015;149:155–162. doi: 10.1016/j.actatropica.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Galvão A.F., Favre T.C., Guimaraes R.J., Pereira A.P., Zani L.C., Felipe K.T., Domingues A.L., Carvalho O.S., Barbosa C.S., Pieri O.S. Spatial distribution of Schistosoma mansoni infection before and after chemotherapy with two praziquantel doses in a community of Pernambuco, Brazil. Mem. Inst. OswaldoCruz. 2010;105:555–562. doi: 10.1590/s0074-02762010000400035. [DOI] [PubMed] [Google Scholar]
- Gazzinelli M.F., Reis D.C., Kloos H., Velasquez-Melendez G., Dutra I.R., Gazzinelli A. The impact of two education methods on knowledge of schistosomiasis transmission and prevention among schoolchildren in a rural community in northern Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2006;101(Suppl. 1):45–53. doi: 10.1590/s0074-02762006000900008. [DOI] [PubMed] [Google Scholar]
- Gazzinelli M.F., Lobato L., Andrade G., Matoso L.F., Diemert D.J., Gazzinelli A. Improving the understanding of schistosomiasis among adolescents in endemic areas in Brazil: a comparison of educational methods. Patient Educ. Couns. 2016;99:1657–1662. doi: 10.1016/j.pec.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli A., Oliveira-Prado R., Matoso L.F., Veloso B.M., Andrade G., Kloos H., Bethony J.M., Assuncao R.M., Correa-Oliveira R. Schistosoma mansoni reinfection: analysis of risk factors by classification and regression tree (CART) modeling. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailu T., Alemu M., Abera B., Mulu W., Yizengaw E., Genanew A., Bereded F. Multivariate analysis of factors associated with Schistosoma mansoni and hookworm infection among primary school children in rural Bahir Dar, Northwest Ethiopia. Trop. Dis. Travel. Med. Vaccines. 2018;4:4. doi: 10.1186/s40794-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G.H., Hu J., Song K.Y., Lin D.D., Zhang J., Cao C.L., Xu J., Li D., Jiang W.S. The role of health education and health promotion in the control of schistosomiasis: experiences from a 12-year intervention study in the Poyang Lake area. Acta Trop. 2005;96:232–241. doi: 10.1016/j.actatropica.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Hurlimann E., Silue K.D., Zouzou F., Ouattara M., Schmidlin T., Yapi R.B., Houngbedji C.A., Dongo K., Kouadio B.A. Effect of an integrated intervention package of preventive chemotherapy, community-led total sanitation and health education on the prevalence of helminth and intestinal protozoa infections in cote d’Ivoire. Parasit. Vectors. 2018;11:115. doi: 10.1186/s13071-018-2642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inobaya M.T., Chau T.N., Ng S.K., MacDougall C., Olveda R.M., Tallo V.L., Landicho J.M., Malacad C.M., Aligato M.F. Mass drug administration and the sustainable control of schistosomiasis: an evaluation of treatment compliance in the rural Philippines. Parasit. Vectors. 2018;11:441. doi: 10.1186/s13071-018-3022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz N., Chaves A., Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S., Person B., Ame S.M., Ali S.M., Muhsin J., Juma S., Khamis I.S., Rabone M., Blair L. Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: a cross-sectional survey. Parasit. Vectors. 2016;9:5. doi: 10.1186/s13071-015-1244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-e-Costa M.F., Guerra H.L., Firmo J.O.A., Uchôa E. Um estudo epidemiológico da efetividade de um programa educativo para o controle da esquistossomose em Minas Gerais. Rev Brasileira Epidemio. 2002;5:107–119. [Google Scholar]
- Lwanga S.K., Lemeshow S. World Health Organization; Geneva: 1991. Sample-Size Determination in Health Studies: A Practical Manual. [Google Scholar]
- Massara C.L., Schall V.T. A pedagogical approach of schistosomiasis – an experience in health education in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2004;99:113–119. doi: 10.1590/s0074-02762004000900021. [DOI] [PubMed] [Google Scholar]
- Massara C.L., Peixoto S.V., Enk M.J., da Silva Barros H., dos Santos Carvalho O., Sakurai E., Schall V. Evaluation of an improved approach using residences of schistosomiasis-positive school children to identify carriers in an area of low endemicity. Am. J. Trop. Med. Hyg. 2006;74:495–499. [PubMed] [Google Scholar]
- McManus D.P., Dunne D.W., Sacko M., Utzinger J., Vennervald B.J., Zhou X.N. Schistosomiasis. Nat. Rev. Dis. Primers. 2018;4(1):13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- Munisi D.Z., Buza J., Mpolya E.A., Angelo T., Kinung’hi S.M. Knowledge, attitude, and practices on intestinal schistosomiasis among primary schoolchildren in the Lake Victoria basin, Rorya District, North-Western Tanzania. BMC Public Health. 2017;17:731. doi: 10.1186/s12889-017-4767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath T.C., Adnan M.R., Sultana N., Husna A., Ndossi B.A. Integration of health education intervention to improve the compliance to mass drug administation for soil-tansmitted helmith infection in Bangladesh:an implementation research. Parasite Epidemiol. Control. 2020;11 doi: 10.1016/j.parepi.2020.e00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Diaye M., Dioukhane E.M., Ndao B., Diedhiou K., Diawara L., Talla I., Vernet C., Bessin F., Barbier D. Schistosomiasis sustained control program in ethnic groups around Ninefescha (eastern Senegal) Am. J. Trop. Med. Hyg. 2016;95:614–622. doi: 10.4269/ajtmh.15-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro P.L., Vaillant M.T., Belizario V.J., Lwambo N.J.S., Ouldabdallahi M. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl. Trop. Dis. 2011;5(6) doi: 10.1371/journal.pntd.0001165. e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeirim M.S., Hürlimann E., Knopp S., Speich B., Belizario V., Jr., Joseph S.A. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: a systematic review, meta-analysis and individual patient data analysis. PLoS Negl. Trop. Dis. 2018;12(4) doi: 10.1371/journal.pntd.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi S., Mazigo H.D., Kreibich S., Puchner K., Kasang C., Mueller A. Factors associated with relevant knowledge of intestinal schistosomiasis and intention to participate in treatment campaigns: a cross sectional survey among school children at Ijinga Island on Lake Victoria, North-Western Tanzania. BMC Public Health. 2019;19(1):1762. doi: 10.1186/s12889-019-8091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A.P., Favre T.C., Galvao A.F., Beck L., Barbosa C.S., Pieri O.S. The prevalence of schistosomiasis in school-aged children as an appropriate indicator of its prevalence in the community. Mem. Inst. Oswaldo Cruz. 2010;105:563–569. doi: 10.1590/s0074-02762010000400036. [DOI] [PubMed] [Google Scholar]
- Price A., Verma A., Welfare W. Are health education interventions effective for the control and prevention of urogenital schistosomiasis in sub-Saharan Africa? A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2015;109:239–244. doi: 10.1093/trstmh/trv008. [DOI] [PubMed] [Google Scholar]
- Reis D.C., Kloos H., King C., Quites H.F.O., Matoso L.F.M., Coelho K.R., Gazzinelli A. Accessibility to and utilisation of schistosomiasis-related health services in a rural area of state of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2010;105(4):587–597. doi: 10.1590/s0074-02762010000400039. [DOI] [PubMed] [Google Scholar]
- Reta B., Erko B. Efficacy and side effects of praziquantel in the treatment for Schistosoma mansoni infection in school children in Senbete town, northeastern Ethiopia. Tropical Med. Int. Health. 2013;18(11):1338–1343. doi: 10.1111/tmi.12187. [DOI] [PubMed] [Google Scholar]
- Rollinson D., Knopp S., Levitz S., Stothard J.R., Tchuem Tchuente L.A., Garba A., Mohammed K.A., Schur N., Person B. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Sacolo H., Chimbari M., Kalinda C. Knowledge, attitudes and practices on Schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect. Dis. 2018;18:46. doi: 10.1186/s12879-017-2923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sady H., Al-Mekhlafi H.M., Mahdy M.A., Lim Y.A., Mahmud R., Surin J. Prevalence and associated factors of Schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L., Albonico M., Engels D., Montresor A. Progress in the prevention and control of schistosomiasis and soil-transmitted helminthiasis. Parasitol. Int. 2004;53(2):103–113. doi: 10.1016/j.parint.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Schall V.T. Health education for children in the control of schistosomiasis. Mem. Inst. Oswaldo Cruz. 1987;82(Suppl. 4):285–292. doi: 10.1590/s0074-02761987000800054. [DOI] [PubMed] [Google Scholar]
- Schall V.T., Jurberg P., Almeida E.M., Casz C., Cavalcante F.G., Bagno S. Health education for 1st grade students. Evaluation of teaching materials and prevention of schistosomiasis. Rev Saúde Pública. 1987;21(5):387–404. doi: 10.1590/s0034-89101987000500005. [DOI] [PubMed] [Google Scholar]
- Schall V.T., Dias A.G., Malaquias M.L., Dos Santos M.G. Health education in 1st grade public schools at the periphery of Belo Horizonte, MG, Brazil. I. Evaluation of the program relative to schistosomiasis. Rev. Inst. Med. Trop. Sao Paulo. 1993;35(6):563–572. [PubMed] [Google Scholar]
- Schall V.T., Massara C.L., Enk M.J., Barros H.S. 1a. ed. IRR FIOCRUZ; Belo Horizonte: 2007. Os caminhos da esquistossomose no meio ambiente.https://www.arca.fiocruz.br/bitstream/icict/16096/2/VIRGINIA_SCHALL_et_al_CPqRR_2007.pdf accessed: 13/07/2020. [Google Scholar]
- Schall V.T., Massara C.L., Enk M.J., Barros H.S. 1a.ed. IRR FIOCRUZ; Belo Horizonte: 2007. Os caminhos da esquistossomose em nosso corpo.https://www.arca.fiocruz.br/bitstream/icict/16087/2/VIRGINIA_SCHALL_et_al_CPqRR_2007.pdf accessed: 13/07/2020. [Google Scholar]
- Shiff C. Why reinvent the wheel? Lessons in schistosomiasis control from the past. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R., Rohlf F.J. 3rd ed. W.H. Freeman and Company; New York: 1995. Biometry: The Principles and Practice of Statistics inBiological Research. [Google Scholar]
- Stothard J.R., Khamis A.N., Khamis I.S., Neo C.H., Wei I., Rollinson D. Health education and the control of urogenital schistosomiasis assessing the impact of the Juma na Kichocho comic-Stip medical booklet in Zanzibar. J. Biosoc. Sci. 2016;48(Suppl. 1):S40–S55. doi: 10.1017/S0021932016000122. [DOI] [PubMed] [Google Scholar]
- Sun L.P., Wang W., Hong Q.B., Li S.Z., Liang Y.S., Yang H.T., Zhou X.N. Approaches being used in the national schistosomiasis elimination programme in China: a review. Infect Dis Poverty. 2017;6:55. doi: 10.1186/s40249-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi R., Njenga S.M., Ichinose Y., Kaneko S., Estrada C.A., Kobayashi J. Is there a gap between health education content and practice toward schistosomiasis prevention among schoolchildren along the shores of Lake Victoria in Kenya? PLoS Negl. Trop. Dis. 2019;13(8) doi: 10.1371/journal.pntd.0007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem-Tchuenté L.A., Rollinson D., Stothard J.R., Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect. Dis. Poverty. 2017;6:42. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quites H.F., Abreu M.N., Matoso L.F., Gazzinelli A. Evaluation of schistosomiasis control activities in the Family Health Strategy in municipalities in the Jequitinhonha Valley, Minas Gerais, Brazil. Rev Bras Epidemiol. 2016;19(2):375–389. doi: 10.1590/1980-5497201600020014. [DOI] [PubMed] [Google Scholar]
- Uchoa E., Barreto S.M., Firmo J.O., Guerra H.L., Pimenta F.G., Jr., Lima-e-Costa M.F. The control of schistosomiasis in Brazil: an ethnoepidemiological study of the effectiveness of a community mobilization program for health education. Soc. Sci. Med. 2000;51(10):1529–1541. doi: 10.1016/s0277-9536(00)00052-6. [DOI] [PubMed] [Google Scholar]
- WHO . Coordinated Use of Antheliminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. World Health Organization; Geneva: 2006. Preventive chemotherapy in human helminthiasis. [Google Scholar]
- WHO . World Health Organization; Geneva: 2011. Helminth Control in School Age Children: A Guide for Managers of Control Programmes. [Google Scholar]
- WHO Schistosomiasis: population requiring preventive chemotherapy and number of people treated in 2010. Wkly Epidemiol. Rec. 2012;87:37–44. [PubMed] [Google Scholar]
- WHO . WHO; Geneva: 2013. Schistosomiasis: Progress Report 2001–2011 and Strategic Plan 2012–2020.http://apps.who.int/iris/handle/10665/78074 [Google Scholar]
- WHO . WHO; Geneva: 2013. Assessing the Efficacy of Anthelminthic Drugs against Schistosomiasis and Soil-Transmitted Helminthiasis. [Google Scholar]
- WHO Schistosomiasis. 2020. http://www.who.int/mediacentre/factsheets/fs115/en/ accessed: 13/07/2020.
- Zhou X., Chen R.S., Zheng D.R., Li J.R., Cheng X.B., Ao J.Q., Liu H.X. Effect of health education intervention in schools of Yanrui town, Yushan County. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25(6):675–676. [PubMed] [Google Scholar]