Abstract
Sertraline, an antidepressant, is commonly used to manage mental health symptoms related to depression, anxiety disorders, and obsessive–compulsive disorder. The use of sertraline has been associated with rare but severe hepatotoxicity. Previous research demonstrated that mitochondrial dysfunction, apoptosis, and endoplasmic reticulum stress were involved in sertraline-associated cytotoxicity. In this study, we reported that after a 24-h treatment in HepG2 cells, sertraline caused cytotoxicity, suppressed topoisomerase I and IIα, and damaged DNA in a concentration-dependent manner. We also investigated the role of cytochrome P450 (CYP)-mediated metabolism in sertraline-induced toxicity using our previously established HepG2 cell lines individually expressing 14 CYPs (1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7). We demonstrated that CYP2D6, 2C19, 2B6, and 2C9 metabolize sertraline, and sertraline-induced cytotoxicity was significantly decreased in the cells expressing these CYPs. Western blot analysis demonstrated that the induction of ɣH2A.X (a hallmark of DNA damage) and topoisomerase inhibition were partially reversed in CYP2D6-, 2C19-, 2B6-, and 2C9-overexpressing HepG2 cells. These data indicate that DNA damage and topoisomerase inhibition are involved in sertraline-induced cytotoxicity and that CYPs-mediated metabolism plays a role in decreasing the toxicity of sertraline.
Keywords: Sertraline, Liver toxicity, DNA damage, Topoisomerase I, Topoisomerase II, CYP2D6, CYP2C19, CYP2B6, CYP2C9
Introduction
Sertraline (Zoloft), a selective serotonin reuptake inhibitor (SSRI), is the most prescribed psychiatric medication in the United States, with over 37 million prescriptions per year (clincalc.com 2019; Grohol 2018). It is also used for the treatment of premenstrual syndrome (Marjoribanks et al. 2013) and premature ejaculation (Balon 1996; Mendels et al. 1995; Yi et al. 2019). Although generally considered a safe drug, sertraline-associated hepatic toxicity has been documented in preclinical studies and clinical case reports. In a recently published in vivo study, considerable morphometric and hepatic histological alternations, such as hepatocyte necrosis and hydropic degeneration, were observed in rabbits treated orally with sertraline for 9 weeks (Almansour et al. 2018). Clinically, multiple adverse hepatic events, including severe hepatitis, have been reported during sertraline therapy (Collados Arroyo et al. 2008; Collados et al. 2010; Fartoux-Heymann et al. 2001; Galan Navarro 2001; Hautekeete et al. 1998; Kim et al. 1999; Persky and Reinus 2003; Verrico et al. 2000). Our previous in vitro studies demonstrated that mitochondrial dysfunction, apoptosis, and endoplasmic reticulum (ER) stress are the underlying mechanisms of sertraline-induced hepatic cytotoxicity (Chen et al. 2014a, b; Li et al. 2012).
Thus far, it is not well known whether metabolism plays a role in sertraline-induced toxicity and if so, which enzymes are responsible for such actions. In a study by Almansour and colleagues, the observed hepatotoxicity in rabbits was suggested to be associated with sertraline metabolites from CYP oxidation in the hepatic tissues (Almansour et al. 2018). However, the contribution of the metabolites in sertraline’s toxicity is debatable because we previously found that cytotoxicity of sertraline was lower in metabolically competent rat primary hepatocytes than in HepG2 cells that have limited drug metabolism capability (Chen et al. 2014b) and that SKF525-A, a general inhibitor of CYP, exacerbated sertraline-induced toxicity in rat primary hepatocytes. Our results imply that the metabolism of sertraline catalyzed by CYP may contribute to the detoxification of sertraline.
In recognition of the role of metabolism in sertraline-associated cytotoxicity, the identification of drug metabolizing enzymes that are responsible for sertraline’s metabolism is of interest. Several studies have investigated the metabolism of sertraline using different cellular systems; however, the results have displayed some inconsistencies. for example, using microsomes isolated from human liver and human B-lymphoblastoid cell lines expressing CYP isoforms (CYP1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4), a study reported that CYP2D6, 2C9, 2B6, 2C19, and 3A4 were the five isoforms of CYP that metabolized sertraline with the percent contributions of 35, 29, 14, 13, and 9%, respectively (Kobayashi et al. 1999). These five CYPs were the main enzymes that contribute to the N-demethylation of sertraline, the first and major reaction in metabolism of sertraline. In contrast, in the study by Xu et al. (1999), CYP2D6 and 3A4 were reported not to be involved in N-demethylation of sertraline.
In the current study, we used our previously established HepG2 cell lines that stably overexpress individual CYPs to conduct a systematic analysis to understand better the involvement of drug metabolizing enzymes in sertraline-induced toxicity. These HepG2-derived cell lines, which stably express 14 human CYPs individually, including 1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7, have been characterized and their applications in metabolism-associated toxicity studies have been demonstrated (Chen et al. 2018b; Wu et al. 2016; Xuan et al. 2016). Using mass spectrometry analysis, the involvement of each CYP in the metabolism of sertraline was characterized in each cell line by measuring the decrease of parent drug (C17H18Cl3N) and the production of the major metabolite, N-desmethylsertraline (C16H15Cl2N). In addition, the mechanisms of sertraline-induced toxicity were investigated after a 24-h exposure. Since we have previously reported that treatment of sertraline altered the expression of 124 genes in the cell cycle pathway based on pathway analysis (Chen et al. 2014a), we further studied the effect of sertraline on cell cycle progression. We also investigated the effect of sertraline on the occurrence of DNA damage which alters cell cycle progression.
Materials and methods
Chemicals and reagents
Sertraline, dimethylsulfoxide (DMSO), Williams’ medium E, and propidium iodide were from Sigma-Aldrich (St. Louis, MO, USA). N-Desmethylsertraline and N-desmethylsertraline-d4 were purchased from Toronto Research Chemicals Inc. (North York, Canada). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA, USA). Antibiotic–antimycotic was from Life Technologies (Grand Island, NY, USA). For Western blotting assays, a primary antibody against the γ-H2A.X (Ser139) was purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies for topoisomerase I, CYP2B6, CYP2C9, and CYP2C19 were obtained from Abcam (Cambridge, MA, USA). Antibodies for topoisomerase IIα, CYP2D6, and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and treatment with sertraline
The HepG2 human hepatoma cell line was from the American Type Culture Collection (ATCC; Manassas, VA, USA). HepG2 cells were cultured in Williams’ medium E complete media containing 10% FBS and 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL Amphotericin B at 37 °C in a humidified atmosphere with 5% CO2. The passage number did not exceed 10. Unless otherwise specified, cells were seeded at a density of 2.5 × 105 cells/mL in a volume of 100 μL per well in 96-well plates, or in a volume of 8 mL in 60 mm tissue culture dishes, or 16 mL in 100 mm tissue culture dishes. Cells were cultured for 24 h and then treated with sertraline or the DMSO vehicle control. The final concentration of DMSO was 0.1%.
Mass spectrometry analyses of sertraline and N-desmethylsertraline
After a 24-h exposure to 5 μM sertraline, cells and culture media from each individual cell line were collected separately. Cell lysates were obtained by adding 100 μL Nanopure water, followed by three cycles of freezing and thawing. The protein concentrations of the cell lysates were determined using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The supernatants were diluted using four volumes of acetonitrile containing 100 ng/mL of the internal standard N-desmethylsertraline-d4, vortexed and then sonicated for 10 min. Samples were centrifuged at 12,000 rpm for 5 min and the supernatants were loaded into sample vials for UPLC–MS analysis. Five-μL portions of the supernatants were injected onto a Waters ACQUITY UPLC System coupled with a Waters Quattro Premier XE mass spectrometer. Sertraline, N-desmethylsertraline, and the N-desmethylsertraline-d4 were eluted on an ACQUITY UPLC HSS T3 column (2.1 mm × 50 mm, 1.8 μm) at 40 °C with mobile phases of LC–MS grade water (A) and acetonitrile (B), both containing 0.1% formic acid, at a flow rate of 0.3 mL/min. Elution started with 3% solvent B followed by a linear gradient of 3–95% solvent B in 2 min, returning to 3% B in 0.1 min, and maintained for 0.9 min to re-equilibrate the column. The LC eluent from 0.5 to 2.5 min was introduced to mass spectrometry with an electrospray ion source operating in the positive ion mode (ESI+) using multiple reaction monitoring (MRM) mode. The monitored MRM transitions were m/z 306.3–159.3 for sertraline, m/z 292.3–159.2 for N-desmethylsertraline, and m/z 296.3–160.2 for N-desmethylsertraline-d4. Sertraline and N-desmethylsertraline were quantified with calibration curves using Waters MassLynx 4.1 software. The results were expressed as ng analyte/mg protein or ng analyte/mL media.
MTS cell viability assay
Cell viability was determined using a CellTiter 96® AQueous One Solution Reagent (MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, Promega Corporation, Madison, WI, USA). Briefly, after treatment of sertraline at the concentrations of 5–15 μM, the supernatants were aspirated and then 10 μL of MTS reagent, pre-mixed with 90 μL serum-free media, was added to each well. After 1 h incubation in a tissue culture incubator, the absorbance at 490 nm was measured with a Cytation 5 Cell Imaging Reader (BioTek, Winooski, VT, USA). Background absorbance signals, determined in a set of cell-free wells, were subtracted from sample signals. The cell viabilities were calculated by comparing the absorbance of the treated cells to that of the DMSO controls.
Lactate dehydrogenase assay
The cytotoxicity of sertraline was assessed using a lactate dehydrogenase (LDH) assay as described previously (Chen et al. 2018a).
Cell cycle analysis
HepG2 cells were seeded in 6-well plates at a density of 1 × 106 cells/well and cultured for 24 h and then treated with sertraline or the DMSO vehicle control. Cell cycle phase distribution was determined by propidium iodide (PI; Sigma-Aldrich) staining and followed by flow cytometry analysis as described previously (Chen et al. 2018a).
Caspase-3/7 activity measurement
The activity of caspase-3/7 was measured using a luminescent assay kit (Caspase-Glo® 3/7 Assay Systems, Promega, Madison, WI, USA). Briefly, 2.5 × 104 per well HepG2 cells were seeded in white-walled 96-well plates for 24 h and then treated with sertraline or DMSO control. After treatment of sertraline for 24 h, the supernatants were aspirated and then 5 μL of Caspase-Glo® 3/7 reagent, pre-mixed with 45 μL serum-free media, was added to each well. After incubation at room temperature for 30 min, luminescence was measured using a Cytation 5 Cell Imaging Reader (BioTek). Background luminescence signals, determined in a set of cell-free wells, were subtracted from sample signals. The activity of caspase-3/7 was calculated by comparing the luminescence of the treated cells to that of the DMSO controls.
Western blot analysis
Cells were plated in 60- or 100-mm tissue culture dishes and treated with sertraline. After treating for specified times and concentrations, whole-cell lysates were prepared using RIPA buffer containing Halt Protease Inhibitor Cocktail (ThermoFisher Scientific, Waltham, MA, USA). The protein concentrations of the samples were determined using a Bio-Rad Protein Assay. Standard Western blots were performed. Depending on the proteins of interest, antibodies were selected against topoisomerase I, topoisomerase IIα, γ-H2A.X (Ser139), CYP2B6, CYP2C9, CYP2C19, and CYP2D6, and followed by an incubation with a secondary antibody conjugated with horseradish peroxidase (HRP) (Santa Cruz Biotechnology). GAPDH was used as the internal control. The protein signals were determined with a FluorChem E System and quantified with AlphaView software (ProteinSimple, San Jose, CA, USA).
Statistical analyses
Data are presented as the mean ± standard deviation (SD) of at least three independent experiments. Analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined by one-way analysis of variance (ANOVA) followed by the Dunnett’s test for pairwise-comparisons or two-way ANOVA followed by the Bonferroni post-test. The difference was considered statistically significant when p was less than 0.05.
Results
Sertraline causes cytotoxicity and cell cycle G2/M arrest
As shown in Fig. 1a, HepG2 cells treated with sertraline showed a concentration-dependent decrease in cell viability when measured using a MTS assay. At 7.5 μM, sertraline decreased the cell viability to about 83% of that of the DMSO control. Moreover, HepG2 viability detected using the MTS assay dropped to about 23% when cells were treated with 15 μM of sertraline, indicating that significant cellular growth inhibition and injury occurred. A LDH release assay was then used to evaluate the severity of cell death caused by sertraline. The extent of LDH release was concentration-dependent and significant release was observed at 7.5 μM. A 35% release of LDH occurred at 15 μM sertraline treatment indicating that more profound cell death occurred at the higher concentrations (Fig. 1b).
Fig. 1.
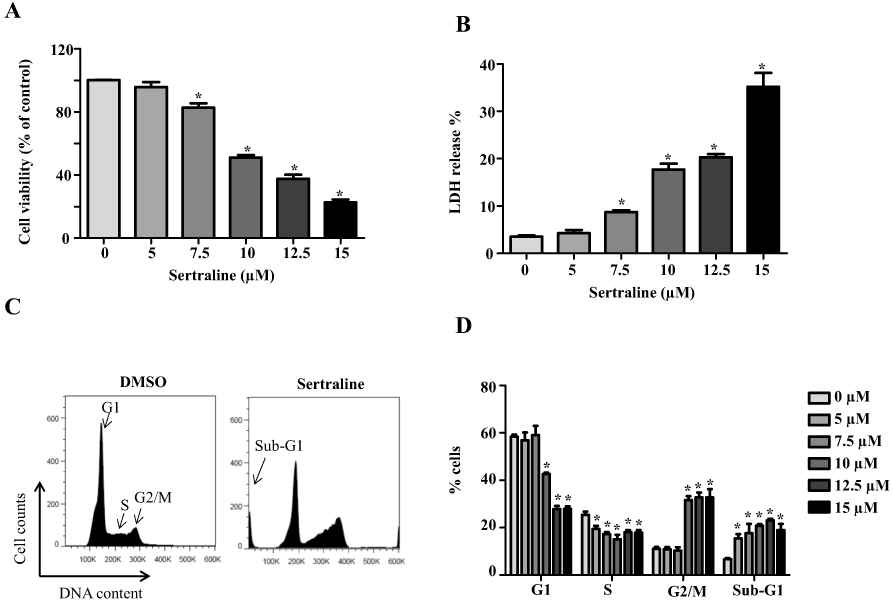
Sertraline induces cellular damage and cell cycle arrest. HepG2 cells were exposed to sertraline at 5, 7.5, 10, 12.5, and 15 μM for 24 h, with DMSO as the vehicle control. The cytotoxicity was measured using MTS assay (a) and LDH assay (b). c Cell cycle distribution was analyzed by flow cytometry. Histograms shown were DNA content analyses for HepG2 cells treated with sertraline for 24 h. d Bar graph depicts the mean percentage of each cell cycle phase ± SD from three independent experiments. *p < 0.05 compared with DMSO vehicle control
Our previous study using microarray analysis showed that cell cycle signaling was the most affected pathway upon sertraline exposure when mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Chen et al. 2014a). Both cell growth inhibition (Fig. 1a, b) and gene expression results prompted us to study further the possible mechanisms for sertraline caused cellular growth inhibition. Cell cycle arrest at the G2/M checkpoint and a concurrent reduction of cell numbers in G1 and S phase were found with increased concentrations of sertraline by cell cycle analysis using flow cytometry. Consistent with our previous findings, apoptosis was also detected upon a 24-h treatment of sertraline as evidenced by the increased ratio of cells with sub-diploid nucleus formation (sub-G1 phase) (Fig. 1c, d). The induction of apoptosis was further confirmed by the concentration-dependent increase of caspase 3/7 activity (Fig. 2a). These data showed that a 24-h exposure of sertraline caused cellular damage, cell cycle disturbance, and apoptosis.
Fig. 2.

Sertraline causes caspase3/7 activation, DNA damage, and topoisomerase suppression. a Cellular caspase 3/7 activity of sertraline-treated HepG2 cells for 24 h was expressed as fold change to DMSO control-treated cells. Total cellular proteins were extracted at 24 h after sertraline treatment at indicated concentrations, and levels of γH2A.X (b) and topoisomerase (topo) I and IIα (c) were determined by Western blotting. GAPDH was used as a loading control. Similar results were obtained from three independent experiments. Intensities of bands were analyzed using AlphaView software and normalized to the amount of GAPDH. *p < 0.05 compared with DMSO vehicle control
Sertraline causes DNA damage and topoisomerases suppression
The occurrence of cell cycle arrest in G2/M phase could be a result of DNA damage (Kaufmann 1998); thus we investigated whether or not sertraline induced DNA damage by measuring histone H2A.X phosphorylation at serine139 (γ-H2A.X), a hallmark of double-strand DNA breakage in cells (Sharma et al. 2012). As shown in Fig. 2b, starting at 7.5 μM, sertraline caused a concentration-dependent increase of γ-H2A.X expression, indicating DNA damage was induced upon sertraline exposure.
The generation of reactive oxygen species (ROS) is generally recognized to induce DNA damage, so intracellular ROS level was measured using H2DCFDA staining. No significant increase in ROS levels was observed in the cells treated with sertraline 5–15 μM for up to 24 h (data not shown). In addition, Western blotting showed that the levels of ROS-related proteins including catalase, glutathione reductase (GR), glutathione S-transfaerase A1, heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), γ-glutamate-cysteine synthetase (γ-GCSc), superoxide dismutase 1 (SOD1), and SOD2 were not altered (data not shown). All these results implied that neither ROS generation nor oxidative stress was involved in sertraline-induced toxicity.
The inhibition of topoisomerases is considered to be one of the classic DNA-damaging mechanisms (Larsen et al. 2003). Studies have shown that hepatic toxicity by drugs and chemicals (dronedarone, trovafloxacin, benzoquinone, ginkgo biloba leaf extract, and goldenseal extract) can be attributed to topoisomerase inhibition (Chen et al. 2013, 2018a, b; Holmes and Winn 2019; Poulsen et al. 2014; Zhang et al. 2015). Thus, the involvement of DNA topoisomerases was investigated. As displayed in Fig. 2c, the expression of topoisomerases I and IIα significantly decreased in a concentration-dependent manner. The inhibition was more severe with topoisomerase IIα as the expression of topoisomerase IIα became undetectable starting at 12.5 μM.
Metabolism of sertraline in 14 HepG2 cell lines overexpressing individual CYPs
As stated in the “Introduction”, metabolism may affect cytotoxicity of sertraline; thus, we established an analytical method utilizing UPLC-tandem-mass spectrometry (UPLC-MS/MS) to screen the CYPs that are responsible for the metabolism of sertraline. N-demethylation of sertraline is the first and major reaction in metabolism of sertraline, so the metabolism of sertraline was determined by measuring the amount of parent sertraline and its major metabolite N-desmethylsertraline in cells exposed to 5 μM sertraline, a non-toxic concentration. The retention time for sertraline was 1.84 min while N-desmethylsertraline and N-desmethylsertraline-d4 eluted at 1.81 min. The linear quantification ranged from 3.2 to 2000 ng/mL, with excellent linearity of R2 = 0.99 for both sertraline and N-desmethylsertraline. It is worth noting that we attempted to identify sertraline ketone, a minor metabolite of CYP-involved metabolism; but we did not detect it possibly due to the limit of our detection system.
We took advantage of our previously established 14 HepG2 cell lines, each of which overexpresses a human CYP isoform, including CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7 (Xuan et al. 2016), to investigate which human CYP isoforms contribute to the metabolism of sertraline. The overexpression of each CYP was previously confirmed by various parameters, including real-time PCR, Western blots, and CYP enzymatic activities (Xuan et al. 2016). The contribution of CYP was characterized by determining the reduction of parent drug and the production of the major metabolite (N-desmethylsertraline). Figure 3a shows the representative UPLC–MS/MS chromatograms of sertraline and N-desmethylsertraline detected in CYP2D6-overexpressing HepG2 cells and empty vector-transduced control cells after incubation with 5 μM sertraline for 24 h. Significantly decreased levels of the parent sertraline were observed in cell lysates from four CYP-overexpressing HepG2 cell lines (CYP2D6, 2C19, 2B6, and 2C9), indicating that these CYPs were responsible for metabolizing sertraline (Fig. 3b). Parent sertraline decreased to 22, 28, 40, and 73% in CYP2D6, 2C19, 2B6, and 2C9-overexpressing cells when compared to the empty vector control under the same experimental conditions. Meanwhile, significantly increased levels of N-desmethylsertraline were found in CYP2D6, 2C19, 2B6, and 2C9-overexpressing cell lines (Fig. 3c). Significant increased level of N-desmethylsertraline or decreased level of parent sertraline was not observed in the cells overexpressing the rest of 10 CYPs (Fig. 3b, c). These data indicated that CYP2D6, 2C19, 2B6, and 2C9 were the major enzymes involved in the N-demethylation of sertraline and the contributions of other CYPs were minimum.
Fig. 3.
Metabolism of sertraline in individual CYP-overexpressing HepG2 cells. a UPLC-QDa mass spectrometry chromatograms for sertraline and its metabolite N-desmethylsertraline in the extracts from HepG2-empty vector control (EV) and HepG2-CYP2D6 overexpressing cells treated with 5 μM sertraline for 24 h. b, c Fourteen CYP-overexpressing HepG2 cell lines were exposed to 5 μM sertraline for 24 h. The total amount of sertraline (b) and its metabolite N-desmethylsertraline (c) in cell lysate were quantified with LC–MS. The results shown are relative values normalized to EV control. Data represent mean ± SD from three independent experiments. *p < 0.05 compared with that of EV control
CYP2D6, 2C19, 2B6, and 2C9-mediated metabolism suppresses sertraline-induced cytotoxicity, DNA damage, and topoisomerase depletion
The effect of CYP2D6, 2C19, 2B6, and 2C9-mediated metabolism on cytotoxicity of sertraline was investigated by comparing the cytotoxicity of sertraline at concentrations that ranged from 5 to 15 μM in empty vector control cells and CYP2D6, 2C19, 2B6, and 2C9-overexpressing cells. As shown in Fig. 4, the metabolism catalyzed by CYP2D6, 2C19, 2B6, and 2C9 significantly attenuated sertraline-induced toxicity as measured by MTS assays and LDH release, implying that the metabolism of sertraline is closely associated with the detoxification of sertraline.
Fig. 4.

Effect of metabolism mediated by CYP2D6, 2C19, CYP2B6, and 2C9 on sertraline-induced cytotoxicity. Empty vector (EV)-transduced or CYP2D6, 2C19, CYP2B6, and 2C9-overexpressing HepG2 cells were treated with the indicated concentrations of sertraline for 24 h. Cytotoxicity was measured using MTS assay (a) and LDH assay (b). The results shown are mean ± SD from three independent experiments. *p < 0.05 compared with that of EV control
We then investigated the contribution of metabolism in sertraline-induced DNA damage and in inhibition of topoisomerases. DNA damage, as determined by the induction of γ-H2A.X, was significantly attenuated in the cells overexpressing CYP2D6, 2C19, 2B6, and 2C9 (Fig. 5). The inhibition of topoisomerase I was also partially recovered in the cells over-expressing CYP2D6, 2C19, 2B6, and 2C9 (Fig. 5). The depletion of topoisomerase IIα caused by 10 μM sertraline treatment was partially recovered in the cells over-expressing CYP2D6, 2C19, and 2B6; however, the recovery was not significant in CYP2C9-expressing cells (Fig. 5). This may explain why LDH release was not significantly attenuated in CYP2C9-expressing cells for the sertraline treatments at higher concentrations of 12.5–15 μM (Fig. 4b). Nevertheless, these data suggested that CYP2D6, 2C19, 2B6, and 2C9 convert sertraline to N-demethylsertraline and this results in a decrease in DNA damage and topoisomerase suppression.
Fig. 5.

Effect of CYP2D6-, 2C19-, 2B6-, and 2C9-mediated metabolism on sertraline-induced DNA damage and topoisomerases suppression. Empty vector (EV)-transduced or a CYP2D6-, b CYP2C19-, c CYP2B6-, and d CYP2C9-overexpressing HepG2 cells were treated with 10 μM sertraline for 24 h. Total cellular proteins were extracted after sertraline treatment. The expression levels of ɣ-H2A.X, topoisomerase I and II were determined by Western blotting. GAPDH was used as a loading control. Similar results were obtained from three independent experiments. Intensities of bands were analyzed using AlphaView software and normalized to the amount of GAPDH. *p < 0.05 versus treatment of EV control
Discussion
Although sertraline is widely prescribed as a first-line antidepressant medication for people with major depression (Cipriani et al. 2008), idiosyncratic liver toxicity caused by sertraline treatment has been documented. We have studied previously the molecular mechanisms underlying sertraline’s liver toxicity. Genome-wide microarray and pathway analysis suggested that the cell cycle pathway was the most affected and a biochemical analysis revealed that apoptosis occurred. In this study, we confirmed the occurrence of cell cycle arrest and investigated further the cause of cell cycle arrest and apoptosis. We found that sertraline induced DNA damage, a trigger of cell cycle arrest, which eventually led to cell apoptosis.
Sertraline has been reported to induce ROS in an aquatic organism B. koreanus (Byeon et al. 2019); however, ROS production is unlikely a major mechanism for sertraline associated DNA damage in our cell system because (1) no significant increase in ROS levels was observed (data not shown), and (2) the levels of ROS-related proteins were not altered (data not shown).
We next focused on the inhibition of DNA topoisomerase since the role of topoisomerase inhibition in DNA damage response and drug/chemical induced liver toxicity has been documented (Chen et al. 2013, 2018a, b; Holmes and Winn 2019; Poulsen et al. 2014; Zhang et al. 2015). Comparing to other inducers of DNA damage, one major feature of topoisomerase inhibitors is that they can directly bind to key cell cycle regulatory proteins and mediate cell cycle arrest (Larsen et al. 2003; Meyer et al. 1997). DNA topoisomerases are classified into two categories, I and II, based on their different properties. Mammalian cells have two topoisomerase II isozymes, namely IIα and IIβ. Since IIβ plays insignificant role in cell proliferation and growth (Austin et al. 2018), our research focused on topoisomerases I and IIα. It has been reported that the activation of unfolded protein response (UPR) is directly linked to the loss of topoisomerase IIα; activation of UPR is essential to reduce the protein level of topoisomerase IIα, but not IIβ (Gray et al. 2005). Interestingly, the results on cell cycle arrest from this current study (Fig. 1c, d) and ER stress and UPR activation from our previous study (Chen et al. 2014a) suggested that the inhibition of topoisomerases could be responsible for DNA damage induced by sertraline, in line with previous studies from our lab and other researchers (Chen et al. 2013, 2018a, b; Holmes and Winn 2019; Poulsen et al. 2014; Zhang et al. 2015). Our data showed that sertraline treatment significantly inhibited the expression of topoisomerases I and IIα (Fig. 2c), suggesting the significance of topoisomerases I and IIα in drug-induced liver toxicity.
We previously found that the cytotoxicity of sertraline was lower in metabolically competent rat primary hepatocytes than in HepG2 cells lacking sufficient drug metabolism capability (Chen et al. 2014b), suggesting that the metabolism may contribute to detoxification of sertraline. Using our previously established CYP-overexpressing HepG2 cells, we confirmed that four CYP isoforms (2D6, 2C19, 2B6, and 2C9) participated in the catalyzation and consequently resulted in the detoxification of sertraline. CYP3A4 has been reported to carry out N-demethylation of sertraline; however, we did not observe CYP3A4-dependent catalysis after 24-h incubation in our cell-based system (Fig. 3). To confirm our observation, we extended the incubation time with CYP3A4-expressing cells to 48 h and found no increased conversion of sertraline compared to the EV-control cells (data not shown). The explanation for this could be that CYP3A4 contributes to the least extent compared to other identified CYP isoforms (CYP2D6, 2C19, 2B6, and 2C9), as supported by a study using human liver microsomes (Kobayashi et al. 1999). Another possible reason is that sertraline itself is a CYP3A4 inhibitor (Masubuchi and Kawaguchi 2013; Riesenman 1995); the enzyme activity of CYP3A4 could be diminished by addition of sertraline. Taken together, these results indicate that the contribution of CYP3A4 is minor or negligible in the metabolism of sertraline, at least under our experimental conditions.
A key finding of our study is that four CYP isoforms (CYP2D6, 2C19, 2B6, and 2C9) are responsible for the metabolism of sertraline and the metabolism mediated by these CYPs rescued the cytotoxicity of sertraline (Figs. 4 & 5). These findings suggest that individuals with lower levels of these enzymes may have a higher risk of hepatic exposure to the parental form of sertraline and which may consequently increase their susceptibility to sertraline-induced liver toxicity. Many factors, including genetic (Saiz-Rodriguez et al. 2018; Yang et al. 2013; Yuce-Artun et al. 2016), epigenetic (Dluzen and Lazarus 2015), environmental factors, and disease/health status of the individuals, impact the inter-individual variability in the expression of the CYP enzymes among humans. As to genetic polymorphisms, 2B6*6 or 2B6*9 variants had a significant decreasing effect on sertraline metabolism in patients with major depression (Yuce-Artun et al. 2016). Patients carrying the T allele of CYP2B6*6 G516T SNP have reduced metabolism and higher serum concentrations of sertraline (Saiz-Rodriguez et al. 2018). In addition, some disease status influence the function of CYPs. For instance, patients with hypoxemia caused by chronic pulmonary disease or heart failure have decreased CYP2B6, 2C9, and 2C19. It has been reported that CYP2B6- and 2C9-mediated clearance of a nonsteroidal anti-inflammatory drug antipyrine was decreased in patients with hypoxemia (Fradette and Du Souich 2004). In patients with chronic kidney disease, CYP2D6-mediated clearance of drugs such as antipsychotic medicine risperidone and antidepressant paroxetine was decreased in parallel with the severity of chronic kidney disease (Yoshida et al. 2016). CYP2B6, 2D6, 2C9, and 2C19 can also be inhibited by a number of drugs, foods, and dietary supplements. For example, dietary supplements, such as green tea extract and curcumin (a component of turmeric root), have been reported to inhibit CYP2B6, 2C9, and 2D6 (Albassam and Markowitz 2017; Sasaki et al. 2017). Grapefruit juice and its main constituents have shown inhibitory effects on CYP3A4, 2C19, 2B6, 2D6, and 2C9 (Holmberg et al. 2014; Lin et al. 2005; Tassaneeyakul et al. 2000). The intake of grapefruit juice has been reported to significantly increase the plasma sertraline levels in healthy volunteers and patients with a history of depression (Lee et al. 1999; Ueda et al. 2009). Thus, precautions should be taken to avoid the factors that can inhibit these CYP isoforms, since they may cause an elevated serum level of sertraline which increases the likelihood of sertraline-induced liver toxicity.
In summary, our study indicated that cell cycle arrest and topoisomerase inhibition-induced DNA damage play important roles in sertraline-caused hepatic cytotoxicity. CYP2D6, 2C19, 2B6, and 2C9-mediated metabolism is responsible for the detoxification of sertraline. The parental form of sertraline is likely the main cause of sertraline-induced liver toxicity.
Acknowledgements
XL and DL were supported by appointments to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science Education (ORISE) through an interagency agreement between the U.S. Department of Energy and the U.S. FDA. MF was supported by NCTR Summer Science Research Program administered by ORISE.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Publisher′s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albassam AA, Markowitz JS (2017) An appraisal of drug–drug interactions with green tea (Camellia sinensis). Planta Med 83(6):496–508. 10.1055/s-0043-100934 [DOI] [PubMed] [Google Scholar]
- Almansour MI, Jarrar YB, Jarrar BM (2018) In vivo investigation on the chronic hepatotoxicity induced by sertraline. Environ Toxicol Pharmacol 61:107–115. 10.1016/j.etap.2018.05.021 [DOI] [PubMed] [Google Scholar]
- Austin CA, Lee KC, Swan RL et al. (2018) TOP2B: the first thirty years. Int J Mol Sci 19:9. 10.3390/ijms19092765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balon R (1996) Antidepressants in the treatment of premature ejaculation. J Sex Marital Ther 22(2):85–96. 10.1080/00926239608404912 [DOI] [PubMed] [Google Scholar]
- Byeon E, Park JC, Hagiwara A, Han J, Lee JS (2019) Two antidepressants fluoxetine and sertraline cause growth retardation and oxidative stress in the marine rotifer Brachionus koreanus. Aquat Toxicol 218:105337. 10.1016/j.aquatox.2019.105337 [DOI] [PubMed] [Google Scholar]
- Chen S, Wan L, Couch L et al. (2013) Mechanism study of goldenseal-associated DNA damage. Toxicol Lett 221(1):64–72. 10.1016/j.toxlet.2013.05.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xuan J, Couch L et al. (2014a) Sertraline induces endoplasmic reticulum stress in hepatic cells. Toxicology 322:78–88. 10.1016/j.tox.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xuan J, Wan L et al. (2014b) Sertraline, an antidepressant, induces apoptosis in hepatic cells through the mitogen-activated protein kinase pathway. Toxicol Sci 137(2):404–415. 10.1093/toxsci/kft254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang Z, Qing T et al. (2017) Activation of the Nrf2 signaling pathway in usnic acid-induced toxicity in HepG2 cells. Arch Toxicol 91(3):1293–1307. 10.1007/s00204-016-1775-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ren Z, Yu D, Ning B, Guo L (2018a) DNA damage-induced apoptosis and mitogen-activated protein kinase pathway contribute to the toxicity of dronedarone in hepatic cells. Environ Mol Mutagen 59(4):278–289. 10.1002/em.22173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wu Q, Ning B, Bryant M, Guo L (2018b) The role of hepatic cytochrome P450s in the cytotoxicity of dronedarone. Arch Toxicol 92(6):1969–1981. 10.1007/s00204-018-2196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Geddes JR et al. (2008) Does randomized evidence support sertraline as first-line antidepressant for adults with acute major depression? A systematic review and meta-analysis. J Clin Psychiatry 69(11):1732–1742. 10.4088/jcp.v69n1108 [DOI] [PubMed] [Google Scholar]
- clincalc.com (2019) The Top 300 of 2019. https://clincalc.com/DrugStats/Top300Drugs.aspx
- Collados Arroyo V, Plaza Aniorte J, Hallal H, Perez-Cuadrado E (2008) Sertraline-induced hepatotoxicity. Farm Hosp 32(1):60–61 [DOI] [PubMed] [Google Scholar]
- Collados V, Hallal H, Andrade RJ (2010) Sertraline hepatotoxicity: report of a case and review of the literature. Dig Dis Sci 55(6):1806–1807. 10.1007/s10620-010-1192-7 [DOI] [PubMed] [Google Scholar]
- DeVane CL, Liston HL, Markowitz JS (2002) Clinical pharmacokinetics of sertraline. Clin Pharmacokinet 41(15):1247–1266. 10.2165/00003088-200241150-00002 [DOI] [PubMed] [Google Scholar]
- Dluzen DF, Lazarus P (2015) MicroRNA regulation of the major drug-metabolizing enzymes and related transcription factors. Drug Metab Rev 47(3):320–334. 10.3109/03602532.2015.1076438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Cooke MS (2004) Factors contributing to the outcome of oxidative damage to nucleic acids. BioEssays 26(5):533–542. 10.1002/bies.20027 [DOI] [PubMed] [Google Scholar]
- Fartoux-Heymann L, Hezode C, Zafrani ES, Dhumeaux D, Mallat A (2001) Acute fatal hepatitis related to sertraline. J Hepatol 35(5):683–684. 10.1016/s0168-8278(01)00159-3 [DOI] [PubMed] [Google Scholar]
- Fradette C, Du Souich P (2004) Effect of hypoxia on cytochrome P450 activity and expression. Curr Drug Metab 5(3):257–271. 10.2174/1389200043335577 [DOI] [PubMed] [Google Scholar]
- Galan Navarro JL (2001) Acute cholestatic hepatitis probably caused by sertraline. Rev Esp Enferm Dig 93(12):822. [PubMed] [Google Scholar]
- Gray MD, Mann M, Nitiss JL, Hendershot LM (2005) Activation of the unfolded protein response is necessary and sufficient for reducing topoisomerase IIalpha protein levels and decreasing sensitivity to topoisomerase-targeted drugs. Mol Pharmacol 68(6):1699–1707. 10.1124/mol.105.014753 [DOI] [PubMed] [Google Scholar]
- Grohol JM (2018) Top 25 psychiatric medications for 2016. Psych Central https://psychcentral.com/blog/top-25-psychiatric-medications-for-2016/ [Google Scholar]
- Guo L, Li Q, Xia Q, Dial S, Chan PC, Fu P (2009) Analysis of gene expression changes of drug metabolizing enzymes in the livers of F344 rats following oral treatment with kava extract. Food Chem Toxicol 47(2):433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautekeete ML, Colle I, van Vlierberghe H, Elewaut A (1998) Symptomatic liver injury probably related to sertraline. Gastroenterol Clin Biol 22(3):364–365 [PubMed] [Google Scholar]
- Holmberg MT, Tornio A, Neuvonen M, Neuvonen PJ, Backman JT, Niemi M (2014) Grapefruit juice inhibits the metabolic activation of clopidogrel. Clin Pharmacol Ther 95(3):307–313. 10.1038/clpt.2013.192 [DOI] [PubMed] [Google Scholar]
- Holmes TH, Winn LM (2019) DNA damage and perturbed topoisomerase iialpha as a target of 1,4-benzoquinone toxicity in murine fetal liver cells. Toxicol Sci. 10.1093/toxsci/kfz158 [DOI] [PubMed] [Google Scholar]
- Jin Y, Yu D, Tolleson WH et al. (2016) MicroRNA hsa-miR-25-3p suppresses the expression and drug induction of CYP2B6 in human hepatocytes. Biochem Pharmacol 113:88–96. 10.1016/j.bcp.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WK (1998) Human topoisomerase II function, tyrosine phosphorylation and cell cycle checkpoints. Proc Soc Exp Biol Med 217(3):327–334. 10.3181/00379727-217-44240 [DOI] [PubMed] [Google Scholar]
- Kim KY, Hwang W, Narendran R (1999) Acute liver damage possibly related to sertraline and venlafaxine ingestion. Ann Pharmacother 33(3):381–382. 10.1345/aph.18155 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ishizuka T, Shimada N, Yoshimura Y, Kamijima K, Chiba K (1999) Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos 27(7):763–766 [PubMed] [Google Scholar]
- Larsen AK, Escargueil AE, Skladanowski A (2003) From DNA damage to G2 arrest: the many roles of topoisomerase II. Prog Cell Cycle Res 5:295–300 [PubMed] [Google Scholar]
- Lee AJ, Chan WK, Harralson AF, Buffum J, Bui BC (1999) The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther 21(11):1890–1899. 10.1016/S0149-2918(00)86737-5 [DOI] [PubMed] [Google Scholar]
- Li Y, Couch L, Higuchi M, Fang JL, Guo L (2012) Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol Sci 127(2):582–591. 10.1093/toxsci/kfs100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Kent UM, Hollenberg PF (2005) The grapefruit juice effect is not limited to cytochrome P450 (P450) 3A4: evidence for bergamottin-dependent inactivation, heme destruction, and covalent binding to protein in P450s 2B6 and 3A5. J Pharmacol Exp Ther 313(1):154–164. 10.1124/jpet.104.079608 [DOI] [PubMed] [Google Scholar]
- Marjoribanks J, Brown J, O’Brien PM, Wyatt K (2013) Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev 6:CD001396. 10.1002/14651858.CD001396.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi Y, Kawaguchi Y (2013) Time-dependent inhibition of CYP3A4 by sertraline, a selective serotonin reuptake inhibitor. Biopharm Drug Dispos 34(8):423–430. 10.1002/bdd.1857 [DOI] [PubMed] [Google Scholar]
- Mendels J, Camera A, Sikes C (1995) Sertraline treatment for premature ejaculation. J Clin Psychopharmacol 15(5):341–346. 10.1097/00004714-199510000-00006 [DOI] [PubMed] [Google Scholar]
- Meyer KN, Kjeldsen E, Straub T et al. (1997) Cell cycle-coupled relocation of types I and II topoisomerases and modulation of catalytic enzyme activities. J Cell Biol 136(4):775–788. 10.1083/jcb.136.4.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky S, Reinus JF (2003) Sertraline hepatotoxicity: a case report and review of the literature on selective serotonin reuptake inhibitor hepatotoxicity. Dig Dis Sci 48(5):939–944. 10.1023/a:1023007831047 [DOI] [PubMed] [Google Scholar]
- Poulsen KL, Olivero-Verbel J, Beggs KM, Ganey PE, Roth RA (2014) Trovafloxacin enhances lipopolysaccharide-stimulated production of tumor necrosis factor-alpha by macrophages: role of the DNA damage response. J Pharmacol Exp Ther 350(1):164–170. 10.1124/jpet.114.214189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP (2010) Update information on drug metabolism systems—2009, part II: summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr Drug Metab 11(1):4–84. 10.2174/138920010791110917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP (2012) Summary of information on the effects of ionizing and non-ionizing radiation on cytochrome P450 and other drug metabolizing enzymes and transporters. Curr Drug Metab 13(6):787–814. 10.2174/138920012800840356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger JK, Reutter S, Hofmann U, Schwab M, Zanger UM (2015) Inflammation-associated microRNA-130b down-regulates cytochrome P450 activities and directly targets CYP2C9. Drug Metab Dispos 43(6):884–888. 10.1124/dmd.114.062844 [DOI] [PubMed] [Google Scholar]
- Riesenman C (1995) Antidepressant drug interactions and the cytochrome P450 system: a critical appraisal. Pharmacotherapy 15(6 Pt 2):84S–99S [PubMed] [Google Scholar]
- Saiz-Rodriguez M, Belmonte C, Roman M et al. (2018) Effect of polymorphisms on the pharmacokinetics, pharmacodynamics and safety of sertraline in healthy volunteers. Basic Clin Pharmacol Toxicol 122(5):501–511. 10.1111/bcpt.12938 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Sato Y, Kumagai T, Yoshinari K, Nagata K (2017) Effect of health foods on cytochrome P450-mediated drug metabolism. J Pharm Health Care Sci 3:14. 10.1186/s40780-017-0083-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Singh K, Almasan A (2012) Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol 920:613–626. 10.1007/978-1-61779-998-3_40 [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y (2000) Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys 378(2):356–363. 10.1006/abbi.2000.1835 [DOI] [PubMed] [Google Scholar]
- Ueda N, Yoshimura R, Umene-Nakano W et al. (2009) Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry 10(4 Pt 3):832–835. 10.1080/15622970802688069 [DOI] [PubMed] [Google Scholar]
- Verrico MM, Nace DA, Towers AL (2000) Fulminant chemical hepatitis possibly associated with donepezil and sertraline therapy. J Am Geriatr Soc 48(12):1659–1663. 10.1111/j.1532-5415.2000.tb03879.x [DOI] [PubMed] [Google Scholar]
- Wu Q, Ning B, Xuan J, Ren Z, Guo L, Bryant MS (2016) The role of CYP 3A4 and 1A1 in amiodarone-induced hepatocellular toxicity. Toxicol Lett 253:55–62. 10.1016/j.toxlet.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZH, Wang W, Zhao XJ et al. (1999) Evidence for involvement of polymorphic CYP2C19 and 2C9 in the N-demethylation of sertraline in human liver microsomes. Br J Clin Pharmacol 48(3):416–423. 10.1046/j.1365-2125.1999.00023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan J, Chen S, Ning B, Tolleson WH, Guo L (2016) Development of HepG2-derived cells expressing cytochrome P450s for assessing metabolism-associated drug-induced liver toxicity. Chem Biol Interact 255:63–73. 10.1016/j.cbi.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Price ET, Chang CW et al. (2013) Gene expression variability in human hepatic drug metabolizing enzymes and transporters. PLoS ONE 8(4):e60368. 10.1371/journal.pone.0060368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi ZM, Chen SD, Tang QY, Tang HL, Zhai SD (2019) Efficacy and safety of sertraline for the treatment of premature ejaculation: systematic review and meta-analysis. Medicine (Baltimore) 98(23):e15989. 10.1097/MD.0000000000015989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Sun B, Zhang L et al. (2016) Systematic and quantitative assessment of the effect of chronic kidney disease on CYP2D6 and CYP3A4/5. Clin Pharmacol Ther 100(1):75–87. 10.1002/cpt.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Green B, Marrone A et al. (2015a) Suppression of CYP2C9 by microRNA hsa-miR-128-3p in human liver cells and association with hepatocellular carcinoma. Sci Rep 5:8534. 10.1038/srep08534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Green B, Tolleson WH et al. (2015b) MicroRNA hsa-miR-29a-3p modulates CYP2C19 in human liver cells. Biochem Pharmacol 98(1):215–223. 10.1016/j.bcp.2015.08.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuce-Artun N, Baskak B, Ozel-Kizil ET et al. (2016) Influence of CYP2B6 and CYP2C19 polymorphisms on sertraline metabolism in major depression patients. Int J Clin Pharm 38(2):388–394. 10.1007/s11096-016-0259-8 [DOI] [PubMed] [Google Scholar]
- Zeng L, Chen Y, Wang Y et al. (2017) MicroRNA hsa-miR-370-3p suppresses the expression and induction of CYP2D6 by facilitating mRNA degradation. Biochem Pharmacol 140:139–149. 10.1016/j.bcp.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen S, Mei H et al. (2015) Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci Rep 5:14633. 10.1038/srep14633 [DOI] [PMC free article] [PubMed] [Google Scholar]



