Abstract
Skin wound healing is an intractable problem that represents an urgent clinical need. To solve this problem, a large number of studies have focused on the use of exosomes (EXOs) derived from adipose‐derived stem cells (ADSCs). This review describes the mechanisms whereby ADSCs‐EXOs regulate wound healing and their clinical application. In the wound, ADSCs‐EXOs modulate immune responses and inflammation. They also promote angiogenesis, accelerate proliferation and re‐epithelization of skin cells, and regulate collagen remodelling which inhibits scar hyperplasia. Compared with ADSCs therapeutics, ADSCs‐EXOs have highly stability and are easily stored. Additionally, they are not rejected by the immune system and have a homing effect and their dosage can be easily controlled. ADSCs‐EXOs can improve fat grafting and promote wound healing in patients with diabetes mellitus. They can also act as a carrier and combined scaffold for treatment, leading to scarless cutaneous repair. Overall, ADSCs‐EXOs have the potential to be used in the clinic to promote wound healing.
Keywords: adipose‐derived stem cells, angiogenesis, exosomes, inflammation, skin wound healing
This review describes the mechanisms whereby exosomes (EXOs) derived from adipose‐derived stem cells (ADSCs) regulate wound healing and their clinical application. In the wound, ADSCs‐EXOs modulate immune responses and inflammation, promote angiogenesis, accelerate proliferation and re‐epithelization of skin cells and regulate collagen remodelling, which inhibits scar hyperplasia. ADSCs‐EXOs have the potential to be used in the clinic, as they can improve fat grafting, promote wound healing of diabetic patients and act as a carrier and combined scaffold for treatment, leading to scarless cutaneous repair.
1. INTRODUCTION
Wound healing is a complex biological process that takes place in skin tissue after injury by trauma, burn or diabetic ulcers. 1 Chronic skin wounds are difficult to heal, for example, in diabetic or long‐term bedridden patients. 2 Therefore, wound healing is one of the most intractable problems for clinicians and a heavy burden for patients, both physically and financially. Conventional wound care methods, with the risk of atrophic scars and pigmentary abnormalities, include skin grafting, skin flap transplantation, laser therapy and biological stents. 3 , 4 Also, biological scaffolds are costly and slow, and they are not suitable to treat large scale trauma. 5 , 6
Other treatments include local application of specific growth factors 7 and gene therapy. 8 However, local growth factors are easily degraded in body fluids, whereas dosage cannot be easily controlled at the wound site. 9 Hence, there is a crucial and urgent need for alternative efficient and safe methods to promote wound healing.
Recently, stem cell therapy has flourished because of its pluripotency, self‐renewal and the ability to promote secretion of regenerative cytokines. 10 Pluripotent stem cells are considered safe and overcome moral concerns associated with embryonic stem cells. However, stem cell therapy may present both problems of storage and transportation and risks of induced tumorigenesis and deformity. 11 Stem cells have been proposed to promote wound healing in a paracrine way by (1) regulating macrophages, 12 T cells, B cells and others 13 , 14 , 15 to reduce inflammation, (2) secreting VEGF to promote angiogenesis, 16 (3) promoting proliferation and differentiation of fibroblasts and keratinocyte‐forming cells, (4) producing anti‐fibrosis cytokines and (5) transforming into microvascular endothelial cells and keratinocytes. 13 , 17
Exosomes are one of the components of paracrine and the main contributor to stem cells efficacy. 18 They are small, single membranous, secretory organelles rich in proteins, lipids, nucleic acids and carbohydrate conjugates. They are also thought to have a wide variety of activities, such as remodelling the extracellular matrix and delivering signals and molecules to other cells. Their usage avoids many of the shortcomings of stem cells, since they are stable and easily stored. In addition, they are not rejected by the immune system, have a homing effect, and dosage can be easily controlled. 19 , 20
Exosomes derived from adipose‐derived stem cells (ADSCs‐EXOs) have become a hot topic in the field of skin wound repairing and treatment. Adipose‐derived stem cells (ADSCs) are derived from adipose tissue, where they are nearly 500 times more abundant than in an equivalent amount of bone. 21 , 22 The abundance and the simple methods of sampling of ADSCs make it safer against trauma and other adverse reactions.
2. BIOLOGICAL CHARACTERISTICS OF EXOS
2.1. Biogenesis and release
Exos, as a subtype of extracellular vesicles (EVs), are derived from endosome and plasma membranes through endocytosis, fusion and budding processes. 23 There modes of EXOs and other EVs biogenesis and release are as follows (Figure 1). In the first, the primary endosomes produced by phagosomes and plasma membrane are further acidified to form secondary endosomes. These subsequently bud inward to form multivesicular bodies (MVBs). Some MVBs entering lysosomes are degraded, whereas the rest release EVs when they fuse to the plasma membrane. This last step has been shown using genetically encoded, pH‐sensitive CD63–pHluorin fusion proteins. 24 In the second, EVs directly bud from the plasma membrane, as shown by atomic force microscopy experiments which demonstrated that the budding of EVs at the plasma membrane of stem cells occurs at rates equal to their production. 25 Moreover, findings of earlier electron microscope and electron microscopy experiments also prove this mode. 26 , 27 , 28 , 29 There may exist other modes of EXOs biogenesis. Recent researches illustrate that EXOs can be released in delayed by deep invaginations of certain cell types at the plasma membrane, which are indistinguishable from MVBs by conventional transmission electron microscopy. 30 , 31 , 32 These intracellular plasma membrane–connected compartments (IPMCs) form a continuum with the extracellular milieu via necks, where vesicles can be stored and released in a pulsatile form. 30
FIGURE 1.
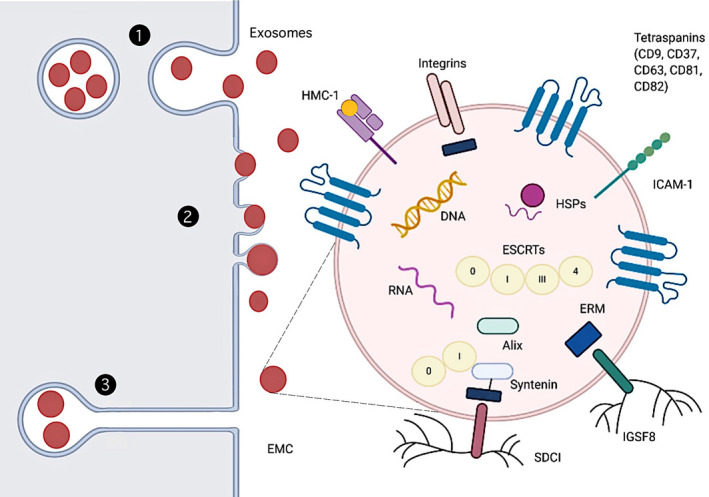
EXOs bud from endosome and plasma membranes. Exosome biogenesis may use three mechanisms: (1) vesicle budding into discrete endosomes that mature into multivesicular bodies, releasing EXOs upon plasma membrane fusion; (2) direct budding from plasma membrane and (3) delayed release by budding at intracellular plasma membrane–connected compartments (IPMCs) followed by deconstriction of IPMC neck(s). We note that this is not a comprehensive list and it is just to illustrate some of the mechanisms. Abbreviations: ECM, extracellular matrix; ERM, ezrin‐radixin‐moesin; ESCRTs, endosomal sorting complexes required for transport; MHC, major histocompatibility complex; IGSF8, immunoglobulin superfamily member 8; ICAM‐1, intercellular adhesion molecule‐1; SDC1, syndecans 1; HSPs, heat shock proteins
Exosomes biogenesis and release is regulated by several factors.
Rab proteins determine organelle membrane identity and mediate organelle dynamics 33 but also regulate EXOs biogenesis that take place via endosomes and the plasma membrane. Rab27 proteins can mediate MVB maturation and traffic to the plasma membrane 34 , 35 and regulate plasma membrane PIP2 dynamics to organize microdomains involved in budding, 28 together with Rab35. 36 Rab11 proteins may affect EXOs release via a calcium‐induced homotypic MVBs fusion/maturation process. 37
EXOs release can be promoted by the binding of the vacuolar protein sorting factor 4 (Vps4) and the endosomal sorting complex, required for transport (ESCRT), to ubiquitination protein. The ESCRT machinery contributes to membrane deformation, sealing and repairing in a wide array of processes that include MVB biogenesis. 38 Therefore, EXOs biogenesis is likely to be ESCRT‐dependent, 39 although this mechanism depends on other factors such as VPS4 ATPase. 38 , 40
EXOs biogenesis is also regulated by the Ral family of small GTPases. Their inhibition produced accumulation of MVBs near the plasma membrane and a marked reduction in secreted EXOs and EXOs marker proteins. 41 The small GTPase Arf6 and the phospholipase PLD2 of the Ral family are also implicated in promoting EXO biogenesis. 42 PLD2 seems to be dependent on a pair of exosomal scaffolds, syntenin and Alix.
Autophagy‐related (Atg) proteins coordinate initiation, nucleation, and elongation during autophagosome biogenesis. A decrease in EXOs secretion was observed in cancer cells lacking ATG5, 43 whereas secretion of EXOs and EXOs related proteins increased in CRISPR/Cas9‐mediated neuronal cells knockout of Atg5. 44
UV radiation, oxygen‐free radical stimulation, changes in calcium levels or cholesterol content may all contribute to variations in EXOs secretion. 45 , 46
2.2. Structure and composition
Exosomes, released by various types of cells to the extracellular space, are small (30‐150 nm) lipidic vesicles (LVs). 35 , 47 , 48 , 49 , 50 , 51 Since they are approximately 5‐ to 10‐fold smaller than the wavelength of visible light, they can be detected by conventional fluorescence microscopy when fluorescently labelled instead of conventional light microscopy. 28 Cryo‐electron microscopy shows that EXOs display a spheroid morphology, although a small proportion has multiple membranes or presents elongated, tubule‐like morphologies. The latter may be generated by biological processes, by physical force‐induced fragmentation and mechanical resealing. 52 EXOs have a density of ~1.1‐1.2 g/mL. 53 Homogeneity is affected by protein, lipid ratio, expression of a specific single exosomal cargo protein 28 , 29 or exosomal metabolic pathways (eg, as a consequence of hydroxyapatite crystallization by osteogenic EXOs 54 ).
Several tetraspanin proteins are highly enriched in EXOs, for example CD81, CD82, CD37 and CD63, with CD63 and CD81 are the least and the most enriched in B lymphocytes, respectively. 55 In general studies, the successful isolation of EXOs from ADSCs is based on the detection of EXO markers (CD9, CD63 and CD81). Since that initial report, CD81 and CD63 have come to be among the most commonly used exosomal marker proteins, together with CD9, 56 also detected in large vesicles. 57 EXOs also have other transmembrane signal proteins acting as signal molecules, 58 and carry cytokines, fibronectin, tenascin C and other extracellular matrix proteins (ECM). These suggest they participate in multiple signal platforms for autocrine and paracrine signaling. 59 , 60 , 61 The inner cortex of exosomes is rich in scaffolding proteins Syntenin and Alix. In addition, a large number of tetrameric associated chaperones, including major histocompatibility complex (MHC), 53 , 55 immunoglobulin superfamily member 8 (IGSF8), 62 intercellular adhesion molecule‐1 (ICAM‐1), 63 syndecans 1 (SDC1) 64 and integrins, 65 are also present in the endothelium of EXOs. The presence of heat shock proteins (HSPs) in EXOs was first reported by Mathew et al. 66
Exosomes membrane riches (or riched) in cholesterol and sphingomyelin, with a small amount of lecithin and phosphatidylethanolamine. 67 Also, the outermost surface of the EXOS consists of a glycan canopy, interrogated by lectin panning and other approaches. 68 , 69
Exosomes also contain nucleic acids include single‐stranded, genomic double‐stranded, mitochondrial or reverse‐transcribed complementary DNAs. 70 , 71 , 72 RNAs include microRNA, ribosomal RNA and long non‐coding RNA, which can be transferred in functional form to other cells and tissues. 73 , 74 , 75 , 76
Adipose tissue is not only a reservoir of fat, but also an indispensable endocrine and immune organ. EXOs have been found in adipose tissue, 77 adipocytes 78 , 79 and adipose‐derived mesenchymal stem cells (AMSCs). 80 EXOs derived from ADSCs were larger than common EXOs, 81 but expression of EXOs markers such as CD63 and HSP‐70 was positive, indicating that the size range of EXOs could be changed with different cell types. Adipocyte‐derived EVs distributed into large extracellular vesicles (lEV) and small extracellular vesicles (sEVs), respectively, 48 with different protein composition. LEVs presented a high content of phosphatidylserine, whereas sEVs were rich in cholesterol, which also confirmed that lipid composition depends on cell source. 48 , 82
2.3. Isolation and identification technology
Common methods used to characterize EXOs include traditional fluorescence microscopy, 28 super‐resolution microscopy, 83 dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), tunable resistive pulse sensing (TRPS) and single‐particle interferometric reflectance (SPIR). The latter can detect the presence and abundance of specific lipids, proteins, carbonic acids and carbohydrates. 84
Exosomes are enriched by differential centrifugation, size‐exclusion chromatography, field flow fractionation, microfluidic filtration or contact‐free sorting immunoaffinity enrichment. Common methods for detecting EXOs‐labelled proteins include conventional protein analysis (Western blotting and ELISA, mass spectrometry), flow cytometry and newer protein analysis techniques, such as micro particle flow cytometry, micro‐nuclear magnetic resonance, nanoplasmonic EXO (nPLEX) sensor, integrated magnetic‐electrochemical EXO (iMEX) sensor and ExoScreen. 85 Of these, differential centrifugation not only obtains more EXOs, but also avoid the influence of polyethylene glycol when using transmission electron microscopy. 86
3. PHYSIOLOGICAL PROCESS AND MECHANISM OF WOUND HEALING
Wound healing is a complex dynamic physiological process, which can be generally divided into four stages: haemostasis, inflammation, proliferation and remodelling. 87 Initial injury causes endothelial damage and basement membrane exposure, along with subsequent spillover of blood components. The immediate response to injury is vasoconstriction caused by the release of thromboxane and prostaglandins. Meanwhile, platelets adhere to exposed collagen and release the contents of their granules, whereas tissue factor activates both platelets and coagulation cascades. 88 Blood clots formed by collagen, platelets, thrombin and fibronectin not only control haemorrhages but protect the wound and provide matrix and soluble factors to promote adhesion. They also concentrate growth factors that serve as wound healing scaffolds. 89 , 90 Blood clots also appear to be inducers of cell lineage differentiation during wound healing. 91 , 92 , 93
During the inflammatory phase, vasodilation and capillary permeability results in oedema. Bone marrow‐derived immune cells prepare for wound healing by clearing pathogens, apoptotic cells, cell debris and damage mechanisms at the wound site. 87 Cytokines and other factors attract granulocytes to wounds. 94 , 95 Subsequently, neutrophils digest debris and injured tissues by secreting proteases. And clear microbial pathogens through oxygen‐dependent mechanisms. Local monocytes also migrate into the wound and become macrophages, which can phagocytose apoptotic cells and cell debris and secrete a large number of growth factors. 96 Lymphocytes are also involved in inflammation. Interestingly, CD4+ T cells are associated with healing, whereas CD8‐T cells negatively affect this process. 97 Inflammation eventually promotes transformation of M1 macrophages to M2 macrophages. 98
In the proliferative phase, re‐epithelization occurs. This relies on migration of epithelial cells from the wound margins and any remaining adnexal structures in the dermis. Epithelial migration and proliferation continue until the wound is completely covered and an intact epithelial barrier is reestablished. 99 M2 macrophages promote tissue regeneration and a mass production of extracellular matrix by regulating the proliferation and migration of keratinocytes, fibroblasts and endothelial cells. 91 Fibroblasts begin to secrete high levels of immature collagen type III into the matrix. 100
In the last remodelling period, fibroblasts continue to secret collagen 101 , 102 and over time collagen type III decreases and is replaced by collagen type I. Collagen fibres gradually become organized and the tensile strength of the wound increases. 103 Collagen breakdown and structural adjustment of the neonatal extracellular matrix results in reduced wound thickness, degradation of newly formed capillaries and narrowing of the wound edge through contraction of the subvascular connective tissue. 87 , 93 , 104
4. ADSCs‐EXOs MEDIATE WOUND HEALING
4.1. Regulations of immune response and inflammation
Inflammation is the body's self‐defence mechanism in response to harmful stimuli. Wound healing can be delayed by chronic and excessive inflammation, therefore a well‐regulated inflammation guarantees wound healing. 105 Activated T regulatory cells can promote wound healing by reducing both production of interferon alpha (IFN‐α) and aggregation capacity of M1 macrophages. 106 ADSCs‐EXOs play an immunosuppressive role by reducing IFN‐α secretion, thus inhibiting activation of T cells. 107 Furthermore, ADSCs‐EXOs contain immunoregulatory proteins such as TNF‐a, macrophage colony‐stimulating factor (MCSF) and RBP‐4. 108 The role of ADSCs‐EXOs in promoting monocyte differentiation into M1 macrophages was confirmed by Kranendonk et al 108 It was also found that miR‐155 in ADSCs‐EXOs can induce adipocyte‐derived macrophages from obese mice to differentiate into M1, causing chronic inflammation with an imbalance in the M1‐to‐M2 macrophage ratio in adipose tissue. 109 ADSCs‐EXOs can also up‐regulate the expression of macrophage inflammatory protein‐1α and monocyte chemoattractant protein‐1, promoting early inflammation. 110
4.2. Promoting angiogenesis in wounds
Angiogenesis provides blood supply for wound healing, facilitating the transport of nutrients and metabolic waste products. 89 ADSCs‐EXOs promote the proliferation and migration of vascular endothelial cells, thereby enhancing angiogenesis. 111 Human adipose stem cells (hADSCs)–derived EXOs are rich in miRNA‐125a and miRNA‐31, which can be transferred to vascular endothelial cells to stimulate proliferation and promote angiogenesis. Transfer of miR‐125a to endothelial cells has been demonstrated in vitro and in animal experiments. 112 MSCs‐EXOs could inhibit expression of angiogenesis inhibitor (DLL4), thus promoting migration and sprouting vascular endothelial tip cells. Transfer of miRNA‐31 to endothelial cells has also been shown, 113 where hADSCs‐EXOs inhibited expression of the anti‐angiogenesis gene HIF1 in vascular endothelial cells, promoting migration and enhancing angiogenesis in human umbilical vein endothelial cells. ADSCs‐EXOs may also promote the survival of skin flaps and increase capillary density, playing a role in repairing ischaemia‐reperfusion injury. 114
4.3. Speeding up proliferation and re‐epithelialization of skin cells
During the proliferative phase, fibroblasts proliferate to produce ECM, whereas epithelial cells proliferate and migrate towards the wound centre to promote wound healing. Thus, proliferation and re‐epithelization of skin cells are important for skin regeneration. 89 ADSCs‐EXOs are internalized by fibroblasts and stimulate proliferation, migration and collagen synthesis in a dose‐dependent manner. 115 ADSCs‐EXOs accelerate cutaneous wound healing by optimizing fibroblast properties, as shown in in vivo experiments. 113 Finally, hADSCs‐EXOs up‐regulated 199 miRNAs and down‐regulated 93 miRNAs to promote dermal fibroblast proliferation and differentiation that sped up skin regeneration. 116
4.4. Regulating collagen remodelling to inhibit scar hyperplasia
Scar hyperplasia is a morphological and histopathological change of skin and soft tissue after wound healing. Severe trauma and extensive burn usually lead to scar proliferation, affecting aesthetic appearance and impairing organ function. 89 ADSCs‐EXOs can regulate collagen remodelling to inhibit scar hyperplasia. In an early stage, EXOs promote collagen remodelling through synthesis of type Ⅰ and Ⅲ, whereas they reduce scarring in the late stage by inhibiting collagen formation. 115 In addition, ADSCs‐EXOs can stimulate the reconstruction of extracellular matrix by regulating fibroblast differentiation and gene expression, thereby promoting wound healing and preventing scar proliferation. Wang et al 117 found that ADSCs‐EXOs prevented the differentiation of fibroblasts into myofibroblasts but increased the ratio of transforming growth factor‐β3 (TGF‐β3) to TGF‐β1 in vivo. ADSCs‐EXOs also increased the matrix metalloproteinases‐3 (MMP3) expression in skin dermal fibroblasts, resulting in a high ratio of MMP3 to tissue inhibitor of matrix metalloproteinases‐1 (TIMP1). This is beneficial for the remodelling of extracellular matrix (ECM), reducing scaring. Instead, in diabetic mice, ADSCs‐EXOs promoted collagen deposition, which increased in the late stage of wound healing. 118 However, this leads to scar hyperplasia, which is not conducive to healing. 18 These controversial results may be due to the complex function of collagen and EXOs during different stages of wound healing. More studies on the effect of ADSCs‐EXOs on collagen deposition and their association with scar proliferation need to be performed (Figure 2).
FIGURE 2.
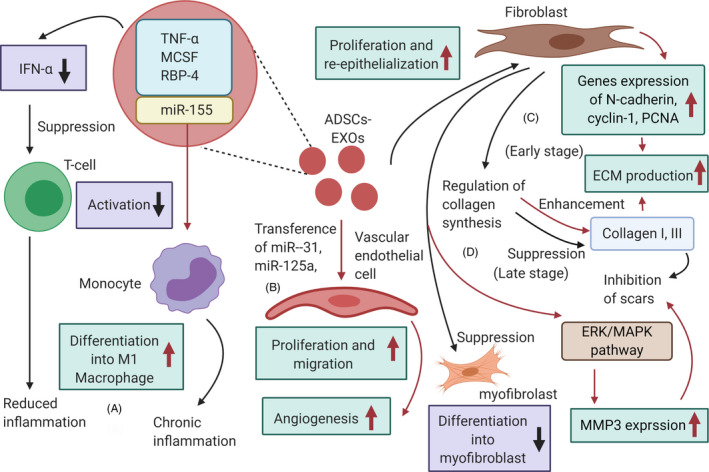
Mechanisms by which ADSCs‐EXOs may promote wound healing. (A) adipose‐derived stem cells (ADSCs)‐EXOs contain immunoregulatory proteins and reduce the secretion of IFN‐α, subsequently inhibiting activation of T cells, resulting in reduced inflammation. Additionally, miR‐155 in ADSCs‐EXOs can induce monocyte differentiation into M1 macrophages, causing chronic inflammation; (B) ADSCs‐EXOs can transfer miRNA‐125a and miRNA‐31 to vascular endothelial cells, stimulating proliferation and migration to promote angiogenesis; (C) In the early stages, ADSCs‐EXOs may promote proliferation, migration and collagen synthesis in fibroblasts, stimulating N‐cadherin, cyclin‐1, PCNA and collagen I, III expression and increasing ECM production; (D) in late stages, ADSCs‐EXOs prevent the differentiation of fibroblasts into myofibroblasts, and reduce scarring by inhibition of the formation of collagen and activation the ERK/MAPK pathway to increase MMP3 expression. Abbreviations: ECM, extracellular matrix; MCSF, macrophage colony‐stimulating factor
5. CLINICAL APPLICATION OF ADSCs‐EXOs FOR WOUND REPAIR AND TREATMENT
5.1. Improving fat grafting
Fat grafting is used in cosmetic surgery, for example in the treatment of hemifacial atrophy, depressed scars and breast reconstruction. 119 , 120 Adequate blood supply in the early stage after transplantation is required for successful grafting 121 which highlights the importance of promoting angiogenesis. ADSCs‐EXOs can promote proliferation and migration of vascular endothelial cells, thus promoting angiogenesis. 122 They can also raise fat graft volume retention in adipose‐derived mesenchymal stem cells. Indeed, EVs from ADSCs could improve fat graft volume retention by stimulating angiogenesis and regulating inflammatory response 123 and EXOs were found comparable to source ADSCs in fat graft retention, up‐regulating early inflammation and angiogenesis 110 ; thus, it is clear that ADSCs‐EXOs may play an important role in the improvement of fat grafting in the clinic. Lastly, EXOs from hypoxia‐treated human adipose‐derived mesenchymal stem cells possessed a higher capacity to enhance angiogenesis in fat grafting. 124 The latter may help develop new strategies to improve the survival of fat grafts.
5.2. Promoting wound healing of diabetic patients
According to the World Health Organization (WHO), the number of diabetes patients will reach 592 million by 2035, 125 and one of the most challenging complications of diabetes mellitus (DM) is delayed wound healing. 126 Lack of ideal treatments among all available ones appeals many scientists to develop new therapies. 127 Wang et al 118 demonstrated that ADSCs‐EXOs could promote wound healing in diabetic mice by promoting angiogenesis, proliferation and migration of fibroblasts and collagen synthesis. The capacity of ADSCs‐EXOs to promote wound healing in diabetic foot patients was also reported. 128 The latter study showed that ADSCs‐EXOs can simultaneously express antioxidant receptors (Nrf2), laying an experimental foundation for clinical application of EXOs to treat chronic diabetic wounds. In diabetic mice, ADSCs‐EXOs also promoted increased collagen deposition in the late stage of wound healing, 118 but excessive collagen deposition may be unfavourable as it leads to scar hyperplasia. 18 This controversy remains unsolved and therefore more research is required on the effect of ADSCs‐EXOs on collagen deposition and their association with scar proliferation.
5.3. EXOs as a carrier and combined scaffold for treatment
The natural biocompatibility and cell‐targeting features equip EXOs for carring (delivering) drugs. 18 To stabilize their concentration following local application, hydrogel or fibrin can be used as scaffolds to delay EXOs release and enhancing their wound healing ability. 18 Shilan et al 129 used EXOs loaded in alginate gel as a bioactive scaffold in an in vivo study. This showed that this active wound dressing technique could significantly promote wound healing, collagen synthesis and local angiogenesis, providing a new strategy for the composite structure of alginate hydrogel to speed up the healing process.
5.4. Promoting scarless cutaneous repair
Scar formation after skin wounds have healed is an intractable medical problem, affecting both aesthetic appearance and organ function. 89 In murine incisional wounds, intravenous injection of ADSCs‐Exos decreased the size of scars, increased the ratio of collagen III to collagen I and regulated fibroblast differentiation and gene expression 117 ; thus, ADSCs‐EXOs may be a new treatment for scarless cutaneous repair.
6. DISCUSSION
Adipose‐derived stem cells‐EXOs have a great potential in the clinic for wound repair and regeneration (Figure 3). They can participate in the regulation of the immune response and wound inflammation and promote angiogenesis by transferring miRNA‐125a and miRNA‐31 to vascular endothelial cells. Also, ADSCs‐EXOs can stimulate the proliferation of fibroblasts and keratinocytes and regulate collagen remodelling. This inhibits scar hyperplasia by activating the ERK/MAPK pathway that regulates the secretion of related cytokines. These properties make them an optimal tool to improve fat grafting application, promote wound healing of diabetic patients and scarless cutaneous repair and also to act as a carrier for combined scaffolds used for treatment. Recently, more attention is given to self‐derived and free‐cell auxiliary agents, especially ADSC‐Exos. Oral wound repair may use free‐cell therapies to promote oral mucosa defects healing 130 and reduce inflammatory process in wound after tooth extraction. 131 Moreover, these therapies are also used in the healings of acute and chronic ulcers, 132 postoperative hand wounds, 133 chronic lower‐extremity wounds. 134 We have every reason to believe there is more potential in the use of ADSC‐Exos in free‐cell therapies to be discovered.
FIGURE 3.
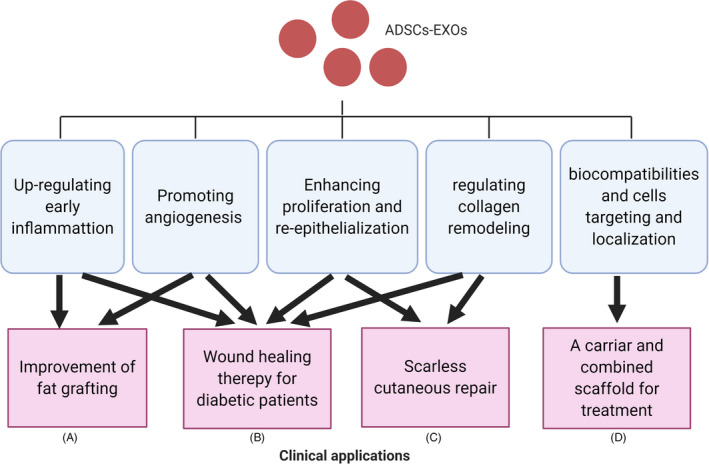
Potential clinical applications of adipose‐derived stem cells (ADSCs)‐EXOs: improvement of fat grafting (A), wound healing therapy for diabetic patients (B), scarless repair (C) and carrier and combined scaffold for treatment (D)
However, although their efficacy has been proved, the mechanism is not yet clear. There remains a burning question in this field about the pro‐ or anti‐cancer status of ADSCS‐EXOs. Thus, safety and efficacy of ADSCs‐EXOs cannot be guaranteed. The problems of lacking of easy process of extraction and purification of EXOs and standard methods for identifying EXOs from specific cell sources also need to be solved. To better isolate and identify ADSCS‐EXOs and understand their mechanism of action, further research is needed in the expect of more efficient ADSCs‐EXOs products and boarder applications in clinical practice.
7. CONCLUSION
In summary, ADSCs‐EXOs are a highly promising therapeutic for wound repair and regeneration. In the wound, ADSCs‐EXOs modulate immune responses and inflammation, promote angiogenesis, accelerate proliferation and re‐epithelization of skin cells and regulate collagen remodelling which inhibits scar hyperplasia. ADSCs‐EXOs can improve fat grafting, promote wound healing of diabetic patients and act as a carrier and combined scaffold for treatment, leading to scarless cutaneous repair. ADSCs‐EXOs have a board applications in clinical practice and are likely to achieve the best fictionally skin wound healing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
JW and DL designed the study; SL and YA wrote the first draft of the manuscript; XT critically revised the manuscript; SZ, FN, YZ, LG, CZ, BW and WW prepared the figures. All authors approved the final version of the manuscript for submission.
An Y, Lin S, Tan X, et al. Exosomes from adipose‐derived stem cells and application to skin wound healing. Cell Prolif. 2021;54:e12993. 10.1111/cpr.12993
Yang An, Shuyan Lin and Xiaojie Tan contributed equally to this work
Funding information
This work was supported by the National Nature Science Foundation of China (No. 81873939), the Scientific Research Staring Foundation for the Returned Overseas Chinese Scholars, Peking University Third Hospital (No. BYSYLXHG2019001), and Key Clinical Projects of Peking University Third Hospital (No. BYSYZD2019013).
Contributor Information
Yang An, Email: anyangdoctor@163.com.
Dong Li, Email: lidong9@sina.com.
Junhao Wu, Email: anyangdoctor@163.com, Email: lidong9@sina.com, Email: huaxiwjh@163.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med J Br Diabet Assoc. 2006;23(6):594‐608. 10.1111/j.1464-5491.2006.01773.x [DOI] [PubMed] [Google Scholar]
- 2. Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmoulière A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2014;22(1):23‐33. 10.1111/wrr.12135 [DOI] [PubMed] [Google Scholar]
- 3. Goodarzi P, Alavi‐Moghadam S, Sarvari M, et al. Adipose tissue‐derived stromal cells for wound healing. Adv Exp Med Biol. 2018;1119:133‐149. 10.1007/5584_2018_220 [DOI] [PubMed] [Google Scholar]
- 4. Orgill DP, Ogawa R. Discussion: the embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg. 2014;133(2):406‐407. [DOI] [PubMed] [Google Scholar]
- 5. Turner NJ, Badylak SF. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv Wound Care. 2015;4(8):490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaghardoost R, Momeni M, Kazemikhoo N, et al. Effect of low‐level laser therapy on the healing process of donor site in patients with grade 3 burn ulcer after skin graft surgery (a randomized clinical trial). Lasers Med Sci. 2018;33(3):603‐607. 10.1007/s10103-017-2430-4 [DOI] [PubMed] [Google Scholar]
- 7. Martino MM, Briquez PS, Güç E, et al. Growth factors engineered for super‐affinity to the extracellular matrix enhance tissue healing. Science. 2014;343(6173):885‐888. 10.1126/science.1247663 [DOI] [PubMed] [Google Scholar]
- 8. Andreadis ST, Geer DJ. Biomimetic approaches to protein and gene delivery for tissue regeneration. Trends Biotechnol. 2006;24(7):331‐337. 10.1016/j.tibtech.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 9. Bernuzzi G, Petraglia F, Pedrini MF, et al. Use of platelet‐rich plasma in the care of sports injuries: our experience with ultrasound‐guided injection. Blood Transfus Trasfus Sangue. 2014;12(Suppl 1):S229‐S234. 10.2450/2013.0293-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Behr B, Ko SH, Wong VW, Gurtner GC, Longaker MT. Stem cells. Plast Reconstr Surg. 2010;126(4):1163‐1171. 10.1097/PRS.0b013e3181ea42bb [DOI] [PubMed] [Google Scholar]
- 11. Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11‐22. 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 12. Cho D‐I, Kim MR, Jeong H, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow‐derived macrophages. Exp Mol Med. 2014;46:e70. 10.1038/emm.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC. Mesenchymal stromal cells and cutaneous wound healing: a comprehensive review of the background, role, and therapeutic potential. Stem Cells Int. 2018;2018:e6901983. 10.1155/2018/6901983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan Z, Zhao J, Chen Y, Yang Z, Cui W, Zheng Q. Regulating inflammation using acid‐responsive electrospun fibrous scaffolds for skin scarless healing. Mediators Inflamm. 2014;2014:e858045. 10.1155/2014/858045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Miguel MP, Fuentes‐Julián S, Blázquez‐Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12(5):574‐591. 10.2174/156652412800619950 [DOI] [PubMed] [Google Scholar]
- 16. Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585‐596. 10.1002/stem.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3(3):20. 10.1186/scrt111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taverna S, Pucci M, Alessandro R. Extracellular vesicles: small bricks for tissue repair/regeneration. Ann Transl Med. 2017;5(4):83. 10.21037/atm.2017.01.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell‐free therapy. Front Physiol. 2012;3:359. 10.3389/fphys.2012.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic Cph Den. 2002;3(5):321‐330. 10.1034/j.1600-0854.2002.30502.x [DOI] [PubMed] [Google Scholar]
- 21. Toyserkani NM, Christensen ML, Sheikh SP, Sørensen JA. Adipose‐derived stem cells: new treatment for wound healing? Ann Plast Surg. 2015;75(1):117‐123. 10.1097/SAP.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 22. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150‐154. 10.1016/j.tibtech.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 23. Yáñez‐Mó M, Siljander PR‐M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:e27066. 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907‐1920. 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 25. Casado S, Lobo MDVT, Paíno CL. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci Rep. 2017;7(1):6767. 10.1038/s41598-017-07265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia‐reperfusion injury by microRNA‐dependent reprogramming of resident renal cells. Kidney Int. 2012;82(4):412‐427. 10.1038/ki.2012.105 [DOI] [PubMed] [Google Scholar]
- 27. Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci J Virtual Libr. 2005;10:822‐837. 10.2741/1576 [DOI] [PubMed] [Google Scholar]
- 28. Booth AM, Fang Y, Fallon JK, Yang J‐M, Hildreth JEK, Gould SJ. Exosomes and HIV Gag bud from endosome‐like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923‐935. 10.1083/jcb.200508014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher‐order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5(6):e158. 10.1371/journal.pbio.0050158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nkwe DO, Pelchen‐Matthews A, Burden JJ, Collinson LM, Marsh M. The intracellular plasma membrane‐connected compartment in the assembly of HIV‐1 in human macrophages. BMC Biol. 2016;14:50. 10.1186/s12915-016-0272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse‐Locker J, Kräusslich H‐G. HIV‐1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3(3):e36. 10.1371/journal.ppat.0030036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deneka M, Pelchen‐Matthews A, Byland R, Ruiz‐Mateos E, Marsh M. In macrophages, HIV‐1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177(2):329‐341. 10.1083/jcb.200609050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128(17):3171‐3176. 10.1242/jcs.166074 [DOI] [PubMed] [Google Scholar]
- 34. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19‐30; sup pp 1–13. 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- 35. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255‐289. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 36. Klinkert K, Echard A. Rab35 GTPase: a central regulator of phosphoinositides and F‐actin in endocytic recycling and beyond. Traffic Cph Den. 2016;17(10):1063‐1077. 10.1111/tra.12422 [DOI] [PubMed] [Google Scholar]
- 37. Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium‐dependent manner. Traffic Cph Den. 2005;6(2):131‐143. 10.1111/j.1600-0854.2004.00257.x [DOI] [PubMed] [Google Scholar]
- 38. Radulovic M, Stenmark H. ESCRTs in membrane sealing. Biochem Soc Trans. 2018;46(4):773‐778. 10.1042/BST20170435 [DOI] [PubMed] [Google Scholar]
- 39. Gould SJ, Booth AM, Hildreth JEK. The Trojan exosome hypothesis. Proc Natl Acad Sci USA. 2003;100(19):10592‐10597. 10.1073/pnas.1831413100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77‐91. 10.1016/j.devcel.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 41. Hyenne V, Apaydin A, Rodriguez D, et al. RAL‐1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211(1):27‐37. 10.1083/jcb.201504136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghossoub R, Lembo F, Rubio A, et al. Syntenin‐ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. 10.1038/ncomms4477 [DOI] [PubMed] [Google Scholar]
- 43. Guo H, Chitiprolu M, Roncevic L, et al. Atg5 disassociates the V1V0‐ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43(6):716‐730.e7. 10.1016/j.devcel.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 44. Abdulrahman BA, Abdelaziz DH, Schatzl HM. Autophagy regulates exosomal release of prions in neuronal cells. J Biol Chem. 2018;293(23):8956‐8968. 10.1074/jbc.RA117.000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267‐283. 10.1586/epr.09.17 [DOI] [PubMed] [Google Scholar]
- 46. Caby M‐P, Lankar D, Vincendeau‐Scherrer C, Raposo G, Bonnerot C. Exosomal‐like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879‐887. 10.1093/intimm/dxh267 [DOI] [PubMed] [Google Scholar]
- 47. Conde‐Vancells J, Rodriguez‐Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7(12):5157‐5166. 10.1021/pr8004887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Durcin M, Fleury A, Taillebois E, et al. Characterisation of adipocyte‐derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6(1):e1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49(1):3‐13. 10.1111/cpr.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell‐derived extracellular vesicles: toward cell‐free therapeutic applications. Mol Ther J Am Soc Gene Ther. 2015;23(5):812‐823. 10.1038/mt.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676‐705. 10.1124/pr.112.005983 [DOI] [PubMed] [Google Scholar]
- 52. Claude A. The constitution of protoplasm. Science. 1943;97(2525):451‐456. 10.1126/science.97.2525.451 [DOI] [PubMed] [Google Scholar]
- 53. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen‐presenting vesicles. J Exp Med. 1996;183(3):1161‐1172. 10.1084/jem.183.3.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bakhshian Nik A, Hutcheson JD, Aikawa E. Extracellular vesicles as mediators of cardiovascular calcification. Front Cardiovasc Med. 2017;4:78. 10.3389/fcvm.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B‐lymphocytes. J Biol Chem. 1998;273(32):20121‐20127. 10.1074/jbc.273.32.20121 [DOI] [PubMed] [Google Scholar]
- 56. Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397‐422. 10.1146/annurev.cellbio.19.111301.153609 [DOI] [PubMed] [Google Scholar]
- 57. Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1(1):18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487‐514. 10.1146/annurev-biochem-013118-111902 [DOI] [PubMed] [Google Scholar]
- 59. Atay S, Gercel‐Taylor C, Taylor DD. Human trophoblast‐derived exosomal fibronectin induces pro‐inflammatory IL‐1β production by macrophages. Am J Reprod Immunol. 2011;66(4):259‐269. 10.1111/j.1600-0897.2011.00995.x [DOI] [PubMed] [Google Scholar]
- 60. Santasusagna S, Moreno I, Navarro A, et al. Proteomic analysis of liquid biopsy from tumor‐draining vein indicates that high expression of exosomal ECM1 is associated with relapse in stage I‐III colon cancer. Transl Oncol. 2018;11(3):715‐721. 10.1016/j.tranon.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng J, Hernandez JM, Doussot A, et al. Extracellular matrix proteins and carcinoembryonic antigen‐related cell adhesion molecules characterize pancreatic duct fluid exosomes in patients with pancreatic cancer. HPB. 2018;20(7):597‐604. 10.1016/j.hpb.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liang Y, Eng WS, Colquhoun DR, Dinglasan RR, Graham DR, Mahal LK. Complex N‐linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J Biol Chem. 2014;289(47):32526‐32537. 10.1074/jbc.M114.606269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Segura E, Nicco C, Lombard B, et al. ICAM‐1 on exosomes from mature dendritic cells is critical for efficient naive T‐cell priming. Blood. 2005;106(1):216‐223. 10.1182/blood-2005-01-0220 [DOI] [PubMed] [Google Scholar]
- 64. Baietti MF, Zhang Z, Mortier E, et al. Syndecan‐syntenin‐ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677‐685. 10.1038/ncb2502 [DOI] [PubMed] [Google Scholar]
- 65. Rieu S, Géminard C, Rabesandratana H, Sainte‐Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267(2):583‐590. 10.1046/j.1432-1327.2000.01036.x [DOI] [PubMed] [Google Scholar]
- 66. Mathew A, Bell A, Johnstone RM. Hsp‐70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J. 1995;308(Pt 3):823‐830. 10.1042/bj3080823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Subra C, Grand D, Laulagnier K, et al. Exosomes account for vesicle‐mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51(8):2105‐2120. 10.1194/jlr.M003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Batista BS, Eng WS, Pilobello KT, Hendricks‐Muñoz KD, Mahal LK. Identification of a conserved glycan signature for microvesicles. J Proteome Res. 2011;10(10):4624‐4633. 10.1021/pr200434y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shimoda A, Tahara Y, Sawada S‐I, Sasaki Y, Akiyoshi K. Glycan profiling analysis using evanescent‐field fluorescence‐assisted lectin array: importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem Biophys Res Commun. 2017;491(3):701‐707. 10.1016/j.bbrc.2017.07.126 [DOI] [PubMed] [Google Scholar]
- 70. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 71. Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470‐1476. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pegtel DM, Cosmopoulos K, Thorley‐Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107(14):6328‐6333. 10.1073/pnas.0914843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. 10.1038/ncomms1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kahlert C, Melo SA, Protopopov A, et al. Identification of double‐stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869‐3875. 10.1074/jbc.C113.532267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thakur BK, Zhang H, Becker A, et al. Double‐stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766‐769. 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sansone P, Savini C, Kurelac I, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy‐resistant breast cancer. Proc Natl Acad Sci USA. 2017;114(43):E9066‐E9075. 10.1073/pnas.1704862114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Deng Z, Poliakov A, Hardy RW, et al. Adipose tissue exosome‐like vesicles mediate activation of macrophage‐induced insulin resistance. Diabetes. 2009;58(11):2498‐2505. 10.2337/db09-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity‐related liver disease. J Surg Res. 2014;192(2):268‐275. 10.1016/j.jss.2014.06.050 [DOI] [PubMed] [Google Scholar]
- 79. Ogawa R, Tanaka C, Sato M, et al. Adipocyte‐derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010;398(4):723‐729. 10.1016/j.bbrc.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 80. Lin R, Wang S, Zhao RC. Exosomes from human adipose‐derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13‐20. 10.1007/s11010-013-1746-z [DOI] [PubMed] [Google Scholar]
- 81. Katsuda T, Tsuchiya R, Kosaka N, et al. Human adipose tissue‐derived mesenchymal stem cells secrete functional neprilysin‐bound exosomes. Sci Rep. 2013;3:1197. 10.1038/srep01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30‐41. 10.1016/j.plipres.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 83. Golfetto O, Wakefield DL, Cacao EE, et al. A platform to enhance quantitative single molecule localization microscopy. J Am Chem Soc. 2018;140(40):12785‐12797. 10.1021/jacs.8b04939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Daaboul GG, Gagni P, Benussi L, et al. Digital detection of exosomes by interferometric imaging. Sci Rep. 2016;6:e37246. 10.1038/srep37246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917‐1950. 10.1021/acs.chemrev.7b00534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2:e20389. 10.3402/jev.v2i0.20389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Monaco JL, Lawrence WT. Acute wound healing an overview. Clin Plast Surg. 2003;30(1):1‐12. 10.1016/s0094-1298(02)00070-6 [DOI] [PubMed] [Google Scholar]
- 88. Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938‐949. 10.1056/NEJMra0801082 [DOI] [PubMed] [Google Scholar]
- 89. Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276(5309):75‐81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- 90. Kurkinen M, Vaheri A, Roberts PJ, Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Investig J Tech Methods Pathol. 1980;43(1):47‐51. [PubMed] [Google Scholar]
- 91. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528‐1542. 10.1177/147323000903700531 [DOI] [PubMed] [Google Scholar]
- 92. Broughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S‐34S. 10.1097/01.prs.0000225430.42531.c2 [DOI] [PubMed] [Google Scholar]
- 93. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76(5):2003‐2011. 10.1172/JCI112200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pohlman TH, Stanness KA, Beatty PG, Ochs HD, Harlan JM. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor‐alpha increases neutrophil adherence by a CDw18‐dependent mechanism. J Immunol. 1986;136(12):4548‐4553. [PubMed] [Google Scholar]
- 96. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958‐969. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187(5A):11S‐16S. 10.1016/S0002-9610(03)00296-4 [DOI] [PubMed] [Google Scholar]
- 98. Mills RE, Taylor KR, Podshivalova K, McKay DB, Jameson JM. Defects in skin gamma delta T cell function contribute to delayed wound repair in rapamycin‐treated mice. J Immunol. 2008;181(6):3974‐3983. 10.4049/jimmunol.181.6.3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grotendorst GR, Soma Y, Takehara K, Charette M. EGF and TGF‐alpha are potent chemoattractants for endothelial cells and EGF‐like peptides are present at sites of tissue regeneration. J Cell Physiol. 1989;139(3):617‐623. 10.1002/jcp.1041390323 [DOI] [PubMed] [Google Scholar]
- 100. Bailey AJ, Sims TJ, Lous LE, Bazin S. Collagen polymorphism in experimental granulation tissue. Biochem Biophys Res Commun. 1975;66(4):1160‐1165. 10.1016/0006-291x(75)90480-5 [DOI] [PubMed] [Google Scholar]
- 101. Diegelmann RF. Analysis of collagen synthesis. Methods Mol Med. 2003;78:349‐358. 10.1385/1-59259-332-1:349 [DOI] [PubMed] [Google Scholar]
- 102. Madden JW, Smith HC. The rate of collagen synthesis and deposition in dehisced and resutured wounds. Surg Gynecol Obstet. 1970;130(3):487‐493. [PubMed] [Google Scholar]
- 103. Levenson SM, Geever EF, Crowley LV, Oates JF, Berard CW, Rosen H. The healing of rat skin wounds. Ann Surg. 1965;161:293‐308. 10.1097/00000658-196502000-00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2005;31(6):674‐686; discussion 686. 10.1111/j.1524-4725.2005.31612 [DOI] [PubMed] [Google Scholar]
- 105. Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls it s onset? Front Immunol. 2016;7:160. 10.3389/fimmu.2016.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2(9):764‐766. 10.1038/ni0901-764 [DOI] [PubMed] [Google Scholar]
- 107. Blazquez R, Sanchez‐Margallo FM, de la Rosa O, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556. 10.3389/fimmu.2014.00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kranendonk MEG, Visseren FLJ, van Balkom BWM, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obes Silver Spring Md. 2014;22(5):1296‐1308. 10.1002/oby.20679 [DOI] [PubMed] [Google Scholar]
- 109. Zhang Y, Mei H, Chang X, Chen F, Zhu Y, Han X. Adipocyte‐derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR‐155. J Mol Cell Biol. 2016;8(6):505‐517. 10.1093/jmcb/mjw040 [DOI] [PubMed] [Google Scholar]
- 110. Chen B, Cai J, Wei Y, et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up‐regulating early inflammation and angiogenesis. Plast Reconstr Surg. 2019;144(5):816e‐827e. 10.1097/PRS.0000000000006175 [DOI] [PubMed] [Google Scholar]
- 111. Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose‐derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8(1):125. 10.1186/s13287-017-0578-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR‐125a. J Cell Sci. 2016;129(11):2182‐2189. 10.1242/jcs.170373 [DOI] [PubMed] [Google Scholar]
- 113. Kang T, Jones TM, Naddell C, et al. Adipose‐derived stem cells induce angiogenesis via microvesicle transport of miRNA‐31. Stem Cells Transl Med. 2016;5(4):440‐450. 10.5966/sctm.2015-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pu C‐M, Liu C‐W, Liang C‐J, et al. Adipose‐derived stem cells protect skin flaps against ischemia/reperfusion injury via IL‐6 expression. J Invest Dermatol. 2017;137(6):1353‐1362. 10.1016/j.jid.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 115. Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:e32993. 10.1038/srep32993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose‐derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. 2018;27(10):1170‐1172. 10.1111/exd.13451 [DOI] [PubMed] [Google Scholar]
- 117. Wang L, Hu L, Zhou X, Xiong Z, Chen L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7(1):e13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang J, Yi Y, Zhu Y, et al. Effects of adipose‐derived stem cell released exosomes on wound healing in diabetic mice. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi Zhongguo Xiufu Chongjian Waike Zazhi Chin J Reparative Reconstr Surg. 2020;34(1):124‐131. 10.7507/1002-1892.201903058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ko M‐S, Jung J‐Y, Shin I‐S, et al. Effects of expanded human adipose tissue‐derived mesenchymal stem cells on the viability of cryopreserved fat grafts in the nude mouse. Int J Med Sci. 2011;8(3):231‐238. 10.7150/ijms.8.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shim YH, Zhang RH. Literature review to optimize the autologous fat transplantation procedure and recent technologies to improve graft viability and overall outcome: a systematic and retrospective analytic approach. Aesthetic Plast Surg. 2017;41(4):815‐831. [DOI] [PubMed] [Google Scholar]
- 121. Li L, Pan S, Ni B, Lin Y. Improvement in autologous human fat transplant survival with SVF plus VEGF‐PLA nano‐sustained release microspheres. Cell Biol Int. 2014;38(8):962‐970. 10.1002/cbin.10284 [DOI] [PubMed] [Google Scholar]
- 122. Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell‐assisted lipotransfer: a systematic review and meta‐analysis. Plast Reconstr Surg. 2016;137(1):44e‐57e. 10.1097/PRS.0000000000001981 [DOI] [PubMed] [Google Scholar]
- 123. Mou S, Zhou M, Li Y, et al. Extracellular vesicles from human adipose‐derived stem cells for the improvement of angiogenesis and fat‐grafting application. Plast Reconstr Surg. 2019;144(4):869‐880. 10.1097/PRS.0000000000006046 [DOI] [PubMed] [Google Scholar]
- 124. Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia‐treated human adipose‐derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF‐R. Int J Biochem Cell Biol. 2019;109:59‐68. 10.1016/j.biocel.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 125. Krug EG. Trends in diabetes: sounding the alarm. Lancet Lond Engl. 2016;387(10027):1485‐1486. 10.1016/S0140-6736(16)30163-5 [DOI] [PubMed] [Google Scholar]
- 126. Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30(1):37‐45. 10.1016/s0094-1298(02)00066-4 [DOI] [PubMed] [Google Scholar]
- 127. Hyldig K, Riis S, Pennisi CP, Zachar V, Fink T. Implications of extracellular matrix production by adipose tissue‐derived stem cells for development of wound healing therapies. Int J Mol Sci. 2017;18(6):1167. 10.3390/ijms18061167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li X, Xie X, Lian W, et al. Exosomes from adipose‐derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):29. 10.1038/s12276-018-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shafei S, Khanmohammadi M, Heidari R, et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full‐thickness skin wounds: an in vivo study. J Biomed Mater Res A. 2020;108(3):545‐556. 10.1002/jbm.a.36835 [DOI] [PubMed] [Google Scholar]
- 130. Lin SL, Wu SL, Tsai CC, Ko SY, Chiang WF, Yang JW. The use of solid‐phase concentrated growth factors for surgical defects in the treatment of dysplastic lesions of the oral mucosa. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. Published online. 2016;74(12):2549‐2556. 10.1016/j.joms.2016.06.183 [DOI] [PubMed] [Google Scholar]
- 131. Asmael HM, Jamil FA, Hasan AM. Novel application of platelet‐rich fibrin as a wound healing enhancement in extraction sockets of patients who smoke. J Craniofac Surg. 2018;29:e794‐e797. 10.1097/SCS.0000000000004818 [DOI] [PubMed] [Google Scholar]
- 132. Kim S‐A, Ryu H‐W, Lee K‐S, Cho J‐W. Application of platelet‐rich plasma accelerates the wound healing process in acute and chronic ulcers through rapid migration and upregulation of cyclin A and CDK4 in HaCaT cells. Mol Med Rep. 2013;7(2):476‐480. 10.3892/mmr.2012.1230 [DOI] [PubMed] [Google Scholar]
- 133. Chignon‐Sicard B, Georgiou CA, Fontas E, et al. Efficacy of leukocyte‐ and platelet‐rich fibrin in wound healing: a randomized controlled clinical trial. Plast Reconstr Surg. 2012;130(6):819e‐829e. 10.1097/PRS.0b013e31826d1711 [DOI] [PubMed] [Google Scholar]
- 134. McAleer JP, Kaplan E, Persich G. Efficacy of concentrated autologous platelet‐derived growth factors in chronic lower‐extremity wounds. J Am Podiatr Med Assoc. 2006;96(6):482‐488. 10.7547/0960482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


