Key Points
Question
Is short-course antibiotic therapy (5 days of high-dose amoxicillin) inferior to standard care (10 days of high-dose amoxicillin) for the treatment of children aged 6 months to 10 years diagnosed with community-acquired pneumonia in an outpatient setting?
Findings
In this 2-center, blinded randomized clinical trial, children treated with short-course antibiotic therapy had comparable rates of clinical cure at 14 to 21 days after enrollment compared with standard care (85.7% vs 84.1%).
Meaning
Results of this study suggest that short-course therapy for pediatric community-acquired pneumonia not requiring hospitalization offers more benefit than harm and should be considered for inclusion in treatment guidelines.
This equivalence and noninferiority randomized clinical trial assesses whether 5 days of high-dose amoxicillin for community-acquired pneumonia was associated with noninferior rates of clinical cure compared with 10 days of high-dose amoxicillin among children 10 years or younger.
Abstract
Importance
Community-acquired pneumonia (CAP) is a common occurrence in childhood; consequently, evidence-based recommendations for its treatment are required.
Objective
To determine whether 5 days of high-dose amoxicillin for CAP was associated with noninferior rates of clinical cure compared with 10 days of high-dose amoxicillin.
Design, Setting, and Participants
The SAFER (Short-Course Antimicrobial Therapy for Pediatric Respiratory Infections) study was a 2-center, parallel-group, noninferiority randomized clinical trial consisting of a single-center pilot study from December 1, 2012, to March 31, 2014, and the follow-up main study from August 1, 2016, to December 31, 2019 at the emergency departments of McMaster Children’s Hospital and the Children’s Hospital of Eastern Ontario. Research staff, participants, and outcome assessors were blinded to treatment allocation. Eligible children were aged 6 months to 10 years and had fever within 48 hours, respiratory symptoms, chest radiography findings consistent with pneumonia as per the emergency department physician, and a primary diagnosis of pneumonia. Children were excluded if they required hospitalization, had comorbidities that would predispose them to severe disease and/or pneumonia of unusual origin, or had previous β-lactam antibiotic therapy. Data were analyzed from March 1 to July 8, 2020.
Interventions
Five days of high-dose amoxicillin therapy followed by 5 days of placebo (intervention group) vs 5 days of high-dose amoxicillin followed by a different formulation of 5 days of high-dose amoxicillin (control group).
Main Outcomes and Measures
Clinical cure at 14 to 21 days.
Results
Among the 281 participants, the median age was 2.6 (interquartile range, 1.6-4.9) years (160 boys [57.7%] of 279 with sex listed). Clinical cure was observed in 101 of 114 children (88.6%) in the intervention group and in 99 of 109 (90.8%) in the control group in per-protocol analysis (risk difference, −0.016; 97.5% confidence limit, −0.087). Clinical cure at 14 to 21 days was observed in 108 of 126 (85.7%) in the intervention group and in 106 of 126 (84.1%) in the control group in the intention-to-treat analysis (risk difference, 0.023; 97.5% confidence limit, −0.061).
Conclusions and Relevance
Short-course antibiotic therapy appeared to be comparable to standard care for the treatment of previously healthy children with CAP not requiring hospitalization. Clinical practice guidelines should consider recommending 5 days of amoxicillin for pediatric pneumonia management in accordance with antimicrobial stewardship principles.
Trial Registration
ClinicalTrials.gov Identifier: NCT02380352
Introduction
Community-acquired pneumonia (CAP) commonly occurs in children.1,2,3,4 Unfortunately, the optimal duration of antimicrobial therapy for pediatric CAP is unclear; both the Infectious Disease Society of America5 and the Canadian Pediatric Society6 note that current treatment duration recommendations are based on sparse evidence.
Randomized clinical trials (RCTs) have shown that 5-day courses of antibiotics for adults with CAP are as effective as longer courses, even for severe disease.7,8 In contrast, few RCTs of short-course antibiotic therapy for CAP have been performed in children, and those that exist have important limitations, including small size,9 lack of blinding,10 inappropriate study design,11 and loss of effective blinding12 because of zero clinical failures in a noninferiority study.13 We conducted an RCT to determine whether, in previously healthy children diagnosed with CAP in the emergency department (ED), 5 days of high-dose amoxicillin led to noninferior rates of clinical cure at 14 to 21 days after enrollment compared with the current standard, 10 days of high-dose amoxicillin.
Methods
Study Design
The SAFER (Short-Course Antimicrobial Therapy for Pediatric Respiratory Infections) study was a 2-center, blinded, noninferiority RCT conducted in the EDs of McMaster Children’s Hospital (MCH), Hamilton, Ontario, Canada, and the Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada, with the support of Pediatric Emergency Research Canada, after the successful completion of a pilot study at MCH. The complete trial protocol is found in Supplement 1. Both academic hospitals have EDs staffed predominantly by pediatric specialists. The purpose of the pilot study was to ensure feasibility of a subsequent larger trial; in its assessment, measurement of feasibility outcomes did not require identification of treatment assignment. Ethics approval was obtained from the Hamilton Integrated Research Ethics Board and the Children’s Hospital of Ontario Research Ethics Board. Written informed consent was obtained from each participant’s parent or guardian. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Children aged 6 months to 10 years with CAP well enough to be treated as outpatients were eligible. We defined CAP by all of the following criteria, similar to other studies14,15,16,17:
Fever (temperature >37.5 °C axillary, >37.7 °C oral, or >38 °C rectal) in the 48 hours before presentation;
Tachypnea (>60 breaths/min if younger than 1 year, >50 breaths/min if aged 1-2 years, >40 breaths/min if aged 2-4 years, and >30 breaths/min if older than 4 years); increased work of breathing on examination (scalene muscle use or suprasternal recessions/indrawing or intercostal retractions or subcostal recessions/indrawing); or auscultatory findings (eg, focal crackles) consistent with CAP;
Chest radiography findings consistent with CAP as per the ED physician; and
Primary diagnosis of CAP as per the ED physician.
Children were excluded if they had any of the following that would predispose them to severe disease and/or pneumonia with atypical microbiology (ie, unusual causative pathogens): empyema or necrotizing pneumonia, preexisting pulmonary disease, congenital heart disease, history of aspiration, malignant neoplasm, immunodeficiency, or kidney dysfunction. Those who received more than 24 hours of β-lactam antibiotic therapy at presentation, at least a 5-day course of β-lactam therapy less than 72 hours before presentation, or intravenous cephalosporin or azithromycin in the ED were not eligible, because it would be difficult to give these children short-course treatment. The Canadian amoxicillin monograph18 has a precaution against coadministration with warfarin or tetracyclines, so children receiving those drugs were excluded, and children with suspected infectious mononucleosis were excluded because of the possibility of rash. We also excluded children with a prolonged admission (>48 hours) to the hospital in the prior 2 months, CAP diagnosed in the previous month, or lung abscess in the previous 6 months, to avoid enrolling children with hospital-acquired or complicated pneumonia. Those with penicillin allergy were not enrolled. Children were not eligible to participate more than once. In Ontario, pneumococcal vaccination coverage (assessed at 7 years of age) has varied from 74.1% to 79.7% during the past 6 years.19
Procedures
A 1:1 randomization scheme, stratified by site, was developed using a random number generator with variable block sizes of 2, 4, and 6. Only the study pharmacists, who did not recruit or follow-up participants, had access to randomization lists. On recruitment, the research assistants (including R.C.) contacted the study pharmacists, who assigned a unique study identifier in sequential order. These identifiers corresponded with entries in the randomization tables, which indicated either 5 days of amoxicillin plus 5 days of placebo (intervention group) or 5 days of amoxicillin plus 5 days of amoxicillin (control group). The appearance and taste of the placebo and the amoxicillin for the second 5 days were similar to each other and different than the amoxicillin used for the first 5 days of treatment to preserve blinding in both groups. Participants, caregivers, clinicians, outcome assessors, and study investigators were blinded to treatment assignment.
All laboratory testing was optional. If blood work was ordered by nonstudy clinicians, the results of complete blood cell count and/or serum C-reactive protein level measurement were documented; no additional blood draws were requested. Nasopharyngeal swabs (NPSs) from participants recruited at 1 site (MCH) were assayed prospectively using a laboratory-developed multiplex respiratory virus panel.20 The NPS specimens from all consenting participants were stored and batch tested after the study period using a laboratory-developed and validated multiplex polymerase chain reaction assay to detect Mycoplasma pneumoniae and Chlamydia pneumoniae. Saliva was sampled for C-reactive protein level measurement using a commercially available assay (Salimetrics, LLC).
Caregivers were asked to complete a daily diary documenting temperature, respiratory symptoms, school and/or daycare attendance, caregiver absenteeism from work, adverse drug reactions, and missed medication doses. Caregivers were telephoned once at days 3 to 5 and once at days 7 to 10. Any participant with persistent fever at 96 hours after enrollment received an additional 5 days of open-label amoxicillin after the initial 5 days (ie, was not permitted to potentially receive short-course treatment) and was considered a clinical failure. Any participant who clinically deteriorated was asked to return for assessment. Participants all returned at days 14 to 21 for primary outcome measurement. Caregivers were telephoned 1 month after enrollment.
Outcomes
The primary outcome, clinical cure, was defined by all of the following:
Initial improvement during the first 4 days after enrollment (including defervescence);
Significant improvement in dyspnea and increased work of breathing and no recorded tachypnea at the 14- to 21-day follow-up visit;
No more than 1 fever spike (as previously defined) as a result of possible bacterial respiratory illness from day 4 up to and including the 14- to 21-day follow-up visit; and
Lack of a requirement for additional antibacterials or admission to hospital because of persistent or progressive lower respiratory illness before the 14- to 21-day follow-up visit.
The primary outcome was measured using caregiver-report items at the 3- to 5-day and 7- to 10-day follow-ups, daily diary entries, and the physical examination at the 14- to 21-day visit.
In this RCT, our aim was to measure relevant outcomes. While conducting the study, we realized that there were participants with responses to treatment that were categorized as clinical failures but—outside an RCT—might not be categorized as such in the course of typical care (eg, children with 2 spikes of fever after finishing antibiotics but no other worrisome signs/symptoms). Consequently, post hoc, we created another new secondary cure outcome, clinical cure not requiring additional intervention. This was defined as initial improvement during the first 4 days after enrollment (including defervescence), plus the lack of a requirement for additional antibacterials or admission to hospital because of persistent/progressive lower respiratory illness (ie, only criteria 1 and 4 of standard clinical cure). Other secondary outcomes included the number of days the participant was absent from school or daycare, the total number of days that caregiver work was disrupted, the number of days of mild adverse reactions to the drug, the incidence of serious adverse reactions to the drug (including anaphylaxis), participant adherence to the study medications, and recurrence of presumed bacterial respiratory illness after the primary outcome visit in the month after enrollment.
Statistical Analysis
Data were analyzed from March 1 to July 8, 2020. We estimated the success rate of standard therapy to be 95%, consistent with previous pediatric trials.11 The noninferiority margin was set at 7.5% (1-sided 97.5% confidence limit [CL]) and, with α = .025 and β = 0.2, 135 participants per group were required.
Descriptive statistics were used to describe demographic characteristics and outcomes by treatment group. Binomial regression was used to estimate treatment effects of clinical cure and other binary outcomes, adjusting for site (because randomization was stratified by site). For the primary analysis, results were reported using adjusted risk differences (RDs) and the lower limit of the 97.5% CLs. For secondary analyses of binary outcomes, results were reported using adjusted RDs and 95% CIs. Poisson regression was used to compare secondary count outcomes; results were reported using adjusted incident rate ratios with 95% CIs. The primary outcome was assessed using intention-to-treat (ITT) analysis, per-protocol (PP) analysis including those participants adherent to medications (ie, >80% of antimicrobial doses taken and no additional antibiotics taken for nonpneumonia infections), and strict PP analysis including only those in the PP group whose radiographs had radiologist-confirmed pneumonia. To be conservative,12 we specified a priori that the PP analysis would be primary.21 Secondary outcomes were analyzed using PP and ITT approaches.
Three different subgroup analyses were investigated: age (<5 vs ≥5 years), salivary C-reactive protein level (<30 vs ≥30 pg/mL [to convert to mg/L, divide by 1012]), and detection of virus or Mycoplasma species at baseline (negative vs positive test results). Subgroup analyses (using ITT) were conducted including an interaction term between treatment group and the subgroup. The interaction P value was used to determine whether there were significant differences between subgroup rates. These analyses were exploratory and were not adjusted for multiple comparisons. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc).
The data safety management board oversaw a single interim analysis of the data halfway through enrollment in the main study. The trial would have been prematurely terminated if the proportion of treatment failures in the intervention arm was statistically significantly greater (P < .0001) than 7.5% more than the proportion of treatment failures in the reference arm. Statistical significance was set at P < .05 for 2-sided analysis and P < .025 for 1-sided analysis.
Results
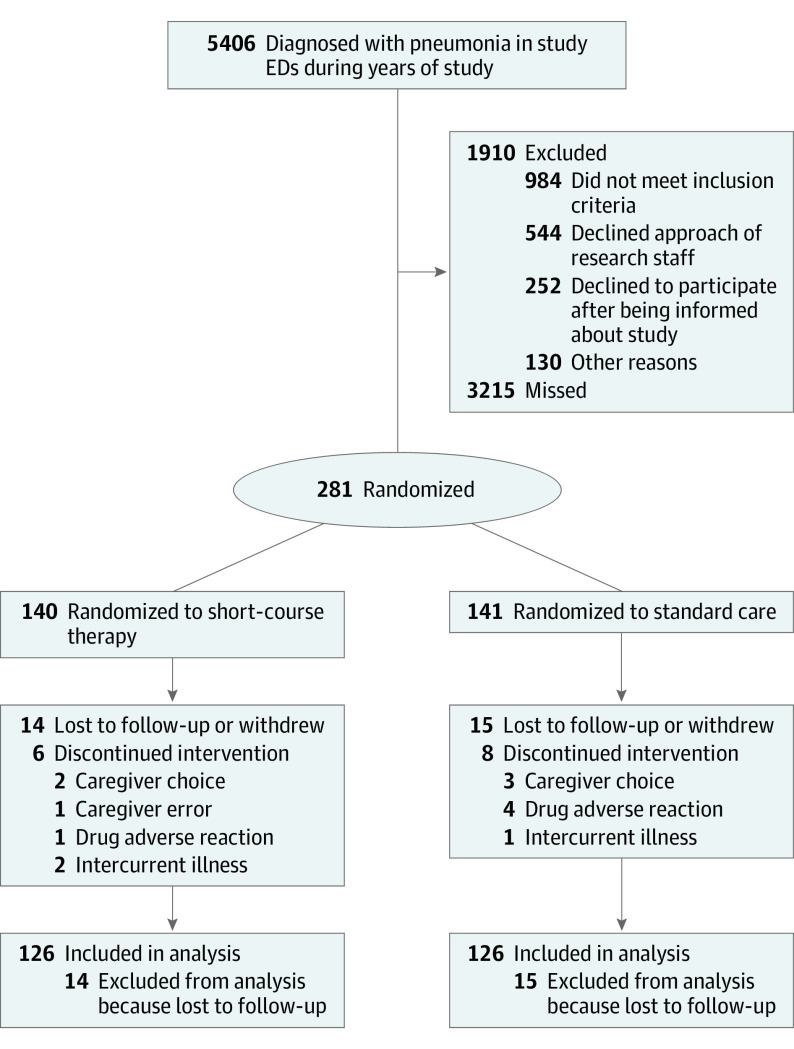
Sixty participants were enrolled in the pilot phase from December 1, 2012, to March 31, 2014, at MCH. The main study started enrolling 1 August 1, 2016, at MCH and January 1, 2017, at Children’s Hospital of Eastern Ontario; recruitment stopped December 31, 2019, because of funding limitations. A total of 281 participants were recruited and randomized; 160 were boys [57.3%] and 119 were girls (42.7%) (with data not documented for 2) and had a median (interquartile range [IQR]) age of 2.6 (1.6-4.9) years. Fourteen patients in the intervention group and 15 in the control group were lost to or unavailable for follow-up, so that 252 of 281 (89.7%) had outcomes documented (Figure).
Figure. Study Flow Diagram.
EDs indicates emergency departments.
The study participants are described in Table 1. The median (IQR) age of participants in each treatment group were similar (2.6 [1.6-4.5] years in the intervention group vs 2.6 [IQR, 1.6-5.1] years in the control group) as was the mean (SD) triage respiratory rate (30.6 [9.1] breaths/min in the intervention group vs 31.5 [10.9] breaths/min in the control group). One hundred participants in the intervention group (71.4%) and 108 in the control group (76.6%) were reported to have features consistent with pneumonia according to the attending radiologist. Only 13 participants (4.6%) underwent venipuncture.
Table 1. Baseline Characteristics.
| Characteristic | Patient groupa | |
|---|---|---|
| Intervention (n = 140) | Control (n = 141) | |
| Sex | ||
| Male | 70 (50.7%) | 90 (63.8%) |
| Female | 68 (49.3) | 51 (36.2) |
| Missing data, No. | 2 | 0 |
| Age, median (IQR), y | 2.55 (1.55-4.49) | 2.61 (1.62-5.11) |
| Missing data, No. | 1 | 0 |
| Respiratory rate at admission, mean (SD), breaths/min | 30.6 (9.1) | 31.5 (10.9) |
| Missing data, No. | 7 | 4 |
| Pneumonia reported by radiologist | 100 (71.4) | 108 (76.6) |
| Baseline salivary CRP level, median (IQR), pg/mL | 16.4 (8.46-76.0) | 15.2 (6.90-64.1) |
| Missing data, No. | 73 | 65 |
| Baseline NPS test result positive for a respiratory virusb | ||
| RSV | 21 (21.9) | 25 (25.8) |
| Rhinovirus/enterovirus | 18 (18.8) | 16 (16.5) |
| Metapneumovirus | 12 (12.5) | 7 (7.2) |
| Influenza | 7 (7.3) | 6 (6.2) |
| Parainfluenza | 7 (7.3) | 4 (4.1) |
| Adenovirus | 4 (4.2) | 6 (6.2) |
| Negative for all | 35 (36.5) | 37 (38.1) |
| Missing data, No. | 44 | 44 |
| Baseline NPS positive for Mycoplasma pneumoniae | 5 (5.5) | 7 (7.5) |
| Missing data, No. | 49 | 48 |
Abbreviations: CRP, C-reactive protein; IQR, interquartile range; NPS, nasopharyngeal swabs; RSV, respiratory syncytial virus.
SI conversion factor: To convert CRP to mg/L, divide by 1012.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
Column percentages add to more than 100% because individuals can have more than 1 virus.
Clinical Cure
The primary outcome, overall clinical cure at 14 to 21 days after enrollment (ITT analysis), was similar between the 2 groups; cure was reported in 108 of 126 participants (85.7%) in the intervention group and 106 of 126 (84.1%) in the control group (RD, 0.023; 97.5% CL, −0.061) (Table 2). The PP analysis results were similar, with cure in 101 of 114 patients (88.6%) in the intervention group and 99 of 109 (90.8%) in the control group (RD, −0.016; 97.5% CL, −0.087). In the strict PP analysis, clinical cure was documented in 73 of 82 patients (89.0%) allocated to the intervention group and in 74 of 83 (89.1%) allocated to the control group (RD, −0.011; 97.5% CL, −0.096).
Table 2. Clinical Cure Outcomes.
| Outcome | Intention-to-treat analysis | Per protocol analysis (adherent to medications) | Strict per protocol analysis (adherent to medications and consolidation on radiograph) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient groupa | RD (97.5% 1-sided CL) | Patient groupa | RD (97.5% 1-sided CL) | Patient groupa | RD (97.5% 1-sided CL) | ||||
| Intervention (n = 140) | Control (n = 141) | Intervention (n = 122) | Control (n = 114) | Intervention (n = 86) | Control (n = 87) | ||||
| Clinical cure (primary) | 108 (85.7) | 106 (84.1) | 0.023 (−0.061 to ∞) | 101 (88.6) | 99 (90.8) | −0.016 (−0.087 to ∞) | 73 (89.0) | 74 (89.2) | −0.011 (−0.096 to ∞) |
| Missing data, No. | 14 | 15 | 29 | 8 | 5 | 13 | 4 | 4 | 8 |
| Clinical cure not requiring additional intervention (secondary) | 116 (93.5) | 113 (90.4) | 0.028 (−0.038 to ∞) | 107 (95.5) | 104 (95.4) | −0.006 (−0.055 to ∞) | 76 (95.0) | 78 (94.0) | −0.004 (−0.071 to ∞) |
| Missing data, No. | 16 | 16 | 32 | 10 | 5 | 15 | 6 | 4 | 10 |
Abbreviations: CL, confidence limit; RD, risk difference.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
The proportion of participants who experienced clinical cure not requiring additional intervention was more consistent with that of the original cure estimates and was similar between groups. Clinical cure not requiring additional intervention was documented in 116 of 124 patients (93.5%) in the intervention group and 113 of 125 (90.4%) in the control group in the ITT analysis (RD, 0.028; 97.5% CL, −0.038), in 107 of 112 (95.5%) in the intervention group and 104 of 109 (95.4%) in the control group in the PP analysis (RD, −0.006; 97.5% CL, −0.055), and in 76 of 80 (95.0%) in the intervention group and 78 of 83 (94.0%) in the control group in the strict PP analysis (RD, −0.004; 97.5% CL, −0.071).
Nonclinical Cure Secondary Outcomes
Caregiver work absenteeism was significantly lower in the intervention group than in the control group (median [IQR], 2 [0-4] days vs 3 [IQR, 1-6] days; incident rate ratio, 0.76; 95% CI, 0.66-0.87; P < .001 in PP analysis). All other secondary outcomes were similar between groups (Table 3).
Table 3. Secondary Outcomes.
| Outcome | Intention-to-treat analysis | Per-protocol analysis | ||||
|---|---|---|---|---|---|---|
| Patient group | Estimate (95% CI) (n = 282)a | Patient group | Estimate (95% CI)a | |||
| Intervention (n = 140) | Control (n = 141) | Intervention (n = 126) | Control (n = 118) | |||
| Caregiver absenteeism, median (IQR), d | 2 (0-4) | 3 (0-6) | IRR, 0.74 (0.65 to 0.84) | 2 (0-4) | 3 (1-6) | IRR, 0.76 (0.66 to 0.87) |
| Missing data, No. | 16 | 19 | 35 | 11 | 11 | 22 |
| Child absenteeism, median (IQR), d | 1 (0-1) | 1 (0-1) | IRR, 0.95 (0.71 to 1.27) | 1 (0-1) | 1 (0-1) | IRR, 0.93 (0.68 to 1.27) |
| Mild drug adverse reactions, median (IQR), d | 1 (0-3) | 1 (0-3) | IRR, 0.91 (0.76 to 1.08) | 1 (0-3) | 1 (0-3) | IRR, 0.89 (0.74 to 1.07) |
| Missing data, No. | 16 | 19 | 35 | 11 | 11 | 22 |
| Anaphylaxis and other severe drug adverse reactions, No. (%) | 0 | 0 | NA | 0 | 0 | NA |
| Adherence to study medications, No. (%) | 122 (90.4) | 114 (86.4) | RD, 0.059 (−0.015 to 0.13) | NA (per-protocol defined by adherence) | NA (per-protocol defined by adherence) | NA (per-protocol defined by adherence) |
| Missing data, No. | 5 | 9 | 14 | |||
| Recurrence of respiratory illness after primary outcome visit but before 30-d follow-up, No. (%) | 11 (8.5) | 12 (9.6) | RD, −0.0070 (−0.078 to 0.064) | 11 (9.3) | 10 (9.0) | RD, 0.014 (−0.062 to 0.089) |
| Missing data, No. | 11 | 16 | 27 | 8 | 7 | 15 |
Abbreviations: IQR, interquartile range; IRR, incident rate ratio; NA, not applicable; RD, risk difference.
Estimates adjusted for center; 95% CIs are 2 sided.
Discussion
Our study recruited and randomized previously healthy children diagnosed with CAP who did not require hospitalization to either 5 or 10 days of amoxicillin therapy. In the PP analysis of the primary outcome, because the 1-sided 97.5% CL around the risk difference crossed the 7.5% noninferiority margin, a formal conclusion of noninferiority could not be made; however, in the ITT analysis, short-course treatment was indeed found to be statistically noninferior. In addition, short-course treatment was found to be statistically noninferior to the reference standard in all analyses (PP, ITT, and strict PP) when a potentially more relevant outcome, clinical cure not requiring additional intervention, was evaluated. Furthermore, we documented that the caregivers of participants receiving short-course treatment had significantly less work absenteeism than caregivers of those receiving standard care, although we cannot fully explain the mechanism of this difference. Consequently, we judge that the results of the present study are evidence that short-course antibiotic treatment for children with CAP who do not require hospitalization is comparable to standard care. Our findings are consistent with those of RCTs of short-course antibiotic treatment for the treatment of adults with nonsevere7 or severe8 CAP, who are both less likely than children to have viral disease and more likely to have poor outcomes.22,23
The overall clinical cure rate was lower than we expected. However, only 7 participants were hospitalized because of progressive bacterial respiratory illness; 6 were hospitalized in the first 5 days and 1 was hospitalized on day 7 after being switched to open-label amoxicillin owing to persistent fever. Most of the other clinical failures were attributable to fever that persisted more than 96 hours after enrollment, recurrent fever after initial defervescence, and/or prescription of additional antibiotics to children who otherwise did not deteriorate clinically; some of these children were then classified as having treatment failure but would not have been judged so outside of the context of a clinical trial. To address this issue, we created a new outcome (clinical cure not requiring additional intervention) that is probably more relevant to clinicians; by this measure, the cure rate was in line with initial projections. We attempted to bring participants with persistent or recurrent fever back to the study sites for reevaluation, but if they sought medical care elsewhere, they were often reflexively prescribed additional or broader-spectrum antimicrobials. We have observed this practice outside the clinical trial setting and hypothesize that it is owing to underappreciation of how commonly pediatric CAP is caused by pathogens unaffected by amoxicillin (most commonly respiratory viruses and M pneumoniae). This practice may be partially preventable via baseline NPS testing of children diagnosed with CAP. Of 130 participants enrolled during the main trial at MCH who had a baseline NPS taken and were followed up successfully to 10 days after enrollment, 17 (13.1%) either had persistent or recurrent fever but no other evidence of clinical deterioration. Of those participants, 14 (82.4%) had either M pneumoniae detected or a respiratory virus that had not been present previously detected on repeated NPS testing results; in this context, in the absence of clinical worsening, the detection of these pathogens implicated them as the probable cause of persistent or recurrent fever. For children diagnosed with CAP, therefore, results of the present study suggest that it may be prudent to perform nasopharyngeal testing to document viral or atypical coinfections at baseline to guard against needless escalation of antibiotic therapy should fever persist or recur. The antimicrobial stewardship benefits of routine nasopharyngeal testing should be further explored and more precisely quantified in future studies of pediatric CAP to verify whether they would outweigh the potential costs of this strategy.
In this era of widespread antimicrobial resistance,24 which may well worsen as a result of the coronavirus disease 2019 pandemic,25,26 it is important that antibiotic treatment durations for common infections are as short as possible and based on evidence rather than custom.27,28 It seems likely that reducing antimicrobial exposure will likely result in less circulating resistance in the community,29 which has prompted widespread efforts to do just that.24,30 There are other good reasons to minimize antibiotic prescriptions; even narrow-spectrum agents, such as amoxicillin, cost families money and can have adverse effects.31 In addition, a number of diverse and important conditions, such as obesity,32,33,34,35,36,37 atopy/asthma,38,39,40,41 arthritis,42 neurodevelopmental disorders,43,44 necrotizing enterocolitis,45 and, more recently, appendicitis,46 have been associated with antimicrobial exposure, likely mediated through changes in the human microbiome. For all of these reasons, the minimum amount of antibiotics necessary to treat bacterial infections should be prescribed, and no more.
Limitations
A limitation of the study is that we could not definitively establish the presence of bacterial pneumonia in the study participants; the inclusion of participants with purely viral respiratory disease would have interfered with the trial’s ability to detect a potential benefit of 10 days of amoxicillin compared with short-course therapy for children with bacterial CAP. However, we emphasize that it is difficult for clinicians to reliably discriminate among children with viral, atypical, and bacterial CAP in any context.47 The Infectious Disease Society of America and British Thoracic Society actively discourage blood sampling for children with nonsevere CAP,5,48 and a cohort study has recently demonstrated that biomarkers cannot reliably distinguish between nonsevere and severe CAP in children.49 The fact that most study participants had viral respiratory pathogens detected in their nasopharynges does not imply that only a minority truly had bacterial infections given how commonly viral pathogens are detected in patients hospitalized with CAP, including those requiring intensive care.23 We found no significant differences in clinical cure rates among those with respiratory viruses or M pneumoniae detected in their nasopharynges compared with those without (eTables 1-4 in Supplement 2). It is suboptimal that some study participants were not believed to have radiographic findings consistent with pneumonia by the attending radiologist; however, substantial interobserver variability in chest radiograph interpretation has been well documented.50,51,52,53,54 In addition, although the Canadian Pediatric Society states that chest radiographs “should usually be obtained,”6(p2) the Infectious Disease Society of America states that they are “not necessary,”5(pe5) and the British Thoracic Society actively discourages chest imaging for nonsevere illness.48 As a result, many physicians assessing children with suspected CAP in North America and Europe may not order chest radiographs. Cure rates in those participants with radiologist-confirmed pneumonia were very similar to those without. Furthermore, 2 other large RCTs evaluating treatment strategies for pediatric CAP55,56 did not use any radiographic criteria whatsoever for inclusion. We designed this pragmatic trial to enroll children similar to those diagnosed every day with CAP by ED physicians in the current era; consequently, our findings should be broadly generalizable. We did not design the study to evaluate the variability in interrater chest radiograph interpretation or to explore whether there were systematic differences between ED physician and radiologist interpretations; further studies might be useful to define the issue more precisely and could permit targeted educational interventions.
We were unable to bring 29 study participants (10.3%) back for complete follow-up. It seems unlikely that, among those unavailable for follow-up, there would have been sufficiently fewer clinical cures in participants randomized to the intervention group than in those randomized to the control group to result in a significant change to study results; however, we cannot state this definitively. Had we not had losses to follow-up, we might have had sufficient power to demonstrate noninferiority of the primary outcome in the PP analysis. Finally, it should be emphasized that these results may not be generalizable to children diagnosed with CAP in low- and middle-income countries, where assessment and management algorithms of children with respiratory illness often differ from those commonly used in North America.57,58,59
Conclusions
This noninferiority RCT found that, in a population of previously healthy children diagnosed with CAP in Canadian EDs, outcomes associated with the use of 5 days of high-dose amoxicillin were comparable to those associated with the use of 10 days of high-dose amoxicillin. Clinical practice guidelines should consider recommending 5 days of amoxicillin for pediatric pneumonia management in accordance with antimicrobial stewardship principles.
Trial protocol
eTable 1. Baseline Characteristics (Per-Protocol Population)
eTable 2. Subgroup Analyses of Primary Outcome by Subgroups: Age, Salivary CRP, and Detection of Virus/Mycoplasma Species
eTable 3. Subgroup Analyses of Secondary Outcomes (Binary)
eTable 4. Subgroup Analyses of Secondary Outcomes (Count)
Data sharing statement
References
- 1.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346(6):429-437. doi: 10.1056/NEJMra011994 [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408-416. doi: 10.2471/BLT.07.048769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Pneumonia fact sheet No . 331. Published August 2, 2019. Accessed January 28, 2021. https://www.who.int/mediacentre/factsheets/fs331/en/index.html
- 4.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet. 2006;368(9541):1048-1050. doi: 10.1016/S0140-6736(06)69334-3 [DOI] [PubMed] [Google Scholar]
- 5.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the PIDS and IDSA. Clin Infect Dis. 2011;53(7):e25-e76. doi: 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Saux N, Robinson JL; Canadian Paediatric Society, Infectious Diseases and Immunization Committee . Uncomplicated pneumonia in healthy Canadian children and youth: practice points for management. Paediatr Child Health. 2015;20(8):441-450. doi: 10.1093/pch/20.8.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37(6):752-760. doi: 10.1086/377539 [DOI] [PubMed] [Google Scholar]
- 8.Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265. doi: 10.1001/jamainternmed.2016.3633 [DOI] [PubMed] [Google Scholar]
- 9.Kogan R, Martínez MA, Rubilar L, et al. Comparative randomized trial of azithromycin versus erythromycin and amoxicillin for treatment of community-acquired pneumonia in children. Pediatr Pulmonol. 2003;35(2):91-98. doi: 10.1002/ppul.10180 [DOI] [PubMed] [Google Scholar]
- 10.Wubbel L, Muniz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J. 1999;18(2):98-104. doi: 10.1097/00006454-199902000-00004 [DOI] [PubMed] [Google Scholar]
- 11.Harris JA, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17(10):865-871. doi: 10.1097/00006454-199810000-00004 [DOI] [PubMed] [Google Scholar]
- 12.Oczkowski SJ. A clinician’s guide to the assessment and interpretation of noninferiority trials for novel therapies. Open Med. 2014;8(2):e67-e72. [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. doi: 10.1097/INF.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 14.Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851-858. doi: 10.1001/jamapediatrics.2013.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukrafka JL, Fuchs SC, Fischer GB, Flores JA, Fachel JM, Castro-Rodriguez JA. Chest physiotherapy in paediatric patients hospitalised with community-acquired pneumonia: a randomised clinical trial. Arch Dis Child. 2012;97(11):967-971. doi: 10.1136/archdischild-2012-302279 [DOI] [PubMed] [Google Scholar]
- 16.Chappuy H, Keitel K, Gehri M, et al. Nasopharyngeal carriage of individual Streptococcus pneumoniae serotypes during pediatric radiologically confirmed community acquired pneumonia following PCV7 introduction in Switzerland. BMC Infect Dis. 2013;13:357. doi: 10.1186/1471-2334-13-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson M, Lakhanpaul M, Smyth A, et al. Comparison of oral amoxicillin and intravenous benzyl penicillin for community acquired pneumonia in children (PIVOT trial): a multicentre pragmatic randomised controlled equivalence trial. Thorax. 2007;62(12):1102-1106. doi: 10.1136/thx.2006.074906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Canada . Amoxicillin monograph. Accessed February 3, 2021. https://health-products.canada.ca/dpd-bdpp/dispatch-repartition.do;jsessionid=360CF0A7606C30DB2713C80A608EA10A
- 19.Public Health Ontario . Immunization coverage report for school pupils in Ontario: 2018-19 school year. Queen’s Printer for Ontario. Published August 10, 2020. Accessed January 28, 2021. https://www.publichealthontario.ca/en/health-topics/immunization/vaccine-coverage
- 20.Ali M, Han S, Gunst CJ, Lim S, Luinstra K, Smieja M. Throat and nasal swabs for molecular detection of respiratory viruses in acute pharyngitis. Virol J. 2015;12:178. doi: 10.1186/s12985-015-0408-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pernica J, Harman S, Kam A, et al. Short-course antimicrobial therapy for paediatric respiratory infections (SAFER): study protocol for a randomized controlled trial. Trials. 2018;19(1):83. doi: 10.1186/s13063-018-2457-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415-427. doi: 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among US children. N Engl J Med. 2015;372(9):835-845. doi: 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services; 2019. [Google Scholar]
- 25.Yam ELY. COVID-19 will further exacerbate global antimicrobial resistance. J Travel Med. 2020;27(6):taaa098. doi: 10.1093/jtm/taaa098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwlaat R, Mbuagbaw L, Mertz D, et al. COVID-19 and antimicrobial resistance: parallel and interacting health emergencies. Clin Infect Dis. 2020;ciaa773. doi: 10.1093/cid/ciaa773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spellberg B, Rice LB. Duration of antibiotic therapy: shorter is better. Ann Intern Med. 2019;171(3):210-211. doi: 10.7326/M19-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wald-Dickler N, Spellberg B. Short-course antibiotic therapy-replacing constantine units with “shorter is better”. Clin Infect Dis. 2019;69(9):1476-1479. doi: 10.1093/cid/ciy1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Elsevier; 2020. [Google Scholar]
- 30.World Health Organization . Global Action Plan on Antimicrobial Resistance. World Health Organization; 2019. [Google Scholar]
- 31.Gillies M, Ranakusuma A, Hoffmann T, et al. Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication. CMAJ. 2015;187(1):E21-E31. doi: 10.1503/cmaj.140848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández E, Bargiela R, Diez MS, et al. Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes. 2013;4(4):306-315. doi: 10.4161/gmic.25321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab. 2013;17(6):883-894. doi: 10.1016/j.cmet.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168(11):1063-1069. doi: 10.1001/jamapediatrics.2014.1539 [DOI] [PubMed] [Google Scholar]
- 35.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617-626. doi: 10.1542/peds.2014-3407 [DOI] [PubMed] [Google Scholar]
- 36.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705-721. doi: 10.1016/j.cell.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621-626. doi: 10.1038/nature11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patrick DM, Sbihi H, Dai DLY, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020;8(11):1094-1105. doi: 10.1016/S2213-2600(20)30052-7 [DOI] [PubMed] [Google Scholar]
- 39.Panzer AR, Lynch SV. Influence and effect of the human microbiome in allergy and asthma. Curr Opin Rheumatol. 2015;27(4):373-380. doi: 10.1097/BOR.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 40.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. ; CHILD Study Investigators . Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271 [DOI] [PubMed] [Google Scholar]
- 41.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17(5):592-602. doi: 10.1016/j.chom.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton DB, Scott FI, Haynes K, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics. 2015;136(2):e333-e343. doi: 10.1542/peds.2015-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509-518. doi: 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 44.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373-403. doi: 10.1007/978-1-4939-0897-4_17 [DOI] [PubMed] [Google Scholar]
- 45.Silverman MA, Konnikova L, Gerber JS. Impact of antibiotics on necrotizing enterocolitis and antibiotic-associated diarrhea. Gastroenterol Clin North Am. 2017;46(1):61-76. doi: 10.1016/j.gtc.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonsen J, Hansen S, Morgen CS, Jess T, Jorgensen LN, Allin KH. Antibiotics during childhood and development of appendicitis: a nationwide cohort study. Aliment Pharmacol Ther. 2020;53(1):87-93. doi: 10.1111/apt.16084 [DOI] [PubMed] [Google Scholar]
- 47.Meyer Sauteur PM. Challenges and progress towards determining pneumonia etiology. Clin Infect Dis. 2020;71(3):514-516. doi: 10.1093/cid/ciz879 [DOI] [PubMed] [Google Scholar]
- 48.Harris M, Clark J, Coote N, et al. ; British Thoracic Society Standards of Care Committee . British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1-ii23. doi: 10.1136/thoraxjnl-2011-200598 [DOI] [PubMed] [Google Scholar]
- 49.Florin TA, Ambroggio L, Brokamp C, et al. Biomarkers and disease severity in children with community-acquired pneumonia. Pediatrics. 2020;145(6):e20193728. doi: 10.1542/peds.2019-3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuman MI, Lee EY, Bixby S, et al. Variability in the interpretation of chest radiographs for the diagnosis of pneumonia in children. J Hosp Med. 2012;7(4):294-298. doi: 10.1002/jhm.955 [DOI] [PubMed] [Google Scholar]
- 51.Elemraid MA, Muller M, Spencer DA, et al. ; North East of England Paediatric Respiratory Infection Study Group . Accuracy of the interpretation of chest radiographs for the diagnosis of paediatric pneumonia. PLoS One. 2014;9(8):e106051. doi: 10.1371/journal.pone.0106051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson J, Kline JA. Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs. Emerg Radiol. 2010;17(4):285-290. doi: 10.1007/s10140-009-0854-2 [DOI] [PubMed] [Google Scholar]
- 53.Andronikou S, Lambert E, Halton J, et al. Guidelines for the use of chest radiographs in community-acquired pneumonia in children and adolescents. Pediatr Radiol. 2017;47(11):1405-1411. doi: 10.1007/s00247-017-3944-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xavier-Souza G, Vilas-Boas AL, Fontoura MS, et al. ; PNEUMOPAC-Efficacy Study Group . The inter-observer variation of chest radiograph reading in acute lower respiratory tract infection among children. Pediatr Pulmonol. 2013;48(5):464-469. doi: 10.1002/ppul.22644 [DOI] [PubMed] [Google Scholar]
- 55.Lyttle MD, Bielicki JA, Barratt S, et al. ; PERUKI, GAPRUKI and the CAP-IT trial team . Efficacy, safety and impact on antimicrobial resistance of duration and dose of amoxicillin treatment for young children with community-acquired pneumonia: a protocol for a randomised controlled Trial (CAP-IT). BMJ Open. 2019;9(5):e029875. doi: 10.1136/bmjopen-2019-029875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ClinicalTrials.gov. A Phase IV Double-Blind, Placebo-Controlled, Randomized Trial to Evaluate Short Course vs.Standard Course Outpatient Therapy of Community Acquired Pneumonia in Children (SCOUT-CAP). NCT02891915. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02891915
- 57.World Health Organization . Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities: Evidence of Summaries. World Health Organization; 2014:46. [PubMed] [Google Scholar]
- 58.World Health Organization . Recommendations for Management of Common Childhood Conditions: Evidence for Technical Update of Pocket Book Recommendations: Newborn Conditions, Dysentery, Pneumonia, Oxygen Use and Delivery, Common Causes of Fever, Severe Acute Malnutrition and Supportive Care. World Health Organization; 2012. [PubMed] [Google Scholar]
- 59.World Health Organization . Integrated Management of Childhood Illness Chart Booklet. World Health Organization; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Baseline Characteristics (Per-Protocol Population)
eTable 2. Subgroup Analyses of Primary Outcome by Subgroups: Age, Salivary CRP, and Detection of Virus/Mycoplasma Species
eTable 3. Subgroup Analyses of Secondary Outcomes (Binary)
eTable 4. Subgroup Analyses of Secondary Outcomes (Count)
Data sharing statement