Abstract
This cohort study measures Δ-9-tetrahydrocannabinol levels in breast milk and serum samples from women who were newly abstinent from marijuana and had just delivered an infant.
Δ-9-Tetrahydrocannabinol (THC) crosses the placenta, is highly lipophilic, and can be detected in breast milk.1 National guidelines recommend abstinence from marijuana use during pregnancy and lactation, with limited data regarding the presence of THC in breast milk.2,3,4 We aimed to estimate the amount and duration of THC excretion in breast milk among women with known prenatal marijuana use.
Methods
We performed a prospective, observational pharmacokinetic study of women with prenatal marijuana use who delivered their infants between November 1, 2016, and June 30, 2019. This study was approved by the Colorado Multiple Institutional Review Board, and the National Institutes of Health provided a certificate of confidentiality. All women aged 18 to 42 years presenting for delivery were screened for prenatal marijuana exposure by medical record review, which was verified by a drugs-of-abuse assay (Beckman-Coulter) with a threshold of 50 ng/mL for carboxy THC glucuronide for a positive THC exposure result. Inclusion criteria were an intention to breastfeed and marijuana abstention for 6 weeks. After written informed consent was obtained, participants reported substance use patterns and provided paired maternal plasma and breast milk samples 2 to 5 times per week, which were frozen for periodic batched analysis.5 High-performance liquid chromatography tandem mass spectrometry assays (ABSciex API5000 MS/MS [Sciex]) were used to quantify THC and metabolites.6 Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado Denver through the Colorado Clinical and Translational Sciences Institute. The sample size was determined based on classic laboratory testing models to investigate the pharmacokinetics of THC in an observational analysis. Elimination rate constants for disappearance of THC from breast milk were determined by semilog regression of concentration vs time among participants with biological evidence of abstention. The THC half-life elimination values were determined from the elimination rate constant determined for each person; values of maximum drug concentrations across time, from inspection of concentration vs time data; and milk:plasma ratios, from comparison of breast milk and plasma THC concentration values collected concomitantly. Data analysis was completed with R version 3.1.1 (R Foundation for Statistical Computing).
Results
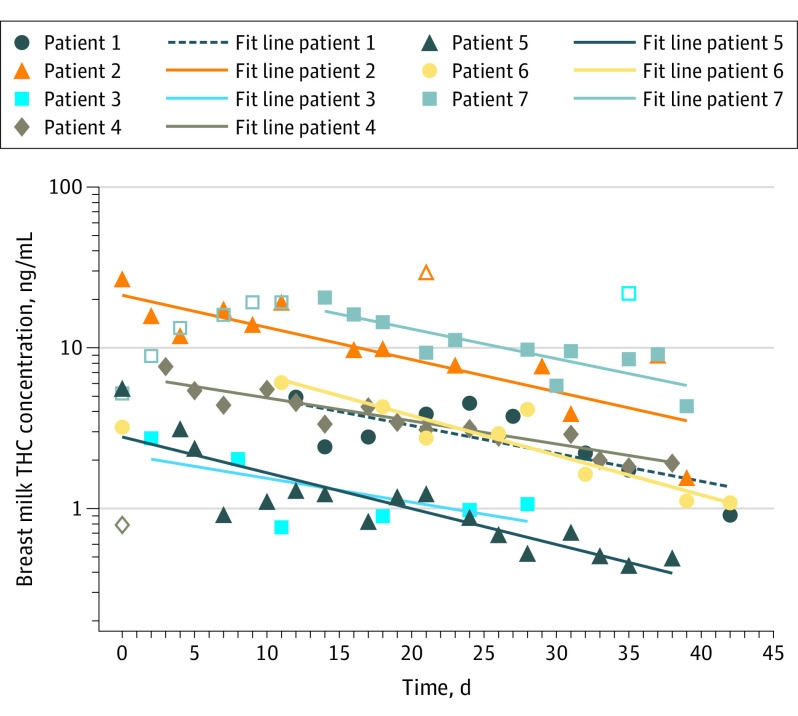
Of 394 women screened, 105 were eligible, and 25 enrolled; 12 of 25 self-reporting abstinence from marijuana were abstinent based on plasma analysis results (median [interquartile range (IQR)] maternal age, 26 [20-28] years; Hispanic, 8 [32%]; White and African American, 9 [36%] each; other race/ethnicity, 7 [28%]). Most had completed high school (n = 10 [40%]) or some college/professional education (n = 10 [40%]). Women with continued marijuana use were younger than the overall sample (median [IQR], 21 [20-27] years) and less likely to have attended college (3 of 13 [23%]) than women who abstained (7 of 12 [58%]). Surveys of prenatal use revealed primarily inhalational consumption more than 2 times weekly. Analysis was performed on 402 matched plasma-milk samples; THC was the predominant compound found in breast milk, with negligible concentrations of hydrophilic THC metabolites. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 (IQR, 1.2-6.8) ng/mL within the first week post partum, increased to 5.5 (IQR, 4.4-16.0) ng/mL at 2 weeks, and declined to 1.9 (IQR, 1.1-4.3) ng/mL at 6 weeks. The milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1) (Table). Among 7 women who abstained more than 5 weeks, the estimated mean (SD) half-life of THC in breast milk was 17 (3.3) days, with a projected time to elimination greater than 6 weeks (Figure).
Table. Pharmacokinetic Analysis of Breast Milk Δ-9-Tetrahydrocannabinol (THC) Concentrations After Marijuana Cessationa.
| Characteristic | Pharmacokinetic analysis of selected participants who abstained from marijuana use | ||||||
|---|---|---|---|---|---|---|---|
| Participant | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Maximum drug concentration across time interval, ng/mL | 4.93 | 26.14 | 2.81 | 7.64 | 5.58 | 6.07 | 20.57 |
| Calculated elimination rate constant/d | 0.04 | 0.046 | 0.034 | 0.033 | 0.051 | 0.057 | 0.043 |
| Time for 50% decrease of drug concentration, d | 17.4 | 15 | 20.2 | 21 | 13.5 | 12.2 | 16.3 |
| Milk:plasma ratiob | 4.3 | 5.6 | No ratioc | 4.8 | No ratioc | 7 | 6.5 |
| Daily infant dosage, median (interquartile range), μgb | 2 (1.6-2.7) | 6.7 (5.3-10.8) | 0.7 (0.7-1.3) | 2.4 (2.0-3.1) | 0.6 (0.5-0.9) | 2.0 (1.1-2.9) | 6.8 (6.2-11.2) |
Participants with survey reports, laboratory evidence of THC abstention, and study completion.
Plasma THC concentrations less than the limit of quantification (0.39 ng/mL), in which case a ratio cannot be calculated; plasma tetrahydrocannabinol carboxylic acid and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid glucuronide were present.
Based on the mean daily milk intake for newborns (700 mL); the milk:plasma ratios were calculated by concomitant milk and plasma samples.
Figure. Pharmacokinetic Modeling for the Estimated Time to Elimination of Δ-9-Tetrahydrocannabinol (THC) in Breast Milk Following Delivery.
Values represented with open shapes were omitted from the time-to-elimination analysis of THC in breast milk. The initial values for patient 7 were omitted until a peak THC level in breast milk was measured, and subsequent declining values were included in the time-to-elimination analysis.
Discussion
We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use. These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.
Recent studies appear to be limited to an isolated milk analysis after maternal 1-time marijuana use, showing peak concentrations in the first 4 hours,3 and cross-sectional analysis of milk samples from a research repository, reporting THC detection up to 6 days after last use.4 Furthermore, the concentration of THC in breast milk relative to plasma has only been previously described in 1 mother,2 to our knowledge. Our longitudinal findings and multiple biomarker data suggest there are varying patterns of THC transport in breast milk following delivery: increasing THC concentration in the first 2 weeks followed by a prolonged period of decreasing concentrations over several weeks. This phenomenon may be attributable to changing fat composition in breast milk in the early postpartum period, individual metabolism, and differences in marijuana use patterns (frequency, dosage, and mode). Our limitations include an inability to quantify the THC dosage by participants’ consumption and difficulties in abstention, which are recognized challenges in marijuana research.
Pharmacokinetic data are not intended to assess safety, particularly in newborns, who have increased vulnerabilities. To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum. We found recruitment challenging, and only half of the participants who intended cessation were successful. This may be multifactorial: self-medication for medical disorders, normalization of use because of legalization, and suboptimal resources for treatment of cannabis use disorder.1 Critical research is needed to determine the effect that other methods of consumption may have on THC in breast milk and infant metabolism and the significance these concentrations have on neurocognitive and health outcomes in children.
References
- 1.Metz TD, Borgelt LM. Marijuana use in pregnancy and while breastfeeding. Obstet Gynecol. 2018;132(5):1198-1210. doi: 10.1097/AOG.0000000000002878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 1982;307(13):819-820. doi: 10.1056/NEJM198209233071311 [DOI] [PubMed] [Google Scholar]
- 3.Baker T, Datta P, Rewers-Felkins K, Thompson H, Kallem RR, Hale TW. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131(5):783-788. doi: 10.1097/AOG.0000000000002575 [DOI] [PubMed] [Google Scholar]
- 4.Bertrand KA, Hanan NJ, Honerkamp-Smith G, Best BM, Chambers CD. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics. 2018;142(3):e20181076. doi: 10.1542/peds.2018-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sempio C, Wymore E, Palmer C, et al. Detection of cannabinoids by LC-MS-MS and ELISA in breast milk. J Anal Toxicol. 2020;bkaa142. doi: 10.1093/jat/bkaa142 [DOI] [PubMed] [Google Scholar]
- 6.Klawitter J, Sempio C, Mörlein S, et al. An atmospheric pressure chemical ionization ms/MS assay using online extraction for the analysis of 11 cannabinoids and metabolites in human plasma and urine. Ther Drug Monit. 2017;39(5):556-564. doi: 10.1097/FTD.0000000000000427 [DOI] [PMC free article] [PubMed] [Google Scholar]