Abstract
Our ability to perceive meaningful action events involving objects, people, and other animate agents is characterized in part by an interplay of visual and auditory sensory processing and their cross-modal interactions. However, this multisensory ability can be altered or dysfunctional in some hearing and sighted individuals, and in some clinical populations. The present meta-analysis sought to test current hypotheses regarding neurobiological architectures that may mediate audio-visual multisensory processing. Reported coordinates from 82 neuroimaging studies (137 experiments) that revealed some form of audio-visual interaction in discrete brain regions were compiled, converted to a common coordinate space, and then organized along specific categorical dimensions to generate activation likelihood estimate (ALE) brain maps and various contrasts of those derived maps. The results revealed brain regions (cortical “hubs”) preferentially involved in multisensory processing along different stimulus category dimensions, including 1) living versus nonliving audio-visual events, 2) audio-visual events involving vocalizations versus actions by living sources, 3) emotionally valent events, and 4) dynamic-visual versus static-visual audio-visual stimuli. These meta-analysis results are discussed in the context of neurocomputational theories of semantic knowledge representations and perception, and the brain volumes of interest are available for download to facilitate data interpretation for future neuroimaging studies.
Keywords: categorical perception, embodied cognition, multisensory integration, neuroimaging, sensory-semantic categories
Introduction
The perception of different categories of visual (unisensory) object and action forms are known to differentially engage distinct brain regions or networks in neurotypical individuals, such as when observing or identifying faces, body parts, living things, houses, fruits and vegetables, and outdoor scenes, among other proposed categories (Martin et al. 1996; Tranel et al. 1997; Caramazza and Mahon 2003; Martin 2007). Distinct semantic categories of real world sound-producing (unisensory) events are also known or thought to recruit different brain networks, such as nonliving environmental and mechanical sounds (Lewis et al. 2012), nonvocal action events produced by nonhuman animal sources (Engel et al. 2009; Lewis, Talkington, et al. 2011), as well as the more commonly studied categories of living things (especially human conspecifics) and vocalizations (notably speech) (Dick et al. 2007; Saygin et al. 2010; Goll et al. 2011; Trumpp et al. 2013; Brefczynski-Lewis and Lewis 2017). Extending beyond unisensory category-specific percepts, the neurobiological representations of multisensory events are thought to develop based on complex combinations of sensory and sensory-motor information, with some dependence on differences with individual observers’ experiences throughout life, such as with handedness (Lewis et al. 2006). One may have varying experiences with, for instance, observing and hearing a construction worker hammering a nail, or feeling a warm purring gray boots breed cat on a sofa. Additionally, while watching television, or a smart phone device, one can readily accept the illusion that the synchronized audio (speakers) and video movements (the screen) are emanating from a single animate or object source, leading to stable, unified multisensory percepts. Psychological literature indicates that perception of multisensory events can manifest as well-defined category-specific objects and action representations that build on past experiences (Rosch 1973; Vygotsky 1978; McClelland and Rogers 2003; Miller et al. 2003; Martin 2007). However, the rules that may guide the organization of cortical network representations that mediate multisensory perception of real-world events, and whether any taxonomic organizations for such representations exist at a categorical level, remain unclear.
The ability to organize information to attain a sense of global coherence, meaningfulness, and possible intention behind every-day observable events may fail to fully or properly develop, as for some individuals with autism spectrum disorder (ASD) (Jolliffe and Baron-Cohen 2000; Happe and Frith 2006; Kouijzer et al. 2009; Powers et al. 2009; Marco et al. 2011; Pfeiffer et al. 2011, 2018; Ramot et al. 2017; Webster et al. 2020) and possibly for some individuals with various forms of schizophrenia (Straube et al. 2014; Cecere et al. 2016; Roa Romero et al. 2016; Vanes et al. 2016). Additionally, brain damage, such as with stroke, has been reported to lead to deficits in multisensory processing (Van der Stoep et al. 2019). Thus, further understanding the organization of the multisensory brain has been becoming a topic of increasing clinical relevance.
At some processing stages or levels, the central nervous system is presumably “prewired” to readily develop an organized architecture that can rapidly and efficiently extract meaningfulness from multisensory events. This includes audio-visual event encoding and decoding that enables a deeper understanding of one’s environment, thereby conferring a survival advantage through improvements in perceived threat detection and in social communication (Hewes 1973; Donald 1991; Rilling 2008; Robertson and Baron-Cohen 2017). An understanding of multisensory neuronal processing mechanisms, however, may in many ways be better understood through models of semantic knowledge processing rather than models of bottom-up signal processing, which is prevalent in unisensory fields of literature. One set of theories behind semantic knowledge representation includes distributed-only views, wherein auditory, visual, tactile, and other sensory-semantic systems are distributed neuroanatomically with additional task-dependent representations or convergence-zones in cortex that link knowledge (Damasio 1989a; Languis and Miller 1992; Damasio et al. 1996; Tranel et al. 1997; Ghazanfar and Schroeder 2006; Martin 2007). A distributed-plus-hub view further posits the existence of additional task-independent representations (or “hubs”) that support the interactive activation of representations in all modalities, and for all semantic categories (Patterson et al. 2007).
More recent neurocomputational theories of semantic knowledge learning entails a sensory-motor framework wherein action perception circuits (APCs) are formed through sensory experiences, which manifest as specific distributions across cortical areas (Pulvermuller 2013, 2018; Tomasello et al. 2017). In this construct, combinatorial knowledge is thought to become organized by connections and dynamics between APCs, and cognitive processes can be modeled forthright. Such models have helped to account for the common observation of cortical hubs or “connector hubs” for semantic processing (Damasio 1989b; Sporns et al. 2007; van den Heuvel and Sporns 2013), which may represent multimodal, supramodal, or amodal mechanisms for representing knowledge. From this connector hub theoretical perspective, it remains unclear whether or how different semantic categories of multisensory perceptual knowledge might be organized, potentially including semantic hubs that link, for instance, auditory and visual unisensory systems at a category level.
Here, we addressed the issue of global neuronal organizations that mediate different aspects of audio-visual categorical perception by using activation likelihood estimate (ALE) meta-analyses of a diverse range of published studies to date that reported audio-visual interactions of some sort in the human brain. We defined the term “interaction” to include measures of neuronal sensitivity to temporal and/or spatial correspondence, response facilitation or suppression, inverse effectiveness, an explicit comparison of information from different modalities that pertained to a distinct object, and cross-modal priming (Stein and Meredith 1990; Stein and Wallace 1996; Calvert and Lewis 2004). These interaction effects were assessed in neurotypical adults (predominantly, if not exclusively, right-handed) using hemodynamic blood flow measures (functional magnetic resonance imaging [fMRI], or positron emission tomography [PET]) or magnetoencephalography (MEG) methodologies as whole brain neuroimaging techniques.
The resulting descriptive compilations and analytic contrasts of audio-visual interaction sites across different categories of audio-visual stimuli were intended to meet three main goals: The first goal was to reveal a global set of brain regions (cortical and noncortical) with significantly high probability of cross-sensory interaction processing regardless of variations in methods, stimuli, tasks, and experimental paradigms. The second goal was to validate and refine earlier multisensory processing concepts borne out of image-based meta-analyses of audio-visual interaction sites (Lewis 2010) that used a subset of the paradigms included in the present study, but here taking advantage of coordinate-based meta-analyses and more rigorous statistical approaches now that additional audio-visual interaction studies have subsequently been published.
The third goal, as a special focus, was to test recent hypotheses regarding putative brain architectures mediating multisensory categorical perception that were derived from unisensory auditory object perception literature (Fig. 1), which encompassed theories to explain how real-world natural sounds are processed to be perceived as meaningful events to the observer (Brefczynski-Lewis and Lewis 2017). This hearing perception model entailed four proposed tenets that may shape brain organizations for processing real-world natural sounds, helping to explain “why” certain category-preferential representations appear in the human brain (and perhaps more generally in the brains of all mammals with hearing ability). These tenets for hearing perception included: 1) parallel hierarchical pathways process increasing information content, 2) metamodal operators guide sensory and multisensory processing network organizations, 3) natural sounds are embodied when possible, and 4) categorical perception emerges in neurotypical listeners.
Figure 1.
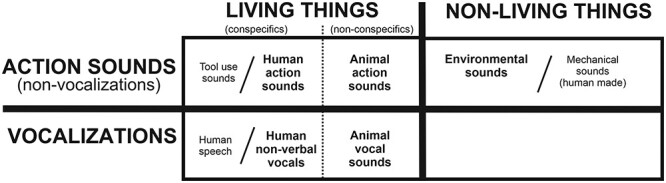
A taxonomic category model of the neurobiological organization of the human brain for processing and recognizing different acoustic–semantic categories of natural sounds (from Brefczynski-Lewis and Lewis 2017). Bold text in the boxed regions depict rudimentary sound categories, including living versus nonliving things and vocalizations versus nonvocal action sounds, which are categories being tested in the present audio-visual meta-analyses. Other subcategories are also indicated, including human speech, tool use sounds, and human-made machinery sounds. Vocal and instrumental music sounds/events are regarded as higher forms of communication, which rely on other networks and are thus outside the scope of the present study. Refer to text for other details.
After compiling the numerous multisensory human neuroimaging studies that employed different types of audio-visual stimuli, tasks, and imaging modalities, we sought to test three hypotheses relating to the above mentioned tenets and neurobiological model. The first two hypotheses effectively tested for support of the major taxonomic boundaries depicted in Figure 1: The first hypothesis being 1) that there will be a double-dissociation of brain systems for processing living versus nonliving audio-visual events, and the second hypothesis 2) that there will be a double-dissociation for processing vocalizations versus action audio-visual events produced by living things.
In the course of compiling neuroimaging literature, there was a clear divide between studies using static visual images (iconic representations) versus video with dynamic motion stimuli that corresponded with aspects of the auditory stimuli. The production of sound necessarily implies dynamic motion of some sort, which in many of the studies’ experimental paradigms also correlated with viewable object or agent movements. Thus, temporal and/or spatial intermodal invariant cues that physically correlate visual motion (“dynamic-visual”) with changes in acoustic energy are typically uniquely present in experimental paradigms using video (Stein and Meredith 1993; Lewkowicz 2000; Bulkin and Groh 2006). Conversely, static or iconic visual stimuli (“static-visual”) must be learned to be associated and semantically congruent with characteristic sounds, and with varying degrees of arbitrariness. Thus, a third hypothesis emerged 3) that the processing of audio-visual stimuli that entailed dynamic-visual motion stimuli versus static-visual stimuli will also reveal a double-dissociation of cortical processing pathways in the multisensory brain. The identification and characterization of any of these hypothesized neurobiological processing categories at a meta-analysis level would newly inform neurocognitive theories, specifying regions or network hubs where certain types of information may merge or in some way interact across sensory systems at a semantic category level. Thus, the resulting ALE maps are expected to facilitate the generation of new hypotheses regarding multisensory interaction and integration mechanisms in neurotypical individuals. They should also contribute to providing a foundation for ultimately understanding “why” multisensory processing networks develop the way they typically do, and why they may develop aberrantly, or fail to recover after brain injury, in certain clinical populations.
Materials and Methods
This work was performed in accordance with the PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions (Moher et al. 2009). Depicted in the PRISMA flow-chart (Fig. 2), original research studies were identified by PubMed and Google Scholar literature searches with keyword combinations “auditory + visual,” “audiovisual,” “multisensory,” and “fMRI” or “PET” or “MEG,” supplemented through studies identified through knowledge of the field published between 1999 through early 2020. Studies involving drug manipulations, patient populations, children, or nonhuman primates were excluded unless there was a neurotypical adult control group with separately reported outcomes. Of the included studies, reported coordinates for some paradigms had to be estimated from figures. Additionally, some studies did not use whole-brain imaging, but rather incorporated imaging to 50–60 mm slabs of axial brain slices so as to focus, for instance, on the thalamus or basal ganglia. These studies were included despite their being a potential violation of assumptions made by ALE analyses (see below) because the emphasis of the present study was to reveal proof of concept regarding differential audio-visual processing at a semantic category level. This yielded inclusion of 82 published fMRI, PET and MEG studies including audio-visual interaction(s) of some form (Table 1). The compiled coordinates, after converting to afni-TLRC coordinate system, derived from these studies are included in Appendix A, and correspond directly to Table 1.
Figure 2.
PRISMA table illustrating the flow of information through the different phases of the meta-analysis review.
Table 1.
List of all studies used in the subsequent subsets of audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | Congruent versus Incong | Living versus Nonliving | Emotional stimuli/task | Vocalizations versus Not | Dynamic (video) versus static | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 82 | 137 | First author | Year | Experimental code and abbreviated task | 1285 | 376 | 338 | 714 | Brief description of experimental paradigm | 2B | 2C | 2C | 2D | 2E | |
| 1 | 1 | Adams | 2002 | Expt 1 Table 3 A + V (aud coords only) | 12 | 5 | 1 | 6 | A and V commonly showing subordinate > basic object name verification (words with pictures or environmental sounds) | 1 | 2 | 2 | 2 | ||
| 2 | 2 | Alink | 2008 | Table 1c spheres move to drum sounds | 10 | 4 | 6 | 10 | Visual spheres and drum sounds moving: crossmodal dynamic capture versus conflicting motion | 1 | 2 | 2 | 1 | ||
| 3 | 3 | Balk | 2010 | Figure 2 asynchronous versus simultaneous | 14 | 2 | 1 | 3 | Natural asynchronous versus simultaneous AV speech synchrony (included both contrasts as interaction effects) | 1 | 1 | 1 | |||
| 4 | 4 | Baumann | 2007 | Table 1B coherent V + A versus A | 12 | 2 | 1 | 3 | Visual dots 16% coherent motion and in-phase acoustic noise > stationary acoustic sound | 1 | 2 | 2 | 2 | 1 | |
| 5 | Baumann | 2007 | Table 2B | pooled | 15 | 12 | 27 | Moving acoustic noise and visual dots 16% in-phase coherent > random dot motion | 1 | 2 | 2 | 2 | 1 | ||
| 5 | 6 | Baumgaertner | 2007 | Table 3 Action > nonact sentence+video | 19 | 3 | 0 | 3 | Conjunction spoken sentences (actions > nonactions) AND videos (actions > nonactions) | 1 | 1 | 1 | 1 | ||
| 6 | 7 | Beauchamp | 2004a | Figure 3J and K, Table 1 first 2 foci only | 26 | 2 | 0 | 2 | See photographs of tools, animals and hear corresponding sounds versus scrambled images and synthesized rippled sounds | 1 | 1 | 2 | 2 | 2 | |
| 7 | 8 | Beauchamp | 2004b | Expt 1 coordinates | 8 | 1 | 1 | 2 | High resolution version of 2004a study: AV tool videos versus unimodal (AV > A,V) | 1 | 1 | 2 | 2 | 1 | |
| 8 | 9 | Belardinelli | 2004 | Table 1 AV semantic congruence | 13 | 6 | 6 | 12 | Colored images of tools, animals, humans and semantically congruent versus incongruent sounds | 1 | 1 | 2 | 2 | ||
| 10 | Belardinelli | 2004 | Table 2 AV semantic incongruent | pooled | 2 | 3 | 5 | Colored images of tools, animals, humans and semantically incongruent versus congruent sounds | 2 | 0 | 0 | 0 | 0 | ||
| 9 | 11 | Biau | 2016 | Table 1A Interaction; speech synchronous | 17 | 8 | 0 | 8 | Hand gesture beats versus cartoon disk and speech interaction: synchronous versus asynchronous | 1 | 1 | 1 | 1 | ||
| 10 | 12 | Bischoff | 2007 | Table 2A only P < 0.05 included | 19 | 2 | 1 | 3 | Ventriloquism effect: gray disks and tones, synchronous (P < 0.05 corrected) | 1 | 2 | ||||
| 11 | 13 | Blank | 2013 | Figure 2 | 19 | 1 | 0 | 1 | Visual-speech recognition correlated with recognition performance | 1 | 1 | 1 | 1 | ||
| 12 | 14 | Bonath | 2013 | pg 116 congruent thalamus | 18 | 1 | 0 | 1 | Small checkerboards and tones: spatially congruent versus incongruent (thalamus) | 1 | 2 | 2 | 2 | ||
| 15 | Bonath | 2013 | pg 116 incongruent | pooled | 1 | 1 | 2 | Small checkerboards and tones: spatially incongruent versus congruent (thalamus) | 2 | 0 | 0 | 0 | 0 | ||
| 13 | 16 | Bonath | 2014 | Table 1A illusory versus not | 20 | 1 | 5 | 6 | Small checkerboards and tones: temporal > spatial congruence | 1 | 2 | 2 | 2 | ||
| 17 | Bonath | 2014 | Table 1B synchronous > no illusion | pooled | 3 | 0 | 3 | Small checkerboards and tones: spatial > temporal congruence | 1 | 2 | 2 | 2 | |||
| 14 | 18 | Bushara | 2001 | Table 1A (Fig. 2) AV-Control | 12 | 1 | 3 | 4 | Tones (100 ms) and colored circles synchrony: detect Auditory then Visual presentation versus Control | 1 | 2 | 2 | |||
| 19 | Bushara | 2001 | Table 1B (VA-C) five coords | pooled | 2 | 3 | 5 | Tones (100 ms) and colored circles synchrony: detect Visual then Auditory presentation versus Control | 1 | 2 | 2 | ||||
| 20 | Bushara | 2001 | Table 2A interact w/Rt Insula | pooled | 2 | 4 | 6 | Tones and colored circles: correlated functional connections with (and including) the right insula | 1 | 2 | 2 | ||||
| 15 | 21 | Bushara | 2003 | Table 2A collide > pass, strong A-V interact | 7 | 5 | 3 | 8 | Tone and two visual bars moving: tone synchrony induce perception they collide (AV interaction) versus pass by | 1 | 2 | 2 | 1 | ||
| 16 | 22 | Callan | 2014 | Table 5 AV-Audio (AV10-A10)-(AV6-A6) | 16 | 4 | 4 | 8 | Multisensory enhancement to visual speech in noise correlated with behavioral results | 1 | 1 | 1 | 1 | ||
| 23 | Callan | 2014 | Table 6 AV—Visual only | pooled | 1 | 1 | 2 | Multisensory enhancement to visual speech audio-visual versus visual only | 1 | 1 | 1 | 1 | |||
| 17 | 24 | Calvert | 1999 | Table 1 (Fig. 1) | 5 | 3 | 4 | 7 | View image of lower face and hear numbers 1 through 10 versus unimodal conditions (AV > Photos, Auditory) | 1 | 1 | 2 | 1 | 2 | |
| 18 | 25 | Calvert | 2000 | Figure 2 superadditive+subadditive AVspeech | 10 | 1 | 0 | 1 | Speech and lower face: supra-additive plus subadditive effects (AV-congruent > A,V > AV-incongruent) | 1 | 1 | 2 | 1 | 1 | |
| 26 | Calvert | 2000 | Table 1 supradditive AVspeech | pooled | 4 | 5 | 9 | Speech and lower face: supra-additive AV enhancement | 1 | 1 | 2 | 1 | 1 | ||
| 27 | Calvert | 2000 | Table 2 incongruent subadditive AVspeech | pooled | 3 | 3 | 6 | Speech and lower face: subadditive AV response to incongruent AV inputs | 2 | 0 | 0 | 0 | 0 | ||
| 19 | 28 | Calvert | 2001 | Table 2 superadditive and response depression | 10 | 4 | 11 | 15 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; supradditive and response depression | 1 | 2 | 2 | |||
| 29 | Calvert | 2001 | Table 3A superadditive only | pooled | 6 | 4 | 10 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; supradditive only | 1 | 2 | 2 | ||||
| 30 | Calvert | 2001 | Table 3B response depression only | pooled | 3 | 4 | 7 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; response depression only | 1 | 2 | 2 | ||||
| 20 | 31 | Calvert | 2003 | Table 2A (Fig. 3 blue) | 8 | 13 | 8 | 21 | Speech and lower face: Moving dynamic speech (phonemes) versus stilled speech frames | 1 | 1 | 2 | 1 | 1 | |
| 21 | 32 | DeHaas | 2013 | Table 1A AVcong—Visual | 15 | 3 | 3 | 6 | Video clips of natural scenes (animals, humans): AV congruent versus Visual | 1 | 1 | 1 | |||
| 33 | DeHaas | 2013 | Table 1B V-AV incongruent | pooled | 2 | 0 | 2 | Video clips of natural scenes (animals, humans): Visual versus AV incongruent | 2 | 0 | 0 | 0 | 0 | ||
| 22 | 34 | Erickson | 2014 | Table 1A Congruent AV speech | 10 | 2 | 2 | 4 | McGurk effect (phonemes): congruent AV speech: AV > A and AV > V | 1 | 1 | 2 | 1 | 1 | |
| 35 | Erickson | 2014 | Table 1B McGurk speech | pooled | 2 | 0 | 2 | McGurk speech effect (phonemes) | 1 | 1 | 2 | 1 | 1 | ||
| 23 | 36 | Ethofer | 2013 | Table 1C emotion | 23 | 1 | 2 | 3 | Audiovisual emotional face-voice integration | 1 | 1 | 1 | 1 | 1 | |
| 24 | 37 | Gonzalo | 2000 | Table 1 AV > AVincon music and Chinese ideograms | 14 | 1 | 1 | 2 | Learn novel Kanji characters and musical chords, activity increases over time for consistent AV pairings | 1 | 2 | 2 | |||
| 38 | Gonzalo | 2000 | Table 2 inconsistent AV | pooled | 4 | 4 | 8 | Learn novel Kanji characters and musical chords, activity increases over time to inconsistent pairings | 2 | 0 | 0 | 0 | 0 | ||
| 39 | Gonzalo | 2000 | Table 3 AV consistent versus Aud | pooled | 1 | 1 | 2 | Learn novel Kanji characters and musical chords, learn consistent (vs. inconsistent) pairings versus auditory only | 1 | 2 | 2 | ||||
| 25 | 40 | Green | 2009 | Table 1 incongruent > congruent gesture-speech | 16 | 4 | 5 | 9 | Incongruent versus congruent gesture-speech | 2 | 1 | 1 | 1 | ||
| 41 | Green | 2009 | Table 4A Congruent gesture-speech > gesture or speech | pooled | 1 | 0 | 1 | Congruent gesture-speech versus gesture with unfamiliar speech and with familiar speech | 1 | 1 | 1 | 1 | |||
| 26 | 42 | Hagan | 2013 | Table 1 AV emotion, novel over time | 18 | 5 | 3 | 8 | Affective audio-visual speech: congruent AV emotion versus A, V; unique ROIs over time (MEG) | 1 | 1 | 1 | 1 | ||
| 43 | Hagan | 2013 | Table 2 AV emotion incongruent | pooled | 1 | 5 | 6 | Affective audio-visual speech: incongruent AV emotion versus A, V; unique ROIs over time (MEG) | 2 | 0 | 0 | 0 | 0 | ||
| 27 | 44 | Hasegawa | 2004 | Table 1A (well trained piano) AV induced by V-only | 26 | 12 | 6 | 18 | Piano playing: well trained pianists, mapping hand movements to sequences of sound | 1 | 1 | 2 | 1 | ||
| 28 | 45 | Hashimoto | 2004 | Table 1G (Fig. 4B, red) Learning Hangul letters to sounds | 12 | 2 | 1 | 3 | Unfamiliar Hangul letters and nonsense words, learn speech versus tone/noise pairings | 1 | 2 | 1 | 2 | ||
| 29 | 46 | He | 2015 | Table 3C AV speech foreign (left MTG focus) | 20 | 1 | 0 | 1 | Intrinsically meaningful gestures with German speech: Gesture-German > Gesture-Russian, German speech only | 1 | 1 | 1 | 1 | ||
| 30 | 47 | He | 2018 | Table 2 gestures and speech integration | 20 | 1 | 0 | 1 | Gesture-speech integration: Bimodal speech-gesture versus unimodal gesture with foreign speech and versus unimodal speech | 1 | 1 | 0 | 1 | 1 | |
| 31 | 48 | Hein | 2007 | Figure 2A AV incongruent | 18 | 0 | 2 | 2 | Familiar animal images and incorrect (incongruent) vocalizations (dog: meow) versus correct pairs | 2 | 0 | 0 | 0 | 0 | |
| 49 | Hein | 2007 | Figure 2B AV-artificial/nonliving | pooled | 0 | 1 | 1 | B/W images of artificial objects (“fribbles”) and animal vocalizations versus unimodal A, V | 1 | 2 | |||||
| 50 | Hein | 2007 | Figure 2C pSTS, pSTG, mSTG AV-cong | pooled | 0 | 3 | 3 | Familiar animal images and correct vocalizations (dog: woof-woof) | 1 | 1 | 1 | 2 | |||
| 51 | Hein | 2007 | Figure 3A incongruent | pooled | 4 | 0 | 4 | AV familiar incongruent versus unfamiliar artificial (red foci 1, 5, 6, 9) | 2 | 0 | 0 | 0 | 0 | ||
| 52 | Hein | 2007 | Figure 3B Foci 2, 3, 4 (blue) artificial/nonliving | pooled | 3 | 0 | 3 | Visual “Fribbles” and backward/underwater distorted animal sounds, learn pairings (blue foci 2,3,4) | 1 | 1 | 2 | ||||
| 53 | Hein | 2007 | Figure 3C congruent living (green) | pooled | 3 | 0 | 3 | Familiar congruent living versus artificial AV object features and animal sounds (green foci 7, 8, 10) | 1 | 1 | 2 | ||||
| 32 | 54 | Hocking | 2008 | pg 2444 verbal | 18 | 2 | 0 | 2 | (pSTS mask) Color photos, written names, auditory names, environmental sounds conceptually matched “amodal” | 1 | 1 | 2 | |||
| 55 | Hocking | 2008 | Table 3 incongruent simultaneous matching | pooled | 8 | 10 | 18 | Incongruent sequential AV pairs (e.g., see drum, hear bagpipes) versus congruent pairs | 2 | 0 | 0 | 0 | 0 | ||
| 33 | 56 | Hove | 2013 | pg 316 AV interaction putamen | 14 | 0 | 1 | 1 | Interaction between (beep > flash) versus (siren > moving bar); left putamen focus | 1 | 2 | ||||
| 34 | 57 | James | 2003 | Figure 2 | 12 | 0 | 1 | 1 | Activation by visual objects (“Greebles”) associated with auditory features (e.g., buzzes, screeches); (STG) | 1 | 2 | ||||
| 35 | 58 | James | 2011 | Table 1A bimodal (vs. scrambled) | 12 | 4 | 2 | 6 | Video of human manual actions (e.g., sawing): Auditory and Visual intact versus scrambled, AV event selectivity | 1 | 1 | 2 | 2 | 1 | |
| 36 | 59 | Jessen | 2015 | Table 1A emotion > neutral AV enhanced | 17 | 1 | 1 | 2 | Emotional multisensory whole body and voice expressions: AV emotion (anger and fear) > neutral expressions | 1 | 1 | 1 | 1 | 1 | |
| 60 | Jessen | 2015 | Table 1D fear > neutral AV enhanced | pooled | 2 | 1 | 3 | Emotional multisensory whole body and voice expressions: AV fear > neutral expressions | 1 | 1 | 1 | 1 | 1 | ||
| 37 | 61 | Jola | 2013 | Table 1C AVcondition dance | 12 | 3 | 3 | 6 | Viewing unfamiliar dance performance (tells a story by gesture) with versus without music: using intersubject correlation | 1 | 1 | 1 | 2 | 1 | |
| 38 | 62 | Kim | 2015 | Table 2A AV > C speech semantic match | 15 | 2 | 0 | 2 | Moving audio-visual speech perception versus white noise and unopened mouth movements | 1 | 1 | 1 | 1 | ||
| 39 | 63 | Kircher | 2009 | Figure 3B gesture related activation increase | 14 | 3 | 1 | 4 | Bimodal gesture-speech versus gesture and versus speech | 1 | 1 | 1 | 1 | ||
| 40 | 64 | Kreifelts | 2007 | Table 1 voice-face emotion | 24 | 1 | 2 | 3 | Facial expression and intonated spoken words, judge emotion expressed (AV > A,V; P < 0.05 only) | 1 | 1 | 1 | 1 | 1 | |
| 65 | Kreifelts | 2007 | Table 5 AV increase effective connectivity | pooled | 2 | 4 | 6 | Increased effectiveness connectivity with pSTS and thalamus during AV integration of nonverbal emotional information | 1 | 1 | 1 | 1 | 1 | ||
| 41 | 66 | Lewis | 2000 | Table 1 | 7 | 2 | 3 | 5 | Compare speed of tone sweeps to visual dot coherent motion: Bimodal versus unimodal | 1 | 2 | 2 | 1 | ||
| 42 | 67 | Matchin | 2014 | Table 1 AV > Aud only (McGurk) | 20 | 2 | 7 | 9 | McGurk audio-visual speech: AV > A only | 1 | 1 | 2 | 1 | 1 | |
| 68 | Matchin | 2014 | Table 2 AV > Video only | pooled | 9 | 6 | 15 | McGurk audio-visual speech: AV > V only | 1 | 1 | 2 | 1 | 1 | ||
| 69 | Matchin | 2014 | Table 3 MM > AV McGurk | pooled | 7 | 4 | 11 | McGurk Mismatch > AV speech integration | 2 | 0 | 0 | 0 | 0 | ||
| 43 | 70 | McNamara | 2008 | Table (BA44 and IPL) | 12 | 2 | 2 | 4 | Videos of meaningless hand gestures and synthetic tone sounds: Increases in functional connectivity with learning | 1 | 2 | 1 | |||
| 44 | 71 | Meyer | 2007 | Table 3 paired A + V versus null | 16 | 3 | 3 | 6 | Paired screen red flashes with phone ring: paired V (conditioned stimulus) and A (unconditioned) versus null events | 1 | 2 | 2 | |||
| 72 | Meyer | 2007 | Table 4 CS+, learned AV association with V-only | pooled | 4 | 6 | 10 | Paired screen flashes with phone ring: View flashes after postconditioned versus null events | 1 | 2 | 2 | ||||
| 45 | 73 | Muller | 2012 | Table S1 effective connectivity changes | 27 | 4 | 3 | 7 | Emotional facial expression (groaning, laughing) AV integration and gating of information | 1 | 1 | 1 | 1 | 2 | |
| 46 | 74 | Murase | 2008 | Figure 4 discordant > concordant AVinteraction | 28 | 1 | 0 | 1 | Audiovisual speech (syllables) showing activity to discordant versus concordant stimuli: left mid-STS | 2 | 1 | 2 | 1 | 1 | |
| 47 | 75 | Naghavi | 2007 | Figure 1C | 23 | 0 | 3 | 3 | B/W pictures (animals, tools, instruments, vehicles) and their sounds: Congruent versus Incongruent | 1 | 3 | 3 | 2 | ||
| 48 | 76 | Naghavi | 2011 | Figure 2A cong = incon | 30 | 1 | 0 | 1 | B/W drawings of objects (living and non) and natural sounds (barking, piano): congruent = incongruent encoding | 0 | 2 | ||||
| 77 | Naghavi | 2011 | Figure 2B congruent > incongruent | pooled | 0 | 1 | 1 | B/W drawings of objects (living and non) and natural sounds (barking, piano): congruent > incongruent encoding | 1 | 2 | |||||
| 78 | Naghavi | 2011 | Figure 2C incongruent > congruent | pooled | 1 | 1 | 2 | B/W drawings of objects (living and non) and natural sounds (barking, piano): incongruent > congruent encoding | 2 | 0 | 0 | 0 | 0 | ||
| 49 | 79 | Nath | 2012 | pg 784 | 14 | 1 | 0 | 1 | McGurk effect (phonemes): congruent AV speech correlated with behavioral percept | 1 | 1 | 2 | 1 | 1 | |
| 50 | 80 | Naumer | 2008 | Figure 2 Table 1A max contrast | 18 | 8 | 6 | 14 | Images of “Fribbles” and learned artificial sounds (underwater animal vocals): post training versus max contrast | 1 | 1 | 2 | |||
| 81 | Naumer | 2008 | Figure 3 Table 1B pre–post | pooled | 5 | 6 | 11 | Images of “Fribbles” and learned corresponding artificial sounds: Post- versus Pre-training session | 1 | 1 | 2 | ||||
| 82 | Naumer | 2008 | Figure 4 Table 2 | pooled | 1 | 1 | 2 | Learn of “Freebles” and distorted sounds as incongruent > congruent pairs | 2 | 0 | 0 | 0 | 0 | ||
| 51 | 83 | Naumer | 2011 | Figure 3C | 10 | 1 | 0 | 1 | Photographs of objects (living and non) and related natural sounds | 1 | 2 | ||||
| 52 | 84 | Noppeny | 2008 | Table 2 AV incongruent > congruent | 17 | 5 | 2 | 7 | Speech sound recognition through AV priming, environmental sounds and spoken words: Incongruent > congruent | 2 | 0 | 0 | 0 | 0 | |
| 85 | Noppeny | 2008 | Table 3 AV congruent sounds/words | pooled | 4 | 0 | 4 | Speech sound recognition through AV priming, environmental sounds and spoken words: Congruent > incongruent | 1 | 2 | |||||
| 53 | 86 | Ogawa | 2013a | Table 1 (pg 162 data) | 13 | 1 | 0 | 1 | AV congruency of pure tone and white dots moving on screen (area left V3A) | 1 | 2 | 2 | |||
| 54 | 87 | Ogawa | 2013b | Table 1 3D > 2D and surround > monaural effects | 16 | 3 | 4 | 7 | Cinematic 3D > 2D video and surround sound > monaural while watching a movie (“The Three Musketeers”) | 1 | 1 | 0 | 1 | ||
| 55 | 88 | Okada | 2013 | Table 1 AV > A | 20 | 5 | 4 | 9 | Video of AV > A speech only | 1 | 1 | 1 | 1 | ||
| 56 | 89 | Olson | 2002 | Table 1A synchronized AV > static Vis-only | 10 | 7 | 4 | 11 | Whole face video and heard words: Synchronized AV versus static V | 1 | 1 | 1 | 1 | ||
| 90 | Olson | 2002 | Table 1C synchronized AV > desynchronized AV speech | pooled | 2 | 0 | 2 | Whole face video and heard words: Synchronized versus desynchronized | 1 | 1 | 1 | 1 | |||
| 57 | 91 | Plank | 2012 | pg 803 AV congruent effect | 15 | 0 | 1 | 1 | AV spatially congruent > semantically matching images of natural objects and associated sounds (right STG) | 1 | 3 | 3 | 2 | ||
| 92 | Plank | 2012 | Table 2A spatially congruent-baseline | pooled | 5 | 5 | 10 | Images of natural objects and associated sounds, spatially congruent versus baseline | 1 | 3 | 3 | 2 | |||
| 58 | 93 | Raij | 2000 | Table 1B letters and speech sounds | 9 | 2 | 3 | 5 | Integration of visual letters and corresponding auditory phonetic expressions (MEG study) AV versus (A + V) | 1 | 2 | 1 | 2 | ||
| 59 | 94 | Regenbogen | 2017 | Table 2A degraded > clear Multisensory versus unimodal input | 29 | 5 | 6 | 11 | Degraded > clear AV versus both visual and auditory unimodal visual real-world object-in-action recognition | 1 | 0 | 2 | 1 | ||
| 60 | 95 | Robins | 2008 | Table 2 (Fig. 2) AV integration (AV > A and AV > V) | 10 | 2 | 1 | 3 | Face speaking sentences: angry, fearful, happy, neutral (AV > A,V) | 1 | 1 | 1 | |||
| 96 | Robins | 2008 | Table 4A (Fig. 5) AV integration and emotion | pooled | 1 | 4 | 5 | AV faces and spoken sentences expressing fear or neutral valence: AV integration (AV > A,V conditions) | 1 | 1 | 1 | 1 | |||
| 97 | Robins | 2008 | Table 4B emotion effects | pooled | 2 | 0 | 2 | AV faces and spoken sentences expressing fear or neutral valence: Emotional AV-fear > AV-neutral | 1 | 1 | 1 | 1 | 1 | ||
| 98 | Robins | 2008 | Table 4C (Fig. 5) fearful AV integration | pooled | 1 | 5 | 6 | AV faces and spoken sentences expressing fear or neutral valence: Fearful-only AV integration | 1 | 1 | 1 | 1 | 1 | ||
| 99 | Robins | 2008 | Table 4D AV-only emotion | pooled | 1 | 3 | 4 | AV faces and spoken sentences expressing fear or neutral valence: AV-only emotion | 1 | 1 | 1 | 1 | 1 | ||
| 61 | 100 | Scheef | 2009 | Table 1 cartoon jump + boing | 16 | 1 | 2 | 3 | Video of cartoon person jumping and “sonification” of a tone, learn correlated pairings: AV-V and AV-A conjunction | 1 | 1 | 2 | 2 | 1 | |
| 62 | 101 | Schmid | 2011 | Table 2E A effect V (Living and nonliving, pictures) | 12 | 3 | 4 | 7 | Environmental sounds and matching pictures: reduced activity by A | 1 | 3 | 3 | 2 | ||
| 102 | Schmid | 2011 | Table 2F V competition effect A (reduced activity by a visual object) | pooled | 2 | 2 | 4 | Environmental sounds and matching pictures: reduced activity by V | 1 | 3 | 3 | 2 | |||
| 103 | Schmid | 2011 | Table 2G AV crossmodal interaction × auditory attention | pooled | 2 | 3 | 5 | Environmental sounds and matching pictures: cross-modal interaction and auditory attention | 1 | 3 | 3 | 2 | |||
| 63 | 104 | Sekiyama | 2003 | Table 3 (fMRI nAV-AV) | 8 | 1 | 0 | 1 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (fMRI) | 1 | 1 | 2 | 1 | 1 | |
| 105 | Sekiyama | 2003 | Table 4 (PET nAV-AV) | pooled | 1 | 3 | 4 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (PET) | 1 | 1 | 2 | 1 | 1 | ||
| 64 | 106 | Sestieri | 2006 | Table 1 (Fig. 3), AV location match versus semantic | 10 | 2 | 5 | 7 | B/W images (animal, weapons) and environmental sounds: Match location > recognition | 1 | 1 | 2 | 2 | ||
| 107 | Sestieri | 2006 | Table 2 AV semantic recognition versus localization | pooled | 2 | 1 | 3 | B/W pictures and environmental sounds: congruent semantic recognition > localization task | 1 | 3 | 3 | 2 | |||
| 65 | 108 | Stevenson | 2009 | Table 1B AVtools > AVspeech | 11 | 1 | 1 | 2 | Hand tools in use video: inverse effectiveness (degraded AV tool > AV speech) | 1 | 1 | 2 | 3 | 1 | |
| 109 | Stevenson | 2009 | Table 1C (Fig. 8) AVspeech > AVtools | pooled | 1 | 1 | 2 | Face and speech video: inverse effectiveness (degraded AV speech > AV tool use) | 1 | 1 | 1 | 1 | |||
| 66 | 110 | Straube | 2011 | Table 3A and B iconic/metaphoric speech-gestures versus speech, gesture | 16 | 2 | 2 | 4 | Integration of Iconic and Metaphoric speech-gestures versus speech and gesture | 1 | 1 | 1 | 1 | ||
| 67 | 111 | Straube | 2014 | p939 Integration foci | 16 | 3 | 0 | 3 | Integration of iconic hand gesture-speech > unimodal speech and unimodal gesture (healthy control group) | 1 | 1 | 1 | 1 | ||
| 68 | 112 | Szycik | 2009 | Table 1 AV incongruent > AV congruent face+speech | 11 | 7 | 2 | 9 | Incongruent AV face-speech versus congruent AV face-speech | 2 | 1 | 1 | 1 | ||
| 69 | 113 | Tanabe | 2005 | Table 1A AV; A then V; not VA | 15 | 10 | 10 | 20 | Amorphous texture patterns and modulated white noises: Activation during learning delay period (AV) | 1 | 2 | 2 | 2 | 2 | |
| 114 | Tanabe | 2005 | Table 2A+2B (Fig. 5A) AV and VA | pooled | 5 | 6 | 11 | Amorphous texture patterns and modulated white noises: changes after feedback learning (AV and VA) | 1 | 2 | 2 | 2 | 2 | ||
| 115 | Tanabe | 2005 | Table 3A+3B (Fig. 6) AV and VA; delay period | pooled | 9 | 1 | 10 | Amorphous texture patterns and modulated white noises: sustained activity throughout learning (AV and VA) | 1 | 2 | 2 | 2 | 2 | ||
| 70 | 116 | Taylor | 2006 | pg 8240 AV incongruent | 15 | 1 | 0 | 1 | Color photos (V), environmental sounds (A), spoken words: Incongruent (living objects) | 2 | 0 | 0 | 0 | 0 | |
| 117 | Taylor | 2006 | Figure 1A and B, Figure 1C and D (living > nonliving) | pooled | 2 | 0 | 2 | Color photos (V), environmental sounds and spoken words (A): Cong AV versus Incong (living objects) | 1 | 2 | |||||
| 71 | 118 | Van Atteveldt | 2004 | Table 1A letters and speech sounds | 16 | 3 | 1 | 4 | Familiar letters and their speech sounds: Congruent versus not and Bimodal versus Unimodal | 1 | 1 | 2 | |||
| 72 | 119 | Van Atteveldt | 2007 | Table 2A+B (Fig. 2) | 12 | 3 | 2 | 5 | Single letters and their speech sounds (phonemes): Congruent > Incong; Passive perception, blocked and event-related design | 1 | 1 | 2 | |||
| 120 | Van Atteveldt | 2007 | Table 3 (Fig. 2) passive | pooled | 1 | 1 | 2 | Single letters and their speech sounds (phonemes): Congruent > Incong, active perception task | 1 | 1 | 2 | ||||
| 121 | Van Atteveldt | 2007 | Table 4 (Fig. 6) active condition, incongruent | pooled | 1 | 6 | 7 | Single letters and their speech sounds (phonemes): Incongruent > Congruent | 2 | 0 | 0 | 0 | 0 | ||
| 73 | 122 | Van Atteveldt | 2010 | Table 1B STS; specific adaptation congruent > incong | 16 | 3 | 1 | 4 | Letter and speech sound pairs (vowels, consonants): Specific adaptation effects | 1 | 1 | 2 | |||
| 74 | 123 | Van der Wyk | 2010 | Table 2 AV interaction effects oval/circles+speech/nonspeech | 16 | 3 | 3 | 6 | Geometric shape modulate with speech (sentences) | 1 | 1 | 1 | |||
| 75 | 124 | Von Kriegstein | 2006 | Figure 4B after > before voice-face | 14 | 0 | 4 | 4 | Face and object photos with voice and other sounds: Voice-Face association learning | 1 | 1 | 1 | 2 | ||
| 76 | 125 | Watkins | 2006 | Figure 4 illusory multisensory interaction | 11 | 0 | 2 | 2 | Two brief tone pips leads to illusion of two screen flashes (annulus with checkerboard) when only one flash present | 1 | 2 | 2 | |||
| 126 | Watkins | 2006 | Table 1 (A enhances V in general) | pooled | 5 | 3 | 8 | Single brief tone pip leads to illusion of single screen flash (annulus with checkerboard) when two flashes present | 1 | 2 | 2 | ||||
| 77 | 127 | Watkins | 2007 | Figure 3 2 flashes +1 beep illusion | 10 | 0 | 1 | 1 | Two visual flashes and single audio bleep leads to the illusion of a single flash | 1 | 2 | 2 | |||
| 78 | 128 | Watson | 2014a | Table 1A AV-adaptation effect (multimodal localizer) | 18 | 0 | 1 | 1 | Videos of emotional faces and voice: multisensory localizer | 1 | 1 | 1 | 1 | ||
| 129 | Watson | 2014a | Table 1C AV-adaptation effect, cross-modal adaptation effect | pooled | 0 | 1 | 1 | Videos of emotional faces and voice: crossmodal adaptation effects | 1 | 1 | 1 | 1 | 1 | ||
| 79 | 130 | Watson | 2014b | Table 1 AV > baseline (Living and nonliving) | 40 | 3 | 5 | 8 | Moving objects and videos of faces with corresponding sounds: AV > baseline | 1 | 1 | ||||
| 131 | Watson | 2014b | Table 4A integrative regions (Living and nonliving) | pooled | 2 | 2 | 4 | Moving objects and videos of faces with corresponding sounds: Integrative regions (AV > A,V) | 1 | 1 | |||||
| 132 | Watson | 2014b | Table 4B integrative regions (Living and nonliving) | pooled | 0 | 1 | 1 | Moving objects and videos of faces with corresponding sounds: People-selective integrative region | 1 | 1 | 1 | 1 | |||
| 80 | 133 | Werner | 2010 | Table 1 superadditive (AV-salience effect) | 21 | 0 | 3 | 3 | Categorize movies of actions with tools or musical instruments (degraded stimuli); AV interactions both tasks | 1 | 1 | 2 | 2 | 1 | |
| 134 | Werner | 2010 | Table 2 AV interactions predict behavior | pooled | 1 | 2 | 3 | Categorize movies of actions with tools or musical instruments; AV interactions predicted by behavior | 1 | 1 | 2 | 2 | 1 | ||
| 135 | Werner | 2010 | Table 3C superadditive AV due to task | pooled | 3 | 0 | 3 | Categorize movies of actions with tools or musical instruments; Subadditive AV to task | 1 | 1 | 2 | 2 | 1 | ||
| 81 | 136 | Willems | 2007 | Table 3C and D mismatch hand gestures and speech | 16 | 2 | 1 | 3 | Mismatch of hand gesture (no face) and speech versus correct | 2 | 1 | 1 | 1 | ||
| 82 | 137 | Wolf | 2014 | Table 1 face cartoons + phonemes | 16 | 1 | 1 | 2 | Drawing of faces with emotional expressions: Supramodal effects with emotional valence | 1 | 1 | 1 | 1 | 2 |
Notes: The first column denotes the 82 included studies, and the second column the 137 experimental paradigms of those studies. The next columns depict first author (alphabetically), the year, and abbreviated description of the data table (T) or figure (F) used, followed by number of subjects. The column labeled “Multiple experiments” indicates that the multiple experimental paradigms where subject numbers were pooled from that study for the meta-analysis, such as for the single study ALE meta-analysis depicted in Figure 3A (purple). The number of reported foci in the left and right hemispheres and their sum is also indicated. This is followed by a brief description of the experimental paradigm: B/W = black and white, A = audio, AV = audio-visual, V = visual, VA = visual-audio. The rightmost columns show the coding of experimental paradigms that appear in subsequent meta-analyses and Tables, with correspondence to the results illustrated in Figure 3: 0 = not used in contrast, 1 = included, 2 = included as the contrast condition, blank cell = uncertain of clear category membership and not used in that contrast condition. See text for other details.
Activation Likelihood Estimate Analyses
The ALE analysis consists of a coordinate-based, probabilistic meta-analytic technique for assessing the colocalization of reported activations across studies (Turkeltaub et al. 2002, 2012; Eickhoff et al. 2009, 2012, 2016; Laird et al. 2009, 2010; Muller et al. 2018). Whole-brain probability maps were initially created across all the reported foci in standardized stereotaxic space (Talairach “T88,” being converted from, for example, Montreal Neurological Institute “MNI” format) using GingerALE software (Brainmap GingerALE version 2.3.6; Research Imaging Institute; http://brainmap.org). This software was also used to create probability maps, where probabilities were modeled by 3D Gaussian density distributions that took into account sample size variability by adjusting the full-width half-max (FWHM) for each study (Eickhoff et al. 2009; Eickhoff et al. 2016). For each voxel, GingerALE estimated the cumulative probabilities that at least one study reported activation for that locus for a given experimental paradigm condition. Assuming and accounting for spatial uncertainty across reports, this voxel-wise procedure generated statistically thresheld ALE maps, wherein the resulting ALE values reflected the probability of reported activation at that locus. Using a random effects model, the values were tested against the null hypothesis that activation was independently distributed across all studies in a given meta-analysis.
To determine the likely spatial convergence of reported activations across studies, activation foci coordinates from experimental paradigms were transferred manually and compiled into one spreadsheet on two separate occasions by two different investigators (coauthors). To avoid (or minimize) the potential for errors (e.g., transformation from MNI to TAL, sign errors, duplicates, omissions, etc.) an intermediate stage of data entry involved logging all the coordinates and their transformations into one spreadsheet (Appendix A) where they were coded by Table/Figure and number of subjects (Table 1), facilitating inspection and verification relative to hard copy printouts of all included studies. A third set of files (text files) were then constructed from that spreadsheet of coordinates and entered as input files for the various meta-analyses using GingerALE software. This process enabled a check-sums of number of left and right hemisphere foci and the number of subjects for all of the meta-analyses reported herein. When creating single study data set analysis ALE maps, coordinates from experimental paradigms of a given study (using the same participants in each paradigm) were pooled together, thereby avoiding potential violations of assumed subject-independence across maps, which could negatively impact the validity of the meta-analytic results (Turkeltaub et al. 2012). After pooling, there were 1285 participants (Table 1, column 6). Some participants could conceivably have been recruited in more than one study (such as from the same laboratory). However, we had no means for assessing this and assumed that these were all unique individuals. All single study data set ALE maps were thresheld at P < 0.05 with a voxel-level family-wise error (FWE) rate correction for multiple comparisons (Muller et al. 2018) using 10 000 Monte Carlo threshold permutations. For all “contrast” ALE meta-analysis maps, cluster-level thresholds were derived using the single study corrected FWE datasets and then further thresheld for contrast at an uncorrected P < 0.05, and using 10 000 permutations. Minimum cluster sizes were used to further assess rigorousness of clusters, which are included in the tables and addressed in Results.
Guided by earlier meta-analyses of hearing perception and audio-visual interaction sites, several hypothesis driven contrasts were derived as addressed in the Introduction (Lewis 2010; Brefczynski-Lewis and Lewis 2017). A minimum of 17–20 studies was generally recommended to achieve sufficient statistical power to detect smaller effects and make sure that results were not driven by single experiments (Eickhoff et al. 2016; Muller et al. 2018). However, 2 of the 10 subsets of meta-analysis were performed despite there being relatively few numbers of studies (i.e., n = 13 in Table 9; n = 9 in Table 10), and thus their outcomes would presumably only reveal the larger effect sizes and merit future study. For visualization purposes, resulting maps were initially projected onto the N27 atlas brain using AFNI software (Cox 1996) to assess and interpret results, and onto the population-averaged, landmark-, and surface-based (PALS) atlas cortical surface models (in AFNI-Talairach space) using Caret software (http://brainmap.wustl.edu) for illustration of the main findings (Van Essen et al. 2001; Van Essen 2005).
Table 9.
Studies included in the Actions category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 19 | First author | Year | Experimental code and abbreviated task | 205 | 50 | 50 | 100 | Brief description of experimental paradigm | |
| 6 | 7 | Beauchamp | 2004a | Figure 3J and K, Table 1 first 2 foci only | 26 | 2 | 0 | 2 | See photographs of tools, animals and hear corresponding sounds versus scrambled images and synthesized rippled sounds | |
| 7 | 8 | Beauchamp | 2004b | Expt 1 coordinates | 8 | 1 | 1 | 2 | High-resolution version of 2004a study: AV tool videos versus unimodal (AV > A,V) | |
| 8 | 9 | Belardinelli | 2004 | Table 1 AV semantic congruence | 13 | 6 | 6 | 12 | Colored images of tools, animals, humans and semantically congruent versus incongruent sounds | |
| 27 | 44 | Hasegawa | 2004 | Table 1A (well-trained piano) AV induced by V-only | 26 | 12 | 6 | 18 | Piano playing: well trained pianists, mapping hand movements to sequences of sound | |
| 35 | 58 | James | 2011 | Table 1A bimodal (vs. scrambled) | 12 | 4 | 2 | 6 | Video of human manual actions (e.g., sawing): Auditory and Visual intact versus scrambled, AV event selectivity | |
| 37 | 61 | Jola | 2013 | Table 1C AVcondition dance | 12 | 3 | 3 | 6 | Viewing unfamiliar dance performance (tells a story by gesture) with versus without music: using intersubject correlation | |
| 47 | 75 | Naghavi | 2007 | Figure 1C | 23 | 0 | 3 | 3 | B/W pictures (animals, tools, instruments, vehicles) and their sounds: Cong versus Incong | |
| 57 | 91 | Plank | 2012 | pg 803 AV congruent effect | 15 | 0 | 1 | 1 | AV spatially congruent > semantically matching images of natural objects and associated sounds (right STG) | |
| 92 | Plank | 2012 | Table 2A spatially congruent-baseline | pooled | 5 | 5 | 10 | Images of natural objects and associated sounds, spatially congruent versus baseline | ||
| 61 | 100 | Scheef | 2009 | Table 1 cartoon jump + boing | 16 | 1 | 2 | 3 | Video of cartoon person jumping and “sonification” of a tone, learn correlated pairings: AV-V and AV-A conjunction | |
| 62 | 101 | Schmid | 2011 | Table 2E A effect V (Living and nonliving, pictures) | 12 | 3 | 4 | 7 | Environmental sounds and matching pictures: reduced activity by A | |
| 102 | Schmid | 2011 | Table 2F V competition effect A (reduced activity by a visual object) | pooled | 2 | 2 | 4 | Environmental sounds and matching pictures: reduced activity by V | ||
| 103 | Schmid | 2011 | Table 2G AV crossmodal interaction × auditory attention | pooled | 2 | 3 | 5 | Environmental sounds and matching pictures: cross-modal interaction and auditory attention | ||
| 64 | 106 | Sestieri | 2006 | Table 1 (Fig. 3), AV location match versus semantic | 10 | 2 | 5 | 7 | B/W images (animal, weapons) and environmental sounds: Match location > recognition | |
| 107 | Sestieri | 2006 | Table 2 AV semantic recognition versus localization | pooled | 2 | 1 | 3 | B/W pictures and environmental sounds: congruent semantic recognition > localization task | ||
| 65 | 108 | Stevenson | 2009 | Table 1B AVtools > AVspeech | 11 | 1 | 1 | 2 | Hand tools in use video: inverse effectiveness (degraded AV tool > AV speech) | |
| 80 | 133 | Werner | 2010 | Table 1 superadditive (AV-salience effect) | 21 | 0 | 3 | 3 | Categorize movies of actions with tools or musical instruments (degraded stimuli); AV interactions both tasks | |
| 134 | Werner | 2010 | Table 2 AV interactions predict behavior | pooled | 1 | 2 | 3 | Categorize movies of actions with tools or musical instruments; AV interactions predicted by behavior | ||
| 135 | Werner | 2010 | Table 3C superadditive AV due to task | pooled | 3 | 0 | 3 | Categorize movies of actions with tools or musical instruments; Subadditive AV to task |
Table 10.
Studies included in the Emotional audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 13 | First author | Year | Experimental code and abbreviated task | 160 | 24 | 29 | 53 | Brief description of experimental paradigm | |
| 23 | 36 | Ethofer | 2013 | Table 1C emotion | 23 | 1 | 2 | 3 | Audiovisual emotional face-voice integration | |
| 26 | 42 | Hagan | 2013 | Table 1 AV emotion, novel over time | 18 | 5 | 3 | 8 | Affective audio-visual speech: congruent AV emotion versus A, V; unique ROIs over time (MEG) | |
| 36 | 59 | Jessen | 2015 | Table 1A emotion > neutral AV enhanced | 17 | 1 | 1 | 2 | Emotional multisensory whole body and voice expressions: AV emotion (anger and fear) > neutral expressions | |
| 60 | Jessen | 2015 | Table 1D fear > neutral AV enhanced | pooled | 2 | 1 | 3 | Emotional multisensory whole body and voice expressions: AV fear > neutral expressions | ||
| 37 | 61 | Jola | 2013 | Table 1C AVcondition dance | 12 | 3 | 3 | 6 | Viewing unfamiliar dance performance (tells a story by gesture) with versus without music: using intersubject correlation | |
| 40 | 64 | Kreifelts | 2007 | Table 1 voice-face emotion | 24 | 1 | 2 | 3 | Facial expression and intonated spoken words, judge emotion expressed (AV > A,V; P < 0.05 only) | |
| 65 | Kreifelts | 2007 | Table 5 AV increase effective connectivity | pooled | 2 | 4 | 6 | Increased effectiveness connectivity with pSTS and thalamus during AV integration of nonverbal emotional information | ||
| 45 | 73 | Muller | 2012 | Supplementary Table 1 effective connectivity changes | 27 | 4 | 3 | 7 | Emotional facial expression (groaning, laughing) AV integration and gating of information | |
| 60 | 97 | Robins | 2008 | Table 4B emotion effects | 5 | 2 | 0 | 2 | AV faces and spoken sentences expressing fear or neutral valence: Emotional AV-fear > AV-neutral | |
| 98 | Robins | 2008 | Table 4C (Fig. 5) fearful AV integration | pooled | 1 | 5 | 6 | AV faces and spoken sentences expressing fear or neutral valence: Fearful-only AV integration | ||
| 99 | Robins | 2008 | Table 4D AV-only emotion | pooled | 1 | 3 | 4 | AV faces and spoken sentences expressing fear or neutral valence: AV-only emotion | ||
| 78 | 129 | Watson | 2014a | Table 1C AV-adaptation effect, cross-modal adaptation effect | 18 | 0 | 1 | 1 | Videos of emotional faces and voice: crossmodal adaptation effects | |
| 82 | 137 | Wolf | 2014 | Table 1 face cartoons + phonemes | 16 | 1 | 1 | 2 | Drawing of faces with emotional expressions: Supramodal effects with emotional valence |
Results
The database search for audio-visual experiments reporting interaction effects yielded 137 experimental paradigms from 82 published articles (Fig. 2; PRISMA flow-chart). Experiments revealing an effect of audio-visual stimuli (Table 1) included 1285 subjects (though see Materials and Methods) and 714 coordinate brain locations (376 left hemisphere, 338 right). ALE meta-analysis of all these reported foci (congruent plus incongruent audio-visual interaction effects) revealed a substantial expanse of activated brain regions (Fig. 3A, purple hues; projected onto both fiducial and inflated brain model images). Note that this unthresholded map revealed foci reported as demonstrating audio-visual interactions that were found to be significant in at least one of the original studies, thereby illustrating the substantial global expanse of reported brain territories involved in audio-visual interaction processing in general. This included subcortical in addition to cortical regions, such as the thalamus and basal ganglia (Fig. 3A insets), and cerebellum (not illustrated). However, subcortical regions are only approximately illustrated here since they did not survive threshold criteria imposed in the below single study and contrast ALE brain maps. Each study contained one or multiple experimental paradigms. For each experimental paradigm, several neurobiological subcategories of audio, visual, and/or audio-visual stimuli were identified. The subcategories are coded in Table 1 (far right columns) as either being excluded (0), included (1), included as a contrast condition (2), or deemed as uncertain for inclusion (blank cells) for use in different meta-analyses. Volumes resulting from the meta-analyses (depicted in Fig. 3) are available in Supplementary Material.
Figure 3.
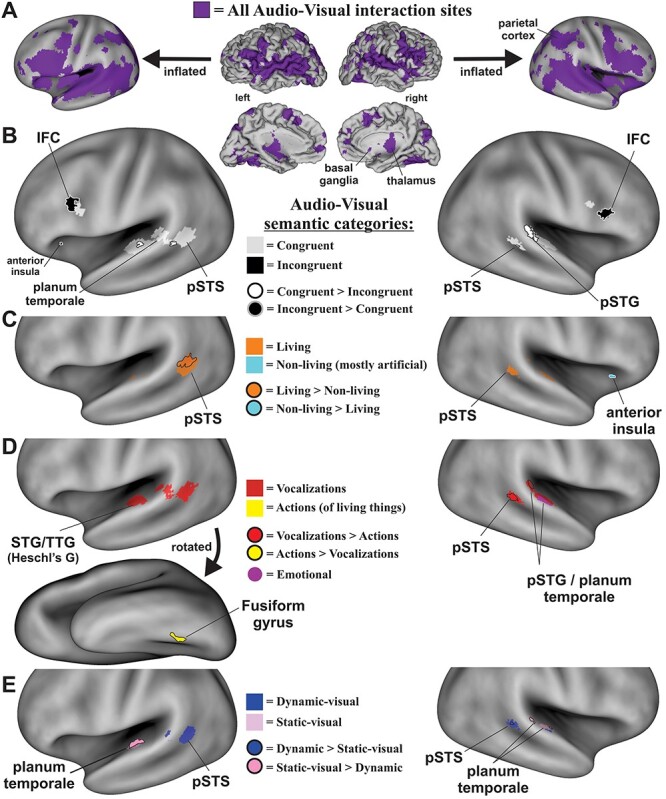
ALE maps of audio-visual interaction sites. (A) Cortical maps derived from all studies (Table 1; purple hues, unthresholded) to illustrate global expanses of cortices involved. Data were projected onto the fiducial (lateral and medial views) and inflated (lateral views only) PALS atlas model of cortex. (B) Illustration of maps derived from single study congruent paradigms (white hues) plus superimposed maps of single study incongruent audio-visual paradigms (black). Outlined foci depict ROIs surviving after direct contrasts (e.g., congruent > incongruent). (C) ALE map revealing single study living (orange) contrasted with single study nonliving (cyan) categories of audio-visual paradigms and outlined foci that survived after direct contrasts. (D) ALE maps revealing audio-visual interactions involving single study vocalizations (red) versus single study action (mostly nonvocal) living source sounds (yellow), and outlined foci that survived after direct contrasts. A single study ALE map for paradigms using emotionally valent audio-visual stimuli, predominantly involving human vocalizations, is also illustrated (violet). (E) ALE maps showing ROIs preferentially recruited using single study dynamic-visual (blue hues) relative to single study static-visual (pink hues) audio-visual interaction foci, and outlined foci that survived after direct contrasts. All single study ALE maps were at corrected FWE P < 0.05, and subsequently derived contrast maps were at uncorrected P < 0.05. IFC = inferior frontal cortex, pSTS = posterior superior temporal sulcus, TTG = transverse temporal gyrus. Refer to text for other details.
Congruent versus Incongruent Audio-Visual Stimuli
The first set of meta-analyses examined reported activation foci specific to when audio-visual stimuli were perceived as congruent spatially, temporally, and/or semantically (Table 2; 79 studies, 117 experimental paradigms, 1235 subjects, 608 reported foci—see Table captions) versus those regions more strongly activated when the stimuli were perceived as incongruent (Table 3). Brain maps revealing activation when processing only congruent audio-visual pairings (congruent single study; corrected FWE P < 0.05) revealed several regions of interest (ROIs) (Fig. 3B, white hues; Table 4A coordinates), including the bilateral posterior superior temporal sulci (pSTS) that extended into the bilateral planum temporale and transverse temporal gyri (left > right), and the bilateral inferior frontal cortices (IFC). Brain maps revealing activation when processing incongruent audio-visual pairings (Fig. 3B, black hues; incongruent single study; corrected FWE P < 0.05; Table 4B) revealed bilateral IFC foci that were located immediately anterior to the IFC foci for congruent stimuli, plus a small left anterior insula focus.
Table 2.
Studies included in the Congruent category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 79 | 117 | First author | Year | Experimental code and abbreviated task | 1235 | 320 | 288 | 608 | Brief description of experimental paradigm | |
| 1 | 1 | Adams | 2002 | Expt 1 Table 3 A + V (aud coords only) | 12 | 5 | 1 | 6 | A and V commonly showing subordinate > basic object name verification (words with pictures or environmental sounds) | |
| 2 | 2 | Alink | 2008 | Table 1c spheres move to drum sounds | 10 | 4 | 6 | 10 | Visual spheres and drum sounds moving: crossmodal dynamic capture versus conflicting motion | |
| 3 | 3 | Balk | 2010 | Figure 2 asynchronous versus simultaneous | 14 | 2 | 1 | 3 | Natural asynchronous versus simultaneous AV speech synchrony (included both contrasts as interaction effects) | |
| 4 | 4 | Baumann | 2007 | Table 1B coherent V + A versus A | 12 | 2 | 1 | 3 | Visual dots 16% coherent motion and in-phase acoustic noise > stationary acoustic sound | |
| 5 | Baumann | 2007 | Table 2B | pooled | 15 | 12 | 27 | Moving acoustic noise and visual dots 16% in-phase coherent > random dot motion | ||
| 5 | 6 | Baumgaertner | 2007 | Table 3 Action > nonact sentence+video | 19 | 3 | 0 | 3 | Conjunction spoken sentences (actions > nonactions) AND videos (actions > nonactions) | |
| 6 | 7 | Beauchamp | 2004a | Figure 3J and K, Table 1 first 2 foci only | 26 | 2 | 0 | 2 | See photographs of tools, animals and hear corresponding sounds versus scrambled images and synthesized rippled sounds | |
| 7 | 8 | Beauchamp | 2004b | Expt 1 coordinates | 8 | 1 | 1 | 2 | High resolution version of 2004a study: AV tool videos versus unimodal (AV > A,V) | |
| 8 | 9 | Belardinelli | 2004 | Table 1 AV semantic congruence | 13 | 6 | 6 | 12 | Colored images of tools, animals, humans and semantically congruent versus incongruent sounds | |
| 9 | 11 | Biau | 2016 | Table 1A Interaction; speech synchronous | 17 | 8 | 0 | 8 | Hand gesture beats versus cartoon disk and speech interaction: synchronous versus asynchronous | |
| 10 | 12 | Bischoff | 2007 | Table 2A only P < 0.05 included | 19 | 2 | 1 | 3 | Ventriloquism effect: gray disks and tones, synchronous (P < 0.05 corrected) | |
| 11 | 13 | Blank | 2013 | Figure 2 | 19 | 1 | 0 | 1 | Visual-speech recognition correlated with recognition performance | |
| 12 | 14 | Bonath | 2013 | pg 116 congruent thalamus | 18 | 1 | 0 | 1 | Small checkerboards and tones: spatially congruent versus incongruent (thalamus) | |
| 13 | 16 | Bonath | 2014 | Table 1A illusory versus not | 20 | 1 | 5 | 6 | Small checkerboards and tones: temporal > spatial congruence | |
| 17 | Bonath | 2014 | Table 1B synchronous > no illusion | pooled | 3 | 0 | 3 | Small checkerboards and tones: spatial > temporal congruence | ||
| 14 | 18 | Bushara | 2001 | Table 1A (Fig. 2) AV-Control | 12 | 1 | 3 | 4 | Tones (100 ms) and colored circles synchrony: detect Auditory then Visual presentation versus Control | |
| 19 | Bushara | 2001 | Table 1B (VA-C) five coords | pooled | 2 | 3 | 5 | Tones (100 ms) and colored circles synchrony: detect Visual then Auditory presentation versus Control | ||
| 20 | Bushara | 2001 | Table 2A interact w/Rt Insula | pooled | 2 | 4 | 6 | Tones and colored circles: correlated functional connections with (and including) the right insula | ||
| 15 | 21 | Bushara | 2003 | Table 2A collide > pass, strong A-V interact | 7 | 5 | 3 | 8 | Tone and two visual bars moving: Tone synchrony induce perception they collide (AV interaction) versus pass by | |
| 16 | 22 | Callan | 2014 | Table 5 AV-Audio (AV10-A10)-(AV6-A6) | 16 | 4 | 4 | 8 | Multisensory enhancement to visual speech in noise correlated with behavioral results | |
| 23 | Callan | 2014 | Table 6 AV—Visual only | pooled | 1 | 1 | 2 | Multisensory enhancement to visual speech audio-visual versus visual only | ||
| 17 | 24 | Calvert | 1999 | Table 1 (Fig. 1) | 5 | 3 | 4 | 7 | View image of lower face and hear numbers 1 through 10 versus unimodal conditions (AV > Photos, Auditory) | |
| 18 | 25 | Calvert | 2000 | Figure 2 superadd+subadd AVspeech | 10 | 1 | 0 | 1 | Speech and lower face: supra-additive plus subadditive effects (AV-congruent > A,V > AV-incongruent) | |
| 26 | Calvert | 2000 | Table 1 supradd AVspeech | pooled | 4 | 5 | 9 | Speech and lower face: supra-additive AV enhancement | ||
| 19 | 28 | Calvert | 2001 | Table 2 superadditive and resp depression | 10 | 4 | 11 | 15 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; supradditive and response depression | |
| 29 | Calvert | 2001 | Table 3A superadditive only | pooled | 6 | 4 | 10 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; supradditive only | ||
| 30 | Calvert | 2001 | Table 3B response depression only | pooled | 3 | 4 | 7 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; response depression only | ||
| 20 | 31 | Calvert | 2003 | Table 2A (Fig. 3 blue) | 8 | 13 | 8 | 21 | Speech and lower face: Moving dynamic speech (phonemes) versus stilled speech frames | |
| 21 | 32 | DeHaas | 2013 | Table 1A AVcong—Visual | 15 | 3 | 3 | 6 | Video clips of natural scenes (animals, humans): AV congruent versus Visual | |
| 22 | 34 | Erickson | 2014 | Table 1A Congruent AV speech | 10 | 2 | 2 | 4 | McGurk effect (phonemes): congruent AV speech: AV > A and AV > V | |
| 35 | Erickson | 2014 | Table 1B McGurk speech | pooled | 2 | 0 | 2 | McGurk speech effect (phonemes) | ||
| 23 | 36 | Ethofer | 2013 | Table 1C emotion | 23 | 1 | 2 | 3 | Audiovisual emotional face-voice integration | |
| 24 | 37 | Gonzalo | 2000 | Table 1 AV > AVincon music and Chinese ideograms | 14 | 1 | 1 | 2 | Learn novel Kanji characters and musical chords, activity increases over time for consistent AV pairings | |
| 39 | Gonzalo | 2000 | Table 3 AV consistent versus Aud | pooled | 1 | 1 | 2 | Learn novel Kanji characters and musical chords, learn consistent (vs inconsistent) pairings versus auditory only | ||
| 25 | 41 | Green | 2009 | Table 4A Congruent gesture-speech > gesture or speech | 16 | pooled | 1 | 0 | 1 | Congruent gesture-speech versus gesture with unfamiliar speech and with familiar speech |
| 26 | 42 | Hagan | 2013 | Table 1 AV emotion, novel over time | 18 | 5 | 3 | 8 | Affective audio-visual speech: congruent AV emotion versus A, V; unique ROIs over time (MEG) | |
| 27 | 44 | Hasegawa | 2004 | Table 1A (well trained piano) AV induced by V-only | 26 | 12 | 6 | 18 | Piano playing: well trained pianists, mapping hand movements to sequences of sound | |
| 28 | 45 | Hashimoto | 2004 | Table 1G (Fig 4B, red) Learning Hangul letters to sounds | 12 | 2 | 1 | 3 | Unfamiliar Hangul letters and nonsense words, learn speech versus tone/noise pairings | |
| 29 | 46 | He | 2015 | Table 3C AV speech foreign (left MTG focus) | 20 | 1 | 0 | 1 | Intrinsically meaningful gestures with German speech: Gesture-German > Gesture-Russian, German speech only | |
| 30 | 47 | He | 2018 | Table 2, GSI, left MTG, gestures and speech integration | 20 | 1 | 0 | 1 | Gesture-speech integration: Bimodal speech-gesture versus unimodal gesture with foreign speech and versus unimodal speech | |
| 31 | 49 | Hein | 2007 | Figure 2B AV-artificial/nonliving | 18 | 0 | 1 | 1 | B/W images of artificial objects (“fribbles”) and animal vocalizations versus unimodal A, V | |
| 50 | Hein | 2007 | Figure 2C pSTS, pSTG, mSTG AV-cong | pooled | 0 | 3 | 3 | Familiar animal images and correct vocalizations (dog: woof-woof) | ||
| 52 | Hein | 2007 | Figure 3B Foci 2, 3, 4 (blue) artificial/nonliving | pooled | 3 | 0 | 3 | Visual “Fribbles” and backward/underwater distorted animal sounds, learn pairings (blue foci 2,3,4) | ||
| 53 | Hein | 2007 | Figure 3C congruent living (green) | pooled | 3 | 0 | 3 | Familiar congruent living versus artificial AV object features and animal sounds (green foci 7, 8, 10) | ||
| 32 | 54 | Hocking | 2008 | pg 2444 verbal | 18 | 2 | 0 | 2 | (pSTS mask) Color photos, written names, auditory names, environmental sounds conceptually matched “amodal” | |
| 33 | 56 | Hove | 2013 | pg 316 AV interaction putamen | 14 | 0 | 1 | 1 | Interaction between (beep > flash) versus (siren > moving bar); left putamen focus | |
| 34 | 57 | James | 2003 | Figure 2 | 12 | 0 | 1 | 1 | Activation by visual objects (“Greebles”) associated with auditory features (e.g., buzzes, screeches); (STG) | |
| 35 | 58 | James | 2011 | Table 1A bimodal (vs. scrambled) | 12 | 4 | 2 | 6 | Video of human manual actions (e.g., sawing): Auditory and Visual intact versus scrambled, AV event selectivity | |
| 36 | 59 | Jessen | 2015 | Table 1A emotion > neutral AV enhanced | 17 | 1 | 1 | 2 | Emotional multisensory whole body and voice expressions: AV emotion (anger and fear) > neutral expressions | |
| 60 | Jessen | 2015 | Table 1D fear > neutral AV enhanced | pooled | 2 | 1 | 3 | Emotional multisensory whole body and voice expressions: AV fear > neutral expressions | ||
| 37 | 61 | Jola | 2013 | Table 1C AVcondition dance | 12 | 3 | 3 | 6 | Viewing unfamiliar dance performance (tells a story by gesture) with versus without music: using intersubject correlation | |
| 38 | 62 | Kim | 2015 | Table 2A AV > C speech semantic match | 15 | 2 | 0 | 2 | Moving audio-visual speech perception versus white noise and unopened mouth movements | |
| 39 | 63 | Kircher | 2009 | Figure 3B: gesture related activation increase | 14 | 3 | 1 | 4 | Bimodal gesture-speech versus gesture and versus speech | |
| 40 | 64 | Kreifelts | 2007 | Table 1 voice-face emotion | 24 | 1 | 2 | 3 | Facial expression and intonated spoken words, judge emotion expressed (AV > A,V; P < 0.05 only) | |
| 65 | Kreifelts | 2007 | Table 5 AV increase effective connectivity | pooled | 2 | 4 | 6 | Increased effectiveness connectivity with pSTS and thalamus during AV integration of nonverbal emotional information | ||
| 41 | 66 | Lewis | 2000 | Table 1 | 7 | 2 | 3 | 5 | Compare speed of tone sweeps to visual dot coherent motion: Bimodal versus unimodal | |
| 42 | 67 | Matchin | 2014 | Table 1 AV > Aud only (McGurk) | 20 | 2 | 7 | 9 | McGurk audio-visual speech: AV > A only | |
| 68 | Matchin | 2014 | Table 2 AV > Video only | pooled | 9 | 6 | 15 | McGurk audio-visual speech: AV > V only | ||
| 43 | 70 | McNamara | 2008 | Table (BA44 and IPL) | 12 | 2 | 2 | 4 | Videos of meaningless hand gestures and synthetic tone sounds: Increases in functional connectivity with learning | |
| 44 | 71 | Meyer | 2007 | Table 3 paired A + V versus null | 16 | 3 | 3 | 6 | Paired screen red flashes with phone ring: paired V (conditioned stimulus) and A (unconditioned) versus null events | |
| 72 | Meyer | 2007 | Table 4 CS+, learned AV association with V-only | pooled | 4 | 6 | 10 | Paired screen flashes with phone ring: View flashes after postconditioned versus null events | ||
| 45 | 73 | Muller | 2012 | Supplementary Table 1 effective connectivity changes | 27 | 4 | 3 | 7 | Emotional facial expression (groaning, laughing) AV integration and gating of information | |
| 47 | 75 | Naghavi | 2007 | Figure 1C | 23 | 0 | 3 | 3 | B/W pictures (animals, tools, instruments, vehicles) and their sounds: congruent versus incongruent | |
| 48 | 77 | Naghavi | 2011 | Figure 2B congruent > incongruent | 30 | 0 | 1 | 1 | B/W drawings of objects (living and non) and natural sounds (barking, piano): congruent > incongruent encoding | |
| 49 | 79 | Nath | 2012 | pg 784 | 14 | 1 | 0 | 1 | McGurk effect (phonemes): congruent AV speech correlated with behavioral percept | |
| 50 | 80 | Naumer | 2008 | Figure 2 Table 1A max contrast | 18 | 8 | 6 | 14 | Images of “Fribbles” and learned artificial sounds (underwater animal vocals): posttraining versus max contrast | |
| 81 | Naumer | 2008 | Figure 3 Table 1B pre–post | pooled | 5 | 6 | 11 | Images of “Fribbles” and learned corresponding artificial sounds: Post- versus pretraining session | ||
| 51 | 83 | Naumer | 2011 | Figure 3C | 10 | 1 | 0 | 1 | Photographs of objects (living and non) and related natural sounds | |
| 52 | 85 | Noppeny | 2008 | Table 3 AV congruent sounds/words | 17 | 4 | 0 | 4 | Speech sound recognition through AV priming, environmental sounds and spoken words: Congruent > incongruent | |
| 53 | 86 | Ogawa | 2013a | Table 1 (pg 162 data) | 13 | 1 | 0 | 1 | AV congruency of pure tone and white dots moving on screen (area left V3A) | |
| 54 | 87 | Ogawa | 2013b | Table 1 3D > 2D and surround > monaural effects | 16 | 3 | 4 | 7 | Cinematic 3D > 2D video and surround sound > monaural while watching a movie (“The Three Musketeers”) | |
| 55 | 88 | Okada | 2013 | Table 1 AV > A | 20 | 5 | 4 | 9 | Video of AV > A speech only | |
| 56 | 89 | Olson | 2002 | Table 1A synchronized AV > static Vis-only | 10 | 7 | 4 | 11 | Whole face video and heard words: Synchronized AV versus static V | |
| 90 | Olson | 2002 | Table 1C synchronized AV > desynchronized AV speech | pooled | 2 | 0 | 2 | Whole face video and heard words: Synchronized versus desynchronized | ||
| 57 | 91 | Plank | 2012 | pg 803 AV congruent effect | 15 | 0 | 1 | 1 | AV spatially congruent > semantically matching images of natural objects and associated sounds (right STG) | |
| 92 | Plank | 2012 | Table 2A spatially congruent-baseline | pooled | 5 | 5 | 10 | Images of natural objects and associated sounds, spatially congruent versus baseline | ||
| 58 | 93 | Raij | 2000 | Table 1B letters and speech sounds | 9 | 2 | 3 | 5 | Integration of visual letters and corresponding auditory phonetic expressions (MEG study) AV versus (A + V) | |
| 59 | 94 | Regenbogen | 2017 | Table 2A degraded > clear Multisensory versus unimodal input | 29 | 5 | 6 | 11 | Degraded > clear AV versus both visual and auditory unimodal visual real-world object-in-action recognition | |
| 60 | 95 | Robins | 2008 | Table 2 (Fig. 2) AV integration (AV > A and AV > V) | 10 | 2 | 1 | 3 | Face speaking sentences: angry, fearful, happy, neutral (AV > A,V) | |
| 96 | Robins | 2008 | Table 4A (Fig. 5) AV integration and emotion | 5 | 1 | 4 | 5 | AV faces and spoken sentences expressing fear or neutral valence: AV integration (AV > A,V conditions) | ||
| 97 | Robins | 2008 | Table 4B emotion effects | pooled | 2 | 0 | 2 | AV faces and spoken sentences expressing fear or neutral valence: Emotional AV-fear > AV-neutral | ||
| 98 | Robins | 2008 | Table 4C (Fig. 5) fearful AV integration | pooled | 1 | 5 | 6 | AV faces and spoken sentences expressing fear or neutral valence: Fearful-only AV integration | ||
| 99 | Robins | 2008 | Table 4D AV-only emotion | pooled | 1 | 3 | 4 | AV faces and spoken sentences expressing fear or neutral valence: AV-only emotion | ||
| 61 | 100 | Scheef | 2009 | Table 1 cartoon jump + boing | 16 | 1 | 2 | 3 | Video of cartoon person jumping and “sonification” of a tone, learn correlated pairings: AV-V and AV-A conjunction | |
| 62 | 101 | Schmid | 2011 | Table 2E A effect V (Living and nonliving, pictures) | 12 | 3 | 4 | 7 | Environmental sounds and matching pictures: reduced activity by A | |
| 102 | Schmid | 2011 | Table 2F V competition effect A (reduced activity by a visual object) | pooled | 2 | 2 | 4 | Environmental sounds and matching pictures: reduced activity by V | ||
| 103 | Schmid | 2011 | Table 2G AV crossmodal interaction x auditory attention | pooled | 2 | 3 | 5 | Environmental sounds and matching pictures: cross-modal interaction and auditory attention | ||
| 63 | 104 | Sekiyama | 2003 | Table 3 (fMRI nAV-AV) | 8 | 1 | 0 | 1 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (fMRI) | |
| 105 | Sekiyama | 2003 | Table 4 (PET nAV-AV) | pooled | 1 | 3 | 4 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (PET) | ||
| 64 | 106 | Sestieri | 2006 | Table 1 (Fig. 3), AV location match versus semantic | 10 | 2 | 5 | 7 | B/W images (animal, weapons) and environmental sounds: Match location > recognition | |
| 107 | Sestieri | 2006 | Table 2 AV semantic recognition versus localization | pooled | 2 | 1 | 3 | B/W pictures and environmental sounds: congruent semantic recognition > localization task | ||
| 65 | 108 | Stevenson | 2009 | Table 1B 2 AVtools > AVspeech | 11 | 1 | 1 | 2 | Hand tools in use video: inverse effectiveness (degraded AV tool > AV speech) | |
| 109 | Stevenson | 2009 | Table 1C (Fig. 8) AVspeech > AVtools | pooled | 1 | 1 | 2 | Face and speech video: inverse effectiveness (degraded AV speech > AV tool use) | ||
| 66 | 110 | Straube | 2011 | Table 3A and B iconic/metaphoric speech-gestures versus speech, gesture | 16 | 2 | 2 | 4 | Integration of Iconic and Metaphoric speech-gestures versus speech and gesture | |
| 67 | 111 | Straube | 2014 | p939 Integration foci | 16 | 3 | 0 | 3 | Integration of iconic hand gesture-speech > unimodal speech and unimodal gesture (healthy control group) | |
| 69 | 113 | Tanabe | 2005 | Table 1A AV; A then V; not VA | 15 | 10 | 10 | 20 | Amorphous texture patterns and modulated white noises: Activation during learning delay period (AV) | |
| 114 | Tanabe | 2005 | Table 2A+2B (Fig. 5a) AV and VA | pooled | 5 | 6 | 11 | Amorphous texture patterns and modulated white noises: changes after feedback learning (AV and VA) | ||
| 115 | Tanabe | 2005 | Table 3A+3B (Fig. 6) AV and VA; delay period | pooled | 9 | 1 | 10 | Amorphous texture patterns and modulated white noises: sustained activity throughout learning (AV and VA) | ||
| 70 | 117 | Taylor | 2006 | Figure 1A and B, Figure 1C and D (living > nonliving) | 15 | 2 | 0 | 2 | Color photos (V), environmental sounds and spoken words (A): Congruent AV versus Incongruent (living objects) | |
| 71 | 118 | Van Atteveldt | 2004 | Table 1a letters and speech sounds | 16 | 3 | 1 | 4 | Familiar letters and their speech sounds: Congruent versus not and Bimodal versus Unimodal | |
| 72 | 119 | Van Atteveldt | 2007 | Table 2A+B (Fig. 2) | 12 | 3 | 2 | 5 | Single letters and their speech sounds (phonemes): Congruent > Incong; Passive perception, blocked and event-related design | |
| 120 | Van Atteveldt | 2007 | Table 3 (Fig. 2) passive | pooled | 1 | 1 | 2 | Single letters and their speech sounds (phonemes): Congruent > Incong, active perception task | ||
| 73 | 122 | Van Atteveldt | 2010 | Table 1B STS; specific adaptation congruent > incong | 16 | 3 | 1 | 4 | Letter and speech sound pairs (vowels, consonants): Specific adaptation effects | |
| 74 | 123 | Van der Wyk | 2010 | Table 2 AV interaction effects oval/circles +speech/nonspeech | 16 | 3 | 3 | 6 | Geometric shape modulate with speech (sentences) | |
| 75 | 124 | Von Kriegstein | 2006 | Figure 4B after > before voice-face | 14 | 0 | 4 | 4 | Face and object photos with voice and other sounds: Voice-Face association learning | |
| 76 | 125 | Watkins | 2006 | Figure 4 illusory multisensory interaction | 11 | 0 | 2 | 2 | Two brief tone pips leads to illusion of two screen flashes (annulus with checkerboard) when only one flash present | |
| 126 | Watkins | 2006 | Table 1 (A enhances V in general) | pooled | 5 | 3 | 8 | Single brief tone pip leads to illusion of single screen flash (annulus with checkerboard) when two flashes present | ||
| 77 | 127 | Watkins | 2007 | Figure 3 2 flashes +1 beep illusion | 10 | 0 | 1 | 1 | Two visual flashes and single audio bleep leads to the illusion of a single flash | |
| 78 | 128 | Watson | 2014a | Table 1A AV-adaptation effect (multimodal localizer) | 18 | 0 | 1 | 1 | Videos of emotional faces and voice: multisensory localizer | |
| 129 | Watson | 2014a | Table 1C AV-adaptation effect, cross-modal adaptation effect | pooled | 0 | 1 | 1 | Videos of emotional faces and voice: crossmodal adaptation effects | ||
| 79 | 130 | Watson | 2014b | Table 1 AV > baseline (Living and nonliving) | 40 | 3 | 5 | 8 | Moving objects and videos of faces with corresponding sounds: AV > baseline | |
| 131 | Watson | 2014b | Table 4A integrative regions (Living and nonliving) | pooled | 2 | 2 | 4 | Moving objects and videos of faces with corresponding sounds: Integrative regions (AV > A,V) | ||
| 132 | Watson | 2014b | Table 4B integrative regions (Living and nonliving) | pooled | 0 | 1 | 1 | Moving objects and videos of faces with corresponding sounds: People-selective integrative region | ||
| 80 | 133 | Werner | 2010 | Table 1 superadditive (AV-salience effect) | 21 | 0 | 3 | 3 | Categorize movies of actions with tools or musical instruments (degraded stimuli); AV interactions both tasks | |
| 134 | Werner | 2010 | Table 2 AV interactions predict behavior | pooled | 1 | 2 | 3 | Categorize movies of actions with tools or musical instruments; AV interactions predicted by behavior | ||
| 135 | Werner | 2010 | Table 3C superadditive AV due to task | pooled | 3 | 0 | 3 | Categorize movies of actions with tools or musical instruments; Subadditive AV to task | ||
| 82 | 137 | Wolf | 2014 | Table 1 face cartoons + phonemes | 16 | 1 | 1 | 2 | Drawing of faces with emotional expressions: Supramodal effects with emotional valence |
Note: This meta-analysis included 79 of the studies with 117 experiments (first and second column). The column “multiple experiments” indicates paradigms where the same set of participants were included, and so all coordinates were pooled together as one study to avoid biases related to violation the assumption of subject independence (refer to Materials and Methods). Results of Congruent meta-analyses are shown in Figure 3B white hues. Refer to Table 1 and text for other details.
Table 3.
Studies included in the Incongruent category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 18 | 19 | First author | Year | Experimental code and abbreviated task | 307 | 55 | 50 | 105 | Brief description of experimental paradigm | |
| 8 | 10 | Belardinelli | 2004 | Table 2 AV semantic incongruent | 13 | 2 | 3 | 5 | Colored images of tools, animals, humans and semantically incongruent versus congruent sounds | |
| 12 | 15 | Bonath | 2013 | pg 116 incongruent | 18 | 1 | 1 | 2 | Small checkerboards and tones: spatially incongruent versus congruent (thalamus) | |
| 18 | 27 | Calvert | 2000 | Table 2 incongruent subadditive AVspeech | 10 | 3 | 3 | 6 | Speech and lower face: subadditive AV response to incongruent AV inputs | |
| 21 | 33 | DeHaas | 2013 | Table 1B V- AV incongruent | 15 | 2 | 0 | 2 | Video clips of natural scenes (animals, humans): Visual versus AV incongruent | |
| 24 | 38 | Gonzalo | 2000 | Table 2 inconsistent AV | 14 | 4 | 4 | 8 | Learn novel Kanji characters and musical chords, activity increases over time to inconsistent pairings | |
| 25 | 40 | Green | 2009 | Table 1 incongruent > congruent gesture-speech | 16 | 4 | 5 | 9 | Incongruent versus congruent gesture-speech | |
| 26 | 43 | Hagan | 2013 | Table 2 AV emotion incongruent | 18 | 1 | 5 | 6 | Affective audio-visual speech: incongruent AV emotion versus A, V; unique ROIs over time (MEG) | |
| 31 | 48 | Hein | 2007 | Figure 2A AV incongruent | 18 | 0 | 2 | 2 | Familiar animal images and incorrect (incongruent) vocalizations (dog: meow) versus correct pairs | |
| 51 | Hein | 2007 | Figure 3A incongruent | 4 | 0 | 4 | AV familiar incongruent versus unfamiliar artificial (red foci 1,5,6,9) | |||
| 32 | 55 | Hocking | 2008 | Table 3 incongruent simultaneous matching | 18 | 8 | 10 | 18 | Incongruent sequential AV pairs (e.g., see drum, hear bagpipes) versus congruent pairs | |
| 42 | 69 | Matchin | 2014 | Table 3 MM > AV McGurk | 20 | 7 | 4 | 11 | McGurk Mismatch > AV speech integration | |
| 46 | 74 | Murase | 2008 | Figure 4 discordant > concordant AVinteraction | 28 | 1 | 0 | 1 | Audiovisual speech (syllables) showing activity to discordant versus concordant stimuli: left mid-STS | |
| 48 | 78 | Naghavi | 2011 | Figure 2C incongruent > congruent | 30 | 1 | 1 | 2 | B/W drawings of objects (living and non) and natural sounds (barking, piano): incongruent > congruent encoding | |
| 50 | 82 | Naumer | 2008 | Figure 4 Table 2 | 18 | 1 | 1 | 2 | Learn of “Freebles” and distorted sounds as incongruent > congruent pairs | |
| 52 | 84 | Noppeny | 2008 | Table 2 AV incongruent > congruent | 17 | 5 | 2 | 7 | Speech sound recognition through AV priming, environmental sounds and spoken words: incongruent > congruent | |
| 68 | 112 | Szycik | 2009 | Table 1 AV incongruent > AV congruent face+speech | 11 | 7 | 2 | 9 | Incongruent AV face-speech versus congruent AV face-speech | |
| 70 | 116 | Taylor | 2006 | pg 8240 AV incongruent | 15 | 1 | 0 | 1 | Color photos (V), environmental sounds (A), spoken words (W): Incongruent (living objects) | |
| 72 | 121 | Van Atteveldt | 2007 | Table 4 (Fig. 6) active condition, incongruent | 12 | 1 | 6 | 7 | Single letters and their speech sounds (phonemes): incongruent > congruent | |
| 81 | 136 | Willems | 2007 | Table 3C and D mismatch hand gestures and speech | 16 | 2 | 1 | 3 | Mismatch of hand gesture (no face) and speech versus correct |
Table 4.
Locations of significant clusters from the meta-analyses involving Congruent and Incongruent audio-visual paradigms
| Condition | Region | Major contributing studies | x | y | z | Volume | ALE value |
|---|---|---|---|---|---|---|---|
| A. Congruent audio-visual stimuli single study | |||||||
| 1 | L Posterior Superior Temporal Sulcus/Gyrus | 32 (3–4,6,7,10,13,18,19,22,27,31, 32,40,42,45,49,50,54,57,60,63,65–67,69,71–74,76.79,82) | −51 | −36 | 9 | 4824 | 0.055 |
| 2 | R Posterior Superior Temporal Sulcus (pSTS) | 27 (4,7,13,16,20,23,31,34,40,42,45, 50,54,55, 57,60,60,65,66,71, 72,73,74,78–80) | 51 | −29 | 10 | 4064 | 0.045 |
| 3 | L Inferior Frontal Cortex (posterior IFC) | 4 (50,52,78,80) | −42 | 7 | 25 | 312 | 0.035 |
| 4 | R Inferior Frontal Cortex (posteriorIFC) | 3 (27,44,50) | 46 | 6 | 31 | 216 | 0.034 |
| B. Incongruent audio-visual stimuli single study | |||||||
| 1 | R Middle Frontal Gyrus/anterior IFC | 3 (31,52,68) | 45 | 14 | 25 | 320 | 0.024 |
| 2 | L Middle Frontal Gyrus/anterior IFC | 3 (8,52,72) | −40 | 11 | 29 | 216 | 0.022 |
| C. Contrast Congruent > Incongruent audio-visual stimuli | |||||||
| 1 | R Posterior Superior Temporal Gyrus | 8 (20,40,55,60,65,66) | 52 | −33 | 13 | 1112 | 2.820 |
| 2 | L Posterior Superior Temporal Gyrus | −51 | −42 | 8 | 168 | 2.149 | |
| 3 | L Posterior Superior Temporal Gyrus | −49 | −25 | 5 | 72 | 1.862 | |
| D. Contrast Incongruent > Congruent audio-visual stimuli | |||||||
| 1 | R Inferior Frontal Cortes (Middle Frontal Gyrus) | 4 (31,52,68,72) | 45 | 13 | 25 | 416 | 3.540 |
| 2 | L Inferior Frontal Cortex (area 9) | 2 (8,72) | −41 | 12 | 28 | 392 | 2.911 |
| 3 | L Inferior Frontal Cortex (area 13) | 2 (42,52) | −32 | 20 | 4 | 56 | 1.932 |
Note: Also indicated are major contributing studies to the ALE meta-analysis clusters, weighted centers of mass (x, y, and z) in Talairach coordinates, brain volumes (mm3), and ALE values. (A) Single study Congruent clusters, and (B) single study Incongruent clusters (both corrected FWE P < 0.05); plus contrast meta-analyses maps revealing (C) Congruent > Incongruent and (D) Incongruent > Congruent audio-visual interaction sites (both uncorrected P < 0.05). The coordinates correspond to foci illustrated in Figure 3B (black and white hues). Refer to text for other details (from Tables 2 and 3).
A contrast meta-analysis of congruent > incongruent audio-visual stimuli (Fig. 3B, white with black outlines; Table 4C, uncorrected P < 0.05) revealed significant involvement of the left and right posterior temporal gyri (pSTG) and pSTS regions. Conversely, a contrast map of brain regions showing significant preferential involvement in processing incongruent > congruent audio-visual stimuli (Fig. 3B, black with white outlines; Table 4D, uncorrected P < 0.05) included bilateral IFC, which extended along inferior portions of the middle frontal gyri in locations immediately anterior to those resulting from the congruent > incongruent contrast. Because both contrast ALE maps revealed functionally dissociated ROIs, these results are herein regarded as providing evidence for a “double-dissociation” of processing along this dimension.
Living versus Nonliving Audio-Visual Stimuli
A major categorical distinction in the neurobiological organization mediating auditory perception is that for sounds produced by living versus nonliving sources (Fig. 1). This potential categorical processing boundary was tested in the multisensory realm by comparing reported activation foci from audio-visual interaction paradigms that involved living versus nonliving sources. The living category paradigms included visual and/or sound-source stimuli such as talking faces, hand/arm gesture with speech, body movements, tool use, and nonhuman animals (Table 5; see brief descriptions). A single study ALE meta-analysis of experimental paradigms using living stimuli revealed portions of the bilateral pSTS/pSTG regions (Fig. 3C, orange hues; Table 7A, corrected FWE P < 0.05). The nonliving visual and sound-source stimuli (Table 6) predominantly included artificial, as opposed to natural, audio-visual events such as flashing checkerboards, coherent dot motion, geometric objects (plus a study depicting natural environmental events), which were paired with sounds such as tones, sirens, or mechanical sounds produced by inanimate sources. A single study ALE meta-analysis of experiments using nonliving stimuli (mostly artificial stimuli) revealed the right anterior insula as a region significantly recruited (Fig. 3C, cyan contained within the white outline; Table 7B, corrected FWE P < 0.05: also see contrast below).
Table 5.
Studies included in the Living category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 43 | 58 | First author | Year | Experimental code and abbreviated task | 699 | 145 | 126 | 271 | Brief description of experimental paradigm | |
| 5 | 6 | Baumgaertner | 2007 | Table 3 Action > nonact sentence+video | 19 | 3 | 0 | 3 | Conjunction spoken sentences (actions>nonactions) AND videos (actions > nonactions) | |
| 6 | 7 | Beauchamp | 2004a | Figure 3J and K, Table 1 first 2 foci only | 26 | 2 | 0 | 2 | See photographs of tools, animals and hear corresponding sounds versus scrambled images and synthesized rippled sounds | |
| 7 | 8 | Beauchamp | 2004b | Expt 1 coordinates | 8 | 1 | 1 | 2 | High resolution version of 2004a study: AV tool videos versus unimodal (AV > A,V) | |
| 8 | 9 | Belardinelli | 2004 | Table 1 AV semantic congruence | 13 | 6 | 6 | 12 | Colored images of tools, animals, humans and semantically congruent versus incongruent sounds | |
| 9 | 11 | Biau | 2016 | Table 1A Interaction; speech synchronous | 17 | 8 | 0 | 8 | Hand gesture beats versus cartoon disk and speech interaction: synchronous versus asynchronous | |
| 11 | 13 | Blank | 2013 | Figure 2 | 19 | 1 | 0 | 1 | Visual-speech recognition correlated with recognition performance | |
| 16 | 22 | Callan | 2014 | Table 5 AV-Audio (AV10-A10)-(AV6-A6) | 16 | 4 | 4 | 8 | Multisensory enhancement to visual speech in noise correlated with behavioral results | |
| 23 | Callan | 2014 | Table 6 AV—Visual only | pooled | 1 | 1 | 2 | Multisensory enhancement to visual speech audio-visual versus visual only | ||
| 17 | 24 | Calvert | 1999 | Table 1 (Fig. 1) | 5 | 3 | 4 | 7 | View image of lower face and hear numbers 1 through 10 versus unimodal conditions (AV > Photos, Auditory) | |
| 18 | 25 | Calvert | 2000 | Figure 2 superadditive+subadditive Avspeech | 10 | 1 | 0 | 1 | Speech and lower face: supra-additive plus subadditive effects (AV-congruent > A,V > AV-incongruent) | |
| 26 | Calvert | 2000 | Table 1 supradditive AVspeech | pooled | 4 | 5 | 9 | Speech and lower face: supra-additive AV enhancement | ||
| 20 | 31 | Calvert | 2003 | Table 2A (Fig. 3 blue) | 8 | 13 | 8 | 21 | Speech and lower face: Moving dynamic speech (phonemes) versus stilled speech frames | |
| 21 | 32 | DeHaas | 2013 | Table 1A AVcong—Visual | 15 | 3 | 3 | 6 | Video clips of natural scenes (animals, humans): AV congruent versus Visual | |
| 22 | 34 | Erickson | 2014 | Table 1A Congruent AV speech | 10 | 2 | 2 | 4 | McGurk effect (phonemes): congruent AV speech: AV > A and AV > V | |
| 35 | Erickson | 2014 | Table 1B McGurk speech | pooled | 2 | 0 | 2 | McGurk speech effect (phonemes) | ||
| 23 | 36 | Ethofer | 2013 | Table 1C emotion | 23 | 1 | 2 | 3 | Audiovisual emotional face-voice integration | |
| 25 | 41 | Green | 2009 | Table 4A Congruent gesture-speech > gesture or speech | 16 | 1 | 0 | 1 | Congruent gesture-speech versus gesture with unfamiliar speech and with familiar speech | |
| 27 | 44 | Hasegawa | 2004 | Table 1A (well trained piano) AV induced by V-only | 26 | 12 | 6 | 18 | Piano playing: well trained pianists, mapping hand movements to sequences of sound | |
| 29 | 46 | He | 2015 | Table 3C AV speech foreign (left MTG focus) | 20 | 1 | 0 | 1 | Intrinsically meaningful gestures with German speech: Gesture-German > Gesture-Russian, German speech only | |
| 30 | 47 | He | 2018 | Table 2 gestures and speech integration (left MTG) | 20 | 1 | 0 | 1 | Gesture-speech integration: Bimodal speech-gesture versus unimodal gesture with foreign speech and versus unimodal speech | |
| 31 | 50 | Hein | 2007 | Figure 2C pSTS, pSTG, mSTG AV-cong | 18 | 0 | 3 | 3 | Familiar animal images and correct vocalizations (dog: woof-woof) | |
| 32 | 54 | Hocking | 2008 | pg 2444 verbal | 18 | 2 | 0 | 2 | (pSTS mask) Color photos, written names, auditory names, environmental sounds conceptually matched “amodal” | |
| 35 | 58 | James | 2011 | Table 1A bimodal (vs. scrambled) | 12 | 4 | 2 | 6 | Video of human manual actions (e.g., sawing): Auditory and Visual intact versus scrambled, AV event selectivity | |
| 36 | 59 | Jessen | 2015 | Table 1A emotion > neutral AV enhanced | 17 | 1 | 1 | 2 | Emotional multisensory whole body and voice expressions: AV emotion (anger and fear) > neutral expressions | |
| 60 | Jessen | 2015 | Table 1D fear > neutral AV enhanced | pooled | 2 | 1 | 3 | Emotional multisensory whole body and voice expressions: AV fear > neutral expressions | ||
| 37 | 61 | Jola | 2013 | Table 1C AVcondition dance | 12 | 3 | 3 | 6 | Viewing unfamiliar dance performance (tells a story by gesture) with versus without music: using intersubject correlation | |
| 38 | 62 | Kim | 2015 | Table 2A AV > C speech semantic match | 15 | 2 | 0 | 2 | Moving audio-visual speech perception versus white noise and unopened mouth movements | |
| 39 | 63 | Kircher | 2009 | Figure 3B gesture-related activation increase | 14 | 3 | 1 | 4 | Bimodal gesture-speech versus gesture and versus speech | |
| 40 | 64 | Kreifelts | 2007 | Table 1 voice-face emotion | 24 | 1 | 2 | 3 | Facial expression and intonated spoken words, judge emotion expressed (AV > A,V; P < 0.05 only) | |
| 65 | Kreifelts | 2007 | Table 5 AV increase effective connectivity | pooled | 2 | 4 | 6 | Increased effectiveness connectivity with pSTS and thalamus during AV integration of nonverbal emotional information | ||
| 42 | 67 | Matchin | 2014 | Table 1 AV > Aud only (McGurk) | 20 | 2 | 7 | 9 | McGurk audio-visual speech: AV > A only | |
| 68 | Matchin | 2014 | Table 2 AV > Video only | pooled | 9 | 6 | 15 | McGurk audio-visual speech: AV > V only | ||
| 45 | 73 | Muller | 2012 | Table S1 effective connectivity changes | 27 | 4 | 3 | 7 | Emotional facial expression (groaning, laughing) AV integration and gating of information | |
| 49 | 79 | Nath | 2012 | pg 784 | 14 | 1 | 0 | 1 | McGurk effect (phonemes): congruent AV speech correlated with behavioral percept | |
| 54 | 87 | Ogawa | 2013b | Table 1 3D > 2D and surround > monaural effects | 16 | 3 | 4 | 7 | Cinematic 3D > 2D video and surround sound > monaural while watching a movie (“The Three Musketeers”) | |
| 55 | 88 | Okada | 2013 | Table 1 AV > A | 20 | 5 | 4 | 9 | Video of AV > A speech only | |
| 56 | 89 | Olson | 2002 | Table 1A synchronized AV > static Vis-only | 10 | 7 | 4 | 11 | Whole face video and heard words: Synchronized AV versus static V | |
| 90 | Olson | 2002 | Table 1C synchronized AV > desynchronized AV speech | pooled | 2 | 0 | 2 | Whole face video and heard words: Synchronized versus desynchronized | ||
| 60 | 96 | Robins | 2008 | Table 4A (Fig. 5) AV integration and emotion | 5 | 1 | 4 | 5 | AV faces and spoken sentences expressing fear or neutral valence: AV integration (AV > A,V conditions) | |
| 97 | Robins | 2008 | Table 4B emotion effects | pooled | 2 | 0 | 2 | AV faces and spoken sentences expressing fear or neutral valence: Emotional AV-fear > AV-neutral | ||
| 98 | Robins | 2008 | Table 4C (Fig. 5) fearful AV integration | pooled | 1 | 5 | 6 | AV faces and spoken sentences expressing fear or neutral valence: Fearful-only AV integration | ||
| 99 | Robins | 2008 | Table 4D AV-only emotion | pooled | 1 | 3 | 4 | AV faces and spoken sentences expressing fear or neutral valence: AV-only emotion | ||
| 61 | 100 | Scheef | 2009 | Table 1 cartoon jump + boing | 16 | 1 | 2 | 3 | Video of cartoon person jumping and “sonification” of a tone, learn correlated pairings: AV-V and AV-A conjunction | |
| 63 | 104 | Sekiyama | 2003 | Table 3 (fMRI nAV-AV) | 8 | 1 | 0 | 1 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (fMRI) | |
| 105 | Sekiyama | 2003 | Table 4 (PET nAV-AV) | pooled | 1 | 3 | 4 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (PET) | ||
| 64 | 106 | Sestieri | 2006 | Table 1 (Fig. 3), AV location match versus semantic | 10 | 2 | 5 | 7 | B/W images (animal, weapons) and environmental sounds: Match location > recognition | |
| 65 | 108 | Stevenson | 2009 | Table 1B AVtools > AVspeech | 11 | 1 | 1 | 2 | Hand tools in use video: inverse effectiveness (degraded AV tool > AV speech) | |
| 109 | Stevenson | 2009 | Table 1C (Fig. 8) AVspeech > AVtools | pooled | 1 | 1 | 2 | Face and speech video: inverse effectiveness (degraded AV speech > AV tool use) | ||
| 66 | 110 | Straube | 2011 | Table 3A and B iconic/metaphoric speech-gestures versus speech, gesture | 16 | 2 | 2 | 4 | Integration of Iconic and Metaphoric speech-gestures versus speech and gesture | |
| 67 | 111 | Straube | 2014 | p939 Integration foci | 16 | 3 | 0 | 3 | Integration of iconic hand gesture-speech > unimodal speech and unimodal gesture (healthy control group) | |
| 75 | 124 | Von Kriegstein | 2006 | Figure 4B after > before voice-face | 14 | 0 | 4 | 4 | Face and object photos with voice and other sounds: Voice-Face association learning | |
| 78 | 128 | Watson | 2014a | Table 1A AV-adaptation effect (multimodal localizer) | 18 | 0 | 1 | 1 | Videos of emotional faces and voice: multisensory localizer | |
| 129 | Watson | 2014a | Table 1C AV-adaptation effect, cross-modal adaptation effect | pooled | 0 | 1 | 1 | Videos of emotional faces and voice: crossmodal adaptation effects | ||
| 79 | 132 | Watson | 2014b | Table 4B integrative regions (living and nonliving) | 40 | 0 | 1 | 1 | Moving objects and videos of faces with corresponding sounds: People-selective integrative region | |
| 80 | 133 | Werner | 2010 | Table 1 superadditive (AV-salience effect) | 21 | 0 | 3 | 3 | Categorize movies of actions with tools or musical instruments (degraded stimuli); AV interactions both tasks | |
| 134 | Werner | 2010 | Table 2 AV interactions predict behavior | pooled | 1 | 2 | 3 | Categorize movies of actions with tools or musical instruments; AV interactions predicted by behavior | ||
| 135 | Werner | 2010 | Table 3C superadditive AV due to task | pooled | 3 | 0 | 3 | Categorize movies of actions with tools or musical instruments; Subadditive AV to task | ||
| 82 | 137 | Wolf | 2014 | Table 1 face cartoons + phonemes | 16 | 1 | 1 | 2 | Drawing of faces with emotional expressions: Supramodal effects with emotional valence |
Table 7.
Locations of significant clusters from the meta-analyses involving Congruent and Incongruent audio-visual experimental paradigms (from Tables 5 and 6), indicating major contributing studies to the ALE meta-analysis clusters, weighted centers of mass (x, y, and z) in Talairach coordinates, brain volumes (mm3), and ALE values
| Condition | Region | Major contributing studies | x | y | z | Volume | ALE value |
|---|---|---|---|---|---|---|---|
| A. Living audio-visual stimuli single study | |||||||
| 1 | L Superior Temporal Sulcus, posterior (pSTS) | 8 (6,18,32,40,63,65–67) | −50 | −51 | 10 | 1448 | 0.042 |
| 2 | R Superior Temporal Sulcus | 8 (20,31,40,60,65,66,78,79) | 48 | −37 | 12 | 1280 | 0.035 |
| 3 | R superior Temporal Gyrus (pSTG) | 3 (40,42,45) | 55 | −19 | 7 | 256 | 0.025 |
| 4 | L Superior Temporal Gyrus | 2 (45,49) | −53 | −23 | 7 | 144 | 0.024 |
| B. Nonliving audio-visual stimuli single study | |||||||
| 1 | R Anterior Insula | 1 (44) | 31 | 19 | 6 | 32 | 0.019 |
| C. Living > Nonliving audio-visual stimuli | |||||||
| 1 | L Posterior Superior Temporal Sulcus | 2 (40,66) | −50 | −52 | 12 | 408 | 2.054 |
| 2 | R Posterior Superior Temporal Sulcus | 1 (66) | 51 | −35 | 12 | 48 | 1.779 |
| D. Nonliving > Living audio-visual stimuli | |||||||
| 1 | R Anterior Insula | 1 (44) | 31 | 19 | 6 | 32 | 3.195 |
Note: (A) Single study ALE maps for Living (corrected FWE P < 0.05) and (B) Nonliving audio-visual interaction sites (corrected FWE P < 0.05); plus contrast meta-analyses maps revealing (C) Living > Nonliving, and (D) Nonliving > Living audio-visual interaction sites (uncorrected P < 0.05). The coordinates correspond to foci illustrated in Figure 3C (orange and cyan).
Table 6.
Studies included in the Nonliving category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 25 | First author | Year | Experimental code and abbreviated task | 187 | 93 | 93 | 186 | Brief description of experimental paradigm | |
| 1 | 1 | Adams | 2002 | Expt 1 Table 3 A + V (aud coords only) | 12 | 5 | 1 | 6 | A and V commonly showing subordinate > basic object name verification (words with pictures or environmental sounds) | |
| 2 | 2 | Alink | 2008 | Table 1c spheres move to drum sounds | 10 | 4 | 6 | 10 | Visual spheres and drum sounds moving: crossmodal dynamic capture versus conflicting motion | |
| 4 | 4 | Baumann | 2007 | Table 1B coherent V + A versus A | 12 | 2 | 1 | 3 | Visual dots 16% coherent motion and in-phase acoustic noise > stationary acoustic sound | |
| 5 | Baumann | 2007 | Table 2B | pooled | 15 | 12 | 27 | Moving acoustic noise and visual dots 16% in-phase coherent > random dot motion | ||
| 12 | 14 | Bonath | 2013 | pg 116 congruent thalamus | 18 | 1 | 0 | 1 | Small checkerboards and tones: spatially congruent versus incongruent (thalamus) | |
| 13 | 16 | Bonath | 2014 | Table 1A illusory versus not | 20 | 1 | 5 | 6 | Small checkerboards and tones: temporal > spatial congruence | |
| 17 | Bonath | 2014 | Table 1B synchronous > no illusion | pooled | 3 | 0 | 3 | Small checkerboards and tones: spatial > temporal congruence | ||
| 14 | 18 | Bushara | 2001 | Table 1A (Fig. 2) AV-Control | 12 | 1 | 3 | 4 | Tones (100 ms) and colored circles synchrony: detect Auditory then Visual presentation versus Control | |
| 19 | Bushara | 2001 | Table 1B (VA-C) five coords | pooled | 2 | 3 | 5 | Tones (100 ms) and colored circles synchrony: detect Visual then Auditory presentation versus Control | ||
| 20 | Bushara | 2001 | Table 2A interact w/Rt Insula | pooled | 2 | 4 | 6 | Tones and colored circles: correlated functional connections with (and including) the right insula | ||
| 15 | 21 | Bushara | 2003 | Table 2A collide > pass, strong A-V interact | 7 | 5 | 3 | 8 | Tone and two visual bars moving: Tone synchrony induce perception they collide (AV interaction) versus pass by | |
| 19 | 28 | Calvert | 2001 | Table 2 superadditive and response depression | 10 | 4 | 11 | 15 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; supradditive and response depression | |
| 29 | Calvert | 2001 | Table 3A superadditive only | pooled | 6 | 4 | 10 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; supradditive only | ||
| 30 | Calvert | 2001 | Table 3B response depression only | pooled | 3 | 4 | 7 | B/W visual checkerboard reversing and white noise bursts: Synchronous versus not; response depression only | ||
| 33 | 56 | Hove | 2013 | pg 316 AV interaction putamen | 14 | 0 | 1 | 1 | Interaction between (beep > flash) versus (siren > moving bar); left putamen focus | |
| 41 | 66 | Lewis | 2000 | Table 1 | 7 | 2 | 3 | 5 | Compare speed of tone sweeps to visual dot coherent motion: Bimodal versus unimodal | |
| 44 | 71 | Meyer | 2007 | Table 3 paired A + V versus null | 16 | 3 | 3 | 6 | Paired screen red flashes with phone ring: paired V (conditioned stimulus) and A (unconditioned) versus null events | |
| 72 | Meyer | 2007 | Table 4 CS+, learned AV association with V-only | pooled | 4 | 6 | 10 | Paired screen flashes with phone ring: View flashes after postconditioned versus null events | ||
| 53 | 86 | Ogawa | 2013a | Table 1 (pg 162 data) | 13 | 1 | 0 | 1 | AV congruency of pure tone and white dots moving on screen (area left V3A) | |
| 69 | 113 | Tanabe | 2005 | Table 1A AV; A then V; not VA | 15 | 10 | 10 | 20 | Amorphous texture patterns and modulated white noises: Activation during learning delay period (AV) | |
| 114 | Tanabe | 2005 | Table 2A+2B (Fig. 5a) AV and VA | pooled | 5 | 6 | 11 | Amorphous texture patterns and modulated white noises: changes after feedback learning (AV and VA) | ||
| 115 | Tanabe | 2005 | Table 3A+3B (Fig. 6) AV and VA; delay period | pooled | 9 | 1 | 10 | Amorphous texture patterns and modulated white noises: sustained activity throughout learning (AV and VA) | ||
| 76 | 125 | Watkins | 2006 | Figure 4 illusory multisensory interaction | 11 | 0 | 2 | 2 | Two brief tone pips leads to illusion of two screen flashes (annulus with checkerboard) when only one flash present | |
| 126 | Watkins | 2006 | Table 1 (A enhances V in general) | pooled | 5 | 3 | 8 | Single brief tone pip leads to illusion of single screen flash (annulus with checkerboard) when two flashes present | ||
| 77 | 127 | Watkins | 2007 | Figure 3 2 flashes +1 beep illusion | 10 | 0 | 1 | 1 | Two visual flashes and single audio bleep leads to the illusion of a single flash |
A contrast ALE meta-analysis of maps living > nonliving events revealed bilateral pSTS foci as showing significant differential responsiveness (Fig. 3C, orange with outline [visible only in left hemisphere model]; Table 7C, uncorrected P < 0.05). The contrast meta-analysis of nonliving > living congruent audio-visual events revealed the right anterior insula as a common hub of activation (Fig. 2C, cyan with white outline; Table 7D, uncorrected P < 0.05). A main contributing study to this right anterior insula ROI (study #44 Meyer et al. 2007) included screen flashes paired with phone rings as part of a conditioned learning paradigm.
In visual perception literature, a prominent dichotomy of stimulus processing involves “what versus where” streams (Ungerleider et al. 1982; Goodale et al. 1994; Ungerleider and Haxby 1994), which has also been explored in the auditory system (Rauschecker 1998; Kaas and Hackett 1999; Rauschecker and Tian 2000; Clarke et al. 2002; Rauschecker and Scott 2015). A few of the audio-visual interaction studies examined in the present meta-analyses either explicitly or implicitly tested that organization (Sestieri et al. 2006; Plank et al. 2012). However, there were insufficient numbers of studies germane to that dichotomy for conducting a proper meta-analysis along this dimension.
Vocalization versus Action Event Audio-Visual Interaction Sites
Another stimulus category boundary derived from auditory categorical perception literature was that for processing vocalizations versus action sounds (Fig. 1). To be consistent with that neurobiological model, this category boundary was tested using only living audio-visual sources. This analysis included vocalizations by human or animal sources (Table 8) versus action events (Table 9) including sounds produced by, for example, hand tool use, bodily actions, and persons playing musical instruments. An ALE single study map for experiments using vocalizations revealed four ROIs along the pSTG/pSTS region (Fig. 3D, red hues; Table 11A, corrected FWE P < 0.05). The action event category was initially restricted to using only nonvocalizations (by living things) as auditory stimuli. This initially yielded nine studies that showed audio-visual interaction foci, and no clusters survived the single study ALE meta-analysis map voxel-wise thresholding. Adjusting the study restrictions to include studies that reported using a mix of action events together with some nonliving visual stimuli and some vocalizations as auditory (nonverbal) event stimuli yielded 13 studies (Table 9). A single study ALE map for these action events, which were predominantly nonvocal and depicting living things, revealed one ROI along the left fusiform gyrus (Table 11B, corrected FWE P < 0.05).
Table 8.
Studies included in the Vocalizations category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 | 57 | First author | Year | Experimental code and abbreviated task | 647 | 146 | 117 | 263 | Brief description of experimental paradigm | |
| 3 | 3 | Balk | 2010 | Figure 2 asynchronous versus simultaneous | 14 | 2 | 1 | 3 | Natural asynchronous versus simultaneous AV speech synchrony (included both contrasts as interaction effects) | |
| 5 | 6 | Baumgaertner | 2007 | Table 3 Action > nonact sentence+video | 19 | 3 | 0 | 3 | Conjunction spoken sentences (actions>nonactions) AND videos (actions > nonactions) | |
| 9 | 11 | Biau | 2016 | Table 1A Interaction; speech synchronous | 17 | 8 | 0 | 8 | Hand gesture beats versus cartoon disk and speech interaction: synchronous versus asynchronous | |
| 11 | 13 | Blank | 2013 | Figure 2 | 19 | 1 | 0 | 1 | Visual-speech recognition correlated with recognition performance | |
| 16 | 22 | Callan | 2014 | Table 5 AV-Audio (AV10-A10)-(AV6-A6) | 16 | 4 | 4 | 8 | Multisensory enhancement to visual speech in noise correlated with behavioral results | |
| 23 | Callan | 2014 | Table 6 AV—Visual only | pooled | 1 | 1 | 2 | Multisensory enhancement to visual speech audio-visual versus visual only | ||
| 17 | 24 | Calvert | 1999 | Table 1 (Fig. 1) | 5 | 3 | 4 | 7 | View image of lower face and hear numbers 1 through 10 versus unimodal conditions (AV > Photos, Auditory) | |
| 18 | 25 | Calvert | 2000 | Figure 2 superadd+subadd AVspeech | 10 | 1 | 0 | 1 | Speech and lower face: supra-additive plus subadditive effects (AV-congruent > A,V > AV-incongruent) | |
| 26 | Calvert | 2000 | Table 1. supradd AVspeech | pooled | 4 | 5 | 9 | Speech and lower face: supra-additive AV enhancement | ||
| 20 | 31 | Calvert | 2003 | Table 2A (Fig. 3 blue) | 8 | 13 | 8 | 21 | Speech and lower face: Moving dynamic speech (phonemes) versus stilled speech frames | |
| 22 | 34 | Erickson | 2014 | Table 1A Congruent AV speech | 10 | 2 | 2 | 4 | McGurk effect (phonemes): congruent AV speech: AV > A and AV > V | |
| 35 | Erickson | 2014 | Table 1B McGurk speech | pooled | 2 | 0 | 2 | McGurk speech effect (phonemes) | ||
| 23 | 36 | Ethofer | 2013 | Table 1C emotion | 23 | 1 | 2 | 3 | Audiovisual emotional face-voice integration | |
| 25 | 41 | Green | 2009 | Table 4A Congruent gesture-speech > gesture or speech | 16 | 1 | 0 | 1 | Congruent gesture-speech versus gesture with unfamiliar speech and with familiar speech | |
| 26 | 42 | Hagan | 2013 | Table 1 AV emotion, novel over time | 18 | 5 | 3 | 8 | Affective audio-visual speech: congruent AV emotion versus A, V; unique ROIs over time (MEG) | |
| 28 | 45 | Hashimoto | 2004 | Table 1G (Fig. 4B, red) Learning Hangul letters to sounds | 12 | 2 | 1 | 3 | Unfamiliar Hangul letters and nonsense words, learn speech versus tone/noise pairings | |
| 29 | 46 | He | 2015 | Table 3C AV speech foreign (left MTG focus) | 20 | 1 | 0 | 1 | Intrinsically meaningful gestures with German speech: Gesture-German > Gesture-Russian, German speech only | |
| 30 | 47 | He | 2018 | Table 2 gestures and speech integration | 20 | 1 | 0 | 1 | Gesture-speech integration: Bimodal speech-gesture versus unimodal gesture with foreign speech and versus unimodal speech | |
| 31 | 50 | Hein | 2007 | Figure 2C pSTS, pSTG, mSTG AV-cong | 18 | 0 | 3 | 3 | Familiar animal images and correct vocalizations (dog: woof-woof) | |
| 52 | Hein | 2007 | Figure 3B Foci 2, 3, 4 (blue) artificial/nonliving | pooled | 3 | 0 | 3 | Visual “Fribbles” and backward/underwater distorted animal sounds, learn pairings (blue foci 2,3,4) | ||
| 53 | Hein | 2007 | Figure 3C congruent living (green) | pooled | 3 | 0 | 3 | Familiar congruent living versus artificial AV object features and animal sounds (green foci 7, 8, 10) | ||
| 36 | 59 | Jessen | 2015 | Table 1A emotion > neutral AV enhanced | 17 | 1 | 1 | 2 | Emotional multisensory whole body and voice expressions: AV emotion (anger and fear) > neutral expressions | |
| 60 | Jessen | 2015 | Table 1D fear > neutral AV enhanced | pooled | 2 | 1 | 3 | Emotional multisensory whole body and voice expressions: AV fear > neutral expressions | ||
| 38 | 62 | Kim | 2015 | Table 2A AV > C speech semantic match | 15 | 2 | 0 | 2 | Moving audio-visual speech perception versus white noise and unopened mouth movements (AV > C) | |
| 39 | 63 | Kircher | 2009 | Figure 3B: gesture related activation increase | 14 | 3 | 1 | 4 | Bimodal gesture-speech versus gesture and versus speech | |
| 40 | 64 | Kreifelts | 2007 | Table 1 voice-face emotion | 24 | 1 | 2 | 3 | Facial expression and intonated spoken words, judge emotion expressed (AV > A,V; P < 0.05 only) | |
| 65 | Kreifelts | 2007 | Table 5 AV increase effective connectivity | pooled | 2 | 4 | 6 | Increased effectiveness connectivity with pSTS and thalamus during AV integration of nonverbal emotional information | ||
| 42 | 67 | Matchin | 2014 | Table 1 AV > Aud only (McGurk) | 20 | 2 | 7 | 9 | McGurk audio-visual speech: AV > A only | |
| 68 | Matchin | 2014 | Table 2 AV > Video only | pooled | 9 | 6 | 15 | McGurk audio-visual speech: AV > V only | ||
| 45 | 73 | Muller | 2012 | Supplementary Table 1 effective connectivity changes | 27 | 4 | 3 | 7 | Emotional facial expression (groaning, laughing) AV integration and gating of information | |
| 49 | 79 | Nath | 2012 | pg 784 | 14 | 1 | 0 | 1 | McGurk effect (phonemes): congruent AV speech correlated with behavioral percept | |
| 50 | 80 | Naumer | 2008 | Figure 2 Table 1A max contrast | 18 | 8 | 6 | 14 | Images of “Fribbles” and learned artificial sounds (underwater animal vocals): post training versus max contrast | |
| 81 | Naumer | 2008 | Figure 3 Table 1B pre–post | pooled | 5 | 6 | 11 | Images of “Fribbles” and learned corresponding artificial sounds: Post- versus pretraining session | ||
| 55 | 88 | Okada | 2013 | Table 1 AV > A | 20 | 5 | 4 | 9 | Video of AV > A speech only | |
| 56 | 89 | Olson | 2002 | Table 1A synchronized AV > static Vis-only | 10 | 7 | 4 | 11 | Whole face video and heard words: Synchronized AV versus static V | |
| 90 | Olson | 2002 | Table 1C synchronized AV > desynchronized AV speech | pooled | 2 | 0 | 2 | Whole face video and heard words: Synchronized versus desynchronized | ||
| 58 | 93 | Raij | 2000 | Table 1B letters and speech sounds | 9 | 2 | 3 | 5 | Integration of visual letters and corresponding auditory phonetic expressions (MEG study) AV versus (A + V) | |
| 60 | 95 | Robins | 2008 | Table 2 (Fig. 2) AV integration (AV > A and AV > V) | 10 | 2 | 1 | 3 | Face speaking sentences: angry, fearful, happy, neutral (AV > A,V) | |
| 96 | Robins | 2008 | Table 4A (Fig. 5) AV integration and emotion | 5 | 1 | 4 | 5 | AV faces and spoken sentences expressing fear or neutral valence: AV integration (AV > A,V conditions) | ||
| 97 | Robins | 2008 | Table 4B emotion effects | pooled | 2 | 0 | 2 | AV faces and spoken sentences expressing fear or neutral valence: Emotional AV-fear > AV-neutral | ||
| 98 | Robins | 2008 | Table 4C (Fig. 5) fearful AV integration | pooled | 1 | 5 | 6 | AV faces and spoken sentences expressing fear or neutral valence: Fearful-only AV integration | ||
| 99 | Robins | 2008 | Table 4D AV-only emotion | pooled | 1 | 3 | 4 | AV faces and spoken sentences expressing fear or neutral valence: AV-only emotion | ||
| 63 | 104 | Sekiyama | 2003 | Table 3 (fMRI nAV-AV) | 8 | 1 | 0 | 1 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (fMRI) | |
| 105 | Sekiyama | 2003 | Table 4 (PET nAV-AV) | pooled | 1 | 3 | 4 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (PET) | ||
| 65 | 109 | Stevenson | 2009 | Table 1C (Fig. 8) AVspeech > AVtools | 11 | 1 | 1 | 2 | Face and speech video: inverse effectiveness (degraded AV speech > AV tool use) | |
| 66 | 110 | Straube | 2011 | Table 3A and B iconic/metaphoric speech-gestures versus speech, gesture | 16 | 2 | 2 | 4 | Integration of Iconic and Metaphoric speech-gestures versus speech and gesture | |
| 67 | 111 | Straube | 2014 | p939 Integration foci | 16 | 3 | 0 | 3 | Integration of iconic hand gesture-speech > unimodal speech and unimodal gesture (healthy control group) | |
| 71 | 118 | Van Atteveldt | 2004 | Table 1a letters and speech sounds | 16 | 3 | 1 | 4 | Familiar letters and their speech sounds: Congruent versus not and Bimodal versus Unimodal | |
| 72 | 119 | Van Atteveldt | 2007 | Table 2A+B (Fig. 2) | 12 | 3 | 2 | 5 | Single letters and their speech sounds (phonemes): Congruent > Incong; Passive perception, blocked and event-related design | |
| 120 | Van Atteveldt | 2007 | Table 3 (Fig. 2) passive | pooled | 1 | 1 | 2 | Single letters and their speech sounds (phonemes): Congruent > Incong, active perception task | ||
| 73 | 122 | Van Atteveldt | 2010 | Table 1B STS; specific adaptation congruent > incong | 16 | 3 | 1 | 4 | Letter and speech sound pairs (vowels, consonants): Specific adaptation effects | |
| 74 | 123 | Van der Wyk | 2010 | Table 2 AV interaction effects oval/circles +speech/nonspeech | 16 | 3 | 3 | 6 | Geometric shape modulate with speech (sentences) | |
| 75 | 124 | Von Kriegstein | 2006 | Figure 4B after > before voice-face | 14 | 0 | 4 | 4 | Face and object photos with voice and other sounds: Voice-Face association learning | |
| 78 | 128 | Watson | 2014a | Table 1A AV-adaptation effect (multimodal localizer) | 18 | 0 | 1 | 1 | Videos of emotional faces and voice: multisensory localizer | |
| 129 | Watson | 2014a | Table 1C AV-adaptation effect, cross-modal adaptation effect | pooled | 0 | 1 | 1 | Videos of emotional faces and voice: crossmodal adaptation effects | ||
| 79 | 132 | Watson | 2014b | Table 4B integrative regions (Living and nonliving) | 40 | 0 | 1 | 1 | Moving objects and videos of faces with corresponding sounds: People-selective integrative region | |
| 82 | 137 | Wolf | 2014 | Table 1 face cartoons + phonemes | 16 | 1 | 1 | 2 | Drawing of faces with emotional expressions: Supramodal effects with emotional valence |
Table 11.
Locations of significant clusters from the meta-analyses involving Vocalizations and Nonvocal audio-visual experimental paradigms, indicating major contributing studies to the ALE meta-analysis clusters, weighted centers of mass (x, y, and z) in Talairach coordinates, brain volumes (mm3), and ALE values
| Condition | Region | Major contributing studies | x | y | z | Volume | ALE value |
|---|---|---|---|---|---|---|---|
| A. Vocal audio-visual stimuli single study | |||||||
| 1 | R Superior Temporal Sulcus | 19 (20,23,26,31,40,42,45,50,55, 58,60,60,65,66,71,72,74,78,79) | 50 | −32 | 11 | 3040 | 0.041 |
| 2 | L Superior Temporal Sulcus (posterior), BA 22 | 9 (18,22,40,63,66,67,71,73,74) | −54 | −47 | 11 | 1328 | 0.034 |
| 3 | L Superior Temporal Sulcus, BA 41 | 7 (3,22,31,42,45,50,72) | −49 | −21 | 7 | 1200 | 0.035 |
| 4 | L Superior Temporal Gyrus (posterior), BA 41 | 4 (45,50,60,65) | −47 | −37 | 11 | 376 | 0.030 |
| B. Nonvocal (living) audio-visual stimuli single study | |||||||
| 1 | L Fusiform Gyrus (inferior-medial) | 1 (62) | −28 | −54 | −14 | 8 | 0.017 |
| C. Vocal > Nonvocal audio-visual stimuli | |||||||
| R Posterior Superior Temporal Sulcus | 7 (31,40,60,65,66,78,79) | 46 | −37 | 13 | 976 | 2.530 | |
| R Posterior Superior Temporal Gyrus | 54 | −26 | 8 | 8 | 1.672 | ||
| D. Nonvocal > Vocal audio-visual stimuli | |||||||
| 1 | L Fusiform Gyrus (inferior-medial) | 1 (62) | −28 | −54 | −14 | 8 | 2.400 |
| E. Emotionally valent (mostly vocal) > Nonemotional stimuli | |||||||
| R Posterior Superior Temporal Gyrus | 3 (26,37,45) | 58 | −21 | 8 | 152 | 2.391 | |
Note: (A) Single study ALE maps for Vocalizations (corrected FWE P < 0.05) and (B) Action stimuli (corrected FWE P < 0.05), plus (C) contrast maps revealing interaction sites involving Vocalization > Action and (D) Action > Vocalization auditory stimuli (both uncorrected P < 0.05). A subset of the Vocal/Living audio-visual stimuli also entailed (E) emotionally valent audio-visual stimuli, which was conducted as a single study ALE map (corrected FWE P < 0.05). TTG = transverse temporal gyrus (aka HG = Heschl’s gyrus). The coordinates correspond to foci illustrated in Figure 3D (red, yellow, and violet hues) (from Tables 8–10).
The contrast meta-analysis of vocalizations > actions revealed right pSTS and pSTG foci as being preferential for vocalizations (Fig. 3D, red with black outlines; Table 11C, uncorrected P < 0.05). Conversely, the contrast meta-analysis of action > vocalization audio-visual interactions revealed the left fusiform gyrus ROIs (Fig. 3D, yellow with black outline; Table 11D, uncorrected P < 0.05). This left fusiform ROI had a volume of 8 mm3, both in the single study and contrast ALE meta-analysis maps. This cluster size fell below some criteria for rigor depending on theoretical interpretation when group differences are diffuse (Tench et al. 2014). Nonetheless, this theoretical processing dissociation existed in at least some single studies, in the single ALE map, and in the contrast ALE map, and was thus at least suggestive of a double-dissociation. A main contributing study to this fusiform ROI (study #62, Schmid et al. 2011) employed a relatively simple task of determining if a colored picture included a match to a presented sound, or vice versa, which involved a wide variety of nonliving but a few living real-world object images. This ROI was consistent in location with the commonly reported fusiform foci involved in functions pertaining to high-level visual object processing (Gauthier et al. 1999; Bar et al. 2001; James and Gauthier 2003).
A subset of the paradigms involving living things and/or vocalizations included emotionally valent stimuli (Table 10). This predominantly including emotional faces with voice (expressing fear, anger, sadness, happiness, and laughter), but also whole body and dance expressions rated for emotional content. These emotionally valent paradigms preferentially activated a portion of the right pSTG (Fig. 3D, violet hues; Table 11E), when analyzed as a single study ALE map (corrected FWE P < 0.05), but also as a contrast meta-analysis with nonemotionally valent paradigms involving living things (mostly control conditions from the same or similar paradigms; data not shown).
Dynamic Visual Motion versus Static Images in Audio-Visual Interactions
We next sought to determine if the use of dynamic visual motion versus static visual images in audio-visual interaction paradigms might reveal differences in processing organizations in the brain. Studies using dynamic-visual stimuli (Table 12), included talking faces, the McGurk effect, hand gestures, bodily gestures, and geometric shapes modulating synchronously with vocals, plus nonvocal drum sounds, musical instruments (e.g., piano), hand tool sounds, tone sweeps, and synthetic tones. Studies using static-visual images (Table 13) involved the matching of pictures of human faces or animals to characteristically associated vocal sounds, plus other forms of photos or drawings (in color, grayscale, or black and white) of faces, animals, objects, or written word/character forms, while excluding stimuli such as flashing screens or light emitting diodes (LEDs). ALE single study maps for experiments utilizing dynamic-visual stimuli (Fig. 3E, blue hues; Table 14A, corrected FWE P < 0.05) and static-visual stimuli (pink hues; Table 14B, corrected FWE P < 0.05) were constructed. A contrast ALE meta-analysis of dynamic-visual > static-visual revealed significantly greater activation of the right pSTS region (Fig. 3E, blue with black outline; Table 14C, uncorrected P < 0.05). Conversely, the contrast ALE meta-analysis of static-visual > dynamic-visual paradigms preferentially activated the bilateral planum temporale and STG regions (Fig. 3E, pink with black outlines; Table 14D, uncorrected P < 0.05).
Table 12.
Studies included in the Dynamic-visual stimuli category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 43 | 62 | First author | Year | Experimental code and abbreviated task | 682 | 177 | 148 | 325 | Brief description of experimental paradigm | |
| 2 | 2 | Alink | 2008 | Table 1c spheres move to drum sounds | 10 | 4 | 6 | 10 | Visual spheres and drum sounds moving: crossmodal dynamic capture versus conflicting motion | |
| 3 | 3 | Balk | 2010 | Figure 2 asynchronous versus simultaneous | 14 | 2 | 1 | 3 | Natural asynchronous versus simultaneous AV speech synchrony (included both contrasts as interaction effects) | |
| 4 | 4 | Baumann | 2007 | Table 1B coherent V + A versus A | 12 | 2 | 1 | 3 | Visual dots 16% coherent motion and in-phase acoustic noise > stationary acoustic sound | |
| 5 | Baumann | 2007 | Table 2B | pooled | 15 | 12 | 27 | Moving acoustic noise and visual dots 16% in-phase coherent > random dot motion | ||
| 5 | 6 | Baumgaertner | 2007 | Table 3 Action > nonact sentence+video | 19 | 3 | 0 | 3 | Conjunction spoken sentences (actions > nonactions) AND videos (actions > nonactions) | |
| 7 | 8 | Beauchamp | 2004b | Expt 1 coordinates | 8 | 1 | 1 | 2 | High-resolution version of 2004a study: AV tool videos versus unimodal (AV > A,V) | |
| 9 | 11 | Biau | 2016 | Table 1A Interaction; speech synchronous | 17 | 8 | 0 | 8 | Hand gesture beats versus cartoon disk and speech interaction: synchronous versus asynchronous | |
| 11 | 13 | Blank | 2013 | Figure 2 | 19 | 1 | 0 | 1 | Visual-speech recognition correlated with recognition performance | |
| 15 | 21 | Bushara | 2003 | Table 2A collide > pass, strong A-V interact | 7 | 5 | 3 | 8 | Tone and two visual bars moving: Tone synchrony induce perception they collide (AV interaction) versus pass by | |
| 16 | 22 | Callan | 2014 | Table 5 AV-Audio (AV10-A10)-(AV6-A6) | 16 | 4 | 4 | 8 | Multisensory enhancement to visual speech in noise correlated with behavioral results | |
| 23 | Callan | 2014 | Table 6 AV—Visual only | pooled | 1 | 1 | 2 | Multisensory enhancement to visual speech audio-visual versus visual only | ||
| 18 | 25 | Calvert | 2000 | Figure 2 superadd+subadd AVspeech | 10 | 1 | 0 | 1 | Speech and lower face: supra-additive plus subadditive effects (AV-congruent > A,V > AV-incongruent) | |
| 26 | Calvert | 2000 | Table 1. supradd AVspeech | pooled | 4 | 5 | 9 | Speech and lower face: supra-additive AV enhancement | ||
| 20 | 31 | Calvert | 2003 | Table 2A (Fig. 3 blue) | 8 | 13 | 8 | 21 | Speech and lower face: Moving dynamic speech (phonemes) versus stilled speech frames | |
| 21 | 32 | DeHaas | 2013 | Table 1A AVcong—Visual | 15 | 3 | 3 | 6 | Video clips of natural scenes (animals, humans): AV congruent versus Visual | |
| 22 | 34 | Erickson | 2014 | Table 1A Congruent AV speech | 10 | 2 | 2 | 4 | McGurk effect (phonemes): congruent AV speech: AV > A and AV > V | |
| Erickson | 2014 | Table 1B McGurk speech | pooled | 2 | 0 | 2 | McGurk speech effect (phonemes) | |||
| 23 | 36 | Ethofer | 2013 | Table 1C emotion | 23 | 1 | 2 | 3 | Audiovisual emotional face-voice integration | |
| 25 | 41 | Green | 2009 | Table 4A Congruent gesture-speech > gesture or speech | 16 | 1 | 0 | 1 | Congruent gesture-speech versus gesture with unfamiliar speech and with familiar speech | |
| 26 | 42 | Hagan | 2013 | Table 1 AV emotion, novel over time | 18 | 5 | 3 | 8 | Affective audio-visual speech: congruent AV emotion versus A, V; unique ROIs over time (MEG) | |
| 27 | 44 | Hasegawa | 2004 | Table 1A (well trained piano) AV induced by V-only | 26 | 12 | 6 | 18 | Piano playing: well trained pianists, mapping hand movements to sequences of sound | |
| 29 | 46 | He | 2015 | Table 3C AV speech foreign (left MTG focus) | 20 | 1 | 0 | 1 | Intrinsically meaningful gestures with German speech: Gesture-German > Gesture-Russian, German speech only | |
| 30 | 47 | He | 2018 | Table 2, GSI, left MTG, gestures and speech integration | 20 | 1 | 0 | 1 | Gesture-speech integration: Bimodal speech-gesture versus unimodal gesture with foreign speech and versus unimodal speech | |
| 35 | 58 | James | 2011 | Table 1A bimodal (vs scrambled) | 12 | 4 | 2 | 6 | Video of human manual actions (e.g., sawing): Auditory and Visual intact versus scrambled, AV event selectivity | |
| 36 | 59 | Jessen | 2015 | Table 1A emotion > neutral AV enhanced | 17 | 1 | 1 | 2 | Emotional multisensory whole body and voice expressions: AV emotion (anger and fear) > neutral expressions | |
| 60 | Jessen | 2015 | Table 1D fear > neutral AV enhanced | pooled | 2 | 1 | 3 | Emotional multisensory whole body and voice expressions: AV fear > neutral expressions | ||
| 37 | 61 | Jola | 2013 | Table 1C AVcondition dance | 12 | 3 | 3 | 6 | Viewing unfamiliar dance performance (tells a story by gesture) with versus without music: using intersubject correlation | |
| 38 | 62 | Kim | 2015 | Table 2A AV > C speech semantic match | 15 | 2 | 0 | 2 | Moving audio-visual speech perception versus white noise and unopened mouth movements | |
| 39 | 63 | Kircher | 2009 | Figure 3B: gesture-related activation increase | 14 | 3 | 1 | 4 | Bimodal gesture-speech versus gesture and versus speech | |
| 40 | 64 | Kreifelts | 2007 | Table 1 voice-face emotion | 24 | 1 | 2 | 3 | Facial expression and intonated spoken words, judge emotion expressed (AV > A,V; P < 0.05 only) | |
| 65 | Kreifelts | 2007 | Table 5 AV increase effective connectivity | pooled | 2 | 4 | 6 | Increased effectiveness connectivity with pSTS and thalamus during AV integration of nonverbal emotional information | ||
| 41 | 66 | Lewis | 2000 | Table 1 | 7 | 2 | 3 | 5 | Compare speed of tone sweeps to visual dot coherent motion: Bimodal versus unimodal | |
| 42 | 67 | Matchin | 2014 | Table 1 AV > Aud only (McGurk) | 20 | 2 | 7 | 9 | McGurk audio-visual speech: AV > A only | |
| 68 | Matchin | 2014 | Table 2 AV > Video only | pooled | 9 | 6 | 15 | McGurk audio-visual speech: AV > V only | ||
| 43 | 70 | McNamara | 2008 | Table (BA44 and IPL) | 12 | 2 | 2 | 4 | Videos of meaningless hand gestures and synthetic tone sounds: Increases in functional connectivity with learning | |
| 49 | 79 | Nath | 2012 | pg 784 | 14 | 1 | 0 | 1 | McGurk effect (phonemes): congruent AV speech correlated with behavioral percept | |
| 54 | 87 | Ogawa | 2013b | Table 1 3D > 2D and surround > monaural effects | 16 | 3 | 4 | 7 | Cinematic 3D > 2D video and surround sound > monaural while watching a movie (“The Three Musketeers”) | |
| 55 | 88 | Okada | 2013 | Table 1 AV > A | 20 | 5 | 4 | 9 | Video of AV > A speech only | |
| 56 | 89 | Olson | 2002 | Table 1A synchronized AV > static Vis-only | 10 | 7 | 4 | 11 | Whole face video and heard words: Synchronized AV versus static V | |
| 90 | Olson | 2002 | Table 1C synchronized AV > desynchronized AV speech | pooled | 2 | 0 | 2 | Whole face video and heard words: Synchronized versus desynchronized | ||
| 59 | 94 | Regenbogen | 2017 | Table 2A degraded > clear Multisensory versus unimodal input | 29 | 5 | 6 | 11 | Degraded > clear AV versus both visual and auditory unimodal visual real-world object-in-action recognition | |
| 60 | 95 | Robins | 2008 | Table 2 (Fig. 2) AV integration (AV > A and AV > V) | 10 | 2 | 1 | 3 | Face speaking sentences: angry, fearful, happy, neutral (AV > A,V) | |
| 96 | Robins | 2008 | Table 4A (Fig. 5) AV integration and emotion | pooled | 1 | 4 | 5 | AV faces and spoken sentences expressing fear or neutral valence: AV integration (AV > A,V conditions) | ||
| 97 | Robins | 2008 | Table 4B emotion effects | pooled | 2 | 0 | 2 | AV faces and spoken sentences expressing fear or neutral valence: Emotional AV-fear > AV-neutral | ||
| 98 | Robins | 2008 | Table 4C (Fig. 5) fearful AV integration | pooled | 1 | 5 | 6 | AV faces and spoken sentences expressing fear or neutral valence: Fearful-only AV integration | ||
| 99 | Robins | 2008 | Table 4D AV-only emotion | pooled | 1 | 3 | 4 | AV faces and spoken sentences expressing fear or neutral valence: AV-only emotion | ||
| 61 | 100 | Scheef | 2009 | Table 1 cartoon jump + boing | 16 | 1 | 2 | 3 | Video of cartoon person jumping and “sonification” of a tone, learn correlated pairings: AV-V and AV-A conjunction | |
| 63 | 104 | Sekiyama | 2003 | Table 3 (fMRI nAV-AV) | 8 | 1 | 0 | 1 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (fMRI) | |
| 105 | Sekiyama | 2003 | Table 4 (PET nAV-AV) | pooled | 1 | 3 | 4 | AV speech, McGurk effect with phonemes (ba, da, ga) and noise modulation: noise-AV > AV (PET) | ||
| 65 | 108 | Stevenson | 2009 | Table 1B 2 AVtools > AVspeech | 11 | 1 | 1 | 2 | Hand tools in use video: inverse effectiveness (degraded AV tool > AV speech) | |
| 109 | Stevenson | 2009 | Table 1C (Fig. 8) AVspeech > AVtools | pooled | 1 | 1 | 2 | Face and speech video: inverse effectiveness (degraded AV speech > AV tool use) | ||
| 66 | 110 | Straube | 2011 | Table 3A and B iconic/metaphoric speech-gestures versus speech, gesture | 16 | 2 | 2 | 4 | Integration of Iconic and Metaphoric speech-gestures versus speech and gesture | |
| 67 | 111 | Straube | 2014 | p939 Integration foci | 16 | 3 | 0 | 3 | Integration of iconic hand gesture-speech > unimodal speech and unimodal gesture (healthy control group) | |
| 74 | 123 | Van der Wyk | 2010 | Table 2 AV interaction effects oval/circles+speech/nonspeech | 16 | 3 | 3 | 6 | Geometric shape modulate with speech (sentences) | |
| 78 | 128 | Watson | 2014a | Table 1A AV-adaptation effect (multimodal localizer) | 18 | 0 | 1 | 1 | Videos of emotional faces and voice: multisensory localizer | |
| 129 | Watson | 2014a | Table 1C AV-adaptation effect, cross-modal adaptation effect | pooled | 0 | 1 | 1 | Videos of emotional faces and voice: crossmodal adaptation effects | ||
| 79 | 130 | Watson | 2014b | Table 1 AV > baseline (Living and nonliving) | 40 | 3 | 5 | 8 | Moving objects and videos of faces with corresponding sounds: AV > baseline | |
| 131 | Watson | 2014b | Table 4A integrative regions (Living and nonliving) | pooled | 2 | 2 | 4 | Moving objects and videos of faces with corresponding sounds: Integrative regions (AV > A,V) | ||
| 132 | Watson | 2014b | Table 4B integrative regions (Living and nonliving) | pooled | 0 | 1 | 1 | Moving objects and videos of faces with corresponding sounds: People-selective integrative region | ||
| 80 | 133 | Werner | 2010 | Table 1 superadditive (AV-salience effect) | 21 | 0 | 3 | 3 | Categorize movies of actions with tools or musical instruments (degraded stimuli); AV interactions both tasks | |
| 134 | Werner | 2010 | Table 2 AV interactions predict behavior | pooled | 1 | 2 | 3 | Categorize movies of actions with tools or musical instruments; AV interactions predicted by behavior | ||
| 135 | Werner | 2010 | Table 3C superadditive AV due to task | pooled | 3 | 0 | 3 | Categorize movies of actions with tools or musical instruments; Subadditive AV to task |
Table 13.
Studies included in the Static-visual stimuli category for audio-visual interaction site meta-analyses
| Study # | Experiment # | # Subjects | Multiple experiments | Left hem foci | Right hem foci | Number of foci | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 39 | First author | Year | Experimental code and abbreviated task | 405 | 106 | 89 | 195 | Brief description of experimental paradigm | |
| 1 | 1 | Adams | 2002 | Expt 1 Table 3 A + V (aud coords only) | 12 | 5 | 1 | 6 | A and V commonly showing subordinate > basic object name verification (words with pictures or environmental sounds) | |
| 6 | 7 | Beauchamp | 2004a | Figure 3J and K, Table 1 first 2 foci only | 26 | 2 | 0 | 2 | See photographs of tools, animals and hear corresponding sounds versus scrambled images and synthesized rippled sounds | |
| 8 | 9 | Belardinelli | 2004 | Table 1 AV semantic congruence | 13 | 6 | 6 | 12 | Colored images of tools, animals, humans and semantically congruent versus incongruent sounds | |
| 17 | 24 | Calvert | 1999 | Table 1 (Fig. 1) | 5 | 3 | 4 | 7 | View image of lower face and hear numbers 1 through 10 versus unimodal conditions (AV > Photos, Auditory) | |
| 24 | 37 | Gonzalo | 2000 | Table 1 AV > AVincon music and Chinese ideograms | 14 | 1 | 1 | 2 | Learn novel Kanji characters and musical chords, activity increases over time for consistent AV pairings | |
| 39 | Gonzalo | 2000 | Table 3 AV consistent versus Aud | pooled | 1 | 1 | 2 | Learn novel Kanji characters and musical chords, learn consistent (vs inconsistent) pairings versus auditory only | ||
| 28 | 45 | Hashimoto | 2004 | Table 1G (Fig. 4B, red) Learning Hangul letters to sounds | 12 | 2 | 1 | 3 | Unfamiliar Hangul letters and nonsense words, learn speech versus tone/noise pairings | |
| 31 | 49 | Hein | 2007 | Figure 2B AV-artificial/nonliving | 18 | 0 | 1 | 1 | B/W images of artificial objects (“fribbles”) and animal vocalizations versus unimodal A, V | |
| 50 | Hein | 2007 | Figure 2C pSTS, pSTG, mSTG AV-cong | pooled | 0 | 3 | 3 | Familiar animal images and correct vocalizations (dog: woof-woof) | ||
| 52 | Hein | 2007 | Figure 3B Foci 2, 3, 4 (blue) artificial/nonliving | pooled | 3 | 0 | 3 | Visual “Fribbles” and backward/underwater distorted animal sounds, learn pairings (blue foci 2,3,4) | ||
| 53 | Hein | 2007 | Figure 3C congruent living (green) | pooled | 3 | 0 | 3 | Familiar congruent living versus artificial AV object features and animal sounds (green foci 7, 8, 10) | ||
| 32 | 54 | Hocking | 2008 | pg 2444 verbal | 18 | 2 | 0 | 2 | (pSTS mask) Color photos, written names, auditory names, environmental sounds conceptually matched “amodal” | |
| 34 | 57 | James | 2003 | Figure 2 | 12 | 0 | 1 | 1 | Activation by visual objects (“Greebles”) associated with auditory features (e.g., buzzes, screeches); (STG) | |
| 45 | 73 | Muller | 2012 | Table S1 effective connectivity changes | 27 | 4 | 3 | 7 | Emotional facial expression (groaning, laughing) AV integration and gating of information | |
| 47 | 75 | Naghavi | 2007 | Figure 1C | 23 | 0 | 3 | 3 | B/W pictures (animals, tools, instruments, vehicles) and their sounds: Cong versus Incong | |
| 48 | 76 | Naghavi | 2011 | Figure 2A cong = incon | 30 | 1 | 0 | 1 | B/W drawings of objects (living and non) and natural sounds (barking, piano): congruent = incongruent encoding | |
| 77 | Naghavi | 2011 | Figure 2B congruent > incongruent | pooled | 0 | 1 | 1 | B/W drawings of objects (living and non) and natural sounds (barking, piano): congruent > incongruent encoding | ||
| 50 | 80 | Naumer | 2008 | Figure 2 Table 1A max contrast | 18 | 8 | 6 | 14 | Images of “Fribbles” and learned artificial sounds (underwater animal vocals): post training versus max contrast | |
| 81 | Naumer | 2008 | Figure 3 Table 1B pre–post | pooled | 5 | 6 | 11 | Images of “Fribbles” and learned corresponding artificial sounds: Post- versus Pretraining session | ||
| 51 | 83 | Naumer | 2011 | Figure 3C | 10 | 1 | 0 | 1 | Photographs of objects (living and non) and related natural sounds | |
| 52 | 85 | Noppeny | 2008 | Table 3 AV congruent sounds/words | 17 | 4 | 0 | 4 | Speech sound recognition through AV priming, environmental sounds and spoken words: Congruent > incongruent | |
| 57 | 91 | Plank | 2012 | pg 803 AV congruent effect | 15 | 0 | 1 | 1 | AV spatially congruent > semantically matching images of natural objects and associated sounds (right STG) | |
| 92 | Plank | 2012 | Table 2A spatially congruent-baseline | pooled | 5 | 5 | 10 | Images of natural objects and associated sounds, spatially congruent versus baseline | ||
| 58 | 93 | Raij | 2000 | Table 1B letters and speech sounds | 9 | 2 | 3 | 5 | Integration of visual letters and corresponding auditory phonetic expressions (MEG study) AV versus (A + V) | |
| 62 | 101 | Schmid | 2011 | Table 2E A effect V (Living and nonliving, pictures) | 12 | 3 | 4 | 7 | Environmental sounds and matching pictures: reduced activity by A | |
| 102 | Schmid | 2011 | Table 2F V competition effect A (reduced activity by a visual object) | pooled | 2 | 2 | 4 | Environmental sounds and matching pictures: reduced activity by V | ||
| 103 | Schmid | 2011 | Table 2G AV crossmodal interaction × auditory attention | pooled | 2 | 3 | 5 | Environmental sounds and matching pictures: cross-modal interaction and auditory attention | ||
| 64 | 106 | Sestieri | 2006 | Table 1 (Fig. 3), AV location match versus semantic | 10 | 2 | 5 | 7 | B/W images (animal, weapons) and environmental sounds: Match location > recognition | |
| 107 | Sestieri | 2006 | Table 2 AV semantic recognition versus localization | pooled | 2 | 1 | 3 | B/W pictures and environmental sounds: congruent semantic recognition > localization task | ||
| 69 | 113 | Tanabe | 2005 | Table 1A AV; A then V; not VA | 15 | 10 | 10 | 20 | Amorphous texture patterns and modulated white noises: Activation during learning delay period (AV) | |
| 114 | Tanabe | 2005 | Table 2A+2B (Fig. 5a) AV and VA | pooled | 5 | 6 | 11 | Amorphous texture patterns and modulated white noises: changes after feedback learning (AV and VA) | ||
| 115 | Tanabe | 2005 | Table 3A+3B (Fig. 6) AV and VA; delay period | pooled | 9 | 1 | 10 | Amorphous texture patterns and modulated white noises: sustained activity throughout learning (AV and VA) | ||
| 70 | 117 | Taylor | 2006 | Figure 1A and B, Figure 1C and D (living > nonliving) | 15 | 2 | 0 | 2 | Color photos (V), environmental sounds and spoken words (A): Cong AV versus Incong (living objects) | |
| 71 | 118 | Van Atteveldt | 2004 | Table 1a letters and speech sounds | 16 | 3 | 1 | 4 | Familiar letters and their speech sounds: Congruent versus not and Bimodal versus Unimodal | |
| 72 | 119 | Van Atteveldt | 2007 | Table 2A+B (Fig. 2) | 12 | 3 | 2 | 5 | Single letters and their speech sounds (phonemes): Congruent > Incong; Passive perception, blocked and event-related design | |
| 120 | Van Atteveldt | 2007 | Table 3 (Fig. 2) passive | pooled | 1 | 1 | 2 | Single letters and their speech sounds (phonemes): Congruent > Incongruent, active perception task | ||
| 73 | 122 | Van Atteveldt | 2010 | Table 1B STS; specific adaptation congruent > incong | 16 | 3 | 1 | 4 | Letter and speech sound pairs (vowels, consonants): Specific adaptation effects | |
| 75 | 124 | Von Kriegstein | 2006 | Figure 4B after > before voice-face | 14 | 0 | 4 | 4 | Face and object photos with voice and other sounds: Voice-Face association learning | |
| 82 | 137 | Wolf | 2014 | Table 1 face cartoons + phonemes | 16 | 1 | 1 | 2 | Drawing of faces with emotional expressions: Supramodal effects with emotional valence |
Table 14.
Locations of significant clusters from the meta-analyses involving Dynamic-visual and Static-visual audio-visual experimental paradigms, indicating major contributing studies to the ALE meta-analysis clusters, weighted centers of mass (x, y, and z) in Talairach coordinates, brain volumes (mm3), and ALE values
| Condition | Region | Major contributing studies | x | y | z | Volume | ALE value |
|---|---|---|---|---|---|---|---|
| A. Dynamic-visual audio-visual stimuli single study | |||||||
| 1 | R Posterior Superior Temporal Sulcus | 9 (20,40,60,60,65,66,74,78,79) | 48 | −36 | 11 | 1312 | 0.037 |
| 2 | L Posterior Superior Temporal Sulcus | 6 (18,40,65,66,67,79) | −51 | −49 | 10 | 928 | 0.035 |
| 3 | L Posterior Superior Temporal Gyrus | 2 (22,74) | −58 | −38 | 12 | 136 | 0.027 |
| 4 | R Superior Temporal Gyrus | 58 | −17 | 8 | 32 | 0.024 | |
| B. Static-visual audio-visual stimuli single study | |||||||
| 1 | L Transverse Temporal Gyrus/Planum Temporale | 5 (31,45,50,57,72) | −47 | −22 | 7 | 552 | 0.031 |
| 2 | R Superior Temporal Gyrus/Planum Temporale | 2 (45,72) | 53 | −20 | 8 | 288 | 0.023 |
| 3 | R Superior Temporal Gyrus | 58 | −29 | 11 | 120 | 0.021 | |
| C. Dynamic-visual > Static-visual audio-visual stimuli | |||||||
| 1 | R Superior Temporal Gyrus/Sulcus | 4 (40,60,60,66) | 46 | −37 | 12 | 392 | 2.287 |
| D. Static-visual > Dynamic-visual audio-visual stimuli | |||||||
| 1 | L Superior Temporal Gyrus/Planum Temporale/TTG | 4 (31,50,57,72) | −46 | −22 | 7 | 480 | 2.620 |
| 2 | R Superior Temporal Gyrus (posterior) | 58 | −28 | 11 | 128 | 2.308 | |
| 3 | R Superior Temporal Gyrus/Sulcus | 52 | −20 | 3 | 64 | 2.254 | |
| 4 | R Transverse Temporal Gyrus | 50 | −18 | 8 | 24 | 1.739 | |
| 5 | R Transverse Temporal Gyrus | 52 | −18 | 12 | 8 | 1.863 | |
Note: Single study ALE maps for (A) Dynamic-visual stimuli (corrected FWE P < 0.05) and (B) Static-visual stimuli (nonmoving images) (corrected FWE P < 0.05), plus (C) contrast maps of interaction sites revealing Dynamic-visual > Static-visual, and (D) Static-visual > Dynamic-visual audio-visual stimuli (both uncorrected P < 0.05). The coordinates correspond to foci illustrated in Figure 3E (blue and pink hues) (from Tables 12 and 13).
Analyses of the dynamic-visual versus static-visual were further conducted separately for those experimental paradigms using artificial versus natural stimuli. With the exception of natural dynamic-visual studies (n = 37 of the 43 in Table 12), the other subcategories had too few studies for the recommended 17–20 study minimum for meta-analysis. Nonetheless, the artificial static-visual (n = 12) meta-analysis revealed clusters that overlapped with the outcomes using the respective full complement of studies, while the natural dynamic-visual (n = 37) meta-analysis similarly revealed clusters that overlapped with the respective full complement of studies. Thus, audio-visual events involving dynamic visual motion (and mostly natural stimuli) generally recruited association cortices situated roughly between auditory and visual cortices, while audio-visual interactions involving static (iconic) visual images (and mostly artificial stimuli) generally recruited regions located closer to auditory cortex proper along the pSTG and planum temporale bilaterally.
Discussion
The present meta-analyses examined a wide variety of published human neuroimaging studies that revealed some form of audio-visual “interaction” in the brain, entailing responses beyond or different from the corresponding unisensory auditory and/or visual stimuli alone. One objective was to test several tenets regarding potential brain organizations or architectures that may develop for processing different categories of audio-visual event types at a semantic level. The tenets were borne out of recent ethologically derived unisensory hearing perception literature (Brefczynski-Lewis and Lewis 2017). This included a taxonomic model of semantic categories of natural sound-producing events (i.e., Fig. 1), but here being applied to testing specific hypotheses in the realm of multisensory (audio-visual) processing. The category constructs were derived with the idea of identifying putative cortical “hubs” that could be further applied to, and tested by, various neurocomputational models of semantic knowledge and multisensory processing.
Providing modest support for our first hypothesis, contrast ALE meta-analyses revealed a double-dissociation of brain regions preferential for the processing of living versus nonliving (mostly artificial sources) audio-visual interaction events at a category level (Fig. 3C, orange vs. cyan). These results implicated the bilateral pSTS complexes versus the right anterior insula as processing hubs, respectively, which are further addressed below in Embodied Representations of Audio-Visual Events. Providing modest support for our second hypothesis, contrast ALE meta-analyses revealed a double-dissociation of brain regions preferential for the processing of audio-visual interaction events involving vocalizations versus actions, respectively (Fig. 3D, red vs. yellow). These results implicated the bilateral planum temporale, pSTG, and pSTS complexes versus the left fusiform cortex, respectively, which is also further addressed in Embodied Representations of Audio-Visual Events below.
Providing strong support for our third hypothesis, different cortices were preferential for processing audio-visual interactions that involved dynamic-visual (video) versus static-visual (iconic images) as visual stimuli (Fig. 3E, blue vs. pink). This finding is addressed further below in the context of parallel multisensory processing hierarchies in the section ``Dynamic-Visual versus Static-Visual Images and Audio-Visual Interaction Processing''. The original volumes of the ROIs identified herein (comprising clusters in Tables 4,7,11, and14, and depicted in Fig. 3) are available in Supplementary Material. These ROI volumes should facilitate the generation and testing of new hypotheses, especially as they pertain to neurocomputational theories of semantic knowledge representation, which is a topic addressed in below in the section ``Semantic processing and neurocomputational models of cognition''. This is followed by a discussion that considers various limitations of the present meta-analysis studies.
Upon inspection of Figure 3C–E, only ventral cortices, as opposed to dorsal cortices (e.g., superior to the lateral sulcus), revealed activation foci that were preferential for any of the different semantic categories of audio-visual events. In particular, neither the bilateral IFC foci for congruent versus incongruent audio-visual interactions (Fig. 3B, black/white), nor the frontal or parietal cortices (Fig. 3A, purple), revealed any differential activation along the semantic category dimensions tested. This was generally consistent with the classic ventral “what is it” (perceptual identification of objects) versus dorsal “where is it” (sensory transformations for guided actions directed at objects) dichotomy observed in both vision and auditory neuroimaging and primate literature (Goodale and Milner 1992; Ungerleider and Haxby 1994; Belin and Zatorre 2000; Sestieri et al. 2006). While dorsal cortical regions such a bilateral parietal cortices and noncortical regions such as the cerebellum were reported to be revealing audio-visual interaction effects by many studies, their involvement appeared to relate more to task demands, spatial processing, and task difficulty rather than semantic category of the audio-visual events per se. Dorsal cortical networks are also implicated in various components of attention. While some form of sensory attention was involved in nearly all of the experimental paradigms, the specific effects of different types or degrees of sensory attention was not a measurable dimension across the studies, and thus fell outside the scope of the present study.
Embodied Representations of Audio-Visual Events
One of the tenets regarding the taxonomic category model of real-world hearing perception was that “natural sounds are embodied when possible” (Brefczynski-Lewis and Lewis 2017), and this tenet appears to also apply to the context of cortical organizations for processing audio-visual interactions at a semantic category level. This is further addressed below by region in the context of the pSTS complexes for embodiable representations, and of the right anterior insula focus for nonembodiable nonliving and artificial audio-visual event perception.
The pSTS complexes and audio-visual motion processing
The bilateral pSTS complexes were significantly more involved with processing audio-visual interactions associated with events by living things, by stimuli involving vocalizations, and by dynamic-visual (vs static-visual image) audio-visual events (cf. Fig. 3C–E, orange, red, and blue). Although these respective foci were derived by several overlapping studies, the meta-analysis results support the notion that the lateral temporal cortices are the primary loci for complex natural motion processing (Calvert et al. 2000; Beauchamp, Argall, et al. 2004; Beauchamp, Lee, et al. 2004; Calvert and Lewis 2004; Lewis et al. 2004; Taylor et al. 2006, 2009; Martin 2007): More specifically, the pSTS complexes are thought to play a prominent perceptual role in transforming the spatially and temporally dynamic features of natural auditory and visual action information together into a common neural code, which may then facilitate cross-modal interactions and integration from a “bottom-up” intermodal invariant sensory perspective. An earlier image-based (as opposed to coordinate-based) meta-analysis using a subset of these paradigms (Lewis 2010) further highlighted the idea that the pSTS complexes may form a temporal reference frame for probabilistically comparing the predicted or expected incoming auditory and/or visual information based on what actions have already occurred.
From a “top-down” cognitive perspective, however, words and phrases that depict human actions, and even imagining complex audio and/or visual actions, are reported to lead to activation of the pSTS regions (Kellenbach et al. 2003; Tettamanti et al. 2005; Kiefer et al. 2008; Noppeney et al. 2008). Furthermore, the pSTS complexes are known to be recruited by a variety of sensory-perceptual tasks in congenitally blind and in congenitally deaf individuals (Burton et al. 2004; Pietrini et al. 2004; Amedi et al. 2007; Patterson et al. 2007; Capek et al. 2008, 2010; Lewis, Frum, et al. 2011), suggesting that aspects of their basic functional roles are not dependent on bimodal sensory input outright. To reconcile these findings, one hypothesis was that some cortical regions may develop to perform amodal or metamodal operations (Pascual-Leone and Hamilton 2001). More specifically, different patches of cortex, such as the pSTS, may innately develop to contain circuitry predisposed to compete for the ability to perform particular types of operations or computations useful to the observer regardless of the modality (or presence) of sensory input. Thus, the organization of the multisensory brain may be influenced as much, if not more, by internal processing factors than by specific external sensory experiences per se. This interpretation reflects another tenet regarding the taxonomic category model of real-world hearing perception that “metamodal operators guide sound processing network organizations” (Brefczynski-Lewis and Lewis 2017), but here applying to the processing of audio-visual interactions at a semantic category level.
Another interpretation regarding the functions of the bilateral pSTS complexes is that they are more heavily recruited by living and dynamic audio-visual events simply because of their greater life-long familiarity in adult observers. They may reflect an individual’s experiences and habits of extracting subtle nuances from day-to-day real-world interactions, including other orally communicating people as prevalent sources of multisensory events. Ostensibly, this experiential multisensory process would start from the time of birth when there becomes a critical need to interact with human caretakers. Consistent with this interpretation is that the pSTS complexes have prominent roles in social cognition, wherein reading subtleties of human expressions and body language is often highly relevant for conveying information that guides effective social interactions (Pelphrey et al. 2004; Jellema and Perrett 2006; Zilbovicius et al. 2006).
Embodied cognition models (also called grounded cognition) posit that perception of natural events (social or otherwise) is at least in part dependent on modal simulations, bodily states, and situated actions (Barsalou 2008). The discovery of mirror neuron systems (MNS) and echo-mirror neuron (ENS) systems (Rizzolatti and Arbib 1998; Rizzolatti and Craighero 2004; Molenberghs et al. 2012) have been recognized as having major implications for explaining many cognitive functions, including action understanding, imitation and empathy. Such neuronal systems, which often include the bilateral pSTS complexes, are proposed to mediate elements of the perception of sensory events as they relate to one’s own repertoire of dynamic visual action-producing and sound-producing motoric events (Gazzola et al. 2006; Lahav et al. 2007; Galati et al. 2008; Engel et al. 2009; Lewis et al. 2018). Thus, the pSTS complexes may reflect metamodal cortices that typically develop to process natural multisensory events, which especially include dynamic actions by living things (including vocalizations) that are interpreted for meaningfulness (and possibly intent) based on embodiment strategies by the brain. Notwithstanding, the dynamic viewable motions and sounds produced by nonliving things and artificial stimulus events are arguably less embodiable or mimicable than those by living things. The pSTG/pSTS complexes were not preferentially activated by nonliving and artificial multisensory events. Rather, this event category preferentially recruited the right anterior insula, as addressed next.
The right anterior insula and nonliving/artificial audio-visual interaction processing
The right anterior insula emerged as a cortical hub that was preferentially involved in processing nonliving and largely artificial audio-visual sources, which are typically deemed as being nonembodiable. Moreover, unlike the pSTS complexes, the right anterior insula did not show significant sensitivity to the dynamic-visual versus static-visual image stimulus dimension, suggesting that intermodal invariant cues were not a major driving factor in its recruitment. Interestingly, the mirror opposite left anterior insula showed preferential activation for incongruent versus congruent audio-visual stimuli (cf. Fig. 3B,C).
On a technical note, portions of the claustrum are located very close to the anterior insulae, and activation of the claustrum may have contributed to the anterior insula foci in several neuroimaging studies, and thus also in this meta-analysis. The enigmatic claustrum is reported to have a role in integrative processes that require the analysis of the “content” of the stimuli, and in coordinating the rapid integration of object attributes across different modalities that lead to coherent conscious percepts (Crick and Koch 2005; Naghavi et al. 2007).
Embodiment encoding functions have been ascribed to the anterior insula in representing “self” versus “nonself.” For instance, the anterior insulae, which receive input from numerous cortical areas, have reported roles in multimodal integrative functions, rerepresentation of interoceptive awareness of bodily states, cognitive functions, and metacognitive functions (Craig 2009, 2010; Menon and Uddin 2010), and in social emotions that may function to help establish “other-related” states (Singer et al. 2004; Lamm and Singer 2010). The right lateralized anterior insula activation has further been reported to be recruited during nonverbal empathy-related processing such as with compassion meditation, which places an emphasis on dissolving the “self-versus-other” boundary (Lutz et al. 2008). Moreover, dysfunction of the anterior insulae has been correlated with an inability to differentiate the self from the nonself in patients with schizophrenia (Cascella et al. 2011; Shura et al. 2014).
Although the anterior insula territories are commonly associated with affective states, visceral responses, and the processing of feelings (Damasio 2001; Critchley et al. 2004; Dalgleish 2004; Mutschler et al. 2009; Cacioppo 2013), the emotionally valent paradigms in this meta-analysis did not yield significant differential audio-visual interaction effects in the right (or left) insula, but rather only along the right pSTG. Though speculative, the anterior insula(e) may be subserving the mapping of events that are heightened by relatively “nonembodiable” multisensory events (notably nonliving and artificial sources) with differential activation depending on the perceived relatedness to self. This outcome will likely be a topic of interest for future studies, including neurocomputational modeling of cognition, which is addressed in a later section after first considering parallel multisensory processing hierarchies.
Dynamic-Visual versus Static-Visual Images and Audio-Visual Interaction Processing
The double-dissociation of cortical hubs for processing dynamic-visual versus static-visual audio-visual interactions was consistent with notion of parallel processing hierarchies. The experimental paradigms using video typically included dynamic intermodal invariant cross-modal cues (mostly by living things), where the audio and visual stimuli were either perceived to be coming from roughly the same region of space, moving along similar spatial trajectories, and/or had common temporal synchrony and modulations in stimulus intensity or change. These correlated physical changes in photic and acoustic energy attributes are likely to serve to naturally bind audio-visual interactions, consistent with bottom-up Hebbian-like learning mechanisms. Such stimuli preferentially recruited circuitry of the bilateral pSTS complexes (Fig. 3E, blue vs. pink), as addressed earlier.
In direct contrast to dynamic-visual stimuli, static-visual images (e.g., pictures, characters, and drawings) can have symbolic congruence with sound that must be learned to be associated with, and having few or no cross-modal invariant cues, thereby placing greater emphasis on declarative memory and related semantic-level matching mechanisms. The dynamic versus static visual stimulus dimension was further assessed using a subset of natural-only versus artificial stimuli. While there were insufficient numbers of studies in three of the subgroups for definitive meta-analysis results (data not shown), the outcomes suggested a bias for the dynamic-visual stimuli clusters being driven by natural stimuli while the static-visual stimuli clusters may have been driven more by images involving relatively artificial stimuli (e.g., checkerboards, dots, circles, texture patterns). Regardless, a double-dissociation was evident.
Another consideration regarding the dynamic/natural versus static/artificial processing was depth-of-encoding. The greater depth required for encoding for subordinate versus basic level information is reported to recruit greater expanses of cortices along the anterior temporal lobes (Adams and Janata 2002; Tranel et al. 2003; Tyler et al. 2004). For instance, associating a picture of an iconic dog to the sound “woof” represents a “basic” level of semantic matching, while matching the specific and more highly familiar image of one’s pet Tibetan terrier to her particular bark to be let outside would represent a “subordinate” level of matching that is regarded as having greater depth in its encoding. Neuroimaging and neuropsychological studies of semantically congruent cross-modal processing has led to a Conceptual Structure Account model (Tyler and Moss 2001; Taylor et al. 2009), suggesting that objects in different categories can be characterized by the number and statistical properties of their constituent features (i.e., its depth), and this model points to the anterior temporal poles as “master binders” of such audio-visual information.
Correlating static-visual images with sound could be argued to require a more cognitive learning process than perceptually observing dynamic-visual events as they unfold and provide more intermodal-invariant information correlated with ongoing acoustic information. Thus, it was somewhat surprisingly that the static-visual stimuli preferentially recruited of the bilateral planum temporale (Fig. 3E, pink hues), in locations close to secondary auditory cortices, rather than in the temporal poles. However, this may relate to depth-of-encoding issues. The audio-visual stimuli used in many of the included studies used a relatively basic level of semantic matching (stimuli and tasks), which may have masked more subtle or widespread activation in inferotemporal cortices (e.g., temporal poles).
One possibility is that the pSTS complexes may represent intermediate processing stages that convey dynamically matched audio-visual congruent interaction information to the temporal poles, while the bilateral planum temporale regions may represent parallel intermediate processing stages that convey semantically congruent audio-visual information derived from learned associations of sound with static (iconic) images referring to their matching source. Overall, this interpretation supports the tenet from unisensory systems “that parallel hierarchical pathways process increasing information content,” but here including two parallel multisensory processing pathways mediating the perception of audio-visual interaction information as events that are physically matched from a bottom-up perspective versus learned to be semantically congruent.
Semantic Processing and Neurocomputational Models of Cognition
Several mechanistic models regarding how and why semantic knowledge formation might develop in the brain includes the concept of hubs (and connector hubs) in brain networks (Damasio 1999; Sporns et al. 2007; Pulvermuller 2018), which are thought to allow for generalizations and the formation of categories. As such, the roughly six basic ROIs emerging from the present meta-analysis study (left and right pSTS complexes, left and right planum temporale, left fusiform, and right anterior insula) were of particular interest.
With regard to double-dissociations of cortical function, the right anterior insula and left fusiform ROIs had relatively small volumes, and thus may be considered less robust by some meta-analysis standards (also see Limitations). Nonetheless, these preliminary findings provide at least moderate support for a taxonomic neurobiological model for processing different categories of real-world audio-visual events (Fig. 1), which is readily amenable to testing with neurocomputational models and future hypothesis-driven multisensory processing studies. For instance, one might directly assess whether the different ROIs have functionally distinct characteristics as connector hubs for semantic processing with activity dynamics that are functionally linking action perception circuits (APCs) at a category level (Pulvermuller 2018). Additionally, one may test for functional connectivity pattern differences across these ROIs (e.g., resting state functional connectivity MRI) in neurotypical individuals relative to various clinical populations. Overall, the results indicating that different semantic categories of audio-visual interaction events may be differentially processed along different brain regions supports the tenet that “categorical perception emerges in neurotypical listeners [observers],” but here applying to the realm of cortical representations mediating multisensory object information. It remains unclear, however, whether this interpretation regarding categorical perception would provide greater support for domain-specific theoretical models, as proposed for some vision-dominated categories, such as the processing of faces, tools, fruits and vegetables, animals, and body parts (Damasio et al. 1996; Caramazza and Shelton 1998; Pascual-Leone and Hamilton 2001; Mahon and Caramazza 2005; Mahon et al. 2009) or for sensory-motor property-based models that develop because of experience (Lissauer 1890/1988; Barsalou et al. 2003; Martin 2007; Barsalou 2008), or perhaps some combination of both.
Limitations
While this meta-analysis study revealed significant dissociations of cortical regions involved in different aspects of audio-visual interaction processing, at a more detailed or refined level there were several limitations to consider. As with most meta-analyses, the reported results were confined only to published “positive” results, and tended to be biased in examining topics (in this case sensory stimulus categories) that typically have greater rationale for being studied (and funded). In particular, the categories of living things (humans) and/or vocalizations (speech) are simply more thoroughly studied as socially- and health-relevant topics relative to the categories of nonliving and nonvocal audio-visual stimuli, as evident in the numbers of studies listed in the provided tables. Because there were fewer numbers of studies in some semantic categories, double-dissociation differences could only be observed in some contrast meta-analyses when using uncorrected P-values, a statistical correction process that to date remains somewhat contentious in the field of meta-analyses. The biases in stimuli commonly used also led to the limitation that there would be greater heterogeneity of, for instance, nonliving audio-visual sources and action events devoid of any vocalizations. This precluded examination of subcategories such as environmental sources, mechanical (human-made) audio-visual sources, versus “artificial” events (being computer-derived or involving illusory sources), which limited a more thorough testing of the taxonomic model (Fig. 1) being investigated.
At a more technical level, other potential limitations included methodological differences across study designs, such as 1) differences in alignment methods, 2) imaging large swaths of brain rather than truly “whole brain” imaging, and 3) potential inclusion of participants in more than one published study (which was not accessible information). Together, these limitations may constitute violations of assumptions by the ALE meta-analysis processes. Nonetheless, the modest support for our first two hypotheses and strong support for our third hypothesis should merit future study to validate and/or refine these basic cortical organization tenets and neurobiological taxonomic model.
Conclusion
This study summarized evidence derived from meta-analyses across 137 experimental paradigms to test for brain organizations for representing putative taxonomic boundaries related to perception of audio-visual events at a category-level. The semantic categories tested were derived from an ethologically and evolutionarily inspired taxonomic neurobiological model of real-world auditory event perception. The outcomes provided novel, though tentative support for the existence of double-dissociations mediating processing and perception around semantic categories, including 1) living versus nonliving (artificial) audio-visual events, and 2) vocalization versus action audio-visual events. The outcomes further provided strong support for a double-dissociation for processing 3) dynamic-visual (mostly natural events) versus static-visual (including artificial) audio-visual interactions. Together, these findings were suggestive of parallel hierarchical pathways for processing and representing different semantic categories of multisensory event types, with embodiment strategies as potential underlying neuronal mechanisms. Overall, the present findings highlighted where and how auditory and visual perceptual representations interact in the brain, including the identification of a handful of cortical hubs in Figure 3C–E that are amenable to future neurocomputational modeling and testing of semantic knowledge representation mechanisms. Exploration of these and other potential multisensory hubs will be important for future studies addressing why specific brain regions may typically develop to process different aspects of audio-visual information, and thereby establish and maintain the “multisensory brain,” which ultimately subserves many of the complexities of human communication and social behavior.
Supplementary Material
Appendix A
List of all 137 experimental coordinates from the 82 studies after converting all to afni-TLRC coordinates using GingerALE software. The number of subjects are also indicated. The coordinate sets were used to derive all of the meta analyses of the present study.
Contributor Information
Matt Csonka, Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University, Morgantown, WV 26506, USA.
Nadia Mardmomen, Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University, Morgantown, WV 26506, USA.
Paula J Webster, Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University, Morgantown, WV 26506, USA.
Julie A Brefczynski-Lewis, Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University, Morgantown, WV 26506, USA.
Chris Frum, Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University, Morgantown, WV 26506, USA.
James W Lewis, Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University, Morgantown, WV 26506, USA.
Funding
National Institute of General Medical Sciences of the National Institutes of Health (NIGMS) Centers of Biomedical Research Excellence (COBRE) grant GM103503 to the Centers for Neuroscience of West Virginia University, and affiliated WVU Summer Undergraduate Research Internships, plus an Internship for Medical Students through the National Institute of General Medical Sciences, U54GM104942-02.
Availability of Data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Adams RB, Janata P. 2002. A comparison of neural circuits underlying auditory and visual object categorization. Neuroimage. 16(2):361–377. [DOI] [PubMed] [Google Scholar]
- Alink A, Singer W, Muckli L. 2008. Capture of auditory motion by vision is represented by an activation shift from auditory to visual motion cortex. J Neurosci. 28(11):2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi A, Stern WM, Camprodon JA, Bermpohl F, Merabet L, Rotman S, Hemond C, Meijer P, Pascual-Leone A. 2007. Shape conveyed by visual-to-auditory sensory substitution activates the lateral occipital complex. Nat Neurosci. 10(6):687–689. [DOI] [PubMed] [Google Scholar]
- Balk MH, Ojanen V, Pekkola J, Autti T, Sams M, Jaaskelainen IP. 2010. Synchrony of audio-visual speech stimuli modulates left superior temporal sulcus. Neuroreport. 21(12):822–826. doi: 10.1097/WNR.0b013e32833d138f. [DOI] [PubMed] [Google Scholar]
- Bar M, Tootell RBH, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM. 2001. Cortical mechanisms specific to explicit visual object recognition. Neuron. 29(2):529–535. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. 2008. Grounded cognition. Annu Rev Psychol. 59:617–645. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Kyle Simmons W, Barbey AK, Wilson CD. 2003. Grounding conceptual knowledge in modality-specific systems. Trends Cogn Sci. 7(2):84–91. [DOI] [PubMed] [Google Scholar]
- Baumann O, Greenlee MW. 2007. Neural correlates of coherent audiovisual motion perception. Cereb Cortex. 17(6):1433–1443. [DOI] [PubMed] [Google Scholar]
- Baumgaertner A, Buccino G, Lange R, McNamara A, Binkofski F. 2007. Polymodal conceptual processing of human biological actions in the left inferior frontal lobe. Eur J Neurosci. 25(3):881–889. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. 2004. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nat Neurosci. 7:1190–1192. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KM, Argall BD, Martin A. 2004. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 41:809–823. [DOI] [PubMed] [Google Scholar]
- Belardinelli MO, Sestieri C, Di Matteo R, Delogu F, Del Gratta C, Ferretti A, Caulo M, Tartaro A, Romani, GL. 2004. Audio-visual crossmodal interactions in environmental perception: an fMRI investigation. Cogn Process. 5:167–174. [Google Scholar]
- Belin P, Zatorre R. 2000. 'What', 'where' and 'how' in auditory cortex. Nat Neurosci. 3(10):965–966. [DOI] [PubMed] [Google Scholar]
- Biau E, Moris Fernandez L, Holle H, Avila C, Soto-Faraco S. 2016. Hand gestures as visual prosody: BOLD responses to audio-visual alignment are modulated by the communicative nature of the stimuli. Neuroimage. 132:129–137. doi: 10.1016/j.neuroimage.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Walter B, Blecker CR, Morgen K, Vaitl D, Sammer G. 2007. Utilizing the ventriloquism-effect to investigate audio-visual binding. Neuropsychologia. 45(3):578–586. doi: 10.1016/j.neuropsychologia.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Blank H, von Kriegstein K. 2013. Mechanisms of enhancing visual-speech recognition by prior auditory information. Neuroimage. 65:109–118. doi: 10.1016/j.neuroimage.2012.09.047. [DOI] [PubMed] [Google Scholar]
- Bonath B, Noesselt T, Krauel K, Tyll S, Tempelmann C, Hillyard SA. 2014. Audio-visual synchrony modulates the ventriloquist illusion and its neural/spatial representation in the auditory cortex. Neuroimage. 98:425–434. doi: 10.1016/j.neuroimage.2014.04.077. [DOI] [PubMed] [Google Scholar]
- Bonath B, Tyll S, Budinger E, Krauel K, Hopf JM, Noesselt T. 2013. Task-demands and audio-visual stimulus configurations modulate neural activity in the human thalamus. Neuroimage. 66:110–118. doi: 10.1016/j.neuroimage.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lewis JW. 2017. Auditory object perception: a neurobiological model and prospective review. Neuropsychologia. 105:223–242. doi: 10.1016/j.neuropsychologia.2017.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulkin DA, Groh JM. 2006. Seeing sounds: visual and auditory interactions in the brain. Curr Opin Neurobiol. 16(4):415–419. [DOI] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Raichle ME. 2004. Default brain functionality in blind people. Proc Natl Acad Sci U S A. 101(43):15500–15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushara KO, Grafman J, Hallett M. 2001. Neural correlates of auditory-visual stimulus onset asynchrony detection. J Neurosci. 21(1):300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushara KO, Hanakawa T, Immisch I, Toma K, Kansaku K, Hallett M. 2003. Neural correlates of cross-modal binding. Nat Neurosci. 6(2):190–195. [DOI] [PubMed] [Google Scholar]
- Cacioppo S. 2013. Selective decision-making deficit in love following damage to the anterior insula. Front Hum Neurosci. 7:15–19. doi: 10.3389/fnhum.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan DE, Jones JA, Callan A. 2014. Multisensory and modality specific processing of visual speech in different regions of the premotor cortex. Front Psychol. 5:389. doi: 10.3389/fpsyg.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA, Brammer MJ, Bullmore ET, Campbell R, Iversen SD, David AS. 1999. Response amplification in sensory-specific cortices during crossmodal binding. Neuroreport. 10(12):2619–2623. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Campbell R. 2003. Reading speech from still and moving faces: the neural substrates of visible speech. J Cogn Neurosci. 15(1):57–70. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Campbell R, Brammer MJ. 2000. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr Biol. 10:649–657. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Hansen PC, Iversen SD, Brammer MJ. 2001. Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. Neuroimage. 14(2):427–438. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Lewis JW. 2004. Hemodynamic studies of audio-visual interactions. In: Calvert GA, Spence C, Stein B, editors. Handbook of multisensory processing. Cambridge (MA): MIT Press. p. 483–502. [Google Scholar]
- Capek CM, Macsweeney M, Woll B, Waters D, McGuire PK, David AS, Brammer MJ, Campbell R. 2008. Cortical circuits for silent speechreading in deaf and hearing people. Neuropsychologia. 46(5):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capek CM, Woll B, MacSweeney M, Waters D, McGuire PK, David AS, Brammer MJ, Campbell R. 2010. Superior temporal activation as a function of linguistic knowledge: insights from deaf native signers who speechread. Brain Lang. 112(2):129–134. doi: 10.1016/j.bandl.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ. 2003. The organization of conceptual knowledge: the evidence from category-specific semantic deficits. Trends Cogn Sci. 7(8):354–361. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. 1998. Domain-specific knowledge systems in the brain the animate-inanimate distinction. J Cogn Neurosci. 10(1):1–34. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Gerner GJ, Fieldstone SC, Sawa A, Schretlen DJ. 2011. The insula-claustrum region and delusions in schizophrenia. Schizophr Res. 133(1–3):77–81. doi: 10.1016/j.schres.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Cecere R, Gross J, Thut G. 2016. Behavioural evidence for separate mechanisms of audiovisual temporal binding as a function of leading sensory modality. Eur J Neurosci. 43(12):1561–1568. doi: 10.1111/ejn.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S, Thiran AB, Maeder P, Adriani M, Vernet O, Regli L, Cuisenaire O, Thiran J-P. 2002. What and where in human audition: selective deficits following focal hemispheric lesions. Exp Brain Res. 147:8–15. [DOI] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 10(1):59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2010. The sentient self. Brain Struct Funct. 214(5–6):563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. 2005. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 360(1458):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat Neurosci. 7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. 2004. The emotional brain. Nat Rev Neurosci. 5(7):583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Damasio A. 2001. Fundamental feelings. Nature. 413(6858):781. doi: 10.1038/35101669. [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1989a. The brain binds entities and events by multiregional activation from convergence zones. Neural Comput. 1:123–132. [Google Scholar]
- Damasio AR. 1989b. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1999. How the brain creates the mind. Sci Am. 281(6):112–117. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio RD. 1996. A neural basis for lexical retrieval. Nature. 380:499–505. [DOI] [PubMed] [Google Scholar]
- de Haas B, Schwarzkopf DS, Urner M, Rees G. 2013. Auditory modulation of visual stimulus encoding in human retinotopic cortex. Neuroimage. 70:258–267. doi: 10.1016/j.neuroimage.2012.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick F, Saygin AP, Galati G, Pitzalis S, Bentrovato S, D'Amico S, Wilson S, Bates E, Pizzamiglio L. 2007. What is involved and what is necessary for complex linguistic and nonlinguistic auditory processing: evidence from functional magnetic resonance imaging and lesion data. J Cogn Neurosci. 19(5):799–816. [DOI] [PubMed] [Google Scholar]
- Donald M. 1991. Origins of the modern mind: Three stages in the evolution of culture and cognition. USA: Harvard University Press. https://www.amazon.com/Origins-Modern-Mind-Evolution-Cognition/dp/0674644840#reader_0674644840. [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage. 59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR. 2016. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LR, Frum C, Puce A, Walker NA, Lewis JW. 2009. Different categories of living and non-living sound-sources activate distinct cortical networks. Neuroimage. 47(4):1778–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson LC, Zielinski BA, Zielinski JE, Liu G, Turkeltaub PE, Leaver AM, Rauschecker JP. 2014. Distinct cortical locations for integration of audiovisual speech and the McGurk effect. Front Psychol. 5:534. doi: 10.3389/fpsyg.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Wiethoff S, Bisch J, Schlipf S, Wildgruber D, Kreifelts B. 2013. Functional responses and structural connections of cortical areas for processing faces and voices in the superior temporal sulcus. Neuroimage. 76:45–56. doi: 10.1016/j.neuroimage.2013.02.064. [DOI] [PubMed] [Google Scholar]
- Galati G, Committeri G, Spitoni G, Aprile T, Di Russo F, Pitzalis S, Pizzamiglio L. 2008. A selective representation of the meaning of actions in the auditory mirror system. Neuroimage. 40(3):1274–1286. doi: 10.1016/j.neuroimage.2007.12.044. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. 1999. Activation of the middle fusiform 'face area' increases with expertise in recognizing novel objects. Nat Neurosci. 2(6):568–573. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. 2006. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 16(18):1824–1829. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. 2006. Is neocortex essentially multisensory? Trends Cogn Sci. 10(6):278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Goll JC, Crutch SJ, Warren JD. 2011. Central auditory disorders: toward a neuropsychology of auditory objects. Curr Opin Neurol. 23(6):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo D, Shallice T, Dolan R. 2000. Time-dependent changes in learning audiovisual associations: a single-trial fMRI study. Neuroimage. 11(3):243–255. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Meenan JP, Bulthoff HH, Nicolle DA, Murphy KJ, Racicot CI. 1994. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr Biol. 4(7):604–610. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15:20–25. [DOI] [PubMed] [Google Scholar]
- Green A, Straube B, Weis S, Jansen A, Willmes K, Konrad K, Kircher T. 2009. Neural integration of iconic and unrelated coverbal gestures: a functional MRI study. Hum Brain Mapp. 30(10):3309–3324. doi: 10.1002/hbm.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CC, Woods W, Johnson S, Green GG, Young AW. 2013. Involvement of right STS in audio-visual integration for affective speech demonstrated using MEG. PLoS One. 8(8):e70648. doi: 10.1371/journal.pone.0070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Frith U. 2006. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Matsuki K, Ueno T, Maeda Y, Matsue Y, Konishi Y, Sadato N. 2004. Learned audio-visual cross-modal associations in observed piano playing activate the left planum temporale. An fMRI study. Brain Res Cogn Brain Res. 20(3):510–518. doi: 10.1016/j.cogbrainres.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. 2004. Learning letters in adulthood: direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 42(2):311–322. [DOI] [PubMed] [Google Scholar]
- He Y, Gebhardt H, Steines M, Sammer G, Kircher T, Nagels A, Straube B. 2015. The EEG and fMRI signatures of neural integration: an investigation of meaningful gestures and corresponding speech. Neuropsychologia. 72:27–42. doi: 10.1016/j.neuropsychologia.2015.04.018. [DOI] [PubMed] [Google Scholar]
- He Y, Steines M, Sommer J, Gebhardt H, Nagels A, Sammer G, Kircher T, Straube B. 2018. Spatial-temporal dynamics of gesture-speech integration: a simultaneous EEG-fMRI study. Brain Struct Funct. 223(7):3073–3089. doi: 10.1007/s00429-018-1674-5. [DOI] [PubMed] [Google Scholar]
- Hein G, Doehrmann O, Muller NG, Kaiser J, Muckli L, Naumer MJ. 2007. Object familiarity and semantic congruency modulate responses in cortical audiovisual integration areas. J Neurosci. 27(30):7881–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewes GW. 1973. Primate communication and the gestural origin of language. Curr Anthropol. 14:5–24. doi: 10.1086/204019. [DOI] [Google Scholar]
- Hocking J, Price CJ. 2008. The role of the posterior superior temporal sulcus in audiovisual processing. Cereb Cortex. 18(10):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove MJ, Fairhurst MT, Kotz SA, Keller PE. 2013. Synchronizing with auditory and visual rhythms: an fMRI assessment of modality differences and modality appropriateness. Neuroimage. 67:313–321. doi: 10.1016/j.neuroimage.2012.11.032. [DOI] [PubMed] [Google Scholar]
- James TW, Gauthier I. 2003. Auditory and action semantic features activate sensory-specific perceptual brain regions. Curr Biol. 13(20):1792–1796. [DOI] [PubMed] [Google Scholar]
- James TW, VanDerKlok RM, Stevenson RA, James KH. 2011. Multisensory perception of action in posterior temporal and parietal cortices. Neuropsychologia. 49(1):108–114. doi: 10.1016/j.neuropsychologia.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellema T, Perrett DI. 2006. Neural representations of perceived bodily actions using a categorical frame of reference. Neuropsychologia. 44(9):1535–1546. [DOI] [PubMed] [Google Scholar]
- Jessen S, Kotz SA. 2015. Affect differentially modulates brain activation in uni- and multisensory body-voice perception. Neuropsychologia. 66:134–143. doi: 10.1016/j.neuropsychologia.2014.10.038. [DOI] [PubMed] [Google Scholar]
- Jola C, McAleer P, Grosbras MH, Love SA, Morison G, Pollick FE. 2013. Uni- and multisensory brain areas are synchronised across spectators when watching unedited dance recordings. Iperception. 4(4):265–284. doi: 10.1068/i0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. 2000. Linguistic processing in high-functioning adults with autism or Asperger's syndrome. Is global coherence impaired? Psychol Med. 30(5):1169–1187. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. 1999. 'What' and 'where' processing in auditory cortex. Nat Neurosci. 2(12):1045–1047. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. 2003. Actions speak louder than functions: the importance of manipulability and action in tool representation. J Cogn Neurosci. 15(1):30–46. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Sim EJ, Herrnberger B, Grothe J, Hoenig K. 2008. The sound of concepts: four markers for a link between auditory and conceptual brain systems. J Neurosci. 28(47):12224–12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Hahm J, Lee H, Kang E, Kang H, Lee DS. 2015. Brain networks engaged in audiovisual integration during speech perception revealed by persistent homology-based network filtration. Brain Connect. 5(4):245–258. doi: 10.1089/brain.2013.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Straube B, Leube D, Weis S, Sachs O, Willmes K, Konrad K, Green A. 2009. Neural interaction of speech and gesture: differential activations of metaphoric co-verbal gestures. Neuropsychologia. 47(1):169–179. doi: 10.1016/j.neuropsychologia.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Kouijzer MEJ, de Moor JMH, Gerrits BJL, Congedo M, van Schie HT. 2009. Neurofeedback improves executive functioning in children with autism spectrum disorders. Res Autism Spectr Disord. 3(1):145–162. doi: 10.1016/j.rasd.2008.05.001. [DOI] [Google Scholar]
- Kreifelts B, Ethofer T, Grodd W, Erb M, Wildgruber D. 2007. Audiovisual integration of emotional signals in voice and face: an event-related fMRI study. Neuroimage. 37(4):1445–1456. [DOI] [PubMed] [Google Scholar]
- Lahav A, Saltzman E, Schlaug G. 2007. Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J Neurosci. 27(2):308–314. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT, et al. 2009. ALE meta-analysis workflows via the Brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird N, Fitzmaurice G, Ding X. 2010. Comments on 'Empirical vs natural weighting in random effects meta-analysis'. Stat Med. 29(12):1266–1267 discussion 1272–1281. doi: 10.1002/sim.3657. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. 2010. The role of anterior insular cortex in social emotions. Brain Struct Funct. 214(5–6):579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Languis ML, Miller DC. 1992. Luria's theory of brain functioning: a model for research in cognitive psychophysiology. Educ Psychol. 27(4):493–511. doi: 10.1207/s15326985ep2704_6. [DOI] [Google Scholar]
- Lewis JW. 2010. Audio-visual perception of everyday natural objects—hemodynamic studies in humans. In: Naumer MJ, Kaiser J, editors. Multisensory object perception in the primate brain. Oxford University Press: Springer, New York. p. 155–190. [Google Scholar]
- Lewis JW, Beauchamp MS, DeYoe EA. 2000. A comparison of visual and auditory motion processing in human cerebral cortex. Cerebral Cortex. 10(9):873–888. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Frum C, Brefczynski-Lewis JA, Talkington WJ, Walker NA, Rapuano KM, Kovach AL. 2011. Cortical network differences in the sighted versus early blind for recognition of human-produced action sounds. Human Brain Mapping. 32(12):2241–2255. doi: 10.1002/hbm.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Phinney RE, Brefczynski-Lewis JA, DeYoe EA. 2006. Lefties get it "right" when hearing tool sounds. J Cogn Neurosci. 18(8):1314–1330. doi: 10.1162/jocn.2006.18.8.1314. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Silberman MJ, Donai JJ, Frum CA, Brefczynski-Lewis JA. 2018. Hearing and orally mimicking different acoustic-semantic categories of natural sound engage distinct left hemisphere cortical regions. Brain Lang. 183:64–78. doi: 10.1016/j.bandl.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Talkington WJ, Puce A, Engel LR, Frum C. 2011. Cortical networks representing object categories and high-level attributes of familiar real-world action sounds. J Cogn Neurosci. 23(8):2079–2101. doi: 10.1162/jocn.2010.21570. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Talkington WJ, Tallaksen KC, Frum CA. 2012. Auditory object salience: human cortical processing of non-biological action sounds and their acoustic signal attributes. Front Syst Neurosci. 6(27):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Wightman FL, Brefczynski JA, Phinney RE, Binder JR, DeYoe EA. 2004. Human brain regions involved in recognizing environmental sounds. Cerebral Cortex. 14:1008–1021. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. 2000. The development of intersensory temporal perception: an epigenetic systems/limitations view. Psychol Bull. 126:281–308. [DOI] [PubMed] [Google Scholar]
- Lissauer H. 1890/1988. A case of visual agnosia with a contribution to theory. Cogn Neuropsychol. 5:157–192. [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. 2008. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS One. 3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. 2009. Category-specific organization in the human brain does not require visual experience. Neuron. 63(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. 2005. The orchestration of the sensory-motor systems: clues from neuropsychology. Cogn Neuropsychol. 22:480–494. [DOI] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. 2011. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 69(5 Pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. 2007. The representation of object concepts in the brain. Annu Rev Psychol. 58:25–45. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. 1996. Neural correlates of category-specific knowledge. Nature. 379(6566):649–652. [DOI] [PubMed] [Google Scholar]
- Matchin W, Groulx K, Hickok G. 2014. Audiovisual speech integration does not rely on the motor system: evidence from articulatory suppression, the McGurk effect, and fMRI. J Cogn Neurosci. 26(3):606–620. doi: 10.1162/jocn_a_00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. 2003. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 4(4):310–322. [DOI] [PubMed] [Google Scholar]
- McNamara A, Buccino G, Menz MM, Glascher J, Wolbers T, Baumgartner A, Binkofski F. 2008. Neural dynamics of learning sound-action associations. PLoS One. 3(12):e3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Baumann S, Marchina S, Jancke L. 2007. Hemodynamic responses in human multisensory and auditory association cortex to purely visual stimulation. BMC Neurosci. 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Nieder A, Freedman DJ, Wallis JD. 2003. Neural correlates of categories and concepts. Curr Opin Neurobiol. 13(2):198–203. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 151(4):264–269 W264. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. 2012. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Muller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, et al. 2018. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller VI, Cieslik EC, Turetsky BI, Eickhoff SB. 2012. Crossmodal interactions in audiovisual emotion processing. Neuroimage. 60(1):553–561. doi: 10.1016/j.neuroimage.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase M, Saito DN, Kochiyama T, Tanabe HC, Tanaka S, Harada T, Aramaki Y, Honda M, Sadato N. 2008. Cross-modal integration during vowel identification in audiovisual speech: a functional magnetic resonance imaging study. Neurosci Lett. 434(1):71–76. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. 2009. Functional organization of the human anterior insular cortex. Neurosci Lett. 457(2):66–70. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Eriksson J, Larsson A, Nyberg L. 2007. The claustrum/insula region integrates conceptually related sounds and pictures. Neurosci Lett. 422(1):77–80. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Eriksson J, Larsson A, Nyberg L. 2011. Cortical regions underlying successful encoding of semantically congruent and incongruent associations between common auditory and visual objects. Neurosci Lett. 505(2):191–195. doi: 10.1016/j.neulet.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Nath AR, Beauchamp MS. 2012. A neural basis for interindividual differences in the McGurk effect, a multisensory speech illusion. Neuroimage. 59(1):781–787. doi: 10.1016/j.neuroimage.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumer MJ, Doehrmann O, Muller NG, Muckli L, Kaiser J, Hein G. 2008. Cortical plasticity of audio-visual object representations. Cereb Cortex. 19(7):1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumer MJ, van den Bosch JJ, Wibral M, Kohler A, Singer W, Kaiser J, van de Ven V, Muckli L. 2011. Investigating human audio-visual object perception with a combination of hypothesis-generating and hypothesis-testing fMRI analysis tools. Exp Brain Res. 213(2–3):309–320. doi: 10.1007/s00221-011-2669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Hocking J, Price CJ, Friston KJ. 2008. The effect of prior visual information on recognition of speech and sounds. Cereb Cortex. 18(3):598–609. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Bordier C, Macaluso E. 2013. Audio-visual perception of 3D cinematography: an fMRI study using condition-based and computation-based analyses. PLoS One. 8(10):e76003. doi: 10.1371/journal.pone.0076003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Macaluso E. 2013. Audio-visual interactions for motion perception in depth modulate activity in visual area V3A. Neuroimage. 71:158–167. doi: 10.1016/j.neuroimage.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Venezia JH, Matchin W, Saberi K, Hickok G. 2013. An fMRI study of audiovisual speech perception reveals multisensory interactions in auditory cortex. PLoS One. 8(6):e68959. doi: 10.1371/journal.pone.0068959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Gatenby JC, Gore JC. 2002. A comparison of bound and unbound audio-visual information processing in the human cerebral cortex. Brain Res Cogn Brain Res. 14(1):129–138. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Hamilton R. 2001. The metamodal organization of the brain. Prog Brain Res. 134:427–445. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 8(12):976–987. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. 2004. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 16(10):1706–1716. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Clark GF, Arbesman M. 2018. Effectiveness of cognitive and occupation-based interventions for children with challenges in sensory processing and integration: a systematic review. Am J Occup Ther. 72(1):7201190020p7201190021–7201190020p7201190029. doi: 10.5014/ajot.2018.028233. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BA, Koenig K, Kinnealey M, Sheppard M, Henderson L. 2011. Effectiveness of sensory integration interventions in children with autism spectrum disorders: a pilot study. Am J Occup Ther. 65(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini P, Furey ML, Ricciardi E, Gobbini MI, Wu WH, Cohen L, Guazzelli M, Haxby JV. 2004. Beyond sensory images: object-based representation in the human ventral pathway. Proc Natl Acad Sci U S A. 101(15):5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank T, Rosengarth K, Song W, Ellermeier W, Greenlee MW. 2012. Neural correlates of audio-visual object recognition: effects of implicit spatial congruency. Hum Brain Mapp. 33(4):797–811. doi: 10.1002/hbm.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AR 3rd, Hillock AR, Wallace MT. 2009. Perceptual training narrows the temporal window of multisensory binding. J Neurosci. 29(39):12265–12274. doi: 10.1523/JNEUROSCI.3501-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F. 2013. How neurons make meaning: brain mechanisms for embodied and abstract-symbolic semantics. Trends Cogn Sci. 17(9):458–470. doi: 10.1016/j.tics.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. 2018. Neural reuse of action perception circuits for language, concepts and communication. Prog Neurobiol. 160:1–44. doi: 10.1016/j.pneurobio.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Raij T, Uutela K, Hari R. 2000. Audiovisual integration of letters in the human brain. Neuron. 28(2):617–625. [DOI] [PubMed] [Google Scholar]
- Ramot M, Kimmich S, Gonzalez-Castillo J, Roopchansingh V, Popal H, White E, Gotts SJ, Martin A. 2017. Direct modulation of aberrant brain network connectivity through real-time NeuroFeedback. Elife. 6:1–23. doi: 10.7554/eLife.28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. 1998. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 3(2–3):86–103. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. 2015. Pathways and streams in the auditory cortex. In: Hickok GS, Small SL, editors. Neurobiology of language. London: Elsevier. p. 287–298. https://books.google.com/books?hl=en&lr=&id=9c2cBAAAQBAJ&oi=fnd&pg=PP1&dq=In:+Hickok+GS,+Small+SL,+editors.+Neurobiology+of+language.+Elsevier.+location&ots=jsYzD1YNjZ&sig=uf3bLczJDywc5fzs4zhUP8G6g3Q#v=onepage&q&f=false. [Google Scholar]
- Rauschecker JP, Tian B. 2000. Mechanisms and streams for processing of ``what'' and ``where'' in auditory cortex. Proc Natl Acad Sci U S A. 97(22):11800–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenbogen C, Seubert J, Johansson E, Finkelmeyer A, Andersson P, Lundstrom JN. 2018. The intraparietal sulcus governs multisensory integration of audiovisual information based on task difficulty. Hum Brain Mapp. 39(3):1313–1326. doi: 10.1002/hbm.23918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK. 2008. Neuroscientific approaches and applications within anthropology. Am J Phys Anthropol. 51Suppl (47):2–32. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. 1998. Language within our grasp. Trends Neurosci. 21(5):188–194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu Rev Neurosci. 27:169–192. [DOI] [PubMed] [Google Scholar]
- Roa Romero Y, Keil J, Balz J, Gallinat J, Senkowski D. 2016. Reduced frontal theta oscillations indicate altered crossmodal prediction error processing in schizophrenia. J Neurophysiol. 116(3):1396–1407. doi: 10.1152/jn.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Baron-Cohen S. 2017. Sensory perception in autism. Nat Rev Neurosci. 18(11):671–684. doi: 10.1038/nrn.2017.112. [DOI] [PubMed] [Google Scholar]
- Robins DL, Hunyadi E, Schultz RT. 2009. Superior temporal activation in response to dynamic audio-visual emotional cues. Brain Cogn. 69(2):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch EH. 1973. Natural categories. Cogn Psychol. 4:328–350. [Google Scholar]
- Saygin AP, Leech R, Dick F. 2010. Nonverbal auditory agnosia with lesion to Wernicke's area. Neuropsychologia. 48(1):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Boecker H, Daamen M, Fehse U, Landsberg MW, Granath DO, Mechling H, Effenberg AO. 2009. Multimodal motion processing in area V5/MT: evidence from an artificial class of audio-visual events. Brain Res. 1252:94–104. [DOI] [PubMed] [Google Scholar]
- Schmid C, Buchel C, Rose M. 2011. The neural basis of visual dominance in the context of audio-visual object processing. Neuroimage. 55(1):304–311. doi: 10.1016/j.neuroimage.2010.11.051. [DOI] [PubMed] [Google Scholar]
- Sekiyama K, Kanno I, Miura S, Sugita Y. 2003. Auditory-visual speech perception examined by fMRI and PET. Neurosci Res. 47(3):277–287. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Di Matteo R, Ferretti A, Del Gratta C, Caulo M, Tartaro A, Olivetti Belardinelli M, Romani GL. 2006. "what" versus "where" in the audiovisual domain: an fMRI study. Neuroimage. 33(2):672–680. [DOI] [PubMed] [Google Scholar]
- Shura RD, Hurley RA, Taber KH. 2014. Insular cortex: structural and functional neuroanatomy. J Neuropsychiatry Clin Neurosci. 26(4):276–282. doi: 10.1176/appi.neuropsych.260401. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. 2004. Empathy for pain involves the affective but not sensory components of pain. Science. 303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. 2007. Identification and classification of hubs in brain networks. PLoS One. 2(10):e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. 1990. Multisensory integration. Ann N Y Acad Sci. 608(608):51–70. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. 1993. The merging of the senses. Cambridge (MA): MIT Press. [Google Scholar]
- Stein BE, Wallace MT. 1996. Comparisons of cross-modality integration in midbrain and cortex. Prog Brain Res. 112:289–299. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, James TW. 2009. Audiovisual integration in human superior temporal sulcus: inverse effectiveness and the neural processing of speech and object recognition. Neuroimage. 44(3):1210–1223. [DOI] [PubMed] [Google Scholar]
- Straube B, Green A, Bromberger B, Kircher T. 2011. The differentiation of iconic and metaphoric gestures: common and unique integration processes. Hum Brain Mapp. 32(4):520–533. doi: 10.1002/hbm.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B, Green A, Sass K, Kircher T. 2014. Superior temporal sulcus disconnectivity during processing of metaphoric gestures in schizophrenia. Schizophr Bull. 40(4):936–944. doi: 10.1093/schbul/sbt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szycik GR, Jansma H, Munte TF. 2009. Audiovisual integration during speech comprehension: an fMRI study comparing ROI-based and whole brain analyses. Hum Brain Mapp. 30(7):1990–1999. doi: 10.1002/hbm.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe HC, Honda M, Sadato N. 2005. Functionally segregated neural substrates for arbitrary audiovisual paired-association learning. J Neurosci. 25(27):6409–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. 2006. Binding crossmodal object features in perirhinal cortex. Proc Natl Acad Sci U S A. 103(21):8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KI, Stamatakis EA, Tyler LK. 2009. Crossmodal integration of object features: voxel-based correlations in brain-damaged patients. Brain. 132:671–683. [DOI] [PubMed] [Google Scholar]
- Tench CR, Tanasescu R, Auer DP, Cottam WJ, Constantinescu CS. 2014. Coordinate based meta-analysis of functional neuroimaging data using activation likelihood estimation; full width half max and group comparisons. PLoS One. 9(9):e106735. doi: 10.1371/journal.pone.0106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D, et al. 2005. Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci. 17(2):273–281. [DOI] [PubMed] [Google Scholar]
- Tomasello R, Garagnani M, Wennekers T, Pulvermuller F. 2017. Brain connections of words, perceptions and actions: a neurobiological model of spatio-temporal semantic activation in the human cortex. Neuropsychologia. 98:111–129. doi: 10.1016/j.neuropsychologia.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. 1997. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 35(10):1319–1327. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Eichhorn GR, Grabowski TJ, Ponto LLB, Hichwa RD. 2003. Neural correlates of naming animals from their characteristic sounds. Neuropsychologia. 41:847–854. [DOI] [PubMed] [Google Scholar]
- Trumpp NM, Kliese D, Hoenig K, Haarmeier T, Kiefer M. 2013. Losing the sound of concepts: damage to auditory association cortex impairs the processing of sound-related concepts. Cortex. 49(2):474–486. doi: 10.1016/j.cortex.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 16(3 Pt 1):765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. 2012. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Moss HE. 2001. Towards a distributed account of conceptual knowledge. Trends Cogn Sci. 5(6):244–252. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE. 2004. Processing objects at different levels of specificity. J Cogn Neurosci. 16(3):351–362. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. 1994. 'What' and 'where' in the human brain. Curr Opin Neurobiol. 4(2):157–165. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M, Goodale MA, Mansfield RJW. 1982. Two cortical visual systems. In: Ingle DJ, editor. Analysis of visual behavior. Cambridge (MA): MIT Press. p. 549–586. [Google Scholar]
- van Atteveldt N, Formisano E, Goebel R, Blomert L. 2004. Integration of letters and speech sounds in the human brain. Neuron. 43(2):271–282. [DOI] [PubMed] [Google Scholar]
- van Atteveldt NM, Blau VC, Blomert L, Goebel R. 2010. fMR-adaptation indicates selectivity to audiovisual content congruency in distributed clusters in human superior temporal cortex. BMC Neurosci. 11:11. doi: 10.1186/1471-2202-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Atteveldt NM, Formisano E, Goebel R, Blomert L. 2007. Top-down task effects overrule automatic multisensory responses to letter-sound pairs in auditory association cortex. Neuroimage. 36(4):1345–1360. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn Sci. 17(12):683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Van der Stoep N, Van der Stigchel S, Van Engelen RC, Biesbroek JM, Nijboer TCW. 2019. Impairments in multisensory integration after stroke. J Cogn Neurosci. 31(6):885–899. doi: 10.1162/jocn_a_01389. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. 2005. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 28(3):635–662. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. 2001. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 8(5):443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wyk BC, Ramsay GJ, Hudac CM, Jones W, Lin D, Klin A, Lee SM, Pelphrey KA. 2010. Cortical integration of audio-visual speech and non-speech stimuli. Brain Cogn. 74(2):97–106. doi: 10.1016/j.bandc.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanes LD, White TP, Wigton RL, Joyce D, Collier T, Shergill SS. 2016. Reduced susceptibility to the sound-induced flash fusion illusion in schizophrenia. Psychiatry Res. 245:58–65. doi: 10.1016/j.psychres.2016.08.016. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Giraud AL. 2006. Implicit multisensory associations influence voice recognition. PLoS Biol. 4(10):e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L. 1978. Mind in society: the development of higher psychological processes. Cambridge (MA): Harvard University Press. [Google Scholar]
- Watkins S, Shams L, Josephs O, Rees G. 2007. Activity in human V1 follows multisensory perception. Neuroimage. 37(2):572–578. [DOI] [PubMed] [Google Scholar]
- Watkins S, Shams L, Tanaka S, Haynes JD, Rees G. 2006. Sound alters activity in human V1 in association with illusory visual perception. Neuroimage. 31(3):1247–1256. [DOI] [PubMed] [Google Scholar]
- Watson R, Latinus M, Charest I, Crabbe F, Belin P. 2014. People-selectivity, audiovisual integration and heteromodality in the superior temporal sulcus. Cortex. 50:125–136. doi: 10.1016/j.cortex.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R, Latinus M, Noguchi T, Garrod O, Crabbe F, Belin P. 2014. Crossmodal adaptation in right posterior superior temporal sulcus during face-voice emotional integration. J Neurosci. 34(20):6813–6821. doi: 10.1523/JNEUROSCI.4478-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster PJ, Frum C, Kurowski-Burt A, Wen S, Bauer CE, Ramadan J, Baker K, Lewis JW. 2020. Processing of real-world, dynamic natural stimuli in autism is linked to corticobasal function. Autism Res. 13(4):539–549. doi: 10.1002/aur.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Noppeney U. 2010. Distinct functional contributions of primary sensory and association areas to audiovisual integration in object categorization. J Neurosci. 30(7):2662–2675. doi: 10.1523/JNEUROSCI.5091-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Ozyurek A, Hagoort P. 2007. When language meets action: the neural integration of gesture and speech. Cereb Cortex. 17(10):2322–2333. doi: 10.1093/cercor/bhl141. [DOI] [PubMed] [Google Scholar]
- Wolf D, Schock L, Bhavsar S, Demenescu LR, Sturm W, Mathiak K. 2014. Emotional valence and spatial congruency differentially modulate crossmodal processing: an fMRI study. Front Hum Neurosci. 8:659. doi: 10.3389/fnhum.2014.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. 2006. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 29(7):359–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


