Abstract
Hematopoietic stem cell (HSC) numbers collected in cord blood (CB) at the birth of a baby is a limiting factor for efficacious use of CB in hematopoietic cell transplantation (HCT). We now demonstrate that collecting and processing of human CB at 4°C within minutes of the baby’s birth results in significantly enhanced numbers of rigorously defined phenotypic HSC and self-renewing NSG immune-deficient mouse engrafting and SCID-repopulating cells. This was associated with decreased numbers of hematopoietic progenitor cells (HPC), as noted previously for hypoxia collected/processed cells blocking ambient air induced differentiation of HSC to HPC. We have thus defined a simple, cost-effective, means to collect increased numbers of CB HSC, of potential use for clinical CB HCT.
Keywords: cord blood, hematopoietic stem cells, hematopoietic progenitor cells, hematopoietic cell transplantation
Graphical Abstract
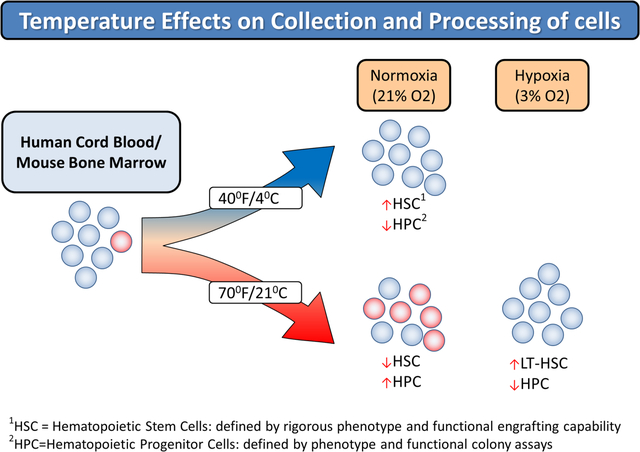
Introduction
Cord blood (CB) has been used as a source of transplantable hematopoietic stem (HSC) and progenitor (HPC) cells for hematopoietic cell transplantation (HCT) since 1988.1–3 Since the first successful transplant1 treating and curing a young boy with HLA-matched sibling CB cells, there have been over 40,000 CB HCT performed3–5 to treat the same malignant and non-malignant disorders by bone marrow (BM) and mobilized peripheral blood (mPB) using allogeneic cells. CB for HCT has advantages and disadvantages compared to BM and mPB.3–5 A limitation of CB for HCT is lower numbers of HSC in single CB collections compared to BM and mPB. While double CB unit HCT has been in practice for years in attempts to compensate for low HSC numbers in single CB units,3–6 it is known that in majority of double CB unit HCT efforts only one of the infused CB units survives.3–6 Thus, there may not be an advantage using double versus single CB units for HCT.7 Increased use of single CB units for HCT may be due in part to better selected (e.g. bigger) CB units, and improved CB HCT recipient care. Other attempts to enhance efficacy of single unit CB HCT, most of which are still laboratory-based, include: enhancing homing efficiency of CB HSC to BM of recipient patients, ex-vivo expansion of CB HSC, and collecting more cells from the CB at the birth of the baby.8,9 However, there are much needed efforts to collect more CB HSC with simple/minimal manipulation.
We reported that collection and processing of CB in ambient air results in “Extra Physiological Oxygen Shock/Stress (EPHOSS)” and collection/processing CB in hypoxic conditions (e.g. at 3% oxygen compared to ambient air [e.g. ~21% oxygen]), so that the cells are never exposed to ambient air oxygen levels, greatly enhanced numbers of collected phenotypically and functionally defined HSC.10 Such collections may not be logistically and cost-effectively feasible. Other means that may in part mimic hypoxia collection/processing are needed. One means is to use cyclosporine A when cells are collected in ambient air, but this process has logistical concerns.11 We have also used combinations of antioxidants and/or epigenetic regulators (as each alone does not work) to enhance collection of mouse BM HSC in ambient air,12 but it is not clear if this would work for human CB cells. In ongoing efforts to elucidate means for collecting more HSC in single CB unit collections, we evaluated the influence of temperature on collection of human CB HSC. We found enhanced numbers of both phenotypically and functionally defined self-renewing HSC when collection and processing of CB at the birth of a baby was performed at chilled temperatures on ice.
Materials and Methods
Collection of human CB mononuclear and CD34+ cells
Human CB was harvested at birth of babies at Eskenazi Hospital in accordance with Indiana University School of Medicine (IUSM) Institutional Review Board guidelines (IRB protocol # 1011002987) as previously reported.10 Collection of CB was performed within 5 minutes of placental delivery through a single venipuncture, 20–30ml of blood was harvested into a 60cc syringe, inverted 1x to mix, and immediately divided into two containers. One container holding 50 ml of ice cold (~4°C) PBS with heparin (Sigma; catalog #H3393) at 1000 units/ml and subsequently maintained on ice, and the other containing room temperature (RT) PBS with heparin. Cells were transported from delivery room to laboratory at either RT or on ice (~4°C). After allowing cells to remain at RT or on ice for one hour, all subsequent processing was performed at 4°C which included mononuclear and CD34+ cell enrichment to eliminate all further processing variables and influences. The only difference between samples was the temperature less than 10 minutes after time of collection. Mononuclear cells from human CB were isolated by density gradient centrifugation with Ficoll-Paque Plus (GE Healthcare) at 4°C. CD34+ cells were collected using an immunomagnetic selection kit according to manufacturer’s instructions (Miltenyi Biotec; catalog #130-046-702) at 4°C. This yielded CD34+ cells with a purity of 90–98%. All procedures were performed at 4°C (i.e., staining of cells for flow cytometry, etc.) and for all experiments centrifuges were set to 4°C. See Fig. S1 for experimental design. While procedures were not performed in a cold room and running temperatures were not actively monitored, all buffers for enrichment, collection tubes and other plastics used were either submersed and maintained in an ice bath, placed in a 4°C refrigerator, or rinsed with ice cold buffer prior to use to maintain temperatures as close to 4°C as possible throughout the procedure.
Mice
C57BL/6J, Boy/J, B6×Boy/J F1 (referred to as F1), and immunodeficient NSG (NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ) mice (6–10 weeks old) were obtained from on-site breeding core facility at IUSM. Animals were maintained under temperature- and light-controlled conditions (21–24°C, 12-hour light/12-hour dark cycle) and were group-housed according to age, sex, and genotype. Mice were fed ad libitum. Mice were matched by age and sex. All animal experiments followed protocols approved by The Institutional Animal Care and Use Committee of IUSM.
Flow Cytometry
Immediately after the 1 hour acclimation time to RT or 4°C, cells were placed in flow tubes at concentration of approximately 2×106 cells per tube, incubated in fluorescently conjugated anti-human antibody cocktail for 20 minutes at ~4°C, washed in cold PBS, and fixed in cold 1% formaldehyde. Samples were analyzed on an LSR II flow cytometer (BD Biosciences). Single-color compensation and isotype controls were included for each experiment. Data analysis was performed using FlowJo 7.6.3 software (Tree Star). Gates were determined using fluorescence minus one controls. Percent of each population was used to calculate numbers/106 CB cells. Human phenotyping markers included: FITC- human lineage cocktail 1 (CD3, CD14, CD19, CD20, CD56; catalog #340546; BD Biosciences), FITC or APC-anti-CD34 (clone 581; BD Biosciences), PE-anti-CD38 (clone HIT2; BD Biosciences), PE-CF594-anti-CD45RA (clone HI100; BD Biosciences), PE-Cy7-anti-CD90 (clone 5E10; BD Biosciences), PerCp-Cy5.5-anti-CD49f (clone WM53; BD Biosciences), APC-anti-CD45 (clone HI30; BD Biosciences) and APC-anti-CD184 (CXCR4; clone 12G5; BD Biosciences). HSCs were defined as lineage− CD34+ CD38− CD45RA− CD90+ CD49f+, multipotent progenitors (MPPs) as lineage− CD34+ CD38− CD45RA− CD90− CD49f−, and progenitors as lineage− CD34+ CD38+.13 See supplementary materials for mouse immunophenotyping.
Functional assessment of human CB CD34+ cells by engraftment into NSG mice.
Frequency of human SCID repopulating cells (SRCs) was analyzed by limiting dilution analysis.14 Increasing doses of CD34+ cells (500, 2500, or 10000 cells) were intravenously injected into sub-lethally irradiated (300 cGy) NSG recipients. At 1, 2, 4 and 5 months post transplantation, cell chimerism was determined in peripheral blood (PB) by immunostaining and flow cytometry. At 5 months, recipient mice were euthanized and chimerism in the BM was examined. For assessment of self-renewal capacity of donor cells, secondary transplants were done with 5×106 BM cells from primary recipients of the 10,000 cell engrafted group. SCID Repopulating Cell (SRC; HSC) frequency was calculated using L-Calc software (Stem Cell Technologies Inc, Vancouver, BC, Canada) and plotted using ELDA software (bioinf.wehi.edu.au/software/elda/).14
Human CB HPC by colony assay
Immediately following one hour acclimation to RT or 4°C, 5×104 human CB mononuclear cells were plated in 1 ml of colony assay media containing Iscove’s Modified Dulbecco’s Medium (IMDM, Lonza), 1% methylcellulose, 30% fetal bovine serum (FBS, Hyclone), 50 ng recombinant human (rhu) stem cell factor (SCF, R&D Systems), 10 ng rhu granulocyte-macrophage-colony stimulating factor (GM-CSF, R&D Systems), 10 ng rhu interleukin 3 (IL-3, R&D Systems), 1 unit rhu erythropoietin (EPO, Amgen) in a 37°C 5% CO2/O2 environment. Colony forming units (CFU) granulocyte-macrophage (GM) and CFU- granulocyte/erythroid/macrophage/megakaryocyte (GEMM) were distinguished by morphology after 13–14 days incubation. Total colonies per ml of CB were calculated.15 Essentially all erythroid cell containing CB colonies obtained after stimulation with the combination of SCF, GM-CSF, IL-3, and EPO at 5% O2 are CFU-GEMM. Few or no BFU-E or CFU-E colonies form, thus the reason for not including data on BFU-E or CFU-E.16
Statistics
Data are mean values ± standard error mean (SEM). Statistical analysis was performed using Microsoft Excel and GraphPad Prism 5.0. Two-tailed Student’s t -tests were performed for statistical analysis between two groups. P-value less than 0.05 was considered as statistically significant. For limiting dilution analysis, statistics were performed by indicated software.
Results and Discussion
Recent studies suggested that collection/processing of CB immediately at the birth of a baby in an hypoxic atmosphere of 3% O2 vs. that at ambient air (~21%), such that the cells were never exposed to ambient air O2 levels, resulted in greatly increased numbers of both phenotypically- and functionally-defined HSC, with concomitant decreases in HPC.10 Hypoxia collected/processed CB blocked ambient air EPHOSS-related effects that resulted in decreased HSC and increased HPC numbers. These effects were not due to cell death mechanisms, but rather to hypoxia ameliorating cell differentiation through mechanisms involving a p53-mitochondrial permeability transition pore-cyclophilin D axis, which was associated with hypoxia inducing factor-1 and hypoxamir, miR210.10 We could by-pass hypoxia collection if cells were immediately collected in ambient air in the presence of cyclosporine A.10 But this method would not be logistically of help.11 To increase collections of HSC, it is not likely that hypoxia collection would be used by CB collection centers as most will not have immediate access to a hypoxic chamber. Use of cyclosporine A had batch to batch differences in cyclosporine A creating a need to define its optimal concentrations with every new batch. Also cyclosporine A is difficult to get into solution. Hence, we have evaluated more easily used means to enhance collections of increased CB HSC numbers. We reasoned from our previously identified mechanistic insights into enhanced hypoxia CB HSC collections,10 that metabolism was involved and perhaps collecting CB at ~4°C (cold), rather than RT, might be advantageous to enhanced collection of CB HSC. This turned out to be a quick and easy means to collect more CB HSC.
Human CB Collections
Collection/processing of cord blood at 4°C, in contrast to RT, demonstrated a doubling of the collection of rigorously-defined HSC (Fig. 1A), with no significant changes in MPP and more mature progenitor numbers (Figs. 1B&C). Since phenotype of HSC does not always recapitulate numbers of functional HSC,17 we evaluated engrafting capacity of CB cells in sub-lethally irradiated immune deficient NSG mice. This demonstrated enhanced collection of functional HSC as determined by chimerism of human CB engraftment of CD34+ cells into NSG mice in a primary transplant (Fig. 1D & E), as well as in a secondary transplant setting (Fig. 1F). Calculations of functional human CB SCID repopulating cells (SRC; functional HSC) numbers via limiting dilution analysis in sub-lethally irradiated NSG mice demonstrated highly significant increases in functional CB HSC in cold collected and processed cells (Fig. 1G & H and S. Table 1). Thus, cold-collected/processed cells manifested increased long-term repopulating self-renewing human CB HSC numbers, as for hypoxia collection/processing.10 As previously noted by us under hypoxia collection/processing,10 this increase in HSC numbers was associated with significant decreases in functional populations of CFU-GM and CFU-GEMM HPC (Fig. 2), due to presumptive blocking of air EPHOSS-induced differentiation of HSC to HPC.10
Figure 1. Isolating human cord blood (CB) at 4°C (cold) increases phenotypically-defined hematopoietic stem cell (HSC) numbers and enhances CB CD34+ cell engraftment in NSG mice compared to CB at room temperature (RT).
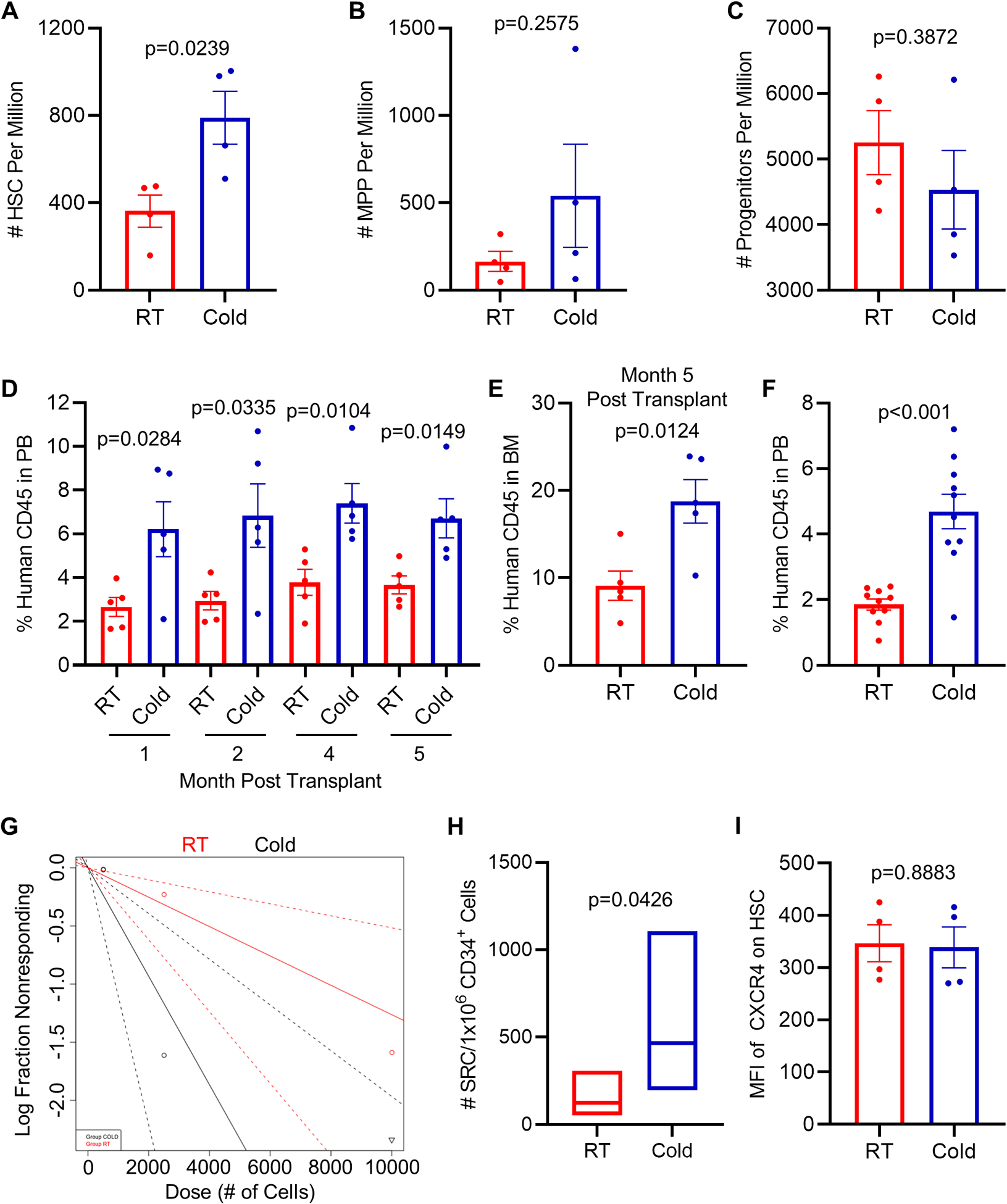
CB mononuclear cells were analyzed by flow cytometry for HSC (A), multipotent progenitor (B) and later progenitor cells (C) numbers. (A-C) Data represents average mean ± SEM of 4 separate CBs. (D) Percentage of human CD45+ cells in peripheral blood (PB) of NSG mice at indicated time points after transplantation with 10,000 CB CD34+ cells. (n=5 mice per group). (E) Percentage of human CD45+ cells in bone marrow (BM) of recipient mice from (D) at 5 months post transplantation. (F) Human CD45+ cell chimerism in the PB (month 4) of secondary recipient NSG mice which had been transplanted with 5×106 BM cells from primary recipient NSG mice (n=10 mice per group). (G-H) Frequency of human SRCs in CB CD34+ cells acclimated to RT vs. left at 4°C, as determined by limiting dilution analysis at 5 months after transplantation (n=5 mice per group, see Supplementary Table 1). (G) Poisson statistical analysis of data from S. Table 1. Shapes (circle or triangle in the plot) represent percentage of negative mice for each dose of cells. Inverted triangles indicate that all tested mice were positive in this group. Solid lines indicate best-fit linear model for each data set. Red line indicates RT group, black line cold group. Dotted lines represent 95% confidence intervals. (H) HSC frequencies (line in the boxes) and 95% confidence intervals (box) presented as numbers of SRCs in 1×106 CD34+ cells. HSC frequency was calculated, and statistical tests performed using L-Calc and ELDA software. (I) Quantification of mean fluorescence intensity (MFI) of CXCR4 within HSC populations. Data represents mean ± SEM of 4 tubes from 2 CB samples (2 tubes per cord). Statistical analysis used Student’s t test.
Figure 2. Isolating CB at 4°C results in fewer functional progenitor cells compared to CB isolated at RT.
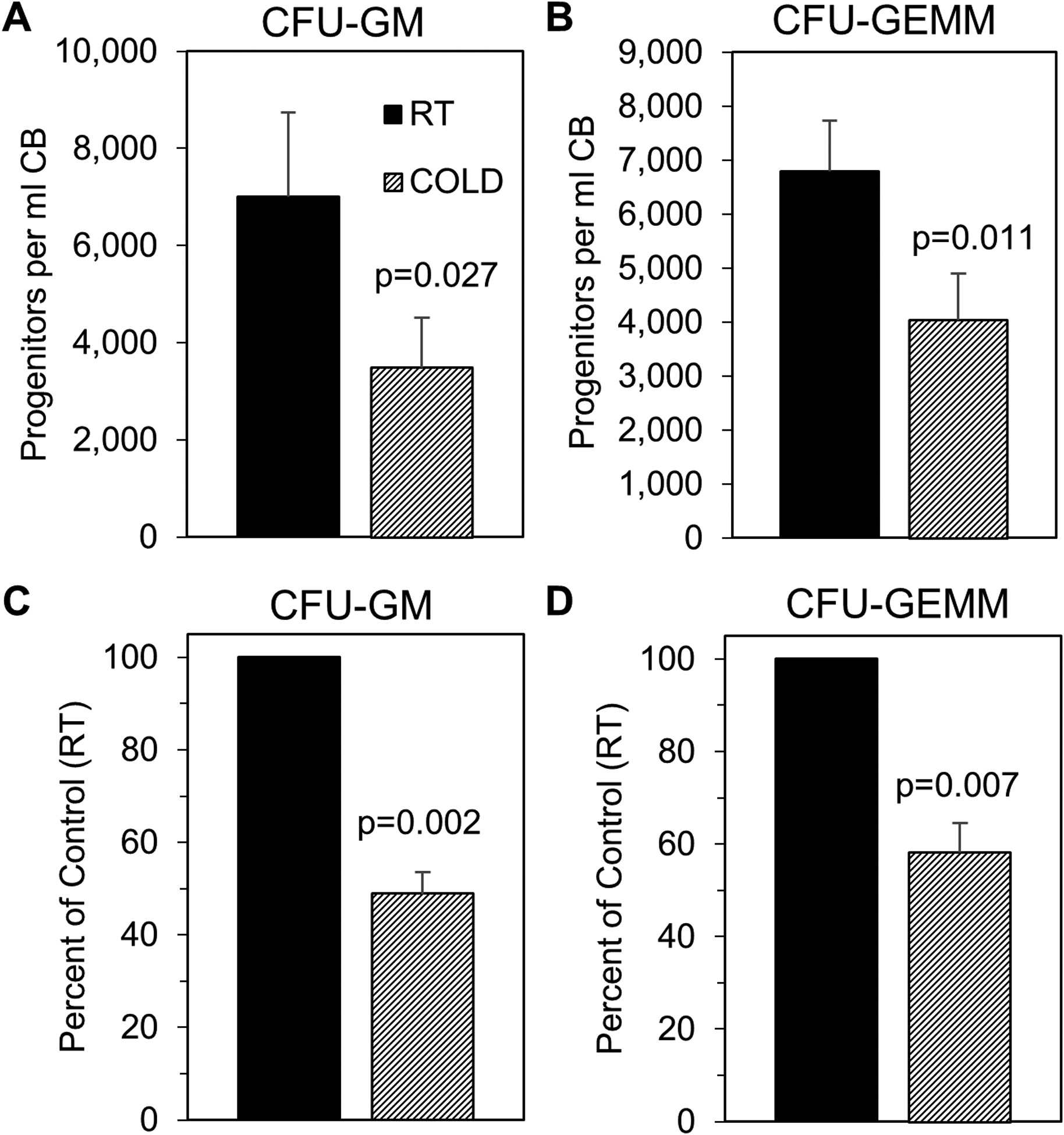
Human CB mononuclear cells were collected as described in Fig. S1. Mononuclear cells were then utilized for hematopoietic progenitor colony forming assays. The number of colony-forming units- granulocyte, macrophage (CFU-GM; A) and CFU- granulocyte, erythrocyte, macrophage, megakaryocyte (CFU-GEMM; B) per ml CB was determined. Percent of control (RT) was also calculated (C-D). Data are average mean ± SEM of 4 separate CBs. Statistical analysis was performed using Student’s t test.
Mouse BM Collections
We determined influences of cold collection and processing on numbers of mouse BM HSC and HPC. Cold collection/processing demonstrated significantly increased numbers of rigorously phenotypically defined LT-HSC and ST-HSC, without effects on phenotypically-defined HPC populations of MPP, CMP, GMP, MEP, and CLP (S. Fig. 2A–H), and with significantly decreased functionally assessed HPC (CFU-GM, BFU-E and CFU-GEMM (S. Fig. 3A–C). However, HSC engrafting capability of cold collected mouse BM did not result in increased engraftment in a competitive repopulating situation using congenic mouse recipients (B6 × Boy/J F1 mice; CD45.1+ CD45.2+), C57BL/6 (CD45.1− CD45.2+) donor and Boy/J CD45.1+ CD45.2−) competitor mouse BM cells (S. Fig. 3D).
To determine why cold collected HSC from mouse BM cells failed to enhance engraftment, we examined whether cold induced decreases in expression of CXCR4 (S. Fig. 3E) and decreased chemotaxis of cold collected HSC to CXCL12 (a.k.a. stromal cell factor-1/SDF-1; S. Fig. 3F). We found significantly decreased cold collected HSC CXCR4 expression and chemotactic responses to CXCL12. Since the CXCL12-CXCR4 axis has been implicated in homing of HSC,9 this may be one reason that mouse BM HSC, shown to be increased in phenotypically-defined numbers of HSC, did not manifest in increased numbers of engrafting HSC. When we checked effects of cold collections of CB HSC on expression of CXCR4, there was no down-regulation of CXCR4 induced by cold treatment (Fig. 1I). It is not clear why CXCR4 was down-regulated on collection/processing of mouse BM cells, but not on cold collected human CB cells. It is possible that future mouse studies may be able to enhance the engraftment of cold collected mouse BM HSCs by enhancing the expression of CXCR4 and their homing capabilities, although some methods to increase homing of human CB HSCs do not work on mouse BM cells.9 Most importantly this was not a problem for the human CB HSCs.
These findings suggest that the use of cold temperature to collect human CB HSC for CB banking and CB HCT certainly warrants further investigation and the possible use of this simple form of collection to increase CB HSC numbers. Even though this and hypoxic collection of CB cells results in decreased numbers of HPC,10 there are still many HPCs collected in hypoxia or with cold maneuvers. These lower numbers of HPC will not likely effect outcomes of HCT using cold collected cells, as seen with human CB engraftment studies in NSG mice (Fig. 1D–H). It is possible that information shown in this paper will be able to enhance the efficacy of CB HCT via the retention of existing HSC in harvested samples. This method of collecting CB in colder temperatures to enhance functional HSC numbers would not only be simple and clinically feasible but should be cost efficient as well.
Supplementary Material
Significance Statement.
Numbers of collected cord blood hematopoietic stem cells at the birth of a baby is a limiting factor in use of cord blood for hematopoietic cell transplantation. We now demonstrate, by collecting and processing cord blood at 4°C, that we can attain significantly enhanced numbers of cord blood hematopoietic stem cells for potential use in clinical cord blood hematopoietic cell transplantation, in a simple and cost-effective manner.
Acknowledgments
These studies were supported by US Public Health Service grants from the NIH: R35 HL 139599 to HEB, R01 DK 109188 to HEB and MLC, and U54 DK 106846 to HEB. Special thanks to David Haas, M.D. and Brittney Yeley, B.S., C.C.R.P of the Obstetrics and Gynecology Research team at IUSM and Eskenazi Hospital for helping procurement of human CB.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Author Disclaimers: Hal E. Broxmeyer: has nothing to disclaim. Scott Cooper: has nothing to disclaim. Maegan L. Capitano: has nothing to disclaim.
References
- 1.Gluckman E, Broxmeyer HE, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989;321:1174–1178. [DOI] [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA 1989;86:3828–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broxmeyer HE, Farag SS, Rocha V. Cord blood hematopoietic cell transplantation. In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR (Eds.), Thomas’ Hematopoietic Cell Transplantation, 5th Edition, John Wiley & Sons, Ltd: Oxford, England, 2016:437–455. [Google Scholar]
- 4.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation- the first 25 years and beyond. Blood 2013;122: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayani H, Wagner JE, Broxmeyer HE. Cord blood research, banking and transplantation: Achievements, challenges, and perspective. Bone Marrow Transplant 2020;55:48–61. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005;105:1343–1347. [DOI] [PubMed] [Google Scholar]
- 7.Milano F, Delaney C, Storb R. Graft-versus-host-disease after double cord blood transplantation: a new look at its characteristics. Biol Blood Marrow Transplant 2013;19:847–848. [DOI] [PubMed] [Google Scholar]
- 8.Ballen K Umbilical cord blood transplantation: Challenges and future directions. Stem Cells Transl Med 2017;6:1312–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Broxmeyer HE. Progress towards improving homing and engraftment of hematopoietic stem cells for clinical transplantation. Curr Opin Hematol 2019;26:266–272. [DOI] [PubMed] [Google Scholar]
- 10.Mantel CR, O’Leary HA, Chitteti BR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 2015;161:1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broxmeyer HE, O’Leary HA, Huang X, et al. The importance of hypoxia and EPHOSS for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex-vivo. Curr Opin in Hematol 2015;22:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Q, Capitano M, Huang X, et al. Combinations of antioxidants and/or of epigenetic enzyme inhibitors allow for enhanced collection of mouse bone marrow hematopoietic stem cells in ambient air. Blood Cells Mol Dis 2018;71:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doulatov S, Notta F, Laurenti E, et al. 2012. Hematopoiesis: a human perspective. Cell Stem Cell 2012;10:120–136. [DOI] [PubMed] [Google Scholar]
- 14.Guo B, Huang X, Cooper S, et al. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat Med 2017;23:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med 2012;18:1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation of adults. Proc. Natl. Acad. Sci. USA 1992;89:4109–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Yao C, Teng Y, et al. Phorbol ester induced ex vivo expansion of rigorously-defined phenotypic but not functional human cord blood hematopoietic stem cells: a cautionary tale demonstrating that phenotype does not always recapitulate stem cell function. Leukemia. 2019;33:2962–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


