Abstract
Background
In the developed world, 5‐years survival of childhood acute myeloid leukaemia (AML) has improved to 70%. However, the survival rates in the developing world are below 40%. The main contributing factors to these reduced survival rates are a late presentation, malnutrition and high treatment‐related mortality.
Aim
To document the factors affecting treatment outcome of childhood AML at a tertiary care facility of Pakistan.
Methods and Results
All newly registered cases of AML under 18 years of age from January 1, 2012 onwards who completed their treatment before November 30, 2019 were included. Data of 219 cases of AML containing 140 (63.9%) males and 79 (36.1%) females was analyzed. The mean age was 6.30 ± 3.66 years. Pallor was the commonest presenting features in 180 (82.2%) and M2 was the commonest French American‐British (FAB) subtype in 103 (47.0%) cases. In univariate analysis, high white blood cells (WBC) count at presentation (P = .006), poor nutritional status (P = .005), unfavourable cytogenetics (P = .019), certain types of FAB AML subtype (P = .005), and use of etoposide in induction chemotherapy (P = .042) significantly adversely affected overall survival (OS). Neutropenic sepsis and bleeding were the major causes of treatment‐related mortality. Response to induction chemotherapy was the most significant prognostic factor in the multivariate analysis (P = <.001). After a median follow‐up of 40.96 ± 26.23 months, 5‐year OS and DFS of the cohort were 40.6% and 38.3% respectively.
Conclusions
In this largest cohort of childhood AML from Pakistan, high WBC count at presentation, malnutrition, unfavourable cytogenetics and use of etoposide during induction chemotherapy were associated with decreased OS and DFS rates. Response to the induction chemotherapy was the most significant prognostic factor.
Keywords: mortality, nutritional status, paediatric acute myeloid Leukaemia, Pakistan, treatment outcome
1. INTRODUCTION
Childhood acute myeloid leukaemia (AML) accounts for 15% to 25% of leukaemia in children. In recent years, the outcome of childhood AML has improved substantially resulting in 5‐year survival to 70% in the developed world. This better survival is attributed to the risk‐based intensive chemotherapy regimens, hematopoietic stem cell transplantation (HSCT) and improved supportive care measures. 1 , 2 , 3 However, most children live in low‐and middle‐income countries (LMICs), where survival rates are below 40%. 4 The main contributing factors to these reduced survival rates are a late presentation, malnutrition and suboptimal supportive and intensive care resulting in high treatment‐related mortality (TRM). 5 , 6 , 7 , 8
Pakistan is a middle‐income country and majority of the cases of childhood AML are not treated because of unaffordability of treatment cost, high TRM and relapse rate and limited treatment facilities for oncology patients in the country. There is a paucity of published data on childhood AML from this part of the world. Pediatric oncology department at Combined Military Hospital (CMH) Rawalpindi is treating all types of paediatric AML cases coming from all over the country. This retrospective study describes the prognostic factors affecting treatment outcome of childhood AML in Pakistani children.
2. PATIENTS AND METHODS
The study was carried out at the Pediatric Oncology department of CMH Rawalpindi, Pakistan. CMH is a military hospital primarily responsible for treating army personnel and their dependents. However, because of the scarcity of dedicated facilities for haematology and oncology in the country, a large number of civilians, especially from northern Pakistan, are also treated here.
Medical records of all newly diagnosed cases of de novo AML younger than 18 years of age, registered since January 2012 and finished their treatment before November 30, 2019, were studied. Patients with Acute Promyelocytic Leukaemia (APL), treatment‐related AML and having prior chemotherapy or leaving during treatment were excluded. Study data included: age, sex, blood counts at presentation, weight‐for‐age, French American‐British (FAB) classification, genetic abnormalities, chemotherapy protocol, treatment outcome, last follow up, and cause of mortality if applicable. Detailed medical history and clinical examination were performed at the first admission. Diagnosis of AML was made on blast morphology in bone marrow (BM) aspiration sample and immunophenotyping by flow cytometry. The diagnostic workup incorporated complete blood picture with differential blood cell counts, biochemical profile consisting of hepatic and renal function tests, uric acid, and assessment of cardiac status with echocardiography before the start of chemotherapy. Response to chemotherapy was assessed by performing BM aspiration after recovery of blood counts. Cases were labeled as having CR, PR and RD as per the following definitions. Complete Remission (CR); BM blasts <5%; absence of blasts with Auer rods; absence of extramedullary disease; independence of red cell transfusions; absolute neutrophil count (ANC) >1 × 109/L; platelet count >100 × 109/L. Refractory Disease (RD) was defined as a failure to achieve CR after one cycle of induction chemotherapy, less than a 50% reduction in blasts with >15% residual blasts and failure to achieve CR after two courses of induction chemotherapy. Partial remission (PR) was defined as a failure to achieve CR after one cycle of induction chemotherapy having >5% but <15% residual blasts but achieved CR after two courses of induction chemotherapy. 9 , 10
3. THERAPY
Chemotherapy was based on AML17 Pediatric version adopted from the AML17 Trial protocol of Medical Research Council, United Kingdome. ADE chemotherapy was the main treatment protocol used during the initial 3 years of the study period. After analysis of treatment outcome with ADE, input from haematologists treating adult AML without Etoposide and literature review, AD chemotherapy was adopted for pediatric AML. There were no preferences for ADE or AD group before starting treatment. Both groups were similar in terms of white blood cells (WBC), cytogenetic risk, malnutrition etc.
3.1. Induction therapy
Two courses of Anthracycline‐based chemotherapy either ADE (Daunorubicin 50 mg/m2 daily by one‐hour intravenous infusion [on days 1, 3 and 5] three doses), Cytarabine 100 mg/m2 every 12 hours by an intravenous push on days 1 to 10 (20 doses) and Etoposide 100 mg/m2 daily by 4‐hour infusion on days 1 to 5 (five doses) or AD (Daunorubicin and Cytarabine only without Etoposide) were used as first induction chemotherapy course. Second induction chemotherapy was identical to the first course except Cytarabine was given for 8 days.
3.2. Consolidation therapy
Two courses of high dose Cytarabine based chemotherapy (HiDAC; Cytarabine 3000 mg/m2 over 4 hours, given every 12 hours on days 1, 3 and 5 (total six doses) were used as consolidation therapy. Patients showing a partial response after induction chemotherapy received FLA‐Ida (Fludarabine 30 mg/m2 daily on days 1‐5, Cytarabine 2000 mg/m2 daily on days 1‐5 and Idarubicin 10 mg/m2 by one‐hour intravenous infusion on days 4, 5 and 6) as consolidation therapy.
3.3. Haematopoietic stem cell transplantation
Patients with high‐risk disease, having HLA matched sibling donor available, underwent allogeneic HSCT. The conditioning regimen used consisted of Busulfan, Cyclophosphamide, and Melphalan (BuCyMel) with Methotrexate and Cyclosporine for graft‐vs‐host disease (GVHD) prophylaxis.
4. SUPPORTIVE CARE
All patients were hospitalized for the initiation of induction chemotherapy. Tumour lysis prophylaxis with hyper‐hydration and allopurinol was commenced 24 hours before the start of chemotherapy and continued for at least 4 days. Intake, output and electrolytes were monitored carefully.
All subsequent chemotherapy courses were given as inpatient. However, clinically stable patients were discharged after chemotherapy and admitted immediately in case of fever or any other problem. Patients not admitted in the hospital were reviewed at least twice weekly in outpatient clinics. No prophylactic antimicrobials and colony‐stimulating factors were used during the neutropenic period. However, all cases of febrile neutropenia were treated as an inpatient with broad‐spectrum intravenous antibiotics. Fever was defined as a single oral temperature of >38°C or two readings >37.5°C at least 2 hours apart. Neutropenia was defined as an ANC of <1000 cells/μL. Febrile patients with ANC < 1000 cells/μL, were treated with a combination of Piperacillin‐Tazobactam and Amikacin as per the hospital guidelines, based upon recommendations of microbiology and The Infectious Diseases Society of America (IDSA). Vancomycin or Teicoplanin was added if the central venous line infection was suspected. Piperacillin‐Tazobactam was swapped with Meropenem if fever continued after 48 hours. Anti‐fungal Amphotericin B was added empirically if fever continued beyond 96 hours. Blood and blood products transfusion was given on as and when required basis. Haemoglobin transfusion threshold was 8.0 g/dL. Thresholds for Platelet transfusion were 10 × 109 cells/L for asymptomatic and 20 × 109/L for febrile patients. TRM was defined as death occurring after the start of chemotherapy due to chemotoxicity, in the absence of progressive cancer.
5. STATISTICAL ANALYSIS
SPSS 23.0 software was used for statistical analysis. t‐test and chi‐square tests were used for comparison between continuous and categorical variables. Frequencies and percentages were calculated for categorical variables. The time from the date of morphological CR until progression of disease or relapse was defined as Event‐free survival (EFS). The time from the day of diagnosis to the day of last follow‐up or death was defined as overall survival (OS). Survival analyses were performed using the Kaplan‐Meier method and survival differences were compared with Log‐rank tests, and P‐value ≤.05 was defined as statistically significant. Multivariate analysis by logistic regression was performed with prognostic factors having P ≤ .05 in univariate analysis.
6. RESULTS
During the study period total, 272 new cases of paediatric AML were registered at the pediatric oncology department of CMH Rawalpindi. Forty cases of APL, six patients who died before starting treatment, two cases who left during treatment and five cases still on treatment at the time of analysis, were excluded from the study.
6.1. Patient characteristics
Data of 219 cases of De novo AML was analyzed. Only 32 (14.6%) patients were children of army personnel. There were 140 (63.9%) males and 79 (36.1%) females. Age at diagnosis ranged from 9 months to 15 years with the mean 6.30 ± 3.66 years. The mean duration of symptoms before reporting to the oncologist was 54.89 ± 59.62 days with a range from 1 to 425 days. The most common presenting symptom was pallor in 180 (82.2%) followed by fever in 170 (77.6%) and bruising/bleeding in 128 (58.4%). Physical examination revealed pallor in 180 (82.2%) and visceromegaly in 167 (76.2%) cases. Proptosis was seen in 32 (14.6%) cases. The mean WBC count was 53.28 ± 68.27 × 109/L and ranged from 1.1 to 408 × 109/L. Initial WBC of >50 × 109/L was seen in 74 (33.8%) patients. The mean haemoglobin was 7.66 ± 2.55 g/dL, and the mean platelets count was 58.41 ± 81.68 × 109/L. Central nervous system (CNS) disease was documented in 10 (4.4%) cases. The most common FAB subtype was M2 in 103 (47.0%), followed by M4 in 27 (12.3%) cases. Results of the genetic analysis were available in 139 (63.5%) cases and 81 (58.3%) of them had intermediate‐risk (normal cytogenetics), 42 (30.2%) favorable, 15 (10.8%) unfavorable abnormalities and 1 (0.7%) had Trisomy 21.
6.2. Prognostic factors and treatment outcome
ADE chemotherapy was given to 110 (50.2%) and AD to 109 (49.8%) cases. TRM was 42 (19.2%) in the first course of induction chemotherapy; 24 (21.8%) in ADE and 18 (16.5%) in AD group (P = .391). Initial response to treatment was assessed after recovery of blood counts, by performing BM aspiration and 130/177 (73.4%) cases achieved morphological CR whereas 24/177 (13.6%) cases had a PR and 23 (13.0%) cases had the RD. Four cases showing no response to the first course of chemotherapy were offered palliative care and 173 cases had the second course of chemotherapy. TRM was 12 (6.9%) in second chemotherapy. After two courses of induction chemotherapy; TRM was 54 (24.7%), CR was 144 (87.3%); 68 (86.1%) in ADE and 76 (88.4%) in AD group and RD was 21 (12.7%); 11 (13.9%) in ADE and 10 (11.6%) in AD group (P = .658). Six patients with RD did not receive any further chemotherapy and 155 received the third course of chemotherapy and 146 cases received the fourth and last course of chemotherapy. Only three cases underwent matched sibling BM transplant and all of them are alive without any complication. The commonest side effect of chemotherapy was neutropenic fever, seen in 209 (95.4%) and 141 (81.5%) cases in first and second induction chemotherapy courses.
In the present cohort overall, non‐relapse mortality (NRM) was 60 (27.4%) including 42/219 (19.2%), 12 /173 (6.9%) and 6/155 (3.9%) during first, second and third chemotherapy courses respectively. No patient died during the fourth course of chemotherapy. The major causes of NRM were neutropenic sepsis and bleeding. Seventy‐five (43.2%) cases had a refractory or relapsed disease and 70 (93.3%) of them also expired. After a median follow‐up of 40.96 ± 26.23 months, 5‐year OS and EFS the cohort was 89 (40.6%) and 84 (38.3%) respectively.
We also looked at various factors influencing the induction mortality, OS and EFS. Nutritional status and AML subtype had a statistically significant influence in induction mortality. See Table 1. WBC counts at presentation, nutritional status, cytogenetics, use of etoposide in induction chemotherapy, remission status after first chemotherapy and AML subtype had a statistically significant impact on treatment outcomes. OS was 47.6% in children having WBC count <50 × 109/L and decreased to 27.0% in children having WBC count >50 × 109/L (P = .006). DFS was 44.1% and 27.0% in children having WBC count <50 × 109/L and >50 × 109/L respectively (P = .011). OS was 45.8% in well‐nourished children and decreased to 38.0% in moderately malnourished and 26.3% in severely malnourished children (P = .005). OS was 66.7% in children having favorable cytogenetics, 39.5% in intermediate‐risk cytogenetics and 33.3% in unfavorable cytogenetics (P = .019). OS was 29.1% and 52.3% in cases having induction chemotherapy with and without etoposide, respectively (P = .042). OS was 59.2% in cases achieving CR and decreased to 21.7% in cases having RD (P = <.001). Similarly, DFS was 56.9% in cases achieving CR and decreased to 13.0% in RD cases (P = <.001). AML subtypes as per FAB classification also showed a significant difference in OS among various AML subtypes; OS was 49.5% in M2 and 17.6% in M0 (P = .005). Multivariate analysis was performed for the above variables and BM remission status after the first course of chemotherapy was found to be the most statistically significant predictor of OS and DFS. (Table 2 and Figures 1, 2, 3, 4).
TABLE 1.
Predictors of induction mortality in paediatric AML (n = 219)
| TRM: Number (%) | Alive: Number (%) | Total: Number (%) | P‐value | |
|---|---|---|---|---|
| Categorical variables | 54 (24.7) | 165 (75.3) | 219 (100) | |
| Age groups | .367 | |||
|
24 (27.9) | 62 (72.1) | 86 (100) | |
|
22 (25.6) | 64 (74.4) | 86 (100) | |
|
8 (17.0) | 39 (83.0) | 47 (100) | |
| Sex | .367 | |||
|
33 (23.6) | 107 (76.4) | 140 (100) | |
|
21 (26.6) | 58 (73.4) | 79 (100) | |
| Nutritional status | .007 | |||
|
26 (19.8) | 105 (80.2) | 131 (100) | |
|
11 (22.0) | 39 (78.0) | 50 (100) | |
|
17 (44.7) | 21 (55.3) | 38 (100) | |
| WBC count at presentation | .141 | |||
|
22 (29.7) | 52 (70.3) | 74 (100) | |
|
32 (22.1) | 113 (77.9) | 145 (100) | |
| Platelets count at presentation | .205 | |||
|
31 (22.5) | 107 (77.5) | 138 (100) | |
|
23 (28.4) | 58 (71.6) | 81 (100) | |
| Hemoglobulin at presentation | .160 | |||
|
12 (32.4) | 25 (67.6) | 37 (100) | |
|
42 (23.1) | 140 (76.9) | 182 (100) | |
| AML subtypes | <.001 | |||
|
8 (47.1) | 9 (52.9) | 17 (100) | |
|
3 (12.5) | 21 (87.5) | 24 (100) | |
|
17 (16.5) | 86 (83.5) | 103 (100) | |
|
5 (18.5) | 22 (81.5) | 27 (100) | |
|
4 (30.8) | 9 (69.2) | 13 (100) | |
|
2 (40.0) | 3 (60.0) | 5 (100) | |
|
1 (20) | 4 (80) | 5 (100) | |
|
14 (58.3) | 10 (41.7) | 24 (100) | |
| Etoposide use in induction chemotherapy | .145 | |||
|
31 (28.2) | 79 (71.8) | 110 (100) | |
|
23 (21.1) | 86 (78.9) | 109 (100) | |
Abbreviations: AML, acute myeloid leukaemia; TRM, treatment‐related mortality.
TABLE 2.
Results of statistical tests of association between OS and EFS and study variables in acute myeloid leukemia cases (n = 219)
| Variable | OS | EFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Log Rank | P | Value | 95% CI | Log Rank | P | |
| Age groups | ||||||||
| <5 years | 45.3% | 32.76‐50.83 | .497 | .780 | 43.0% | 29.66‐47.70 | .770 | .681 |
| From 5 to 10 years | 37.2% | 28.49‐46.97 | 33.7% | 24.51‐42.76 | ||||
| ≥ 10 years | 38.3% | 20.46‐39.91 | 38.3% | 18.28‐38.97 | ||||
| Sex | ||||||||
| Male | 40.7% | 30.74‐45.11 | .001 | .973 | 38.6% | 28.00‐42.18 | .003 | .959 |
| Female | 40.5% | 30.66‐50.51 | 38.0% | 27.43‐47.17 | ||||
| Reporting time to oncologist | ||||||||
| <1 month | 37.1% | 29.44‐46.30 | .996 | .608 | 36.2% | 27.17‐44.13 | .705 | .703 |
| 1‐3 months | 44.8% | 29.22‐45.76 | 41.4% | 25.57‐42.09 | ||||
| >3 months | 40.7% | 18.91‐45.51 | 37.0% | 14.55‐40.25 | ||||
| WBC count at Presentation | ||||||||
| >50 × 109/L | 27.0% | 20.16‐38.27 | 7.580 | .006 | 27.0% | 18.02‐36.68 | 6.546 | .011 |
| <50 × 109/L | 47.6% | 35.69‐49.73 | 44.1% | 32.20‐46.10 | ||||
| PLTs count at Presentation | ||||||||
| >20 × 109/L | 39.1% | 31.20‐46.17 | .154 | .695 | 36.2% | 28.61‐47.58 | .391 | .532 |
| <20 × 109/L | 43.2% | 31.08‐50.08 | 42.0% | 27.59‐42.45 | ||||
| Hb at presentation | ||||||||
| >7 g/dL | 21.6% | 13.09‐33.50 | 6.80 | .009 | 21.6% | 9.60‐30.00 | 6.062 | .014 |
| <7 g/dL | 44.5% | 36.29‐49.78 | 41.8% | 33.32‐46.66 | ||||
| Nutritional status | ||||||||
| No malnutrition | 45.8% | 37.76‐53.41 | 10.42 | .005 | 44.3% | 35.31‐51.01 | 9.47 | .009 |
| Moderate malnutrition | 38.0% | 21.91‐45.55 | 34.0% | 18.66‐41.67 | ||||
| Severe malnutrition | 26.3% | 11.26‐33.27 | 23.7% | 8.14‐29.49 | ||||
| Cytogenetics | ||||||||
| Favorable | 66.7% | 46.35‐71.80 | 7.939 | .019 | 66.7% | 45.90‐71.03 | 11.31 | .003 |
| Intermediate risk | 39.5% | 26.28‐42.61 | 37.0% | 22.31‐38.57 | ||||
| Unfavorable | 33.3% | 12.38‐49.74 | 26.7% | 6.40‐41.86 | ||||
| Treatment group | ||||||||
| ADE | 29.1% | 26.70‐41.73 | 4.117 | 0.042 | 28.2% | 22.92‐38.16 | 4.599 | .032 |
| AD | 52.3% | 32.12‐47.51 | 48.6% | 29.83‐44.34 | ||||
| Remission after first chemo | ||||||||
| CR | 59.2% | 48.99‐64.79 | 28.30 | .000 | 56.9% | 46.46‐62.20 | 56.593 | .000 |
| PR | 29.2% | 16.57‐36.12 | 29.2% | 11.38‐32.61 | ||||
| RD | 21.7% | 6.598‐23.72 | 13.0% | 0.450‐15.65 | ||||
| AML subtypes (FAB class) | ||||||||
| M0 | 17.6% | 5.95‐22.70 | 20.225 | .005 | 11.8% | 2.40‐14.80 | 20.55 | .004 |
| M1 | 41.7% | 23.42‐53.57 | 37.5% | 15.89‐46.02 | ||||
| M2 | 49.5% | 37.42‐54.20 | 48.5% | 36.09‐52.96 | ||||
| M4 | 40.7% | 19.78‐46.57 | 40.7% | 18.62‐47.64 | ||||
| M5 | 38.5% | 10.86‐38.85 | 30.8% | 5.04‐28.63 | ||||
| M6 | 40.0% | .000‐32.17 | 20.0% | .000‐25.69 | ||||
| M7 | 20.0% | 5.45‐16.54 | 20.0% | .000‐13.95 | ||||
| NOS | 20.8% | 4.44‐31.48 | 20.8% | 3.42‐33.21 | ||||
| AMLDS | 25.0% | .000‐23.60 | 25.0% | .000‐20.90 | ||||
Abbreviations: AD, Ara‐C and Daunorubicin; ADE, Ara‐C, Daunorubicin and Etoposide; AML, acute myeloid leukaemia; CR, Complete remission; EFS, Event‐free survival; FAB, French American‐British; OS, overall survival; PR, Partial remission; RD, Resistant disease.
FIGURE 1.
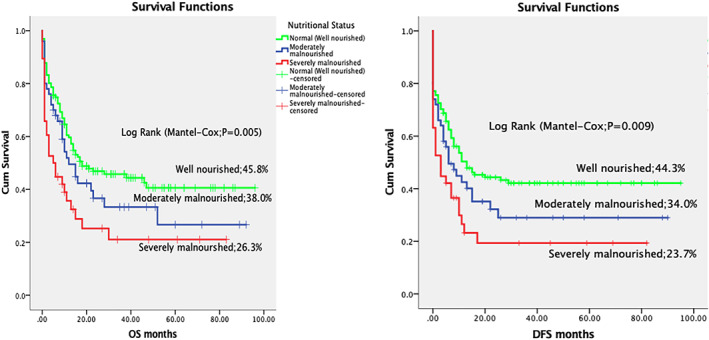
Overall and disease‐free survival in acute myeloid leukaemia, according to the nutritional status
FIGURE 2.
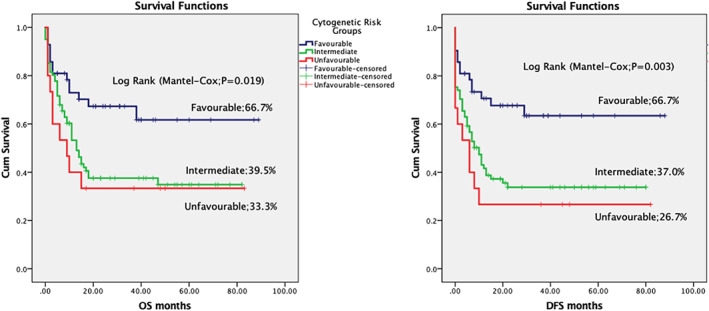
Overall and disease‐free survival in acute myeloid leukaemia, according to the cytogenetics
FIGURE 3.
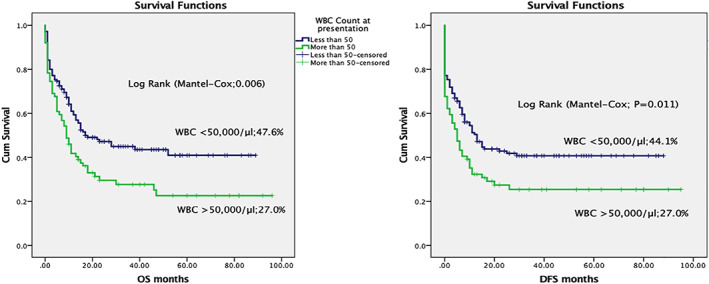
Overall and disease‐free survival in acute myeloid leukaemia, according to the WBC count at presentation
FIGURE 4.
Overall and disease‐free survival in acute myeloid leukaemia, according to the response to first chemotherapy course
7. DISCUSSION
Published data are scarce on childhood AML from Pakistan. The importance of the present study is that it is reporting on the impact of various clinical and laboratory variable affecting OS and EFS in the largest cohort of cases form the country. We investigated the relationship of several patients' clinical characteristics and disease features such as age, sex, nutritional status, reporting time to the oncologist, blood counts, AML subtypes and cytogenetics. There is no cancer registry in Pakistan, so the exact number of AML in the country is unknown, however, the incidence of newly diagnosed childhood AML cases in this hospital‐based study was 23% of acute leukaemia and 11.5% of total new cases. This incidence corresponds with the international reported incidence of 25% of pediatric leukaemia. 1
Higher WBC count at presentation is a poor prognostic factor and is associated with a lower CR rate and worse OS. 11 In the first induction chemotherapy, TRM was 17.2% and 23.0% and CR was 76.7% and 66.7% respectively (P = .337) in patients having WBC less than and more than 50 × 109/L. OS and DFS were statistically significantly better in patients with low WBC count. This finding of better CR, OS and DFS with low WBC at presentation is similar to the results of the Medical Research Council AML12 trial in children with AML. 11
In the present study, like other LMICs, TRM was quite high, especially in severely undernourished children. Induction mortality was 19.8% in well‐nourished children and increased to 44.7% in severely malnourished patients (P = .007). Infection alone or with associated bleeding and respiratory failure were the main causes of induction mortality. This association of infection‐related high mortality with malnutrition supports the findings reported by various previous studies. 12 , 13 Higher TRM in underweight patients has also been reported from developed counties. 14 This high mortality can be explained based on the fact that malnutrition worsens in cancer patients because of an increased metabolic rate due to disease and neutropenic fever. 15 Moreover, anorexia secondary to chemotherapy‐induced nausea and vomiting, and mucositis along with decreased availability of the food of choice in the hospital results in decreased oral intake. Malnutrition in Malawian patients with Wilms tumour and other LMICs has been managed with nutritional interventions. 16 Parenteral nutrition for paediatric patients was not available in our hospital. Though commercially available oral nutritional supplements were advised to children, but many of them were not very eager to take them. Malnutrition results in decreased complement, cytokines, and immunoglobulins, leading to impaired immune system functions. Correction of nutrition deficiencies, infusion of supplemental immunoglobulin and prophylactic antibiotics are recommended for undernourished children undergoing chemotherapy for AML. 14 OS and DFS were statistically significantly lower in malnourished patients. This was mainly due to increased TRM in malnourished children. However, relapse‐related mortality was found to be inversely proportionate to the degree of malnutrition. This may raise a hypothesis for future researches that malnutrition decreases the chances of relapse‐related deaths in paediatric AML patients.
A series of genetic changes in the haematopoietic precursor cells and specific cytogenetic abnormalities play a role in the pathogenesis of AML. The cytogenetic abnormalities have prognostic significance and affect treatment planning. AML can be stratified into favorable, intermediate, and adverse risk based on cytogenetic and molecular profiles. Certain chromosomal rearrangements, such as t(8;21), t(15;17), or inv 16 are associated with a more favorable prognosis, whereas the presence of other cytogenetic profiles, such as complex karyotypic changes, monosomy 5 and 7 are associated with less‐favorable prognosis. AML with normal cytogenetics typically constitutes an intermediate prognostic risk and accounts for approximately 50% of AML cases. 17 Cytogenetic abnormalities had prognostic significance and a statistically significant difference between different cytogenetic risk groups were documented in the present study. OS was 66.7%, 39.5% and 33.3% in favorable, intermediate‐risk and unfavorable cytogenetics (P = .019) and DFS was 66.7%, 37.0% and 26.7% in favorable, intermediate‐risk and unfavorable cytogenetics (P = .003).
In the present study, ADE and AD were compared to see any advantage of incorporating etoposide in the induction chemotherapy for pediatric AML. However, no advantage of etoposide was documented. There were more episodes of neutropenic fever in etoposide group. Induction mortality was higher, albeit not significantly, in the ADE group (28.2%) than the AD group (21.1%). Moreover, etoposide did not improve the remission rate. After first induction chemotherapy, the CR rate was 73.3% with etoposide and 73.6% without etoposide. Several other publications have reported no benefit of etoposide in AML treatment. 18 , 19 Relapse is a major challenge and is reported in 21% to 40% of cases of AML. 20 , 21 Use of etoposide in induction chemotherapy also did not provide any survival benefit. Rather it resulted in decreased OS and DFS. OS was 29.1% and 52.3% with and without etoposide (P = .042) and DFS was 28.2% and 48.6% with and without etoposide (P = .032). Though etoposide group had delayed relapse, the relapse rate was higher in the etoposide group. The relapse rate was 41.8% and 26.6% with and without etoposide (P = .023). Response to induction therapy is a major prognostic factor in AML and was clearly illustrated in the Medical Council Research (MRC) AML 10 trial (n = 1711; ages 0‐55 years). 22 , 23 In the present study, the response to the first course of chemotherapy was the most significant prognostic factor on univariate and multivariate analysis. We also investigated various other prognostic factors described in the literature. However, no statistically significant association of OS and EFS was found with age, sex, time‐lapse before the start of treatment, and platelets count at the time of presentation.
8. CONCLUSION
In this largest cohort of childhood AML from Pakistan, neutropenic sepsis and bleeding are the two major causes of TRM. High WBC count at presentation, poor nutritional status and use of etoposide during induction chemotherapy were associated with decreased OS and DFS rates. Response to induction chemotherapy was the most significant prognostic factor.
CONFLICT OF INTEREST
The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
AUTHORS' CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, T.G.; Formal Analysis, T.G., I.S.; Data Curation, T.G., S.K., T.F., S.A., I.S.; Writing—Original Draft, T.G.; Writing—Review & Editing, S.K., T.F., S.A., I.S.; Supervision, T.G.
ETHICAL STATEMENT
The study was approved by the ethics committee and was performed according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All procedures followed were according to the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. All persons gave their informed consent before their inclusion in the study. Details that might disclose the identity of the subjects under study were omitted. Informed consent was obtained from all patients for being included in the study.
ACKNOWLEDGEMENTS
Pediatric oncology physicians, pharmacists, medical teams, nurses and data manager at the Department of Pediatric Oncology, Combined Military Hospital, Rawalpindi, Pakistan.
Ghafoor T, Khalil S, Farah T, Ahmed S, Sharif I. Prognostic Factors in Childhood Acute Myeloid Leukemia; Experience from A Developing Country. Cancer Reports. 2020;3:e1259. 10.1002/cnr2.1259
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Taga T, Tomizawa D, Takahashi H, Adachi S. Acute myeloid leukemia in children: current status and future directions. Pediatr Int. 2016;58(2):71‐80. [DOI] [PubMed] [Google Scholar]
- 2. Wennström L, Edslev PW, Abrahamsson J, et al. Acute myeloid leukemia in adolescents and young adults treated in pediatric and adult Departments in the Nordic countries. Pediatr Blood Cancer. 2016;63(1):83‐92. [DOI] [PubMed] [Google Scholar]
- 3. Klein K, de Haas V, Kaspers GJL. Clinical challenges in de novo pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2018;18(3):277‐293. [DOI] [PubMed] [Google Scholar]
- 4. Lins MM, Mello MJG, Ribeiro RC, De Camargo B, de Fátima Pessoa Militão de Albuquerque M, Thuler LCS. Survival and risk factors for mortality in pediatric patients with acute myeloid leukemia in a single reference center in low‐middle‐income country. Ann Hematol. 2019;98(6):1403‐1411. [DOI] [PubMed] [Google Scholar]
- 5. Gupta S, Bonilla M, Valverde P, et al. Treatment‐related mortality in children with acute myeloid leukaemia in Central America: incidence, timing and predictors. Eur J Cancer. 2012;48(9):1363‐1369. [DOI] [PubMed] [Google Scholar]
- 6. Fadoo Z, Mushtaq N, Alvi S, Ali M. Acute myeloid leukaemia in children: experience at a tertiary care facility of Pakistan. J Pak Med Assoc. 2012;62(2):125‐128. [PubMed] [Google Scholar]
- 7. Asim M, Zaidi A, Ghafoor T, Qureshi Y. Death analysis of childhood acute lymphoblastic leukaemia; experience at Shaukat Khanum memorial cancer hospital and research centre. Pakistan J Pak Med Assoc. 2011;61(7):666‐670. [PubMed] [Google Scholar]
- 8. Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer. 2012;48(2):243‐252. [DOI] [PubMed] [Google Scholar]
- 9. Ravandi F. Primary refractory acute myeloid leukaemia ‐ in search of better definitions and therapies. Br J Haematol. 2011;155(4):413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferguson P, Hills RK, Grech A, et al. An operational definition of primary refractory acute myeloid leukemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica. 2016;101(11):1351‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibson BES, Webb DKH, Howman AJ, et al. Results of a randomized trial in children with acute myeloid Leukaemia: Medical Research Council AML12 trial. Br J Haematol. 2011;155(3):366‐376. [DOI] [PubMed] [Google Scholar]
- 12. Pribnow AK, Ortiz R, Báez LF, Mendieta L, Luna‐Fineman S. Effects of malnutrition on treatment‐related morbidity and survival of children with cancer in Nicaragua. Pediatr Blood Cancer. 2017;64(11):e26590. [DOI] [PubMed] [Google Scholar]
- 13. Barr RD, Gomez‐Almaguer D, Jaime‐Perez JC, Ruiz‐Argüelles GJ. Importance of nutrition in the treatment of leukemia in children and adolescents. Arch Med Res. 2016;47(8):585‐592. [DOI] [PubMed] [Google Scholar]
- 14. Inaba H, Surprise HC, Pounds S, et al. Effect of body mass index on the outcome of children with acute myeloid leukemia. Cancer. 2012;118(23):5989‐5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brinksma A, Huizinga G, Sulkers E, Kamps W, Roodbol P, Tissing W. Malnutrition in childhood cancer patients: a review on its prevalence and possible causes. Crit Rev Oncol Hematol. 2012;83(2):249‐275. [DOI] [PubMed] [Google Scholar]
- 16. Israëls T, Borgstein E, Jamali M, de Kraker J, Caron HN, Molyneux EM. Acute malnutrition is common in Malawian patients with a Wilms tumour: a role for peanut butter. Pediatr Blood Cancer. 2009;53(7):1221‐1226. [DOI] [PubMed] [Google Scholar]
- 17. De Kouchkovsky I, Abdul‐Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441–e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the Medical Research Council AML15 trial. J Clin Oncol. 2013;31(27):3360‐3368. [DOI] [PubMed] [Google Scholar]
- 19. Luger SM. How can one optimize induction therapy in AML? Best Pract Res Clin Haematol. 2017;30(4):301‐305. [DOI] [PubMed] [Google Scholar]
- 20. Alexander TB, Wang L, Inaba H, et al. Decreased relapsed rate and treatment‐related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer. 2017;123(19):3791‐3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubnitz JE, Inaba H. Childhood acute myeloid leukaemia. Br J Haematol. 2012;159(3):259‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radhi M, Meshinchi S, Gamis A. Prognostic factors in pediatric acute myeloid leukemia. Curr Hematol Malig Rep. 2010;5(4):200‐206. [DOI] [PubMed] [Google Scholar]
- 23. Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk‐directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's adult and childhood Leukaemia working parties. Br J Haematol. 1999;107(1):69‐79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


