Abstract
Background
Tyrosine‐kinase inhibitor (TKI) drugs have been considered first line treatment for metastatic renal cell cancer (RCC) for over a decade. TKI‐induced hypertension is a common adverse‐event in patients treated for metastatic RCC.
Aim
This study was aimed at investigating an association between TKI‐induced hypertension and treatment outcomes for metastatic RCC patients.
Methods and results
Retrospective data pertaining to patients with histologically or radiologically confirmed metastatic RCC treated with sunitinib, pazopanib, sorafenib, axitinib or cabozantinib between June 2012 and May 2019 were evaluated. Clinical information and serial blood pressure measurements were extracted from medical records for each patient. We compared objective response rate, progression‐free survival (PFS), and 12‐month survival between TKI‐induced hypertension (TIH) and non‐TIH groups using χ 2 and Mann‐Whitney tests.
Out of 72 patients screened, 52 met study eligibility criteria. The median age at diagnosis was 61 years (range: 42‐85 years) with a clear male predominance. The majority of patients had a history of nephrectomy with clear cell pathology. Almost all patients were on first‐line TKI therapy with sunitinib or pazopanib. Median follow‐up was 11 months. About half of patients developed TKI‐induced hypertension (grade 2‐3). In the TIH group 82% were commenced on an antihypertensive agent. Median PFS measured 30.5 weeks for TIH group compared to 22.2 weeks for non‐TIH (P = .05). The 6 month and 12 month survival rates for TIH were 82% and 56%, respectively, as compared to 76% and 44% for non‐TIH.
Conclusion
The occurrence of TKI‐induced hypertension was found to be a positive prognostic factor for progression in patients with metastatic RCC.
Keywords: hypertension, renal cancer, TKI
1. INTRODUCTION
Renal cell cancer (RCC) is one of the most common cancers worldwide that primarily affects men. It is estimated that the age‐standardized incidence rate of RCC in Australia will be 13 cases per 100 000 persons in 2019. 1 The most common histological subtype of RCC is clear cell carcinoma, which accounts for up to 80% of all RCCs. 2 Approximately one‐fourth of patients with RCC have metastatic disease at the time of initial diagnosis. 3 Further, a significant proportion of patients who were initially diagnosed with localized disease will eventually recur despite radical treatment.
Metastatic clear cell RCC is considered incurable and the estimated mortality rate in Australia is 3.5 per 100 000. 4 Antiangiogenic agents have been used in first‐line and usually subsequent‐line to treat metastatic RCC for almost a decade. Antiangiogenic drugs targeting vascular endothelial growth factor (VEGF) such as bevacizumab and oral tyrosine‐kinase inhibitors (TKIs) such as sunitinib, sorafenib, pazopanib, cabozantinib, lenvatinib, and axitinib are the standard United States Food and Drug Administration (FDA) approved and available treatments that have demonstrated improvement in PFS in randomized trials. 5 In recent years, immunotherapy has demonstrated significant clinical activity in metastatic RCC in both first‐line and second‐line setting . 6 Several combinational studies with immunotherapy and TKIs are currently underway with some encouraging data already available that could potentially change the future therapeutic landscape of metastatic RCC. 7
TKIs act by disrupting multiple signaling pathways and produce dual‐action antiangiogenic and antiproliferative effects. 8 Clinical trials have shown an objective response rate (ORR) of 30% to 40% to TKIs. 9 Unfortunately TKIs are associated with several adverse‐events, having a detrimental effect on quality of life, but do not benefit every patient. 10 TKIs related adverse‐events are due to the inhibition of VEGF receptors as well as off target interactions with other TKI receptors. 11 Hypertension is a common adverse event in patients with metastatic RCC who are treated with TKIs. Clinical trials of various TKIs have shown that the incidence of all‐grade hypertension ranged between 22% and 55%. 11
There are no known prospectively validated clinical or genomic factors that could predict which patients with metastatic clear cell RCC would benefit from TKIs.
Previous studies have indicated that the occurrence of drug‐induced adverse‐events may have a role as clinical biomarkers in patients with metastatic RCC that are treated with TKI therapy. 9 , 11 , 12 The purpose of the current study is to review medical records of patients with newly diagnosed metastatic RCC who were treated at a single institution with first‐line TKIs and examine for the presence (if any) of an association between TKI‐induced hypertension and treatment outcomes.
2. METHODS
The major inclusion criteria were histologically or radiologically confirmed metastatic RCC patients who were treated with sunitinib, pazopanib, sorafenib, axitinib or cabozantinib. Patients with essential hypertension were included only if their hypertension (with the use of an antihypertensive agent) at the time of commencement of TKI was < grade 1. Patients with baseline hypertension of > grade 1 and those with incomplete records were excluded. This analysis also includes patients who were initially diagnosed at our institution but followed up elsewhere. Every possible effort was made to obtain an update on such patients and those who were lost to follow‐up from their treating oncologist.
A retrospective analysis was performed from the medical records of 52 patients treated by the Medical Oncology Department of The Canberra Hospital, Australian Capital Territory (ACT), between June 2012 and May 2019. Our oncology department is the main tertiary referral center for the Australian Capital Territory and southern New South Wales servicing a population of over 500 000. This population is predominantly of white Australian or European ancestry. The project was approved by the ACT Health and Australian National University Human Research Ethics Committees.
The cases were identified from individual specialist's databases, electronic medical records, and the records of hospital pharmacies. The necessary data for this study were obtained by review of eligible patients' medical records. More specifically, the information gathered included date of initial diagnosis, histological subtype, history of nephrectomy, dates of commencement and cessation of TKI, reasons for discontinuing TKI, occurrence (and grade) of TKI related hypertension, commencement of antihypertensive if any, any other drug related toxicity, dose of TKI reduced if any, best RECIST defined treatment response, dates of disease progression and death (if applicable). Follow‐up data up to May 2019 were included.
Blood pressure of each patient in this study was measured prior to commencement of TKI therapy (baseline). Further blood pressure measurements were recorded on each monthly visit to the Cancer Center over a 6‐month period. Blood pressure readings were classified using National Heart Foundation of Australia guidelines. 13 The highest blood pressure recording was used to determine the grade of hypertension experienced.
The end points of this study were ORR, progression‐free survival (PFS) and 12‐month survival. ORR and PFS were assessed using CT scans (frequency being standard of care) and according to Response Evaluation Criteria in Solid Tumors, version 1.1. Each patient's best treatment response (complete or partial response, stable disease or progressive disease) was documented. PFS was defined as the interval between the commencement of TKI and first documentation of disease progression (assessed by treating physician) or death from any cause. Six and 12‐month survival data was also obtained. Routine standard of care safety evaluation was performed, and adverse‐event severity was assessed by the treating physician with the use of the National Cancer Institute Common Terminology Criteria for adverse‐events.
Above outcomes were assessed and compared in patients with TKI‐induced hypertension “TIH” (grade 2 and grade 3) and those who did not develop TKI‐induced hypertension “non‐TIH” (grade 0 and grade 1) using χ 2 and Mann‐Whitney test. A significance level of P ≤ .05 was used to assess statistical significance.
3. RESULTS
Of 72 patients that were treated with oral TKIs at our site from June 2012 to 20 March 2019 patients were excluded due to incomplete blood pressure records and 52 patients met the eligibility criteria for this study. The median follow‐up period was 11 months (range: 1 month to 5.6 years). No patient was lost to follow‐up, reflecting the centralized nature of the medical services in this region.
The results for the entire cohort of 52 patients are as below. The median age at diagnosis was 61 years (range: 42‐85 years) with a clear male predominance. The majority of patients had a history of nephrectomy (56%) with clear cell pathology (94%). Almost all patients were on first‐line TKI therapy with sunitinib or pazopanib. Seventeen patients had hypertension at baseline and were stable on antihypertensive therapy, of these, nine patients (53%) went on to develop TKI induced hypertension. Twenty‐seven patients (52%) developed TKI‐induced hypertension including 19 patients with grade 2 (70%) and eight patients with grade 3 hypertension (30%). Of those with TKI‐induced hypertension, 22 patients (82%) were commenced on an antihypertensive agent, and 7 (26%) had their TKI treatment held temporarily or dose reduced (due to hypertension).
The baseline characteristics of both the TIH and non‐TIH group are similar, except for the nephrectomy rate, as presented in Table 1. The nephrectomy rate was high at 70% in TIH as compared to 40% in non‐TIH. The rate of other adverse‐events such as diarrhea, mucositis, hand‐foot syndrome was similar between both groups. The ORR as well as stable disease rates in TIH group was high at 44% and 33%, respectively, when compared to 12% and 52% in non‐TIH group. The median PFS in TIH was 30.4 weeks compared to 22.2 weeks in non‐TIH (Figure 1). This difference between the two groups was statistically significant (P = 0.05).
TABLE 1.
Patient characteristics
| Patients with TKI‐induced hypertension (N = 27) | Patients without TKI‐induced hypertension (N = 25) | |
|---|---|---|
| Median age (years) | 61.6 | 60.8 |
| Male: female | 24:3 | 18:7 |
| History of nephrectomy | 19 (70%) | 10 (40%) |
| Histology | ||
| Clear cell | 25 (93%) | 24 (96%) |
| Other | 2 (7%) | 1 (4%) |
| Type of TKI | ||
| Sunitinib | 11 (41%) | 14 (56%) |
| Pazopanib | 14 (52%) | 11 (44%) |
| Sorafenib | 1 (4%) | 0 |
| Cabozantinib | 1 (4%) | 0 |
| Axitinib | 0 | 0 |
| Stable HTN at baseline | 9 (33%) | 8 (32%) |
| TKI‐induced hypertension | ||
| Grade 2 | 19 (70%) | NA |
| Grade 3 | 8 (30%) | NA |
| TKI dose reduced/stopped due to HTN | 7 (26%) | NA |
| Antihypertensive commenced | 22 (82%) | NA |
| Other adverse‐events | ||
| Diarrhea | 7 (26%) | 5 (20%) |
| Hand‐foot syndrome | 2 (7%) | 5 (20%) |
| Mucositis | 3 (11%) | 1 (4%) |
| Abnormal blood | 1 (4%) | 1 (4%) |
| Other | 6 (22%) | 3 (12%) |
| Treatment response | ||
| Partial | 12 (44%) | 3 (12%) |
| Stable | 9 (33%) | 13 (52%) |
| Progression | 4 (15%) | 7 (28%) |
| Median PFS (weeks) | 30.46 | 22.22 |
| 6‐month survival | 22 (82%) | 19 (76%) |
| 12‐month survival | 15 (56%) | 11 (44%) |
Abbreviations: HTN, hypertension, NA, not applicable, PFS, progression‐free survival, TKI, tyrosine‐kinase inhibitor.
FIGURE 1.
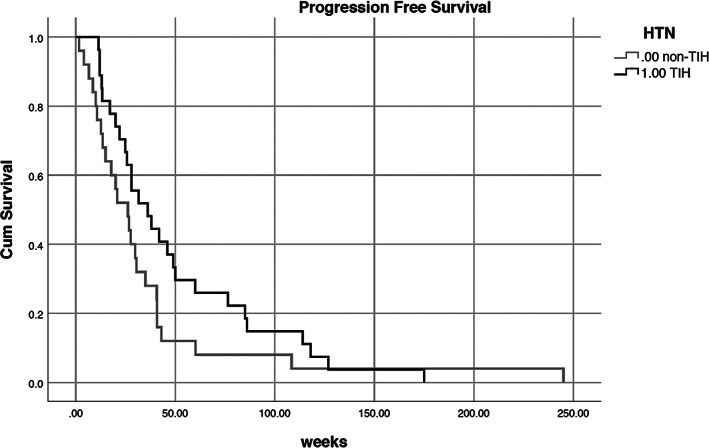
Progression‐free survival for TKI‐induced hypertension (TIH) and non‐TKI‐induced hypertension (non‐TIH) P = 0.05
In total, 19 patients died during study period; the cause of death was metastatic disease in the majority. The 6 month and 12 month survival rates for TIH were 81% and 55%, respectively, as compared to 76% and 44% (P = 0.62) (Figure 2).
FIGURE 2.
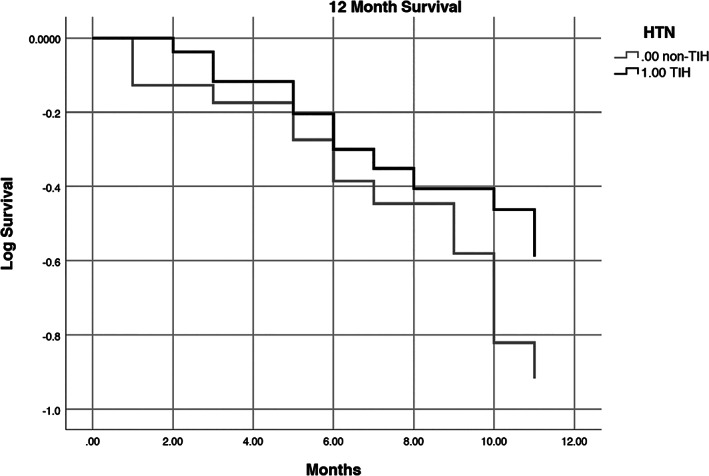
12‐month survival for TKI‐induced hypertension (TIH) and non‐TKI‐induced hypertension (non‐TIH) P = 0.62
4. DISCUSSION
TKI‐induced hypertension is considered to be caused by inhibition of VEGF that results in decreased nitric oxide and increased endothelin production thus leading to vasoconstriction. In this study, we have demonstrated potential association between TKI‐induced hypertension and patient outcomes for PFS and 6 month and 12 month survival. Consistent with previous studies, approximately half of the patients developed TKI‐induced hypertension. 9 , 14 Due to a large proportion of patients still alive at the time of data collection (63%), overall survival (OS) was not able to be evaluated. Six‐ and 12‐month survival, while improved for TKI‐induced hypertension, was not statistically significant, likely due to a small sample size.
A number of small studies have previously reported a correlation between the development of hypertension with TKIs and treatment efficacy. 9 TKI‐induced hypertension is an early phenomenon and normally develops within the first few weeks of commencement of TKIs. In all the published studies, hypertension was easily managed with the introduction of an antihypertensive agent and rarely required discontinuation of TKI. 9 , 14 The best pharmacological therapy for TKI‐induced hypertension is unclear and it is also unknown if it should be managed any differently from essential hypertension. There are no standard guidelines to assist with evaluation, diagnosis, monitoring, and management of TKI‐induced hypertension.
As patients with metastatic RCC in the modern era could expect survival of a few years, the long‐term systemic effects of hypertension are unknown. Although it is difficult to be certain of favorable signification of hypertension from TKI treatment, our study showed that the absence of hypertension might be predictive of unfavorable progression‐free and possibly OS.
In the last two decades of oncology drug development, the occurrence of some drug‐induced adverse‐events has been used as surrogate marker of a drug's clinical activity and predicting treatment outcomes. Clinical trials studying receptor tyrosine‐kinase EGFR‐inhibitors in patients with lung cancer indicated that skin rash could be used as a reliable clinical marker for predicting the treatment response. 15 It was also observed that patients with skin rash have a better PFS and OS. 15 Similarly predictive and prognostic value of skin rash has been observed with monoclonal antibodies against EGFR (such as cetuximab) in colorectal cancer. 16 Furthermore, improved clinical outcomes have been reported in patients with colorectal cancer who developed bevacizumab‐induced hypertension. 17 Despite extensive research and the exploration of a number of candidate biomarkers in metastatic RCC, unfortunately none has transitioned to routine usage in the clinic. The goal of current biomarker research is discovery of a reliable clinical or genomic biomarker that will be valuable in offering most appropriate treatment to an individual patient, while monitoring for early signs of treatment benefit in an efficient and effective manner.
Although this study makes several important contributions, our results must be evaluated within the context of the limitations of its small sample size, single institutional conduct and retrospective methodology. Nevertheless, these results and limitations should be seen as critical avenues for future research. Further research is required to see if prevalence of TKI‐induced hypertension is higher in any particular RCC risk group and evaluate its predictive and prognostic role in a large sample size. Future studies should also try to investigate if the outcomes vary depending upon the grade of hypertension.
5. CONCLUSION
Within our study, half of metastatic RCC patients developed TKI‐induced hypertension. These patients also experienced a statistically significant improvement in PFS. Due to small sample size, 6 month and 12 month survival was statistically insignificant between the two groups.
AUTHOR CONTRIBUTIONS
Gane Pranavan: Data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; writing‐original draft; writing‐review and editing.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ETHICS STATEMENT
Ethics approval for this research was obtained from ACT Health and Australian National University Human Research Ethics Committees. Patient consent waived. Anonymised data stored and analyzed on password‐protected computer, which were only accessible to the authors.
ACKNOWLEDGMENT
Authors would like to thank the staff at the Cancer Center, The Canberra Hospital for their assistance with this project.
Gadd M, Pranavan G, Malik L. Association between tyrosine‐kinase inhibitor induced hypertension and treatment outcomes in metastatic renal cancer. Cancer Reports. 2020;3:e1275. 10.1002/cnr2.1275
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Australian Institute of Health and Welfare . Cancer in Australia 2019. Canberra: AIHW; 2019. [Google Scholar]
- 2. Dunnick NR. Renal cell carcinoma: staging and surveillance. Abdo Radiol. 2016;41(6):1079‐1085. [DOI] [PubMed] [Google Scholar]
- 3. Janzen NKKH, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843‐852. [DOI] [PubMed] [Google Scholar]
- 4. Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med. 2017;376(4):354‐366. [DOI] [PubMed] [Google Scholar]
- 6. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med. 2015;373(19):1803‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rini BIPE, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380(12):1116‐1127. [DOI] [PubMed] [Google Scholar]
- 8. Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193‐205. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Zhou L, Chen Y, et al. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta‐analysis. BMC Urol. 2019;19(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Escudier B, Porta C, Bono P, et al. Randomized, controlled, double‐blind, cross‐over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J Clin Oncol. 2014;32(14):1412‐1418. [DOI] [PubMed] [Google Scholar]
- 11. Ravaud A, Schmidinger M. Clinical biomarkers of response in advanced renal cell carcinoma. Ann Oncol. 2013;24(12):2935‐2942. [DOI] [PubMed] [Google Scholar]
- 12. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Heart Foundation of Australia . Guideline for the diagnosis and management of hypertension in adults 2016. Melbourne: National Heart Foundation of Australia; 2016. [Google Scholar]
- 14. Nakai K, Fujii H, Kono K, Goto S, Nishi S. Hypertension induced by tyrosine‐kinase inhibitors for the treatment of renal cell carcinoma in hemodialysis patients: a single‐center experience and review of the literature. Ther Apher Dial. 2017;21(4):320‐325. [DOI] [PubMed] [Google Scholar]
- 15. Liu HB, Wu Y, Lv TF, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non‐small cell lung cancer: a systematic review and meta‐analysis. PLoS One. 2013;8(1):55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saltz LHH, Tchekmeydian NS. Acne‐like rash predicts response in patients treated with cetuximab (IMC‐C225) plus irinotecan (CPT‐11) in CPT‐11‐refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Proc NCI‐AACR‐EORTC. 2001;559. [Google Scholar]
- 17. Dionisio de Sousa IJ, Ferreira J, Rodrigues J, et al. Association between bevacizumab‐related hypertension and response to treatment in patients with metastatic colorectal cancer. ESMO Open. 2016;1(3):12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


